

Clinical Medicine Research
- © 2018
- Mieczyslaw Pokorski 0
Opole Medical School, Opole, Poland
You can also search for this editor in PubMed Google Scholar
- Advancing new ideas and knowledge
- Innovative and insightful clinical research
- Dissemination of best clinical practice
Part of the book series: Advances in Experimental Medicine and Biology (AEMB, volume 1116)
Part of the book sub series: Clinical and Experimental Biomedicine (CLEXBI)
11k Accesses
81 Citations
77 Altmetric
This is a preview of subscription content, log in via an institution to check access.
Access this book
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
About this book
This book presents an update on new trends and developments in broadly defined medical disciplines. The whole range of multidisciplinary topics are tackled, regarded as being important for advancing the understanding of disease pathogenicity, diagnostic methods, and patient management. The topics include a holistic approach to physiotherapy, with proprioceptive neuromuscular facilitation at the core of it, potential ways to protect kidneys during ischemic coronary interventions, and psychosocial aspects in cancer survivors. Other topics deal with growth hormone deficiency in short children and responses of molecular markers of bone metabolism to growth hormone replacement therapy and with the modern use of transcranial laser-induced photobiomodulation showing surprising benefits in autism disorder. The expert contributions take on the challenges presented to medical professionals by ever growing medical knowledge and various individual and contextual issues that require a multidisciplinary approach in patient management. The authors present a bench-to-bed clinical research to make useful additions to the knowledge on contemporary diagnostic procedures, therapy, and quality of life of patients. The book aims to provide stimulus for new research ideas and to give new perspectives on practical clinical issues. The book is intended for primary care clinicians, family physicians, medical scholars, and other clinicians who treat and manage patients.
Similar content being viewed by others

Photobiomodulation combination therapy as a new insight in neurological disorders: a comprehensive systematic review

State of the Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating Children with Cerebral Palsy
Neurologic complications of Down syndrome: a systematic review
- Bioprogressive treatment
- Cancer research
- Cognitive function
- Coronary disease
- Diagnostic markers
- Growth hormone
- Health care
- Occupational endotoxins
- Physiotherapy
Table of contents (12 chapters)
Front matter, bioprogressive paradigm in physiotherapeutic and antiaging strategies: a review.
- Mieczyslaw Pokorski, Giovanni Barassi, Rosa G. Bellomo, Loris Prosperi, Matteo Crudeli, Raoul Saggini
Influence of Proprioceptive Neuromuscular Facilitation on Lung Function in Patients After Coronary Artery Bypass Graft Surgery
- Małgorzata Bujar-Misztal, Andrzej Chciałowski
Remote Ischemic Preconditioning in Renal Protection During Elective Percutaneous Coronary Intervention
- Małgorzata Wojciechowska, Maciej Zarębiński, Piotr Pawluczuk, Dagmara Gralak-Łachowska, Leszek Pawłowski, Wioletta Loska et al.
Prognostic Impact of Extracapsular Lymph Node Invasion on Survival in Non-small-Cell Lung Cancer: A Systematic Review and Meta-analysis
- Seyed Vahid Tabatabaei, Christoph Nitche, Maximilian Michel, Kurt Rasche, Khosro Hekmat
Influence of Transurethral Resection of Bladder Cancer on Sexual Function, Anxiety, and Depression
- Wojciech Krajewski, Urszula Halska, Sławomir Poletajew, Radosław Piszczek, Bartosz Bieżyński, Mateusz Matyjasek et al.
Cognitive Predictors of Cortical Thickness in Healthy Aging
- Patrycja Naumczyk, Angelika K. Sawicka, Beata Brzeska, Agnieszka Sabisz, Krzysztof Jodzio, Marek Radkowski et al.
Osteoprotegerin, Receptor Activator of Nuclear Factor Kappa B Ligand, and Growth Hormone/Insulin-Like Growth Factor-1 Axis in Children with Growth Hormone Deficiency
- Ewelina Witkowska-Sędek, Małgorzata Rumińska, Anna Stelmaszczyk-Emmel, Maria Sobol, Urszula Demkow, Beata Pyrżak
Inhibition of Cross-Reactive Carbohydrate Determinants in Allergy Diagnostics
- Maciej Grzywnowicz, Emilia Majsiak, Józef Gaweł, Karolina Miśkiewicz, Zbigniew Doniec, Ryszard Kurzawa
Effects of Glutathione on Hydrolytic Enzyme Activity in the Mouse Hepatocytes
- Iwona Stanisławska, Bożena Witek, Marek Łyp, Danuta Rochon-Szmejchel, Adam Wróbel, Wojciech Fronczyk et al.
Adaptation to Occupational Exposure to Moderate Endotoxin Concentrations: A Study in Sewage Treatment Plants in Germany
- M. A. Rieger, V. Liebers, M. Nübling, T. Brüning, B. Brendel, F. Hoffmeyer et al.
Effects of Low-Level Laser Therapy in Autism Spectrum Disorder
- Gerry Leisman, Calixto Machado, Yanin Machado, Mauricio Chinchilla-Acosta
Epidemiology of Granulomatosis with Polyangiitis in Poland, 2011–2015
- Krzysztof Kanecki, Aneta Nitsch-Osuch, Paweł Gorynski, Patryk Tarka, Magdalena Bogdan, Piotr Tyszko
Editors and Affiliations
Mieczyslaw Pokorski
About the editor
Bibliographic information.
Book Title : Clinical Medicine Research
Editors : Mieczyslaw Pokorski
Series Title : Advances in Experimental Medicine and Biology
DOI : https://doi.org/10.1007/978-3-030-04837-2
Publisher : Springer Cham
eBook Packages : Biomedical and Life Sciences , Biomedical and Life Sciences (R0)
Copyright Information : The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG 2018
Hardcover ISBN : 978-3-030-04836-5 Published: 04 December 2018
eBook ISBN : 978-3-030-04837-2 Published: 23 November 2018
Series ISSN : 0065-2598
Series E-ISSN : 2214-8019
Edition Number : 1
Number of Pages : VI, 138
Number of Illustrations : 13 b/w illustrations, 6 illustrations in colour
Topics : Neurochemistry , Cancer Research , Physiotherapy , Cytokines and Growth Factors
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Clinical trials articles from across Nature Portfolio
A clinical trial involves the study of the safety, efficacy and/or dosage regimen of a therapeutic intervention (such as a drug) in humans selected according to predetermined criteria of eligibility (such as a defined severity of a specific disease), who are observed for predefined evidence of favourable and unfavourable effects.
Related Subjects
- Adaptive clinical trial
- Biostatistics
- Phase 0 trials
- Phase I trials
- Phase II trials
- Phase III trials
- Phase IV trials
Latest Research and Reviews
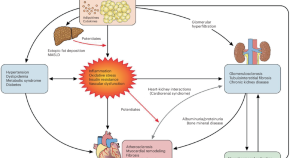
Integrating liver endpoints in clinical trials of cardiovascular and kidney disease
This Perspective calls for inclusion of patients with MASLD and measurement of liver outcomes in cardio–kidney–metabolic trials, when data suggest mechanistically plausible benefits and clinical safety—and outlines considerations for trial design and regulatory approval.
- Faiez Zannad
- Arun J. Sanyal
- Stephen A. Harrison
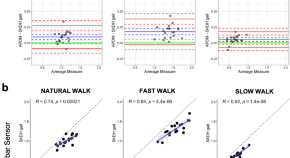
In-Clinic and Natural Gait Observations master protocol (I-CAN-GO) to validate gait using a lumbar accelerometer
- Miles Welbourn
- Paul Sheriff
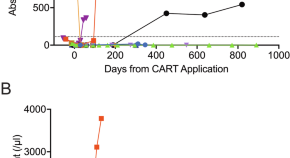
Third-generation anti-CD19 CAR T cells for relapsed/refractory chronic lymphocytic leukemia: a phase 1/2 study
- Patrick Derigs
- Maria-Luisa Schubert
- Michael Schmitt
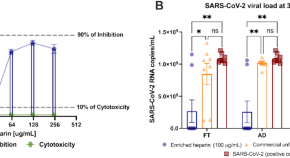
Nebulized enriched heparin improves respiratory parameters in patients with COVID-19: a phase I/II randomized and triple-blind clinical trial
- Vinicius Tadeu Ramos da Silva Grillo
- Matheus Bertanha
- Carlos Magno Castelo Branco Fortaleza
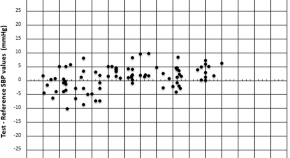
Validation of combiomed hipermax-BF model A7101 automatic oscillometric upper-arm sphygmomanometer in general population: AAMI/ESH/ISO universal standard (ISO 81060-2:2018/Amd 1:2020)
- Damaris Hernández Véliz
- Yamilé Valdés González
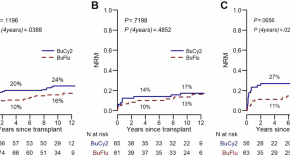
Busulfan-fludarabine versus busulfan-cyclophosphamide for allogeneic transplant in acute myeloid leukemia: long term analysis of GITMO AML-R2 trial
- Gianluca Cavallaro
- Anna Grassi
- Alessandro Rambaldi
News and Comment
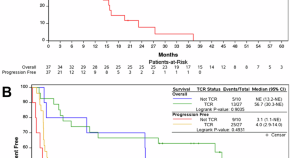
Phase II trial of elotuzumab with pomalidomide and dexamethasone for daratumumab-refractory multiple myeloma
- Ricardo D. Parrondo
- Betsy R. LaPlant
- Sikander Ailawadhi
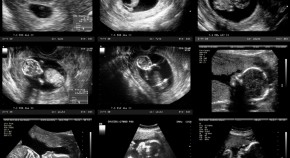
Simplifying obstetric sonography with AI
Researchers developed an AI-enabled, battery-operated tool that can be operated by clinicians with no sonography experience — and that measures gestational age as accurately as high-specification ultrasound.
- Karen O’Leary

Decentralized trial reveals home-testing behaviors for respiratory infections
In a digital-only study, people were more likely to take a home test if prompted to do so on the basis of self-reported symptoms rather than by wearable sensor data, with implications for public health responses and pandemic planning.
IL-23 inhibitors go head to head in Crohn’s disease
Risankizumab outperformed ustekinumab for the treatment of moderate to severe Crohn’s disease in patients refractory to anti-TNF therapy.

HIV prevention through PrEP in cisgender women
A large randomized controlled trial with cisgender women highlights the challenges of adherence to a daily oral preexposure prophylaxis regimen and demonstrates that twice-yearly injection of lenacapavir can maintain effective HIV prevention levels over 6 months.
- Sonia Muliyil
Risankizumab versus ustekinumab for Crohn’s disease: a phase IIIb study
- Eleni Kotsiliti
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Journals | Policy | Permission Journal of Clinical Medicine Research
INFORMATION
- For Authors
- For Reviewers
- For Editors
- For Readers
- Admin-login
| MOST READ |
(For the last 6-month published articles)

Original Article Review Case Report Short Communication Letter to the Editor Editorial
| INDEXED IN |
|
|
|
|
| |
| QUICK LINKS | |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
| BROWSE JOURNALS | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

| ARTICLE STATISTICS |
| Submission to First Decision |
| 23 Days |
|
|
| 26% |
|
|
| 19 Days |
| Average article statistics from the last 12 months data |
- Online-First
Journal of Clinical Medicine Research
|
|
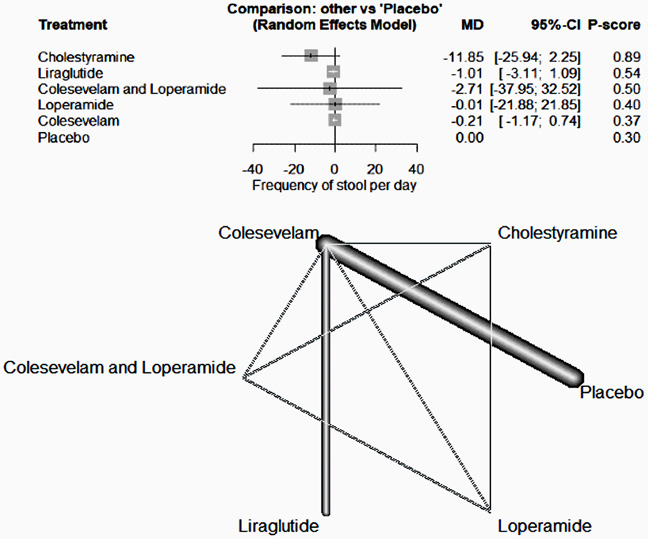
Bile acid malabsorption (BAM) is characterized by chronic watery diarrhea resulting from excessive bile acids in the feces. BAM is often an overlooked cause of chronic diarrhea, with its prevalence not being sufficiently researched.
The field of kidney transplantation is being revolutionized by the integration of artificial intelligence (AI) and machine learning (ML) techniques. AI equips machines with human-like cognitive abilities, while ML enables computers to learn from data.
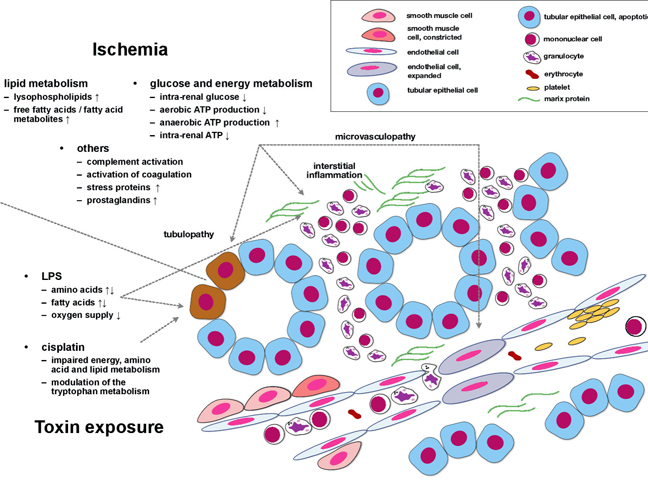
Acute kidney injury (AKI) affects increasing numbers of in-hospital patients in Central Europe and the USA, the prognosis remains poor. Although substantial progress has been achieved in the identification of molecular/cellular processes that induce and perpetuate AKI, more integrated pathophysiological perspectives are missing.

Subjects with mild cognitive impairment (MCI) can progress to dementia. Studies have shown that neuropsychological tests, biological or radiological markers individually or in combination have helped to determine the risk of conversion from MCI to dementia.
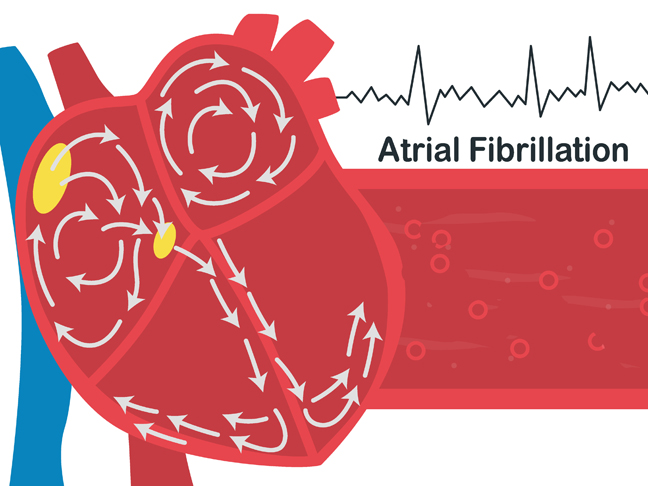
Atrial fibrillation (AF) is the most common arrhythmia with a growing prevalence worldwide, especially in the elderly population. Patients with AF are at higher risk of serious life-threatening events and complications that may lead to long-term sequelae and reduce quality of life.
Viewpoints | � | � | Featured | � |
| Acute kidney injury (AKI) affects up to 30% of all hospitalized patients in Central Europe and the USA. New biomarker molecules have been identified in recent years; most studies performed so far however aimed to identify markers for diagnostic purposes. | | |||
Key Clinical Image |
| |
Current Issue | � |
Vol. 16, No. 6, Jun 2024
Table of contents.
| Marta Maci , Carlotta Fanelli, Mauro Lorusso, Donatella Ferrara, Marino Caroprese, Michele Laurenziello, Michele Tepedino, Domenico Ciavarella | 273-283 |
| doi: https://doi.org/10.14740/jocmr5202 |
Original Article
| Sengottaian Sivakumar, Roni Mendonca, Michael Girshin | 284-292 |
| doi: https://doi.org/10.14740/jocmr5159 |
| Chiara Rosato, Marilena Greco, Giovanni Marciante, Roberta Assunta Lazzari, Floriano Indino, Giambattista Lobreglio | 293-301 |
| doi: https://doi.org/10.14740/jocmr5070 |
| Charlotte Mund, Katharina Asmus, Wajima Safi, Oliver Ritter, Dominique Petrus, Susann Patschan, Daniel Patschan | 302-309 |
| doi: https://doi.org/10.14740/jocmr5190 |
| Anas Elgenidy, Mohammed Al-Mahdi Al-Kurdi, Hoda Atef Abdelsattar Ibrahim , Eman F. Gad, Ahmed K. Awad, Rebecca Caruana, Sheriseane Diacono, Aya Sherif, Tasneem Elattar, Islam E. Al-Ghanam, Asmaa M. Eldmaty, Tareq M. Abubasheer, Ahmed M. Afifi, Amira Elhoufey, Hamad Ghaleb Dailah, Amira M. Osman, Mohamed Ezzat, Doaa Ali Gamal, Rady Elmonier, Ahmed El-Sayed Hammour, Maged T. Abougabal, Khaled Saad | 310-318 |
| doi: https://doi.org/10.14740/jocmr5205 |
Case Report
| Ashley Smith, Sidhant Kalsotra, Joseph D. Tobias | 319-323 |
| doi: https://doi.org/10.14740/jocmr5175 |
Information
InitiativesYou are accessing a machine-readable page. In order to be human-readable, please install an RSS reader. All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess . Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers. Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal. Original Submission Date Received: .
 Journal DescriptionJournal of clinical medicine.
Latest Articles Graphical abstract  Journal Menu
Special Issues
Conferences
Journal Browser
Highly Accessed ArticlesLatest books, e-mail alert.  Topical CollectionsFurther information, mdpi initiatives, follow mdpi.  Subscribe to receive issue release notifications and newsletters from MDPI journals  Clinical Medicine & ResearchClinical Medicine & Research is a peer-reviewed journal of original medical researc h relevant to a broad audience of medical researchers and health care professionals. Clinical Medicine & Research is owned and published by Marshfield Clinic Health System, and is edited within Marshfield Clinic Research Institute. Current Indexing and Archiving US National Library of Medicine's Index Medicus /MEDLINE (PubMed) database, EMBASE, Scopus, EBSCOhost EJS, Chemical Abstracts Service (CAS), Cumulative Index of Nursing and Allied Health Literature (CINAHL) database, Emerging Sources Citation Index, Index Copernicus, PubMed Central, and PubMed Central International. Impact Clinical Medicine & Research has an H index of 62 (2023), an SJR of 0.334 (2023) with a 2-year Cites per Doc of 1.017 (2023). Please visit Scimago's page for Clinical Medicine & Research to learn more about the definition of these journal measures of scientific influence. Ethics Statement Clinical Medicine & Research follows the International Committee of Medical Journal Editors' (ICMJE) Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals , the Council of Science Editors' (CSE) White Paper on Promoting Integrity in Scientific Journal Publications , and the Committee on Publication Ethics' (COPE) Core Practices for Promoting Integrity in Research and Its Publication guidance for editors, reviewers, and authors. Data Privacy Policy The Clinical Medicine & Research data privacy policy for authors and reviewers is available to view/download here: Data Privacy Policy .  Access Full-text articles are available from the Clinical Medicine & Research web site , and the journal's PubMed Central digital archive. Manuscript submission Articles must be submitted using the journal's online manuscript submission system, Editorial Manager . Editorial Office Marshfield Clinic Research Institute 1000 North Oak Avenue (ML9) Marshfield, WI 54449 Email: [email protected] Editor-in-Chief Adedayo A. Onitilo, MD, PhD, MSCR, FACP Marshfield Clinic Weston, WI USA Senior Editor Sherry A. Salzman Scott, MS Marshfield Clinic Research Institute Marshfield, Wisconsin USA Associate Editors Jamiu O. Busari, MD, PhD, MHPE Zuyderland Medical Center Heerleen, The Netherlands Rohit Sharma, MD, FACS Marshfield Clinic Marshfield, Wisconsin USA Board Members Kelley P. Anderson, MD Marshfield Clinic Marshfield, Wisconsin USA Erin G. Brooks, MD University of Wisconsin School of Medicine & Public Health Madison, Wisconsin USA Deanna S. Cross, PhD University of North Texas Health Sciences Center Fort Worth, Texas USA Suhail A.R. Doi, MBBS, FRCP, PhD Qatar University Doha, Qatar Jessica M. Engel, DNP, RN Marshfield Clinic Cancer Services Stevens Point, Wisconsin USA David D. Gutterman, MD Medical College of Wisconsin Milwaukee, Wisconsin USA Abel N. Kho, MD, MSc Northwestern University Chicago, Illinois USA John Lazarchick, MD Medical University of South Carolina Charleston, South Carolina USA Michael S. Pulia, MD, FACEP, FAAEM University of Wisconsin School of Medicine & Public Health Madison, Wisconsin USA Marc H. Scheetz, PharmD, MSc, BCPS AQ-ID Northwestern Memorial Hospital Chicago, Illinois USA Kit N. Simpson, DrPH Medical University of South Carolina Charleston, South Carolina USA Editor Emeriti Richard Dart, MD (Chief Editor, 2003-2006) Marshfield Clinic Marshfield, WI USA Kurt D. Reed, MD, MGIS (Chief Editor, 2007-2014) University of Wisconsin School of Medicine & Public Health Madison, WI USA

You are hereNih clinical research trials and you. The NIH Clinical Trials and You website is a resource for people who want to learn more about clinical trials. By expanding the below questions, you can read answers to common questions about taking part in a clinical trial. What are clinical trials and why do people participate?Clinical research is medical research that involves people like you. When you volunteer to take part in clinical research, you help doctors and researchers learn more about disease and improve health care for people in the future. Clinical research includes all research that involves people. Types of clinical research include: 
What are clinical trials and why would I want to take part?Clinical trials are part of clinical research and at the heart of all medical advances. Clinical trials look at new ways to prevent, detect, or treat disease. Clinical trials can study:
The goal of clinical trials is to determine if these treatment, prevention, and behavior approaches are safe and effective. People take part in clinical trials for many reasons. Healthy volunteers say they take part to help others and to contribute to moving science forward. People with an illness or disease also take part to help others, but also to possibly receive the newest treatment and to have added (or extra) care and attention from the clinical trial staff. Clinical trials offer hope for many people and a chance to help researchers find better treatments for others in the future Why is diversity and inclusion important in clinical trials?People may experience the same disease differently. It’s essential that clinical trials include people with a variety of lived experiences and living conditions, as well as characteristics like race and ethnicity, age, sex, and sexual orientation, so that all communities benefit from scientific advances. See Diversity & Inclusion in Clinical Trials for more information.  How does the research process work?The idea for a clinical trial often starts in the lab. After researchers test new treatments or procedures in the lab and in animals, the most promising treatments are moved into clinical trials. As new treatments move through a series of steps called phases, more information is gained about the treatment, its risks, and its effectiveness. What are clinical trial protocols?Clinical trials follow a plan known as a protocol. The protocol is carefully designed to balance the potential benefits and risks to participants, and answer specific research questions. A protocol describes the following:
A clinical trial is led by a principal investigator (PI). Members of the research team regularly monitor the participants’ health to determine the study’s safety and effectiveness. What is an Institutional Review Board?Most, but not all, clinical trials in the United States are approved and monitored by an Institutional Review Board (IRB) to ensure that the risks are reduced and are outweighed by potential benefits. IRBs are committees that are responsible for reviewing research in order to protect the rights and safety of people who take part in research, both before the research starts and as it proceeds. You should ask the sponsor or research coordinator whether the research you are thinking about joining was reviewed by an IRB. What is a clinical trial sponsor?Clinical trial sponsors may be people, institutions, companies, government agencies, or other organizations that are responsible for initiating, managing or financing the clinical trial, but do not conduct the research. What is informed consent?Informed consent is the process of providing you with key information about a research study before you decide whether to accept the offer to take part. The process of informed consent continues throughout the study. To help you decide whether to take part, members of the research team explain the details of the study. If you do not understand English, a translator or interpreter may be provided. The research team provides an informed consent document that includes details about the study, such as its purpose, how long it’s expected to last, tests or procedures that will be done as part of the research, and who to contact for further information. The informed consent document also explains risks and potential benefits. You can then decide whether to sign the document. Taking part in a clinical trial is voluntary and you can leave the study at any time. What are the types of clinical trials?There are different types of clinical trials. 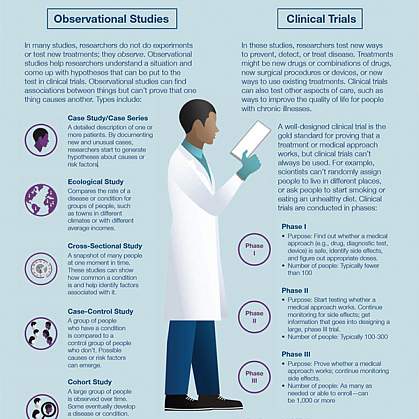
What are the phases of clinical trials?Clinical trials are conducted in a series of steps called “phases.” Each phase has a different purpose and helps researchers answer different questions.
What do the terms placebo, randomization, and blinded mean in clinical trials?In clinical trials that compare a new product or therapy with another that already exists, researchers try to determine if the new one is as good, or better than, the existing one. In some studies, you may be assigned to receive a placebo (an inactive product that resembles the test product, but without its treatment value). Comparing a new product with a placebo can be the fastest and most reliable way to show the new product’s effectiveness. However, placebos are not used if you would be put at risk — particularly in the study of treatments for serious illnesses — by not having effective therapy. You will be told if placebos are used in the study before entering a trial. Randomization is the process by which treatments are assigned to participants by chance rather than by choice. This is done to avoid any bias in assigning volunteers to get one treatment or another. The effects of each treatment are compared at specific points during a trial. If one treatment is found superior, the trial is stopped so that the most volunteers receive the more beneficial treatment. This video helps explain randomization for all clinical trials . " Blinded " (or " masked ") studies are designed to prevent members of the research team and study participants from influencing the results. Blinding allows the collection of scientifically accurate data. In single-blind (" single-masked ") studies, you are not told what is being given, but the research team knows. In a double-blind study, neither you nor the research team are told what you are given; only the pharmacist knows. Members of the research team are not told which participants are receiving which treatment, in order to reduce bias. If medically necessary, however, it is always possible to find out which treatment you are receiving. Who takes part in clinical trials?Many different types of people take part in clinical trials. Some are healthy, while others may have illnesses. Research procedures with healthy volunteers are designed to develop new knowledge, not to provide direct benefit to those taking part. Healthy volunteers have always played an important role in research. Healthy volunteers are needed for several reasons. When developing a new technique, such as a blood test or imaging device, healthy volunteers help define the limits of "normal." These volunteers are the baseline against which patient groups are compared and are often matched to patients on factors such as age, gender, or family relationship. They receive the same tests, procedures, or drugs the patient group receives. Researchers learn about the disease process by comparing the patient group to the healthy volunteers. Factors like how much of your time is needed, discomfort you may feel, or risk involved depends on the trial. While some require minimal amounts of time and effort, other studies may require a major commitment of your time and effort, and may involve some discomfort. The research procedure(s) may also carry some risk. The informed consent process for healthy volunteers includes a detailed discussion of the study's procedures and tests and their risks. A patient volunteer has a known health problem and takes part in research to better understand, diagnose, or treat that disease or condition. Research with a patient volunteer helps develop new knowledge. Depending on the stage of knowledge about the disease or condition, these procedures may or may not benefit the study participants. Patients may volunteer for studies similar to those in which healthy volunteers take part. These studies involve drugs, devices, or treatments designed to prevent,or treat disease. Although these studies may provide direct benefit to patient volunteers, the main aim is to prove, by scientific means, the effects and limitations of the experimental treatment. Therefore, some patient groups may serve as a baseline for comparison by not taking the test drug, or by receiving test doses of the drug large enough only to show that it is present, but not at a level that can treat the condition. Researchers follow clinical trials guidelines when deciding who can participate, in a study. These guidelines are called Inclusion/Exclusion Criteria . Factors that allow you to take part in a clinical trial are called "inclusion criteria." Those that exclude or prevent participation are "exclusion criteria." These criteria are based on factors such as age, gender, the type and stage of a disease, treatment history, and other medical conditions. Before joining a clinical trial, you must provide information that allows the research team to determine whether or not you can take part in the study safely. Some research studies seek participants with illnesses or conditions to be studied in the clinical trial, while others need healthy volunteers. Inclusion and exclusion criteria are not used to reject people personally. Instead, the criteria are used to identify appropriate participants and keep them safe, and to help ensure that researchers can find new information they need. What do I need to know if I am thinking about taking part in a clinical trial? Risks and potential benefitsClinical trials may involve risk, as can routine medical care and the activities of daily living. When weighing the risks of research, you can think about these important factors:
Most clinical trials pose the risk of minor discomfort, which lasts only a short time. However, some study participants experience complications that require medical attention. In rare cases, participants have been seriously injured or have died of complications resulting from their participation in trials of experimental treatments. The specific risks associated with a research protocol are described in detail in the informed consent document, which participants are asked to consider and sign before participating in research. Also, a member of the research team will explain the study and answer any questions about the study. Before deciding to participate, carefully consider risks and possible benefits. Potential benefitsWell-designed and well-executed clinical trials provide the best approach for you to:
Risks to taking part in clinical trials include the following:
What questions should I ask if offered a clinical trial?If you are thinking about taking part in a clinical trial, you should feel free to ask any questions or bring up any issues concerning the trial at any time. The following suggestions may give you some ideas as you think about your own questions.
Risks and possible benefits
Participation and care
Personal issues
Cost issues
Tips for asking your doctor about trials
This information courtesy of Cancer.gov. How is my safety protected? Ethical guidelinesThe goal of clinical research is to develop knowledge that improves human health or increases understanding of human biology. People who take part in clinical research make it possible for this to occur. The path to finding out if a new drug is safe or effective is to test it on patients in clinical trials. The purpose of ethical guidelines is both to protect patients and healthy volunteers, and to preserve the integrity of the science. Informed consentInformed consent is the process of learning the key facts about a clinical trial before deciding whether to participate. The process of providing information to participants continues throughout the study. To help you decide whether to take part, members of the research team explain the study. The research team provides an informed consent document, which includes such details about the study as its purpose, duration, required procedures, and who to contact for various purposes. The informed consent document also explains risks and potential benefits. If you decide to enroll in the trial, you will need to sign the informed consent document. You are free to withdraw from the study at any time. Most, but not all, clinical trials in the United States are approved and monitored by an Institutional Review Board (IRB) to ensure that the risks are minimal when compared with potential benefits. An IRB is an independent committee that consists of physicians, statisticians, and members of the community who ensure that clinical trials are ethical and that the rights of participants are protected. You should ask the sponsor or research coordinator whether the research you are considering participating in was reviewed by an IRB. Further readingFor more information about research protections, see:
For more information on participants’ privacy and confidentiality, see:
For more information about research protections, see: About Research Participation What happens after a clinical trial is completed?After a clinical trial is completed, the researchers carefully examine information collected during the study before making decisions about the meaning of the findings and about the need for further testing. After a phase I or II trial, the researchers decide whether to move on to the next phase or to stop testing the treatment or procedure because it was unsafe or not effective. When a phase III trial is completed, the researchers examine the information and decide whether the results have medical importance. Results from clinical trials are often published in peer-reviewed scientific journals. Peer review is a process by which experts review the report before it is published to ensure that the analysis and conclusions are sound. If the results are particularly important, they may be featured in the news, and discussed at scientific meetings and by patient advocacy groups before or after they are published in a scientific journal. Once a new approach has been proven safe and effective in a clinical trial, it may become a new standard of medical practice. Ask the research team members if the study results have been or will be published. Published study results are also available by searching for the study's official name or Protocol ID number in the National Library of Medicine's PubMed® database . How does clinical research make a difference to me and my family? Only through clinical research can we gain insights and answers about the safety and effectiveness of treatments and procedures. Groundbreaking scientific advances in the present and the past were possible only because of participation of volunteers, both healthy and those with an illness, in clinical research. Clinical research requires complex and rigorous testing in collaboration with communities that are affected by the disease. As research opens new doors to finding ways to diagnose, prevent, treat, or cure disease and disability, clinical trial participation is essential to help us find the answers. This page last reviewed on October 3, 2022 Connect with Us
Masks Strongly Recommended but Not Required in MarylandRespiratory viruses continue to circulate in Maryland, so masking remains strongly recommended when you visit Johns Hopkins Medicine clinical locations in Maryland. To protect your loved one, please do not visit if you are sick or have a COVID-19 positive test result. Get more resources on masking and COVID-19 precautions .
Research at Johns HopkinsResearch begins in the lab, which is why we prioritize lab facilities that drive discovery and advancement in research.  Meet Our Research FacultyOur faculty members expand what's possible through biomedical research.  Clinical TrialsClinical trials provide important research for a wide range of conditions. Find out more about clinical trials at Johns Hopkins Medicine.  Understanding Clinical TrialsAt Johns Hopkins Medicine, we believe that clinical research is key to improve care for people in our community and around the world. Once you understand more about clinical research, you may appreciate why it’s important to participate—for yourself and the community. 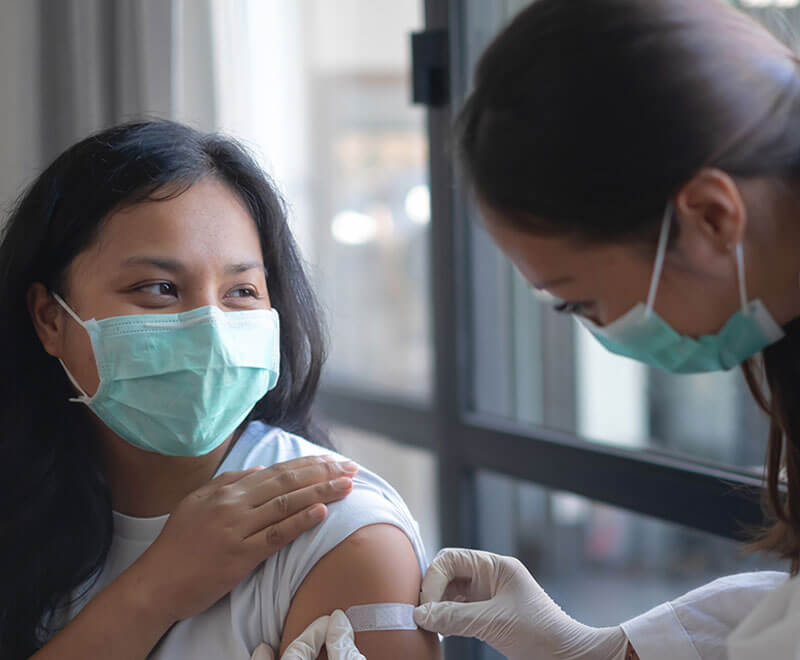 Research TopicsAt the foundation of Johns Hopkins Medicine is research — from basic research, where scientists study cells and mechanisms, to clinical research that builds on those findings using trials, to translational research that takes information learned from trials to the patient bedside. The Latest in Research NewsPress releases and other breaking news from Johns Hopkins Medicine. An official website of the United States government The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site. The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||




COMMENTS
Current Issue Highlights: Cardiovascular Death and Liver Disease: Mortality Trends and Disparities. COVID-19-Related Treatment Cancellations and Oncology Patients' Psychological Health in Nigeria. Assessment of the Prophylactic Effects of Probiotics, Prebiotics, and Synbiotics Against COVID-19: A Systematic Review of Randomized Controlled Trials.
Clinical Medicine is a peer-reviewed journal of the Royal College of Physicians that publishes advances and reviews in medicine. It offers open access articles on various topics, special issues, and a discounted article publishing charge until 31 December 2024.
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. Moreover, any research that evaluates the aspects of a ...
What is clinical research, and is it right for you? Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health.
Clinical Medicine & Research is a peer reviewed publication of original scientific medical research that is relevant to a broad audience of medical researchers and healthcare professionals.Clinical Medicine & Research publishes collections of articles in quarterly issues of the journal and, upon acceptance, author manuscripts are published electronically in the RAPID RELEASE section of this ...
E.D. Gaier and Others. Images in Clinical Medicine. Aug 17, 2024. J.M. Hernández Pérez. The New England Journal of Medicine (NEJM) is a weekly general medical journal that publishes new medical ...
The concept of medical analog patient-specific 'digital twins' is an emerging area of research that has the potential to combine polynomial data (mechanistic data, medical history, with the ...
R.P. Walensky and OthersN Engl J Med 2024;391:845-852. One hundred years ago, a 24-year-old man was admitted to the hospital because of fever, cough, and shortness of breath. The patient died on ...
This book covers various topics in medical disciplines, such as physiotherapy, coronary disease, cancer, and autism. It aims to provide new insights and perspectives on clinical research and practice for clinicians and scholars.
Case Studies in Social Medicine; Childhood Cancer; Childhood Diseases; Chronic Kidney Disease; Climate Change General; Climate Crisis and Health; Clinical Medicine General; Clinical Trials Series ...
Office of Inspector General. USA.gov. NIH…Turning Discovery Into Health ®. National Institutes of Health, 9000 Rockville Pike, Bethesda, Maryland 20892. U.S. Department of Health and Human Services. Clinical research occurs in many formats and can involve anyone. Learn how you can participate and contribute to medical advances.
Clinical trials articles from across Nature Portfolio. ... Research Highlights 29 Aug 2024 Nature Medicine. ... Research Highlights 05 Aug 2024 Nature Reviews Gastroenterology & Hepatology.
The JH-OCT brings together three groups in the school of medicine Office of Research Administration that provide essential services to efficiently move clinical trials contracts and budgets forward: clinical research contracting, clinical research support services and clinical research billing compliance. JH-OCT is the gateway for clinical ...
A peer-reviewed journal that publishes original research and reviews on various topics in clinical medicine. Find articles on bile acid malabsorption, kidney transplantation, acute kidney injury, dementia, atrial fibrillation, and more.
Journal of Clinical Medicine is an international, peer-reviewed, open access journal of clinical medicine, published semimonthly online by MDPI.The International Bone Research Association (IBRA), Italian Resuscitation Council (IRC), Spanish Society of Hematology and Hemotherapy (SEHH), Japan Association for Clinical Engineers (JACE), European Independent Foundation in Angiology/ Vascular ...
Clinical Medicine & Research has an H index of 62 (2023), an SJR of 0.334 (2023) with a 2-year Cites per Doc of 1.017 (2023). Please visit Scimago's page for Clinical Medicine & Research to learn more about the definition of these journal measures of scientific influence.
Clinical research includes all research that involves people. Types of clinical research include: Epidemiology, which improves the understanding of a disease by studying patterns, causes, and effects of health and disease in specific groups. Behavioral, which improves the understanding of human behavior and how it relates to health and disease.
Clinical Medicine & Research is a peer-reviewed medical research journal presenting relevant, credible information that addresses topics of interest to a multi-specialty audience in medicine, preventive medicine, translational medicine, epidemiology, research, and basic science. Clinical Medicine & Research will consider original manuscripts ...
At the foundation of Johns Hopkins Medicine is research — from basic research, where scientists study cells and mechanisms, to clinical research that builds on those findings using trials, to translational research that takes information learned from trials to the patient bedside. Browse Research Topics.
Articles from Clinical Medicine & Research are provided here courtesy of Marshfield Clinic. Follow NCBI. Connect with NLM. National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894. Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. NLM; NIH; HHS; USA.gov ...
Clinical Medicine & Research is an open-access, peer-reviewed, academic journal of clinical medicine published by the Marshfield Clinic Research Foundation. The journal is currently edited by Adedayo A. Onitilo (Marshfield Clinic). Abstracting and indexing. The journal is abstracted and indexed in the following bibliographic databases:
The Blue Ridge Institute for Medical Research currently has the department ranked third in funding for orthopedic departments of public medical schools. ... Even the most impactful findings from clinical research studies can take years to make it into widespread clinical practice. Cutting that lag time and smoothing the path to uptake is the ...
Residents have the discretion of tailoring their research in the form of biological laboratory research, clinical research or medical physics research under the supervision of a designated mentor. Basic research opportunities will not be limited to radiation research, but will be open to any cancer-related laboratory programs associated within ...
This Project Manager will directly lead multiple MPrOVE projects that build research practice partnerships to improve patient care. These will include collaborative quality improvement and research projects aimed to reduce low-value care and bridge the gap between health services research and clinical operations at Michigan Medicine.
Clinical Medicine & Research is committed to providing its readership with relevant, rigorously peer reviewed, original scientific medical re-search that substantially improves human health and well-being. Clinical Medicine & Research is available in print and electronic formats. Full
Job Type: Officer of Administration Regular/Temporary: Regular Hours Per Week: 35 Salary Range: $62,400 - $65,000 The salary of the finalist selected for this role will be set based on a variety of factors, including but not limited to departmental budgets, qualifications, experience, education, licenses, specialty, and training. The above hiring range represents the University's good faith ...
Job Summary: The Department of Radiology, University of Wisconsin - Madison, School of Medicine & Public Health is seeking a Clinical Research Coordinator (CRC) to help advance exciting medical imaging and disease-focused projects! The CRC works as part of a team, which includes physicians, PhD researchers, imaging staff, and other research support staff, to support and advance a portfolio of ...
Directly interacts with clinical research participants, as required, for the research study. Interacts via telephone, telehealth or in-person. ... we're committed to providing rigorous scientific training in both basic and clinical research to scholars across the Medical Center and community. You can take advantage or help support a number of ...