Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 02 March 2017

Hepatitis C virus infection
- Michael P. Manns 1 , 2 , 3 ,
- Maria Buti 4 ,
- Ed Gane 5 ,
- Jean-Michel Pawlotsky 6 , 7 ,
- Homie Razavi 8 ,
- Norah Terrault 9 &
- Zobair Younossi 10
Nature Reviews Disease Primers volume 3 , Article number: 17006 ( 2017 ) Cite this article
22k Accesses
342 Citations
84 Altmetric
Metrics details
- Hepatitis C
- Hepatitis C virus
- Interferons
- Molecular medicine
Hepatitis C virus (HCV) is a hepatotropic RNA virus that causes progressive liver damage, which might result in liver cirrhosis and hepatocellular carcinoma. Globally, between 64 and 103 million people are chronically infected. Major risk factors for this blood-borne virus infection are unsafe injection drug use and unsterile medical procedures (iatrogenic infections) in countries with high HCV prevalence. Diagnostic procedures include serum HCV antibody testing, HCV RNA measurement, viral genotype and subtype determination and, lately, assessment of resistance-associated substitutions. Various direct-acting antiviral agents (DAAs) have become available, which target three proteins involved in crucial steps of the HCV life cycle: the NS3/4A protease, the NS5A protein and the RNA-dependent RNA polymerase NS5B protein. Combination of two or three of these DAAs can cure (defined as a sustained virological response 12 weeks after treatment) HCV infection in >90% of patients, including populations that have been difficult to treat in the past. As long as a prophylactic vaccine is not available, the HCV pandemic has to be controlled by treatment-as-prevention strategies, effective screening programmes and global access to treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
111,21 € per year
only 111,21 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Breakthroughs in hepatitis C research: from discovery to cure

Global burden of hepatitis B virus: current status, missed opportunities and a call for action

Prevalence, incidence, and outcomes of hepatitis E virus coinfection in patients with chronic hepatitis C
Stanaway, J. D. et al . The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 388 , 1081–1088 (2016).
Google Scholar
Gower, E., Estes, C., Blach, S., Razavi-Shearer, K. & Razavi, H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 61 , S45–S57 (2014).
Mohd Hanafiah, K., Groeger, J., Flaxman, A. D. & Wiersma, S. T. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 57 , 1333–1342 (2013).
Lohmann, V. et al . Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285 , 110–113 (1999). This paper establishes the HCV replicon system, which is a methodological breakthrough for drug development in HCV infection.
Hoofnagle, J. H. et al . Treatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon. A preliminary report. N. Engl. J. Med. 315 , 1575–1578 (1986). This is the first study to use IFN in the treatment of hepatitis C before HCV was discovered when the disease was still called non-A, non-B hepatitis.
Lamarre, D. et al . An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature 426 , 186–189 (2003). This is the first study to successfully use and provide proof of concept for a NS3/4A protease inhibitor as the first DAA for HCV infection.
Wakita, T. et al . Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11 , 791–796 (2005). This study establishes an in vitro HCV infection in tissue culture.
Lok, A. S. et al . Preliminary study of two antiviral agents for hepatitis C genotype 1. N. Engl. J. Med. 366 , 216–224 (2012). This study provides proof of concept that a combination of different classes of DAAs without IFN can cure chronic HCV infection.
Choo, Q. L. et al . Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244 , 359–362 (1989). This paper was the first to discover HCV.
Manns, M. P. & von Hahn, T. Novel therapies for hepatitis C — one pill fits all? Nat. Rev. Drug Discov. 12 , 595–610 (2013).
Manns, M. P. et al . Long-term clearance of hepatitis C virus following interferon alpha-2b or peginterferon alpha-2b, alone or in combination with ribavirin. J. Viral Hepat. 20 , 524–529 (2013).
Swain, M. G. et al . A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology 139 , 1593–1601 (2010).
Younossi, Z. M. et al . Effects of sofosbuvir-based treatment, with and without interferon, on outcome and productivity of patients with chronic hepatitis C. Clin. Gastroenterol. Hepatol. 12 , 1349–1359.e13 (2014).
Pawlotsky, J. M. Hepatitis C virus resistance to direct-acting antiviral drugs in interferon-free regimens. Gastroenterology 151 , 70–86 (2016). This review defines and explains the relevance, diagnosis and management of drug resistance and RASs of DAAs.
The Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol. Hepatol. 2 , 161–176 (2017).
Nerrienet, E. et al . Hepatitis C virus infection in Cameroon: a cohort-effect. J. Med. Virol. 76 , 208–214 (2005).
Njouom, R. et al . Phylogeography, risk factors and genetic history of hepatitis C virus in Gabon, Central Africa. PLoS ONE 7 , e42002 (2012).
Sharvadze, L., Nelson, K. E., Imnadze, P., Karchava, M. & Tsertsvadze, T. Prevalence of HCV and genotypes distribution in general population of Georgia. Georgian Med. News 165 , 71–77 (2008).
Baatarkhuu, O. et al . Prevalence and genotype distribution of hepatitis C virus among apparently healthy individuals in Mongolia: a population-based nationwide study. Liver Int. 28 , 1389–1395 (2008).
Qureshi, H., Bile, K. M., Jooma, R., Alam, S. E. & Afridi, H. U. Prevalence of hepatitis B and C viral infections in Pakistan: findings of a national survey appealing for effective prevention and control measures. East. Mediterr. Health J. 16 , S15–S23 (2010).
Ruzibakiev, R. et al . Risk factors and seroprevalence of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus infection in Uzbekistan. Intervirology 44 , 327–332 (2001).
Arafa, N. et al . Changing pattern of hepatitis C virus spread in rural areas of Egypt. J. Hepatol. 43 , 418–424 (2005).
Ministry of Health and Population, El-Zanaty and Associates & ICF International. Egypt health issues survey 2015. DHS Program https://dhsprogram.com/pubs/pdf/FR313/FR313.pdf (2015).
Razavi, H. et al . The present and future disease burden of hepatitis C virus (HCV) infection with today's treatment paradigm. J. Viral Hepat. 21 (Suppl. 1), 34–59 (2014).
Hatzakis, A. et al . The present and future disease burden of hepatitis C virus (HCV) infections with today's treatment paradigm — volume 2. J. Viral Hepat. 22 (Suppl. 1), 26–45 (2015).
Alter, M. J., Kuhnert, W. L., Finelli, L. & Centers for Disease Control and Prevention. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. Centers for Disease Control and Prevention. MMWR Recomm. Rep. 52 , 1–16 (2003).
Schmidt, A. J. et al . Prevalence of hepatitis C in a Swiss sample of men who have sex with men: whom to screen for HCV infection? BMC Public Health 14 , 3 (2014).
Dalgard, O. et al . Risk factors for hepatitis C among injecting drug users in Oslo. Tidsskr. Nor. Laegeforen. 129 , 101–104 (in Norwegian) (2009).
Duberg, A., Janzon, R., Back, E., Ekdahl, K. & Blaxhult, A. The epidemiology of hepatitis C virus infection in Sweden. Euro Surveill. 13 , 18882 (2008).
Mann, A. G. et al . Diagnoses of, and deaths from, severe liver disease due to hepatitis C in England between 2000 and 2005 estimated using multiple data sources. Epidemiol. Infect. 137 , 513–518 (2009).
Public Health Agency of Canada. A study to characterize the epidemiology of hepatitis C infection in Canada, 2002. Public Health Agency Canada http://publications.gc.ca/collections/collection_2009/aspc-phac/HP40-31-2008E.pdf (2008).
[No authors listed.] Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm. Rep. 47 , 1–39 (1998).
U.S. Preventive Services Task Force. Hepatitis C: screening. U.S. Preventive Services Task Force http://www.uspreventiveservicestaskforce.org/uspstf/uspshepc.htm (2013).
Osaki, Y. & Nishikawa, H. Treatment for hepatocellular carcinoma in Japan over the last three decades: our experience and published work review. Hepatol. Res. 45 , 59–74 (2015).
Alfaleh, F. Z. et al . Strategies to manage hepatitis C virus infection disease burden — volume 3. J. Viral Hepat. 22 (Suppl. 4), 42–65 (2015).
Gane, E. et al . Strategies to manage hepatitis C virus (HCV) infection disease burden — volume 2. J. Viral Hepat. 22 (Suppl. 1), 46–73 (2015).
Wedemeyer, H. et al . Strategies to manage hepatitis C virus (HCV) disease burden. J. Viral Hepat. 21 (Suppl. 1), 60–89 (2014).
Negro, F. et al . Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology 149 , 1345–1360 (2015).
van der Meer, A. J. et al . Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 308 , 2584–2593 (2012). This study provides proof that cure of hepatitis C can reduce liver and overall mortality.
Burbelo, P. D. et al . Serology-enabled discovery of genetically diverse hepaciviruses in a new host. J. Virol. 86 , 6171–6178 (2012).
Kapoor, A. et al . Characterization of a canine homolog of hepatitis C virus. Proc. Natl Acad. Sci. USA 108 , 11608–11613 (2011).
Kapoor, A. et al . Identification of rodent homologs of hepatitis C virus and pegiviruses. mBio 4 , e00216-13 (2013).
Lyons, S. et al . Nonprimate hepaciviruses in domestic horses, United Kingdom. Emerg. Infect. Dis. 18 , 1976–1982 (2012).
Simmonds, P. The origin of hepatitis C virus. Curr. Top. Microbiol. Immunol. 369 , 1–15 (2013).
Penin, F., Dubuisson, J., Rey, F. A., Moradpour, D. & Pawlotsky, J. M. Structural biology of hepatitis C virus. Hepatology 39 , 5–19 (2004).
Andre, P. et al . Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J. Virol. 76 , 6919–6928 (2002).
Zeisel, M. B., Felmlee, D. J. & Baumert, T. F. Hepatitis C virus entry. Curr. Top. Microbiol. Immunol. 369 , 87–112 (2013).
Timpe, J. M. et al . Hepatitis C virus cell–cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology 47 , 17–24 (2008).
Honda, M., Beard, M. R., Ping, L. H. & Lemon, S. M. A phylogenetically conserved stem-loop structure at the 5' border of the internal ribosome entry site of hepatitis C virus is required for cap-independent viral translation. J. Virol. 73 , 1165–1174 (1999).
Niepmann, M. Hepatitis C virus RNA translation. Curr. Top. Microbiol. Immunol. 369 , 143–166 (2013).
Moradpour, D. & Penin, F. Hepatitis C virus proteins: from structure to function. Curr. Top. Microbiol. Immunol. 369 , 113–142 (2013).
Lohmann, V. Hepatitis C virus RNA replication. Curr. Top. Microbiol. Immunol. 369 , 167–198 (2013).
Scheel, T. K. & Rice, C. M. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat. Med. 19 , 837–849 (2013).
Lindenbach, B. D. Virion assembly and release. Curr. Top. Microbiol. Immunol. 369 , 199–218 (2013).
Manns, M. P. & Cornberg, M. Sofosbuvir: the final nail in the coffin for hepatitis C? Lancet Infect. Dis. 13 , 378–379 (2013).
Janssen, H. L. et al . Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 368 , 1685–1694 (2013).
Pawlotsky, J. M. Hepatitis C virus population dynamics during infection. Curr. Top. Microbiol. Immunol. 299 , 261–284 (2006).
Khakoo, S. I. et al . HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305 , 872–874 (2004).
Yu, M. Y. et al . Neutralizing antibodies to hepatitis C virus (HCV) in immune globulins derived from anti-HCV-positive plasma. Proc. Natl Acad. Sci. USA 101 , 7705–7710 (2004).
Pestka, J. M. et al . Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc. Natl Acad. Sci. USA 104 , 6025–6030 (2007).
Gerlach, J. T. et al . Recurrence of hepatitis C virus after loss of virus-specific CD4 + T-cell response in acute hepatitis C. Gastroenterology 117 , 933–941 (1999).
Schulze Zur Wiesch, J. et al . Broadly directed virus-specific CD4 + T cell responses are primed during acute hepatitis C infection, but rapidly disappear from human blood with viral persistence. J. Exp. Med. 209 , 61–75 (2012).
Day, C. L. et al . Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J. Clin. Invest. 112 , 831–842 (2003).
Grakoui, A. et al . HCV persistence and immune evasion in the absence of memory T cell help. Science 302 , 659–662 (2003).
Bowen, D. G. & Walker, C. M. Mutational escape from CD8 + T cell immunity: HCV evolution, from chimpanzees to man. J. Exp. Med. 201 , 1709–1714 (2005).
Heim, M. H. & Thimme, R. Innate and adaptive immune responses in HCV infections. J. Hepatol. 61 , S14–S25 (2014).
Hengst, J. et al . Nonreversible MAIT cell-dysfunction in chronic hepatitis C virus infection despite successful interferon-free therapy. Eur. J. Immunol. 46 , 2204–2210 (2016).
Rehermann, B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat. Med. 19 , 859–868 (2013).
Radziewicz, H. et al . Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 81 , 2545–2553 (2007).
Kroy, D. C. et al . Liver environment and HCV replication affect human T-cell phenotype and expression of inhibitory receptors. Gastroenterology 146 , 550–561 (2014).
Park, S. H. & Rehermann, B. Immune responses to HCV and other hepatitis viruses. Immunity 40 , 13–24 (2014).
Moradpour, D., Grakoui, A. & Manns, M. P. Future landscape of hepatitis C research — basic, translational and clinical perspectives. J. Hepatol. 65 , S143–S155 (2016). This review describes the future landscape of HCV research.
Yamane, D., McGivern, D. R., Masaki, T. & Lemon, S. M. Liver injury and disease pathogenesis in chronic hepatitis C. Curr. Top. Microbiol. Immunol. 369 , 263–288 (2013).
Neumann-Haefelin, C. & Thimme, R. Adaptive immune responses in hepatitis C virus infection. Curr. Top. Microbiol. Immunol. 369 , 243–262 (2013).
Nishitsuji, H. et al . Hepatitis C virus infection induces inflammatory cytokines and chemokines mediated by the cross talk between hepatocytes and stellate cells. J. Virol. 87 , 8169–8178 (2013).
Bonilla, N. et al . Interferon gamma-secreting HCV-specific CD8 + T cells in the liver of patients with chronic C hepatitis: relation to liver fibrosis — ANRS HC EP07 study. J. Viral Hepat. 13 , 474–481 (2006).
Franceschini, D. et al . Polyfunctional type-1, -2, and -17 CD8 + T cell responses to apoptotic self-antigens correlate with the chronic evolution of hepatitis C virus infection. PLoS Pathog. 8 , e1002759 (2012).
El-Serag, H. B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142 , 1264–1273.e1 (2012).
Rusyn, I. & Lemon, S. M. Mechanisms of HCV-induced liver cancer: what did we learn from in vitro and animal studies?. Cancer Lett. 345 , 210–215 (2014).
Boyer, T. D., Manns, M. P. & Sanyal, A. J. (eds) Zakim and Boyer's Hepatology, A Textbook of Liver Disease (Elsevier Saunders Philadelphia, 2012).
Chevaliez, S., Rodriguez, C. & Pawlotsky, J. M. New virologic tools for management of chronic hepatitis B and C. Gastroenterology 142 , 1303–1313.e1 (2012).
Takaki, A. et al . Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat. Med. 6 , 578–582 (2000).
Lee, S. R. et al . Evaluation of a new, rapid test for detecting HCV infection, suitable for use with blood or oral fluid. J. Virol. Methods 172 , 27–31 (2011).
Kania, D. et al . Combining rapid diagnostic tests and dried blood spot assays for point-of-care testing of human immunodeficiency virus, hepatitis B and hepatitis C infections in Burkina Faso, West Africa. Clin. Microbiol. Infect. 19 , E533–E541 (2013).
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. J. Hepatol. 63 , 199–236 (2015).
Chevaliez, S., Bouvier-Alias, M., Brillet, R. & Pawlotsky, J. M. Hepatitis C virus (HCV) genotype 1 subtype identification in new HCV drug development and future clinical practice. PLoS ONE 4 , e8209 (2009).
Bouvier-Alias, M. et al . Clinical utility of total HCV core antigen quantification: a new indirect marker of HCV replication. Hepatology 36 , 211–218 (2002).
Chevaliez, S., Soulier, A., Poiteau, L., Bouvier-Alias, M. & Pawlotsky, J. M. Clinical utility of hepatitis C virus core antigen quantification in patients with chronic hepatitis C. J. Clin. Virol. 61 , 145–148 (2014).
US Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. FDA http://www.fda.gov/downloads/Drugs/Guidances/UCM193282 (2009).
Wolffram, I. et al . Prevalence of elevated ALT values, HBsAg, and anti-HCV in the primary care setting and evaluation of guideline defined hepatitis risk scenarios. J. Hepatol. 62 , 1256–1264 (2015).
Mahajan, R., Liu, S. J., Klevens, R. M. & Holmberg, S. D. Indications for testing among reported cases of HCV infection from enhanced hepatitis surveillance sites in the United States, 2004–2010. Am. J. Public Health 103 , 1445–1449 (2013).
Easterbrook, P. J. & WHO Guidelines Development Group. Who to test and how to test for chronic hepatitis C infection — 2016 WHO testing guidance for low- and middle-income countries. J. Hepatol. 65 , S46–S66 (2016).
World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. WHO www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/ (2016).
Baumert, T. F., Fauvelle, C., Chen, D. Y. & Lauer, G. M. A prophylactic hepatitis C virus vaccine: a distant peak still worth climbing. J. Hepatol. 61 , S34–S44 (2014).
Ball, J. K., Tarr, A. W. & McKeating, J. A. The past, present and future of neutralizing antibodies for hepatitis C virus. Antiviral Res. 105 , 100–111 (2014).
Chapman, L. E. et al . Recommendations for postexposure interventions to prevent infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus, and tetanus in persons wounded during bombings and other mass-casualty events — United States, 2008: recommendations of the Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 57 , 1–21 (2008).
Corey, K. E. et al . Pilot study of postexposure prophylaxis for hepatitis C virus in healthcare workers. Infect. Control Hosp. Epidemiol. 30 , 1000–1005 (2009).
Grebely, J. et al . Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: towards a vaccine. Lancet Infect. Dis. 12 , 408–414 (2012).
Petta, S. & Craxi, A. Current and future HCV therapy: do we still need other anti-HCV drugs? Liver Int. 35 (Suppl. 1), 4–10 (2015).
Deterding, K. et al . Ledipasvir plus sofosbuvir fixed-dose combination for 6 weeks in patients with acute hepatitis C virus genotype 1 monoinfection (HepNet Acute HCV IV): an open-label, single-arm, phase 2 study. Lancet Infect. Dis. 17 , 215–222 (2017).
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C. EASL http://www.easl.eu/medias/cpg/HCV2016/Summary.pdf (2016). These are the HCV Clinical Practice Guidelines of the EASL, which are regularly updated online (www.easl.eu).
AASLD–IDSA. Recommendations for testing, managing, and treating hepatitis C. HCV Guidelines http://www.hcvguidelines.org (accessed 1 Dec 2016). These are the joint HCV Clinical Practice Guidelines by the AASLD and the IDSA, which are regularly updated online (www.hcvguidelines.org).
Jaeckel, E. et al . Treatment of acute hepatitis C with interferon alfa-2b. N. Engl. J. Med. 345 , 1452–1457 (2001). This paper highlights that early treatment of acute HCV infection can prevent chronicity.
Wiegand, J. et al . Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology 43 , 250–256 (2006).
Deterding, K. et al . Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non-inferiority trial. Lancet Infect. Dis. 13 , 497–506 (2013).
Deterding, K. et al . Six weeks of sofosbuvir/ledipasvir (SOF/LDV) are sufficient to treat acute hepatitis C virus genotype 1 monoinfection: the HepNet acute HCV IV study. J. Hepatol. 64 , S211 (2016).
Rockstroh, J. K. et al . Ledipasvir/sofosbuvir for 6 weeks in HIV-infected patients with acute HCV infection. CROI Conference http://www.croiconference.org/sessions/ledipasvirsofosbuvir-6-weeks-hiv-infected-patients-acute-hcv-infection (2016).
Zeuzem, S. et al . Grazoprevir–elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis c virus genotype 1, 4, or 6 infection: a randomized trial. Ann. Intern. Med. 163 , 1–13 (2015).
Feld, J. J. et al . Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N. Engl. J. Med. 373 , 2599–2607 (2015).
Foster, G. R. et al . Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N. Engl. J. Med. 373 , 2608–2617 (2015).
Curry, M. P. et al . Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N. Engl. J. Med. 373 , 2618–2628 (2015).
George Lau, M. et al . Efficacy and safety of 3-week response-guided triple direct-acting antiviral therapy for chronic hepatitis C infection: a phase 2, open-label, proof-of-concept study. Lancet Gastroenterol. Hepatol. 1 , 97–104 (2016).
Kim, A. Y. & Chung, R. T. Coinfection with HIV-1 and HCV — a one–two punch. Gastroenterology 137 , 795–814 (2009).
Qurishi, N. et al . Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet 362 , 1708–1713 (2003).
Townsend, K. S. et al . High efficacy of sofosbuvir/ledipasvir for the treatment of HCV genotype 1 in patients coinfected with HIV on or off antiretroviral therapy: results from the NIAID ERADICATE trial. Hepatology 60 , 240a–241a (2014).
Wyles, D. L. et al . TURQUOISE-I: 94% SVR12 in HCV/HIV-1 coinfected patients treated with ABT-450/r/ombitasvir, dasabuvir and ribavirin. Hepatology 60 , 1136a–1137a (2014).
Wyles, D. et al. Sofosbuvir/velpatasvir fixed dose combination for 12 weeks in patients co-infected with HCV and HIV-1: the phase 3 ASTRAL-5 study. J. Hepatol. 64 , S188 (2016).
Nakata, S. et al . Hepatitis-C and hepatitis-B virus-infections in populations at low or high-risk in Ho-Chi-Minh and Hanoi, Vietnam. J. Gastroenterol. Hepatol. 9 , 416–419 (1994).
Conway, M. et al . Prevalence of antibodies to hepatitis-C in dialysis patients and transplant recipients with possible routes of transmission. Nephrol. Dial. Transplant. 7 , 1226–1229 (1992).
Blackmore, T. K., Stace, N. H., Maddocks, P. & Hatfield, P. Prevalence of antibodies to hepatitis-C virus in patients receiving renal replacement therapy, and in the staff caring for them. Aust. N. Z. J. Med. 22 , 353–357 (1992).
Chung, R. T. et al . Hepatitis C guidance: AASLD–IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 62 , 932–954 (2015).
Charlton, M. et al . Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology 148 , 108–117 (2015).
Manns, M. et al . Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect. Dis. 16 , 685–697 (2016).
Hezode, C. et al . Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology 147 , 132–142.e4 (2014).
Flamm, S. L. et al . Ledipasvir/sofosbuvir with ribavirin for the treatment of HCV in patients with decompensated cirrhosis: preliminary results of a prospective, multicenter study. Hepatology 60 , 320a–321a (2014).
Charlton, M. et al . Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 149 , 649–659 (2015).
Pellicelli, A. M. et al . Sofosbuvir plus daclatasvir for post-transplant recurrent hepatitis C: potent antiviral activity but no clinical benefit if treatment is given late. Dig. Liver Dis. 46 , 923–927 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02097966 (2016).
Thuluvath, P. J. et al . Liver transplantation in the United States, 1999–2008. Am. J. Transplant. 10 , 1003–1019 (2010).
Berenguer, M. et al . HCV-related fibrosis progression following liver transplantation: increase in recent years. J. Hepatol. 32 , 673–684 (2000).
Terrault, N. Liver transplantation in the setting of chronic HCV. Best Pract. Res. Clin. Gastroenterol. 26 , 531–548 (2012).
Berenguer, M. et al . Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am. J. Transplant. 8 , 679–687 (2008).
Terrault, N. A. & Berenguer, M. Treating hepatitis C infection in liver transplant recipients. Liver Transpl. 12 , 1192–1204 (2006).
Price, J. C. & Terrault, N. A. Treatment of hepatitis C in liver transplant patients: interferon out, direct antiviral combos in. Liver Transpl. 21 , 423–434 (2015).
Fernández-Carrillo, E. A. Treatment of hepatitis C virus in patients with advanced cirrhosis: always justified? Analysis of the Hepa-C Registry. J. Hepatol. 64 , S133 (2016).
Wiesner, R. H., Sorrell, M., Villamil, F. & International Liver Transplantation Society Expert Panel. Report of the first international Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 9 , S1–S9 (2003).
Curry, M. P. et al . Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology 148 , 100–107.e1 (2015).
Manns, M. et al . High efficacy of ledipasvir/sofosbuvir plus ribavirin among patients with decompensated cirrhosis who underwent liver transplant during participation in the SOLAR-1 and -2 studies. NATAP http://www.natap.org/2016/EASL/EASL_71.htm (2016).
Forns, X. et al . Sofosbuvir compassionate use program for patients with severe recurrent hepatitis C including fibrosing cholestatic hepatitis following liver transplantation. Hepatology 61 , 1485–1494 (2014).
Spiegel, B. M. et al . The impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Gastroenterology 128 , A749–A750 (2005).
Ware, J. E. & Kosinski, M. Interpreting SF-36 summary health measures: a response. Qual. Life Res. 10 , 405–413 (2001).
Younossi, Z. M., Guyatt, G., Kiwi, M., Boparai, N. & King, D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut 45 , 295–300 (1999).
Gnanasakthy, A. et al . A review of patient-reported outcome labels in the United States: 2006 to 2010. Value Health 15 , 437–442 (2012).
Younossi, Z. M. et al . The impact of hepatitis C burden: an evidence-based approach. Aliment. Pharmacol. Ther. 39 , 518–531 (2014).
Afendy, A. et al . Predictors of health-related quality of life in patients with chronic liver disease. Aliment. Pharmacol. Ther. 30 , 469–476 (2009).
Kallman, J. et al . Fatigue and health-related quality of life (HRQL) in chronic hepatitis C virus infection. Dig. Dis. Sci. 52 , 2531–2539 (2007).
Gerber, L. et al . Effects of viral eradication with ledipasvir and sofosbuvir, with or without ribavirin, on measures of fatigue in patients with chronic hepatitis C virus infection. Clin. Gastroenterol. Hepatol. 14 , 156–164.e3 (2016).
Younossi, Z. M. Chronic liver disease and health-related quality of life. Gastroenterology 120 , 305–307 (2001).
Younossi, Z. M. et al . Sofosbuvir and velpatasvir combination improves outcomes reported by patients with HCV infection, without or with compensated or decompensated cirrhosis. Clin. Gastroenterol. Hepatol. http://dx.doi.org/10.1016/j.cgh.2016.10.037 (2016).
Younossi, Z. M. et al . Association of work productivity with clinical and patient-reported factors in patients infected with hepatitis C virus. J. Viral Hepat. 23 , 623–630 (2016).
Younossi, I., Weinstein, A., Stepanova, M., Hunt, S. & Younossi, Z. M. Mental and emotional impairment in patients with hepatitis C is related to lower work productivity. Psychosomatics 57 , 82–88 (2016).
Patel, K. & McHutchison, J. G. Initial treatment for chronic hepatitis C: current therapies and their optimal dosing and duration. Cleve. Clin. J. Med. 71 , S8–S12 (2004).
Shehab, T. M. et al . Effectiveness of interferon alpha-2b and ribavirin combination therapy in the treatment of naive chronic hepatitis C patients in clinical practice. Clin. Gastroenterol. Hepatol. 2 , 425–431 (2004).
Bruno, R. et al . OPERA: responses to peginterferon and ribavirin therapy in a subgroup of interferon-naive patients with HIV/HCV genotype 2/3 co-infection in Italy. Liver Int. 35 , 120–129 (2015).
Younossi, Z. M., Singer, M. E., Mir, H. M., Henry, L. & Hunt, S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J. Hepatol. 60 , 530–537 (2014).
Younossi, Z. M. et al . Sofosbuvir and ledipasvir improve patient-reported outcomes in patients co-infected with hepatitis C and human immunodeficiency virus. J. Viral Hepat. 23 , 857–865 (2016).
John-Baptiste, A. A. et al . Sustained responders have better quality of life and productivity compared with treatment failures long after antiviral therapy for hepatitis C. Am. J. Gastroenterol. 104 , 2439–2448 (2009).
Reilly, M. C., Zbrozek, A. S. & Dukes, E. M. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 4 , 353–365 (1993).
Escorpizo, R. et al . Worker productivity outcome measures in arthritis. J. Rheumatol. 34 , 1372–1380 (2007).
Tang, K. et al . Worker productivity outcome measures: OMERACT filter evidence and agenda for future research. J. Rheumatol. 41 , 165–176 (2014).
Younossi, Z. et al . Sustained virologic response with ledipasvir (LDV) and sofosbuvir (SOF) regimens leads to substantial improvement in patient-reported outcomes (PROs) among chronic hepatitis C (CHC) patients with early hepatic fibrosis as well as those with advanced hepatic fibrosis. Hepatology 60 , 892a–893a (2014).
Baran, R. W., Xie, W. G., Liu, Y., Cohen, D. E. & Gooch, K. L. Health-related quality of life (HRQoL), health state, function and wellbeing of chronic HCV patients treated with interferon-free, oral DAA regimens: patient reported outcome (PRO) results from the AVIATOR study. Hepatology 58 , 750a–751a (2013).
Scott, J. et al . Fatigue during treatment for hepatitis C virus: results of self-reported fatigue severity in two phase IIb studies of simeprevir treatment in patients with hepatitis C virus genotype 1 infection. BMC Infect. Dis. 14 , 465 (2014).
Loria, A. et al . Multiple factors predict physical performance in people with chronic liver disease. Am. J. Phys. Med. Rehabil. 93 , 470–476 (2014).
Younossi, Z. M. et al . Patient-reported outcomes assessment in chronic hepatitis C treated with sofosbuvir and ribavirin: the VALENCE study. J. Hepatol. 61 , 228–234 (2014).
Younossi, Z. M. et al . Sofosbuvir/velpatasvir improves patient-reported outcomes in HCV patients: results from ASTRAL-1 placebo-controlled trial. J. Hepatol. 65 , 33–39 (2016).
Younossi, Z. M. et al . The impact of hepatitis C virus outside the liver: evidence from Asia. Liver Int. 37 , 159–172 (2017).
Bouliere, M. Sofosbuvir/velpatasvir/voxilaprevir for 12 weeks as a salvage regimen in NS5A inhibitor-experienced patients with genotype 1–6 infection: the phase 3 POLARIS-1 study. Hepatology 64 , 102AA (2016).
Zeuzem, S. A. Randomized, controlled, phase 3 trial of sofosbuvir/velpatasvir/voxilaprevir or sofosbuvir/velpatasvir for 12 weeks in direct acting antiviral-experienced patients with genotype 1–6 HCV infection: the POLARIS-4 study. Hepatology 64 , 59A (2016).
Freeman, J. et al. High sustained virological response rates using generic direct acting antiviral treatment for hepatitis c, imported into Australia. J. Hepatol. 64 , S209 (2016).
Reig, M. et al . Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 65 , 719–726 (2016).
Child, C. G. & Turcotte, J. G. Surgery and portal hypertension. Major Probl. Clin. Surg. 1 , 1–85 (1964).
Pugh, R. N., Murray-Lyon, I. M., Dawson, J. L., Pietroni, M. C. & Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 60 , 646–649 (1973).
Cornpropst, M. T. et al . The effect of renal impairment and end stage renal disease on the single-dose pharmacokinetics of Psi-7977. J. Hepatol. 56 , S433 (2012).
Gane, E. J. et al . Safety, anti-viral efficacy and pharmacokinetics (PK) of sofosbuvir (SOF) in patients with severe renal impairment. Hepatology 60 , 667a (2014).
Garimella, T. et al . The effect of renal impairment on single-dose pharmacokinetics of daclatasvir, an HCV NS5A inhibitor. J. Viral Hepatitis 21 , 32 (2014).
Khatri, A. et al . The pharmacokinetics and safety of the direct acting antiviral regimen of ABT-450/r, ombitasvir with/without dasabuvir in subjects with mild, moderate and severe renal impairment compared to subjects with normal renal function. Hepatology 60 , 320a (2014).
Roth, D. et al . Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet 386 , 1537–1545 (2015).
Hoofnagle, J. H. Toward universal vaccination against hepatitis B virus. N. Engl. J. Med. 321 , 1333–1334 (1989).
McHutchison, J. G. et al . Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N. Engl. J. Med. 339 , 1485–1492 (1998). This paper establishes IFN-α2b plus ribavirin as a new standard of care between 1998 and 2001.
Poynard, T. et al . Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet 352 , 1426–1432 (1998). This study establishes IFN-α2b plus ribavirin as standard of care for patients outside the United States.
Zeuzem, S. et al . Peginterferon alfa-2a in patients with chronic hepatitis C. N. Engl. J. Med. 343 , 1666–1672 (2000). This study provides proof of concept that PEG-IFN-α2a is superior to non-PEG-IFN.
Fried, M. W. et al . Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347 , 975–982 (2002).
Manns, M. P. et al . Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358 , 958–965 (2001). This study establishes PEG-IFN-α2b plus ribavirin as a new standard of care between 2001 and 2011.
Saraswat, V. et al . Historical epidemiology of hepatitis C virus (HCV) in select countries — volume 2. J. Viral Hepatitis 22 , 6–25 (2015).
Attaullah, S., Khan, S. & Ali, I. Hepatitis C virus genotypes in Pakistan: a systemic review. Virol. J. 8 , 433 (2011).
Abdel-Hamid, M. et al . Genetic diversity in hepatitis C virus in Egypt and possible association with hepatocellular carcinoma. J. Gen. Virol. 88 , 1526–1531 (2007).
El-Zayadi, A., Simmonds, P., Dabbous, H. & Selim, O. Hepatitis C virus genotypes among HCV-chronic liver disease patients in Egypt: a leading trial. J. Egypt Public Health Assoc. 69 , 327–334 (1994).
Ray, S. C., Arthur, R. R., Carella, A., Bukh, J. & Thomas, D. L. Genetic epidemiology of hepatitis C virus throughout Egypt. J. Infect. Dis. 182 , 698–707 (2000).
Prabdial-Sing, N., Puren, A. J., Mahlangu, J., Barrow, P. & Bowyer, S. M. Hepatitis C virus genotypes in two different patient cohorts in Johannesburg, South Africa. Arch. Virol. 153 , 2049–2058 (2008).
Smuts, H. E. & Kannemeyer, J. Genotyping of hepatitis C virus in South Africa. J. Clin. Microbiol. 33 , 1679–1681 (1995).
Rao, H. et al . Distribution and clinical correlates of viral and host genotypes in Chinese patients with chronic hepatitis C virus infection. J. Gastroenterol. Hepatol. 29 , 545–553 (2014).
Akkarathamrongsin, S. et al . Seroprevalence and genotype of hepatitis C virus among immigrant workers from Cambodia and Myanmar in Thailand. Intervirology 54 , 10–16 (2011).
Leung, N., Chu, C. & Tam, J. S. Viral hepatitis C in Hong Kong. Intervirology 49 , 23–27 (2006).
Hubschen, J. M. et al . High genetic diversity including potential new subtypes of hepatitis C virus genotype 6 in Lao People's Democratic Republic. Clin. Microbiol. Infect. 17 , E30–E34 (2011).
Lwin, A. A. et al . Hepatitis C virus genotype distribution in Myanmar: predominance of genotype 6 and existence of new genotype 6 subtype. Hepatol. Res. 37 , 337–345 (2007).
Pham, V. H. et al . Very high prevalence of hepatitis C virus genotype 6 variants in southern Vietnam: large-scale survey based on sequence determination. Jpn J. Infect. Dis. 64 , 537–539 (2011).
Download references
Acknowledgements
The authors thank S. Hardtke and P. Solbach, Hannover Medical School, Hannover, Germany, for editorial assistance and M. Cornberg, Hannover Medical School for helpful discussions.
Author information
Authors and affiliations.
Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Carl-Neuberg-Str. 1, Hannover, 30625, Germany
Michael P. Manns
German Center for Infection Research (DZIF), Partner Site Hannover-Braunschweig, Germany
Helmholtz Centre for Infection Research (HZI), Braunschweig, Germany
Liver Unit, Hospital Universitari Vall d'Hebron and CIBEREHD del Instituto Carlos III, Barcelona, Spain
New Zealand Liver Transplant Unit, Auckland City Hospital, Auckland, New Zealand
Department of Virology, National Reference Center for Viral Hepatitis B, C and D, Hôpital Henri Mondor, Université Paris-Est, Créteil, France
Jean-Michel Pawlotsky
INSERM U955, Créteil, France
Center for Disease Analysis, Lafayette, Colorado, USA
Homie Razavi
Division of Gastroenterology, Viral Hepatitis Center, University of California at San Francisco, San Francisco, California, USA
Norah Terrault
Beatty Center for Integrated Research, Falls Church, Virginia, USA
Zobair Younossi
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (M.P.M.); Epidemiology (H.R.); Mechanisms/pathophysiology (J.-M.P.); Diagnosis, screening and prevention (J.-M.P. and M.B.); Management (M.P.M., E.G. and N.T.); Quality of life (Z.Y.); Outlook (M.P.M.); Overview of Primer (M.P.M.).
Corresponding author
Correspondence to Michael P. Manns .
Ethics declarations
Competing interests.
M.P.M. has received research grants and or served as an adviser for Roche, Bristol-Myers Squibb (BMS), Gilead, Boehringer Ingelheim, Novartis, Merck, Janssen, GlaxoSmithKline (GSK), Biotest and AbbVie. M.B. has served as a speaker and/or adviser of AbbVie, Gilead, Janssen, Merck and BMS. E.G. has served as an adviser for Roche, Gilead, Janssen, Novira, AbbVie, Novartis, Achillion, Merck and Alios. J.-M.P. has received research grants from Gilead Sciences and AbbVie. He has served as an adviser for AbbVie, BMS, Gilead, Janssen and Merck. H.R. has received research funds from Gilead and AbbVie. N.T. has received research grants and/or served as an adviser for Gilead, Cocrystal Pharma, BMS, AbbVie, Merck and Echosens North America Inc. She received royalty from UpToDate and is involved in continuing medical education and the development of educational material for CCO Hepatitis, Practice Point Communications and Focus Medical Communications. Z.Y. has received research funds from Gilead, BMS and AbbVie and is a consultant or an adviser to BMS, Gilead, GSK, Intercept and Tobira.
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2, powerpoint slide for fig. 3, powerpoint slide for fig. 4, powerpoint slide for fig. 5, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Manns, M., Buti, M., Gane, E. et al. Hepatitis C virus infection. Nat Rev Dis Primers 3 , 17006 (2017). https://doi.org/10.1038/nrdp.2017.6
Download citation
Published : 02 March 2017
DOI : https://doi.org/10.1038/nrdp.2017.6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Leveraging oncovirus-derived antigen against the viral malignancies in adoptive cell therapies.
Biomarker Research (2024)
Unraveling the dynamic mechanisms of natural killer cells in viral infections: insights and implications
- Arash Letafati
- Omid Salahi Ardekani
- Sayed-Hamidreza Mozhgani
Virology Journal (2024)
Trends in hepatitis C virus seroprevalence and associated risk factors among msm in Pakistan: insights from a community-based study
- Raza Tirmizi
- Rimsha Munir
- Nousheen Zaidi
Scientific Reports (2024)
Viral hepatitis–induced acute liver failure
- Sagnik Biswas
- Ramesh Kumar
- Subrat Kumar Acharya
Indian Journal of Gastroenterology (2024)
Therapeutic potential of oleanolic acid in liver diseases
- Yongxin Wang
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Supplement Archive
- Cover Archive
- IDSA Guidelines
- IDSA Journals
- The Journal of Infectious Diseases
- Open Forum Infectious Diseases
- Photo Quizzes
- State-of-the-Art Reviews
- Voices of ID
- Author Guidelines
- Open Access
- Why Publish
- IDSA Journals Calls for Papers
- Advertising and Corporate Services
- Advertising
- Journals Career Network
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- About Clinical Infectious Diseases
- About the Infectious Diseases Society of America
- About the HIV Medicine Association
- IDSA COI Policy
- Editorial Board
- Self-Archiving Policy
- For Reviewers
- For Press Offices
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Testing, evaluation, and monitoring, initial treatment, retreatment, management of unique and key populations, hepatitis c guidance 2023 update: american association for the study of liver diseases– infectious diseases society of america recommendations for testing, managing, and treating hepatitis c virus infection.
HCV Guidance Panel members and their affiliations are listed in the Notes Section.
Potential conflicts of interest . D. B. reports research grants, paid to institution, from Gilead Sciences, Inc. J. C. P. reports research grants, paid to institution, from Gilead Sciences, Inc and Merck & Co, Inc (spouse has interest in stock that is independently managed in AbbVie, Inc, Bristol Myers Squibb Co, Johnson & Johnson, and Merck & Co, Inc). J. J. F. reports serving as a scientific consultant to AbbVie, Inc, Bluejay Therapeutics, Inc, Deep Genomics, Inc, Gilead Sciences, Inc, GSK Plc, and Janssen Pharmaceutical Companies of Johnson & Johnson and grants, paid to institution, from AbbVie, Inc, Alexion AstraZeneca Rare Disease, Altimmune, Inc, Eiger Biopharmaceuticals, Inc, Enanta Pharmaceuticals, Inc, Gilead Sciences, Inc, GSK Plc, Janssen Pharmaceutical Companies of Johnson & Johnson, and Fujifilm Wako Chemicals Corp. S. C. G. reports research grants, paid to institution, from AbbVie, Inc, Gilead Sciences, Inc, and Merck & Co, Inc. R. J. reports participation on the AstraZeneca/Sanofi Advisory Board, being an expert panel member for Moderna, being an advisory board member/consultant for Seqirus, being a consultant for AstraZeneca and Dynavax, royalties from UpToDate, an editorial stipend from PIDS, and a research grant from GSK Plc. M. M. J. reports serving as a scientific consultant to Gilead Sciences, Inc and research grants, paid to institution, from AbbVie, Inc, Gilead Sciences, Inc, F. Hoffmann-La Roche AG, and Merck & Co, Inc. J. J. K. reports research grants, paid to institution, from Gilead Sciences, Inc. T. R. M. reports research grants, paid to institution, from AbbVie, Inc, Genfit, Gilead Sciences, Inc, and Merck & Co, Inc. K. R. R. reports serving on scientific advisory boards for Spark Therapeutics, Novo Nordisk, Genfit, BioVie, and Mallinckrodt; serving on a data and safety monitoring board (DSMB) for Novartis and AstraZeneca; grants, paid to institution, from Bristol Myers Squibb Co, Exact Sciences Corp, Grifols, Intercept Pharmaceuticals, Inc, BioVie, Mallinckrodt Pharmaceuticals, Merck & Co, Inc, Sequana Medical Co, HCC-TARGET, and NASH-TARGET. A. R.'s organization was awarded educational grants from AbbVie, Inc and Gilead Sciences, Inc. J. D. S. reports personal financial relationships with Gilead Sciences, Inc and Premera Blue Cross. G. S.'s organization was awarded grants from AbbVie, Inc, Gilead Sciences, Inc, and Merck & Co, Inc. N. A. T. reports serving on the DSMB of Moderna, Inc and research grants, paid to institution, from Gilead Sciences, Inc, GSK Plc, Helio Health Group, and F. Hoffmann-La Roche AG (Genentech). E. C. V. reports serving on an advisory board for Gilead Sciences, Inc and research grants, paid to institution, from Salix Pharmaceuticals, Inc. J. B. W. is a member of the US Preventive Services Task Force. K. W. reports research grants, paid to institution, from Gilead Sciences, Inc. D. L. W. reports research grants, paid to institution, from Gilead Sciences, Inc. J. P. reports grant support, paid to institution, from AbbVie, Inc, Zydus, and Genentech. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
- Article contents
- Figures & tables
- Supplementary Data
Debika Bhattacharya, Andrew Aronsohn, Jennifer Price, Vincent Lo Re, the American Association for the Study of Liver Diseases–Infectious Diseases Society of America HCV Guidance Panel , Hepatitis C Guidance 2023 Update: American Association for the Study of Liver Diseases– Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection, Clinical Infectious Diseases , 2023;, ciad319, https://doi.org/10.1093/cid/ciad319
- Permissions Icon Permissions
The Infectious Diseases Society of America and the American Association for the Study of Liver Diseases have collaboratively developed evidence-based guidance regarding the diagnosis, management, and treatment of hepatitis C virus (HCV) infection since 2013. A panel of clinicians and investigators with extensive infectious diseases or hepatology expertise specific to HCV infection periodically reviews evidence from the field and update existing recommendations or introduce new recommendations as evidence warrants.
This update focuses on changes to the guidance since the previous 2020 published update, including ongoing emphasis on recommended universal screening; management recommendations for incomplete treatment adherence; expanded eligibility for simplified chronic HCV infection treatment in adults with minimal monitoring; updated treatment and retreatment recommendations for children as young as 3 years; management and treatment recommendations in the transplantation setting; and screening, treatment, and management recommendations for unique and key populations.
The Infectious Diseases Society of America (IDSA) and the American Association for the Study of Liver Diseases (AASLD) collaboratively initiated the Hepatitis C Virus (HCV) Guidance Project in 2013 to provide clinicians with evidence-based, unbiased, timely guidance regarding diagnosis, treatment, and management of HCV infection. The project includes the web-based HCV guidance platform ( www.hcvguidelines.org ) to enable rapid, accessible dissemination of new and/or updated information and recommendations in response to the latest data from the field. The HCV guidance website (hereafter, the HCV guidance) has been highly successful. From the launch of the HCV guidance in January 2014 through April 2022, the site has been accessed by more than 2 million unique users generating more than 4 million page views. In 2021, the site had more the 194 000 unique users from 201 countries and territories, with most visits originating from the United States, India, Russia, Canada, and Pakistan. Under the umbrella of the HCV guidance, the AASLD-IDSA HCV Guidance Panel (hereafter, the Guidance Panel) also issues regular, periodic published updates to review new or updated data and recommendations as well as an overview of the ever-changing landscape of the HCV epidemic.
Recognizing that viral hepatitis poses a public health threat on par with human immunodeficiency virus (HIV), malaria, and tuberculosis, in June 2016, the World Health Organization (WHO) published its first global health sector strategy and set forth the goal of elimination of viral hepatitis as a major public health threat by 2030 [ 1 ]. Specific HCV elimination targets include a 90% reduction in incidence and prevalence, treatment of 80% of eligible persons with chronic infection, a 65% reduction in HCV-related deaths, and universal access to key prevention and treatment services [ 1 ]. In response to the WHO's call to action, the National Academies of Sciences, Engineering, and Medicine developed a US strategic plan for viral hepatitis elimination [ 2 ]. The US Centers for Disease Control and Prevention (CDC) [ 3 ] and the US Department of Health and Human Services (DHHS) [ 4 ] subsequently developed national implementation strategies and targets commensurate with those set forth by the WHO. Notably, the new and updated recommendations highlighted and discussed in this update both independently and collectively support, promote, and advance accomplishment of HCV elimination.
Major changes in the HCV guidance since the previous 2020 publication [ 5 ] featured in this update include an ongoing emphasis on universal HCV screening; new recommendations that address the management of incomplete treatment adherence; updated recommendations regarding simplified treatment with minimal monitoring and expanded eligibility; management and treatment recommendations for solid organ transplant recipients; newly expanded treatment and retreatment recommendations for children and adolescents; and screening, management, and treatment recommendations for unique and key populations. In addition, we highlight key issues critical to HCV management with the mission of HCV elimination in mind. See Figure 1 for key points in this HCV guidance update.

Key points in HCV guidance summary. Abbreviations: DAA, direct-acting antiviral; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
The HCV guidance was developed and is regularly updated by a volunteer panel of more than 30 infectious diseases and hepatology clinicians and investigators with HCV expertise representing IDSA and AASLD, respectively. Four co-chairs (2 from each society) oversee the work of the Guidance Panel. The HCV guidance undergoes major biannual updates based on a rigorous literature review that encompasses peer-reviewed, published literature and relevant abstracts from national and international scientific conferences. The data are reviewed by section leads, with points of discussion resolved during section and full panel remote meetings.
New or updated recommendations are evaluated using a modified scale adapted from the American College of Cardiology and the American Heart Association practice guidelines [ 6 , 7 ] (see the HCV guidance for further details). All new and updated recommendations are reviewed and approved by the IDSA and AASLD governing boards prior to online release or print publication.
Implementation of Universal HCV Screening
The Guidance Panel first recommended universal HCV screening for all adults aged ≥18 years in 2019 [ 5 ], concomitant with congruous draft recommendations from the US Preventive Services Task Force (USPSTF) and the CDC. The USPSTF subsequently recommended universal HCV screening for adults aged 18 to 79 years in March 2020 [ 8 ]. In April 2020, the CDC recommended HCV screening at least once in all adults aged ≥18 years and for all pregnant persons during each pregnancy, except in settings where HCV prevalence is <0.1% [ 9 ]. The rationale for universal HCV screening includes cost-effectiveness [ 10–13 ]; improved HCV case finding [ 8 , 9 ]; shifting epidemiology of HCV infection with incident infections occurring primarily in young adults [ 14–16 ]; and the availability of safe, cost-effective direct-acting antiviral (DAA) treatment [ 17 ]. Universal screening is a crucial and necessary component of any HCV elimination strategy [ 1–4 ] because it is the entry point into the HCV continuum of care [ 18 , 19 ]. For initial HCV testing, the Guidance Panel recommends HCV antibody screening with reflex HCV RNA testing to establish the presence of active infection (as opposed to spontaneous or treatment-induced viral clearance).
Recommendations without rigorous implementation, however, are ineffectual. HCV screening, diagnosis, and treatment were significantly adversely affected by the coronavirus disease 2019 (COVID-19) pandemic [ 20 ]. The number of HCV antibody and HCV RNA tests processed by a large US, multicenter, commercial clinical laboratory decreased precipitously beginning in mid-March 2020 [ 21 ], coincident with the US federal government declaring a national state of emergency due to COVID-19 [ 22 ]. HCV RNA–positive test results decreased 62% in March 2020 and remained 39% below baseline in July 2020, with a concomitant decline in the number of DAA prescriptions dispensed [ 21 ]. Investigators who conducted a similar study in Ontario, Canada, reported comparable decreases in HCV antibody screening and confirmative HCV RNA testing during each of the first 3 waves of the COVID-19 pandemic [ 23 ]. The reduced level of HCV testing negatively affecting initiation of HCV treatment appears corroborated by findings from a US national, retrospective study wherein only 23% of people on Medicaid with a positive HCV RNA test between 30 January 2019 and 31 October 2020 initiated DAA treatment within 360 days of diagnosis [ 24 ]. A survey conducted among European Association for the Study of the Liver members representing 48 clinical centers also demonstrated decreased HCV testing, diagnosis, and treatment in 2020 compared with 2019 (prepandemic) [ 25 ]. Collectively, these findings underscore the critical importance of ongoing, rigorous, universal HCV screening for case identification and linkage to care. In addition, monitoring the proportion of persons who meet steps in the HCV cascade of care will be critical to assessing the quality of HCV care.
Management of Incomplete DAA Adherence
Incomplete medication adherence is well known, even in the highly structured clinical trial setting [ 26 , 27 ]. Recognizing that incomplete DAA treatment may occur in clinical practice and potentially contribute to treatment failure, the HCV guidance includes a new algorithm for the management of incomplete adherence as part of DAA treatment monitoring ( Figure 2 ). The algorithm is applicable only to DAA treatment–naive persons and, generally, the same patient populations who are eligible for the simplified treatment algorithms described in the following section. Excluded persons with incomplete adherence should be managed in consultation with a specialist in HCV management.

Recommended management of DAA treatment interruptions for treatment-naive patients without cirrhosis or with compensated cirrhosis receiving glecaprevir/pibrentasvir or sofosbuvir/velpatasvir. Abbreviations: DAA, direct-acting antiviral; HCV, hepatitis C virus; SVR12, sustained virologic response 12 weeks after completion of therapy. a Extend duration of therapy such that the patient receives the total planned dosage (ie, the total number of daily pills). For example, if a patient missed 10 days of a planned 8-week course of therapy, treatment would be extended to 8 weeks plus 10 days.
Although few studies have examined incomplete medication adherence in the DAA era, data suggest that it is relatively common, occurring in 11% to 40% of persons on treatment [ 28–31 ]. Most episodes of nonadherence appear short-lived. One study demonstrated that 61% of nonadherent episodes lasted 1 to 2 days [ 31 ]. These short periods of nonadherence were not associated with virologic failure. Sustained virologic response (SVR) 12 weeks after the completion of treatment (SVR12) was 94% among both adherent and nonadherent participants, where nonadherence was defined as taking <90% of the total prescribed dosage [ 31 ]. Longer periods of nonadherence, however, may adversely affect SVR. Investigators who examined the relationship between premature discontinuation of DAA therapy and SVR found that among study participants with F0 to F3 liver disease, SVR12 was 50% in persons who received <4 weeks of DAA treatment compared with 99% SVR12 in those who received ≥4 weeks of treatment [ 32 ]. Among participants with compensated cirrhosis, SVR12 rates were 83% and 95% in those who completed <8 weeks of DAA therapy compared with ≥8 weeks of treatment, respectively [ 32 ].
Based on these limited findings and the expert consensus of the Guidance Panel, a management algorithm that considers the timing and duration of the nonadherence, as well as specific patient factors (ie, genotype 3 infection and presence of compensated cirrhosis), is recommended (see Figure 2 ). Additional large-scale studies in clinical practice settings that examine the relationship between DAA adherence and SVR12, including the threshold level of adherence below which SVR12 is adversely affected, are sorely needed.
Simplified HCV Treatment for Treatment-Naive Adults
The Guidance Panel continues to strongly recommend universal DAA treatment for all people with acute or chronic HCV infection (except those with a short life expectancy that cannot be remediated by HCV therapy, liver transplantation, or another directed therapy). A key aspect of facilitating the implementation of this recommendation/goal is expanding the pool of clinicians who provide HCV treatment, thereby boosting accessibility and delivery of care. Accordingly and coincident with the accumulation of real-world data and experience with the pangenotypic DAA regimens, the HCV guidance first introduced the simplified treatment algorithms for treatment-naive persons (without cirrhosis or with compensated cirrhosis) in 2019 [ 5 ]. The current update to the simplified treatment algorithms features reduced pretreatment and on-treatment clinician intervention and expanded eligibility of persons who can be treated using these approaches.
Recent data from a global sample of persons undergoing DAA treatment for chronic HCV infection suggest that a minimal on-treatment monitoring approach is safe and effective and leads to an SVR rate that is comparable to that realized with standard monitoring [ 33 ]. The minimal monitoring (MINMON) approach was examined in an international, phase 4, open-label, single-arm trial. Four hundred treatment-naive participants aged ≥18 years with active HCV infection were enrolled from 38 sites in Brazil, South Africa, Thailand, Uganda, and the United States. Participants included persons with compensated cirrhosis and HIV coinfection. Key exclusion criteria were pregnancy, breastfeeding, and chronic hepatitis B virus (HBV) infection (hepatitis B surface antigen [HBsAg] positive; due to possible risk of HBV reactivation). However, participants with resolved HBV infection (hepatitis B core antibody [anti-HBc] positive, with or without hepatitis B surface antibodies [anti-HBs]) were eligible. Of the 400 enrolled participants, 399 initiated a planned 12-week course of once-daily sofosbuvir (400 mg)/velpatasvir (100 mg). At entry, 42% (166) were living with HIV, 9% (34) had compensated cirrhosis, and 32% (121 of 374) with an available HBV panel had resolved HBV infection. The 4 components of minimal monitoring included no pretreatment genotyping, dispensing the entire treatment course at entry, no scheduled on-treatment visits or laboratory monitoring, and remote contact at week 4 to assess DAA adherence and at week 22 to schedule SVR assessment at week 24. SVR was achieved by 95% (379 of 399) of those who initiated treatment. Fourteen participants experienced a serious adverse event between treatment initiation and week 28; none were treatment-related or led to treatment discontinuation or death [ 33 ].
Given the findings of this minimal monitoring study, treatment-naive persons with HIV/HCV coinfection are newly eligible for a simplified HCV treatment approach. Figure 3 shows the eligibility and exclusion criteria for the simplified HCV treatment approaches. Figure 4 provides an overview of the simplified HCV treatment algorithm for treatment-naive adults without cirrhosis. Figure 5 reviews the simplified treatment algorithm for HCV treatment-naive adults with compensated cirrhosis.

Inclusion and exclusion criteria for simplified HCV treatment algorithm. Abbreviations: eGFR, estimated glomerular filtration rate; FIB-4, fibrosis-4 index for liver fibrosis; HCV, hepatitis C virus. a Noninvasive serologic tests include HCV FibroSure or enhanced liver fibrosis test. b Child–Pugh score based on presence of ascites, hepatic encephalopathy, total bilirubin >2.0 mg/dL, albumin ≤3.5 g/dL, or international normalized ratio ≥1.7.
![literature review of hepatitis c Simplified algorithm for HCV treatment among HCV treatment-naive adults without cirrhosis. Recommended DAA regimens for this simplified treatment approach include either 8 weeks of glecaprevir (300 mg)/pibrentasvir (120 mg) taken with food or 12 weeks of sofosbuvir (400 mg)/velpatasvir (100 mg). More detailed descriptions of the patient evaluation process and antivirals used for HCV treatment can be found on the HCV guidance website. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBC, complete blood count; DAA, direct-acting antiviral; eGFR, estimated glomerular filtration rate; FIB-4, fibrosis-4 index for liver fibrosis; HCV, hepatitis C virus; HIV, human immunodeficiency virus; INR, international normalized ratio; SVR, sustained virologic response. aFIB-4 is a noninvasive measure of hepatic fibrosis that is calculated by: (age [years] × AST [U/L]) ÷ (platelet count [109/L] × (ALT1/2 [U/L]). bA patient is presumed to have cirrhosis if they have a FIB-4 score >3.25 or if they have any of the following from a previously performed test: transient elastography indicating cirrhosis (ie, liver stiffness >12.5 kPa), noninvasive serologic test above the proprietary cutoff indicating cirrhosis (eg, FibroSure, enhanced liver fibrosis test), clinical evidence of cirrhosis (eg, liver nodularity and/or splenomegaly on imaging, platelet count <150 000/mm3), or prior liver biopsy showing cirrhosis. cMedication reconciliation should record currently prescribed medications, over-the-counter drugs, and herbal/dietary supplements. dDrug–drug interaction assessment should be performed using the table in the Monitoring Section of the HCV Guidance website or the University of Liverpool drug interaction checker.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/PAP/10.1093_cid_ciad319/2/m_ciad319f4.jpeg?Expires=1728494988&Signature=m58jKhlq3dhqGeH3pOUPoMqmMqypcDqvujWUNAUcEfOzw9sm9Znm~GnX~3FedO5MqNcGf3DqVi~eiw8liKFbGXn-2ZAPXNokGSgDX-IZVqYBn9KcVrIEdFYmK0PmCP434ZU5115frgHOxwR6LNGo-K~V~T3MAJhPR1XRfiJlr7~Ya8xz8kApAwBk5k~iBb5-pjm5CS0vmYS7hvPxf-3HjBN0Scz9HPlsqMN-47f3mPaOsKKJABtXPrdu0ECPLPYvPbkqD2qx3kPuIei3R16FqbUK498M-~wdsfha6HQBubrwWv3aEy3B~X4WZvXkAbTcaNOtUSz92wdfirYFThg5-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Simplified algorithm for HCV treatment among HCV treatment-naive adults without cirrhosis. Recommended DAA regimens for this simplified treatment approach include either 8 weeks of glecaprevir (300 mg)/pibrentasvir (120 mg) taken with food or 12 weeks of sofosbuvir (400 mg)/velpatasvir (100 mg). More detailed descriptions of the patient evaluation process and antivirals used for HCV treatment can be found on the HCV guidance website. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBC, complete blood count; DAA, direct-acting antiviral; eGFR, estimated glomerular filtration rate; FIB-4, fibrosis-4 index for liver fibrosis; HCV, hepatitis C virus; HIV, human immunodeficiency virus; INR, international normalized ratio; SVR, sustained virologic response. a FIB-4 is a noninvasive measure of hepatic fibrosis that is calculated by: (age [years] × AST [U/L]) ÷ (platelet count [109/L] × (ALT1/2 [U/L]). b A patient is presumed to have cirrhosis if they have a FIB-4 score >3.25 or if they have any of the following from a previously performed test: transient elastography indicating cirrhosis (ie, liver stiffness >12.5 kPa), noninvasive serologic test above the proprietary cutoff indicating cirrhosis (eg, FibroSure, enhanced liver fibrosis test), clinical evidence of cirrhosis (eg, liver nodularity and/or splenomegaly on imaging, platelet count <150 000/mm3), or prior liver biopsy showing cirrhosis. c Medication reconciliation should record currently prescribed medications, over-the-counter drugs, and herbal/dietary supplements. d Drug–drug interaction assessment should be performed using the table in the Monitoring Section of the HCV Guidance website or the University of Liverpool drug interaction checker.

Simplified algorithm for HCV treatment among HCV treatment-naive adults with compensated cirrhosis. Recommended DAA regimens for this simplified treatment approach include either 8 weeks of glecaprevir (300 mg)/pibrentasvir (120) mg taken with food for genotypes 1 through 6 or 12 weeks of sofosbuvir (400 mg)/velpatasvir (100 mg) for genotypes 1, 2, 4, 5, or 6. More detailed descriptions of the patient evaluation process and antivirals used for HCV treatment can be found on the HCV Guidance website. Abbreviations: AASLD, American Association for the Study of Liver Diseases; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBC, complete blood count; DAA, direct-acting antiviral; eGFR, estimated glomerular filtration rate; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HIV, human immunodeficiency virus; INR, international normalized ratio; SVR, sustained virologic response. a Child–Pugh score based on presence of ascites, hepatic encephalopathy, total bilirubin >2.0 mg/dL, albumin ≤3.5 g/dL, or INR ≥1.7. Patients with a Child–Pugh score ≥7 (ie, Child–Pugh B or C) have decompensated cirrhosis; this simplified treatment approach is not recommended for patients with decompensated cirrhosis. b Obtain liver ultrasound within 6 months prior to initiating antiviral treatment to exclude hepatocellular carcinoma and subclinical ascites. This simplified treatment approach is not recommended for patients with hepatocellular carcinoma and/or decompensated cirrhosis. c Medication reconciliation should record currently prescribed medications, over-the-counter drugs, and herbal/dietary supplements. d Drug–drug interaction assessment should be performed using the table in the Monitoring Section of the HCV Guidance website or the University of Liverpool drug interaction checker. e Development of jaundice, ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy may suggest hepatic decompensation. Patients should be referred to a specialist if they develop worsening liver blood tests (eg, total bilirubin, AST, ALT, INR), jaundice, ascites, encephalopathy, or new liver-related symptoms). f Ultrasound surveillance for hepatocellular carcinoma (with or without alpha-fetoprotein testing) every 6 months is recommended for patients with cirrhosis, in accordance with AASLD guidance. g See AASLD guidance for recommendations regarding the evaluation and management of varices.
The inclusion of persons living with HIV in the simplified HCV treatment algorithm is consistent with the DHHS Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV [ 34 ]. In this guidance, the decision to expand eligibility to include persons living with HIV was informed by the comparable SVR12 rates in those with and without HIV coinfection in the MINMON study [ 33 ], the availability of integrase strand transfer inhibitor–based antiretroviral regimens that mitigate concerns of drug–drug interactions between HIV and HCV medications, and the need to expand treatment access, particularly in the COVID-19 pandemic era.
Initial Treatment Regimens
In the current DAA era of hepatitis C treatment, therapy is safe, effective, of relatively short duration, and curative in most people [ 1 , 17 ]. Widespread use of recommended initial treatment regimens has the potential to substantially reduce hepatitis C prevalence. Given the many benefits of virologic cure, including reduced risk of cirrhosis, hepatocellular carcinoma, liver-related mortality [ 35 ], and all-cause mortality [ 35–37 ], expanded use of DAA treatment and the associated probable cure has the capacity to reduce HCV-related disease burden at individual, national, and potentially global levels.
Since the last published update [ 5 ], genotypic activity has been added to the hierarchical ranking of treatment regimens (in addition to recommended or alternative, evidence level, and alphabetical order). Table 1 presents a summary of initial treatment recommendations for treatment-naive adults. Shortening the duration of glecaprevir/pibrentasvir therapy to 8 weeks for persons with compensated cirrhosis is a notable change. The updated recommendation is supported by the findings from the international, single-arm, open-label, phase 3b EXPEDITION-8 trial, titled “Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated cirrhosis” [ 38 ]. Investigators enrolled 343 treatment-naive participants aged ≥18 years with chronic HCV infection (genotypes 1 through 6) and compensated cirrhosis. Key exclusion criteria included coinfection with HIV and/or HBV or a history of hepatic decompensation. Participants received an 8-week course of once-daily glecaprevir (300 mg)/pibrentasvir (120 mg). SVR12 was 98% (335 of 343) in the intention-to-treat (ITT) population. Seven participants experienced a serious adverse event, only 1 of which was treatment-related. One participant who had low baseline leukocyte and neutrophil counts experienced grade 3 leukopenia and neutropenia that presented on posttreatment day 29, which the investigator considered treatment-related. No adverse event led to treatment discontinuation or death [ 38 ].
Recommendations for Initial Treatment of Hepatitis C Virus–Infected Adults
| Regimen . | Genotype . | Classification . | Duration . | Rating . | Caveats and Other Considerations . |
|---|---|---|---|---|---|
| Treatment-naive without cirrhosis or with compensated cirrhosis Glecaprevir/pibrentasvir | 1–6 | Recommended | 8 wk | I, A | |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | I, A | For genotype 3 infection with compensated cirrhosis, NS5A RAS testing is recommended. If baseline NS5A RAS Y93H is present, add weight-based ribavirin or choose another recommended regimen. |
| Ledipasvir/sofosbuvir | 1, 4, 5, 6 | Recommended | 12 wk | I, A | Not recommended for genotype 6e infection if subtype is known. |
| 1 without cirrhosis | Recommended | 8 wk | I, B | Applicable to patients without cirrhosis who are not living with human immunodeficiency virus and whose HCV RNA is <6 million IU/mL. | |
| Elbasvir/grazoprevir | 1b, 4 | Recommended | 12 wk | I, A | |
| 1a | Alternative | 12 wk | I, A | For genotype 1a infection, NS5A RAS testing is recommended. If baseline RASs are present (ie, substitutions at amino acid positions 28, 30, 31, or 93), another recommended regimen should be used. | |
| Sofosbuvir/velpatasvir + weight-based ribavirin | 3 | Alternative | 12 wk | IIa, A | Applicable to genotype 3 infection with compensated cirrhosis and baseline NS5a Y93 RAS. |
| Sofosbuvir/velpatasvir/voxilaprevir | Alternative | 12 wk | IIa, B | Applicable to genotype 3 infection with compensated cirrhosis and baseline NS5a Y93 RAS. | |
| Treatment-naive with decompensated cirrhosis | |||||
| Sofosbuvir/velpatasvir + weight-based ribavirin | 1–6 | Recommended | 12 wk | I, A | Low initial dose of ribavirin (600 mg) is recommended for patients with CTP class C cirrhosis; increase as tolerated. |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 24 wk | I, A | Applicable to patients who are ribavirin ineligible. |
| Ledipasvir/sofosbuvir + weight-based ribavirin | 1, 4, 5, 6 | Recommended | 12 wk | I, A | Low initial dose of ribavirin (600 mg) is recommended for patients with CTP class C cirrhosis; increase as tolerated. |
| Ledipasvir/sofosbuvir | 1, 4, 5, 6 | Recommended | 24 wk | I, A | Applicable to patients who are ribavirin ineligible. |
| Regimen . | Genotype . | Classification . | Duration . | Rating . | Caveats and Other Considerations . |
|---|---|---|---|---|---|
| Treatment-naive without cirrhosis or with compensated cirrhosis Glecaprevir/pibrentasvir | 1–6 | Recommended | 8 wk | I, A | |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | I, A | For genotype 3 infection with compensated cirrhosis, NS5A RAS testing is recommended. If baseline NS5A RAS Y93H is present, add weight-based ribavirin or choose another recommended regimen. |
| Ledipasvir/sofosbuvir | 1, 4, 5, 6 | Recommended | 12 wk | I, A | Not recommended for genotype 6e infection if subtype is known. |
| 1 without cirrhosis | Recommended | 8 wk | I, B | Applicable to patients without cirrhosis who are not living with human immunodeficiency virus and whose HCV RNA is <6 million IU/mL. | |
| Elbasvir/grazoprevir | 1b, 4 | Recommended | 12 wk | I, A | |
| 1a | Alternative | 12 wk | I, A | For genotype 1a infection, NS5A RAS testing is recommended. If baseline RASs are present (ie, substitutions at amino acid positions 28, 30, 31, or 93), another recommended regimen should be used. | |
| Sofosbuvir/velpatasvir + weight-based ribavirin | 3 | Alternative | 12 wk | IIa, A | Applicable to genotype 3 infection with compensated cirrhosis and baseline NS5a Y93 RAS. |
| Sofosbuvir/velpatasvir/voxilaprevir | Alternative | 12 wk | IIa, B | Applicable to genotype 3 infection with compensated cirrhosis and baseline NS5a Y93 RAS. | |
| Treatment-naive with decompensated cirrhosis | |||||
| Sofosbuvir/velpatasvir + weight-based ribavirin | 1–6 | Recommended | 12 wk | I, A | Low initial dose of ribavirin (600 mg) is recommended for patients with CTP class C cirrhosis; increase as tolerated. |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 24 wk | I, A | Applicable to patients who are ribavirin ineligible. |
| Ledipasvir/sofosbuvir + weight-based ribavirin | 1, 4, 5, 6 | Recommended | 12 wk | I, A | Low initial dose of ribavirin (600 mg) is recommended for patients with CTP class C cirrhosis; increase as tolerated. |
| Ledipasvir/sofosbuvir | 1, 4, 5, 6 | Recommended | 24 wk | I, A | Applicable to patients who are ribavirin ineligible. |
Recommendations are listed by recommended vs alternative and by genotypic activity, evidence level, and alphabetically.
Abbreviations: CTP, Child–Turcotte–Pugh score; HCV, hepatitis C virus; NS5A, hepatitis C virus nonstructural protein 5A; RAS, resistance-associated substitution.
The level of evidence rating is I, B for persons with compensated cirrhosis.
The level of evidence rating is I, B for persons with genotype 5 or 6 infection.
The level of evidence rating is IIa, B for persons with genotype 5 or 6 infection and those with genotype 4 infection and compensated cirrhosis.
The level of evidence rating is IIa, B for persons with genotype 4 infection and compensated cirrhosis.
Only available data for genotype 6 infection are in persons with compensated cirrhosis.
Only available data for genotypes 5 or 6 infection are in a small number of persons with compensated cirrhosis.
Another significant change is the recommendation that sofosbuvir/velpatasvir/voxilaprevir may be used as an alternative regimen for persons with genotype 3 infection and compensated cirrhosis. This new recommendation is based on findings from the international, open-label, randomized, phase 3 POLARIS-3 clinical trial, titled “Efficacy of 8 weeks of sofosbuvir, velpatasvir, and voxilaprevir in patients with chronic HCV infection: 2 phase 3 randomized trials” and acknowledges limited access to resistance-associated substitution (RAS) testing in some settings [ 39 ]. Investigators enrolled 220 DAA treatment–naive participants with genotype 3 infection and compensated cirrhosis who were randomized to 8 weeks of once-daily sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) or 12 weeks of once-daily sofosbuvir (400 mg)/velpatasvir (100 mg). SVR12 was 96% in both treatment arms [ 39 ].
Initial treatment using elbasvir/grazoprevir for genotype 1a infection was changed from a recommended to an alternative regimen because of the need for baseline RAS testing. Additionally, several regimens are no longer recommended because the therapeutics are either no longer available in the United States and/or the regimens have inferior SVR rates compared with currently recommended DAA regimens. These include sofosbuvir and daclatasvir; sofosbuvir and ribavirin; paritaprevir/ritonavir/ombitasvir/dasabuvir; and sofosbuvir, telaprevir, or boceprevir with pegylated interferon and ribavirin.
Although DAA therapy is curative for most persons [ 1 , 17 ], the small percentage of those in whom treatment fails to result in SVR12 require retreatment. Updated retreatment recommendations focus on DAA treatment failures, specifically, sofosbuvir-based regimen failure; glecaprevir/pibrentasvir failure; and multiple DAA failure, including sofosbuvir/velpatasvir/voxilaprevir or sofosbuvir plus glecaprevir/pibrentasvir ( Table 2 ). Retreatment recommendations for sofosbuvir-based or HCV nonstructural protein 5A (NS5A) inhibitor-based treatment failures in persons with decompensated cirrhosis are also noted in Table 2 .
Recommendations for Retreatment of Hepatitis C Virus–Infected Adults by Prior Exposure
| Regimen . | Genotype . | Classification . | Duration . | Rating . | Caveats and Other Considerations . |
|---|---|---|---|---|---|
| Sofosbuvir-based treatment failure without cirrhosis or with compensated cirrhosis | |||||
| Sofosbuvir/velpatasvir/voxilaprevir | 1–6 | Recommended | 12 wk | I, A | For genotype 3 infection with compensated cirrhosis, add weight-based ribavirin if there are no contraindications. |
| Glecaprevir/pibrentasvir | 1, 2, 4, 5, 6 | Alternative | 16 wk | I, A | Not recommended for patients with prior exposure to an NS5A inhibitor plus NS3/4A protease inhibitor regimen (eg, elbasvir/grazoprevir). |
| Glecaprevir/pibrentasvir treatment failure without cirrhosis or with compensated cirrhosis | |||||
| Glecaprevir/pibrentasvir + sofosbuvir + weight-based ribavirin | 1–6 | Recommended | 16 wk | IIa, B | |
| Sofosbuvir/velpatasvir/voxilaprevir | 1–6 | Recommended | 12 wk | IIa, B | For patients with compensated cirrhosis, addition of weight-based ribavirin is recommended (rating IIa, C). |
| Sofosbuvir/velpatasvir/voxilaprevir or sofosbuvir + glecaprevir/pibrentasvir treatment failure without cirrhosis or with compensated cirrhosis | |||||
| Glecaprevir/pibrentasvir + sofosbuvir + weight-based ribavirin | 1–6 | Recommended | 16 wk | IIa, B | Extension to 24 wk should be considered in extremely difficult cases (eg, genotype 3 infection with compensated cirrhosis) or failure following sofosbuvir + glecaprevir/pibrentasvir therapy. |
| Sofosbuvir/velpatasvir/voxilaprevir + weight-based ribavirin | 1–6 | Recommended | 24 wk | IIa, B | |
| Sofosbuvir- or NS5A inhibitor–based treatment failure with decompensated cirrhosis | |||||
| Sofosbuvir/velpatasvir + weight-based ribavirin | 1–6 | Recommended | 24 wk | II, C | Low initial dose of ribavirin (600 mg) is recommended for patients with CTP class C cirrhosis; increase as tolerated. |
| Ledipasvir/sofosbuvir + weight-based ribavirin | 1, 4, 5, 6 | Recommended | 24 wk | II, C | Low initial dose of ribavirin (600 mg) is recommended for patients with CTP class C cirrhosis; increase as tolerated. |
| Regimen . | Genotype . | Classification . | Duration . | Rating . | Caveats and Other Considerations . |
|---|---|---|---|---|---|
| Sofosbuvir-based treatment failure without cirrhosis or with compensated cirrhosis | |||||
| Sofosbuvir/velpatasvir/voxilaprevir | 1–6 | Recommended | 12 wk | I, A | For genotype 3 infection with compensated cirrhosis, add weight-based ribavirin if there are no contraindications. |
| Glecaprevir/pibrentasvir | 1, 2, 4, 5, 6 | Alternative | 16 wk | I, A | Not recommended for patients with prior exposure to an NS5A inhibitor plus NS3/4A protease inhibitor regimen (eg, elbasvir/grazoprevir). |
| Glecaprevir/pibrentasvir treatment failure without cirrhosis or with compensated cirrhosis | |||||
| Glecaprevir/pibrentasvir + sofosbuvir + weight-based ribavirin | 1–6 | Recommended | 16 wk | IIa, B | |
| Sofosbuvir/velpatasvir/voxilaprevir | 1–6 | Recommended | 12 wk | IIa, B | For patients with compensated cirrhosis, addition of weight-based ribavirin is recommended (rating IIa, C). |
| Sofosbuvir/velpatasvir/voxilaprevir or sofosbuvir + glecaprevir/pibrentasvir treatment failure without cirrhosis or with compensated cirrhosis | |||||
| Glecaprevir/pibrentasvir + sofosbuvir + weight-based ribavirin | 1–6 | Recommended | 16 wk | IIa, B | Extension to 24 wk should be considered in extremely difficult cases (eg, genotype 3 infection with compensated cirrhosis) or failure following sofosbuvir + glecaprevir/pibrentasvir therapy. |
| Sofosbuvir/velpatasvir/voxilaprevir + weight-based ribavirin | 1–6 | Recommended | 24 wk | IIa, B | |
| Sofosbuvir- or NS5A inhibitor–based treatment failure with decompensated cirrhosis | |||||
| Sofosbuvir/velpatasvir + weight-based ribavirin | 1–6 | Recommended | 24 wk | II, C | Low initial dose of ribavirin (600 mg) is recommended for patients with CTP class C cirrhosis; increase as tolerated. |
| Ledipasvir/sofosbuvir + weight-based ribavirin | 1, 4, 5, 6 | Recommended | 24 wk | II, C | Low initial dose of ribavirin (600 mg) is recommended for patients with CTP class C cirrhosis; increase as tolerated. |
Abbreviations: CTP, Child–Turcotte–Pugh score; NS3/4A, hepatitis C virus nonstructural protein 3–4A; NS5A, hepatitis C virus nonstructural protein 5A.
Sofosbuvir-based Regimen Failure
Generally, persons who have experienced treatment failure with a sofosbuvir-based regimen should be retreated with 12 weeks of sofosbuvir/velpatasvir/voxilaprevir. The exception is persons with genotype 3 infection and compensated cirrhosis for whom the addition of weight-based ribavirin to the regimen is recommended. This recommendation is supported by data from clinical trials [ 40 , 41 ] and real-world cohorts [ 42–45 ]. Glecaprevir/pibrentasvir for 16 weeks can be used as an alternative retreatment regimen [ 46–48 ]. This regimen, however, has not been evaluated in persons with genotype 3 infection and prior sofosbuvir/NS5A inhibitor exposure and is therefore not recommended for these individuals.
Glecaprevir/Pibrentasvir Failure
For persons with a prior glecaprevir/pibrentasvir treatment failure, retreatment with glecaprevir/pibrentasvir plus ribavirin and sofosbuvir is a recommended retreatment option. This recommendation is supported by findings from the MAGELLAN-3 clinical trial, titled “Retreatment of patients who failed glecaprevir/pibrentasvir treatment for hepatitis C virus infection” [ 49 ]. This open-label, phase 3b study evaluated the efficacy and safety of once-daily glecaprevir (300 mg)/pibrentasvir (120 mg) plus sofosbuvir (400 mg) and twice daily weight-based ribavirin for retreatment of persons with a prior glecaprevir/pibrentasvir treatment failure. Participants with non–genotype 3 infection without cirrhosis and naive to HCV nonstructural protein 3-4A (NS3/4A) protease inhibitors and NS5A inhibitors received 12 weeks of treatment. Those with genotype 3 infection and/or compensated cirrhosis, and/or prior exposure to NS3/4A protease inhibitors and/or NS5A inhibitors received 16 weeks of treatment. SVR12 was 96% (22 of 23) in the ITT population. One patient experienced a serious adverse event unrelated to treatment. No treatment discontinuations or deaths occurred [ 49 ].
Treatment with sofosbuvir/velpatasvir/voxilaprevir for 12 weeks is another recommended option in the setting of prior glecaprevir/pibrentasvir treatment failure. Findings from a prospective, nonrandomized, observational study support this recommendation. Investigators enrolled 31 participants with a history of virologic failure with glecaprevir/pibrentasvir therapy. Participants with compensated cirrhosis were included; those with HBV and/or HIV coinfection were excluded. SVR12 was 94% (29 of 31) with 12 weeks of once-daily sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg). Two participants relapsed at week 4 after completion of therapy. No serious adverse events, treatment discontinuations, or deaths occurred [ 50 ]. Although the addition of ribavirin was not evaluated in this study, based on prior studies of DAA failures, addition of weight-based ribavirin to the regimen is recommended for persons with compensated cirrhosis.
Multiple DAA Failures, Including Sofosbuvir/Velpatasvir/Voxilaprevir or Sofosbuvir Plus Glecaprevir/Pibrentasvir
The MAGELLAN-3 clinical trial demonstrated the efficacy (96% SVR12; 22 of 23) of glecaprevir/pibrentasvir plus sofosbuvir and weight-based ribavirin for heavily DAA-experienced patients, although no sofosbuvir/velpatasvir/voxilaprevir failures were included [ 49 ]. Among patients with a prior sofosbuvir/velpatasvir/voxilaprevir treatment failure, 16 weeks of glecaprevir/pibrentasvir plus sofosbuvir and weight-based ribavirin is recommended based on the improved resistance profile of pibrentasvir and high response rate seen with this duration of therapy among genotype 3–infected participants in the MAGELLAN-3 trial [ 49 ]. Extension to 24 weeks or longer with this regimen should be considered for persons with factors that may reduce the likelihood of achieving SVR (eg, genotype 3 infection with cirrhosis or prior treatment failure with glecaprevir/pibrentasvir plus sofosbuvir). While there are case report data that use this treatment duration [ 51–54 ], no clinical trial data are available to support such an approach.
A 24-week course of sofosbuvir/velpatasvir/voxilaprevir plus weight-based ribavirin is also recommended for persons with a prior sofosbuvir/velpatasvir/voxilaprevir treatment failure. Although there are currently no published clinical trial data that examine retreatment with sofosbuvir/velpatasvir/voxilaprevir for patients in whom initial therapy with the same regimen failed, a small retrospective, observational study of persons with an initial DAA treatment failure and a subsequent retreatment failure with sofosbuvir/velpatasvir/voxilaprevir included 4 persons who received 24 weeks of sofosbuvir/velpatasvir/voxilaprevir rescue therapy (1 with the addition of ribavirin). SVR12 was 100% (4 of 4) in this small group of extensively DAA-experienced patients [ 53 ]. The recommendation to extend duration of therapy to 24 weeks in conjunction with weight-based ribavirin when retreating with the same DAA regimen (sofosbuvir/velpatasvir/voxilaprevir) is predominantly based on extrapolation from prior studies that have shown benefit with this strategy in different populations [ 55 ].
Retreatment in Patients With Decompensated Cirrhosis
Retreatment of persons with decompensated cirrhosis and a history of DAA-based treatment failure is limited by the inability to use an NS3/4A protease inhibitor (eg, glecaprevir, grazoprevir, voxilaprevir) in the setting of decompensated cirrhosis. Recommendations to retreat with a 24-week course of either sofosbuvir/velpatasvir plus weight-based ribavirin or ledipasvir/sofosbuvir plus weight-based ribavirin are based on the relatively favorable SVR rates (91% to 100%) with these regimens among patients with compensated cirrhosis and prior DAA failure [ 55–57 ].
The HCV guidance stresses the importance of addressing the special considerations and unmet needs of unique and key populations to achieve significant reductions in the burden of HCV-related disease. This approach aligns with the WHO strategy for achieving hepatitis C elimination targets, which also emphasizes the importance of focusing efforts on populations disproportionately affected by HCV infection, specifically HIV/HCV-coinfected persons, people who inject drugs (PWID), men who have sex with men (MSM), and incarcerated persons [ 1 ]. The HCV guidance additionally focuses on the special considerations and unmet needs of other unique or key populations, namely, individuals with acute HCV infection, pregnant persons, children and adolescents, and solid organ transplant recipients. Recommendations for these populations aim to maximize the potential benefits of often missed opportunities to reduce hepatitis C infection incidence and prevalence, personal and societal disease burden, and HCV-related morbidity and mortality.
HIV/HCV Coinfection
Treatment-naive persons living with HIV and HCV (without cirrhosis or with compensated cirrhosis) are newly eligible for DAA therapy using a simplified treatment algorithm (see Figures 4 and 5 ). This recommendation is supported by findings from the MINMON clinical trial, titled “A minimal monitoring approach for the treatment of hepatitis C virus infection (ACTG A5360 [MINMON]): a phase 4, open-label, single-arm trial”. Among the 166 study participants living with HIV and HCV, 95% (157 of 166) achieved SVR12 [ 33 ]. Given that people living with HIV are disproportionately affected by HCV infection [ 58 ], the reduction in treatment barriers benefits the affected individuals while furthering the goal of HCV elimination.
Acute HCV Infection
The Guidance Panel reiterates the recommendation that persons with confirmed acute HCV infection (HCV RNA–positive) should be treated the same as those with chronic HCV infection without awaiting possible spontaneous clearance (ie, a test-and-treat approach). Given that the incidence of acute hepatitis C in the United States increased 124% from 2013 through 2020 [ 59 ], treatment of this key population is critical to both HCV prevention and elimination.
Findings from studies that evaluated the efficacy of an abbreviated 6 weeks of therapy for acute HCV infection with various DAA regimens, including ledipasvir/sofosbuvir [ 60 , 61 ], glecaprevir/pibrentasvir [ 62 ], and sofosbuvir/velpatasvir [ 63 ], have demonstrated largely inferior response rates compared with the standard of care. As such, an abbreviated course of DAA therapy is not recommended for acute HCV infection.
HCV in Pregnancy
Following the 2018 HCV guidance recommendation for universal hepatitis C screening during pregnancy [ 64 ], the USPSTF and CDC issued largely concurrent recommendations in 2020 [ 8 , 9 ]. In May 2021, the American College of Obstetricians and Gynecologists issued a practice advisory recommending hepatitis screening for all pregnant persons during each pregnancy [ 65 ]. The Society for Maternal-Fetal Medicine endorsed that practice advisory and published a concurring recommendation in September 2021 [ 66 ]. Given that the DHHS viral hepatitis national strategic plan specifies expanded implementation of universal hepatitis C screening during pregnancy as an important strategy for actualizing HCV elimination [ 4 ], the coalescence of screening recommendations for this key population is an important step toward achieving that goal. Treatment recommendations during pregnancy are largely unchanged from the previous update [ 5 ]. Although there have been no published large-scale clinical trials to evaluate the safety of DAA therapy during pregnancy, smaller studies and case series have not demonstrated any safety concerns [ 67–71 ]. The Guidance Panel suggests that DAA treatment may be considered during pregnancy on a case-by-case basis after a discussion of potential risks and benefits.
HCV in Children
Strategies to reduce the burden of HCV-related disease have historically focused on the adult population [ 72 ]. Data from a recent modeling study indicate that at least 3.26 million children and adolescents (aged ≤18 years) are living with HCV infection worldwide [ 73 ]. National hepatitis C incidence and prevalence data among children and adolescents in the United States are sparse and/or outdated [ 73 ]. However, with the recent increase in HCV infection among women of childbearing age [ 15 , 74–78 ] comes a coincident risk of increased cases of mother-to-child transmission [ 76 ], the primary route of HCV transmission in children [ 79 , 80 ].
Treatment for HCV infection in children has been revolutionized in recent years, beginning with the US Food and Drug Administration approval of the first DAAs for adolescents in April 2017 [ 81 , 82 ] to the June 2021 approval of 2 pangenotypic regimens (glecaprevir/pibrentasvir and sofosbuvir/velpatasvir) for children as young as 3 years [ 83 , 84 ]. Efficacy and safety data from therapeutic DAA clinical trials conducted in children are largely comparable to those from studies conducted in adults [ 85–90 ]. As such, the Guidance Panel reaffirms its recommendation to treat all HCV-infected children and adolescents aged ≥3 years with an approved DAA regimen regardless of disease severity. Treatment and retreatment recommendations for children are shown in Tables 3 and 4 , respectively.
Recommendations for Initial Treatment of Hepatitis C Virus–Infected Pediatric Patients Without Cirrhosis or With Compensated Cirrhosis
| Regimen . | Genotype . | Classification . | Duration . | Rating . |
|---|---|---|---|---|
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 8 wk | I, B |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | I, B |
| Ledipasvir/sofosbuvir | 1, 4, 5, 6 | Recommended | 12 wk | I, B |
| Regimen . | Genotype . | Classification . | Duration . | Rating . |
|---|---|---|---|---|
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 8 wk | I, B |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | I, B |
| Ledipasvir/sofosbuvir | 1, 4, 5, 6 | Recommended | 12 wk | I, B |
Recommendations are listed by genotypic activity, evidence level, and alphabetically.
Recommendations for Retreatment of Hepatitis C Virus–Infected Pediatric Patients by Prior Exposure and Cirrhosis Status
| Regimen . | Genotypes . | Classification . | Duration . | Rating . | Cirrhosis Status . |
|---|---|---|---|---|---|
| Interferon-based regimen (±ribavirin) and/or sofosbuvir treatment failure without NS3/4A protease inhibitor or NS5A inhibitor exposure | |||||
| Glecaprevir/pibrentasvir | 1, 2, 4, 5, 6 | Recommended | 8 wk | I, C | No cirrhosis |
| Glecaprevir/pibrentasvir | 1, 2, 4, 5, 6 | Recommended | 12 wk | I, C | Compensated cirrhosis |
| Glecaprevir/pibrentasvir | 3 | Recommended | 16 wk | I, C | Without cirrhosis or with compensated cirrhosis |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | I, C | Without cirrhosis or with compensated cirrhosis |
| Sofosbuvir/velpatasvir + weight-based ribavirin | 1–6 | Recommended | 12 wk | I, C | Decompensated cirrhosis |
| NS3/4A protease inhibitor treatment failure without NS5A inhibitor exposure | |||||
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 12 wk | I, C | Without cirrhosis or with compensated cirrhosis |
| NS5A inhibitor treatment failure without NS3/4A protease inhibitor exposure | |||||
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 16 wk | I, C | Without cirrhosis or with compensated cirrhosis |
| Interferon (± ribavirin) plus a hepatitis C virus protease inhibitor treatment failure | |||||
| Ledipasvir/sofosbuvir | 4, 5, 6 | Recommended | 12 wk | I, C | Without cirrhosis or with compensated cirrhosis |
| Ledipasvir/sofosbuvir | 1 | Recommended | 12 wk | I, C | No cirrhosis |
| Ledipasvir/sofosbuvir | 1 | Recommended | 24 wk | I, C | Compensated cirrhosis |
| Regimen . | Genotypes . | Classification . | Duration . | Rating . | Cirrhosis Status . |
|---|---|---|---|---|---|
| Interferon-based regimen (±ribavirin) and/or sofosbuvir treatment failure without NS3/4A protease inhibitor or NS5A inhibitor exposure | |||||
| Glecaprevir/pibrentasvir | 1, 2, 4, 5, 6 | Recommended | 8 wk | I, C | No cirrhosis |
| Glecaprevir/pibrentasvir | 1, 2, 4, 5, 6 | Recommended | 12 wk | I, C | Compensated cirrhosis |
| Glecaprevir/pibrentasvir | 3 | Recommended | 16 wk | I, C | Without cirrhosis or with compensated cirrhosis |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | I, C | Without cirrhosis or with compensated cirrhosis |
| Sofosbuvir/velpatasvir + weight-based ribavirin | 1–6 | Recommended | 12 wk | I, C | Decompensated cirrhosis |
| NS3/4A protease inhibitor treatment failure without NS5A inhibitor exposure | |||||
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 12 wk | I, C | Without cirrhosis or with compensated cirrhosis |
| NS5A inhibitor treatment failure without NS3/4A protease inhibitor exposure | |||||
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 16 wk | I, C | Without cirrhosis or with compensated cirrhosis |
| Interferon (± ribavirin) plus a hepatitis C virus protease inhibitor treatment failure | |||||
| Ledipasvir/sofosbuvir | 4, 5, 6 | Recommended | 12 wk | I, C | Without cirrhosis or with compensated cirrhosis |
| Ledipasvir/sofosbuvir | 1 | Recommended | 12 wk | I, C | No cirrhosis |
| Ledipasvir/sofosbuvir | 1 | Recommended | 24 wk | I, C | Compensated cirrhosis |
Abbreviations: NS3/4A, hepatitis C virus nonstructural protein 3–4A; NS5A, hepatitis C virus nonstructural protein 5A.
Management of HCV After Solid Organ Transplantation
Clinical trial and real-world data provide robust evidence supporting the safety and efficacy of HCV DAA treatment in patients who have undergone solid organ transplantation [ 91–95 ]. Discussion of specific clinical scenarios follows. Table 5 shows HCV treatment recommendations posttransplantation.
Recommendations for Hepatitis C Virus Treatment Posttransplantation
| Regimen . | Genotypes . | Classification . | Duration . | Rating . | Caveats and Other Considerations . |
|---|---|---|---|---|---|
| Recurrent HCV post liver transplant without cirrhosis | |||||
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 12 wk | I, B | |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | I, B | |
| Ledipasvir/sofosbuvir | 1, 4, 5, 6 | Recommended | 12 wk | I, B | |
| Recurrent HCV post liver transplant with compensated cirrhosis | |||||
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | I, B | |
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 12 wk | I, C | |
| Ledipasvir/sofosbuvir | 1, 4, 5, 6 | Recommended | 12 wk | I, A | |
| Recurrent HCV post kidney transplant without cirrhosis or with compensated cirrhosis | |||||
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 12 wk | I, A IIa, C | |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | IIa, C | |
| Ledipasvir/sofosbuvir | 1, 4, 5, 6 | Recommended | 12 wk | I, A | |
| Elbasvir/grazoprevir | 1, 4 | Alternative | 12 wk | I, B | Limited to patients without baseline NS5A RASs for elbasvir. |
| HCV-uninfected recipients of liver grafts from HCV-viremic donors | |||||
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 12 wk | I, C | Timing: initiate treatment within the first 2 wk posttransplant, preferably within the first week. |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | I, C | Timing: initiate treatment within the first 2 wk posttransplant, preferably within the first week. |
| HCV-uninfected recipients of non-liver solid organs from HCV-viremic donors | |||||
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 8 wk | I, C | Timing: initiate treatment prior to HCV RNA results, immediately pretransplant or day 0 posttransplant, if possible. Otherwise, begin on day 0 to within the first week posttransplant when clinically stable. |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | I, C | Timing: initiate treatment prior to HCV RNA results, immediately pretransplant or day 0 posttransplant, if possible. Otherwise, begin on day 0 to within the first week posttransplant when clinically stable. |
| Regimen . | Genotypes . | Classification . | Duration . | Rating . | Caveats and Other Considerations . |
|---|---|---|---|---|---|
| Recurrent HCV post liver transplant without cirrhosis | |||||
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 12 wk | I, B | |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | I, B | |
| Ledipasvir/sofosbuvir | 1, 4, 5, 6 | Recommended | 12 wk | I, B | |
| Recurrent HCV post liver transplant with compensated cirrhosis | |||||
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | I, B | |
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 12 wk | I, C | |
| Ledipasvir/sofosbuvir | 1, 4, 5, 6 | Recommended | 12 wk | I, A | |
| Recurrent HCV post kidney transplant without cirrhosis or with compensated cirrhosis | |||||
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 12 wk | I, A IIa, C | |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | IIa, C | |
| Ledipasvir/sofosbuvir | 1, 4, 5, 6 | Recommended | 12 wk | I, A | |
| Elbasvir/grazoprevir | 1, 4 | Alternative | 12 wk | I, B | Limited to patients without baseline NS5A RASs for elbasvir. |
| HCV-uninfected recipients of liver grafts from HCV-viremic donors | |||||
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 12 wk | I, C | Timing: initiate treatment within the first 2 wk posttransplant, preferably within the first week. |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | I, C | Timing: initiate treatment within the first 2 wk posttransplant, preferably within the first week. |
| HCV-uninfected recipients of non-liver solid organs from HCV-viremic donors | |||||
| Glecaprevir/pibrentasvir | 1–6 | Recommended | 8 wk | I, C | Timing: initiate treatment prior to HCV RNA results, immediately pretransplant or day 0 posttransplant, if possible. Otherwise, begin on day 0 to within the first week posttransplant when clinically stable. |
| Sofosbuvir/velpatasvir | 1–6 | Recommended | 12 wk | I, C | Timing: initiate treatment prior to HCV RNA results, immediately pretransplant or day 0 posttransplant, if possible. Otherwise, begin on day 0 to within the first week posttransplant when clinically stable. |
Abbreviations: HCV, hepatitis C virus; NS5A, hepatitis C virus nonstructural protein 5A; RAS, resistance associated substitution.
Rating is based on evidence for persons without cirrhosis.
Rating is based on evidence for persons with compensated cirrhosis.
If treatment initiation is delayed beyond the first week after transplant, treatment should be extended to 12 weeks.
Treatment of Recurrent HCV Infection Post Liver and Kidney Transplantation
The phase 3, single-arm, open-label MAGELLAN-2 trial, titled “Glecaprevir/pibrentasvir treatment in liver or kidney transplant patients with hepatitis C virus infection” evaluated a 12-week course of once-daily glecaprevir (300 mg)/pibrentasvir (120 mg) for the treatment of HCV infection (genotypes 1 through 6) among patients without cirrhosis who had undergone liver or kidney transplantation and were ≥3 months posttransplantation. Those whose immunosuppressive regimen included cyclosporine >100 mg/d or prednisone >10 mg/d were excluded. Treatment-naive and treatment-experienced (genotypes 1, 2, 4, 5, 6; prior treatment with interferon-based therapy or sofosbuvir plus ribavirin with or without pegylated interferon) participants were included. Treatment-experienced persons with genotype 3 infection were excluded. Overall, SVR12 was 98% (98 of 100). No treatment-related serious adverse events were reported [ 91 ].
Sofosbuvir-based regimens have also shown efficacy in persons who have undergone liver or kidney transplantation [ 93–96 ]. Investigators who conducted a real-world observational study to evaluate the efficacy and safety of DAA therapy in 179 liver, kidney, or dual liver and kidney transplant recipients reported an SVR12 of 94% (169 of 179) among participants treated with ledipasvir/sofosbuvir. Adverse events, including acute cellular rejection, were rare [ 93 ]. A phase 2, open-label study that evaluated 12 weeks of daily sofosbuvir (400 mg)/velpatasvir (100 mg) in 79 HCV-infected (genotypes 1, 2, 3, 4) liver transplant recipients demonstrated a similar response rate with an SVR12 of 96% (76 of 79). No treatment-related serious adverse events, transplant rejection episodes, or deaths occurred during the study period [ 94 ].
Important drug–drug interactions unique to the posttransplant setting should be addressed prior to initiation of DAA therapy. Cyclosporine significantly increases the area under the curve of elbasvir/grazoprevir [ 97 , 98 ] as well as sofosbuvir/velpatasvir/voxilaprevir [ 99 ] and should not be coadministered with these regimens. Coadministration of glecaprevir/pibrentasvir and cyclosporine >100 mg/d is also not recommended [ 83 ].
Treatment of HCV-Uninfected Transplant Recipients Receiving Organs From HCV-Viremic Donors
A large disparity persists among people in need of solid organ transplantation and available deceased donor organs [ 100 ]. Given that available data support the safety and efficacy of DAA therapy in the posttransplant setting, many transplant centers have begun using solid organs from HCV-positive donors for HCV-negative recipients to increase the pool of available organs [ 101–106 ]. The pool of HCV-positive donors includes both HCV-viremic donors (ie, HCV RNA–positive) and HCV-seropositive donors (ie, HCV antibody–positive, HCV RNA–negative [nonviremic]). The use of HCV-positive organs has been shown to be an effective strategy for increasing access to transplantation and reducing wait-list time and overall mortality [ 107–110 ].
Timing and Treatment of HCV-Viremic Liver Grafts in Nonviremic Recipients
Emerging data support HCV treatment as early as possible when transplanting an HCV-viremic liver graft into an HCV-seronegative recipient [ 111 ]. In a recent multicenter prospective study, 34 HCV-seronegative liver transplant patients underwent transplantation using organs from HCV-positive donors (20 viremic, 14 nonviremic). All recipients of grafts from HCV-viremic donors became viremic by day 3 posttransplantation. DAA treatment was initiated in these graft recipients a median of 27.5 days after transplantation. SVR12 was 100% (20 of 20). One patient developed acute HCV-related membranous nephropathy on postoperative day 18 (prior to initiation of DAA therapy), ultimately resulting in end-stage renal disease requiring dialysis despite achieving SVR12 [ 112 ]. This case highlights the importance of early initiation of DAA therapy posttransplantation to avoid HCV-related complications. The Guidance Panel recommends initiating therapy at least within 2 weeks after transplantation but preferably within 1 week when the patient is clinically stable.
An abbreviated duration of DAA therapy is currently not recommended for recipients of organs from HCV-viremic donors due to lack of data demonstrating efficacy. The large reservoir of HCV in a transplanted liver graft may be responsible for the lack of efficacy.
Timing and Treatment of HCV-Viremic Non-Liver Grafts in Nonviremic Recipients
HCV treatment should occur as early as possible in HCV-seronegative patients who undergo transplantation with a non-liver graft from an HCV-viremic donor. This strategy reduces the likelihood of hepatic and extrahepatic HCV-related complications in the immediate posttransplant period. The phase 4, open-label, multicenter MYTHIC clinical trial evaluated the efficacy and safety of 8 weeks of once-daily glecaprevir (300 mg)/pibrentasvir (120 mg) in 30 HCV-negative kidney transplant recipients who underwent transplantation using a graft from an HCV-viremic donor [ 113 ]. Treatment initiation occurred 2 days to 5 days posttransplantation (target was 3 days). All 30 participants achieved SVR12; no HCV-related serious adverse events were reported [ 113 ]. Based on this study and others showing benefit(s) associated with early HCV treatment [ 113–116 ], use of a prophylactic (immediately prior to transplantation or day 0 posttransplantation) or preemptive (day 0 to day 7 posttransplantation; as soon as the patient is clinically stable) strategy for initiation of DAA treatment is recommended for HCV-negative recipients of a non-liver solid organ graft from an HCV-viremic donor. Note that neither approach requires demonstration of HCV viremia in the transplant recipient.
Shorter durations of DAA-based therapy in this setting are currently under investigation with promising results. These practices, however, are currently not recommended outside of a clinical trial [ 115 , 117 , 118 ].
Outcomes and Process in Transplantation Using HCV-Viremic Donor Grafts in HCV-Seronegative Recipients
Data evaluating longer-term patient outcomes after transplantation with an HCV-viremic donor organ have shown encouraging results. Among 51 dual heart/kidney transplant recipients undergoing transplantation with organs from HCV-viremic donors, 1-year survival was comparable to survival in those who received organs from nonviremic donors [ 119 ]. Another study that evaluated outcomes among multiorgan transplant recipients (heart/kidney, heart/lung, heart/liver) demonstrated similar 1-year survival among recipients of organs from HCV-viremic donors compared with those who received organs from HCV-negative donors [ 106 ].
In an analysis of the United Network for Organ Sharing database, HCV-negative liver transplant patients who received the graft from an HCV-positive donor (viremic and nonviremic) were shown to have superior 1-year graft survival rates compared with those who received a graft from an HCV-negative donor [ 120 ]. Notably, HCV-positive donors were statistically significantly younger than their HCV-negative counterparts. Multivariate analysis demonstrated that donor age, but not donor HCV status, was an independent predictor of 1-year graft survival [ 120 ].
Extensive informed consent, as recommended by the American Society of Transplantation Consensus Panel [ 121 ], and shared decision-making between the patient and clinical team should occur prior to transplantation of an HCV-viremic organ into an HCV-negative recipient. Patients should understand the risk of HCV infection, risk to caregivers from needlestick exposures, as well as success rates and risks of DAA-based therapy [ 115 , 121–125 ]. Given the breadth of safety and efficacy data now available, institutional review board–approved protocols are no longer required. However, based on the unique factors noted, transplant centers should have a specific HCV consent and follow-up process in place.
People Who Inject Drugs
Injection drug use (IDU) is the most common risk factor for HCV infection in North America and Europe. The HCV seroprevalence among PWID ranges from 18% to 88%, depending on geographic location [ 126 ] and duration of IDU exposure [ 127 , 128 ]. IDU accounts for approximately 70% of new HCV infections [ 59 ]. Thus, the growing opioid epidemic has become an important force in the perpetuation of the HCV epidemic [ 1 , 2 , 4 , 14 , 16 , 59 ]. Consequently, achieving the goal of HCV elimination depends heavily on diagnosing and treating HCV infection in PWID and on implementing harm reduction strategies to prevent future infections [ 1 , 2 , 4 , 122 , 129–132 ]. Data from Australia support the efficacy of the treatment-as-prevention approach among PWID. After implementation of unrestricted access to DAA therapy in 2016, the proportion of PWID diagnosed with active HCV infection who were treated increased from 3% to 47%, while the proportion of those with HCV viremia declined from 44% to 17% [ 133 ].
Annual HCV testing is recommended for PWID with ongoing IDU regardless of either no prior testing or past negative testing. Substance use disorder treatment programs and needle/syringe exchange programs should offer routine, opt-out HCV antibody testing with confirmatory HCV RNA testing and linkage to care for those determined to be HCV-infected [ 132 , 134 ]. PWID with HCV infection should be counseled about measures to reduce the risk of transmission to others and offered linkage to harm reduction services, including intranasal naloxone, needle/syringe service programs, medications for opioid use disorder, and other substance use disorder treatment programs.
Clinical trials and observational studies of PWID reporting current IDU at the start of HCV treatment and/or continued use during therapy demonstrate SVR12 rates approaching 95% [ 135–140 ]. The Guidance Panel strongly asserts that active or recent drug use or concern for reinfection is not a contraindication to HCV treatment. At least annual HCV RNA testing is recommended for PWID with recent IDU after they have spontaneously cleared HCV infection or have been successfully treated [ 141–144 ].
Men Who Have Sex With Men Not Living With HIV
While the increased risk of HCV infection among MSM living with HIV is well known [ 145 ], acute HCV infections have also been reported among MSM not living with HIV who present for HIV preexposure prophylaxis (PrEP) [ 146 , 147 ]. HCV testing at HIV PrEP initiation and at least annually thereafter (while on PrEP) is recommended for MSM not living with HIV. All MSM should be counseled about the risk of sexual HCV transmission with high-risk sexual and drug use practices and educated about measures to prevent HCV infection or transmission [ 148 , 149 ].
Antiviral treatment for HCV-infected MSM should be coupled with ongoing counseling about the risk of HCV reinfection and education about methods to reduce HCV reinfection risk after cure [ 150 ]. At least annual (and risk-based, if indicated) HCV RNA testing is recommended for all high-risk sexually active MSM after successful treatment or spontaneous clearance of HCV infection [ 151 , 152 ].
Persons in Correctional Settings
Recent cross-sectional surveys suggest that the HCV seroprevalence among incarcerated populations in the United States ranges from 3.0% to 34.6% [ 153 ], which exceeds the 1.7% HCV seroprevalence in the general population [ 154 ]. More than 90% of these persons are eventually released and reenter the general population where they can contribute to HCV spread in the community [ 155 , 156 ] and may have little contact with the healthcare system [ 157 , 158 ]. Given the high HCV prevalence among persons in the US correctional system, the success of the US HCV elimination effort depends on identifying infected individuals in jails and prisons, linking these persons to medical care for HCV management, and providing access to antiviral treatment [ 2 , 159 ]. Jails and prisons should therefore implement opt-out HCV testing that consists of HCV antibody testing followed by confirmatory HCV RNA testing if antibody-positive. Universal opt-out testing of incarcerated persons for chronic HCV is highly cost-effective and has been shown to reduce ongoing HCV transmission and the incidence of advanced liver disease [ 160 ].
DAA treatment for chronic HCV infection is feasible within jail and prison settings and would aid the HCV elimination effort [ 161 , 162 ]. Chronically infected persons residing in jails should receive counseling about HCV infection and be provided linkage to follow-up community healthcare for evaluation of liver disease and treatment upon release [ 163–166 ]. Those whose jail sentence is sufficiently long to complete a recommended course of DAA therapy should receive that treatment while incarcerated [ 161 ]. Chronically infected individuals in prison should receive DAA therapy according to AASLD–IDSA guidance while incarcerated [ 162 , 167 ]. Jails and prisons should facilitate continuation of HCV therapy for persons on HCV treatment at the time of incarceration. HCV treatment in correctional settings is cost-effective because DAAs halt progression of HCV-related liver disease and decrease the risk of cirrhosis, hepatic decompensation, and hepatocellular carcinoma, offsetting future healthcare costs from liver and non-liver complications [ 168 ].
Upon release from a correctional facility, HCV-infected persons with advanced hepatic fibrosis or cirrhosis should be provided linkage to community healthcare for surveillance for HCV-related complications. To prevent HCV reinfection and reduce the risk of progression of HCV-associated liver disease, correctional facilities should provide harm reduction and evidence-based treatment for underlying substance use disorders [ 169 ]. Addressing hazardous alcohol use among persons with chronic HCV in a correctional setting may help slow liver disease progression, decrease HCV transmission, and might reduce recidivism.
Acknowledgments. The panel thanks the able staff of the Infectious Diseases Society of America (IDSA) and the American Association for the Study of Liver Diseases (AASLD), particularly Jon Heald, Genet Demisashi, Elizabeth Durzy, Audrey Davis-Owino, and Sheila Tynes for project management and administrative support of the Hepatitis C Virus (HCV) Guidance Project, and Dr Tina M. St. John for technical and editorial support of the HCV Guidance Project and manuscript preparation assistance.
Disclaimer. This article does not necessarily represent the views and policies of the US Preventive Services Task Force.
Financial support. The work for the HCV Guidance Project is supported exclusively by the AASLD and the IDSA.
AASLD–IDSA HCV Guidance Panel members and authors.
Andrew I. Aronsohn, MD, Department of Medicine, Section of Gastroenterology, University of Chicago Pritzker School of Medicine, Chicago, IL, USA; Debika Bhattacharya, MD, Department of Medicine, Division of Infectious Diseases, University of California–Los Angeles David Geffen School of Medicine, Los Angeles, CA, USA; Vincent Lo Re, MD, MSCE, Department of Medicine, Division of Infectious Diseases, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA; and Jennifer C. Price, MD, PhD, Department of Medicine, Division of Gastroenterology, University of California–San Francisco School of Medicine, San Francisco, CA, USA
Panel Members
Jordan J. Feld, MD, MPH, University of Toronto, Toronto, ON, Canada; Stuart C. Gordon, MD, Henry Ford Health System, Division of Hepatology, Detroit, MI, USA; Theo Heller, MD, National Institute of Diabetes and Digestive and Kidney Diseases, US National Institutes of Health, Bethesda, MD, USA; Ravi Jhaveri, MD, FIDSA, FPIDS, FAAP, Department of Pediatrics, Division of Pediatric Infectious Diseases, Feinberg Northwestern School of Medicine, Chicago, IL, USA; Maureen M. Jonas, MD, Division of Gastroenterology, Children's Hospital of Boston, Harvard Medical School, Boston, MA, USA; Jennifer J. Kiser, PharmD, PhD, Center for Translational Pharmacokinetics and Pharmacogenomics, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, CO, USA; Benjamin P. Linas, MD, MPH, Department of Medicine, Center for Health Economics of Treatment Interventions for Substance Use Disorders, HCV, and HIV, Boston University Chobanian & Avedisian School of Medicine, Boston, MA, USA; Timothy R. Morgan, MD, Department of Medicine, Veterans Affairs Long Beach Healthcare System, Long Beach, CA, USA, and University of California Irvine School of Medicine, Orange, CA, USA; K. Rajender Reddy, MD, Division of Gastroenterology and Hepatology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA;
Andrew Reynolds, Hepatitis C Wellness Manager, San Francisco AIDS Foundation, San Francisco, CA, USA; John D. Scott, MD, MSc, FIDSA, Department of Medicine, Division of Allergy and Infectious Diseases, University of Washington School of Medicine, Seattle, WA, USA; Gloria Searson, ACSW, Founding Director and Executive Director, Coalition on Positive Health Empowerment, New York, NY, USA; Philip Spradling, MD, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Division of Viral Hepatitis, US Centers for Disease Control and Prevention, Atlanta, GA, USA; Norah A. Terrault, MD, Department of Medicine, Division of Gastroenterology and Liver Diseases, Keck School of Medicine at the University of Southern California, Los Angeles, CA, USA; Elizabeth C. Verna, MD, MS, Department of Medicine, Division of Digestive and Liver Diseases, Columbia University Vagelos College of Physicians and Surgeons, New York, NY, USA; John B. Wong, MD, Department of Medicine, Division of Clinical Decision Making, Tufts University School of Medicine, Boston, MA, USA;
Ann E. Woolley, MD, MPH, Department of Medicine, Division of Infectious Diseases, Harvard Medical School, Boston, MA, USA; Kimberley A. Workowski, MD, Department of Medicine, Division of Infectious Diseases, Emory University School of Medicine, Atlanta, GA, USA; and David L. Wyles, MD, Department of Medicine, Division of Infectious Diseases, Denver Health, University of Colorado School of Medicine, Denver, CO, USA.
World Health Organization . Global health sector strategy on viral hepatitis, 2016–2021 . Published June 2016. Available at : https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf . Accessed 28 November 2022.
National Academies of Sciences, Engineering, and Medicine . A national strategy for the elimination of hepatitis B and C: phase two report . Washington, DC : National Academies Press , 2017 .
Google Scholar
Google Preview
US Centers for Disease Control and Prevention . Healthy people 2030, infectious disease . Available at : https://health.gov/healthypeople/objectives-and-data/browse-objectives/infectious-disease . Accessed 21 November 2022.
US Department of Health and Human Services . Viral hepatitis national strategic plan for the United States: a roadmap to elimination (2021–2025) . Published 7 January 2021 . Available at : https://www.hhs.gov/hepatitis/viral-hepatitis-national-strategic-plan/index.html . Accessed 25 November 2022.
Ghany MG , Morgan TR . Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection . Hepatology 2020 ; 71 : 686 – 721 .
American Heart Association . Methodology manual and policies from the ACCF/AHA Task Force on Practice Guidelines . Dallas, TX : American College of Cardiology Foundation and American Heart Association, Inc , 2010 .
Shiffman RN , Shekelle P , Overhage JM , Slutsky J , Grimshaw J , Deshpande AM . Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization . Ann Intern Med 2003 ; 139 : 493 – 8 .
Owens DK , Davidson KW , Krist AH , et al. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement . JAMA 2020 ; 323 : 970 – 5 .
Schillie S , Wester C , Osborne M , Wesolowski L , Ryerson AB . CDC recommendations for hepatitis C screening among adults—United States, 2020 . MMWR Recomm Rep 2020 ; 69 : 1 – 17 .
Eckman MH , Ward JW , Sherman KE . Cost effectiveness of universal screening for hepatitis C virus infection in the era of direct-acting, pangenotypic treatment regimens . Clin Gastroenterol Hepatol 2019 ; 17 : 930 – 939.e9 .
Buti M , Domínguez-Hernández R , Casado MA , Sabater E , Esteban R . Healthcare value of implementing hepatitis C screening in the adult general population in Spain . PLoS One 2018 ; 13 : e0208036 .
Wong WWL , Tu HA , Feld JJ , Wong T , Krahn M . Cost-effectiveness of screening for hepatitis C in Canada . CMAJ 2015 ; 187 : E110 – 21 .
Barocas JA , Tasillo A , Eftekhari Yazdi G , et al. Population-level outcomes and cost-effectiveness of expanding the recommendation for age-based hepatitis C testing in the United States . Clin Infect Dis 2018 ; 67 : 549 – 56 .
Zibbell JE , Iqbal K , Patel RC , et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012 . MMWR Morb Mortal Wkly Rep 2015 ; 64 : 453 – 8 .
Ly KN , Jiles RB , Teshale EH , Foster MA , Pesano RL , Holmberg SD . Hepatitis C virus infection among reproductive-aged women and children in the United States, 2006 to 2014 . Ann Intern Med 2017 ; 166 : 775 – 82 .
Suryaprasad AG , White JZ , Xu F , et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012 . Clin Infect Dis 2014 ; 59 : 1411 – 9 .
Falade-Nwulia O , Suarez-Cuervo C , Nelson DR , Fried MW , Segal JB , Sulkowski MS . Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review . Ann Intern Med 2017 ; 166 : 637 – 48 .
Ferrante ND , Newcomb CW , Forde KA , et al. The hepatitis C care cascade during the direct-acting antiviral era in a United States commercially insured population . Open Forum Infect Dis 2022 ; 9 : ofac445 .
Yehia BR , Schranz AJ , Umscheid CA , Lo Re V 3rd . The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis . PLoS One 2014 ; 9 : e101554 .
Yeo YH , Gao X , Wang J , et al. The impact of COVID-19 on the cascade of care of HCV in the US and China . Ann Hepatol 2022 ; 27 : 100685 .
Kaufman HW , Bull-Otterson L , Meyer WA 3rd , et al. Decreases in hepatitis C testing and treatment during the COVID-19 pandemic . Am J Prev Med 2021 ; 61 : 369 – 76 .
US Centers for Disease Control and Prevention . CDC museum COVID-19 timeline. Last reviewed 16 August 2022 . Available at : https://www.cdc.gov/museum/timeline/covid19.html . Accessed 29 November 2022.
Mandel E , Peci A , Cronin K , et al. The impact of the first, second and third waves of COVID-19 on hepatitis B and C testing in Ontario, Canada . J Viral Hepat 2022 ; 29 : 205 – 8 .
Thompson WW , Symum H , Sandul A , et al. Vital signs: hepatitis C treatment among insured adults—United States, 2019–2020 . MMWR Morb Mortal Wkly Rep 2022 ; 71 : 1011 – 7 .
Kondili LA , Buti M , Riveiro-Barciela M , et al. Impact of the COVID-19 pandemic on hepatitis B and C elimination: an EASL survey . JHEP Rep 2022 ; 4 : 100531 .
Breckenridge A , Aronson JK , Blaschke TF , Hartman D , Peck CC , Vrijens B . Poor medication adherence in clinical trials: consequences and solutions . Nat Rev Drug Discov 2017 ; 16 : 149 – 50 .
Nieuwlaat R , Wilczynski N , Navarro T , et al. Interventions for enhancing medication adherence . Cochrane Database Syst Rev 2014 ; 2014 : Cd000011 .
Serper M , Evon DM , Stewart PW , et al. Medication non-adherence in a prospective, multi-center cohort treated with hepatitis C direct-acting antivirals . J Gen Intern Med 2020 ; 35 : 1011 – 20 .
Akiyama MJ , Norton BL , Arnsten JH , Agyemang L , Heo M , Litwin AH . Intensive models of hepatitis C care for people who inject drugs receiving opioid agonist therapy: a randomized controlled trial . Ann Intern Med 2019 ; 170 : 594 – 603 .
Cunningham EB , Hajarizadeh B , Amin J , et al. Adherence to once-daily and twice-daily direct-acting antiviral therapy for hepatitis C infection among people with recent injection drug use or current opioid agonist therapy . Clin Infect Dis 2020 ; 71 : e115 – e24 .
Cunningham EB , Amin J , Feld JJ , et al. Adherence to sofosbuvir and velpatasvir among people with chronic HCV infection and recent injection drug use: the SIMPLIFY study . Int J Drug Policy 2018 ; 62 : 14 – 23 .
Fabbiani M , Lombardi A , Colaneri M , et al. High rates of sustained virological response despite premature discontinuation of directly acting antivirals in HCV-infected patients treated in a real-life setting . J Viral Hepat 2021 ; 28 : 558 – 68 .
Solomon SS , Wagner-Cardoso S , Smeaton L , et al. A minimal monitoring approach for the treatment of hepatitis C virus infection (ACTG A5360 [MINMON]): a phase 4, open-label, single-arm trial . Lancet Gastroenterol Hepatol 2022 ; 7 : 307 – 17 .
Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV . Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV . National Institutes of Health, Centers for Disease Control and Prevention, and the HIV Medicine Association of the Infectious Disease Society of America , 2023 . Available at: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection .
Carrat F , Fontaine H , Dorival C , et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study . Lancet 2019 ; 393 : 1453 – 64 .
Kalidindi Y , Jung J , Feldman R , Riley T 3rd . Association of direct-acting antiviral treatment with mortality among Medicare beneficiaries with hepatitis C . JAMA Netw Open 2020 ; 3 : e2011055 .
Chou R , Dana T , Fu R , et al. Screening for hepatitis C virus infection in adolescents and adults: a systematic review update for the U.S. Preventive Services Task Force [Internet] . Rockville, MD : Agency for Healthcare Research and Quality (US) , 2020 .
Brown RS Jr , Buti M , Rodrigues L , et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naive patients with chronic HCV genotypes 1–6 and compensated cirrhosis: the EXPEDITION-8 trial . J Hepatol 2020 ; 72 : 441 – 9 .
Jacobson IM , Lawitz E , Gane EJ , et al. Efficacy of 8 weeks of sofosbuvir, velpatasvir, and voxilaprevir in patients with chronic HCV infection: 2 phase 3 randomized trials . Gastroenterology 2017 ; 153 : 113 – 22 .
Bourlière M , Gordon SC , Schiff ER , et al. Deferred treatment with sofosbuvir-velpatasvir-voxilaprevir for patients with chronic hepatitis C virus who were previously treated with an NS5A inhibitor: an open-label substudy of POLARIS-1 . Lancet Gastroenterol Hepatol 2018 ; 3 : 559 – 65 .
Bourlière M , Gordon SC , Flamm SL , et al. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection . N Engl J Med 2017 ; 376 : 2134 – 46 .
Da BL , Lourdusamy V , Kushner T , Dieterich D , Saberi B . Efficacy of sofosbuvir/velpatasvir/voxilaprevir in direct-acting antiviral experienced patients with hepatitis C virus . Eur J Gastroenterol Hepatol 2021 ; 33 : 859 – 61 .
Belperio PS , Shahoumian TA , Loomis TP , Backus LI . Real-world effectiveness of sofosbuvir/velpatasvir/voxilaprevir in 573 direct-acting antiviral experienced hepatitis C patients . J Viral Hepat 2019 ; 26 : 980 – 90 .
Degasperi E , Spinetti A , Lombardi A , et al. Real-life effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in hepatitis C patients with previous DAA failure . J Hepatol 2019 ; 71 : 1106 – 15 .
Llaneras J , Riveiro-Barciela M , Lens S , et al. Effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in patients with chronic hepatitis C previously treated with DAAs . J Hepatol 2019 ; 71 : 666 – 72 .
Poordad F , Pol S , Asatryan A , et al. Glecaprevir/pibrentasvir in patients with hepatitis C virus genotype 1 or 4 and past direct-acting antiviral treatment failure . Hepatology 2018 ; 67 : 1253 – 60 .
Poordad F , Felizarta F , Asatryan A , et al. Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct-acting antiviral treatment . Hepatology 2017 ; 66 : 389 – 97 .
Lok AS , Sulkowski MS , Kort JJ , et al. Efficacy of glecaprevir and pibrentasvir in patients with genotype 1 hepatitis C virus infection with treatment failure after NS5A inhibitor plus sofosbuvir therapy . Gastroenterology 2019 ; 157 : 1506 – 17.e1 .
Wyles D , Weiland O , Yao B , et al. Retreatment of patients who failed glecaprevir/pibrentasvir treatment for hepatitis C virus infection . J Hepatol 2019 ; 70 : 1019 – 23 .
Pearlman B , Perrys M , Hinds A . Sofosbuvir/velpatasvir/voxilaprevir for previous treatment failures with glecaprevir/pibrentasvir in chronic hepatitis C infection . Am J Gastroenterol 2019 ; 114 : 1550 – 2 .
Bernhard B , Stickel F . Successful fourth line treatment of a relapse patient with chronic hepatitis C virus infection genotype 3a using sofosbuvir, glecaprevir/pibrentasvir, and ribavirin: a case report . Z Gastroenterol 2020 ; 58 : 451 – 5 .
Fierer DS , Wyles DL . Re-treatment of hepatitis C infection after multiple failures of direct-acting antiviral therapy . Open Forum Infect Dis 2020 ; 7 : ofaa095 .
Dietz J , Di Maio VC , de Salazar A , et al. Failure on voxilaprevir, velpatasvir, sofosbuvir and efficacy of rescue therapy . J Hepatol 2021 ; 74 : 801 – 10 .
Trudeau S , Mendiratta V , Dababneh Y , Hollingsworth J , Gordon SC . Letter to the editor: successful treatment of multidrug resistant hepatitis C after >12 months of continuous therapy with direct-acting antivirals . Hepatology 2023 ; 77 : E9 – E10 .
Gane EJ , Shiffman ML , Etzkorn K , et al. Sofosbuvir-velpatasvir with ribavirin for 24 weeks in hepatitis C virus patients previously treated with a direct-acting antiviral regimen . Hepatology 2017 ; 66 : 1083 – 9 .
Osinusi A , Kohli A , Marti MM , et al. Re-treatment of chronic hepatitis C virus genotype 1 infection after relapse: an open-label pilot study . Ann Intern Med 2014 ; 161 : 634 – 8 .
Wyles D , Pockros P , Morelli G , et al. Ledipasvir-sofosbuvir plus ribavirin for patients with genotype 1 hepatitis C virus previously treated in clinical trials of sofosbuvir regimens . Hepatology 2015 ; 61 : 1793 – 7 .
Platt L , Easterbrook P , Gower E , et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis . Lancet Infect Dis 2016 ; 16 : 797 – 808 .
US Centers for Disease Control and Prevention . Viral hepatitis surveillance report—United States, 2020, hepatitis C . Published September 2022 . Available at : https://www.cdc.gov/hepatitis/statistics/2020surveillance/hepatitis-c.htm . Accessed 19 November 2022.
Deterding K , Spinner CD , Schott E , et al. Ledipasvir plus sofosbuvir fixed-dose combination for 6 weeks in patients with acute hepatitis C virus genotype 1 monoinfection (HepNet acute HCV IV): an open-label, single-arm, phase 2 study . Lancet Infect Dis 2017 ; 17 : 215 – 22 .
Rockstroh JK , Bhagani S , Hyland RH , et al. Ledipasvir-sofosbuvir for 6 weeks to treat acute hepatitis C virus genotype 1 or 4 infection in patients with HIV coinfection: an open-label, single-arm trial . Lancet Gastroenterol Hepatol 2017 ; 2 : 347 – 53 .
Martinello M , Orkin C , Cooke G , et al. Short-duration pan-genotypic therapy with glecaprevir/pibrentasvir for 6 weeks among people with recent hepatitis C viral infection . Hepatology 2020 ; 72 : 7 – 18 .
Matthews GV , Bhagani S , Van der Valk M , et al. Sofosbuvir/velpatasvir for 12 vs. 6 weeks for the treatment of recently acquired hepatitis C infection . J Hepatol 2021 ; 75 : 829 – 39 .
AASLD-IDSA HCV Guidance Panel . Hepatitis C guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection . Clin Infect Dis 2018 ; 67 : 1477 – 92 .
American College of Obstetricians and Gynecologists . Practice advisory: routine hepatitis C virus screening in pregnant individuals . Reaffirmed October 2022 . Available at : https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/05/routine-hepatitis-c-virus-screening-in-pregnant-individuals . Accessed 25 November 2022.
Dotters-Katz SK , Kuller JA , Hughes BL . Society for Maternal-Fetal Medicine consult series #56: hepatitis C in pregnancy—updated guidelines: replaces consult number 43, November 2017 . Am J Obstet Gynecol 2021 ; 225 : B8 – B18 .
Kushner T , Lange M , Sperling R , Dieterich D . Treatment of women with hepatitis C diagnosed in pregnancy: a co-located treatment approach . Gastroenterology 2022 ; 163 : 1454 – 6 .e1 .
Zeng QL , Yu ZJ , Lv J , et al. Sofosbuvir-based therapy for late pregnant women and infants with severe chronic hepatitis C: a case series study . J Med Virol 2022 ; 94 : 4548 – 53 .
AbdAllah M , Alboraie M , Abdel-Razek W , et al. Pregnancy outcome of anti-HCV direct-acting antivirals: real-life data from an Egyptian cohort . Liver Int 2021 ; 41 : 1494 – 7 .
Chappell CA , Scarsi KK , Kirby BJ , et al. Ledipasvir plus sofosbuvir in pregnant women with hepatitis C virus infection: a phase 1 pharmacokinetic study . Lancet Microbe 2020 ; 1 : e200 – e8 .
Yattoo GN . Treatment of chronic hepatitis C with ledipasvir/sofosbuvir combination during pregnancy [abstract] . Hepatol Int 2018 ; 12 : S292 – 3 .
Malik F , Bailey H , Chan P , et al. Where are the children in national hepatitis C policies? A global review of national strategic plans and guidelines . JHEP Rep 2021 ; 3 : 100227 .
Schmelzer J , Dugan E , Blach S , et al. Global prevalence of hepatitis C virus in children in 2018: a modelling study . Lancet Gastroenterol Hepatol 2020 ; 5 : 374 – 92 .
Rossi RM , Wolfe C , Brokamp R , et al. Reported prevalence of maternal hepatitis C virus infection in the United States . Obstet Gynecol 2020 ; 135 : 387 – 95 .
Ko JY , Haight SC , Schillie SF , Bohm MK , Dietz PM . National trends in hepatitis C infection by opioid use disorder status among pregnant women at delivery hospitalization—United States, 2000–2015 . MMWR Morb Mortal Wkly Rep 2019 ; 68 : 833 – 8 .
Schillie SF , Canary L , Koneru A , et al. Hepatitis C virus in women of childbearing age, pregnant women, and children . Am J Prev Med 2018 ; 55 : 633 – 41 .
Koneru A , Nelson N , Hariri S , et al. Increased hepatitis C virus (HCV) detection in women of childbearing age and potential risk for vertical transmission—United States and Kentucky, 2011–2014 . MMWR Morb Mortal Wkly Rep 2016 ; 65 : 705 – 10 .
Watts T , Stockman L , Martin J , Guilfoyle S , Vergeront JM . Increased risk for mother-to-infant transmission of hepatitis C virus among Medicaid recipients—Wisconsin, 2011–2015 . MMWR Morb Mortal Wkly Rep 2017 ; 66 : 1136 – 9 .
Indolfi G , Easterbrook P , Dusheiko G , et al. Hepatitis C virus infection in children and adolescents . Lancet Gastroenterol Hepatol 2019 ; 4 : 477 – 87 .
Benova L , Mohamoud YA , Calvert C , Abu-Raddad LJ . Vertical transmission of hepatitis C virus: systematic review and meta-analysis . Clin Infect Dis 2014 ; 59 : 765 – 73 .
US Food and Drug Administration . Harvoni prescribing information . Updated April 2017 . Available at : https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205834s017lbl.pdf . Accessed 25 November 2022.
US Food and Drug Administration . Solvaldi prescribing information . Updated April 2017 . Available at : https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/204671s006lbl.pdf . Accessed 25 November 2022.
US Food and Drug Administration . Mavyret prescribing information . Updated June 2021 . Available at : https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/209394s014,215110s001lbl.pdf . Accessed 25 November 2022.
US Food and Drug Administration . Epclusa prescribing information . Updated June 2021 . Available at : https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208341s017lbl.pdf . Accessed 25 November 2022.
Jonas MM , Rhee S , Kelly DA , et al. Pharmacokinetics, safety, and efficacy of glecaprevir/pibrentasvir in children with chronic HCV: part 2 of the DORA study . Hepatology 2021 ; 74 : 19 – 27 .
Jonas MM , Squires RH , Rhee SM , et al. Pharmacokinetics, safety, and efficacy of glecaprevir/pibrentasvir in adolescents with chronic hepatitis C virus: part 1 of the DORA study . Hepatology 2020 ; 71 : 456 – 62 .
Jonas MM , Romero R , Sokal EM , et al. Safety and efficacy of sofosbuvir/velpatasvir in pediatric patients 6 to <18 years old with chronic hepatitis C infection [abstract 748]. The Liver Meeting. Boston, Massachusetts ; 2019 .
Balistreri WF , Murray KF , Rosenthal P , et al. The safety and effectiveness of ledipasvir-sofosbuvir in adolescents 12–17 years old with hepatitis C virus genotype 1 infection . Hepatology 2017 ; 66 : 371 – 8 .
Schwarz KB , Rosenthal P , Murray KF , et al. Ledipasvir-sofosbuvir for 12 weeks in children 3 to <6 years old with chronic hepatitis C . Hepatology 2020; 2:422-30.
Murray KF , Balistreri WF , Bansal S , et al. Safety and efficacy of ledipasvir-sofosbuvir with or without ribavirin for chronic hepatitis C in children ages 6–11 . Hepatology 2018 ; 68 : 2158 – 66 .
Reau N , Kwo PY , Rhee S , et al. Glecaprevir/pibrentasvir treatment in liver or kidney transplant patients with hepatitis C virus infection . Hepatology 2018 ; 68 : 1298 – 307 .
Ueda Y , Kobayashi T , Ikegami T , et al. Efficacy and safety of glecaprevir and pibrentasvir treatment for 8 or 12 weeks in patients with recurrent hepatitis C after liver transplantation: a Japanese multicenter experience . J Gastroenterol 2019 ; 54 : 660 – 6 .
Saxena V , Khungar V , Verna EC , et al. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: results from the HCV-TARGET study . Hepatology 2017 ; 66 : 1090 – 101 .
Agarwal K , Castells L , Müllhaupt B , et al. Sofosbuvir/velpatasvir for 12 weeks in genotype 1–4 HCV-infected liver transplant recipients . J Hepatol 2018 ; 69 : 603 – 7 .
Colombo M , Aghemo A , Liu H , et al. Treatment with ledipasvir-sofosbuvir for 12 or 24 weeks in kidney transplant recipients with chronic hepatitis C virus genotype 1 or 4 infection: a randomized trial . Ann Intern Med 2017 ; 166 : 109 – 17 .
Sawinski D , Kaur N , Ajeti A , et al. Successful treatment of hepatitis C in renal transplant recipients with direct-acting antiviral agents . Am J Transplant 2016 ; 16 : 1588 – 95 .
Feng HP , Caro L , Fandozzi CM , et al. Pharmacokinetic interactions between elbasvir/grazoprevir and immunosuppressant drugs in healthy volunteers . J Clin Pharmacol 2018 ; 58 : 666 – 73 .
US Food and Drug Administration . Zepatier prescribing information . Updated December 2021 . Available at : https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208261s007lbl.pdf . Accessed 26 November 2022.
US Food and Drug Administration . Vosevi prescribing information . Updated November 2019 . Available at : https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/209195s003lbl.pdf . Accessed 26 November 2022.
United Network for Organ Sharing . Updated 14 November 2022 . Available at : https://www.unos.org/ . Accessed 14 November 2022.
Chang SH , Merzkani M , Lentine KL , et al. Trends in discard of kidneys from hepatitis C viremic donors in the United States . Clin J Am Soc Nephrol 2021 ; 16 : 251 – 61 .
Cotter TG , Aronsohn A , Reddy KG , Charlton M . Liver transplantation of HCV-viremic donors into HCV-negative recipients in the United States: increasing frequency with profound geographic variation . Transplantation 2021 ; 105 : 1285 – 90 .
Cotter TG , Paul S , Sandıkçı B , et al. Increasing utilization and excellent initial outcomes following liver transplant of hepatitis C virus (HCV)-viremic donors into HCV-negative recipients: outcomes following liver transplant of HCV-viremic donors . Hepatology 2019 ; 69 : 2381 – 95 .
Madan S , Patel SR , Rahgozar K , et al. Utilization rates and clinical outcomes of hepatitis C positive donor hearts in the contemporary era . J Heart Lung Transplant 2019 ; 38 : 907 – 17 .
Potluri VS , Goldberg DS , Mohan S , et al. National trends in utilization and 1-year outcomes with transplantation of HCV-viremic kidneys . J Am Soc Nephrol 2019 ; 30 : 1939 – 51 .
Madan S , Patel SR , Vlismas P , et al. Increasing multiorgan heart transplantation with hepatitis C virus donors in the current era . J Heart Lung Transplant 2021 ; 40 : 1382 – 6 .
Sageshima J , Troppmann C , McVicar JP , Santhanakrishnan C , de Mattos AM , Perez RV . Impact of willingness to accept hepatitis C seropositive kidneys among hepatitis C RNA-positive waitlisted patients . Transplantation 2018 ; 102 : 1179 – 87 .
Sawinski D , Forde KA , Lo Re V 3rd , et al. Mortality and kidney transplantation outcomes among hepatitis C virus-seropositive maintenance dialysis patients: a retrospective cohort study . Am J Kidney Dis 2019 ; 73 : 815 – 26 .
Shelton BA , Sawinski D , Mehta S , Reed RD , MacLennan PA , Locke JE . Kidney transplantation and waitlist mortality rates among candidates registered as willing to accept a hepatitis C infected kidney . Transpl Infect Dis 2018 ; 20 : e12829 .
Altshuler PJ , Helmers MR , Schiazza AR , et al. HCV-positive allograft use in heart transplantation is associated with increased access to overdose donors and reduced waitlist mortality without compromising outcomes . J Card Fail 2022 ; 28 : 32 – 41 .
Terrault NA , Burton J , Ghobrial M , et al. Prospective multicenter study of early antiviral therapy in liver and kidney transplant recipients of HCV-viremic donors . Hepatology 2021 ; 73 : 2110 – 23 .
Aqel B , Wijarnpreecha K , Pungpapong S , et al. Outcomes following liver transplantation from HCV-seropositive donors to HCV-seronegative recipients . J Hepatol 2021 ; 74 : 873 – 80 .
Sise ME , Goldberg DS , Kort JJ , et al. Multicenter study to transplant hepatitis C-infected kidneys (MYTHIC): an open-label study of combined glecaprevir and pibrentasvir to treat recipients of transplanted kidneys from deceased donors with hepatitis C virus infection . J Am Soc Nephrol 2020 ; 31 : 2678 – 87 .
Gidea CG , Narula N , Reyentovich A , et al. Increased early acute cellular rejection events in hepatitis C-positive heart transplantation . J Heart Lung Transplant 2020 ; 39 : 1199 – 207 .
Woolley AE , Singh SK , Goldberg HJ , et al. Heart and lung transplants from HCV-infected donors to uninfected recipients . N Engl J Med 2019 ; 380 : 1606 – 17 .
Smith DE , Chen S , Fargnoli A , et al. Impact of early initiation of direct-acting antiviral therapy in thoracic organ transplantation from hepatitis C virus positive donors . Semin Thorac Cardiovasc Surg 2021 ; 33 : 407 – 15 .
Feld JJ , Cypel M , Kumar D , et al. Short-course, direct-acting antivirals and ezetimibe to prevent HCV infection in recipients of organs from HCV-infected donors: a phase 3, single-centre, open-label study . Lancet Gastroenterol Hepatol 2020 ; 5 : 649 – 57 .
Ramirez-Sanchez C , Kozuch J , Shah MM , et al. A pilot trial for prevention of hepatitis C virus transmission from donor to organ transplant recipient with short-course glecaprevir/pibrentasvir . Open Forum Infect Dis 2022 ; 9 : ofac550 .
Diaz-Castrillon CE , Huckaby LV , Witer L , et al. National trends and outcomes of heart-kidney transplantation using hepatitis C positive donors . Clin Transplant 2022 ; 36 : e14581 .
Bekki Y , Crismale JF , Myers B , Schiano TD , Florman S . Varying utilization rates but superior outcomes in liver transplantation from hepatitis C-positive donors in the United States: an analysis of the OPTN/UNOS database . Transplantation 2022 ; 106 : 1787 – 98 .
Levitsky J , Formica RN , Bloom RD , et al. The American Society of Transplantation consensus conference on the use of hepatitis C viremic donors in solid organ transplantation . Am J Transplant 2017 ; 17 : 2790 – 802 .
Kim M , Stern J , Robalino R , et al. Caregiver exposure to hepatitis C virus following transplantation with hepatitis C viremic donor organs: a case series . Transpl Infect Dis 2022 ; 24 : e13775 .
Karkout KA , Al Sherif S , Hussein Q , Albawardi A , Boobes Y . Possible acute rejection associated with the use of the new anti-hepatitis C virus medications . Avicenna J Med 2019 ; 9 : 32 – 4 .
Kwong AJ , Wall A , Melcher M , et al. Liver transplantation for hepatitis C virus (HCV) non-viremic recipients with HCV viremic donors . Am J Transplant 2019 ; 19 : 1380 – 7 .
Zaky Z , Herlitz L , Augustine J . The impact of direct antiviral therapy for hepatitis C (DAA) on acute rejection and donor specific antibody formation in kidney transplant recipients, evidence from surveillance biopsies . Transplantation 2018 ; 102 : S325 .
Degenhardt L , Peacock A , Colledge S , et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review . Lancet Glob Health 2017 ; 5 : e1192 – e207 .
Mateu-Gelabert P , Sabounchi NS , Guarino H , et al. Hepatitis C virus risk among young people who inject drugs . Front Public Health 2022 ; 10 : 835836 .
Amon JJ , Garfein RS , Ahdieh-Grant L , et al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994–2004 . Clin Infect Dis 2008 ; 46 : 1852 – 8 .
Martin NK , Hickman M , Hutchinson SJ , Goldberg DJ , Vickerman P . Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy . Clin Infect Dis 2013 ; 57 : S39 – 45 .
Fraser H , Martin NK , Brummer-Korvenkontio H , et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe . J Hepatol 2018 ; 68 : 402 – 11 .
Fraser H , Zibbell J , Hoerger T , et al. Scaling-up HCV prevention and treatment interventions in rural United States — model projections for tackling an increasing epidemic . Addiction 2018 ; 113 : 173 – 82 .
Palmateer NE , McAuley A , Dillon JF , et al. Reduction in the population prevalence of hepatitis C virus viraemia among people who inject drugs associated with scale-up of direct-acting anti-viral therapy in community drug services: real-world data . Addiction 2021 ; 116 : 2893 – 907 .
Iversen J , Dore GJ , Starr M , et al. Estimating the consensus hepatitis C cascade of care among people who inject drugs in Australia: pre and post availability of direct acting antiviral therapy . Int J Drug Policy 2020 ; 83 : 102837 .
Harris KA Jr , Arnsten JH , Litwin AH . Successful integration of hepatitis C evaluation and treatment services with methadone maintenance . J Addict Med 2010 ; 4 : 20 – 6 .
Dore GJ , Altice F , Litwin AH , et al. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial . Ann Intern Med 2016 ; 165 : 625 – 34 .
Grebely J , Dalgard O , Conway B , et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial . Lancet Gastroenterol Hepatol 2018 ; 3 : 153 – 61 .
Norton BL , Fleming J , Bachhuber MA , et al. High HCV cure rates for people who use drugs treated with direct acting antiviral therapy at an urban primary care clinic . Int J Drug Policy 2017 ; 47 : 196 – 201 .
Scherz N , Bruggmann P , Brunner N . Direct-acting antiviral therapy for hepatitis C infection among people receiving opioid agonist treatment or heroin assisted treatment . Int J Drug Policy 2018 ; 62 : 74 – 7 .
Macías J , Morano LE , Téllez F , et al. Response to direct-acting antiviral therapy among ongoing drug users and people receiving opioid substitution therapy . J Hepatol 2019 ; 71 : 45 – 51 .
Messina V , Onorato L , Di Caprio G , et al. Directly acting antiviral-based treatment for HCV-infected persons who inject drugs: a multicenter real-life study . Life (Basel) 2020 ; 11 : 17 .
Huang P , Wang Y , Yue M , et al. The risk of hepatitis C virus recurrence in hepatitis C virus-infected patients treated with direct-acting antivirals after achieving a sustained virological response: a comprehensive analysis . Liver Int 2021 ; 41 : 2341 – 57 .
Hajarizadeh B , Cunningham EB , Valerio H , et al. Hepatitis C reinfection after successful antiviral treatment among people who inject drugs: a meta-analysis . J Hepatol 2020 ; 72 : 643 – 57 .
Midgard H , Weir A , Palmateer N , et al. HCV epidemiology in high-risk groups and the risk of reinfection . J Hepatol 2016 ; 65 : S33 – 45 .
Simmons B , Saleem J , Hill A , Riley RD , Cooke GS . Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis . Clin Infect Dis 2016 ; 62 : 683 – 94 .
Hagan H , Jordan AE , Neurer J , Cleland CM . Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men . AIDS 2015 ; 29 : 2335 – 45 .
Hoornenborg E , Coyer L , Boyd A , et al. High incidence of HCV in HIV-negative men who have sex with men using pre-exposure prophylaxis . J Hepatol 2020 ; 72 : 855 – 64 .
Hoornenborg E , Achterbergh RCA , Schim van der Loeff MF , et al. MSM starting preexposure prophylaxis are at risk of hepatitis C virus infection . AIDS 2017 ; 31 : 1603 – 10 .
Pufall EL , Kall M , Shahmanesh M , et al. Sexualized drug use (“chemsex”) and high-risk sexual behaviours in HIV-positive men who have sex with men . HIV Med 2018 ; 19 : 261 – 70 .
Jin F , Dore GJ , Matthews G , et al. Prevalence and incidence of hepatitis C virus infection in men who have sex with men: a systematic review and meta-analysis . Lancet Gastroenterol Hepatol 2021 ; 6 : 39 – 56 .
Ingiliz P , Martin TC , Rodger A , et al. HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe . J Hepatol 2017 ; 66 : 282 – 7 .
Boerekamps A , van den Berk GE , Lauw FN , et al. Declining hepatitis C virus (HCV) incidence in Dutch human immunodeficiency virus-positive men who have sex with men after unrestricted access to HCV therapy . Clin Infect Dis 2018 ; 66 : 1360 – 5 .
Adu PA , Rossi C , Binka M , et al. HCV reinfection rates after cure or spontaneous clearance among HIV-infected and uninfected men who have sex with men . Liver Int 2021 ; 41 : 482 – 93 .
Busschots D , Kremer C , Bielen R , et al. Hepatitis C prevalence in incarcerated settings between 2013–2021: a systematic review and meta-analysis . BMC Public Health 2022 ; 22 : 2159 .
Hofmeister MG , Rosenthal EM , Barker LK , et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013–2016 . Hepatology 2019 ; 69 : 1020 – 31 .
Macalino GE , Vlahov D , Sanford-Colby S , et al. Prevalence and incidence of HIV, hepatitis B virus, and hepatitis C virus infections among males in Rhode Island prisons . Am J Public Health 2004 ; 94 : 1218 – 23 .
Rich JD , Allen SA , Williams BA . Responding to hepatitis C through the criminal justice system . N Engl J Med 2014 ; 370 : 1871 – 4 .
Fox RK , Currie SL , Evans J , et al. Hepatitis C virus infection among prisoners in the California state correctional system . Clin Infect Dis 2005 ; 41 : 177 – 86 .
Rich JD , Chandler R , Williams BA , et al. How health care reform can transform the health of criminal justice-involved individuals . Health Aff (Millwood) 2014 ; 33 : 462 – 7 .
Winter RJ , Holmes JA , Papaluca TJ , Thompson AJ . The importance of prisons in achieving hepatitis C elimination: insights from the Australian experience . Viruses 2022 ; 14 : 497 .
He T , Li K , Roberts MS , et al. Prevention of hepatitis C by screening and treatment in U.S. prisons . Ann Intern Med 2016 ; 164 : 84 – 92 .
MacDonald R , Akiyama MJ , Kopolow A , et al. Feasibility of treating hepatitis C in a transient jail population . Open Forum Infect Dis 2017 ; 4 : ofx142 .
Spaulding AS , Kim AY , Harzke AJ , et al. Impact of new therapeutics for hepatitis C virus infection in incarcerated populations . Top Antivir Med 2013 ; 21 : 27 – 35 .
Akiyama MJ , Kaba F , Rosner Z , Alper H , Holzman RS , MacDonald R . Hepatitis C screening of the “birth cohort” (born 1945–1965) and younger inmates of New York City jails . Am J Public Health 2016 ; 106 : 1276 – 7 .
Beckwith CG , Kurth AE , Bazerman LB , et al. A pilot study of rapid hepatitis C virus testing in the Rhode Island Department of Corrections . J Public Health (Oxf) 2016 ; 38 : 130 – 7 .
Schoenbachler BT , Smith BD , Seña AC , et al. Hepatitis C virus testing and linkage to care in North Carolina and South Carolina jails, 2012–2014 . Public Health Rep 2016 ; 131 Suppl 2 : 98 – 104 .
de la Flor C , Porsa E , Nijhawan AE . Opt-out HIV and hepatitis C testing at the Dallas County Jail: uptake, prevalence, and demographic characteristics of testers . Public Health Rep 2017 ; 132 : 617 – 21 .
Liu S , Watcha D , Holodniy M , Goldhaber-Fiebert JD . Sofosbuvir-based treatment regimens for chronic, genotype 1 hepatitis C virus infection in U.S. incarcerated populations: a cost-effectiveness analysis . Ann Intern Med 2014 ; 161 : 546 – 53 .
Ogawa E , Chien N , Kam L , et al. Association of direct-acting antiviral therapy with liver and nonliver complications and long-term mortality in patients with chronic hepatitis C . JAMA Intern Med 2023 ; 183 : 97 – 105 .
Volkow ND , Frieden TR , Hyde PS , Cha SS . Medication-assisted therapies—tackling the opioid-overdose epidemic . N Engl J Med 2014 ; 370 : 2063 – 6 .
Author notes
- liver diseases
- hepatitis c
- hepatitis c virus
- infectious diseases society of america
| Month: | Total Views: |
|---|---|
| May 2023 | 910 |
| June 2023 | 2,533 |
| July 2023 | 2,180 |
| August 2023 | 4,649 |
| September 2023 | 4,291 |
| October 2023 | 3,753 |
| November 2023 | 6,452 |
| December 2023 | 5,382 |
| January 2024 | 6,179 |
| February 2024 | 5,684 |
| March 2024 | 6,111 |
| April 2024 | 5,760 |
| May 2024 | 5,802 |
| June 2024 | 4,675 |
| July 2024 | 5,012 |
| August 2024 | 5,172 |
Email alerts
More on this topic, related articles in pubmed, citing articles via, looking for your next opportunity.
- Recommend to your Library
Affiliations
- Online ISSN 1537-6591
- Print ISSN 1058-4838
- Copyright © 2024 Infectious Diseases Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Research article
- Open access
- Published: 17 January 2015
Achieving sustained virologic response in hepatitis C: a systematic review of the clinical, economic and quality of life benefits
- Jayne Smith-Palmer 1 ,
- Karin Cerri 2 &
- William Valentine 1
BMC Infectious Diseases volume 15 , Article number: 19 ( 2015 ) Cite this article
10k Accesses
137 Citations
6 Altmetric
Metrics details
The goal of chronic hepatitis C treatment is to remove the virus to avoid progression of HCV-related disease. Sustained virologic response (SVR) is the most widely used efficacy endpoint in clinical studies of hepatitis C, and represents the eradication of HCV from the body. The aim of the current review was to examine the long-term clinical, economic and quality of life benefits associated with achieving SVR.
A systematic literature review was performed using the PubMed, EMBASE and Cochrane library databases to identify articles examining the clinical, economic and quality of life benefits associated with SVR, published in English language from 2002–2013. For inclusion studies were required to enroll ≥100 patients and to report clinical endpoints including hepatocellular carcinoma, overall- or liver-related mortality, or progression of disease/complications (e.g. portal hypertension, esophageal varices). Review of economic studies on cost/cost-effectiveness of achieving SVR were focused on studies assessing boceprevir/telaprevir plus pegIFN and ribavirin as this represents the current standard of care in several jurisdictions worldwide. Quality of life evidence was required to use validated quality of life instruments and provide a quantitative analysis of the impact of SVR versus no treatment or treatment failure.
SVR is durable with late relapse rates over 4–5 year periods being in the range of 1–2%. Patients who achieve SVR frequently demonstrate some regression of fibrosis/cirrhosis and have a substantially reduced risk for hepatocellular carcinoma (relative risk [RR] 0.1–0.25), liver-related mortality (RR 0.03–0.2) and overall mortality (RR 0.1–0.3) in comparison with no treatment or treatment failure. In the 5 years post-treatment, medical costs for patients achieving SVR are 13-fold lower than patients not achieving SVR. Patients who achieve SVR also have health state utility values that are 0.05 to 0.31 higher than non-responders to treatment.
Conclusions
SVR represents the fundamental goal of antiviral treatment for patients infected with chronic HCV, so as to reduce risk of liver disease progression. Achievement of SVR has implications beyond those of clearing viral infection; it is associated with improved long-term clinical outcomes, economic benefits and improved health-related quality of life.
Peer Review reports
On a global level over 2% of the population are estimated to be infected with the hepatitis C virus (HCV), which corresponds to a prevalent population of >180 million people with chronic infection [ 1 ]. For many patients who become chronically infected, HCV causes slow, progressive damage to the liver and represents one of the leading causes of cirrhosis and hepatocellular carcinoma (HCC) [ 2 ]. Moreover, the slow insidious nature of disease progression means that many patients are unaware of their status until the later stages of disease.
Six major genotypes of HCV exist in many regions and the current standard of care for patients with HCV genotype 1 is therapy with a direct acting antiviral (DAA) in combination with ribavirin alone or combined with pegIFN or a combination of two DAAs (with or without ribavirin). The present study focuses on HCV genotype 1 as this genotype occurs in all regions and is the predominant genotype in many regions.
The effectiveness of antiviral treatment, the extent to which treatment can clear viral infection is assessed according to the proportion of patients achieving sustained virologic response (SVR). SVR is the fundamental goal of treatment and is defined as undetectable (or below the lower limit of quantification) HCV RNA at 12–24 weeks after cessation of treatment [ 3 , 4 ]. SVR rates with a DAA in combination with pegIFN plus ribavirin (PR) currently range from approx. 80–90% for treatment-naïve patients [ 5 - 8 ], whilst SVR rates of up to 99% have been reported with combinations of two DAAs [ 9 ]. Similarly, SVR rates of up to 99% have been reported in treatment-experienced (non-responders and relapsers) patients treated with two-DAA combinations [ 10 ].
Although considered a surrogate endpoint (a biomarker indicative of viral clearance rather than a finite endpoint such as presence/absence of disease or mortality), SVR is widely accepted as the best available indicator of viral clearance and a subject with SVR is generally considered cured [ 11 ]. Rates of late relapse are extremely low and long-term (up to 4 years) studies of patients treated with pegIFN have shown that SVR is durable, with approximately 99% of patients remaining virus-free, although the patient is still at risk of subsequent reinfection [ 12 ].
To date, the vast majority of clinical trials in HCV, including phase III trials of boceprevir and telaprevir, have used SVR at 24 weeks after the planned end of treatment (SVR24) as the primary endpoint. However, research in the field of HCV is currently advancing at a rapid pace and SVR 12 weeks after the end of treatment is now used as the primary endpoint in most clinical studies. The concordance between SVR12 and SVR24 rates has been investigated, and a high level of concordance was observed, suggesting that SVR12 represents a valid clinical endpoint [ 13 , 14 ]. Specifically, analysis was performed by the FDA in which data from fifteen Phase 2 and 3 trials (n = 12,000 patients) were combined to assess the concordance between SVR24 and SVR12. This analysis showed that concordance was observed between SVR12 and SVR24 for all treatments: 98% of patients with SVR212 had SVR24 [ 15 ].
As mentioned, SVR is the most commonly used endpoint in clinical trials in hepatitis C because the use of incidence of HCC or liver-related mortality as an endpoint is impractical within the context of a clinical trial. Patients with SVR following 24–48 weeks of treatment are generally considered to be permanently cured. While long-term follow-up is still required to fully assess the impact of SVR on hard clinical endpoints such as the progression to compensated or decompensated cirrhosis, HCC and liver-related mortality, it has been shown that patients who achieve SVR have a considerably reduced incidence of liver-related complications in comparison with those who fail treatment. As well as clinical implications, SVR rates can be anticipated to have an impact on the economic burden and humanistic burden of disease. HCV-related complications, such as HCC or liver-transplantation are associated with high direct medical costs and high levels of healthcare resource utilization [ 16 ], therefore any reduction in the incidence of HCV-related complications may have a considerable long-term economic benefit. This also extends to work productivity, as patients with SVR have higher post-treatment employment rates than those who fail treatment [ 17 , 18 ]. However, in the short-term improvements in SVR rates may be associated with increased pharmacy costs; for example, in an analysis in the French setting Deuffic-Burban et al . projected that the introduction of triple therapy would lead to a 3–4 fold increase in the number of genotype 1 patients receiving treatment at a cost of EUR 497–638 million [ 19 ]. As HCV is a transmissible disease, from a public health perspective, benefits of improved SVR rates include a reduced prevalent population and therefore the potential for lower transmission and incidence rates.
The objectives of the current study were to perform a literature review to understand the link between the clinical implications of achievement of SVR with the economic and patient quality of life implications by, firstly, exploring the clinical validity of SVR as an endpoint in terms of the impact of SVR on the incidence of liver-related complications including mortality and HCC and secondly, to assess the impact of attainment of SVR in terms of long-term economic outcomes and quality of life in patients infected with chronic HCV infection.
The search strategy for the literature review was designed using high level Medical Subject Heading (MeSH) terms and supplemented with free text terms and adapted for the PubMed, EMBASE and Cochrane Library databases as required; all initial searches were run on 08 January 2013 (subsequent searches with the same search terms were run on 29 April 2014 to capture studies published since the initial review was performed). For the PubMed searches, MeSH terms used included Hepatitis C [MeSH] OR Hepacivirus [MeSH]; free-text terms were used to identify articles focusing on sustained virologic response (wildcards were used to capture variations in terminology). For the EMBASE searches, MeSH terms were mapped to EMBASE equivalents using the “map term” functionality.
The review was limited to articles published in the last 10 years and for inclusion, studies were required to be published in English and have a minimum enrollment of 100 patients (Table 1 ) (a minimum cohort size of 100 patients was chosen to focus on relatively large scale studies that could detect relatively small differences in outcomes and to preclude small scale pilot studies conducted in highly selective patient populations). The focus of the review was on patients with HCV genotype 1, and studies exclusively in patients with HCV genotypes 2 and 3, or in patients with HIV coinfection, were excluded. Clinical studies were also required to have a minimum follow up of 1-year post-cessation of treatment, compare outcomes in patients with SVR versus either untreated patients or those failing to achieve SVR, and report hard clinical endpoints; studies reporting biochemical parameters only, such as alanine amino transferase levels, were excluded. Cost-effectiveness studies were also limited to studies incorporating analyses of protease inhibitor-based triple therapy regimens; studies evaluating pegIFN plus ribavirin in comparison with pegIFN or IFN alone or no treatment were excluded. Studies reporting on health-related quality of life evidence were required to use validated quality of life instruments and provide a quantitative analysis of the impact of SVR versus no treatment or treatment failure.
The literature searches across the three databases identified a total of 4,206 unique hits after two rounds of screening (first round screening by title and abstract only and second round full-text screening of short-listed articles.) A total of 44 clinical studies (including 4 meta-analyses), 15 quality of life studies (one additional quality of life was identified in supplementary hand searches) and 2 economic studies were included in the final analysis (Figure 1 ). The review was performed in line with PRISMA guidance and a schematic diagram of the literature review process is shown in the Additional file 1 . Updated searches performed in April 2014 identified an additional 20 clinical studies and 3 cost/cost-effectiveness studies.
Diagram of literature review process. Note: the original literature searches were re-run in April 2014 to capture publications published since the original searches. A total of twenty additional clinical articles and three additional economic studies were identified.
Clinical benefits
The literature review process identified a large number of studies that examined the impact of SVR on the long-term risk of a number of clinical outcomes including incidence of HCC, liver transplantation, liver-related mortality and overall mortality in populations with differing levels of severity. Data were captured from a large range of patient populations in terms of relative prevalence of different HCV genotypes, severity of liver disease at baseline and treatment type.
Hepatocellular carcinoma
On a global level, HCV is one of the leading causes of HCC, and is typically associated with a poor prognosis. A total of 34 studies [ 20 - 48 ] including five meta-analyses [ 49 - 53 ] that examined the impact of SVR on risk of HCC were identified (Table 2 ). The overwhelming consensus of the results of the studies included was that patients who achieve SVR have a considerably reduced risk for HCC in comparison with untreated patients or those who fail to achieve SVR. However, the magnitude of this effect varied, with reported RRs for HCC in patients with SVR versus non-responders or untreated patients ranging from 0.09–0.35.
The 2010 meta-analysis by Singal et al . showed that patients who had SVR (following treatment with IFN alone or IFN plus ribavirin) had a RR (95% CI) for HCC of 0.35 (0.26–0.46) in comparison with non-responders [ 49 ]. Similarly, the meta-analysis by Kimer et al . reported a RR (95% CI) for HCC of 0.15 (0.05–0.45); however, the comparator group was untreated patients, rather than non-responders to therapy [ 51 ]. Notably, the analysis by Singal et al . included only studies in patients with cirrhosis, whereas the analysis by Kimer et al . included two studies in mixed or non-cirrhotic patients.
Studies examining the impact of SVR on risk for HCC in Japan are of particular interest owing to the high relative prevalence of HCV genotype 1b, (which is associated with a higher incidence of HCC than genotype 1a) and high incidence of HCV-associated HCC in this setting. For HCV patients with cirrhosis the annual probability of HCC is 1–4%, although this increases to 5–8% for patients with HCV genotype 1b [ 54 , 55 ]. Japan-based studies also showed that SVR was associated with reduced risk for HCC versus non-response, although as expected the absolute risk in both SVR and non-SVR population was increased with advanced age and increased severity of fibrosis. For example, Yoshida et al . determined SVR-related gain in HCC-free survival as both a function of age and fibrosis level (as measured by METAVIR F0–F4) [ 36 ]. For male patients with F0/F1 stage disease the gain in HCC-free survival with SVR was 2.48 years for patients aged 30 years, reducing to 0.15 years for patients aged 80 years. For patients with F4 stage disease SVR-induced gain in HCC free survival was 15.98 years at age 30 years, but only 2.38 years at age 80 years [ 36 ]. In another Japanese study by Imazeki et al ., in the overall treated HCV population they report an annual HCC incidence of 0.5% for those with SVR versus 2.6%; whereas in patients with cirrhosis, the corresponding figures were 1.4% and 5.9%, respectively [ 46 ]. Similar findings were reported in other studies in the Japanese setting [ 22 , 24 , 29 ]. Only two studies (out of thirteen) from the Japanese setting reported no difference in the incidence of HCC for patients achieving SVR versus those without SVR [ 34 ].
Liver-related mortality
Analysis of clinical studies also showed that patients who achieve SVR have a substantially lower risk of liver-related mortality and overall mortality than non-responders to treatment, irrespective of genotype, setting or disease severity level, with a considerable proportion of studies showing that this reduction in risk was statistically significant (Table 3 ). In individual studies the RR for overall mortality for patients with SVR versus non-response or no treatment ranged from 0.14–0.70, whilst the corresponding figures for liver-related mortality were 0.03–0.22. As with HCC studies, the magnitude of the effect of SVR on mortality risk varied considerably between studies, which may be attributable in part to differences in patient characteristics such as mean age and disease stage prior to treatment. A 2010 meta-analysis reported a RR (95% CI) for liver-related mortality of 0.23 (0.10–0.52) for SVR patients compared with treatment failures, although if only patients with advanced fibrosis/cirrhosis were included this figure decreased to 0.13 (0.06–0.29) [ 50 ]. These findings were echoed in individual studies. For example, a large scale (N = 1,215 treatment-naïve patients), UK-based retrospective study reported a multivariate HR (95% CI) for liver-related death for SVR patients of 0.22 (0.09–0.58) (p < 0.01) [ 56 ]. Similarly, an Italian study of HCV patients (with no cirrhosis) reported that not achieving SVR (versus SVR) increased the HR (95% CI) for liver-related death to 6.97 (1.70–28.42) [ 44 ]. Additionally, studies in the Japanese setting reported similar findings, with two studies reporting RRs for liver-related mortality of 0.03–0.04 for patients achieving SVR versus untreated patients [ 57 , 58 ].
The benefits of SVR in terms of reduced risk for liver-related mortality were apparent regardless of baseline severity. A multicenter study by van der Meer et al . [ 38 ] with over 8 years of follow up was conducted exclusively in patients with advanced fibrosis or cirrhosis at baseline. Van der Meer showed that SVR led to a 3-fold reduction in the overall mortality rate (1.01 [0.46–1.56] per 100 patient years for SVR versus 2.93 [2.36–3.51] per 100 patient years for those without SVR; p < 0.001) and a 30-fold reduction in liver-related mortality or transplant (0.23 [0.01–0.50] per 100 patient years for SVR versus 3.20 [2.58–3.82] per 100 patient years for those without SVR; p < 0.001) [ 38 ].
Overall mortality
Achievement of SVR has also been shown to reduce the risk of overall mortality (Table 3 ). For example, in a US-based study, SVR was associated with a HR (95% CI) versus non SVR for all-cause mortality (for genotype 1 only) was 0.70 (0.59–0.83) (p < 0.0001) [ 65 ]. Other studies report a much lower figure, with Morgan et al . reporting a HR (95% CI) for all-cause mortality or liver transplant of 0.17 (0.06–0.46) [ 41 ].
Other complications
Four studies identified in the review (conducted in Japan, Spain and the United States), showed that patients with SVR had a reduced risk for new onset diabetes in comparison with those not achieving SVR; in patients who achieved SVR the risk of developing diabetes was approximately 2-fold lower than for patients who failed treatment (Table 4 ). In all four studies investigating this associated the reduced risk for type 2 diabetes with SVR was statistically significant [ 69 - 72 ].
Economic implications
The incidence of late stage complications associated with HCV (e.g. HCC, decompensated cirrhosis and liver transplant) is a major contributor to the economic burden associated with HCV. In the US alone, direct annual costs associated with HCV exceed USD 1 billion [ 82 ], with annual per patient costs exceeding USD 50,000 for HCC and USD 110,000 for a single liver transplant [ 83 ]. Similarly, in Europe, a 5-country study by Vietri et al . showed that HCV patients have a high level of medical resource utilization leading to high direct costs as well as a high degree of absenteeism and presenteeism leading to high indirect costs. Indeed, Vietri et al . report direct annual costs of EUR 1,147 and indirect costs of EUR 7,533 per patient [ 84 ]. New antiviral treatment regimens that increase the SVR rate have the potential to influence future complication rates and therefore the overall economic burden; however, as triple therapy regimens are also associated with increased pharmacy costs in comparison with pegIFN plus ribavirin alone, cost-effectiveness analyses are required in order to quantify the estimated long-term clinical and economic benefits. The initial literature review and update captured a total of five studies that specifically assessed the economic benefits of treatment in terms of cost per SVR achieved or cost of SVR versus failure (Table 5 ) [ 85 - 89 ].
One 2013 US-based study Manos et al . examined follow up costs for patients achieving SVR versus non-responders over a 5-year period [ 89 ]. They report that patients with SVR (all genotypes) have mean annual costs (2007 USD) of USD 6,301 versus USD 10,149 for non-SVR patients, with the difference attributed to higher hospital costs (USD 5,167 versus USD 2,641) and outpatient costs (USD 4,983 versus USD 3,661). A similar UK-based analysis reported that costs in the 5 years post-treatment were 13-fold higher for patients who failed treatment versus those who achieved SVR, which increased to 56-fold for patients who initially failed treatment and were then retreated [ 85 ].
Three cost-effectiveness analyses presented results in terms of cost or incremental cost per SVR achieved [ 86 - 88 ]. In an Italian-based analysis Camma et al . reported an incremental cost per SVR achieved (versus pegIFN plus ribavirin) of EUR 60,500 per SVR for boceprevir IL28B guided therapy and EUR 74,600 per SVR for telaprevir IL28B guided therapy (2011 EUR) for treatment-naïve patients with HCV genotype 1. However, a key limitation of this analysis is that US pharmacy costs were used as Italian costs were not available at the time of the analysis, which may have led to under- or over-estimation of the true cost-effectiveness [ 87 ]. Another analysis from the Greek setting showed that for the overall HCV genotype 1 population (including treatment naïve patients and prior non-responders and relapsers), that telaprevir-based triple therapy was dominant to pegIFN plus ribavirin in terms of cost per SVR gained (telaprevir was associated with a cost-saving of EUR 10,403 per SVR gained) [ 88 ].
Quality of life
The literature review process identified a total of 15 studies that examined HRQoL in patients with SVR [ 17 , 90 - 103 ], and a further study was identified via searches of the bibliographic sections of included studies [ 18 ]. The most commonly used instrument in HRQoL studies was the SF-36, and studies that used this almost universally showed that patients with SVR had better scores than non-responder/relapser/untreated populations, both in terms of sub-domains and physical and mental component summary scores, with a large proportion of between group differences achieving statistical significance. On an individual domain level, in studies that used the SF-36, the largest differences between patients with SVR and those without were reported for general health followed by role physical [ 18 , 103 ].
A total of seven studies (including two cost-effectiveness analyses of triple therapy), reported utility values for SVR using a number of different methods including standard gamble, time trade off (TTO) and the Health Utilities Index Mark 3 (HUI3) (Table 6 ). In one cost-effectiveness analysis by Liu et al . the mean utility value associated with SVR was dependent upon whether the subject had mild fibrosis or cirrhosis [ 104 ]. Previous studies have shown that HRQoL is influenced by disease severity, but the study by Liu et al . is one of the few studies to suggest the quality of life benefit of SVR is influenced by baseline disease severity. Utility values associated with the SVR state were strongly influenced by the method of assessment used and were typically highest using the TTO (ranging from 0.88–0.89) and standard gamble methods (0.86) (Table 6 ) and lowest using the SF-6D (0.71) and visual analog scale methods (0.74). Additionally, assessment of utility values using the EQ-5D valuation index led to values of 0.83–0.84 for SVR in comparison with 0.70–0.76 for non-response/relapse (Table 6 ) [ 95 , 99 ].
Most quality of life studies included in the review assessed HRQoL within the first year following treatment; however two studies assessed the impact of SVR at >3 years after completion of antiviral therapy. Both Mauss et al . [ 17 ] and John-Baptiste et al . [ 18 ] reported that the HRQoL benefits of SVR persist over >3 years, with both studies showing that patients with SVR had significantly better scores in all eight domains of the SF-36 in comparison with those who had failed treatment. Both Mauss et al . [ 17 ] and John-Baptiste et al . [ 18 ] also showed that SVR was associated with long-term benefits in terms of work productivity. Mauss et al . reported that a significantly higher proportion of patients who achieved SVR were employed (56%) in comparison with non-SVR patients (41%; p < 0.0001) [ 17 ]. Similarly, John-Baptiste et al . reported employment figures of 67% for patients with SVR versus 51% for those who failed treatment (p = 0.02). This analysis also showed that long-term work and leisure capacity were significantly compromised in treatment failures in comparison with the SVR group. Treatment failures had a mean (SD) reduction in work capacity of 5.8 (18)%, versus 1.1 (6)% for SVR; the corresponding figures for reduction in leisure capacity were 10.7 (24)% and 3.3 (13%), respectively [ 18 ].
The overarching aim of the present review was to consolidate published findings relating to the clinical, economic and quality of life benefits associated with achieving SVR and draw together these data to assess how clinical and quality of life benefits translate into economic benefits on both a per-patient and system-wide level. Previous research has largely focused on individual clinical, economic or quality of life aspects of SVR and has not examined how these benefits overlap and interact within a larger framework. For example, on an individual patient level, attainment of SVR is associated with lower risk of progression, HCC and liver-related mortality, less time spent in hospitals and improved symptoms and quality of life. However, when scaled up to a system wide level, SVR translates into substantial direct cost-savings for the payer due to costly complications avoided, as well as lower indirect costs due to lost productivity through absenteeism and presenteeism.
SVR is widely regarded as a cure and has been shown to be durable with rates of late relapse being in the region of 1–2%. In addition to halting progression of liver damage, SVR-induced regression of fibrosis and even cirrhosis has been reported. For example in a meta-analysis of 8 European studies, Veldt et al . reported regression of fibrosis in approximately one third of patients achieving SVR [ 106 ]. Additionally, risk factors such as heavy alcohol use or co-infection with hepatitis B may lead to progression of liver disease even in the presence of SVR. The clinical implications of potential low level viral persistence are not well characterized and it remains largely unknown whether it influences post-SVR progression of liver disease.
There is extensive evidence relating to the clinical benefits of SVR. A reduced risk for progression to cirrhosis, HCC, liver transplantation and liver-related mortality is evident regardless of setting, age, HCV subtype or level of fibrosis (Tables 2 , 3 , 4 and 7 ). However, the magnitude of the impact of SVR in terms of its impact on mortality rates varied notably between studies identified in this review, with some studies suggesting that following SVR the risk for liver-related mortality is comparable to that of the general population, whilst others suggest that mortality risk, although lower than for treatment failures, remains elevated in comparison with the general population. A contributing factor in this disparity may be heterogeneity in populations studied. Some studies excluded patients with advanced fibrosis or cirrhosis, whilst others were conducted exclusively in cirrhotic patients; there were also differences between patient populations in terms of age, previous treatment history, and the relative prevalence of different HCV genotypes.
The absolute risk and the magnitude of benefit does appear to be highly dependent on age and pre-treatment level of fibrosis. One study by Yoshida et al . [ 36 ] in the Japanese setting assessed the gain in HCC-free survival (defined as the difference in expected HCC-free survival with SVR versus without) according to age and fibrosis level. They report that the gain in HCC-free survival was greater when the subject was younger and had advanced fibrosis at baseline. For example for patients with stage F2 fibrosis the RR (95% CI) for HCC were 1.76 (0.47–6.67) for SVR versus 2.86 (1.59–5.13) for non-SVR, whereas for patients with F4 fibrosis the RRs (95% CI) increase to 4.78 (1.13–20.18) and 12.23 (6.81–21.95), respectively. A large proportion of the HCC studies identified in the current review (n = 11/24) were conducted in the Japanese setting, which has among the highest incidence of HCV-related HCC in the world, with an estimated 30,000 deaths per year attributable to HCC [ 36 ] and a mean annual treatment cost of USD 42,360 in Japan (2010 USD) [ 16 ]. As such, even a modest reduction in HCC, such as 100 cases avoided per year, would lead to savings of over USD 4 million for the payer.
The underlying reason for the high HCC rate in Japan is thought to be partly due to the high relative prevalence of genotype 1b (which is associated with a higher risk for HCC development in comparison with other genotypes [ 108 ]) relative to the US and Europe, and also to the fact that the spread of HCV is thought to have begun earlier in Japan than in Europe and North America, [ 109 ] therefore leading to an older prevalent population, with more advanced disease and therefore higher risk for developing HCC.
The clinical benefits of SVR are not limited to HCC. Patients with SVR have reduced risk of progression, liver-related mortality, liver transplantation and overall mortality in comparison with those not achieving SVR. Liver transplantation has a mean (global) cost of USD 146,960 in the year of transplant [ 16 ], so again even small reductions in the number of liver transplants required translate into substantial savings for the payer. The risk of overall mortality is reduced by approximately 5-fold, and liver related mortality approximately 10-fold, versus non-SVR, although this is influenced by age and level of fibrosis prior to treatment.
Patients with HCV have been shown to be at elevated risk for co-morbid conditions including type 2 diabetes [ 110 ]. Three studies showed that patients with SVR had a lower incidence of new onset diabetes versus non-responders. The mechanism for this is not clear, although hypotheses include elevated insulin resistance caused by pro-inflammatory cytokines [ 71 ]. It is has also proposed that insulin resistance may influence the likelihood of achieving SVR, rather than SVR influencing diabetogenic processes [ 111 ]. HCV is associated with a number of other extra-hepatic complications, although there are a lack of data on the impact of SVR on these.
The clinical benefits associated with SVR due to complications avoided translate into economic benefits from a third party perspective. The magnitude of economic benefit is difficult to quantify, due to uncertainty of prevalence estimates and continued advances in therapy leading to ongoing improvements in SVR rates but owing to the high cost, even a small reduction in the incidence of HCC would have considerable economic implications. In addition to direct costs, the attainment of SVR also has implications on indirect costs such as lost productivity, with evidence to suggest that employment rates are higher amongst patients with SVR versus those without [ 17 , 18 ].
The clinical benefits associated with the achievement of SVR translate into clinically meaningful benefits for patients by improving symptoms, functioning and health related quality of life, compared with those not able to achieve SVR. The findings of quality of life studies consistently showed that patients with SVR had higher utility values and SF-36 and EQ-5D scores in comparison with those who did not respond to treatment. However, in the literature review it was noted that there is a paucity of quality of life studies with long-term follow-up (≥5 years). Although SVR leads to improved quality of life in the short-term, data relating to whether or not this improvement persists in the long term are lacking.
Although the scope of the present review was such that the endpoints of fatigue and depression were not assessed directly, SVR is also associated with other benefits in terms of patient reported outcomes including fatigue and depression, which are common side effects associated with antiviral treatment. The Fatigue Severity Scale (FSS) is a commonly used instrument to assess fatigue in HCV studies. The FSS has good reliability, validity and responsiveness and a total score ≥4 is indicative of severe fatigue. In addition to improved SVR, protease inhibitors are associated with benefits in terms of reduced fatigue. Published data relating to the magnitude of change in FSS score required to constitute a minimally important difference are lacking. However, analysis of phase III simeprevir trial data indicate that a clinically meaningful change (improvement or worsening) may be as small as 0.33–0.34 and that patients with SVR have significant improvements in FSS score versus non-responders (Janssen, data on file).
The current study has several limitations that should be acknowledged with regard to interpretation of the findings. In particular, the review included studies that compare SVR groups with both untreated groups and non-responder groups. Several studies in Japan have shown that risk of HCC and overall mortality are reduced, although not significantly in patients who receive treatment but fail to achieve SVR in comparison with untreated patients, although the mechanism behind this is poorly understood [ 57 , 112 ]. Moreover, in a considerable proportion of the studies reported here no distinction is made by the authors in the non-SVR groups in terms of null-response, partial response or relapse following treatment. The potential benefits of SVR in relapsers is an area that warrants further investigation as two studies included here suggested that patients who relapse have lower risks for overall mortality and HCC in comparison with true non-responders [ 37 ]. Similarly, whether benefits of SVR are different across different sub-populations, such as patients with hepatitis B or HIV coinfection, or is influenced by genotype, is an issue for future analysis. A further limitation of the current review is that no formal quality assessment of included studies was performed.
While this systematic literature review attempted to be as holistic as possible in capturing the impact of achieving SVR in patients chronically infected with hepatitis C, it was not possible to capture all possible consequences. For example, the benefits associated with reduced infection risk were not considered, and therefore represent a limitation of the review. Additionally, during the literature search it was noted that an aspect of HCV that is often overlooked in the literature is the stigma associated with HCV and the impact of this on patients’ quality of life, disclosure practices and treatment-seeking behavior. Stigma may be subtle and is inherently difficult to quantify. One of the key factors in stigma arises due to fear of transmission, which although limited to blood-borne routes, does not prevent stigma. Patients with SVR are no longer at risk of transmitting HCV to others, therefore the stigma associated with HCV should be removed. Another aspect to consider is the public health benefit associated with a lower population prevalence; a reduced population prevalence means that there are fewer people from whom HCV can be transmitted to others.
In conclusion, review of the literature has shown that achievement of SVR in patients with chronic HCV infection is associated with significant clinical, economic and quality of life benefits. Patients who achieve SVR, including those with advanced disease, have a substantially reduced risk of progression to cirrhosis, development of HCC and both liver-related and all cause mortality. This reduced risk of late stage complications also leads to economic benefits. Post-treatment, patients with SVR also have lower healthcare resource utilization versus non-responders, which also translates into substantial economic benefits from a healthcare payer perspective. Finally, the attainment of SVR is also associated with improved quality of life.
Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–42.
Article PubMed Google Scholar
Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–44.
Article CAS PubMed Google Scholar
Jacobson IM, Poordad F, Brown Jr RS, Kwo PY, Reddy KR, Schiff E. Standardization of terminology of virological response in the treatment of chronic hepatitis C: panel recommendations. J Viral Hepat. 2012;19:236–43.
Wedemeyer H, Jensen DM, Godofsky E, Mani N, Pawlotsky JM, Miller V, et al. Recommendations for standardized nomenclature and definitions of viral response in trials of hepatitis C virus investigational agents. Hepatology. 2012;56:2398–403.
Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;S0140–6736(14):60494–3.
Google Scholar
Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;S0140–6736(14):60538–9.
Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, DeMicco M, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naïve patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381:2100–7.
Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–87.
Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–98.
Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–93.
Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis. 2011;52:889–900.
Swain MG, Lai MY, Shiffman ML, Cooksley WG, Zeuzem S, Dieterich DT, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593–601.
Martinot-Peignoux M, Stern C, Maylin S, Ripault MP, Boyer N, Leclere L, et al. Twelve weeks posttreatment follow-up is as relevant as 24 weeks to determine the sustained virologic response in patients with hepatitis C virus receiving pegylated interferon and ribavirin. Hepatology. 2010;51:1122–6.
Campos-Varela I, Castells L, Esteban JI, Bes M, Rodríguez-Frías F, Sapisochin G, et al. Twelve-week posttreatment follow-up to predict sustained virologic response for recurrent hepatitis C infection in liver recipients. Transplantation. 2012;93:450–3.
Chen J, Florian J, Carter W, Fleischer RD, Hammerstrom TS, Jadhav PR, et al. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology. 2013;144:1450–5.
El Khoury AC, Wallace C, Klimack WK, Razavi H. Economic burden of hepatitis C-associated diseases: Europe, Asia Pacific, and the Americas. J Med Econ. 2012;15:887–96.
Mauss S, Petersen J, Witthoeft T, Busch HW, Christensen S, Zehnter E, et al. Sustained Responders have Lower Rates of Liver-Related Events and a Better Quality of Life and Productivity Compared with Non-Responders/Relapsers after Antiviral Treatment of Chronic Hepatitis C [abstract] Hepatology. 63rd Annual Meeting of the American Association for the Study of Liver Diseases: The Liver Meeting 2012 Boston, MA United States. 9–13 November 2012 pp 5
John-Baptiste AA, Tomlinson G, Hsu PC, Krajden M, Heathcote EJ, Laporte A, et al. Sustained responders have better quality of life and productivity compared with treatment failures long after antiviral therapy for hepatitis C. Am J Gastroenterol. 2009;104:2439–48.
Deuffic-Burban S, Mathurin P, Pol S, Larsen C, Roudot-Thoraval F, Desenclos JC, et al. Impact of hepatitis C triple therapy availability upon the number of patients to be treated and associated costs in France: a model-based analysis. Gut. 2012;61:290–6.
Van Der Meer AJP, Veldt BJ, Feld JJ, Wedemeyer H, Dufour J-F, Lammert F, et al. Improvement of interferon-based therapy substantially reduced the number needed to treat to prevent HCC among HCV genotype 1 infected cirrhotics [abstract]. Amsterdam, Netherlands: 48th Annual Meeting of the European Association for the Study of the Liver; 2013.
Wang C-H, Chang K-K, Lin R-C, Kuo J-J. Insights into hepatocellular carcinoma occurrence and long-term outcomes in patients with chronic hepatitis C infection after successful antiviral treatment [abstract]. Singapore: 23rd Conference of the Asian Pacific Association for the Study of the Liver; 2013.
Sasaki R, Abiru S, Yamasaki K, Komori A, Yatsuhashi H. Risk factors for hepatocellular carcinoma developed after sustained virological response in hepatitis C patients [abstract]. Brisbane, Australia: Abstract presented at the 23rd Conference of the Asian Pacific Association for the Study of the Liver; 2014.
Calvaruso V, Bavetta MG, Ferraro D, Grimaudo S, Conte E, Pipitone RM, et al. Risk of disease decompensation and HCC in patients with HCV cirrhosis non responders to PEG IFN plus RBV [abstract]. Bologna, Italy: 19th National Congress of Digestive Diseases, Italian Federation of Societies of Digestive Diseases; 2013.
Hara T, Fukushima T, Kawamura Y, Sezaki H, Hosaka T, Akuta N, et al. Sustained viral response reduces liver complications and total mortality among Japanese elderly with hepatitis C virus infection [abstract]. Brisbane, Australia: 23rd Conference of the Asian Pacific Association for the Study of the Liver; 2014.
Ogawa E, Furusyo N, Kajiwara E, Takahashi K, Nomura H, Maruyama T, et al. Efficacy of pegylated interferon alpha-2b and ribavirin treatment on the risk of hepatocellular carcinoma in patients with chronic hepatitis C: a prospective, multicenter study. J Hepatol. 2013;58:495–501.
Aleman S, Rahbin N, Weiland O, Davidsdottir L, Hedenstierna M, Rose N, et al. A risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosis. Clin Infect Dis. 2013;57:230–6.
Pellicelli AM, Vignally P, Romano M, Miglioresi L, Mazzoni E, Mecenate F, et al. Impact of liver fibrosis in development of hepatocellular carcinoma in genotype 1 chronic hepatitis C patients treated with antiviral therapy: Long term follow up study [abstract]. 48th Annual Meeting of the European Association for the Study of the Liver, International Liver Congress 2013 Amsterdam Netherlands. 24–28 April 2013
Imai Y, Tamura S, Tanaka H, Hiramatsu N, Kiso S, Doi Y, et al. Reduced risk of hepatocellular carcinoma after interferon therapy in aged patients with chronic hepatitis C is limited to sustained virological responders. J Viral Hepat. 2010;17:185–91.
Kobayashi S, Takeda T, Enomoto M, Tamori A, Kawada N, Habu D, et al. Development of hepatocellular carcinoma in patients with chronic hepatitis C who had a sustained virological response to interferon therapy: a multicenter, retrospective cohort study of 1124 patients. Liver Int. 2007;27:186–91.
Hung CH, Lee CM, Lu SN, Wang JH, Hu TH, Tung HD, et al. Long-term effect of interferon alpha-2b plus ribavirin therapy on incidence of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. J Viral Hepat. 2006;13:409–14.
Moriyama M, Matsumura H, Aoki H, Shimizu T, Yamagami H, Shioda A, et al. Decreased risk of hepatocellular carcinoma in patients with chronic hepatitis C whose serum alanine aminotransferase levels became less than twice the upper limit of normal following interferon therapy. Liver Int. 2005;25:85–90.
Watanabe S, Enomoto N, Koike K, Izumi N, Takikawa H, Hashimoto E, et al. Cancer preventive effect of pegylated interferon α-2b plus ribavirin in a real-life clinical setting in Japan: PERFECT interim analysis. Hepatol Res. 2011;41:955–64.
Wang CH, Mo LR, Chang KK, Lin RC, Kuo JJ. A cohort study to investigate hepatocellular carcinoma risk in hepatitis C patients. Hepatogastroenterology. 2011;58:904–8.
Sasaki M, Yoshida K, Yoshimatsu S, Setoyama H, Chiyonaga S, Narita R, et al. Hepatocarcinogenesis after SVR by interferon therapy in chronic hepatitis C patients [abstract]. Journal of Gastroenterology and Hepatology. Asian Pacific Digestive Week 2011 Singapore. 1–4 October 2011 pp 169
Ikeda K, Arase Y, Saitoh S, Kobayashi M, Someya T, Hosaka T, et al. Anticarcinogenic impact of interferon on patients with chronic hepatitis C: a large-scale long-term study in a single center. Intervirology. 2006;49:82–90.
Yoshida H, Tateishi R, Arakawa Y, Sata M, Fujiyama S, Nishiguchi S, et al. Benefit of interferon therapy in hepatocellular carcinoma prevention for individual patients with chronic hepatitis C. Gut. 2004;53:425–30.
Article CAS PubMed PubMed Central Google Scholar
Ogawa E, Furusyo N, Kajiwara E, Takahashi K, Nomura H, Tanabe Y, et al. Complete Hepatitis C virus elimination during pegylated interferon a2B and ribavirin treatment reduces the risk of progression to hepatocellular carcinoma [abstract]. Journal of Hepatology. 47th Annual Meeting of the European Association for the Study of the Liver, International Liver Congress 2012 Barcelona Spain. 18–22 April 2012
van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–93.
Velosa J, Serejo F, Marinho R, Nunes J, Glória H. Eradication of hepatitis C virus reduces the risk of hepatocellular carcinoma in patients with compensated cirrhosis. Dig Dis Sci. 2011;56:1853–61.
Maruoka D, Imazeki F, Arai M, Kanda T, Fujiwara K, Yokosuka O. Long-term cohort study of chronic hepatitis C according to interferon efficacy. J Gastroenterol Hepatol. 2012;27:291–9.
Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833–44.
Cardoso AC, Moucari R, Figueiredo-Mendes C, Ripault MP, Giuily N, Castelnau C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol. 2010;52:652–7.
Braks RE, Ganne-Carrie N, Fontaine H, Paries J, Grando-Lemaire V, Beaugrand M, et al. Effect of sustained virological response on long-term clinical outcome in 113 patients with compensated hepatitis C-related cirrhosis treated by interferon alpha and ribavirin. World J Gastroenterol. 2007;13:5648–53.
Article PubMed PubMed Central Google Scholar
Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnù L, Mazzella G, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–87.
Yu ML, Lin SM, Chuang WL, Dai CY, Wang JH, Lu SN, et al. A sustained virological response to interferon or interferon/ribavirin reduces hepatocellular carcinoma and improves survival in chronic hepatitis C: a nationwide, multicentre study in Taiwan. Antivir Ther. 2006;11:985–94.
CAS PubMed Google Scholar
Imazeki F, Yokosuka O, Fukai K, Kawai S, Kanda T, Kojima H, et al. Lower incidence of hepatic failure than hepatocellular carcinoma in Japanese patients with chronic hepatitis C. Liver Int. 2005;25:772–8.
Coverdale SA, Khan MH, Byth K, Lin R, Weltman M, George J, et al. Effects of interferon treatment response on liver complications of chronic hepatitis C: 9-year follow-up study. Am J Gastroenterol. 2004;99:636–44.
Shih K, Su W-W, Hsu Y-C, Yen H-H, Wu S-S, Soon M-S Interferon-based treatment reduced hepatocellular carcinoma development and liver related death incidence in chronic hepatitis C patients V Single institution experience [abstract]. Hepatology International. 22nd Conference of the Asian Pacific Association for the Study of the Liver, APASL 2012 Taipei Taiwan (Republic of China). 16–19 February 2012
Singal AK, Singh A, Jaganmohan S, Guturu P, Mummadi R, Kuo YF, et al. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol. 2010;8:192–9.
Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280–8.
Kimer N, Dahl EK, Gluud LL, Krag A. Antiviral therapy for prevention of hepatocellular carcinoma in chronic hepatitis C: systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2012;2(5):1–7.
Article Google Scholar
Ng V, Saab S. Effects of a sustained virologic response on outcomes of patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2011;9:923–30.
Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–37.
Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52.
Namiki I, Nishiguchi S, Hino K, Suzuki F, Kumada H, Itoh Y, et al. Management of hepatitis C; Report of the Consensus Meeting at the 45th Annual Meeting of the Japan Society of Hepatology (2009). Hepatol Res. 2010;40:347–68.
Innes HA, Hutchinson SJ, Allen S, Bhattacharyya D, Bramley P, Delahooke TE, et al. Excess liver-related morbidity of chronic hepatitis C patients, who achieve a sustained viral response, and are discharged from care. Hepatology. 2011;54:1547–58.
Kasahara A, Tanaka H, Okanoue T, Imai Y, Tsubouchi H, Yoshioka K, et al. Interferon treatment improves survival in chronic hepatitis C patients showing biochemical as well as virological responses by preventing liver-related death. J Viral Hepat. 2004;11:148–56.
Imazeki F, Yokosuka O, Fukai K, Saisho H. Favorable prognosis of chronic hepatitis C after interferon therapy by long-term cohort study. Hepatology. 2003;38:493–502.
Selzner N, Renner EL, Selzner M, Adeyi O, Kashfi A, Therapondos G, et al. Antiviral treatment of recurrent hepatitis C after liver transplantation: predictors of response and long-term outcome. Transplantation. 2009;88:1214–21.
Tanaka T, Selzner N, Therapondos G, Renner EL, Lilly LB. Virological response for recurrent hepatitis C improves long-term survival in liver transplant recipients. Transpl Int. 2013;26:42–9.
Van Der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour J-F, Lammert F, et al. Sustained virological response improves overall survival in chronic hepatitis C patients with advanced fibrosis [abstract]. Hepatology. 63rd Annual Meeting of the American Association for the Study of Liver Diseases: The Liver Meeting 2012 Boston, MA United States. 9–13 November 2012
Aguilera V, Garcia M, Rubin A, Navarro L, Prieto M, Berenguer M. Improved outcome after anti-hcv therapy is less marked when therapy is started at advanced stages of fibrosis [abstract]. Journal of Hepatology. 47th Annual Meeting of the European Association for the Study of the Liver, International Liver Congress 2012 Barcelona Spain. 18–22 April 2012
Kutala BK, Duval X, Guedj J, Asselah T, Marcellin P. Impact of antiviral therapy on survival in patients with advanced fibrosis - Experience of Beaujon Hospital 2000 to 2010 [abstract]. Amsterdam, Netherlands: 48th Annual Meeting of the European Association for the Study of the Liver; 2013.
Uenishi T, Nishiguchi S, Tanaka S, Yamamoto T, Takemura S, Kubo S. Response to interferon therapy affects risk factors for postoperative recurrence of hepatitis C virus-related hepatocellular carcinoma. J Surg Oncol. 2008;98:358–62.
Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–16.
Cozen ML, Ryan JC, Shen H, Lerrigo R, Yee RM, Sheen E, et al. Nonresponse to interferon-α based treatment for chronic hepatitis C infection is associated with increased hazard of cirrhosis. PLoS One. 2013;8:e61568.
Dieperink E, Pocha C, Thuras P, Knott A, Colton S, Ho SB. All-cause mortality and liver-related outcomes following successful antiviral treatment for chronic hepatitis C. Dig Dis Sci. 2014;59:872–80.
Singal AG, Dharia TD, Malet PF, Alqahtani S, Zhang S, Cuthbert JA. Long-term benefit of hepatitis C therapy in a safety net hospital system: a cross-sectional study with median 5-year follow-up. BMJ Open. 2013;3(9):e003231.
Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739–44.
Simó R, Lecube A, Genescà J, Esteban JI, Hernández C. Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care. 2006;29:2462–6.
Oni OA, Rao G, Pandya PK. Impact of sustained virologic response on incident diabetes in chronic hepatitis C [abstract] Hepatology. 62nd Annual Meeting of the American Association for the Study of Liver Diseases: The Liver Meeting 2011 San Francisco, CA United States. 4–8 November 2011
Hyder SM, Krishnan S, Promrat K. Sustained virological response prevents the development of new type 2 diabetes in patients with chronic hepatitis C [abstract]. Gastroenterology. 2013;144(5 Suppl 1):S951. Conference: Digestive Disease Week 2013, DDW 2013 Orlando, FL United States. 18–21 May 2013.
Abergel A, Darcha C, Chevallier M, Ughetto S, Henquell C, Pol S, et al. Histological response in patients treated by interferon plus ribavirin for hepatitis C virus-related severe fibrosis. Eur J Gastroenterol Hepatol. 2004;16:1219–27.
Poynard T, Moussalli J, Munteanu M, Thabut D, Lebray P, Rudler M, et al. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J Hepatol. 2013;59:675–83.
Roche B, Sebagh M, Canfora ML, Antonini T, Roque-Afonso AM, Delvart V, et al. Hepatitis C virus therapy in liver transplant recipients: response predictors, effect on fibrosis progression, and importance of the initial stage of fibrosis. Liver Transpl. 2008;14:1766–77.
Wiese M, Fischer J, Löbermann M, Göbel U, Grüngreiff K, Güthoff W, et al. Evaluation of liver disease progression in the German hepatitis C virus (1b)-contaminated anti-D cohort at 35 years after infection. Hepatology. 2014;59:49–57.
Annicchiarico BE, Siciliano M, Santonocito C, Zocco MA, Avolio AW, Barbaro F. Long-term outcome of hepatitis C-related liver cirrhosis at different stages of portal hypertension after sustained virological response [abstract] Hepatology. 63rd Annual Meeting of the American Association for the Study of Liver Diseases: The Liver Meeting 2012 Boston, MA United States. 9–13 November 2012
Bruno S, Crosignani A, Facciotto C, Rossi S, Roffi L, Redaelli A, et al. Sustained virologic response prevents the development of esophageal varices in compensated, Child-Pugh class A hepatitis C virus-induced cirrhosis. A 12-year prospective follow-up study. Hepatology. 2010;51:2069–76.
D’Ambrosio R, Aghemo A, Rumi MG, Primignani M, Dell’Era A, Lampertico P, et al. The course of esophageal varices in patients with hepatitis C cirrhosis responding to interferon/ribavirin therapy. Antivir Ther. 2011;16:677–84.
Lee SJ, Yeon JE, Lee HJ, Yoon EL, Suh SJ, Kim JH, et al. Risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis C; long term outcome and prognostic factors [abstract]. Singapore: 23rd conference of the Asian Pacific Association for the Study of the Liver; 2013.
Canete N, Garcia M, Ojanguren I, Cirera I, Garcia-Retortillo M, Carrion JA, et al. Long-term evolution of liver fibrosis in mild-moderate chronic hepatitis C: Study with paired biopsies [abstract]. Journal of Hepatology. 48th Annual Meeting of the European Association for the Study of the Liver, International Liver Congress 2013 Amsterdam Netherlands. 18–24 April 2013
Basseri B, Yamini D, Chee G, Enayati PD, Tran T, Poordad F. Comorbidities associated with the increasing burden of hepatitis C infection. Liver Int. 2010;30:1012–8.
McAdam-Marx C, McGarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: a managed care perspective. J Manag Care Pharm. 2011;17:531–46.
PubMed Google Scholar
Vietri J, Prajapati G, El Khoury AC. The burden of hepatitis C in Europe from the patients’ perspective: a survey in 5 countries. BMC Gastroenterol. 2013;13:16.
Backx M, Lewszuk A, White JR, Cole J, Sreedharan A, van Sanden S, et al. The cost of treatment failure: resource use and costs incurred by hepatitis C virus genotype 1-infected patients who do or do not achieve sustained virological response to therapy. J Viral Hepat. 2014;21:208–15.
Morais AD, Pereira ML. Cost per cure of telaprevir and boceprevir in treatment-naive genotype 1 hepatitis c patients with F2 fibrosis in Brazil [abstract]. Dublin, Ireland: ISPOR 16th Annual European Congress; 2013.
Cammà C, Petta S, Enea M, Bruno R, Bronte F, Capursi V, et al. Cost-effectiveness of boceprevir or telaprevir for untreated patients with genotype 1 chronic hepatitis C. Hepatology. 2012;56:850–60.
Yfantopoulos J, Paparouni K, D’Angelo ER. A cost-effectiveness analysis of telaprevir versus boceprevir in the treatment of hepatitis C: A greek national health system perspective [abstract]. Berlin Germany: ISPOR 15th Annual European Congress; 2012. p. A394.
Manos MM, Darbinian J, Rubin J, Ray GT, Shvachko V, Denis B, et al. The effect of hepatitis C treatment response on medical costs: a longitudinal analysis in an integrated care setting. J Manag Care Pharm. 2013;19:438–47.
Bini EJ, Mehandru S. Sustained virological response rates and health-related quality of life after interferon and ribavirin therapy in patients with chronic hepatitis C virus infection and persistently normal alanine aminotransferase levels. Aliment Pharmacol Ther. 2006;23:777–85.
Arora S, O’Brien C, Zeuzem S, Shiffman ML, Diago M, Tran A, et al. Treatment of chronic hepatitis C patients with persistently normal alanine aminotransferase levels with the combination of peginterferon alpha-2a (40 kDa) plus ribavirin: impact on health-related quality of life. J Gastroenterol Hepatol. 2006;21:406–12.
Spiegel BM, Younossi ZM, Hays RD, Revicki D, Robbins S, Kanwal F. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology. 2005;41:790–800.
Thein HH, Krahn M, Kaldor JM, Dore GJ. Estimation of utilities for chronic hepatitis C from SF-36 scores. Am J Gastroenterol. 2005;100:643–51.
Hassanein T, Cooksley G, Sulkowski M, Smith C, Marinos G, Lai MY, et al. The impact of peginterferon alfa-2a plus ribavirin combination therapy on health-related quality of life in chronic hepatitis C. J Hepatol. 2004;40:675–81.
Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98:630–8.
Bonkovsky HL, Snow KK, Malet PF, Back-Madruga C, Fontana RJ, Sterling RK, et al. Health-related quality of life in patients with chronic hepatitis C and advanced fibrosis. J Hepatol. 2007;46:420–31.
Mathew A, Peiffer LP, Rhoades K, McGarrity TJ. Improvement in quality of life measures in patients with refractory hepatitis C, responding to re-treatment with Pegylated interferon alpha -2b and ribavirin. Health Qual Life Outcomes. 2006;4:30.
Hollander A, Foster GR, Weiland O. Health-related quality of life before, during and after combination therapy with interferon and ribavirin in unselected Swedish patients with chronic hepatitis C. Scand J Gastroenterol. 2006;41:577–85.
Van Rooijen EM, Hotho D, Agthoven M, Van Der Kolk A, Hansen BE, Knegt R, Uyl-De Groot CA. The cost and quality of life of hepatitis C in the Netherlands [abstract]. Value in Health. ISPOR 14th Annual European Congress Madrid Spain. 5–8 November 2011. pp A394
Papastergiou V, Skorda L, Lisgos P, Hletsos M, Ketikoglou I, Zamanis C, Karatapanis S. Health-related quality of life in patients with chronic hepatitis C. The impact of antiviral therapy. European Journal of Internal Medicine. 10th Congress of the European Federation of Internal Medicine Athens Greece. 5–8 November 2011 pp S46
Björnsson E, Verbaan H, Oksanen A, Frydén A, Johansson J, Friberg S, et al. Health-related quality of life in patients with different stages of liver disease induced by hepatitis C. Scand J Gastroenterol. 2009;44:878–87.
Younossi Z, Aggarwal J, Martin M, Hernandez N, Donepudi M, Bayliss M, et al. Health-related quality-of-life among genotype 1 treatment-naive chronic Hepatitis C patients receiving telaprevir combination treatment: Post-hoc analyses of data from the advance trial [abstract]. Journal of Hepatology. 47th Annual Meeting of the European Association for the Study of the Liver, International Liver Congress 2012 Barcelona Spain. 18–22 April 2012
Hsu PC, Federico CA, Krajden M, Yoshida EM, Bremner KE, Anderson FH, et al. Health utilities and psychometric quality of life in patients with early- and late-stage hepatitis C virus infection. J Gastroenterol Hepatol. 2012;27:149–57.
Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156:279–90.
Chhatwal J, Ferrante SA, Brass C, El Khoury AC, Burroughs M, Bacon B, et al. Cost-effectiveness of boceprevir in patients previously treated for chronic hepatitis C genotype 1 infection in the United States. Value Health. 2013;16:973–86.
Veldt BJ, Saracco G, Boyer N, Cammà C, Bellobuono A, Hopf U, et al. Long term clinical outcome of chronic hepatitis C patients with sustained virological response to interferon monotherapy. Gut. 2004;53:1504–8.
Almasio PL, Venezia G, Craxì A. The impact of antiviral therapy on the course of chronic HCV infection. A systematic review. Panminerva Med. 2003;45:175–82.
Raimondi S, Bruno S, Mondelli MU, Maisonneuve P. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: a meta-analysis. J Hepatol. 2009;50:1142–54.
Tanaka Y, Hanada K, Orito E, Akahane Y, Chayama K, Yoshizawa H, et al. Molecular evolutionary analyses implicate injection treatment for schistosomiasis in the initial hepatitis C epidemics in Japan. J Hepatol. 2005;42:47–53.
Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133(8):592–9.
Romero-Gómez M, Del Mar VM, Andrade RJ, Salmerón J, Diago M, Fernández-Rodríguez CM, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–41.
Shiratori Y, Ito Y, Yokosuka O, Imazeki F, Nakata R, Tanaka N, et al. Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann Intern Med. 2005;142:105–14.
Download references
Grant support
This study was supported by funding from Janssen Pharmaceutica NV.
Author information
Authors and affiliations.
Ossian Health Economics and Communications, Ossian Health Economics and Communications GmbH, Bäumleingasse 20, 4051, Basel, Switzerland
Jayne Smith-Palmer & William Valentine
Janssen Pharmaceutica NV, Beerse, Belgium
Karin Cerri
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jayne Smith-Palmer .
Additional information
Competing interests.
This manuscript was funded by Janssen Pharmaceutica NV .JSP and WV are current employees of Ossian Health Economics and Communications, which has received consulting fees from Janssen Pharmaceutica NV. KC is a current employee of Janssen Pharmaceutica NV.
Authors’ contributions
JSP performed data acquisition and analysis and prepared first and subsequent drafts of the manuscript. KC was involved in the conception and design of the review, contributed to first and subsequent drafts of the manuscript and was involved in the critical review of all drafts of the manuscript. WV contributed to data acquisition and analysis and was involved in the critical review of all drafts of the manuscript. All authors read and approved the final manuscript.
Additional file
Additional file 1:.
PRISMA Flow Diagram: Achieving sustained virologic response in hepatitis C: a review of the clinical, economic and quality of life benefits.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/4.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Smith-Palmer, J., Cerri, K. & Valentine, W. Achieving sustained virologic response in hepatitis C: a systematic review of the clinical, economic and quality of life benefits. BMC Infect Dis 15 , 19 (2015). https://doi.org/10.1186/s12879-015-0748-8
Download citation
Received : 19 August 2014
Accepted : 07 January 2015
Published : 17 January 2015
DOI : https://doi.org/10.1186/s12879-015-0748-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sustained virologic response
- Chronic hepatitis C, Cost, Quality of life, Utility, Morbidity, Mortality
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
- Research article
- Open access
- Published: 18 August 2012
The ongoing impacts of hepatitis c - a systematic narrative review of the literature
- Emma R Miller 1 ,
- Stephen McNally 2 ,
- Jack Wallace 2 &
- Marisa Schlichthorst 3
BMC Public Health volume 12 , Article number: 672 ( 2012 ) Cite this article
11k Accesses
34 Citations
11 Altmetric
Metrics details
Many countries have developed, or are developing, national strategies aimed at reducing the harms associated with hepatitis C infection. Making these strategies relevant to the vast majority of those affected by hepatitis C requires a more complete understanding of the short and longer term impacts of infection. We used a systematic approach to scope the literature to determine what is currently known about the health and psychosocial impacts of hepatitis C along the trajectory from exposure to ongoing chronic infection, and to identify what knowledge gaps remain.
PubMed, Current Contents and PsychINFO databases were searched for primary studies published in the ten years from 2000–2009 inclusive. Two searches were conducted for studies on hepatitis C in adult persons focusing on: outcomes over time (primarily cohort and other prospective designs); and the personal and psychosocial impacts of chronic infection. All retrieved studies were assessed for eligibility according to specific inclusion/exclusion criteria, data completeness and methodological coherence. Outcomes reported in 264 included studies were summarized, tabulated and synthesized.
Injecting drug use (IDU) was a major risk for transmission with seroconversion occurring relatively early in injecting careers. Persistent hepatitis C viraemia, increasing age and excessive alcohol consumption independently predicted disease progression. While interferon based therapies reduced quality of life during treatment, improvements on baseline quality of life was achieved post treatment – particularly when sustained viral response was achieved. Much of the negative social impact of chronic infection was due to the association of infection with IDU and inflated assessments of transmission risks. Perceived discrimination was commonly reported in health care settings, potentially impeding health care access. Perceptions of stigma and experiences of discrimination also had direct negative impacts on wellbeing and social functioning.
Conclusions
Hepatitis C and its management continue to have profound and ongoing impacts on health and social well being. Biomedical studies provided prospective information on clinical aspects of infection, while the broader social and psychological studies presented comprehensive information on seminal experiences (such as diagnosis and disclosure). Increasing the focus on combined methodological approaches could enhance understanding about the health and social impacts of hepatitis C along the life course.
Peer Review reports
Hepatitis C infection is now acknowledged as an issue of major public health importance for most countries in the world [ 1 ]. In Australia, hepatitis C is one of the most commonly notified communicable diseases with an estimated prevalence approaching 1.5% [ 2 ]. Nationally, there have been over 230,000 notifications since 1995 (when mandatory notification was established in all jurisdictions) and the annual number of new notifications has averaged around 12,000 for the past five years [ 3 ]. Recent estimates put the global hepatitis C prevalence at around 2.4%, with up to 170 million people now thought to be chronically infected [ 4 ]. Between 70% and 85% of those initially infected fail to clear the virus [ 5 – 7 ]. Hepatitis C treatments are available, but uptake remains low even in countries such as Australia, where treatment is available at low cost to eligible patients [ 8 , 9 ]. Thus, most people with hepatitis C infection face the prospect of lifelong chronic infection.
The personal impacts of a diagnosis of hepatitis C infection are known to be significant. The direct effects of the virus and its management on wellbeing can lead to people making significant lifestyle changes including reducing work hours or alcohol consumption [ 10 , 11 ], which may in turn influence economic status and social participation. There can be negative social implications for people living within the context of a broader community who may be largely uneducated about hepatitis C transmission. In most cultures around the world there is prevailing marginalization of people who inject drugs – the major risk factor for infection [ 12 – 14 ]. Disclosure of hepatitis C status can result in alienation from family and friends as well as perceived and actual discrimination in health services and workplaces [ 12 ]. As with other chronic diseases, experiences of diagnosis and management are shaped by a multitude of physical and psychosocial forces. Such forces influence the dynamics of adaptation to illness and impact on well being, often without direct linearity, over time [ 15 ]. Given the size of the affected population, having an understanding of these forces in relation to adapting to, and living with, hepatitis C is critical for effective health policy planning.
Many countries have developed, or are currently developing, national strategies aimed at reducing the harms associated with hepatitis C. For example, the soon to be released National Liver Strategy represents the second strategy for the United Kingdom, while the Third National Hepatitis C Strategy (covering the period 2010 to 2013) has now been implemented in Australia. Making these strategies relevant to the vast majority of those affected by hepatitis C, particularly those for whom treatment does not appear to be either useful or desirable, would seem to require a greater understanding of the ongoing impacts of hepatitis C diagnoses. Using a systematic approach, we undertook a scoping exercise of the biomedical and social literature published in the ten years from 2000 on the ongoing health and social consequences of diagnoses with hepatitis C infection. We searched for studies on hepatitis C in adult persons in which the health or social outcomes of infection were investigated (including studies of hepatitis C transmission). We specifically searched for cohort studies (and other longitudinal designs) as well as studies using qualitative methodologies. The objective of this study was to determine what is currently known about the health and social impacts of hepatitis C along the trajectory from exposure to ongoing chronic infection, and to identify what knowledge gaps remain.
We developed an inclusive search strategy aimed at including studies using both qualitative and quantitative approaches. We searched the health literature in the PubMed (incorporating Medline), Current Contents and PsychINFO data bases for the years 2000 to 2009 inclusive, using two different strategies. The first search used the terms “(hepatitis C OR HCV) AND (cohort study OR follow up study OR longitudinal study OR prospective study OR retrospective study OR concurrent study)”; and the second search used the terms “(hepatitis C OR HCV) AND (quality of life OR social impact OR socioeconomic impact OR psychological well being).” We limited the search to primary studies in adults and excluded investigations that focused solely on evaluating the performance of diagnostic tools and experimental interventions without providing retrievable data on patient health outcome beyond biochemical or serological endpoints (Table 1 ).
To enhance the relevance of reviewed studies to the current context of hepatitis C and its management, we excluded studies published before January 2000, limited to the end of the last full calendar year at the time of the review – December 2009. Resource and time restrictions limited the search to English language journal publications. Studies were excluded if specific data on hepatitis C outcomes were not retrievable (for example, in a study of liver disease in general). We also excluded studies that solely focused on quantifying hepatitis C treatment side effects, unless the personal impact of the symptoms was also investigated – for instance, their effect on quality of life, social function or reported health status. The specific inclusion and exclusion criteria were as follows:
Inclusion criteria
Primary studies
Adult participants
Hepatitis C specific data provided
Publication between January 1, 2000 and December 31, 2009
English language
Exclusion criteria
Data not provided on participant health outcome (beyond serologic endpoints)
Relevant outcome data not retrievable
Non-English language
Previously published analyses of the same data
All identified abstracts were scanned, before relevant articles were retrieved and reviewed. When the same cohort was described in successive publications, only the latest publication was reviewed except where subsequent analyses focused on different outcomes. The reference lists of reviewed articles were also scrutinized for any additional items relevant to the review. Records of all articles retrieved were stored and managed in EndNote (version X3.0.1).
As this review aimed to determine if it was possible to build a picture of the trajectory of a hepatitis C diagnosis by summarizing the evidence from a broad range of eligible studies (using both quantitative and qualitative approaches), the quality assessment was restricted to the completeness of information provided by the authors. Studies were required to provide sufficient data to characterize the participants (e.g. by age, sex, and population group), the follow up period (if relevant to the design), the methodological approach (including any instruments utilized), and outcomes (including appropriate measures of effect such as Relative Risk and Hazard Ratios, in the case of quantitative designs). Inclusion and exclusion criteria were developed by consensus of the authors as were the categories assigned to the reviewed studies. Eligible studies were then summarized and reviewed by the research team, before coding into the specified categories for narrative synthesis. See Additional file 1 for the full list of included articles, as well as their tabulated summaries.
We identified 140 cohort (or follow up) studies with our first strategy (see Figure 1 ) and 133 studies with our second strategy. With the exception of eight studies (all of which investigated health related quality of life), there was little duplication between the results obtained using the two sets of search terms.

Flow chart of included and excluded studies identified in search strategy 1.
Temporal experience of hepatitis C
The cohort studies provided comprehensive information on the clinical and epidemiological impacts of hepatitis C over time and covered the broad categories of: transmission; natural history; health related quality of life during the course of antiviral treatment; and health outcomes after antiviral treatment or liver transplant (see Table 2 ). With the exception of eight studies in people undergoing antiviral treatment, no cohort studies investigated the personal or social experiences, of living with hepatitis C.
Transmission of Hepatitis C
We identified 42 cohort studies investigating the transmission of hepatitis C in various community and hospital based populations. Eighteen of these followed up community-based injecting drug users (IDU) and two studies also included non-injecting participants (see Additional file 1 : Table S1). In thirteen, the IDU cohort was seronegative for hepatitis C at baseline, with rates of new infection ranging from 8.1 to 45.8 per 100 person-years. While all reported incidence rates were significantly higher than in non-IDU, rates appeared to be unrelated to the period of follow up. This may be due to infection of susceptible populations relatively early in their injecting careers. Roy et al. [ 16 ] noted that around 50% of a cohort of IDU in Canada had seroconvert within the first four years of injecting, and Maher et al. [ 17 ] found that being within the first year of injecting independently predicted hepatitis C seroconversion after adjusting for a range of demographic factors and risk behaviours. Re-infection rates among IDU previously achieving viral clearance tended to be lower than new infection rates – suggesting a role for host related factors.
Twenty four studies investigated incidence rates in the general population and its subpopulations (see Additional file 1 : Table S2). Studies undertaken in general population samples in Chile, Italy and Japan indicate tha the incidence of hepatitis C general populations overall was low compared to IDU populations – ranging from 0.01 to 0.4 per 100 person years in four identified studies. A higher general population rate was reported by Grebely et al. [ 18 ] – 7.4 per 100 person years – who sampled an inner city population that included a high proportion of IDU. Reported rates were higher in renal patients undergoing long term hemodialysis in some countries, although reductions were observed in Italy and Germany in line with improving infection control practices.
There is evidence that perinatal transmission is associated with maternal hepatitis C viraemia, and is significantly more frequent in maternal HIV co-infection. While up to 31 percent of babies born to mothers with hepatitis C were reported to be seropositive shortly after birth, Sbebl et al. [ 19 ] found that only 2.4% remain so by three years of age. Hepatitis C transmission in people already infected with HIV, and in HIV negative men who have sex with men, was relatively low – and occurred mainly through injecting drug use. Four studies in the UK and Australia demonstrated an elevated transmission rate in prisoner populations – particularly in prisoners reporting injection drug use whilst incarcerated. Dolan et al. [ 20 ] estimated a rate of 21.3 per 100 person years in imprisoned heroin users, while a rate of 4.6 per 100 person years was observed in the South Australian prison population [ 21 ] – although community exposure couldn’t be ruled out in the latter study.
Natural history of chronic hepatitis C infection
We identified 69 cohort studies investigating the natural history of chronic hepatitis C infection (see Additional file 1 : Table S3). Of these, 42 studies focused on health outcomes for people mono-infected with hepatitis C, while the remainder (27 studies) compared outcomes in hepatitis C, hepatitis B or HIV mono- and co-infection. Mortality, disease progression and development of hepatocellular carcinoma (HCC) were outcomes investigated by the majority of all studies, and a small number focused on viral clearance. Two studies investigated behavioral outcomes – one looking at clinical factors associated with treatment uptake and the other looking at general practitioner (GP) patterns of management and referral to specialists in hepatitis C.
The studies in people with untreated chronic hepatitis C monoinfection had follow up periods ranging from one to 35 years in samples of clinical groups as small as 17 to sample sizes approaching half a million from large clinical data bases. The evidence for fibrotic changes and development of liver cirrhosis, development of HCC and increased liver-specific mortality in chronic hepatitis C over time was relatively consistent. Several studies found that the more serious complications of chronic infection were predicted by the presence of persistent viraemia, moderate to high alcohol consumption and increasing age. Age was not as important in a study in elderly Italians with chronic hepatitis C, which found deaths to non-liver related causes occurred more frequently than liver-specific deaths over ten years of follow up. Studies investigating health outcomes for people with persistently normal alanine amino transferase (ALT) levels suggest that liver cirrhosis and HCC do occur, but the frequency and rate of disease progression tends to be low relative to those with consistently high ALT levels. Despite the apparent importance of this indicator, Yawn et al. [ 22 ] found that less than half of primary care physicians monitored ALT in their patients with hepatitis C. While unlikely to have direct impact on the experience of living with hepatitis C, a lack of ongoing ALT monitoring might eventually have implications for health outcome in their patients. Yawn et al. also found there was only limited and inconsistent management and referral with respect to potential accelerators of progression such as excessive alcohol consumption and viral co-infections.
Twenty seven studies compared the longer term effects of chronic mono-infection with hepatitis C and hepatitis B or HIV, and/or the impact of co-infection with any of these. Fifteen studies investigated the independent or combined effects of hepatitis B and C for between 3.4 and 23 years of follow up. While studies varied in the order of risk for hepatitis B or C monoinfection, co-infection was relatively consistently associated with greater incidence of HCC and lower survival than mono-infection. In their 23 year follow up study, Zampino et al. [ 23 ] found that earlier age at infection was associated with a lower disease progression in both mono- and co-infected patients.
Eleven studies were in cohorts infected with either HIV or hepatitis C or both. Most of these compared the independent effects of infection with each virus – with outcomes such as AIDS, liver cirrhosis, and overall and specific mortality. An exception was Grebely et al. [ 24 ] who investigated hepatitis C viral clearance (rather than disease outcome) in a community sample in Canada, and found that co-infection with HIV and continuing injecting drug use were associated with hepatitis C viral persistence. In other studies, HIV-hepatitis C co-infection was found to have a synergistic effect in which the progression to AIDS and end stage liver disease and liver-related death (mostly due to HCC) was accelerated. Improvements in survival were calculated for HIV patients in hepatitis C-related end stage liver disease under active anti-retroviral treatment (HAART), although this finding was not supported by other studies in this group (see García-Garcia et al. [ 25 ] for example). In their study of antiviral treatment initiation for hepatitis C in Denmark, Hansen et al. [ 26 ] found that 33% of patients commenced treatment within five years and this was predicted by higher ALT, HCV genotype 2 or 3 (the most favourable for successful treatment outcome) and HIV negative status.
We identified two cohort studies investigating the combined and/or independent effects of infection with hepatitis B, hepatitis C and HIV. Bonacini et al. [ 27 ] found liver-related mortality in HIV patients was increased regardless of the hepatitide with which they were co-infected, and was more common when both were present. In a cohort of haemophilia patients with HIV infection, Melendez-Morales et al. [ 28 ] found an 11-fold increase in hepatitis C viral clearance in the presence of hepatitis B. The authors suggest that this may be due to ‘mutual interference in viral replications’ – as is thought to occur in the presence of hepatitis D.
Health related quality of life during the course of antiviral treatment
Eight cohort studies investigated the impact of hepatitis C treatment on health related quality of life (QoL) (see Additional file 1 : Table S4). Six of these studies used the 36-item Medical Outcomes Study Short-Form 36 (SF-36) or the 12 item version (SF-12), in wide use around the world to measure health status across eight domains relevant to physical, mental and emotional health. The SF-36 and various measures to establish depression levels and other social and emotional dimensions, uniformly demonstrated reduced health related quality of life during treatment but improving post treatment – particularly when sustained viral responses were achieved. Evon et al. [ 29 ] found the prevalence of depression was relatively high in patients prior to treatment (12%) and this predicted early exit from treatment. Depression newly diagnosed during treatment had a lesser impact on early exit.
Health outcomes after antiviral treatment or liver transplant
Twenty eight studies investigated health outcomes following interferon based treatments (generally in combination with Ribavirin) or after liver transplant (see Additional file 1 : Table S5). Fifteen studies were in patients with no viral co-infection who were followed up from six months to 14 years after treatment. In most populations, sustained viral responses were attained in between 20% and up to 80% depending on the viral genotype (with genotypes other than 1 and 4 considered the most favourable), and sustained viral responses were associated with a lower incidence of complications such as HCC and lower mortality. Dalgard et al. [ 30 ] demonstrated that the comparable outcomes were achievable in people who inject drugs, despite ongoing concerns about compliance with treatment schedules and ongoing drug use.
Four studies investigated treatment-related outcomes in hepatitis C patients co-infected with either hepatitis B or HIV. Ikeda et al. [ 31 ] found that any viral response to hepatitis C treatment (transient or sustained) was protective for the development of HCC, but not in the case of co-infection with hepatitis B. Co-infection with HIV appeared to be a barrier to evaluation for, and uptake of, hepatitis C treatment although sustained response rates (per genotype) have been reported to be similar to those seen in hepatitis C mono-infection. Furthermore, hepatitis C disease progression may be slowed with treatment even when sustained response is not achieved.
We identified nine cohort studies investigating health outcomes after liver transplantation. Four of these studies compared health outcomes in organ recipients with and without markers for hepatitis C. In all cases, hepatitis C was associated with lower survival and reduced overall health and function. In their seven-year retrospective study of patients following hepatitis C related liver transplants, Gallegos-Orozco et al. [ 32 ] estimated a median survival time of 3.5 years. According to Bizollon et al., [ 33 ] sustained response to hepatitis C treatments provided post-transplantation is associated with improved graft survival although not necessarily with improved survival.
The personal and psychosocial impacts of hepatitis C
Our second strategy identified 133 studies investigating the personal experience and social impacts of hepatitis C – in terms of individual wellbeing and psychosocial function (see Figure 2 ). The studies could be grouped into four broad categories: two categories focusing on either the QoL impacts of ongoing infection or its treatment; one category exploring the psychosocial experience of living with hepatitis C; and a final category focusing on experiences of diagnosis and management of hepatitis C (see Table 3 ). Most of the QoL studies used a quantitative approach, while qualitative approaches were more commonly taken in the broader social research. Very few studies were longitudinal in design, with the majority focusing on analyzing data collected at a single point in time.
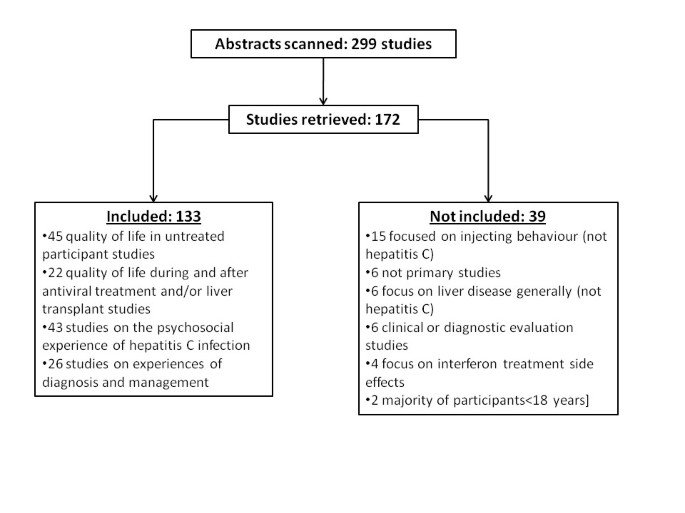
Flow chart of included and excluded studies identified in search strategy 2.
Quality of life in untreated hepatitis C
Sixty six studies investigated quality of life (QoL), including eight cohort studies in people undergoing treatment also identified in the biomedical literature. Forty five of these studies measured QoL impacts in untreated chronic infection in community-based and clinic populations, former or current IDU, people with viral co-infections, patients with various stages of disease progression, and patients with co morbidities (see Additional file 1 : Table S6). Again, the SF-36 (or SF-12) was the main instrument used to assess health related QoL, and several studies used other validated instruments measuring mental health and psychosocial status. The studies provide relatively consistent evidence of reduced QoL in untreated hepatitis C in most populations. The exception was provided by Bailey et al., [ 34 ] who observed moderate to high levels of QoL in US hepatitis patients, upon whom the main negative impact was uncertainty surrounding prognosis. In Australia, Thein et al. [ 35 ] found little difference in QoL between HIV and hepatic C mono-infected, co-infected and un-infected patients – although the authors pointed out the numbers in each group may have been too small to demonstrate clear differences.
There was substantial variation among studies about the relative impact of a range of co-factors on QoL. Several studies concluded reduced QoL in hepatitis C was independent of disease activity, liver histology and drug and alcohol use. Gunasekera et al., [ 36 ] however, found that QoL was reduced in hepatitis C patients recruited from rural drug and alcohol services when compared to rural primary care or hepatitis C centres. Continued drug use was associated with reduced QoL in two other studies, but not in others analyzing the role of substance use. Two studies led by Kramer [ 37 , 38 ] both found reduced QoL in hepatitis C which was associated with fatigue, but not with observed slight neuro-cognitive impairment. Fatigue and depression accounted for the majority of reduced QoL in a study of HIV-hepatitis C coinfected patients in France [ 39 ].
Several studies proposed an emotional or psychosocial basis for reduced QoL. Dalgard et al. [ 40 ] and Shwarzinger et al. [ 41 ], for example, found that awareness of hepatitis status explained much of the reduction in QoL. Depression and psychiatric co morbidities were found to be important in some studies, although others found that the co-existence of mental illness failed to fully explain the QoL differentials in those with and without hepatitis C. While several studies found little association between QoL and ALT or liver fibrosis in clinic populations, advanced liver disease did appear to be closely correlated with reduced QoL in hepatitis C.
Quality of life related to hepatitis C treatment
Twenty two studies investigated health related QoL in patients undergoing antiviral treatment or liver transplantation, with the majority using the SF-36 (see Additional file 1 : Table S7). The studies were mainly cross-sectional, with seven utilizing a follow up design (as previously described). Most of the eleven studies in patients undergoing interferon-based antiviral treatment noted further reductions in health related QoL during the treatment, with depression playing a significant role. Improvements were noted post treatment in most studies, particularly where sustained virological clearance was achieved. Fontana et al. [ 42 ] compared QoL in patients who were accessing treatment and those who were not. They found that emotional distress, which was strongly associated with reduced QoL, was highest in the untreated group – particularly in those who were anticipating a fatal outcome to their chronic hepatitis C infection. One study found no difference in QoL between treated and untreated groups but did find that concurrent treatment had a negative impact on cognitive abilities [ 43 ]. Studies of patients post treatment suggest that the benefits of successful treatment can be sustained over time, as can the deleterious effects of less successful treatment. In their study of untreated patients, patients who had relapsed after treatment and patients who did not respond to treatment, Taliani et al. [ 44 ] found that non-responders had the lowest QoL. Low quality of life scores were independently associated with co morbidity and non-response to treatment. They suggest that treatment expectations may be important modifiers of QoL.
Three studies investigated QoL following hepatitis C related liver transplantation. Two quantitative studies found significant impairments in QoL in recurrent hepatitis C (occurring in 36% to 62% of the study populations) in the years following transplants, while non-recurrence was associated with improved functional performance and quality of life over time. In a qualitative study involving in-depth interviews with eight post transplant patients, Dudley et al. [ 45 ] concluded that stigma and disease uncertainty in hepatitis C continues after liver transplants and may further impact adversely on QoL in hepatitis C patients.
Psychosocial experience of living with hepatitis C infection
We identified 43 studies investigating the impact of hepatitis C on social functioning, lifestyle, health and well-being (see Additional file 1 : Table S8). In a majority of studies, diagnosis with hepatitis C was reported to have profound impacts on social functioning. The perceived stigma associated with infection led to high levels of anxiety, and exaggerated fears of transmission and was the main driver of social isolation and reduced intimacy in relationships [ 46 – 48 ]. Grundy and Beeching [ 46 ] found that fear of transmission – particularly in intimate relationships, child birth and child rearing – led to concerns about being able to fulfil gender roles in a group of women with hepatitis C. There is further evidence that the social experience of living with hepatitis C differs between women and men. For instance, Temple-Smith et al. [ 49 ] found substantial gender differences in health seeking behaviours and notions of social support.
Fear of transmission, where the perceived risk is often unnecessarily inflated, also appeared to result in unnecessary changes in everyday practices, such as refraining from sharing towels and drinking glasses or not taking part in food preparation [ 50 ]. Changes in intimate and/or sexual practices, sometimes exhibiting as sexual dysfunction, have been observed in association with depression and anxiety about transmission [ 51 ]. Other lifestyle impacts of hepatitis C include reductions in alcohol consumption, reductions in smoking and modification of dietary intake [ 52 ]. In the context of competing priorities including housing, employment and legal implications of injecting, hepatitis C was viewed to be of less consequence by some study participants who injected drugs, but modification of risk behaviours (such as cleaning of syringes and spoons, and less sharing of injecting paraphernalia) was reported [ 53 , 54 ].
Infection with hepatitis C has been found by a number of studies to have substantial impacts on health and well-being, often in relation to fear and uncertainty about prognosis [ 55 ]. One study found that feelings of hopelessness in conjunction with uncertainty were experienced by people with either hepatitis C or HIV, but tended to be heightened in hepatitis C [ 56 ]. Perceived stigma and discrimination impeded adaptation to the hepatitis diagnosis and was a common source of anxiety in people with chronic hepatitis C [ 56 , 57 ]. Hopwood and Treloar [ 58 ] found that the psychosocial stress associated with hepatitis C was less in those with a history of IDU, which may arise from greater resilience in coping with hepatitis C stemming from experiences of marginalization for people who inject drugs. A number of studies investigated specific symptoms of hepatitis C infection – with fatigue being the most common, followed by depression and other mental health issues. Bodily pain, particularly in the form of myalgia and a degree of cognitive impairment was also identified by several authors. The majority of studies found symptoms were independent of disease activity or disease severity, but associated with depression, anxiety and other psychosocial factors. The direction of causation might prove difficult to untangle, particularly where specific symptoms are tightly clustered. Golden et al., [ 59 ] for instance found that mood disorders were highly prevalent in hepatitis C, and depression was associated with experiences of illness – including stigma, poor adjustment to the diagnosis and physical symptoms. There were three studies that suggested a biological cause for a slight cognitive impairment observed in hepatitis C [ 60 – 62 ].
Responses to diagnosis and management of hepatitis C
Finally, we identified 26 studies investigating experiences related to diagnosis and treatment (see Additional file 1 : Table S9). With some degree of overlap, these studies focused on the immediate impact of diagnosis and perceptions of stigma and discrimination in relation to it, accessing health services and making decisions about treatment. Diagnosis with hepatitis C could be a stressful event, characterized by feelings of shock and devastation that transitioned into enduring emotional, psychosocial and even physical effects [ 63 ]. For some, the time of diagnosis was an event equivalent to the stress of events such as moving cities, losing a job, marital breakdown and divorce [ 64 ]. Some studies, however, described a more dynamic response that was mediated by changes in social context [ 65 ]. For instance, the threat of HIV and issues related to substance use was considered a higher priority in some groups [ 66 ].
The perception of stigma is generally an internalized phenomenon resulting from individually held understandings and interpretations, or arising in response to actual or perceived discrimination. For instance, study participants described perceptions of stigma resulting from feelings of contamination and fear of disclosure to others, from whom they anticipated rejection, much of which was on the basis of fear of disease transmission [ 67 ]. Golden et al. [ 68 ] found perceived stigma was associated with decreased acceptance of illness, decreased social adjustment and increased reported symptoms in hepatitis C.
Discrimination can flow from the beliefs and attitudes of others and ultimately shape perceptions of stigma through the differential treatment of people with particular conditions. As Paterson et al. [ 69 ] describe, how illnesses are constructed by health providers influences not only the care offered, but also feeds into the self perception of the affected person. Many study participants reporting negative experiences in health care settings in relation to perceived discrimination. In one study of over 500 people with hepatitis C, 65% reported having experienced health care discrimination, which was associated with pessimism about future health and decreased social interaction among other things [ 70 ]. Perceived discrimination was also found to be a significant barrier to health treatment access, to the extent that refusal of treatment by providers was reported [ 71 ]. Harris [ 72 ] found that reports of refusal or withdrawal of health care were common and contributed to a reluctance to disclose to health professionals even in the context of a perceived obligation to do so.
Five studies investigated treatment decision making, confirming that concern about side effects remains an important reason for declining or adhering to treatment. McNally et al. [ 8 ] proposed that confidence in treatment efficacy was the main consideration in deciding to undertake treatment. Underscoring the contextual influences involved in making treatment decisions, Ogawa and Bova [ 73 ] suggested that treatment decisions in former IDU were complicated by fears that self injecting interferon could reintroduce the use of syringes in a way that might threaten their control over their injecting drug use. Treloar and Hopwood [ 74 ] encountered what they defined as ‘unrealistic optimism’ in both patients and health care providers, in which the applicability of information about possible side effects was underestimated. This may have implications for delay in treatment for mental health issues that might arise during antiviral treatment (Table 4 ).
In this paper we reviewed the biomedical and social literature on the ongoing clinical and psychosocial impacts of diagnoses with hepatitis C infection. The published literature provides useful information on selected aspects of living with hepatitis C. Identified cohort studies provided prospective information on clinical aspects of chronic infection in the longer term, with some studies involving very large numbers of patients who were followed up for considerable lengths of time. The social research presented in depth information on some of the social and personal ramifications of living with hepatitis C in the form of comprehensive and contextual ‘snapshots’ of seminal experiences, such as diagnosis and disclosure to others. Yet questions still remain if we are to develop a comprehensive understanding about what happens to people once they are diagnosed with hepatitis C.
The findings summarized here contribute to the knowledge base and could inform the continuing development, and revision, of national strategies aimed at reducing the harms associated with hepatitis C around the world. That the findings are synthesized from a wide range of methodological and discipline related perspectives could potentially enhance their relevance to strategy development and health service planning into the future. Yet the picture still remains fragmented and incomplete. The sometimes substantial impact of hepatitis C (and its management) on QoL has been frequently investigated, but only relatively short term follow up of changes in QoL during the course of treatment has been reported. Over the longer term people may experience fluctuations in disease activity, modification of alcohol or other drug use or changing social circumstances and it is likely that these dynamic forces would influence QoL in a manner that cannot be explored using traditional cross-sectional approaches.
Planning for effective health and community service provision for the growing number of people affected by hepatitis C requires a good understanding of potential trajectories, and the physiological and psychosocial forces underlying them, from hepatitis C diagnosis into the future. As people adapt to their diagnoses (as described in the social research), what proportion go on to find hepatitis has little or no impact on their lives, and what proportion find hepatitis C exerts a significant impact? At which points along their trajectory do people engage with hepatitis C treatment or community support services, and do those services meet their expectations and needs? Do people reporting discrimination at work or in the health system continue to have negative experiences? Is it possible to map improvements in the level of discrimination reported with increasing awareness of hepatitis C in the broader population? How does health status change over time, and how is health status linked with changes in lifestyle factors (drinking, smoking, other drug use) or with the social support people receive, or discrimination they perceive, at different points in time. The research identifies a number of factors that contribute to low uptake of antiviral treatment for hepatitis C. Decisions about treatment may be mediated by a range of health and social factors that may vary over time. It will become important to know how to overcome identified barriers and effectively target information as new treatments [ 75 ] and new management strategies become available.
This study used a systematic approach to the search strategy but may have been limited by incomplete retrieval of potentially relevant studies. Resource and time restrictions limited the search to English Language publications and did not allow for a search of unpublished data. It is possible that some ongoing studies of the health and social impacts of hepatitis infection were not identified. While our search of non-intervention studies may have lessened the potential for publication bias, there remains the possibility that our assessment of the combined data may have overestimated the health and social impacts of hepatitis C. To minimize these potential source of bias, we searched three major data bases using an inclusive search strategy, and all potentially eligible studies were retrieved in full.
This narrative review has provided a useful update on many aspects of living with hepatitis C infection but has also highlighted a number of important research gaps that may have implications for hepatitis C strategy development and implementation around the world. Many of these gaps appear to be due to a methodological and content gulf existing between the biomedical and social research literature. Typically, cohort designs have been used primarily in investigations of clinical or health outcome – with exploration of social parameters primarily focused on identifying their effect on outcome. In broader social research, complex psychosocial phenomena are more comprehensively explored, but temporal change is often not a critical factor. Bridging the research gaps will ultimately require a combined approach, both content and methodology, to study the health and social impacts of hepatitis C along the life course.
Lavanchy D: Evolving epidemiology of hepatitis C virus. Clin Microbial Infect. 2011, 17: 107-115. 10.1111/j.1469-0691.2010.03432.x.
Article CAS Google Scholar
Dore GJ, MacDonald M, Law MG, Kaldor JM: Epidemiology of hepatitis C virus infection in Australia, Chapter 1 in Hepatitis C: an update. Aust Fam Physician. 2003, 32: 2-5. Special feature
Google Scholar
Canberra: Department of Health and Ageing: Commonwealth of Australia: National Notifiable Diseases Surveillance System [on line database]. , http://www9.health.gov.au/cda/Source/CDA-index.cfm ,
Geneva: WHO: World Health Organization: Hepatitis C - Fact sheet N°164. 2012, http://www.who.int/mediacentre/factsheets/fs164/en/ ,
Batey RG: Chronic Hepatitis C, Chapter 4 in Hepatitis C an update. Aust Fam Physician. 2003, 32: 15-20. Special feature
Eyre N, Beard M: HCV virology. Hepatitis C: an expanding perspective. Edited by: Dore G, Temple-Smith M, Lloyd A. 2009, IP Communications, Melbourne, 3-26.
Farrell GC: Hepatitis C, other liver disorders, and liver health. 2002, MacLennan and Petty, NSW
McNally S, Temple-Smith M, Sievert W, Pitts MK: Now, later or never? Challenges associated with hepatitis treatment. Aust N Z J Public Health. 2006, 30: 422-427. 10.1111/j.1467-842X.2006.tb00457.x.
Article CAS PubMed Google Scholar
National Centre for HIV Epidemiological and Clinical Research: HIV, viral hepatitis and sexually transmissible infections in Australia Annual Surveillance Report 2010. 2010, National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, Sydney, NSW
Flamm SL: Chronic hepatitis C virus infection. J Am Med Assoc. 2003, 289: 2413-2417. 10.1001/jama.289.18.2413.
Article Google Scholar
Su J, Brook RA, Kleinman NL, Corey-Lisle P: The impact of hepatitis C virus infection on work absence, productivity, and healthcare benefit costs. Hepatology. 2010, 52: 436-442. 10.1002/hep.23726.
Article PubMed Google Scholar
Anti-Discrimination Board of New South Wales: C-change: the report of the enquiry into hepatitis C related discrimination. 2001, Anti-Discrimination Board of New South Wales, Sydney
O'Brien AP, Cross WM, Higgs P, Munro I, Bloomer MJ, Chou KR: Australians living with and managing hepatitis C. Issues Ment Health Nurs. 2010, 31: 520-524. 10.3109/01612841003629532.
Waller L: Living with hepatitis C: from self-loathing to advocacy. Medical Journal of Australia. 2004, 180: 293-294.
PubMed Google Scholar
Stanton AL, Revenson TA, Tennen H: Health psychological adjustment to chronic disease. Annu Rev Psychol. 2007, 58: 565-592. 10.1146/annurev.psych.58.110405.085615.
Roy E, Boudreau J-F, Boivin J-F: Hepatitis C virus incidence among young street-involved IDUs in relation to injection experience. Drug Alcohol Depend. 2009, 102: 158-161. 10.1016/j.drugalcdep.2009.01.006.
Maher L, Li J, Jalaludin B, Chant KG, Kaldor JM: High hepatitis C incidence in new injecting drug users: a policy failure?. Aust N Z J Public Health. 2007, 31: 30-35. 10.1111/j.1753-6405.2007.00007.x.
Grebely J, Raffa JD, Lai C, Krajden M, Kerr T, Fischer B, Tyndall MW: Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepat. 2009, 16: 352-358. 10.1111/j.1365-2893.2009.01080.x.
Sbebl FM, El-Kamary SS, Saleh DA, Abdel-Hamid M, Mikhail N, Allam A, El-Arabi H, Elhenawy I, El-Kafrawy S, El-Daly M, et al: Prospective cohort study of mother-to-infant infection and clearance of hepatitis C in rural Egyptian villages. J Med Virol. 2009, 81: 1024-1031. 10.1002/jmv.21480.
Dolan KA, Shearer J, White B, Zhou J, Kaldor J, Wodak AD: Four-year follow-up of imprisoned male heroin users and methadone treatment: mortality, re-incarceration and hepatitis C infection. Addiction. 2005, 100: 820-828. 10.1111/j.1360-0443.2005.01050.x.
Miller ER, Bi P, Ryan P: HCV infection in South Australian prisoners: seroprevalence, seroconversion and risk factors. Int J Infect Dis. 2009, 13: 201-208. 10.1016/j.ijid.2008.06.011.
Yawn BP, Wollan P, Gazzuola L, Kim RW: Diagnosis and 10-year follow-up of a community-based hepatitis C cohort. J Fam Pract. 2002, 51: 135-140.
Zampino R, Marrone A, Merola A, Trani B, Cirillo G, Karayiannis P, Coppola N, Zappalà R, Utili R, GRuggiero G, Adinolfi LE: Long-term outcome of hepatitis B and hepatitis C virus co-infection and single HBV infection acquired in youth. J Med Virol. 2009, 81: 2012-2020. 10.1002/jmv.21560.
Grebely J, Raffa JD, Lai C, Krajden M, Conway B, Tyndall MW: Factors associated with spontaneous clearance of hepatitis C virus among illicit drug users. Can J Gastroenterol. 2007, 21: 447-451.
Article PubMed PubMed Central Google Scholar
García-García JA, Romero-Gómez M, Girón-González JA, Rivera-Irigoin R, Torre-Cisneros J, Montero JL, González-Serrano M, Andrade RJ, Aguilar-Guisado M, Grilo I, et al: Incidence of and factors associated with hepatocellular carcinoma among hepatitis C virus and Human Immunodeficiency virus coinfected patients with decompensated cirrhosis. AIDS Res Hum Retroviruses. 2006, 22: 1236-1241. 10.1089/aid.2006.22.1236.
Hansen N, Obel N, Christensen PB, Krarup H, Laursen AL, Clausen MR, Lunding S, Moller A, Schlichting P, Kromann-Andersen H, et al: Predictors of antiviral treatment initiation in hepatitis C virus-infected patients: a Danish cohort study. J Viral Hepat. 2009, 16: 659-665. 10.1111/j.1365-2893.2009.01126.x.
Bonacini M, Louie S, Bzowej N, Wohl AR: Survival in patients with HIV infection and viral hepatitis B or C: a cohort study. AIDS. 2004, 18: 2039-2045. 10.1097/00002030-200410210-00008.
Melendez-Morales L, Konkle BA, Preiss L, Zhang M, Mathew P, Eyster ME, Goedert JJ: Chronic hepatitis B and other correlates of spontaneous clearance of hepatitis C virus among HIV-infected people with hemophilia. AIDS. 2007, 21: 1631-1636. 10.1097/QAD.0b013e32826fb6d9.
Evon DM, Ramcharran D, Belle SH, Terrault NA, Fontana RJ, Fried MW, the Virahep-C Study Group: American Journal of Gastroenterology. Prospective analysis of depression during peginterferon and ribavirin therapy of chronic hepatitis C: result of the Virahep-C Study. 2009, Advance on line publication, 29 September 2009
Dalgard O, Bjøro K, Hellum K, Myrvang B, Skaug K, Gutigard G, Belle H, the Construct Group: Treatment of chronic hepatitis C in injecting drug users: 5 years' follow-up. Eur Addict Res. 2002, 8: 45-49. 10.1159/000049487.
Ikeda K, Marusawa H, Osaki Y, Nakamura T, Kitajima N, Yamashita Y, Kudo M, Sato T, Chiba T: Antibody to hepatitis B core antigen and risk for hepatitis C-related hepatocellular carcinoma: a prospective study. Ann Intern Med. 2007, 146: 649-656.
Gallegos-Orozco JF, Yosephy A, Noble B, Aqel BA, Byrne TJ, Williams JW, Rakela J, Vargas HE: Natural history of post-liver transplantation hepatitis C: a review of factors that may influence its course. Liver Transpl. 2009, 15: 1872-1881. 10.1002/lt.21954.
Bizollon T, Pradat P, Mabrut JY, Chevallier M, Adham M, Radenne S, Souquet JC, Ducerf C, Baulieux J, Zoulim F, Trepo C: Benefit of sustained virological response to combination therapy on graft survival of liver transplanted patients with recurrent chronic hepatitis C. Am J Transplant. 2005, 5: 1909-1913. 10.1111/j.1600-6143.2005.00976.x.
Bailey DE, Landerman L, Barroso J, Bixby P, Mishel MH, Muir AJ, Strickland L, Clipp E: Uncertainty, symptoms, and quality of life in persons with chronic hepatitis. Psychosomatics. 2009, 50: 138-146. 10.1176/appi.psy.50.2.138.
Thein HH, Maruff P, Krahn M, Kaldor JM, Koorey DJ, Brew BJ, Dore GJ: Cognitive function, mood and health-related quality of life in hepatitis C virus (HCV)-monoinfected and HIV/HCV-coinfected individuals commencing HCV treatment. HIV Med. 2007, 8: 192-202. 10.1111/j.1468-1293.2007.00452.x.
Gunasekera S, Fraser J, Alexander C: Quality of life in Hepatitis C virus infection: assessment of rural patients living in north-western New South Wales. Aust J Rural Health. 2008, 16: 213-220. 10.1111/j.1440-1584.2008.00983.x.
Kramer L, Bauer E, Funk G, Hofer H, Jessner W, Steindl-Munda P, Wrba F, Madl C, Gangl A, Ferenci P: Subclinical impairment of brain function in chronic hepatitis C infection. J Hepatol. 2002, 37: 349-354. 10.1016/S0168-8278(02)00172-1.
Kramer L, Hofer H, Bauer E, Funk G, Formann E, Steindl-Munda P, Ferenci P: Relative impact of fatigue and subclinical cognitive brain dysfunction on health-related quality of life in chronic hepatitis C infection. AIDS. 2005, 19: S85-S92. 10.1097/01.aids.0000192075.26314.87.
Marcellin F, Preau M, Ravaux I, Dellamonica P, Spire B, Carrieri MP: Self-reported fatigue and depressive symptoms as main indicators of the quality of life (QOL) of patients living with HIV and hepatitis C: Implications for clinical management and future research. HIV Clin Trials. 2007, 8: 320-327. 10.1310/hct0805-320.
Dalgard O, Egeland A, Skaug K, Vilimas K, Steen T: Health-related quality of life in active injecting drug users with and without chronic hepatitis C virus infection. Hepatology. 2004, 39: 74-80. 10.1002/hep.20014.
Schwarzinger M, Dewedar S, Rekacewicz C, Abd Elaziz KM, Fontanet A, Carrat F, Mohamed MK: Chronic hepatitis C virus infection: Does it really impact health-related quality of life? A study in rural Egypt. Hepatology. 2004, 40: 1434-1441. 10.1002/hep.20468.
Fontana RJ, Hussain KB, Schwartz SM, Moyer CA, Su GL, Lok ASF: Emotional distress in chronic hepatitis C patients not receiving antiviral therapy. J Hepatol. 2002, 36: 401-407. 10.1016/S0168-8278(01)00280-X.
Hilsabeck RC, Hassanein TI, Perry W: Biopsychosocial predictors of fatigue in chronic hepatitis C. J Psychosom Res. 2005, 58: 173-178. 10.1016/j.jpsychores.2004.07.003.
Taliani G, Rucci P, Biliotti E, Cirrincione L, Aghemo A, Alberti A, Almasio PL, Bartolozzi D, Caporaso N, Coppola R, et al: Therapy expectations and physical co morbidity affect quality of life in chronic hepatitis C virus infection. J Viral Hepat. 2007, 14: 875-882.
CAS PubMed Google Scholar
Dudley T, Chaplin D, Clifford C, Mutimer DJ: Quality of life after liver transplantation for hepatitis C infection. Qual Life Res. 2007, 16: 1299-1308. 10.1007/s11136-007-9244-y.
Grundy G, Beeching N: Understanding social stigma in women with hepatitis C. Nurs Stand. 2004, 19: 35-39.
Janke EA, McGraw S, Garcia-Tsao G, Fraenkel L: Psychosocial issues in hepatitis C: a qualitative analysis. Psychosomatics. 2008, 49: 494-501. 10.1176/appi.psy.49.6.494.
Sgorbini M, O'Brien L, Jackson D: Living with hepatitis C and treatment: the personal experiences of patients. J Clin Nurs. 2009, 18: 2282-2291. 10.1111/j.1365-2702.2009.02806.x.
Temple-Smith M, Gifford S, Stoové M: The lived experience of men and women with hepatitis C: implications for support needs and health information. Aust Health Rev. 2004, 27: 46-56. 10.1071/AH042720046.
Zacks S, Beavers K, Theodore D, Dougherty K, Batey B, Shumaker J, Galanko J, Shrestha R, Fried MW: Social stigmatization and hepatitis C virus infection. Journal of Clinincal Gastroenterology. 2006, 40: 220-224. 10.1097/00004836-200603000-00009.
Castera L, Constant A, Bernard PH, de Ledinghen V, Couzigou P: Lifestyle changes and beliefs regarding disease severity in patients with chronic hepatitis C. J Viral Hepat. 2006, 13: 482-488. 10.1111/j.1365-2893.2005.00719.x.
Scognamiglio P, Galati V, Navarra A, Longo MA, Aloisi MS, Antonini MG, Puoti M, Almasio PL, Ippolito G, Girardi E: Impact of hepatitis C virus infection on lifestyle. World J Gastroenterol. 2007, 13: 2722-2726.
Roy E, Nonn É, Haley N, Cox J: Hepatitis C meaning and preventive strategies among street-involved young injection drug users in Montréal. Int J Drug Policy. 2007, 18: 397-405. 10.1016/j.drugpo.2007.02.005.
Wright NMJ, Tompkins CNE, Jones L: Exploring risk perceptions and behaviour of homeless injecting drug users diagnosed with hepatitis C. Health Soc Care Community. 2005, 13: 75-83. 10.1111/j.1365-2524.2005.00552.x.
Conrad S, Garrett LE, Cooksley WGE, Dunne MP, MacDonald GA: Living with chronic hepatitis C means 'you just haven't got a normal life any more'. Chronic Ilness. 2006, 2: 121-131.
CAS Google Scholar
Grassi L, Satriano J, Serra A, Biancosino B, Zotos S, Sighinolfi L, Ghinelli F: Emotional stress, psychosocial variables and coping associated with hepatitis C virus and human immunodeficiency virus infections in intravenous drug users. Psychother Psychosom. 2002, 71: 342-349. 10.1159/000065993.
Groessl EJ, Weingart KR, Kaplan RM, Clark JA, Gifford AL, Ho SB: Living with hepatitis C: qualitative interviews with hepatitis C-infected veterans. J Gen Intern Med. 2008, 23: 1959-1965. 10.1007/s11606-008-0790-y.
Hopwood M, Treloar C: Resilient coping: applying adaptive responses to prior adversity during treatment for hepatitis C infection. J Health Psychol. 2008, 13: 17-27. 10.1177/1359105307084308.
Golden J, O'Dwyer AM, Conroy RM: Depression and anxiety in patients with hepatitis C: prevalence, detection rates and risk factors. Gen Hosp Psychiatry. 2005, 27: 431-438. 10.1016/j.genhosppsych.2005.06.006.
Forton DM, Thomas HC, Murphy CA, Allsop JM, Foster GR, Main J, Wesnes KA, Taylor-Robinson SD: Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology. 2002, 35: 433-439. 10.1053/jhep.2002.30688.
McAndrews MP, Farcnik K, Carlen P, Damyanovich A, Mrkonjic M, Jones S, Heathcoate EJ: Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology. 2005, 41: 801-808. 10.1002/hep.20635.
Piche T, Gelsi E, Schneider SM, Hebuterne X, Giudicelli J, Ferrua B, Laffont C, Benzaken S, Hastier P, Montoya ML, et al: Fatigue is associated with high circulating leptin levels in chronic hepatitis C. Gut. 2002, 51: 434-439. 10.1136/gut.51.3.434.
Article CAS PubMed PubMed Central Google Scholar
Tompkins CN, Wright NM, Jones L: Impact of a positive hepatitis C diagnosis on homeless injecting drug users: a qualitative study. Br J Gen Pract. 2005, 55: 263-268.
PubMed PubMed Central Google Scholar
Castera L, Constant A, Bernard PH, de Ledinghen V, Couzigou P: Psychological impact of chronic hepatitis C: Comparison with other stressful life events and chronic diseases. World J Gastroenterol. 2006, 12: 1545-1550.
Sutton R, Treloar C: Chronic illness experiences, clinical markers and living with hepatitis C. J Health Psychol. 2007, 12: 330-340. 10.1177/1359105307074278.
Harris M: Troubling biographical disruption: narratives of unconcern about hepatitis C diagnosis. Sociol Health Illn. 2009, 31: 1028-1042. 10.1111/j.1467-9566.2009.01172.x.
Crockett B, Gifford SM: "Eyes Wide Shut": narratives of women living with hepatitis C in Australia. Women Health. 2004, 39: 117-137. 10.1300/J013v39n04_07.
Golden J, Conroy RM, O'Dwyer AM, Golden D, Hardouin JB: Illness-related stigma, mood and adjustment to illness in persons with hepatitis C. Soc Sci Med. 2006, 63: 3188-3198. 10.1016/j.socscimed.2006.08.005.
Paterson BL, Butt G, McGuinness L, Moffat B: The construction of hepatitis C as a chronic illness. Clin Nurs Res. 2006, 15: 209-224. 10.1177/1054773806288569.
Hopwood M, Treloar C, Bryant J: Hepatitis C and injecting-related discrimination in New South Wales, Australia. Drugs-Education Prevention and Policy. 2006, 13: 61-75. 10.1080/09687630500481150.
Butt G, Paterson BL, McGuinness LK: Living with the stigma of hepatitis C. West J Nurs Res. 2008, 30: 204-221.
Harris M: Living with hepatitis C: the medical encounter. N Z Sociol. 2005, 20: 4-19.
Ogawa LM, Bova C: HCV treatment decision-making substance use experiences and hepatitis C treatment decision-making among HIV/HCV Coinfected Adults. Subst Use Misuse. 2009, 44: 915-933. 10.1080/10826080802486897.
Treloar C, Hopwood M: "Look, I'm fit, I'm positive and I'll be all right, thank you very much": coping with hepatitis C treatment and unrealistic optimism. Psychol Health Med. 2008, 13: 360-366. 10.1080/13548500701477532.
Seden K, Back D, Saye Khoo S: New directly acting antivirals for hepatitis C: potential for interaction with antiretrovirals. J Antimicrob Chemother. 2010, 65: 1079-1085. 10.1093/jac/dkq086.
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2458/12/672/prepub
Download references
Acknowledgements
The authors would like to thank Professor Marian Pitts and Professor Anthony Smith who provided solid general support for the project and provided valuable advice in the development of the search strategy.
Author information
Authors and affiliations.
Discipline of General Practice, School of Population Health, University of Adelaide, Adelaide, 5005, South Australia
Emma R Miller
Australian Research Centre in Sex, Health & Society, La Trobe University, Melbourne, Australia
Stephen McNally & Jack Wallace
School of Population Health, University of Melbourne, Melbourne, Australia
Marisa Schlichthorst
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Emma R Miller .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
EM designed the search strategy; retrieved, reviewed, summarized and tabulated the papers; and drafted the manuscript. SM participated in the coordination of the search strategy; reviewed summary data; and critically revised subsequent drafts of the manuscript. JW and MS both participated in the critical revision of the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
12889_2012_4528_moesm1_esm.pdf.
Additional file 1: Table S1. Hepatitis C transmission in injecting drug users. Table S2. Hepatitis C transmission in various initially hepatitis C negative population groups. Table S3. Studies investigating the health outcomes in hepatitis C mono-infection and co-infection with other blood borne viruses. Table S4. Health related quality of life associated with antiviral treatment. Table S5. Studies investigating the health outcomes after treatment. Table S6. Health related quality of life in untreated chronic hepatitis C infection. Table S7. Health related quality of life during and after treatment/transplant for hepatitis C infection. Table S8. Psychosocial experience of living with hepatitis C infection. Table S9. Diagnosis, management and treatment of chronic hepatitis C infection. (PDF 413 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions

About this article
Cite this article.
Miller, E.R., McNally, S., Wallace, J. et al. The ongoing impacts of hepatitis c - a systematic narrative review of the literature. BMC Public Health 12 , 672 (2012). https://doi.org/10.1186/1471-2458-12-672
Download citation
Received : 20 January 2012
Accepted : 13 August 2012
Published : 18 August 2012
DOI : https://doi.org/10.1186/1471-2458-12-672
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hepatitis C
- Health outcome
- Social impact
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
- Open access
- Published: 27 December 2022
A review on hepatitis C virus: role of viral and host-cellular factors in replication and existing therapeutic strategies
- Fatima Butt 1 ,
- Muhammad Shahid 1 ,
- Muhammad Hassan 1 ,
- Farah Tawakkal 1 ,
- Iram Amin 1 ,
- Samia Afzal 1 ,
- Rashid Bhatti 1 ,
- Rabia Nawaz 2 &
- Muhammad Idrees 1
Egyptian Liver Journal volume 12 , Article number: 71 ( 2022 ) Cite this article
2930 Accesses
4 Citations
Metrics details
Hepatitis C virus, a member of Flaviviridae is a single-stranded positive-sense RNA virus infecting 62–79 million people around the globe. This blood-borne virus is one of the leading causes of liver diseases worldwide. This review aims to identify novel potential genes linked to cellular host factors, as well as revise the roles of each gene in hepatitis C Virus infection. This review also aims to provide a comprehensive insight into therapeutic advancements against HCV.
For this review article, 190 articles were searched via PubMed Central, Bio-One, National Academy of Science, Google Scholar, and Worldwide Science. 0ut of these 190 studies, 55 articles were selected for this review. The inclusion of articles was done on the criteria of high citation and Q1 ranking.
The information gathered from previously published articles highlighted a critical link between host-cellular factors that are important for HCV infection.
Although many advancements in HCV treatment have been made like DAAs and HTAs, the development of a completely effective HCV therapy is still a challenge. Further research on combinations of DAAs and HTAs can help in developing a better therapeutic alternative. Keywords: Hepatitis C virus, Replication cycle, Non-structural proteins, Host-cellular factors, Treatment strategies
Hepatitis C virus (HCV) is a single-stranded blood-borne RNA virus infecting 62–79 million people around the globe. It is one of the leading causes of liver diseases worldwide. Almost 3% of the world population has the chronic infection of HCV that leads to fibrosis, cirrhosis, and eventually the carcinoma of hepatic cells in most cases [ 1 ]. In Pakistan, nearly 1 in every 20 individuals is a victim of this viral infection [ 2 , 3 ]. To date, six different genotypes of HCV are reported. Due to the high mutation rate, all six genotypes further possess many different subtypes. Of these, four subtypes (1a, 1b, 2a, and 3a) are reported repeatedly across the globe, but the epidemics of others are still bound to specific geographic distributions [ 4 ] Genotype 4 is reported as the highly prevalent genotype in Middle East countries including Saudi Arabia and Central Africa but very little is known about its genotypic pandemic background at the subtype level in the region [ 5 ]. However, a recent virologic report on HCV has predicted the molecular phylogeny of 4a genotype with Egyptian prototype strain, while 1a isolates were closely related phylogenetically to North American and European countries [ 6 ]. A recent study in Iran has shown a remarkable growth of genotype 3a as a dominant infectious agent of HCV followed by 1a [ 7 ]. This high rate of mutations in the HCV virus has been a major hindrance in the development of an effective vaccine against its infection. The 9.6 kb genome of this enveloped virus consists of a single large open reading frame (ORF) and encodes 3 structural and 7 non-structural proteins. Structural proteins include core protein, envelope protein 1, and envelope protein 2. Non-structural proteins (NS) include p7, NS2, NS3 and NS4A, NS4B, NS5A, and NS5B (RNA-dependent RNA-polymerase [ 3 ].
Our genome is transcribed into two categories of transcriptional products, i.e., coding RNAs and non-coding RNAs. Coding RNAs are translated into proteins that play crucial biological functions in our body. Many of these protein-coding genes have been reported to be potential biomarkers for HCV infection. In this review, we have not only described the role of the coding region of the genome in HCV prognosis and diagnosis but have also highlighted the importance of the non-coding region. Non-coding RNAs once referred as “transcriptional noise”, are now being reported not only as good therapeutic targets but also as potential biomarkers. The non-coding RNAs play a significant regulatory role in the expression of gene networks [ 8 ]. Circular RNAs (circRNA) are a subset of specialized non-coding RNA. Interestingly, circRNAs have been reported to hinder the progress of tumor formation in HCV-related carcinoma by playing a regulatory role in cell division, growth, migration, and apoptosis. Thus, circular RNAs are not only potential therapeutic targets but can also serve as biomarkers for the diagnosis and prognosis of HCV infection and HCV-related hepatocarcinoma [ 9 , 10 , 11 ]. Moreover, MicroRNAs (miRNA) is another host factor reported to be associated with the progression of HCV infection [ 8 , 12 ].
Methodology
This article was prepared with the aim to review the role of viral and cellular host factors in infection of hepatitis C Virus. For this review article, 190 literature papers were searched via PubMed Central, Bio-One, National Academy of Science, Google Scholar, and Worldwide Science using the following keywords: ‘HCV’, ‘Life cycle of HCV’, ‘Translation of HCV genome’, ‘Host-cellular factors crucial for HCV infection’, ‘HCV receptors’, and ‘Immunity against HCV’. These 190 studies were then subjected to initial screening in which duplicated, and review articles were removed. Following an initial screening, the final screening was performed independently by two authors (Fatima Butt and Muhammad Shahid) on 135 studies. During the final screening, studies were subjected to our inclusion/exclusion criteria. Consequently, 104 articles were selected considering the following factors: (1) high citation and (2) Q1 ranking. Out of these 104 studies, 80 full-length articles were selected for eligibility testing. Subsequently, 55 articles were selected to be included in this review (Fig. 1 ).
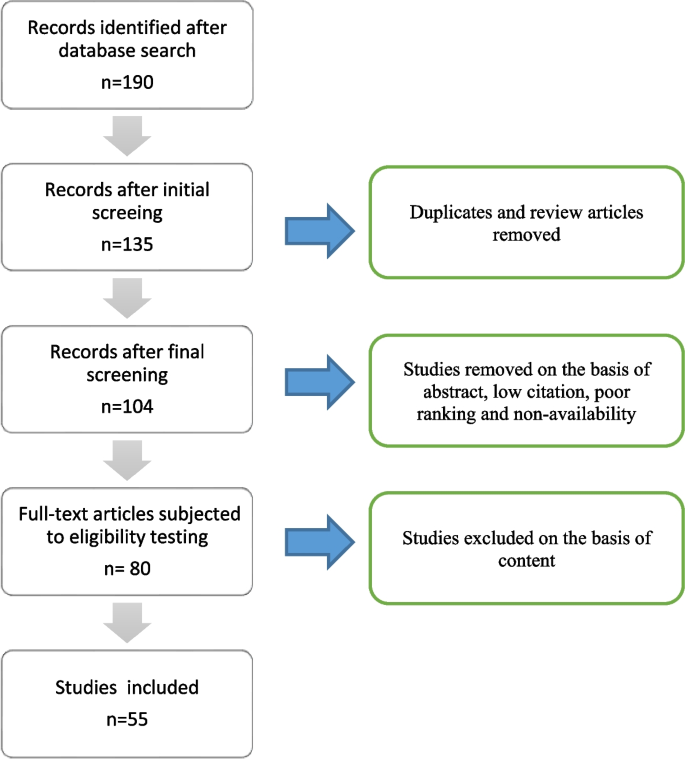
Flowchart describing the steps of the methodology
Role of different viral and cellular factors in the life cycle of HCV
Attachment and entry.
The presence of certain specific receptors and additional host entry factors at the hepatocytes make them susceptible and permissive cells for HCV infection as shown in Fig. 2 . These blood-borne viral particles reach the basolateral surface of liver cells via blood flow after passing through sinusoids. The two transmembranes envelop proteins, E1 and E2, facilitating the binding of HCV to the host cell receptors. The outer surface of hepatocytes carries four receptors of HCV, i.e., the scavenger receptor class B type I (SCARB1), occluding (OCLN), cluster of differentiation 81(CD81), and claudin 1 (CLDN1) are present [ 13 ]. Along with these receptors, other additional factors like attachment factors glycosaminoglycans, low-density lipoproteins LDL-R receptors, Niemann-Pick C1-Like 1 (NPC1L1), and transferrin receptor 1 are also believed to contribute to the susceptibility of hepatocytes for HCV infection [ 1 ]. The association of HCV with lipoproteins and apolipoproteins makes its unique structure in the Flaviviridae family. This association helps in camouflaging HCV particles and prevents them from eliciting an immune response. However, the viral-lipoprotein association is also believed to facilitate the entry of HCV into host cells via LDL receptors only to onset a pathway leading to the degradation of these foreign viral particles by the host cell.
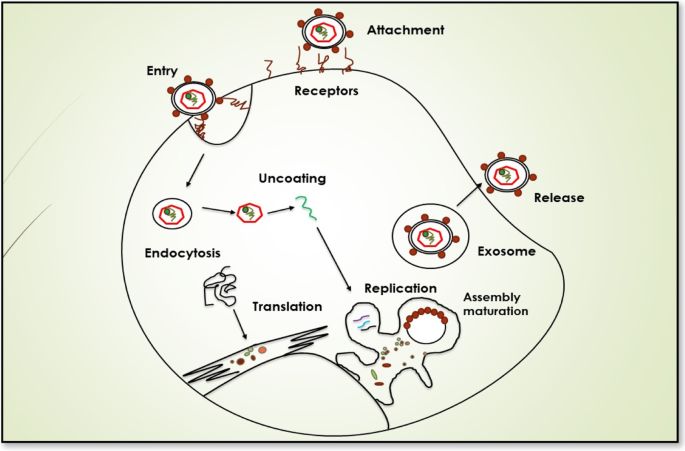
HCV entry into the host cell
The first interaction for attachment between viral particles and hepatocytes is usually via SCARB1 which is an integral membrane protein that acts as one of the receptors for HCV particles. To date, several hypotheses regarding the HCV-SCARB1 association have been suggested. The earliest studies reported that viral glycoproteins are responsible for the initial interaction between HCV and host liver cells. The binding between the HVR1 region of E2 glycoproteins and the SCARB1 receptor of hepatocytes can be the first attachment signal stimulating the entry of the virus in host cells [ 14 ]. However, some modern studies also propose the involvement of associated apoprotein E in initial HCV-SCARB1 binding. Likewise, some other studies suggest that interaction between HRV1 and SCARB1 can also assist the binding of E2 with the CD81 receptor of host cells. CD81, a transmembrane protein, containing an extracellular domain for interaction with E2 of HCV particles is also considered one of the receptors for HCV particles [ 15 , 16 ]. The HCV interaction with CD81 is considered to be a significant event in facilitating the viral entry into host cells [ 17 ]. CD81 is also believed to interact with another receptor of HCV known as CLDN1 [ 18 ]. CLDN1 is a tight junction protein that is expressed in many cells and tissues including liver cells. In hepatocytes, they play their role by separating bile from blood. The interaction between CD81 and CLDN1 is seemed to be assisted by EGFR (epidermal growth factor receptor). EGFR stimulates HRAS activation which results in CD81 diffusion, thus helping in the formation of the CD81-CLDN1 complex that enters the hepatocytes along with HCV particles [ 19 ]. Similarly, another tight junction protein, OCLN is one of the key receptors for HCV particles and plays a significant part in determining tissue tropism of HCV. However, the mechanism of OCLN action in the viral entry is still not known [ 14 ].
In addition to these receptors, some other host factors like factors glycosaminoglycans, Transferrin receptor 1, and NPC1L1 have also been shown to contribute to viral attachment and entry, but the exact mechanism of their role is still unknown. After attachment, the next step in the viral life cycle is the entry of these HCV particles into hepatocytes. These HCV particles enter hepatocytes via clathrin-dependent endocytosis. Following the entry into the cells in form of endosomes, pH-dependent fusion takes place between the endosomal membrane and viral envelope [ 20 ].
Translation and replication
The positive-strand RNA of HCV is released into the cytosol and its replication starts directly at ER via ribosomes of host hepatocytes. Essentially, only one large polyprotein is expressed from the single ORF of the viral genome which is then cleaved into 10 different proteins exploiting both host and viral proteases. The ORF carries the flanking regions 5′ and 3′ UTRs (Un-translated regions) that are essential for the translation and replication of viral RNA. 5′ UTR region includes an internal ribosome binding site (IRES) that instigates the translation of viral RNA into one large polyprotein. Proteolytic cleavage of this polyprotein results in the formation of ten different proteins. Among these 10 proteins, core protein, envelope protein 1, and envelope protein 2 are structural proteins while non-structural proteins include p7, NS2, NS3 and NS4A, NS4B, NS5A, and NS5B. Enzymes (non-structural protein) encoded by its genome viz. cysteine protease (NS2), serine protease (NS3-4A), helicase (NS3), and RNA-dependent RNA-polymerase (NS5B) are recruited during the replication process [ 21 ].
Signal peptide region E1 transports polyprotein to the ER membrane. Once the polyprotein reaches the membrane of ER, core protein is cleaved via subsequent host cell signal peptidase (SP) SP and signal peptide peptidase (SPP) cleavage. SP cleavage is important for the release of infectious viral particles from cells while SPP cleavage helps in viral displacement to lipid droplets. Owing to its cysteine proteolytic activity, NS2 is responsible for the cleavage of NS 2-3 junction. Likewise, NS3-assisted cleavage separates NS4A from NS3 and NS4B that ensues the NS3-4A association which can, in turn, modulate the cleavage of NS4B-5A and NS5B-5A junctions. After post-translational cleavages and polypeptide processing, non-structural HCV proteins are recruited for replication of viral RNA. A key event in this process is the formation of a negative-strand RNA intermediate by NS5B. After replication, the newly formed RNA molecules again go through the mechanisms of translation and replication and are ultimately packaged into capsids to form infectious viral particles. A specialized compartment called “membranous web” mainly composed of double-membrane vesicles (DMVs) is created in the cytosol for the replication of the HCV genome. The formation of this web is induced by viral proteins; however, the exact mechanism of induction is still not completely understood [ 21 ].
Several host-cellular factors are shown to be crucial for the replication of the HCV genome including micro-RNA 122, phosphatidyl-inositol-4-kinase-III (PI4KIII), ADP ribosylation factor GTPase activating protein 1 (ARFGAP1), and cyclophilin A (CypA) [ 22 ]. MicroRNA 122 is a liver enzyme that prevents the enzymatic degradation of viral RNA by attaching the Argonaute 2 enzyme at the 5′ UTR site thus stabilizing the viral genome. The interaction between PI4KIII and NS5A has also been shown to assist the maintenance of membranous web structure by mediating the accumulation of phosphatidylinositol-4-phosphate (PI4P) in this cytosolic web [ 23 ]. Furthermore, NS5A has also been reported to interact with CypA assisting in viral protein configuration and folding. Interestingly, inhibition of Cyp A has been shown to prevent the formation of double-membrane vesicles of the membranous web. Moreover, NS5A also interacts with ARFGAP1 for maintaining a high concentration of P1P4P at the membranous web [ 24 ]. Additionally, some other cellular molecules like proteins of intracellular transport, lipids including cholesterol, and vesicle-associated membrane proteins (VAP-A and VAP-B) have also been reported to favor the replication of HCV inside host cells.
Another key player in favoring HCV replication and translation is lipid droplets (LDs). LD-associated proteins, TIP47, and Rab18 stimulate LD interaction with NS5A and thus modulate viral replication [ 25 ]. Moreover, according to an ex vivo study, the presence of double-stranded RNA molecules of HCV adjacent to LD-rich sites illustrates the significance of LDs for the replication of the virus [ 26 ].
Assembly and release
Following genome translation and replication, assembly and release of infectious virions take place. The viral core proteins after undergoing SPP cleavage, are transported to LDs at which they get attached via their C terminal domain. The exact mechanism of HCV packaging and release is unclear. Consequently, different models have been proposed regarding its assembly and release. According to the most widely accepted model, the packaging of the viral genome into the capsids takes place in surrounding sites of LDs. Next, these nucleocapsids are trafficked to the ER from where they acquire their glycoprotein-containing envelope through budding. Another model proposes that E1 and E2 glycoproteins are dislocated to LD surrounding sites where viral particles get assembled. This dislocation is shown to be modulated by the interplay between NS2 and other viral proteins namely E1, E2, and p7 [ 27 , 28 ]. Following assembly, HCV particles either undergo maturation or degradation depending upon their quality. Viral protein NS5A is crucial for virion assembly since phosphorylation of its C-terminal domain modulates viral packaging [ 29 ]. This C-terminal domain also interacts p7-NS2 complex [ 30 ]. Likewise, NS3-4 A complex also has been reported to be crucial for packaging HCV particles [ 31 ]. Moreover, mutations in p7, NS2, and NS5B proteins have been shown to cause improper packaging of viral particles elucidating the significance of these proteins in HCV morphogenesis [ 32 ].
Mature HCV particles exit the cells via very low density lipoprotein (VLDL) secretory pathway and circulate in the blood in close association with host lipoproteins. Thus, a host protein required for VLDL secretory pathway called MTP (microsomal triglyceride transfer protein) is also vital for the synthesis of infectious HCV particles. Similarly, another host protein Y-box-binding protein 1 (YB-1) interacts with the NS3-4A complex and has proved to be crucial for HCV particle synthesis. Additionally, two host enzymes namely DGAT1 (diacylglycerol acyltransferase 1) and PLA2GA4 (cytosolic phospholipase A2) are shown to play a key role in the assembly and release of HCV [ 33 ]. DGAT1 is essential for the interaction between viral core and lipid droplets while PLA2GA4 makes favorable changes in fluidity and curvature of membrane for proper assembly of HCV particles [ 34 ]. Moreover, glucosidase enzymes present in ER of the host modulate the proper folding of viral E1 and E2 proteins. Likewise, apoproteins (majorly apo E) have been reported to be crucial for the synthesis of infectious HCV particles [ 35 ]. Surprisingly, in vitro studies have also reported direct transfer of HCV infection from one cell to another known as cell-to-cell contact-mediated (CCCM) transfer for which all HCV receptors along with actin cytoskeleton are needed. However, this process remains unclear in vivo [ 36 ].
Therapeutic strategies
On account of the high mutation rate of this virus, no preventative vaccine is available for the hepatitis C virus. However, extensive research on therapeutic measures is being done. Currently, the most commonly used antiviral agents against HCV are direct antiviral drugs (DAAs) which target viral proteins. The most widely targeted proteins include NS3-4 complex owing to its proteolytic activity and NS5B protein due to its RNA-dependent RNA polymerase activity. The first DAA that was licensed against HCV used NS3-4 complex as the target. A study in 2019 has shown that DAAs have improved survival rates in patients having HCV-related cirrhosis. Chronic infections by all 6 major genotypes of HCV have been reported to be treated safely and efficiently using a combination of two DAAs targeting NS3-4 complex (Glecaprevir) and NS5A (pibrentasvir) [ 36 ].
Sofosbuvir (SOF) is a very efficient and effective pan-genotypic drug, especially for 1-4 HCV genotypes. It has great potential to act as an antiviral agent and has suitable pharmacokinetic properties [ 37 ]. This drug is safe for consumption and there is minimal risk of drug resistance. It is a NS5B nucleotide inhibitor that prevents the formation of HCV nucleotide (RNA) synthesis by the termination of RNA chains [ 38 ].
Likewise, another pan-genotypic drug, MAVYRET (combination of Glecaprevir and Pibrentasvir) has been approved by FDA in 2017 for treating chronic hepatitis C infections. EPCLUSA (combination of sofosbuvir and velpatasvir) is another pan-genotypic drug that has been approved for treating adults infected with chronic HCV infection. VOSEVI, a remarkable drug which is the combination of drugs from three separate antiviral groups, have been approved in 2017 for HCV genotypes 1–6 [ 37 ].
However, genetic diversity among variants due to the high mutation rate of HCV is a major challenge to the broad-term efficacy of DAAs. Moreover, serious side-effects and the development of resistance in patients are also some of the major shortcomings of DAAs. One of the possible reasons for this resistance could be the cell-to-cell-mediated transferability of HCV [ 39 ].
Viral genotype assessment is required in nations without pan-genotypic programs to customize medication and provide affordable therapies [ 40 ]. The drug named HARVONI which is a combination of ledipasvir and sofosbuvir was approved in 2014 for treatment of HCV genotype 1 in patients having liver cirrhosis. It is more effective drug as compared to traditional Ribavirin or peg-interferone therapy. It is a non-invasive treatment method having less side effects, high SVR rates, and the brief length of therapy [ 40 ]. Zepatier is an excellent drug for treatment of HCV genotypes 1 and 4 infection. It is a combination of grazoprevir and elbasvir. It yields high SVR rates and is favorable in terms of safety and efficacy. Patients with liver cirrhosis can also be treated using Zepatier [ 41 ]. Daclatasvir is an efficient antiviral agent used for the treatment of HCV genotypes 2 and 3. It is an inhibitor of NS5A protein. Daclatasvir is used in combination with sofosbuvir for treating HCV infections with SVR rates of 90% in patients having cirrhosis and > 90% for patients without cirrhosis. This drug has high safety and tolerability properties and can effectively treat HCV infections [ 42 ].
A combination of sofosbuvir and velpatasvir is approved for the treatment of patients infected with genotypes 5 and 6 for 4 months. High SVR rates are achieved in case of patients receiving treatment and for those who have not been treated before. Patients having cirrhosis are also effectively treated using this drug [ 43 ].
Now with the advancements in tissue culture models for HCV, host factors have also been shown to be potential targets against HCV infection that resulted in the development of host targeting agents (HTAs). An effective way to control HCV infection is to inhibit the entry of HCV in hepatocytes. This can be achieved by targeting host receptors and additional entry factors. One of the potential targets is the SCARB1 receptor against which ITX 5061 is available as HTA. However, a mutation in E2 glycoprotein has been reported as a result of long-term use of ITX 5061, indicating the possibility of HTA resistance in patients. Remarkably, neutralizing antibodies have also been proved to be effective as in HCV clearance. In addition to entry factors, other factors of host required at later steps of HCV cycle can also be used as targets for HTAs like CypA, apo E, miR-122, MTTP, PI4KIIIα, and DGAT1. For instance, Avasimibe, an inhibitor of Apo B and Apo E secretion, is a clinically approved HTA and can be used against all 6 major genotypes of HCV [39]. Likewise, ASP5286, a potential inhibitor of HCV has been discovered that targets cyclophilin [ 44 ].
The non-coding RNAs (ncRNAs) is a novel class of potential genes that can serve as potential targets for drug development or discovery. These genes not only regulate the expression and functioning of normal genes but also aid in disease progression. Understanding the mechanism of action of these genes and their classification can aid in designing customized therapies for the treatment of HCV and other infections [ 8 ].
Circular RNAs (circRNA) are a subtype of non-coding RNAs that despite their low abundance, are very stable molecules and are found to be associated with the progression of several diseases. In the case of HCV infection, some circRNAs are found to be upregulated while others are reported to be downregulated. One example is of circPSD3 whose deregulation was found to be associated with the pathogenesis of HCV [ 9 ]
Moreover, downregulation of circ-ZEB1.33 is found to be associated with HCC progression. On contrary, some circRNAs hinder the progression of HCV-related carcinoma, e.g., circFBLIMI and circSETD3. Thus, the potential of circRNAs as biomarkers can be evaluated by analyzing their expression upon HCV infection [ 10 ].
Interestingly a study has reported upregulation of circCMTM3 in HCC cells. This circ RNA has been shown to regulate the expression of EZH2 and thus can serve as a therapeutic target in HCV infection [ 11 ]
MicroRNA ‘miR-122’ is abundantly present and highly expressed in liver cells. It facilitates the synthesis of HCV RNA by efficiently binding with 5′-UTR of HCV RNA. Targeting this microRNA, the replication of HCV RNA can be inhibited. Miravirsen, an anti-miR-122 is developed to prevent miRNA-122 role in promoting HCV infection [ 8 ]. Miravirsen injections have been given to hepatitis patients and a substantial amount of decreased miRNA levels have been observed in the plasma of these patients [ 12 ].
MicroRNA (MiR-155) is responsible for cancer formation due to its oncogenic potential. It causes inflammation leading to the formation of tumors at sites. The levels of MiR-155 are upregulated in the case of hepatocellular carcinoma. MiR-155 promotes cell migration, cell invasion and cell proliferation in hepatic cells (hepatocytes) [ 8 ].
Some other novel genes include TAF1 and HNF4A can serve as potential biomarkers for the assessment of liver fibrosis. These can also be targeted to design therapy for liver treatment. The CALM2 gene levels are found to be inversely proportional to the degree of liver fibrosis. Upregulation of the CALM2 genes can be helpful for designing liver cancer treatment [ 45 ].
A potential vaccine candidate against HCV could be a disulfide motif of broadly neutralizing antibodies that interacts with E2 protein [ 46 ]. Similarly, neutralizing antibodies against the AR3 epitope of HCV have been reported to promote viral clearance [ 47 ]. Interestingly, it has been proposed that combinations of different neutralizing antibodies can be an effective and broad-term therapy against the hepatitis C virus. Moreover, a peptide vaccine containing 6 different epitopes has been shown to induce a very strong immune response in mice models. Likewise, antibodies against host factor OCLN has been reported to completely inhibit HCV infection in a mouse [ 48 ]. A study has reported that recombinant E1E2 glycoproteins of the HCV envelope can induce an insufficient anti-body response in humans and non-human primates [ 49 ]. In addition to entry factors, other factors of host required at later steps of the HCV cycle can also be used as targets for HTAs like CypA, apo E, miR-122, MTTP, PI4KIIIα, and DGAT1 [ 49 ].
During the last two decades, extensive research on the hepatitis C virus has provided significant information regarding its structure and life cycle that not only revealed its molecular nature but also gave insight into better therapeutic strategies. Although many advancements in HCV treatment have been made like DAAs and HTA s, the development of a completely effective HCV therapy is still a challenge. Further research on combinations of DAAs and HTAs can help in developing a better therapeutic alternative. Furthermore, many viral and host-cellular factors involved in different stages of the HCV life cycle have been discovered; however, the complete mechanism is still unknown. Extensive studies in this area would not only contribute to the knowledge concerning HCV infection but would also help in the development of novel and modern treatment strategies against this blood-borne virus.
Availability of data and materials
Stanaway JD et al (2016) The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 388(10049):1081–1088
Article Google Scholar
Al Kanaani Z et al (2018) The epidemiology of hepatitis C virus in Pakistan: systematic review and meta-analyses. R Soc Open Sci 5(4):180257
Morozov VA, Lagaye S (2018) Hepatitis C virus: Morphogenesis, infection and therapy. World J Hepatol 10(2):186
Mostafa A et al (2016) Excess mortality rate associated with hepatitis C virus infection: a community-based cohort study in rural Egypt. J Hepatol 64(6):1240–1246
Khan A et al (2017) Tracing the epidemic history of hepatitis C virus genotypes in Saudi Arabia. Infect Genet Evol 52:82–88
AlMalki WH et al (2021) Virological surveillance, molecular phylogeny, and evolutionary dynamics of hepatitis C virus subtypes 1a and 4a isolates in patients from Saudi Arabia. Saudi J Biol Sci 28(3):1664–1677
Article CAS Google Scholar
Rezaee N, Babaeekhou L, Ghane M (2020) Hepatitis C virus in Iran; transmission routes, growth in 3a genotype distribution, and lack of liver marker relation with genotypes. Int J Res Med Sci 25
Wang X et al (2021) MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut 70(4):784–795
Chen T-C et al (2020) Host-derived circular RNAs display proviral activities in Hepatitis C virus-infected cells. PLoS Pathog 16(8):e1008346
Song C et al (2019) The competing endogenous circular RNA ADAMTS14 suppressed hepatocellular carcinoma progression through regulating microRNA-572/regulator of calcineurin 1. J Cell Physiol 234(3):2460–2470
Lin T et al (2019) Silencing Of hsa_circ_0008450 represses hepatocellular carcinoma progression through regulation of microRNA-214-3p/EZH2 Axis. Cancer Manag Res 11:9133–9143
Qadir MI et al (2020) RNA therapeutics: identification of novel targets leading to drug discovery. J Cell Biochem 121(2):898–929
Pileri P et al (1998) Binding of hepatitis C virus to CD81. Science 282(5390):938–941
Ploss A et al (2009) Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 457(7231):882–886
Bankwitz D et al (2010) Hepatitis C virus hypervariable region 1 modulates receptor interactions, conceals the CD81 binding site, and protects conserved neutralizing epitopes. J Virol 84(11):5751–5763
Scarselli E et al (2002) The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J 21(19):5017–5025
Fénéant L, Levy S, Cocquerel L (2014) CD81 and hepatitis C virus (HCV) infection. Viruses 6(2):535–572
Harris HJ et al (2010) Claudin association with CD81 defines hepatitis C virus entry. J Biol Chem 285(27):21092–21102
Iwamoto M et al (2020) The machinery for endocytosis of epidermal growth factor receptor coordinates the transport of incoming hepatitis B virus to the endosomal network. J Biol Chem 295(3):800–807
Blanchard E et al (2006) Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol 80(14):6964–6972
Moradpour D, Penin F (2013) Hepatitis C virus proteins: from structure to function. Hepatitis C virus: from molecular virology to antiviral therapy, pp 113–142
Book Google Scholar
Germain M-A et al (2014) Elucidating novel hepatitis C virus–host interactions using combined mass spectrometry and functional genomics approaches. Mol Cell Proteomics 13(1):184–203
Reiss S et al (2011) Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe 9(1):32–45
Li H et al (2014) Hepatitis C virus NS5A hijacks ARFGAP1 to maintain a phosphatidylinositol 4-phosphate-enriched microenvironment. J Virol 88(11):5956–5966
Salloum S et al (2013) Rab18 binds to hepatitis C virus NS5A and promotes interaction between sites of viral replication and lipid droplets. PLoS Pathog 9(8):e1003513
Targett-Adams P, Boulant S, McLauchlan J (2008) Visualization of double-stranded RNA in cells supporting hepatitis C virus RNA replication. J Virol 82(5):2182–2195
Jirasko V et al (2010) Structural and functional studies of nonstructural protein 2 of the hepatitis C virus reveal its key role as organizer of virion assembly. PLoS Pathog 6(12):e1001233
Ma Y et al (2011) Hepatitis C virus NS2 protein serves as a scaffold for virus assembly by interacting with both structural and nonstructural proteins. J Virol 85(1):86–97
Tellinghuisen TL, Foss KL, Treadaway J (2008) Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog 4(3):e1000032
Scheel TK et al (2012) Analysis of functional differences between hepatitis C virus NS5A of genotypes 1–7 in infectious cell culture systems. PLoS Pathog 8 (5):e1002696
Phan T et al (2011) The acidic domain of hepatitis C virus NS4A contributes to RNA replication and virus particle assembly. J Virol 85(3):1193–1204
Gouklani H et al (2012) Hepatitis C virus nonstructural protein 5B is involved in virus morphogenesis. J Virol 86(9):5080–5088
Herker E et al (2010) Efficient hepatitis C virus particle formation requires diacylglycerol acyltransferase-1. Nat Med 16(11):1295–1298
Menzel N et al (2012) MAP-kinase regulated cytosolic phospholipase A2 activity is essential for production of infectious hepatitis C virus particles. PLoS Pathog 8(7):e1002829
Weller R et al (2017) Hepatitis C virus strain-dependent usage of apolipoprotein E modulates assembly efficiency and specific infectivity of secreted virions. J Virol 91(18):e00422–e00417
Liu Z, He JJ (2013) Cell-cell contact-mediated hepatitis C virus (HCV) transfer, productive infection, and replication and their requirement for HCV receptors. J Virol 87(15):8545–8558
Desnoyer A et al (2016) Pharmacokinetics, safety and efficacy of a full dose sofosbuvir-based regimen given daily in hemodialysis patients with chronic hepatitis C. J Hepatol 65(1):40–47
Ahmed H et al (2018) Safety and efficacy of sofosbuvir plus velpatasvir with or without ribavirin for chronic hepatitis C virus infection: a systematic review and meta-analysis. J Infect Public Health 11(2):156–164
Xiao F et al (2014) Hepatitis C virus cell-cell transmission and resistance to direct-acting antiviral agents. PLoS Pathog 10(5):e1004128
Aboras SI et al (2021) A review on analytical strategies for the assessment of recently approved direct acting antiviral drugs. Crit Rev Anal Chem:1–23
Papudesu C, Kottilil S, Bagchi S (2017) Elbasvir/grazoprevir for treatment of chronic hepatitis C virus infection. Hepatol Int 11:152–160. https://doi.org/10.1007/s12072-016-9761-2
Manolakopoulos S et al (2016) Safety and efficacy of daclatasvir in the management of patients with chronic hepatitis C. Ann Gastroenterol 29(3):282
Google Scholar
Feld JJ et al (2015) Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 373(27):2599–2607
Makino T et al (2020) Discovery of ASP5286: A novel non-immunosuppressive cyclophilin inhibitor for the treatment of HCV. Bioorg Med Chem Lett 30(16):127308
Ji D et al (2019) Identification of TAF1, HNF4A, and CALM2 as potential therapeutic target genes for liver fibrosis. J Cell Physiol 234(6):9045–9051
Flyak AI et al (2020) An ultralong CDRH2 in HCV neutralizing antibody demonstrates structural plasticity of antibodies against E2 glycoprotein. Elife 9
Merat SJ et al (2019) Cross-genotype AR3-specific neutralizing antibodies confer long-term protection in injecting drug users after HCV clearance. J Hepatol 71(1):14–24
Shimizu Y et al (2018) Monoclonal antibodies against occludin completely prevented hepatitis C virus infection in a mouse model. J Virol 92(8):e02258–e02217
Wakita T et al (2005) Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 11(7):791–796
Download references
Acknowledgements
All authors contributed to the main writing and editing of the manuscript. All authors have read and approved the final manuscript.
No funding was received.
Author information
Authors and affiliations.
Divison of Molecular Virology, National Centre of Excellence in Molecular Biology (CEMB), University of the Punjab, Lahore, Pakistan
Fatima Butt, Muhammad Shahid, Muhammad Hassan, Farah Tawakkal, Iram Amin, Samia Afzal, Rashid Bhatti & Muhammad Idrees
Department of Allied Health Sciences, The Superior University, Lahore, Pakistan
Rabia Nawaz
You can also search for this author in PubMed Google Scholar
Contributions
FB, MH, and MS analyzed and interpreted patient data and drafted the manuscript. FT, IA, SA RB, RN, and MI participated, coordinated, and analyzed the clinical data. The authors read and approved the final manuscript.
Corresponding author
Correspondence to Muhammad Shahid .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
All the authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Butt, F., Shahid, M., Hassan, M. et al. A review on hepatitis C virus: role of viral and host-cellular factors in replication and existing therapeutic strategies. Egypt Liver Journal 12 , 71 (2022). https://doi.org/10.1186/s43066-022-00232-w
Download citation
Received : 25 February 2022
Accepted : 13 December 2022
Published : 27 December 2022
DOI : https://doi.org/10.1186/s43066-022-00232-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Hepatitis C - Science topic

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
A clinical review of viral hepatitis
Loader, Michelle MPAS, PA-C; Moravek, Rudolph MPAS, PA-C; Witowski, Sarah E. MPAS, PA-C; Driscoll, Lynette M. MA, PA-C
At the University of Colorado School of Medicine in Aurora, Colo., Michelle Loader is a senior instructor in the Department of Medicine, Division of Hospital Gastroenterology and Hepatology; Rudolph Moravek and Sarah E. Witowski are instructors in the Department of Medicine, Division of Hospital Medicine; and Lynette M. Driscoll is an instructor in the Department of Surgery, Division of Transplant Surgery. The authors have disclosed no potential conflicts of interest, financial or otherwise.
Earn Category I CME Credit by reading both CME articles in this issue, reviewing the post-test, then taking the online test at http://cme.aapa.org . Successful completion is defined as a cumulative score of at least 70% correct. This material has been reviewed and is approved for 1 hour of clinical Category I (Preapproved) CME credit by the AAPA. The term of approval is for 1 year from the publication date of November 2019.
Viral hepatitis remains a significant public health problem in the United States, despite advances in antiviral therapy and effective vaccines. According to the CDC, about 20,000 deaths each year are attributed to viral hepatitis, and 5 million people are chronically infected and at risk for serious liver disease and hepatocellular cancer. This article reviews the three most common causes of viral hepatitis, screening guidelines, clinical features, medical management, approaches for primary prevention, and the natural history of untreated disease.

Despite advances in antiviral therapy and access to effective vaccines, viral hepatitis remains a significant public health problem in the United States. The condition is most commonly caused by infection from the hepatitis A virus (HAV), hepatitis B virus (HBV), or hepatitis C virus (HCV). Liver injury can result from nearly any viral infection with systemic involvement, however, including cytomegalovirus and herpes simplex virus. Clinical features of viral hepatitis, including risk for progression to chronic infection with development of cirrhosis, vary considerably and are virus-specific. As frontline healthcare providers, physician assistants (PAs) can reduce the burden of disease through infection prevention, early detection, and collaborative care with specialists.
HEPATITIS A
HAV is an RNA virus transmitted person-to-person via the fecal-oral route, typically after consuming contaminated food or handling contaminated objects. Risk factors for HAV exposure include traveling to countries with high endemic rates of infection as well as being in close contact with HAV-infected persons. The overall incidence of HAV has declined since the HAV vaccine was added to the childhood immunization schedule. Peak outbreaks of the disease occurred in the United States in 2012-2013 and again in 2015-2016. 1 In 2016, more than 2,000 cases were reported to the CDC, although the actual number was estimated to be closer to 4,000. 1 Recent nationwide outbreaks have been linked to imported food, but state health departments have reported that person-to-person transmission affecting homeless persons and those who use illicit drugs is becoming more widespread. 2

Because HAV has a low prevalence, self-limited clinical course, and is associated with lifelong immunity after exposure, routine HAV screening of the general population is not recommended ( Table 1 ).

Clinical features
After the average incubation period of 28 days, HAV may cause fatigue, abdominal discomfort, vomiting, pruritus, or fever. Patients with severe illness may develop jaundice and dark-colored urine. Most adults are symptomatic, but the condition is asymptomatic in about 70% of infections among children under age 6 years.
Diagnosis and management
Clinical suspicion for acute hepatitis should prompt laboratory testing for the HAV immunoglobulin M antibody (IgM anti-HAV) ( Table 1 ). Associated laboratory abnormalities may include elevated alanine transaminase (ALT) and aspartate transaminase (AST) enzymes; total serum bilirubin and alkaline phosphatase will be normal or mildly elevated. Cases of viral hepatitis due to HAV, HBV, and HCV are nationally notifiable conditions, though local and state-specific reporting requirements may vary.
Management is largely supportive because symptoms typically resolve within several weeks. Advise patients to avoid alcohol and medications associated with potential hepatotoxicity, such as acetaminophen, until they have recovered. Encourage good hand hygiene and administration of the HAV vaccine for unvaccinated caregivers and close contacts.
Older adults and patients with underlying chronic liver disease are at increased risk for severe infection. Fewer than 1% of patients develop acute liver failure and need to be considered for emergency transplant evaluation. 3 Acute hepatitis due to HAV does not progress to chronic infection ( Table 1 ).
Vaccination provides long-term protection and should be offered to groups at increased risk for infection, such as military personnel, travelers to endemic areas, IV drug users, persons with chronic liver disease, and those with occupational risks for infection or who are in close contact with international adoptees. Also consider administering vaccine to any unvaccinated person who requests immunity. In 2018, the CDC's Advisory Committee on Immunization Practices (ACIP) updated its guidelines with recommendations to vaccinate infants ages 6 to 11 months who are traveling internationally. 4
A single dose of HAV immune globulin (IG) administered IM offers short-term protection and is recommended for postexposure prophylaxis (PEP) for all immunocompetent patients age 12 months or older. 4 Positive HAV immunoglobulin G antibody (IgG anti-HAV) testing indicates immunity ( Table 1 ).
HEPATITIS B
HBV is a DNA virus transmitted by percutaneous or perinatal exposure, or from direct mucosal contact with HBV-infected blood or body fluid.
In 2016, more than 3,000 new cases of HBV hepatitis were reported to the CDC, considerably lower than the estimate of 20,000 cases. 1 In the same year, the number of patients with hepatitis due to chronic HBV was projected at 850,000 to 2.2 million. 1
All pregnant women regardless of vaccination status, and patients at increased risk for HBV infection ( Table 2 ) , should be screened for the HBV surface antigen (HBsAg) and HBV surface antibody (anti-HBs) ( Table 1 ). 5 A positive HBsAg test indicates acute or chronic infection regardless of HBV DNA level. Even if immunity has been established, testing for the antibody to the HBV core antigen (IgG anti-HBc) is recommended for patients who need immunosuppressive therapy and would be at risk for HBV disease reactivation. 6

Following HBV transmission and an incubation period ranging from 4 weeks to 6 months, 30% to 50% of immunocompetent persons age 5 years and older will develop clinical signs and symptoms of infection. 7 Symptoms may include fever, fatigue, loss of appetite, vomiting, abdominal pain, dark-colored urine, clay-colored stools, arthralgias, and jaundice. Symptoms generally are present for several weeks but can persist for up to 6 months. As with HAV, severe illness is more prevalent among older adults. The rate of progression to acute liver failure is estimated at 1% to 2%, and without transplantation, the prognosis is poor. 7
The risk for developing chronic infection is inversely related to patient age at the time of transmission, with fewer than 5% of immunocompetent adults developing chronic infection, compared with 90% of newborns. 7
Serologic testing differentiates acute versus chronic HBV infection and identifies patients who have acquired immunity, either through vaccination or natural immune clearance. Positive HBsAg and immunoglobulin M antibody to the HBV core antigen (IgM anti-HBc) establishes acute infection. If clinical suspicion for infection is high, perform repeat testing to exclude a window period where both HBsAg and anti-HBs are undetectable. Chronic infection is confirmed by the presence of HBsAg for at least 6 months. If HBV e-antigen also is positive, active viral replication is occurring and the patient is considered highly contagious. An isolated positive anti-HBs generally is consistent with immunity conferred by vaccination; the additional finding of positive IgG anti-HBc indicates recovery from previous infection ( Table 1 ).
All patients diagnosed with chronic hepatitis due to HBV should be counseled about the importance of lifelong monitoring with serial laboratory testing, practicing universal precautions to reduce the risk of disease transmission, and testing for HCV coinfection. Additionally, sexual and household contacts should be offered vaccination against HBV. Although HBV cannot be cured, viral suppressive therapy is highly effective. The goal of treatment is to reduce HBV viral load and the risk for histologic progression of liver disease. The decision to initiate pharmacologic therapy is based on review of HBeAg status, ALT level, HBV DNA, and evidence of advanced liver fibrosis. 6 Antiviral therapy is recommended for all patients with previous HBV exposure who are undergoing chemotherapy or other immunosuppressive therapy, because they need prophylaxis against HBV reactivation. 6
First-line antiviral agents include tenofovir and entecavir, although medication selection may be individualized according to anticipated treatment duration, resistance patterns, and adverse reaction profile.
The first dose of the HBV vaccine should be administered at birth as part of the routine series and immunity typically is achieved within 18 months. 5 The HBV vaccine also is recommended for anyone who screens negative for anti-HBs. In 2018, Heplisav-B was approved by the FDA for adults age 18 years and older; the two doses of the vaccine are administered 4 weeks apart. Testing for serologic evidence of immunity postvaccination is not routinely performed and is only indicated if knowing the patient's immune status will affect clinical management (for example, in the case of healthcare workers who are at risk for exposure to HBV-infected body fluids).
HEPATITIS C (HCV)
HCV is an RNA virus transmitted by exposure to HCV-infected blood or blood-containing body fluid (for example, by sharing HCV-contaminated needles during IV drug use). Cases of HCV infection have been associated with occupational injuries, perinatal transmission, sexual transmission, receiving piercings or tattoos from unregulated body art studios, and transfusion of unscreened blood before 1992.
In 2016, nearly 3,000 new cases of acute hepatitis due to HCV were reported to the CDC, and the number of chronic cases was estimated at 3.5 million. 1 An estimated 50% of patients with chronic hepatitis due to HCV are unaware that they are infected; this figure includes more than 800,000 people who are institutionalized, incarcerated, or homeless. 8
One-time HCV screening with HCV antibody (anti-HCV) testing is recommended for the following groups:
- Anyone born between 1945 and 1965, regardless of risk factors
- Anyone with recent or remote history of IV drug use
- Anyone with increased risk for HCV exposure, such as persons on chronic hemodialysis, children born to mothers with HCV infection, patients who received an organ transplant or blood transfusion before 1992, patients with HIV infection, and those with unexplained ALT abnormalities. 9
A positive anti-HCV indicates previous exposure and does not confirm immunity. Perform both anti-HCV and HCV RNA testing if the patient was exposed to the virus within the past 6 months ( Table 1 ). Patients who continue to engage in high-risk behaviors or have ongoing risk exposures should also be screened annually, even if they have been previously treated for HCV. 10
Acute hepatitis due to HCV often is unrecognized because patients who are infected frequently are asymptomatic. Regardless, 75% to 85% of these patients will go on to develop chronic infection after the 6-month acute phase. 11 Chronic infection due to HCV also is asymptomatic; the diagnosis is typically established through routine screening or diagnostic workup of an incidental abnormal finding on routine laboratory tests. Chronic infection is associated with increased risk for hepatic fibrosis, and 10% to 20% of patients with HCV infection eventually develop cirrhosis. 11
Extrahepatic conditions associated with HCV infection include cryoglobulinemia vasculitis, membranoproliferative glomerulonephritis, porphyria cutanea tarda, and B-cell non-Hodgkin lymphoma.
Qualitative HCV RNA testing usually confirms the presence of hepatitis C, which is detectable within 21 days of an exposure ( Table 1 ). Of the six known HCV genotypes, genotype 1 is the most common in patients from the United States. HCV viral load does not correlate with disease severity or prognosis. Like HBV infection, evidence of coexisting hepatic fibrosis can be assessed by a clinical examination revealing stigmata of cirrhosis, serum biomarkers, vibration-controlled transient elastography, or cross-sectional imaging. Liver biopsy is not needed to assess the extent of fibrosis but can be considered when there are discordant results.
HCV treatment has evolved significantly in the last decade with the development of direct-acting antiviral (DAA) medications. The primary goal of therapy is to achieve an undetectable HCV RNA level or sustained viral response at 12 weeks post-treatment, which is associated with reduced morbidity and mortality. 12 DAA regimens are pan-genotypic and well tolerated with minimal drug interactions. FDA-approved medications include ledipasvir-sofosbuvir, sofosbuvir-velpatasvir, elbasvir-grazoprevir, sofosbuvir-velpatasvir-voxilaprevir, and glecaprevir-pibrentasvir. Once sustained viral response has been achieved, repeat HCV RNA testing is not recommended unless there is clinical concern for reinfection.
The American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) have partnered to develop HCV Guidance , an online resource that offers guidelines about medication selection and treatment duration, including recommendations for specific populations such as pregnant patients and those with decompensated cirrhosis, coinfections with HBV or HIV, or renal impairment. 10 The online resource also provides recommendations for when to refer a patient to a liver specialist.
No approved vaccines are protective against HCV, and PEP is not recommended.
HIV COINFECTION
An estimated 25% of patients with HCV infection and 10% of patients with HBV infection are coinfected with HIV, a fact that highlights the importance of HBV/HCV screening in patients with HIV infection. 10 In the era of DAA therapy, treating HCV in HIV/HCV-coinfected patients is now comparable to HCV monotherapy. 10 Liver transplant also can be considered if the patient has satisfactory immune function and persistent HIV viral suppression. In 2013, the HIV Organ Policy Equity (HOPE) Act was enacted so that HIV-positive organs can be offered to patients who are HIV-positive. 13
RISK OF HEPATOCELLULAR CANCER
Hepatocellular cancer is a leading cause of cancer-related death in the United States, and its incidence has steadily increased over the past 20 years. 14,15 Risk factors associated with the development of hepatocellular cancer include cirrhosis (regardless of etiologic factors) and chronic hepatitis due to HBV or HCV. Patients diagnosed with cirrhosis due to chronic HCV have an estimated 1% to 5% annual risk for developing hepatocellular cancer. 16 Screening is recommended for patients who have cirrhosis or chronic HBV with a family history of hepatocellular cancer and who are likely to tolerate hepatocellular cancer treatment. 16 AASLD guidelines recommend screening for hepatocellular cancer with abdominal ultrasound, with or without alfa-fetoprotein (AFP) level, every 6 months. 16 Having a sustained viral response does not eliminate the need for hepatocellular cancer surveillance in patients with cirrhosis due to chronic HCV.
HBV and HCV account for almost all cases of chronic viral hepatitis. Repeated inflammatory insults and injury to the liver parenchyma cause structural changes that lead to fibrosis. The degree of fibrosis is described by stage, using one of several histopathology scoring systems (for example, METAVIR or Batts-Ludwig) and ranges from no fibrosis (stage 0) to cirrhosis (stage 4). 17
The features and complications of cirrhosis arise from decreased hepatic function (altered protein synthesis, metabolic disturbances) as well as structural changes to the liver resulting in progressive portal hypertension. Recognizing patients who have cirrhosis can be challenging because often they are asymptomatic. As a result, patients are often diagnosed after seeking care for symptoms of cirrhosis decompensated by jaundice, ascites, hepatic encephalopathy, or variceal bleeding.
- Jaundice is the yellowing of the skin due to increased concentrations of serum bilirubin.
- Ascites results from portal fluid leaking into the peritoneal cavity due to increased resistance to hepatic flow secondary to fibrosis and reduced albumin synthesis. Management includes strict adherence to a low-sodium diet, diuretic therapy, and therapeutic paracentesis as clinically indicated. A transjugular intrahepatic portosystemic shunt (TIPS) procedure can be considered for patients with refractory ascites.
- Hepatic encephalopathy is a disturbance in brain function related to an accumulation of toxic nitrogenous byproducts crossing the blood-brain barrier in patients with reduced filtration due to hepatic dysfunction. Hepatic encephalopathy is treated with lactulose, a nonabsorbable disaccharide that titrated to effect, may be given with rifaximin, a nonabsorbable antibiotic. Secondary prophylaxis is recommended after the first episode of hepatic encephalopathy. 18
- Esophageal varices form in response to increased intraportal pressures with splanchnic vascular dilation. Varices can spontaneously rupture and are associated with life-threatening gastrointestinal bleeding. Patients with newly diagnosed cirrhosis should undergo a screening endoscopy to assess for esophageal varices. Timing of ongoing surveillance varies and depends on endoscopy findings.
Patients also may seek consultation for dermatologic manifestations of liver disease such as spider angioma, palmar erythema, or pruritus.
Diagnostic approach
Although liver biopsy is considered the gold standard test, it is possible to diagnose cirrhosis caused by chronic viral hepatitis by review of viral serologies, physical examination notable for stigmata of chronic liver disease, and/or cross-sectional imaging. As liver disease progresses, laboratory results consistent with decreased synthetic function of the liver may include an elevated International Normalized Ratio with hypoalbuminemia. Thrombocytopenia is suggestive of hypersplenism due to portal hypertension and decreased thrombopoietin produced by the liver. Normal liver function tests do not exclude cirrhosis.
Nonsurgical management
With few exceptions, management of cirrhosis is independent of cause. A comprehensive approach involves counseling patients about their disease and prognosis, using evidence-based guidelines to screen for complications, and employing strategies to minimize further decompensation. Patients with cirrhosis also should receive vaccinations against HAV and HBV when applicable, and receive counseling about the importance of avoiding alcohol and hepatotoxic medications.
Liver transplantation
According to AASLD guidelines, patients with chronic viral hepatitis should be referred for liver transplant evaluation if they develop decompensated cirrhosis, hepatocellular cancer (depending on size and number of lesions), or have a calculated Model for End-Stage Liver Disease (MELD) score of 15 or greater. 13 The MELD score, calculated using serum INR, total bilirubin, creatinine, and sodium values, was originally developed to predict 3-month mortality for patients with cirrhosis undergoing TIPS, and is used to assess the severity of disease.
For patients undergoing transplant for HCV who have not achieved virologic cure, recurrent infection in the graft is universal. DAA therapy can be considered post-transplant despite immunosuppression. Following transplant for cirrhosis due to chronic HBV, lifelong (suppressive) antiviral therapy is recommended to reduce the risk for development of HBV hepatitis in the allograft.
The World Health Organization recently announced a goal of eliminating viral hepatitis as a major public health threat by 2030. 19 Because PAs practice in nearly every clinical setting and specialty, they likely will encounter patients with liver disease due to viral hepatitis, and are appropriately positioned to drive change to achieve this goal. In addition to contributing to public awareness campaigns that support elimination efforts, PAs can promote safe and effective vaccinations that provide long-term protection against HAV and HBV, use screening tools to detect HCV earlier to mitigate patient risk for cirrhosis, and collaborate with specialists to initiate DAA therapy to achieve virologic cure in select patients with HCV.
- Cited Here |
- View Full Text | PubMed | CrossRef
- PubMed | CrossRef
hepatitis; antiviral; public health; hepatocellular cancer; liver transplant; cirrhosis
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Colorectal cancer screening: the role of the noninvasive options, acute kidney injury, the interservice physician assistant program: the veterans' solution, when exercise causes exertional rhabdomyolysis, cholelithiasis.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
CDC Recommendations for Hepatitis C Screening Among Adults — United States, 2020
Recommendations and Reports / April 10, 2020 / 69(2);1–17
Sarah Schillie, MD 1 ; Carolyn Wester, MD 1 ; Melissa Osborne, PhD 1 ; Laura Wesolowski, PhD 1 ; A. Blythe Ryerson, PhD 1 ( View author affiliations )
Views: Views equals page views plus PDF downloads
Introduction, recommendations, future directions, acknowledgments.
- Updated CDC Recommendations for Universal Hepatitis C Virus Screening Among Adults and Pregnant Women: Implications for Clinical Practice
- MMWR Article PDF
Hepatitis C virus (HCV) infection is a major source of morbidity and mortality in the United States. HCV is transmitted primarily through parenteral exposures to infectious blood or body fluids that contain blood, most commonly through injection drug use. No vaccine against hepatitis C exists and no effective pre- or postexposure prophylaxis is available. More than half of persons who become infected with HCV will develop chronic infection. Direct-acting antiviral treatment can result in a virologic cure in most persons with 8–12 weeks of all-oral medication regimens. This report augments (i.e., updates and summarizes) previously published recommendations from CDC regarding testing for HCV infection in the United States ( Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rec 2012;61[No. RR-4] ). CDC is augmenting previous guidance with two new recommendations: 1) hepatitis C screening at least once in a lifetime for all adults aged ≥18 years, except in settings where the prevalence of HCV infection is <0.1% and 2) hepatitis C screening for all pregnant women during each pregnancy, except in settings where the prevalence of HCV infection is <0.1%. The recommendation for HCV testing that remains unchanged is regardless of age or setting prevalence, all persons with risk factors should be tested for hepatitis C, with periodic testing while risk factors persist. Any person who requests hepatitis C testing should receive it, regardless of disclosure of risk, because many persons might be reluctant to disclose stigmatizing risks.
Hepatitis C is the most commonly reported bloodborne infection in the United States ( 1 ), and surveys conducted during 2013 – 2016 indicated an estimated 2.4 million persons (1.0%) in the nation were living with hepatitis C ( 2 ). Percutaneous exposure is the most efficient mode of hepatitis C virus (HCV) transmission, and injection drug use (IDU) is the primary risk factor for infection ( 1 ). National surveillance data revealed an increase in reported cases of acute HCV infection every year from 2009 through 2017 ( 1 ). The highest rates of acute infection are among persons aged 20 – 39 years ( 1 ). As new HCV infections have increased among reproductive aged adults, rates of HCV infection nearly doubled during 2009 – 2014 among women with live births ( 3 ). In 2015, 0.38% of live births were delivered by mothers with hepatitis C ( 4 ).
This report augments (i.e., updates and summarizes) previous CDC recommendations for testing of hepatitis C among adults in the United States published in 1998 and 2012 ( 5 , 6 ). The recommendations in this report do not replace or modify previous recommendations for hepatitis C testing that are based on known risk factors or clinical indications. Previously published recommendations for hepatitis C testing of persons with risk factors and alcohol use screening and intervention for persons identified as infected with HCV remain in effect ( 5 , 6 ). This report is intended to serve as a resource for health care professionals, public health officials, and organizations involved in the development, implementation, delivery, and evaluation of clinical and preventive services.
Epidemiology
In 2017, a total of 3,216 cases (1.0 per 100,000 population) of acute HCV infection were reported to CDC ( 1 ). The reported number of cases in any given year likely represents less than 10% of the actual number of cases because of underascertainment and underreporting ( 7 ). An estimated 44,700 new cases of HCV infection occurred in 2017. The rate of reported acute HCV infections increased from 0.7 cases per 100,000 population in 2013 to 1.0 in 2017 ( Figure 1 ) ( 1 ). In 2017, acute HCV incidence was greatest for persons aged 20 – 29 years (2.8) and 30 – 39 years (2.3) ( 1 ). Persons aged ≤19 years had the lowest incidence (0.1) ( 1 ). Incidence was slightly greater for males than females (1.2 cases and 0.9, respectively) ( 1 ). During 2006 – 2012, the combined incidence of acute HCV infection in four states (Kentucky, Tennessee, Virginia, and West Virginia) increased 364% among persons aged ≤30 years. Among cases in these states with identified risk information, IDU was most commonly reported (73%). Those infected were primarily non-Hispanic white persons from nonurban areas ( 8 ).
On the basis of 2013 – 2016 National Health and Nutrition Examination Survey (NHANES) data and considering populations not sampled in NHANES, an estimated 1.0% of all adults in the United States, or 2,386,100 persons, were living with HCV infection (HCV RNA positive) ( 2 ). Nine states comprise 51.9% of all persons living with HCV infection: California, Florida, New York, North Carolina, Michigan, Ohio, Pennsylvania, Tennessee, and Texas ( Figure 2 ) ( 9 ).
Virus Description and Transmission
HCV is a small, single-stranded, enveloped RNA virus in the flavivirus family with a high degree of genetic heterogeneity. Seven distinct HCV genotypes have been identified. Genotype 1 is the most prevalent genotype in the United States and worldwide, accounting for approximately 75% and 46% of cases, respectively ( 10 , 11 ). Geographic differences in global genotype distribution are important because some treatment options are genotype specific ( 11 , 12 ). High rates of mutation in the HCV RNA genome are believed to play a role in the pathogen’s ability to evade the immune system ( 11 ). Prior infection with HCV does not protect against subsequent infection with the same or different genotypes.
HCV is primarily transmitted through direct percutaneous exposure to blood. Mucous membrane exposures to blood also can result in transmission, although this route is less efficient. HCV can be detected in saliva, semen, breast milk, and other body fluids, although these body fluids are not believed to be efficient vehicles of transmission ( 11 , 13 ).
Persons at Risk for HCV Infection
IDU is the most common means of HCV transmission in the United States. Invasive medical procedures (e.g., injections and hemodialysis) pose risks for HCV infection when standard infection-control practices are not followed ( 14 , 15 ). Health care – related hepatitis C outbreaks also stem from drug diversion (e.g., tampering with fentanyl syringes) ( 16 , 17 ). Although HCV infection is primarily associated with IDU, high-risk behaviors (e.g., anal sex without using a condom), primarily among persons with HIV, are also important risk factors for transmission ( 18 ). Other possible exposures include sharing personal items contaminated with blood (e.g., razors or toothbrushes), unregulated tattooing, needlestick injuries among health care personnel, and birth to a mother with hepatitis C. Receipt of donated blood, blood products, and organs was once a common means of transmission but is now rare in the United States ( 19 ).
Before implementing universal blood product testing in 1992, children acquired hepatitis C predominantly through blood transfusion. Because of the increasing incidence of HCV infection among women of childbearing age, perinatal transmission (intrauterine or intrapartum) has become an increasingly important mode of HCV transmission ( 20 , 21 ). Among pregnant women from 2011 to 2016, hepatitis C virus testing increased by 135% (from 5.7% to 13.4%), and positivity increased by 39% (from 2.6% to 3.6%) ( 4 ). The risk for perinatal transmission is informed by a systematic review and meta-analysis of studies conducted in multiple countries and is 5.8% for infants born to mothers infected with HCV but not with HIV and doubles for infants born to mothers co-infected with HCV and HIV. Perinatal HCV transmission is almost always confined to infants born to mothers with detectable HCV RNA ( 22 ). Only approximately 20% of infants with perinatally acquired hepatitis C clear the infection, 50% have chronic asymptomatic infection, and 30% have chronic active infection ( 23 ). HCV-related liver disease rarely causes complications during childhood. Because fibrosis increases with disease duration, perinatally infected persons might develop severe disease as young adults ( 20 , 21 ).
Clinical Features and Natural History
Persons with acute HCV infection are typically either asymptomatic or have a mild clinical illness like that of other types of viral hepatitis ( 24 ). Jaundice might occur in 20%–30% of persons, and nonspecific symptoms (e.g., anorexia, malaise, or abdominal pain) might be present in 10%–20% of persons. Fulminant hepatic failure following acute hepatitis C is rare. The average time from exposure to symptom onset is 2–12 weeks (range: 2–26 weeks) ( 25 , 26 ). HCV antibodies (anti-HCV) can be detected 4–10 weeks after infection and are present in approximately 97% of persons by 6 months after exposure. HCV RNA can be detected as early as 1–2 weeks after exposure. The presence of HCV RNA indicates current infection ( 27 – 29 ).
Historically, approximately 15%–25% of persons were believed to resolve their acute infection without sequelae ( 30 ); however, more recent data suggest that spontaneous clearance might be as high as 46%, varying by age at the time of infection ( 31 ). Spontaneous clearance is lower among persons co-infected with HIV ( 11 ). Predictors of spontaneous clearance include jaundice; elevated alanine aminotransferase (ALT) level; hepatitis B virus surface antigen (HBsAg) positivity; female sex; younger age; HCV genotype 1; and host genetic polymorphisms, most notably those near the IL28B gene ( 27 – 29 ). Chronic HCV infection develops when viral replication evades the host immune response. The course of chronic liver disease is usually insidious, progressing slowly without symptoms or physical signs in most persons during the first 20 years or more following infection. Approximately 5%–25% of persons with chronic hepatitis C will develop cirrhosis over 10–20 years ( 30 ). Those with cirrhosis experience a 1%–4% annual risk for hepatocellular carcinoma ( 30 ). Persons who are male, aged >50 years, use alcohol, have nonalcoholic fatty liver disease, have hepatitis B virus (HBV) or HIV coinfection, and who are undergoing immunosuppressive therapy have increased rates of progression to cirrhosis. Extrahepatic manifestations of chronic HCV infection might occur and include membranoproliferative glomerulonephritis, essential mixed cryoglobulinemia, porphyria cutanea tarda ( 27 – 29 ), and lymphoma ( 32 ).
Diagnosis and Hepatitis C Elimination
In one report, the National Academies of Sciences, Engineering, and Medicine explored the feasibility of hepatitis C elimination and concluded that hepatitis C could be eliminated as a public health problem in the United States, but that substantial obstacles exist ( 33 ). In another report, specific actions were recommended to achieve elimination considering information, interventions, service delivery, financing, and research ( 34 ). These reports were the culmination of decades of progress in the development of HCV infection diagnostic and therapeutic tools.
In 1990, serologic tests to detect immunoglobulin G anti-HCV by enzyme immunoassay were licensed and became commercially available in the United States, and U.S. blood banks voluntarily began testing donations for anti-HCV ( 35 ). In 1991, U.S. Public Health Service issued interagency guidelines addressing hepatitis C screening of blood, organs, and tissues ( 35 ). These guidelines recommended hepatitis C testing for all donations of whole blood and components for transfusion, as well as testing serum/plasma from donors of organs, tissues, or semen intended for human use ( 35 ).
In 1998, CDC expanded the interagency guidelines to provide recommendations for preventing transmission of HCV; identifying, counseling, and testing persons at risk for hepatitis C; and providing appropriate medical evaluation and management of persons with hepatitis C ( 6 ). The guidelines recommended testing on the basis of risk factors for HCV infection for persons who ever injected drugs and shared needles, syringes, or other drug preparation equipment, including those who injected once or a few times many years ago and do not consider themselves as drug users; with selected medical conditions, including those who received clotting factor concentrates produced before 1987; who were ever on chronic hemodialysis (maintenance hemodialysis); with persistently abnormal ALT levels; who were prior recipients of transfusions or organ transplants, including those who were notified that they received blood from a donor who later tested positive for HCV infection; who received a transfusion of blood or blood components before July 1992, or who received an organ transplant before July 1992; and with a recognized exposure, including health care, emergency medical, and public safety workers after a needlestick injury, sharps injury, or mucosal exposure to blood infected with hepatitis C or children born to mothers infected with hepatitis C ( 6 ). In 1999, the U.S. Public Health Service and Infectious Diseases Society of America (IDSA) guidelines recommended hepatitis C testing for persons with HIV ( 36 ).
Because of the limited effectiveness of risk-based hepatitis C testing, CDC considered strategies to increase the proportion of infected persons who are aware of their status and are linked to care ( 5 ). In 2012, CDC augmented its guidance to recommend one-time hepatitis C screening for persons born during 1945–1965 (birth cohort) without ascertainment of risk ( 5 ). With an anti-HCV positivity prevalence of 3.25%, persons born in the 1945–1965 birth year cohort accounted for approximately three fourths of chronic HCV infections among U.S. adults during 1999–2008 ( 37 ). Approximately 45% of persons infected with HCV do not recall or report having specific risk factors ( 38 ). Included in the 2012 guidelines were recommendations for alcohol use screening and intervention for persons identified with HCV infection ( 5 ). This report expands hepatitis C screening to at least once in a lifetime for all adults aged >18 years, except in settings where the prevalence of HCV infection is <0.1%.
The 2012 CDC guidelines recommended that pregnant women be tested for hepatitis C only if they have known risk factors ( 5 ). However, in 2018, universal hepatitis C screening during pregnancy was recommended by the American Association for the Study of Liver Diseases and IDSA ( 39 ). This report expands hepatitis C screening for all pregnant women during each pregnancy, except in settings where the prevalence of HCV infection is <0.1%.
Existing strategies for hepatitis C testing have had limited success. The 2013–2016 surveys indicate only approximately 56% of persons with HCV infection reported having ever been told they had hepatitis C ( 38 ). Therefore, strengthened guidance for universal hepatitis C testing is warranted. Models to address barriers related to access to direct-acting antiviral (DAA) treatment are needed to ensure health care equity and the success of expanded hepatitis C screening. The recommendation for HCV testing that remains unchanged is regardless of age or setting prevalence, all persons with risk factors should be tested for hepatitis C, with periodic testing while risk factors persist. Any person who requests hepatitis C testing should receive it regardless of disclosure of risk because many persons might be reluctant to disclose stigmatizing risks.
Clinical Management and Treatment
The treatment for HCV infection has evolved substantially since the introduction of DAA agents in 2011. DAA therapy is better tolerated, of shorter duration, and more effective than interferon-based regimens used in the past ( 39 , 40 ). The antivirals for hepatitis C treatment include next-generation DAAs, categorized as either protease inhibitors, nucleoside analog polymerase inhibitors, or nonstructural (NS5A) protein inhibitors. Many agents are pangenotypic, meaning they have antiviral activity against all genotypes ( 20 , 21 , 40 ). A sustained virologic response (SVR) is indicative of cure and is defined as the absence of detectable HCV RNA 12 weeks after completion of treatment. Approximately 90% of HCV-infected persons can be cured of HCV infection with 8–12 weeks of therapy, regardless of HCV genotype, prior treatment experience, fibrosis level, or presence of cirrhosis ( 39 – 41 ).
Despite their favorable safety profile, DAAs are not yet approved for use in pregnancy. Safety data during pregnancy are preliminary and larger studies are required. A small study of seven pregnant women treated with ledipasvir/sofosbuvir identified no safety concerns ( 42 ). Until DAAs become available for use during pregnancy, testing women during pregnancy for HCV infection still has benefits to both the mother and the infant. Many women only have access to health care during pregnancy and the immediate postpartum period. In 2017, 12.4% of women aged 19–44 years were not covered by public or private health insurance ( 43 ). Pregnancy is an opportune time for women to receive a hepatitis C test while simultaneously receiving other prenatal pathogen testing such as for HIV or hepatitis B. The postpartum period might represent a unique time to transition women who have had HCV infection diagnosed during pregnancy to treatment with DAAs. Treatment during the interconception (interpregnancy) period reduces the transmission risk for subsequent pregnancies. Identification of HCV infection during pregnancy also can inform pregnancy and delivery management issues that might reduce the likelihood of HCV transmission to the infant. The Society for Maternal-Fetal Medicine recommends a preference for amniocentesis over chorionic villus sampling when needed, and for avoiding internal fetal monitoring, prolonged rupture of the membranes, and episiotomy among HCV-infected women, unless it is unavoidable ( 44 ).
Testing during pregnancy allows for simultaneous identification of infected mothers and infants who should receive testing at a pediatric visit. Testing of infants consists of HCV RNA testing at or after age 2 months or anti-HCV testing at or after age 18 months ( 39 ). Although DAA treatment is not approved for children aged <3 years, infected children aged <3 years should be monitored. In 2017, ledipasvir/sofosbuvir became the first DAA approved for use in persons aged 12–17 years ( 20 , 21 ). In 2019 glecaprevir/pibrentasvir became approved for use in persons aged ≥12 years ( 45 ), and ledipasvir/sofosbuvir became approved for use in persons aged ≥3 years ( 46 ).
No vaccine against hepatitis C exists and no effective pre- or postexposure prophylaxis (e.g., immune globulin) is available. Prenatal treatment options and/or infant antiviral postexposure prophylaxis might become available to prevent perinatal transmission. HCV infection is not an indication for Cesarean delivery and is not a contraindication to breastfeeding if nipples are not bleeding or cracked ( 44 ).
To inform these recommendations, comprehensive systematic reviews of the literature were conducted, analyzed, and assessed in two stages. These reviews examined the availability of evidence regarding HCV infection prevalence and the health benefits and harms associated with one-time hepatitis C screening for persons unaware of their status.
CDC determined that the new recommendations constituted scientific information that will have a clear and substantial impact on important public policies and private sector decisions. Therefore, the Information Quality Act required peer review by specialists in the field who were not involved in the development of these recommendations. CDC solicited nominations for reviewers from the American Association for the Study of Liver Diseases (AASLD), IDSA, and the American College of Obstetricians and Gynecologists (ACOG). Six clinicians with expertise in hepatology, gastroenterology, internal medicine, infectious diseases and/or obstetrics and gynecology provided structured peer reviews. In addition, feedback from the public was solicited through a Federal Register notice released on October 28, 2019, announcing the availability of the draft recommendations for public comment through December 27, 2019. CDC received 69 public comments on the draft document from academia, professional organizations, industry, and the public. Many of the comments from both peer reviewers and the public were in support of the recommendations. For those comments that proposed changes, the majority related to screening for hepatitis C in every pregnancy or removing the prevalence threshold for universal screening. Feedback attained during both the peer review process and the public comment period was reviewed by CDC. Ultimately, no changes to the recommendations were made; however, additional references and justification for the recommendation to screen during every pregnancy and maintaining the prevalence threshold were added to the document.
To facilitate the systematic review of the evidence, two research questions were formulated to guide the development of the recommendations:
- Does universal screening for hepatitis C virus infection among adults aged ≥18 years, compared with risk-based screening, reduce morbidity and mortality?
- Does universal screening for hepatitis C virus infection among pregnant women, compared with risk-based screening, reduce morbidity and mortality among mothers and their children?
An analytic framework describing the chain of indirect evidence was developed:
- How would universal screening for hepatitis C affect the number (and composition) of persons who screen positive for HCV infection?
- How many additional persons would be linked to care?
- Do desirable treatment effects outweigh undesirable effects?
Key questions (KQ) were formulated for each link of the chain (Supplementary Table 1, https://stacks.cdc.gov/view/cdc/85840 ):
- K.Q.1.a. What is the prevalence of HCV infection in the United States in the general population and by risk groups?
- K.Q.2.a. What is the diagnostic accuracy of HCV antibody testing?
- K.Q.2.b. What are the harms of hepatitis C screening?
- K.Q.2.c. What proportion of persons who screen positive for HCV infection are linked to care?
- K.Q.3.a. What is the effect of DAA treatment on HCV viral load?
- K.Q.3.b. What is the effect of DAA treatment on morbidity (including cirrhosis and hepatocellular carcinoma)?
- K.Q.3.c. What is the effect of DAA treatment on mortality (HCV-specific and all-cause)?
- K.Q.3.d. What are the adverse effects of DAA treatment?
Because the diagnostic accuracy of anti-HCV testing and treatment effects have been described previously, K.Q.2.a. and K.Q.3.a.–d. key questions were not included in this review.
Literature Review
Systematic reviews were conducted to examine benefits and harms of hepatitis C screening. The systematic review process for these recommendations was separated into two stages: 1) a review of evidence to inform the hepatitis C screening strategy among all adults and 2) a review of the evidence to inform the hepatitis C screening strategy among pregnant women.
Systematic reviews were conducted for literature published worldwide in Medline (OVID), Embase (OVID), CINAHL (EBSCO), Scopus, and Cochrane Library. For the all-adult review, the beginning search date was 2010 to capture studies reflecting the changing epidemiology of HCV infection and the availability of DAAs, and the end date was the run date of August 6, 2018 (Supplementary Table 2, https://stacks.cdc.gov/view/cdc/85840 ). For the pregnancy review, the beginning search date was 1998 to capture studies published since previous recommendations were issued in 1998, and the end date was the run date of July 2, 2018 (Supplementary Table 3, https://stacks.cdc.gov/view/cdc/85840 ). Duplicates were identified using the Endnote (Clarivate Analytics, Philadelphia, Pennsylvania, United States) automated “find duplicates” function with preference set to match on title, author, and year. Duplicates were removed from the Endnote library.
Following the initial collection of results from the search, titles/abstracts were independently reviewed by two persons. For papers in which the title indicated the study was irrelevant to the research question, abstracts were not reviewed.
Titles/abstracts for the all-adult review were independently reviewed by two reviewers, one of whom was always a senior abstractor (and author LW or SS). Conflicts were resolved by SS. If a conflict arose from a study whose title/abstract was reviewed only by both LW and SS, that study was retrieved for the full text review. All full texts were screened by both MO and LW. SS made the final decision regarding conflicts. Information from the full texts was extracted for the evidence review. A systematic review software program (Covidence; Melbourne, Victoria, Australia) was used to facilitate the all-adult review process.
Titles/abstracts for the pregnancy review were independently reviewed by two senior abstractors (LW or SS). Studies that either abstractor deemed as potentially relevant were retrieved for full text review. All full texts were screened by both senior abstractors. Information from the full texts was extracted for the evidence review.
Studies were excluded if they were conducted in a correctional facility because separate CDC guidance for hepatitis C screening in correctional facilities is under development. Other reasons for exclusion were: if prevalence data from 2010 forward could not be abstracted (all-adult review only); if the study reported estimated, projected, or self-reported data; if data were only available from a conference abstract, or if the study population was non-U.S. based, unless the study examined outcomes related to harms of screening. Studies related to harms of screening were included broadly to help ensure all potential harms were captured in the review. When multiple studies reported data for the same patients (e.g., when results of an initial pilot study were reported or when multiple studies reported outcomes of the CDC-funded Hepatitis Testing and Linkage to Care Project) ( 47 ), only the study with the most complete data was included. Linkage-to-care data were abstracted from 2010 forward from studies formally assessing linkage-to-care and reporting arrangement of or attendance at a follow-up appointment with a provider with special training for hepatitis C management. HCV RNA testing alone was not deemed linkage-to-care for purposes of this review, and studies did not have to report achievement of SVR to be included in the linkage-to-care review. Study design and setting were abstracted for all applicable studies. After the formal literature review was conducted, relevant studies identified through reference lists and those that were newly published were added for review.
To capture recently published studies, a supplementary literature search was conducted on November 15, 2019 for all adults (Supplementary Table 4, https://stacks.cdc.gov/view/cdc/85840 ) and on October 29, 2019 for pregnant women (Supplementary Table 5, https://stacks.cdc.gov/view/cdc/85840 ). The search strategy was the same as for the original searches. Titles/abstracts were independently reviewed by BR and SS. In the case of a conflict, the study was kept for full text review. Full texts were independently reviewed by two reviewers, one of whom was either MO, BR, or SS for the all-adult review and BR or SS for the pregnant women review. Information from the full texts was abstracted and added to the original review.
Summary of the Literature
For the all-adult review, the initial literature search yielded 4,867 studies. Twenty-nine duplicates were identified. Of 4,838 unique studies, 4,170 (86.2%) were deemed irrelevant by title/abstract screening, resulting in 668 (13.8%) full texts for review. Among these, 368 studies had data available to extract.
For the pregnancy review, the initial literature search yielded 1,500 studies. Two duplicates were identified. Of 1,498 unique studies, 1,412 (94.3%) were deemed irrelevant by title/abstract screening, resulting in 86 (5.7%) full texts for review.
The supplementary review yielded an additional 1,038 and 195 studies among all adults and pregnant women, respectively. Of these, 912 (87.9%) and 168 (86.2%), respectively, were deemed irrelevant by title/abstract screening, resulting in 126 (12.1%) and 27 (13.9%), respectively, full texts for review. One study was added to the pregnant women review outside of the formal literature search (i.e., the study was not among the retrieved studies but was known by the authors) ( 3 ).
Considering all 104 applicable studies, the median anti-HCV positivity prevalence (indicative of past or current infection) among all adults was 6.6% (range: 0.0%–76.1%) ( Table ). Median anti-HCV positivity prevalence was 1.7% (range: 0.02%–7.9%) for the general population (nine studies) (Supplementary Table 6, https://stacks.cdc.gov/view/cdc/85840 ), 7.5% (range: 0.5%–25.8%) for ED patients (19 studies) (Supplementary Table 7, https://stacks.cdc.gov/view/cdc/85840 ), 3.3% (range: 0.0%–43.5%) for birth cohort members (31 studies) (Supplementary Table 8, https://stacks.cdc.gov/view/cdc/85840 ), 9.3% (range: 1.6%–76.1%) for others/multiple risk factors (23 studies) (Supplementary Table 9, https://stacks.cdc.gov/view/cdc/85840 ), 54.2% (range: 12.7%–67.1%) for persons who use drugs (11 studies) (Supplementary Table 10, https://stacks.cdc.gov/view/cdc/85840 ), 5.2% (range: 1.2%–32.9%) for persons with HIV or sexual risk (eight studies) (Supplementary Table 11, https://stacks.cdc.gov/view/cdc/85840 ), and 4.7% (range: 3.4%–7.5%) for immigrants (three studies) (Supplementary Table 12, https://stacks.cdc.gov/view/cdc/85840 ). Considering 26 applicable studies among pregnant women, median anti-HCV positivity prevalence was 1.2% (range: 0.1%–70.8%) (Supplementary Table 13, https://stacks.cdc.gov/view/cdc/85840 ).
Considering all 61 applicable studies, the median rate of HCV RNA positivity (indicative of viremia) among those who were anti-HCV positive was 68.7% (range: 20.0%–100%) (Table). Median HCV RNA positivity was 55.2% (range: 36.8%–83.0%) for the general population (six studies) (Supplementary Table 6), 69.0% (range: 42.5%–90.5%) for ED patients (12 studies) (Supplementary Table 7), 62.7% (20.0%–95.3%) for birth cohort members (21 studies) (Supplementary Table 8), 74.1% (range: 47.0%–100%) for others/multiple (14 studies) (Supplementary Table 9), 73.8% (range: 69.9%–100%) for persons who use drugs (three studies) (Supplementary Table 10), 63.4% (range: 41.4%–83.8%) for persons with HIV or sexual risk (four studies) (Supplementary Table 11), and 81.8% for immigrants (one study) (Supplementary Table 12). Median HCV RNA positivity was 66.1% (range: 61.3%–77.2%) for pregnant women (four studies) (Supplementary Table 13).
One primary study ( 2 ) and one follow-up modeling study ( 9 ) examined nationally representative anti-HCV and HCV RNA data for adults from the 2013–2016 NHANES as well as data from the literature to estimate prevalence among populations not sampled by NHANES. The national estimate for anti-HCV positivity among adults was 1.7% (95% confidence interval [CI] = 1.4–2.0) ( 2 ). The HCV RNA prevalence estimate among adults was 1.0% (95% CI = 0.8%–1.1%) ( 2 ). Forty-two studies informed linkage-to-care among adults. Follow-up appointments or referrals were made for a median of 76.0% of HCV RNA positive patients (range: 25%–100%) (23 studies). A median of 73.9% of patients attended their first follow-up appointment (range: 0.0%–100%) (25 studies). This excludes self-reported data and studies that reported patients who were “linked to care” without explicitly stating the patient attended an appointment. A median of 39.0% of those attending a follow-up appointment received treatment (range: 21.5%–76.1%) (13 studies). Among those who received treatment, a median of 85.2% of patients achieved SVR (range: 66.7%–100%) (14 studies) (Supplementary Tables 6–12, https://stacks.cdc.gov/view/cdc/85840 ). Because DAAs are not approved for use during pregnancy, linkage-to-care was not assessed for pregnant women.
Harms associated with hepatitis C screening were initially informed by 21 and 12 studies from the all-adult and pregnancy review, respectively, including U.S.-based and non-U.S.-based studies. The supplementary literature search identified five studies from the all-adult review and one study from the pregnancy review informing harms. No study compared harms systematically using comparison groups associated with different screening approaches. Harms informed by the all-adult review included physical harms of screening (two studies) ( 48 , 49 ); anxiety/stress related to testing or waiting for results (five studies) ( 49 – 53 ); cost (one study) ( 54 ); anxiety related to receiving positive results (one study) ( 55 ); interpersonal outcomes (e.g., problems related to family, friends from learning HCV infection status) (five studies) ( 51 , 55 – 58 ); attitudes toward persons with hepatitis C, including stigma (11 studies) ( 49 , 55 , 57 – 65 ); time for screening (two studies) ( 49 , 66 ); and false-positive results, including among left ventricular assist device patients, possibly precluding heart transplantation (six studies) ( 67 – 72 ). Harms informed by the pregnancy review included physical harms of screening (one study) ( 73 ), anxiety (five studies) ( 74 – 78 ), stigma (one study) ( 77 ), psychological issues (two studies) ( 73 , 79 ), fears related to sexual relationships (one study) ( 80 ), legal ramifications and potential loss of infant custody (one study) ( 81 ), decreased quality of life (one study) ( 82 ), social repercussions (one study) ( 83 ), reluctance to disclose illegal risky behaviors because potential impact on mother or newborn (one study) ( 84 ), expense (two studies) ( 78 , 85 ), and false-positive results (one study) ( 73 ). Other plausible harms associated with hepatitis C screening identified outside of these studies (i.e., by subject matter experts, from the peer review process, or among studies not captured through the formal literature review) include harms associated with undergoing a liver biopsy (e.g., pain, bleeding, intestinal perforation, and death), insurability and employability issues, treatment adverse effects, the need to wait or return for test results, difficulty accessing treatment, and unnecessary Cesarean deliveries and unnecessary avoidance of breastfeeding. CDC concluded that identified or potential harms did not outweigh the benefits of screening.
These literature reviews are subject to at least three limitations. First, heterogeneity of individual study results might not be comparable across studies. For example, regarding anti-HCV positivity, some studies reported the proportion of persons testing positive out of the number of persons tested, while other studies reported the total population as the denominator. Other examples of heterogeneity between studies include varying definitions for follow-up (e.g., variations in provider types [specialist versus primary care provider] for which linkage-to-care was considered and varying definitions of “treated” [e.g., treatment initiated versus completed or not specified]). Second, limitations of the included studies also exist and could carry over into the systematic review findings. For example, recall bias and low response rates might have occurred within individual studies, potentially contributing to similar bias in the overall systematic review results. In addition, studies performed in high-burden areas might not be representative of the general populations and could impact external validity of the systematic review. Finally, publication bias might favor publication of studies reporting high disease prevalence, also potentially impacting external validity.
Cost-Effectiveness Considerations
Certain recent economic analyses provide information on the cost-effectiveness of hepatitis C screening. One analysis determined universal screening for persons aged ≥18 years, using a health care perspective, and yielded an incremental cost-effectiveness ratio (ICER) of $11,378 per quality-adjusted life year (QALY) gained when compared with 1945–1965 birth cohort screening, using a base case hepatitis C prevalence of 2.6% and 0.29% for birth cohort members and nonbirth cohort members, respectively ( 86 ). ICER remained below $50,000 per QALY gained, a threshold sometimes considered as a cut-off for determining cost-effectiveness, until the anti-HCV positivity prevalence dropped below 0.07% among nonbirth cohort members. Another analysis calculated an ICER of $28,000/QALY gained under a health care perspective for a strategy of screening all persons aged ≥18 years compared with birth cohort screening, with an additional 280,000 cures and 4,400 fewer cases of hepatocellular carcinoma ( 87 ). When the national hepatitis C prevalence was halved from the base case of 0.84%, ICER increased to $39,400. ICER remained below $100,000 per QALY gained when varying key parameters across broad ranges (e.g., when there was no improvement in quality of life and costs decreased following early-stage cure, when cost of early-stage disease was $0, when treatment costs varied, and when there was no mortality benefit from SVR). A third analysis reported an ICER of $7,900/QALY gained for one-time general population hepatitis C screening of persons aged 20–69 years compared with risk-based screening using a societal perspective and a base case hepatitis C prevalence of 1.6% ( 88 ). ICER was $5,400/QALY gained for screening persons born during 1945–1965 compared with risk-based screening with a hepatitis C prevalence of 3.3% for persons in the birth cohort. Birth cohort screening dominated general population screening, although the model also included treatment with ribavirin and pegylated interferon; protease inhibitor therapy was modeled for treatment naïve genotype 1 patients at costs ranging from $61,773–$88,248. Studies using higher treatment costs would be expected to calculate ICERs higher than those with lower treatment costs. Several other studies provide similar cost effectiveness estimates of a universal screening strategy for adults, with ICERs ranging from cost saving to $71,000/QALY gained ( 89 – 91 ).
Analyses focusing on pregnant women have yielded similar results. One analysis calculated an ICER of $2,826 for universal screening of pregnant women under the health care perspective, compared with risk-based screening at an HCV RNA positivity prevalence of 0.73%; sensitivity analyses generated an ICER of $50,000 per QALY gained or less until the prevalence of chronic hepatitis C infection dropped to 0.03%–0.04% ( 92 ).
Although real-world data informing screening during each pregnancy are lacking, a modeled analysis suggests that hepatitis C screening during each pregnancy would be cost-effective. Using a hepatitis C prevalence of 0.38% among pregnant women, as determined from national birth certificate data, the analysis found that universal hepatitis C screening during the first trimester of each pregnancy under a health care perspective compared with the current practice of risk-based screening had an ICER of $41,000/QALY gained ( 93 ). The model assumed no hepatitis C treatment would be offered until after 6 months postpartum and that 25% of women would be linked to care, with 92% of those linked initiating treatment. Only current injecting drug users were deemed at risk for new HCV infection or reinfection after cure. Universal screening reduced HCV-attributable mortality by 16% and more than doubled the proportion of infants born to mothers with hepatitis C who were identified as HCV-exposed, from 44% to 92%. ICER remained at or below $100,000 per QALY gained if hepatitis C prevalence was higher than 0.16%. This study did not account for any cost savings associated with prevention of risks for subsequent pregnancies or the potential benefits to early detection and management of infected infants.
Hepatitis C Testing Strategy
The goal of hepatitis C screening is to identify persons who are currently infected with HCV. Hepatitis C testing should be initiated with a U.S. Food and Drug Administration (FDA)-approved anti-HCV test. Persons who test anti-HCV positive are either currently infected or had past infection that has resolved naturally or with treatment. Immunocompetent persons without hepatitis C risks who test anti-HCV negative are not infected and require no further testing. Persons testing anti-HCV positive should have follow-up testing with an FDA-approved nucleic acid test (NAT) for detection of HCV RNA. NAT for HCV RNA detection determines viremia and current HCV infection. Persons who test anti-HCV positive but HCV RNA negative do not have current HCV infection. CDC encourages use of reflex HCV RNA testing, in which specimens testing anti-HCV positive undergo HCV RNA testing immediately and automatically in the laboratory, using the same sample from which the anti-HCV test was conducted. Hepatitis C testing should be provided on-site when feasible.
Determining the Prevalence Threshold for the Recommendations
The recommended HCV RNA prevalence threshold of 0.1% was determined based, in part, on review of published ICERs, as a function of hepatitis C prevalence, and the most up-to-date estimated prevalence of hepatitis C within states. In general, cost analyses determined that for all adults, ICER would be approximately $50,000 per QALY gained or less at current treatment costs (approximately $25,000 per course of treatment) at an anti-HCV positivity prevalence of 0.07% in the nonbirth cohort, which is similar to the HCV RNA prevalence in all adults. At a hepatitis C prevalence of 0.1%, ICER would be approximately $36,000 per QALY gained ( 86 ). Certain economists use $50,000 as a conservative threshold to determine cost-effectiveness. As treatment costs decrease, ICERs also will decrease, assuming other parameters remain stable. According to modeling results using NHANES data, no state has a hepatitis C prevalence in adults below 0.1% ( 9 ). Similarly, for universal testing in pregnant women, ICER would be approximately $50,000 per QALY gained or less at an HCV RNA positivity prevalence of 0.05%; at a prevalence of 0.1%, ICER would be approximately $15,000 per QALY gained ( 92 ). ICERs might be higher for testing in subsequent pregnancies when testing during the index pregnancy identifies women with hepatitis C who receive treatment following pregnancy, resulting in a decrease in hepatitis C prevalence among women with more than one pregnancy. According to birth certificate data (likely an underestimate of current maternal HCV infections), only three states were below the 0.1% prevalence among pregnant women ( 4 ).
Although the intent of public health screening is usually to identify undiagnosed disease, many persons previously diagnosed with hepatitis C are not appropriately linked to care and are not cured of their HCV infection, thereby representing an ongoing source of transmission. Therefore, the prevalence threshold of 0.1% should be determined on the basis of estimates of chronic hepatitis C prevalence, regardless of whether hepatitis C has been diagnosed previously.
The following recommendations for hepatitis C screening augment those issued by CDC in 2012 ( 5 ). The recommendations issued by CDC in 1998 remain in effect ( 6 ). CDC recommends ( Box 1 ):
- Hepatitis C screening at least once in a lifetime for all adults aged ≥18 years, except in settings where the prevalence of HCV infection (HCV RNA-positivity) is <0.1%
- Hepatitis C screening for all pregnant women during each pregnancy, except in settings where the prevalence of HCV infection (HCV RNA-positivity) is <0.1%
- Persons with HIV
- Persons who ever injected drugs and shared needles, syringes, or other drug preparation equipment, including those who injected once or a few times many years ago
- Persons with selected medical conditions, including persons who ever received maintenance hemodialysis and persons with persistently abnormal ALT levels
- Prior recipients of transfusions or organ transplants, including persons who received clotting factor concentrates produced before 1987, persons who received a transfusion of blood or blood components before July 1992, persons who received an organ transplant before July 1992, and persons who were notified that they received blood from a donor who later tested positive for HCV infection
- Health care, emergency medical, and public safety personnel after needle sticks, sharps, or mucosal exposures to HCV-positive blood
- Children born to mothers with HCV infection
- Persons who inject drugs and share needles, syringes, or other drug preparation equipment
- Persons with selected medical conditions, including persons who ever received maintenance hemodialysis
- Any person who requests hepatitis C testing should receive it, regardless of disclosure of risk, because many persons might be reluctant to disclose stigmatizing risks
Determining Prevalence
In the absence of existing data for hepatitis C prevalence, health care providers should initiate universal hepatitis C screening until they establish that the prevalence of HCV RNA positivity in their population is <0.1%, at which point universal screening is no longer explicitly recommended but might occur at the provider’s discretion. Hepatitis C screening can be conducted in a variety of settings or programs that serve populations at different risk and with varying hepatitis C prevalence. Regardless of the provider, organization, or program providing testing, health care providers should initiate universal screening for adults and pregnant women unless the prevalence of HCV infection (HCV RNA positivity prevalence) in their patients has been documented to be <0.1%. There are statistical challenges with determining a “number needed to screen” to detect a relatively rare disease in lower-risk settings; therefore, providers and program directors are encouraged to consult their state or local health departments or CDC to determine a reasonable estimate of baseline prevalence in their setting or a methodology for determining how many persons they need to screen before confidently establishing that the prevalence is <0.1%. As a general guide: as HCV RNA prevalence is predicated on first testing for anti-HCV, and according to the most current serologic data in the United States, approximately 59% of anti-HCV positive persons are HCV RNA positive ( 2 ), an estimated 507 randomly selected patients in a setting of any size would need to be tested using any of the available anti-HCV tests ( 94 ) to detect an anti-HCV prevalence positivity of ≤0.17%, corresponding to an expected HCV RNA positivity prevalence of 0.1% with 95% confidence and 5% tolerance ( 95 ) ( https://epitools.ausvet.com.au ).
Patient Follow-up After Hepatitis C Testing
Providers and patients can discuss hepatitis C screening as part of a person’s preventive health care. For persons identified with current HCV infection, CDC recommends that they receive appropriate care, including hepatitis C-directed clinical preventive services (e.g., screening and intervention for alcohol or drug use, hepatitis A and hepatitis B vaccination, and medical monitoring of disease).
Recommendations are available to guide management of persons infected with HCV ( Box 2 ). Persons infected with HCV can benefit from treatment, prevention, and other counseling messages.
- Persons with negative anti-HCV test results should be informed of their test results and reassured that they are not infected, unless they were recently exposed to infection (e.g., recent IDU). Repeat testing should occur for persons with ongoing risk behaviors. Persons with negative anti-HCV and positive HCV RNA test results have recent HCV infection.
- Persons with positive anti-HCV and negative HCV RNA test results should be informed that they do not have current HCV infection. Test results indicate either a resolved past infection or a false-positive anti-HCV test result. Additional testing might be warranted to determine the patient’s status.
- At the time when positive test results are communicated to patients, health care providers should evaluate the patient’s level of alcohol and drug use and provide a brief alcohol or drug use intervention, if clinically indicated ( 5 ).
Testing Considerations
Universal hepatitis C screening was compared with risk-based screening for adults and pregnant women. As such, the marginal benefits and harms of universal screening compared with birth cohort screening was not directly assessed. For the purposes of this literature review, the birth cohort was deemed a risk group, and studies comparing birth cohort with universal screening strategies were eligible for inclusion. The incidence of acute hepatitis C is greatest among persons younger than birth cohort members ( 5 ). Because most pregnant women are younger than persons born during the 1945–1965 birth cohort, hepatitis C testing among pregnant women has previously been based on the presence of risk factors. The new recommendations apply to all pregnant women, including those aged <18 years.
Data informing the optimal time during pregnancy for hepatitis C testing are lacking. If DAA treatment becomes available for use during pregnancy, testing at an early prenatal visit would allow for identification of women who could benefit from treatment. Testing early in pregnancy also could inform pregnancy and delivery management per the Society for Maternal-Fetal Medicine recommendations for a preference for amniocentesis over chorionic villus sampling and for avoiding internal fetal monitoring, prolonged rupture of the membranes, and episiotomy ( 44 ). Testing at an early prenatal visit harmonizes testing for hepatitis C with testing for other infectious diseases during pregnancy; however, this strategy might miss women who acquire HCV infection later during pregnancy. Pregnant women with ongoing risk factors tested early in pregnancy could undergo repeat testing later in pregnancy to identify those who acquired HCV infection later in pregnancy. Hepatitis C screening during pregnancy should be an opportunity to promote a dialogue between the pregnant woman and her medical provider about hepatitis C transmission and risk factors.
Hepatitis C prevalence in U.S. correctional settings is high because of high incarceration rates among persons who use drugs ( 96 ). Two recent systematic reviews estimated average anti-HCV positivity prevalence in correctional settings at 16.1% and 23% ( 2 , 97 ). Hepatitis C prevalence varies across individual correctional jurisdictions based on factors including underlying community prevalence, sentencing standards for drug-related offenses, and type of institution. These estimates exceed both the general population prevalence of 1.7% ( 2 ) and the target threshold of ≥0.1% at which these guidelines recommend universal hepatitis C testing in other settings. Therefore, the well-documented prevalence of HCV infection in a variety of correctional jurisdictions supports the application of these guidelines to prisons and jails. Universal hepatitis C testing in correctional facilities can be expected to yield higher infection identification rates compared with the risk-based testing practices that many jurisdictions employ ( 98 , 99 ) and to support broader hepatitis C elimination efforts ( 34 , 100 , 101 ).
Cases of hepatitis C should be reported to the appropriate state or local health jurisdiction in accordance with requirements for reporting acute, perinatal, and chronic HCV infection. Case definitions for the classification of reportable cases of HCV infection have been published previously by the Council of State and Territorial Epidemiologists ( 102 ).
Recommendations of Other Organizations
Recommendations in this report for hepatitis C screening among certain groups differ somewhat from the recommendations of other organizations. The U.S. Preventive Services Task Force ( 103 ) and AASLD and IDSA ( 39 ) also make recommendations for hepatitis C testing.
CDC will review and possibly revise these recommendations as new epidemiology or other information related to hepatitis C becomes available, including potential availability of DAA treatments for pregnant women, infants, and younger children, and the experience gained from the implementation of these recommendations. A review of the evidence regarding infant testing is needed to inform future recommendations for an infant testing algorithm. Evidence should examine the benefits and harms of HCV RNA testing beginning at age 2 months compared with anti-HCV testing at or after age 18 months. The greater expense of HCV RNA testing might be justified as earlier testing will likely minimize loss to follow-up. Additional data on the safety of DAA use during pregnancy are needed to inform treatment during pregnancy, which might reduce the risk for perinatal transmission. Finally, for expanded screening to be effective in reducing the morbidity and mortality of hepatitis C in the United States, models to address barriers related to access to DAA treatment are needed.
CDC recommends hepatitis C screening of all adults aged ≥18 years once in their lifetimes, and screening of all pregnant women (regardless of age) during each pregnancy. The recommendations include an exception for settings where the prevalence of HCV infection is demonstrated to be <0.1%; however, few settings are known to exist with a hepatitis C prevalence below this threshold ( 2 , 9 ). The recommendation for testing of persons with risk factors remains unchanged; those with ongoing risk factors should be tested regardless of age or setting prevalence, including continued periodic testing as long as risks persist. These recommendations can be used by health care professionals, public health officials, and organizations involved in the development, implementation, delivery, and evaluation of clinical and preventive services.
Peter Havens, MD, Medical College of Wisconsin/Children’s Hospital of Wisconsin, Milwaukee, Wisconsin; Jean Anderson, MD, Johns Hopkins Medicine, Baltimore, Maryland; Susan Clark, MPH, Alycia Downs, MPH, Liesl Hagan, MPH, Cynthia Jorgensen, DrPH, Maja Kodani, PhD, Elizabeth McClune, MSW, MPA, Matthew Pauly, PhD, Alexandra Tejada-Strop, MS, Nicholas Wiese, PhD, Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC; Randy Elder, PhD, Office of Science, CDC; Shanna Cox, MSPH, Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC; Kakoli Roy, PhD, National Center for Chronic Disease Prevention and Health Promotion, CDC; Joanna Taliano, MLS, Office of Library Science, CDC.
Corresponding author: Sarah Schillie, MD, Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC. Telephone: 404-718-8608; E-mail: [email protected] .
1 Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC
Conflict of Interest
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
- CDC. Viral hepatitis surveillance—United States, 2017. Atlanta, GA: US Department of Health and Human Services, CDC; 2019. https://www.cdc.gov/hepatitis/statistics/2017surveillance/index.htm
- Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013–2016. Hepatology 2019;69:1020–31. CrossRef PubMed
- Patrick SW, Bauer AM, Warren MD, Jones TF, Wester C. Hepatitis C virus infection among women giving birth—Tennessee and United States, 2009–2014. MMWR Morb Mortal Wkly Rep 2017;66:470–3. CrossRef PubMed
- Schillie SF, Canary L, Koneru A, et al. Hepatitis C virus in women of childbearing age, pregnant women, and children. Am J Prev Med 2018;55:633–41. CrossRef PubMed
- Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep 2012;61(RR-4):1–32. PubMed
- CDC. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep 1998;47(RR-19):1–39. PubMed
- Klevens RM, Liu S, Roberts H, Jiles RB, Holmberg SD. Estimating acute viral hepatitis infections from nationally reported cases. Am J Public Health 2014;104:482–7. CrossRef PubMed
- Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mortal Wkly Rep 2015;64:453–8. PubMed
- Rosenberg ES, Rosenthal EM, Hall EW, et al. Prevalence of hepatitis C virus infection in US states and the District of Columbia, 2013 to 2016. JAMA Netw Open 2018;1:e186371. CrossRef PubMed
- Collier MF, Holtzman D, Holmberg SD. Hepatitis C virus [Chapter 220]. In: Long SS, Prober CG, Fisher M, eds. Principles and practice of pediatric infectious diseases. 5th ed. Philadelphia, PA: Elsevier; 2018:1135–41.
- Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 2000;284:450–6. CrossRef PubMed
- Wang LS, D’Souza LS, Jacobson IM. Hepatitis C-A clinical review. J Med Virol 2016;88:1844–55. CrossRef PubMed
- CDC. Updated U.S. Public Health Service Guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001;50(RR-11):1–52. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5011a1.htm
- Perz JF, Grytdal S, Beck S, et al. Case-control study of hepatitis B and hepatitis C in older adults: Do healthcare exposures contribute to burden of new infections? Hepatology 2013;57:917–24. CrossRef PubMed
- Guh AY, Thompson ND, Schaefer MK, Patel PR, Perz JF. Patient notification for bloodborne pathogen testing due to unsafe injection practices in the US health care settings, 2001-2011. Med Care 2012;50:785–91. CrossRef PubMed
- Schaefer MK, Perz JF. Outbreaks of infections associated with drug diversion by US health care personnel. Mayo Clin Proc 2014;89:878–87. CrossRef PubMed
- Njuguna HN, Stinson D, Montgomery P, et al. Hepatitis C virus potentially transmitted by opioid drug diversion from a nurse—Washington, August 2017–March 2018. MMWR Morb Mortal Wkly Rep 2019;68:374–6. CrossRef PubMed
- Chan DP, Sun HY, Wong HT, Lee SS, Hung CC. Sexually acquired hepatitis C virus infection: a review. Int J Infect Dis 2016;49:47–58. CrossRef PubMed
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol 2014;61(Suppl):S58–68. CrossRef PubMed
- Nwaohiri A, Schillie S, Bulterys M, Kourtis AP. Towards elimination of hepatitis C virus infection in children. Lancet Child Adolesc Health 2018;2:235–7. CrossRef PubMed
- Nwaohiri A, Schillie S, Bulterys M, Kourtis AP. Hepatitis C virus infection in children: How do we prevent it and how do we treat it? Expert Rev Anti Infect Ther 2018;16:689–94. CrossRef PubMed
- Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis 2014;59:765–73. CrossRef PubMed
- European Paediatric Hepatitis C Virus Network. Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clin Infect Dis 2005;41:45–51. CrossRef PubMed
- Hoofnagle JH. Hepatitis C: the clinical spectrum of disease. Hepatology 1997;26(Suppl 1):15S–20S. CrossRef PubMed
- Marcellin P. Hepatitis C: the clinical spectrum of the disease. J Hepatol 1999;31(Suppl 1):9–16. CrossRef PubMed
- Maheshwari A, Ray S, Thuluvath PJ. Acute hepatitis C. Lancet 2008;372:321–32. CrossRef PubMed
- Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin North Am 2015;44:717–34. CrossRef PubMed
- Lee MH, Yang HI, Yuan Y, L’Italien G, Chen CJ. Epidemiology and natural history of hepatitis C virus infection. World J Gastroenterol 2014;20:9270–80. CrossRef PubMed
- CDC/National Center for HIV/AIDS. Viral Hepatitis, STD, and TB Prevention. Hepatitis C. Atlanta, GA: US Department of Health and Human Services, CDC; 2019. https://www.cdc.gov/hepatitis/hcv/index.htm
- Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis 2005;9:383–98, vi. CrossRef PubMed
- Seo S, Silverberg MJ, Hurley LB, et al. Prevalence of spontaneous clearance of hepatitis C virus infection doubled from 1998 to 2017. Clin Gastroenterol Hepatol 2020;18:511–3. CrossRef PubMed
- Masarone M, Persico M. Hepatitis C virus infection and non-hepatocellular malignancies in the DAA era: A systematic review and meta-analysis. Liver Int 2019;39:1292–306. CrossRef PubMed
- National Academies of Sciences, Engineering, and Medicine. Eliminating the public health problem of hepatitis B and C in the United States: phase one report. Buckley GJ, Strom BL, eds. Washington, DC: The National Academies Press; 2016. http://www.nap.edu/catalog/23407
- National Academies of Sciences, Engineering, and Medicine. A national strategy for the elimination of hepatitis B and C: phase two report. Strom BL, Buckley GJ, eds. Washington, DC: The National Academies Press; 2017. http://www.nap.edu/catalog/24731
- CDC. Public Health Service inter-agency guidelines for screening donors of blood, plasma, organs, tissues, and semen for evidence of hepatitis B and hepatitis C. MMWR Recommend Rep 1991;40(No. RR–4):1–17.
- USPHS/IDSA Prevention of Opportunistic Infections Working Group. 1999 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. Clin Infect Dis 2000;30(Suppl 1):S29–65. CrossRef PubMed
- Smith BD, Patel N, Beckett GA, Jewett A, Ward JW. Hepatitis C virus antibody prevalence, correlates and predictors among persons born from 1945 through 1965, United States, 1999–2008 [Abstract]. Presented at the American Association for the Study of Liver Disease meeting, San Francisco, California, November 6, 2011.
- Kim HS, Yang JD, El-Serag HB, Kanwal F. Awareness of chronic viral hepatitis in the United States: An update from the National Health and Nutrition Examination Survey. J Viral Hepat 2019;26:596–602. CrossRef PubMed
- American Association for the Study of Liver Diseases (AASLD); Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. Alexandria and Arlington, VA: AASLD and IDSA; 2019. https://www.hcvguidelines.org
- Terrault N, Monto A, Stinchon MR, Rusie E, Moreo K. New therapies, evidence, and guidance in hepatitis C management: expert practices and insights from an educational symposium at the AMCP 27th annual meeting expo. J Manag Care Spec Pharm 2015;21(9 Suppl):S1–17. CrossRef PubMed
- Jones CR, Flower BF, Barber E, Simmons B, Cooke GS. Treatment optimisation for hepatitis C in the era of combination direct-acting antiviral therapy: a systematic review and meta-analysis. Wellcome Open Res 2019;4:132. CrossRef PubMed
- Chappell CA, Krans EE, Bunge K, et al. A phase 1 study of ledipasvir/sofosbuvir in pregnant women with hepatitis C virus [Abstract 87]. Presented at the Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, March 4–7, 2019. http://www.croiconference.org/sessions/phase-1-study-ledipasvirsofosbuvir-pregnant-women-hepatitis-c-virus
- United Health Foundation. America’s health rankings: uninsured women. Minneapolis, MN: United Health Group; 2020. https://www.americashealthrankings.org/explore/health-of-women-and-children/measure/Uninsured_women/state/ALL
- Hughes BL, Page CM, Kuller JA. Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol 2017;217:B2–12. CrossRef
- AbbVie, Inc. Highlights of prescribing information: Mavyret™ North Chicago, IL: AbbVie, Inc.; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/209394s006lbl.pdf
- Sciences G. Inc. Highlights of prescribing information: Harvoni. Foster City, CA: Gilead Sciences, Inc.; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212477s000lbl.pdf
- Ramirez G, Cabral R, Patterson M, et al. Early identification and linkage to care for people with chronic HBV and HCV infection: the HepTLC initiative. Public Health Rep 2016;131(Suppl 2):5–11. CrossRef PubMed
- Poll R. Preventing ill-health: assessing the potential impact of NICE guidance to promote and offer hepatitis C testing within drug services [Chapter 11]. In: Tod AM, Hirst J, eds. Health and inequality: applying public health research to policy and practice. New York, NY: Routledge; 2014: 87–96.
- Shehata N, Austin T, Ha S, Timmerman K. Barriers to and facilitators of hepatitis C virus screening and testing: A scoping review. Can Commun Dis Rep 2018;44:166–72. CrossRef PubMed
- Bottero J, Boyd A, Gozlan J, et al. Simultaneous human immunodeficiency virus-hepatitis B-hepatitis C point-of-care tests improve outcomes in linkage-to-care: results of a randomized control trial in persons without healthcare coverage. Open Forum Infect Dis 2015;2:ofv162. CrossRef PubMed
- Jones L, Atkinson A, Bates G, et al. Views and experiences of hepatitis C testing and diagnosis among people who inject drugs: systematic review of qualitative research. Int J Drug Policy 2014;25:204–11. CrossRef PubMed
- Reynolds GL, Fisher DG, Brocato J, van Otterloo L, Khahlil K, Huckabay L. Stressful point-of-care rapid testing for human immunodeficiency virus, hepatitis C virus, and syphilis. Int J STD AIDS 2017;28:975–84. CrossRef PubMed
- White DA, Anderson ES, Pfeil SK, Trivedi TK. Hepatitis C virus antibody testing: result availability at time of discharge for emergency department patients. J Acquir Immune Defic Syndr 2016;71:e82–4. CrossRef PubMed
- McCarty TR, Lombard A, Lim JK. Advances in the care of patients with chronic hepatitis C infection in Connecticut: epidemiology, screening, and treatment. Connecticut Medicine 2018;82:197–205.
- Turner BJ, Craig K, Makanji VS, Flores BE, Hernandez L. Improving support and education of low-income baby boomers diagnosed with chronic hepatitis C virus infection through universal screening. J Clin Nurs 2017;26:4605–12. CrossRef PubMed
- Brener L, Wilson H, Jackson LC, Johnson P, Saunders V, Treloar C. Experiences of diagnosis, care and treatment among Aboriginal people living with hepatitis C. Aust N Z J Public Health 2016;40(Suppl 1):S59–64. CrossRef PubMed
- Deacon RM, Mooney-Somers J, Treloar C, Maher L. At the intersection of marginalised identities: lesbian, gay, bisexual and transgender people’s experiences of injecting drug use and hepatitis C seroconversion. Health Soc Care Community 2013;21:402–10. CrossRef PubMed
- Ly W, Cocohoba J, Chyorny A, Halpern J, Auerswald C, Myers J. Perspectives on integrated HIV and hepatitis C virus testing among persons entering a northern California jail: a pilot study. J Acquir Immune Defic Syndr 2018;78:214–20. CrossRef PubMed
- Brener L, Ellard J, Murphy D, Callander D. Perceptions and deflections: associations between attitudes towards people with hepatitis C and testing for hepatitis C among Australian gay and bisexual men. Sex Health 2013;10:268–74. CrossRef PubMed
- Brener L, Murphy DA, Cama EJ, Ellard J. Hepatitis C risk factors, attitudes and knowledge among HIV-positive, HIV-negative and HIV-untested gay and bisexual men in Australia. Sex Health 2015;12:411–7. CrossRef PubMed
- Joukar F, Mansour-Ghanaei F, Soati F, Meskinkhoda P. Knowledge levels and attitudes of health care professionals toward patients with hepatitis C infection. World J Gastroenterol 2012;18:2238–44. CrossRef PubMed
- Skeer MR, Ladin K, Wilkins LE, Landy DM, Stopka TJ. ‘Hep C’s like the common cold’: understanding barriers along the HCV care continuum among young people who inject drugs. Drug Alcohol Depend 2018;190:246–54. CrossRef PubMed
- Talal AH, Dimova RB, Seewald R, et al. Assessment of methadone clinic staff attitudes toward hepatitis C evaluation and treatment. J Subst Abuse Treat 2013;44:115–9. CrossRef PubMed
- Kempf MC, Ott C, Wise JM, et al. Universal screening for HIV and hepatitis C infection: a community-based pilot project. Am J Prev Med 2018;55(Suppl 1):S112–21. CrossRef PubMed
- Zou B, Yeo YH, Le MH, et al. Prevalence of viremic hepatitis C virus infection by age, race/ethnicity, birthplace and disease awareness among viremic persons in the United States, 1999–2016. J Infect Dis 2020;221:408–18. CrossRef PubMed
- Plant A, Snow EG, Montoya JA, Young S, Javanbakht M, Klausner JD. Test4HepC: promoting hepatitis C testing to baby boomers using social media. Health Promot Pract 2019;1524839919833987:1524839919833987. CrossRef PubMed
- Durand CM, Marr KA, Ostrander D, et al. False-positive hepatitis C virus serology after placement of a ventricular assistance device. Transpl Infect Dis 2016;18:146–9. CrossRef PubMed
- Heinrichs A, Antoine M, Steensels D, Montesinos I, Delforge ML. HCV false positive immunoassays in patients with LVAD: A potential trap! J Clin Virol 2016;78:44–6. CrossRef PubMed
- Minamoto GY, Lee D, Colovai A, et al. False positive hepatitis C antibody test results in left ventricular assist device recipients: increased risk with age and transfusions. J Thorac Dis 2017;9:205–10. CrossRef PubMed
- Moorman AC, Drobenuic J, Kamili S. Prevalence of false-positive hepatitis C antibody results, National Health and Nutrition Examination Study (NHANES) 2007-2012. J Clin Virol 2017;89:1–4. CrossRef PubMed
- Sindermann JR, Holthaus AJ, Schepers M, Schlüter B, Martens S, Scherer M. False-positive hepatitis C testing in long-term LVAD support. ASAIO J 2015;61:e19. CrossRef PubMed
- Srivastava AV, Hrobowski T, Krese L, et al. High rates of false-positive hepatitis C antibody tests can occur after left ventricular assist device implantation. ASAIO J 2013;59:660–1. CrossRef PubMed
- Pembrey L, Newell ML, Peckham C. Is there a case for hepatitis C infection screening in the antenatal period? J Med Screen 2003;10:161–8. CrossRef PubMed
- U.S. Preventive Services Task Force. Screening for Hepatitis C virus infection in adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2013;159:03. CrossRef
- U.S. Preventive Services Task Force. Summaries for patients: screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013;159:I-32. CrossRef PubMed
- Baig S. Antenatal screening practice for hepatitis B and C. J Coll Physicians Surg Pak 2009;19:137–8. PubMed
- Moyer VA. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013;159:349–57. CrossRef PubMed
- Wilson E, Beckmann M. Antenatal screening for hepatitis C: Universal or risk factor based? Aust N Z J Obstet Gynaecol 2015;55:318–22. CrossRef PubMed
- Sheikh SM. Hepatitis B and C: value of universal antenatal screening. J Coll Physicians Surg Pak 2009;19:179–82. PubMed
- Krans EE, Zickmund SL, Rustgi VK, Park SY, Dunn SL, Schwarz EB. Screening and evaluation of hepatitis C virus infection in pregnant women on opioid maintenance therapy: A retrospective cohort study. Subst Abus 2016;37:88–95. CrossRef PubMed
- Pergam SA, Hawes SE, Gardella CM, Wang CC. HCV and pregnancy: Is now the time for universal testing? Future Virol 2008;3:1–5. CrossRef
- Plunkett BA, Grobman WA. Routine hepatitis C virus screening in pregnancy: a cost-effectiveness analysis. Am J Obstet Gynecol 2005;192:1153–61. CrossRef PubMed
- Waruingi W, Mhanna MJ, Kumar D, Abughali N. Hepatitis C Virus universal screening versus risk based selective screening during pregnancy. J Neonatal Perinatal Med 2016;8:371–8. CrossRef PubMed
- Gowda C, Kennedy S, Glover C, Prasad MR, Wang L, Honegger JR. Enhanced identification of maternal hepatitis C virus infection using existing public health surveillance systems. Paediatr Perinat Epidemiol 2018;32:401–10. CrossRef PubMed
- Yeung CY, Lee HC, Chan WT, Jiang CB, Chang SW, Chuang CK. Vertical transmission of hepatitis C virus: Current knowledge and perspectives. World J Hepatol 2014;6:643–51. CrossRef PubMed
- Eckman MH, Talal AH, Gordon SC, Schiff E, Sherman KE. Cost-effectiveness of screening for chronic hepatitis C infection in the United States. Clin Infect Dis 2013;56:1382–93. CrossRef PubMed
- Barocas JA, Tasillo A, Eftekhari Yazdi G, et al. Population-level outcomes and cost-effectiveness of expanding the recommendation for age-based hepatitis C testing in the United States. Clin Infect Dis 2018;67:549–56 . CrossRef PubMed
- Coffin PO, Scott JD, Golden MR, Sullivan SD. Cost-effectiveness and population outcomes of general population screening for hepatitis C. Clin Infect Dis 2012;54:1259–71. CrossRef PubMed
- Younossi Z, Blissett D, Blissett R, et al. In an era of highly effective treatment, hepatitis C screening of the United States general population should be considered. Liver Int 2018;38:258–65. CrossRef PubMed
- Linthicum MT, Gonzalez YS, Mulligan K, et al. Value of expanding HCV screening and treatment policies in the United States. Am J Manag Care 2016;22(6 Spec No.):SP227–35.
- Assoumou SA, Tasillo A, Leff JA, et al. Cost-effectiveness of one-time hepatitis C screening strategies among adolescents and young adults in primary care settings. Clin Infect Dis 2018;66:376–84. CrossRef PubMed
- Chaillon A, Rand EB, Reau N, Martin NK. Cost-effectiveness of universal hepatitis C virus screening of pregnant women in the United States. Clin Infect Dis 2019;69:1888–95. CrossRef PubMed
- Tasillo A, Eftekhari Yazdi G, Nolen S, et al. Short-term effects and long-term cost-effectiveness of universal hepatitis C testing in prenatal care. Obstet Gynecol 2019;133:289–300. CrossRef PubMed
- Chapko MK, Dufour DR, Hatia RI, Drobeniuc J, Ward JW, Teo CG. Cost-effectiveness of strategies for testing current hepatitis C virus infection. Hepatology 2015;62:1396–404. CrossRef PubMed
- Humphry RW, Cameron A, Gunn GJ. A practical approach to calculate sample size for herd prevalence surveys. Prev Vet Med 2004;65:173–88. CrossRef PubMed
- Varan AK, Mercer DW, Stein MS, Spaulding AC. Hepatitis C seroprevalence among prison inmates since 2001: still high but declining. Public Health Rep 2014;129:187–95. CrossRef PubMed
- Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology 2015;62:1353–63. CrossRef PubMed
- Assoumou SA, Wang J, Tasillo A, et al. Hepatitis C testing and patient characteristics in Washington state’s prisons between 2012 and 2016. Am J Prev Med 2019;56:8–16. CrossRef PubMed
- de la Flor C, Porsa E, Nijhawan AE. Opt-out HIV and hepatitis C testing at the Dallas County jail: uptake, prevalence, and demographic characteristics of testers. Public Health Rep 2017;132:617–21. CrossRef PubMed
- He T, Li K, Roberts MS, et al. Prevention of hepatitis C by screening and treatment in U.S. prisons. Ann Intern Med 2016;164:84–92. CrossRef PubMed
- Larney S, Beckwith C, Zaller N, Montague B, Rich J. “Seek, test, treat and retain” for hepatitis C in the United States criminal justice system. Int J Prison Health 2014;10:164–71. CrossRef PubMed
- National Notifiable Diseases Surveillance System (NNDSS). Surveillance case definitions for current and historical conditions. Atlanta, GA: US Department of Health and Human Services, CDC; 2017. https://wwwn.cdc.gov/nndss/conditions/
- Owens DK, Davidson KW, Krist AH, et al. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA 2020; Epub ahead of print. CrossRef PubMed
FIGURE 1 . Incidence rates* of reported acute hepatitis C cases ── United States, 2000–2017
Source : CDC, National Notifiable Diseases Surveillance System. * Per 100,000 population.
FIGURE 2 . Estimated prevalence of hepatitis C virus RNA positivity among all adults* and hepatitis C among pregnant women, † by state §
Abbreviations: HCV = hepatitis C virus; RNA = ribonucleic acid; NHANES = National Health and Nutrition Examination Survey.
* State estimates of HCV RNA positivity among all adults are based on a statistical model that allocated nationally representative hepatitis C prevalence from the 2013–2016 NHANES and additional previously published data for populations not sampled in NHANES to states according to the spatial demographics and distributions of 1999–2016 hepatitis C mortality and narcotic overdose deaths in the National Vital Statistics System.
† Data are from an analysis of 2015, National Center for Health Statistics birth certificate data (live births) (Schillie SF, Canary L, Koneru A, et al. Hepatitis C virus in women of childbearing age, pregnant women, and children. Am J Prev Med 2018;55:633–41).
§ Connecticut did not report maternal HCV infection on 2015 birth certificates and New Jersey reported infections from only a limited number of facilities; therefore, women residing in these two states were not included in the analysis.
| Population | No. studies informing anti-HCV positivity prevalence | Median anti-HCV prevalence (range) | No. studies informing HCV RNA positivity prevalence | Median HCV RNA positivity (range) |
|---|---|---|---|---|
| General population | 9 | 1.7% (0.02%–7.9%) | 6 | 55.2% (36.8%–83.0%) |
| Emergency department patients | 19 | 7.5% (0.5%–25.8%) | 12 | 69.0% (42.5%–90.5%) |
| Birth cohort | 31 | 3.3% (0.0%–43.5%) | 21 | 62.7% (20.0%–95.3%) |
| Others/multiple | 23 | 9.3% (1.6%–76.1%) | 14 | 74.1% (47.0%–100.0%) |
| Persons who use drugs | 11 | 54.2% (12.7%–67.1%) | 3 | 73.8% (69.9%–100.0%) |
| Persons with HIV or sexual risks | 8 | 5.2% (1.2%–32.9%) | 4 | 63.4% (41.4%–83.8%) |
| Immigrants | 3 | 4.7% (3.4%–7.5%) | 1 | 81.8% |
Abbreviations: anti-HCV = hepatitis C virus antibody; HCV = hepatitis C virus; NHANES = National Health and Nutrition Examination Survey; RNA = ribonucleic acid; SVR = sustained virologic response;
BOX 1 . Persons recommended for hepatitis C testing
BOX 2 . Management of persons with HCV infection
| ) or obese (BMI ≥30kg/m ) |
Suggested citation for this article: Schillie S, Wester C, Osborne M, Wesolowski L, Ryerson AB. CDC Recommendations for Hepatitis C Screening Among Adults — United States, 2020. MMWR Recomm Rep 2020;69(No. RR-2):1–17. DOI: http://dx.doi.org/10.15585/mmwr.rr6902a1 .
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services. Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services. References to non-CDC sites on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. URL addresses listed in MMWR were current as of the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version ( https://www.cdc.gov/mmwr ) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Epidemiology, risk factors, and pathogenesis associated with a superbug: A comprehensive literature review on hepatitis C virus infection
Affiliations.
- 1 Department of Microbiology, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad, Pakistan.
- 2 BreathMAT Lab, IAD, Pakistan Institute of Nuclear Science and Technology (PINSTECH), Islamabad, Pakistan.
- 3 Institute of Microbiology and Molecular Genetics, University of the Punjab, Lahore, Punjab, Pakistan.
- PMID: 35795865
- PMCID: PMC9252020
- DOI: 10.1177/20503121221105957
Viral hepatitis is a major public health concern. It is associated with life threatening conditions including liver cirrhosis and hepatocellular carcinoma. Hepatitis C virus infects around 71 million people annually, resultantly 700,000 deaths worldwide. Extrahepatic associated chronic hepatitis C virus accounts for one fourth of total healthcare load. This review included a total of 150 studies that revealed almost 19 million people are infected with hepatitis C virus and 240,000 new cases are being reported each year. This trend is continually rising in developing countries like Pakistan where intravenous drug abuse, street barbers, unsafe blood transfusions, use of unsterilized surgical instruments and recycled syringes plays a major role in virus transmission. Almost 123-180 million people are found to be hepatitis C virus infected or carrier that accounts for 2%-3% of world's population. The general symptoms of hepatitis C virus infection include fatigue, jaundice, dark urine, anorexia, fever malaise, nausea and constipation varying on severity and chronicity of infection. More than 90% of hepatitis C virus infected patients are treated with direct-acting antiviral agents that prevent progression of liver disease, decreasing the elevation of hepatocellular carcinoma. Standardizing the healthcare techniques, minimizing the street practices, and screening for viral hepatitis on mass levels for early diagnosis and prompt treatment may help in decreasing the burden on already fragmented healthcare system. However, more advanced studies on larger populations focusing on mode of transmission and treatment protocols are warranted to understand and minimize the overall infection and death stigma among masses.
Keywords: Direct-acting antiviral agents; Pakistan; epidemiology/public health; hepatitis C virus; infectious diseases; pathogenesis; risk factors.
© The Author(s) 2022.
PubMed Disclaimer
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Graphical depiction of HCV life…
Graphical depiction of HCV life cycle.
Similar articles
- Burden of pediatric hepatitis C. El-Shabrawi MH, Kamal NM. El-Shabrawi MH, et al. World J Gastroenterol. 2013 Nov 28;19(44):7880-8. doi: 10.3748/wjg.v19.i44.7880. World J Gastroenterol. 2013. PMID: 24307782 Free PMC article. Review.
- NIH Consensus Statement on Management of Hepatitis C: 2002. [No authors listed] [No authors listed] NIH Consens State Sci Statements. 2002 Jun 10-12;19(3):1-46. NIH Consens State Sci Statements. 2002. PMID: 14768714 Review.
- Tuberculosis. Bloom BR, Atun R, Cohen T, Dye C, Fraser H, Gomez GB, Knight G, Murray M, Nardell E, Rubin E, Salomon J, Vassall A, Volchenkov G, White R, Wilson D, Yadav P. Bloom BR, et al. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. PMID: 30212088 Free Books & Documents. Review.
- Hepatitis C Virus and Interferon-Free Antiviral Therapeutics Revolution: Implications for Pakistan. Afzal MS. Afzal MS. Viral Immunol. 2017 May;30(4):252-257. doi: 10.1089/vim.2016.0164. Epub 2017 Jan 24. Viral Immunol. 2017. PMID: 28118096 Review.
- NIH consensus development statement on management of hepatitis B. Belongia EA, Costa J, Gareen IF, Grem JL, Inadomi JM, Kern ER, McHugh JA, Petersen GM, Rein MF, Sorrell MF, Strader DB, Trotter HT. Belongia EA, et al. NIH Consens State Sci Statements. 2008 Oct 22-24;25(2):1-29. NIH Consens State Sci Statements. 2008. PMID: 18949020
- Alterations in the "Gut-Liver Axis" on Rats with Immunological Hepatic Fibrosis. Qi Z, Qi X, Xu Y, Sun H, Li D, Liu J, Cong M, Liu T. Qi Z, et al. J Immunol Res. 2023 Sep 21;2023:5577850. doi: 10.1155/2023/5577850. eCollection 2023. J Immunol Res. 2023. PMID: 37781475 Free PMC article.
- Alter MJ, Margolis HS, Krawczynski K, et al.. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med 1992; 327: 1899–1905. - PubMed
- Williams IT, Bell BP, Kuhnert W, et al.. Incidence and transmission patterns of acute hepatitis C in the United States, 1982-2006. Arch Intern Med 2011; 171: 242–248. - PubMed
- Feinstone SM, Kapikian AZ, Purcell RH, et al.. Transfusion-associated hepatitis not due to viral hepatitis type A or B. N Engl J Med 1975; 292: 767–770. - PubMed
- Gower E, Estes C, Blach S, et al.. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014; 61(Suppl. 1): S45–S57. - PubMed
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol 2013; 10: 553–562. - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- PubMed Central

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Election 2024
- Entertainment
- Newsletters
- Photography
- AP Buyline Personal Finance
- AP Buyline Shopping
- Press Releases
- Israel-Hamas War
- Russia-Ukraine War
- Global elections
- Asia Pacific
- Latin America
- Middle East
- Election results
- Google trends
- AP & Elections
- U.S. Open Tennis
- Paralympic Games
- College football
- Auto Racing
- Movie reviews
- Book reviews
- Financial Markets
- Business Highlights
- Financial wellness
- Artificial Intelligence
- Social Media
Book Review: Matt Haig extols the magic of Ibiza in ‘The Life Impossible’
This cover image released by Viking shows “The Life Impossible” by Matt Haig. (Viking via AP)
- Copy Link copied
“Reality is not always probable, or likely.” That’s the quote from the late Argentine poet Jorge Luis Borges that prefaces Matt Haig’s new novel, “The Life Impossible.” If you fundamentally take issue with it, don’t bother turning the page.
But if you’re willing to suspend disbelief when reading fiction, this is an engaging story. Some readers, like my teenage daughter who devoured Haig’s bestselling book, “The Midnight Library,” may not vibe as well with the septuagenarian narrator recovering from varicose vein surgery, but the book’s plot takes care of her physical deterioration soon enough.
The action is set in Ibiza, the Spanish island famous for its nightclubs. When the narrator, Grace Winters, suddenly inherits a rundown house there, she leaves behind her tragic life as a childless and widowed mathematics teacher in England for an adventure. And, oh, what an adventure! As Grace pieces together the fate of a collegiate acquaintance, Christina, who gifted her the house, she meets Alberto Ribas, a “once respected marine biologist” who now gives diving tours in the Mediterranean and who Grace describes as “not so much of a pirate but a castaway, with the unkempt hair and the beard escaping his face in every direction.” On one of those dives, Grace’s life is forever altered by a blue phosphorescent light she swims toward under the water. “La Presencia,” or “The Presence,” imbues her with actual superpowers, the details of which are too much fun to spoil here.
And while at this point the plot proudly strays from reality, it’s not embarrassed by it. Grace is a reliable narrator and the structure of the novel is her telling her story to a former student. “Mathematics is… as mysterious and enigmatic as the whole of life, and expecting it — or anything — to confirm to what I wanted it to be was a mistake,” she writes. Grace’s reawakening to the wonders of the natural world forms the second half of the story, as she and a cast of characters work to save parts of Ibiza from development.
The entire book will take an average reader just a few hours to read. Really short chapters — some just a sentence long — help the pages fly. And while some may finish the last sentence shaking their head at the implausibility of it all, Grace’s realization that everything on Earth is worthy of admiration and preservation is a message the whole world can get behind.
AP book reviews: https://apnews.com/hub/book-reviews
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PMC11142523

A systematic review of barriers and facilitators to antenatal screening for HIV, syphilis or hepatitis B in Asia: Perspectives of pregnant women, their relatives and health care providers
Lucie sabin.
Institute for Global Health, University College London, London, United Kingdom
Hassan Haghparast-Bidgoli
Faith miller, naomi saville, associated data.
All relevant data are within the manuscript and its Supporting Information files.
Despite improvements, the prevalence of HIV, syphilis, and hepatitis B remains high in Asia. These sexually transmitted infections (STIs) can be transmitted from infected mothers to their children. Antenatal screening and treatment are effective interventions to prevent mother-to-child transmission (MTCT), but coverage of antenatal screening remains low. Understanding factors influencing antenatal screening is essential to increase its uptake and design effective interventions. This systematic literature review aims to investigate barriers and facilitators to antenatal screening for HIV, syphilis, and hepatitis B in Asia.
We conducted a systematic review by searching Ovid (MEDLINE, Embase, PsycINFO), Scopus, Global Index Medicus and Web of Science for published articles between January 2000 and June 2023, and screening abstracts and full articles. Eligible studies include peer-reviewed journal articles of quantitative, qualitative and mixed-method studies that explored factors influencing the use of antenatal screening for HIV, syphilis or hepatitis B in Asia. We extracted key information including study characteristics, sample, aim, identified barriers and facilitators to screening. We conducted a narrative synthesis to summarise the findings and presented barriers and facilitators following Andersen’s conceptual model.
The literature search revealed 23 articles suitable for inclusion, 19 used quantitative methods, 3 qualitative and one mixed method. We found only three studies on syphilis screening and one on hepatitis B. The analysis demonstrates that antenatal screening for HIV in Asia is influenced by many barriers and facilitators including (1) predisposing characteristics of pregnant women (age, education level, knowledge) (2) enabling factors (wealth, place of residence, husband support, health facilities characteristics, health workers support and training) (3) need factors of pregnant women (risk perception, perceived benefits of screening).
Knowledge of identified barriers to antenatal screening may support implementation of appropriate interventions to prevent MTCT and help countries achieve Sustainable Development Goals’ targets for HIV and STIs.
Introduction
Human immunodeficiency virus (HIV), syphilis and hepatitis B are sexually transmitted infections (STIs) that, if left undiagnosed and untreated, can lead to serious complications and death. Despite improvements in the last decade, their prevalence remains high in Asia [ 1 , 2 ]. In 2017, 5.2 million people were living with HIV in the Asia Pacific region [ 3 ] and 123,000 people died from HIV-related causes in 2021 [ 4 ]. The regional prevalence of HIV was 0.2% [ 4 ]. In 2012, an estimated 1.8 million women were infected with syphilis in the South-East Asia region [ 5 ] and 39 million people with hepatitis B with a prevalence of 2.0% [ 6 ].
These STIs can be transmitted from infected mothers to their children during pregnancy and childbirth, resulting in significant morbidity and mortality. The rate of mother-to-child transmission of HIV in Asia and the Pacific is relatively high, at 17%, among the estimated 61,000 women living with HIV who gave birth in the region in 2017 [ 3 ] and 1.3 million pregnant women are at risk of transmitting HBV to their newborns each year [ 7 ]. The global number of adverse pregnancy events attributable to maternal syphilis infection was estimated to be 52,307 in the South-East Asia Region and 13,472 in the Western Pacific Region [ 8 ].
Mother-to-child transmission (MTCT), also called vertical transmission, can be prevented with simple and effective interventions, including antenatal screening and treatment, prevention of male-to-female transmission during sexual intercourse, and improving community awareness. Antenatal screening is an essential tool to enable women to find out if they are infected and to take the necessary steps to access preventive treatment if they test positive in order to avoid MTCT [ 9 ]. Since 2010, an estimated 7,400 new HIV infections among children in the Asia Pacific region were averted because of interventions aimed at reducing the MTCT of HIV [ 3 ]. However, due to limited availability and access to these interventions [ 10 ], antenatal screening for STIs in Asia remains low [ 11 ]. Only three of the 17 reporting countries in the Asia-Pacific region met the global target of over 95% coverage for knowledge of HIV status among women receiving ANC in 2017 and six countries (Bangladesh, Timor-Leste, Papuz New Guinea, Lao People’s Democratic Republic, Indonesia, Singapore) reported coverage below 40% [ 11 ]. Only thirteen countries currently out of 17 countries have a policy of screening for hepatitis B during pregnancy, and very little data on hepatitis B screening coverage is currently available [ 10 ]. Most Asian countries also have no data on syphilis screening for pregnant women. Of the 28 countries in Asia and Pacific regions (according to WHO definitions of regions) reporting antenatal screening coverage for syphilis between 2010 and 2017, four countries reported coverage between 20% and 49% (India, Myanmar, Vanuatu, Papua New Guinea) and three reported coverage below 5% (Afghanistan, Indonesia, Solomon Islands) [ 11 ]. Yet unknowingly infected people can transmit infections to their sexual partners and infected women to their children through MTCT. This also prevents them from accessing timely treatment leading to long-term complications that generate significant costs for the health system. In addition, low uptake of STIs screening services can exacerbate existing health disparities, with vulnerable populations, such as marginalised communities or migrant populations, facing additional barriers to accessing screening services.
To guide a path towards triple elimination of MTCT of HIV, syphilis, and hepatitis B in Asia and the Pacific, the WHO developed a regional framework [ 10 ]. This framework aims to eliminate these three infections in newborns and infants by 2030 in Asia. The key recommendations emphasise an integrated approach to triple elimination, recognising the interconnectedness of the three diseases and the potential for resource optimisation and highlights the importance of strengthening health systems to effectively deliver comprehensive services and achieve universal health coverage. The framework focuses on building capacity, improving laboratory and diagnostic services, ensuring a reliable supply chain for medicines and commodities, and improving reporting systems. It recognises the need for collaboration between different sectors beyond the health sector and the importance of sustainable financing mechanisms to support the implementation of elimination programmes. Meanwhile, it encourages the participation of women living with HIV, women affected by syphilis, and mothers with hepatitis B, men and communities in the design, implementation, and evaluation of programmes and policies.
Understanding barriers and facilitators influencing antenatal screening for STIs is essential to design effective screening interventions. The information will also be useful to help countries to achieve a key health target of the Sustainable Development Goals (SDGs), i.e., “end the epidemics of AIDS, tuberculosis, malaria and neglected tropical diseases and combat hepatitis, water-borne diseases, and other communicable diseases by 2030”. A systematic review conducted by Blackstone et al. [ 12 ] investigated the barriers and facilitators to routine antenatal HIV screening in sub-Saharan Africa, using literature published between 2000 and 2015. They identified the fear of the screening results, perceived stigma towards HIV-positive people, fear of the partner’s reaction in case of a positive test result, and perceived partner disapproval of the test as barriers to antenatal HIV screening. A high level of education, good knowledge of MTCT and HIV, and partner involvement in antenatal care were favourable factors for screening. Health system and provider issues affected the acceptance of antenatal screening. Good patient-provider communication, counselling to improve knowledge of pregnant women of the benefits of screening through counselling, and the perception that HIV screening is mandatory were facilitators to screening.
Barriers are likely to change over time, as societies evolve, beliefs change, or targeted interventions are put in place. There is no literature review summarising the evidence on barriers and facilitators to antenatal screening for HIV, syphilis, and hepatitis B in the Asian context. Factors affecting screening are likely to be different from those in the African context due to cultural and contextual differences. This hinders the development of targeted strategies and interventions to overcome barriers and improve the effectiveness of antenatal screening programmes. It also limits the application of the WHO framework towards triple elimination of MTCT of HIV, syphilis and hepatitis B. Health care providers in Asia may also lack guidance on how to effectively implement and improve antenatal screening programmes for STIs. Barriers preventing vulnerable communities from accessing screening are not known, which may contribute to disparities in health outcomes, with potentially negative impacts on maternal and child health.
In order to fill this evidence gap, this review aimed to investigate the barriers and facilitators to antenatal screening for HIV, syphilis, or hepatitis B for women in Asia. Its specific objectives were to identify available evidence and underline possible gaps in the research knowledge base surrounding this subject.
Methods and analysis
The review and its reporting comply with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist ( S1 Table ) and the protocol has been published on PROSPERO (registration number CRD42023435483).
Search strategy
We conducted a comprehensive search of electronic databases including Ovid (MEDLINE, Embase, PsycINFO), Scopus, Global Index Medicus, and Web of Science was conducted to identify relevant studies published between 2000 and June 2023. The first search was conducted on 13 December 2021 and repeated on 10 June 2023 by LS. The keyword search was divided into five main groups: “barriers or facilitators”, “antenatal screening”, “HIV or syphilis or hepatitis B”, and “Asian countries”. The finalised search terms were developed through a trial-and-error process for use on Scopus and adapted to the different databases. The full key words used are shown in S1 File .
We used forward and backward citation searching to capture resources either citing or being cited by the included literature and searched the websites of the WHO, the World Bank and UNAIDS for reports.
Inclusion criteria
The eligibility criteria for study inclusion were developed using the acronym SPlDER: S sample; P phenomenon of interest; D design; E evaluation; R research type [ 13 ] ( Table 1 ).
| Pregnant women or women of childbearing age in Asian countries, their family members, health workers and decision-makers (the search term including all the Asian countries as defined by the United Nations is provided in ). | |
| Barriers and facilitators to antenatal screening and factors influencing screening uptake. Barriers were defined as factors discouraging or impeding screening uptake. Facilitators were defined as factors or resources enhancing screening uptake. Factors may also relate to the implementation and effectiveness of antenatal screening. | |
| Primary or secondary research studies, including quantitative, qualitative, and mixed-methods studies. | |
| Antenatal screening programs or interventions related to the screening of HIV, syphilis, or hepatitis B during pregnancy. | |
| Peer-reviewed journal articles in English conducted between 2000 and June 2023. |
Study selection
Following the initial search, LS collated records and uploaded them into Rayyan [ 14 ] to facilitate screening. After removal of duplicates, two independent reviewers (LS and FM) screened titles and abstracts for relevance and assessed full text of potentially relevant article using the inclusion criteria. Those meeting inclusion criteria at full-text screen were included in our results. Any discrepancies were resolved through discussion or consultation with a third reviewer (NS) when needed.
Data extraction
We used a standard form to extract key information including study characteristics (author, year, country, urban/rural setting, diseases considered), study design, sample, aim, identified significant barriers and facilitators to screening (e.g., odds ratios at the 95% confidence interval, p-value < 0.05). We thematically analysed qualitative articles through an iterative process of reading and coding them using Andersen’s framework [ 15 ]. This theoretical framework widely used in literature reviews on healthcare utilisation [ 16 ] provides understanding of how individuals and environmental factors influence health behaviours. The framework categorises predictors of health service use as i) Predisposing characteristics including demographic factors, social structure, and health beliefs that influence health services use. ii) Enabling factors allowing the individual to seek health services if needed. iii) Need factors including perceived needs of healthcare services use.
Quality assessment
LS and FM assessed the quality of included studies using tools appropriate to the study design. The quality of the studies included was evaluated based on Von Elm et al’s [ 17 ] checklist for observational studies and O’Brien et al’s [ 18 ] checklist for qualitative studies. S2 and S3 Tables present the quality appraisal checklists for the considered studies. We scored each paper based on how many checklist items were met. Overall, papers that met over 75% of the checklist items were considered to be of high quality, those meeting 50% to 75% of the checklist were regarded as moderate quality, and those meeting less than 50% poor quality. Because the aim was to describe and synthesise a body of the literature and not determine an effect size, studies were not excluded based on quality.
Data analysis and presentation
Descriptive characteristics of research studies were presented in tables. A narrative synthesis (Popay et al. 2006) was conducted to summarize the findings of the included studies. We did not combine quantitative estimates because of the heterogeneity of approaches and findings. Themes and patterns related to factors influencing screening uptake were identified and analysed and the final set of barriers and facilitators categorised according to Andersen [ 15 ]’s conceptual model.
After the selection process, 23 articles met the eligibility criteria and were included in the review. The PRISMA diagram provides an overview of the selection process ( Fig 1 ).

General study characteristics
Details about the articles included are presented in Table 2 . Most included studies were on HIV screening, one was on syphilis screening [ 19 ], one on HIV and syphilis [ 20 ] and one on HIV, syphilis and hepatitis B [ 21 ]. Eight out of the 23 studies used data collected after 2015 [ 20 , 22 – 28 ]. Six of the studies were conducted in Vietnam, five in India, three in Indonesia, two in Cambodia, and one each in Hong Kong, Mongolia, China, Afghanistan and Thailand. Nineteen of the studies (83%) used quantitative methods, three (15%) used qualitative methods, and one (2%) used mixed methods.
| Citation | Date | Country | Urban/ rural | Disease | Sample | Study type | Aim |
|---|---|---|---|---|---|---|---|
| Dinh [ ] | 2005 | Vietnam | Urban | HIV | 500 pregnant women 18 aged years and older who were first-time antenatal care (ANC) visitors and had never been tested or were unaware of their results | Quantitative | Identify the factors associated with declining HIV antenatal screening and the failure to return for results |
| Nguyen [ ] | 2010 | Vietnam | Urban | HIV | 300 women who had recently delivered | Quantitative | Describe the uptake of antenatal HIV screening |
| Hạnh [ ] | 2011 | Vietnam | Urban/ rural | HIV | 1108 nursing mothers | Quantitative | Assess early uptake of HIV screening and the provision of HIV counselling among pregnant women |
| Pharris [ ] | 2011 | Vietnam | Urban | HIV | 1108 pregnant women who attend antenatal care at primary and higher-level health facilities | Quantitative | Assess early uptake of HIV testing and the provision of HIV counselling among pregnant women |
| Khuu [ ] | 2018 | Vietnam | Urban | HIV | 320 women who were tested during ANC | Quantitative | Identify reasons for late HIV screening among pregnant women |
| Chu [ ] | 2019 | Vietnam | Urban/ rural | HIV | 1484 women aged 15 to 49 years having a live birth within the last 2 years | Quantitative | Assess the socioeconomic inequalities in HIV screening during ANC |
| Bharucha [ ] | 2005 | India | Urban | HIV | 6,702 pregnant women presenting in labour | Quantitative | Explore factors affecting the eligibility and acceptability of voluntary counselling and rapid HIV testing |
| Rogers [ ] | 2006 | India | Rural | HIV | 202 pregnant women attending a rural ANC clinic | Quantitative | Investigate HIV-related knowledge, attitudes toward infant feeding practices, and perceived benefits and risks of HIV screening |
| Sinha [ ] | 2008 | India | Rural | HIV | 400 women that have gave birth in the previous 12 months | Quantitative | Investigate HIV screening among rural women during pregnancy |
| Sarin [ ] | 2013 | India | Rural | HIV | 357 women who had given birth in the last two years | Quantitative | Examine the prevalence and the barriers to HIV screening among pregnant women vulnerable to HIV due to their spouses’ risky behaviours |
| Sharma [ ] | 2022 | India | Urban/ rural | HIV | 122,351 women aged 15–49 | Quantitative | Determine the factor associated with HIV screening during ANC |
| Lubis [ ] | 2019 | Indonesia | Urban/ rural | HIV | 20 private midwives | Qualitative | Examine midwives’ perceptions of barriers and enabling factors about referring pregnant women for HIV screening |
| Wulandari [ ] | 2019 | Indonesia | Urban/ rural | HIV | 619 women to voluntary HIV counselling and screening clinics | Quantitative | Examine the rates of HIV screening uptake among pregnant women attending private midwife clinics |
| Baker [ ] | 2020 | Indonesia | Rural | HIV, syphilis | 3382 pregnant women and 40 health workers involved in screening | Mixed-methods | Explore current practice, barriers and facilitators in the delivery of antenatal testing for anaemia, HIV and syphilis |
| Pakki [ ] | 2020 | Indonesia | Rural | HIV | 42 health workers managers | Quantitative | Investigate the influence of training given to health workers on HIV testing uptake by pregnant women |
| Setiyawati [ ] | 2021 | Indonesia | Urban | HIV | 350 housewives in districts that already implemented prevention mother-to-child transmission program | Quantitative | Assess the factors that influence the housewife attitude toward HIV testing |
| Kakimoto [ ] | 2007 | Cambodia | Urban | HIV | 315 mothers who came to a childhood immunization with a child aged 6–24 months | Quantitative | Assess predictive determinants for HIV testing |
| Sasaki [ ] | 2010 | Cambodia | Urban | HIV | 600 eligible mothers who were admitted to the hospital after delivery | Quantitative | Assess the prevalence of and barriers to HIV screening |
| Lee [ ] | 2005 | Hong Kong | Urban | HIV | 3,500 pregnant women attending their first ANC visit | Quantitative | Investigate acceptance of universal HIV antibody screening programme |
| Munkhuu [ ] | 2006 | Mongolia | Urban | Syphilis | 150 ANC providers and 27 senior doctors | Qualitative | Assess ANC providers’ practices and opinions toward antenatal syphilis screening |
| Todd [ ] | 2008 | Afghanistan | Urban | HIV, syphilis, hepatitis B | 114 doctors and midwives | Quantitative | Determine attitudes toward and utilization of testing for HIV, syphilis, and hepatitis B among obstetric care providers |
| Crozier [ ] | 2013 | Thailand | Urban | HIV | 38 migrant pregnant women who had been through the HIV screening process 2013and 26 health personnel | Qualitative | Explore factors that relate to HIV screening decisions for migrant women |
| Li [ ] | 2014 | China | Urban | HIV | 500 pregnant women recruited during their antenatal visit | Quantitative | Assess the prevalence of the willingness for HIV testing among pregnant women and cognitive factors associated with it |
In the four studies that used qualitative methods, pregnant women were interviewed as well as other individuals such as health providers, district managers, husbands, and mothers. Sample sizes in quantitative studies ranged from 114 to 122,351 pregnant women, most often recruited during ANC visits. The quantitative studies were all cross-sectional except one from Indonesia, which was longitudinal [ 25 ]. Most quantitative studies used logistic regression models to determine the association between potential barriers and the outcome of interest.
Overviews of the barriers and the facilitators identified
The barriers and facilitators identified in the included articles are presented based on the categories of the Andersen’s conceptual model ( Table 3 and Fig 2 ).

| Citation | Date | Country | Diseases | Predisposing characteristics | Enabling factors | Need factors |
|---|---|---|---|---|---|---|
| Bharucha [ ] | 2005 | India | HIV | Facilitators: • Being older • Living closer to the hospital | Barriers: • Being too far along in the birth delivery process when the opportunity to test arises Facilitators: • Having had antenatal care in the hospital rather than in other health facilities | |
| Dinh [ ] | 2005 | Vietnam | HIV | Barriers: • Being a housewife • Low level of education | Barriers: • Fear of husband’s disapproval • Perception of poor healthcare availability | Barriers: • Low-risk perception |
| Lee [ ] | 2005 | Hong Kong | HIV | Facilitators: • High level of education • Good HIV knowledge • Access to HIV information by means of posters, pamphlets, videos and group talks | Facilitators: • Healthcare workers’ recommendations to be screened | Barriers: • No or low-risk perception Facilitators: • Good perceived benefits of screening |
| Rogers [ ] | 2006 | India | HIV | Barriers: • Low knowledge of HIV | Barriers: • Fear of negative reactions from husbands, parents, and community • Fear of stigma and discrimination | |
| Munkhuu [ ] | 2006 | Mongolia | Syphilis | Barriers: • Low knowledge of syphilis • Being poor • Long travel distance to get tested | Barriers: • Limited time for screening due to antenatal visits starting late in pregnancy • Complexity of testing service system • Undersupplied screening materials • Healthcare workers not in favour of screening | Barriers: • Reporting previous sexually transmitted diseases |
| Kakimoto [ ] | 2007 | Cambodia | HIV | Facilitators: • Basic knowledge of HIV transmission • High partner education level | Barriers: • Need to obtain husband’s approval to be tested | |
| Sinha [ ] | 2008 | India | HIV | Barriers: • Low awareness of existing HIV testing facilities | Barriers: • Never received HIV counselling before | |
| Todd [ ] | 2008 | Afghanistan | HIV, syphilis, hepatitis B | Facilitators: • High acceptance of screening by providers Barriers: • Providers’ perceptions that infections were rare • Provider’s low perceived likelihood of infection based on healthy appearance • Stigma toward infected individuals • Need to obtain husband’s approval to be tested | ||
| Nguyen [ ] | 2010 | Vietnam | HIV | Barriers: • High distance to the hospital | ||
| Sasaki [ ] | 2010 | Cambodia | HIV | Barriers: • Low knowledge of HIV | Barriers: • Lack of access to antenatal care services • Need to obtain husband’s approval to be tested | |
| Hạnh [ ] | 2011 | Vietnam | HIV | Facilitators: • First antenatal check-up at primary health facilities rather than at district and provincial health facilities | ||
| Pharris [ ] | 2011 | Vietnam | HIV | Facilitators: • Younger age • Residence in a semi-urban area • Higher economic status | Barriers: • Low perception of risk | |
| Crozier [ ] | 2013 | Thailand | HIV | Barriers: • Low knowledge of HIV and mother-to-child transmission | Barriers: • Language differences between health worker and pregnant women • Concern about the reactions of health workers • Financial barriers • Costs and time of transportation • Provider’s lack of time to inform women properly • Having only one antenatal check-up • Lack of support from husband | Barriers: • Low perception of risk |
| Sarin [ ] | 2013 | India | HIV | Facilitators: • More than six years of education • Good knowledge of HIV | Facilitators: • Discussions with husband about HIV • Seeking antenatal care in government district hospitals and private clinics as opposed to community health centres (not equipped with either HIV counselling or testing facilities) | |
| Li [ ] | 2014 | China | HIV | Facilitators: • Good knowledge of HIV | Facilitators: • Less perception of social stigma | Facilitators: • High perception of risk |
| Khuu [ ] | 2018 | Vietnam | HIV | Barriers: • Younger than 30 years old • Nine or fewer years of education • Working as a homemaker or worker/farmer • Living 20km or more from the hospital | Barriers: • Having received antenatal care at private clinic/hospital only | Barriers: • Low perceived benefits of screening |
| Chu [ ] | 2019 | Vietnam | HIV | Barriers: • Belonging to ethnic minorities • Having primary or less education • Being poor • Living in rural areas | ||
| Lubis [ ] | 2019 | Indonesia | HIV | Facilitators: • Free HIV screening • Reward and punishment system to motivate providers • Training for health workers Barriers: • Fear of stigma • Limited voluntary counselling and testing opening hours do not cater for those in employment • Not a one-roof for ANC and VCT services • Providers disguising or not revealing purpose of the blood testing for fear of causing offense | ||
| Wulandari [ ] | 2019 | Indonesia | HIV | Facilitators: • Living in urban area | ||
| Baker [ ] | 2020 | Indonesia | HIV, syphilis | Barriers: • National policy on testing not widely disseminated • Testing not seen as a priority intervention • Multiple small-scale funding sources • Tests seen as expensive by pregnant women • Lack of knowledge and training of providers • Shortage of laboratory personnel • Shortage of tests and laboratory resources • Stigma amongst providers and community • Lack of time from pregnant women • Fear of the results | Barriers: • Perceived low prevalence | |
| Pakki [ ] | 2020 | Indonesia | HIV | Facilitators: • Health workers training on predisposing factors of provider-initiated testing and counselling of HIV | ||
| Setiyawati [ ] | 2021 | Indonesia | HIV | Barriers: • Pregnant women’s beliefs that their husbands have a bad attitude towards HIV testing | Barriers: • Low perceived benefits of screening | |
| Sharma [ ] | 2022 | India | HIV | Barriers: • Low educational level • Low knowledge of HIV • Being poor • Living in rural area • Low exposure to mass media |
Predisposing characteristics
Several predisposing characteristics were reported as either barriers or facilitators to antenatal screening for HIV and syphilis. In three studies conducted in Vietnam and India, age was associated with antenatal screening of HIV [ 22 , 32 , 33 ]. Pharris et al. [ 32 ] found that younger Vietnamese women were more likely to be screened while Bharucha et al. [ 33 ] found the opposite result in India. Khuu et al. [ 22 ] identified being younger than 30 years old as a barrier to antenatal screening.
Low education status of pregnant women was a barrier to antenatal screening in three studies conducted in Vietnam [ 22 , 23 , 29 ] and one in India [ 28 ]. Similarly, one study conducted in Hong Kong [ 39 ] and one in India [ 36 ] identified higher education as a facilitator to antenatal screening. However, the level of education associated with a positive likelihood of being screened varied between studies. For example, Khuu et al. [ 22 ] showed that nine or more years of education was associated with more acceptance of screening in Vietnam, whereas Sarin et al. [ 36 ] showed that this was true at more than six years of education in rural India.
Pregnant women’s knowledge about HIV and PMTCT was associated with antenatal screening decisions. Lack of knowledge about HIV amongst pregnant women [ 28 , 34 , 36 , 38 ], about the MTCT services [ 34 ], and about the availability of HIV testing facilities [ 35 ] were identified as barriers to screening in four studies in India, one in Cambodia and one in Thailand. Similarly, three studies conducted in Cambodia, Hong Kong and China found that a better knowledge of HIV amongst pregnant women was associated with a higher screening uptake [ 37 , 39 , 41 ]. Moreover, Munkhuu et al. [ 19 ] found similar results for syphilis in their study conducted in Mongolia. Lack of knowledge about syphilis amongst pregnant women was associated with lower screening uptake. A study conducted in India [ 28 ] found that low exposure to mass media was associated with lower HIV screening uptake. Similarly in Hong Kong, Lee et al. [ 39 ] identified access to HIV information by means of posters, pamphlets, videos, and group talks as a facilitator to screening.
Enabling factors
The role of enabling factors such as wealth, place of residence, husbands and health workers’ roles, social and cultural norms or screening cost has been discussed in several articles.
Low household wealth or socio-economic status was a barrier even in countries where antenatal screening was free of charge. Three studies conducted in Mongolia, Vietnam, and India found low socio-economic status as being a barrier to antenatal screening for HIV [ 19 , 23 , 28 ]. Pharris et al. [ 32 ] identified higher economic status as a facilitator to antenatal screening for HIV in Vietnam.
Various studies have shown that the place of residence was associated with antenatal screening for HIV [ 22 , 23 , 25 , 28 , 30 , 32 , 33 ] and syphilis [ 19 ]. A study conducted in Vietnam [ 23 ] and another conducted in India [ 28 ] identified living in a rural area as a barrier to antenatal screening for HIV. Similarly, Wulandari et al. [ 25 ] and Pharris et al. [ 32 ] found that living in an urban area and a semi-urban area were facilitators to antenatal screening of HIV in Vietnam and Indonesia respectively. Proximity to the hospital is also a factor influencing antenatal screening uptake. Khuu et al. [ 22 ] and Nguyen, Christoffersen, and Rasch [ 30 ] found that living further away from the hospital (over 20km in the case of Khuu et al.) was a barrier to antenatal screening for HIV. Similar results were found by Munkhuu et al. [ 19 ] in Mongolia for the antenatal screening of syphilis. Meanwhile, Bharucha et al. [ 33 ] identified living closer to the hospital as a facilitator for antenatal screening of HIV in India.
Two studies conducted in Vietnam found a significant effect of occupation on the decision to be tested. For example, housewives, or labourers/farmers were less likely to be tested for HIV [ 22 , 29 ]. Kakimoto et al. [ 37 ] identified high partner education level as a facilitator to antenatal screening in Cambodia. Meanwhile, Chu, Vo [ 23 ] found a negative association between belonging to ethnic minorities and being tested during pregnancy.
Several articles identified that their husband play a key role in women’s decision to be screened. Fear of negative reactions from their husbands [ 34 ], husband’s disapproval [ 29 ] and lack of support [ 40 ], and beliefs that their husbands have a bad attitude towards HIV testing [ 27 ] were identified as barriers to screening in India, Thailand, Indonesia and Vietnam respectively. Two studies conducted in Cambodia [ 37 , 38 ] found that the perceived need to obtain partner’s authorisation is a barrier to screening for HIV. Similar findings were found in Afghanistan by Todd et al. [ 21 ] for antenatal screening of syphilis and hepatitis B. Similarly, Sarin et al. [ 36 ] reported that having discussions with spouses about HIV in India encouraged women’s screening for HIV.
Various studies have shown that social and cultural factors were key barriers to antenatal screening for HIV, syphilis or hepatitis B. Todd et al. [ 21 ] identified stigma toward infected people as a barrier to antenatal screening for HIV, syphilis, and hepatitis B in Afghanistan. Similar results were found by Baker et al. [ 20 ] in Indonesia for the screening of HIV and syphilis, and Lubis et al. [ 24 ] and Rogers et al. [ 34 ] for the screening of HIV. This last article also identified the fear of negative reactions from parents and community as a barrier. Similarly, Li et al. [ 41 ] found that lower perception of social stigma was associated with higher screening uptake.
Time was also associated with antenatal screening decisions for HIV and syphilis. It was a barrier both from the supply and the demand side. Working pregnant women reported that limited opening hours of screening centres were a major health-facility related barrier to antenatal screening for HIV in Indonesia [ 24 ]. Limited time to inform women properly about HIV during pregnancy and antenatal screening [ 40 ] as well as limited time to perform screening for syphilis [ 19 ] were barriers to antenatal screening in Thailand and Mongolia. From the demand side, long travel time to access antenatal screening services was associated with lower HIV screening uptake in Thailand [ 40 ]. Similarly, lack of time was identified as a barrier to screening for HIV and syphilis in Indonesia by Baker et al. [ 20 ]. Meanwhile, Bharucha et al. [ 33 ] found that being offered testing too late in pregnancy as associated with lower screening uptake for HIV.
The type of screening provider was a factor associated with screening in various studies. Hạnh, Gammeltoft, and Rasch [ 31 ] showed that, in Vietnam, having the first antenatal check-up at a commune health station was a factor associated with an increased probability of being tested, compared with district and provincial health facilities. Similarly and in the same country, having received ANC only at a private clinic/hospital was found to be a barrier [ 22 ]. However, in India, Sarin et al. [ 36 ] found that seeking ANC at government district hospitals and private clinics, as opposed to community health centres not equipped with either HIV counselling or testing facilities, had a positive effect on the probability of receiving HIV screening. Similar results were found by Bharuch et al. [ 33 ] in India. Some facilities lack screening materials and this was associated with lower screening of syphilis in Mongolia [ 19 ] and lower screening of HIV and syphilis in Indonesia [ 20 ]. In addition, a study carried out in Indonesia [ 24 ] revealed that the lack of antenatal care and screening services in the same building was a barrier to HIV screening. In Cambodia, the lack of access to ANC services outside the capital city was a barrier to screening for HIV [ 38 ].
Healthcare workers play a key role in screening decisions. In Vietnam, Dinh, Detels and Nguyen [ 29 ] found that a poor perception of healthcare availability was negatively associated with screening for HIV. Fear that healthcare workers would become impatient with them or that their questions would not be considered important was a barrier in Thailand [ 40 ], and concern that healthcare workers were opposed to antenatal screening for syphilis impeded testing in Mongolia [ 19 ]. Similarly, Lee et al. [ 39 ] identified health worker recommending HIV testing as a facilitator of screening. A study conducted in Vietnam [ 32 ] identified never having received antenatal HIV counselling as a barrier to screening and another identified a language barrier between health workers and women as barriers [ 40 ]. High acceptance of screening for HIV, syphilis and hepatitis B was also a factor increasing screening uptake in Afghanistan [ 21 ]. Pakki et al. [ 26 ] and Lubis et al. [ 24 ] found that, in Indonesia, health worker training as well as reward and punishment system to motivate them was associated with higher antenatal HIV screening. This is consistent with findings reported in Indonesia for HIV and syphilis screening [ 20 ]. Todd et al. [ 21 ] found that provider perceptions of low infection rates and assumptions on a person’s likelihood of infection based on a healthy appearance were associated with lower screening uptake of HIV, syphilis and hepatitis B in Afghanistan. Baker et al. [ 20 ] also identified shortage of laboratory personnel as a barrier to screening.
Costs of screening was also identified as factor influencing HIV and syphilis screening uptake. Tests being seen as expensive by pregnant women was identified as a barrier to HIV and syphilis screening in Indonesia [ 20 ]. Similarly, Crozier et al. [ 40 ] found that costs of screening and transportation represent barriers to screening of HIV and syphilis in Thailand.
At the national-level, enabling factors were identified by two studies in Mongolia and Indonesia [ 19 , 20 ]. Munkhuu et al. [ 19 ] identified the complexity of the syphilis testing service system as a barrier to antenatal screening. Similarly, Baker et al. [ 20 ] found that poor dissemination of national policy on screening, not seeing screening as a priority intervention, and funding consisting of multiple small-scale sources were barriers to HIV and syphilis screening in Indonesia.
Finally, Crozier, Chotiga et Pfeil [ 40 ] showed that having only one ANC check-up was associated with low screening uptake.
Need factors
Few need factors were identified as barriers or facilitators in antenatal screening for HIV and syphilis. Four studies conducted in Hong Kong, Vietnam and Thailand found that low perceived risk of HIV was associated with low screening [ 29 , 32 , 39 , 40 ]. Similarly, Lee, Yang, and Kong [ 41 ] found that, in China, high perceived risk of HIV was associated with high screening. In a study investigating barriers and facilitators in the delivery of antenatal testing for anaemia, HIV, and syphilis, Baker et al. [ 20 ] identified perceived low prevalence of HIV and syphilis as barriers to antenatal screening in Indonesia. Two studies found that believing that HIV testing was not important during pregnancy was associated with a lower screening uptake in Indonesia and Vietnam [ 22 , 27 ]. Similar Lee et al. [ 39 ] identified the perception of the benefits of HIV screening as a factor facilitating it. Finally, Munkhuu et al. [ 19 ] found that women who previously reported STIs were less likely to be screened in Mongolia.
This study is the first to provide a narrative synthesis of the current literature on barriers and facilitators to antenatal screening for HIV, syphilis and hepatitis B in Asia. This systematic review of qualitative, quantitative and mixed-method studies shows that there are research gaps into the factors influencing screening for syphilis and hepatitis B, with most of the studies reviewed focusing on HIV. This review therefore effectively allows conclusions to be drawn about HIV alone.
Antenatal screening for HIV in Asia is influenced by a range of factors including predisposing characteristics (age, education level, wealth, place of residence, knowledge about HIV), enabling factors (husband support, health facilities characteristics, health workers’ support and training) and need factors (risk perception, perceived benefits of screening). These factors are similar to those identified in a review conducted by Blackstone et al. [ 12 ] in sub-Saharan Africa. In our literature review, as in the sub-Saharan African context, being better-off and highly educated were identified as facilitators. In both contexts, pregnant women’s lack of knowledge about HIV appears to be a significant barrier to antenatal HIV screening. Our results suggest that antenatal screening could be improved by facilitating access to information for women, their husbands and health workers. Most studies have emphasised the importance of improving dissemination of information about HIV and HIV testing in order to improve uptake of antenatal screening. Unlike Blackstone et al.’s review of the literature in the sub-Saharan African context [ 12 ], our review did not identify fear of results as such as a barrier to testing, but more broadly fear of partner reactions and potential violence in the event of a positive result. We did not find that cultural gender norms to be barrier, such as "testing is a woman’s business", as found by Blackstone et al. [ 12 ]. However, women in this review mentioned the need to obtain a husband’s approval to undergo screening. In both African and Asian contexts, societal stigma towards HIV-positive people proved to be a major barrier to HIV testing. Our findings, and those of Blackstone et al. [ 12 ], suggest that antenatal screening could be improved by strengthening the health care system. Both reviews highlighted the role of healthcare and communication professionals in increasing antenatal screening rates. In the sub-Saharan African context the perception of screening being mandatory was a barrier to screening, but this did not emerge in our literature review.
Although the studies we reviewed were all conducted in Asia, they spanned very different contexts. It is reasonable to assume that the barriers to antenatal screening will differ between Hong Kong and India for instance. Guidelines about screening and adherence to guidelines differ between countries. A review of maternal health care policies in eight countries in the Western Pacific region [ 42 ] found that WHO recommendations on antenatal HIV screening were not included in antenatal care guidelines in two countries. In 2018, 37 countries in the Asia Pacific region promoted antiretroviral therapy for all pregnant and breastfeeding women living with HIV, but in six of these countries, the policy is being implemented in less than 50% of all maternal and child health sites [ 43 ]. Reported barriers in the Hong Kong study were mainly focused on the demand side [ 39 ], whereas the Mongolia study identified many supply-side barriers [ 19 ]. This highlights the need for qualitative studies in Asian contexts to investigate context-dependent factors that may be missed in quantitative studies.
As stigmatisation of people with STDs is one of the main factors preventing pregnant women from being screened, interventions should provide information and counselling to pregnant women and their husbands, tailored to low-literacy populations to help reduce stigma and increase uptake [ 36 , 38 , 39 ]. Raising awareness within communities of the importance of male partner involvement, the benefits of screening and adherence to treatment could increase demand for antenatal screening services. However, studies on awareness campaigns about HIV in Vietnam [ 44 ] and Thailand [ 45 ] showed that the stigma attached to social judgement is difficult to reduce. Various studies recommended the integration of HIV screening into community level ANC services [ 23 , 25 , 30 , 31 , 39 ] and the development of opt-out approaches for those who prefer not to test [ 29 , 35 ], as recommended in sub-Saharan Africa by Blackstone et al. [ 12 ]. We found that husbands play a key role in encouraging pregnant women to undergo screening. Interventions to improve husbands’ knowledge and involvement in maternal and newborn health had a positive impact on maternal health behaviour in Bangladesh [ 46 ] and Nepal [ 47 ]. To reduce social and financial barriers to antenatal screening, screening should be offered to pregnant women universally free of cost [ 32 , 39 ]. Currently, national budgets do not cover all the costs associated with antenatal screening in all Asian countries. In the 17 Asian countries for which data on the cost of screening pregnant women for HIV, syphilis and hepatitis B were available in 2017, HIV screening of pregnant women was free in all of these countries, syphilis screening in 14 countries and hepatitis B screening was free in eight countries [ 11 ]. Finally, the quality of services depends on the availability and capacity of healthcare workers. To reduce the persistence of inappropriate healthcare practices in pregnancy, interventions need to develop health worker training programmes on STIs and pregnancy screening. A successful initiative in Cambodia in decreasing risky sexual intercourse and improving the access to sexual and reproductive health care services has focused on training community health workers in sexual and reproductive, maternal, neonatal, child and adolescent health [ 48 ].
Adolescent pregnancy is still common in the region with 3.7 million births to adolescent girls aged 15–19 every year in Asia and the Pacific [ 49 ]. Pregnant adolescents are very vulnerable and are known to have poor outcomes for both mother and child [ 50 ]. This systematic review of the literature highlighted a lack of age-specific data, particularly in relation to adolescent pregnancy, and confirmed the need to fill this research gap. Similarly, a systematic literature review of interventions addressing health outcomes for pregnant adolescents in low- and middle-income countries highlighted the need to develop studies to design high-quality care and services for pregnant adolescents [ 51 ].
Several limitations to this study should be noted. Firstly, most studies sampled pregnant women through ANC services. However, women who have not sought ANC may face the greatest barriers to testing. Due to resource constraints, only articles in English were reviewed, which may limit access to the grey literature and studies published in other languages (especially Chinese). Finally, different studies were undertaken in different contexts and using different methods. This heterogeneity limits our ability to compare between studies. However, this systematic review follows a rigorous method of article selection and analysis. It complements existing literature reviews on barriers to antenatal screening, particularly in sub-Saharan Africa [ 12 , 52 ].
The main barriers to antenatal screening in this systematic review were stigmatisation of infected individuals, lack of involvement of husbands and healthcare system factors. To improve uptake of antenatal screening interventions to improve community and husband involvement, awareness campaigns with communities and health workers, and training of health workers on STI issues are needed. While countries vary in their contexts and implementation of international recommendations on integrated antenatal screening for STIs, in all settings the planning, implementation, reporting and monitoring of interventions to eliminate mother-to-child transmission require coordination between different health system stakeholders at national, regional and local levels to avoid gaps or duplication. Global, regional and national guidelines need to be harmonised to avoid gaps and duplication between disease-specific and maternal and child health programs and guidelines. Integration of services for different diseases should be prioritised where possible. However, studies to examine the barriers and facilitators to antenatal screening for syphilis and hepatitis B and to examine the behavioural determinants of antenatal screening in Asia are still needed.
Supporting information
Funding statement.
The authors received no specific funding for this work.
Data Availability
- PLoS One. 2024; 19(5): e0300581.
Decision Letter 0
19 May 2023
PONE-D-23-03027Barriers and facilitators to antenatal screening for sexually transmitted diseases in Asia: a scoping review.PLOS ONE
Dear Dr. Sabin,
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process.
==============================
Carefully consider both reviewers' comments especially with regards to the framework that was used for the study and also labelling of the study as a scoping review. The various components and processes that are necessary for a scoping review ought to be presented in the manuscript.
Please submit your revised manuscript by Jul 03 2023 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp . When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please include the following items when submitting your revised manuscript:
- A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
- A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
- An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: https://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols . Additionally, PLOS ONE offers an option for publishing peer-reviewed Lab Protocol articles, which describe protocols hosted on protocols.io. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols .
We look forward to receiving your revised manuscript.
Kind regards,
Gifty Dufie Ampofo, M.D., Ph.D
Academic Editor
Journal requirements:
When submitting your revision, we need you to address these additional requirements.
1. Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming. The PLOS ONE style templates can be found at
https://journals.plos.org/plosone/s/file?id=wjVg/PLOSOne_formatting_sample_main_body.pdf and
https://journals.plos.org/plosone/s/file?id=ba62/PLOSOne_formatting_sample_title_authors_affiliations.pdf .
2. Please include a copy of Table 4 and 5 which you refer to in your text on page 6.
3. Please include captions for your Supporting Information files at the end of your manuscript, and update any in-text citations to match accordingly. Please see our Supporting Information guidelines for more information: http://journals.plos.org/plosone/s/supporting-information .
[Note: HTML markup is below. Please do not edit.]
Reviewers' comments:
Reviewer's Responses to Questions
Comments to the Author
1. Is the manuscript technically sound, and do the data support the conclusions?
The manuscript must describe a technically sound piece of scientific research with data that supports the conclusions. Experiments must have been conducted rigorously, with appropriate controls, replication, and sample sizes. The conclusions must be drawn appropriately based on the data presented.
Reviewer #1: No
Reviewer #2: No
2. Has the statistical analysis been performed appropriately and rigorously?
Reviewer #1: N/A
Reviewer #2: N/A
3. Have the authors made all data underlying the findings in their manuscript fully available?
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
Reviewer #1: Yes
Reviewer #2: Yes
4. Is the manuscript presented in an intelligible fashion and written in standard English?
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please note any specific errors here.
5. Review Comments to the Author
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Reviewer #1: The topic is of great interest, however the manuscript seems hurriedly put together. Concepts involved such as Andersen's model are not clearly and coherently applied in the course of the write-up.
The title for example does not capture the full essence of the review - it seems to be based on the "regional framework for the triple elimination of MTCT of HIV, Hep B and syphillis in Asia and Pacific" (lines 99 - 101)- that should have reflected clearly in the title. Such an understanding should have been provided briefly in the "Background" section of the script.
Secondly, the target population of the review was not clear right from the "title". Who did the review focus on - pregnant women, healthcare providers, relatives, etc. or all of them? That is not clear from the manuscript.
The objectives of the review as stated in lines 74 and 75 are not meant for a scoping review, but some other approaches that would generate clear evidence such as a review of qualitative evidence.
The methods section has lots of lapses.
a. A key concept such as the "population of the review" is not defined. The "search strategy" should have been systematically presented - e.g., keywords/ subject headings/ index terms that were used, example of a search strategy of at least one of the databases should have been placed in the appendices; the search should have been as exhaustive as possible - searching two databases does not sound exhaustive enough.
b. The "inclusion and exclusion criteria" should have been clearly defined each criterion - this subsection at best is confusing to the reader as it does not serve its purpose of clearly pointing out how studies were included in the review. Studies of experimental designs were excluded with no apparent reasons as to why they were.
c. Under "Data extraction", the Andersen's framework was said to have been the guide - it would have been great to have this clearly "tabulated" with "findings" related to each component of the framework duely and systematically presented. The quality of studies was assessed, but this outcome did not feature further into any decision-making or discussion as to how study quality influenced the review process.
d. Andersen's model as defined in lines 123 - 124 does not seem to be in sync with the cited publication (11), i.e., predisposing, enabling, and needs factors. "Enabling factors" were not given the prominence required.
Aside the mention of "facilitators" on line 147, this key component of the review was hardly addressed. Furthermore in the results section, the nature of the findings as were presented did not necessarily aptly fit the defined factors under the Andersen model.
In its current form, this manuscript in my view is not fit for publication.
Reviewer #2: Review Comments on manuscript PONE-D-23-03027
…..perhaps the title should be restricted to HIV, Syphilis and Hepatitis B rather than ‘sexually transmitted diseases’
Despite being a scoping review, a little more detail will improve the Background and situate arguments in better context.
• Lines 50/51…….the authors can provide recent data on the morbidity and mortality they refer to…..first globally and in the Asian context
Quantify the prevalence of these STDs in Asia (at least present data from some Asian countries)
• Lines 58/61…..can the authors assign, at least, an estimate of how many children are born to these STDs? Can they quantify antenatal screening for STDs in Asia? Can they give us an idea of how low is ‘low’.
I think it would be useful to give a brief overview of this WHO regional framework and what different prescriptions it gave compared to whatever existed before its formation
• Lines 68/70……The authors may want to give more meaning to the listed ‘barriers’ and how they relate to uptake of HIV screening services.
It would be particularly interesting to see what the story is for “health system and health care provider issues”
In many jurisdictions in sub-Saharan Africa, HIV screening is part of the antenatal care package and is offered using the opt-out model.
• There is no literature review summarising the research-based evidence on barriers and facilitators to antenatal screening for STDs in the Asian context.
Why is the statement above a problem? What is the burden of maternal and child morbidity and mortality in Asia in the context of HIV, Syphilis, Hepatitis C? What are the fall-outs from the supposed low uptake of available screening services? What do we stand to lose if this review is not done to better understand barriers and facilitators that can help inform useful interventions?
• Can the authors rewrite the Methods section without ‘We’?
• Line 84. We used a very inclusive search strategy to ensure that no item was missed…..Can you give a 100% guarantee no item was missed?
• There appears to be something in Line 92 that is not supposed to be there
Inclusion and Exclusion Criteria
The inclusion and exclusion criteria appear to be ‘all over the place’. They can be made more focused.
We did not include studies investigating antenatal screening for other STDs……..This does not qualify as an exclusion criterion because you have earlier specified that you are dealing with HIV, Hep B and Syphilis
• The data extraction sub-section has nothing on “facilitators”
• Line 121…..the authors may want to justify the choice of Andersen’s conceptual model over other models they could have used.
• For Table 1, I think the year the study was conducted ought to be in separate column by itself. This will enable readers to relate them to implementation of the MDGs and contextualize them.
• I have some questions relating to Fig.1;
……you mentioned Google Scholar but I don’t see in the flow diagram
…….I am struggling to understand how you excluded 546 articles because the studies were not conducted in Asia when ‘Asia’ should have been a key part of your search strategy. Kindly elaborate on how this happened.
• Lines 210/211…….please give more meaning to ‘perceptions of poor healthcare support’ and ‘concerns about the reactions of healthcare workers
Secondly, of the 16 articles, 15 were on HIV and 1 was on Syphilis with nothing on Hepatitis B. In this context, I fail to see how you can make any reasonable pronouncements on Syphilis and Hepatitis B screening uptake.
On this basis, I suggest you drop off Syphilis and Hep B and make your work entirely about uptake of HIV screening than about STDs and appropriately reorient your discussion to that effect.
I look forward to reading a new version of your work.
6. PLOS authors have the option to publish the peer review history of their article ( what does this mean? ). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy .
Reviewer #1: Yes: Yeetey ENUAMEH
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/ . PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at gro.solp@serugif . Please note that Supporting Information files do not need this step.
Author response to Decision Letter 0
Dear Doctor Ampofo,
We thank you and the reviewer for your time and generous comments provided on our manuscript PLONE-D-23-03027 entitled "Barriers and facilitators to antenatal screening for sexually transmitted diseases in Asia: a scoping review", and we thank you for the opportunity to address these comments. After consideration of your comments, we have made several improvements.
The comments provided are shown in bold below, with our responses in italics.
Editor's Comments to the Author:
1. Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming.
Response: We have ensured that the manuscript meets the stylistic requirements of PLOS ONE.
2. Please include a copy of Tables 4 and 5 which you refer to in your text on page 6.
Response: A copy of tables 4 and 5 has been included under the name S2 Table and S3 Table.
3. Please include captions for your Supporting Information files at the end of your manuscript, and update any in-text citations to match accordingly.
Response: Captions for our Supporting Information files have been included at the end of the manuscript.
Reviewers’ Comments to the Author:
Reviewer #1:
The topic is of great interest, however the manuscript seems hurriedly put together. Concepts involved such as Andersen's model are not clearly and coherently applied in the course of the write-up.
Response: Thank you for your comment. We now define Andersen’s model (lines 148-153) and clearly and systematically apply the concepts cited. We have added Table 3 and systematically structured our results according to each component of Andersen’s model. The "facilitators" have been taken into account appropriately in the review.
The title for example does not capture the full essence of the review - it seems to be based on the "regional framework for the triple elimination of MTCT of HIV, Hep B and syphilis in Asia and Pacific" (lines 99 - 101)- that should have reflected clearly in the title. Such an understanding should have been provided briefly in the "Background" section of the script.
Response: We have changed the initial title from “Barriers and facilitators to antenatal screening for sexually transmitted diseases in Asia: a scoping review” to “A systematic review of barriers and facilitators to antenatal screening for HIV, syphilis or hepatitis B in Asia: perspectives of pregnant women, their relatives and health care providers.” The role of the WHO framework as the basis for this study has also been clarified in the background section (lines 76-89).
Response: On the basis of all the reviewers' comments, we decided to carry out a systematic analysis of the literature rather than a scoping review, using a second person to screen and review articles in duplicate and by searching other databases. We adapted the objectives to those of a systematic literature review (lines 113-115).
a. A key concept such as the "population of the review" is not defined. The "search strategy" should have been systematically presented - e.g., keywords/ subject headings/ index terms that were used, an example of a search strategy of at least one of the databases should have been placed in the appendices; the search should have been as exhaustive as possible - searching two databases does not sound exhaustive enough.
Response: Thank you for this comment. To make the search as exhaustive as possible and in line with other reviews of this nature, we searched Ovid (MEDLINE, Embase, PsycINFO), Scopus, Global Index Medicus and Web of Science. Our database search strategy has been included in S1 File.
Response: The eligibility criteria for the inclusion of studies have been rewritten and tabulated in Table 2 using the acronym SPlDER: S sample; P phenomenon of interest; D design; E evaluation; R research type. Our original inclusion/exclusion criteria were unchanged except that we also decided to include experimental design studies.
c. Under "Data extraction", the Andersen's framework was said to have been the guide - it would have been great to have this clearly "tabulated" with "findings" related to each component of the framework duly and systematically presented. The quality of studies was assessed, but this outcome did not feature further into any decision-making or discussion as to how study quality influenced the review process.
Response: We agree that a more systematic application of Andersen’s framework improves the paper so have rewritten the results section to clearly reflect each of the elements of the Andersen framework and added Table 3. As the aim of the review was to describe and synthesise a body of literature and not to determine effect size, we did not exclude studies on the basis of their quality assessment (lines 160-161) but we have summarised the quality assessment in S2 and S3 Tables to enable readers to see the quality of the evidence included in the review.
Response: We agree with this comment and have now given more explanation of the ‘enabling factors’ of antenatal screening (lines 151-152).
Response: As explained above we have now explicitly defined the "facilitators" in Table 1 and entirely rewritten the results section to follow the factors defined in Andersen’s model.
Response: Thank you for your detailed comments. We hope that the changes you have made will enable the study to be published.
Reviewer #2:
Title…..perhaps the title should be restricted to HIV, Syphilis and Hepatitis B rather than ‘sexually transmitted diseases’.
Response: Thank you. We have changed the title to “A systematic review of barriers and facilitators to antenatal screening for HIV, syphilis or hepatitis B in Asia: perspectives of pregnant women, their relatives and health care providers.”
Introduction: Despite being a scoping review, a little more detail will improve the Background and situate arguments in better context.
Response: We have expanded the introduction to better place the study in context. We have also changed from a scoping to a systematic review.
Lines 50/51…….the authors can provide recent data on the morbidity and mortality they refer to…..first globally and in the Asian context Quantify the prevalence of these STDs in Asia (at least present data from some Asian countries).
Response: Data on mother-to-child transmission of HIV, syphilis and hepatitis B were added to the introduction. The prevalence of the STDs considered was quantified in the introduction section.
Lines 58/61…..can the authors assign, at least, an estimate of how many children are born to these STDs? Can they quantify antenatal screening for STDs in Asia? Can they give us an idea of how low is ‘low’.
Response: We have added an estimate of the number of children born with these STDs (lines 54-59), as well as a quantification of antenatal screening for STDs in Asia (lines 63-68).
Response: Thank you for this suggestion. We have added an overview of the WHO regional framework and its main prescriptions (lines 76-87).
Lines 68/70……The authors may want to give more meaning to the listed ‘barriers’ and how they relate to uptake of HIV screening services. It would be particularly interesting to see what the story is for “health system and health care provider issues”. In many jurisdictions in sub-Saharan Africa, HIV screening is part of the antenatal care package and is offered using the opt-out model.
Response: Thank you for this suggestion. The obstacles listed have been detailed for greater clarity.
There is no literature review summarising the research-based evidence on barriers and facilitators to antenatal screening for STDs in the Asian context. Why is the statement above a problem? What is the burden of maternal and child morbidity and mortality in Asia in the context of HIV, Syphilis, Hepatitis C? What are the fall-outs from the supposed low uptake of available screening services? What do we stand to lose if this review is not done to better understand barriers and facilitators that can help inform useful interventions?
Response: We have clarified and expanded the introduction by highlighting the importance of this review (lines 88-92) and giving the reasons why a literature review in Asia is needed (lines 102-112). We now detail, in the introduction, the burden of maternal and infant morbidity and mortality in Asia within the limits of available data. We have also explained the consequences of the low uptake of screening services (lines 70-75).
Can the authors rewrite the Methods section without ‘We’?
Response: We decided to keep this section written in active voice as it is easier to understand and saves words. It is nowadays recommended for scientific writing in biomedical journals.
Line 84. We used a very inclusive search strategy to ensure that no item was missed…..Can you give a 100% guarantee no item was missed?
Response: We removed this sentence from the manuscript.
There appears to be something in Line 92 that is not supposed to be there.
Response: We removed this from the manuscript.
Inclusion and Exclusion Criteria: The inclusion and exclusion criteria appear to be ‘all over the place’. They can be made more focused. We did not include studies investigating antenatal screening for other STDs……..This does not qualify as an exclusion criterion because you have earlier specified that you are dealing with HIV, Hep B and Syphilis.
Response: We agree and have rewritten the eligibility criteria for study inclusion using the acronym SPlDER: S sample; P phenomenon of interest; D design; E evaluation; R research type.
The data extraction sub-section has nothing on “facilitators”
Response: In the methods, we have added a subsection on the extraction of data and explained how we focus upon both barriers and facilitators to antenatal screening within the structure of Andersen’s framework (Table 3).
Line 121…..the authors may want to justify the choice of Andersen’s conceptual model over other models they could have used.
Response: Thank you for your suggestion. We chose the Andersen conceptual model because it provides an understanding of how individuals and environmental factors influence health behaviours. This theoretical framework is widely used in literature reviews on healthcare utilisation. This has been justified in lines 147-150.
For Table 1, I think the year the study was conducted ought to be in separate column by itself. This will enable readers to relate them to implementation of the MDGs and contextualize them.
Responses: We agree with this suggestion and a separate column for the date has been added to Table 1.
I have some questions relating to Fig.1;
Responses: We have modified Figure 1 to reflect the new search strategy. With the new search strategy, only four articles were found to have been conducted outside Asia because the term "Asia" appeared in their abstracts.
Lines 210/211…….please give more meaning to ‘perceptions of poor healthcare support’ and ‘concerns about the reactions of healthcare workers
Response: We agree and have clarified the “perception of poor healthcare support” and the “concerns about the reactions of healthcare workers” (lines 276-291).
Secondly, of the 16 articles, 15 were on HIV and 1 was on Syphilis with nothing on Hepatitis B. In this context, I fail to see how you can make any reasonable pronouncements on Syphilis and Hepatitis B screening uptake. On this basis, I suggest you drop off Syphilis and Hep B and make your work entirely about the uptake of HIV screening than about STDs and appropriately reorient your discussion to that effect.
Response: We agree with your suggestion with respect to the discussion and conclusions and have rewritten these sections with respect to HIV only. However, the new search showed up three papers on syphilis and one on hepatitis B, so we believe it is important to highlight this gap and summarise the limited evidence in the results.
We hope that you will be satisfied with the amendments made. If there are any further issues do not hesitate to get in touch. We would like to thank you again for your time and consideration of our manuscript.
Yours sincerely,
Lucie Sabin (on behalf of all co-authors)
Submitted filename: response_reviewers.docx
Decision Letter 1
PONE-D-23-03027R1A systematic review of barriers and facilitators to antenatal screening for HIV, syphilis or hepatitis B in Asia: perspectives of pregnant women, their relatives and health care providers.PLOS ONE
Dear Dr. Sabin, Thank you for resubmitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. The manuscript was sent for further review - an initial reviewer advised minor revisions (not accept) and a third (new) reviewer has indicated major revision. The original second reviewer was not available for re-review. You have the benefit then of three careful reviews. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process.
Please submit your revised manuscript by Feb 16 2024 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp . When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Stephen Michael Graham, FRACP, PhD
Additional Editor Comments:
This submission was reviewed for a second time - and again a decision of Major Revision has been made. If you decide to resubmit, then there will need to be clear evidence that you have addressed all concerns of the reviewers for it to be considered for re-review - and then further review will be required anyway.
1. If the authors have adequately addressed your comments raised in a previous round of review and you feel that this manuscript is now acceptable for publication, you may indicate that here to bypass the “Comments to the Author” section, enter your conflict of interest statement in the “Confidential to Editor” section, and submit your "Accept" recommendation.
Reviewer #2: (No Response)
Reviewer #3: (No Response)
2. Is the manuscript technically sound, and do the data support the conclusions?
Reviewer #2: Partly
Reviewer #3: Yes
3. Has the statistical analysis been performed appropriately and rigorously?
4. Have the authors made all data underlying the findings in their manuscript fully available?
Reviewer #3: No
5. Is the manuscript presented in an intelligible fashion and written in standard English?
6. Review Comments to the Author
Reviewer #2: Comments on manuscript PONE-D-23-03027R1
I am grateful to the authors for incorporating the previous comments made. The manuscript looks more refined now but will still need to be improved in some aspects as listed below.
After they have worked on these comments, I believe the work can be accepted for publication
• Line 21………I am wondering if Antenatal screening alone is enough for PMTCT. Shouldn’t it be accompanied by treatment?
• Line 26…..read it again and rectify the grammatical error there….similar error in Line 30/31
• Line 27……..what you sought for in those published articles should be in this line.
• There is nothing about “barriers” in the Results section. If there is, it needs to be made more explicit
• Line 73/74……can the authors show proof that there is a low uptake of STDs screening?
• Who or which persons conducted the initial search? (show with initials)
• Who is the third reviewer?
• Line 133……what is the use of the boldened Error statement there?
• In SPIDER, you defined SAMPLE to include women of childbearing age. I reckoned this work was about Antenatal Screening of Pregnant women. Please clarify the need to include women of childbearing age.
• Review the Data Extraction section for some grammatical omissions and errors. Line 150 is missing “of”. Line 151 …..’categorizes’ instead of ‘categories’.
• Line 160……’that’ instead of ‘who’
Line 321…..rephrase to read “…….allow conclusions to be drawn effectively about HIV alone”
Line 340/341…….there is free screening of HIV, Hep B and Syphilis in many parts of sub-Saharan Africa already.
Line 348……On heterogeneity……there were 19 quantitative studies and you could have evaluated heterogeneity statistically to enable you make a more refined pronouncement on the subject
The Conclusion can be better written…..with emphasis on what specifically needs to be done and by which organization or department or health agency
Reviewer #3: GENERAL COMMENTS:
This review deals with an important topic that could be very useful in the prevention of HIV, syphilis and hepatitis B and transmission of these infections to infants. However, there are several major limitations to the quality of the review which hinder its relevance and applicability. The initial most striking feature of this review is that all four authors are affiliated with only one institute and this institute is in a high-income country that is not in Asia (used as general term here as the authors do not define Asia in their manuscript). Do any of these authors have lived experience of ANC, or healthy policy or practice in the region they are reviewing? If so, it would be helpful to have this information somewhere. Furthermore, the review only includes manuscripts published in English, a major limitation given the region being considered. there are excellent research Institutes throughout Asia, and no doubt this review would be enhanced if it included some collaborations within Asia to increase available grey literature and other studies that may not have been in their search methods.
DETAILED COMMENTS:
“Despite improvements” is vague, some time reference of stats would be helpful here.
STIs is more commonly used now, rather than STDs.
“Antenatal screening” (Sabin, p. 1)
• and treatment. without treatment, screening won’t prevent transmission.
Methods paragraph. typo ‘conducted’ included twice in 1st and last sentence.
What is the definition of ‘Asia’? This should be included in the abstract.
Results section: please define in predisposing characteristics, enabling factors and need factors who you are referring to. The pregnant woman? The health worker?
INTRODUCTION:
“antenatal screening” (Sabin, p. 12) Line 62. And treatment, screening alone will not prevent transmission.
“infected women may transmit infections to their sexual partners or children” (Sabin, p. 13) Line 71. Please rephrase. Women are often infected by their partner, only saying women may give it to their partners overemphasizes their responsibility. What do you mean by infecting their children? Do you mean by MTCT? If so please be specific.
“Meanwhile, it encourages the participation of women living with HIV” (Sabin, p. 13) Line 86. prevention of MTCT of HIV, syphilis and hepatitis B is a shared responsibility, men and communities should also be encouraged to participate.
Preventing male transmission to women during sex, as well as preventing community transmission, of HIV, Syphilis and Hep B is also an effective method of preventing neonatal and infant infections. Whilst I recognise this is not the focus of the review, it should at least be mentioned to prevent misunderstandings and reduce stigma. Overly focusing on pregnant women being the source of transmission to their infants misses an opportunity to emphasise that they are not always the original source of the infection and may not have been able to negotiate appropriate protection for themselves in order to avoid infection.
“An estimated 10,000 new HIV infections occurred 56 among children aged 0–14 years in the Asia Pacific region in 2017” (Sabin, p. 12) Line 56/57. What is the number of infants infected with HIV due to MTCT? You mentioned 10,000 children infected between 0 and 14 years, but clearly not all of these are necessarily due to MTCT.
Line 122, word repetition.
Research type. Why were the articles limited to English? Given most countries in Asia have a primary language other than English this seems a big problem / barrier to identifying relevant research.
Table 3. It would be helpful to also have a column of disease studied in this table.
Line 208. Whose knowledge are you referring to?
Paragraph re male partner’s opinion. In some countries mentioned it may be impossible for a woman to be screened without the express permission of the husband. It would be useful to contrast findings against legal framework for relevant countries as the approach to overcoming this barrier would be very different.
DISCUSSION:
Line 317. Given this review was limited to the English language I do not agree with it being referred to as a “comprehensive synthesis.”
Terminology used is not consistent regarding if this is a scoping review, narrative review or systematic review.
Paragraph 2. Part of the justification for this review was that findings in Asia may differ from that already published in sub-Saharan Africa. Given this, it would be interesting to understand the similarities and differences in more detail in this paragraph.
Line 330/331. Can you reference other differences in ANC screening or barriers that may support this statement?
Line 336/337. It is likely that training programs already exist, could you please highlight what efforts are already made in these settings before suggesting interventions. Again line 340/341 calls for free screening, this would be more helpful if information regarding whether this does or does not exist in the areas included in studies would be more meaningful.
The limitations paragraph needs to mention the limitation of including only English language and the apparent lack of inclusion of experts from the region.
CONCLUSION:
“and STDs” (Sabin, p. 30) Line 454. Please rephrase, you do not address all sexually transmitted infections.
“systematic review” (Sabin, p. 30) Line 354. Be consistent with use of terms narrative or systematic review.
In terms of translating these findings into practice it would be helpful, if possible, to comment in the conclusion as to which factors appeared to be the largest barriers. It may be that this varies in different countries, or at the sub district level. In addition to reviewing studies that look at implementing screening (and treatment), it would be more helpful to also know/contrast this with which countries have policies for ANC screening and treatment and if this is meant to be free or fee for service.
7. PLOS authors have the option to publish the peer review history of their article ( what does this mean? ). If published, this will include your full peer review and any attached files.
Submitted filename: Review of PONE-D-23-03027_R1.docx
Author response to Decision Letter 1
21 Feb 2024
Decision Letter 2
22 Feb 2024
PONE-D-23-03027R2A systematic review of barriers and facilitators to antenatal screening for HIV, syphilis or hepatitis B in Asia: perspectives of pregnant women, their relatives and health care providers.PLOS ONE Dear Dr. Sabin, Thank you for resubmitting your manuscript to PLOS ONE. After careful consideration, we feel that you have addressed comments and suggestions of previous reviewers. I request that you consider comments below about clarity on age ranges and representativeness of the populations studied - if possible.
Please submit your revised manuscript by Apr 07 2024 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp . When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
- A brief letter that responds to the recent point raised by the academic editor. You should upload this letter as a separate file labeled 'Response to Reviewers'.
Journal Requirements:
Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article’s retracted status in the References list and also include a citation and full reference for the retraction notice.
Thanks for resubmitting and addressing the comments of the reviewers so comprehensively.
I support publication - however, would it be possible to improve the reporting of these populations by age groups that they represent? Is that something that could be added in a specific column in Table for each study: age range OR what proportion were adolescent pregnancy for example.
Adolescent pregnancy is still common in the region and a very vulnerable population with known poorer outcomes for mother and baby. V neglected population and as at risk for such infections as other pregnant women but perhaps even less likely to be screened? There is a data gap.
If this is not possible, it may still be worth a comment in discussion to highlight lack of data by age, especially in this vulnerable group. a suggested ref for this would be Sabet F, et al. The forgotten girls: .....Lancet. 2023;402:1580-1596.
For journal use only: PONEDEC3
Author response to Decision Letter 2
29 Feb 2024
Dear Dr Graham,
We thank you and the reviewer for your time and comments provided on our manuscript PLONE-D-23-03027 entitled " A systematic review of barriers and facilitators to antenatal screening for HIV, syphilis or hepatitis B in Asia: perspectives of pregnant women, their relatives and health care providers", and we thank you for the opportunity to address these comments. After consideration of your comments, we have made several improvements.
Response: The list of references has been examined. It is complete and correct, and no changes were required.
Response: Thank you for highlighting the importance of this research gap. Unfortunately, it was not possible to report the proportion of adolescent pregnancies in each study, as this information was not always included in the articles. However, we have added a paragraph in the discussion section on the lack of age-specific data, particularly for this vulnerable group of pregnant women, and the importance of filling this data gap (lines 388 to 394).
Decision Letter 3
A systematic review of barriers and facilitators to antenatal screening for HIV, syphilis or hepatitis B in Asia: perspectives of pregnant women, their relatives and health care providers.
PONE-D-23-03027R3
Dear Dr Sabin,
We’re pleased to inform you that your manuscript has been judged scientifically suitable for publication and will be formally accepted for publication once it meets all outstanding technical requirements.
Within one week, you’ll receive an e-mail detailing the required amendments. When these have been addressed, you’ll receive a formal acceptance letter and your manuscript will be scheduled for publication.
An invoice for payment will follow shortly after the formal acceptance. To ensure an efficient process, please log into Editorial Manager at http://www.editorialmanager.com/pone/ , click the 'Update My Information' link at the top of the page, and double check that your user information is up-to-date. If you have any billing related questions, please contact our Author Billing department directly at gro.solp@gnillibrohtua .
If your institution or institutions have a press office, please notify them about your upcoming paper to help maximize its impact. If they’ll be preparing press materials, please inform our press team as soon as possible -- no later than 48 hours after receiving the formal acceptance. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information, please contact gro.solp@sserpeno .
Additional Editor Comments (optional):
Acceptance letter
10 May 2024
I'm pleased to inform you that your manuscript has been deemed suitable for publication in PLOS ONE. Congratulations! Your manuscript is now being handed over to our production team.
At this stage, our production department will prepare your paper for publication. This includes ensuring the following:
* All references, tables, and figures are properly cited
* All relevant supporting information is included in the manuscript submission,
* There are no issues that prevent the paper from being properly typeset
If revisions are needed, the production department will contact you directly to resolve them. If no revisions are needed, you will receive an email when the publication date has been set. At this time, we do not offer pre-publication proofs to authors during production of the accepted work. Please keep in mind that we are working through a large volume of accepted articles, so please give us a few weeks to review your paper and let you know the next and final steps.
Lastly, if your institution or institutions have a press office, please let them know about your upcoming paper now to help maximize its impact. If they'll be preparing press materials, please inform our press team within the next 48 hours. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information, please contact gro.solp@sserpeno .
If we can help with anything else, please email us at gro.solp@eracremotsuc .
Thank you for submitting your work to PLOS ONE and supporting open access.
PLOS ONE Editorial Office Staff
on behalf of
Dr. Stephen Michael Graham

IMAGES
VIDEO
COMMENTS
Introduction. In the United States, hepatitis C virus (HCV) infection is a leading cause of liver-related deaths, cirrhosis, and hepatocellular carcinoma [1•, 2•].HCV was discovered in 1989, but it was not until 1992 that the blood supply was screened for HCV; therefore, prior to 1992, contaminated blood products had been a primary cause of infection [3-5•].
First diagnosed in 1989, the hepatitis C virus (HCV) is a significant public health problem affecting 58 million people worldwide. The percentage of people who are seropositive for anti-HCV antibodies worldwide is estimated to have increased from 2.3% to 2.8% between 1990 to 2005. Most patients (80% to 85%) who become acutely infected cannot clear the virus and progress to chronic infection ...
Hepatitis C is a global health problem, and an estimated 71·1 million individuals are chronically infected with hepatitis C virus (HCV). The global incidence of HCV was 23·7 cases per 100 000 population (95% uncertainty interval 21·3-28·7) in 2015, with an estimated 1·75 million new HCV infections diagnosed in 2015. Globally, the most common infections are with HCV genotypes 1 (44% of ...
Hepatitis C virus (HCV) is a hepatotropic RNA virus that can cause acute and chronic hepatitis, with progressive liver damage resulting in cirrhosis, decompensated liver disease, and hepatocellular carcinoma. In 2016, WHO called for the elimination of HCV infection as a public health threat by 2030. Despite some progress, an estimated 57 million people were living with HCV infection in 2020 ...
Hepatitis C virus (HCV) is a major cause of liver diseases including liver cirrhosis and hepatocellular carcinoma. Approximately 3% of the world population is infected with HCV. Thus, HCV infection is considered a public healthy challenge. It is worth mentioning, that the HCV prevalence is dependent on the countries with infection rates around ...
Hepatitis C virus (HCV) is a hepatotropic RNA virus that causes progressive liver damage, which might result in liver cirrhosis and hepatocellular carcinoma. Globally, between 64 and 103 million ...
Recent advances in hepatitis C virus (HCV) therapies have transformed the treatment landscape for this disease. However, efficacy of current treatments depends on HCV genotype and individual patient characteristics. This review aimed to appraise observational studies reporting epidemiological outcom …
Introduction. Hepatitis C is a complex disease of liver. Due its widespread nature and global burden, this disease has always attracted attention for insight into its causative agent, hepatitis C virus (HCV), and for the development of new therapeutic approaches. Even after twenty four years of its discovery, HCV continues to be a major cause ...
1. Background. Chronic Hepatitis C Virus (HCV) is a growing public health concern. HCV is transmitted through exposure to infected blood and cannot be spread through intact skin or mucous membranes [].Globally, there are approximately 170 million people diagnosed with chronic HCV [2, 3], with 3 to 4 million new cases per year [].It has been estimated that approximately 30% of individuals ...
We used a systematic approach to scope the literature to determine what is currently known about the health and psychosocial impacts of hepatitis C along the trajectory from exposure to ongoing chronic infection, and to identify what knowledge gaps remain. Methods: PubMed, Current Contents and PsychINFO databases were searched for primary ...
The HCV guidance undergoes major biannual updates based on a rigorous literature review that encompasses peer-reviewed, published literature and relevant abstracts from national and international scientific conferences. ... National hepatitis C incidence and prevalence data among children and adolescents in the United States are sparse and/or ...
Introduction. Hepatitis C is caused by infection with the hepatitis C virus (HCV). 60-80% of patients with acute hepatitis C develop the chronic form when the virus overcomes host innate and adaptive immune defenses [[1], [2], [3], [4]].HCV can be classified into seven confirmed genotypes and 67 subtypes [5].These genotypes lead to differential prognosis of hepatitis C disease and influence ...
The goal of chronic hepatitis C treatment is to remove the virus to avoid progression of HCV-related disease. Sustained virologic response (SVR) is the most widely used efficacy endpoint in clinical studies of hepatitis C, and represents the eradication of HCV from the body. The aim of the current review was to examine the long-term clinical, economic and quality of life benefits associated ...
Many countries have developed, or are developing, national strategies aimed at reducing the harms associated with hepatitis C infection. Making these strategies relevant to the vast majority of those affected by hepatitis C requires a more complete understanding of the short and longer term impacts of infection. We used a systematic approach to scope the literature to determine what is ...
Hepatitis C virus, a member of Flaviviridae is a single-stranded positive-sense RNA virus infecting 62-79 million people around the globe. This blood-borne virus is one of the leading causes of liver diseases worldwide. This review aims to identify novel potential genes linked to cellular host factors, as well as revise the roles of each gene in hepatitis C Virus infection.
This systematic review and meta-analysis provide detailed information on the prevalence of SBP among hepatitis B virus (HBV) and hepatitis C virus (HCV)-related liver cirrhosis globally.
Hepatitis C is a global health problem, and an estimated 71·1 million individuals are chronically infected with hepatitis C virus (HCV). The global incidence of HCV was 23·7 cases per 100 000 population (95% uncertainty interval 21·3-28·7) in 2015, with an estimated 1·75 million new HCV infections diagnosed in 2015. Globally, the
Hepatitis C is a major cause of chronic liver disease. It has been recognized as a global health problem because of the progression to cirrhosis and hepatocellular cancer. Chronic hepatitis C is usually asymptomatic but can cause considerable liver damage before its recognition. This review discusses the natural history, clinical features ...
Although these recommendations included a systematic review of approximately 20 years of literature during 2001-2022 on hepatitis C in pregnancy and children exposed perinatally, multiple gaps in the literature exist. ... Median and range of number of studies in literature review related to perinatal hepatitis C testing, prevalence, and ...
Figure Box 1. Despite advances in antiviral therapy and access to effective vaccines, viral hepatitis remains a significant public health problem in the United States. The condition is most commonly caused by infection from the hepatitis A virus (HAV), hepatitis B virus (HBV), or hepatitis C virus (HCV). Liver injury can result from nearly any viral infection with systemic involvement, however ...
Introduction. Hepatitis C is the most commonly reported bloodborne infection in the United States (1), and surveys conducted during 2013-2016 indicated an estimated 2.4 million persons (1.0%) in the nation were living with hepatitis C (2).Percutaneous exposure is the most efficient mode of hepatitis C virus (HCV) transmission, and injection drug use (IDU) is the primary risk factor for ...
This review included a total of 150 studies that revealed almost 19 million people are infected with hepatitis C virus and 240,000 new cases are being reported each year. This trend is continually rising in developing countries like Pakistan where intravenous drug abuse, street barbers, unsafe blood transfusions, use of unsterilized surgical ...
This review included a total of 150 studies that revealed almost 19 million people are infected with hepatitis C virus and 240,000 new cases are being reported each year. This trend is continually rising in developing countries like Pakistan where intravenous drug abuse, street barbers, unsafe blood transfusions, use of unsterilized surgical ...
Hepatitis C virus (HCV) burden is higher among people in prison given high prevalence of injecting drug use. This study evaluated direct-acting antiviral (DAA) treatment outcome in prisons. Methods. The Surveillance and Treatment of Prisoners with hepatitis C (SToP-C) study enrolled individuals incarcerated in four Australian prisons (2017-2019).
Book Review: Ellen Hopkins' new novel 'Sync' is a stirring story of foster care through teens' eyes. Book Review: 'Swallow the Ghost' a promising but uneven exploration of memory in internet age. The entire book will take an average reader just a few hours to read. Really short chapters — some just a sentence long — help the ...
This systematic literature review aims to investigate barriers and facilitators to antenatal screening for HIV, syphilis, and hepatitis B in Asia. Methods We conducted a systematic review by searching Ovid (MEDLINE, Embase, PsycINFO), Scopus, Global Index Medicus and Web of Science for published articles between January 2000 and June 2023, and ...