- Open access
- Published: 15 February 2023

Literature review of stroke assessment for upper-extremity physical function via EEG, EMG, kinematic, and kinetic measurements and their reliability
- Rene M. Maura ORCID: orcid.org/0000-0001-6023-9038 1 ,
- Sebastian Rueda Parra 4 ,
- Richard E. Stevens 2 ,
- Douglas L. Weeks 3 ,
- Eric T. Wolbrecht 1 &
- Joel C. Perry 1
Journal of NeuroEngineering and Rehabilitation volume 20 , Article number: 21 ( 2023 ) Cite this article
7812 Accesses
24 Citations
Metrics details
Significant clinician training is required to mitigate the subjective nature and achieve useful reliability between measurement occasions and therapists. Previous research supports that robotic instruments can improve quantitative biomechanical assessments of the upper limb, offering reliable and more sensitive measures. Furthermore, combining kinematic and kinetic measurements with electrophysiological measurements offers new insights to unlock targeted impairment-specific therapy. This review presents common methods for analyzing biomechanical and neuromuscular data by describing their validity and reporting their reliability measures.
This paper reviews literature (2000–2021) on sensor-based measures and metrics for upper-limb biomechanical and electrophysiological (neurological) assessment, which have been shown to correlate with clinical test outcomes for motor assessment. The search terms targeted robotic and passive devices developed for movement therapy. Journal and conference papers on stroke assessment metrics were selected using PRISMA guidelines. Intra-class correlation values of some of the metrics are recorded, along with model, type of agreement, and confidence intervals, when reported.
A total of 60 articles are identified. The sensor-based metrics assess various aspects of movement performance, such as smoothness, spasticity, efficiency, planning, efficacy, accuracy, coordination, range of motion, and strength. Additional metrics assess abnormal activation patterns of cortical activity and interconnections between brain regions and muscle groups; aiming to characterize differences between the population who had a stroke and the healthy population.
Range of motion, mean speed, mean distance, normal path length, spectral arc length, number of peaks, and task time metrics have all demonstrated good to excellent reliability, as well as provide a finer resolution compared to discrete clinical assessment tests. EEG power features for multiple frequency bands of interest, specifically the bands relating to slow and fast frequencies comparing affected and non-affected hemispheres, demonstrate good to excellent reliability for populations at various stages of stroke recovery. Further investigation is needed to evaluate the metrics missing reliability information. In the few studies combining biomechanical measures with neuroelectric signals, the multi-domain approaches demonstrated agreement with clinical assessments and provide further information during the relearning phase. Combining the reliable sensor-based metrics in the clinical assessment process will provide a more objective approach, relying less on therapist expertise. This paper suggests future work on analyzing the reliability of metrics to prevent biasedness and selecting the appropriate analysis.
Stroke is one of the leading causes of death and disability in developed countries. In the United States, a stroke occurs every 40 s, ranking stroke as the fifth leading cause of death and the first leading cause of disability in the country [ 1 ]. The high prevalence of stroke, coupled with increasing stroke survival rates, puts a growing strain on already limited healthcare resources; the cost of therapy is elevated [ 2 ] and restricted mostly to a clinical setting [ 3 ], leading to 50% of survivors that reach the chronic stage experiencing severe motor disability for upper extremities [ 4 ]. This highlights the need for refined (improved) assessment which can help pair person-specific impairment with appropriately targeted therapeutic strategies.
Rehabilitation typically starts with a battery of standardized tests to assess impairment and function. This initial evaluation serves as a baseline of movement capabilities and usually includes assessment of function during activities of daily living (ADL). Because these clinical assessments rely on trained therapists as raters, the scoring scale is designed to be discrete and, in some cases, bounded. While this improves the reliability of the metric [ 5 ] (i.e., raters more likely to agree), it also reduces the sensitivity of the scale. Furthermore, those assessment scales that are bounded, such as the Fugl-Meyer Assessment (FMA) [ 6 ], Ashworth or Modified Ashworth (MA) Scale [ 7 ], and Barthel Index [ 8 ], suffer from floor/ceiling effects where the limits of the scales become insensitive to the extremes of impairment and function. It is therefore important to develop new clinical assessment methods that are objective, quantifiable, reliable, and sensitive to change over the full range of function and impairment.
Over the last several decades, robotic devices have been designed and studied for administering post-stroke movement therapy. These devices have begun being adopted into clinical rehabilitation practice. More recently, researchers have proposed and studied the use of robotic devices to assess stroke-related impairments as an approach to overcome the limitations of existing clinical measures previously discussed [ 9 , 10 , 11 , 12 ]. Robots may be equipped with sensitive measurement devices that can be used to rate the person’s performance in a predefined task. These devices can include measuring kinematic (position/velocity), kinetic (force/torque), and/or neuromuscular (electromyography/electroencephalography) output from the subject during the task. Common sensor-based robotic metrics for post-stroke assessment included speed of response, planning time, movement planning, smoothness, efficiency, range, and efficacy [ 13 , 14 ]. Figure 1 demonstrates an example method for comprehensive assessment of a person who has suffered a stroke with data acquired during robotically administered tests. Furthermore, there is potential for new and more comprehensive knowledge to be gained from a wider array of assessment methods and metrics that combine the benefits of biomechanical (e.g., kinematic and kinetic) and neurological (e.g., electromyographic and electroencephalographic) measures [ 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 ].
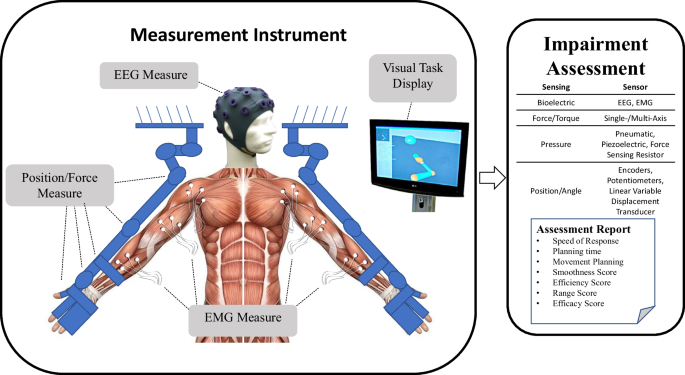
Example of instrument for upper extremities bilateral biomechanical and neuromuscular assessment. From this data, a wide variety of measures and metrics for assessment of upper-extremity impairment and function may be reported
- Biomechanical assessment
Many classical methods of assessing impairment or function involve manual and/or instrumented quantification of performance through measures of motion (i.e., kinematic) and force (i.e., kinetic) capabilities. These classical methods rely on the training of the therapist to evaluate the capabilities of the person through keen observation (e.g., FMA [ 6 ] and MA [ 7 ]). The quality of kinematic and kinetic measures can be improved with the use of electronic-based measurements [ 23 ]. Robotic devices equipped with electronic sensors have the potential to improve the objectivity, sensitivity, and reliability of the assessment process by providing a means for more quantitative, precise, and accurate information [ 9 , 10 , 11 , 12 , 24 , 25 , 26 , 27 , 28 ]. Usually, the electronic sensors on a rehabilitation robotic device are used for control purposes [ 29 , 30 , 31 ]. Robotics can also measure movement outputs, such as force or joint velocities, which the clinician may not be able to otherwise measure as accurately (or simultaneously) using existing clinical assessment methods [ 23 ]. With accurate and repeatable measurement of forces and joint velocities, sensor-based assessments have the potential to assess the person’s movement in an objective and quantifiable way. This article reviews validity and reliability of biomechanical metrics in relationship to assessment of motor function for upper extremities.
Electrophysiological features for assessment
Neural signals that originate from the body can be measured using non-invasive methods. Among others, electroencephalograms (EEG) measure cortical electrical activity, and electromyograms (EMG) measure muscle electrical activity. The relative low cost, as well as the noninvasive nature of these technologies make them suitable for studying changes in cortical or muscle activation caused by conditions or injuries of the brain, such as the ones elicited by stroke lesions [ 32 ].
Initially, EMG/EEG were used strictly as clinical diagnostic tools [ 33 , 34 ]. Recent improvements in signal acquisition hardware and computational processing methods have increased their use as viable instruments for understanding and treating neuromuscular diseases and neural conditions [ 32 ]. Features extracted from these signals are being researched to assess their relationship to motor and cognitive deficits [ 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ] and delayed ischemia [ 34 , 43 ], as well as to identify different uses of the signals that could aid rehabilitation [ 44 ]. Applications of these features in the context of stroke include: (1) commanding robotic prostheses [ 45 , 46 ], exoskeletons [ 21 , 47 , 48 ], and brain-machine interfaces [ 44 , 49 , 50 , 51 ]; and (2) bedside monitoring for sub-acute patients and thrombolytic therapy [ 52 , 53 , 54 ]. Here we review the validity and reliability of metrics derived from electrophysiological signals in relationship to stroke motor assessment for upper extremity.
Reliability of metrics
Robotic or sensor-based assessment tools have not gained widespread clinical acceptance for stroke assessment. Numerous barriers to their clinical adoption remain, including demonstrating their reliability and providing sufficient validation of robotic metrics with respect to currently accepted assessment techniques [ 55 ]. In the assessment of motor function with sensor-based systems, several literature reviews reveal a wide spectrum of sensor-based metrics to use for stroke rehabilitation and demonstrate their validity [ 13 , 42 , 56 , 57 , 58 , 59 , 63 , 64 ]. However, in addition to demonstrating validity, new clinical assessments must also demonstrate good or excellent reliability in order to support their adoption in the clinical field. This is achieved by: (1) comparing multiple measurements on the same subject (test–retest reliability), and (2) checking agreement between multiple raters of the same subject (inter-rater reliability). Reliability quantifies an assessment’s ability to deliver scores that are free from measurement error [ 65 ]. Previous literature reviews have presented limited, if any, information on the reliability of the biomechanical robotic metrics. Murphy and Häger [ 66 ], Wang et al. [ 56 ], and Shishov et al. [ 67 ] reviewed reliability, but omitted some important aspects of intra-class correlation methods used in the study (e.g., the model type and/or the confidence interval), which are required when analyzing intra-class correlation methods for reliability [ 68 ]. If the reliability is not properly analyzed and reported, the study runs the risk of having a biased result. Murphy and Häger [ 66 ] also found a lack of studies determining the reliability of metrics in 2015. Since electronic-based assessments require the use of a therapist or an operator to administer the test, an inter-observer reliability test should be investigated to observe the effect of the test administrators on the assessment process. Therefore, both test–retest and inter-observer reliability in biomechanical and electrophysiological metrics are reviewed to provide updated information on the current findings of the metrics’ reliability.
Integrated metrics
Over the past 50 years, numerous examples of integrated metrics have provided valuable insight into the inner workings of human arm function. In the 1970s EMG was combined with kinematic data in patients with spasticity to understand muscle patterns during ballistic arm reach movements [ 69 ], the affects of pharmacological intervention on spastic stretch reflexes during passive vs. voluntary movement [ 70 ], and in the 1990s EMG was combined with kinetic data to understand the effects of abnormal synergy patterns on reach workspace when lifting the arm against gravity [ 71 ]. This work dispelled long-standing theories of muscular weakness and spasticity alone being the major contributors to arm impairment. More recently, quantified aspects of processed EEG and EMG signals are being combined with kinematic data to investigate the compensatory role, and relation to shoulder-related abnormal muscle synergies of the contralesional secondary sensorimotor cortex, in a group of chronic stroke survivors [ 72 ]. These and other works demonstrate convincingly the value of combined metrics and the insights they can uncover that isolated metrics cannot discover alone.
To provide further information on the stroke severity and the relearning process during stroke therapy, researchers are investigating a multi-modal approach using biomechanical and neuromuscular features [ 15 , 16 , 18 , 19 , 21 , 22 ]. Combining both neuromuscular and biomechanical metrics will provide a comprehensive assessment of the person’s movement starting from motor planning to the end of motor execution. Neuromuscular output provides valuable information on the feedforward control and the movement planning phase [ 22 ]. However, neuromuscular signals provides little information on the movement quality that is often investigated with movement function tests or biomechanical output [ 21 ]. Also, using neuromuscular data will provide information to therapist on the neurological status and nervous system reorganization of the person that biomechanical information cannot provide [ 73 ]. The additional information can assist in developing more personalized care for the person with stroke, as well as offer considerable information on the changes that occur at the physiological level.
Paper overview
This paper reviews published sensor-based methods, for biomechanical and neuromuscular assessment of impairment and function after neurological damage, and how the metrics resulting from the assessments, both alone and in combination, may be able to provide further information on the recovery process. Specifically, methods and metrics utilizing digitized kinematic, kinetic, EEG, and EMG data were considered. The “Methods” section explains how the literature review was performed. In “Measures and methods based on biomechanical performance” section, prevailing robotic assessment metrics are identified and categorized including smoothness, resistance, efficiency, accuracy, efficacy, planning, range-of-motion, strength, inter-joint coordination, and intra-joint coordination. In “Measures and methods based on neural activity using EEG/EMG” section, EEG- and EMG-derived measures are discussed by the primary category of analysis performed to obtain them, including frequency power and coherence analyses. The relationship of each method and metric to stroke impairment and/or function is also discussed. Section “Reliability of measures” discusses the reliability of sensor-based metrics and some of the complications in demonstrating the effectiveness of the metrics. Section “Integrated metrics” reviews previous studies on combining biomechanical and neuromuscular data to provide further information on the changes occurring during assessment and training. Finally, Section “Discussions and conclusions” concludes the paper with a discussion on the advantages of combining multi-domain data, which of the metrics from the earlier sections should be considered in future robotic applications, as well as the ones that still require more investigation for either validity and/or reliability.
A literature review was performed following PRISMA guidelines [ 74 ] on biomechanical and neuromuscular assessment in upper-limb stroke rehabilitation. The review was composed of two independent searches on (1) biomechanical robotic devices, and (2) electrophysiological digital signal processing. Figures 2 and 3 show the selection process of the electrophysiological and biomechanical papers, respectively. Each of these searches applied the following steps: In step 1, each researcher searched in Google Scholar for papers between 2000 and 2021 (see Table 1 for search terms and prompts). In step 2, resulting titles and abstracts were screened to remove duplicates, articles in other languages, and articles not related to the literature review. In step 3, researchers read the full texts of articles screened in step 2, papers qualifying for inclusion using the Literature Review Criteria in Table 1 were selected. Finally, in step 4, selected articles from independent review process were read by the other researcher. Uncertainties in determining if a paper should be included/excluded were discussed with the whole research group. Twenty-four papers focus on biomechanical measures (kinematic and kinetic), thirty-three focus on electrophysiological measures (EEG/EMG), and six papers on multimodal approaches combining biomechanical and neuromuscular measures to assess stroke. Three of the six multimodal papers are also reported in the biomechanical section and 3 papers were hand-picked. A total of 60 papers are reviewed and reported.
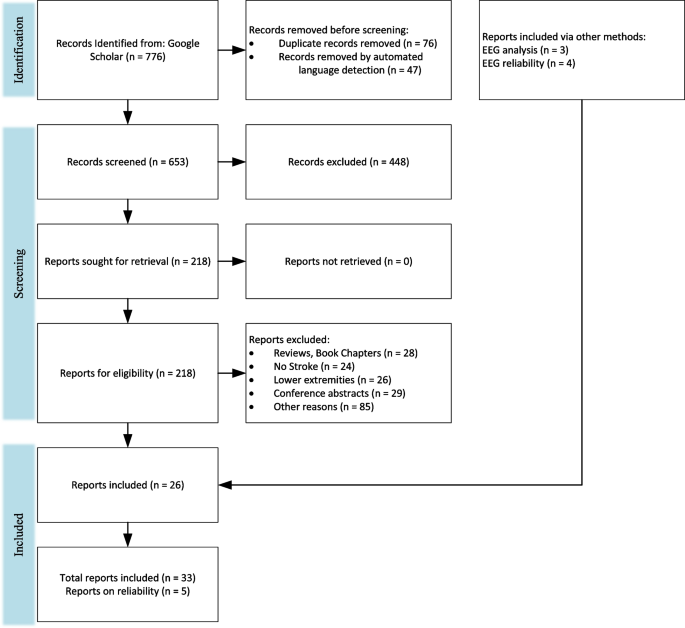
PRISMA flowchart on the selection for electrophysiological papers
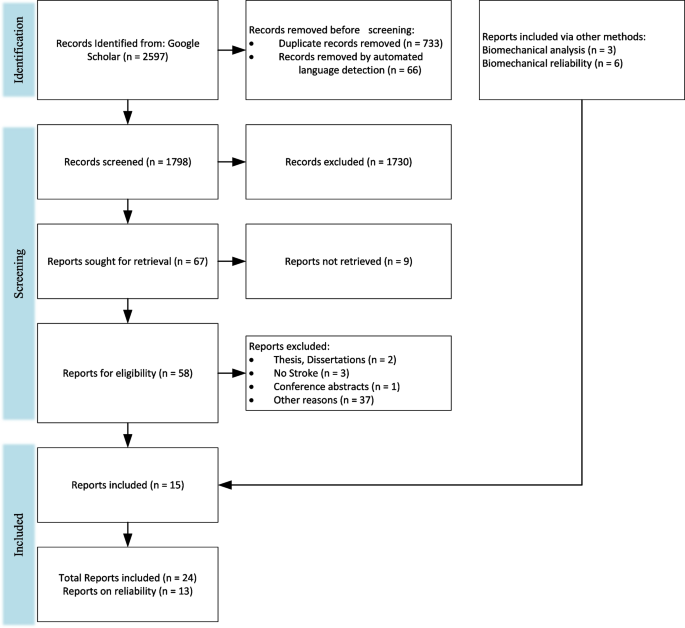
PRISMA flow chart for the selection for biomechanical papers
Measures and methods based on biomechanical performance
This review presents common robotic metrics which have been previously used to assess impairment and function after stroke. Twenty-five biomechanical papers are reviewed, which used both sensor-based and traditional clinical metrics to assess upper-extremity impairment and function. The five common metrics included in the reviewed studies measured the number of velocity peaks (~ 9 studies), path-length ratio (~ 8 studies), the max speed of the arm (~ 7 studies), active range of motion (~ 7 studies), and movement time (~ 7 studies). The metrics are often compared to an established clinical assessment to determine validity of the metric. The sensor-based metrics can be categorized by the aspect in which they evaluate movement quality similar to De Los Reyes-Guzmán et al.: smoothness, efficiency, efficacy, accuracy, coordination, or range of motion [ 14 ]. Resistance, Movement Planning, Coordination, and Strength are included as additional categories since some of the reviewed sensor-based metrics best evaluate those movement aspects. Examples of common evaluation activities and specific metrics that have been computed to quantify movement quality are outlined in Table 2 .
Lack of arm movement smoothness is a key indicator of underlying impairment [ 79 ]. Traditional therapist-administered assessments do not computationally measure smoothness leaving therapists unable to determine the degree to which disruption to movement smoothness is compromising motor function and, therefore, ADL. Most metrics that have been developed to quantify smoothness are based on features of the velocity profile of an arm movement, such as speed [ 80 , 81 ], speed arc length [ 79 ], local minima of velocity [ 10 ], velocity peaks [ 75 , 76 , 81 ], tent [ 80 ], spectral [ 25 ], spectral arc length [ 25 , 81 ], modified spectral arc length [ 79 ], and mean arrest period ratio [ 76 ]. Table 3 summarizes the smoothness metrics and their corresponding equations with equation numbers for reference. The speed metric is expressed as a ratio between the mean speed and the peak speed (Eq. 1). The speed arc length is the temporal length of the velocity profile (Eq. 2). Local minima of velocity and the velocity peaks metrics are measured by counting the number of minimum (Eq. 3) or maximum (Eq. 4) peaks in the velocity profile, respectively. The tent metric is a graphical approach that divides the area under the velocity curve by the area of a single peak velocity curve (Eq. 5). The spectral metric is the summation of the maximal Fourier transformed velocity vector (Eq. 6). The spectral arc-length metric is calculated from the frequency spectrum of the velocity profile by performing a fast Fourier transform operation and then computing the length (Eq. 7). The modified spectral arc length adapts the cutoff frequency according to a given threshold velocity and an upper-bound cutoff frequency (Eq. 8). The modified spectral arc length is then independent of temporal movement scaling. The mean arrest period ratio is the time portion that movement speed exceeds a given percentage of peak speed (Eq. 9).
Another commonly used approach is to analyze the jerk (i.e., the derivative of acceleration) profile. The common ways to assess smoothness using the jerk profile are root mean square jerk, mean rectified jerk, normalized jerk, and the logarithm of dimensionless jerk. The root mean square jerk takes the root-mean-square of the jerk that is then normalized by the movement duration [ 82 ] (Eq. 10). The mean rectified jerk (normalized mean absolute jerk) is the mean of the magnitude jerk normalized or divided by the peak velocity [ 80 , 82 ] (Eq. 11). The normalized jerk (dimensionless-squared jerk) is the square of the jerk times the duration of the movement to the fifth power over the length squared (Eq. 12). It is then integrated over the duration and square rooted. The normalized jerk can be normalized by mean speed, max speed, or mean jerk [ 80 ]. The logarithm of dimensionless jerk (Eq. 13) is the logarithm of normalized jerk defined in Eq. 12 [ 81 ].
It has yet to be determined which smoothness metric is more effective for characterizing recovery of smooth movement. According to Rohrer et al. [ 80 ], the metrics of speed, local minima of velocity, peaks, tent, and mean arrest period ratio showed increases in smoothness for inpatient recovery from stroke, but the mean rectified jerk metric seemed to show a decrease in smoothness as survivors of stroke recovered. Rohrer et al. warned that a low smoothness factor in jerk does not always mean the person is highly impaired. The spectral arc-length metric showed a consistent increase in smoothness as the number of sub-movements decreased [ 25 ], whereas the other metrics showed sudden changes in smoothness. For example, the mean arrest period ratio and the speed metric showed an increase in smoothness with two or more sub-movements, but when two sub-movements started to merge, the smoothness decreased. As a result, the spectral arc-length metric appears to capture change over a wider range of movement conditions in recovery in comparison to other metrics.
The presence of a velocity-dependent hyperactive stretch reflex is referred to as spasticity [ 83 ]. Spasticity results in a lack of smoothness during both passive and active movements and is more pronounced with activities that involve simultaneous shoulder abduction loading and extension of the elbow, wrist, or fingers [ 83 ], which are unfortunately quite common in ADL. A standard approach to assessing spasticity by a therapist involves moving a subject’s passive arm at different velocities and checking for the level of resistance. While this manual approach is subjective, electronic sensors have the potential to assess severity of spasticity in much more objective ways. Centen et al. report a method to assess the spasticity of the elbow using an upper-limb exoskeleton [ 84 ] involving the measurement of peak velocity, final angle, and creep. Sin et al., similarly performed a comparison study between a therapist moving the arm versus a robot moving the arm. An EMG sensor was used to detect the catch and compared with a torque sensor to detect catch angle for the robotic motion [ 85 ]. The robot moving the arm seemed to perform better with the inclusion of either an EMG or a torque sensor than with the therapist moving the arm and the robot simply recording the movement. A related measure that may be correlated with spasticity is the assessment of joint resistance torques during passive movement [ 76 ]. This can provide an assessment of the velocity-dependent resistance to movement that arises following stroke.
Efficiency measures movement fluency in terms of both task completion times and spatial trajectories. In point-to-point reaching, people who have suffered a stroke commonly display inefficient paths in comparison to their healthy side or compared to subjects who are unimpaired [ 10 ]. During the early phases of recovery after stroke, subjects may show slow overall movement speed resulting in longer task times. As recovery progresses, overall speed tends to increase and task times decrease, indicating more effective and efficient motor planning and path execution. Therapists usually observe the person’s efficiency in completing a task and then rate the person’s ability in completing a task in a timely manner. Therefore, both task time (or movement time) [ 10 , 76 , 77 , 86 , 87 ] and mean speed [ 25 , 75 , 77 , 81 , 86 ] are effective ways to assess temporal efficiency. Similar measures used by Wagner et al. include peak-hand velocity and time to peak-hand velocity [ 87 ]. To measure spatial efficiency of movement, both Colombo et al. [ 75 ], Mostafavi [ 77 ], and Germanotta [ 86 ] calculated the movement path length and divided it by the straight-line distance between the start and end points. This is known as the path-length ratio.
Movement planning
Movement planning is associated with feedforward sensorimotor control, elements that occur before the initial phase of movement. A common approach is to use reaction time to assess the duration of the planning phase. In a typical clinical assessment, a therapist can only observe/quantify whether movement can be initiated or not, but has no way to quantify the lag between the signal to initiate movement and initiation of movement. Keller et al., Frisoli et al., and Mostafavi et al. quantified the reaction time to assess movement planning [ 10 , 76 , 77 ] in subjects who have suffered a stroke. Mostafavi assessed movement planning in three additional ways by assessing characteristics of the actual movement: change in direction, movement distance ratio, and maximum speed ratio [ 77 ]. The change in direction is the angular deviation between the initial movement vector and the straight line between the start and end points. The first-movement-distance ratio is the ratio between the distance the hand traveled during the initial movement and the total distance between start and end points. The first-movement-maximum speed ratio is the ratio of the maximum hand speed during the initial phase of the movement divided by the global hand speed for the entire movement task.
Movement efficacy
Movement efficacy measures the person’s ability to achieve the desired task without assistance. While therapists can assess the number of completed repetitions, they have no means to kinetically quantify amount of assistance required to perform a given task. Movement efficacy is quantified by robot sensor systems that can measure: (a) person-generated movement, and/or (b) the amount of work performed by the robot to complete the movement (e.g., when voluntary person-generated movement fails to achieve a target). Hence, movement efficacy can involve both kinematic and kinetic measures. A kinematic metric that can be used to represent movement efficacy is the active movement index, which is calculated by dividing the portion of the distance the person is to complete by the total target distance for the task [ 75 ]. An example metric based on kinetic data is the amount of assistance metric, proposed by Balasubramanian et al. [ 25 ]. It is calculated by estimating the work performed by the robot to assist voluntary movement, and then dividing it by the work performed by the robot as if the person performs the task without assistance from the robot. A similar metric obtained by Germanotta et al. calculates the total work by using the movement’s path length, but Germanotta et al. also calculate the work generated towards the target [ 86 ].
Movement accuracy
Movement accuracy has been characterized by the error in the end-effector trajectory compared to a theoretical trajectory. It measures the person’s ability to follow a prescribed path, whereas movement efficiency assesses the person’s ability to find the most ideal path to reach a target. Colombo et al. measured movement accuracy in people after stroke by calculating the mean-absolute value of the distance, which is the mean absolute value of the distance between each point on the person’s path and the theoretical path [ 75 ]. Figure 4 demonstrates the difference between path-length ratio and mean-absolute value of the distance. The mean-absolute value of the distance computes the error between a desired trajectory and the actual, and the path-length ratio computes the total path length the person’s limb has traveled. Another similar metric is the average inter-quartile range, which quantifies the average “spread” among several trajectories [ 15 ]. Balasubramanian et al. characterized movement accuracy as a measure of the subject’s ability to achieve a target during active reaching. They refer to the metric as movement synergy [ 25 ], and calculate it by finding the distance between the end-effector’s final location and the target location.
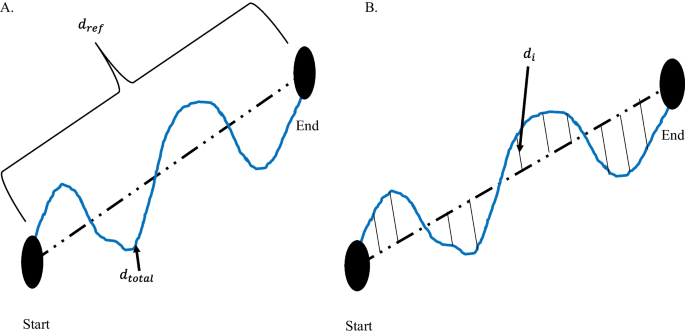
Difference between path-length ratio and mean absolute value of the distance. A Path-length ratio. \(d_{ref}\) is the theoretical distance the hand should travel between the start and end point. \(d_{total}\) is the total distance the hand travelled from Start to End. B Mean absolute value of the distance. \(d_{i}\) is the distance between the theoretical path and the actual hand path
Intra-limb coordination
Intra-limb (inter-joint) coordination is a measure of the level of coordination achieved by individual joints of a limb or between multiple joints of the same limb (i.e., joint synergy) when performing a task. Since the upper limb consists of kinematic redundancies, the human arm can achieve a desired outcome in multiple ways. For example, a person might choose to move an atypical joint in order to compensate for a loss of mobility in another joint. Frisoli et al. and Bosecker et al. used the shoulder and elbow angle to find a linear correlation between the two angles in a movement task that required multi-joint movement [ 10 , 78 ]. In terms of clinical assessment, joint angle correlations can illustrate typical or atypical contribution of a joint while performing a multi-joint task.
Inter-limb coordination
Inter-limb coordination refers to a person’s ability to appropriately perform bilateral movements with affected and unaffected arms. Therapists observe the affected limb by often comparing to the unaffected limb during a matching task, such as position matching. Matching can either be accomplished with both limbs moving simultaneously or sequentially, and typically without the use of vision. Dukelow et al. used position matching to obtain measures of inter-limb coordination [ 24 ], including trial-to-trial variability, spatial contraction/expansion, and systematic shifts. Trial-to-trial variability is the standard deviation of the matching hand’s position for each location in the x (distal/proximal), y (anterior/posterior), and both in x and y in the transverse plane. Spatial contraction/expansion is the ratio of the 2D work area of the target hand to the 2D work area of the matching hand during a matching task. Systematic shifts were found by calculating the mean absolute position error between the target and matching hand for each target location.
Semrau et al. analyzed the performance of subjects in their ability to match their unaffected arm with the location of their affected arm [ 88 ]. In the experiment, a robot moved the affected arm to a position and the person then mirrored the position with the unaffected side. The researchers compared the data when the person was able to see the driven limb versus when they were unable to see the driven limb. The initial direction error, path length ratio, response latency, peak speed ratio, and their variabilities were calculated to assess the performance of the person’s ability to perform the task.
Range of motion
Range of motion is a measure of the extent of mobility in one or multiple joints. Traditionally, range of motion can be measured with the use of a goniometer [ 89 ]. The goniometer measures the individual joint range of motion, which takes considerable time. Range of motion can be expressed as a 1-DOF angular measure [ 76 , 89 ], a 2-DOF planar measure (i.e., work area) [ 82 ], or a 3-DOF spatial measure (i.e., workspace) [ 77 ]. Individual joints are commonly measured in joint space, whereas measures of area or volume are typically given in Cartesian space. In performing an assessment of work area or workspace with a robotic device, the measure can be estimated either by: (a) measuring individual joint angles with an exoskeleton device and then using these angles to compute the region swept out by the hand, or (b) directly measuring the hand or fingertips with a Cartesian (end-effector) device. The measurement of individual joint range of motion (ROM) as well as overall workspace have significant clinical importance in assessing both passive (pROM) and active (aROM) range of motion. To measure pROM, the robot drives arm movement while the person remains passive. The pROM is the maximum range of motion the person has with minimal or no pain. For aROM, a robot may place the arm in an initial position/orientation from which the person performs unassisted joint movements to determine the ROM of particular joints [ 76 ], or the area or volume swept by multiple joints. Lin et al. quantified the work area of the elbow and shoulder using potentiometers and derived test–retest reliability [ 89 ]. The potentiometer measurements were then compared to therapist measurements to determine validity.
Measures of strength evaluate a person’s ability to generate a force in a direction or a torque about a joint. Strength measurements may involve single or multiple joints. At the individual joint level, strength is typically measured from a predefined position of a person’s arm and/or hand. The person then applies a contraction to produce a torque at the assessed joint [ 76 , 78 ]. Multi-joint strength may also be measured by assessing strength and/or torque in various directions at distal locations along the arm, such as the hand. Lin et al. compared the grip strength obtained from load cells to a clinical method using precise weights, which showed excellent concurrent validity [ 89 ].
Measures and methods based on neural activity using EEG/EMG
Although much information can be captured and analyzed using the kinematic and kinetic measures listed above, their purview is limited. These measures provide insight into the functional outcomes of neurological system performance but provide limited perspective on potential contributing sources of measured impairment [ 90 ]. For a deeper look into the neuromuscular system, measures based on neurological activation are often pursued. As a complement to biomechanical measures, methods based on quantization of neural activity like EEG and EMG have been used to characterize the impact of stroke and its underlying mechanisms of impairments [ 91 , 92 ]. Over the past 20 years, numerous academic research studies have used these measures to explore the effects of stroke, therapeutic interventions, or time on the evolution of abnormal neural activity [ 91 ]. Groups with different levels of neurological health are commonly compared (e.g., chronic/acute/subacute stroke vs. non-impaired, or impairment level) or other specific experimental characteristics (e.g., different rehabilitation paradigms [ 93 , 94 ]). With this evidence, the validity of these metrics has been tested; however, the study of reliability of these metrics is needed to complete the jump from academic to clinical settings.
Extracting biomarkers from non-invasive neural activity requires careful decomposition and processing of raw EEG and EMG recordings [ 32 ]. Various methods have been used, and the results have produced a growing body of evidence for the validity of these biomarkers in providing insight on the current and future state of motor, cognitive, and language skills in people after stroke [ 38 , 95 ]. Some of the biomarkers derived from EEG signals include: power-related band-specific information [ 34 , 35 , 43 , 47 , 53 , 54 , 96 , 97 , 98 , 99 , 100 , 101 ], band frequency event-related synchronization and desynchronization (ERS/ERD) [ 22 , 51 , 102 , 103 ], intra-cortical coherence or functional connectivity [ 39 , 59 , 73 , 94 , 104 , 105 , 106 , 107 , 108 , 109 ], corticomuscular coherence (CMC) [ 37 , 110 , 111 , 112 , 113 ], among others [ 114 , 115 ]. Biomarkers extracted from EEG can be used to assess residual functional ability [ 38 , 54 , 73 , 97 , 98 , 99 ], derive prognostic indicators [ 34 , 43 , 104 ], or categorize people into groups (e.g., to better match impairments with therapeutic strategies) [ 39 , 47 , 58 , 116 ].
In the following subsections, valid biomarkers derived mostly from EEG signal features (relationship with motor outcome for a person after stroke) will be discussed and introduced theoretically. Distinctions will be made about the stage after stroke when signals were taken. Findings are reported from 33 studies that have examined the relationship between extracted neural features and motor function for different groups of people after stroke. These records are grouped by quantization methods used including approaches based on measures of frequency spectrum power (n = 9), inter-regional coherence (n = 10 for cortical coherence and n = 9 for CMC), and reliability (n = 5).
Frequency spectrum power
Power measures the amount of activity within a signal that occurs at a specific frequency or range of frequencies. Power can be computed in absolute or relative terms (i.e., with respect to other signals). It is often displayed as a power density spectrum where the magnitudes of signal power can be seen across a range of frequencies. In electro-cognitive research, the representation of power within specific frequency bands has been useful to explain brain activity and to characterize abnormal oscillatory activity due to regional neurological damage [ 32 , 117 ].
Frequency bands in EEG content
Electrical activity in the brain is dominated primarily by frequencies from 0–100 Hz where different frequency bands correspond with different states of activity: Delta (0–4 Hz) is associated with deep sleep, Theta (4–8 Hz) with drowsiness, Alpha (8–13 Hz) with relaxed alertness and important motor activity [ 117 ], and Beta (13–31 Hz) with focused alertness. Gamma waves (> 32 Hz) are also seen in EEG activity; however, their specific relationship to level of alertness or consciousness is still debated [ 32 , 117 ]. Important cognitive tasks have been found to trigger activity in these bands in different ways. Levels of both Alpha and Delta activity have also been shown to be affected by stroke and can therefore be examined as indicators of prognosis or impairment in sub-acute and chronic stroke [ 52 , 100 , 118 ].
Power in acute and sub-acute stroke
For individuals in the early post-stroke (i.e., sub-acute) phase, abnormal power levels can be an indicator of neurological damage [ 98 ]. Attenuation of activity in Alpha and Beta bands have been observed in the first hours after stroke [ 100 ] preceding the appearance of abnormally high Delta activity. Tolonen et al. reported a high correlation between Delta power and regional Cerebral Blood Flow (rCBF). This relationship appears during the sub-acute stroke phase and has been used to predict clinical, cognitive, and functional outcomes [ 119 ]. Delta activity has also been shown to positively correlate with 1-month National Institutes of Health Stroke Scale (NIHSS) [ 52 ] and 3-month Rankin scale [ 36 ] assessments.
Based on these findings, several QEEG (Quantitative Electroencephalography) metrics involving ratios of abnormal slow (Delta) and abnormal fast (Alpha and Beta) activity have been developed. The Delta-Alpha Ratio (DAR), Delta-Theta Ratio (DTR), and (Delta + Theta)/(Alpha + Beta) Ratio (DTABR also known as PRI for Power Ratio Index) relate amount of abnormal slow activity with the activity from faster bands and have been shown to provide valuable insight into prognosis of stroke outcome and thrombolytic therapy monitoring [ 98 ]. Increased DAR and DTABR have been repeatedly found to be the QEEG indices that best predict worse outcome for the following: comparing with the Functional Independence Measure and Functional Assessment Measure (FIM-FAM) at 105 days [ 53 ], Montreal Cognitive Assessment (MoCa) at 90 days [ 54 ], NIHSS at 1 month [ 35 ], modified ranking scale (mRS) at 6 months [ 105 ], NIHSS evolution at multiple times [ 120 ], and NIHSS at 12 months [ 96 ]. DAR was also used to classify people in the acute phase and healthy subjects with an accuracy of 100% [ 58 ].
The ability of basic EEG monitoring to derive useful metrics during the early stage of stroke has made EEG collection desirable for people who have suffered a stroke in intensive care settings. The derived QEEG indices have proven to be helpful to determine Delayed Cerebral Ischemia (DCI), increased DAR [ 43 ], and increased Delta power [ 34 , 118 ]. However, finding the electrode montage with the least number of electrodes that still reveals the necessary information for prognoses is one of the biggest challenges for this particular use of EEG. Comparing DAR from 19 electrodes on the scalp with 4 electrodes on the frontal cortex suggests that DAR from 4 frontal electrodes may be enough to detect early cognitive and functional deficits [ 53 ]. Studies explored the possibility of a single-electrode montage over the Fronto-Parietal area (FP1); the DAR and DTR from this electrode might be a valid predictor of cognitive function after stroke when correlated with the MoCA [ 54 ], relative power in Theta band correlated with mRS and modified Barthel Index (mBI) 30 and 90 days after stroke [ 121 ].
Power in chronic stroke
The role of power-related QEEG indices during chronic stroke and progression of motor functional performance have been examined with respect to rehabilitation therapies, since participants have recovered their motion to a certain degree [ 4 ]. Studies have shown that therapy and functional activity improvements correlate with changes of the shape and delay of event-related desynchronization and synchronization (ERD-ERS) for time–frequency power features when analyzing Alpha and Beta bands on the primary motor cortex for ipsilesional and contralesional hemispheres [ 21 , 22 , 122 ]. Therapies with better outcome tend to have reduced Delta rhythms and increased Alpha rhythms [ 122 ].
Bertolucci [ 47 ] compared starting power spectrum density in different bands for both hemispheres with changes in WMFT and FMA over time. Increased global Alpha and Beta activity was shown to correlate with better WMFT evolution while, increase in contralesional Beta activity was shown to be correlated with FMA evolution. Metrics combining slow and fast activity have also been tested in the chronic stage of stroke, significant negative correlation between DTABR (PRI) at the start of therapy was related to FMA change during robotic therapy [ 99 ]. This finding suggests that DTABR may have promise as prognostic indicators for all stages of stroke.
Brain Symmetry Index (BSI) is a generalized measure of “left to right” (affected to non-affected) power symmetry of mean spectral power per hemisphere. These inter-hemispheric relationships of power have been used as prognostic measures during all stages of stroke. Baseline BSI (during the sub-acute stage) was found to correlate with the FMA at 2 months [ 73 ], mRS at 6 months [ 123 ], and FM-UE predictor when using only theta band BSI for patients in the chronic stage [ 124 ]. BSI can be modified to account for the direction of asymmetry, the directed BSI at Delta and Theta bands proved meaningful to describe evolution from acute to chronic stages of upper limb impairment as measured by FM-UE [ 120 , 125 ]. Table 4 and Table 11 in Appendix 1 communicate power-derived metrics across different stages of stroke documented in this section and their main reported relationships with motor function. Findings are often reported in terms of correlation with clinical tests of motor function.
Brain connectivity (cortical coherence)
Brain connectivity is a measure of interaction and synchronization between distributed networks of the brain and allows for a clearer understanding of brain function. Although cortical damage from ischemic stroke is focal, cortical coherence can explain abnormalities in functionality of remote zones that share functional connections to the stroke-affected zone [ 59 ].
Several estimators of connectivity have been proposed in the literature. Coherency, partial coherence (pCoh) [ 125 ], multiple coherence (mCoh), imaginary part of coherence (iCoh) [ 126 ], Phase Lagged Index (PLI), weighted Phase Lagged Index (wPLI) [ 127 ], and simple ratios of power at certain frequency bands [ 73 ] describe synchronic symmetric activity between ROIs and are referred to as non-directed or functional connectivity [ 128 ]. Estimators based on Granger’s prediction such as partial directed coherence (PDC) [ 129 , 130 , 131 ], or directed transfer Function (DTF) [ 132 , 133 ] and any of their normalizations describe causal relationships between variables and are referred to as directed or effective connectivity [ 134 ]. Connectivity also allows the analysis of brain activity as network topologies, borrowing methods from graph theory [ 32 , 134 ]. Network features such as complexity, linearity, efficiency, clustering, path length, node hubs, and more can be derived from graphs [ 128 ]. Comparisons of these network features among groups with impairment and healthy controls have proven to be interesting tools to understand and characterize motor and functional deficits after stroke [ 108 ].
Studies have used intra- and inter-cortical coherence to expand the clinical understanding of the neural reorganization process [ 59 , 106 , 107 , 108 , 109 ], as a clinical motor and cognitive predictor [ 38 , 94 , 104 , 135 , 136 ], and as a tool to predict the efficacy of rehabilitation therapy [ 94 ]. Table 5 and Table 12 in Appendix 2 briefly summarize the main metrics discussed in this section and their results that are related with motor function assessment. In general, studies have shown that motor deficits in stroke survivors are related to less connectivity to main sensory motor areas [ 38 , 94 , 104 , 137 ], weak interhemispheric sensorimotor connectivity [ 109 , 138 ], less efficient networks [ 106 , 135 ], with less “small world” network patterns [ 108 , 134 ] (small-world networks are optimized to integrate specialized processes in the whole network and are known as an important feature of healthy brain networks).
Survivors of stroke tend to exhibit more modular (i.e., more clustered, less integrated) and less efficient networks than non-impaired controls with the biggest difference occurring in the Beta and Gamma bands [ 106 ]. Modular networks are less “small-world” [ 134 ]; small-world networks are optimized to integrate specialized processes in the whole network and are known as an important feature of healthy brain networks. Such a transition to a less small-world network was observed during the acute stage of stroke (first hours after stroke) and documented to be bilaterally decreased in the Delta band and bilaterally increased in the high Alpha band (also known as Alpha2: 10.5–13 Hz) [ 108 ].
Global connectivity with the ipsilesional primary motor cortex (M1) is the most researched biomarker derived from connectivity and has been studied in longitudinal experiments as a plasticity indicator leading to future outcome improvement [ 38 ], motor and therapy gains [ 94 ], upper limb gains during the sub-acute stage [ 137 ], and as a feature that characterizes stroke survivors’ cognitive deficits [ 104 ]. Pietro [ 38 ] used iCoh to test the weighted node degree (WND), a measure that quantifies the importance of a ROI in the brain, for M1 and reported that Beta-band features are linearly related with motor improvement as measured by FM-UE and Nine-Hole-Peg Test. Beta-band connectivity to ipsilesional M1, as measured by spectral coherence, can be used as a therapy outcome predictor, and more than that, results point heavily toward connectivity between M1 and ipsilesional frontal premotor area (PM) to be the most important variable as a therapy gain predictor; predictions can be further improved by using lesion-related information such as CST or MRI to yield more accurate results [ 94 ]. Comparisons between groups of people with impairment and controls showed significant differences on Alpha connectivity involving ipsilesional M1, this value showed a relation with FMA 3 months for the group with impairment due to stroke [ 104 ].
The relationship between interhemispheric ROI connectivity and motor impairment has been studied. The normalized interhemispheric strength (nIHS) from PDC was used to quantify the coupling between structures in the brain, Beta- and lower Gamma-band features of this quantity in sensorimotor areas exhibited linear relationships with the degree of motor impairment measured by CST [ 136 ]. A similar measure, also derived from PDC used to measure ROI interhemispheric importance named EEG-PDC was used in [ 109 ]; here the results show that Mu-band (10–12 Hz) and Beta-band features could be used to explain results for hand motor function from FM-UE. In another study, Beta debiased weighted phase lag index (dwPLI), correlated with outcome measured by Action Research Arm Test (ARAT) and FM-UE [ 138 ].
Global and local network efficiency for Beta and Gamma bands seem to be significantly decreased in the population who suffered from a stroke compared to healthy controls as reported in [ 106 ]. Newer results, such as the ones pointed out by [ 135 ] found statistically significant relationships between Beta network efficiency, network intradensity derived using a non-parametric method (named Generalized Measure of Association), and functional recovery results given by FM-UE. Global maximal coherence features in the Alpha band have been recently recognized as FM-UE predictors, where coherence was computed using PLI and related to motor outcome by means of linear regression [ 139 ].
Corticomuscular coherence
Corticomuscular coherence (CMC) is a measure of the amount of synchronous activity between signals in the brain (i.e., EEG or MEG) and associated musculature (i.e., EMG) of the body [ 92 ]. Typically measured during voluntary contractions [ 110 ], the presence of coherence demonstrates a direct relationship between cortical rhythms in the efferent motor commands and the discharge of neurons in the motor cortex [ 140 ]. CMC is computed as correlation between EEG and EMG signals at a given frequency. Early CMC research found synchronous (correlated) activity in Beta and low Gamma bands [ 40 , 41 , 42 ]. CMC is strongest in the contralateral motor cortex [ 141 ]. This metric seems to be affected by stroke-related lesions, and thus provides an interesting tool to assess motor recovery [ 111 , 142 , 143 , 144 ]. The level of CMC is lower in the chronic stage of stroke than in healthy subjects [ 112 , 145 ], with chronic stroke survivors showing lower peak CMC frequency [ 146 ], and topographical patterns that are more widespread than in healthy people; highlighting a connection to muscle synergies [ 142 , 147 , 148 ]. CMC has been shown to increase with training [ 37 , 112 , 144 ].
Corticomuscular coherence has been proposed as a tool to: (a) identify the functional contribution of reorganized cortical areas to motor recovery [ 37 , 112 , 141 , 144 , 146 ]; (b) understand functional remapping [ 93 , 142 , 145 ]; and (c) study the mechanisms underlying synergies [ 147 , 148 ]. CMC has shown increased abnormal correlation with deltoid EMG during elbow flexion for people who have motor impairment [ 147 ], and the best muscles to target with rehabilitative interventions [ 148 ]. Changes in CMC have been shown to correlate with motor improvement for different stages of stroke, although follow-up scores based on CMC have not shown statistically significant correlations when compared to clinical metrics [ 37 , 93 ]. Results summarizing CMC on stroke can be found in Table 6 and Table 13 in Appendix 3.
Reliability of measures
Each of the aforementioned measures have the potential to be integrated into robotic devices for upper-limb assessment. However, to improve the clinical acceptability of robotic-assisted assessment, the measurements and derived metrics must meet reliability standards in a clinical setting [ 55 ]. Reliability can be defined as the degree of consistency between measurements or the degree to which a measurement is free of error. A common method to represent the relative reliability of a measurement process is the intraclass correlation coefficient (ICC) [ 150 ]. Koo and Li suggest a guideline on reporting ICC values for reliability that includes the ICC value, analysis model (one-way random effects, two-way random effects, two-way fixed effects, or two-way mixed effects), the model type per Shrout and Fleiss (individual trials or mean of k trials), model definition (absolute agreement or consistency), and confidence interval [ 68 ]. Koo and Li also provide a flowchart in selecting the appropriate ICC based on the type of reliability and rater information. An ICC value below 0.5 indicates poor reliability, 0.5 to 0.75 moderate reliability, 0.75 to 0.9 good reliability, and above 0.9 excellent reliability. The reviewed papers will be evaluated based on these guidelines. For reporting the ICC, the Shrout and Fleiss convention is used [ 68 ]. The chosen reliability studies are included in the tables if the chosen ICC model, type, definition, and confidence interval are identifiable, and the metrics have previously been used in electronic-based metrics. For studies that report multiple ICC scores due to assessment of test–retest reliability for multiple raters, the lowest ICC reported is included to avoid bias in the reported results.
In the assessment of reliability of data from robotic sensors, common ways to assess reliability are to correlate multiple measurements in a single session (intra-session) and correlate multiple measurements between different sessions (inter-session) measurements (i.e., test–retest reliability) [ 151 ]. Checking for test–retest reliability determines the repeatability of the robotic metric. The repeatability is the ability to reproduce the same measurements under the same conditions. Table 7 shows the test–retest reliability of several robotic metrics. For metrics checking for test–retest reliability, a two-way mixed-effects model with either single or multiple measurements may be used [ 68 ]. Since the same set of sensors will be used to assess subjects, the two-way mixed model is used. The test–retest reliability should be checking for absolute agreement. Checking for absolute agreement (y = x) rather than consistency (y = x + b) determines the reliability without a bias or systematic error. For example, in Fig. 5 , for a two-way random effect with a single measurement checking for agreement gives a score of 0.18. When checking for consistency, the ICC score reaches to 1.00. In other words, the bias has no effect on the ICC score when checking for consistency. Therefore, when performing test–retest reliability, it is important to check for absolute agreement to prevent bias in the test–retest result.
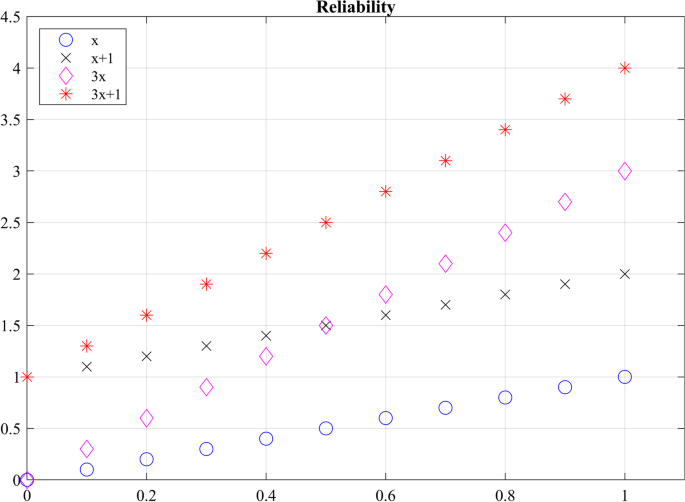
Checking agreement versus consistency among ratings. For y = x, the absolute ICC score is 1 and the consistency ICC score is 1.00. For y = x + 1, the agreement ICC score is 0.18 and the consistency ICC score is 1.00. For y = 3x, the absolute ICC score is 0.32 and the consistency ICC score is 0.60. For y = 3x + 1, the absolute ICC score is 0.13 and the consistency ICC score is 0.60
Not only should a robotic metric demonstrate repeatability, it should also be reproducible when different operators are using the same device. Reproducibility evaluates the change in measurements when conditions have changed. Inter-rater reliability tests have been performed to determine the effect raters have when collecting measurements when two or more raters perform the same experimental protocol [ 68 ]. To prevent a biased result, raters should have no knowledge of the evaluations given by other raters, ensuring that raters’ measurements are independent from one another. Table 8 shows the reproducibility of several robotic biomechanical metrics. All the included studies have used two raters to check for reproducibility. The researchers performed a two-way random effects analysis with either a single measurement or multiple measurements to check for agreement.
Measurement reliability of robotic biomechanical assessment
Of the 24 papers reviewed for biomechanical metrics, 13 papers reported on reliability. 6 papers reported reproducibility and 9 papers reported on repeatability. Overall, the metrics seem to demonstrate good to moderate reliability for both repeatability and reproducibility. However, caution should be exercised in determining which robotic metric is more effective in assessing movement quality based on reliability studies. The quality of measurements is highly dependent on the quality of the robotic device and sensors [ 85 ]. Having a completely transparent robot with a sensitive and accurate sensor will further improve assessment of reliability. Also, the researchers have used different versions of the ICC, as seen in Tables 7 and 8 , which complicates direct comparisons of the metrics.
Reliability of electrophysiological signal features
Of the 33 papers reviewed for electrophysiological metrics, 5 papers reported on reliability. 6 papers reported on repeatability. Convenience of acquiring electrophysiological signals non-invasively is relatively new. Metrics for assessment of upper limb motor impairment in stroke, derived from these signals have shown to be valid in academic settings, but most of these valid metrics have yet to be tested for intra- and inter-session reliability to be used in clinical and rehabilitation settings. Few studies found as a result of our systematic search have looked at test–retest reliability of these metrics. Therefore, we found and manually added records reporting on intra- and inter-session reliability on metrics based on electrophysiological features described in section “Measures and methods based on neural activity using EEG/EMG”, even if reliability was not assessed on people with stroke. Relevant results are illustrated in Table 9 .
Spectral power features of EEG signals have been tested during rest [ 153 , 154 ] and task (cognitive and motor) conditions for different cohorts of subjects [ 102 , 103 ]. Some of the spectral features observed during these experiments are related to timed behavior of oscillatory activity due to cued experiments, such as event-related desynchronization of the Beta band (ERD and Beta rebound) [ 102 ] and topographical patterns of Alpha activity R = 0.9302, p < 0.001 [ 103 ].
Test–retest reliability for rest EEG functional connectivity has been explored for few of the estimators listed in section “Measures and methods based on neural activity using EEG/EMG”: (1) for a cohort of people with Alzheimer by means of the amplitude envelope correlation (AEC), phase lag index (PLI) and weighted phase lag index (wPLI) [ 155 ]; (2) in healthy subjects using iCoh and PLI [ 156 ]; and (3) in infants, by studying differences of inter-session PLI graph metrics such as path length, cluster coefficient, and network “small-worldness” [ 60 ]. Reliability for upper limb CMC has not yet been documented (at least to our knowledge). However, an experiment involving testing reliability of CMC for gait reports low CMC reliability in groups with different ages [ 61 ].
EEG and EMG measurements could be combined with kinematic and kinetic measurements to provide additional information about the severity of impairment and decrease the number of false positives from individual measurements [ 21 ]. This could further be used to explain abnormal relationships between brain activation, muscle activation and movement kinematics, as well as provide insight about subject motor performance during therapy [ 15 ]. The availability of EEG and EMG measures can also enhance aspects of biofeedback given during tests or be used to complement other assessments to provide a more holistic picture of an individual’s neurological function.
It has been shown that combining EEG, EMG, and kinematic data using a multi-domain approach can produce correlations to traditional clinical assessments, a summary of some of the reviewed studies is presented in Table 10 . Belfatto et al. have assessed people’s ROM for shoulder and elbow flexion, task time, and computed jerk to measure people’s smoothness, while the EMG was used to measure muscle synergies, and EEG detected ERD and a lateralization coefficient [ 21 ]. Comani et al. used task time, path length, normalized jerk, and speed to measure motor performance while observing ERD and ERS during motor training [ 22 ]. Pierella et al. gathered kinematic data from an upper-limb exoskeleton, which assessed the mean tangential velocity, path-length ratio, the number of speed peaks, spectral arc length, the amount of assistance, task time, and percentage of workspace, while observing EEG and EMG activity [ 18 ]. Mazzoleni et al. used the InMotion2 robot system to capture the movement accuracy, movement efficiency, mean speed, and the number of velocity peaks, while measuring brain activity with EEG [ 16 ]. However, further research is necessary to determine the effectiveness of the chosen metrics and methods compared to other more promising methods to assess function. Furthermore, greater consensus in literature is needed to support the clinical use of more reliable metrics. For example, newer algorithms to estimate smoothness such as spectral arc length have been shown to provide greater validity and reliability than the commonly used normalized jerk metric. Despite this evidence, normalized jerk remains a widely accepted measure of movement smoothness.
Discussions and conclusions
In this paper we reviewed studies that used different sensor-acquired biomechanical and electrophysiological signals to derive metrics related to neuromuscular impairment for stroke survivors; such metrics are of interest for robotic therapy and assessment applications. To assess the ability of a given measure to relate with impairment or motor outcome, we looked for metrics where results have been demonstrated to correlate or predict scores from established clinical assessment metrics for impairment and function (validity). Knowing that a metric has some relationship with impairment and function (i.e., that it is valid) is not enough for it to be used in clinical settings if those results are not repeatable (reliable). Thus, we also reviewed the reliability of metrics and related signal features looking for metrics which produce similar results for the same subject during different test sessions and for different raters. With this information, researchers can aim to use metrics that not only seem to be related with stroke, but also can be trusted, with less bias, and with a simpler interpretation. The main conclusions of this review paper are presented as answers to the following research questions.
Which biomechanical-based metrics show promise for valid assessment of function and impairment?
Metrics derived from kinematic (e.g., position & velocity) and kinetic (e.g., force & torque) sensors affixed to robotic and passive mechanical devices have successfully been used to measure biomechanical aspects of upper-extremity function and impairment in people after stroke. The five common metrics included in the reviewed studies measured the number of velocity peaks (~ 9 studies), path-length ratio (~ 8 studies), the maximum speed of the arm (~ 7 studies), active range of motion (~ 7 studies), and movement time (~ 7 studies). The metrics are often compared to an established clinical assessment to determine validity of the metric. According to the review study by Murphy and Häger, the Fugl-Meyer Assessment for Upper Extremity had significant correlation with movement time, movement smoothness, peak velocity, elbow extension, and shoulder flexion [ 66 ]. The movement time and smoothness showed strong correlation with the Action Research Arm Test, whereas speed, path-length ratio, and end-point error showed moderate correlation. Tran et al. reviewed specifically validation of robotic metrics with clinical assessments [ 57 ]. The review found mean speed, number of peak velocities, movement accuracy, and movement duration to be most promising metrics based on validation with clinical assessments. However, the review mentioned that some studies seem to conflict on the correlation between the robotic metric and clinical measures, which could be due to assessment task, subject characteristics, type of intervention, and robotic device. For further information about the validation of sensor-based metrics, please refer to the previously mentioned literature reviews [ 57 , 66 ].
Which biomechanical-based metrics show promise for repeatable assessment?
Repeatable measures, in which measurement taken by a single instrument and/or person produce low variation within a single task, are a critical requirement for assessment of impairment and function. The biomechanical based metrics that show the most promise for repeatability are range of motion, mean speed, mean distance, normal path length, spectral arc length, number of peaks, and task time. Two or more studies used these metrics and demonstrated good and excellent reliability, which implies the metric is robust against measurement noise and/or disturbances. Since the metrics have been used on different measuring instruments, the sensors’ resolution and signal-to-noise ratio appear to have a minimal impact on the reliability. However, more investigation is needed to confirm this robustness. In lieu of more evidence, it is recommended that investigators choose sensors similar or superior in quality to those used in the measuring devices presented in Tables 7 and 8 to achieve the same level of reliability.
What aspects of biomechanical-based metrics lack evidence or require more investigation?
Although many metrics (see previous section) demonstrate good or excellent repeatability across multiple studies, the evidence for reproducibility is limited to single studies. When developing a novel device capable of robotic assistance and assessment, researchers have typically focused their efforts to create a device capable of repeatable and reliable measurements. However, since the person administering the test is using the device to measure the subject’s performance, the reproducibility of the metric must also be considered. The reproducibility of a metric is affected by the ease-of-use of the device; if the device is too complicated to setup and use, there is an increased probability that different operators will observe different measurements. Also, the operator’s instructions to the subject affects the reproducibility, especially in the initial sessions, which may lead to different learning effects, and different assessment results. More studies are needed across multiple sites and operators to determine the reproducibility of the biomechanical metrics reviewed in this paper.
Which neural activity-based metrics (EEG & EMG) show the most promise for reliable assessment?
Electrical neurological signals such as EEG and EMG have successfully been used to understand changes in motor performance and outcome variability across all stages of post-stroke recovery including the first few hours after onset. Experimental results have shown that metrics derived from slow frequency power (delta power, relative delta power, and theta power), and power ratio between slow and fast EEG frequency bands like DAR and DTABR convey useful information both about current and future motor capabilities, as presented in Table 4 and Table 11 in Appendix 1. Multimodal studies using robotic tools for assessment of motor performance have expanded the study of power signal features in people who suffered a stroke in the chronic recovery stage by studying not only rest EEG activity but also task-related activity [ 19 , 21 , 122 ]; ERD-ERS features like amplitude and latency along with biomechanical measures have been shown to correlate with clinical measures of motor performance and to predict a person’s response to movement therapies. EEG power features in general have been found to have good to excellent reliability for test–retest conditions among different populations, across all frequency bands of interest (see Table 9 ).
Functional connectivity (i.e., non-directed connectivity) expands the investigative capacity of EEG measurements, enabling analyzing the brain as a network system by investigating the interactions between regions of interest in the brain while resting or during movement tasks. Inter-hemispheric interactions (interactions between the same ROI in both hemispheres) and global interactions (interactions between the entire brain and an ROI) reported as power or graph indices in Beta and Gamma bands have fruitfully been used to explain motor outcome scores. Although results seem promising, connectivity reliability is still debated with results ranging mostly between moderate to good reliability only for a few connectivity estimators ( PLI, wPLI and iCoh ).
Which neural activity-based metrics (EEG and EMG) lack evidence or require more investigation?
EEG and EMG provide useful non-invasive insight into the human neuromuscular system allowing researchers to make conjectures about its function and structure; however, interpretation of results based on these measures solely must be carefully analyzed within the frame of experimental conditions. Overall, the field needs more studies involving cohorts of stroke survivors to determine the reliability (test–retest) of metrics derived from EEG and EMG signal features that have already shown validity in academic studies.
Metrics calculated from power imbalance between interhemispheric activity like BSI , pwBSI and PRI [ 62 , 73 , 124 ] are a great premise to measure how the brain relies on foreign regions to accomplish tasks related with affected areas. A battery of diverse estimators for connectivity, especially those of effective (directed) connectivity, open the door to investigations into the relationship between abnormal communication of regions of interest and impairment (see Table 5 and Table 12 in Appendix 2). These metrics, although valid have yet to be tested in terms of reliability in clinical use. Reliability for connectivity metrics should specify which estimator was used to derive the metric.
CMC is another exciting neural-activity-based metric lacking sufficient evidence to support its significance. CMC considers and bridges two of the most affected domains for motor execution in neuromuscular system, making it a good candidate for robotic-based therapy and assessment of survivors of stroke [ 147 ]. Although features in the Beta and Gamma bands seem to be related to motor impairment, there is still not agreement about which one is most closely related to motor outcomes. Studies reviewed in this paper considered cortical spatial patterns of maximum coherence, peak frequency shift when compared to healthy controls, latency for peak coherence, among others (see Table 6 and Table 13 in Appendix 3). However, when comparing to motor outcomes, results are not always significant, and test–retest reliability for this metric is yet (to our knowledge) to be documented for the upper extremity (see [ 61 ] for a lower-extremity study).
What standards should be adopted for reporting biomechanical and neural activity-based metrics and their reliability?
For metrics to be accepted as reliable in the clinical field, researchers are asked to follow the guidelines presented in Koo and Li [ 68 ], which provide guidance on which ICC model to use depending on the type of reliability study and what should be reported (e.g., the software they used to compute the ICC and confidence interval). In the papers reviewed, some investigated the learning effects of the assessment task and checked for consistency rather than agreement (see Table 7 ). However, the learning effects should be minimal in a clinical setting between each session, and potential effects should be taken into consideration during protocol design; common practices to minimize the implications of learning effects is to allow practice runs by the patients [ 99 , 122 ] and to remove the first experimental runs [ 81 , 85 ]. By removing this information, signal analysis focuses performance of learned tasks with similar associated behaviors. Therefore, to demonstrate test–retest reliability (i.e., repeatability), the researcher should be checking for absolute agreement. Also, as can be seen in Tables 7 and 8 , there does not seem to be a standard on reporting ICC values. Some researchers report the confidence interval of the ICC value, while others do not. It was also difficult to determine the ICC model used in some of the studies. Therefore, a standard on reporting ICC values is needed to help readers understand the ICC used and prevent bias (see [ 68 ] for suggestive guideline on how to report ICC scores). Also, authors are asked to include the means of each individual session or rater would provide additional information on the variation of the means between the groups. The variation between groups can be shown with Bland–Altman plot, but readers are unable to perform other forms of analysis. To help with this, data from studies should be made publicly available to allow results to be verified and enable further analysis in the future.
When is it advantageous to combine biomechanical and neural activity-based metrics for assessment?
Biomechanical and neural activity provide distinct but complementary information about the neuro-musculoskeletal system, potentially offering a more complete picture of impairment and function after stroke. Metrics derived from kinematic/kinetic information assess motor performance based on motor execution; however, compensatory strategies related to stroke may mask underlying neural deficits (i.e., muscle synergies line up to complete a given task) [ 18 , 21 , 69 , 70 , 71 , 72 , 122 ]. Information relevant to these compensatory strategies can be obtained when analyzing electrophysiological activity, as has been done using connectivity [ 59 , 107 ], CMC [ 147 , 148 ] and brain cortical power [ 91 ].
Combining signals from multiple domains, although beneficial in the sense that it would allow a deeper understanding of a subject’s motor ability, is still a subject of exploration. Experimental paradigms play an important role that influences the decision of feature selection; increasing the dimensionality of signals may provide more useful information for analysis, but comes at the expense of experimental costs (e.g., hardware) and time (e.g., subject setup). With all this in mind, merging information from different domains in the hierarchy of the neuro-musculoskeletal system may provide a more comprehensive quantitative profile of a person’s impairment and performance. Examples of robotic multidomain methods such as the ones in [ 18 , 21 ], highlight the importance of this type of assessment for monitoring and understanding the impact of rehabilitation in chronic stroke survivors. In both cases, these methodologies allowed pairing of observed behavioral changes in task execution (i.e., biomechanical data) with corresponding functional recovery, instead of adopted compensation strategies.
What should be the focus of future investigations of biomechanical and/or neural activity-based metrics?
Determining the reliability and validity of sensor-based metrics requires carefully designed experiments. In future investigations, experiments should be conducted that calculate multiple metrics from multiple sensors and device combinations, allowing the effect of sensor type and quality on the measure’s reliability to be quantified. After the conclusion of such experiments, researchers are strongly encouraged to make their anonymized raw data public to allow other researchers to compute different ICCs. Performing comparison studies on the reliability of metrics will produce reliability data to expand Tables 7 , 8 , 9 and improve our ability to compare similar sensor-based metrics. Additional reliability studies should also be performed that include neural features of survivors of stroke, with increased focus on modeling the interactions between these domains (biomechanical and neural activity). It is also important to understand how to successfully combine data from multimodal experiments; many of the studies reviewed in this paper recorded multidimensional data, but performed analysis for each domain separately.
Availability of data and materials
Not applicable.
Abbreviations
Activities of daily living
Amplitude envelope correlation
Action research arm test
Active range of motion
Autism spectrum disorder
Box and Blocks test
Brain Symmetry Index
Canonical correlation analysis
Cortico-spinal tract
Delta-alpha ratio
Delayed cerebral ischemia
Direct directed transfer function
Degree of freedom
(Delta + Theta)/(Alpha + Beta)
Directed transfer function
Delta-theta ratio
- Electroencephalography
Electromyography
Event related desynchronization
Event related synchronization
Full frequency directed transfer function
Functional independence measure and functional assessment measure
Fugl-Meyer assessment for upper extremity
Generalized Measure of Association
Generalized partial directed coherence
Intra-class correlations
Imaginary part of coherence
Primary motor cortex
Modified Ashworth
Modified Barthel Index
Multiple coherence
Motricity Index
Montreal Cognitive Assessment
Movement related beta desynchronization
Magnetic resonance imaging
Modified Ranking Scale
Normalized interhemispheric strength
National Institutes of Health Stroke Scale
Non-negative matrix factorization algorithm
Principal component analysis
Partial coherence
Partial directed coherence
Phase lag index, weight phase lag index, debiased weighted phase lag index
Premotor area
Post movement beta rebound
Power Ratio Index
Passive range of motion
Quantitative EEG
Regional cerebral blood flow
Region of interest
Renormalized partial directed coherence
Singular value decomposition
Wolf motor function
Weighted Node Degree Index
Stroke Facts. 2020. https://www.cdc.gov/stroke/facts.htm . Accessed 26 Mar 2020.
Ottenbacher KJ, Smith PM, Illig SB, Linn RT, Ostir GV, Granger CV. Trends in length of stay, living setting, functional outcome, and mortality following medical rehabilitation. JAMA. 2004;292(14):1687–95. https://doi.org/10.1001/jama.292.14.1687 .
Article CAS PubMed Google Scholar
Lang CE, MacDonald JR, Gnip C. Counting repetitions: an observational study of outpatient therapy for people with hemiparesis post-stroke. J Neurol Phys Ther. 2007;31(1). https://journals.lww.com/jnpt/Fulltext/2007/03000/Counting_Repetitions__An_Observational_Study_of.4.aspx .
Gresham GE, Phillips TF, Wolf PA, McNamara PM, Kannel WB, Dawber TR. Epidemiologic profile of long-term stroke disability: the Framingham study. Arch Phys Med Rehabil. 1979;60(11):487–91.
CAS PubMed Google Scholar
Duncan EA, Murray J. The barriers and facilitators to routine outcome measurement by allied health professionals in practice: a systematic review. BMC Health Serv Res. 2012;12(1):96.
Article PubMed PubMed Central Google Scholar
Sullivan KJ, Tilson JK, Cen SY, Rose DK, Hershberg J, Correa A, et al. Fugl-meyer assessment of sensorimotor function after stroke: standardized training procedure for clinical practice and clinical trials. Stroke. 2011;42(2):427–32.
Article PubMed Google Scholar
Ansari NN, Naghdi S, Arab TK, Jalaie S. The interrater and intrarater reliability of the Modified Ashworth Scale in the assessment of muscle spasticity: limb and muscle group effect. NeuroRehabilitation. 2008;23:231–7.
Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability? Int Disabil Stud. 1988;10(2):64–7.
Maggioni S, Melendez-Calderon A, van Asseldonk E, Klamroth-Marganska V, Lünenburger L, Riener R, et al. Robot-aided assessment of lower extremity functions: a review. J Neuroeng Rehabil. 2016;13(1):72. https://doi.org/10.1186/s12984-016-0180-3 .
Frisoli A, Procopio C, Chisari C, Creatini I, Bonfiglio L, Bergamasco M, et al. Positive effects of robotic exoskeleton training of upper limb reaching movements after stroke. J Neuroeng Rehabil. 2012;9(1):36. https://doi.org/10.1186/1743-0003-9-36 .
Groothuis-Oudshoorn CGM, Prange GB, Hermens HJ, Ijzerman MJ, Jannink MJA. Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke. J Rehabil Res Dev. 2006;43(2):171.
Harwin WS, Murgia A, Stokes EK. Assessing the effectiveness of robot facilitated neurorehabilitation for relearning motor skills following a stroke. Med Biol Eng Comput. 2011;49(10):1093–102.
Nordin N, Xie SQ, Wünsche B. Assessment of movement quality in robot- assisted upper limb rehabilitation after stroke: a review. J NeuroEngineering Rehabil. 2014;11:137. https://doi.org/10.1186/1743-0003-11-137 .
Article Google Scholar
De Los Reyes-Guzman A, Dimbwadyo-Terrer I, Trincado-Alonso F, Monasterio-Huelin F, Torricelli D, Gil-Agudo A. Quantitative assessment based on kinematic measures of functional impairments during upper extremity movements: a review. Clin Biomech. 2014;29(7):719–27. https://doi.org/10.1016/j.clinbiomech.2014.06.013 .
Molteni E, Preatoni E, Cimolin V, Bianchi AM, Galli M, Rodano R. A methodological study for the multifactorial assessment of motor adaptation: integration of kinematic and neural factors. 2010 Annu Int Conf IEEE Eng Med Biol Soc EMBC’10. 2010;4910–3.
Mazzoleni S, Coscia M, Rossi G, Aliboni S, Posteraro F, Carrozza MC. Effects of an upper limb robot-mediated therapy on paretic upper limb in chronic hemiparetic subjects: a biomechanical and EEG-based approach for functional assessment. 2009 IEEE Int Conf Rehabil Robot ICORR 2009. 2009;92–7.
Úbeda A, Azorín JM, Chavarriaga R, Millán JdR. Classification of upper limb center-out reaching tasks by means of EEG-based continuous decoding techniques. J Neuroeng Rehabil. 2017;14(1):1–14.
Pierella C, Pirondini E, Kinany N, Coscia M, Giang C, Miehlbradt J, et al. A multimodal approach to capture post-stroke temporal dynamics of recovery. J Neural Eng. 2020;17(4): 045002.
Steinisch M, Tana MG, Comani S. A post-stroke rehabilitation system integrating robotics, VR and high-resolution EEG imaging. IEEE Trans Neural Syst Rehabil Eng. 2013;21(5):849–59. https://doi.org/10.1596/978-1-4648-1002-2_Module14 .
Úbeda A, Hortal E, Iáñez E, Perez-Vidal C, Azorín JM. Assessing movement factors in upper limb kinematics decoding from EEG signals. PLoS ONE. 2015;10(5):1–12.
Belfatto A, Scano A, Chiavenna A, Mastropietro A, Mrakic-Sposta S, Pittaccio S, et al. A multiparameter approach to evaluate post-stroke patients: an application on robotic rehabilitation. Appl Sci. 2018;8(11):2248.
Comani S, Schinaia L, Tamburro G, Velluto L, Sorbi S, Conforto S, et al. Assessing Neuromotor Recovery in a stroke survivor with high resolution EEG, robotics and virtual reality. In: Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2015. p. 3925–8.
Kwon HM, Yang IH, Lee WS, Yu ARL, Oh SY, Park KK. Reliability of intraoperative knee range of motion measurements by goniometer compared with robot-assisted arthroplasty. J Knee Surg. 2019;32(3):233–8.
Dukelow SP, Herter TM, Moore KD, Demers MJ, Glasgow JI, Bagg SD, et al. Quantitative assessment of limb position sense following stroke. Neurorehabil Neural Repair. 2010;24(2):178–87.
Balasubramanian S, Wei R, Herman R, He J. Robot-measured performance metrics in stroke rehabilitation. In: 2009 ICME International Conference on Complex Medical Engineering, CME 2009. 2009.
Otaka E, Otaka Y, Kasuga S, Nishimoto A, Yamazaki K, Kawakami M, et al. Clinical usefulness and validity of robotic measures of reaching movement in hemiparetic stroke patients. J Neuroeng Rehabil. 2015;12(1):66.
Singh H, Unger J, Zariffa J, Pakosh M, Jaglal S, Craven BC, et al. Robot-assisted upper extremity rehabilitation for cervical spinal cord injuries: a systematic scoping review. Disabil Rehabil Assist Technol. 2018;13(7):704–15. https://doi.org/10.1080/17483107.2018.1425747 .
Molteni F, Gasperini G, Cannaviello G, Guanziroli E. Exoskeleton and end-effector robots for upper and lower limbs rehabilitation: narrative review. PMR. 2018;10(9):174–88.
Jutinico AL, Jaimes JC, Escalante FM, Perez-Ibarra JC, Terra MH, Siqueira AAG. Impedance control for robotic rehabilitation: a robust markovian approach. Front Neurorobot. 2017;11(AUG):1–16.
Google Scholar
Li Z, Huang Z, He W, Su CY. Adaptive impedance control for an upper limb robotic exoskeleton using biological signals. IEEE Trans Ind Electron. 2017;64(2):1664–74.
Marchal-Crespo L, Reinkensmeyer DJ. Review of control strategies for robotic movement training after neurologic injury. J NeuroEngineering Rehabil. 2009;6:20. https://doi.org/10.1186/1743-0003-6-20 .
Cohen MX. Analyzing neural time series data: theory and practice. Cambridge: MIT Press; 2014.
Book Google Scholar
Stafstrom CE, Carmant L. Seizures and epilepsy: an overview. Cold Spring Harb Perspect Med. 2015;5(6):65–77.
Machado C, Cuspineda E, Valdãs P, Virues T, Liopis F, Bosch J, et al. Assessing acute middle cerebral artery ischemic stroke by quantitative electric tomography. Clin EEG Neurosci. 2004;35(3):116–24.
Finnigan SP, Walsh M, Rose SE, Chalk JB. Quantitative EEG indices of sub-acute ischaemic stroke correlate with clinical outcomes. Clin Neurophysiol. 2007;118(11):2525–31.
Cuspineda E, Machado C, Galán L, Aubert E, Alvarez MA, Llopis F, et al. QEEG prognostic value in acute stroke. Clin EEG Neurosci. 2007;38(3):155–60.
Belardinelli P, Laer L, Ortiz E, Braun C, Gharabaghi A. Plasticity of premotor cortico-muscular coherence in severely impaired stroke patients with hand paralysis. NeuroImage Clin. 2017;14:726–33.
Di PM, Schnider A, Nicolo P, Rizk S, Guggisberg AG. Coherent neural oscillations predict future motor and language improvement after stroke. Brain. 2015;138(10):3048–60.
Chen CC, Lee SH, Wang WJ, Lin YC, Su MC. EEG-based motor network biomarkers for identifying target patients with stroke for upper limb rehabilitation and its construct validity. PLoS ONE. 2017;12(6):1–20. https://doi.org/10.1371/journal.pone.0178822 .
Article CAS Google Scholar
Conway BA, Halliday DM, Farmer SF, Shahani U, Maas P, Weir AI, et al. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J Physiol. 1995;489(3):917–24.
Article CAS PubMed PubMed Central Google Scholar
Salenius S, Portin K, Kajola M, Salmelin R, Hari R. Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol. 1997;77(6):3401–5.
Mima T, Hallett M. Electroencephalographic analysis of cortico-muscular coherence: reference effect, volume conduction and generator mechanism. Clin Neurophysiol. 1999;110(11):1892–9.
Claassen J, Hirsch LJ, Kreiter KT, Du EY, Sander Connolly E, Emerson RG, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004;115(12):2699–710.
Sullivan JL, Bhagat NA, Yozbatiran N, Paranjape R, Losey CG, Grossman RG, et al. Improving robotic stroke rehabilitation by incorporating neural intent detection: preliminary results from a clinical trial. In: 2017 International Conference on Rehabilitation Robotics (ICORR). IEEE; 2017. p. 122–7.
Muralidharan A, Chae J, Taylor DM. Extracting attempted hand movements from EEGs in people with complete hand paralysis following stroke. Front Neurosci. 2011. https://doi.org/10.3389/fnins.2011.00039 .
Nam C, Rong W, Li W, Xie Y, Hu X, Zheng Y. The effects of upper-limb training assisted with an electromyography-driven neuromuscular electrical stimulation robotic hand on chronic stroke. Front Neurol. 2017. https://doi.org/10.3389/fneur.2017.00679 .
Bertolucci F, Lamola G, Fanciullacci C, Artoni F, Panarese A, Micera S, et al. EEG predicts upper limb motor improvement after robotic rehabilitation in chronic stroke patients. Ann Phys Rehabil Med. 2018;61:e200–1.
Cantillo-Negrete J, Carino-Escobar RI, Carrillo-Mora P, Elias-Vinas D, Gutierrez-Martinez J. Motor imagery-based brain-computer interface coupled to a robotic hand orthosis aimed for neurorehabilitation of stroke patients. J Healthc Eng. 2018;3(2018):1–10.
Bhagat NA, Venkatakrishnan A, Abibullaev B, Artz EJ, Yozbatiran N, Blank AA, et al. Design and optimization of an EEG-based brain machine interface (BMI) to an upper-limb exoskeleton for stroke survivors. Front Neurosci. 2016;10(MAR):122.
PubMed PubMed Central Google Scholar
Biasiucci A, Leeb R, Iturrate I, Perdikis S, Al-Khodairy A, Corbet T, et al. Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nat Commun. 2018;9(1):1–13. https://doi.org/10.1038/s41467-018-04673-z .
Ang KK, Guan C, Chua KSG, Ang BT, Kuah C, Wang C, et al. Clinical study of neurorehabilitation in stroke using EEG-based motor imagery brain-computer interface with robotic feedback. Annu Int Conf IEEE Eng Med Biol. 2010. pp. 5549–52.
Finnigan SP, Rose SE, Walsh M, Griffin M, Janke AL, Mcmahon KL, et al. Correlation of quantitative EEG in acute ischemic stroke with 30-day NIHSS score: comparison with diffusion and perfusion MRI. Stroke. 2004;35(4):899–903.
Schleiger E, Sheikh N, Rowland T, Wong A, Read S, Finnigan S. Frontal EEG delta / alpha ratio and screening for post-stroke cognitive de fi cits: the power of four electrodes. Int J Psychophysiol. 2014;94(1):19–24. https://doi.org/10.1016/j.ijpsycho.2014.06.012 .
Aminov A, Rogers JM, Johnstone SJ, Middleton S, Wilson PH. Acute single channel EEG predictors of cognitive function after stroke. PLoS ONE. 2017;12(10): e0185841.
Andresen EM. Criteria for assessing the tools of disability outcomes research. Arch Phys Med Rehabil. 2000. https://doi.org/10.1053/apmr.2000.20619 .
Wang Q, Markopoulos P, Yu B, Chen W, Timmermans A. Interactive wearable systems for upper body rehabilitation: a systematic review. J Neuroeng Rehabil. 2017;14(1):1–21.
Tran VD, Dario P, Mazzoleni S. Kinematic measures for upper limb robot-assisted therapy following stroke and correlations with clinical outcome measures: a review. Med Eng Phys. 2018;53:13–31. https://doi.org/10.1016/j.medengphy.2017.12.005 .
Finnigan S, Wong A, Read S. Defining abnormal slow EEG activity in acute ischaemic stroke: Delta/alpha ratio as an optimal QEEG index. Clin Neurophysiol. 2016;127(2):1452–9. https://doi.org/10.1016/j.clinph.2015.07.014 .
Carter AR, Shulman GL, Corbetta M. Why use a connectivity-based approach to study stroke and recovery of function? Neuroimage. 2012;62(4):2271–80.
van der Velde B, Haartsen R, Kemner C. Test-retest reliability of EEG network characteristics in infants. Brain Behav. 2019;9(5):1–10.
Gennaro F, de Bruin ED. A pilot study assessing reliability and age-related differences in corticomuscular and intramuscular coherence in ankle dorsiflexors during walking. Physiol Rep. 2020;8(4):1–12.
Brihmat N, Loubinoux I, Castel-Lacanal E, Marque P, Gasq D. Kinematic parameters obtained with the ArmeoSpring for upper-limb assessment after stroke: a reliability and learning effect study for guiding parameter use. J Neuroeng Rehabil. 2020;17(1):130. https://doi.org/10.1186/s12984-020-00759-2 .
Dewald JPA, Ellis MD, Acosta AM, McPherson JG, Stienen AHA. Implementation of impairment- based neurorehabilitation devices and technologies following brain injury. Neurorehabilitation technology, 2nd edn. 2016. 375–392 p.
Subramanian SK, Yamanaka J, Chilingaryan G, Levin MF. Validity of movement pattern kinematics as measures of arm motor impairment poststroke. Stroke. 2010;41(10):2303–8.
Fayers PM, Machin D. Quality of life: the assessment, analysis and reporting of patient‐reported outcomes . John Wiley & Sons, Incorporated. 2016;3:89-124.
Alt Murphy M, Häger CK. Kinematic analysis of the upper extremity after stroke—how far have we reached and what have we grasped? Phys Ther Rev. 2015;20(3):137–55.
Shishov N, Melzer I, Bar-Haim S. Parameters and measures in assessment of motor learning in neurorehabilitation; a systematic review of the literature. Front Hum Neurosci. 2017. https://doi.org/10.3389/fnhum.2017.00082 .
Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–63. https://doi.org/10.1016/j.jcm.2016.02.012 .
Angel RW. Electromyographic patterns during ballistic movement of normal and spastic limbs. Brain Res. 1975;99(2):387–92.
McLellan DL. C0-contraction and stretch reflexes in spasticity during treatment with baclofen. J Neurol Neurosurg Psychiatry. 1977;40(1):30–8.
Dewald JPA, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118(2):495–510. https://doi.org/10.1093/brain/118.2.495 .
Wilkins KB, Yao J, Owen M, Karbasforoushan H, Carmona C, Dewald JPA. Limited capacity for ipsilateral secondary motor areas to support hand function post-stroke. J Physiol. 2020;598(11):2153–67. https://doi.org/10.1113/JP279377 .
Agius Anastasi A, Falzon O, Camilleri K, Vella M, Muscat R. Brain symmetry index in healthy and stroke patients for assessment and prognosis. Stroke Res Treat. 2017;30(2017):1–9.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700.
Colombo R, Pisano F, Micera S, Mazzone A, Delconte C, Carrozza MC, et al. Assessing mechanisms of recovery during robot-aided neurorehabilitation of the upper limb. Neurorehabil Neural Repair. 2008;22(1):50–63. https://doi.org/10.1177/1545968307303401 .
Keller U, Schölch S, Albisser U, Rudhe C, Curt A, Riener R, et al. Robot-assisted arm assessments in spinal cord injured patients: a consideration of concept study. PLoS One. 2015;10(5):e0126948. https://doi.org/10.1371/journal.pone.0126948 .
Mostafavi SM. Computational models for improved diagnosis and prognosis of stroke using robot-based biomarkers. 2016. http://hdl.handle.net/1974/14563 .
Bosecker C, Dipietro L, Volpe B, Krebs HI. Kinematic robot-based evaluation scales and clinical counterparts to measure upper limb motor performance in patients with chronic stroke. Neurorehabil Neural Repair. 2010;24(1):62–9.
Balasubramanian S, Melendez-Calderon A, Roby-Brami A, Burdet E. On the analysis of movement smoothness. J Neuroeng Rehabil. 2015;12(1):112. https://doi.org/10.1186/s12984-015-0090-9 .
Rohrer B, Fasoli S, Krebs HI, Hughes R, Volpe B, Frontera WR, et al. Movement smoothness changes during stroke recovery. J Neurosci. 2002;22(18):8297–304.
Mobini A, Behzadipour S, Saadat M. Test-retest reliability of Kinect’s measurements for the evaluation of upper body recovery of stroke patients. Biomed Eng Online. 2015;14(1):1–14.
Zariffa J, Myers M, Coahran M, Wang RH. Smallest real differences for robotic measures of upper extremity function after stroke: implications for tracking recovery. J Rehabil Assist Technol Eng. 2018;5:205566831878803. https://doi.org/10.1177/2055668318788036 .
Elovic E, Brashear A. Spasticity : diagnosis and management. New York: Demos Medical; 2011. http://ida.lib.uidaho.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=e000xna&AN=352265&site=ehost-live&scope=site .
Centen A, Lowrey CR, Scott SH, Yeh TT, Mochizuki G. KAPS (kinematic assessment of passive stretch): a tool to assess elbow flexor and extensor spasticity after stroke using a robotic exoskeleton. J Neuroeng Rehabil. 2017;14(1):1–13.
Sin M, Kim WS, Cho K, Cho S, Paik NJ. Improving the test-retest and inter-rater reliability for stretch reflex measurements using an isokinetic device in stroke patients with mild to moderate elbow spasticity. J Electromyogr Kinesiol. 2017;2018(39):120–7. https://doi.org/10.1016/j.jelekin.2018.01.012 .
Germanotta M, Cruciani A, Pecchioli C, Loreti S, Spedicato A, Meotti M, et al. Reliability, validity and discriminant ability of the instrumental indices provided by a novel planar robotic device for upper limb rehabilitation. J Neuroeng Rehabil. 2018;15(1):1–14.
Wagner JM, Rhodes JA, Patten C. Reproducibility and minimal detectable change of three-dimensional kinematic analysis of reaching tasks in people with hemiparaesis after stroke. 2008. https://doi.org/10.2522/ptj.20070255 .
Semrau JA, Herter TM, Scott SH, Dukelow SP. Inter-rater reliability of kinesthetic measurements with the KINARM robotic exoskeleton. J Neuroeng Rehabil. 2017;14(1):1–10.
Lin CH, Chou LW, Wei SH, Lieu FK, Chiang SL, Sung WH. Validity and reliability of a novel device for bilateral upper extremity functional measurements. Comput Methods Programs Biomed. 2014;114(3):315–23. https://doi.org/10.1016/j.cmpb.2014.02.012 .
Wolf S, Butler A, Alberts J, Kim M. Contemporary linkages between EMG, kinetics and stroke. J Electromyogr Kinesiol. 2005;15(3):229–39.
Iyer KK. Effective assessments of electroencephalography during stroke recovery : contemporary approaches and considerations. J Neurophysiol. 2017;118(5):2521–5.
Liu J, Sheng Y, Liu H. Corticomuscular coherence and its applications: a review. Front Hum Neurosci. 2019;13(March):1–16.
Pan LLH, Yang WW, Kao CL, Tsai MW, Wei SH, Fregni F, et al. Effects of 8-week sensory electrical stimulation combined with motor training on EEG-EMG coherence and motor function in individuals with stroke. Sci Rep. 2018;8(1):1–10.
Wu J, Quinlan EB, Dodakian L, McKenzie A, Kathuria N, Zhou RJ, et al. Connectivity measures are robust biomarkers of cortical function and plasticity after stroke. Brain. 2015;138(8):2359–69.
Mrachacz-Kersting N, Jiang N, Thomas Stevenson AJ, Niazi IK, Kostic V, Pavlovic A, et al. Efficient neuroplasticity induction in chronic stroke patients by an associative brain-computer interface. J Neurophysiol. 2016;115(3):1410–21.
Bentes C, Peralta AR, Viana P, Martins H, Morgado C, Casimiro C, et al. Quantitative EEG and functional outcome following acute ischemic stroke. Clin Neurophysiol. 2018;129(8):1680–7.
Leon-carrion J, Martin-rodriguez JF, Damas-lopez J, Manuel J, Dominguez-morales MR. Delta–alpha ratio correlates with level of recovery after neurorehabilitation in patients with acquired brain injury. Clin Neurophysiol. 2009;120(6):1039–45. https://doi.org/10.1016/j.clinph.2009.01.021 .
Finnigan S, van Putten MJAM. EEG in ischaemic stroke: qEEG can uniquely inform (sub-)acute prognoses and clinical management. Clin Neurophysiol. 2013;124(1):10–9.
Trujillo P, Mastropietro A, Scano A, Chiavenna A, Mrakic-Sposta S, Caimmi M, et al. Quantitative EEG for predicting upper limb motor recovery in chronic stroke robot-assisted rehabilitation. IEEE Trans Neural Syst Rehabil Eng. 2017;25(7):1058–67.
Jordan K. Emergency EEG and continuous EEG monitoring in acute ischemic stroke. Clin Neurophysiol. 2004;21(5):341–52.
Comani S, Velluto L, Schinaia L, Cerroni G, Serio A, Buzzelli S, et al. Monitoring neuro-motor recovery from stroke with high-resolution EEG, robotics and virtual reality: a proof of concept. IEEE Trans Neural Syst Rehabil Eng. 2015;23(6):1106–16.
Espenhahn S, de Berker AO, van Wijk BCM, Rossiter HE, Ward NS. Movement-related beta oscillations show high intra-individual reliability. Neuroimage. 2017;147:175–85. https://doi.org/10.1016/j.neuroimage.2016.12.025 .
Vázquez-Marrufo M, Galvao-Carmona A, Benítez Lugo ML, Ruíz-Peña JL, Borges Guerra M, Izquierdo AG. Retest reliability of individual alpha ERD topography assessed by human electroencephalography. PLoS ONE. 2017;12(10):1–16.
Dubovik S, Ptak R, Aboulafia T, Magnin C, Gillabert N, Allet L, et al. EEG alpha band synchrony predicts cognitive and motor performance in patients with ischemic stroke. In: Behavioural Neurology. Hindawi Limited; 2013. p. 187–9.
Sheorajpanday RVAA, Nagels G, Weeren AJTMTM, Putten MJAMV, Deyn PPD, van Putten MJAM, et al. Quantitative EEG in ischemic stroke: correlation with functional status after 6 months. Clin Neurophysiol. 2011;122(5):874–83. https://doi.org/10.1016/j.clinph.2010.07.028 .
De Vico Fallani F, Astolfi L, Cincotti F, Mattia D, La Rocca D, Maksuti E, et al. Evaluation of the brain network organization from EEG signals: a preliminary evidence in stroke patient. In: Anatomical Record. 2009. p. 2023–31.
Westlake KP, Nagarajan SS. Functional connectivity in relation to motor performance and recovery after stroke. Front Syst Neurosci. 2011;18(5):8.
Caliandro P, Vecchio F, Miraglia F, Reale G, Della Marca G, La Torre G, et al. Small-world characteristics of cortical connectivity changes in acute stroke. Neurorehabil Neural Repair. 2017;31(1):81–94.
Eldeeb S, Akcakaya M, Sybeldon M, Foldes S, Santarnecchi E, Pascual-Leone A, et al. EEG-based functional connectivity to analyze motor recovery after stroke: a pilot study. Biomed Signal Process Control. 2019;49:419–26.
Myers LJ, O’Malley M. The relationship between human cortico-muscular coherence and rectified EMG. In: International IEEE/EMBS Conference on Neural Engineering, NER. IEEE Computer Society; 2003. p. 289–92.
Braun C, Staudt M, Schmitt C, Preissl H, Birbaumer N, Gerloff C. Crossed cortico-spinal motor control after capsular stroke. Eur J Neurosci. 2007;25(9):2935–45.
Larsen LH, Zibrandtsen IC, Wienecke T, Kjaer TW, Christensen MS, Nielsen JB, et al. Corticomuscular coherence in the acute and subacute phase after stroke. Clin Neurophysiol. 2017;128(11):2217–26.
Ang KK, Chua KSG, Phua KS, Wang C, Chin ZY, Kuah CWK, et al. A randomized controlled trial of EEG-based motor imagery brain-computer interface robotic rehabilitation for stroke. Clin EEG Neurosci. 2015;46(4):310–20.
Liu S, Guo J, Meng J, Wang Z, Yao Y, Yang J, et al. Abnormal EEG complexity and functional connectivity of brain in patients with acute thalamic ischemic stroke. Comput Math Methods Med. 2016;14(2016):1–9.
CAS Google Scholar
Sun R, Wong W, Wang J, Tong RK. Changes in electroencephalography complexity using a brain computer interface-motor observation training in chronic stroke patients : a fuzzy approximate entropy analysis. Front Hum Neurosci. 2017;5(11):444.
Auriat AM, Neva JL, Peters S, Ferris JK, Boyd LA. A review of transcranial magnetic stimulation and multimodal neuroimaging to characterize post-stroke neuroplasticity. Front Neurol. 2015;6:1–20.
Niedermeyer E, Schomer DL, Lopes da Silva FH. Niedermeyer’s electroencephalography: basic principles, clinical applications, and related fields, 6th edn. Philadelphia: Lippincott Williams & Wilkins.; 2011.
Foreman B, Claasen J. Update in intensive care and emergency medicine. Update in intensive care and emergency medicine. Springer Berlin Heidelberg; 2012.
Tolonen U, Ahonen A, Kallanranta T, Hokkanen E. Non-invasive external regional measurement of cerebral circulation time changes in supratentorial infarctions using pertechnetate. Stroke. 1981;12(4):437–44.
Saes M, Zandvliet SB, Andringa AS, Daffertshofer A, Twisk JWR, Meskers CGM, et al. Is resting-state EEG longitudinally associated with recovery of clinical neurological impairments early poststroke? A prospective cohort study. Neurorehabil Neural Repair. 2020;34(5):389–402.
Rogers J, Middleton S, Wilson PH, Johnstone SJ. Predicting functional outcomes after stroke: an observational study of acute single-channel EEG. Top Stroke Rehabil. 2020;27(3):161–72. https://doi.org/10.1080/10749357.2019.1673576 .
Sale P, Infarinato F, Lizio R, Babiloni C. Electroencephalographic markers of robot-aided therapy in stroke patients for the evaluation of upper limb rehabilitation. Rehabil Res. 2015;38(4):294–305.
Sheorajpanday RVA, Nagels G, Weeren AJTM, De Surgeloose D, De Deyn PP, De DPP. Additional value of quantitative EEG in acute anterior circulation syndrome of presumed ischemic origin. Clin Neurophysiol. 2010;121(10):1719–25.
Saes M, Meskers CGM, Daffertshofer A, van Wegen EEH, Kwakkel G. Are early measured resting-state EEG parameters predictive for upper limb motor impairment six months poststroke? Clin Neurophysiol. 2021;132(1):56–62. https://doi.org/10.1016/j.clinph.2020.09.031 .
Saes M, Meskers CGM, Daffertshofer A, de Munck JC, Kwakkel G, van Wegen EEH. How does upper extremity Fugl-Meyer motor score relate to resting-state EEG in chronic stroke? A power spectral density analysis. Clin Neurophysiol. 2019;130(5):856–62. https://doi.org/10.1016/j.clinph.2019.01.007 .
Nolte G, Bai O, Mari Z, Vorbach S, Hallett M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin Neurophysiol. 2004;115(10):2292–307.
Stam CJ, Nolte G, Daffertshofer A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum Brain Mapp. 2007;28(11):1178–93.
Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186–98.
Baccalá LA, Sameshima K. Partial directed coherence: a new concept in neural structure determination. Biol Cybern. 2001;84(6):463–74.
Baccalá LA, Sameshima K, Takahashi D. Generalized partial directed coherence. Int Conf Digit Signal Process. 2007;3:163–6.
Schelter B, Timmer J, Eichler M. Assessing the strength of directed influences among neural signals using renormalized partial directed coherence. J Neurosci Methods. 2009;179(1):121–30.
Kamiński M, Ding M, Truccolo WA, Bressler SL. Evaluating causal relations in neural systems: Granger causality, directed transfer function and statistical assessment of significance. Biol Cybern. 2001;85(2):145–57.
Korzeniewska A, Mańczak M, Kamiński M, Blinowska KJ, Kasicki S. Determination of information flow direction among brain structures by a modified directed transfer function (dDTF) method. J Neurosci Methods. 2003;125(1–2):195–207.
Fornito A, Bullmore ET, Zalesky A. Fundamentals of brain network analysis. Cambridge: Academic Press; 2016.
Philips GR, Daly JJ, Príncipe JC. Topographical measures of functional connectivity as biomarkers for post-stroke motor recovery. J Neuroeng Rehabil. 2017;14(1):67.
Pichiorri F, Petti M, Caschera S, Astolfi L, Cincotti F, Mattia D. An EEG index of sensorimotor interhemispheric coupling after unilateral stroke: clinical and neurophysiological study. Eur J Neurosci. 2018;47(2):158–63.
Hoshino T, Oguchi K, Inoue K, Hoshino A, Hoshiyama M. Relationship between upper limb function and functional neural connectivity among motor related-areas during recovery stage after stroke. Top Stroke Rehabil. 2020;27(1):57–66. https://doi.org/10.1080/10749357.2019.1658429 .
Hordacre B, Goldsworthy MR, Welsby E, Graetz L, Ballinger S, Hillier S. Resting state functional connectivity is associated with motor pathway integrity and upper-limb behavior in chronic stroke. Neurorehabil Neural Repair. 2020;34(6):547–57.
Riahi N, Vakorin VA, Menon C. Estimating Fugl-Meyer upper extremity motor score from functional-connectivity measures. IEEE Trans Neural Syst Rehabil Eng. 2020;28(4):860–8.
Gwin JT, Ferris DP. Beta- and gamma-range human lower limb corticomuscular coherence. Front Hum Neurosci. 2012;11(6):258.
Zheng Y, Peng Y, Xu G, Li L, Wang J. Using corticomuscular coherence to reflect function recovery of paretic upper limb after stroke: a case study. Front Neurol. 2018;10(8):728.
Rossiter HE, Eaves C, Davis E, Boudrias MH, Park CH, Farmer S, et al. Changes in the location of cortico-muscular coherence following stroke. NeuroImage Clin. 2013;2(1):50–5.
Mima T, Toma K, Koshy B, Hallett M. Coherence between cortical and muscular activities after subcortical stroke. Stroke. 2001;32(11):2597–601.
Krauth R, Schwertner J, Vogt S, Lindquist S, Sailer M, Sickert A, et al. Cortico-muscular coherence is reduced acutely post-stroke and increases bilaterally during motor recovery: a pilot study. Front Neurol. 2019;20(10):126.
Bao SC, Wong WW, Leung TW, Tong KY. Low gamma band cortico-muscular coherence inter-hemisphere difference following chronic stroke. In: Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS. Institute of Electrical and Electronics Engineers Inc.; 2018. p. 247–50.
von Carlowitz-Ghori K, Bayraktaroglu Z, Hohlefeld FU, Losch F, Curio G, Nikulin VV. Corticomuscular coherence in acute and chronic stroke. Clin Neurophysiol. 2014;125(6):1182–91.
Chen X, Xie P, Zhang Y, Chen Y, Cheng S, Zhang L. Abnormal functional corticomuscular coupling after stroke. NeuroImage Clin. 2018;19:147–59. https://doi.org/10.1016/j.nicl.2018.04.004 .
Curado MR, Cossio EG, Broetz D, Agostini M, Cho W, Brasil FL, et al. Residual upper arm motor function primes innervation of paretic forearm muscles in chronic stroke after brain-machine interface (BMI) training. PLoS ONE. 2015;10(10):1–18.
Guo Z, Qian Q, Wong K, Zhu H, Huang Y, Hu X, et al. Altered corticomuscular coherence (CMCoh) pattern in the upper limb during finger movements after stroke. Front Neurol. 2020. https://doi.org/10.3389/fneur.2020.00410 .
Bruton A, Conway JH, Holgate ST. Reliability: what is it, and how is it measured? Physiotherapy. 2000;86(2):94–9.
Colombo R, Cusmano I, Sterpi I, Mazzone A, Delconte C, Pisano F. Test-retest reliability of robotic assessment measures for the evaluation of upper limb recovery. IEEE Trans Neural Syst Rehabil Eng. 2014;22(5):1020–9.
Costa V, Ramírez Ó, Otero A, Muñoz-García D, Uribarri S, Raya R. Validity and reliability of inertial sensors for elbow and wrist range of motion assessment. PeerJ. 2020;8: e9687.
Gasser T, Bächer P, Steinberg H. Test-retest reliability of spectral parameters of the EEG. Electroencephalogr Clin Neurophysiol. 1985;60(4):312–9.
Levin AR, Naples AJ, Scheffler AW, Webb SJ, Shic F, Sugar CA, et al. Day-to-day test-retest reliability of EEG profiles in children with autism spectrum disorder and typical development. Front Integr Neurosci. 2020;14:1–12.
Briels CT, Briels CT, Schoonhoven DN, Schoonhoven DN, Stam CJ, De Waal H, et al. Reproducibility of EEG functional connectivity in Alzheimer’s disease. Alzheimer’s Res Ther. 2020;12(1):1–14.
Marquetand J, Vannoni S, Carboni M, Li Hegner Y, Stier C, Braun C, et al. Reliability of magnetoencephalography and high-density electroencephalography resting-state functional connectivity metrics. Brain Connect. 2019;9(7):539–53.
Lowrey CR, Blazevski B, Marnet J-L, Bretzke H, Dukelow SP, Scott SH. Robotic tests for position sense and movement discrimination in the upper limb reveal that they each are highly reproducible but not correlated in healthy individuals. J Neuroeng Rehabil. 2020;17(1):103. https://doi.org/10.1186/s12984-020-00721-2 .
Simmatis LER, Early S, Moore KD, Appaqaq S, Scott SH. Statistical measures of motor, sensory and cognitive performance across repeated robot-based testing. J Neuroeng Rehabil. 2020;17(1):86. https://doi.org/10.1186/s12984-020-00713-2 .
Download references
Acknowledgements
The authors would like to thank Stephen Goodwin and Aaron I. Feinstein for their contributions to the collection and organization of references on robotic systems, measurements, and metrics.
This work was funded by the National Science Foundation (Award#1532239) and the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (Award#K12HD073945). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Science Foundation nor the National Institutes of Health.
Author information
Authors and affiliations.
Mechanical Engineering Department, University of Idaho, Moscow, ID, USA
Rene M. Maura, Eric T. Wolbrecht & Joel C. Perry
Engineering and Physics Department, Whitworth University, Spokane, WA, USA
Richard E. Stevens
College of Medicine, Washington State University, Spokane, WA, USA
Douglas L. Weeks
Electrical Engineering Department, University of Idaho, ID, Moscow, USA
Sebastian Rueda Parra
You can also search for this author in PubMed Google Scholar
Contributions
RM, and SRP drafted the manuscript and performed the literature search. EW, JP, RS, and DW provided concepts, edited, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Rene M. Maura .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
See Table 11 .
See Table 12 .
See Table 13 .
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Maura, R.M., Rueda Parra, S., Stevens, R.E. et al. Literature review of stroke assessment for upper-extremity physical function via EEG, EMG, kinematic, and kinetic measurements and their reliability. J NeuroEngineering Rehabil 20 , 21 (2023). https://doi.org/10.1186/s12984-023-01142-7
Download citation
Received : 27 May 2021
Accepted : 19 January 2023
Published : 15 February 2023
DOI : https://doi.org/10.1186/s12984-023-01142-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Reliability
- Robot-assisted therapy
- Exoskeleton
- Neurological assessment
- Rehabilitation
- Motor function
Journal of NeuroEngineering and Rehabilitation
ISSN: 1743-0003
- Submission enquiries: [email protected]
- Open access
- Published: 20 December 2021
Role of imaging in early diagnosis of acute ischemic stroke: a literature review
- Mohammad Amin Akbarzadeh 1 ,
- Sarvin Sanaie 2 , 3 ,
- Mahshid Kuchaki Rafsanjani 3 &
- Mohammad-Salar Hosseini ORCID: orcid.org/0000-0003-2765-5018 1 , 2 , 4
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery volume 57 , Article number: 175 ( 2021 ) Cite this article
11k Accesses
10 Citations
Metrics details
Stroke is a serious health condition that is responsible for more than 5% of total deaths. Near 20% of patients experiencing stroke die every year, resulting in the stroke being at the top of the list of preventable causes of death. Once an acute stroke is suspected, a golden hour of less than an hour is available to prevent the undesirable consequences. Since neuroimaging is mandatory in the diagnosis of stroke, the proper use of neuroimaging could help saving time and planning the right treatment for the patient. Some of the available imaging methods help us with rapid results, while others benefit us from a more accurate diagnosis. Hereby, we aim to provide a clinical review of the advantages and disadvantages of different available neuroimaging methods in approaching acute stroke to help clinicians choose the best method according to the settings.
Introduction
Cerebrovascular diseases are serious health conditions that can affect the quality of life by leading to disabling consequences, such as dysarthria, paralysis, and amnesia [ 1 , 2 ]. A stroke happens when the brain’s blood-supplying vessels are ruptured or blocked, usually along with thrombus formation, displaced embolism, stenosis in cerebral arteries, and hemorrhage in the brain parenchyma [ 3 ]. These events can lead to a significant decrease in blood flow and oxygen to the brain, resulting in a stroke. Stroke is responsible for more than 10% of deaths worldwide [ 4 ], and one in five stroke patients die each year, putting stroke at the top of the list of preventable causes of death [ 5 ]. Risk factors attributable to stroke are mainly the ones that are also common in other non-communicable diseases, such as diabetes, blood pressure, being obesity, smoking, and alcohol consumption; although, the prevalence of them may vary between different countries [ 6 , 7 ].
In the management of acute stroke, a “golden time” of less than an hour is available from the onset of the symptoms, where making prudent diagnostic and therapeutic choices may result in fewer complications [ 8 , 9 ]. Sense of weakness or numbness, visual loss or blurred vision, sensory disturbance, impaired consciousness, dizziness and loss of balance, dysphagia, headache, and speech problems are the most common findings in favor of stroke [ 10 ]. Like other life-threatening emergencies, an accurate and rapid diagnosis is crucial. Delay in diagnosis could result in irreversible damages, since the brain tissue is lost for each minute delay. Several stroke assessment scales [such as Face Arm Speech Time (FAST), Cincinnati Prehospital Stroke Scale (CPSS), Los Angeles Prehospital Stroke Scale (LAPSS), and Melbourne Ambulance Stroke Scale (MASS)], and severity grading scores [such as Los Angeles Motor Scale (LAMS), Kurashiki Prehospital Stroke Scale (KPSS), and National Institutes of Health Stroke Scale (NIHSS)] have been developed, trying to facilitate the rapid diagnosis of stroke through different criteria. Simpler scales such as CPSS and FAST have been shown to have enough sensitivity for clinical purposes, while the more complex scales might have lower sensitivity and, therefore, miss more cases [ 11 ]. Although some important clinical manifestations, such as face drooping, arm weakness, and speech difficulty, may suggest stroke in the initial assessment, the definitive diagnosis is primarily determined based on imaging [ 12 ]. Therefore, an intelligent choice of imaging technique could result in an early, lifesaving diagnosis of acute stroke. This article aims to review the role of different imaging methods in the diagnosis of acute ischemic stroke and determine the advantages and disadvantages of each.
Etiology and pathophysiology of ischemic stroke
The main pathology of ischemic stroke is loss or abruption of blood circulation in specific parts of the brain, which could be due to the thrombotic or embolic occlusion [ 13 ]. The thrombotic ischemic strokes are generally associated with atherosclerotic accidents and are classified based on the size of the involved vessels [ 14 ]. Depending on the speed of the plaque rupture, it can be presented with sudden devastating damages or subtle pathologic changes that manifest slowly [ 15 ]. In the latter situation, collateral circulation would partly compensate the circulation loss. The small-vessel ischemic stroke could occur as a consequence of systemic and chronic diseases, such as diabetes [ 16 ]. Rarely, vasculitis can predispose the vessel walls to narrowing and obstruction [ 17 ]. On the other hand, the embolic ischemic stroke is mainly caused by atrial fibrillation [ 18 ]. In addition, several monogenic disorders (such as Ehlers–Danlos, Fabry’s disease, and Marfan syndrome) are associated with ischemic stroke [ 19 , 20 ].
Regardless of the etiology, the abruption in blood circulation, a cascade of ischemia-related events initiate in both cellular level and macroscopic scale [ 13 , 21 ]. In the first place, the energy supply by mitochondria gets interrupted—even in the absence of complete blood occlusion—leading to loss of function of the membranous proteins and an impaired gradient between intracellular and extracellular space, which eventually results in swelled neurons and glial cells (also referred to as cytotoxic edema) [ 22 , 23 ]. Besides, the release of excitatory neurotransmitters adds extra stress in terms of energy supply by the cells, ectopic activation of destructive enzymes, such as lipases and proteases, destruction of vital organelles in neurons, and production of oxygen free radicals, eventually leading to necrotic death of the neurons in the core of the ischemic region, and triggering the cascade of apoptotic events in the peripheral neurons [ 24 , 25 , 26 ].
Importance of imaging in approach to stroke
For the following reasons, the suspicion of ischemic stroke should be followed by early neuroimaging:
Exclude the presence of hemorrhage
Apart from stabilizing the patient, the initial management of a patient with stroke depends on the type and etiology of stroke [ 27 ]. While thrombolytic therapy benefits ischemic strokes, it is contraindicated in hemorrhagic ones [ 28 ]. This process should be done fast, as there is a golden time of 4.5 h available from the symptom onset to safely use Tissue Plasminogen Activators (tPA), such as Alteplase [ 29 , 30 ].
Confirmation of the stroke
Although a stroke is generally accompanied by clinical signs and symptoms, such as face drooping on one side, arm weakness, and difficulty in speech, this diagnosis should be confirmed by detecting signs of infarction and early signs of ischemia (such as presence of hyperdense area in a vessel—mainly in the middle cerebral artery—which is a common sign of intravascular thrombus or embolus) through imaging [ 31 , 32 ]. In addition, although the collateral flow tends to compensate the lack of blood flow in the affected area, early signs such as hypoattenuation and loss of grey–white matter differentiation would be evident [ 33 ].
Determining the status of brain-supplying arteries
The status of brain-supplying arteries should be assessed in the work-up to specify the possible etiologies, indicate the damage severity, and determine the recurrence risk in some cases [ 34 ].
Rule out the stroke mimics
Tumors are stroke-mimics that should be ruled out. Migraines and seizures are other diseases that could present with stroke-like symptoms and require imaging for ruling out the stroke [ 27 , 35 , 36 , 37 ].
Common imaging modalities in healthcare settings
Ranging from Magnetic Resonance Imaging (MRI) to Computed Tomography Perfusion (CTP), there are various imaging techniques available [ 38 , 39 ]. Different modalities of Computed Tomography (CT) include CT Angiography of head and neck (CTA), CTP, and noncontrast CT. As for MRI modalities, various measures can be useful, such as Apparent Diffusion Coefficient (ADC) Diffusion-Weighted Imaging (DWI), Gradient Recalled Echo (GRE), Fluid-Attenuated Inversion Recovery (FLAIR), and MR Angiography of head and neck (MRA) [ 40 , 41 , 42 ]. Although CT is still the preferred imaging modality in the early management of acute stroke, MRI with DW measure is more sensitive than noncontrast CT for the very early detection of acute ischemia and is shown to be more efficient than the first CT, second CT and MRI in the evaluation of patients who are presented within the 24 h [ 43 ]. The main reason for this accuracy is the capability of the DW imaging to show the slight differences in diffusion of the water molecules across the membranes during the cytotoxic edema; therefore, more visually available contrast will be presented in contrast to CT imaging which requires significant amount water molecule activity and retention and more time to exhibit detectable contrasts [ 44 , 45 ].
However, not all medical centers have convenient access to MRI, and due to patient contraindications and intolerance to MRI scans, the amount of time it takes, and despite providing more accurate insight in tissue and vascular pathology, CT is considered the first-line imaging study at most centers [ 46 ]. Widespread availability, rapid scanning features, and ease of detecting intracranial hemorrhage make noncontract CT of the head an appealing choice in early acute stroke evaluation [ 47 ]. Although, approaches including multimodal CT/MRI protocols seem to be valuable tools in prevention, diagnosis and tracking the pathologic course of stroke and therapeutic response of interventions [ 48 ]. In addition, after the initial treatment is given and the acute emergency phase has passed, carotid ultrasound should be performed to evaluate the vessels’ status in terms of artery occlusion, decreased blood flow, and potential sources of embolism. The characteristics of the brain infarct (including the size and the location) are key findings to determine the subtype of stroke (Intracerebral hemorrhage, subarachnoid hemorrhage, and brain ischemia) [ 49 ].
Early signs of ischemic stroke in CT are chiefly described as hypoattenuation, loss of differentiation between the grey and the white matter, parenchymal swelling and edema, hyperattenuation of the MCA (middle cerebral artery), infarction in the territory of the brain-supplying arteries, and cortical sulcal effacement on the affected side [ 50 , 51 , 52 ]. The Alberta Stroke Program Early CT Score (ASPECTS) is a quantitative scoring system that evaluates early changes of MCA stroke, based on the topographic involvement of the MCA-supplying territories of the brain [ 53 ]. For each area involved, one point is deducted from the total of ten points. Some clinical variations of ASPECTS have also been introduced, such as pc-ASPECTS which evaluates the stroke of the posterior circulation [ 54 ]. On the other hand, speaking of MRI, an increase in DWI signals is evident from the first hours of the ischemic stroke [ 55 ]. While the ADC mapping shows reduced signals, T1- and T2-weighted sequences do not show significant changes in the early hyperacute phase of the ischemic stroke (0–6 h after stroke) [ 56 ]. T1-weighted MRI usually shows low signal intensity after 16 h, and T2-weighted MRI is usually high-signal after 8 h [ 56 ]. Therefore, the presentations of different MRI sequences could determine the age of the infarct.
Angiography modalities
Non-invasive methods such as CT Angiography (CTA) or Magnetic Resonance Angiography (MRA) are accessible and typically used for screening [ 57 , 58 ]. Although non-invasive imaging modalities prevent a significant number of stroke cases, choosing the right strategy may differ based on the time that has been passed from the transient ischemic attack [ 59 ]. With intravenous administration of contrast, CTA can illustrate a precise anatomical picture of the vascular system with sufficient details [ 60 ]. On the other hand, MRA is an appropriate modality to evaluate the dynamic changes, since the technique used in this imaging is based on changes in the vasculature flow [ 61 ]. Four-dimensional CTA and MRA are also less invasive alternatives to determine the clot burden and grading the collateral blood flow in large vessel occlusions [ 62 ]. CTA could be considered as the most appropriate approach to diagnose stenosis and occlusions [ 63 ]. However, as the injection of contrast material can cause kidney failure, it requires a thorough history, which is not always possible and time permitting in an acute stroke situation [ 64 , 65 ].
Role of ultrasonography in early identification of stroke
Ultrasound methods are also available as non-invasive measures, though they are practically used only for the evaluation of non-acute cerebrovascular accidents [ 66 ]. In addition, strokes of large arteries and patients with transient ischemic attack (TIA) can possibly benefit from Carotid Duplex Ultrasound (mostly extracranial vessels) and Transcranial Doppler (mostly intracranial vessels), where both of them are non-invasive [ 43 , 67 ]. Transcranial Doppler (TCD) relies on the physical phenomenon known as the Doppler effect [ 68 ]. In this case, changes in sound wave frequencies secondary to the motion of the blood in the arteries are measured and evaluated usually by calculating the Doppler shift. In addition, The peak systolic, diastolic, and mean flow velocities, along with the Gosling pulsatility index are also usually calculated, providing vital information about blood flow and whether it is altered or absent, or there is a change in pulsatility of the concerned vessel [ 69 , 70 ]. Detecting these changes helps the clinicians to find out about the presence of occlusion and determine if the vessel has been recanalized after the initiation of treatment or not. The previous studies have demonstrated that prehospital diagnosis of middle cerebral artery occlusion with or without microbubble contrast agents in stroke patients has a significant amount of sensitivity and specificity, and therefore, prehospital assessment with ultrasonography may provide therapeutic benefits for stroke patients [ 71 ]. Especially utilizing imaging techniques that describe the morphologic characteristics of lesions that lead to embolic events is useful in the early stages of stroke pathology, with ultrasound being the leading modality for monitoring the plaque rupture and dynamic changes in the vessels [ 72 ]. Doppler ultrasound may provide prognostic value that may guide the therapeutic approach in stroke patients and better risk scoring for stroke patients; As an example, previous studies reported that asymptomatic embolic signals, detected in patients with symptomatic carotid artery stenosis, may be useful in developing better risk score system for prognostic purposes and efficient antithrombotic therapy [ 73 , 74 , 75 ]. Table 1 provides a summary of the benefits and limitations of MRI, CT, and ultrasound.
Telemedicine and neuroimaging
Since the health burden of stroke has led to the development of specialized centers and units for providing stroke-related care, an increasing need for expanding a constant and reliable communication between these centers and the primary healthcare units is tangible. Therefore, new concepts such as telehealth and telemedicine have become bold, especially in emergencies, introducing even more specific terms, such as teleneurology and telestroke [ 76 , 77 , 78 ]. Telestroke aims to virtually examine the suspected stroke patient by evaluating the imaging results and providing clinical recommendations [ 79 ]. Neuroimaging is the fundamental element of telediagnosis for stroke, and appropriate imaging would indicate the best treatment approach for each patient.
Not all stroke patients need a transfer to a comprehensive stroke center (CSC). Several indications have been defined for the patients in need of CSC transfer, including qualification for tPA administration (ischemic stroke who would remain in the golden time after the transfer), endovascular thrombectomy, large-vessel occlusion, and massive stroke [ 80 , 81 ]. Apart from making the final decision for which patients should be transferred, the patients’ transfer time will also decrease significantly by implementing the telestroke strategies. From the several available modalities, CT is an essential for the primary units and local hospitals, since it benefits from availability in most emergency departments and rapid results. Based on the patient’s presentations, results of CT imaging, and the facilities of the primary unit, the stroke specialists might request the local team to perform vascular imaging, such as CTA, MRA, or Digital Subtraction Angiography (DSA) [ 82 ].
Providing a standard and detailed protocol for the application of telestroke could help the patients with a rapid and more successful management, and the healthcare system by reducing the patient burden.
Conclusions
According to the clinical importance of stroke and the advantages of early management, imaging plays a vital role in the patients’ survival. MRI is more accurate in ruling out the intracranial hemorrhage and MRI with DWI is more precise in the detection of acute ischemic stroke. However, due to the availability and lower acquisition time, CT is preferred in most healthcare settings. Being cognizant of the early warning signs of a stroke, alongside developing rapid, simple, and handy imaging techniques could help us improve the outcomes and overcome the burden of stroke.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
Apparent Diffusion Coefficient
Alberta Stroke Program Early CT Score
Cincinnati Prehospital Stroke Scale
Comprehensive stroke center
Computed tomography
Computed tomography angiography
Computed Tomography Perfusion Imaging
Digital Subtraction Angiography
Diffusion-Weighted Imaging
Face Arm Speech Time
Fluid-Attenuated Inversion Recovery
Gradient Recalled Echo
Kurashiki Prehospital Stroke Scale
Los Angeles Motor Scale
Los Angeles Prehospital Stroke Scale
Melbourne Ambulance Stroke Scale
Middle cerebral artery
Magnetic resonance angiography
Magnetic Resonance Imaging
National Institutes of Health Stroke Scale
Transient ischemic attack
Transcranial Doppler
Tissue Plasminogen Activators
Geisler F, Ali SF, Ebinger M, Kunz A, Rozanski M, Waldschmidt C, et al. Evaluation of a score for the prehospital distinction between cerebrovascular disease and stroke mimic patients. Int J Stroke. 2019;14(4):400–8. https://doi.org/10.1177/1747493018806194 .
Article PubMed Google Scholar
Chiaramonte R, Pavone P, Vecchio M. Speech rehabilitation in dysarthria after stroke: a systematic review of the studies. Eur J Phys Rehabil Med. 2020;56(5):547–62. https://doi.org/10.23736/s1973-9087.20.06185-7 .
Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):70. https://doi.org/10.1038/s41572-019-0118-8 .
Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120(3):439–48. https://doi.org/10.1161/CIRCRESAHA.116.308413 .
Article CAS PubMed Google Scholar
Yang Q, Tong X, Schieb L, Vaughan A, Gillespie C, Wiltz JL, et al. Vital signs: recent trends in stroke death rates—United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;66(35):933.
Article Google Scholar
Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009;8(4):345–54.
Almobarak A, Badi S, Elmadhoun W, Tahir H, Ahmed M. The prevalence and risk factors of stroke among Sudanese individuals with diabetes: cross-sectional survey. Brain Circ. 2020;6(1):26–30. https://doi.org/10.4103/bc.bc_15_19 .
Article PubMed PubMed Central Google Scholar
Advani R, Naess H, Kurz MW. The golden hour of acute ischemic stroke. Scand J Trauma Resusc Emerg Med. 2017;25(1):54.
Saver JL, Smith EE, Fonarow GC, Reeves MJ, Zhao X, Olson DM, et al. The “golden hour” and acute brain ischemia: presenting features and lytic therapy in >30,000 patients arriving within 60 minutes of stroke onset. Stroke. 2010;41(7):1431–9. https://doi.org/10.1161/strokeaha.110.583815 .
Di Stefano V, De Angelis MV, Montemitro C, Russo M, Carrarini C, di Giannantonio M, et al. Clinical presentation of strokes confined to the insula: a systematic review of literature. Neurol Sci. 2021. https://doi.org/10.1007/s10072-021-05109-1 .
Purrucker JC, Hametner C, Engelbrecht A, Bruckner T, Popp E, Poli S. Comparison of stroke recognition and stroke severity scores for stroke detection in a single cohort. J Neurol Neurosurg Psychiatry. 2015;86(9):1021–8.
Brunser AM, Hoppe A, Illanes S, Díaz V, Muñoz P, Cárcamo D, et al. Accuracy of diffusion-weighted imaging in the diagnosis of stroke in patients with suspected cerebral infarct. Stroke. 2013;44(4):1169–71.
Xing C, Arai K, Lo EH, Hommel M. Pathophysiologic cascades in ischemic stroke. Int J Stroke. 2012;7(5):378–85.
Alqahtani SA, Stemer AB, McCullough MF, Bell RS, Mai J, Liu A-H, et al. Endovascular management of stroke patients with large vessel occlusion and minor stroke symptoms. Cureus. 2017;9(6):e1355.
PubMed PubMed Central Google Scholar
Tziotzios C, Dawson J, Walters M. Stroke in practice: from diagnosis to evidence-based management. Boca Raton: CRC Press; 2017.
Book Google Scholar
Umemura T, Kawamura T, Hotta N. Pathogenesis and neuroimaging of cerebral large and small vessel disease in type 2 diabetes: a possible link between cerebral and retinal microvascular abnormalities. J Diabetes Investig. 2017;8(2):134–48.
Article CAS Google Scholar
Younger DS. Stroke due to vasculitis in children and adults. Neurol Clin. 2019;37(2):279–302.
Perera KS, Vanassche T, Bosch J, Swaminathan B, Mundl H, Giruparajah M, et al. Global survey of the frequency of atrial fibrillation-associated stroke: embolic stroke of undetermined source Global Registry. Stroke. 2016;47(9):2197–202.
Majersik JJ, Skalabrin EJ. Single-gene stroke disorders. Seminars in neurology. New York: Thieme Medical Publishers Inc.; 2006.
Google Scholar
Rosenberg RN, Pascual JM. Rosenberg’s molecular and genetic basis of neurological and psychiatric disease, vol. 2. Cambridge: Academic press; 2020.
Zhao X-J, Larkin TM, Lauver MA, Ahmad S, Ducruet AF. Tissue plasminogen activator mediates deleterious complement cascade activation in stroke. PLoS ONE. 2017;12(7):e0180822.
Gerriets T, Walberer M, Ritschel N, Tschernatsch M, Mueller C, Bachmann G, et al. Edema formation in the hyperacute phase of ischemic stroke. J Neurosurg. 2009;111(5):1036–42.
Wang P, Shao B-Z, Deng Z, Chen S, Yue Z, Miao C-Y. Autophagy in ischemic stroke. Prog Neurobiol. 2018;163:98–117.
Endres M, Dirnagl U, Moskowitz MA. The ischemic cascade and mediators of ischemic injury. Handb Clin Neurol. 2008;92:31–41.
Rodrigo R, Fernández-Gajardo R, Gutiérrez R, Manuel Matamala J, Carrasco R, Miranda-Merchak A, et al. Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol Disord-Drug Targets (Formerly Curr Drug Targets-CNS Neurol Disord). 2013;12(5):698–714.
CAS Google Scholar
Guo Y, Li P, Guo Q, Shang K, Yan D, Du S, et al. Pathophysiology and biomarkers in acute ischemic stroke—a review. Trop J Pharm Res. 2013;12(6):1097–105.
Kamalian S, Lev MH. Stroke imaging. Radiol Clin N Am. 2019;57(4):717–32. https://doi.org/10.1016/j.rcl.2019.02.001 .
Chugh C. Acute ischemic stroke: management approach. Indian J Crit Care Med Peer-rev. 2019;23(Suppl 2):S140.
Alonso de Leciñana M, Egido JA, Casado I, Ribó M, Dávalos A, Masjuan J, et al. Guidelines for the treatment of acute ischaemic stroke. Neurologia (Barcelona, Spain). 2014;29(2):102–22. https://doi.org/10.1016/j.nrl.2011.09.012 .
Liao XL, Wang CX, Wang YL, Wang CJ, Zhao XQ, Zhang LQ, et al. Implementation and outcome of thrombolysis with alteplase 3 to 4.5 h after acute stroke in Chinese patients. CNS Neurosci Ther. 2013;19(1):43–7. https://doi.org/10.1111/cns.12031 .
Merino JG, Warach S. Imaging of acute stroke. Nat Rev Neurol. 2010;6(10):560.
Chrzan R, Gleń A, Urbanik A. Hyperdense middle cerebral artery sign as the only radiological manifestation of hyperacute ischemic stroke in computed tomography. Neurol Neurochir Pol. 2017;51(1):33–7.
Nakano S, Iseda T, Kawano H, Yoneyama T, Ikeda T, Wakisaka S. Correlation of early CT signs in the deep middle cerebral artery territories with angiographically confirmed site of arterial occlusion. AJNR Am J Neuroradiol. 2001;22(4):654–9.
CAS PubMed PubMed Central Google Scholar
Weimar C. Stroke of undetermined cause: workup and secondary prevention. Curr Opin Neurol. 2016;29(1):4–8. https://doi.org/10.1097/wco.0000000000000280 .
Ifergan H, Amelot A, Ismail M, Gaudron M, Cottier JP, Narata AP. Stroke-mimics in stroke-units. Evaluation after changes imposed by randomized trials. Arq Neuropsiquiatr. 2020;78(2):88–95. https://doi.org/10.1590/0004-282x20190154 .
Quenardelle V, Lauer-Ober V, Zinchenko I, Bataillard M, Rouyer O, Beaujeux R, et al. Stroke mimics in a stroke care pathway based on MRI screening. Cerebrovasc Dis (Basel, Switzerland). 2016;42(3–4):205–12. https://doi.org/10.1159/000445956 .
Psychogios K, Kargiotis O, Safouris A, Magoufis G, Gelagoti M, Bonakis A, et al. Perfusion imaging averting intravenous thrombolysis in stroke mimics. Neurol Sci. 2021;42(6):2591–4. https://doi.org/10.1007/s10072-021-05090-9 .
Provost C, Soudant M, Legrand L, Ben Hassen W, Xie Y, Soize S, et al. Magnetic resonance imaging or computed tomography before treatment in acute ischemic stroke. Stroke. 2019;50(3):659–64. https://doi.org/10.1161/strokeaha.118.023882 .
Zhang W, Cheng J, Zhang Y, Wang K, Jin H. Analysis of CT and MRI combined examination for the diagnosis of acute cerebral infarction. J Coll Physicians Surg Pak. 2019;29(9):898–9. https://doi.org/10.29271/jcpsp.2019.09.898 .
Malhotra K, Liebeskind D. Overview of neuroimaging of stroke. In: Welch KM, Caplan LR, Siesjo BK, Weir B, Reis DJ, editors. Primer on cerebrovascular diseases. Amsterdam: Elsevier; 2017. p. 676–85.
Chapter Google Scholar
Wang Y, Zhou Z, Ding S. FLAIR vascular hyperintensity-DWI mismatch most likely to benefit from recanalization and good outcome after stroke. Medicine. 2020;99(2):e18665. https://doi.org/10.1097/md.0000000000018665 .
Boss SM, Moustafa RR, Moustafa MA, El Sadek A, Mostafa MM, Aref HM. Lesion homogeneity on diffusion-weighted imaging is a marker of outcome in acute ischemic stroke. Egypt J Neurol Psychiatry Neurosurg. 2019;55(1):59. https://doi.org/10.1186/s41983-019-0101-z .
Oliveira-Filho J, WJ K. Initial assessment and management of acute stroke. UpToDate. Waltham: UpToDate Retrieved January. 2010.
Mullins ME, Schaefer PW, Sorensen AG, Halpern EF, Ay H, He J, et al. CT and conventional and diffusion-weighted MR imaging in acute stroke: study in 691 patients at presentation to the emergency department. Radiology. 2002;224(2):353–60. https://doi.org/10.1148/radiol.2242010873 .
van Gelderen P, de Vleeschouwer MHM, DesPres D, Pekar J, van Zijl PCM, Moonen CTW. Water diffusion and acute stroke. Magn Reson Med. 1994;31(2):154–63. https://doi.org/10.1002/mrm.1910310209 .
Almandoz JED, Romero JM. Advanced CT imaging in the evaluation of hemorrhagic stroke. Neuroimaging Clin. 2011;21(2):197–213.
Zerna C, Thomalla G, Campbell BC, Rha J-H, Hill MD. Current practice and future directions in the diagnosis and acute treatment of ischaemic stroke. Lancet. 2018;392(10154):1247–56.
Liebeskind DS, Alexandrov AV. Advanced multimodal CT/MRI approaches to hyperacute stroke diagnosis, treatment, and monitoring. Ann N Y Acad Sci. 2012;1268:1.
Kang DW, Jeong HG, Kim DY, Yang W, Lee SH. Prediction of stroke subtype and recanalization using susceptibility vessel sign on susceptibility-weighted magnetic resonance imaging. Stroke. 2017;48(6):1554–9. https://doi.org/10.1161/strokeaha.116.016217 .
Wardlaw JM, Mielke O. Early signs of brain infarction at CT: observer reliability and outcome after thrombolytic treatment—systematic review. Radiology. 2005;235(2):444–53. https://doi.org/10.1148/radiol.2352040262 .
Lövblad KO, Altrichter S, Mendes Pereira V, Vargas M, Marcos Gonzalez A, Haller S, et al. Imaging of acute stroke: CT and/or MRI. J Neuroradiol. 2015;42(1):55–64. https://doi.org/10.1016/j.neurad.2014.10.005 .
Guberina N, Dietrich U, Radbruch A, Goebel J, Deuschl C, Ringelstein A, et al. Detection of early infarction signs with machine learning-based diagnosis by means of the Alberta Stroke Program Early CT score (ASPECTS) in the clinical routine. Neuroradiology. 2018;60(9):889–901. https://doi.org/10.1007/s00234-018-2066-5 .
Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet (Lond, Engl). 2000;355(9216):1670–4. https://doi.org/10.1016/s0140-6736(00)02237-6 .
Pallesen LP, Gerber J, Dzialowski I, van der Hoeven EJ, Michel P, Pfefferkorn T, et al. Diagnostic and prognostic impact of pc-ASPECTS applied to perfusion CT in the Basilar Artery International Cooperation Study. J Neuroimaging. 2015;25(3):384–9. https://doi.org/10.1111/jon.12130 .
Lansberg MG, Albers GW, Beaulieu C, Marks MP. Comparison of diffusion-weighted MRI and CT in acute stroke. Neurology. 2000;54(8):1557–61. https://doi.org/10.1212/wnl.54.8.1557 .
Allen LM, Hasso AN, Handwerker J, Farid H. Sequence-specific MR imaging findings that are useful in dating ischemic stroke. Radiographics. 2012;32(5):1285–97. https://doi.org/10.1148/rg.325115760 ( discussion 97-9 ).
Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015;275(2):510–20.
Eastman AL, Muraliraj V, Sperry JL, Minei JP. CTA-based screening reduces time to diagnosis and stroke rate in blunt cervical vascular injury. J Trauma. 2009;67(3):551–6. https://doi.org/10.1097/TA.0b013e3181b84408 ( discussion 5-6 ).
Wardlaw JM, Stevenson MD, Chappell F, Rothwell PM, Gillard J, Young G, et al. Carotid artery imaging for secondary stroke prevention. Stroke. 2009;40(11):3511–7. https://doi.org/10.1161/STROKEAHA.109.557017 .
Liebeskind DS, Kosinski AS, Saver JL, Feldmann E, Investigators S. Computed tomography angiography in the stroke outcomes and neuroimaging of intracranial atherosclerosis (SONIA) study. Interv Neurol. 2013;2(4):153–9.
Liebeskind DS, Kosinski AS, Lynn MJ, Scalzo F, Fong AK, Fariborz P, et al. Noninvasive fractional flow on MRA predicts stroke risk of intracranial stenosis. J Neuroimaging. 2015;25(1):87–91. https://doi.org/10.1111/jon.12101 .
Kilburg C, McNally JS, de Havenon A, Taussky P, Kalani MYS, Park MS. Advanced imaging in acute ischemic stroke. Neurosurg Focus. 2017;42(4):E10.
Latchaw RE, Alberts MJ, Lev MH, Connors JJ, Harbaugh RE, Higashida RT, et al. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40(11):3646–78.
Myung JW, Kim JH, Cho J, Park I, Kim HY, Beom JH. Contrast-induced acute kidney injury in radiologic management of acute ischemic stroke in the emergency setting. AJNR Am J Neuroradiol. 2020;41(4):632–6. https://doi.org/10.3174/ajnr.A6472 .
Article CAS PubMed PubMed Central Google Scholar
Hopyan JJ, Gladstone DJ, Mallia G, Schiff J, Fox AJ, Symons SP, et al. Renal safety of CT angiography and perfusion imaging in the emergency evaluation of acute stroke. AJNR Am J Neuroradiol. 2008;29(10):1826–30. https://doi.org/10.3174/ajnr.A1257 .
Connolly F, Röhl JE, Guthke C, Wengert O, Valdueza JM, Schreiber SJ. Emergency room use of “fast-track” ultrasound in acute stroke: an observational study. Ultrasound Med Biol. 2019;45(5):1103–11. https://doi.org/10.1016/j.ultrasmedbio.2019.01.001 .
Buskens E, Nederkoorn PJ, Buijs-Van Der Woude T, Mali WP, Kappelle LJ, Eikelboom BC, et al. Imaging of carotid arteries in symptomatic patients: cost-effectiveness of diagnostic strategies. Radiology. 2004;233(1):101–12. https://doi.org/10.1148/radiol.2331030863 .
Purkayastha S, Sorond F. Transcranial Doppler ultrasound: technique and application. Semin Neurol. 2012;32(4):411–20. https://doi.org/10.1055/s-0032-1331812 .
de Riva N, Budohoski KP, Smielewski P, Kasprowicz M, Zweifel C, Steiner LA, et al. Transcranial Doppler pulsatility index: what it is and what it isn’t. Neurocrit Care. 2012;17(1):58–66. https://doi.org/10.1007/s12028-012-9672-6 .
Robba C, Cardim D, Sekhon M, Budohoski K, Czosnyka M. Transcranial Doppler: a stethoscope for the brain-neurocritical care use. J Neurosci Res. 2018;96(4):720–30. https://doi.org/10.1002/jnr.24148 .
Schlachetzki F, Herzberg M, Hölscher T, Ertl M, Zimmermann M, Ittner KP, et al. Transcranial ultrasound from diagnosis to early stroke treatment—part 2: prehospital neurosonography in patients with acute stroke—The Regensburg Stroke Mobile Project. Cerebrovasc Dis. 2012;33(3):262–71.
Giannoni MF, Vicenzini E, Sbarigia E, Di Piero V, Lenzi GL, Speziale F. Early ultrasound imaging of carotid arteries in the acute ischemic cerebrovascular patients. Perspect Med. 2012;1(1–12):108–11.
Markus HS, MacKinnon A. Asymptomatic embolization detected by Doppler ultrasound predicts stroke risk in symptomatic carotid artery stenosis. Stroke. 2005;36(5):971–5. https://doi.org/10.1161/01.STR.0000162717.62684.40 .
Prabhakaran S. Imaging markers of stroke risk in asymptomatic carotid artery stenosis. Brain Circ. 2015;1(1):38–46. https://doi.org/10.4103/2394-8108.166373 .
Tang Y, Wang M-Y, Wu T-T, Zhang J-Y, Yang R, Zhang B, et al. The role of carotid stenosis ultrasound scale in the prediction of ischemic stroke. Neurol Sci. 2020;41(5):1193–9. https://doi.org/10.1007/s10072-019-04204-8 .
Mahmoodpoor A, Akbarzadeh MA, Sanaie S, Hosseini MS. Role of telehealth in outbreaks—where the classical healthcare systems fail. Infect Control Hosp Epidemiol. 2020;41(8):992–4. https://doi.org/10.1017/ice.2020.120 .
Birns J, Bhalla A, Rudd A. Telestroke: a concept in practice. Age Ageing. 2010;39(6):666–7. https://doi.org/10.1093/ageing/afq125 .
Hubert GJ, Corea F, Schlachetzki F. The role of telemedicine in acute stroke treatment in times of pandemic. Curr Opin Neurol. 2021;34(1):22–6. https://doi.org/10.1097/wco.0000000000000887 .
Agarwal S, Warburton EA. Teleneurology: is it really at a distance? J Neurol. 2011;258(6):971–81. https://doi.org/10.1007/s00415-011-5920-5 .
Laghari FJ, Hammer MD. Telestroke imaging: a review. J Neuroimaging. 2017;27(1):16–22. https://doi.org/10.1111/jon.12402 .
Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet (Lond, Engl). 2016;387(10029):1723–31. https://doi.org/10.1016/s0140-6736(16)00163-x .
Pedragosa A, Alvarez-Sabín J, Rubiera M, Rodriguez-Luna D, Maisterra O, Molina C, et al. Impact of telemedicine on acute management of stroke patients undergoing endovascular procedures. Cerebrovasc Dis (Basel, Switzerland). 2012;34(5–6):436–42. https://doi.org/10.1159/000345088 .
Download references
Acknowledgements
Author information, authors and affiliations.
Student Research Committee, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Mohammad Amin Akbarzadeh & Mohammad-Salar Hosseini
Neurosciences Research Center, Tabriz University of Medical Sciences, Golgasht Street, 5166/15731, Tabriz, EA, Iran
Sarvin Sanaie & Mohammad-Salar Hosseini
Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
Sarvin Sanaie & Mahshid Kuchaki Rafsanjani
Emergency Medicine Research Team, Tabriz University of Medical Sciences, Tabriz, Iran
Mohammad-Salar Hosseini
You can also search for this author in PubMed Google Scholar
Contributions
SS and MSH developed the idea, MAA, MSH, and MKR carried out the data gathering, MAA and MSH prepared the manuscript, and SS and MKR revised the manuscript critically. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Mohammad-Salar Hosseini .

Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Akbarzadeh, M.A., Sanaie, S., Kuchaki Rafsanjani, M. et al. Role of imaging in early diagnosis of acute ischemic stroke: a literature review. Egypt J Neurol Psychiatry Neurosurg 57 , 175 (2021). https://doi.org/10.1186/s41983-021-00432-y
Download citation
Received : 11 June 2021
Accepted : 03 December 2021
Published : 20 December 2021
DOI : https://doi.org/10.1186/s41983-021-00432-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Brain infarction
- Cerebrovascular disorders
- Diagnostic imaging
- Intravenous thrombolysis
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Biomed Res Int

Systematic Review of Existing Stroke Guidelines: Case for a Change
Tissa wijeratne.
1 School of Applied Health, Department of Psychology, RMIT University, Melbourne, Australia
2 Department of Medicine, Faculty of Medicine, University of Rajarata, Saliyapura, Anuradhapura, Sri Lanka
3 Department of Neurology, Western Health & University of Melbourne, AIMSS, Level Three, WHCRE, Sunshine Hospital, St Albans, 3021, Australia
Carmela Sales
Chanith wijeratne.
4 Monash Medical School, Clayton, Victoria, Australia
Leila Karimi
5 Faculty of Social and Political Sciences, Tbilisi State University, Georgia
Mihajlo Jakovljevic
6 Institute of Advanced Manufacturing Technologies, Peter the Great St. Petersburg Polytechnic University, St. Petersburg, Russia
7 Institute of Comparative Economic Studies, Hosei University Chiyoda, Japan
8 Department of Global Health Economics and Policy, University of Kragujevac, Serbia
Associated Data
Data that support the findings of this study are available from the corresponding author, [TW], upon reasonable request.
We systematically searched for guideline recommendation on the day-to-day use of peripheral inflammatory markers such as NLR published in the English language between January 1, 2005, and October 2020. Any other evidence of system biology-based approach or recommendation was explored within the selected guidelines for this scoping review. Only the latest guideline per writing group was selected. Each guideline was analyzed independently by 2 to 4 authors to determine clinical scenarios explained/given, scientific evidence used, and recommendations presented in the context of system biology.
The scoping review found 2,911 titles at the beginning of the search. Final review included with 15 guidelines. Stroke-related organizations wrote sixty-five percent of the guidelines while national ministries wrote a fewer number of guidelines. We were primarily interested in recommendations for acute management in AIS published in the English language. Fifteen eligible guidelines were identified from 15 different countries/regions. None of the guidelines recommended the routine use of peripheral markers of inflammation, such as NLR, among their acute assessment and management recommendations. None of the existing guidelines explored the system biology approach to one of the most complex diseases affecting the human brain, stroke.
Conclusions
This systematic review has identified a significant evidence-practice gap in all existing national stroke guidelines published in English medium as of October 2020. These guidelines included the only current “living stroke guidelines,” stroke guidelines from Australia with a real opportunity to modernize the living stroke guidelines with systems biology approach, and provide 2020 vision towards better stroke care globally. Investigation of complex disease such as stroke is best served through a systems biology approach. One of the easiest places to start is simple blood tests such as total white cell count and NLR. Systems biology approach point us towards simple tools such immune-inflammatory index (SII) and serial systemic immune inflammatory indices (SSIIi) which should pave the way for the stroke physician community address the challenges in systems biology approach in stroke care. These challenges include translating bench research to the bedside, managing big data (continuous pulse, blood pressure, sleep, oxygen saturation, progressive changes in NLR, SII, SSIIi, etc.). Working with an interdisciplinary team also provides a distinct advantage. Recent adoption of historic WHO-IGAP calls for immediate action. The 2022 World Brain Day campaign on Brain Health for All is the perfect opportunity to raise awareness and start the process.
1. Introduction
Evidence-based medicine calls for the utilization of widely available clinical guidelines especially for the management of common conditions which have an impact on mortality and morbidity such as acute ischemic stroke (AIS). The first of this kind was published in 1974 which was entitled “Prologue to Guidelines for Stroke Care,” a compendium of articles compiled by neurologists on the management of cerebrovascular disease [ 1 ] It was not until more than 20 years later that the Cochrane Collaboration Stroke Review Group convened and initiated the task of constructing a systematic guideline for the management of acute stroke [ 2 ].
Clinical guidelines are essential tools to improve the quality of healthcare systems. Factors which are crucial for a clinical guideline to be successfully crafted are team collaboration and multidisciplinary engagement [ 3 , 4 ]. Furthermore, these should be tailor-fitted to individual country needs, hence, the nonexistence of a universally implemented guideline [ 4 ]. The use of tools to assess the quality of evidence also aids clinicians to interpret the recommendations according to the weight of evidence [ 5 ]. Potential barriers to nonadherence include unfamiliarity, lack of agreement, and outcome expectancy, as well as the significant impact of the precedent guideline [ 6 ].
Perhaps one of the game changers in the history of medicine is the development of clinical guidelines for the management of AIS. The wealth of data from clinical trials on reperfusion therapies paved the way for the American Heart Association (AHA) and the Canadian Stroke Consortium to publish their respective recommendations on the acute intervention of cerebrovascular ischemia [ 7 , 8 ]. Through time, various versions of clinical guidelines have also been published in different languages with the primary objective of implement ability according to the resources available in each country. While constructs behind these standard procedures are anchored on the same theory, some degree of variability still occurs [ 9 ]. To date, there are no studies which specifically look at the differences in the clinical guidelines on acute ischemic stroke globally. It is in this light that this study was conceived.
2. Methodology
The authors of this review used the Arksey and O'Malley methodology to identify and extract useful literature. The steps undertaken include (1) research question identification; (2) relevant literature identification; (3) screening and selection of relevant literature; (4) data charting; and (5) analyzing, summarizing, and reporting results.
MEDLINE, Cochrane, and CINHAL databases were searched to identify useful keywords. Subsequently, the identified keywords were used to search the same databases for relevant studies. Literature were first screened at the title and the abstract level and then the full text articles.
Following search terms were employed based on the PICO strategy. Topic = “country name” AND TOPIC = “guideline” OR” clinical protocols” OR “recommendations” OR” standards” AND TOPIC = stroke OR cerebrovascular disorder OR cerebrovascular accident.
Guideline repositories such as the National Guideline Clearinghouse, the Scottish Intercollegiate Guidelines Network (SIGN), and Professional stroke societies were also searched. Individual bibliographies were also manually searched. Studies were included if they met the following criteria: (a) published after year 2000, (b) guidelines on stroke and/or poststroke rehabilitation, (c) graded recommendations, and (d) written in English. Titles and abstracts were initially screened (TW), and any full-text articles were further appraised (TW, CS). Any disagreement was adjudicated by an independent reviewer (LK). Guidelines which were updated in a modular format and published over separate papers were treated as one guideline.
3.1. Guideline Characteristics
Figure 1 shows the diagram on available stroke guidelines worldwide. Figure 2 shows the PRISMA diagram of the process. Majority of the countries have no available published national guidelines while a number have guidelines but no graded recommendations. A significant majority also have guidelines published in their own language while 14 countries have their own published, graded, English clinical guidelines, with the one from the European Stroke Organization as a separate entity.

Acute ischemic stroke guidelines worldwide.

PRISMA diagram.
A total of 2897 titles were identified in the electronic search. Fourteen additional records were identified through other sources. After removal of duplicates and screening at the title level, 255 articles were further reviewed at the abstract level. Hundred and eighty-one papers were thoroughly assessed by the two authors (TW and CS) for eligibility. A total of 15 guidelines were included in this scoping review.
Table 1 outlines the characteristics of the 15 clinical guidelines included in this study.
Summary of acute ischemic stroke clinical guidelines.
| Country | Name of guideline | Year of first published version | Subsequent revisions |
|---|---|---|---|
| Australia/New Zealand [ ] | Clinical Guidelines for Stroke Management | 2007 | 2010, 2019 |
| Brazil [ ] | Guidelines for Acute Ischemic Stroke Treatment | 2001 | 2012 |
| Canada [ ] | Canadian Best Practice Recommendations for Acute Stroke Management | 1998 | 2006, 2008, 2010, 2015, 2018 |
| China [ ] | The Chinese Stroke Association scientific statement: intravenous thrombolysis in acute ischemic stroke | 2012 | 2014, 2017 |
| ESO [ ] | European Stroke Organisation–Karolinska Stroke Update | 2003 | 2015, 2017, 2019 |
| Italy [ ] | The Italian guidelines for stroke prevention | 2000 | |
| Japan [ ] | Japanese Guidelines for the Management of Stroke | 2004 | 2009 |
| Malaysia [ ] | Clinical practice guidelines, management of ischemic stroke | 2003 | 2006 |
| Qatar [ ] | Clinical Guidelines for the State of Qatar, the Diagnosis and Management of Stroke and TIA | 2016 | |
| Scotland [ ] | Management of patients with stroke and TIA: assessment, investigation, immediate management, and secondary prevention | 2008 | |
| Singapore [ ] | Stroke and TIA: assessment, investigation, immediate management, and secondary prevention | 2009 | 2011 |
| South Africa [ ] | South African guideline for management of ischemic stroke and transientischemic attack 2010: a guideline from the South African Stroke Society (SASS) and the SASS Writing Committee | 2000 | 2010 |
| Sri Lanka [ ] | Clinical practice guidelines, management of stroke | 2017 | |
| UK [ ] | National clinical guideline for stroke | 2000 | 2016, 2017 |
| USA [ ] | Guidelines for the early Management of Patients with Acute Ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke | 1994 | 1996, 2003, 2005, 2007, 2013, 2015, 2018/2019 |
3.2. Regionalization and Adaptation from Other Clinical Guidelines
Most countries worldwide have no available published national guidelines. However, this does not translate to lack of systematic processes and workflows in the management of acute ischemic stroke. The European Stroke Organization has successfully implemented the ESO Stroke Guidelines which is being operationalized by countries in the European region [ 14 ]. A unified approach is also being implemented in Australia and New Zealand as they both adapt the Australian Clinical Guideline for Stroke Management published in 2017 [ 25 ]. More recently, the Middle East and North Africa Stroke and Interventional Neurotherapies Organization has also created a consolidated plan to manage stroke in the midst of the pandemic [ 26 ].
Most of the conceptualized guidelines have been adapted from existing ones, usually from high-income countries [ 27 ]. A systematic review comparing stroke clinical practice guidelines (CPGs) from low- and high-income countries revealed a degree of compromise in terms of the quality on the former [ 27 ]. It is in this reason that in 2014, the World Stroke Organization conceived the WSO Global Stroke Services Guideline and Action Plan [ 28 ]. This initiative aims to aid country-level health authorities to set up or improve existing stroke frameworks to achieve high-quality, evidence-based recommendations and ensure that outcomes are measured to foster a milieu for continuous improvement [ 27 ].
3.3. The Need for Grading Recommendations
While countries have their own specific treatment recommendations, grading of evidence with the use of standardized systems is lacking. It is essential for guidelines to incorporate these as it ensures transparency and some level of confidence as these recommendations are translated into clinical practice [ 29 ]. Various country-specific guidelines make use of their own grading systems in assessing the weight and level of evidence of the recommended guidelines [ 12 , 14 , 24 ]. It is also essential for grading systems to be customized accordingly for low-income countries. Epidemiologists suggest the adaptation of internationally recognized approaches with efforts to integrate local evidence and weigh in appropriate resources [ 30 ].
3.4. Clinical Trials That Changed the Guidelines
In 1994, the AHA published the first clinical guideline on the management of acute ischemic stroke [ 31 ]. While the efficacy of thrombolytic therapy was already being recognized then, it remained to be in the sidelines for safety concerns [ 31 , 32 ]. With the encouraging results of the NINDs trial and the subsequent approval of alteplase by the US-FDA for systemic reperfusion, the AHA guidelines were updated, and it was also within this period that the Canadian Stroke Guidelines were conceived [ 7 , 8 , 33 ]. Other major clinical guidelines from different parts of the world were also published subsequently.
With the aim of further improving stroke care, further modifications of then existing guidelines have been made. With the promising results of the ECASS3 trial, the time period for thrombolysis has been extended from three to 4.5 hours [ 34 , 35 ]. The results of the J-ACT trial in 2006 has also resulted in the approval for use of the 0.6 mg/kg dose of alteplase as mandated by the revised 2009 Japanese guidelines for stroke [ 16 , 36 ].
The results of five clinical trials on endovascular therapy from 2012 to 2014 have also revolutionized the landscape of stroke management in the year 2015. The MR CLEAN, ESCAPE, EXTEND-IA, SWIFT-PRIME, and the REVASCAT trials showed statistically significant improvement in clinical and radiologic outcomes after endovascular therapy (EVT) for large vessel occlusion [ 37 – 41 ]. Clinical guidelines were revised so that patients within six hours from onset of symptoms were deemed eligible for EVT [ 10 , 12 , 24 ]. A few years later, this time period was extended to 16-24 hours based on perfusion imaging parameters, as demonstrated by the DAWN and the DEFUSE 3 trials [ 42 , 43 ]. Various clinical trials are still in the pipeline and are expected to make significant changes in existing guidelines worldwide in the future.
3.5. Initial Assessment
Guidelines included in the study are represented from all parts of the world including Asia, Australia, Europe, Africa, and America ( Table 1 ). Sections of clinical guidelines are subdivided into initial assessment, supportive treatment, reperfusion therapy, management of complications, and rehabilitation. In most guidelines, prehospital and preventive strategies are usually included, but these are not discussed in this study. It is noted that some degree of variability in grading exists with the appraisal of different clinical guidelines.
In terms of initial assessment, there is unanimity in the clinical strategies that all patients suspected to have stroke should have neuroimaging urgently. This received the strongest recommendation among most of the countries with only ones from USA and Qatar, putting significant weight on aiming less than 20 minutes for it to be accomplished. High-income countries who have established facilities for endovascular thrombectomy also put the priority on neurovascular imaging. On the other hand, only a number of countries emphasize the use of scales for stroke severity assessment.
The importance of neuroimaging cannot be overemphasized in the management of acute stroke. While seamless processes to ensure efficiency in initial brain scanning have already been established in high-income countries, limitations in resources and logistics are still problematic most especially in rural areas of low to middle-income countries [ 44 ]. For example, a tertiary center in India identified that the lack of neuroimaging facilities posed as one of the most important barriers for thrombolysis, with even the out of pocket cost for CT scan contributing to this limitation [ 45 ]. It is also practical for other countries such as Sri Lanka, South Africa, and Malaysia not to put too much weight on neurovascular imaging as inaccessibility to neurointerventionists and comprehensive stroke centers, as well as the high cost of treatment for this sophisticated procedure, is still one of the identified problems in most developing countries [ 46 ]. On the other hand, among countries in which reputable standard operating procedures for neuroimaging are already existent, aiming to shorter door to imaging times are being optimized, as trends to improved clinical outcomes have been observed [ 47 ].
There is also homogeneity among different countries in terms of what ancillary tests are to be performed during the hyperacute management of stroke. Serum blood glucose is being specified as an absolute test to be done prior to thrombolysis in some countries while in some, this is not explicitly identified. There is also unanimity among different countries that troponin, immune cell counts, and ECG should not be deterrents to timely thrombolysis. While obtaining baseline temperature is deemed significant in almost all clinical guidelines, less degree of weight is put in this parameter as opposed to blood glucose.
It has long been recognized that hypo and hyperglycemia are known stroke masquerades [ 48 ]. A study in 2015 among 80 consecutively recruited hypoglycemic patients revealed that 11% had stroke-like presentation with symptoms reversing within one hour of administration of intravenous dextrose [ 49 ]. Furthermore, it is also essential that this parameter be recognized and corrected at an early stage as glycemic aberrations in the perithrombolysis period may significantly impact on clinical outcomes [ 50 ]. While deemed equally important, cardiac investigations should not preclude nor delay thrombolysis. It has been demonstrated in various studies that the presence of strain pattern, t -wave alterations, and QT dispersion may be predictors of poor outcomes among stroke patients [ 51 – 53 ]. Troponin is also essential to exclude the co-occurrence of AIS and acute myocardial infarction. A national registry including more than 800,000 patients with AIS identified that simultaneous occurrence of both only happens in 1.6% of the patients [ 54 ]. While the incidence is significantly low, substantial increase in hospital mortality has been observed [ 54 ].
3.6. General Supportive Care
There is heterogeneity in terms of supportive care among acute stroke patients with most clinical guidelines stressing moderate to strong recommendations on airway protection, correction of fluid imbalances, and treatment of sources of hyperthermia and hypoglycemia. Consensus for blood pressure targets is not uniform, with Caucasian guidelines emphasizing a threshold of 180/105 prior to thrombolysis while some Asian guidelines follow a higher target [ 17 , 20 ].
It is essential that acute stroke units be organized in a manner that caters to the efficient provision of abovementioned parameters as this has been positively associated with good outcomes such as reduction of mortality, length, and cost of hospitalization as well as institutionalization [ 55 , 56 ]. This is particularly problematic in low- to middle-income countries because of concerns for costs, facilities, and hospital staffing. Contrary to this, a recent prospective observational study in a tertiary hospital in South Africa demonstrated that despite the resource limitations, adaptation of the acute stroke response network which integrates organization of an acute stroke unit yields favorable thrombolysis outcomes at par to those observed in developed countries [ 57 , 58 ].
Evidence proves that blood pressure optimization during thrombolysis results in good functional outcomes [ 59 ]. Prospective and retrospective studies as well as clinical trials reveal that blood pressure during thrombolysis ranging from 140 to 160 reduced the odds of poor outcomes [ 60 – 62 ]. To date, no studies have identified the most optimal blood pressure to achieve best outcomes post reperfusion therapy; however, clinical trial targets are set at 180/105; hence, the parameters are set in clinical guidelines [ 24 ].
3.7. Thrombolysis and the Management of Medical and Surgical Complications
There is also agreement between different guidelines that thrombolytic therapy (tissue plasminogen activator, alteplase) at a dose of 0.9 mg/kg be instituted among eligible patients who arrive between three and 4.5 hours from the onset of symptoms. It is only the Japanese guideline which has approved of the use of the lower dose (0.6 mg/kg). Also, only a few guidelines explicitly emphasize recommendations on the management of bleeding and angioedema after treatment. Neurosurgical recommendations for the management of malignant infarcts and obstructive hydrocephalous are also clearly defined in medium and high-income countries.
Majority of the clinical trials which looked at the safety and efficacy of the low-dose alteplase were employed among Asians, specifically Japanese. The favorable results of the J-ACT, ENCHANTED, and THAWS trial support the Japanese recommendations [ 36 , 62 – 64 ]). Aside from practical reasons of the lower cost from the reduced dose of alteplase (which usually just consumes 1 vial per dose), physiologic advantages such as lower levels of fibrinogen and plasminogen activator inhibitor-1(PAI-1) along with less marked genetic polymorphisms that induces a higher state of coagulation compared to Caucasians have also cited by Ueshima and colleagues [ 65 ]. On the other hand, thrombolysis of patients with unclear onset of symptoms but with eligibility according the neuroimaging parameters of the WAKE-UP trial has also made the Australian and the AHA stroke guidelines recommend in favor of the later [ 66 ].
It is also interesting to note that of the guidelines reviewed, only three had explicitly stated recommendations on the management of thrombolysis-related complications such as bleeding and angioedema. More so, of the Asian countries included, only Japan had clear statements with this regard. It is equally important to address these limitations especially in resource-limited regions such as Asia and South America, where there is also a scarcity of stroke intensive care units [ 67 , 68 ].
Encouraging results of various clinical trials for the management of malignant supra and infratentorial infarctions have been instrumental for the increase in confidence for guidelines to recommend these procedures especially for highly eligible patients. While this is of no question for countries with sufficient infrastructure and manpower, it has always been challenging for low- and middle-income countries. In sub-Saharan Africa, it has been previously identified that the ratio of neurosurgeon to population is as low as 1 : 64,000,000 [ 69 ]. Furthermore, a study in 2015 on the economic losses attributed to neurosurgical diseases revealed that stroke was a major contributor to the three trillion macroeconomic deficits particularly in low-income countries [ 70 ]. It is therefore critical that guidelines be crafted according to individually available resources to ensure optimal implementability.
3.8. Poststroke Rehabilitation
Stroke rehabilitation is another key component of stroke clinical guidelines. Majority put significant weight on early rehabilitation while moderate to weak strengths have been tagged for professional dysphagia assessment. The American, Australian, and UK guidelines likewise put high premium on functional assessment while heterogeneity exists on integrating rehabilitation on comprehensive stroke care center as well as the use of intermittent pneumatic compression for deep vein thrombosis. Majority of the guidelines have weak or no recommendations for depression screening and treatment, as well as regular skin assessment.
One of the aspects of stroke care that most clinicians fail to put attention into is postacute rehabilitation. It is important for healthcare systems to adhere to postsstroke rehabilitation guidelines as various studies have shown that compliance is positively correlated with good clinical outcomes [ 71 – 73 ]. It has also been shown that low-cost rehabilitation with focus on exercise-based and brain training interventions, in resource-deprived settings, still translated to good clinical outcomes [ 74 ]. Commensurate rehabilitation initiated within the first seven days of stroke has been shown to initiate complex neurobiological processes which is instrumental in early neurologic recovery as evidenced in various clinical trials [ 75 , 76 ]. Various clinical settings have also confirmed that poststroke dysphagia results in aspiration pneumonia which further complicates hospital outcomes [ 77 , 78 ]. Additionally, evidence-based practices for the prevention of deep vein thrombosis such as the use of IPC should likewise be integrated as it may likewise impact on survival [ 79 ]. Likewise, there should be increased vigilance for poststroke depression among clinicians as it may occur in more than one third of stroke cases [ 80 ]. The need to integrate this in clinical guidelines could not be overemphasized especially in low-income to middle-income countries due to its increasing prevalence [ 81 , 82 ]. Moreover, its impact on the disability-adjusted life-years lost is significantly greater in than in high-income countries [ 82 ].
3.9. Ignored Aspects of Stroke Care
The abundance of sophisticated techniques for stroke care has led clinicians to forget about the basic yet practical aspects of stroke management. It is noted that none of the stroke guidelines incorporate the use of basic immune biomarkers such as the neutrophil to lymphocyte ratio. In the advent of precision medicine nowadays, clinical practice is shifting towards accurate and specific disease characterization, as well as quantifying disease progression and response to therapy, for which biomarkers play critically important role [ 83 ]. The neutrophil to lymphocyte ratio is a cheap, readily available, and easy to interpret immune marker which may provide a diagnostic clue particularly for clinical outcomes poststroke [ 84 – 86 ].
Wijeratne and Wijeratne demonstrated the clinical utility of an easily available, universal biomarker (SSIIi) predicting the Post-Covid-19 Neurological Syndrome [ 87 ]. It is worth exploring the clinical utility of such biomarkers in the context of poststroke recovery trajectory given the shared pathobiology of these two disorders [ 88 ].
While not mentioned in any of the clinical guidelines, the importance of ocular examination in stroke care should not be discounted. Fundus photography is an emerging tool which may assist in differentiating of stroke and TIA from other causes of neurologic deficits, particularly in the emergency setting [ 89 ]. Retinal imaging otherwise known as the “window to the brain” may supplement neuroimaging particularly in providing insights for cerebrovascular neurodegenerative conditions [ 90 ]. Lastly, it may also provide additional information for identifying stroke etiology, especially that of complicated ones [ 91 ]. We have shawn the added value ot low-cost bed side functional vision testing at the bedside in the real world that should be considered in the national and international stroke guidlines [ 92 , 93 ].
4. Discussion
Stroke and poststroke complications culminate in massive health and economic impacts globally. Stroke occurs in a compromised vascular system. The risk factors associated with stroke (both nonmodifiable risk factors such as genetic, age, and gender and modifiable risk factors such as hypertension, diabetes, high cholesterol, sedentary lifestyle, reduced fruits and vegetable intake, obesity, atrial fibrillation, poor air quality, and smoking) are linked with the build-up of low-grade chronic inflammation that perturbs the homeostasis of the vascular bed prior to the index vascular event such as acute stroke. The newly adopted WHO Intersectoral Global Action Plan calls for immediate action by national international gudiline committees in this regard ( https://wfneurology.org/world-brain-day-2022 ) [ 94 ].
The index vascular event leads to a cascade of events that involve bioenergetic failure, disrupted cellular homeostasis, excitotoxicity, acidosis, damaged blood-brain barrier, and cell death very much akin to COVID-19 and brain involvement (Wijeratne and Crewther; https:// http://www1.racgp.org.au/ajgp/coronavirus/covid-19-and-long-term-neurological-problems ).
Contrary to the traditional belief that the brain and immune systems are physically separate systems, the neural and immune systems are intimately linked through sympathetic nervous system (SNS), hypothalamic pituitary adrenal (HPA) axis, and also through glymphatic systems where bidirectional communication does occur regularly [ 14 , 27 , 95 , 96 ].
There were 80.2 million (74.1 to 86.3) prevalent strokes globally in 2016 [ 41 , 97 ]. Poststroke cognitive impairment has been reported over 50% (which is still a gross underestimation) of stroke survivors with worsened disability and quality of life [ 42 , 98 ]. Frequency of anxiety after stroke is very high at 24.2% (21.5%-26.9%) by rating scales [ 43 , 99 ] with likely increased risk of further stroke and downward spiral from the psycho-neuroimmunological PNI point of view. Poststroke depression (PSD) is reported at 18%-33% [ 44 , 100 ](gross under estimation again, see the comprehensive review on pathobiology of PSD Wijeratne and Sales [ 19 ]). Poststroke fatigue (PSF) is reported as one of the worst symptoms by 40% of the stroke survivors with prevalence of PSF that varies from 25% to 85% [ 45 , 46 , 101 , 102 ]. Poststroke apathy (PSA) with a prevalence of 34.6% and central poststroke pain (CPSP) with a prevalence that varies from 8% to 55%,can be added to long list of poststroke neurological complications with a similar psycho-neuroimmunological pathobiology to the PCNS as we elaborated in the experimental chapters.
We suggest the desperate need of systems biology approach to all these complications and conder the complete picture with a view to optimize the best immune response after the index event of acute stroke and revisit the current guidelines as a matter of high priority. Such an approach will help the world to address one of the most disabling brain disorders affecting well over 80 million people with excellent value for money with current management approach and also the potential for individualized therapeutic and management avenues (please note that the first submission of this manuscript was published in a preprint server) [ 103 , 104 ].
5. Conclusion
Stroke management is a dynamic process which has evolved at a very fast pace over the past two decades. With the abundance of clinical trials in this field, it is possible that trends of management now may not be applicable in the future. It is disappointing to see the lack of incorporation of easily accessible, low-cost prognostic markers such as NLR or functional vision assessment at the bed side in any of the published stroke guidelines anywhere in the world. This is despite the fact that large number of publications and metanalyses support the role of NLR in acute stroke as well as in the context of poststroke trajectory. It is therefore imperative for country-specific standard operating procedures to be updated constantly to fit to emerging needs with a systems biology-based approach. Implementability of clinical guidelines is anchored on evidence-based and well-appraised clinical guidelines which are customized according to available resources and to the beliefs of its end-users.
Data Availability
Conflicts of interest.
The authors declare that they have no conflicts of interest.
- Systematic Review
- Open access
- Published: 30 August 2024
A scoping review of stroke services within the Philippines
- Angela Logan 1 , 2 ,
- Lorraine Faeldon 3 ,
- Bridie Kent 1 , 4 ,
- Aira Ong 1 &
- Jonathan Marsden 1
BMC Health Services Research volume 24 , Article number: 1006 ( 2024 ) Cite this article
Metrics details
Stroke is a leading cause of mortality and disability. In higher-income countries, mortality and disability have been reduced with advances in stroke care and early access to rehabilitation services. However, access to such services and the subsequent impact on stroke outcomes in the Philippines, which is a lower- and middle-income countries (LMIC), is unclear. Understanding gaps in service delivery and underpinning research from acute to chronic stages post-stroke will allow future targeting of resources.
This scoping review aimed to map available literature on stroke services in the Philippines, based on Arksey and O’Malley’s five-stage-process.
Summary of review
A targeted strategy was used to search relevant databases (Focused: MEDLINE (ovid), EMBASE (ovid), Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO (ebsco); broad-based: Scopus; review-based: Cochrane Library, International Prospective Register of Systematic Reviews (PROSPERO), JBI (formerly Joanna Briggs Institute) as well as grey literature (Open Grey, Google scholar). The searches were conducted between 12/2022-01/2023 and repeated 12/2023. Literature describing adults with stroke in the Philippines and stroke services that aimed to maximize well-being, participation and function were searched. Studies were selected if they included one or more of: (a) patient numbers and stroke characteristics (b) staff numbers, qualifications and role (c) service resources (e.g., access to a rehabilitation unit) (d) cost of services and methods of payment) (e) content of stroke care (f) duration of stroke care/rehabilitation and interventions undertaken (g) outcome measures used in clinical practice.
A total of 70 papers were included. Articles were assessed, data extracted and classified according to structure, process, or outcome related information. Advances in stroke services, including stroke ready hospitals providing early access to acute care such as thrombectomy and thrombolysis and early referral to rehabilitation coupled with rehabilitation guidelines have been developed. Gaps exist in stroke services structure (e.g., low number of neurologists and neuroimaging, lack of stroke protocols and pathways, inequity of stroke care across urban and rural locations), processes (e.g., delayed arrival to hospital, lack of stroke training among health workers, low awareness of stroke among public and non-stroke care workers, inequitable access to rehabilitation both hospital and community) and outcomes (e.g., low government insurance coverage resulting in high out-of-pocket expenses, limited data on caregiver burden, absence of unified national stroke registry to determine prevalence, incidence and burden of stroke). Potential solutions such as increasing stroke knowledge and awareness, use of mobile stroke units, TeleMedicine, TeleRehab, improving access to rehabilitation, upgrading PhilHealth and a unified national long-term stroke registry representing the real situation across urban and rural were identified.
This scoping review describes the existing evidence-base relating to structure, processes and outcomes of stroke services for adults within the Philippines. Developments in stroke services have been identified however, a wide gap exists between the availability of stroke services and the high burden of stroke in the Philippines. Strategies are critical to address the identified gaps as a precursor to improving stroke outcomes and reducing burden. Potential solutions identified within the review will require healthcare government and policymakers to focus on stroke awareness programs, primary and secondary stroke prevention, establishing and monitoring of stroke protocols and pathways, sustainable national stroke registry, and improve access to and availability of rehabilitation both hospital and community.
What is already known?
Stroke services in the Philippines are inequitable, for example, urban versus rural due to the geography of the Philippines, location of acute stroke ready hospitals and stroke rehabilitation units, limited transport options, and low government healthcare insurance coverage resulting in high out-of-pocket costs for stroke survivors and their families.
What are the new findings?
The Philippines have a higher incidence of stroke in younger adults than other LMICs, which impacts the available workforce and the country’s economy. There is a lack of data on community stroke rehabilitation provision, the content and intensity of stroke rehabilitation being delivered and the role and knowledge/skills of those delivering stroke rehabilitation, unmet needs of stroke survivors and caregiver burden and strain,
What do the new findings imply?
A wide gap exists between the availability of stroke services and the high burden of stroke. The impact of this is unclear due to the lack of a compulsory national stroke registry as well as published data on community or home-based stroke services that are not captured/published.
What does this review offer?
This review provides a broad overview of existing evidence-base of stroke services in the Philippines. It provides a catalyst for a) healthcare government to address stroke inequities and burden; b) development of future evidence-based interventions such as community-based rehabilitation; c) task-shifting e.g., training non-neurologists, barangay workers and caregivers; d) use of digital technologies and innovations e.g., stroke TeleRehab, TeleMedicine, mobile stroke units.
Peer Review reports
Introduction
In the Philippines, stroke is the second leading cause of death, with a prevalence of 0·9% equating to 87,402 deaths per annum [ 1 , 2 ]. Approximately 500,000 Filipinos will be affected by stroke, with an estimated US$350 million to $1·2 billion needed to meet the cost of medical care [ 1 ]. As healthcare is largely private, the cost is borne out-of-pocket by patients and their families. This provides a major obstacle for the lower socio-demographic groups in the country.
Research on implementation of locally and regionally adapted stroke-services and cost-effective secondary prevention programs in the Philippines have been cited as priorities [ 3 , 4 ]. Prior to developing, implementing, and evaluating future context-specific acute stroke management services and community-based models of rehabilitation, it was important to map out the available literature on stroke services and characteristics of stroke in the Philippines.
The scoping review followed a predefined protocol, established methodology [ 5 ] and is reported according to the Preferred Reporting Items for Systematic Review and Meta-Analyses Extension for Scoping Reviews Guidelines (PRISMA-ScR) [ 6 , 7 ]. Healthcare quality will be described according to the following three aspects: structures, processes, and outcomes following the Donabedian model [ 8 , 9 ].The review is based on Arksey and O'Malley’s five stages framework [ 5 ].
Stage 1: The research question:
What stroke services are available for adults within the Philippines? The objective was to systematically scope the literature to describe the availability, structure, processes, and outcome of stroke services for adults within the Philippines.
Stage 2: Identifying relevant studies:
The following databases were searched. Focused: MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO; broad-based: Scopus; review-based: Cochrane Library, Prospero, JBI (formerly Joanna Briggs Institute); Grey literature: Herdin, North Grey, Grey matters, MedRxiv, NIHR health technology assessment, Department of Health Philippines, The Kings Fund, Ethos, Carrot2. Additionally, reference lists of full text included studies were searched.
The targeted search strategy, developed in consultation with an information scientist, was adapted for each database (see supplemental data). Search terms were peer reviewed using the PRESS (Peer Review of Electronic Search Strategies) checklist [ 10 ].
The key search concepts from the Population, Concept and Context (PCC) framework were ≥ 18 years with a stroke living in the Philippines ( population ), stroke services aiming to maximize well-being, participation and function following a stroke ( concept ) and stroke services from acute to chronic including those involving healthcare professionals, non-healthcare related personnel or family or friends ( context ). Search tools such as medical subject headings (MESH) and truncation to narrow or expand searches were used. Single and combined search terms were included (see supplemental data). The search was initially conducted over two weeks in December 2022 and re-run in December 2023.
Studies were selected if they described stroke care in the Philippines in terms of one or more of the following: (a) patient numbers and stroke characteristics (b) staff numbers, qualifications and role (c) service resources (e.g., number of beds/access to a rehabilitation unit, equipment used) (d) cost of services and methods of payment (UHC, Insurance, private) (e) content of stroke care (f) duration of stroke care (hours of personnel contact e.g., Therapy hours per day); interventions undertaken (g) outcome measures used in clinical practice.
Additional criteria:
Context: all environments (home, hospital, outpatients, clinic, academic institute).
Date limits: published between 2002 onwards. This is based on the Philippines Community Rehabilitation Guidelines published in 2009 that would suggest that papers earlier than 2002 may not reflect current practice [ 11 ].
Qualitative and quantitative studies including grey literature.
Language: reported in English or Filipino only.
Publication status: no limit because the level of rigor was not assessed.
Type of study: no limit which included conference abstracts, as the level of rigor was not assessed.
Studies were excluded if they were in non-stroke populations or the full text article could not be obtained. Conference abstracts were excluded if there were insufficient data about methods and results.
Searches of databases were performed by one researcher (JM) and searches of grey literature were performed by one researcher (AO). All retrieved articles were uploaded into Endnote X9 software™, and duplicates identified and removed before transferring them to Rayyan [ 12 ] for screening.
Stage 3: study selection
The title and abstract were selected using eligibility criteria. Two pairs of researchers independently screened abstracts and titles;(Databases: JM and AL and grey literature by AO and LF). Where a discrepancy existed for title and abstract screening, the study was automatically included for full text review and discussed among reviewers.
Two reviewers (JM and AL) undertook full-text screening of the selected studies. Discrepancies were resolved through consensus discussions without the need for a third reviewer. There were no discrepancies that required a third reviewer. Reason for exclusion were documented according to pre-determined eligibility criteria. References of included full text articles were screened by each reviewer independently and identified articles were subjected to the same screening process as per the PRISMA-ScR checklist (Fig. 1 ).

PRISMA-ScR flow diagram
Stage 4: Charting the data
Two reviewers independently extracted the data using a piloted customized and standardized data extraction form including (1) Structure: financial (e.g., costs, insurance, government funding), resources (structure and number of stroke facilities, staff (number, profession/specialism, qualifications etc.), stroke characteristics (2) Process: duration of care, content of stroke care within acute, secondary care, community, outcome measures used; (3) Outcome: survival, function, patient satisfaction, cost (admission and interventions), and (4) year of publication, geographical location (including if Philippines only or multiple international locations) and type of evidence (e.g., policy, review, observational, experimental, clinical guidelines). Critical appraisal of included studies was not undertaken because the purpose of the review was to map available evidence on stroke services available within the Philippines.
Stage 5: Collating, summarising and reporting the results
The search identified 351 records from databases and registers. A total of 70 records are included and reasons for non-inclusion are summarized in Fig. 1 .
Study descriptors
The characteristics of included studies are shown in Supplementary Material Table 1. Of the 70 included studies, 36 were observational with most being based on a retrospective review of case notes ( n = 31), two were audits, eight were surveys or questionnaires, four were consensus opinion and/or guideline development, three were randomized controlled trial (RCT) or feasibility RCT, 1 was a systematic review, two were policy and guidelines, 11 were narrative reviews or opinion pieces, two were case series or reports and one was an experimental study.
Of the 70 studies, 32 (45.7%) were based in a single tertiary hospital site. There were only three papers based in the community (4.3%). Papers that were opinion pieces or reviews were classified as having a national focus. Of the 22 papers classified as having a national focus, 10 (45.5%) were narrative reviews/ opinion pieces (Table 1 ).
The primary focus of the research studies (excluding the 11 narrative reviews and 2 policy documents) were classified as describing structure ( n = 8, 14%); process ( n = 21,36.8%) or outcomes ( n = 29, 49.2%). The structure of acute care was described in seven studies out of eight studies ( n = 7/8 87.5%) whilst neurosurgery structures were described in one out of eight studies (12.5%). Acute care processes were described in 11 out of 21 studies ( n = 11/21 52.3%) whilst rehabilitation processes were described in six out of 21 studies (28.6%), with three out of 21 studies primarily describing outcome measurement (14.3%). The primary focus of the outcomes were stroke characteristics (25 out of 28 papers, 89.2%) in terms of number of stroke (prevalence), mortality or severity of stroke. Measures of stroke quality of life were not reported. Healthcare professional knowledge was described in two studies ( n = 2/28 7.1%) whilst risk factors for stroke were described in one study ( n = 1/28, 3.6%). Carer burden was described in one study ( n = 1/28, 3.6%).
A summary of the findings is presented in Table 2 .
This scoping review describes the available literature on stroke services within the Philippines across the lifespan of an adult (> 18 years) with a stroke. The review has identified gaps in information about structures, processes and outcomes as well as deficits in provision of stroke services and processes as recommended by WHO. These included a low number of specialist clinicians including neurologists, neuro-radiographers and neurosurgeons. The high prevalence of stroke suggests attention and resources need to focus on primary and secondary prevention. Awareness of stroke is low, especially in terms of what a stroke is, the signs/symptoms and how to minimize risk of stroke [ 25 ]. Barriers exist, such as lack of healthcare resources, maldistribution of health facilities, inadequate training on stroke treatment among health care workers, poor stroke awareness, insufficient government support and limited health insurance coverage [ 22 ].
The scoping review also highlighted areas where further work is needed, for example, descriptions and research into the frequency, intensity, and content of rehabilitation services especially in the community setting and the outcome measures used to monitor recovery and impairment. PARM published stroke rehabilitation clinical practice guidelines in 2012, which incorporated an innovative approach to contextualize Western clinical practice guidelines for stroke care to the Philippines [ 42 ]. Unfortunately, availability and equitable access to evidence-based rehabilitation for people with stroke in the Philippines pose significant challenges because of multiple factors impacting the country (e.g., geographical, social, personal, environmental, educational, economic, workforce) [ 25 , 40 , 43 ].
The number of stroke survivors with disability has not been reported previously, thus, the extent and burden of stroke from acute to chronic is unknown. The recent introduction of a national stroke registry across public and private facilities may provide some of this data [ 82 ]. The project started in 2021 and captures data on people hospitalized for transient ischemic attack or stroke in the Philippines. National stroke registries have been identified as a pragmatic solution to reduce the global burden of stroke [ 83 ] through surveillance of incidence, prevalence, and outcomes (e.g., death, disability) of, and quality of care for, stroke, and prevalence of risk factors. For the Philippine government to know the full impact and burden of stroke nationally, identify areas for improvement and make meaningful changes for the benefit of Filipinos, the registry would need to be compulsory for all public and private facilities and include out of hospital data. This will require information technology, trained workforces for data capture, monitoring and sharing, as well as governance and funding [ 83 ].
This scoping review has generated a better understanding of the published evidence focusing on availability of stroke services in the Philippines, as well as the existing gaps through the lens of Donabedian’s Structure , Process and Outcome framework. The findings have helped to inform a wider investigation of current stroke service utilization conducted using survey and interview methods with stroke survivors, carers and key stakeholders in the Philippines, and drive forward local, regional and national policy and service changes.
Conclusions
This scoping review describes the existing evidence-based relating to structure, processes and outcomes of stroke services for adults within the Philippines. The review revealed limited information in certain areas, such as the impact of stroke on functional ability, participation in everyday life, and quality of life; the content and intensity of rehabilitation both in the hospital or community setting; and the outcome measures used to evaluate clinical practice. Developments in stroke services have been identified however, a wide gap exists between the availability of stroke services and the high burden of stroke in the Philippines. Strategies are critical to address the identified gaps as a precursor to improving stroke outcomes and reducing burden. Potential solutions identified within the review will require a comprehensive approach from healthcare policymakers to focus on stroke awareness programs, primary and secondary prevention, establishing and monitoring of stroke protocols and pathways, implementation of a compulsory national stroke registry, use of TeleRehab, TeleMedicine and mobile stroke units and improve access to and availability of both hospital- and community-based stroke rehabilitation. Furthermore, changes in PhilHealth coverage and universal credit to minimize catastrophic out-of-pocket costs.
Limitations
Although a comprehensive search was undertaken, data were taken from a limited number of located published studies on stroke in the Philippines. This, together with data from databases and grey literature, may not reflect the current state of stroke services in the country.
Availability of data and materials
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Navarro JC, Baroque AC, Lokin JK, Venketasubramanian N. The real stroke burden in the Philippines. Int J Stroke. 2014;9(5):640–1.
Article PubMed Google Scholar
Philippines TSSot. Phillipines: stroke 2024. Available from: https://www.strokesocietyphilippines.org/philippines-stroke/#:~:text=Stroke%20is%20the%20Philippines'%20second,or%2014.12%25%20of%20total%20deaths .
Banaag MS, Dayrit MM, Mendoza RU. Health Inequity in the Philippines. In: Batabyal A, Higano Y, Nijkamp P (eds). Disease, Human Health, and Regional Growth and Development in Asia. New Frontiers in Regional Science: Asian Perspectives, vol 38. Singapore: Springer; 2019.
Hodge A, Firth S, Bermejo R, Zeck W, Jimenez-Soto E. Utilisation of health services and the poor: deconstructing wealth-based differences in facility-based delivering in teh Philippines. BMC Public Health. 2016;16:1–12.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32.
Article Google Scholar
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69.
Article PubMed PubMed Central Google Scholar
Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743–8.
Article CAS PubMed Google Scholar
McDonald KM, Sundaram V, Bravata DM, Lewis R, Lin N, Kraft SA, et al. Closing the quality gap: a critical analysis of quality improvement strategies. Tech Rev. 2007;7(9).
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6.
McGlade B, Mendoza VE. Philippines CBR manual: an inclusive development strategy. Philippines: CBM-CBR Coordinating office; 2009.
Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(210). https://doi.org/10.1186/s13643-016-0384-4 .
Baliguas B. Adherence to the clinical practice guidelines of the stroke society of the Philippines in the management of ischemic stroke in young adults admitted in 3 tertiary hospitals in Bacolod City, Philippines from May to October 2010. Neurology. 2018;90(15).
Barcelon EA, Moll MAKDN, Serondo DJ, Collantes MEV. Validation of the Filipino version of national institute of health stroke scale. Clinical Neurology. 2016;56:S379.
Google Scholar
Baticulon RE, Lucena LLN, Gimenez MLA, Sabalza MN, Soriano JA. The Neurosurgical Workforce of the Philippines. Neurosurgery. 2024;94(1):202–11. https://doi.org/10.1227/neu.0000000000002630 .
Berroya RM. Incidence of symptomatic intracerebral hemorrhage after thrombolysis for acute ischemic stroke at St. Luke’s Medical Center-Global City from January 2010 to February 2017. J Neurol Sci. 2010;2017(381):398–9.
Carcel C, Espiritu-Picar R. Circadian variation of ischemic and hemorrhagic strokes in adults at a tertiary hospital: a retrospective study. J Neurol Sci. 2009;285:S174.
Cayco CS, Gorgon EJR, Lazaro RT. Proprioceptive neuromuscular facilitation to improve motor outcomes in older adults with chronic stroke. Neurosciences (Riyadh). 2019;24(1):53–60.
Co COC, Yu JRT, Macrohon-Valdez MC, Laxamana LC, De Guzman VPE, Berroya-Moreno RMM, et al. Acute stroke care algorithm in a private tertiary hospital in the Philippines during the COVID-19 pandemic: a third world country experience. J Stroke Cerebrovasc Dis. 2020;29(9):105059.
Co COC, Yu JRT, Laxamana LC, David-Ona DIA. Intravenous thrombolysis for stroke in a COVID-19 positive Filipino patient, a case report. J Clin Neurosci. 2020;77:234–6.
Article CAS PubMed PubMed Central Google Scholar
Collantes ME. Evaluation of change in stroke care in the Philippines using RES-Q data. Eur Stroke J. 2019;4:318.
Collantes ME. Improving stroke systems of care in lmic: Philippines. Int J Stroke. 2021;16(2):4.
Collantes ME, Navarro J, Belen A, Gan R. Stroke systems of care in the Philippines: addressing gaps and developing strategies. Front Neurol. 2022;13:1046351.
Collantes MEV, Yves Miel H, Zuñiga Uezono DR. Incidence and prevalence of stroke and its risk factors in the Philippines: a systematic review. Acta Medica Philippina. 2022;56:26–34.
Collantes MV, Zuniga YH, Granada CN, Uezono DR, De Castillo LC, Enriquez CG, et al. Current state of stroke care in the Philippines. Front Neurol. 2021;12:665086.
Constantino GA, Soliven JA. Points of in-hospital delays in thrombolytic therapy among patients with acute ischemic stroke: a single center 5-year retrospective study. Neurology. 2020;94(15). https://doi.org/10.1212/WNL.94.15_supplement.2901 .
Constantino GAA, Señga MMA, Soliven JAR, Jocson VED. Emerging Utility of Endovascular Thrombectomy in the Philippines: A Single-center Clinical Experience. Acta Med Philipp [Internet]. 2023;57(5). Available from: https://actamedicaphilippina.upm.edu.ph/index.php/acta/article/view/5113 . [cited 2024 Aug 21].
Dans AL, Punzalan FE, Villaruz MV. National Nutrition and Health Survey (NNHeS): atherosclerosis-related diseases and risk factors. Philipp J Intern Med. 2005;43:103–15.
De Castillo LL, Collantes ME. Thrombolysis for stroke at the Philippine general hospital: a descriptive analysis. Cerebrovasc Dis. 2019;48:54.
de Castillo LLC, Diestro JDB, Tuazon CAM, Sy MCC, Añonuevo JC, San Jose MCZ. J Stroke Cerebrovasc Dis. 2021;30(7):105831.
Delfino JPM, Carandang-Chacon CA. Comparison of acute ischemic stroke care quality before and during the COVID-19 pandemic in a private tertiary hospital in metro Manila, Philippines. Neurol Asia. 2023;28(1):13–7.
Department of Health. Department of Health Administrative Order 2011-0003. 2011. [Accessed online: 12/2022], from the Philippine Department of Health].
Department of Health. The national policy framework on the prevention, control and management of acute stroke in the Philippines. 2020.
Diestro JDB, Omar AT, Sarmiento RJC, Enriquez CAG, Castillo LLC, Ho BL, et al. Cost of hospitalization for stroke in a low-middle-income country: Findings from a public tertiary hospital in the Philippines. Int J Stroke. 2021;16(1):39–42.
Duenas M, Ranoa G, Benjamin VS. Assessment of post-stroke caregivers’ burden through the modified caregivers strain index (MCSI) in a tertiary center in the Philippines: a cross-sectional study. Cerebrovasc Dis. 2019;48:56–7.
Duya JE, Hernandez K, San Jose MC. The evolving clinical and echocardiographic profile of patients admitted for acute cardioembolic stroke at a Tertiary Hospital in the Philippines. J Hong Kong Coll Cardiol. 2019;27(1):58.
Espiritu AI, San Jose MCZ. A call for a stroke referral network between primary care and stroke-ready hospitals in the philippines: a narrative review. Neurologist. 2021;26(6):253–60.
Gambito ED, Gonzalez-Suarez CB, Grimmer KA, Valdecañas CM, Dizon JM, Beredo ME, et al. Updating contextualized clinical practice guidelines on stroke rehabilitation and low back pain management using a novel assessment framework that standardizes decisions. BMC Res Notes. 2015;8:643.
Gelisanga MA, Gorgon EJ. Upright motor control test: interrater reliability, retest reliability, and concurrent validity in adults with subacute stroke. Eur Stroke J. 2017;2(1):357–8.
Gonzalez-Suarez C, Grimmer K, Alipio I, Anota-Canencia EG, Santos-Carpio ML, Dizon JM, et al. Stroke rehabilitation in the Philippines: an audit study. Disabil CBR Inclusive Develop. 2015;26(3):44–67.
Gonzalez-Suarez CB, Grimmer K, Cabrera JTC, Alipio IP, Anota-Canencia EGG, Santos-Carpio MLP, et al. Predictors of medical complications in stroke patients confined in hospitals with rehabilitation facilities: a Filipino audit of practice. Neurology Asia. 2018;23(3):199–208.
Gonzalez-Suarez CB, Grimmer-Somers K, Margarita Dizon J, King E, Lorenzo S, Valdecanas C, et al. Contextualizing Western guidelines for stroke and low back pain to a developing country (Philippines): an innovative approach to putting evidence into practice efficiently. J Healthc Leadersh. 2012;4:141–56.
Gonzalez-Suarez CB, Margarita J, Dizon R, Grimmer K, Estrada MS, Uyehara ED, et al. Implementation of recommendations from the Philippine Academy of Rehabilitation Medicine's Stroke Rehabilitation Guideline: a plan of action. Clin Audit. 2013;5:77–89.
Ignacio KHD, Diestro JDB, Medrano JMM, Salabi SKU, Logronio AJ, Factor SJV, et al. Depression and anxiety after stroke in young adult Filipinos. J Stroke Cerebrovasc Dis. 2022;31(2):106232.
Inting K, Canete MT. Ischemic stroke subtypes: a comparison between causative and phenotypic classifications in a tertiary hospital in the Philippines. Int J Stroke. 2021;16(2):28.
Jaca PKM, Chacon CAC, Alvarez RM. Clinical characteristics of cerebrovascular disease with COVID-19: a single-center study in Manila. Philippines Neurology Asia. 2021;26(1):15–25.
Jamora RDG, Corral EV, Ang MA, Epifania M, Collantes V, Gan R. Stroke recurrence among Filipino patients taking aspirin for first-ever non-cardioembolic ischemic stroke. Neurol Clin Neurosci. 2017;5:1–5.
Jamora RDG, Prado MB Jr, Anlacan VMM, Sy MCC, Espiritu AI. Incidence and risk factors for stroke in patients with COVID-19 in the Philippines: an analysis of 10,881 cases. J Stroke Cerebrovasc Dis. 2022;31(11).
Juangco DN, Mariano GS. Endovascular therapy for acute ischemic stroke: a review of cases and outcomes from a primary stroke center (a 5-year retrospective study). Cerebrovasc Dis. 2016;41:54.
Leochico CFD, Austria EMV, Gelisanga MAP, Ignacio SD, Mojica JAP. Home-based telerehabilitation for community-dwelling persons with stroke during the COVID-19 pandemic: a pilot study. J Rehabil Med. 2023;55:jrm4405.
Loo KW, Gan SH. Burden of stroke in the Philippines. Int J Stroke. 2013;8(2):131–4.
Mansouri A, Ku JC, Khu KJ, Mahmud MR, Sedney C, Ammar A, et al. Exploratory analysis into reasonable timeframes for the provision of neurosurgical care in low- and middle-income countries. World Neurosurg. 2018;117:e679–91.
Mendoza RA. The clinical profile and treatment outcome of acute ischemic stroke patients who underwent thrombolysis with recombinant tissue plasminogen activator therapy, Philippine experience: a retrospective study. J Neurol Sci. 2009;285:S85–6.
Navarro J. Prevalence of stroke: a community survey. Philipp J Neurol. 2005;9(2):11–5.
Navarro JC, Venketasubramanian N. Stroke burden and services in the Philippines. Cerebrovasc Dis Extra. 2021;11(2):52–4.
Navarro JC, Baroque AC 2nd, Lokin JK. Stroke education in the Philippines. Int J Stroke. 2013;8 Suppl A100:114–5.
Navarro JC, Chen CL, Lee CF, Gan HH, Lao AY, Baroque AC, et al. Durability of the beneficial effect of MLC601 (NeuroAiD™) on functional recovery among stroke patients from the Philippines in the CHIMES and CHIMES-E studies. Int J Stroke. 2017;12(3):285–91.
Navarro JC, Escabillas C, Aquino A, Macrohon C, Belen A, Abbariao M, et al. Stroke units in the Philippines: an observational study. Int J Stroke. 2021;16(7):849–54.
Navarro JC, San Jose MC, Collantes E, Macrohon-Valdez MC, Roxas A, Hivadan J, et al. Stroke thrombolysis in the Philippines. Neurol Asia. 2018;23(2):115.
Ng JC, Churojana A, Pongpech S, Vu LD, Sadikin C, Mahadevan J, et al. Current state of acute stroke care in Southeast Asian countries. Interv Neuroradiol. 2019;25(3):291–6.
Ocampo FF, De Leon-Gacrama FRG, Cuanang JR, Navarro JC. Profile of stroke mimics in a tertiary medical center in the Philippines. Neurol Asia. 2021;26(1):35–9.
Pascua R, Hiyadan JH. Outcome of decompressive hemicraniectomy without evacuation of hematoma in supratentorial intracerebral hemorrhage in a tertiary government hospital in the Philippines: a retrospective study. Eur Stroke J. 2023;8(2):586.
Prado M, Jamora RD, Charmaine Sy M, Anlacan M, Espiritu A. Determinants and Outcomes of Cerebrovascular Disease in Patients with COVID19 in the Philippines: An Analysis of 10881 Cases. Neurology. 2022;98(18). https://doi.org/10.1212/WNL.98.18_supplement.2076 .
Qua CV, Tiqui V, Villatima NE, Perales DJ, Rubio SM, Santos ER, et al. A predictive assessment of early neurological deterioration among Filipino acute ischemic stroke patients utilizing hematological, lipid profile, and metabolic parameters in a tertiary hospital in Pampanga. Philippines Cerebrovasc Dis. 2022;51:101.
Que DL, Cuanang J, San Jose MC. Clinical profile, management and outcomes of patients with cerebralvenous thrombosis in atertiary hospital in the Philippines. Int J Stroke. 2020;15(1):511.
Quiles LEP, Diamante PAB, Pascual JLV. Impact of the COVID-19 pandemic in the acute stroke admissions and outcomes in a Philippine Tertiary Hospital. Cerebrovasc Dis Extra. 2022;12(2):76–84.
Roxas AA. The RIFASAF project: a case-control study on risk factors for stroke among Filipinos. Philippine J Neurol. 2002;6(1):1–7.
Roxas AAC, Carabal-Handumon J. Knowledge and perceptions among the barangay health workers in Plaridel, Misamis Occidental. Philipp J Neurol. 2002;6(1):44.
Sasikumar S, Bengzon Diestro JD. Global & community health: acute ischemic stroke in Toronto and Manila: bridging the gap. Neurology. 2020;95(13):604–6.
Senga MM, Reyes JPB. Cerebral venous thrombosis in a single center tertiary hospital of a South East Asian country (CVSTS study)-a retrospective study on the clinical profiles of patients with cerebral venous thrombosis. Neurology. 2019;92(15). https://doi.org/10.1212/WNL.92.15_supplement.P5.3-011 .
Sese LVC, Guillermo MCL. Strengthening stroke prevention and awareness in the Philippines: a conceptual framework. Front Neurol. 2023;14:1258821.
Suwanwela NC, Chen CLH, Lee CF, Young SH, Tay SS, Umapathi T, et al. Effect of combined treatment with MLC601 (NeuroAiDTM) and rehabilitation on post-stroke recovery: the CHIMES and CHIMES-E studies. Cerebrovasc Dis. 2018;46(1–2):82–8.
Talamera AF, Franco DS. Validation study of Siriraj stroke score in Southern Philippines. Cerebrovasc Dis. 2011;32:9.
Tan A, Navarro J. Outcomes and quality of care outcome of patients with primary intracerebral hemorrhage in a single center in the philippines. Int J Stroke. 2014;9:269.
Tangcuangco NC, Bitanga ES, Roxas AA, Pascual JL, Saniel E, Reyes JP, et al. Intravenous recombinant tissue plasminogen activator (IV-rtPA) use in acute ischemic stroke in a private tertiary hospital: a Philippine setting. Int J Stroke. 2010;5:107.
Tsang ACO, Yang IH, Orru E, Nguyen QA, Pamatmat RV, Medhi G, et al. Overview of endovascular thrombectomy accessibility gap for acute ischemic stroke in Asia: a multi-national survey. Int J Stroke. 2020;15(5):516–20.
Vatanagul J, Cantero-Auguis C. Awareness on acute stroke management among family medicine and internal medicine residents in Metro Cebu. Philippines J Neurol Sci. 2015;357:e418–9.
Vatanagul J, Rulona IA. The incidence of post-stroke depression in a tertiary hospital in Cebu City, Philippines. J Neurol Sci. 2015;357:e419.
Vatanagul JAS, Rulona IA, Belonguel NJ. Cerebral venous thrombosis (CVST): study of four Filipino patients and literature review. Cerebrovasc Dis. 2013;36:81.
Venketasubramanian N, Yoon BW, Pandian J, Navarro JC. Stroke epidemiology in south, east, and south-east Asia: a review. J Stroke. 2017;19(3):286–94.
Yu RF, San Jose MC, Manzanilla BM, Oris MY, Gan R. Sources and reasons for delays in the care of acute stroke patients. J Neurol Sci. 2002;199(1–2):49–54.
Philippine Neurological Association One Database - Stroke DsSMG. Multicentre collection of uniform data on patients hospitalised for transient ischaemic attack or stroke in the Philippines: the Philippine Neurological Association One Database-Stroke (PNA1DB-Stroke) protocol. BMJ Open. 2022;12(5):54.
Feigin VL, Owolabi MO, Group WSOLNCSC. Pragmatic solutions to reduce the global burden of stroke: a world stroke organization-lancet neurology commission. Lancet Neurol. 2023;22(12):1160–206.
Download references
Acknowledgements
We acknowledge the TULAY collaborators: Dr Roy Francis Navea, Dr Myrna Estrada, Dr Elda Grace Anota, Dr Maria Mercedes Barba, Dr June Ann De Vera, Dr Maria Elena Tan, Dr Sarah Buckingham and Professor Fiona Jones. We are grateful to Lance de Jesus and Dr Annah Teves, Research Assistants on the TULAY project, for their contribution to some of the data extraction.
This research was funded by the NIHR Global Health Policy and Systems Research Programme (Award ID: NIHR150244) in association with UK aid from the UK Government to support global health research. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the UK’s Department of Health and Social Care.
Author information
Authors and affiliations.
Faculty of Health, Intercity Place, University of Plymouth, Plymouth, Devon, PL4 6AB, UK
Angela Logan, Bridie Kent, Aira Ong & Jonathan Marsden
Royal Devon University Healthcare NHS Foundation Trust, William Wright House, Barrack Road, Exeter, Devon, EX2 5DW, UK
Angela Logan
De La Salle University-Evelyn D. Ang Institute of Biomedical Engineering and Health Technologies, 2401 Taft Avenue, Malate, Manila, 1004, Philippines
Lorraine Faeldon
The University of Plymouth Centre for Innovations in Health and Social Care: A JBI Centre of Excellence, Faculty of Health, Intercity Place, University of Plymouth, Plymouth, Devon, PL4 6AB, UK
Bridie Kent
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualisation, methodology and setting search terms, AL, LF, AO, JM, BK. Searches and screening, AL, JM, LF, AO. Data extraction, AL, LF, AO, JM, LdJ, AT. Original draft preparation, AL, JM. All authors provided substantive intellectual and editorial revisions and approved the final manuscript.
Corresponding author
Correspondence to Angela Logan .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Logan, A., Faeldon, L., Kent, B. et al. A scoping review of stroke services within the Philippines. BMC Health Serv Res 24 , 1006 (2024). https://doi.org/10.1186/s12913-024-11334-z
Download citation
Received : 20 March 2024
Accepted : 22 July 2024
Published : 30 August 2024
DOI : https://doi.org/10.1186/s12913-024-11334-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Stroke care
- Low- middle-income countries
- Developing countries
- Philippines
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Being a stroke patient: a review of the literature
Affiliation.
- 1 Department of Neurology, University Hospital Utrecht, The Netherlands.
- PMID: 9378880
- DOI: 10.1046/j.1365-2648.1997.t01-19-00999.x
The purpose of this paper is to review the research literature on the patient's experience of stroke. Four qualitative studies on how patients experience stroke were identified. The findings of these studies show that the stroke patient often has clear goals for himself in relation to functional abilities, against which he measures all success and forward progress in his rehabilitation. Even though the stroke patient accepts a lower level of functional ability, he is not willing to accept the rehabilitation professionals' prediction of his ultimate functional level if it is lower than his own goal. Furthermore, stroke patients see recovery as a return to the existence they had lived before the stroke, which is different from the health care providers' view. To the health care provider, recovery is measured in terms of isolated and discrete return of movement, whereas in the eyes of the patients, recovery is a return to previously valued activities. Further, studies on psychosocial function after stroke were reviewed. Recent studies show that the psychological impact of the stroke experience is immense and that stroke patients experience stress on a variety of levels. Also, depression exists in a large portion of the stroke population. The impact of stroke also influences the patient's social existence, as studies have shown that stroke patients do manifest diminished social function. However, the reviewed studies are not without limitations. Further studies, with a qualitative design, are needed to throw light on the patient's experience of being ill with stroke, and the process of his recovery.
PubMed Disclaimer
Similar articles
- Assessment and psychologic factors in stroke rehabilitation. Kelly-Hayes M, Paige C. Kelly-Hayes M, et al. Neurology. 1995 Feb;45(2 Suppl 1):S29-32. Neurology. 1995. PMID: 7885588 Review.
- Painting a mural and writing an article: creative rehabilitation strategies. Skinner MK, Nagel PJ. Skinner MK, et al. Rehabil Nurs. 1996 Mar-Apr;21(2):63-6. doi: 10.1002/j.2048-7940.1996.tb01678.x. Rehabil Nurs. 1996. PMID: 8701096
- Social work and the stroke patient. Ue B. Ue B. Clin Orthop Relat Res. 1978 Mar-Apr;(131):101-3. Clin Orthop Relat Res. 1978. PMID: 657605
- Recovering from stroke: a qualitative investigation of the role of goal setting in late stroke recovery. Lawler J, Dowswell G, Hearn J, Forster A, Young J. Lawler J, et al. J Adv Nurs. 1999 Aug;30(2):401-9. doi: 10.1046/j.1365-2648.1999.01086.x. J Adv Nurs. 1999. PMID: 10457242
- Stroke rehabilitation. Lorish TR. Lorish TR. Clin Geriatr Med. 1993 Nov;9(4):705-16. Clin Geriatr Med. 1993. PMID: 8281500 Review.
- Motivation as a Measurable Outcome in Stroke Rehabilitation: A Systematic Review of the Literature. Verrienti G, Raccagni C, Lombardozzi G, De Bartolo D, Iosa M. Verrienti G, et al. Int J Environ Res Public Health. 2023 Feb 26;20(5):4187. doi: 10.3390/ijerph20054187. Int J Environ Res Public Health. 2023. PMID: 36901206 Free PMC article. Review.
- Corticosterone Administration Alters White Matter Tract Structure and Reduces Gliosis in the Sub-Acute Phase of Experimental Stroke. Zalewska K, Hood RJ, Pietrogrande G, Sanchez-Bezanilla S, Ong LK, Johnson SJ, Young KM, Nilsson M, Walker FR. Zalewska K, et al. Int J Mol Sci. 2021 Jun 22;22(13):6693. doi: 10.3390/ijms22136693. Int J Mol Sci. 2021. PMID: 34206635 Free PMC article.
- First-Hand Experience of Severe Dysphagia Following Brainstem Stroke: Two Qualitative Cases. Kjaersgaard A, Pallesen H. Kjaersgaard A, et al. Geriatrics (Basel). 2020 Mar 4;5(1):15. doi: 10.3390/geriatrics5010015. Geriatrics (Basel). 2020. PMID: 32143302 Free PMC article.
- Gateway to Recovery: A Comparative Analysis of Stroke Patients' Experiences of Change and Learning in Norway and Denmark. Pallesen H, Aadal L, Moe S, Arntzen C. Pallesen H, et al. Rehabil Res Pract. 2019 Jan 17;2019:1726964. doi: 10.1155/2019/1726964. eCollection 2019. Rehabil Res Pract. 2019. PMID: 30775038 Free PMC article.
- Should Sabbath Prohibitions Be Overridden to Provide Emotional Support to a Sick Relative? Greenberger C, Mor P. Greenberger C, et al. Rambam Maimonides Med J. 2016 Jul 28;7(3):e0023. doi: 10.5041/RMMJ.10250. Rambam Maimonides Med J. 2016. PMID: 27487314 Free PMC article. Review.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Write for Us
- BMJ Journals
You are here
- Online First
- Importance of training and education for nurses delivering stroke care
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Alison McLoughlin 1 ,
- Lisa Kidd 2
- 1 East Lancashire Hospitals NHS Trust , Blackburn , Lancashire , UK
- 2 Nursing & Community Health , Glasgow Caledonian University , Glasgow , UK
- Correspondence to Professor Lisa Kidd; lisa.kidd{at}gcu.ac.uk
https://doi.org/10.1136/ebnurs-2024-104040
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
- Evidence-Based Nursing
Commentary on : Zhao et al . The impact of education/training on nurses caring for patients with stroke: a scoping review. BMC Nurs 23:90
Implications for practice and research
Nurses working in clinical stroke care could benefit from leadership and management strategies that encourage empowerment and time and space to reflect on current evidence, training and practice.
Comprehensive evaluation strategies are needed to assess the impact and effectiveness of empowerment-based stroke education and training on patient outcomes.
Stroke nursing is widely recognised for its significant role across the whole multidisciplinary stroke care pathway. 1 Ensuring that stroke nurses are equipped with the latest stroke specialist knowledge and skills is fundamental in providing high-quality and safe stroke care. 1 2 However, in spite of the provision of nationally-recognised, quality-assured training and education programmes, for example, the Stroke-Specific Education Framework in the UK, education and training for nursing staff within the current clinical climate remains a challenge and less is known about the impact of such training programmes on patient outcomes.
X @lisakidd22
Competing interests LK is a Deputy Editor for Evidence Based Nursing Journal.
Provenance and peer review Commissioned; internally peer reviewed.
Read the full text or download the PDF:
Advertisement
The 100 Best Books of the 21st Century: A Printable List
By The New York Times Books Staff Aug. 26, 2024
- Share full article
Print this version to keep track of what you’ve read and what you’d like to read. See the full project, including commentary about the books, here.
A PDF version of this document with embedded text is available at the link below:
Download the original document (pdf)
The New York Times Book Review I've I want THE 100 BEST BOOKS OF THE 21ST CENTURY read to it read it 1 My Brilliant Friend, by Elena Ferrante 26 26 Atonement, by lan McEwan 2 The Warmth of Other Suns, by Isabel Wilkerson 27 Americanah, by Chimamanda Ngozi Adichie 3 Wolf Hall, by Hilary Mantel 28 Cloud Atlas, by David Mitchell 4 The Known World, by Edward P. Jones 29 The Last Samurai, by Helen DeWitt 5 The Corrections, by Jonathan Franzen 30 Sing, Unburied, Sing, by Jesmyn Ward 6 2666, by Roberto Bolaño 31 White Teeth, by Zadie Smith 7 The Underground Railroad, by Colson Whitehead 32 The Line of Beauty, by Alan Hollinghurst 8 Austerlitz, by W.G. Sebald 33 Salvage the Bones, by Jesmyn Ward 9 Never Let Me Go, by Kazuo Ishiguro 34 Citizen, by Claudia Rankine 10 Gilead, by Marilynne Robinson 35 Fun Home, by Alison Bechdel 11 The Brief Wondrous Life of Oscar Wao, by Junot Díaz 36 Between the World and Me, by Ta-Nehisi Coates 12 The Year of Magical Thinking, by Joan Didion 37 The Years, by Annie Ernaux 13 The Road, by Cormac McCarthy 38 The Savage Detectives, by Roberto Bolaño 14 Outline, by Rachel Cusk 39 A Visit From the Goon Squad, by Jennifer Egan 15 Pachinko, by Min Jin Lee 40 H Is for Hawk, by Helen Macdonald 16 The Amazing Adventures of Kavalier & Clay, by Michael Chabon 41 Small Things Like These, by Claire Keegan 17 The Sellout, by Paul Beatty 42 A Brief History of Seven Killings, by Marlon James 18 Lincoln in the Bardo, by George Saunders 43 Postwar, by Tony Judt 19 Say Nothing, by Patrick Radden Keefe 44 The Fifth Season, by N.K. Jemisin 20 Erasure, by Percival Everrett 45 The Argonauts, by Maggie Nelson 21 Evicted, by Matthew Desmond 46 The Goldfinch, by Donna Tartt 22 22 Behind the Beautiful Forevers, by Katherine Boo 47 A Mercy, by Toni Morrison 23 Hateship, Friendship, Courtship, Loveship, Marriage, by Alice Munro 48 Persepolis, by Marjane Satrapi 24 The Overstory, by Richard Powers 49 The Vegetarian, by Han Kang 25 25 Random Family, by Adrian Nicole LeBlanc 50 Trust, by Hernan Diaz I've I want read to it read it
The New York Times Book Review I've I want THE 100 BEST BOOKS OF THE 21ST CENTURY read to it read it 51 Life After Life, by Kate Atkinson 52 52 Train Dreams, by Denis Johnson 53 Runaway, by Alice Munro 76 77 An American Marriage, by Tayari Jones 78 Septology, by Jon Fosse Tomorrow, and Tomorrow, and Tomorrow, by Gabrielle Zevin 54 Tenth of December, by George Saunders 55 The Looming Tower, by Lawrence Wright 56 The Flamethrowers, by Rachel Kushner 57 Nickel and Dimed, by Barbara Ehrenreich ཤྲཱ རྒྱ སྐྱ A Manual for Cleaning Women, by Lucia Berlin The Story of the Lost Child, by Elena Ferrante Pulphead, by John Jeremiah Sullivan. Hurricane Season, by Fernanda Melchor 58 Stay True, by Hua Hsu 83 When We Cease to Understand the World, by Benjamín Labatut 59 Middlesex, by Jeffrey Eugenides 84 The Emperor of All Maladies, by Siddhartha Mukherjee 60 Heavy, by Kiese Laymon 85 Pastoralia, by George Saunders 61 Demon Copperhead, by Barbara Kingsolver 86 Frederick Douglass, by David W. Blight 62 10:04, by Ben Lerner 87 Detransition, Baby, by Torrey Peters 63 Veronica, by Mary Gaitskill 88 The Collected Stories of Lydia Davis 64 The Great Believers, by Rebecca Makkai 89 The Return, by Hisham Matar 65 The Plot Against America, by Philip Roth 90 The Sympathizer, by Viet Thanh Nguyen 66 We the Animals, by Justin Torres 91 The Human Stain, by Philip Roth 67 Far From the Tree, by Andrew Solomon 92 The Days of Abandonment, by Elena Ferrante 68 The Friend, by Sigrid Nunez 93 Station Eleven, by Emily St. John Mandel 69 59 The New Jim Crow, by Michelle Alexander 94 On Beauty, by Zadie Smith 10 70 All Aunt Hagar's Children, by Edward P. Jones 95 Bring Up the Bodies, by Hilary Mantel 71 The Copenhagen Trilogy, by Tove Ditlevsen 96 Wayward Lives, Beautiful Experiments, by Saidiya Hartman 72 22 Secondhand Time, by Svetlana Alexievich 97 Men We Reaped, by Jesmyn Ward 73 The Passage of Power, by Robert A. Caro 98 Bel Canto, by Ann Patchett 74 Olive Kitteridge, by Elizabeth Strout 99 How to Be Both, by Ali Smith 75 15 Exit West, by Mohsin Hamid 100 Tree of Smoke, by Denis Johnson I've I want read to it read it

IMAGES
VIDEO
COMMENTS
past two years with publication of various landmark trials that. have the potential to change stroke care guidelines. Recent. advancements in the stroke management mainly include that. of ...
Stroke is the leading cause of long-term disability and is the fifth leading cause of death in the US. Ischemic stroke represents 87% of total strokes in the US, and is currently the main focus of stroke research. This literature review examines the risk factors associated with ischemic stroke, changes in cell morphology and signaling in the ...
In the US in 2005, the average age of incidence of stroke was 69.2 years [ 2, 29, 30 ]. Recent research has indicated that people aged 20-54 years are at increasing risk of stroke, probably due to pre-existing secondary factors [ 31 ]. Women are at equal or greater risk of stroke than men, irrespective of age [ 32 ].
Stroke is a clinically defined syndrome of acute, focal neurological deficit attributed to vascular injury (infarction, haemorrhage) of the central nervous system; in modern clinical practice, neuroimaging is increasingly used to confirm the exact pattern of tissue injury. •. Hypertension is the most important modifiable risk factor for ...
Transient ischemic attack (TIA) is traditionally viewed as a self-resolving episode of neurological change without persistent impairments and without evidence of acute brain injury on neuroimaging. However, emerging evidence suggests that TIA may be associated with lingering cognitive dysfunction. Cognitive impairment is a prevalent and disabling sequela of ischemic stroke, but the clinical ...
In this review, we focus on the pathophysiology of stroke, major advances in the identification of therapeutic targets and recent trends in stroke research. Keywords: stroke; pathophysiology; treatment; neurological deficit; recovery; rehabilitation 1. Introduction Stroke is a neurological disorder characterized by blockage of blood vessels.
Previous systematic reviews have explored the effectiveness of interventions on the health, quality of life, and/or well-being outcomes of stroke caregivers. 9-11 A review by Legg et al. 12 evaluated the effectiveness of interventions targeting informal stroke caregivers on outcomes such as caregiver stress and strain. This review included eight randomized controlled trials and found no ...
Methods and materials: A systematic review was performed using PubMed and Embase for studies including adults. > years, rst-ever ischemic stroke in population-based observational studies or regis-. fi. tries, documented TOAST-criteria and minimum 1-year follow-up. Meta-analysis on stroke recurrence rate was performed.
Acute Ischemic Stroke. •. Treatment for patients with acute ischemic stroke is guided by the time from the onset of stroke, the severity of neurologic deficit, and findings on neuroimaging. By ...
The review identified a number of areas of stroke care where there was strong consensus. However, there was extensive repetition and redundancy in guideline recommendations. ... Aims: To systematically review the literature to identify stroke guidelines (excluding primary stroke prevention and subarachnoid hemorrhage) since 1 January 2011, ...
Stroke is a major cause of death and disability globally. Diagnosis depends on clinical features and brain imaging to differentiate between ischaemic stroke and intracerebral haemorrhage. Non-contrast CT can exclude haemorrhage, but the addition of CT perfusion imaging and angiography allows a positive diagnosis of ischaemic stroke versus mimics and can identify a large vessel occlusion target ...
This was thought to be due to the need to review both the patient message and the AI response to check for potential incorrect or misleading results. The authors also reported significantly increased lengths of reply (17.9% [95% CI, 10.1-26.2]), describing a generally wordier but more empathetic AI-produced text.
By critically evaluating the existing literature, this review aims to provide healthcare professionals, researchers, and policymakers with a better understanding of the effectiveness and optimal delivery of physiotherapy interventions in stroke rehabilitation. Ultimately, the findings of this study have the potential to enhance the quality of ...
Literature Synopsis (Clinical) Large artery atherosclerosis remains a common cause of ischemic stroke. Treatment options for stenotic disease include the best medical therapy, surgical endarterectomy, and stenting. The optimal treatment approach remains a contentious topic. There are theoretical reasons to favor each treatment option, and each ...
Stroke is a leading cause of death and disability globally. The current lifetime risk of stroke is 25% for both men and women. 3 There are 14 million new strokes each year and over 80 million stroke survivors with a strong upwards trajectory given the continuous ageing of societies, population growth, and declining stroke fatality. 4 Exciting recent advances in acute stroke therapy translate ...
The Stroke Action Test [21], Stroke Kno wledge Test [22], and Stroke Preparedne ss Vignettes [23] were the most commonly used tools in the published literature, post-2015. Feasibility of tools
stroke. It is hoped that stroke patients will try to control stroke risk factors. Conclusion: This literature review shows that the risk factors for stroke are. increasing due to a history of ...
A literature review was performed following PRISMA guidelines on biomechanical and neuromuscular assessment in upper-limb stroke rehabilitation. The review was composed of two independent searches on (1) biomechanical robotic devices, and (2) electrophysiological digital signal processing.
A systematic review aims to bring evidence together to answer a pre-defined research question. This involves the identification of all primary research relevant to the defined review question, the critical appraisal of this research, and the synthesis of the findings.13 Systematic reviews may combine data from different.
Stroke Rehabilitation Intensity Literature Review Topic Description and Rationale for Choosing the Topic: Providing effective levels of therapy is a major concern post stroke. Patients spend 60% of their day alone and only 13% of their day on therapeutic activities (Bernhardt et al., 2004). We know that the earlier therapy starts
Stroke is a serious health condition that is responsible for more than 5% of total deaths. Near 20% of patients experiencing stroke die every year, resulting in the stroke being at the top of the list of preventable causes of death. Once an acute stroke is suspected, a golden hour of less than an hour is available to prevent the undesirable consequences. Since neuroimaging is mandatory in the ...
stroke. The high incidence of stroke affects state financing. The article published by the Ministry of Health in 2019 mentioned that in 2016 BPJS spent 1.43 trillion, in 2017 it spent 2.18 trillion and in 2018 again increased to 2.56 trillion for stroke services.4 About 85% of stroke events can be avoided by
We systematically searched for guideline recommendation on the day-to-day use of peripheral inflammatory markers such as NLR published in the English language between January 1, 2005, and October 2020. Any other evidence of system biology-based approach or recommendation was explored within the selected guidelines for this scoping review.
Oral vitamin K antagonists (VKA) have been shown to reduce the risk of stroke by approximately 60% compared with placebo. 13 Non-vitamin K antagonist oral anticoagulants (NOACs) further reduce the risk of stroke or systemic embolism compared with VKA by 19% and are associated with a 10% relative risk reduction in mortality. 14 While NOACs ...
Background Stroke is a leading cause of mortality and disability. In higher-income countries, mortality and disability have been reduced with advances in stroke care and early access to rehabilitation services. However, access to such services and the subsequent impact on stroke outcomes in the Philippines, which is a lower- and middle-income countries (LMIC), is unclear. Understanding gaps in ...
kidney disease, tuberculosis, heart disease, heart failure, obesity, central obesity, atrial fibrillation. and also smoking are declared associated w ith the incidence of stroke. The irreversible ...
Abstract. The purpose of this paper is to review the research literature on the patient's experience of stroke. Four qualitative studies on how patients experience stroke were identified. The findings of these studies show that the stroke patient often has clear goals for himself in relation to functional abilities, against which he measures ...
Commentary on : Zhao et al . The impact of education/training on nurses caring for patients with stroke: a scoping review. BMC Nurs 23:90 ### Implications for practice and research Stroke nursing is widely recognised for its significant role across the whole multidisciplinary stroke care pathway.1 Ensuring that stroke nurses are equipped with the latest stroke specialist knowledge and skills ...
The New York Times Book Review I've I want THE 100 BEST BOOKS OF THE 21ST CENTURY read to it read it 51 Life After Life, by Kate Atkinson 52 52 Train Dreams, by Denis Johnson 53 Runaway, by Alice ...
existing literature. The present review seeks to resolve that need. Previous Reviews There are several literature reviews on e-Portfolios. Bryant and Chittum's (2013) literature review on the effectiveness of e-Portfolios in higher education identified four trends in e-Portfolio research, including theory-based arguments, descriptive accounts,