- Department of Health and Human Services
- National Institutes of Health


COVID-19 Research Studies
More information, about clinical center, clinical trials and you, participate in a study, referring a patient.
About Clinical Research
Research participants are partners in discovery at the NIH Clinical Center, the largest research hospital in America. Clinical research is medical research involving people The Clinical Center provides hope through pioneering clinical research to improve human health. We rapidly translate scientific observations and laboratory discoveries into new ways to diagnose, treat and prevent disease. More than 500,000 people from around the world have participated in clinical research since the hospital opened in 1953. We do not charge patients for participation and treatment in clinical studies at NIH. In certain emergency circumstances, you may qualify for help with travel and other expenses Read more , to see if clinical studies are for you.
Medical Information Disclaimer
Emailed inquires/requests.
Email sent to the National Institutes of Health Clinical Center may be forwarded to appropriate NIH or outside experts for response. We do not collect your name and e-mail address for any purpose other than to respond to your query. Nevertheless, email is not necessarily secure against interception. This statement applies to NIH Clinical Center Studies website. For additional inquiries regarding studies at the National Institutes of Health, please call the Office of Patient Recruitment at 1-800-411-1222

Find NIH Clinical Center Trials
The National Institutes of Health (NIH) Clinical Center Search the Studies site is a registry of publicly supported clinical studies conducted mostly in Bethesda, MD.

- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español

NIH Research Festival
A Celebration of Intramural Science, featuring lectures, exhibits, poster sessions and more.
Learn more »

NIH Loan Repayment Programs
Researchers may be eligible for student loan repayment. Apply through November 21, 2024.

Fetal Alcohol Spectrum Disorders
Prenatal alcohol exposure can result in FASD by interfering with the baby’s development.

August Is Gastroparesis Awareness Month
Gastroparesis is a disorder that slows or stops the movement of food to the stomach or small intestine.

NIH STEM Teaching Resources
Free, innovative NIH-funded content to engage pre-K-12 students in health science.
In the News

CPR for Cardiac Arrest
Black people and women less likely to survive bystanders provide aid.

Vision Care
Study finds gaps in Medicaid coverage of routine vision care.

Differences in most common symptoms identified between children and adolescents.

Parkinson’s Disease
Self-adjusting “brain pacemaker” provides real-time symptom relief.
NIH at a Glance
Virtual-tour-screenshot-square.jpg.

Take the Virtual Tour
Explore the Bethesda campus and how NIH turns discovery into health.
dr-monica-bertagnolli-thumbnail.jpg

The NIH Director
Monica M. Bertagnolli, M.D., is the NIH Director and provides leadership for the 27 Institutes and Centers that make up NIH.
nih-at-a-glance-funding.jpg
Funding for Research
NIH is the largest source of funding for medical research in the world, creating hundreds of thousands of high-quality jobs.
nih-at-a-glance-labs.jpg

Labs at NIH
Scientists conduct research on NIH campuses across the U.S., as part of our Intramural Research Program.
improving-health-collage.jpg

Impact of NIH Research
NIH-supported research has had a major positive impact on nearly all of our lives.
researcher-holding-petri-dish.jpg

Jobs at NIH
The central recruitment point of access to all NIH jobs and training opportunities
Featured Resources & Initiatives
A new science agency proposed by President Joseph Biden as part of NIH to drive biomedical breakthroughs and provide transformative solutions for all patients.
Anti-Sexual Harassment
NIH does not tolerate pervasive or severe harassment of any kind, including sexual harassment.
Ending Structural Racism
Learn more about NIH’s efforts to end structural racism in biomedical research through the UNITE initiative.
All of Us Research Program
A research effort to revolutionize how we improve health and treat disease.
NIH HEAL Initiative
Trans-agency effort to speed scientific solutions to stem the national opioid crisis.
Clinical Trials
Learn about participating in clinical trials and where to find them.
Accelerating Medicines Partnership
A bold venture to help identify new treatments and cures for diseases.
Medical Research Initiatives
Important initiatives aimed at improving medical research.
Training at NIH
NIH provides training opportunities internally, as well as at universities and other institutions across the U.S.
A new research initiative to understand, prevent, and treat the long-term effects of COVID-19.
COVID-19 Research information from NIH
NIH supports research in COVID-19 testing, treatments, and vaccines. También disponible en español.
Climate Change and Health Initiative
Research to reduce health threats from climate change.
Connect with Us
- More Social Media from NIH
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee on Health Research and the Privacy of Health Information: The HIPAA Privacy Rule; Nass SJ, Levit LA, Gostin LO, editors. Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research. Washington (DC): National Academies Press (US); 2009.

Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research.
- Hardcopy Version at National Academies Press
3 The Value, Importance, and Oversight of Health Research
The previous chapter reviewed the value of privacy, while this chapter examines the value and importance of health research. As noted in the introduction to Chapter 2 , the committee views privacy and health research as complementary values. Ideally, society should strive to facilitate both for the benefit of individuals as well as the public.
In addition to defining health research and delineating its value to individuals and society, this chapter provides an overview and historical perspective of federal research regulations that were in place long before the Privacy Rule was implemented. Because a great deal of medical research falls under the purview of multiple federal regulations, it is important to understand how the various rules overlap or diverge. The chapter also explains how the definition of research has become quite complex under the various federal regulations, which make a distinction between research and some closely related health practice activities that also use health data, such as quality improvement initiatives.
The chapter also reviews the available survey data regarding public perceptions of health research and describes the importance of effective communication about health research with patients and the public.
- CONCEPTS AND VALUE OF HEALTH RESEARCH
Definitions
Under both the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule and the Common Rule, “research” is defined as “a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.” This is a broad definition that may include biomedical research, epidemiological studies, 1 and health services research, 2 as well as studies of behavioral, social, and economic factors that affect health.
Perhaps the most familiar form of health research is the clinical trial, in which patients volunteer to participate in studies to test the efficacy and safety of new medical interventions. But an increasingly large portion of health research is now information based. A great deal of research entails the analysis of data and biological samples that were initially collected for diagnostic, treatment, or billing purposes, or that were collected as part of other research projects, and are now being used for new research purposes. This secondary 3 use of data is a common research approach in fields such as epidemiology, health services research, and public health research, and includes analysis of patterns of occurrences, determinants, and natural history of disease; evaluation of health care interventions and services; drug safety surveillance; and some genetic and social studies ( Lowrance, 2002 ; Lowrance and Collins, 2007 ).
The Importance of Health Research
Like privacy, health research has high value to society. It can provide important information about disease trends and risk factors, outcomes of treatment or public health interventions, functional abilities, patterns of care, and health care costs and use. The different approaches to research provide complementary insights. Clinical trials can provide important information about the efficacy and adverse effects of medical interventions by controlling the variables that could impact the results of the study, but feedback from real-world clinical experience is also crucial for comparing and improving the use of drugs, vaccines, medical devices, and diagnostics. For example, Food and Drug Administration (FDA) approval of a drug for a particular indication is based on a series of controlled clinical trials, often with a few hundred to a few thousand patients, but after approval it may be used by millions of people in many different contexts. Therefore, tracking clinical experience with the drug is important for identifying relatively rare adverse effects and for determining the effectiveness in different populations or in various circumstances. It is also vital to record and assess experience in clinical practice in order to develop guidelines for best practices and to ensure high-quality patient care.
Collectively, these forms of health research have led to significant discoveries, the development of new therapies, and a remarkable improvement in health care and public health. 4 Economists have found that medical research can have an enormous impact on human health and longevity, and that the resulting increased productivity of the population contributes greatly to the national economy ( Hatfield et al., 2001 ; Murphy and Topel, 1999 ) in addition to the individual benefits of improved health. If the research enterprise is impeded, or if it is less robust, important societal interests are affected.
The development of Herceptin as a treatment for breast cancer is a prime example of the benefits of research using biological samples and patient records ( Box 3-1 ) ( Slamon et al., 1987 ). Many other examples of findings from medical records research have changed the practice of medicine as well. Such research underlies the estimate that tens of thousands of Americans die each year from medical errors in the hospital, and research has provided valuable information for reducing these medical errors by implementing health information technology, such as e-prescribing ( Bates et al., 1998 ; IOM, 2000b ). This type of research also has documented that disparities in health care and lack of access to care in inner cities and rural areas result in poorer health outcomes ( Mick et al., 1994 ). Furthermore, medical records research has demonstrated that preventive services (e.g., mammography) substantially reduce mortality and morbidity at reasonable costs ( Mandelblatt et al., 2003 ), and has established a causal link between the nursing shortage and patient health outcomes by documenting that patients in hospitals with fewer registered nurses are hospitalized longer and are more likely to suffer complications, such as urinary tract infections and upper gastrointestinal bleeding ( Needleman et al., 2002 ). These findings have all informed and influenced policy decisions at the national level. As the use of electronic medical records increases, the pace of this form of research is accelerating, and the opportunities to generate new knowledge about what works in health care are expanding ( CHSR, 2008 ).
Examples of Important Findings from Medical Database Research. Herceptin and breast cancer: Data were collected from a cohort of more than 9,000 breast cancer patients whose tumor specimens were consecutively received at the University (more...)
Advances in health information technology are enabling a transformation in health research that could facilitate studies that were not feasible in the past, and thus lead to new insights regarding health and disease. As noted by the National Committee on Vital and Health Statistics, “Clinically rich information is now more readily available, in a more structured format, and able to be electronically exchanged throughout the health and health care continuum. As a result, the information can be better used for quality improvement, public health, and research, and can significantly contribute to improvements in health and health care for individuals and populations” ( NCVHS, 2007a ). The informatics grid recently developed with support from the National Cancer Institute (Cancer Biomedical Informatics Grid, or caBIG) is an example of a how information technologies can facilitate health research by enabling broader sharing of health data while still ensuring regulatory compliance and protecting patient privacy ( Box 3-2 ).
caBIG (Cancer Biomedical Informatics Grid). The National Cancer Institute’s caBIG Data Sharing and Intellectual Capital Workspace’s mission is to enable all constituencies in the cancer community—including researchers, physicians, (more...)
Science today is also changing rapidly and becoming more complex, so no single researcher or single site can bring all the expertise to develop and validate medical innovations or to ensure their safety. Thus, efficient sharing of information between institutions has become even more important than in previous eras, when there were fewer new therapies introduced. The expansion of treatment options, as well as the escalating expense of new therapies, mandates greater scrutiny of true effectiveness, 5 once efficacy has been demonstrated. This requires registries of patient characteristics, outcomes, and adverse events. Large populations are required to facilitate comparison of patient populations and to calculate risk/benefit estimates. For example, INTERMACS 6 (Interagency Registry for Mechanically Assisted Circulatory Support) is a national registry for patients who are receiving mechanical circulatory support device therapy to treat advanced heart failure. This registry was devised as a joint effort of the National Heart, Lung and Blood Institute, Centers for Medicare & Medicaid Services, FDA, clinicians, scientists and industry representatives. Analysis of the data collected is expected to facilitate improved patient evaluation and management while aiding in better device development. Registry results are also expected to influence future research and facilitate appropriate regulation and reimbursement of such devices. Similarly, the Extracorporeal Life Support Organization (ELSO), 7 an international consortium of health care professionals and scientists who focus on the development and evaluation of novel therapies for support of failing organ systems, maintains a registry of extracorporeal membrane oxygenation and other novel forms of organ system support. Registry data are used to support clinical practice and research, as well as regulatory agencies. Another example is the database developed by the United Network for Organ Sharing (UNOS) for the collection, storage, analysis and publication of data pertaining to the patient waiting list, organ matching, and transplants. 8 Launched in 1999, this secure Internet-based system contains data regarding every organ donation and transplant event occurring in the United States since 1986.
Information-based research, such as research using health information databases has many advantages (reviewed by Lowrance, 2002 ). It is often faster and less expensive than experimental studies; it can analyze very large sets of data and may detect unexpected phenomena or differences among subpopulations that might not be included in a controlled experimental study; it can often be undertaken when controlled trials are simply not possible for ethical, technical, or other reasons, and it can be used to study effectiveness of a specific test or intervention in clinical practice, rather than just the efficacy as determined by a controlled experimental study. It can also reexamine data accrued in other research studies, such as clinical trials, to answer new questions quickly and inexpensively. However, information-based research does have limitations. Often it has less statistical rigor than controlled clinical studies because it lacks scientific control over the original data collection, quality, and format that prospective experimental research can dictate from the start. In addition to these scientific limitations, because of its relational and often distant physical separation from the data subjects, and the sheer volume of the records involved, obtaining individual consent for the research can be difficult or impossible.
Advances in information-based medical research could also facilitate the movement toward personalized medicine, which will make health research more meaningful to individuals. The goal of personalized medicine is to tailor prevention strategies and treatments to each individual based on his/her genetic composition and health history. In spite of the strides made in improving health through new treatments, it is widely known that most drugs are effective in only a fraction of patients who have the condition for which the drug is indicated. Moreover, a small percentage of patients are likely to have adverse reactions to drugs that are found to be safe for the majority of the population at the recommended dose. Both of these phenomena are due to variability in the patient population. Revolutionary advances in the study of genetics and other markers of health and disease are now making it possible to identify and study these variations, and are leading to more personalized approaches to health care—that is, the ability to give “the appropriate drug, at the appropriate dose, to the appropriate patient, at the appropriate time.” Achieving the goals of personalized medicine will lead to improvements in both the effectiveness and the safety of medical therapies.
Public Perceptions of Health Research
A number of studies have been undertaken to gauge the public’s attitude toward research and the factors that influence individuals’ willingness to participate in research. The surveys reviewed in this chapter focus on interventional clinical trials. A review of survey questions to gauge the public willingness to allow their medical records to be used in research can be found in Chapter 2 .
The Public Values Health Research
A number of studies suggest that most Americans have a positive view of medical research and believe that research is beneficial to society. A recent Harris poll found that nearly 80 percent of respondents were interested in health research findings, consistent with previous survey results ( Westin, 2007 ). A study in 2005 compiled data from 70 state surveys and 18 national surveys and found that the majority of Americans believe maintaining world leadership in health-related research is important. Seventy-eight percent of respondents said that it is very important, and 17 percent said that it is somewhat important. Only 4 percent of Americans reported that maintaining world leadership in health-related research is not impor tant ( Woolley and Propst, 2005 ). Similar results were found in a 2007 survey—76 percent of respondents reported that science plays a very important role in our health, and 78 percent reported that science plays a very important role in our competitiveness ( Research!America, 2007 ).
The Virginia Commonwealth University 2004 Life Sciences Survey also found that most Americans have a positive view of research. In this study, 90 percent of respondents agreed that developments in science have made society better; 92 percent reported that “scientific research is essential for improving the quality of human lives”; and 84 percent agreed that “the benefits of scientific research outweigh the harmful results” ( NSF, 2006 ).
Overall Experience When Participating in Research
Little is known about the attitudes of individuals who have actually participated in medical research. However, the available evidence suggests that most research participants have positive experiences. A recent Harris Poll found that 13 percent of respondents had participated in some form of health research, and 87 percent of those felt comfortable about their experience ( Westin, 2007 ). In a study focused on cancer, 93 percent of respondents who participated in research reported it as a very positive experience; 76 percent said they would recommend participation in a clinical trial to someone with cancer. Most physicians surveyed in this study stated that they believe clinical trial participants receive the best possible care, and have outcomes at least as good as patients receiving standard cancer treatment ( Comis et al., 2000 ). Another study found that 55 percent of individuals who participated in a research study would be willing to participate again in a future research study ( Trauth et al., 2000 ).
Willingness to Participate in Research
Public opinion surveys indicate that a majority of Americans are willing to participate in clinical research studies. In 2001, a compilation of studies commissioned by Research!America found that 63 percent of Americans would be willing to participate in a clinical research study ( Woolley and Propst, 2005 ). This percentage has remained stable over time. A 2007 Research!America survey also found that 63 percent of Americans would be very likely to participate in a clinical research study if asked ( Research!America, 2007 ); 68 percent of respondents reported that their desire to improve their own health or the health of others was a major factor in deciding whether to participate in a clinical research project ( Research!America, 2007 ).
Other surveys also suggest that willingness to participate in research focused on specific diseases is quite high. In one survey, the percentage of respondents indicating a willingness to participate in a medical research study was 88 percent for cancer, 86 percent for heart disease, 83 percent for a noncurable fatal disease, 79 percent for addiction, 78 percent for depression, and 76 percent for schizophrenia ( Trauth et al., 2000 ). Respondents with greater knowledge of how research is conducted were more willing to participate ( Trauth et al., 2000 ). Another study found that 8 of 10 Americans would consider participating in a clinical trial if faced with cancer. More than two-thirds of respondents said they would be willing to participate in a clinical trial designed to prevent cancer ( Comis et al., 2000 ).
Americans also seem to be very supportive of medical research that relies on genetic data. A 2007 survey found that 93 percent of Americans supported the use of genetic testing if the information collected is used by researchers to find new ways to diagnose, prevent, or treat disease ( Genetics & Public Policy Center, 2007 ). Two separate surveys found that 66 percent of Americans would be willing to donate their genetic material for medical research ( Genetics & Public Policy Center, 2007 ; Research!America, 2007 ). However, despite this apparent positive view of genetic research, 92 percent of Americans reported they were concerned about their genetic information being used in a “harmful way” ( Genetics & Public Policy Center, 2007 ).
Many factors, in addition to concerns about privacy and confidentiality ( Genetics & Public Policy Center, 2007 ; Research!America, 2007 ), may influence an individual’s willingness to participate in a medical research study. The Trauth survey found that individuals with higher income levels, with a college or graduate degree, or with children were more likely to participate in research. Age affected willingness to participate: 57 percent of respondents ages 18–34 were willing to participate in research, but only 31 percent of respondents ages 65 or older were willing ( Trauth et al., 2000 ).
Other factors that potentially influence an individual’s willingness to participate in research are race and ethnicity. It is well documented that minorities participate in health research at a much lower percentage than white Americans. Many cultural, linguistic, and socioeconomic barriers could be responsible for this difference ( Giuliano et al., 2000 ), and study results have been variable on this issue. Several studies suggest that the low participation rates by racial and ethnic minority groups are due to their strong distrust of the medical research community compared to the general population ( Braunstein et al., 2008 ; Corbie-Smith et al., 1999 ; Farmer et al., 2007 ; Grady et al., 2006 ; Shavers et al., 2002 ).
However, other evidence suggests that the low percentage of minorities participating in research is related to minority groups’ lack of access to the research community ( Brown et al., 2000 ; Wendler et al., 2006 ; Williams and Corbie-Smith, 2006 ). Thus, it is likely that the low number of minority individuals participating in medical research is at least partly due to recruitment techniques that are ineffective for minority populations.
The survey that focused on cancer research suggests that one of the main reasons why individuals do not participate in research is lack of knowledge about the availability of clinical trials. In a survey of nearly 6,000 cancer patients, 85 percent said they were unaware of the opportunity to participate in a clinical trial. Respondents who did participate said they did so because of one of the following beliefs: (1) trials provide access to the best quality of care (76 percent), (2) their participation would benefit future cancer patients (72 percent), (3) they would receive newer and better treatment (63 percent), and (4) participation would get them more care and attention (40 percent) ( Comis et al., 2000 ).
A recommendation from a physician can also impact participation. In the United States, 48 percent of respondents to one survey reported that a physicians’ recommendation would be a major factor in deciding whether to take part in a research study. Nearly three-fourths of respondents also cited an institution’s reputation as a key factor to consider when deciding whether to participate in a study ( Research!America, 2007 ). Twenty percent of respondents in an Italian public survey indicated that the presence of a physician as a reference during a research study influenced their willingness to participate ( Mosconi et al., 2005 ).
In sum, surveys indicate that the vast majority of Americans have a positive view of medical research, believe that research is beneficial to society, and are interested in health research findings. Although little is known about the attitudes of individuals who have actually participated in medical research, the available evidence suggests that most research participants have positive experiences. Surveys also suggest that a majority of Americans are willing to participate in clinical research studies. Similar to the findings in Chapter 2 , surveys indicate that many factors, in addition to concerns about privacy and confidentiality, can potentially influence an individual’s willingness to participate in medical research, including the type of research and personal characteristics such as health status, age, education, and race. Notably, respondents with greater knowledge of how research is conducted were more willing to participate in research.
- OVERSIGHT OF HEALTH RESEARCH
Historical Development of Federal Protections of Health Information in Research
The development of international codes, federal legislation, and federal regulation of human subjects often occurred in response to past abuses in biomedical experiments (reviewed by Pritts, 2008 ) ( Box 3-3 ). The most well-known examples included (1) reported abuses of concentration camp prisoners in Nazi experiments during World War II, and (2) the Tuskegee syphilis study begun in 1932, in which researchers withheld effective treatment from affected African American men long after a cure for syphilis was found. Most of the current principles and standards for conducting human subjects research were developed primarily to protect against the physical and mental harms that can result from these types of biomedical experiments. Therefore, they focus on the principles of autonomy and consent. Although the standards apply to research that uses identifiable health information, research based solely on information is not their primary focus.
The Basis for Human Subjects Protections in Biomedical Research. Nuremberg Code The Nuremberg Code, created by the international community after the Nazi War Crimes Trials, is generally seen as the first codification (more...)
In the United States, perhaps the most influential inquiry into the protection of human subjects in research was the Belmont Report. The Belmont principles have been elaborated on in many settings, and served as the basis for formal regulation of human subjects research in the United States. In general, states do not directly regulate the activity of most researchers ( Burris et al., 2003 ). However, the Belmont Commission’s recommendations were reflected in the Department of Health and Human Services’ (HHS’s) Policy for Protection of Human Subjects Research, Subpart A of 45 C.F.R. 46 (“Subpart A”) in 1979. 9 These protections were considered a benchmark policy for federal agencies, and in December 1981, the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research recommended 10 that all federal departments and agencies adopt the HHS regulations. 11
In 1982, the President’s Office of Science and Technology Policy appointed a Committee for the Protection of Human Research Subjects to respond to the recommendations of the President’s commission. The committee agreed that uniformity of federal regulations on human subjects protection is desirable to eliminate unnecessary regulations and to promote increased understanding by institutions that conduct federally supported or regulated research. As a result, in 1991, other federal departments and agencies joined HHS in adopting a uniform set of rules for the protection of human subjects of research, identical to Subpart A of 45 C.F.R. 46, which is now informally known as the “Common Rule.” Eighteen federal agencies have now adopted the Common Rule as their own respective regulations.
Overview of the Common Rule
The Common Rule governs most federally funded research conducted on human beings and aims to ensure that the rights of human subjects are protected during the course of a research project. The Common Rule stresses the importance of individual autonomy and consent; requires independent review of research by an Institutional Review Board (IRB); and seeks to minimize physical and mental harm. Privacy and confidentiality protections, although not defined in a detailed and prescriptive manner, are included as important components of risk in research.
The framework for achieving the goal of protecting human subjects is based on two foundational requirements: the informed consent of the research participant and the review of proposed research by an IRB. This section describes some of the basic parameters of the Common Rule (reviewed by Pritts, 2008 ). Particular provisions that interact with the HIPAA Privacy Rule are described in more detail in Chapter 4 .
Scope of the Common Rule
In general, the Common Rule applies only to research on human subjects that is supported by the federal government. 12 As noted previously, research is defined as “a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge.” 13
Under the Common Rule, a “human subject” is defined as “a living individual about whom an investigator … conducting research obtains (1) Data through intervention or interaction with the individual, or (2) Identifiable private information.” Private information is considered to be personally identifiable if the identity of the subject is or may readily be ascertained by the investigator or associated with the information.
The Common Rule applies to most human subjects research conducted using federal funds, but its influence is broader because most institutions that accept federal funds sign an agreement (a Federalwide Assurance or FWA) with HHS to abide by the Common Rule requirements in all research, regardless of funding source. Nonetheless, some privately funded human subjects research is conducted outside the purview of federal regulation ( Goldman and Choy, 2001 ; Williams, 2005 ). Companies and other organizations may voluntarily choose to apply the Common Rule to their research projects, and many do. However, research projects in which compliance is voluntary are not subject to oversight or disciplinary action by HHS ( Goldman and Choy, 2001 ; Williams, 2005 ).
Informed Consent 14
The Common Rule requires that a researcher obtain informed consent (usually in writing) from a person before he/she can be admitted to a study ( Williams, 2005 ). Informed consent is sought through a process in which a person learns key facts about a research study, including the potential risks and benefits, so that he/she can then agree voluntarily to take part or decide against it.
The Common Rule informed consent regulations focus primarily on the elements and documentation of informed consent rather than on the process used to obtain it. As to the process, the regulations require that informed consent be sought only under circumstances that provide the prospective subject with adequate opportunity to consider whether to participate. The Common Rule requires that information pertaining to informed consent be given in language understandable to the subject, and that the consent does not imply that the subject is giving up his/her legal rights or that the investigator is released from liability for negligence during the conduct of the study. 15
The Common Rule also specifies a number of elements that must be provided when informed consent is sought. These elements include:
- an explanation of the purposes of the research,
- the expected duration of the subject’s participation,
- the potential risks and benefits of the research,
- how confidentiality will be maintained,
- the fact that participation is strictly voluntary, and
- who the subject can contact to answer questions about the study or about his/her rights as a research participant.
In certain limited circumstances, the Common Rule allows an informed consent to be for unspecified future research. For example, under the Common Rule an informed consent can be used to obtain a person’s permission to study personally identifiable information maintained in a repository for future, unspecified research purposes ( HHS, 2003 ).
For the most part, the required elements of an informed consent address all types of research, although some are more relevant to biomedical research (e.g., the consent must include a disclosure of appropriate alternative procedures or courses of treatment, if any, that might be advantageous to the subject). One required element of informed consent is particularly relevant to research involving personally identifiable health information. The Common Rule requires an informed consent to include a statement describing the extent, if any, to which confidentiality of records identifying the subject will be maintained. 16
Institutional Review Boards
Adopting the principles of the Belmont Report, the Common Rule requires that protocols for human subjects research be reviewed by an IRB ( Box 3-4 ) before research may begin. 17 The IRB must meet certain membership requirements, including having members with different expertise and at least one member who is not affiliated with the investigator’s institution. The Common Rule specifies which level of IRB review is needed for various types of research and provides criteria for the IRB to consider during the review. Although the Common Rule does not specify the procedures an IRB must follow in its review of protocols, it does require the IRB to have written procedures for how it will review protocols and document IRB decisions.
Institutional Review Boards. According to the Department of Health and Human Services (HHS) Institutional Review Board (IRB) guidebook, “the IRB is an administrative body established to protect the rights and welfare of human research subjects (more...)
The Common Rule requires that an IRB determine the following factors are satisfied to approve proposed research:
- Risks to subjects are minimized;
- Risks to subjects are reasonable in relation to anticipated benefits, if any, to subjects, and the importance of the knowledge that may reasonably be expected to result;
- The selection of subjects is equitable;
- Informed consent will be sought in accordance with the rules and will be documented;
- When appropriate, the research plan makes adequate provision for monitoring the data collected to ensure the safety of subjects; and
- When appropriate, adequate provisions are in place to protect the privacy of subjects and to maintain the confidentiality of data. 18
An IRB may waive the requirement to obtain informed consent or approve an alteration of the consent form for some minimal risk research. The IRB may also waive the requirement for signed consent in certain circumstances. 19
Anonymized Data
As noted above, the Common Rule considers use of “private identifiable information” to be human subjects research. Data are considered personally identifiable if the identity of the subject is or may be readily ascertained by the investigator or associated with the information accessed by the researcher. 20 However, the Common Rule exempts from its requirements research that involves:
[T]he collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. 21
Otherwise identifiable data may be deidentified or “anonymized” for purposes of the Common Rule if it is coded and certain other conditions are met ( HHS, 2004 ). Under Guidance issued by the Office for Human Research Protection, information is “coded” if identifying information (such as name or Social Security number) that would enable the investigator to readily ascertain the identity of the individual to whom the private information or specimens pertain has been replaced with a number, letter, symbol, or combination thereof (the code), and a key to decipher the code exists, enabling linkage of the identifying information to the private information or specimen.
Research involving only coded private information or specimens is not considered to involve human subjects under the Common Rule if the following conditions are met:
- The private information or specimens were not collected specifically for the currently proposed research project through an interaction or intervention with living individuals; and
- —The key to decipher the code is destroyed before the research begins;
- —The investigators and the holder of the key enter into an agreement prohibiting the release of the key to the investigators under any circumstances, until the individuals are deceased;
- —IRB-approved written policies and operating procedures for a repository or data management center prohibit the release of the key to investigators under any circumstances, until the individuals are deceased; or
- —Other legal requirements prohibit the release of the key to the investigators, until the individuals are deceased.
Under this standard, when a researcher accesses or receives data that have been coded and does not have access to the identifying key, the research is not considered human subjects research and is not subject to the Common Rule’s requirements of informed consent or IRB review and approval of protocol.
Enforcement of the Common Rule
The Common Rule requirements for informed consent do not preempt any applicable federal, state, or local laws that require additional information to be disclosed to a subject in order for informed consent to be legally effective. 22
Federal funding can be suspended or withdrawn from an institution when it is found to be in material violation of the Common Rule. 23 There is no authority to impose penalties directly on individual researchers for violations. Neither does the Common Rule expressly provide a research participant with a private right of action. It should be noted, however, that recent cases indicate that courts may be willing to hold an institution liable under common law negligence theories where the approved informed consent form is determined to be less than adequate ( Shaul et al., 2005 ). 24
FDA Protection of Human Research Subjects
Some health research is also subject to FDA regulations. The FDA is charged by statute with ensuring the protection of the rights, safety, and welfare of human subjects who participate in clinical investigations 25 involving articles subject to the Federal Food, Drug, and Cosmetic Act 26 (the Act), as well as clinical investigations that support applications for research or marketing permits for products regulated by the FDA, including drugs, medical devices, and biological products for human use ( Box 3-5 ).
FDA Protection of Human Subjects Regulations. The Food and Drug Administration (FDA) Protection of Human Subjects Regulations aim to protect the rights of human subjects enrolled in research involving products that the FDA regulates (i.e., drugs, medical (more...)
In January 1981, the FDA adopted regulations governing informed consent of human subjects 27 and regulations establishing standards for the composition, operation, and responsibilities of IRBs that review clinical investigations involving human subjects. 28 At the same time, HHS adopted the Common Rule regulations on the protection of human research subjects. 29 The FDA’s regulations were harmonized with the Common Rule in 1991 to the extent permitted by statute. Key differences between FDA and HHS regulations include that the FDA does not allow for waiver or alteration of informed consent and requires that subjects be informed that the FDA may inspect their medical records. In addition, studies of efficacy based solely on medical records research are not permitted to support registration. Remaining differences in the rules are due to differences in the statutory scope or requirements ( Lee, 2000 ).
- DISTINGUISHING HEALTH RESEARCH FROM PRACTICE
The Common Rule and Privacy Rule make a somewhat artificial distinction between health research and some closely related health care practices, such as public health practice, quality improvement activities, program evaluations, 30 and utilization reviews, 31 all of which may involve collection and analysis of personally identifiable health information. However, determining which activities meet the definition of “research” is a major challenge for IRBs, Privacy Boards, 32 investigators, and health care practitioners because neither the regulations nor their interpretations by HHS provide clear guidance on how to distinguish research from activities that use similar techniques to analyze health information ( IOM, 2000a ).
It is important for IRBs and Privacy Boards to correctly distinguish among activities that are or are not subject to the various provisions of the Privacy Rule and the Common Rule. Only research requires formal IRB or Privacy Board review and informed consent. 33 Inappropriate classification of an activity as research can make it difficult or impossible for important health care activities, such as public health practice and quality improvement, to be undertaken. On the other hand, failure to correctly identify an activity as research could potentially allow improper disclosure of personally identifiable health information without sufficient oversight.
Thus, standard criteria are urgently needed for IRBs and Privacy Boards to use when making distinctions between health research and related activities, and the committee recommends that HHS consult with relevant stake holders to develop such standard criteria. HHS is aware of this need, and created a working document titled “What Is Research?” However, the work on this project apparently has been delayed for unknown reasons ( NCURA, 2007 ). 34 As described below, a number of other models have already been proposed to help determine whether activities should be classified as research in the fields of public health and quality improvement, and these could be instructive for developing HHS guidance. Any criteria adopted by HHS should be regularly evaluated to ensure that they are helpful and producing the desired outcomes.
The following sections describe some ongoing efforts to develop such criteria in the fields of public health and quality improvement. The intent of the committee is not to endorse these particular models, but rather to illustrate the challenges associated with making these distinctions and establishing standard criteria.
Public Health Practice Versus Public Health Research
The Belmont Report defined health practice as “interventions designed solely to enhance the well-being of the person, patient or client, and which have reasonable expectation of success” ( CDC, 1999 ). To apply this definition to “public” health practice, the targeted beneficiary of the intervention must be expanded to include benefit to the community, rather than just a particular person. Neither the Common Rule nor the Privacy Rule provides a specific definition for public health research; rather public health research is included in the general definition of research. However, the Privacy Rule regulates public health practice differently from public health research (see Chapter 4 ).
An early model for distinguishing public health research from public health practice focused on the intent for which the activity was designed, noting that the intent of public health research is to “contribute to or generate generalizable knowledge,” while the intent of public health practice is to “conduct programs to prevent disease and injury and improve the health of communities” ( Snider and Stroup, 1997 ). The Centers for Disease Control and Prevention developed a similar method with an expanded assessment of intent. For example, the model posits that in public health research, the intended benefits of the project extend beyond the study participants, and the data collected exceed the requirements for the care of the study participants. But for public health practice, the intended benefits of the project are primarily for the participants in the activity, or for the participants’ community, and the only data collected are those needed to assess or improve a public health program or service, or the health of the participants and their community. The model also assumes that public health practice is based on well-established medical interventions and is nonexperimental ( CDC, 1999 ). However, these models both have been criticized as too subjective and too dependent on the opinion of the person conducting the activity ( Gostin, 2008 ; Hodge, 2005 ).
A new, more comprehensive model incorporating much of the previous two was recently proposed as a more objective checklist to be used by IRBs, Privacy Boards, and interested parties ( Hodge, 2005 ; Hodge and Gostin, 2004 ). The foundations for this model are specific definitions of public health research: “the collection and analysis of identifiable health data by a public health authority for the purpose of generating knowledge that will benefit those beyond the participating community who bear the risks of participation,” and public health practice: “the collection and analysis of identifiable health data by a public health authority for the purpose of protecting the health of a particular community, where the benefits and risks are primarily designed to accrue to the participating community.”
The model is based on two primary assumptions. First, the actor performing the activity in question is a governmental public health official, agent, agency, or entity at the federal, tribal, state, or local level. Second, the activity in question involves the acquisition, use, or disclosure of personally identifiable health data. The model is then divided into two stages. Stage 1 is applied to all activities, and can be used to distinguish practice from research in the easiest cases. Stage 2 is only applied to those cases that are hard to distinguish, and where Stage 1 failed to lead to a definitive IRB/Privacy Board decision ( Box 3-6 ).
A Model for Distinguishing Public Health Practice from Research. Stage 1 Public health practice:
Quality Improvement Versus Health Research
Quality improvement has been defined as “systematic, data-guided activities designed to bring about immediate, positive change in the delivery of health care in a particular setting” ( Baily, 2008 ). Quality improvement activities do not require IRB or Privacy Board approval under the Common Rule or the Privacy Rule, which classify quality improvement as a component of health care operations. 35
However, in many cases, it is difficult for health care providers, IRBs, and Privacy Boards to determine whether a particular activity is purely for quality improvement, or whether it also entails research. One survey 36 exploring opinions in the health care community about the need for IRBs to review various quality-related activities found that physicians conducting quality improvement were less likely than IRB chairs to believe that IRB review was required for a given hypothetical activity, or that informed consent was necessary ( Lindenauer et al., 2002 ). Recently, a highly publicized case has again brought the issue to the forefront for all the stakeholders ( Box 3-7 ).
A Case Study of Quality Improvement and Research. Peter Pronovost of Johns Hopkins University (JHU) led a quality improvement effort at 103 intensive care units (ICUs) in Michigan hospitals to reduce the number of catheter-related bloodstream infections. (more...)
Some members of the health care community have proposed requiring that all prospective quality improvement activities go through external review ( Bellin and Dubler, 2001 ), while others have outlined specific criteria to differentiate quality improvement activities from research.
For example, Casarett and colleagues developed a two-part test to identify quality improvement activities. The first test is whether the majority of patients are expected to benefit directly from “the knowledge to be gained” from the initiative. This means that the patients must actually benefit from the knowledge learned during the evaluation, not just from being a recipient of the protocol itself. If the patients are generally expected to directly benefit from the knowledge gained during the activity, then the activity is quality improvement. If not, the activity is research. The second test is whether the participants would be subjected to additional risks or burdens, including the risk of privacy breach, beyond the usual clinical practice in order to make the results of the initiative generalizable. If yes, then the initiative should be reviewed as research ( Casarett et al., 2000 ).
More recently, the Hastings Center published a report exploring the similarities and differences between research and quality improvement. The report emphasized three fundamental characteristics of quality improvement and three fundamental characteristics of research. The authors argue that individuals have a responsibility to participate in the quality improvement activities because all patients have an interest in receiving high-quality medical care, and the success of a quality improvement activity depends on the cooperation of all patients. In addition, the report notes that quality improvement activities are a low risk to the patient, so there is little justification for not participating. The report also assumes that quality improvement activities are based on existing knowledge about human health and should lead to immediate local improvements in the provision of medical care.
In contrast, the report notes that participation in research should be voluntary, and decisions to participate should be based on researchers’ full disclosure of all the potential risks and benefits. In addition, the authors assert that research is designed to create new knowledge about human health, rather than relying solely on existing knowledge, and that most research does not result in any direct benefit to the institution where the research is being conducted.
The authors concluded that IRBs are not the appropriate body for the ethical oversight of quality improvement activities. They argue that IRBs unnecessarily impose high transaction costs on these activities because of the difference in the way they are conducted compared to research. For example, in research, any changes in methodology require further IRB approval. In contrast, quality improvement activities involve frequent adjustments in the intervention, measurement, and goals of the activity based on the experience of the investigators. Requiring the investigator to revisit an IRB every time a small adjustment is needed in such an activity significantly increases the amount of time and effort required to conduct the initiative and to produce meaningful data. Also, the investigators involved in quality improvement activities ordinarily are already involved in the clinical care of participants and bear responsibility for the quality and safety of an intervention. Thus, the authors argue that there is no need for the additional oversight by an IRB to protect participant safety.
Rather, the report recommended integrating the ethical oversight of quality improvement activities into the ongoing management of an institution’s health care delivery system, suggesting that oversight of quality improvement could be left with the managers of clinical care organizations, and that consent to receive treatment should include consent to participate in any quality improvement project that is minimal risk. However, the report stated that if a project has the characteristics of both quality improvement and research, the project should be reviewed as both human subjects research and quality improvement ( Baily et al., 2006 ; Lynn et al., 2007 ).
In response to the ongoing confusion over when quality improvement rises to the level of research and requires IRB review, the IOM jointly hosted a meeting with the American Board of Internal Medicine in May 2008 to discuss this issue. Key members of the quality improvement community attended, and short- and long-term solutions to this problem were proposed. However, no written report from this meeting was produced and no general consensus was reached.
- THE IMPORTANCE OF EFFECTIVE COMMUNICATION WITH THE PUBLIC
As noted previously in this chapter, surveys indicate that the vast majority of Americans believe that health research is important and are interested in the findings of research studies. The majority of patients also appear to be willing to participate in health research, either by volunteering for a study to test a medical intervention or by allowing access to their medical records or stored biospecimens, under certain conditions. Their willingness to participate depends on trust in researchers to safeguard the rights and well-being of patients, including assurance of privacy and confidentiality, and the belief that it is a worthwhile endeavor that warrants their involvement. Yet patients often lack information about how research is conducted, and are rarely informed about research results that may have a direct impact on their health. The committee’s recommendations in this section are intended to address both the public’s desire for more information about health research and to help fulfill two of the committees overarching goals of the report: (1) improving the privacy and security of health information, and (2) improving the effectiveness of health research.
Disseminating Health Research Results
Ethicists have long suggested greater community involvement in health research studies, including more communication about research results (reviewed by Shalowitz and Miller, 2008a , b ). In addition, the IOM committee identified transparency—the responsibility to disclose clearly how and why personally identifiable information is being collected—as an important component of comprehensive privacy protections. A previous IOM report also recommended improved communication with the public and research participants to ensure that the protection process is open and accessible to all interested parties ( IOM, 2002 ). Effective communication would build the public’s trust of the research community and is consistent with the principles of fair information practices.
When patients consent to the use of their medical records in a particular study, health researchers should make greater efforts at the conclusion of the study to inform study participants about the results, and the relevance and importance of those results. Learning about clinically relevant findings from a study in which a patient has participated could make patients feel more integrated into the process and could encourage more to participate in future studies. A recent United Kingdom report on the use of personal data in health research concluded that public involvement in research is necessary for the success of information-based research, and that a public informed about the value of research is likely to have greater enthusiasm and confidence in research and the research community ( AMS, 2006 ). Moreover, direct feedback with study participants could lead to improved health care for the individuals if the results indicate that an altered course of care is warranted.
Nonetheless, there are multiple impediments, beyond cost, to providing meaningful feedback to participants. A summary of the results alone, while necessary and reasonable, can be seen as a token, and also raises questions about issues such as how best to write summaries, the stage at which results should be disseminated, and how to present research with uninformative outcomes. For example, one recent study found that sharing results directly with study participants was met with overwhelmingly favorable reactions from patients, but the study also revealed some obstacles ( Partridge et al., 2008 ). In a survey of women who had participated in a randomized trial of breast cancer therapy and had received a summary of the study results by mail, 95 percent reported that they were glad they received the results. Most respondents interpreted the results correctly, although incorrect interpretation of the results was associated with increased anxiety, as was dissatisfaction with treatment.
Although some guidelines for providing and explaining study results to research participants have been proposed, they differ in details because limited data are available on this subject, and thus standards are lacking ( Partridge and Winer, 2002 ; Partridge et al., 2008 ; Shalowitz and Miller, 2008b ; Zarin and Tse, 2008 ). Because transparency is best achieved by providing graded levels of information and guidance to interested parties ( IOM, 2002 ), it will be important to develop effective and efficient ways to communicate with various sectors of the population. A commitment to the principles of “plain language” 37 will be important. Broader adoption of electronic medical records may also be helpful in accomplishing this goal.
Research Registries
One way to make information about research studies more broadly available to the public is through registration of trials and other studies in public databases. HHS should encourage such registration of trials and other studies, particularly when research is conducted with an IRB/Privacy Board approved waiver of consent or authorization (see Chapter 4 ). Numerous clinical trial registries already exist, and registration has increased in recent years (reviewed by Zarin and Tse, 2008 ). In 2000, the National Library of Medicine established a clinical trials registry ( ClinicalTrials.gov ), which has expanded to include information from several other trial registries and to serve as the FDA-required site for submissions about clinical trials subject to the FDA databank requirement. The FDA Amendments Act of 2007 38 expanded the scope of required registrations at ClinicalTrials.gov and provided the first federally funded trials results database. It mandates registrations of controlled clinical investigations, except for Phase I trials, of drugs, biologics, and devices subject to FDA regulation.
A policy of the International Committee of Medical Journal Editors (ICMJE), adopted in fall 2005, also requires prospective trial registration as a precondition for publication ( DeAngelis et al., 2004 ). This policy led to a 73 percent increase in trial registrations of all intervention types from around the world ( Zarin et al., 2005 ). Nearly 45,000 trials had been registered by fall 2007.
However, although the development of such registries is an important first step toward providing high-quality clinical trial information to the public, no centralized system currently exists to disseminate information about clinical trials of drugs or other interventions, making it difficult for consumers and their health care providers to identify ongoing studies. The current statutory requirements for registration and data reporting in the United States are not as broad as the transnational policies of the ICMJE or the World Health Organization, which call for the registration of all interventional studies in human beings regardless of intervention type ( Laine et al., 2007 ; Sim et al., 2006 ). Moreover, noninterventional studies, such as observational studies that play an increasingly critical role in biomedical research, are not generally included in these databases. Because many noninterventional studies are conducted with an IRB/Privacy Board approved waiver of consent or authorization, including those studies in a registry could be an important method for increasing public knowledge of such studies.
Informing the Public About the Methods and Value of Research
As noted previously, clinical trials are the most visible of the various types of health research, but a great deal of information-based health research entails analysis of thousands of patient records to better understand human diseases, to determine treatment effectiveness, and to identify adverse side effects of therapies. This form of research is likely to increase in frequency as the availability of electronic records continues to expand. As we move toward the goal of personalized medicine, research results will be even more likely to be directly relevant to patients, but more study subjects will be necessary to derive meaningful results.
However, many patients probably are not aware that their medical records are being used in information-based research. For example, the recent study that used focus groups to examine the views of veterans toward the use of medical records in research found that the majority of participants (75 percent) were not aware that “under some circumstances, [their] medical records could be used in some research studies without [their] permission,” despite the fact that a notice of privacy practices, which included a statement that such research could occur, had been mailed to all participants less than a year prior to the study ( Damschroder et al., 2007 ).
Moreover, surveys show that many patients desire not only notice, but also the opportunity to decide whether to consent to such research with medical records. Those surveys further indicate that patients who wish to be asked for consent for each study are most concerned about the potentially detrimental affects of inappropriate disclosure of their personally identifiable health information, including discrimination in obtaining health or life insurance or employment.
As noted in Chapter 2 , strengthening security protections of health data should reduce the risk of security breaches and their potential negative consequences, and thus should help to alleviate patient concerns in this regard. But educating patients about how health research is conducted, monitored, and reported on could also help to ease patient concerns about privacy and increase patients’ trust in the research community, which as noted above is important for the public’s continued participation in health research. For example, datasets are most often provided to researchers without direct identifiers such as name and Social Security number. Furthermore, identifiers are not included in publications about research results. Also, under both the Privacy Rule and the Common Rule, a waiver of consent and authorization is possible only under the supervision of an IRB or Privacy Board, and a waiver is granted only when the research entails minimal risk and when obtaining individual consent and authorization is impracticable (see the previous section and also Chapter 4 ). Finally, professional ethics dictate that researchers safeguard data and respect privacy.
Conveying the value of medical records research to patients will be important. Surveys show that people are more supportive of research that is relevant to them and their loved ones. At the same time, educational efforts should stress the negative impact of incomplete datasets on research findings. Representative samples are essential to ensure the validity and generalizability of health research ( Box 3-8 ), but datasets will not represent the entire population if some people withhold access to their health information.
Selection Bias in Health Research. When researchers are required to obtain consent or authorization to access each individual’s medical record for a research study, it is likely that individuals’ willingness to grant access will not be (more...)
In addition, an educated public could also decrease the potential for biased research samples. A universal requirement for consent or authorization in medical records research leads to incomplete datasets, and thus to biased results and inaccurate conclusions. Some large medical institutions with a strong research history and reputation (e.g., Mayo Clinic) can obtain authorization and consent rates as high as 80 percent, but the 20 percent who refuse have distinct demographic and health characteristics. In fact, even a refusal rate of less than 5 percent can create selection bias in the data ( Jacobsen et al., 1999 ; see Chapter 5 for more detail). Conveying to the public the importance of health care improvements derived from medical records research and stressing the negative impact of incomplete datasets on research findings may increase the public’s participation in research and their willingness to support information-based research that is conducted with IRB or Privacy Board oversight, under a waiver of patient consent or authorization.
Numerous examples of important research findings from medical records research would not have been possible if direct patient consent and authorization were always required ( Box 3-1 ). For example, analysis of medical records showed that infants exposed to diethylstilbesterol (DES) during the first trimester of pregnancy had an increased risk of breast, vaginal, and cervical cancer as well as reproductive anomalies as adults. Similarly, studies of medical records led to the discovery that folic acid supplementation during pregnancy can prevent neural tube defects.
Thus, HHS and the health research community should work to edu cate the public about how research is done and the value it provides. All stakeholders, including professional organizations, nonprofit funders, and patient organizations, have different interests and responsibilities to make sure that their constituencies are well informed. For example, the American Society of Clinical Oncology and the American Heart Association already have some online resources to help patients gather information about research that may be relevant to their conditions. But coordination and identification of best practices by HHS would be helpful, and research is needed to identify which segments of the population would be receptive to and benefit from various types of information about how research is done and its value in order to create and implement an effective plan.
Greater use of community-based participatory research, in which community-based organizations or groups bring community members into the research process as partners to help design studies and disseminate the knowledge gained, 39 could help achieve this goal. These groups help researchers to recruit research participants by using the knowledge of the community to understand health problems and to design activities that the community is likely to value. They also inform community members about how the research is done and what comes out of it, with the goal of providing immediate community benefits from the results when possible.
- CONCLUSIONS AND RECOMMENDATIONS
Based on its review of the information described in this chapter, the committee agreed on a second overarching principle to guide the formation of recommendations. The committee affirms the importance of maintaining and improving health research effectiveness. Research discoveries are central to achieving the goal of extending the quality of healthy lives. Research into causes of disease, methods for prevention, techniques for diagnosis, and new approaches to treatment has increased life expectancy, reduced infant mortality, limited the toll of infectious diseases, and improved outcomes for patients with heart disease, cancer, diabetes, and other chronic diseases. Patient-oriented clinical research that tests new ideas makes rapid medical progress possible. Today, the rate of discovery is accelerating, and we are at the precipice of a remarkable period of investigative promise made possible by new knowledge about the genetic underpinnings of disease. Genomic research is opening new possibilities for preventing illness and for developing safer, more effective medical care that may eventually be tailored for specific individuals. Further advances in relating genetic information to predispositions to disease and responses to treatments will require the use of large amounts of existing health-related information and stored tissue specimens. The increasing use of electronic medical records will further facilitate the generation of new knowledge through research and accelerate the pace of discovery. These efforts will require broad participation of patients in research and broad data sharing to ensure that the results are valid and applicable to different segments of the population. Collaborative partnerships among communities of patients, their physicians, and teams of researchers to gain new scientific knowledge will bring tangible benefits for people in this country and around the world.
Surveys indicate that the majority of Americans believe that health research is important, are interested in the findings of research studies, and are willing to participate in health research. But patients often lack information about how research is conducted and are rarely informed about research results that may have a direct impact on their health. Effective communication could build the public’s trust of the research community, which is important because trust is necessary for the public’s continued participation in research. Moreover, direct feedback could lead to improved health care for study participants if the results indicate that an altered course of care is warranted.
Thus, the committee recommends that when patients consent to the use of their medical records in a particular study, health researchers should make greater efforts when the study ends to inform study participants about the results, and the relevance and importance of those results. Broader adoption of electronic health records may be helpful in accomplishing this goal, but standards and guidelines for providing and explaining study results to research participants or various sectors of the public are needed.
HHS should also encourage registration of trials and other studies in public databases, particularly when research is conducted with an IRB/Privacy Board approved waiver of consent or authorization, as a way to make information about research studies more broadly available to the public. Numerous clinical trial registries already exist, and registration has increased in recent years, but no centralized system currently exists for disseminating information about clinical trials of drugs or other interventions, making it difficult for consumers and their health care providers to identify ongoing studies. Moreover, noninterventional studies, such as observational studies that play an increasingly critical role in biomedical research, are not generally included in these databases. Because many noninterventional studies are conducted with an IRB/Privacy Board approved waiver of consent or authorization, including such studies in a registry could be an important method for increasing public knowledge of those studies.
Interventional clinical trials are the most visible of the various types of health research, but a great deal of information-based health research entails analysis of thousands of patient records to better understand human diseases, to determine treatment effectiveness, and to identify adverse side effects of therapies. This form of research is likely to increase in frequency as the availability of electronic health records continues to expand. As we move toward the goal of personalized medicine, research results will be even more likely to be directly relevant to patients, but more study participants will be necessary to derive meaningful results.
However, many patients are likely not aware that their medical records are being used in information-based research, and surveys show that many patients desire not only notice, but also the opportunity to decide about whether to consent to such research with medical records. As noted in Chapter 2 , strengthening security protections of health data should reduce the risk of security breaches and their potential negative consequences, and thus should help to alleviate patient concerns in this regard. But educating patients about how health research is conducted, monitored, and reported could also increase patients’ trust in the research community. Thus, HHS and the health research community should work to educate the public about how research is done.
It will also be important for HHS and researchers to convey the value of health care improvements derived from medical records research, and to stress the negative impact of incomplete datasets on research findings. Representative samples are essential to ensure the validity and generalizability of health research, but datasets will not be representative of the entire population if some people withhold access to their health information. A universal requirement for consent or authorization in information-based research may lead to incomplete datasets, and thus to biased results and inaccurate conclusions. Numerous examples of important research findings from medical records research would not have been possible if direct patient consent and authorization were always required.
To ensure that beneficial health research and related activities continue to be undertaken with appropriate oversight under federal regulations, it will be important for HHS to also provide more guidance on how to distinguish the various activities. The Privacy Rule makes a distinction between health research and some closely related endeavors, such as public health and quality improvement activities, which also may involve collection and analysis of personally identifiable health information. Under the Privacy Rule (as well as the Common Rule), these activities, which aim to protect the public’s health and improve the quality of patient care, are considered health care “practice” rather than health research. Therefore, they can be undertaken without consent or authorization, or an IRB/Privacy Board waiver of consent or authorization. However, it can be a challenge for IRBs and Privacy Boards to distinguish among activities that are or are not subject to the various provisions of the Privacy Rule and the Common Rule, and inappropriate decisions may prevent important activities from being undertaken or could potentially allow improper disclosure of personally identifiable health information.
To address these difficulties, a number of models have been proposed that outline the criteria IRBs and Privacy Boards should use to distinguish practice and research. For example, one recent model provides a detailed checklist for IRBs and Privacy Boards to use in determining whether an activity is public health research and required to comply with the research provisions of the Privacy Rule, or public health practice that does not need IRB/Privacy Board review. The committee believes that standardizing the criteria is essential to support the conduct of these important health care activities.
Thus, HHS should convene the relevant stakeholders to develop standard criteria for IRBs and Privacy Boards to use when making decisions about whether protocols entail research or practice. There should be flexibility in the regulation to allow important activities to go forward with appropriate levels of oversight. Also, it will be important to evaluate whether these criteria are effective in aiding IRB/Privacy Board reviews of proposed protocols, and whether they lead to appropriate IRB/Privacy Board decisions.
These changes suggested above could be accomplished without any changes to HIPAA by making them a condition of funding from HHS and other research sponsors and by providing some additional funds to cover the cost.
- AMS (Academy of Medical Sciences). Personal data for public good: Using health information in medical research. 2006. [accessed August 28, 2008]. http://www .acmedsci.ac .uk/images/project/Personal.pdf .
- Baily MA. Harming through protection? New England Journal of Medicine. 2008; 258 (8):768–769. [ PubMed : 18287599 ]
- Baily MA, Bottrell M, Lynn J, Jennings B. The ethics of using QI methods to improve health care quality and safety. A Hastings Center Special Report. 2006; 36 (4):S1–S40. [ PubMed : 16898359 ]
- Bates DW, Leape LL, Cullen DJ, Laird N, Petersen LA, Teich JM, Burdick E, Hickey M, Kleefield S, Shea B, Vander VM, Seger DL. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998; 280 (15):1311–1316. [ PubMed : 9794308 ]
- Bellin E, Dubler NN. The quality improvement-research divide and the need for external oversight. American Journal of Public Health. 2001; 91 (9):1512–1517. [ PMC free article : PMC1446813 ] [ PubMed : 11527790 ]
- Big Health. Big health consortium. 2008. [accessed October 29, 2008]. http://www .bighealthconsortium .org/about/
- Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine. 2008; 87 (1):1–9. [ PubMed : 18204365 ]
- Brown DR, Fouad MN, Basen-Engquist K, Tortolero-Luna G. Recruitment and retention of minority women in cancer screening, prevention and treatment trials. Annals of Epidemiology. 2000; 10 :S13–S21. [ PubMed : 11189088 ]
- Burris S, Gable L, Stone L, Lazzarini Z. The role of state law in protecting human subjects of public health research and practice. Journal of Law, Medicine & Ethics. 2003; 31 :654. [ PubMed : 14968667 ]
- Casarett D, Karlawish J, Sugarman J. Determining when quality improvement initiatives should be considered research: Proposed criteria and potential implications. JAMA. 2000; 284 (7):2275–2280. [ PubMed : 10807388 ]
- Casarett D, Karlawish J, Andrews E, Caplan A. Bioethical issues in pharmacoepidemiological research. In: Strom BL, editor. Pharmacoepidemiology. West Sussex, England: John Wiley & Sons, Ltd.; 2005. pp. 417–432.
- CDC (Centers for Disease Control and Prevention). Guidelines for defining public health research and public health non-research. 1999. [accessed March 4, 2008]. http://www .cdc.gov/od /science/regs/hrpp/researchdefinition .htm .
- CHSR (Coalition for Health Services Research). Framework for health services research policy for 2008. 2008. [accessed August 21, 2008]. http://www .chsr.org/Policy_Priorities .pdf .
- Comis RL, Aldige CR, Stovall EL, Krebs LU, Risher PJ, Taylor HJ. A quantitative survey of public attitudes towards cancer clinical trials. Philadelphia, PA: Coalition of National Cancer Cooperative Groups, Cancer Research Foundation of America, Cancer Leadership Council, and Oncology Nursing Society; 2000.
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans towards participation in medical research. Journal of General Internal Medicine. 1999; 14 :537–546. [ PMC free article : PMC1496744 ] [ PubMed : 10491242 ]
- Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: Patients’ willingness to allow researchers to access their medical records. Social Science & Medicine. 2007; 64 (1):223–235. [ PubMed : 17045717 ]
- DeAngelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, Kotzin S, Laine C, Marusic A, Overbeke AJPM, Schroeder TV, Sox HC, Van Der Weyden MB. Clinical trial registration: A statement from the International Committee of Medical Journal Editors. JAMA. 2004; 292 (11):1363–1364. [ PubMed : 15355936 ]
- Farmer D, Jackson SA, Camacho F, Hall MA. Attitudes of African American and low socioeconomic status white women toward medical research. Journal of Health Care for the Poor and Underserved. 2007; 18 :85–99. [ PubMed : 17337800 ]
- FDA (Food and Drug Administration). Certain estrogens for oral or parenteral use. Drugs for human use; drug efficacy study implementation. Federal Register. 1971; 36 (217):21537–21538.
- FDA. FDA public health advisory, deaths with antipsychotics in elderly patients with behavioral disturbances. 2005. [accessed August 18, 2008]. http://www .fda.gov/cder /drug/advisory/antipsychotics.htm .
- FDA. FDA alert [6/16/2008]: Information for healthcare professionals. Antipsychotics. 2008. [accessed August 18, 2008]. http://www .fda.gov/cder /drug/infosheets/hcp /antipsychotics_conventional.htm .
- Finkelstein EA, Fiebelkorn IC, Wang G. National medical spending attributable to overweight and obesity: How much, and who’s paying? Health Affairs Web Exclusive. 2003. [accessed August 21, 2008]. http://content .healthaffairs .org/cgi/content /abstract/hlthaff.w3.219v1 . [ PubMed : 14527256 ]
- Furrow BR, Greaney TL, Johnson SH, Jost TS, Schwartz RL. Bioethics: Health care law and ethics. St. Paul, MN: Thomson/West; 2004.
- GAO (Government Accountability Office). Scientific research: Continued vigilance critical to protecting human subjects. Washington, DC: GAO; 1996.
- Genetics & Public Policy Center. U.S. public opinion on uses of genetic information and genetic discrimination. 2007. [accessed August 21, 2008]. http://www .dnapolicy .org/resources/GINAPublic _Opinion_Genetic _Information_Discrimination.pdf .
- Gill S, Bronskill S, Normand S, Anderson G, Sykora K, Lam K, Bell C, Lee P, Fischer H, Herrmann N, Gurwitz J, Rochon P. Antipsychotic drug use and mortality in older adults with dementia. Annals of Internal Medicine. 2007; 146 :775–786. [ PubMed : 17548409 ]
- Giuliano AR, Mokuau N, Hughes C, Tortelero-Luna G, Risendal B, Ho RCS, Prewitt TE, McCaskill-Stevens WJ. Participation of minorities in cancer research: The influence of structural, cultural, and linguistic factors. Annals of Epidemiology. 2000; 10 :S22–S34. [ PubMed : 11189089 ]
- Goldman J, Choy A. Ethical and policy issues in research involving human participants. Bethesda, MD: National Bioethics Advisory Commission; 2001. Privacy and confidentiality in health research; pp. C1–C34.
- Gostin LO. Public health law: Power, duty, restraint. Berkeley, CA: University of California Press; 2008. Surveillance and public health research: Personal privacy and the “right to know.”
- Grady C, Hampson LA, Wallen GR, Rivera-Goba MV, Carrington KL, Mittleman BB. Exploring the ethics of clinical research in an urban community. American Journal of Public Health. 2006; 96 (11):1996–2001. [ PMC free article : PMC1751807 ] [ PubMed : 17018826 ]
- Hatfield M, Sonnenschein HF, Rosenberg LE. Exceptional returns: The economic value of America’s investment in medical research. 2001. [accessed August 21, 2008]. http://www .laskerfoundation .org/advocacy/pdf/exceptional.pdf .
- Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. New England Journal of Medicine. 1971; 284 (15):878–881. [ PubMed : 5549830 ]
- HEW (Department of Health, Education and Welfare). The Belmont Report: Ethical principles and guidelines for the protection of human subjects of research. 1979. [accessed August 21, 2008]. http://ohsr .od.nih.gov /guidelines/belmont.html . [ PubMed : 25951677 ]
- HHS (Department of Health and Human Services). Institutional review boards and the HIPAA Privacy Rule. 2003. [accessed August 21, 2008]. http: //privacyruleandresearch .nih.gov/pdf/IRB_Factsheet.pdf .
- HHS. Guidance on research involving coded private information or biological specimens. 2004. [accessed August 21, 2008]. http://www .hhs.gov/ohrp /humansubjects/guidance/cdebiol.pdf .
- Hodge JG Jr. An enhanced approach to distinguishing public health practice and human subjects research. Journal of Law, Medicine & Ethics. 2005; 33 (1):125–141. [ PubMed : 15934670 ]
- Hodge JG, Gostin LO. Public health practice vs. research: A report for public health practitioners including cases and guidance for making distinctions. Atlanta, GA: Council of State and Territorial Epidemiologists; 2004.
- IOM (Institute of Medicine). Health services research: Work force and educational issues. Washington, DC: National Academy Press; 1995.
- IOM. Protecting data privacy in health services research. Washington, DC: National Academy Press; 2000a. [ PubMed : 25057723 ]
- IOM. To err is human: Building a safer health system. Washington, DC: National Academy Press; 2000b. [ PubMed : 25077248 ]
- IOM. Responsible research: A systems approach to protecting research participants. Washington, DC: The National Academies Press; 2002. [ PubMed : 20669487 ]
- Jacobsen S, Xia Z, Campion M, Darby C, Plevak M, Seltman K, Melton L. Potential effect of authorization bias on medical record research. Mayo Clinic Proceedings. 1999; 74 :330–338. [ PubMed : 10221460 ]
- Laine C, Horton R, DeAngelis CD, Drazen JM, Frizelle FA, Godlee F, Haug C, Hébert PC, Kotzin S, Marusic A, Sahni P, Schroeder TV, Sox HC, Van Der Weyden MB, Verheugt FWA. Clinical trial registration—looking back and moving ahead. New England Journal of Medicine. 2007; 356 (26):2734–2736. [ PubMed : 17548427 ]
- Lee B. Comparison of FDA and HHS human subject protection regulations. 2000. [accessed August 21, 2008]. http://www .fda.gov/oc/gcp/comparison .html .
- Lindenauer PK, Benjamin EM, Naglieri-Prescod D, Fitzgerald J, Pekow P. The role of the institutional review board in quality improvement: A survey of quality officers, institutional review board chairs, and journal editors. The American Journal of Medicine. 2002; 113 :575–579. [ PubMed : 12459404 ]
- Lowrance WW. Learning from experience, privacy and the secondary use of data in health research. London: The Nuffield Trust; 2002.
- Lowrance WW, Collins FS. Identifiability in genomic research. Science. 2007; 317 :600–602. [ PubMed : 17673640 ]
- Lynn J, Baily MA, Bottrell M, Jennings B, Levine RJ, Davidoff F, Casarett D, Corrigan J, Fox E, Wynia MK, Agich GJ, Speroff T, Schyve P, Batalden P, Tunis S, Berlinger N, Cronenwett L, Fitzmaurice M, Dubler NN, James B. The ethics of using quality improvement methods in health care. Annals of Internal Medicine. 2007; 146 (6):666–674. [ PubMed : 17438310 ]
- Mandelblatt J, Saha S, Teutsch S, Hoerger T, Siu AL, Atkins D, Klein J, Helfand M. The cost-effectiveness of screening mammography beyond age 65: A systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2003; 139 :835–842. [ PubMed : 14623621 ]
- Mick S, Morlock LL, Salkever D, de Lissovoy G, Malitz F, Wise CG, Jones A. Strategic activity and financial performance of U.S. rural hospitals: A national study, 1983 to 1988. Journal of Rural Health. 1994; 10 (3):150–167. [ PubMed : 10138031 ]
- Mosconi P, Poli P, Giolo A, Apolone G. How Italian health consumers feel about clinical research: A questionnaire survey. European Journal of Public Health. 2005; 15 (4):372–379. [ PubMed : 16014662 ]
- Murphy K, Topel R. The economic value of medical research. Chicago, IL: University of Chicago Press; 1999.
- NCI (National Cancer Institute). Getting connected with caBIG: Data sharing and security framework. 2008. [accessed October 27, 2008]. https://cabig .nci.nih .gov/working_groups /DSIC_SLWG/data_sharing_policy/
- NCURA (National Council of University Research Administrators). Report on research compliance. 2007. [accessed March 4, 2008]. http://www .reportonresearchcompliance .com .
- NCVHS (National Committee on Vital and Health Statistics). Enhanced protections for uses of health data: A stewardship framework for “secondary uses” of electronically collected and transmitted health data. 2007a. [accessed December 19, 2007]. http://ncvhs .hhs.gov/071221lt.pdf .
- NCVHS, Ad Hoc Work Group on Secondary Uses of Health Data. Testimony of the cancer Biomedical Informatics Grid (caBIG) Data Sharing and Intellectual Capital (DSIC) workspace. 2007b August 1, 2007
- Needleman J, Buerhaus P, Mattke S, Stewart M, Zelevinsky K. Nurse-staffing levels and the quality of care in hospitals. New England Journal of Medicine. 2002; 346 (22):1715–1722. [ PubMed : 12037152 ]
- NSF (National Science Foundation). Science and Engineering Indicators 2006. Arlington, VA: National Science Foundation; 2006. Science and technology: Public attitudes and understanding. Chapter 7.
- OHRP (Office for Human Research Protections). IRB guidebook, part I.A. 2008a. [accessed August 21, 2008]. http://www .hhs.gov/ohrp /irb/irb_guidebook.htm .
- OHRP. OHRP concludes case regarding Johns Hopkins University research on hospital infections. 2008b. [accessed August 21, 2008]. http://www .hhs.gov/ohrp/news/recentnews .html#20080215 .
- Partridge A, Winer E. Informing clinical trial participants about study results. JAMA. 2002; 288 :363–365. [ PubMed : 12117402 ]
- Partridge AH, Wolff AC, Marcom PK, Kaufman PA, Zhang L, Gelman R, Moore C, Lake D, Fleming GF, Rugo HS, Atkins J, Sampson E, Collyar D, Winer EP. The impact of sharing results of a randomized breast cancer clinical trial with study participants. Breast Cancer Research and Treatment. 2008 June 10 [ PubMed : 18543100 ]
- Pitkin R. Folate and neural tube defects. American Journal of Clinical Nutrition. 2007; 85 (1):285S–288S. [ PubMed : 17209211 ]
- Pritts J. The importance and value of protecting the privacy of health information: Roles of HIPAA Privacy Rule and the Common Rule in health research. 2008. [accessed March 15, 2008]. http://www .iom.edu/CMS/3740/43729/53160 .aspx .
- Research!America. America speaks: Poll summary. Vol. 7. Alexandria, VA: United Health Foundation; 2007.
- Schneeweiss S, Setoguchi S, Brookhart A, Dormuth C, Wang P. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. Canadian Medical Association Journal. 2007; 176 :672–632. [ PMC free article : PMC1800321 ] [ PubMed : 17325327 ]
- Shalowitz DI, Miller FG. Communicating the results of clinical research to participants: Attitudes, practices, and future directions. PLoS Medicine. 2008a May 13; 5 (5):e91. [ PMC free article : PMC2375946 ] [ PubMed : 18479180 ]
- Shalowitz DI, Miller FG. The search for clarity in communicating research results to study participants. Journal of Medical Ethics. 2008b September; 34 (9):e17. [ PubMed : 18757617 ]
- Shaul RZ, Birenbaum S, Evans M. Legal liability in research: Early lessons from North America. BMC Medical Ethics. 2005; 6 (4):1–4. [ PMC free article : PMC1182131 ] [ PubMed : 15953387 ]
- Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Annals of Epidemiology. 2002; 12 :248–256. [ PubMed : 11988413 ]
- Sim I, Chan A-W, Gülmezoglu AM, Evans T, Pang T. Clinical trial registration: Transparency is the watchword. The Lancet. 2006; 367 (9523):1631–1633. [ PubMed : 16714166 ]
- Slamon D, Clark G, Wong S, Levin W, Ullrich A, McGuire W. Human breast cancer: Correlation of relapse and survival with amplification of the her-2/neu oncogene. Science. 1987; 235 (4785):177–182. [ PubMed : 3798106 ]
- Snider DE, Stroup DF. Defining research when it comes to public health. Public Health Reports. 1997; 112 :29–32. [ PMC free article : PMC1381834 ] [ PubMed : 9018284 ]
- Thorpe KE, Florence CS, Howard DH, Joski P. The impact of obesity in rising medical spending. Health Affairs Web Exclusive. 2004. [accessed August 21, 2008]. http://content .healthaffairs .org/cgi/content /abstract/hlthaff.w4.480v1 . [ PubMed : 15496437 ]
- Trauth JM, Musa D, Siminoff L, Jewell IK, Ricci E. Public attitudes regarding willingness to participate in medical research studies. Journal of Health & Social Policy. 2000; 12 (2):23–43. [ PubMed : 11184441 ]
- Veurink M, Koster M, Berg L. The history of DES, lessons to be learned. Pharmacy World & Science. 2005; 27 (3):139–143. [ PubMed : 16096877 ]
- Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, Pratt LA, Brawley OW, Gross CP, Emanuel E. Are racial and ethnic minorities less willing to participate in health research? PLoS Medicine. 2006; 3 (2):201–210. [ PMC free article : PMC1298944 ] [ PubMed : 16318411 ]
- Westin A. How the public views privacy and health research. 2007. [accessed November 11, 2008]. http://www .iom.edu/Object .File/Master/48 /528/%20Westin%20IOM %20Srvy%20Rept%2011-1107.pdf .
- Williams ED. Federal protection for human research subjects: An analysis of the Common Rule and its interactions with FDA regulations and the HIPAA Privacy Rule, CRS report for Congress. Washington, DC: Congressional Research Service; 2005.
- Williams IC, Corbie-Smith G. Investigator beliefs and reported success in recruiting minority participants. Contemporary Clinical Trials. 2006; 27 :580–586. [ PubMed : 16839822 ]
- Winston FK, Durbin DR, Kallan MJ, Moll EK. The danger of premature graduation to seat belts for young children. Pediatrics. 2000; 105 (6):1179–1183. [ PubMed : 10835054 ]
- WMA (World Medical Association). Declaration of Helsinki: Ethical principles for medical research involving human subjects. 1964. [accessed August 21, 2008]. http://ohsr .od.nih.gov /guidelines/helsinki.html . [ PubMed : 16010903 ]
- Woolley M, Propst SM. Public attitudes and perceptions about health-related research. JAMA. 2005; 294 (11):1380–1384. [ PubMed : 16174697 ]
- Zarin DA, Tse T. Moving toward transparency of clinical trials. Science. 2008; 319 :1340–1342. [ PMC free article : PMC2396952 ] [ PubMed : 18323436 ]
- Zarin DA, Tse T, Ide NC. Trial registration at ClinicalTrials.gov between May and October 2005. New England Journal of Medicine. 2005; 353 (26):2779–2787. [ PMC free article : PMC1568386 ] [ PubMed : 16382064 ]
Epidemiology is the study of the occurrence, distribution, and control of diseases in populations.
Health services research has been defined as a multidisciplinary field of inquiry, both basic and applied, that examines the use, costs, quality, accessibility, delivery, organization, financing, and outcomes of health care services to increase knowledge and understanding of the structure, processes, and effects of health services for individuals and populations ( IOM, 1995 ).
The National Committee on Vital and Health Statistics has noted that “secondary uses” of health data is an ill-defined term, and urges abandoning it in favor of precise description of each use ( NCVHS, 2007a ). Thus, the committee chose to minimize use of the term in this report.
See Standards for Privacy of Individually Identifiable Health Information, 64 Fed. Reg. 59918, 59967 (preamble to rule proposed November 3, 1999) for a discussion on the benefits of health records research.
Effectiveness can be defined as the extent to which a specific test or intervention, when used under ordinary circumstances, does what it is intended to do. Efficacy refers to the extent to which a specific test or intervention produces a beneficial result under ideal conditions (e.g., in a clinical trial).
See http://www .intermacs.org .
See http://www .elso.med.umich.edu .
See http://www .unos.org/Data .
The Department of Health, Education and Welfare (now HHS) had previously issued policy and guidance on the protection of human subjects. See Williams (2005) .
In its report “First Biennial Report on the Adequacy and Uniformity of Federal Rules and Policies, and their Implementation, for the Protection of Human Subjects in Biomedical and Behavioral Research, Protecting Human Subjects.”
45 C.F.R. part 46 (2005).
See 45 C.F.R. § 46.101 (2005).
See 45 C.F.R. § 46.102(d) (2005).
This section on informed consent is based largely on a Congressional Research Service report ( Williams, 2005 ), as adapted by Pritts (2008) .
See 45 C.F.R. § 46.116 (2005).
See 45 C.F.R. § 46.116(b) (2005).
See 45 C.F.R. § 46.103 (2005).
See 45 C.F.R. § 46.111 (2005). There are additional factors if the study includes subjects who are likely to be vulnerable to coercion or undue influence.
See 45 C.F.R. § 46.116(d); 46.117(c) (2005).
See 45 C.F.R. § 46.102(f) (2005).
See 45 C.F.R. § 46.101(b)(4) (2005).
See 45 C.F.R. § 46.116(e) (2005).
See 45 C.F.R. § 46.123 (2005).
See also Grimes v. Kennedy Krieger Institute , 782 A. 2d 807 (Md. Ct. App. 2001); Gelsinger v. University of Pennsylvania (Philadelphia County Court of Common Pleas filed September 18, 2000), available at http://www .sskrplaw.com /links/healthcare2.html .
The FDA has defined “clinical investigation” to be synonymous with “research.”
The Food, Drug, and Cosmetic Act Section 505(i), 507(d), or 520(g) of 21 U.S.C. 355(i), 357(d), or 360j(g) (1972).
See 21 C.F.R. part 50 (2008); 46 Fed. Reg. 8942 (1981).
See 21 C.F.R. part 56 (2008); 46 Fed. Reg. 8958 (1981).
See 45 C.F.R. part 46 (2005); 46 Fed. Reg. 8366 (1981).
The Centers for Disease Control and Prevention defines program evaluation as the “systematic investigation of the merit, worth, or significance of organized public health action,” noting that such evaluations are “systematic ways to improve and account for public health actions by involving procedures that are useful, feasible, ethical, and accurate.” They can be based on goals, processes, outcomes, or value ( http://www .cdc.gov/mmwr /preview/mmwrhtml/rr4811a1.htm ).
The Utilization Review Accreditation Commission defines utilization review as “the evaluation of the medical necessity, appropriateness, and efficiency of the use of health care services, procedures, and facilities under the provisions of the applicable health benefits plans” ( http://www .urac.org/about/ ).
Another type of oversight board defined by the Privacy Rule. See Chapter 4 .
Under the Privacy Rule, consent is referred to as authorization. See Chapter 4 .
Personal communication, C. Heide, Office for Civil Rights, HHS, May 29, 2008.
The Privacy Rule defines the term “health care operations” by listing a number of specific activities that qualify as health care operations. These include “conducting quality assessment and improvement activities, population-based activities relating to improving or reducing health care costs, and case management and care coordination.” See 45 C.F.R. § 164.501 (2006).
A total of 444 surveys were mailed to the medical directors of quality improvement and IRB chairs at hospitals with 400 or more beds that belong to the Council of Teaching Hospitals of the Association of American Medical Colleges, and to the editors of all U.S.-based medical journals that publish original research and appear in the Abridged Index Medicus. 236 surveys were returned, for a 53 percent response rate. The survey consisted of six brief scenarios that asked respondents to determine whether the described project needed IRB review and informed consent.
See http: //plainlanguage.gov/index.cfm .
FDA, Public Law 110–85 § 801 (2007).
See http://www .ahrq.gov/research/cbprrole .htm .
- Cite this Page Institute of Medicine (US) Committee on Health Research and the Privacy of Health Information: The HIPAA Privacy Rule; Nass SJ, Levit LA, Gostin LO, editors. Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research. Washington (DC): National Academies Press (US); 2009. 3, The Value, Importance, and Oversight of Health Research.
- PDF version of this title (1.6M)
In this Page
Other titles in this collection.
- The National Academies Collection: Reports funded by National Institutes of Health
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- The Value, Importance, and Oversight of Health Research - Beyond the HIPAA Priva... The Value, Importance, and Oversight of Health Research - Beyond the HIPAA Privacy Rule
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Skip to Content
Participate in research with Google Health Studies
A new app by google empowering you to contribute to important health research with leading institutions, simply and securely from your phone..
Health research led by scientists, with help from Google
In partnership with doctors, nurses, and health researchers, Google Health is providing secure technology that can help improve our understanding of health. Our partnerships will always be guided by healthcare experts focused on questions that are important to improving well-being.
Improving the health of your community
Just as individuals have specific healthcare needs, so do individual communities. By participating in Google Health Studies research, you can help leading institutions and researchers develop a better understanding of your community’s specific health issues and needs. Your study participation can impact the health of your region — and even the future of healthcare for all.
Digital wellbeing study
Respiratory health study, contribute to an understanding of digital wellbeing.
The second study available is a digital wellbeing study conducted by the Center for Digital Mental Health at the University of Oregon. If you participate in this study, you’ll provide data to help researchers understand how patterns of smartphone use are associated with mental and physical wellbeing.
Dr. Nicholas Allen, Ann Swindells Professor of Clinical Psychology and Director of the Center for Digital Mental Health at the University of Oregon.
“ Digital technologies are transforming every aspect of modern life, but we need to know more about how they interact with human wellbeing. This innovative study can shed new light on this question. ”
Help researchers better understand respiratory diseases
The first study available is a respiratory health study conducted by Boston Children’s Hospital and Harvard Medical School. If you participate in this study, you’ll provide data to help researchers understand how demographics, health history, behavior, and mobility patterns contribute to the spread of respiratory illnesses.
Dr. John Brownstein, professor at Harvard Medical School and Chief Innovation Officer of Boston Children’s Hospital.
“ Google Health Studies provides people with a secure and easy way to take part in medical research, while letting researchers discover novel epidemiological insights into respiratory diseases. ”
Benefit the public, in private
Protecting your information in the respiratory health study.
Your study data stays on your device
After joining a health study, you’ll begin completing weekly surveys. At all times, your individual survey responses, location history and other personally identifiable data stays on your device.
Your device computes statistics based on your study data
During the study, your device receives different queries, computes and summarizes the results based on your individual study data, and encrypts these results for subsequent aggregation with federated analytics.
Participant data gets aggregated
Encrypted summaries from many devices are combined together, using the federated analytics technology. Google and study partners do not receive any individual study data about you.
Research that values your privacy
Combined insights are sent securely to the researchers conducting the study. You can safely contribute to health research knowing your personally identifiable study data will never be available to Google or third parties.
Protecting your health information
Personal health information is extremely sensitive, which is why Google Health Studies uses privacy-preserving methods to keep your data private and protected. If you choose to participate in research with Google Health Studies, Google does not sell your study data and does not use it to show you ads. You must explicitly consent to the purposes for which it will be used. You can easily unenroll from studies at any time. And if you choose to delete the app, all study data will be deleted from your phone and no new information will be collected.
A step toward representative health research
Google Health Studies makes it faster and easier for leading research institutions to connect with study participants by taking care of the technological infrastructure. If you're interested in adding your study to the platform, get notified when the app is available for more studies.
Less than 10% of the U.S. population participates in clinical research
How does racial participation differ by geographic locations?
Whether in the United States or in the rest of the world, clinical trial participants are mostly White. The majority of Asian trial participants were at non-US sites. The representation of Black or African American participants at US sites is similar to the US general population, which is 13% Black or African American (2011 - 2015 Census)
Participate in a Google Health study today
Take one minute a week to participate in health research led and developed by leading research institutions.
- Fact sheets
- Facts in pictures
Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Observatory
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Science Division /
- Research for health
Research for Health
WHO’s goal: Forward looking and prioritized global health research
Research for health is a global endeavour, and WHO has a unique role to play in ensuring that these efforts can help improve health for all.
WHO provides leadership, calling on the wider scientific community to engage behind global health concerns. This is based on a deep understanding of the needs of countries, and rigorous assessment by international experts.
WHO has three key objectives to promote forward-looking and prioritized global health research:

Anticipating scientific, technological, and epidemiological shifts
To stay on top of scientific and technological advancements and epidemiological trends, WHO must anticipate new trends, technologies, research, and discoveries in medical and public health.
Through continuous, rigorous, and systematic horizon scanning, the Science Division assesses and identifies emerging issues, for early identification of potential health benefits or threats. It actively prospects for scientific and technological innovations that could change the equation on advancing health.
Science in action: the WHO Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing
This committee examines the scientific, ethical, social, and legal challenges associated with human genome editing, and makes recommendations on the ethical framework for research and application of this technology.

Setting a global research agenda to address gaps, emerging areas, and country priorities
Truly useful innovations are not simply new; they are designed explicitly with the needs of the user in mind. By analyzing gaps, inequities, emerging areas and country priorities, the WHO research agenda anticipates the complex issues affecting people’s health and supports the discovery of innovative solutions to address them.
Science in action: R&D Blueprint for dementia research.
In 2017, the World Health Assembly adopted a Global Action Plan on the Public Health Response to Dementia. A key component of this plan was a call to action for research and innovation. To move this forward, the Science Division is developing an R&D Blueprint for dementia research.

Strengthening confidence in science
The Science Division supports countries in developing their scientific expertise and research capacities and facilitating the development of new and innovative research methodologies. This will improve understanding of the determinants of health, health systems, and the transformative potential of innovations in health.
Science in action: WHO Science Council
At WHO, Research for Health covers five key functions , which are integrated to apply research and innovation and achieve impact for people’s health around the world.

Foresight and emerging technologies
We try to get ahead of the curve by understanding what is needed to improve health for all in the future, and where the best new ideas are emerging.
Advances in science and technology hold great promise for new ways to address global health and support healthier populations worldwide. WHO engages in horizon scanning across the science and technology landscape. It also supports countries in doing their own futures and foresight exercises to understand their future needs. The aim of foresight is to identify and connect known, new, or emerging issues that could significantly impact global health within the next two decades.
Emerging technologies offer great health opportunities but also pose potentially significant challenges. The WHO Foresight function provides ongoing monitoring of emerging technologies to spot potential risks and come up with strategies for prevention and mitigation.

Research prioritization and support, R&D optimization
We identify gaps in current research priorities, and promote and support research that can best address unmet needs.
WHO has a unique role in supporting research for health , because we can help ensure health research is directed towards the biggest unmet needs in global health. We do this by sharing upstream research information from clinical trials , and research and development pipelines , and by providing guidance for research priority setting exercises.
WHO can determine strategic public health areas and identify key research and development needs. It then produces a clear target product profile to promote research and development that will be of most benefit. By mapping existing target product profiles in the Target Product Profile Directory and developing new ones based on identified public health needs, WHO steers innovation in support of improved health for all.
Product developers seek advice from WHO on whether or not their product likely has value for public health. In this way, WHO, expedites development of health related products, including novel therapeutics, diagnostics, and repurposing existing products.
Research for Health works with researchers and innovators to ensure they are aligned with the Prequalification Team and WHO’s technical departments on the package of evidence that will be needed to secure prequalification or a WHO policy recommendation. This process informs clinical trials on life-saving medical products, technologies and processes. A coordinated scientific advice process is currently in pilot phase.

Health ethics and governance
By putting ethics at the heart of decision-making and providing guidance on governance, WHO promotes this ethos within WHO and throughout the global health community.
In addition to supporting projects conducted by WHO, we are often called upon by development partners at country level for our expertise in global health ethics. Our Health Ethics and Governance unit produces guidance and tools for Member States on ethics in research and public health. Inside and outside WHO, it also helps researchers and public health specialists navigate ethical challenges posed by their projects.

Research policy for access
The best ideas are not just the brightest, but the one that actually get implemented and make an impact. WHO provides leadership on policies in research to ensure access and scale-up.
Having the right research policy is a key step towards ensuring health research has actual impact. This means that research priorities match real-world problems. At WHO, Research for Health works to ensure that the needs of countries are clearly articulated, and then communicated to the research community.
At WHO we promote an end-to-end approach in research policy. Working with local health systems and communities is needed to better understand the delivery and uptake of new products and to achieve widespread and equitable access. WHO can help broker multinational studies, foster regulatory harmonization, and promote dialogue among all stakeholders.

Taking knowledge from evidence to impact
Through a global network for evidence-informed health policy-making and tailored country support, WHO brings together researchers, policy-makers and implementers to translate evidence into improved health policies and programmes.
Public health problems are often complex and require nuanced, context-specific solutions and tailored implementation strategies. To make a difference for patients, communities and medical professionals, reliable evidence on how to tackle a health issue needs to be synthesized, reflected in a local context, and effectively communicated between researchers and decision-makers.
Through a set of field-tested and user-friendly tools, the Evidence to Policy and Impact Unit supports countries in bridging the gap between public health research, policy, and programme. Evidence briefs for policy and rapid response mechanisms put key research findings into context and place them at the fingertips of decision-makers. Policy dialogues provide researchers, policy-makers, and partner organizations with a forum to rally behind evidence-informed policy options and effective health interventions, discuss the findings, and share their own experiences and values. Citizen engagement strategies give voice to the beliefs and perspectives of individuals and communities, upholding accountability and democratic deliberation as core principles of equitable health care.
WHO’s global Evidence-informed Policy Network (EVIPNet) is a key initiative building sustainable and resilient capacity for evidence-informed decision-making and knowledge translation with Member States and in WHO offices at country, regional and international level. With over 15 years of experience and active teams in close to 50 countries, EVIPNet has successfully strengthened national health systems and emergency response capacity around the globe. The network also forms a vivid community of practice, facilitating decentralized peer-support among members and offering a treasure trove of successful strategies in evidence-informed health policy-making.
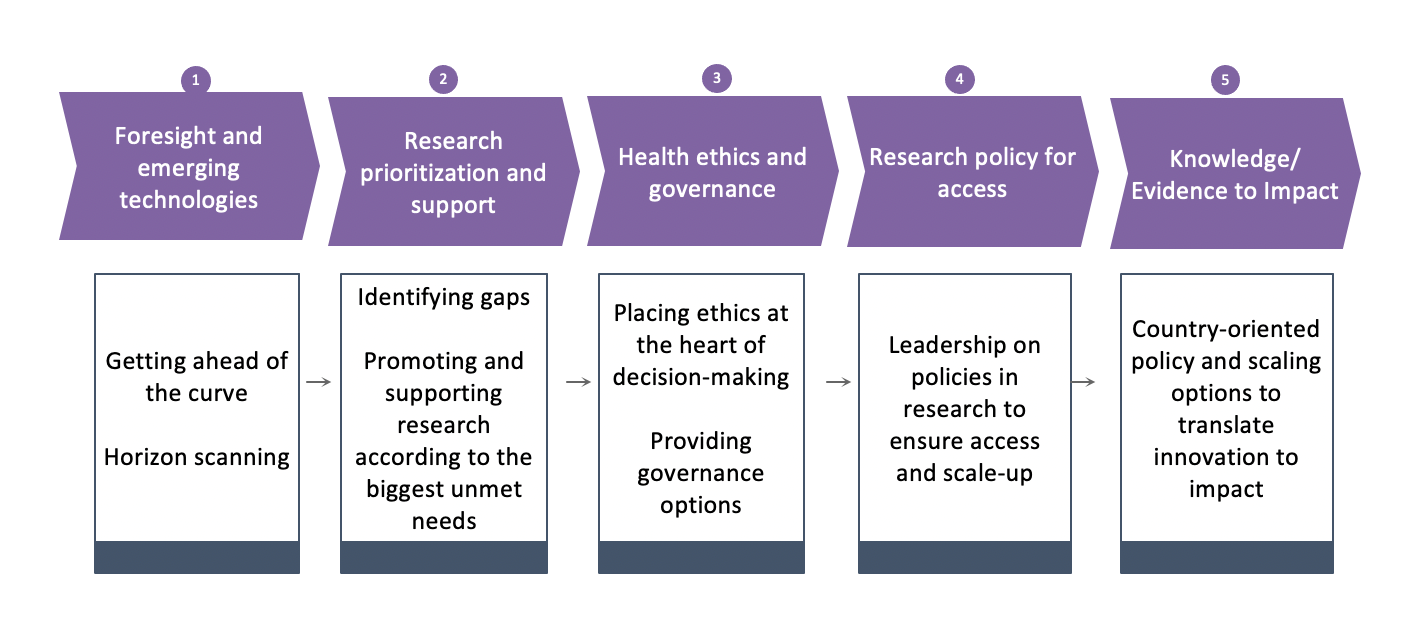
Research for Health within WHO
WHO’s Research for Health Department supports teams and units across the entire organization to establish their own research priorities . It helps people working in different parts of our global network connect the dots and create a better coordinated research response. This in turns helps keep WHO on track, ensuring that the research done within WHO is aligned with the health-related Sustainable Development Goals (SDGs) and our own Triple Billion Targets of 1 billion more people benefitting from universal health coverage, 1 billion more people better protected from health emergencies, and 1 billion more people enjoying better health and well-being.
Research for Health: our role in the global public health research community
WHO’s technical units are just one part of a global web of research for health, encompassing academia, national and regional research bodies, product development partnerships and the private sector. WHO helps to provide global guidance for research priority setting. Our global, regional and country-level reach means we can help to clearly articulate the needs of the countries, and we are uniquely well-placed to broker multinational research efforts.
WHO consults on action plan for sustainable clinical research infrastructure
Pan American Health Organization regional meeting on human genomic research for health held
WHO advisory group convenes its first meeting on responsible use of the life sciences in Geneva
Public consultation
CLOSED - Call for Experts – Technical Advisory Group on the responsible use of the life sciences and dual-use research
Public consultation on WHO guidance for best practices for clinical trials

WHO global research priorities for sexually transmitted infections
Sexually transmitted infections (STIs) are widespread globally and negatively affect sexual and reproductive health. Gaps in evidence and in available...

Report of the sixth meeting of the WHO Diagnostic Technical Advisory Group for Neglected Tropical Diseases:...
The World Health Organization’s Global Neglected Tropical Diseases Programme (WHO/NTD) manages a diverse portfolio of 21 diseases and disease groups,1 ...

Protocol: using data to drive governance
This document introduces a collaboratively developed study protocol to identify what data and information is currently being collected by governments and...


Evidence generation for development of health products: a practical guide for WHO staff
This document describes the main elements that World Health Organization (WHO) technical departments should elaborate on when providing guidance on evidence...
Subscribe to our free Newsletter! →
Home › Health & Medical News
Health & Medical News
Dive into the latest Health & Medical News, with a special emphasis on cutting-edge research studies. Our coverage spans from clinical trials to innovative medical research, providing deep insights into new treatments and healthcare advancements. Stay informed about the scientific developments that are transforming health and medicine.

Longevity breakthrough: Scientists uncover key gene that extends lifespan
September 4, 2024

Nicotine pouches surging in popularity — Because users can’t recognize what they are

Where to Find the Best Healthcare in the World

Microwave myth debunked: These germs survive the reheating process
Will Ozempic lead to depression or suicide? New study reveals mental health risks
September 3, 2024

Smart mask turns every exhale into a mini health check-up while protecting you from germs

Food additive warning: Why it’s hard to trust what’s on your plate

The $1,000 phone call: How a single conversation could slash your medical debt
September 2, 2024

5 Red Light Therapy Devices Experts Actually Recommend
August 28, 2024

Quit before conception: Researchers find ‘no safe period’ to smoke during pregnancy

70% of parents say they failed their kids in this important summer task
August 26, 2024

‘Teflon flu’ cases surge: What you need to know

©2024 Study Finds. All rights reserved. Privacy Policy • Disclosure Policy • Do Not Sell My Personal Information

Research Topics & Ideas: Healthcare

F inding and choosing a strong research topic is the critical first step when it comes to crafting a high-quality dissertation, thesis or research project. If you’ve landed on this post, chances are you’re looking for a healthcare-related research topic , but aren’t sure where to start. Here, we’ll explore a variety of healthcare-related research ideas and topic thought-starters across a range of healthcare fields, including allopathic and alternative medicine, dentistry, physical therapy, optometry, pharmacology and public health.
NB – This is just the start…
The topic ideation and evaluation process has multiple steps . In this post, we’ll kickstart the process by sharing some research topic ideas within the healthcare domain. This is the starting point, but to develop a well-defined research topic, you’ll need to identify a clear and convincing research gap , along with a well-justified plan of action to fill that gap.
If you’re new to the oftentimes perplexing world of research, or if this is your first time undertaking a formal academic research project, be sure to check out our free dissertation mini-course. In it, we cover the process of writing a dissertation or thesis from start to end. Be sure to also sign up for our free webinar that explores how to find a high-quality research topic.
Overview: Healthcare Research Topics
- Allopathic medicine
- Alternative /complementary medicine
- Veterinary medicine
- Physical therapy/ rehab
- Optometry and ophthalmology
- Pharmacy and pharmacology
- Public health
- Examples of healthcare-related dissertations
Allopathic (Conventional) Medicine
- The effectiveness of telemedicine in remote elderly patient care
- The impact of stress on the immune system of cancer patients
- The effects of a plant-based diet on chronic diseases such as diabetes
- The use of AI in early cancer diagnosis and treatment
- The role of the gut microbiome in mental health conditions such as depression and anxiety
- The efficacy of mindfulness meditation in reducing chronic pain: A systematic review
- The benefits and drawbacks of electronic health records in a developing country
- The effects of environmental pollution on breast milk quality
- The use of personalized medicine in treating genetic disorders
- The impact of social determinants of health on chronic diseases in Asia
- The role of high-intensity interval training in improving cardiovascular health
- The efficacy of using probiotics for gut health in pregnant women
- The impact of poor sleep on the treatment of chronic illnesses
- The role of inflammation in the development of chronic diseases such as lupus
- The effectiveness of physiotherapy in pain control post-surgery

Topics & Ideas: Alternative Medicine
- The benefits of herbal medicine in treating young asthma patients
- The use of acupuncture in treating infertility in women over 40 years of age
- The effectiveness of homoeopathy in treating mental health disorders: A systematic review
- The role of aromatherapy in reducing stress and anxiety post-surgery
- The impact of mindfulness meditation on reducing high blood pressure
- The use of chiropractic therapy in treating back pain of pregnant women
- The efficacy of traditional Chinese medicine such as Shun-Qi-Tong-Xie (SQTX) in treating digestive disorders in China
- The impact of yoga on physical and mental health in adolescents
- The benefits of hydrotherapy in treating musculoskeletal disorders such as tendinitis
- The role of Reiki in promoting healing and relaxation post birth
- The effectiveness of naturopathy in treating skin conditions such as eczema
- The use of deep tissue massage therapy in reducing chronic pain in amputees
- The impact of tai chi on the treatment of anxiety and depression
- The benefits of reflexology in treating stress, anxiety and chronic fatigue
- The role of acupuncture in the prophylactic management of headaches and migraines
Topics & Ideas: Dentistry
- The impact of sugar consumption on the oral health of infants
- The use of digital dentistry in improving patient care: A systematic review
- The efficacy of orthodontic treatments in correcting bite problems in adults
- The role of dental hygiene in preventing gum disease in patients with dental bridges
- The impact of smoking on oral health and tobacco cessation support from UK dentists
- The benefits of dental implants in restoring missing teeth in adolescents
- The use of lasers in dental procedures such as root canals
- The efficacy of root canal treatment using high-frequency electric pulses in saving infected teeth
- The role of fluoride in promoting remineralization and slowing down demineralization
- The impact of stress-induced reflux on oral health
- The benefits of dental crowns in restoring damaged teeth in elderly patients
- The use of sedation dentistry in managing dental anxiety in children
- The efficacy of teeth whitening treatments in improving dental aesthetics in patients with braces
- The role of orthodontic appliances in improving well-being
- The impact of periodontal disease on overall health and chronic illnesses

Topics & Ideas: Veterinary Medicine
- The impact of nutrition on broiler chicken production
- The role of vaccines in disease prevention in horses
- The importance of parasite control in animal health in piggeries
- The impact of animal behaviour on welfare in the dairy industry
- The effects of environmental pollution on the health of cattle
- The role of veterinary technology such as MRI in animal care
- The importance of pain management in post-surgery health outcomes
- The impact of genetics on animal health and disease in layer chickens
- The effectiveness of alternative therapies in veterinary medicine: A systematic review
- The role of veterinary medicine in public health: A case study of the COVID-19 pandemic
- The impact of climate change on animal health and infectious diseases in animals
- The importance of animal welfare in veterinary medicine and sustainable agriculture
- The effects of the human-animal bond on canine health
- The role of veterinary medicine in conservation efforts: A case study of Rhinoceros poaching in Africa
- The impact of veterinary research of new vaccines on animal health
Topics & Ideas: Physical Therapy/Rehab
- The efficacy of aquatic therapy in improving joint mobility and strength in polio patients
- The impact of telerehabilitation on patient outcomes in Germany
- The effect of kinesiotaping on reducing knee pain and improving function in individuals with chronic pain
- A comparison of manual therapy and yoga exercise therapy in the management of low back pain
- The use of wearable technology in physical rehabilitation and the impact on patient adherence to a rehabilitation plan
- The impact of mindfulness-based interventions in physical therapy in adolescents
- The effects of resistance training on individuals with Parkinson’s disease
- The role of hydrotherapy in the management of fibromyalgia
- The impact of cognitive-behavioural therapy in physical rehabilitation for individuals with chronic pain
- The use of virtual reality in physical rehabilitation of sports injuries
- The effects of electrical stimulation on muscle function and strength in athletes
- The role of physical therapy in the management of stroke recovery: A systematic review
- The impact of pilates on mental health in individuals with depression
- The use of thermal modalities in physical therapy and its effectiveness in reducing pain and inflammation
- The effect of strength training on balance and gait in elderly patients
Need a helping hand?
Topics & Ideas: Optometry & Opthalmology
- The impact of screen time on the vision and ocular health of children under the age of 5
- The effects of blue light exposure from digital devices on ocular health
- The role of dietary interventions, such as the intake of whole grains, in the management of age-related macular degeneration
- The use of telemedicine in optometry and ophthalmology in the UK
- The impact of myopia control interventions on African American children’s vision
- The use of contact lenses in the management of dry eye syndrome: different treatment options
- The effects of visual rehabilitation in individuals with traumatic brain injury
- The role of low vision rehabilitation in individuals with age-related vision loss: challenges and solutions
- The impact of environmental air pollution on ocular health
- The effectiveness of orthokeratology in myopia control compared to contact lenses
- The role of dietary supplements, such as omega-3 fatty acids, in ocular health
- The effects of ultraviolet radiation exposure from tanning beds on ocular health
- The impact of computer vision syndrome on long-term visual function
- The use of novel diagnostic tools in optometry and ophthalmology in developing countries
- The effects of virtual reality on visual perception and ocular health: an examination of dry eye syndrome and neurologic symptoms
Topics & Ideas: Pharmacy & Pharmacology
- The impact of medication adherence on patient outcomes in cystic fibrosis
- The use of personalized medicine in the management of chronic diseases such as Alzheimer’s disease
- The effects of pharmacogenomics on drug response and toxicity in cancer patients
- The role of pharmacists in the management of chronic pain in primary care
- The impact of drug-drug interactions on patient mental health outcomes
- The use of telepharmacy in healthcare: Present status and future potential
- The effects of herbal and dietary supplements on drug efficacy and toxicity
- The role of pharmacists in the management of type 1 diabetes
- The impact of medication errors on patient outcomes and satisfaction
- The use of technology in medication management in the USA
- The effects of smoking on drug metabolism and pharmacokinetics: A case study of clozapine
- Leveraging the role of pharmacists in preventing and managing opioid use disorder
- The impact of the opioid epidemic on public health in a developing country
- The use of biosimilars in the management of the skin condition psoriasis
- The effects of the Affordable Care Act on medication utilization and patient outcomes in African Americans
Topics & Ideas: Public Health
- The impact of the built environment and urbanisation on physical activity and obesity
- The effects of food insecurity on health outcomes in Zimbabwe
- The role of community-based participatory research in addressing health disparities
- The impact of social determinants of health, such as racism, on population health
- The effects of heat waves on public health
- The role of telehealth in addressing healthcare access and equity in South America
- The impact of gun violence on public health in South Africa
- The effects of chlorofluorocarbons air pollution on respiratory health
- The role of public health interventions in reducing health disparities in the USA
- The impact of the United States Affordable Care Act on access to healthcare and health outcomes
- The effects of water insecurity on health outcomes in the Middle East
- The role of community health workers in addressing healthcare access and equity in low-income countries
- The impact of mass incarceration on public health and behavioural health of a community
- The effects of floods on public health and healthcare systems
- The role of social media in public health communication and behaviour change in adolescents
Examples: Healthcare Dissertation & Theses
While the ideas we’ve presented above are a decent starting point for finding a healthcare-related research topic, they are fairly generic and non-specific. So, it helps to look at actual dissertations and theses to see how this all comes together.
Below, we’ve included a selection of research projects from various healthcare-related degree programs to help refine your thinking. These are actual dissertations and theses, written as part of Master’s and PhD-level programs, so they can provide some useful insight as to what a research topic looks like in practice.
- Improving Follow-Up Care for Homeless Populations in North County San Diego (Sanchez, 2021)
- On the Incentives of Medicare’s Hospital Reimbursement and an Examination of Exchangeability (Elzinga, 2016)
- Managing the healthcare crisis: the career narratives of nurses (Krueger, 2021)
- Methods for preventing central line-associated bloodstream infection in pediatric haematology-oncology patients: A systematic literature review (Balkan, 2020)
- Farms in Healthcare: Enhancing Knowledge, Sharing, and Collaboration (Garramone, 2019)
- When machine learning meets healthcare: towards knowledge incorporation in multimodal healthcare analytics (Yuan, 2020)
- Integrated behavioural healthcare: The future of rural mental health (Fox, 2019)
- Healthcare service use patterns among autistic adults: A systematic review with narrative synthesis (Gilmore, 2021)
- Mindfulness-Based Interventions: Combatting Burnout and Compassionate Fatigue among Mental Health Caregivers (Lundquist, 2022)
- Transgender and gender-diverse people’s perceptions of gender-inclusive healthcare access and associated hope for the future (Wille, 2021)
- Efficient Neural Network Synthesis and Its Application in Smart Healthcare (Hassantabar, 2022)
- The Experience of Female Veterans and Health-Seeking Behaviors (Switzer, 2022)
- Machine learning applications towards risk prediction and cost forecasting in healthcare (Singh, 2022)
- Does Variation in the Nursing Home Inspection Process Explain Disparity in Regulatory Outcomes? (Fox, 2020)
Looking at these titles, you can probably pick up that the research topics here are quite specific and narrowly-focused , compared to the generic ones presented earlier. This is an important thing to keep in mind as you develop your own research topic. That is to say, to create a top-notch research topic, you must be precise and target a specific context with specific variables of interest . In other words, you need to identify a clear, well-justified research gap.

Find The Perfect Research Topic

How To Choose A Research Topic: 5 Key Criteria
How To Choose A Research Topic Step-By-Step Tutorial With Examples + Free Topic...
Research Topics & Ideas: Automation & Robotics
Research Topics & Ideas: Robotics 50 Topic Ideas To Kickstart Your Research...

Research Topics & Ideas: Sociology
Research Topics & Ideas: Sociology 50 Topic Ideas To Kickstart Your Research...

Research Topics & Ideas: Public Health & Epidemiology
Research Topics & Ideas: Public Health 50 Topic Ideas To Kickstart Your Research...

Research Topics & Ideas: Neuroscience
Research Topics & Ideas: Neuroscience 50 Topic Ideas To Kickstart Your Research...
📄 FREE TEMPLATES
Research Topic Ideation
Proposal Writing
Literature Review
Methodology & Analysis
Academic Writing
Referencing & Citing
Apps, Tools & Tricks
The Grad Coach Podcast
18 Comments
I need topics that will match the Msc program am running in healthcare research please
Hello Mabel,
I can help you with a good topic, kindly provide your email let’s have a good discussion on this.
Can you provide some research topics and ideas on Immunology?
Thank you to create new knowledge on research problem verse research topic
Help on problem statement on teen pregnancy
This post might be useful: https://gradcoach.com/research-problem-statement/
can you give me research titles that i can conduct as a school nurse
can you provide me with a research topic on healthcare related topics to a qqi level 5 student
Please can someone help me with research topics in public health ?
Hello I have requirement of Health related latest research issue/topics for my social media speeches. If possible pls share health issues , diagnosis, treatment.
I would like a topic thought around first-line support for Gender-Based Violence for survivors or one related to prevention of Gender-Based Violence
Please can I be helped with a master’s research topic in either chemical pathology or hematology or immunology? thanks
Can u please provide me with a research topic on occupational health and safety at the health sector
Good day kindly help provide me with Ph.D. Public health topics on Reproductive and Maternal Health, interventional studies on Health Education
may you assist me with a good easy healthcare administration study topic
May you assist me in finding a research topic on nutrition,physical activity and obesity. On the impact on children
I have been racking my brain for a while on what topic will be suitable for my PhD in health informatics. I want a qualitative topic as this is my strong area.
Hi, may I please be assisted with research topics in the medical laboratory sciences
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Submit Comment
- Print Friendly
UR Health Research

URMC is currently conducting several coronavirus clinical trials that are in need of volunteers. Learn More »
Participate in health research.
Health Research & Clinical Trials
Interested in COVID-19 Studies?
Learn about studies for all diseases and conditions below.

What is Health Research?
Browse our frequently asked questions for more information.

Find a Study
Search for currently open studies by disease or condition.

Add your name to our registry to be contacted for future studies.
Call (585) 275-2107 or email us with any questions.
The project described in this publication was supported by the University of Rochester CTSA award number UL1 TR002001 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Research News

Follow Us on Facebook
Participating in Health Research Studies
- What is Health Research?
- Is Health Research Safe?
- Is Health Research Right for Me?
Types of Health Research
Behavioral studies.
These are studies that test how people act in different ways.
Clinical Trials
These are studies of a drug, surgery, or medical device in healthy volunteers or people who have a specific disease. See below for more information.
Community-Based Participatory Research (CBPR)
This is research that engages community partners as equal participants in the research.
Genetic Studies
These are studies to find the role of genes in different diseases.
Observational Studies
These are studies in which a group of people is observed for many years.
Physiological Studies
These are studies to better understand how the human body functions.
Prevention Studies
These are studies that test ways to prevent specific conditions or diseases.
Public Health Research
This type of research can be one or a combination of the types of research mentioned above. Public health research tries to improve the health and well-being of people from a population-level perspective.
More Information about Clinical Trials
Clinical trials are often done in a "randomized" way. These are sometimes called RCTs for "randomized clinical trials." In an RCT, some people will be chosen at random to receive a treatment or intervention, such as a new drug. The rest of the participants will be given a "placebo," such as a sugar pill. In other cases, when two interventions are being compared, one group will receive one of the interventions and the other group will be given a different one. Some clinical trials are also "blinded." This means that both the volunteers and the doctors do not know if people are taking the new medicine or the placebo. Only at the end of the study will this be revealed. Since people are chosen at random (similar to a coin toss) in an RCT, people who receive the treatment should be no different than those who do not. For instance, there should be an equal number of males who receive treatment compared with those who do not. This helps reduce bias due to something like gender in a study.
New drugs are first developed in research labs, and then tested in animals. Only then are clinical studies done in humans. Clinical trials of new drugs are done in different phases:
- Phase I studies test a new drug for the first time in a small group of people (about 20-80) to see if it safe, to find the right dose, and to know the side effects.
- Phase II studies are done in more people (about 100-300) to see how well the new drug treats a disease.
- Phase III studies are done in large groups of people (about 1000 to 3000) to see if the new drug works well, has side effects, and how it compares to other drugs.
- Phase IV studies are done after the treatment is approved by the U.S. Food and Drug Administration (FDA).
- << Previous: Is Health Research Right for Me?
- Last Updated: May 27, 2020 3:05 PM
- URL: https://guides.library.harvard.edu/healthresearch
| | |

Be part of tomorrow's healthcare breakthroughs
When you participate in a University of Minnesota study, you can help create a healthier future.

Find a study that's right for you
Advanced search, how you could make a difference.
- Help someone who needs it - Your participation in research could benefit a friend, a family member, or someone across the world.
- Make healthcare better for everyone - Healthcare is safer and more effective for everyone when people from different backgrounds, ages, genders, races and ethnicity participate in health research.
- Help researchers solve health problems - Volunteers play a key role in research and make new discoveries possible. Your participation helps researchers find new ways to prevent, detect, or treat disease.
Featured research opportunities

Research at the CSC
The M Health Fairview Clinics and Surgery Center houses a wide range of specialists all in one easy-to-access location on the U of M campus. Find studies taking place in this unique space where clinical care and research connect.

Healthy volunteers needed
Healthy volunteers play a vital role in research. Some research studies rely on participation from healthy volunteers (people who do not have the condition being studied) to provide data that is used as a comparison for patient groups.

Share your voice
We want to hear what YOU think about health research. If you're 18 or older, take our quick 5-10 minute anonymous survey and shape the future of U of MN studies. Your voice matters!
Research participants have rights
Every study is different. Some studies are looking for people with certain conditions, while others are open to healthy volunteers. Some studies involve visits to a clinic, while others can be done online.
One thing that is common to all research is that the decision to participate is personal and always voluntary. Whether agreeing to share your medical data or consenting to an experimental treatment, we want you to know that research participants have rights and protections.
Click the link below to read the research participants' Bill of Rights and to learn more about how the University of MInnesota reviews, approves and monitors research studies.
Join a national registry!

ResearchMatch
ResearchMatch.org connects volunteers with research studies across the country. Volunteers of any age, race, ethnicity, or health status are invited to join. Log on, register, and receive emails when studies might be a good fit for you.
Here’s how you know
- U.S. Department of Health and Human Services
- National Institutes of Health
Whole Person Health: What It Is and Why It's Important
.header_greentext{color:greenimportant;font-size:24pximportant;font-weight:500important;}.header_bluetext{color:blueimportant;font-size:18pximportant;font-weight:500important;}.header_redtext{color:redimportant;font-size:28pximportant;font-weight:500important;}.header_darkred{color:#803d2fimportant;font-size:28pximportant;font-weight:500important;}.header_purpletext{color:purpleimportant;font-size:31pximportant;font-weight:500important;}.header_yellowtext{color:yellowimportant;font-size:20pximportant;font-weight:500important;}.header_blacktext{color:blackimportant;font-size:22pximportant;font-weight:500important;}.header_whitetext{color:whiteimportant;font-size:22pximportant;font-weight:500important;}.header_darkred{color:#803d2fimportant;}.green_header{color:greenimportant;font-size:24pximportant;font-weight:500important;}.blue_header{color:blueimportant;font-size:18pximportant;font-weight:500important;}.red_header{color:redimportant;font-size:28pximportant;font-weight:500important;}.purple_header{color:purpleimportant;font-size:31pximportant;font-weight:500important;}.yellow_header{color:yellowimportant;font-size:20pximportant;font-weight:500important;}.black_header{color:blackimportant;font-size:22pximportant;font-weight:500important;}.white_header{color:whiteimportant;font-size:22pximportant;font-weight:500important;} what is whole person health.
Whole person health involves looking at the whole person—not just separate organs or body systems—and considering multiple factors that promote either health or disease. It means helping and empowering individuals, families, communities, and populations to improve their health in multiple interconnected biological, behavioral, social, and environmental areas. Instead of just treating a specific disease, whole person health focuses on restoring health, promoting resilience, and preventing diseases across a lifespan.
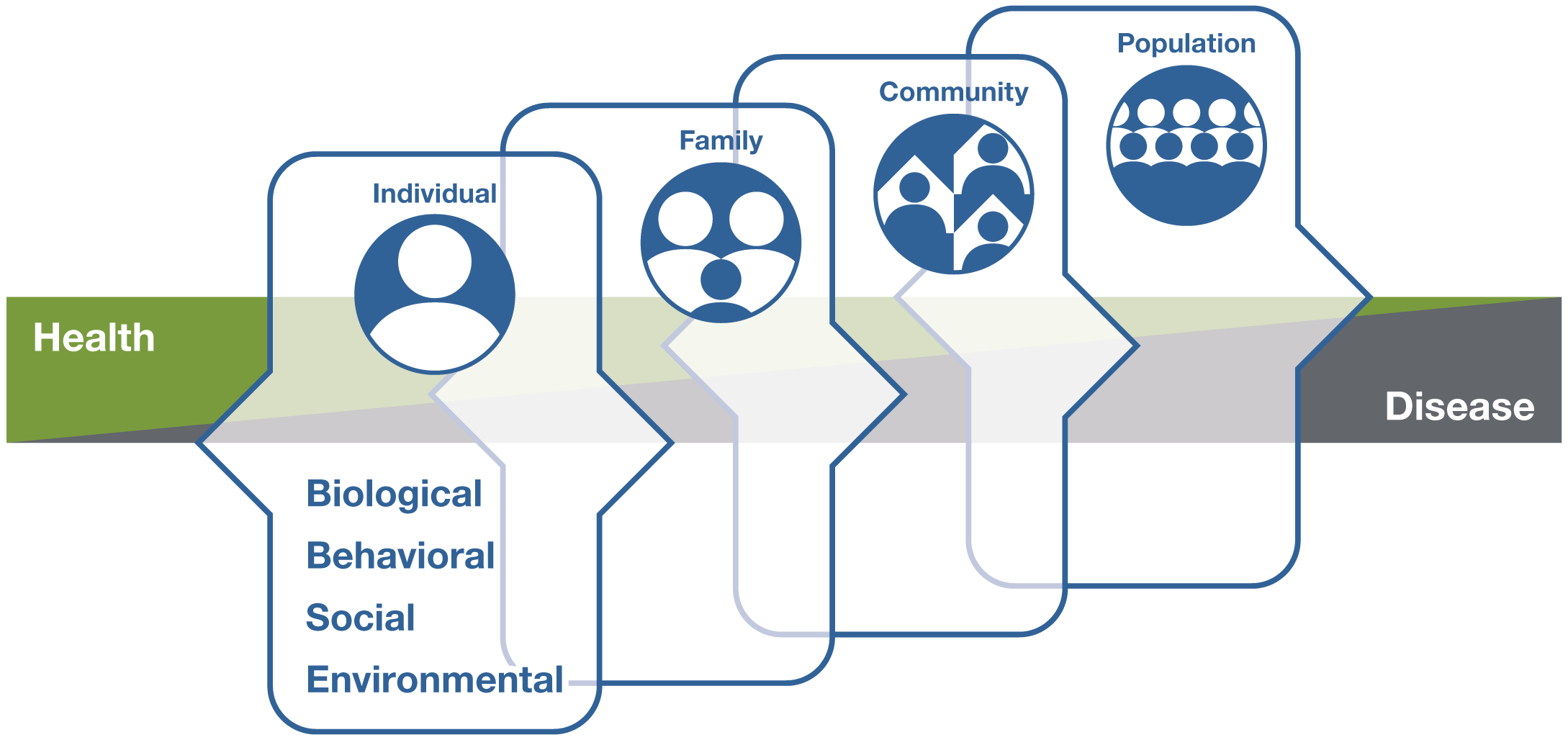
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Why is whole person health important?
Health and disease are not separate, disconnected states but instead occur on a path that can move in two different directions, either toward health or toward disease.
On this path, many factors, including one’s biological makeup; some unhealthy behaviors, such as poor diet, sedentary lifestyle, chronic stress, and poor sleep; as well as social aspects of life—the conditions in which people are born, grow, live, work, and age—can lead to chronic diseases of more than one organ system. On the other hand, self-care, lifestyle, and behavioral interventions may help with the return to health.
Chronic diseases, such as diabetes, cardiovascular disease, obesity, and degenerative joint disease, can also occur with chronic pain, depression, and opioid misuse—all conditions exacerbated by chronic stress. Some chronic diseases increase the immediate and long-term risks with COVID-19 infection. Understanding the condition in which a person has lived, addressing behaviors at an early stage, and managing stress can not only prevent multiple diseases but also help restore health and stop the progression to disease across a person’s lifespan.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Is whole person health being used now in health care?
Some health care systems and programs are now focusing more on whole person health.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} U.S. Department of Veterans Affairs (VA) Whole Health Approach
The VA’s Whole Health System of Care and Whole Health approach aims to improve the health and well-being of veterans and to address lifestyle and environmental root causes of chronic disease. The approach shifts from a disease-centered focus to a more personalized approach that engages and empowers veterans early in and throughout their lives to prioritize healthy lifestyle changes in areas like nutrition, activity, sleep, relationships, and surroundings. Conventional testing and treatment are combined with complementary and integrative health approaches that may include acupuncture, biofeedback, massage therapy, yoga, and meditation.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} U.S. Department of Defense Total Force Fitness Program
The Total Force Fitness program arose within the U.S. Department of Defense Military Health System in response to the need for a more holistic approach—a focus on the whole person instead of separate parts or only symptoms—to the demands of multiple deployments and the strains on the U.S. Armed Forces and their family members. The focus extends the idea of total fitness to include the health, well-being, and resilience of the whole person, family, community, and U.S. military.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Whole Health Institute
Established in 2020, the Whole Health Institute’s Whole Health model helps people identify what matters most to them and build a plan for their journey to whole health. The model provides tools to help people take good care of their body, mind, and spirit, and involves working with a health care team as well as tapping into the support of family, friends, and communities.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} North Carolina Department of Health and Human Services
The North Carolina Department of Health and Human Services has incorporated a whole person health approach into its health care system by focusing on integrating physical, behavioral, and social health. The state has taken steps to encourage collaborative behavioral health care and help resolve widespread inequities in social conditions, such as housing and nutritious food access.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Ornish Program for Reversing Heart Disease
The Ornish Program for Reversing Heart Disease is an intensive cardiac rehabilitation program that has been shown to reverse the progression of coronary heart disease through lifestyle changes, without drugs or surgery. The program is covered by Medicare and some health insurance companies. The program’s lifestyle changes include exercise, smoking cessation, stress management, social support, and a whole-foods, plant-based diet low in total fat. The program is offered by a team of health care professionals who provide the support that individuals need to make and maintain lasting changes in lifestyle.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} What does research show about whole person health?
A growing body of research suggests the benefits of healthy behaviors, environments, and policies to maintain health and prevent, treat, and reverse chronic diseases. This research includes several large, long-term epidemiological studies—such as the Framingham Heart Study, Nurses’ Health Study, and Adventist Health Studies—that have evaluated the connections between lifestyle, diet, genetics, health, and disease.
There is a lack, however, of randomized controlled trials and other types of research on multicomponent interventions and whole person health. Challenges come with conducting this type of research and with finding appropriate ways to assess the evidence. But opportunities are emerging to explore new paths toward reliable and rigorous research on whole person health.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Will the National Center for Complementary and Integrative Health (NCCIH) fund research on whole person health?
Yes, NCCIH plans to fund research on whole person health . (Details can be found in the NCCIH Strategic Plan FY 2021–2025: Mapping a Pathway to Research on Whole Person Health . )
By deepening the scientific understanding of the connections that exist across the different areas of human health, researchers can better understand how conditions interrelate, identify multicomponent interventions that address these problems, and determine the best ways to support individuals through the full continuum of their health experience, including the return to health.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} For More Information
Nccih clearinghouse.
The NCCIH Clearinghouse provides information on NCCIH and complementary and integrative health approaches, including publications and searches of Federal databases of scientific and medical literature. The Clearinghouse does not provide medical advice, treatment recommendations, or referrals to practitioners.
Toll-free in the U.S.: 1-888-644-6226
Telecommunications relay service (TRS): 7-1-1
Website: https://www.nccih.nih.gov
Email: [email protected] (link sends email)
Know the Science
NCCIH and the National Institutes of Health (NIH) provide tools to help you understand the basics and terminology of scientific research so you can make well-informed decisions about your health. Know the Science features a variety of materials, including interactive modules, quizzes, and videos, as well as links to informative content from Federal resources designed to help consumers make sense of health information.
Explaining How Research Works (NIH)
Know the Science: How To Make Sense of a Scientific Journal Article
Understanding Clinical Studies (NIH)
A service of the National Library of Medicine, PubMed® contains publication information and (in most cases) brief summaries of articles from scientific and medical journals. For guidance from NCCIH on using PubMed, see How To Find Information About Complementary Health Approaches on PubMed .
Website: https://pubmed.ncbi.nlm.nih.gov/
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Key References
- Aggarwal M, Ornish D, Josephson R, et al. Closing gaps in lifestyle adherence for secondary prevention of coronary heart disease. American Journal of Cardiology. 2021;145:1-11.
- Centers for Medicare & Medicaid Services. Decision Memo for Intensive Cardiac Rehabilitation (ICR) Program—Dr. Ornish’s Program for Reversing Heart Disease (CAG-00419N). Accessed at https://www.cms.gov/ on April 26, 2021.
- Deuster PA, O’Connor FG. Human performance optimization: culture change and paradigm shift. Journal of Strength and Conditioning Research. 2015;29(suppl 11):S52-S56.
- Gaudet T, Kligler B. Whole health in the whole system of the Veterans Administration: how will we know we have reached this future state? Journal of Alternative and Complementary Medicine. 2019;25(S1):S7-S11.
- Malecki HL, Gollie JM, Scholten J. Physical activity, exercise, whole health, and integrative health coaching. Physical Medicine and Rehabilitation Clinics of North America. 2020;31(4):649-663.
- National Center for Complementary and Integrative Health. NCCIH Strategic Plan FY 2021–2025: Mapping a Pathway to Research on Whole Person Health. National Center for Complementary and Integrative Health website. Accessed at https://www.nccih.nih.gov/about/nccih-strategic-plan-2021-2025 on May 14, 2021.
- North Carolina Department of Health and Human Services website. Healthy Opportunities and Medicaid Transformation. Accessed at https://www.ncdhhs.gov/about/department-initiatives/healthy-opportunities/healthy-opportunities-pilots/healthy on April 26, 2021.
- Military Health System website. Total Force Fitness. Accessed at https://health.mil/Military-Health-Topics/Total-Force-Fitness on April 26, 2021.
- Tilson EC, Muse A, Colville K, et al. Investing in whole person health: working toward an integration of physical, behavioral, and social health. North Carolina Medical Journal. 2020;81(3):177-180.
- U.S. Department of Veterans Affairs website. Whole Health. Accessed at https://www.va.gov/wholehealth/ on April 26, 2021.
- U.S. Department of Veterans Affairs website. Whole Health Library. Accessed at https://www.va.gov/wholehealthlibrary/ on April 26, 2021.
- Vodovotz Y, Barnard N, Hu FB, et al. Prioritized research for the prevention, treatment, and reversal of chronic disease: recommendations from the Lifestyle Medicine Research Summit. Frontiers in Medicine (Lausanne). 2020;7:585744.
- Whitehead AM, Kligler B. Innovations in care: complementary and integrative health in the Veterans Health Administration Whole Health System. Medical Care. 2020;58(9S)(suppl 2):S78-S79.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Other References
- Alborzkouh P, Nabati M, Zainali M, et al. A review of the effectiveness of stress management skills training on academic vitality and psychological well-being of college students. Journal of Medicine and Life. 2015;8(4):39-44.
- Bisht K, Sharma K, Tremblay M-È. Chronic stress as a risk factor for Alzheimer's disease: roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiology of Stress. 2018;9:9-21.
- Buettner D, Skemp S. Blue Zones: lessons from the world’s longest lived. American Journal of Lifestyle Medicine. 2016;10(5):318-321.
- Chen T-L, Chang S-C, Hsieh H-F, et al. Effects of mindfulness-based stress reduction on sleep quality and mental health for insomnia patients: a meta-analysis. Journal of Psychosomatic Research. 2020;135:110144.
- Conversano C, Orrù G, Pozza A, et al. Is mindfulness-based stress reduction effective for people with hypertension? A systematic review and meta-analysis of 30 years of evidence. International Journal of Environmental Research and Public Health. 2021;18(6):2882.
- Katz DL, Karlsen MC, Chung M, et al. Hierarchies of evidence applied to lifestyle medicine (HEALM): introduction of a strength-of-evidence approach based on a methodological systematic review. BMC Medical Research Methodology. 2019;19(1):178.
- Kruk J, Aboul-Enein BH, Bernstein J, et al. Psychological stress and cellular aging in cancer: a meta-analysis. Oxidative Medicine and Cellular Longevity. 2019;2019:1270397.
- Levesque C. Therapeutic lifestyle changes for diabetes mellitus. Nursing Clinics of North America. 2017;52(4):679-692.
- Ni Y, Ma L, Li J. Effects of mindfulness-based stress reduction and mindfulness-based cognitive therapy in people with diabetes: a systematic review and meta-analysis. Journal of Nursing Scholarship. 2020;52(4):379-388.
- Ornish Lifestyle Medicine website. The Ornish Reversal Program: Intensive Cardiac Rehabilitation. Accessed at https://www.ornish.com/intensive-cardiac-rehab/ on April 26, 2021.
- Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annual Review of Clinical Psychology. 2005;1:607-628.
- Seal KH, Becker WC, Murphy JL, et al. Whole Health Options and Pain Education (wHOPE): a pragmatic trial comparing whole health team vs primary care group education to promote nonpharmacological strategies to improve pain, functioning, and quality of life in veterans—rationale, methods, and implementation. Pain Medicine. 2020;21(suppl 2):S91-S99.
- Tamashiro KL, Sakai RR, Shively CA, et al. Chronic stress, metabolism, and metabolic syndrome. Stress. 2011;14(5):468-474.
- Whayne TF Jr, Saha SP. Genetic risk, adherence to a healthy lifestyle, and ischemic heart disease. Current Cardiology Reports. 2019;21(1):1.
- Whole Health Institute website. Accessed at https://www.wholehealth.org/ on May 19, 2021.
Acknowledgments
NCCIH thanks Mary Beth Kester, M.S., and Helene M. Langevin, M.D., NCCIH, for their review of this publication.
This publication is not copyrighted and is in the public domain. Duplication is encouraged.
NCCIH has provided this material for your information. It is not intended to substitute for the medical expertise and advice of your health care provider(s). We encourage you to discuss any decisions about treatment or care with your health care provider. The mention of any product, service, or therapy is not an endorsement by NCCIH.
Related Topics
NCCIH Strategic Plan FY 2021–2025 Mapping a Pathway to Research on Whole Person Health
Methodological Approaches for Whole Person Research Workshop
Transforming Veterans’ Health: Implementing a Whole Health System of Care
Complementary, Alternative, or Integrative Health: What’s In a Name?
- See us on twitter
- See us on youtube
- See us on instagram
IGNITE Firefighters' Health

In partnership with the San Francisco Firefighters Cancer Prevention Foundation , we are conducting a study to determine if a diet low in processed foods, mostly plant-based, and rich in fermented foods can reduce cancer risk in firefighters.
Firefighters face a 9% higher risk of cancer diagnosis and a 14% higher risk of cancer-related death compared to the general population, largely due to exposure to hazardous chemicals.
The study will involve 120 firefighters, split into two groups: one continuing their usual diet and the other adopting a diet based on whole foods, including plant-based options, fish, eggs, and fermented foods. The study will measure cancer risk markers, cardiometabolic risk markers, and changes in microbiome composition over eight weeks, with the goal of reducing inflammation and cancer risk.
Participant recruitment for the study will start once IRB approval is obtained.
Stanford Prevention Research Center

Living in tree-filled neighborhoods may reduce risk of heart disease, study shows
Living in a tree-filled neighborhood may be as beneficial to the heart as regular exercise, new research shows.
Researchers at the University of Louisville designed a clinical trial that followed hundreds of people living in six low- to middle-income neighborhoods in South Louisville, Kentucky. They used blood and other samples to better understand how their heart risks changed before and after the team planted thousands of mature trees near their homes.
Results from the Green Heart Louisville Project ’s HEAL Study , released Tuesday, showed that people living in neighborhoods with twice as many trees and shrubs had lower levels of a blood marker associated with heart disease, diabetes and some types of cancer compared with those who lived in more tree-bare neighborhoods.

“We are trying to see if we can decrease the rates of heart disease in a community,” said Aruni Bhatnagar, a professor of medicine at the University of Louisville, who led the project.
Most previous studies showing the effects of nature on mental and physical health are observational and can’t answer whether people who live in green communities are healthier because they’re wealthier and have access to better health care.
The HEAL study was set up with a control group and an intervention, meaning something measurable that some of the participants were exposed to during the study but not before.

Bhatnagar and his team recruited about 750 people living in a 4-mile area of South Louisville cut by a highway. The residents were 25 to 75 years old.
Nearly 80% were white, and 60% identified as female. Half reported average household incomes of $50,000.
The researchers collected blood, urine, nail and hair samples, as well as health data, from each person before they began their intervention.
Then, from 2019 to 2022, they planted nearly 8,500 evergreen trees, 630 deciduous trees — the type that lose leaves in the fall — and 45 different types of shrubs in parts of the 4-mile study area, leaving others untouched.
Last year and this year, they took new samples from residents living in both areas.
People living in the intervention areas had 13% lower levels of high-sensitivity C-reactive protein , a blood marker associated with heart disease, including stroke, coronary artery disease and heart attack. The drop was similar to starting a regular exercise routine, Bhatnagar said.
“I wouldn’t have expected such a strong biomarker response, and that speaks to maybe something truly is causal here with how trees impact health,” said Peter James, director of the Center for Occupational and Environmental Health at the University of California, Davis School of Medicine, who wasn’t involved in the new research.

How trees can improve physical health
Previous research has shown spending time in green spaces boosts mental health .
The new study showed the connection between living among more trees and physical health.
Trees provide shade and cool the areas where they’re planted, helping quell the urban heat effect that disproportionately affects low-income neighborhoods and neighborhoods of color. Hot weather aggravates heart disease and can cause heatstroke in people without pre-existing conditions.
Trees also buffer noise, which is linked to higher rates of cardiovascular disease, James said.
“They provide areas for people to relax, exercise, and probably more importantly, socialize,” Joan Casey, an environmental epidemiologist and associate professor of environmental and occupational health sciences at the University of Washington, said in an email.
“They also replace other health-harmful land uses, like industrial sites,” she said.
Because one of the city’s major highways cuts through the study area, Bhatnagar and his team believe, trees’ ability to filter air pollution and buffer neighborhoods from constantly breathing in harmful particles could be a primary way the tree-planting intervention appeared to lower inflammation markers in people living in greened areas.
During the study, the project planted trees only in the parts of South Louisville that had the worst air quality. It took air quality samples before the project, and it is still analyzing how the new tree cover has affected pollution. It’s a complex undertaking, because air quality fluctuates based on the weather — a windy day might increase or decrease air pollution in certain areas, depending on the direction of the wind, and air pollution is worse on hotter days.
The project plans to plant trees in the control group neighborhoods in another three or four years if the intervention neighborhoods continue to show positive results. It also wants to determine whether tree cover improves sleep or children’s immune systems by encouraging outside play.
“There is no sort of ultimate proof,” Bhatnagar said. “But this is the strongest evidence of any study that’s ever been done on trees and their relationship to health.”
Growing evidence shows the importance of ensuring green spaces are equitably distributed around cities, which is currently not the case .
Casey said it’s important that city planners be careful not to create “green gentrification” when they create more equitable access to green spaces in cities — that is, when spaces such as water fronts are restored and housing prices increase as a result, making it unaffordable for current residents to continue living there once a green space is completed.
“The take-home message here is that nature is not an amenity; green spaces are not a perk for the wealthy. They are essential for us as human beings,” James said.
Kaitlin Sullivan is a contributor for NBCNews.com who has worked with NBC News Investigations. She reports on health, science and the environment and is a graduate of the Craig Newmark Graduate School of Journalism at City University of New York.
Anne Thompson is NBC News’ chief environmental affairs correspondent.
Queensland research finds young people 'burnt out and in need of help'
By Claudia Williams
Topic: Mental Health
New research shows almost nine out of 10 young Queenslanders have seen a negative change in their health and wellbeing in the past year. ( ABC News: Stephanie Anderson )
It is impossible to ignore the negative impacts of smartphones and social media on the mental health and wellbeing of young people, Queensland’s chief health officer says.
The comments come as new research shows almost nine out of 10 young Queenslanders have seen a negative change in their health and wellbeing in the past year.
The survey of 1,424 young people conducted by the state's prevention agency, Health and Wellbeing Queensland, found more than half of respondents reported feeling stressed or anxious.
Chief Health Officer Dr John Gerrard said while less people were dying from heart disease and strokes, the mental health of young people was "getting worse very rapidly".
"It appears to be a real phenomenon and not the result of better reporting," he said. "I believe this is a very significant concern.
"One of the most dramatic indicators is the instances of hospitalisation due to self-harm in young children aged 10 to 14 has almost [tripled] over the last decade."
John Gerrard says the mental ill-health of young people is a very real phenomenon being seen across the world. ( ABC News: Claudia Williams )
Dr Gerrard said the mental health decline in young people had been seen on a global scale since 2010, in the years following the release of the first smartphone.
He said there were no simple solutions, adding the community at-large has not spoken about "this enough".
"It is not clear at this stage what to do about this specific problem, but I have been meeting with Commonwealth agencies to discuss these issues."
'Burnt out and in need of help'
The research, commissioned by the Queensland government, found more than half of those aged 15 to 24 reported feeling tired for no reason or that everything was an effort in the four weeks prior to being surveyed.
Health and Wellbeing Queensland deputy chief executive Gemma Hodgetts said these were the warning signs of a generation "burnt out and in need of help".
"Young Queenslanders who should be our most vibrant, energetic and hopeful generation are struggling," she said.
Gemma Hodgetts says the research shows young people are struggling. ( ABC News: Claudia Williams )
"Almost one in two Queenslanders will experience mental ill-health in their lifetime ... about 75 per cent of mental disorders emerge before the age of 24 years, so we need to act now."
The research found those experiencing mental health challenges were more likely to rate their health significantly lower.
The report said the findings suggest increased stress, along with poorer diets, may be negatively impacting the mental health of young Queenslanders, particularly young adults.
According to the research, women, girls and mothers are also more likely to experience negative impacts, which may in part be due to their lower activity levels.
Ms Hodgetts said the report laid the foundation for an Australian-first strategy which would take a deliberate wellbeing approach to mental health.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Environmental Factor
Your online source for niehs news, september 2024, researchers chart a path forward for report back.
NIH kicked off program aimed at studying how to responsibly report back research results to environmental health study participants.
By Marla Broadfoot
In environmental health, "report back" refers to the process of reporting research findings back to the individuals and communities involved in a study. This practice is crucial for fostering transparency, building trust, and promoting actionable change. However, debate around how to ethically report back continues to evolve as the research community shifts to engage study participants as full and equal partners.
On July 26, NIEHS, in partnership with the National Institutes of Health (NIH) Office of Science Policy and the National Human Genome Research Institute (NHGRI), hosted a kickoff meeting for recipients of NIH grants focused on charting a responsible path forward for report back. Researchers from across the country presented eight different awarded projects aimed at informing and developing guidelines, educational resources, and community engagement approaches for effective communication of research results.
“Report back is complicated, and there are lots of different variables that have to be considered in our studies,” said NIEHS Director Rick Woychik, Ph.D. “I’m encouraged that we have a diversified portfolio of research strategies to address these complexities so that we can move forward to get the job done.”
Right to know
Recent research indicates that study participants want to receive their environmental research results, even if the implications for disease risk or health implications are still uncertain. A 2018 report by the National Academies of Sciences, Engineering, and Medicine (NASEM) considered the various issues surrounding report back and provided some early guidance for researchers.
“This report stated in no uncertain terms that report back should be the norm when possible going forward,” said NIEHS Health Scientist Administrator Kimberly McAllister, Ph.D.
In other words, people have the “right to know” the information generated by their research participation. The new grant opportunity, which is a partnership between NIEHS, the NIH Office of Science Policy, the All of Us Research Program, and NHGRI, aims to develop tools and approaches for report back and guidance for addressing the ethical, legal, and social challenges that are part of the process.
“There's a lot of concerns about stigma, discrimination, and privacy risks associated with report back, and this is especially of concern for communities that may be carrying the heaviest burden of chemical exposures,” said McAllister.
Right to design

One project funded by the new grant opportunity is exploring how to report back results on both social determinants of health and environmental exposures — such as arsenic, lead, and cadmium levels in soil and dust — in rural and urban communities that are disproportionately affected by pollution.
“One of the key bioethical questions guiding this work is how do we address technical elitism,” asked Mónica Ramírez-Andreotta, Ph.D. , who leads the project from the University of Arizona.
Ramírez-Andreotta has been using an equity-centered community design approach to identify the factors that influence preferred report back strategies.
“The rationale behind this is extending the ‘right to know’ to the ‘right to design’ and introducing democracy-based values into the report back process,” she said.
Right to understand

Experience shows that participants can benefit from report back and concerns about harm have been overstated, according to the 2018 NASEM report. However, researchers still expressed the desire to carefully navigate specific situations to make sure that report back does not cause harm, citing unintended consequences such as declining property values.
Some also voiced the need for trained environmental counselors, analogous to genetic counselors, who can help participants make sense of their research results. Others shared efforts to forge bidirectional communication between researchers and participants, to move the field from the “right to know” to the “right to understand.”
“These studies create opportunities to expand environmental health literacy about the multifactorial causes of disease in a way that empowers communities and individuals to take action,” said Julia Brody, Ph.D. , from the Silent Spring Institute.
Reporting back
Liam O’Fallon , who directs the NIEHS Partnerships for Environmental Public Health, said that plans are underway to convene the group again within the next six months to identify the logical next steps for advancing the specific aims of each of the projects as well as the broader objectives of the overall program.
“Today, I’ve seen many connections forming in various areas, whether it's environmental health literacy scales and measures, hubs and portals for sharing our lessons learned, or the products, knowledge, and frameworks we need to report back effectively,” he said. “With all of us coming together, I think we are going be able to do something pretty spectacular.”
Citation : Creative Reaction Lab [Internet]. 2018. Equity Centered Community Design Field Guide. Available from: https://crxlab.org/our-approach .
(Marla Broadfoot, Ph.D., is a contract writer for the NIEHS Office of Communications and Public Liaison.)

This new grant opportunity will provide a total of more than $4 million in funding over the next four years to support studies on responsibly reporting back environmental health and non-genomic research results . The following researchers presented overviews of their projects at the kickoff meeting.
- Best practices to return results about pesticide exposures in family child care homes (Abbey Alkon, Ph.D., University of California, San Francisco).
- From the cell to the street: Personalized report-back in large cohort studies with multi-level measurements (Julia Brody, Ph.D., Silent Spring Institute).
- Collaborative development of ethical report-back guidelines for household exposure research (Katrina Korfmacher, Ph.D., University of Rochester Medical Center).
- Engaging community Members to Plan for dissemination Of Wastewater Epidemiology Results: the EMPOWER study (Amy McGuire, J.D., Ph.D., Baylor College of Medicine).
- Disentangling the role of culture, life stage, and information design to facilitate equity in data report back (Mónica Ramírez-Andreotta, Ph.D., University of Arizona).
- Translating Research to Action & Knowledge (TRAK) Portal: a web-based platform for report-back of research results (Diana Rohlman, Ph.D., Oregon State University).
- Evaluation of report-back strategies for long-term and short-term exposure information in rural tribal populations (Scott Collingwood, Ph.D., University of Utah School of Medicine).
- Reframing personal and community report back of consumer products by centering intersectionality (Ami Zota, Sc.D., Columbia University Mailman School of Public Health).
Related Articles

Biomedical research program for local teachers is a success

Tribal leaders focus on environmental health, data sovereignty

Advisory Council considers military exposures, community-based research

NIEHS honors Juneteenth with health equity talk, wellness activities

NIEHS Women’s Health Awareness event celebrates 10th anniversary

IMAGES
COMMENTS
The Clinical Center provides hope through pioneering clinical research to improve human health. We rapidly translate scientific observations and laboratory discoveries into new ways to diagnose, treat and prevent disease. More than 500,000 people from around the world have participated in clinical research since the hospital opened in 1953.
Health research entails systematic collection or analysis of data with the intent to develop generalizable knowledge to understand health challenges and mount an improved response to them. The full spectrum of health research spans five generic areas of activity: measuring the health problem; understanding its cause(s); elaborating solutions; translating the solutions or evidence into policy ...
Join a National Registry of Research Volunteers ResearchMatch This is an NIH-funded initiative to connect 1) people who are trying to find research studies, and 2) researchers seeking people to participate in their studies. It is a free, secure registry to make it easier for the public to volunteer and to become involved in clinical research studies that contribute to improved health in the ...
At Mayo Clinic, research is essential to improving patient care. See how discoveries are translated into therapies that improve lives. Find clinical trials.
Objective. This article aims to provide a brief overview and summary of the evidence on: 1) the preventive effects of physical activity on a wide range of mental disorders; 2) the role of physical activity in promoting the physical health of people with mental disorders; 3) the role of exercise as a strategy to manage mental health symptoms in ...
What is Health Research? The term "health research," sometimes also called "medical research" or "clinical research," refers to research that is done to learn more about human health. Health research also aims to find better ways to prevent and treat disease. Health research is an important way to help improve the care and treatment of people worldwide.
A new study from the National Institutes of Health has performed more diverse and extensive biological measurements of people experiencing chronic fatigue syndrome than any previous research. Here's what they found and what it means. Staying Healthy
Qualitative Health Research (QHR) is a peer-reviewed monthly journal that provides an international, interdisciplinary forum to enhance health care and further the development and understanding of qualitative research in health-care settings. QHR is an … | View full journal description. This journal is a member of the Committee on Publication ...
Official website of the National Institutes of Health (NIH). NIH is one of the world's foremost medical research centers. An agency of the U.S. Department of Health and Human Services, the NIH is the Federal focal point for health and medical research. The NIH website offers health information for the public, scientists, researchers, medical professionals, patients, educators, and students ...
The review highlights the (1) concept of preventive psychiatry, including various mental health promotions and prevention approaches, (2) current level of evidence of various mental health preventive interventions, including the novel interventions, and (3) challenges and opportunities in implementing concepts of preventive psychiatry and ...
The previous chapter reviewed the value of privacy, while this chapter examines the value and importance of health research. As noted in the introduction to Chapter 2, the committee views privacy and health research as complementary values. Ideally, society should strive to facilitate both for the benefit of individuals as well as the public.
The Google Health Studies app lets you securely contribute to health research studies with leading institutions, right from your phone.
Research for health is a global endeavour, and WHO has a unique role to play in ensuring that these efforts can help improve health for all. WHO provides leadership, calling on the wider scientific community to engage behind global health concerns. This is based on a deep understanding of the needs of countries, and rigorous assessment by ...
Health & Medical News Dive into the latest Health & Medical News, with a special emphasis on cutting-edge research studies. Our coverage spans from clinical trials to innovative medical research, providing deep insights into new treatments and healthcare advancements. Stay informed about the scientific developments that are transforming health and medicine.
A mega list of research topic ideas in healthcare, including allopathic and alternative medicine, dentistry, rehab, optometry and more.
Health Research & Clinical Trials. Carefully conducted clinical trials are the safest and fastest way to find effective vaccines, treatments, and new ways to improve health. The University of Rochester Medical Center is currently conducting several clinical trials that are in need of volunteers. Learn about studies for all diseases and ...
Public Health Research This type of research can be one or a combination of the types of research mentioned above. Public health research tries to improve the health and well-being of people from a population-level perspective.
Help researchers solve health problems - Volunteers play a key role in research and make new discoveries possible. Your participation helps researchers find new ways to prevent, detect, or treat disease.
What is Health Research? Health research is an investigation of a human health issue to learn more about it. It is usually funded by the government, private foundations, and/or drug companies with the hope that the new information will be useful to patients, the community, and other researchers.
Health research methodology: A guide for training in research methods INTRODUCTION This is a revised version of an earlier manual on Health Research Methodology and deals with the basic concepts and principles of scientific research methods with particular attention to research in the health field. The research process is the cornerstone for ...
This research includes several large, long-term epidemiological studies—such as the Framingham Heart Study, Nurses' Health Study, and Adventist Health Studies—that have evaluated the connections between lifestyle, diet, genetics, health, and disease. ... International Journal of Environmental Research and Public Health. 2021;18(6):2882.
A systematic review into the potential health effects from radio wave exposure has shown mobile phones are not linked to brain cancer. The review was commissioned by the World Health Organization ...
In partnership with the San Francisco Firefighters Cancer Prevention Foundation, we are conducting a study to determine if a diet low in processed foods, mostly plant-based, and rich in fermented foods can reduce cancer risk in firefighters. Firefighters face a 9% higher risk of cancer diagnosis and a 14% higher risk of cancer-related death compared to the general population, largely due to ...
How trees can improve physical health. Previous research has shown spending time in green spaces boosts mental health. The new study showed the connection between living among more trees and ...
Dr John Gerrard has linked the rise in social media with the rise in teenagers' mental health issues. The report said the findings suggest increased stress, along with poorer diets, may be ...
Recent research indicates that study participants want to receive their environmental research results, even if the implications for disease risk or health implications are still uncertain. A 2018 report by the National Academies of Sciences, Engineering, and Medicine (NASEM) considered the various issues surrounding report back and provided ...
Study co-authors included Christopher N. Morrison, Charles C. Branas, Ariana N. Gobaud and Sonali Rajan of Columbia University, and Douglas J. Wiebe of the University of Michigan. This study was funded in part by the National Collaborative on Gun Violence Research and the Arnold Foundation.