A-Level AQA Biology - Biological Molecules
Finish sign up, protein structure.
Proteins are large polymers made up of amino acids. These amino acids are arranged in a series of structures to create the finished 3D protein. There are up to four levels of structural arrangements in a protein, as shown in the image below, which will each be explained fully.

Proteins are polymer chains, or polypeptides, created on the ribosome in cells and are then further folded and modified in the Golgi apparatus.

Primary Structure
The first structure that forms in the creation of a protein is the polypeptide chain. Proteins are all made up of one or more polypeptide chains folded into highly specific 3D shapes. The exact definition of primary structure is: The sequence of amino acids in a polypeptide chain.
This would be a one-mark question, and it is essential to state the word ‘ sequence ’. The order in which the amino acids are bonded is determined by DNA. This specific order of amino acids will alter how the protein further folds and is bonded, enabling unique functions.
There are 20 different amino acids that can form the primary structure. The polypeptide chain is created by a series of condensation reactions occurring between amino acids.
Secondary Structure
The sequence of amino acids causes parts of a protein molecule to bend into α helix shapes or fold into β pleated sheets. Hydrogen bonds between the carboxyl groups of one amino acid and the amino group of another are what hold the secondary structure in place. Hydrogen bonds are individually weak bonds, but when there are large numbers of them, they provide collective strength.

Describing the secondary structure would be a 2-mark question. The two marks would be for stating:
- Folding the primary structure into α helix shapes or fold into β pleated sheets
- Held in place by hydrogen bonds
Tertiary Structure
The secondary structure is bent and folded to form a precise 3D shape. This unique 3D shape is held in place by hydrogen bonds, ionic bonds, and sometimes disulfide bonds. Disulfide bonds (di meaning 2) only form between the R-groups of two amino acids that contain sulfur. Describing the tertiary structure of a protein is a 3-mark question. The 3 marks are typically:
- The further folding of the secondary structure
- To create a unique 3D structure
- Held in place by hydrogen, ionic, and disulfide bonds.
Make sure you always mention the bonds involved when describing protein structures; there are always marks for this because without these bonds, the unique shapes are not maintained.

Quaternary Structure
A protein that is made up of more than one polypeptide chain is a quaternary structure protein. It is still folded into a 3D shape and held by hydrogen, ionic, and disulfide bonds. Hemoglobin is the typical example given. Hemoglobin is made up of 4 polypeptide chains.

In the diagram of hemoglobin, you can also see extra molecules attached that are not part of the polypeptide chains. Any group that is attached to a protein but is not made up of amino acids is known as a prosthetic group. Iron is the prosthetic group in hemoglobin. A protein that has a prosthetic group can be described as a conjugated protein, which simply means a non-protein group is added onto it.
Fibrous & Globular Proteins
The 3D folding in tertiary and quaternary proteins generally results in either a shape that is spherical, called globular proteins, or a long rope-like shape, called fibrous proteins. Fibrous Protein Fact File:
- Polypeptide chains form long twisted strands linked together
- Stable structure
- Insoluble in water
- Strength gives structural function
- e.g., collagen in bone and keratin in hair.

Globular Protein Fact File: Polypeptide chains ‘roll up’ into a spherical shape
- Relatively unstable structure
- Metabolic functions
- e.g., all enzymes, antibodies, some hormones (e.g., insulin), hemoglobin.


Personalised lessons and regular feedback to ensure you ace your exams! Book a free consultation today
100+ Video Tutorials, Flashcards and Weekly Seminars
Gain hands-on experience of how physics is used in different fields. Experience life as a uni student and boost your university application with our summer programme!
- Revision notes >
- A-Level Biology Revision Notes >
- CIE A-level Biology Revision Notes
Proteins and Amino Acids: An Introduction (A-level Biology)
Proteins and amino acids: an introduction.
Proteins are biological molecules that are made up of small monomers i.e. amino acids.
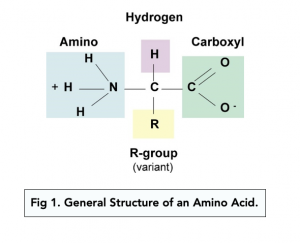
Structure of Amino Acids
- Amino acids are the monomers of proteins .
- There are 20 amino acids and they all have very similar structures . On one end, they contain an amine group (-NH2). This is known as the N-terminus . On the opposite end they contain a carboxyl group (-COOH) which makes them an acid. This is known as the C-terminus .
- The amine group and carboxyl group are covalently bonded to a central carbon atom . The central carbon atom is bonded to one hydrogen (H) atom, and a variable “R” group.
- The variable “R” group is different for each amino acid . This variable group is what gives each amino acid its unique chemical and physical properties. This group is also commonly known as the side chain.
- Different amino acids can be grouped together based on their “R” groups .
Still got a question? Leave a comment
Leave a comment, cancel reply.
Save my name, email, and website in this browser for the next time I comment.
CIE 1 Cell structure
Roles of atp (a-level biology), atp as an energy source (a-level biology), the synthesis and hydrolysis of atp (a-level biology), the structure of atp (a-level biology), magnification and resolution (a-level biology), calculating cell size (a-level biology), studying cells: confocal microscopes (a-level biology), studying cells: electron microscopes (a-level biology), studying cells: light microscopes (a-level biology), life cycle and replication of viruses (a-level biology), cie 10 infectious disease, bacteria, antibiotics, and other medicines (a-level biology), pathogens and infectious diseases (a-level biology), cie 11 immunity, types of immunity and vaccinations (a-level biology), structure and function of antibodies (a-level biology), the adaptive immune response (a-level biology), introduction to the immune system (a-level biology), primary defences against pathogens (a-level biology), cie 12 energy and respiration, anaerobic respiration in mammals, plants and fungi (a-level biology), anaerobic respiration (a-level biology), oxidative phosphorylation and chemiosmosis (a-level biology), oxidative phosphorylation and the electron transport chain (a-level biology), the krebs cycle (a-level biology), the link reaction (a-level biology), the stages and products of glycolysis (a-level biology), glycolysis (a-level biology), the structure of mitochondria (a-level biology), the need for cellular respiration (a-level biology), cie 13 photosynthesis, limiting factors of photosynthesis (a-level biology), cyclic and non-cyclic phosphorylation (a-level biology), the 2 stages of photosynthesis (a-level biology), photosystems and photosynthetic pigments (a-level biology), site of photosynthesis, overview of photosynthesis (a-level biology), cie 14 homeostasis, ectotherms and endotherms (a-level biology), thermoregulation (a-level biology), plant responses to changes in the environment (a-level biology), cie 15 control and co-ordination, the nervous system (a-level biology), sources of atp during contraction (a-level biology), the ultrastructure of the sarcomere during contraction (a-level biology), the role of troponin and tropomyosin (a-level biology), the structure of myofibrils (a-level biology), slow and fast twitch muscles (a-level biology), the structure of mammalian muscles (a-level biology), how muscles allow movement (a-level biology), the neuromuscular junction (a-level biology), features of synapses (a-level biology), cie 16 inherited change, calculating genetic diversity (a-level biology), how meiosis produces variation (a-level biology), cell division by meiosis (a-level biology), importance of meiosis (a-level biology), cie 17 selection and evolution, types of selection (a-level biology), mechanism of natural selection (a-level biology), types of variation (a-level biology), cie 18 biodiversity, classification and conservation, biodiversity and gene technology (a-level biology), factors affecting biodiversity (a-level biology), biodiversity calculations (a-level biology), introducing biodiversity (a-level biology), the three domain system (a-level biology), phylogeny and classification (a-level biology), classifying organisms (a-level biology), cie 19 genetic technology, cie 2 biological molecules, properties of water (a-level biology), structure of water (a-level biology), test for lipids and proteins (a-level biology), tests for carbohydrates (a-level biology), protein structures: globular and fibrous proteins (a-level biology), protein structures: tertiary and quaternary structures (a-level biology), protein structures: primary and secondary structures (a-level biology), protein formation (a-level biology), phospholipid bilayer (a-level biology), cie 3 enzymes, enzymes: inhibitors (a-level biology), enzymes: rates of reaction (a-level biology), enzymes: intracellular and extracellular forms (a-level biology), enzymes: mechanism of action (a-level biology), enzymes: key concepts (a-level biology), enzymes: introduction (a-level biology), cie 4 cell membranes and transport, transport across membranes: active transport (a-level biology), investigating transport across membranes (a-level biology), transport across membranes: osmosis (a-level biology), transport across membranes: diffusion (a-level biology), signalling across cell membranes (a-level biology), function of cell membrane (a-level biology), factors affecting cell membrane structure (a-level biology), structure of cell membranes (a-level biology), cie 5 the mitotic cell cycle, chromosome mutations (a-level biology), cell division: checkpoints and mutations (a-level biology), cell division: phases of mitosis (a-level biology), cell division: the cell cycle (a-level biology), cell division: chromosomes (a-level biology), cie 6 nucleic acids and protein synthesis, transfer rna (a-level biology), transcription (a-level biology), messenger rna (a-level biology), introducing the genetic code (a-level biology), genes and protein synthesis (a-level biology), synthesising proteins from dna (a-level biology), structure of rna (a-level biology), dna replication (a-level biology), dna structure and the double helix (a-level biology), polynucleotides (a-level biology), cie 7 transport in plants, translocation and evidence of the mass flow hypothesis (a-level biology), the phloem (a-level biology), importance of and evidence for transpiration (a-level biology), introduction to transpiration (a-level biology), the pathway and movement of water into the roots and xylem (a-level biology), the xylem (a-level biology), cie 8 transport in mammals, controlling heart rate (a-level biology), structure of the heart (a-level biology), transport of carbon dioxide (a-level biology), transport of oxygen (a-level biology), exchange in capillaries (a-level biology), structure and function of blood vessels (a-level biology), cie 9 gas exchange and smoking, lung disease (a-level biology), pulmonary ventilation rate (a-level biology), ventilation (a-level biology), structure of the lungs (a-level biology), general features of exchange surfaces (a-level biology), understanding surface area to volume ratio (a-level biology), the need for exchange surfaces (a-level biology), edexcel a 1: lifestyle, health and risk, phospholipids – introduction (a-level biology), edexcel a 2: genes and health, features of the genetic code (a-level biology), gas exchange in plants (a-level biology), gas exchange in insects (a-level biology), edexcel a 3: voice of the genome, edexcel a 4: biodiversity and natural resources, edexcel a 5: on the wild side, reducing biomass loss (a-level biology), sources of biomass loss (a-level biology), transfer of biomass (a-level biology), measuring biomass (a-level biology), net primary production (a-level biology), gross primary production (a-level biology), trophic levels (a-level biology), edexcel a 6: immunity, infection & forensics, microbial techniques (a-level biology), the innate immune response (a-level biology), edexcel a 7: run for your life, edexcel a 8: grey matter, inhibitory synapses (a-level biology), synaptic transmission (a-level biology), the structure of the synapse (a-level biology), factors affecting the speed of transmission (a-level biology), myelination (a-level biology), the refractory period (a-level biology), all or nothing principle (a-level biology), edexcel b 1: biological molecules, inorganic ions (a-level biology), edexcel b 10: ecosystems, nitrogen cycle: nitrification and denitrification (a-level biology), the phosphorus cycle (a-level biology), nitrogen cycle: fixation and ammonification (a-level biology), introduction to nutrient cycles (a-level biology), edexcel b 2: cells, viruses, reproduction, edexcel b 3: classification & biodiversity, edexcel b 4: exchange and transport, edexcel b 5: energy for biological processes, edexcel b 6: microbiology and pathogens, edexcel b 7: modern genetics, edexcel b 8: origins of genetic variation, edexcel b 9: control systems, ocr 2.1.1 cell structure, structure of prokaryotic cells (a-level biology), eukaryotic cells: comparing plant and animal cells (a-level biology), eukaryotic cells: plant cell organelles (a-level biology), eukaryotic cells: the endoplasmic reticulum (a-level biology), eukaryotic cells: the golgi apparatus and lysosomes (a-level biology), ocr 2.1.2 biological molecules, introduction to eukaryotic cells and organelles (a-level biology), ocr 2.1.3 nucleotides and nucleic acids, ocr 2.1.4 enzymes, ocr 2.1.5 biological membranes, ocr 2.1.6 cell division, diversity & organisation, ocr 3.1.1 exchange surfaces, ocr 3.1.2 transport in animals, ocr 3.1.3 transport in plants, examples of xerophytes (a-level biology), introduction to xerophytes (a-level biology), ocr 4.1.1 communicable diseases, structure of viruses (a-level biology), ocr 4.2.1 biodiversity, ocr 4.2.2 classification and evolution, ocr 5.1.1 communication and homeostasis, the resting potential (a-level biology), ocr 5.1.2 excretion, ocr 5.1.3 neuronal communication, hyperpolarisation and transmission of the action potential (a-level biology), depolarisation and repolarisation in the action potential (a-level biology), ocr 5.1.4 hormonal communication, ocr 5.1.5 plant and animal responses, ocr 5.2.1 photosynthesis, ocr 5.2.2 respiration, ocr 6.1.1 cellular control, ocr 6.1.2 patterns of inheritance, ocr 6.1.3 manipulating genomes, ocr 6.2.1 cloning and biotechnology, ocr 6.3.1 ecosystems, ocr 6.3.2 populations and sustainability, related links.
- A-Level Biology Past Papers
Boost your A-Level Biology Performance
Get a 9 in A-Level Biology with our Trusted 1-1 Tutors. Enquire now.
100+ Video Tutorials, Flashcards and Weekly Seminars. 100% Money Back Guarantee
Gain hands-on experience of how physics is used in different fields. Boost your university application with our summer programme!
Learn live with other students and gain expert tips and advice to boost your score.

Let's get acquainted ? What is your name?
Nice to meet you, {{name}} what is your preferred e-mail address, nice to meet you, {{name}} what is your preferred phone number, what is your preferred phone number, just to check, what are you interested in, when should we call you.
It would be great to have a 15m chat to discuss a personalised plan and answer any questions
What time works best for you? (UK Time)
Pick a time-slot that works best for you ?
How many hours of 1-1 tutoring are you looking for?
My whatsapp number is..., for our safeguarding policy, please confirm....
Please provide the mobile number of a guardian/parent
Which online course are you interested in?
What is your query, you can apply for a bursary by clicking this link, sure, what is your query, thank you for your response. we will aim to get back to you within 12-24 hours., lock in a 2 hour 1-1 tutoring lesson now.
If you're ready and keen to get started click the button below to book your first 2 hour 1-1 tutoring lesson with us. Connect with a tutor from a university of your choice in minutes. (Use FAST5 to get 5% Off!)
PMT Education is looking for a full-time Customer Support Specialist

AQA A-Level Biology Revision
For each of the papers below, there are revision notes, summary sheets, questions from past exam papers separated by topic and other worksheets.
AS Papers 1 & 2
A-level paper 1, a-level paper 2, a-level paper 3.
- Revision Courses
- Past Papers
- Solution Banks
- University Admissions
- Numerical Reasoning
- Legal Notices
A Level Biology
Instant Access to A Level Biology Revision
Sign up now to get access to the entire library of a level biology resources for all exam boards.
If you're ready to pass your A-Level Biology exams, become a member now to get complete access to our entire library of revision materials.
Join over 22,000 learners who have passed their exams thanks to us!
Sign up below to get instant access!
Or try a sample...
Not ready to purchase the revision kit yet? No problem. If you want to see what we offer before purchasing, we have a free membership with sample revision materials.
Signup as a free member below and you'll be brought back to this page to try the sample materials before you buy.
Functions of Proteins
All enzymes are proteins, functions of proteins: they act as receptors on cell membranes, some hormones are also proteins, proteins act as transport channels in cell membranes, proteins maintain the shape and structure of a cell, proteins are involved in cell division, proteins are required for transport within a cell, proteins are necessary for the transport of various substances in the blood, proteins are involved in muscle contraction, proteins prevent edema, proteins protect our body against diseases, proteins are needed for digestion, proteins also act as storage substances, proteins control the expression of genes, what are some examples of proteins, what are the functions of albumin, what are the functions of collagen, what are the functions of antibodies.
Proteins are the most abundant organic molecules present on earth. They are present abundantly in every living cell. Proteins are the polymers made up of thousands of amino acids linked via peptide bonds. Long chains of amino acids known as polypeptides fold around themselves in several ways to form complex structures called proteins.
Functions performed by proteins can be divided into different categories. Some functions are essential at the cellular level while others are required for the better performance of the body as a whole. Here, we will try to understand different functions performed by proteins in our body through various examples.
Enzymes are the proteins that are required for any chemical reaction to take place in our body. They catalyze the biochemical reaction so that life can proceed.
An example of enzymatic reaction in our body is glycolysis. This is the process by which energy is released from a glucose molecule. This energy is required to carry out several processes taking place within a cell. The process of glycolysis involves around 10 steps each requiring a particular enzyme. The absence of a single enzyme stops the process and energy from glucose cannot be obtained.
Synthesis of proteins also requires specific enzymes. Protein synthesis involves transcription of DNA into mRNA and then translation of mRNA by ribosomes. Both these steps require enzymes that are proteins. For example;
- RNA polymerase is an enzyme required to join RNA nucleotides in the process of transcription.
- Aminoacyl tRNA synthetase is an enzyme that attaches specific amino acids to tRNA so that it can be used in protein synthesis.
Thus, from obtaining energy to making proteins, all chemical processes in living organisms need enzymes, and all enzymes are proteins. The role of proteins as enzymes is the most important and crucial function performed by proteins.
Proteins are essential components of all the cell membranes and membranes of the organelles. One of the functions of these membrane proteins is that they act as receptors. Hormones, neurotransmitters, and other signalling molecules bind to these receptors and convey signals to cells. In this way, proteins play a role in cell signalling that is essential for the coordinated function of all the cells present in our body. Take the following example to understand the role of proteins as receptors.
- Insulin is a hormone that controls the glucose levels in our blood. It performs its function by binding to its receptor that is a protein. Insulin binds to its receptor that sends signals for the opening of glucose channels so that glucose can be taken up from the blood into the liver and muscle cells. If the insulin receptors are not present, the blood glucose levels cannot be regulated.
This and various other examples in our body prove why proteins are necessary for cell signalling and coordination of cellular functions.
Read more about Tests for Proteins
Proteins not only act as cellular receptors but also hormones. Insulin and Glucagon are the two hormones that are protein in nature. Both these hormones are required for the regulation of blood glucose levels. They control the uptake and release of glucose by the cells, glycolysis and gluconeogenesis, as well as the synthesis and degradation of glycogen. The roles of these hormones in our body are listed below;
- Insulin is released by the pancreas when blood glucose levels are high. It promotes glucose uptake by the cells, its breakdown as well as its storage in the form of glycogen. It also inhibits the synthesis of new glucose molecules from non-carbohydrate sources (gluconeogenesis).
- Glucagon is released by the pancreas when the blood glucose levels are low. It promotes the breakdown of glycogen to release glucose. It also promotes gluconeogenesis.
Proteins present in cell membranes also act as transport channels. Substances that are not permeable through membranes due to their size or charge can enter the cell through these protein channels. One protein channel is specific for one or more substances. Examples of protein channels are given below;
- Aquaporins are the protein channels that allow the passage of water molecules through cells
- GLUT (glucose transporter) are the transporters for glucose molecules
- Sodium channels allow the passage of sodium ions within the cell
- Potassium channels allow only potassium ions to pass through them
- Calcium channels are specific for calcium ions only
These are the few examples of protein channels present in membranes.
This is another important cellular function performed by proteins. Cytoskeleton is made up of several interlinked protein filaments. The proteins in the cytoskeleton are organized in the form of microtubules, microfilaments, and intermediate filaments. All these components of the cytoskeleton are arranged in a particular fashion that maintains the shape of a cell. Important proteins that make cytoskeleton include actin and tubulin. In the absence of these proteins, it would not be possible for a cell to maintain its structure.
Cell division is the process by which a mature adult parent cell divides into daughter cells. Proteins are required for this process also.
During cell division, the chromosomes of a cell are divided into two halves by pulling apart. This separation of chromosomes is done by proteins known as spindle fibers.
Proteins are also required for the division of cytoplasm that occurs after the chromosomes have been divided.
Specific transport proteins are needed for intracellular transport of different substances. The different proteins that are involved in intracellular proteins are known as motor proteins. These proteins use energy in the form of ATP and travel along the microtubules to transport various substances within the cytoplasm of a cell. An example of motor proteins is kinesin protein. It is involved in the transport of various substances in axons of neurons.
Proteins are needed for oxygen transport
This function of proteins is essential for the survival of the body as a whole. Two proteins are involved in this process, hemoglobin and myoglobin.
It is a protein present in the red blood cells. Hemoglobin is made up of four polypeptide chains, two alpha chains and two beta chains, that are coiled around each other. Each of these polypeptide chains carries one heme group (containing an iron atom).
This protein is responsible for the transport of oxygen from the lungs to the tissue fluid. One molecule of oxygen can bind to four molecules of oxygen. It binds to oxygen molecules present in the air while passing through the lungs. These oxygen molecules are released when the blood passes through the tissues.
Any deficiency or abnormality of hemoglobin impairs the oxygen transport by the blood. Our cells cannot survive in the absence of oxygen. Any interruption in oxygen supply will result in cell death in the affected tissues.
Myoglobin is another protein involved in the transport of oxygen. It is made up of a single polypeptide chain having a heme group. It is a cytoplasmic protein having a higher affinity for oxygen molecules meaning that it can bind to oxygen even when the concentration of oxygen is high. Its function is to transport oxygen from tissue fluid to the cells.
Because of its high affinity for oxygen, myoglobin releases oxygen at very low concentrations. This feature of myoglobin is responsible for storing oxygen in tissues.
Although blood acts as a transport medium, proteins are necessary to hold and transport some substances that cannot dissolve in blood. This function of proteins is also essential for the proper functioning of the body. Some examples of transport proteins present in blood are as follows.
- Albumin is the major transport protein in blood. It acts as a carrier for fatty acids, steroids, thyroid hormones, lipophilic drugs, heavy metals, calcium ions, and bilirubin
- Prealbumin is another transport protein in blood that carries steroid hormones, thyroxine, and vitamin A
- Haptoglobin is a transport protein that carries any free hemoglobin that is present in plasma
- Thyroxine binding protein is specific for thyroid hormone
- HDL is a lipoprotein that transports cholesterol from tissues to the liver
- LDL is another lipoprotein that transports cholesterol from the liver to the tissues
Muscle contraction is the process that enables us to perform our daily life tasks such as walking, running, sitting, standing, writing and even speaking. This process of muscle contraction is also because of proteins. Contractile proteins are present in muscle fibers. These proteins interact in a particular fashion that enables contraction and relaxation of muscles. The most important contractile present are;
Edema is a condition in which excess fluid leaks from the blood vessels and collects in the tissue spaces. The loss of fluid from blood results in decreased blood pressure. It is a potentially lethal condition that can compromise the effective delivery of blood to body tissues.
Proteins present in blood known as plasma proteins prevent the leakage of fluid through capillaries due to their osmotic effects. The oncotic pressure due to plasma proteins keeps water inside the blood vessels preventing its leakage into the tissue fluids, thus preventing edema. If these proteins are absent, edema develops in different parts of the body.
This function is performed by antibodies. Antibodies or immunoglobulins are the plasma proteins that are produced in response to various disease-causing agents entering our body. They fight against these pathogens and prevent our bodies from their harmful effects. If antibodies are already present in our body against a pathogen, they destroy the pathogen before it causes any disease. This process is known as immunity.
The process of digestion involves breaking down the complex substances present in our diet into simpler ones so that they can be absorbed into the blood. The breakdown of various dietary substances into simpler molecules takes place in our digestive system by enzymes that are proteins in nature.
Proteins are the polymers of amino acids. They act as storage substances that store thousands of amino acids. These amino acids are released from proteins when needed in the body. Examples of storage proteins are;
- Casein present in milk
- Albumin present in egg
These proteins provide the essential amino acids needed in the body to make several proteins. Moreover, in time of starvation, the proteins present in the body can also be used as an energy source to provide the calories needed for carrying out various body functions.
Gene expression is a process by which the information in a particular gene is copied in the form of mRNA and later, this mRNA is used by ribosomes to make the protein coded by that gene.
This process of gene expression is controlled by transcription factors. These transcription factors allow the transcription of genes of only those proteins that are currently required in the body.
The transcription factors are also proteins in nature. Thus, proteins regulate their own synthesis by regulating gene expression.
Proteins are the polymers made up of amino acids. They are involved in almost all the processes taking place in our body. A summary of the functions performed by proteins is as follows;
- As enzymes, proteins are required for all chemical processes in living organisms
- As hormones and cellular receptors, they are needed for cellular signalling and co-ordination
- As transport channels, protein are needed for the entry of ions and larger-sized particles into the cells
- Being components of cytoskeleton, they maintain the shape of cells
- Spindle fibers are protein fibers that are needed for cell division
- Hemoglobin and myoglobin are the proteins required for oxygen transport
- Albumin and other plasma proteins are needed for the transport of lipids, drugs and other substances in the blood
- Contractile proteins are needed for muscle contraction
- Antibodies are the proteins that protect our bodies from harmful disease
- Plasma proteins maintain fluid balance in our body
- They regulate gene expression
- Proteins also provide energy to the body in times of starvation
Frequently Asked Questions
Some examples of proteins include albumin, globulins, collagen, elastin, haemoglobin, etc.
Albumin is the most abundant plasma protein. Its main function is to provide oncotic pressure. It also acts as a carrier for the transportation of hydrophobic substances in the blood. It transports lipids, fatty acids, bilirubin, calcium, heavy metals, etc.
Collagen is a structural protein found in bones, cartilage, ligaments, etc. It provides strength and support to these structures.
Antibodies or immunoglobulins are found in the plasma. They identify antigens and bind to them. They are an essential component of humoral immunity.
- Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipurksy SL, Darnell J (2004). Molecular Cell Biology (5th ed.). New York, New York: WH Freeman and Company
- Zhang C, Kim SH (February 2003). “Overview of structural genomics: from structure to function” . Current Opinion in Chemical Biology. 7 (1): 28–32. doi : 10.1016/S1367-5931(02)00015-7 . PMID 12547423
- Sleator RD (2012). “Prediction of protein functions”. Functional Genomics. Methods in Molecular Biology. 815 . pp. 15–24. doi : 10.1007/978-1-61779-424-7_2 . ISBN 978-1-61779-423-0 . PMID 22130980
- International
- Education Jobs
- Schools directory
- Resources Education Jobs Schools directory News Search

Entry Level AQA Science - Component 1_Bio_Human Body
Subject: Biology
Age range: 14-16
Resource type: Unit of work
Last updated
27 August 2024
- Share through email
- Share through twitter
- Share through linkedin
- Share through facebook
- Share through pinterest

- resource packs and answer for taught content required for exam for AQA Entry Level Science (component 1: biology - The human body) (resource packs contains opportunities to push pupils towards GCSE level)
packs included:
- what is the body made of
- how the body works
- how the body fights disease
- how the body is coordinated
- Practice opportunities for teacher designed assessments (in the packs) and resource pack for a selection of teacher designed assessments. There are multiple teacher designed assessments to allow more than one opportunity to successfully complete task.
Tes paid licence How can I reuse this?
Get this resource as part of a bundle and save up to 17%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.

AQA Entry Level Science Complete Course
Contains work packs and answers for all examined content required for each of the 6 optional components Components: 1. biology - the human body 2. biology - environment, evolution and inheritance 3. chemistry - elements, mixtures and compounds 4. chemistry - chemistry in our world 5. physics - energy, forces and structure of matter 6. physics - electricity, magnetism and waves Each component is broken down into manage sized work packs to make delivery easier for pupils to handle. Work packs also contain practice teacher designed assessments Separate teacher designed assessments have been created. There are multiple teacher designed assessments per component to allow pupils multiple attempts at successful completion
Your rating is required to reflect your happiness.
It's good to leave some feedback.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resource to let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch.
Not quite what you were looking for? Search by keyword to find the right resource:
Protein: Structure ( OCR A Level Biology )
Revision note.

Biology Lead
Levels of Protein Structure
- There are four levels of structure in proteins, three are related to a single polypeptide chain and the fourth level relates to a protein that has two or more polypeptide chains
- Polypeptide or protein molecules can have anywhere from 3 amino acids (Glutathione) to more than 34,000 amino acids (Titan) bonded together in chains
Primary structure
- The sequence of amino acids bonded by covalent peptide bonds is the primary structure of a protein
- The DNA of a cell determines the primary structure of a protein by instructing the cell to add certain amino acids in specific quantities in a certain sequence. This affects the shape and therefore the function of the protein
- The primary structure is specific for each protein (one alteration in the sequence of amino acids can affect the function of the protein)

The primary structure of a protein. The three-letter abbreviations indicate the specific amino acid (there are 20 commonly found in cells of living organisms).
Secondary structure
- The secondary structure of a protein occurs when the weak negatively charged nitrogen and oxygen atoms interact with the weak positively charged hydrogen atoms to form hydrogen bonds
- β-pleated sheet
- The α-helix shape occurs when the hydrogen bonds form between every fourth peptide bond (between the oxygen of the carboxyl group and the hydrogen of the amine group)
- The β-pleated sheet shape forms when the protein folds so that two parts of the polypeptide chain are parallel to each other enabling hydrogen bonds to form between parallel peptide bonds
- Most fibrous proteins have secondary structures (e.g. collagen and keratin)
- The secondary structure only relates to hydrogen bonds forming between the amino group and the carboxyl group (the ‘protein backbone’)
- The hydrogen bonds can be broken by high temperatures and pH changes

The secondary structure of a protein with the α-helix and β-pleated sheet shapes highlighted. The magnified regions illustrate how the hydrogen bonds form between the peptide bonds.
Tertiary structure
- Further conformational change of the secondary structure leads to additional bonds forming between the R groups (side chains)
- Hydrogen (these are between R groups)
- Disulphide (only occurs between cysteine amino acids)
- Ionic (occurs between charged R groups)
- Weak hydrophobic interactions (between non-polar R groups)
- This structure is common in globular proteins

The tertiary structure of a protein with hydrogen bonds, ionic bonds, disulphide bonds and hydrophobic interactions formed between the R groups of the amino acids.
- Disulphide bonds are strong covalent bonds that form between two cysteine R groups (as this is the only amino acid with a sulphur atom)
- These bonds are the strongest within a protein but occur less frequently, and help stabilise the proteins
- These are also known as disulphide bridges
- Disulphide bonds can be broken by oxidation
- This type of bond is common in proteins secreted from cells eg. insulin
- Ionic bonds form between positively charged (amine group -NH 3 + ) and negatively charged (carboxylic acid -COO - ) R groups
- Ionic bonds are stronger than hydrogen bonds but they are not common
- These bonds are broken by pH changes
- Hydrogen bonds form between strongly polar R groups . These are the weakest bonds that form but the most common as they form between a wide variety of R groups
Hydrophobic interactions
- Hydrophobic interactions form between the non-polar (hydrophobic) R groups within the interior of proteins
Tertiary structure determines function
- A polypeptide chain will fold differently due to the interactions (and hence the bonds that form) between R groups
- Each of the twenty amino acids that make up proteins has a unique R group and therefore many different interactions can occur creating a vast range of protein configurations and therefore functions

The interactions that occur between the R groups of amino acids determines the shape and function of a protein. These interactions are found within tertiary structures of proteins.
Quaternary structure
- Quarternary structure exists in proteins that have more than one polypeptide chain working together as a functional macromolecule, for example, haemoglobin
- Each polypeptide chain in the quaternary structure is referred to as a subunit of the protein

The quaternary structure of a protein. This is an example of haemoglobin which contains four subunits (polypeptide chains) working together to carry oxygen.
Summary of Bonds in Proteins Table

Familiarise yourself with the difference between the four structural levels found in proteins, noting which bonds are found at which level. Remember that the hydrogen bonds in tertiary structures are between the R groups whereas in secondary structures the hydrogen bonds form between the amino and carboxyl groups.
You've read 0 of your 10 free revision notes
Get unlimited access.
to absolutely everything:
- Downloadable PDFs
- Unlimited Revision Notes
- Topic Questions
- Past Papers
- Model Answers
- Videos (Maths and Science)
Join the 100,000 + Students that ❤️ Save My Exams
the (exam) results speak for themselves:
Did this page help you?
Author: Lára
Lára graduated from Oxford University in Biological Sciences and has now been a science tutor working in the UK for several years. Lára has a particular interest in the area of infectious disease and epidemiology, and enjoys creating original educational materials that develop confidence and facilitate learning.
Sorghum: Biology, Functional Potential and Sustainable Utilization
Major Millets
- First Online: 29 August 2024
Cite this chapter

- Shalini Choudhary 3 ,
- Karuna Singh 3 ,
- Muskan Chadha 3 &
- Ratnakar Shukla 4
Part of the book series: World Sustainability Series ((WSUSE))
Sorghum is a versatile grain due to its excellent nutrient potential, climate competency, agronomic benefits and sustainable applications in various industrial areas. Sorghum contains good amount of essential nutrients particularly proteins, minerals and dietary fiber. Despite of being low in fats, it contains essential fatty acids like linoleic, oleic, palmitic, stearic, myristic, and hexadecenoic. Apart from this, sorghum possess various health promoting properties as it is a good source of antioxidants phenolic acids, tannins, flavonoids and phytosterols. These bioactive compounds are known to play a curative and preventive role against various lifestyle disorders and that is why sorghum is regarded as a superfood. This chapter delves into sorghum’s structural and functional properties, focusing primarily on its botanical characteristics, nutritive values, and bioactive composition. It also addresses the challenges and opportunities of utilizing sorghum grain as different food products, emphasizing therapeutic benefits and economic sustainability.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Airaodion AI (2019) Antidiabetic effect of ethanolic extract of Carica papaya leaves in alloxan-induced diabetic rats. AJBSR 5:227–234. https://doi.org/10.34297/AJBSR.2019.05.000917
Akintayo I, Sedgo J (2001) Towards sustainable sorghum production, utilization, and commercialization in West and Central Africa proceedings of a technical workshop of the West and Central Africa Sorghum Research Network. International Crops Research Institute for the Semi-Arid Tropics
Google Scholar
Anunciação PC, de Morais Cardoso L, de Cássia Gonçalves Alfenas R et al (2019) Extruded sorghum consumption associated with a caloric restricted diet reduces body fat in overweight men: a randomized controlled trial. Food Res Int 119:693–700. https://doi.org/10.1016/j.foodres.2018.10.048
Arouna N, Gabriele M, Pucci L (2020) The impact of germination on sorghum nutraceutical properties. Foods 9:1218. https://doi.org/10.3390/foods9091218
Article CAS Google Scholar
Bouargalne Y, Ben Mrid R, Bouchmaa N et al (2022) Genetic diversity for agromorphological traits, phytochemical profile, and antioxidant activity in Moroccan sorghum ecotypes. Sci Rep 12:5895. https://doi.org/10.1038/s41598-022-09810-9
Chhikara N, Abdulahi B, Munezero C et al (2018) Exploring the nutritional and phytochemical potential of sorghum in food processing for food security. Nutr Food Sci 49:318–332. https://doi.org/10.1108/NFS-05-2018-0149
Article Google Scholar
Dalton TJ, Hodjo M (2020) Trends in Global Production, Consumption, and Utilization of Sorghum. In: Tonapi VA, Talwar HS, Are AK et al (eds) Sorghum in the 21st Century: Food – Fodder – Feed – Fuel for a Rapidly Changing World. Springer, Singapore, pp 3–15
Dayakar Rao B, Bhaskarachary K, Arlene Christina GD et al (2017) Nutritional and health benefits of millets. ICAR_Indian Institute of Millets Research (IIMR), Rajendranagar, Hyderabad 112:33
De Morais Cardoso L, Pinheiro SS, Martino HSD, Pinheiro-Sant’Ana HM (2017) Sorghum ( Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health. Critical Reviews in Food Science and Nutrition 57:372–390. https://doi.org/10.1080/10408398.2014.887057
Espitia-Hernández P, Chávez González ML, Ascacio-Valdés JA et al (2022) Sorghum (Sorghum bicolor L.) as a potential source of bioactive substances and their biological properties. Crit Rev Food Sci Nutr 62:2269–2280. https://doi.org/10.1080/10408398.2020.1852389
Feyera M (2021) Overview of malting and fermentation role in Sorghum Flour, primarily for antinutrient reduction. Journal of Human Nutrition and Food Science 9:1–9. https://doi.org/10.47739/1138
Ghinea IO, Ionica Mihaila MD, Blaga (Costea) G-V, et al (2021) HPLC-DAD Polyphenolic Profiling and Antioxidant Activities of Sorghum bicolor during Germination. Agronomy 11:417. https://doi.org/10.3390/agronomy11030417
Girard AL, Awika JM (2018) Sorghum polyphenols and other bioactive components as functional and health promoting food ingredients. J Cereal Sci 84:112–124. https://doi.org/10.1016/j.jcs.2018.10.009
Gowda NAN, Siliveru K, Prasad PVV et al (2022) Modern processing of Indian Millets: a perspective on changes in nutritional properties. Foods 11:499. https://doi.org/10.3390/foods11040499
Hassan S, Imran M, Ahmad N, Khan MK (2017) Lipids characterization of ultrasound and microwave processed germinated sorghum. Lipids Health Dis 16:125. https://doi.org/10.1186/s12944-017-0516-4
Hasseldine BPJ, Gao C, Collins JM et al (2017) Mechanical response of common millet ( Panicum miliaceum ) seeds under quasi-static compression: Experiments and modeling. J Mech Behav Biomed Mater 73:102–113. https://doi.org/10.1016/j.jmbbm.2017.01.008
Hossain MS, Islam MN, Rahman MM et al (2022) Sorghum: A prospective crop for climatic vulnerability, food and nutritional security. J Agric Food Res 8:100300. https://doi.org/10.1016/j.jafr.2022.100300
Keyata EO, Tola YB, Bultosa G, Forsido SF (2021) Premilling treatments effects on nutritional composition, antinutritional factors, and in vitro mineral bioavailability of the improved Assosa I sorghum variety (Sorghum bicolor L.). Food Sci Nutr 9:1929–1938. https://doi.org/10.1002/fsn3.2155
Khalid W, Ali A, Arshad MS et al (2022) Nutrients and bioactive compounds of Sorghum bicolor L. used to prepare functional foods: a review on the efficacy against different chronic disorders. Int J Food Prop 25:1045–1062. https://doi.org/10.1080/10942912.2022.2071293
Khoddami A, Messina V, Vadabalija Venkata K et al (2023) Sorghum in foods: Functionality and potential in innovative products. Crit Rev Food Sci Nutr 63:1170–1186. https://doi.org/10.1080/10408398.2021.1960793
Li Z, Zhao X, Zhang X, Liu H (2021) Bioactive compounds and biological activities of sorghum grains. Foods 10:2868. https://doi.org/10.3390/foods10112868
Makanjuola SBL, Ogundaini AO, Ajonuma LC, Dosunmu A (2018) Apigenin and apigeninidin isolates from the Sorghum bicolor leaf targets inflammation via cyclo-oxygenase-2 and prostaglandin-E2 blockade. Int J Rheum Dis 21:1487–1495. https://doi.org/10.1111/1756-185X.13355
Martiwi INA, Nugroho LH, Daryono BS, Susandarini R (2020) Morphological variability and taxonomic relationship of Sorghum bicolor (L.) Moench Accessions Based on Qualitative Characters. Ann Res Rev Biol 35:40–52. https://doi.org/10.9734/arrb/2020/v35i630234
Mbulwe L, Ajayi OC (2020) Case Study—Sorghum Improvement in Zambia: Promotion of Sorghum Open Pollinated Varieties (SOPVs). Eur J Agric Food Sci 2. https://doi.org/10.24018/ejfood.2020.2.5.108
Mohamed H, Fawzi E, Basit A et al (2022) Sorghum: nutritional factors, bioactive compounds, pharmaceutical and application in food systems: a review. Phyton 91:1303–1325. https://doi.org/10.32604/phyton.2022.020642
Mughal M, Fontan Sers C (2020) Cereal production, undernourishment, and food insecurity in South Asia. Rev Dev Econ 24:524–545. https://doi.org/10.1111/rode.12659
Nagy R, Szőllősi E, Molnár PB et al (2021) Condensed tannin content and antioxidant activity of Hungarian sorghum varieties grown at Research Institute in Karcag. Acta Agraria Debreceniensis 155–160. https://doi.org/10.34101/actaagrar/1/8467
Nani M, Krishnaswamy K (2021) Physical and functional properties of ancient grains and flours and their potential contribution to sustainable food processing. Int J Food Prop 24:1529–1547. https://doi.org/10.1080/10942912.2021.1975740
Nithiyanantham S, Kalaiselvi P, Mahomoodally MF et al (2019) Nutritional and functional roles of millets—a review. J Food Biochem 43:e12859. https://doi.org/10.1111/jfbc.12859
Ojha P, Adhikari R, Karki R et al (2018) Malting and fermentation effects on antinutritional components and functional characteristics of sorghum flour. Food Sci Nutr 6:47–53. https://doi.org/10.1002/fsn3.525
Palavecino PM, Bustos MC, Heinzmann Alabí MB et al (2017) Effect of ingredients on the quality of gluten-free sorghum pasta. J Food Sci 82:2085–2093. https://doi.org/10.1111/1750-3841.13821
Przybylska-Balcerek A, Frankowski J, Stuper-Szablewska K (2019) Bioactive compounds in sorghum. Eur Food Res Technol 245:1075–1080. https://doi.org/10.1007/s00217-018-3207-0
Rao PS, Vinutha KS, Kumar GSA et al (2019) Sorghum: A Multipurpose Bioenergy Crop. In: Ciampitti IA, Vara Prasad PV (eds) Agronomy Monographs. Soil Science Society of America, Madison, WI, USA, pp 399–424
Rashwan AK, Yones HA, Karim N, Taha EM, Chen W (2021) Potential processing technologies for developing sorghum-based food products: an update and comprehensive review. Trends Food Sci Technol 110:168–182. https://doi.org/10.1016/j.tifs.2021.01.087
Ravisankar S, Agah S, Kim H et al (2019) Combined cereal and pulse flavonoids show enhanced bioavailability by downregulating phase II metabolism and ABC membrane transporter function in Caco-2 model. Food Chem 279:88–97. https://doi.org/10.1016/j.foodchem.2018.12.006
Ryu J-M, Jang GY, Woo KS et al (2017) Effects of sorghum ethyl-acetate extract on PC3M prostate cancer cell tumorigenicity. J Funct Foods 37:449–459. https://doi.org/10.1016/j.jff.2017.07.063
Saithalavi KM, Bhasin A, Yaqoob M (2021) Impact of sprouting on physicochemical and nutritional properties of sorghum: a review. Food Measure 15:4190–4204. https://doi.org/10.1007/s11694-021-00969-9
Salazar-López NJ, González-Aguilar G, Rouzaud-Sández O, Robles-Sánchez M (2018) Technologies applied to sorghum ( Sorghum bicolor L. Moench): changes in phenolic compounds and antioxidant capacity. Food Sci Technol 38:369–382. https://doi.org/10.1590/fst.16017
Samtiya M, Aluko RE, Dhewa T (2020) Plant food anti-nutritional factors and their reduction strategies: an overview. Food Prod Process Nutr 2:6. https://doi.org/10.1186/s43014-020-0020-5
Sathesh-Prabu C, Murugesan AG (2011) Potential utilization of sorghum field waste for fuel ethanol production employing Pachysolen tannophilus and Saccharomyces cerevisiae . Biores Technol 102:2788–2792. https://doi.org/10.1016/j.biortech.2010.11.097
Tanwar R, Panghal A, Chaudhary G et al (2023) Nutritional, phytochemical and functional potential of sorghum: a review. Food Chem Adv 3:100501. https://doi.org/10.1016/j.focha.2023.100501
Teli MD, Mallick A (2018) Utilization of waste sorghum grain for producing superabsorbent for personal care products. J Polym Environ 26:1393–1404. https://doi.org/10.1007/s10924-017-1044-z
Tuhanioglu A, Lafontaine S, Ubeyitogullari A (2023) Enhancing the aroma of white whole sorghum flour using supercritical carbon dioxide. Future Foods 8:100253. https://doi.org/10.1016/j.fufo.2023.100253
Verma R, Ranwah BR, Bharti B et al (2017) Characterization of Sorghum germplasm for various qualitative traits. J Appl Nat Sci 9:1002–1007. https://doi.org/10.31018/jans.v9i2.1311
Visarada KBRS, Aruna C (2019) Chapter 1—Sorghum: A bundle of opportunities in the 21st century. In: Aruna C, Visarada KBRS, Bhat BV, Tonapi VA (eds) Breeding Sorghum for diverse end uses. Woodhead Publishing, pp 1–14
Xiong Y, Zhang P, Warner RD, Fang Z (2019) Sorghum grain: from genotype, nutrition, and phenolic profile to its health benefits and food applications. Compr Rev Food Sci Food Saf 18:2025–2046. https://doi.org/10.1111/1541-4337.12506
Zheng H, Dang Y, Sui N (2023) Sorghum: a multipurpose crop. J Agric Food Chem 71:17570–17583. https://doi.org/10.1021/acs.jafc.3c04942
Download references
Author information
Authors and affiliations.
Department of Nutrition and Dietetics, Sharda School of Allied Health Sciences, Sharda University, Greater Noida, India
Shalini Choudhary, Karuna Singh & Muskan Chadha
Department of Clinical Research, Sharda School of Allied Health Sciences, Sharda University, Greater Noida, India
Ratnakar Shukla
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Karuna Singh .
Editor information
Editors and affiliations.
Amity Institute of Food Technology, Amity University, Noida, Uttar Pradesh, India
Monika Thakur
Rights and permissions
Reprints and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Choudhary, S., Singh, K., Chadha, M., Shukla, R. (2024). Sorghum: Biology, Functional Potential and Sustainable Utilization. In: Thakur, M. (eds) Millets: The Multi-Cereal Paradigm for Food Sustainability. World Sustainability Series. Springer, Cham. https://doi.org/10.1007/978-3-031-64237-1_6
Download citation
DOI : https://doi.org/10.1007/978-3-031-64237-1_6
Published : 29 August 2024
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-64236-4
Online ISBN : 978-3-031-64237-1
eBook Packages : Earth and Environmental Science Earth and Environmental Science (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

IMAGES
VIDEO
COMMENTS
Proteins: Structures & Functions. There are four levels of structure in proteins, three are related to a single polypeptide chain and the fourth level relates to a protein that has two or more polypeptide chains. Polypeptide or protein molecules can have anywhere from 3 amino acids (Glutathione) to more than 34,000 amino acids (Titan) bonded ...
A LEVEL BIOLOGY: 25 Mark essays. 16 terms. joboyd12. Preview. Importance of cycles in biology essay*** 20+/25. 29 terms. hjungbluth. Preview. Biology definitions. 10 terms. AC22023237. ... Genes, alleles and proteins produced 5) Comparing protein sequences 6) Selection - selective breeding, artificial selection 7) ...
Biology is detailed and comprehensive A-level content, uses appropriate terminology, and is very well written and always clearly explained. No significant errors or irrelevant material. For top marks in the band, the answer shows evidence of reading beyond specification requirements. 16-20. Relational.
This document contains the essay titles and mark schemes used in AQA A-level Biology examinations since 2007. The specifications these exam questions came from are no longer in use, but the marking method has largely remained unchanged. Further guidance on the marking method used with the essay can be found in Paper 3 Essay marking guidance.
Summary. Proteins carry out the majority of the functions of the cell. Proteins have hugely diverse components, sizes, structures, and functions. Proteins are made up of chains of amino acids, which progressively fold to form the final structure of the protein. Primary, secondary, tertiary and quaternary structures classify the distinct layers ...
3.1.4 Proteins. esSPECIFICATION‒ Each enzyme lowers the activation energy of the react. on it catalyses. ‒ The induced-fit model. f enzyme action.‒ The properties of an enzyme relate to the tertiary structure of its active site and its ability to combine with complementary substrate(s) to form an enzyme-s.
Advice for the essay. The levels scheme states that more than two A-level topics need to be addressed to get higher than 10 marks. A minimum of four topics is required to get higher than 15 marks. A topic area is a numbered sub-section in the specification. For example, for the 2017 'diffusion' essay, gas exchange (3.3.2) was a topic area.
Proteins have a variety of functions within all living organisms. In this video we will see the relationship between the 4 levels of protein structure.1. Pri...
A protein that is made up of more than one polypeptide chain is a quaternary structure protein. It is still folded into a 3D shape and held by hydrogen, ionic, and disulfide bonds. Hemoglobin is the typical example given. Hemoglobin is made up of 4 polypeptide chains. In the diagram of hemoglobin, you can also see extra molecules attached that ...
Structure of Amino Acids. Amino acids are the monomers of proteins. There are 20 amino acids and they all have very similar structures. On one end, they contain an amine group (-NH2). This is known as the N-terminus. On the opposite end they contain a carboxyl group (-COOH) which makes them an acid. This is known as the C-terminus.
Study with Quizlet and memorise flashcards containing terms like how a protein is formed, secondary structure of protein, tertiary structure and others. ... AQA A Level Biology - 25 Mark Essays. 23 terms. biotig. Preview. AQA Biology A-Level - Essay Titles and Topics. 15 terms. VicHsu. Preview. Biology i don't know (paper 2) 68 terms ...
There are four levels of structure in proteins, three are related to a single polypeptide chain and the fourth level relates to a protein that has two or more polypeptide chains. Polypeptide or protein molecules can have anywhere from 3 amino acids (Glutathione) to more than 34,000 amino acids (Titan) bonded together in chains.
AQA A Level Biology - 25 Mark Essays. 23 terms. biotig. Preview. AQA A level biology essay plans. 20 terms. Amy_L2004. Preview. biology paper 1 aqa ... • Channel proteins in facilitated diffusion provide pores though which these molecules can diffuse *Enzymes in Respiration • acetate combines with coenzyme A in the link reaction to produce ...
Levels of Protein Structure. There are four levels of structure in proteins. Three are related to a single polypeptide chain. The fourth level relates to a protein that has two or more polypeptide chains. Polypeptide or protein molecules can have anywhere from 3 amino acids (Glutathione) to more than 34,000 amino acids (Titan) bonded together ...
Proteins are complex macromolecules (polymers). They have high molecular weight and are made up of structural units (monomers) called amino acids. Amino acids are the protein's building units. They are organic compounds made up of hydrogen, oxygen, carbon and nitrogen atoms. Amino acids are made up of a basic group (amino group NH2), an ...
Topic 6: Responding to Changes in Environment. Topic 7: Genetics, Populations, Evolution and Ecosystems. Topic 8: Control of Gene Expression. Practical Skills. Practical Skills. Revision for AQA Biology AS and A Level Papers, including summary notes, worksheets and past exam questions for each topic.
Focusing on the essay performance on the 7402 biology specification only, we can see how the 2017-2019 essays have performed in isolation in Figure 2. The 7402 essay has shown performance that skews slightly to the higher end of the mark scale, which is understandable given the mean mark sits just above 50%.
AQA A Level Biology Essay Plans. The importance of proteins in the control of processes and responses in organisms. Click the card to flip it 👆. -Enzymes as catalysts. -Proteins and enzymes in respiration. -Proteins and enzymes in photosynthesis. -Immunology. -Haemoglobin. -DNA.
Membranes are vital structures found in all cells. The cell surface membrane creates an enclosed space separating the internal cell environment from the external environment, and intracellular membranes form compartments within the cell such as the nucleus, mitochondria and RER. Membranes do not only separate different areas but also control ...
Proteins are involved in cell division. Proteins are required for transport within a cell. Proteins are needed for oxygen transport. Hemoglobin. Myoglobin. Proteins are necessary for the transport of various substances in the blood. Proteins are involved in Muscle contraction. Proteins prevent edema.
AQA Entry Level Science Complete Course. Contains work packs and answers for all examined content required for each of the 6 optional components Components: 1. biology - the human body 2. biology - environment, evolution and inheritance 3. chemistry - elements, mixtures and compounds 4. chemistry - chemistry in our world 5. physics - energy, forces and structure of matter 6. physics ...
There are four levels of structure in proteins, three are related to a single polypeptide chain and the fourth level relates to a protein that has two or more polypeptide chains. Polypeptide or protein molecules can have anywhere from 3 amino acids (Glutathione) to more than 34,000 amino acids (Titan) bonded together in chains.
The total protein content of sorghum is 9.97 ± 0.43 of which Prolamins and glutelins are the two main protein components found in sorghum, with prolamins being the most abundant. Kafirin, a protein that resembles maize zein in terms of molecular weight, shape, solubility, and amino acid content, is the prolamin found in sorghum.
The base sequence of amino acids. - Primary protein has a 2D structure. - Peptide bonds. Secondary protein structure & bondings. 3 or more amino acids join together via peptide bonds through condensation reaction to form polypeptide chain. (More hydrogen) so Long polypeptide chain twists and folds to make the 3D shapes : - (Alpha) α helices.
Study with Quizlet and memorise flashcards containing terms like enzymes, antigens, antibodies - immune response, protein channels, carrier proteins - facilitations diffusion, active transport and others. ... AQA A level Biology Essay. 18 terms. Jesscane1. Preview. Biology Essay - The importance of energy transfer within and between organisms ...