Online Help
Our 24/7 cancer helpline provides information and answers for people dealing with cancer. We can connect you with trained cancer information specialists who will answer questions about a cancer diagnosis and provide guidance and a compassionate ear.
Chat live online
Select the Live Chat button at the bottom of the page
Call us at 1-800-227-2345
Available any time of day or night
Our highly trained specialists are available 24/7 via phone and on weekdays can assist through online chat. We connect patients, caregivers, and family members with essential services and resources at every step of their cancer journey. Ask us how you can get involved and support the fight against cancer. Some of the topics we can assist with include:
- Referrals to patient-related programs or resources
- Donations, website, or event-related assistance
- Tobacco-related topics
- Volunteer opportunities
- Cancer Information
For medical questions, we encourage you to review our information with your doctor.
Breast Cancer
- What Is Breast Cancer?
- What Causes Breast Cancer?
- Ductal Carcinoma in Situ (DCIS)
- Invasive Breast Cancer (IDC/ILC)
- Triple-negative Breast Cancer
- Inflammatory Breast Cancer
- Angiosarcoma of the Breast
- Paget Disease of the Breast
- Phyllodes Tumors
- Key Statistics for Breast Cancer

What’s New in Breast Cancer Research?
- Breast Cancer Risk Factors You Cannot Change
- Lifestyle-related Breast Cancer Risk Factors
- Factors with Unclear Effects on Breast Cancer Risk
- Disproven or Controversial Breast Cancer Risk Factors
- Can I Lower My Risk of Breast Cancer?
- Genetic Counseling and Testing for Breast Cancer Risk
- Deciding Whether to Use Medicine to Reduce Breast Cancer Risk
- Tamoxifen and Raloxifene for Lowering Breast Cancer Risk
- Aromatase Inhibitors for Lowering Breast Cancer Risk
- Preventive Surgery to Reduce Breast Cancer Risk
- American Cancer Society Recommendations for the Early Detection of Breast Cancer
- Mammogram Basics
- Tips for Getting a Mammogram
- What Does the Doctor Look for on a Mammogram?
- Getting Called Back After a Mammogram
- Understanding Your Mammogram Report
- Breast Density and Your Mammogram Report
- Limitations of Mammograms
- Mammograms After Breast Cancer Surgery
- Mammograms for Women with Breast Implants
- Breast Ultrasound
- Newer and Experimental Breast Imaging Tests
- Breast Cancer Signs and Symptoms
- Fine Needle Aspiration (FNA) of the Breast
- Core Needle Biopsy of the Breast
- Surgical Breast Biopsy
- Questions to Ask Before a Breast Biopsy
- Finding Breast Cancer During Pregnancy
- Breast Cancer Grades
- Breast Cancer Ploidy and Cell Proliferation
- Breast Cancer Hormone Receptor Status
- Breast Cancer HER2 Status
- Breast Cancer Gene Expression Tests
- Other Breast Cancer Gene, Protein, and Blood Tests
- Imaging Tests to Find Out if Breast Cancer Has Spread
- Breast Cancer Stages
- Breast Cancer Survival Rates
- Questions to Ask Your Doctor About Breast Cancer
- Breast-conserving Surgery (Lumpectomy)
- Lymph Node Surgery for Breast Cancer
- Exercises After Breast Cancer Surgery
- Radiation for Breast Cancer
- Chemotherapy for Breast Cancer
- Hormone Therapy for Breast Cancer
- Targeted Drug Therapy for Breast Cancer
- Immunotherapy for Breast Cancer
- Treatment of Ductal Carcinoma in Situ (DCIS)
- Treatment of Breast Cancer Stages I-III
- Treatment of Stage IV (Metastatic) Breast Cancer
- Treatment of Recurrent Breast Cancer
- Treatment of Triple-negative Breast Cancer
- Treatment of Inflammatory Breast Cancer
- Treating Breast Cancer During Pregnancy
- Should I Get Breast Reconstruction Surgery?
- Breast Reconstruction Alternatives
- Breast Reconstruction After Breast-conserving Surgery
- Breast Reconstruction Using Implants
- Breast Reconstruction Using Your Own Tissues (Flap Procedures)
- Reconstructing the Nipple and Areola After Breast Surgery
- Questions to Ask Your Surgeon About Breast Reconstruction
- Preparing for Breast Reconstruction Surgery
- What to Expect After Breast Reconstruction Surgery
- Follow-up Care After Breast Cancer Treatment
- Can I Lower My Risk of Breast Cancer Progressing or Coming Back?
- Body Image and Sexuality After Breast Cancer
- Pregnancy After Breast Cancer
- Menopausal Hormone Therapy After Breast Cancer
- Second Cancers After Breast Cancer
- If You Have Breast Cancer
- Fibrosis and Simple Cysts
- Hyperplasia (Ductal or Lobular)
- Lobular Carcinoma in Situ (LCIS)
- Fibroadenomas
- Intraductal Papillomas
- Fat Necrosis and Oil Cysts
- Duct Ectasia
- Radial Scars and Other Non-cancerous Breast Conditions
- Breast Cancer Videos
- Breast Cancer Quiz
- Infographic: 7 Things to Know About Getting a Mammogram
- Frequently Asked Questions About the American Cancer Society’s Breast Cancer Screening Guideline
Researchers around the world are working to find better ways to prevent, detect, and treat breast cancer, and to improve the quality of life of patients and survivors.
Research studies
Breast cancer causes, breast cancer prevention, new tests to personalize your treatment, new imaging tests, breast cancer treatment, supportive care.
Current guidance on preventing and treating breast cancer as well as what might cause it (among other things) has come mainly from information discovered from research studies . Research studies can range from studies done in the lab to clinical trials done with hundreds of thousands of people. Clinical trials are carefully controlled studies that can gather specific information about certain diseases as well as explore promising new treatments.
Clinical trials are one way to get the latest cancer treatments that are being investigated. Still, they are not right for everyone. If you would like to learn more about clinical trials that might be right for you, start by asking your doctor if your clinic or hospital conducts clinical trials, or see Clinical Trials to learn more.
Studies continue to look at how certain lifestyle factors, habits, and other environmental factors, as well as inherited gene changes, might affect breast cancer risk. Here are a few examples:
- Several studies are looking at the effects of physical activity, weight gain or loss, and diet on breast cancer risk.
- Some breast cancers run in families, but many of the gene mutations (changes) that cause these breast cancers are not yet known. Research is being done to identify these gene changes.
- Several studies are focusing on the best use of genetic testing for inherited breast cancer gene mutations.
- Scientists are exploring how common gene variants (small changes in genes that are not as significant as mutations) may affect breast cancer risk. Gene variants typically have only a modest effect on risk by themselves, but when combined they could possibly have a large impact.
- Possible environmental causes of breast cancer have also received more attention in recent years. While much of the science on this topic is still in its earliest stages, this is an area of active research.
Researchers are looking for ways to help reduce breast cancer risk, especially for women who are at high risk. Here are some examples:
- Studies continue to look at whether certain levels of physical activity, losing weight, or eating certain foods, groups of foods, or types of diets might help lower breast cancer risk.
- Some hormonal medicines such as tamoxifen, raloxifene, exemestane, and anastrozole have already been shown to help lower breast cancer risk for certain women at higher risk. Researchers continue to study which groups of women might benefit most from these drugs.
- Clinical trials are also looking at whether some non-hormonal drugs might lower breast cancer risk, such as drugs used to treat blood or bone marrow disorders, like ruxolitinib.
- Studies are looking at vaccines that might help prevent certain types of breast cancer in people who are at high risk for breast cancer (due to presence of hereditary gene mutations or breast cancer in the family).
Breast cancer tissue is routinely tested for the biomarkers ER , PR , and HER2 to help make treatment decisions. A biomarker is any gene, protein, or other substance that can be measured in blood, tissues, or other body fluids. Some studies are looking at whether testing for other biomarkers, such as HER3, might also be helpful, but research on this is still in early phases.
Circulating tumor DNA (ctDNA) is DNA that is released into the bloodstream when cancer cells die. Identifying and testing the ctDNA in the blood for biomarkers is a rapidly growing area of study.
Some ways ctDNA might potentially be used in breast cancer include:
- Looking for new biomarkers in the tumor cells that might mean the cancer has become resistant to specific treatments (like chemo or targeted drug therapy)
- Determining if a certain drug will work on a tumor before trying it
- Predicting if the breast cancer will recur (come back) in women with early-stage breast cancer
- Predicting if neoadjuvant treatment is working to destroy the tumor instead of using imaging tests like a CT scan or US
- Determining if breast cancer or a high-risk breast condition is present before changes are found on an imaging test like a mammogram
Newer types of tests are being developed for breast imaging. Some of these are already being used in certain situations, while others are still being studied. It will take time to see if they are as good as or better than those used today. Some of these tests include:
- Scintimammography (molecular breast imaging)
- Positron emission mammography (PEM)
- Electrical impedance imaging (EIT)
- Elastography
- New types of optical imaging tests
For more on these tests, see Newer and Experimental Breast Imaging Tests .
New kinds of treatments for breast cancer are always being studied. For example, in recent years, several new targeted drugs have been approved to treat breast cancer.
But more and better treatment options are needed, especially for cancers like triple-negative breast cancer, where chemotherapy is the main option.
Some areas of research involving breast cancer treatment include:
- Studying if shorter courses of radiation therapy for very early-stage breast cancers are at least as good as the longer courses now often used
- Testing if different types of radiation therapy, such as proton beam radiation, might be better than standard radiation.
- Combining certain drugs (like 2 targeted drugs, a targeted drug with an immunotherapy drug, or a hormone drug with a targeted drug) to see if they work better together
- Trying to find new drugs or drug combinations that might help treat breast cancer that has spread to the brain
- Testing different immunotherapy drugs to treat triple-negative breast cancer
- Giving cancer vaccines to see if this helps keep the cancer from either worsening or coming back after treatment. There are many ways in which cancer vaccines work. For example, protein vaccines stimulate the immune system to recognize and attack specific cancer proteins. DNA vaccines contain DNA instructions so that once the vaccine is given, the DNA will instruct your body to make protein(s) to help the immune system recognize and attack cancer cells.
- Finding new ways to treat women with hereditary breast cancer, since they have a higher chance of the cancer recurring (coming back)
- Determining if chemotherapy is needed to treat every woman with HER2-positive breast cancer
- Finding new treatment options when breast cancer becomes resistant to current treatments
Supportive care helps patients and caregivers manage the symptoms of cancer and side effects of cancer treatment. Clinical trials are looking at different medicines and techniques to try to improve supportive care for people with breast cancer. For example, some studies are investigating:
- If there are better medicines or ways to prevent the damage to nerves that sometimes happen with certain chemotherapy drugs
- If drugs or other treatments might be helpful in limiting memory problems and other brain symptoms after chemotherapy
- If certain heart or blood pressure drugs, can help prevent the heart damage sometimes caused by common breast cancer drugs such as doxorubicin and trastuzumab
- If there are medicines that might be able to help treat the tired feeling that cancer can cause
Breast Cancer Research Highlights
The Society's research program has played a crucial role in saving lives from breast cancer. See examples of our current research.

The American Cancer Society medical and editorial content team
Our team is made up of doctors and oncology certified nurses with deep knowledge of cancer care as well as editors and translators with extensive experience in medical writing.
Chan JCH, Chow JCH, Ho CHM, Tsui TYM, Cho WC. Clinical application of circulating tumor DNA in breast cancer. J Cancer Res Clin Oncol. 2021;147(5):1431-1442. doi:10.1007/s00432-021-03588-5.
Cullinane C, Fleming C, O’Leary DP, et al. Association of Circulating Tumor DNA With Disease-Free Survival in Breast Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3(11):e2026921. doi:10.1001/jamanetworkopen.2020.26921.
Cuzick, J et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. The Lancet. 2014;383 (9922):1041 - 1048.
Goss, P.E., et al., Exemestane for Breast-Cancer Prevention in Postmenopausal Women . New England Journal of Medicine , 2011. 364(25): p. 2381-2391.
Greene LR, Wilkinson D. The role of general nuclear medicine in breast cancer. J Med Radiat Sci . 2015;62(1):54-65.
Henry NL, Bedard PL, and DeMichele A. Standard and Genomic Tools for Decision Support in Breast Cancer Treatment. In Dizon DS, Pennel N, Rugo HS, Pickell LF, eds. 2017 American Society of Clinical Oncology Educational Book. 53 rd Annual Meeting. 2017.
Ignatiadis M, Lee M, and Jeffrey SS. Circulating Tumor Cells and Circulating Tumor DNA: Challenges and Opportunities on the Path to Clinical Utility. Clin Cancer Res ; 21(21); 4786–800.
Litton JK, Burstein HJ, Turner NC. Molecular Testing in Breast Cancer. Am Soc Clin Oncol Educ Book . 2019 Jan;39:e1-e7. doi: 10.1200/EDBK_237715. Epub 2019 May 17.
Magbanua MJM, Swigart LB, Wu HT, et al. Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol . 2021;32(2):229-239. doi:10.1016/j.annonc.2020.11.007.
Mayer IA, Dent R, Tan T, et al. Novel Targeted Agents and Immunotherapy in Breast Cancer. In Dizon DS, Pennel N, Rugo HS, Pickell LF, eds. 2017 American Society of Clinical Oncology Educational Book. 53 rd Annual Meeting. 2017.
National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/clinical-trials/search. A Vaccine (Alpha-Lactalbumin) for the Treatment of Stage II-III Triple-Negative Breast Cancer. Accessed January 19, 2022.
National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/clinical-trials/search. Bexarotene in Preventing Breast Cancer in Patients at High Risk for Breast Cancer. Accessed August 15, 2019.
National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/clinical-trials/search. Donepezil Hydrochloride in Improving Memory Performance in Breast Cancer Survivors after Chemotherapy. Accessed August 15, 2019.
National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/clinical-trials/search. Ruxolitinib in Preventing Breast Cancer in Patients with High Risk and Precancerous Breast Lesions. Accessed August 15, 2019.
National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/clinical-trials/search. Testing the Addition of a Blood Pressure Medication, Carvedilol, to HER-2 Targeted Therapy for Metastatic Breast Cancer to Prevent Cardiac Toxicity. Accessed August 15, 2019.
National Institute of Environmental Health Sciences. Breast Cancer. Last reviewed November 15, 2021. Accessed January 19, 2022. https://www.niehs.nih.gov/health/topics/conditions/breast-cancer/index.cfm.
Rossi G, Mu Z, Rademaker AW, Austin LK, Strickland KS, Costa RLB et al. Cell-Free DNA and Circulating Tumor Cells: Comprehensive Liquid Biopsy Analysis in Advanced Breast Cancer. Clin Cancer Res. 2018 Feb 1;24(3):560-568.
Shoukry M, Broccard S, Kaplan J, Gabriel E. The Emerging Role of Circulating Tumor DNA in the Management of Breast Cancer. Cancers (Basel) . 2021;13(15):3813. Published 2021 Jul 29. doi:10.3390/cancers13153813.
Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst . 2013;105:701-710.
Yu M, Bardia A, Aceto N et al. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science . 2014 Jul 11; 345(6193): 216–220.
Last Revised: February 15, 2024
American Cancer Society medical information is copyrighted material. For reprint requests, please see our Content Usage Policy .
American Cancer Society Emails
Sign up to stay up-to-date with news, valuable information, and ways to get involved with the American Cancer Society.
More in Breast Cancer
- About Breast Cancer
- Risk and Prevention
- Early Detection and Diagnosis
- Understanding a Breast Cancer Diagnosis
- Breast Reconstruction Surgery
- Living as a Breast Cancer Survivor
Help us end cancer as we know it, for everyone.

If this was helpful, donate to help fund patient support services, research, and cancer content updates.
Breast Cancer Research

Breast Cancer Risk Factors
Breast Cancer Research is presenting our Retrospective Collection on "Breast Cancer Risk Factors." Celebrating 'Breast Cancer Awareness Month (1 October- 31 October)', with this Collection, we aim to gain valuable insights into the multifaceted aspects of breast cancer risk to promote awareness, prevention, and early detection.
NEW CROSS-JOURNAL COLLECTIONS Find out more by clicking the links below:
Artif icial Intelligence in Breast Imaging PDGFB in Br east Cancer Initiation,Progression, and Metastasis

Aims and scope
- Most accessed
Validation of an AI-based solution for breast cancer risk stratification using routine digital histopathology images
Authors: Abhinav Sharma, Sandy Kang Lövgren, Kajsa Ledesma Eriksson, Yinxi Wang, Stephanie Robertson, Johan Hartman and Mattias Rantalainen
The SEMA3F-NRP1/NRP2 axis is a key factor in the acquisition of invasive traits in in situ breast ductal carcinoma
Authors: Núria Moragas, Patricia Fernandez-Nogueira, Leire Recalde-Percaz, Jamie L. Inman, Anna López-Plana, Helga Bergholtz, Aleix Noguera-Castells, Pedro J. del Burgo, Xieng Chen, Therese Sorlie, Pere Gascón, Paloma Bragado, Mina Bissell, Neus Carbó and Gemma Fuster
Association of Life’s Essential 8 cardiovascular health with breast cancer incidence and mortality according to genetic susceptibility of breast cancer: a prospective cohort study
Authors: Yan Zhao, Yang Song, Xiangmin Li and Ayao Guo
Micrometastases in axillary lymph nodes in breast cancer, post-neoadjuvant systemic therapy
Authors: Janghee Lee, Seho Park, Soong June Bae, Junghwan Ji, Dooreh Kim, Jee Ye Kim, Hyung Seok Park, Sung Gwe Ahn, Seung Il Kim, Byeong-Woo Park and Joon Jeong
Development of a humanized anti-FABP4 monoclonal antibody for potential treatment of breast cancer
Authors: Jiaqing Hao, Rong Jin, Yanmei Yi, Xingshan Jiang, Jianyu Yu, Zhen Xu, Nicholas J. Schnicker, Michael S. Chimenti, Sonia L. Sugg and Bing Li
Most recent articles RSS
View all articles
Serum thymidine kinase 1 activity as a pharmacodynamic marker of cyclin-dependent kinase 4/6 inhibition in patients with early-stage breast cancer receiving neoadjuvant palbociclib
Authors: Nusayba Bagegni, Shana Thomas, Ning Liu, Jingqin Luo, Jeremy Hoog, Donald W. Northfelt, Matthew P. Goetz, Andres Forero, Mattias Bergqvist, Jakob Karen, Magnus Neumüller, Edward M. Suh, Zhanfang Guo, Kiran Vij, Souzan Sanati, Matthew Ellis…
Choosing the right cell line for breast cancer research
Authors: Deborah L Holliday and Valerie Speirs
Triple-negative breast cancer molecular subtyping and treatment progress
Authors: Li Yin, Jiang-Jie Duan, Xiu-Wu Bian and Shi-cang Yu
Breast asymmetry and predisposition to breast cancer
Authors: Diane Scutt, Gillian A Lancaster and John T Manning
Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer
Authors: Suzanne A Eccles, Eric O Aboagye, Simak Ali, Annie S Anderson, Jo Armes, Fedor Berditchevski, Jeremy P Blaydes, Keith Brennan, Nicola J Brown, Helen E Bryant, Nigel J Bundred, Joy M Burchell, Anna M Campbell, Jason S Carroll, Robert B Clarke, Charlotte E Coles…
Most accessed articles RSS

Editor-in-Chief
Lewis Chodosh , University of Pennsylvania, USA

Trending in the Media
Click here to see the most popular articles published in Breast Cancer Research in the past three months.

BCR's 20th Anniversary
20 years ago Breast Cancer Research published its first articles with BMC. Well-respected in the field, the journal has continually placed in the first quartile of the ‘Oncology’ category of Journal Citation Reports. Over the past decade, Breast Cancer Research (BCR) has also become the highest ranked breast cancer focused title in the field.
Look back at the journal’s milestone achievements and article highlights .

Featured Review - Artificial intelligence in mammographic phenotyping of breast cancer risk: a narrative review
In this review, we provide a useful reference for AI researchers investigating image-based breast cancer risk assessment while indicating key priorities and challenges that, if properly addressed, could accelerate the implementation of AI-assisted risk stratification to future refine and individualize breast cancer screening strategies.
Springer Nature Oncology Portfolio
Discover the range of academic oncology titles at Springer Nature here .

Loving your pup may be a many splendored thing

Aspirin may help cut colorectal cancer risk
Faster ‘in a dish’ model may speed up treatment for Parkinson’s
Estrogen a more powerful breast cancer culprit than we realized.
Getty Images
Ekaterina Pesheva
HMS Communications
Potential path to better testing in findings that identify hormone as ‘a catalyst and a cause’ in disease
In what may turn out to be a long-missing piece in the puzzle of breast cancer, Harvard Medical School researchers have identified the molecular sparkplug that ignites cases of the disease currently unexplained by the classical model of breast-cancer development.
A report on the team’s work is published May 17 in Nature.
“We have identified what we believe is the original molecular trigger that initiates a cascade culminating in breast tumor development in a subset of breast cancers that are driven by estrogen,” said study senior investigator Peter Park , professor of Biomedical Informatics in the Blavatnik Institute at HMS.
The researchers said as many as one-third of breast cancer cases may arise through the newly identified mechanism.
The study also shows that the sex hormone estrogen is the culprit behind this molecular dysfunction because it directly alters a cell’s DNA.
Most, though not all, breast cancers are fueled by hormonal fluctuations . The prevailing view of estrogen’s role in breast cancer is that it acts as a catalyst for cancer growth because it stimulates the division and proliferation of breast tissue, a process that carries the risk for cancer-causing mutations. The new work, however, shows that estrogen causes mischief in a far more direct manner.
“Our work demonstrates that estrogen can directly induce genomic rearrangements that lead to cancer, so its role in breast cancer development is both that of a catalyst and a cause,” said study first author Jake Lee , a former research fellow in the Park lab who is now a medical oncology fellow at Memorial Sloan Kettering Cancer Center.
Although the work has no immediate implications for therapy, it could inform the design of tests that can track treatment response and could help doctors detect the return of tumors in patients with a history of certain breast cancers.
Birth of a cancer cell
The human body is made up of hundreds of trillions of cells. Most of these cells are constantly dividing and replicating, a process that sustains the function of organs day after day, over a lifetime.
With each division, a cell makes a copy of its chromosomes — bundles of tightly compressed DNA — into a new cell. But this process sometimes goes awry, and DNA can break. In most cases, these DNA breaks get swiftly mended by the molecular machinery that guards the integrity of the genome. However, every now and then, the repair of broken DNA gets botched, causing chromosomes to get misplaced or scrambled inside a cell.
Many human cancers arise in this manner during cell division, when chromosomes get rearranged and awaken dormant cancer genes that can trigger tumor growth.
One such chromosomal scramble can occur when a chromosome breaks, and a second copy of the broken chromosome is made before the break gets fixed.
Then, in what ends up being a botched repair attempt, the broken end of one chromosome is fused to the broken end of its sister copy rather than to its original partner. The resulting new structure is a misshapen, malfunctioning chromosome.
During the next cell division, the misshapen chromosome is stretched between the two emerging daughter cells and the chromosome “bridge” breaks, leaving behind shattered fragments that contain cancer genes to multiply and get activated.
“Our work demonstrates that estrogen can directly induce genomic rearrangements that lead to cancer, so its role in breast cancer development is both that of a catalyst and a cause.” Jake Lee, medical oncology fellow at Memorial Sloan Kettering Cancer Center
Certain human cancers, including some breast cancers, arise when a cell’s chromosomes get rearranged in this way. This malfunction was first described in the 1930s by Barbara McClintock , who went on to win the Nobel Prize in physiology or medicine in 1983.
Cancer experts can often identify this particular aberration in tumor samples by using genomic sequencing. Yet, a portion of breast cancer cases do not harbor this mutational pattern, raising the question: What is causing these tumors?
These were the “cold” cases that intrigued study authors Park and Lee. Looking for answers, they analyzed the genomes of 780 breast cancers obtained from patients diagnosed with the disease. They expected to find the classical chromosomal disarray in most of the tumor samples, but many of the tumor cells bore no trace of this classic molecular pattern.
Instead of the classic misshapen and improperly patched-up single chromosome, they saw that two chromosomes had fused, suspiciously near “hot spots” where cancer genes are located.
Just as in McClintock’s model, these rearranged chromosomes had formed bridges, except in this case, the bridge contained two different chromosomes. This distinctive pattern was present in one-third (244) of the tumors in their analysis.
Lee and Park realized they had stumbled upon a new mechanism by which a “disfigured” chromosome is generated and then fractured to fuel the mysterious breast cancer cases.
A new role for estrogen in breast cancer?
When the researchers zoomed onto the hot spots of cancer-gene activation, they noticed that these areas were curiously close to estrogen-binding areas on the DNA.
Estrogen receptors are known to bind to certain regions of the genome when a cell is stimulated by estrogen. The researchers found that these estrogen-binding sites were frequently next to the zones where the early DNA breaks took place.
This offered a strong clue that estrogen might be somehow involved in the genomic reshuffling that gave rise to cancer-gene activation.
Lee and Park followed up on that clue by conducting experiments with breast cancer cells in a dish. They exposed the cells to estrogen and then used CRISPR gene editing to make cuts to the cells’ DNA.
As the cells mended their broken DNA, they initiated a repair chain that resulted in the same genomic rearrangement Lee and Park had discovered in their genomic analyses.
Estrogen is already known to fuel breast cancer growth by promoting the proliferation of breast cells. However, the new observations cast this hormone in a different light.
They show estrogen is a more central character in cancer genesis because it directly alters how cells repair their DNA.
The findings suggest that estrogen-suppressing drugs such as tamoxifen — often given to patients with breast cancer to prevent disease recurrence — work in a more direct manner than simply reducing breast cell proliferation.
“In light of our results, we propose that these drugs may also prevent estrogen from initiating cancer-causing genomic rearrangements in the cells, in addition to suppressing mammary cell proliferation,” Lee said.
The study could lead to improved breast cancer testing. For instance, detecting the genomic fingerprint of the chromosome rearrangement could alert oncologists that a patient’s disease is coming back, Lee said.
A similar approach to track disease relapse and treatment response is already widely used in cancers that harbor critical chromosomal translocations, including certain types of leukemias.
More broadly, the work underscores the value of DNA sequencing and careful data analysis in deepening the biology of cancer development, the researchers said.
“It all started with a single observation. We noticed that the complex pattern of mutations that we see in genome sequencing data cannot be explained by the textbook model,” Park said. “But now that we’ve put the jigsaw puzzle together, the patterns all make sense in light of the new model. This is immensely gratifying.” Additional authors included Youngsook Lucy Jung, Taek-Chin Cheong, Jose Espejo Valle-Inclan, Chong Chu, Doga C. Gulhan,Viktor Ljungstrom, Hu Jin, Vinayak Viswanadham, Emma Watson, Isidro Cortes-Ciriano, Stephen Elledge, Roberto Chiarle, and David Pellman.
This work was funded by grants from Ludwig Center at Harvard, Cancer Grand Challenges, Cancer Research UK, and the Mark Foundation for Cancer Research, National Institutes of Health grant 1R01-CA222598, and with additional support from the Office of Faculty Development/CTREC/BTREC Career Development Fellowship.
Share this article
You might like.

New research suggests having connection to your dog may lower depression, anxiety

New research suggests those with less healthy lifestyles may get highest benefit from regular use

Could result in personalized models to test diagnostic and treatment strategies
Good genes are nice, but joy is better
Harvard study, almost 80 years old, has proved that embracing community helps us live longer, and be happier
Committee named to lead Legacy of Slavery memorial project
University names committee to lead Harvard & the Legacy of Slavery Memorial Project.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
News Release
Tuesday, February 1, 2022
NIH study advances personalized immunotherapy for metastatic breast cancer

An experimental form of immunotherapy that uses an individual’s own tumor-fighting immune cells could potentially be used to treat people with metastatic breast cancer, according to results from an ongoing clinical trial led by researchers at the National Cancer Institute’s (NCI) Center for Cancer Research, part of the National Institutes of Health. Many people with metastatic breast cancer can mount an immune reaction against their tumors, the study found, a prerequisite for this type of immunotherapy, which relies on what are called tumor-infiltrating lymphocytes (TILs).
In a clinical trial of 42 women with metastatic breast cancer, 28 (or 67%) generated an immune reaction against their cancer. The approach was used to treat six women, half of whom experienced measurable tumor shrinkage. Results from the trial appeared Feb. 1, 2022, in the Journal of Clinical Oncology .
“It’s popular dogma that hormone receptor–positive breast cancers are not capable of provoking an immune response and are not susceptible to immunotherapy,” said study leader Steven A. Rosenberg, M.D., Ph.D., chief of the Surgery Branch in NCI’s Center for Cancer Research. “The findings suggest that this form of immunotherapy can be used to treat some people with metastatic breast cancer who have exhausted all other treatment options.”
Immunotherapy is a treatment that helps a person’s own immune system fight cancer. However, most available immunotherapies, such as immune checkpoint inhibitors, have shown limited effectiveness against hormone receptor–positive breast cancers, which are the majority of breast cancers.
The immunotherapy approach used in the trial was pioneered in the late 1980s by Dr. Rosenberg and his colleagues at NCI. It relies on TILs, T cells that are found in and around the tumor.
TILs can target tumor cells that have specific proteins on their surface, called neoantigens, that the immune cells recognize. Neoantigens are produced when mutations occur in tumor DNA. Other forms of immunotherapy have been found to be effective in treating cancers, such as melanoma, that have many mutations, and therefore many neoantigens. Its effectiveness in cancers that have fewer neoantigens, such as breast cancer, however, has been less clear.
The results of the new study come from an ongoing phase 2 clinical trial being carried out by Dr. Rosenberg and his colleagues. This trial was designed to see if the immunotherapy approach could lead to tumor regressions in people with metastatic epithelial cancers, including breast cancer. In 2018, the researchers showed that one woman with metastatic breast cancer who was treated in this trial had complete tumor shrinkage, known as a complete response.
In the trial, the researchers used whole-genome sequencing to identify mutations in tumor samples from 42 women with metastatic breast cancer whose cancers had progressed despite all other treatments. The researchers then isolated TILs from the tumor samples and, in lab tests, tested their reactivity against neoantigens produced by the different mutations in the tumor.
Twenty-eight women had TILs that recognized at least one neoantigen. Nearly all the neoantigens identified were unique to each patient.
“It’s fascinating that the Achilles’ heel of these cancers can potentially be the very gene mutations that caused the cancer,” said Dr. Rosenberg. “Since that 2018 study, we now have information on 42 patients, showing that the majority give rise to immune reactions.”
For the six women treated, the researchers took the reactive TILs and grew them to large numbers in the lab. They then returned the immune cells to each patient via intravenous infusion. All the patients were also given four doses of the immune checkpoint inhibitor pembrolizumab (Keytruda) before the infusion to prevent the newly introduced T cells from becoming inactivated.
After the treatment, tumors shrank in three of the six women. One is the original woman reported in the 2018 study, who remains cancer free to this day. The other two women had tumor shrinkage of 52% and 69% after six months and 10 months, respectively. However, some disease returned and was surgically removed. Those women now have no evidence of cancer approximately five years and 3.5 years, respectively, after their TIL treatment.
The researchers acknowledged that the use of pembrolizumab, which has been approved for some early-stage breast cancers, may raise uncertainties about its influence on the outcome of TIL therapy. However, they said, treatment with such checkpoint inhibitors alone has not led to sustained tumor shrinkage in people with hormone receptor–positive metastatic breast cancer.
Dr. Rosenberg said that with the anticipated opening early this year of NCI’s new building devoted to cell-based therapies, he and his colleagues can begin treating more individuals with metastatic breast cancer as part of the ongoing clinical trial. He noted that this new immunotherapy approach could potentially be used for people with other types of cancer as well.
“We’re using a patient’s own lymphocytes as a drug to treat the cancer by targeting the unique mutations in that cancer,” he said. “This is a highly personalized treatment.”
About the Center for Cancer Research (CCR): CCR comprises nearly 250 teams conducting basic, translational, and clinical research in the NCI intramural program — an environment supporting innovative science aimed at improving human health. CCR’s clinical program is housed at the NIH Clinical Center — the world’s largest hospital dedicated to clinical research. For more information about CCR and its programs, visit ccr.cancer.gov .
About the National Cancer Institute (NCI): NCI leads the National Cancer Program and NIH’s efforts to dramatically reduce the prevalence of cancer and improve the lives of cancer patients and their families, through research into prevention and cancer biology, the development of new interventions, and the training and mentoring of new researchers. For more information about cancer, please visit the NCI website at cancer.gov or call NCI’s contact center, the Cancer Information Service, at 1-800-4-CANCER (1-800-422-6237).
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Connect with Us
- More Social Media from NIH
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Menopausal Med
- v.28(2); 2022 Aug

Hormone Therapy and Risk of Breast Cancer: Where Are We Now?
John p. micha.
1 Women’s Cancer Research Foundation, Laguna Beach, CA, USA.
Mark A. Rettenmaier
Randy d. bohart.
2 Oso Home Care, Inc., Irvine, CA, USA.
Bram H. Goldstein
Several studies have examined the clinical benefits of hormone replacement therapy (HRT). However, because long-term use of HRT has been implicated as a risk factor for the development of breast cancer, some women remain skeptical when considering this therapy to address their vasomotor symptoms. Hence, physicians and nurses should actively engage in constructive discourse with their patients regarding HRT while specifically reviewing the potential risks of its extended use as well as provide the available medical alternatives the patients could potentially use.
INTRODUCTION
Breast cancer is the most common female malignancy in the United States, afflicting 281,500 women in 2020 [ 1 ]. Fortunately, nearly two-thirds of breast cancer patient cases are diagnosed at a localized stage, wherein the 5-year survival rate is favorable. Age (45 to 75 years) is a primary risk factor for breast cancer development [ 2 ], in addition to ovarian hormones, the presence of BRCA1 (BReast CAncer gene 1) and BRCA2 (BReast CAncer gene 2) gene mutations, reproductive history, and previous chest irradiation [ 3 , 4 ].
HORMONE THERAPY AND BREAST CANCER
Since the 1970’s, approximately 600 million women from western countries have used hormone replacement therapy (HRT) [ 5 ]. HRT, encompassing conjugated estrogens alone, or in combination with progestin, is indicated to attenuate vasomotor symptoms, forestall cognitive deficits, and avert cardiovascular disease [ 6 , 7 ]. However, several randomized clinical trials and observational studies have impugned the safety of HRT because of the medication’s putative relationship with breast cancer incidence and mortality [ 5 , 8 , 9 , 10 ].
Estradiol heightens the risk of breast cancer in postmenopausal women and preclinical studies have demonstrated that progestin engenders progenitor cells in both human and breast cancer cells [ 11 , 12 , 13 ]. While estrogen and progesterone levels significantly decline following menopause, HRT-induced blood estrogen levels correlate with an increased incidence of estrogen receptor-positive breast cancer, especially in women with a higher body mass index [ 13 ]. Studies have remarked that perhaps, estrogen-alone is safe, whereas estrogen/progestin ostensibly accords a countervailing or detrimental effect [ 14 , 15 ]. Hence, when evaluating a woman’s lifetime risk for developing breast cancer, one should consider the persistent inclusion of these two agents, either independently or collectively.
WOMEN’S HEALTH INITIATIVE (WHI) AND MILLION WOMEN TRIALS
The WHI initially reported on the controversial benefits and potential complications inherent in HRT in their 2002 landmark study [ 10 ]. The trial comprised 16,608 healthy postmenopausal women with a uterus who underwent treatment with conjugated equine estrogens (0.625 mg/d) and medroxyprogesterone acetate (2.5 mg/d) or placebo for a median duration of 7.2 years. The HRT group was associated with more breast cancer-related deaths compared to the place group (2.6 vs. 1.3 per 10,000 women annually), resulting in clinical trial termination. In 2004, the WHI evaluated 10,739 postmenopausal women with a prior hysterectomy, ages 50–79 years, who underwent either conjugated equine estrogens (0.625 mg/d) or placebo. This study was also prematurely closed due to the elevated risk of stroke in the HRT group although interestingly, the results suggested a possible decreased incidence in breast cancer [ 8 ].
In the Million Women Study, 1,084,110 women from the United Kingdom (U.K.), ages 50–64 years, reported their use of HRT (estrogen alone or progestin-estrogen) and were surveilled to ascertain the subjects’ breast cancer incidence and mortality [ 9 ]. The results suggested that women who underwent estrogen-only or estrogen-progestogen had a 1.3-fold risk or 2-fold risk of developing breast cancer, respectively. Following the results from the WHI and U.K. trials, the use of HRT declined significantly [ 16 ]; and from 2001 to 2004, an 8.6% decrease in breast cancer incidence was reported for women, 50 years or older [ 17 ].
WHI FOLLOW-UP STUDIES
Manson et al. [ 18 ] reported on the updated findings from the two WHI studies, indicating that hormone therapy may be beneficial in terms of all-cause mortality, with fewer risks (e.g., coronary heart disease, stroke) among women 50 through 59 years. Moreover, the risks from conjugated equine estrogens plus medroxyprogesterone were primarily inherent to the intervention phase, and both the risks and benefits were ultimately ephemeral throughout the post-intervention period.
In 2017, the WHI clinical studies [ 15 ] reported that there were 7,489 deaths throughout the treatment and surveillance periods; all-cause mortality was 27.1% for the women who underwent HRT vs. 27.6% in the placebo group (i.e., neither estrogen-alone nor in combination with medroxyprogesterone acetate was associated with an increased risk of cancer mortality). Accordingly, the North American Menopause Society revised its guidelines [ 19 ]; HRT was recommended for women who were younger than 60 or within 10 years of menopause, especially if they were at higher risk for bone loss or fracture.
A prolonged review of the WHI trials in 2019 suggested that estrogen-alone had a countervailing effect on breast cancer incidence, compared to the increased risk of breast cancer from estrogen/progestin therapy [ 14 ]. There was a 23% reduction in breast cancer for the postmenopausal woman who received estrogen therapy-alone, whereas the risk of breast cancer and breast cancer-related death was elevated by 29% for the women treated with estrogen/progestin, an effect that transcended 10-years of discontinued use.
U.K. HRT STUDIES
Despite the findings from the 2019 WHI study, an epidemiological study conducted in the U.K. reported that of the 108,647 postmenopausal subjects who developed breast cancer, 51% had been treated with HRT [ 5 ]. Interestingly, the elevated risk encompassed all types of HRT (vaginal estrogens excepted), particularly combined estrogen/progestin. The increased risk from estrogen/progesterone was evident for patients on therapy for 1–4 years, with a two-fold risk during years 5–14, particularly in women who ultimately developed estrogen receptor-positive breast cancer.
When considering the specific risks according to age, women who used HRT for 5 years, commencing at age 50 years, exhibited a significant increase in risk for breast cancer at ages 50–69 years (half of the elevated risk was attributed during the first 5 years of current HRT use and the other half was ascribed to the subsequent 15 years of prior use) [ 5 ]; this represents an absolute increase of approximately 2% for women undergoing estrogen/progesterone. Additionally, the corresponding risks with 10 years of use starting at age 50 years would be nearly two-fold.
In a subsequently published U.K. nested-control study, the researchers compared the impact of HRT on 98,611 breast cancer patients, ages 50–79, to 457,498 female control subjects [ 20 ]. Overall, HRT use was associated with an increased risk of breast cancer (odds ratio [OR], 1.21; 95% confidence interval [CI], 1.19 to 1.23). Intriguingly, the elevated risk was attributed to both estrogen and progesterone (OR, 1.26; 95% CI, 1.24 to 1.29) and estrogen-only therapy (OR, 1.06; 95% CI, 1.03 to 1.10). They also reported that the associated risk of breast cancer increased with advanced age, potentially attributed to relatively longer HRT use. Moreover, the association between HRT use and breast cancer progressively diminished with increasing years of HRT cessation.
NON-HORMONAL OPTIONS
In accordance with the numerous reported toxicities associated with HRT, women are persistently inquiring about medical alternatives to HRT in endeavoring to attenuate their menopausal symptoms. Neurotransmitter modulators, namely selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors (e.g., venlafaxine, fluoxetine) have been considered viable alternatives to hormone therapy [ 21 , 22 , 23 ]. Gabapentin, an anticonvulsant, and clonidine, an antihypertensive, have also been utilized to mitigate the frequency and severity of menopause-associated vasomotor symptoms by approximately 70% [ 24 , 25 ]; however, the precise mechanism of action inherent in these medications that attenuates patient symptoms remains indeterminate [ 23 ]. Soy and herbs have additionally conferred a reported beneficial effect in managing menopausal symptoms [ 26 ]. Please refer to Table 1 for a list of nonhormonal agents used as therapy for hot flashes [ 27 , 28 , 29 , 30 , 31 , 32 , 33 ].
| Agent | Dose (mg/d) | Duration of dosage | Efficacy | Reference no. |
|---|---|---|---|---|
| Black cohosh | 16–127 | Up to 12 mo | 26% reduction in hot flashes | [ ] |
| Clonidine | 0.1 | 8–12 wk | 20% reduction in hot flashes | [ ] |
| Fluoxetine | 20 | 9 wk | 50% decrease in hot flashes | [ ] |
| Paroxetine | 20–40 | 6–12 wk | 33%–67% reduction in hot flash frequency | [ ] |
| Soy | 40–164 | 7–12 wk | Relatively short; long-term efficacy unknown | [ ] |
| Venlafaxine | 37.5–150 | 4–12 wk | Median hot flash frequency decrease by 7.6 hot flashes/day | [ ] |
| Gabapentin | 300 | 12 wk | 45% reduction in hot flashes | [ ] |
HRT was routinely used in the 1990’s to attenuate menopausal symptoms and currently, greater than 40% of women in the United States are prescribed this treatment [ 34 ]. Moreover, HRT reduces symptoms by 75% in the management of vulvovaginal atrophy, a urogenital condition that occurs in approximately 40% of postmenopausal breast cancer patients following treatment [ 35 ]. The combined results from the aforesaid HRT trials are varied, and thus, confound an unequivocal approach to treating menopausal symptoms. Conversely, when endeavoring to assess the risks associated with HRT, we recognize the disadvantages of untreated vasomotor symptoms, which can impair quality of life and diminish work productivity [ 36 ]. Perhaps, before initiating HRT, the physician and patient should thoughtfully engage in discourse that incorporates the individual’s clinical symptoms and attendant risk profile.
ACKNOWLEDGMENTS
This study was supported by the Women’s Cancer Research Foundation, Joan and Len Rullo, in memory of Elizabeth Johnson, and Susan Berg.
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Breast Cancer Index in Premenopausal Women With Early-Stage Hormone Receptor-Positive Breast Cancer
Affiliations.
- 1 University of Rochester Department of Medicine, Rochester, New York.
- 2 Biotheranostics, A Hologic Company, San Diego, California.
- 3 The University of Chicago Medical Center, Chicago, Illinois.
- 4 The Sir Peter MacCallum Department of Medical Oncology, The University of Melbourne, Parkville, Australia.
- 5 Department of Medical Oncology, Peter MacCallum Cancer Center, Melbourne, Australia.
- 6 St Vincent's Hospital, Melbourne, Australia.
- 7 Breast Cancer Trials Australia & New Zealand, Newcastle, Australia.
- 8 University of Newcastle, Callaghan, Newcastle, Australia.
- 9 International Breast Cancer Study Group, ETOP IBCSG Partners Foundation, Bern, Switzerland.
- 10 International Breast Cancer Study Group Central Pathology Office, European Institute of Oncology IRCCS, Milan, Italy.
- 11 Department of Pathology and Laboratory Medicine, European Institute of Oncology IRCCS, Milan, Italy.
- 12 Clinexpert-Research, Budapest, Hungary.
- 13 Vall d'Hebron Institute of Oncology (VHIO) and Vall d'Hebron University Hospital, Barcelona, Spain.
- 14 SOLTI Breast Cancer Research Cooperative Group, Barcelona, Spain.
- 15 Institut Bergonie Comprehensive Cancer Center, Universite de Bordeaux, INSERM U1312, Bordeaux, France.
- 16 European Organization for Research and Treatment of Cancer (EORTC), Brussels, Belgium.
- 17 Ospedale Papa Giovanni XXIII, Bergamo, Italy.
- 18 Oncology Unit, Department of Oncology, Alessandro Manzoni Hospital, ASST Lecco, Lecco, Italy.
- 19 Operative Unit of Medical Oncology, IRCCS ICS Maugeri, Pavia, Italy.
- 20 Medical Oncology Department, University Hospital 12 de Octubre, Madrid, Spain.
- 21 Gynecologic Oncology and Multidisciplinary Breast Center, University Hospitals UZ Leuven, KU Leuven, Leuven, Belgium.
- 22 Department of Oncology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
- 23 Chilean Cooperative Group for Oncologic Research (GOCCHI), Santiago, Chile.
- 24 Institute of Pathology, Cantonal Hospital St. Gallen, St. Gallen, Switzerland.
- 25 Swiss Group for Clinical Cancer Research (SAKK), Bern, Switzerland.
- 26 Breast Center, Lausanne University Hospital CHUV, Lausanne, Switzerland.
- 27 The Angeles Clinic and Research Institute, Santa Monica, California.
- 28 SWOG Cancer Research Network, San Antonio, Texas.
- 29 University of Pittsburgh Medical Center Hillman Cancer Center Pittsburgh, Pennsylvania.
- 30 NSABP Foundation/NRG Oncology, Pittsburgh, Pennsylvania.
- 31 Division of Medical Oncology, Sunnybrook Odette Cancer Centre, University of Toronto, Toronto, ON, Canada.
- 32 Fred Hutchinson Cancer Center, University of Washington Seattle, Washington.
- 33 ECOG-ACRIN Cancer Research Group, Philadelphia, Pennsylvania.
- 34 Weston Park Hospital, Sheffield, United Kingdom.
- 35 National Cancer Research Institute, Breast Cancer Clinical Studies Group (NCRI-BCSG), London, United Kingdom.
- 36 The Institute for Cancer Research, The Clinical Trials and Statistics Unit (ICR-CTSU), London, United Kingdom.
- 37 Mayo Clinic and Alliance for Clinical Trials in Oncology, Rochester, Minnesota.
- 38 German Breast Group, Neu Isenburg, Germany.
- 39 University Hospital of Schleswig-Holstein, Campus Kiel, Germany.
- 40 Division of Cancer Research, Peter MacCallum Cancer Center, Melbourne Australia.
- 41 Division of Medical Senology, IEO, European Institute of Oncology IRCCS, Milan, Italy.
- 42 IBCSG Statistical Center, Dana-Farber Cancer Institute and Harvard Medical School, Boston, Massachusetts.
- PMID: 39145953
- DOI: 10.1001/jamaoncol.2024.3044
Importance: Adjuvant ovarian function suppression (OFS) with oral endocrine therapy improves outcomes for premenopausal patients with hormone receptor-positive (HR+) breast cancer but adds adverse effects. A genomic biomarker for selecting patients most likely to benefit from OFS-based treatment is lacking.
Objective: To assess the predictive and prognostic performance of the Breast Cancer Index (BCI) for OFS benefit in premenopausal women with HR+ breast cancer.
Design, setting, and participants: This prospective-retrospective translational study used all available tumor tissue samples from female patients from the Suppression of Ovarian Function Trial (SOFT). These individuals were randomized to receive 5 years of adjuvant tamoxifen alone, tamoxifen plus OFS, or exemestane plus OFS. BCI testing was performed blinded to clinical data and outcome. The a priori hypothesis was that BCI HOXB13/IL17BR ratio (BCI[H/I])-high tumors would benefit more from OFS and high BCI portended poorer prognosis in this population. Settings spanned multiple centers internationally. Participants included premenopausal female patients with HR+ early breast cancer with specimens in the International Breast Cancer Study Group tumor repository available for RNA extraction. Data were collected from December 2003 to April 2021 and were analyzed from May 2022 to October 2022.
Main outcomes and measures: Primary end points were breast cancer-free interval (BCFI) for the predictive analysis and distant recurrence-free interval (DRFI) for the prognostic analyses.
Results: Tumor specimens were available for 1718 of the 3047 female patients in the SOFT intention-to-treat population. The 1687 patients (98.2%) who had specimens that yielded sufficient RNA for BCI testing represented the parent trial population. The median (IQR) follow-up time was 12 (10.5-13.4) years, and 512 patients (30.3%) were younger than 40 years. Tumors were BCI(H/I)-low for 972 patients (57.6%) and BCI(H/I)-high for 715 patients (42.4%). Patients with tumors classified as BCI(H/I)-low exhibited a 12-year absolute benefit in BCFI of 11.6% from exemestane plus OFS (hazard ratio [HR], 0.48 [95% CI, 0.33-0.71]) and an absolute benefit of 7.3% from tamoxifen plus OFS (HR, 0.69 [95% CI, 0.48-0.97]) relative to tamoxifen alone. In contrast, patients with BCI(H/I)-high tumors did not benefit from either exemestane plus OFS (absolute benefit, -0.4%; HR, 1.03 [95% CI, 0.70-1.53]; P for interaction = .006) or tamoxifen plus OFS (absolute benefit, -1.2%; HR, 1.05 [95% CI, 0.72-1.54]; P for interaction = .11) compared with tamoxifen alone. BCI continuous index was significantly prognostic in the N0 subgroup for DRFI (n = 1110; P = .004), with 12-year DRFI of 95.9%, 90.8%, and 86.3% in BCI low-risk, intermediate-risk, and high-risk N0 cancers, respectively.
Conclusions and relevance: In this prospective-retrospective translational study of patients enrolled in SOFT, BCI was confirmed as prognostic in premenopausal women with HR+ breast cancer. The benefit from OFS-containing adjuvant endocrine therapy was greater for patients with BCI(H/I)-low tumors than BCI(H/I)-high tumors. BCI(H/I)-low status may identify premenopausal patients who are likely to benefit from this more intensive endocrine therapy.
PubMed Disclaimer
Similar articles
- Absolute Benefit of Adjuvant Endocrine Therapies for Premenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Early Breast Cancer: TEXT and SOFT Trials. Regan MM, Francis PA, Pagani O, Fleming GF, Walley BA, Viale G, Colleoni M, Láng I, Gómez HL, Tondini C, Pinotti G, Price KN, Coates AS, Goldhirsch A, Gelber RD. Regan MM, et al. J Clin Oncol. 2016 Jul 1;34(19):2221-31. doi: 10.1200/JCO.2015.64.3171. Epub 2016 Apr 4. J Clin Oncol. 2016. PMID: 27044936 Free PMC article.
- Ovarian suppression for adjuvant treatment of hormone receptor-positive early breast cancer. Bui KT, Willson ML, Goel S, Beith J, Goodwin A. Bui KT, et al. Cochrane Database Syst Rev. 2020 Mar 6;3(3):CD013538. doi: 10.1002/14651858.CD013538. Cochrane Database Syst Rev. 2020. PMID: 32141074 Free PMC article.
- Absolute Improvements in Freedom From Distant Recurrence to Tailor Adjuvant Endocrine Therapies for Premenopausal Women: Results From TEXT and SOFT. Pagani O, Francis PA, Fleming GF, Walley BA, Viale G, Colleoni M, Láng I, Gómez HL, Tondini C, Pinotti G, Di Leo A, Coates AS, Goldhirsch A, Gelber RD, Regan MM; SOFT and TEXT Investigators and International Breast Cancer Study Group. Pagani O, et al. J Clin Oncol. 2020 Apr 20;38(12):1293-1303. doi: 10.1200/JCO.18.01967. Epub 2019 Oct 16. J Clin Oncol. 2020. PMID: 31618131 Free PMC article.
- Treating HR+/HER2- breast cancer in premenopausal Asian women: Asian Breast Cancer Cooperative Group 2019 Consensus and position on ovarian suppression. Yeo W, Ueno T, Lin CH, Liu Q, Lee KH, Leung R, Naito Y, Park YH, Im SA, Li H, Yap YS, Lu YS; Asian Breast Cancer Cooperative Group. Yeo W, et al. Breast Cancer Res Treat. 2019 Oct;177(3):549-559. doi: 10.1007/s10549-019-05318-5. Epub 2019 Jul 4. Breast Cancer Res Treat. 2019. PMID: 31270763 Review.
- Role of Ovarian Function Suppression in Premenopausal Women with Early Breast Cancer. Park WC. Park WC. J Breast Cancer. 2016 Dec;19(4):341-348. doi: 10.4048/jbc.2016.19.4.341. Epub 2016 Dec 23. J Breast Cancer. 2016. PMID: 28053622 Free PMC article. Review.
LinkOut - more resources
Full text sources.
- Silverchair Information Systems
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Skip to content
Explore CUIMC
Diversity, equity, and inclusion.
At CUIMC, we are committed to continuous improvement in providing culturally inclusive medical education and clinical care.
Columbia Vagelos College of Physicians and Surgeons is dedicated to developing the next generation of leaders in medicine
Patient Care
- Find a Doctor
Search for a provider by specialty, expertise, location and insurance. Schedule an appointment online.
Read the latest news stories about CUIMC faculty, research, and events
Study Suggests Way to Improve Treatment of Hereditary Breast Cancer
Share this page.
- Share on Facebook
- Share on X (formerly Twitter)
- Share on LinkedIn
- Share by email
PARP inhibitors have improved survival of breast cancer patients with BRCA1/2 mutations, but the drugs eventually stop working and the cancer returns.
A new study of cancer in mice now suggests that a second cancer drug may be able to prevent or delay relapse.
“The problem so far has been that the response to PARP inhibitors is not durable,” says study co-leader Swarnali Acharyya , associate professor in the Department of Pathology & Cell Biology and Institute for Cancer Genetics at Columbia University Vagelos College of Physicians and Surgeons. “Sometimes it lasts six months, sometimes a little longer. But oncologists tell me that almost all patients eventually relapse, so it’s important to find out why.”
More than 60% of women who inherit a mutated BRCA1 and BRCA2 gene will get breast cancer in their lifetime, according to the National Cancer Institute. PARP inhibitors work by targeting PARP, a protein that helps cancer cells repair damaged DNA and continue their growth.
Cancer cells are known to use various mechanisms to overcome PARP inhibitors, but the Columbia study is the first to identify a new mechanism that can be thwarted by an existing drug (axitinib, FDA-approved for treating metastatic kidney cancer).
Healthy neighbor cells promote cancer growth
To understand how BRCA cancers develop resistance to PARP inhibitors, the researchers developed new mouse models that respond to PARP inhibitors much the same way patients do. Both experience a striking response to the drugs, before the cancers acquire resistance and recur.
When the researchers examined resistant cancers from these mice, they noticed something curious. “When we took the resistant cancer cells out of the tumor and treated the cancer cells in the lab with PARP inhibitors, they died,” Acharyya says. “So, the next question we had was why do cells that resist the drug in vivo die in vitro.”
The answer was found in the tumor’s microenvironment. When mice get treated with PARP inhibitors, a protein called PGF is secreted by normal cells around the tumor, perhaps as a stress response. This protein then binds to FLT1 receptors on the cancer cells, promoting cancer growth and driving away cancer-fighting T cells.
When researchers blocked the FLT1 receptor either genetically or with a drug called axitinib, the PARP inhibitor started to work again, killing the PARP inhibitor-resistant tumors.
Combination treatment
It’s likely that human breast cancers are developing resistance in much the same way, Acharyya says, because the study also found that patients with high levels of FTL1 have less success with PARP inhibitors and develop resistance more quickly.
Swarnali Acharrya and Anup Biswas lab members. From left: Courtney Coker, Anup K. Biswas, Swarnali Acharyya, Yifan Gu, Yifan Tai, Angela Chow, and Wanchao Ma. Photo courtesy of the Acharrya lab / Columbia University.
“The combination of axitinib with PARP inhibitors could make resistant cancers more responsive to treatment and may even be effective for patients who don’t respond to PARP inhibitors from the beginning,” says study co-leader Anup Biswas , assistant professor of pathology & cell biology.
“Because axitinib is already FDA-approved, our findings could be tested in patients relatively quickly,” Acharyya adds, and the team is now talking with physicians from several institutions interested in launching clinical trials.
High levels of FLT1 have also been reported in ovarian, prostate, and pancreatic cancers treated with PARP inhibitors, suggesting the same drug combination may have potential beyond breast cancer.
Additional information
All authors (from Columbia unless noted): Yifan Tai, Angela Chow, Seoyoung Han, Courtney Coker, Wanchao Ma, Yifan Gu, Valeria Estrada Navarro (Weill Cornell Medicine), Manoj Kandpal (Rockefeller University), Hanina Hibshoosh, Kevin Kalinsky (Emory University), Katia Manova-Todorova (Memorial Sloan Kettering), Anton Safonov (MSK), Elaine M. Walsh (MSK), Mark Robson (MSK), Larry Norton (MSK), Richard Baer, Taha Merghoub (Weill Cornell), Anup K. Biswas, and Swarnali Acharyya
This work was supported by a Department of Defense Breast Cancer Breakthrough Award, Irma T. Hirschl Monique Weill-Caulier Trust Award, American Cancer Society Scholar Award, Susan G. Komen Career Catalyst Award, Pershing Square Sohn Award, METAvivor Early Career Investigator Award, Phi Beta Psi Sorority Cancer Research Award, Columbia University Irving Scholars Program, the National Cancer Institute (grant R01CA231239), and pilot awards from the Herbert Irving Comprehensive Cancer Center and Irving Institute for Clinical and Translational Research (P30CA013696). The research used core facilities at the Herbert Irving Comprehensive Cancer Center, funded in part through the National Cancer Institute (P30CA013696).
The authors declare that they have no competing interests that relate to the research described in this paper.
Top image of breast cancer cells from National Cancer Institute , created by Sheheryar Kabraji and Sridhar Ramaswamy / Dana-Farber Harvard Cancer Center at Massachusetts General Hospital.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 17 August 2024
Trends in incidence, prevalence, and survival of breast cancer in the United Kingdom from 2000 to 2021
- Nicola L. Barclay 1 ,
- Edward Burn 1 ,
- Antonella Delmestri 1 ,
- Talita Duarte-Salles 2 , 3 ,
- Asieh Golozar 4 , 5 ,
- Wai Yi Man 1 ,
- Eng Hooi Tan 1 ,
- Ilona Tietzova 6 ,
- OPTIMA Consortium ,
- Daniel Prieto-Alhambra 1 , 3 &
- Danielle Newby 1
Scientific Reports volume 14 , Article number: 19069 ( 2024 ) Cite this article
Metrics details
- Cancer epidemiology
Breast cancer is the most frequently diagnosed cancer in females globally. However, we know relatively little about trends in males. This study describes United Kingdom (UK) secular trends in breast cancer from 2000 to 2021 for both sexes. We describe a population-based cohort study using UK primary care Clinical Practice Research Datalink (CPRD) GOLD and Aurum databases. There were 5,848,436 eligible females and 5,539,681 males aged 18+ years, with ≥ one year of prior data availability in the study period. We estimated crude breast cancer incidence rates (IR), prevalence and survival probability at one-, five- and 10-years after diagnosis using the Kaplan–Meier method. Analyses were further stratified by age. Crude IR of breast cancer from 2000 to 2021 was 194.4 per 100,000 person-years for females and 1.16 for males. Crude prevalence in 2021 was 2.1% for females and 0.009% for males. Both sexes have seen around a 2.5-fold increase in prevalence across time. Incidence increased with age for both sexes, peaking in females aged 60–69 years and males 90+ . There was a drop in incidence for females aged 70–79 years. From 2003–2019, incidence increased > twofold in younger females (aged 18–29: IR 2.12 in 2003 vs. 4.58 in 2018); decreased in females aged 50–69 years; and further declined from 2015 onwards in females aged 70–89 years. Survival probability for females after one-, five-, and ten-years after diagnosis was 95.1%, 80.2%, and 68.4%, and for males 92.9%, 69.0%, and 51.3%. Survival probability at one-year increased by 2.08% points, and survival at five years increased by 5.39% from 2000–2004 to 2015–2019 for females, particularly those aged 50–70 years. For males, there were no clear time-trends for short-term and long-term survival probability. Changes in incidence of breast cancer in females largely reflect the success of screening programmes, as rates rise and fall in synchronicity with ages of eligibility for such programmes. Overall survival from breast cancer for females has improved from 2000 to 2021, again reflecting the success of screening programmes, early diagnosis, and improvements in treatments. Male breast cancer patients have worse survival outcomes compared to females, highlighting the need to develop male-specific diagnosis and treatment strategies to improve long-term survival in line with females.
Introduction
Female breast cancer has been the leading cause of global cancer incidence in recent years, with an estimated number of new cases of 2.3 million in 2020 alone 1 . Male breast cancer accounts for around 1% of all diagnosed cases 2 , though incidence and survival trends are infrequently investigated given its rarity. Whilst the incidence of female breast cancer is stabilising or decreasing in certain age groups due to earlier detection and improved treatments 3 , male breast cancer has been increasing from the 1980s to 2000s at least in the United Kingdom (UK) and the United States (US) 4 , 5 .
Breast cancer risk increases with age across both sexes, though males tend to be older at the age of diagnosis 5 . Other risk factors in both males and females include family history, the risk for which is doubled in males with a first-degree relative with the disease 6 ; genetic mutations in BRCA 1/2 genes and others 2 , 7 ; elevated estrogen levels 8 , and lifestyle factors such as alcohol consumption 9 , 10 , obesity 11 and radiation exposure 12 .
Compared to females, males with breast cancer are more likely to be estrogen-receptor positive, androgen-receptor positive, Hormone receptor-positive with Human Epidermal Growth Factor 2-negative, and present with regional nodal metastases 2 . Males are also more likely to present at more advanced stage of disease than females 2 , which is likely to impact their survival.
Recent evidence from 500,000 females with early invasive breast cancer in England suggests breast cancer survival has improved over time, with five-year risk of death reducing from 14 to 5% from the 1990s to 2015 13 . Whilst there is relatively little evidence for males, one study shows mortality from breast cancer reduced by nearly 40% in North-Western Europe from the early 2000s to 2017 14 . At least for females, the improved prognosis is likely driven by the success of national screening programmes aiding early detection, whereas the lack of such routine screening in males precludes this explanation. Improvements in males are likely a reflection of improved local and systemic treatments which have substantially improved over recent decades 15 .
A comprehensive assessment of the disease burden and survival of breast cancer across both sexes in the UK will inform future decisions regarding screening, prevention, treatment, and disease management in both females and males. However, much of our understanding of the disease burden of breast cancer has been derived from cancer registries. Cancer registry analyses do not have a general population denominator to estimate incidence, prevalence and survival, but rather use national general population statistics as their denominator population—methods which can introduce biases 16 , 17 .
Therefore, the aim of the present study was to describe the breast cancer trends from 2000 to 2021 in the UK for both females and males in terms of incidence, prevalence and survival probability (at one-, five- and ten-years after diagnosis) using nationally representative, routinely collected electronic health record data from primary care. Additionally, incidence and prevalence analyses were stratified by age, and survival probability estimates were stratified by calendar time to investigate age and time trends.

Ethical approval
The protocol for this research was approved by the Research Data Governance (RDG) Board of the Medicine and Healthcare products Regulatory Agency database research (protocol number 22_001843).
Patient populations and characteristics
There were 5,848,436 and 5,539,681 eligible female and male patients 18 years and older, with at least one year of data availability prior to diagnosis from January 2000 to December 2021 for CPRD GOLD. Attrition tables for this study can be found in the supplementary information (Supplement S2). A summary of study patient characteristics of those with a diagnosis of breast cancer stratified by sex is shown in Table 1 .
Overall, the majority of those with breast cancer were female, with a median age of 63 years across both databases. Males only made up 0.6% of cancer diagnoses with an older median age of 70 years. In females, the highest percentage of patients were those aged 60–69 years, contributing to 26% of diagnosed patients, whereas for males, those aged 70–79 years contributed to 32% of diagnosed patients. Overall, males were more likely to have comorbidities compared to females apart from depressive disorders which were higher in females. The patient characteristics in Aurum can be found in the supplementary information (Supplement S3).
Overall and annualised crude incidence rates for study population stratified by age and sex across databases
Overall crude incidence rates.
Table 2 shows the overall incidence rates (IR) of breast cancer stratified by age and sex. For females, the overall IR per 100,000 person-years (pys) of breast cancer from 2000 to 2021 was 194.4 (95% CI 193.1–195.7) in GOLD, with slightly lower results in Aurum (180.4; 95% CI 179.5–181.3). For males, the overall IR was 1.16 (95% CI 1.07–1.28) in GOLD, with the same results in Aurum. When stratifying by age, the overall IR for females increased with age, peaking in those 60–69 years (IR: 381.0) before dropping in those aged 70–79 years (IR: 343.9), increasing in those aged 80–89 years (IR: 366.9), and with a final decrease in those 90+ years (IR: 357.6). This trend was similar in both databases. For males, overall IR was higher with increasing age up to 90+ years (IR: 6.7) in GOLD and up to 80–89 years (IR: 5.2) in Aurum. The biggest increase in overall IR for females was between those aged 30–39 years (IR: 38.3) to 40–49 years (IR: 143.7) with an increase in IR of 3.75-fold; whereas the biggest difference in IR for males was between those aged 50–59 (IR: 0.9) and 60–69 (IR: 2.2) years with a 2.43-fold increase.
Annualised crude incidence rates
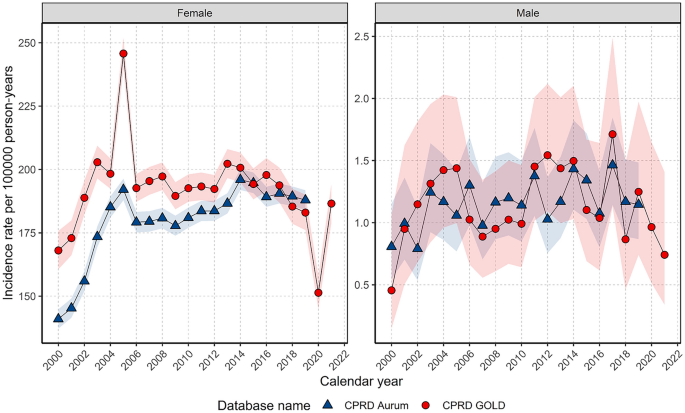
For females, annualised IRs rapidly increased to 2004 before a sharp peak and gradual increase up to 2014 before declining (Fig. 1 ). In GOLD, IRs dropped in 2020 but recovered in 2021. For males, IRs gradually increased to a small extent over the study period but had high variability due to small sample numbers (Fig. 1 ).
Annualised crude incidence rates for breast cancer stratified by database and sex.
When stratifying by age group, annualised IRs over the study period showed different trends in females depending on age of diagnosis (Fig. 2 ). For those aged 18–29 years, despite an initial peak IR of 5.06 in 2000, IRs increased over the study period (from a low of 2.1 in 2003 to a high of 4.6 in 2018), whereas IRs in those aged 30–39 years declined from 2000–2011 (from 42.4 in 2000 to 32.3 in 2011) before a gradual increase from 2012–2019.
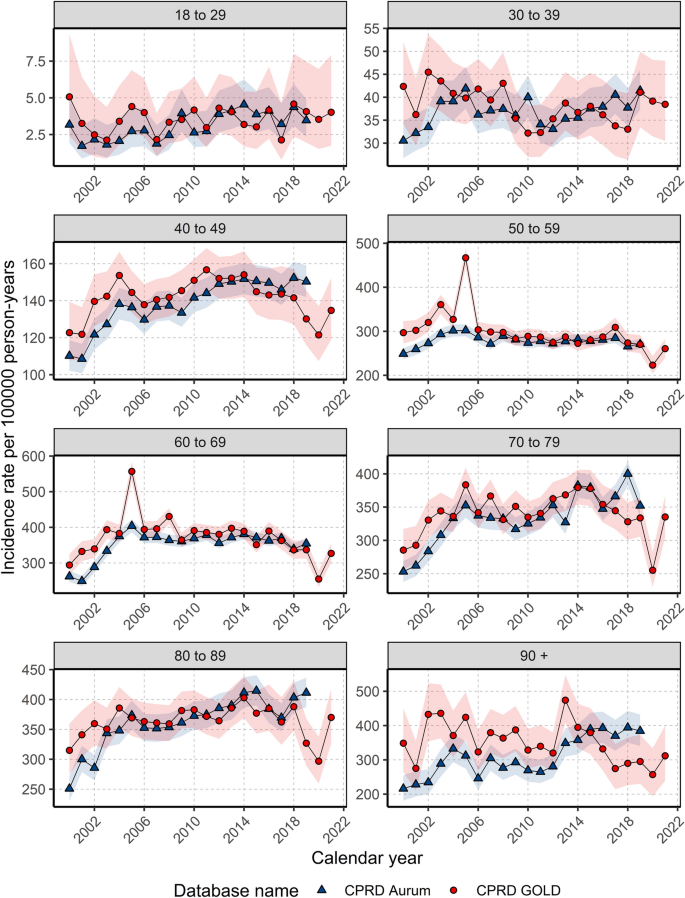
Annualised crude incidence rates for females stratified by database and age group.
For those aged 40–49 and 70–79/80–89 years, IRs increased rapidly between 2000 (and 2004 before gradually increasing and peaking around 2011–2014 (peaking at 156.7 in 2011 for 40–49 years; and peaking at 379.4 in 2014 for 70–79 years; and 402.8 in 2014 for 80–89 years) then declining (to a low of 121.5 in 2020 for 40–49; 255.2 in 2020 for 70–79; 296.8 in 2020 for 80–89 years).
For those aged 50–69 years, IRs increased from 2000–2005 (from 296.8 in 2000 to 467.0 in 2004 for 50–59; and from 294.3 to 556.8 in 2005 for 60–69 years) before a gradual decline from 2006–2020.
For those aged 90+ years, there were differences between the two databases with IRs in GOLD declining but with a peak in 2013 (474.0); whereas IRs in Aurum increased over the study period, peaking in 2018 (394.1). For all age groups, IRs decreased in 2020 before increasing in 2021, apart from those aged 30–39 years. For males, there were not enough cases per age group to assess trends in annualised IR across age groups apart from those aged 70–79 years, which shows the stability of IRs over the study period (Supplement S4).
All results for this study can be found and downloaded in a user-friendly interactive web application: https://dpa-pde-oxford.shinyapps.io/BreastCancerIncPrevSurvShiny/ .
Overall and annualised crude prevalence for the study population stratified by age and sex across databases
Crude prevalence.
In GOLD, the crude prevalence for breast cancer in 2021 was 2.11% (2.09%-2.14%) for females and 0.009% (0.008%-0.011%) for males, which is equivalent to 2,110 cases per million people for females, and 90 cases per million people for males. Similar prevalence was obtained in 2019 when comparing GOLD and Aurum across sexes. When stratifying by age, prevalence in GOLD in 2021 peaked in females aged 70–79 years (5.39%) and in males 90+ years (0.06%) with similar trends in 2019 when comparing both databases (Supplement S5).
Annualised crude prevalence
In GOLD, prevalence increased from 2000–2013 before stabilising to 2018 in females and declining to the end of the study period in males; whereas in Aurum, prevalence increased each year over the study period for females and males (Fig. 3 ). Both sexes have seen around 2.5-fold increase in PP across the study period in both databases (Fig. 3 ). The annual percent change was significantly different from zero across all timepoints, except for between 2013–2018 for males in CPRD GOLD (supplementary figures S6-S9).
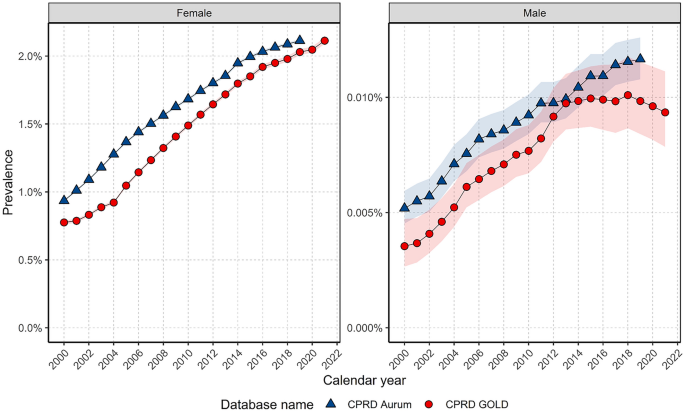
Annualised crude prevalence stratified by database and sex.
When stratifying by age, prevalence trends over time for females showed some differences per age group (Fig. 4 ). Those aged 40–49 and 60–69 years showed increases in prevalence over time until 2014 where prevalence stabilised to the end of the study period, with a small decline in those aged 40–49 years. For all other age groups prevalence increased over the study period.
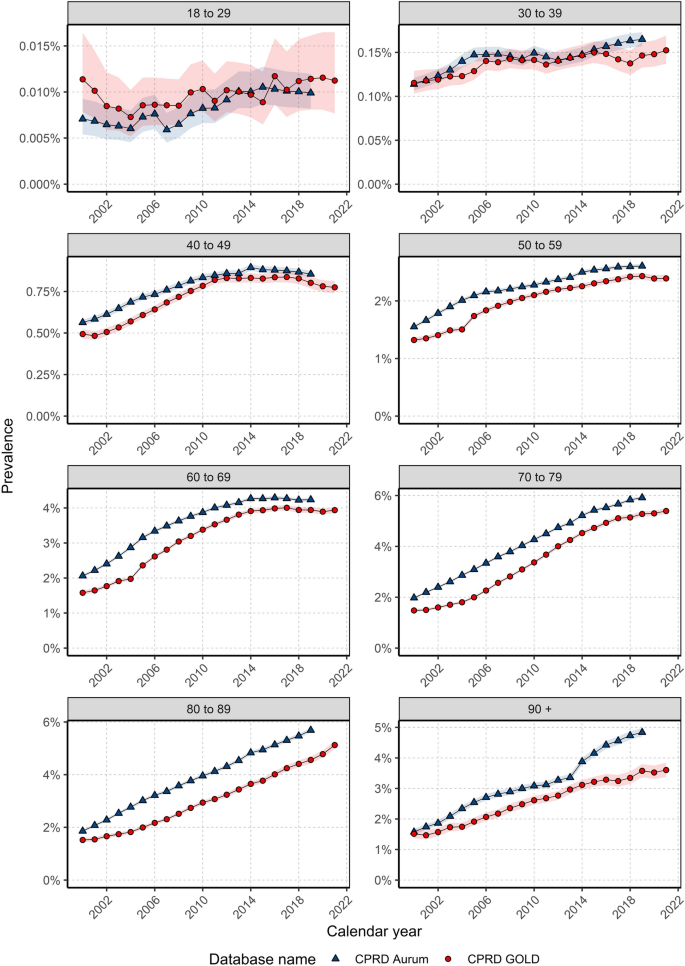
Annualised crude prevalence for females stratified by database and age.
For males, there again were differences in prevalence trends over the study period depending on age (Fig. 5 ). For those aged 40–49 years, prevalence was stable for most of the study period with an increase in prevalence from 2012/14. For those aged 50–79 years, prevalence increased over the study period in both databases. In GOLD, prevalence increased between 2000 and 2018 and declined thereafter; whereas in aurum, prevalence increased over time for those aged 80–89 years. for those aged 30–39 years and 90+ years there was not enough data to assess trends in gold; however, in aurum prevalence trends indicated stability in those aged 30–39 years and an increase in prevalence over time in those aged over 90 years.
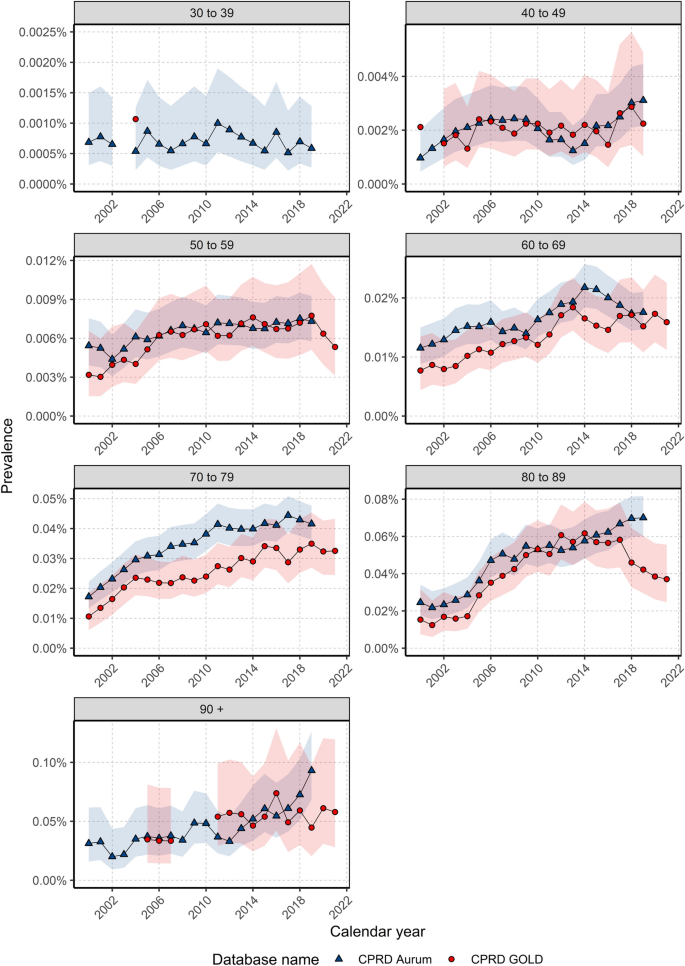
Annualised crude prevalence for males stratified by database and age.
Overall survival probability for breast cancer population stratified by age, sex and calendar year
For females, there were 84,984 patients, 19,974 deaths and a median follow-up of 4.7 years; and for males, there were 505 patients, 173 deaths and a median follow-up of 3.8 years in GOLD (Fig. 6 ). Number of patients at risk, died and censored across the follow-up period is indicated in Supplement S10. Median survival was not reached for females within the specified follow-up period, whereas for males, median survival was between 10–11 years across both databases. What this means is that, on average, females lived beyond the duration of the follow-up period; whereas males survived for a period of time falling within the range of 10 to 11 years during the specified follow-up period. Indeed, survival probability for females after one-, five-, and ten-years after diagnosis was 95.1%, 80.2%, and 68.4%, and for males 92.9%, 69.0%, and 51.3% in GOLD, with similar results in Aurum. Long-term survival probability at five- and ten-years was higher in females than males across both databases (Supplement S11).
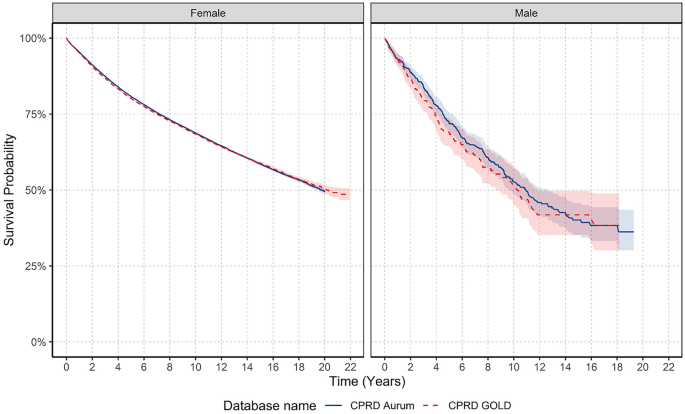
Kaplan–Meier survival curve of breast cancer stratified by database and sex.
For females, when stratifying by age group, for those aged 18–69 years median survival was not reached. For those aged older than 70 years, median survival decreased with increasing age. Median survival decreased from 11–12 years to 2.5 for those older than 90 years. For males, median survival was not achieved in those aged 40–69 and 90+ years. However, median survival was lower in those aged 80–89 years compared to those aged 70–79 years across both databases.
For females, one-year survival probability for those aged 18–69 years was similar (97–98%), peaking in those aged 40–59 years, and declining from 70 years (Table 3 ). After five- and ten-years, survival rates increased from 18–29 years peaking in those aged 50–59 years before declining. For short- and long-term survival probability for males, results indicate that those aged 80 years and older had lower short- and long-term survival probability compared to younger age groups, however, sample numbers were small.
To investigate if survival probability has changed over time, we stratified by calendar time of cancer diagnosis in five-year windows. Figure 7 shows the KM survival curves stratified by sex and calendar year. For females, short- and long-term survival probability increased over calendar time. Survival probability at one-year increased by 2.1%, and survival probability at five-years increased by 5.4% from 2000–2004 to 2015–2019 in GOLD (note that survival data stratified by calendar year was not available for Aurum). For males, when comparing survival probability between those diagnosed in 2000–2004 with those diagnosed in 2015–2019, there was no clear pattern over calendar time for short-term and long-term survival probability due to small sample numbers.
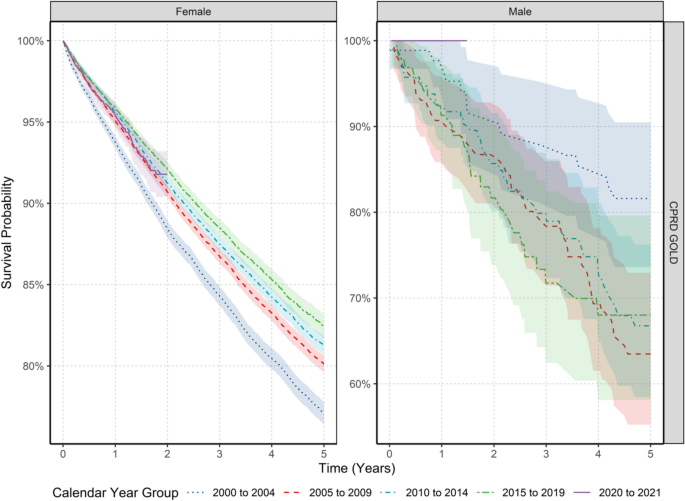
Kaplan–Meier survival curve of breast cancer stratified by sex and calendar year of diagnosis.
Supplement S12 shows the short- and long-term survival probabilities and 95% confidence intervals stratified by calendar year of diagnosis, age and sex. Short-term survival probability in the different age groups showed that in females those aged between 50–69 years had increases in survival probability over time when comparing those diagnosed in 2000–2004 to those diagnosed between 2015–2019 (one-year survival probability of 97.4% vs. 99.0% in those aged 50–59 years; and 95.6% vs. 97.8% in those aged 60–69 years from 2000–2004 vs. 2015–2019). There was a similar pattern of increased survival probability over time for long-term (five-year) survival probability in those aged 50–69 years when comparing those diagnosed in 2000–2004 to those diagnosed between 2015–2019 (five-year survival probability of 88.2% vs. 92.4% in those aged 50–59 years; and 84.5% vs. 89.2% in those aged 60–69 years from 2000–2004 vs. 2015–2019). For those aged 80–89 years results showed an improvement in short- and long-term survival probability over calendar time (one-year survival was 84.3% vs. 88.1% for those diagnosed in 2000–2004 vs. 2015–2019). There was no significant change in short-term survival probability in any age groups in those diagnosed in 2020–2021 compared to previous years. For males, trends stratified by age did not show improvement over time in short- and long-term survival probability, however, sample numbers were small and for certain age groups not enough data to assess secular trends.
This study demonstrates trends of breast cancer incidence, prevalence and survival probability in the UK in females and males. Below is a summary of the key findings in the context of previous research.
Overall incidence and prevalence for study population stratified by age and sex
Overall crude incidence rates of breast cancer in females (IR: 194 per 100,000 person-years) and males (IR: 1.2 per 100,000 person-years) were in line with national statistics (IR: 166 and 1.1 for females and males, respectively, between 2016–2018 from Cancer Research UK) 3 . Incidence of breast cancer increased with age in both females and males peaking in those aged 60–69 years in females and those aged 90 + in males. These estimates deviate slightly with national statistics, which demonstrate a peak in females aged 90+ years and in males 85–89 years 3 . The drop in overall incidence for females aged 70–79 years observed here is likely to coincide with the ending of routine breast cancer screening in the UK (women are eligible for the breast cancer screening programme between the ages of 50 and 70 years 18 ). Thus, this is likely a compensatory decrease in incidence as screening has advanced the detection of cases among women in this age bracket 3 . This also explains why the incidence subsequently reverts to somewhat higher rates among those aged 80–89 years. Overall incidence in those 90+ years is lower than younger ages, which could indicate reduced diagnostic activity, perhaps due to general ill health in this age group.
National data on prevalence of breast cancer is scarce. In this study prevalence of breast cancer at the end of the study period was 2.1% in females, peaking in those aged 70–79 years, and 0.009% in males, peaking in those aged 90+ years. That said, these could be overestimates of population prevalence as in this study anyone with a diagnosis of breast cancer was included until the end of their observation period. Patients with survival over 5–10 years who are discharged would still be contributing to the prevalence estimate. Furthermore, the increase of early-stage breast cancers in the context of screening programmes is likely to drive this overestimation further due to patients surviving longer. However, while many of these cases may be considered cured after five years and no longer being actively treated, people in this survivorship phase may have long-term medical needs and accordingly, it is important to provide accurate counts to allow for healthcare planning.
Trends in incidence and prevalence over time for females and males
In terms of trends over time, incidence increased for females across the study period before dropping dramatically during 2020 – coinciding with the COVID-19 pandemic – and returning to expected levels in 2021. Largely speaking, changes in incidence of breast cancer over time are likely explained by many factors, including increased screening and diagnostic activity, but also increases in risk factors in the population (such as obesity 19 , 20 ). Another time trend of note is the sharp spike in incidence in females in 2004–2005. One possible explanation for this is that the Quality and Outcomes Framework (QOF), which assesses performance of general practices on several key disease areas (including cancer), and provides financial incentives for achieving specified quality targets, was introduced in 2004 (of note, there were substantially more patients from Scotland with a date of diagnosis in April 2004, which is the start of QOF reporting period). Thus, screening, diagnostics and reporting of cancer diagnoses may have been greater during this time-period. Additionally, many screening units in the UK extended the screening of women aged 65–70 years between 2001 and 2004 21 , and it is possible that this extension accounted for the large spike observed.
When examining incidence trends over time by age group, three key findings are highlighted. First is the increase in incidence over the study period for younger women (aged 18–29; and 30–39). Several possible explanations include: increasing awareness of breast symptoms leading to more women being diagnosed; but also risk factor exposures in early life such as earlier age of thelarche (pubertal phase of breast development) and menarche than previous decades 22 , 23 , leading to increased cumulative exposure to oestrogen; and increased use of hormonal contraceptives which pose an elevated risk for breast cancer 24 . Second, the decline in incidence for women aged 50–69 years from 2005 to 2019 may be a reflection of the success of screening programmes; and third, the decline from 2015 onwards for women aged 70–89 years coincides with the launch of the “Be Clear on Cancer” campaign aimed at women in this age group 25 .
Prevalence of breast cancer in females and for most age groups in males increased across the study period, which is likely a reflection of increased survival due to the success of screening programmes (for females at least), early diagnosis and effective treatments 26 , 27 .
Differences in short-term and long-term survival probability in different age groups in females and males
Short-term (one-year) survival probability in females was similar across the age groups from 18–69 years; whereas long-term survival probability at five-years was low in younger age groups, and highest in those aged 50–59 years likely due to the eligibility of females into national breast cancer screening programmes in the UK (which starts at 50 years of age) 18 . It should also be noted that women typically transition through menopause from age 50 years, and breast cancer that develops during menopause typically progresses more slowly and is less aggressive than earlier onset breast cancer, which may account for age-related differences in survival 28 .
Generally speaking, males have lower long-term survival compared to females which is in line with previous studies 29 . This could be due to several factors such as age and disease severity. Males tend to be older when diagnosed compared to females and it is likely that older males present with more comorbidities and medication use making treatment decisions more complex. Males also tend to present with more advanced disease likely due to the rarity of the disease and consequential delays in diagnosis 5 , 30 . Furthermore, as males are underrepresented in trials, treatment recommendations follow those for postmenopausal women 31 . Therefore, the current management of male breast cancer might not be ideal and could explain the lower long-term survival compared to females. Additionally, males lack breast cancer screening and it is clear that at least in females this has contributed a marked decrease in female breast cancer mortality since the late 1980s 32 . The National Comprehensive Cancer Network (NCCN) recommend that males aged 35 years and older with BRCA mutations receive self-examination training for breast cancer alongside annual clinical breast examination 33 . Yet, the sporadic nature of genetic testing for such mutations may impede the practical realisation of this recommendation.
Survival probability over calendar time for whole population and age strata
For females, one-year survival probability increased by 2.08% and five-year survival probability increased by 5.39% from 2000–2004 to 2015–2019. Improvements in survival over the past 20 years are echoed in data from the National Cancer Registration and Analysis Service which showed annual mortality rates of ~ 4% for females diagnosed between 1993–1999 reduced to around 1% for those diagnosed between 2010–2015. Similarly, 5-year cumulative mortality risk reduced from 14.4% for females diagnosed between 1993–1999 to 4.9% for those diagnosed between 2010–2015 13 . In the current study, survival probability particularly improved for females aged 50–70 years, not surprisingly coinciding with eligibility into national screening programmes. Reassuringly, there did not appear to be an effect of the COVID-19 pandemic on short-term survival probability for those diagnosed between 2020–2021 compared to those diagnosed in the years prior. This is somewhat surprising, given data that suggests that screening, diagnosis and treatments were impacted by the pandemic 34 , 35 , 36 .
For males, both short-term and long-term survival probability did not show improvements across calendar periods, but this is likely driven by small sample sizes. Other data shows death rates in North-Western Europe decreased between 10%-40% from 2000–2004 to 2015–2017 14 , and so further data is required before we have clear evidence on the survival trends from male breast cancer in the UK.
Strengths and limitations
The main strength of this study is the use of two large primary care databases covering the whole of the UK. CPRD GOLD covers primary care practices from England, Wales, Scotland and Northern Ireland (with greater representation from Scotland rather than England), whereas CPRD Aurum covers primary care practices in England. The similarity between the results in both databases provides increased generalizability across the UK. Nevertheless, there were a few discrepancies in results between the two databases which can partly be explained by differences in observation period for patients across the study period. In GOLD, the number of people in the database steadily increased from 2000 up to 2006, then remained stable until 2011 before a gradual decline. This gradual decline is likely due to GP practices in England moving EMIS clinical systems. Furthermore, over time the demographic representation of GOLD has changed which could explain differences in results. The advent of the CPRD Aurum database saw some practices transferred from GOLD to Aurum. Across our observation period practices from England and Northern Ireland reduced, whilst practices from Scotland and Wales increased.
Another strength of our study is the inclusion of a complete study population database for the assessment of incidence and prevalence. In contrast, cancer registry studies extrapolate the registry data to the whole population using national population statistics, potentially introducing biases 16 , 17 . The high validity and completeness of mortality data with over 98% accuracy compared to national mortality records 37 allowed us to examine the impact of calendar time on overall survival probability—one of the key outcomes in cancer care.
Our study had limitations. First, we used primary care data without linkage to cancer registry potentially leading to misclassification and delayed recording of diagnoses. However, previous validation studies have shown high accuracy and completeness of cancer diagnoses in primary care records 38 . Second, our use of primary care records precluded us from studying tumour histology, genetic mutations, staging or cancer therapies, which can all impact breast cancer survival. Therefore, our survival probability estimates may overestimate survival in those with higher staging as well those with specific genetic mutations such as BRCA 1/2 39 . Other factors such as socio-economic status and ethnicity could also result in different values for incidence, prevalence and survival 40 , 41 , 42 . Third, in this study we calculated overall survival probability which does not differentiate between deaths caused by cancer vs. other causes. Therefore, it is a broad measure of overall survival probability rather than specifically cancer mortality.
Our study demonstrates that changes in incidence of breast cancer in females largely reflect the success of national breast cancer screening programmes, as rates rise and fall in synchronicity with ages of eligibility for such programmes. Overall survival probability for females from breast cancer has improved from 2000 to 2021, again reflecting the success of screening programmes, early diagnosis, and improvements in treatments. Male breast cancer patients, however, have worse survival outcomes compared with those of female patients. This highlights the need to develop male-specific treatment strategies and promote education and self-examination recommendations of breasts in males, given there are no screening programmes, to improve long-term survival in line with females.
Study design, setting, and data sources
We carried out a population-based cohort study using routinely collected primary care data from the United Kingdom (UK). People with a diagnosis of breast cancer and a background cohort were identified from Clinical Practice Research Datalink (CPRD) GOLD to estimate overall survival probability, incidence, and prevalence. We additionally carried out this study using CPRD Aurum and compared to the results in GOLD. Both these databases contain pseudo-anonymised patient-level information on demographics, lifestyle data, clinical diagnoses, prescriptions, and preventive care provided by GPs and collected by the NHS as part of their care and support. CPRD GOLD contains data from across the UK, representing 6.9% of the UK population 43 , whereas Aurum only contains data from England, but represents 13% of the population of England 44 . CPRD GOLD and Aurum have been mapped to the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM) to facilitate replication of analyses 45 , 46 .
The protocol for this research was approved by the Research Data Governance (RDG) Board of the Medicine and Healthcare products Regulatory Agency database research (protocol number 22_001843). The data is provided by patients to their GPs and collected by the NHS as part of their care and support, and so consent is provided to GPs prior to inclusion in this study. All methods were carried out in accordance with ethical principles outlined in the Declaration of Helsinki; and the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance.
Study participants and time at risk
All participants were required to be aged 18 years or older and have at least one year of data availability prior to diagnosis, and information on age and sex. For the incidence and prevalence analysis, the study cohort consisted of individuals present in the database from 1st January 2000. For CPRD GOLD, these individuals were followed up to whichever came first: diagnosis of breast cancer, exit from the database, date of death, or the 31st of December 2021 (the end of the study period), whereas for Aurum, the end of the study period was 31st of December 2019 (due to data availability). For the survival analysis, only individuals with a newly diagnosed cancer were included. These individuals were followed up from the date of their diagnosis to either date of death, exit from the database, or end of the study period. Any patients whose death date and cancer diagnosis date occurred on the same date were removed from the survival analysis.
Outcome definitions
We used Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT) diagnostic codes to identify breast cancer events (see Supplementary Table S1). Diagnostic codes indicative of either non-malignant cancer or metastasis were excluded (apart from prevalence analyses), as well as diagnosis codes indicative of melanoma, sarcoma, lymphoma, and other tumors not originating from breast tissue. Note that prior diagnoses of other cancers were not excluded. The study outcome cancer definition was reviewed using the CohortDiagnostics R package 47 . This package was used to identify additional codes of interest and to remove those highlighted as irrelevant based on feedback from clinicians with oncology expertise through an iterative process during the initial stages of analysis. For survival analysis, mortality was defined as all-cause mortality based on records of date of death. Mortality data in CPRD GOLD has been previously validated and shown to be over 98% accurate 37 .
Statistical methods
The population characteristics of patients with a diagnosis of breast cancer were summarised on a range of comorbid conditions using standardised SNOMED codes, with median and interquartile range (IQR) used for continuous variables and counts and percentages used for categorical variables.
We calculated the overall and annualised crude incidence rates (IR) and annualised crude prevalence for breast cancer from 2000 to 2021. For incidence, the number of events, the observed time at risk, and the incidence rate per 100,000 person-years (pys) were summarised along with 95% confidence intervals. Annualised incidence rates were calculated as the number of incident cancer cases as the numerator and the recorded number of person-years in the general population within that year as the denominator, whereas overall incidence was calculated from 2000 to 2021.
Prevalence was calculated on 1st January for the years 2000 to 2021 with the number of patients aged ≥ 18 years fulfilling the case definition for breast cancer as the numerator. The denominators were the participants ≥ 18 years on 1st January in the respective years for each database. The number of events and prevalence (%) were summarised along with 95% confidence intervals. Joinpoint regression using the National Cancer Institute's Joinpoint Trend analysis software 48 was utilised to determine the significance of changes in prevalence across time.
For survival probability analysis, we used the Kaplan–Meier (KM) method to estimate the overall survival probability from observed survival times with 95% confidence intervals 49 . We estimated the median survival and survival probability one-, five-, and ten-year after diagnosis.
All results were stratified by database, age (ten-year age bands apart from the first and last age bands which were 18–29 years and 90+ years, respectively) and sex. For survival analysis, we additionally stratified by calendar time of cancer diagnosis (2000–2004, 2005–2009, 2010–2014, 2015–2019 and 2020–2021) allowing a maximum of five years follow-up from cancer diagnosis. To avoid re-identification, we do not report results with fewer than five cases.
To compare with results obtained from GOLD, the same statistical analyses were performed in Aurum using data from 1st January 2000 to 31st December 2019 (note that data from Aurum beyond 2019 was not available).
The statistical software R version 4.2.3 was used for analyses. For calculating incidence and prevalence, we used the IncidencePrevalence R package 50 . For survival analysis we used the survival R package. The analytic code to perform the study is available at https://github.com/oxford-pharmacoepi/EHDENCancerIncidencePrevalence .
Data availability
This study is based in part on data from the Clinical Practice Research Datalink (CPRD) obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data is provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone. Patient level data used in this study was obtained through an approved application-the CPRD (application number 22_001843) and is only available following an approval process-safeguard the confidentiality of patient data. Details on how to apply for data access can be found at https://cprd.com/data-access .
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 71 , 209–249 (2021).
Article Google Scholar
Giordano, S. H. Breast cancer in men. N. Engl. J. Med. 378 , 2311–2320 (2018).
Article PubMed Google Scholar
Cancer Research UK. Breast cancer incidence (invasive) statistics , https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/incidence-invasive#heading-Zero (2021).
Speirs, V. & Shaaban, A. M. The rising incidence of male breast cancer. Breast Cancer Res. Treat. 115 , 429–430 (2009).
Giordano, S. H., Cohen, D. S., Buzdar, A. U., Perkins, G. & Hortobagyi, G. N. Breast carcinoma in men: a population-based study. Cancer 101 , 51–57 (2004).
Brinton, L. A. et al. Prospective evaluation of risk factors for male breast cancer. J. Natl. Cancer Inst. 100 , 1477–1481 (2008).
Article PubMed PubMed Central Google Scholar
Ding, Y. C., Steele, L., Kuan, C.-J., Greilac, S. & Neuhausen, S. L. Mutations in BRCA2 and PALB2 in male breast cancer cases from the United States. Breast Cancer Res. Treat. 126 , 771–778 (2011).
Brinton, L. A. et al. Prediagnostic sex steroid hormones in relation to male breast cancer risk. J. Clin. Oncol. 33 , 2041 (2015).
Macke, A. J. & Petrosyan, A. Alcohol and prostate cancer: Time to draw conclusions. Biomolecules 12 , 375 (2022).
Shield, K. D., Soerjomataram, I. & Rehm, J. Alcohol use and breast cancer: a critical review. Alcohol. Clin. Exp. Res. 40 , 1166–1181 (2016).
Pati, S., Irfan, W., Jameel, A., Ahmed, S. & Shahid, R. K. Obesity and cancer: A current overview of epidemiology, pathogenesis, outcomes, and management. Cancers 15 , 485 (2023).
Ron, E., Ikeda, T., Preston, D. L. & Tokuoka, S. Male breast cancer incidence among atomic bomb survivors. J. Natl. Cancer Inst. 97 , 603–605 (2005).
Taylor, C. et al. Breast cancer mortality in 500 000 women with early invasive breast cancer in England, 1993–2015: Population based observational cohort study. BMJ 381 , 1 (2023).
Google Scholar
Pizzato, M. et al. Trends in male breast cancer mortality: A global overview. Eur. J. Cancer 30 , 472–479 (2021).
Eggemann, H. et al. Survival benefit of tamoxifen in male breast cancer: Prospective cohort analysis. Br. J. Cancer 123 , 33–37 (2020).
Sarfati, D., Blakely, T. & Pearce, N. Measuring cancer survival in populations: Relative survival vs cancer-specific survival. Int. J. Epidemiol. 39 , 598–610 (2010).
Swerdlow, A. Cancer registration in England and Wales: Some aspects relevant to interpretation of the data. J. R. Stat. Soc. 149 , 146–160 (1986).
Cancer Research UK. Breast Screening , https://www.cancerresearchuk.org/about-cancer/breast-cancer/getting-diagnosed/screening-breast#:~:text=Who%20has%20breast%20screening%3F,are%2052%20or%2053%20years . (2023).
Woolcott, O. O. & Seuring, T. Temporal trends in obesity defined by the relative fat mass (RFM) index among adults in the United States from 1999 to 2020: A population-based study. BMJ open 13 , e071295 (2023).
Malik, V. S., Willet, W. C. & Hu, F. B. Nearly a decade on—Trends, risk factors and policy implications in global obesity. Nature Reviews Endocrinology 16 , 615–616 (2020).
Bennett, R., Blanks, R. & Moss, S. Evaluation of extension of breast screening to women aged 65–70 in England using screening performance measures. Br. J. Cancer 100 , 1043–1047 (2009).
Eckert-Lind, C. et al. Worldwide secular trends in age at pubertal onset assessed by breast development among girls: A systematic review and meta-analysis. JAMA Pediat. 174 , e195881–e195881 (2020).
Morris, D. H., Jones, M. E., Schoemaker, M. J., Ashworth, A. & Swerdlow, A. J. Secular trends in age at menarche in women in the UK born 1908–93: Results from the Breakthrough Generations Study. Paediatr. Perinat. Epidemiol. 25 , 394–400 (2011).
Fitzpatrick, D., Pirie, K., Reeves, G., Green, J. & Beral, V. Combined and progestagen-only hormonal contraceptives and breast cancer risk: A UK nested case–control study and meta-analysis. Plos Medicine 20 , e1004188 (2023).
Public Health England. Public Health England launches nationwide breast cancer campaign , https://www.gov.uk/government/news/public-health-england-launches-nationwide-breast-cancer-campaign#:~:text=The%20Be%20Clear%20on%20Cancer%20campaign%20is%20part%20of%20the,us%20to%20improve%20cancer%20survival . (2015).
Arnold, M. et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol 20 , 1493–1505 (2019).
Cancer Research UK. Cancer mortality statistics , https://www.cancerresearchuk.org/health-professional/cancer-statistics/mortality#heading-Zero (2023).
Benz, C. C. Impact of aging on the biology of breast cancer. Crit. Rev. Oncol./Hematol. 66 , 65–74 (2008).
Liu, N., Johnson, K. J. & Ma, C. X. Male breast cancer: An updated surveillance, epidemiology, and end results data analysis. Clin. Breast Cancer 18 , e997–e1002 (2018).
Miao, H. et al. Incidence and outcome of male breast cancer: an international population-based study. J. Clin. Oncol. 29 , 4381–4386 (2011).
Korde, L. A. et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J. Clin. Oncol. 28 , 2114 (2010).
Cancer Research UK. Breast cancer survival statistics , https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/survival#heading-Zero (2014).
NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic , https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf (2023).
Barclay, N. L. et al. The impact of the COVID-19 pandemic on incidence and short-term survival for common solid tumours in the United Kingdom: A cohort analysis. Clin. Epidemiol. 1 , 417–429 (2024).
Barclay, N. L. et al. Collateral effects of the COVID-19 pandemic on endocrine treatments for breast and prostate cancer in the UK: A cohort study. Ther. Adv. Med. Oncol. 16 , 17588359241253116 (2024).
Barclay, N. L. et al. The impact of the UK COVID-19 lockdown on the screening, diagnostics and incidence of breast, colorectal, lung and prostate cancer in the UK: A population-based cohort study. Front. Oncol. 14 , 1370862 (2024).
Gallagher, A. M., Dedman, D., Padmanabhan, S., Leufkens, H. G. & de Vries, F. The accuracy of date of death recording in the Clinical Practice Research Datalink GOLD database in England compared with the Office for National Statistics death registrations. Pharmacoepidemiol. Drug Saf. 28 , 563–569 (2019).
Strongman, H., Williams, R. & Bhaskaran, K. What are the implications of using individual and combined sources of routinely collected data to identify and characterise incident site-specific cancers? A concordance and validation study using linked English electronic health records data. BMJ Open 10 , e037719 (2020).
Huszno, J., Kołosza, Z. & Grzybowska, E. BRCA1 mutation in breast cancer patients: Analysis of prognostic factors and survival. Oncol. Lett. 17 , 1986–1995 (2019).
PubMed Google Scholar
Denu, R. A. et al. Racial and socioeconomic disparities are more pronounced in inflammatory breast cancer than other breast cancers. J. Cancer Epidemiol. 2017 , 1 (2017).
Taheri, M., Tavakol, M., Akbari, M. E., Almasi-Hashiani, A. & Abbasi, M. Relationship of socio economic status, income, and education with the survival rate of breast cancer: a meta-analysis. Iran. J. Public Health 48 , 1428 (2019).
PubMed PubMed Central Google Scholar
Dong, J.-Y. & Qin, L.-Q. Education level and breast cancer incidence: a meta-analysis of cohort studies. Menopause 27 , 113–118 (2020).
Herrett, E. et al. Data resource profile: clinical practice research datalink (CPRD). Int. J. Epidemiol. 44 , 827–836 (2015).
Wolf, A. et al. Data resource profile: Clinical practice research datalink (CPRD) aurum. Int. J. Epidemiol. 48 , 1740–1740g (2019).
Voss, E. A. et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J. Am. Med. Inform. Assoc. 22 , 553–564 (2015).
Hripcsak, G. et al. Observational health data sciences and informatics (OHDSI): Opportunities for observational researchers. Stud. Health Technol. Inform. 216 , 574–578 (2015).
Gilbert, J., Rao, G., Schuemie, M., Ryan, P. & Weaver, J. CohortDiagnostics: Diagnostics for OHDSI Cohorts , https://ohdsi.github.io/CohortDiagnostics , https://github.com/OHDSI/CohortDiagnostics (2023).
National Cancer Institute. Joinpoint Regression Program, Version 5.0.2. Statistical Methodology and Applications Branch, Surveillance Research Program (2023).
Goel, M. K., Khanna, P. & Kishore, J. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 1 , 274 (2010).
Burn, E., Raventos, B., Catala, M., Du, M., Guo, Y., Black, A>, Inberg, G., Lopez, K. IncidencePrevalence: Estimate Incidence and Prevalence using the OMOP Common Data Model. R Package Verion 0.4.0 , https://cran.r-project.org/web/packages/IncidencePrevalence/index.html (2023).
Download references
This activity under the European Health Data & Evidence Network (EHDEN) and OPTIMA has received funding from the Innovative Medicines Initiative 2 (IMI2) Joint Undertaking under grant agreement No 806968 and No. 101034347 respectively. IMI2 receives support from the European Union’s Horizon 2020 research and innovation programme and European Federation of Pharmaceutical Industries and Associations (EFPIA). IMI supports collaborative research projects and builds networks of industrial and academic experts in order to boost pharmaceutical innovation in Europe. The views communicated within are those of OPTIMA. Neither the IMI nor the European Union EFPIA or any Associated Partners are responsible for any use that may be made of the information contained herein. The study funders had no role in the conceptualisation, design, data collection, analysis, interpretation of data, decision to publish, or preparation of the manuscript. Additionally, there was partial support from the Oxford NIHR Biomedical Research Centre. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
A list of authors and their affiliations appears at the end of the paper.
Authors and Affiliations
Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, OX3 7LD, UK
Nicola L. Barclay, Edward Burn, Antonella Delmestri, Wai Yi Man, Eng Hooi Tan, Daniel Prieto-Alhambra & Danielle Newby
Fundació Institut Universitari Per a La Recerca a L’Atenció Primària de Salut Jordi Gol I Gurina (IDIAPJGol), Barcelona, Spain
Talita Duarte-Salles
Department of Medical Informatics, Erasmus University Medical Centre, Rotterdam, The Netherlands
Talita Duarte-Salles & Daniel Prieto-Alhambra
Odysseus Data Service, Cambridge, MA, USA
Asieh Golozar
OHDSI Center at the Roux Institute, Northeastern University, Boston, MA, USA
First Department of Tuberculosis and Respiratory Diseases, First Faculty of Medicine, Charles University, Katerinska 1660/32, 121 08, Prague, Czech Republic
Ilona Tietzova
Stichting European Urological Foundation, Arnhem, The Netherlands
James N’Dow, Wim Witjes, Emma Jane Smith, Carla Bezuidenhout, Sarah Collen, Karin Plass, Torsten Gerriet Blum, Angelika Borkowetz, Peter-Paul Willemse, Philip Cornford, Saeed Dabestani, Maurice Schlief & Juan Gómez Rivas
Lunds Universitet, Lund, Sweden
Anders Bjartell
Erasmus Universitair Medisch Centrum Rotterdam, Rotterdam, The Netherlands
Monique Roobol, Katharina Beyer, Lionne Venderbos, Sebastiaan Remmers, Daan Nieboer & Raoul Boomsma
Association EISBM, Vourles, France
Bertrand De Meulder, Charles Auffray, Nesrine Taibi, Ayman Hijazy, Albert Saporta & Johann Pellet
The University Court of the University of Aberdeen, Aberdeen, UK
Imran Omar, Lesley Anderson, Steven MacLennan, Sara MacLennan, Valerie Speirs, Solveiga Zibaite, Moragh Boyle, Charlotte Murray, Dianne Brown & Demi McDonald
Tartu Ulikool, Tartu, Estonia
Andres Metspalu, Jaak Vilo, Raivo Kolde, Sulev Reisberg, Elena Sügis, Marek Oja & Telver Objartel
Universita Vita-Salute San Raffaele, Milan, Italy
Alberto Briganti, Giorgio Gandaglia, Martina Faticoni & Greta Matteuzzi
Queen Mary University of London, London, UK
Claude Chelala, Louise Jones, Maryam Abdollahyan & Emanuela Gadaleta
Helmholtz-Zentrum Dresden-Rossendorf EV, Dresden, Germany
Guido Juckeland, Michael Bussmann, Daniel Kotik & Artur Yakimovich
Helios Klinikum Emil Von Behring GMBH, Berlin, Germany
Torsten Bauer, Jens Kollmeier, Jessica Werchan, Torsten Blum & Rebecca Graebig-Rancourt
Uppsala Universitet, Uppsala, Sweden
Tobias Sjöblom, Chatarina Larsson & Arvid Widenlou Nordmark
The Chancellor, Masters and Scholars of the University of Oxford, Oxford, UK
Daniel Prieto-Alhambra, Sara Khalid, Edward Burn, Antonella Delmestri, Mahkameh Mafi, Danielle Newby & Cheryl Tan
Universitat Wien, Vienna, Austria
Nikolaus Forgó, Antoni Napieralski, Martina Wimmer, Katharina Haimbuchner, Saskia Kaltenbrunner, Katja Hartl & Kseniia Guliaeva
Istituto Europeo di Oncologia SRL, Milan, Italy
Giuseppe Curigliano, Carmen Criscitiello, Stefania Morganti, Chiara Corti & Elena Dal Zotto
Ludwig-Maximiliansuniversitaet Muenchen, Munich, Germany
Nadia Harbeck & Julian Koch
University College London, London, UK
Neal Navani, Sam Janes & Amyn Bhamani
European Organisation for Research and Treatment of Cancer AISBL, Brussels, Belgium
Stephane Lejeune
Institut de Cancerologie de L’ouest, Angers, France
Mario Campone, Jean-Sebastien Frenel, Kevin Joubel, François Bocquet, Camille Berneur, Marion Laloue, Malvina Dutot, Ludovic Jacob, Delphine Macle, Stéphanie Thauvin, Fanny Seguin & Catherine Le Manach
Universiteit Maastricht, Maastricht, The Netherlands
Philippe Lambin & Anshu Ankolekar
Fundacio Institut Universitari Pera la Recerca A L’atencio Primaria de Salut Jordi Gol I Gurina, Barcelona, Spain
Talita Duarte-Salles & Laura Perez
European Respiratory Society, Lausanne, Switzerland
Valérie Vaccaro, Thomy Tonia, Céline Genton, Wouter van Geffen, Ilona Tietzova, Armin Frille, Vincent Fallet & Adrien Costantini
Deutsche Krebsgesellschaft EV, Berlin, Germany
Simone Wesselmann, Christoph Kowalski, Nora Tabea Sibert & Ellen Griesshammer
European Lung Foundation, Sheffield, UK
Pippa Powell & Clare Williams
ttopstart BV, Rijswijk, The Netherlands
Sigrid van Dorp & Nadia Honing
GMV Soluciones Globales Internet SAU, Madrid, Spain
Javier Téllez, Sandra Garrido, Roberto Galán, Ruben Villoria, Inmaculada Perea Fernández, Paloma López de Arenosa Barbeito, Enric Bousoño Borrull, Laura Tur Giménez, Soralys Hernandez, Pablo Gonzalez Fuente, Juan Miguel Auñón García, José Carlos Barrios González & Alvaro Morandeira Galban
Information Technology for Translational Medicine (ITTM) SA, Esch-Sur-Alzette, Luxembourg
Andreas Kremer, Maria Quaranta, Sebastiano La Ferla, Loic Marc, Nils Christian, Christian Bauer & Mariana Pina
Smart Reporting GMBH, Munich, Germany
Sigrid Auweter, Julia Reichwald, Corinna Zur Bonsen-Thomas, Larissa Tschetsch & Francisco Pinto
Owkin France, Paris, France
Samuel Lesuffleur, Matthieu Blottière, Louise Duflot, David Vallas, Pierre-Olivier Chaudé, Marie Baumier & Daniele Cremonini
Arttic Innovation GMBH, Munich, Germany
Patrizia Torremante, Florian Fromm, Verena von Scharfenberg & Karin Rosenits
Ydeal.Net Software LDA, Santa Maria Da Feira, Portugal
Nuno Azevedo
Pfizer Limited, Sandwich, UK
Marcel Hartig, Waltraud Kantz, Frederic Kube, Amanda Matthews, Bhakti Arondekar, Bruno Gori, Hagen Krüger, Julia Ilinares, Keith Wilner, Lucile Serfass, Lynn McRoy, Robert Miller, Simon Bauer, Sofia Simon, Georgios Papanastasiou, Karen Godbold, Edwina Cahill, Stefan Langhammer, Anne Adams, Sebastian Boie & Florian Reis
Bayer Aktiengesellschaft, Leverkusen, Germany
Susan Evans Axelsson, John-Edward Butler-Ransohoff, Imke Meyer, Selmin Ulusu Saatci, Samu Kurki, Helene Ostojic, Abdelali Majdi, Santiago Villalba, Sai Jasti, Adrian Wolny & Lisa Schneider
Amegen, Diegem, Belgium
Adrian Rousset, Ivo Cleuren, Sandra Eketorp Sylvan, Ellie Paintin, Monika Pokrzepa & Nicolas Pourbaix
F. Hoffmann-LA Roche AG, Basel, Switzerland
Carolin Lorber, Marlene Thomas, Stefanie Morris, Joao Mouta, Martina von Meyenn, Mahesh Shivhare, Thomas Metcalfe, Camille Andre, Tobias Schulte in den Baeumen, Jason Hannon, Alan Mark Hochberg & Kartick Sukumaran
AbbVie Inc, North Chicago, USA
Jie Shen, Nareen Katta, Yilin Xu, Sean Turner & John Ossyra
AstraZeneca AB, Södertälje, Sweden
David Dellamonica, Heather Moses, Yiduo Zhang, Christophe Dufour, Marcus Simon, Maria Teresa Campos, Hassan Naqvi, Jens Ceder & Olga Alekseeva
Mutabor Technologies GMBH, Hamburg, Germany
Burkhard Mueller, Tobias Flosdorf, Ruben Koch & Anastasia Goette
Region Uppsala, Uppsala, Sweden
Gustaf Hedström & Per-Henrik Edqvist
You can also search for this author in PubMed Google Scholar
OPTIMA Consortium
- James N’Dow
- , Wim Witjes
- , Emma Jane Smith
- , Carla Bezuidenhout
- , Sarah Collen
- , Karin Plass
- , Torsten Gerriet Blum
- , Angelika Borkowetz
- , Peter-Paul Willemse
- , Philip Cornford
- , Saeed Dabestani
- , Maurice Schlief
- , Juan Gómez Rivas
- , Anders Bjartell
- , Monique Roobol
- , Katharina Beyer
- , Lionne Venderbos
- , Sebastiaan Remmers
- , Daan Nieboer
- , Raoul Boomsma
- , Bertrand De Meulder
- , Charles Auffray
- , Nesrine Taibi
- , Ayman Hijazy
- , Albert Saporta
- , Johann Pellet
- , Imran Omar
- , Lesley Anderson
- , Steven MacLennan
- , Sara MacLennan
- , Valerie Speirs
- , Solveiga Zibaite
- , Moragh Boyle
- , Charlotte Murray
- , Dianne Brown
- , Demi McDonald
- , Andres Metspalu
- , Jaak Vilo
- , Raivo Kolde
- , Sulev Reisberg
- , Elena Sügis
- , Marek Oja
- , Telver Objartel
- , Alberto Briganti
- , Giorgio Gandaglia
- , Martina Faticoni
- , Greta Matteuzzi
- , Claude Chelala
- , Louise Jones
- , Maryam Abdollahyan
- , Emanuela Gadaleta
- , Guido Juckeland
- , Michael Bussmann
- , Daniel Kotik
- , Artur Yakimovich
- , Torsten Bauer
- , Jens Kollmeier
- , Jessica Werchan
- , Torsten Blum
- , Rebecca Graebig-Rancourt
- , Tobias Sjöblom
- , Chatarina Larsson
- , Arvid Widenlou Nordmark
- , Daniel Prieto-Alhambra
- , Sara Khalid
- , Edward Burn
- , Antonella Delmestri
- , Mahkameh Mafi
- , Danielle Newby
- , Cheryl Tan
- , Nikolaus Forgó
- , Antoni Napieralski
- , Martina Wimmer
- , Katharina Haimbuchner
- , Saskia Kaltenbrunner
- , Katja Hartl
- , Kseniia Guliaeva
- , Giuseppe Curigliano
- , Carmen Criscitiello
- , Stefania Morganti
- , Chiara Corti
- , Elena Dal Zotto
- , Nadia Harbeck
- , Julian Koch
- , Neal Navani
- , Sam Janes
- , Amyn Bhamani
- , Stephane Lejeune
- , Mario Campone
- , Jean-Sebastien Frenel
- , Kevin Joubel
- , François Bocquet
- , Camille Berneur
- , Marion Laloue
- , Malvina Dutot
- , Ludovic Jacob
- , Delphine Macle
- , Stéphanie Thauvin
- , Fanny Seguin
- , Catherine Le Manach
- , Philippe Lambin
- , Anshu Ankolekar
- , Talita Duarte-Salles
- , Laura Perez
- , Valérie Vaccaro
- , Thomy Tonia
- , Céline Genton
- , Wouter van Geffen
- , Ilona Tietzova
- , Armin Frille
- , Vincent Fallet
- , Adrien Costantini
- , Simone Wesselmann
- , Christoph Kowalski
- , Nora Tabea Sibert
- , Ellen Griesshammer
- , Pippa Powell
- , Clare Williams
- , Sigrid van Dorp
- , Nadia Honing
- , Javier Téllez
- , Sandra Garrido
- , Roberto Galán
- , Ruben Villoria
- , Inmaculada Perea Fernández
- , Paloma López de Arenosa Barbeito
- , Enric Bousoño Borrull
- , Laura Tur Giménez
- , Soralys Hernandez
- , Pablo Gonzalez Fuente
- , Juan Miguel Auñón García
- , José Carlos Barrios González
- , Alvaro Morandeira Galban
- , Andreas Kremer
- , Maria Quaranta
- , Sebastiano La Ferla
- , Loic Marc
- , Nils Christian
- , Christian Bauer
- , Mariana Pina
- , Sigrid Auweter
- , Julia Reichwald
- , Corinna Zur Bonsen-Thomas
- , Larissa Tschetsch
- , Francisco Pinto
- , Samuel Lesuffleur
- , Matthieu Blottière
- , Louise Duflot
- , David Vallas
- , Pierre-Olivier Chaudé
- , Marie Baumier
- , Daniele Cremonini
- , Patrizia Torremante
- , Florian Fromm
- , Verena von Scharfenberg
- , Karin Rosenits
- , Nuno Azevedo
- , Marcel Hartig
- , Waltraud Kantz
- , Frederic Kube
- , Amanda Matthews
- , Bhakti Arondekar
- , Bruno Gori
- , Hagen Krüger
- , Julia Ilinares
- , Keith Wilner
- , Lucile Serfass
- , Lynn McRoy
- , Robert Miller
- , Simon Bauer
- , Sofia Simon
- , Georgios Papanastasiou
- , Karen Godbold
- , Edwina Cahill
- , Stefan Langhammer
- , Anne Adams
- , Sebastian Boie
- , Florian Reis
- , Susan Evans Axelsson
- , John-Edward Butler-Ransohoff
- , Imke Meyer
- , Selmin Ulusu Saatci
- , Samu Kurki
- , Helene Ostojic
- , Abdelali Majdi
- , Santiago Villalba
- , Sai Jasti
- , Adrian Wolny
- , Lisa Schneider
- , Adrian Rousset
- , Ivo Cleuren
- , Sandra Eketorp Sylvan
- , Ellie Paintin
- , Monika Pokrzepa
- , Nicolas Pourbaix
- , Carolin Lorber
- , Marlene Thomas
- , Stefanie Morris
- , Joao Mouta
- , Martina von Meyenn
- , Mahesh Shivhare
- , Thomas Metcalfe
- , Camille Andre
- , Tobias Schulte in den Baeumen
- , Jason Hannon
- , Alan Mark Hochberg
- , Kartick Sukumaran
- , Nareen Katta
- , Sean Turner
- , John Ossyra
- , David Dellamonica
- , Heather Moses
- , Yiduo Zhang
- , Christophe Dufour
- , Marcus Simon
- , Maria Teresa Campos
- , Hassan Naqvi
- , Jens Ceder
- , Olga Alekseeva
- , Burkhard Mueller
- , Tobias Flosdorf
- , Ruben Koch
- , Anastasia Goette
- , Gustaf Hedström
- & Per-Henrik Edqvist
Contributions
All authors were involved in the study conception and design, interpretation of the results and the preparation of the manuscript. D.N. carried out data analysis for the manuscript. A.G. and I.T. reviewed the clinical codelist used in this study. N.L.B. and D.N. wrote the initial draft of the manuscript with D.N., E.B. and D.P.A. had access to CPRD. data. All authors were involved in the interpretation of the results, critically reviewed the final manuscript and gave consent for publication.
Corresponding author
Correspondence to Daniel Prieto-Alhambra .
Ethics declarations
Competing interests.
Professor Daniel Prieto-Alhambra’s research group has received research grants from the European Medicines Agency from the Innovative Medicines Initiative from Amgen Chiesi and from UCB Biopharma; and consultancy or speaker fees (paid to his department) from Astellas Amgen Astra Zeneca and UCB Biopharma. NLB is Director of Sleep Universal Limited (though this work is in no way connected to the present manuscript). All other authors declare no conflicts of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Barclay, N.L., Burn, E., Delmestri, A. et al. Trends in incidence, prevalence, and survival of breast cancer in the United Kingdom from 2000 to 2021. Sci Rep 14 , 19069 (2024). https://doi.org/10.1038/s41598-024-69006-1
Download citation
Received : 17 January 2024
Accepted : 30 July 2024
Published : 17 August 2024
DOI : https://doi.org/10.1038/s41598-024-69006-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
You need to enable JavaScript to run this app.

- Health A to Z
- Alternative Medicine
- Blood Disorders
- Bone and Joint
- Cardiovascular Diseases
- Child Health
- Coronavirus
- Dental Care
- Digestive System
- Disabilities
- Drug Center
- Ear, Nose, and Throat
- Environmental Health
- Exercise And Fitness
- First Aid and Emergencies
- General Health
- Health Technology
- Hearing Loss
- Hypertension
- Infectious Disease
- LGBTQ Health
- Liver Health
- Men's Health
- Mental Health
- Pain Management
- Public Health
- Pulmonology
- Senior Health
- Sexual Health
- Skin Health
- Women's Health
- News For Medical Professionals
- Our Products
- Consumer News
- Physician's Briefing
- HealthDay TV
- Wellness Library
- HealthDay Living
- Conference Coverage
- Custom Products
- Health Writing
- Health Editing
- Video Production
- Medical Review
Post-Op Radiation May Give Long-Term Protection Against Breast Cancer's Return
Key takeaways.
Radiation therapy following surgery can keep breast cancer from returning for up to a decade
About 16% of women treated with radiation had their breast cancer return, compared to 36% who didn’t get radiation
However, overall average survival rates were about the same between the two groups
FRIDAY, Aug. 9, 2024 (HealthDay News) -- Radiation therapy following surgery can keep breast cancer from returning for up to 10 years, a new study claims.
The study supports the current standard of care for early-stage breast cancer , which involves surgery followed by radiation therapy, researchers said.
“Our evidence suggests that radiotherapy protects against cancer returning in the same breast for up to 10 years,” said researcher Ian Kunkler , a professor of clinical oncology with the University of Edinburgh’s Institute of Genetics and Cancer.
“It supports the continued use of radiotherapy after breast-conserving surgery for most patients with early breast cancer,” Kunkler added in a university news release.
For the study, researchers analyzed results for 585 Scottish women who received treatment for early-stage breast cancer.
Half received radiation therapy, and half did not. Radiation therapy uses high doses of radiation to destroy any remaining cancer cells after a tumor has been removed from the breast.
After 10 years, 16% of those treated with radiation therapy had their cancer return in the same location, compared with 36% of those who didn’t receive radiation.
Overall average survival rates after 30 years were similar between the two groups – 19.2 years for those who got radiation therapy, and 18.7 for those who didn’t.
There were fewer deaths from breast cancer among those who got radiation therapy, 37% versus 46%.
However, there were more deaths from other cancers in the radiation therapy group, 20% versus 11%.
The study was published Aug. 7 in The Lancet Oncology journal .
“This 30-year study marks the longest follow-up of postoperative radiotherapy in the treatment of early-stage breast cancer,” said lead researcher Dr. Linda Williams , with the University of Edinburgh. “Long-term studies like this, which go beyond 10 years of follow-up, are crucial to fully assess the risks and benefits of treatments.”
More information
The National Breast Cancer Foundation has more about treatment options for breast cancer .
SOURCE: University of Edinburgh, news release, Aug. 7, 2024
What This Means For You
Women with early-stage breast cancer should talk with their doctor about the benefits and risks of radiation therapy.
Related Stories

- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
August 15, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Study suggests way to improve treatment of hereditary breast cancer
by Robin Lally, Columbia University Irving Medical Center

PARP inhibitors have improved survival of breast cancer patients with BRCA1/2 mutations, but the drugs eventually stop working and the cancer returns.
A new study of cancer in mice now suggests that a second cancer drug may be able to prevent or delay relapse. The findings are published in the journal EMBO Molecular Medicine .
"The problem so far has been that the response to PARP inhibitors is not durable," says study co-leader Swarnali Acharyya, associate professor in the Department of Pathology & Cell Biology and Institute for Cancer Genetics at Columbia University Vagelos College of Physicians and Surgeons. "Sometimes it lasts six months, sometimes a little longer. But oncologists tell me that almost all patients eventually relapse, so it's important to find out why."
More than 60% of women who inherit a mutated BRCA1 and BRCA2 gene will get breast cancer in their lifetime, according to the National Cancer Institute. PARP inhibitors work by targeting PARP, a protein that helps cancer cells repair damaged DNA and continue their growth.
Cancer cells are known to use various mechanisms to overcome PARP inhibitors, but the Columbia study is the first to identify a new mechanism that can be thwarted by an existing drug (axitinib, FDA-approved for treating metastatic kidney cancer).
Healthy neighbor cells promote cancer growth
To understand how BRCA cancers develop resistance to PARP inhibitors, the researchers developed new mouse models that respond to PARP inhibitors much the same way patients do. Both experience a striking response to the drugs, before the cancers acquire resistance and recur.
When the researchers examined resistant cancers from these mice, they noticed something curious. "When we took the resistant cancer cells out of the tumor and treated the cancer cells in the lab with PARP inhibitors, they died," Acharyya says. "So, the next question we had was why do cells that resist the drug in vivo die in vitro."
The answer was found in the tumor's microenvironment. When mice get treated with PARP inhibitors, a protein called PGF is secreted by normal cells around the tumor, perhaps as a stress response . This protein then binds to FLT1 receptors on the cancer cells, promoting cancer growth and driving away cancer-fighting T cells.
When researchers blocked the FLT1 receptor either genetically or with a drug called axitinib, the PARP inhibitor started to work again, killing the PARP inhibitor-resistant tumors.
Combination treatment
It's likely that human breast cancers are developing resistance in much the same way, Acharyya says, because the study also found that patients with high levels of FTL1 have less success with PARP inhibitors and develop resistance more quickly.
"The combination of axitinib with PARP inhibitors could make resistant cancers more responsive to treatment and may even be effective for patients who don't respond to PARP inhibitors from the beginning," says study co-leader Anup Biswas, assistant professor of pathology & cell biology.
"Because axitinib is already FDA-approved, our findings could be tested in patients relatively quickly," Acharyya adds, and the team is now talking with physicians from several institutions interested in launching clinical trials.
High levels of FLT1 have also been reported in ovarian, prostate, and pancreatic cancers treated with PARP inhibitors, suggesting the same drug combination may have potential beyond breast cancer .
Explore further
Feedback to editors

Researchers develop new chemical method to enhance drug discovery
4 hours ago

Rare diseases point to connections between metabolism and immunity
Researchers discover novel nanoparticles in blood with potential to transform cancer diagnosis
5 hours ago

Research shows how to reduce inappropriate IV use by more than a third
13 hours ago

Now that mpox is a global health emergency, will it trigger another pandemic?
18 hours ago

Knocking out one key gene leads to autistic traits, mouse study shows

Study: Rare cancer patients nearly three times more likely to develop anxiety and depression than common cancer patients

Intervention for cleaning shared health care equipment could significantly reduce health care–associated infections
19 hours ago

Lip reading activates brain regions similar to real speech, researchers show

Parents' excessive smartphone use could harm children's mental health
Related stories.

Researchers shed light on cancer drug resistance, potential to expand use of PARP inhibitors across cancer types
Jul 31, 2024

Researchers identify what drives PARP inhibitor resistance in advanced breast cancer
May 8, 2024

Researchers pinpoint how PARP inhibitors combat BRCA1 and BRCA2 tumor cells
Aug 12, 2021

Estrogen and PARP inhibitor combination for advanced ER+ breast cancer shows promise in preclinical models
Jul 13, 2023
Existing cancer drugs have potential to benefit thousands more patients, research shows
Aug 1, 2023

Discovery could lead to more effective PARP inhibitor drugs against cancer
Jan 19, 2021
Recommended for you

Can a mouthwash-based test help predict head and neck cancer recurrence?
Aug 15, 2024

AI shows greater efficiency in detecting early signs of bowel cancer

First successful treatment of pediatric high-risk refractory neuroblastoma with PARP inhibition

Study unveils impact of cardiovascular risk factors on genetic predisposition to heart disease
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

Press Releases
IceCure's ProSense® Destroyed 100% of Breast Cancer Tumors in Independent Study of Patients Who Chose Cryoablation Instead of Surgery
- After a median follow-up of 16 months, the complete ablation rate in Luminal A and B breast cancer tumors ≤ 25mm was 100%
- Study concluded that most non-surgical patients with early-stage breast cancer accepted cryoablation when the treatment was offered and that cryoablation is a safe, effective alternative to surgery and well-tolerated as an out-patient procedure
CAESAREA, Israel , Aug. 14, 2024 /PRNewswire/ -- IceCure Medical Ltd. (Nasdaq: ICCM) ("IceCure", "IceCure Medical" or the "Company"), developer of minimally-invasive cryoablation technology that destroys tumors by freezing as an alternative to surgical tumor removal, today announced the publication of an independent study titled "Acceptance and results of cryoablation for the treatment of early breast cancer in non-surgical patients" in the British Journal of Radiology , a publication of the British Institute of Radiology. The single-site study, led by Lucia L. Garna Lopez, PhD, was conducted by researchers in the radiology, oncology, and surgery departments at Hospital Lucus Augusti in Lugo, Spain.

The aim of the study was to evaluate the acceptance of percutaneous cryoablation treatment by patients with early-stage breast cancer who choose not to have surgery. Of the 45 patients offered cryoablation with ProSense®, 43 patients, or 95.6% accepted. 36 of these, representing 39 malignant tumors (median size 24mm), proceeded to undergo cryoablation.
"This study is a good case in point that when women who elect not to have surgery, or are not eligible for surgery, are given the option, they overwhelmingly choose cryoablation to treat their breast cancer," stated IceCure CEO Eyal Shamir. "In addition to providing excellent data on the safety and efficacy of ProSense® in patients who chose not undergo surgery, the study's authors also point to the correlation between a larger aging population, increased risk of breast cancer with age, and the fact that most patients who elect not to have surgery or are not eligible are elderly patients. These factors, we believe, point to increasing demand for ProSense® when it is presented as an option."
The median age of patients treated with cryoablation was 87, with a range of 60-96. After a median follow-up of 16 months, the complete ablation rate in luminal breast cancer with tumors ≤ 25mm was 100%. No major complications were seen.
The study investigators concluded that most non-surgical patients with early-stage breast cancer accepted cryoablation when the treatment was offered and that cryoablation is safe, effective, and well-tolerated as an outpatient procedure. The published article went on to state that outcomes suggest cryoablation could be an alternative to surgery for the management of breast cancer in this group of patients and pointed to financial, physical, and cosmetic benefits.
About ProSense® The ProSense ® Cryoablation System provides a minimally invasive treatment option to destroy tumors by freezing them. The system uniquely harnesses the power of liquid nitrogen to create large lethal zones for maximum efficacy in tumor destruction in benign and cancerous lesions, including breast, kidney, lung, and liver.
ProSense® enhances patient and provider value by accelerating recovery, reducing pain, surgical risks, and complications. With its easy, transportable design and liquid nitrogen utilization, ProSense® opens that door to fast and convenient office-based procedure for breast tumors.
About IceCure Medical IceCure Medical ( Nasdaq : ICCM ) develops and markets advanced liquid-nitrogen-based cryoablation therapy systems for the treatment of tumors (benign and cancerous) by freezing, with the primary focus areas being breast, kidney, bone and lung cancer. Its minimally invasive technology is a safe and effective alternative to hospital surgical tumor removal that is easily performed in a relatively short procedure. The Company's flagship ProSense ® system is marketed and sold worldwide for the indications cleared and approved to date including in the U.S ., Europe and China.
Forward Looking Statements This press release contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995 and other Federal securities laws. Words such as "expects," "anticipates," "intends," "plans," "believes," "seeks," "estimates" and similar expressions or variations of such words are intended to identify forward-looking statements. For example, IceCure is using forward looking statements in this press release when it discusses the belief that the correlation between an aging population, increased risk of breast cancer with age, and the fact that most patients who elect not to have surgery or are not eligible are elderly patients point to increasing demand for ProSense ® when it is presented as an option. Historical results of scientific research and clinical and preclinical trials do not guarantee that the conclusions of future research or trials will suggest identical or even similar conclusions. Important factors that could cause actual results, developments and business decisions to differ materially from those anticipated in these forward-looking statements include, among others: the Company's planned level of revenues and capital expenditures; the Company's available cash and its ability to obtain additional funding; the Company's ability to market and sell its products; legal and regulatory developments in the United States and other countries; the Company's ability to maintain its relationships with suppliers, distributors and other partners; the Company's ability to maintain or protect the validity of its patents and other intellectual property; the Company's ability to expose and educate medical professionals about its products; political, economic and military instability in the Middle East, specifically in Israel; as well as those factors set forth in the Risk Factors section of the Company's Annual Report on Form 20-F for the year ended December 31, 2023 filed with the SEC on April 3, 2024, and other documents filed with or furnished to the SEC which are available on the SEC's website, www.sec.gov . The Company undertakes no obligation to update these statements for revisions or changes after the date of this release, except as required by law
IR Contact: Email: [email protected]
Michael Polyviou Phone: 732-232-6914
Todd Kehrli Phone: 310-625-4462
Logo - https://mma.prnewswire.com/media/2319310/IceCure_Medical_Logo.jpg
View original content: https://www.prnewswire.com/news-releases/icecures-prosense-destroyed-100-of-breast-cancer-tumors-in-independent-study-of-patients-who-chose-cryoablation-instead-of-surgery-302222148.html
SOURCE IceCure Medical
Released August 14, 2024
- Email Alerts
- Company Profile
- RSS News Feed
IceCure Medical HQ: 7 Ha’Eshel St., PO Box 3163, Caesarea, 3079504, Israel
We use cookies to offer you a better browsing experience, including personalized advertising. By continuing to use the site you agree to their use.
Double mastectomies don't increase cancer survival, study suggests
Other types of surgery besides double mastectomies are equally good at lowering death rates in women with cancer in one breast.

Women with cancer in one breast have the option to have both breasts removed, as a precaution. However, new research finds that these patients are no less likely to die from breast cancer than women who have only the affected breast or the tumor inside it removed.
The recent study looked at data from more than 660,000 women of various ethnicities in a large U.S. cancer registry . The women were 58 years old, on average, and all had been diagnosed with unilateral breast cancer, meaning cancer in one breast.
Following diagnosis, each patient had one of three standard surgical procedures: a lumpectomy , to remove only the tumor in the affected breast; a unilateral mastectomy , to remove one breast; or a bilateral mastectomy , to remove both breasts. Otherwise, the women were matched in terms of the clinical features of their cancers.
Researchers tracked whether the women developed breast cancer in their remaining breast — a condition called contralateral breast cancer — over 20 years. This can also happen in people with double mastectomies because there's a chance some leftover breast tissue or cancer cells may recur on the chest wall . The risk of contralateral breast cancer happening is usually around 0.4% per year following a unilateral breast cancer diagnosis.
Related: 'Bionic breast' could restore sensation for cancer survivors
The team also recorded whether any of the women died from breast cancer during this follow-up period.
Overall, women who'd had a double mastectomy had a statistically significant, lower risk of developing contralateral breast cancer than the other groups (0.7% versus 6.9%). However, there was no statistically significant difference in overall death rates between groups — these were 16.3% for lumpectomies and 16.7% for both types of mastectomy.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
This similarity showed up despite the fact that the patients who developed contralateral breast cancer had more than twice the risk of dying than those who didn't (32.1% compared to 14.5%).
A possible explanation for this seemingly counterintuitive finding is that the original tumor is the predominant driver of deaths in these patients, rather than contralateral breast cancer, said Dr. Seema Khan and Masha Kocherginsky , cancer researchers from Northwestern University in Illinois who were not involved in the research.
Patients are diagnosed with their original tumor at a younger age, when the risk of breast-cancer death is higher , and they're treated with "older, less effective regimens," they wrote in an accompanying editorial . For these reasons, the risk of death from contralateral breast cancer may be lower than that from the original tumor. That's especially true "if second cancers are diagnosed at earlier stages in survivors who tend to be more adherent to posttreatment screening," they added.
Therefore, reducing the risk of contralateral breast cancer wouldn't necessarily affect overall survival rates for breast cancer.
These new findings were described in a paper published July 25 in the journal JAMA Oncology . They confirm trends that other research have previously shown but with a larger sample size and a longer follow-up period, the authors said.
Ultimately, though, the decision to have a double mastectomy is extremely personal.
"We are grateful that in 2024, women have options for treating breast cancer," which have equal survival rates, Dr. Mehra Golshan , a cancer surgeon at Yale School of Medicine who was not involved in the research, told Live Science in an email.
— AI predicts 5-year breast cancer risk better than standard tools — but we aren't sure how it works
— Breast cancer screening should start at age 40, expert task force says
— Black patients may need breast cancer screenings earlier than what many guidelines recommend
"Developing a contralateral breast cancer is very traumatic and for some women, removing that second breast is reassuring and they won't have to undergo routine screening," Vasily Giannakeas , lead study author and an epidemiologist at the Women's College Hospital Research Institute in Canada, told Live Science. For others, it may increase their confidence by enhancing the symmetry of their chest. Usually patients are given the choice as to whether they'd also like to have breast reconstructive surgery after a mastectomy.
"What is important is that clinicians support women to make an informed choice about this issue, after they have all the facts," said Dr. Lynda Wyld , a professor of surgical oncology at the University of Sheffield in the U.K. who was not involved in the research. "Papers like this are very valuable in helping clinicians advise their patients about the risks and benefits of this type of surgery," Wyld told Live Science in an email.
This article is for informational purposes only and is not meant to offer medical advice.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun ? Send us your questions about how the human body works to [email protected] with the subject line "Health Desk Q," and you may see your question answered on the website!
Emily is a health news writer based in London, United Kingdom. She holds a bachelor's degree in biology from Durham University and a master's degree in clinical and therapeutic neuroscience from Oxford University. She has worked in science communication, medical writing and as a local news reporter while undertaking journalism training. In 2018, she was named one of MHP Communications' 30 journalists to watch under 30. ( [email protected] )
Sweden reports 1st case of deadlier mpox outside Africa
CRISPR could be used to treat UTIs, early trial hints
Earth’s days were once 2 hours longer — and that may have triggered one of the biggest evolutionary explosions in history, study suggests
Most Popular
- 2 CRISPR could be used to treat UTIs, early trial hints
- 3 Earth’s days were once 2 hours longer — and that may have triggered one of the biggest evolutionary explosions in history, study suggests
- 4 Massive medieval coin hoard worth 'about 150 sheep' discovered in Germany's Black Forest
- 5 Dinosaur-killing asteroid was a rare rock from beyond Jupiter, new study reveals

IMAGES
COMMENTS
This page highlights what's new and recent research in the detection and treatment of breast cancer. New methods to detect breast cancer, such as 3-D mammography, are described. Treatments tailored to breast cancer subtypes are discussed, including targeted therapies.
This study aims to compare an additional support program (text message reminders and/or telephone-based counseling) with usual care in making sure breast cancer patients take their endocrine therapy medication as prescribed (medication adherence).
Find research articles on breast cancer, which may include news stories, clinical trials, blog posts, and descriptions of active studies.
Explore the comprehensive coverage on breast cancer, its diagnosis, treatment, and research in The Lancet Breast Cancer article.
In triple-negative breast cancer, the combination of cancer immunotherapy based on PD-1/PD-L1 immune checkpoint inhibitors with chemotherapy was effective both in advanced and early setting phase ...
To reduce mortality, screening must detect life-threatening disease at an earlier, more curable stage. Effective cancer-screening programs therefore both increase the incidence of cancer detected a...
Among patients with high-risk, HER2-negative early breast cancer and germline BRCA1 or BRCA2 pathogenic or likely pathogenic variants, adjuvant olaparib after completion of local treatment and ...
Research studies Current guidance on preventing and treating breast cancer as well as what might cause it (among other things) has come mainly from information discovered from research studies. Research studies can range from studies done in the lab to clinical trials done with hundreds of thousands of people.
Predicting breast cancer risk in women of African ancestry. The largest genome-wide association study of its kind has identified new markers for risk prediction in women of African ancestry ...
This study provides a comprehensive transcriptional atlas of the cellular architecture of breast cancer.
Development of a humanized anti-FABP4 monoclonal antibody for potential treatment of breast cancer Breast cancer is the most common cancer in women diagnosed in the U.S. and worldwide. Obesity increases breast cancer risk without clear underlying molecular mechanisms. Our studies demonstrate that circulatin...
Breast Cancer Research is an international, peer-reviewed online journal, publishing original research, reviews, editorials and reports. Open access research articles of exceptional interest are published in all areas of biology and medicine relevant to breast cancer, including normal mammary gland biology, with special emphasis on the genetic ...
Genetic testing for breast cancer susceptibility is widely used, but for many genes, evidence of an association with breast cancer is weak, underlying risk estimates are imprecise, and reliable sub...
A Harvard Medical School study shows the sex hormone estrogen — thus far thought to be only a fuel for breast cancer growth — can directly cause tumor-driving genomic rearrangements.
Breast cancer is the most common cancer among women. It is estimated that 2.3 million new cases of BC are diagnosed globally each year. Based on mRNA gene expression levels, BC can be divided into molecular subtypes that provide insights into new treatment ...
An experimental form of immunotherapy that uses an individual's own tumor-fighting immune cells could potentially be used to treat people with metastatic breast cancer, according to results from an ongoing clinical trial led by researchers at the National Cancer Institute's (NCI) Center for Cancer Research, part of the National Institutes of Health. Many people with metastatic breast ...
Breast Cancer: An Overview of Current Therapeutic Strategies, Challenge, and Perspectives. Breast cancer is the most commonly diagnosed cancer and the leading cause of death among female patients, which seriously threatens the health of women in the whole world. The treatments of breast cancer require the cooperation of a multidisciplinary ...
Some modifiable risk factors differ between AYAs and older women. 1, 9 Although obesity increases breast cancer risk among postmenopausal women, several large epidemiologic studies report that breast cancer is more frequent in young women with low or normal compared with higher body mass index. Other studies suggest that body habitus and a higher waist-to-hip ratio are more predictive of AYA ...
Several studies have examined the clinical benefits of hormone replacement therapy (HRT). However, because long-term use of HRT has been implicated as a risk factor for the development of breast cancer, some women remain skeptical when considering this therapy to address their vasomotor symptoms. Hence, physicians and nurses should actively ...
For postmenopausal women with hormone-receptor-positive breast cancer, the most effective duration for adjuvant therapy with an aromatase inhibitor remains unclear. In this prospective, phase 3 ...
35 National Cancer Research Institute, Breast Cancer Clinical Studies Group (NCRI-BCSG), London, ... Participants included premenopausal female patients with HR+ early breast cancer with specimens in the International Breast Cancer Study Group tumor repository available for RNA extraction. Data were collected from December 2003 to April 2021 ...
PARP inhibitors have improved survival of breast cancer patients with BRCA1/2 mutations, but the drugs eventually stop working and the cancer returns. A new study of cancer in mice now suggests that a second cancer drug may be able to prevent or delay relapse. Read the paper
Breast cancer is the most frequently diagnosed cancer in females globally. However, we know relatively little about trends in males. This study describes United Kingdom (UK) secular trends in ...
Braunstein LZ, Wong J, Dillon D, et al. Preliminary report of the PRECISION trial (profiling early breast cancer for radiotherapy omission): a phase II study of breast-conserving surgery without ...
This is a multicenter, open label, nonrandomized, sequential dose escalation/cohort expansion, multiple dose study designed to evaluate the safety, toxicity, and PK as well as preliminary efficacy of BTX-A51 in subjects with advanced solid tumors and breast cancer. The study will be done in two phases, described below.
"This 30-year study marks the longest follow-up of postoperative radiotherapy in the treatment of early-stage breast cancer," said lead researcher Dr. Linda Williams, with the University of Edinburgh. "Long-term studies like this, which go beyond 10 years of follow-up, are crucial to fully assess the risks and benefits of treatments."
PARP inhibitors have improved survival of breast cancer patients with BRCA1/2 mutations, but the drugs eventually stop working and the cancer returns. A new study of cancer in mice now suggests ...
After a median follow-up of 16 months, the complete ablation rate in Luminal A and B breast cancer tumors ≤ 25mm was 100%; Study concluded that most non-surgical patients with early-stage breast cancer accepted cryoablation when the treatment was offered and that cryoablation is a safe, effective alternative to surgery and well-tolerated as an out-patient procedure
Patients who have residual invasive carcinoma after the receipt of neoadjuvant chemotherapy for human epidermal growth factor receptor 2 (HER2)-negative breast cancer have poor prognoses. The ...
This similarity showed up despite the fact that the patients who developed contralateral breast cancer had more than twice the risk of dying than those who didn't (32.1% compared to 14.5%).