
- University of Pretoria Institute for Sustainable Malaria Control
- My UP Login


Vector mosquito collection
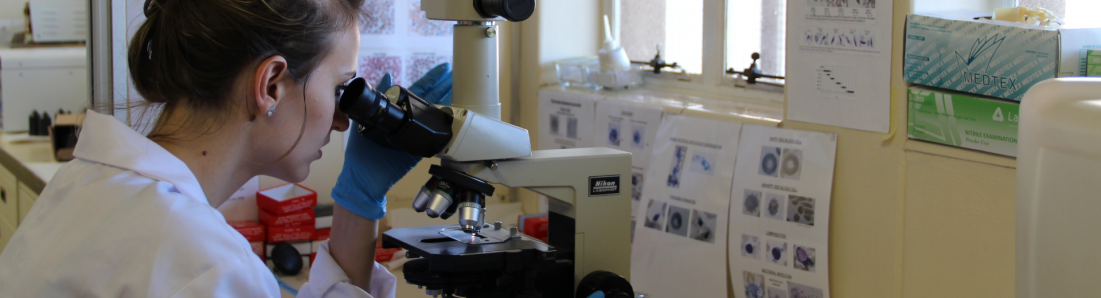
Studying parasites with a microscope

Huts in a rural malaria endemic area

- Theses, Dissertations and Research Reports
| Student | Degree | Type | Title | Supervisors |
| Mr A Sitoe | PhD, Chemical Engineering | Thesis | Controlled-release of mosquito repellents from microporous polymer strands | Prof WW Focke |
| Ms Masego Dilebo | MSc, Biochemistry | Dissertation | Mechanistic investigation of novel artemisinin-based combination that targets parasites | Prof L Birkholtz, Dr D Coertzen, RK Haynes |
| Ms Ashley van Heerden | MSc, Biochemistry | Dissertation | Stratifying antimalarial compounds with similar mode of action using machine learning on chemo-transcriptome profiles | Prof L Birkholtz |
| Ms Suzan Maboane | MSc, Biochemistry | Dissertation | The contribution of genetic diversity to differentiate drug response in gametocytes | Dr J Niemand, Prof L Birkholtz |
| Ms Dore Joubert | MSc, Biotechnology | Dissertation | Development of a constitutive luciferase reporter line for comparable evaluation of antiplasmodial drugs on all life cycle stages of | Prof L Birkholtz |
| Ms Meta Leshabane | MSc, Biochemistry | Dissertation | Novel acid-labile and targeted nanoparticles as possible antimalarial drug-delivery systems | Prof L Birkholtz |
| Ms Engela Theron | MSc, Environmental Health | Dissertation | Associations between DDT and DDE uptake and male reproductive hormones with specific reference to Insulin Like Hormone-3 | Prof MS Bornman, Prof J Wichmann |
| Ms Aamira Vally | BSc Honours, Biochemistry | Research report | The functional importance of adenyly Cyclades alpha in gametocytes | Prof L Birkholtz |
| Ms Eunice Claassen | BSc Honours, Biochemistry | Research report | Generation of atp11-GFP-glms constructs for functional investigation of the putative aminophospholipid transporter in parasites | Dr J Niemand |
| Mr Jaco van Staden | BSc Honours, Biochemistry | Research report | Determining mitochondrial functionality in asexual intraerythricytic parasites | Dr D Coertzen, Prof L Birkholtz |
| Student | Degree | Type | Title | Supervisors |
| Mr S Awandu | PhD, Biochemistry | Thesis | Understanding the contribution of malaria parsites to continued residual malaria in a pre-elimnation setting in South Africa: implications for elimination strategies | Prof L Birkholtz, Dr J Raman, Prof T Bousema |
| Mr AB Mapossa | PhD, Chemical Engineering | Thesis | Slow-release of mosquito repellents from microporous polyolefin strands | Prof WW Focke |
| Mr M Mpofu | PhD, Environmental Health | Thesis | Effectiveness of community larval source management (LSM) as an additional vector control intervention for malaria elimination | Prof C de Jager, Dr K Mudambo |
| Mr AEJ Sitoe | PhD, Chemical Engineering | Thesis | Controlled-Release of mosquito repellents from microporous polymer strands | Prof WW Focke |
| Ms M van der Watt | PhD, Biochemistry | Thesis | Exploring the kinase inhibitor chemical space for dual active and gametocyte-focused antimalarials | Prof L Birkholtz, Dr J Niemand |
| Ms EA Misiani | MSc, Public Health | Dissertation | An assessment of malaria vector control and staff workloads in South Africa: implications for malaria elimination | Prof A Beke, Prof C de Jager |
| Ms Z Mkatshane | MSc, Plant and Soil Science | Dissertation | Antimicrobial and antimalarial activity of Stapf. (Apocynaceae) | Dr MJ Bapela, Dr TE Tshikalange |
| Ms N Sibiya | MSc, Environmental Health | Dissertation | Sperm chromatin integrity is pesticide exposed | Dr N Aneck-Hahn, Prof C de Jager |
| Ms S da Rocha | BSc Honours, Biochemistry | Research report | Investigating the essentiality of putative mitochondrial pyruvate carriers within the intraerythrocytic stages of parasites | Dr J Niemand |
| Mr H Langeveld | BSc Honours, Biochemistry | Research report | Targeted gene disruption of novel putative kinases involved in the cell cycle of parasites | Prof L Birkholtz |
| Ms R Mofokeng | BSc Honours, Biochemistry | Research report | Investigating the role of putative amino acid transporters during the intra-erythrocytic stages | Dr J Niemand |
| Mr D Opperman | BSc Honours, Biochemistry | Research report | Epi-drugs kill asexual parasites through global changes in histone acetylation status and confer transmission blocking | Prof L Birkholtz |
| Ms J Tempies | BSc Honours, Biochemistry | Research report | Exploring a novel family of putative RNA binding proteins potentially driving asexual proliferation in | Prof L Birkholtz |
| Ms A Warima | BSc Honours, Biochemistry | Research report | Characterisation of a recombinant -adenosylmethionine decarboxylase mutant as a novel drug target in the malaria parasite, | Dr D Coertzen, Prof L Birkholtz |
| Student | Degree | Type | Title | Supervisors |
| Ms N Coetzee | PhD, Biochemistry | Thesis | Sexual differentiation of malaria parasites is controlled by unique epigenomic and proteomic cascades as revealed by comparative functional genome analysis | Prof L Birkholtz |
| Ms J Reader | PhD, Biochemistry | Thesis | Interrogation of the chemo- therapeutic and transmission-blocking abilities of metallodrugs against malaria parasites | Prof L Birkholtz, Dr D Meyer |
| Ms T Adebayo-Ojo | MSc, Public Health | Dissertation | Indoor Residual Spraying (IRS) and Climate Variability on Malaria Burden in Vhembe district, Limpopo Province, South Africa from 2005 - 2015 | Prof MS Bornman, Prof J Gaudart |
| Ms DL Kouemo | MSc, Pharmacology | Dissertation | Assessing the efficacy of designed compounds targeting the malarial Q site of cytochrome | Prof L Birkholtz, Prof AD Cromarty, Dr BA Stander |
| Mr TP Madzorera | MSc, Chemical Engineering | Dissertation | A slow-release organophosphate-filled trilayer polyolefin film | Prof WW Focke |
| Ms M Naude | MSc, Biochemistry | Dissertation | Dynamic bioinformatics and isotopic evaluation of the permeome of intraerythrocytic parasites | Prof L Birkholtz, Dr J Niemand |
| Mr AP Roodt | MSc, Chemistry | Dissertation | A new sampling method for human skin volatile analysis by comprehensive gas chromatography and mass spectrometry | Prof E Rohwer, Prof A Stoltz, Dr Y Naude |
| Ms H von Grüning | MSc, Biochemistry ( ) | Dissertation | CRISPR/Cas9 mediated deletion of genes encoding putative cell cycle regulators in | Prof L Birkholtz |
| DF Botha | BSc Honours, Biochemistry | Research report | Functional evaluation of RNA binding proteins in regulating re-initiation of the cell cycle after cell cycle arrest in | Prof L Birkholtz, Dr J Niemand |
| M Freeman | BSc Honours, Biochemistry | Research report | Creating a sex specific gametocyte transmission blocking assay by reverse transcriptase qPCR | Dr BK Brider, Prof L Birkholtz |
| Ms MK Leshabane | BSc Honours, Biochemistry | Research report | Investigation of the possible anti-plasmodial activity of DNA gyrase inhibitors against asexual and sexual intra erythrocytic stages of | Prof L Birkholtz, Dr J Niemand |
| D Moonasamy | BSc Honours, Biochemistry | Research report | Detection and quantification of oxidative stress on multiple stages of parasites | Dr D Coertzen, Prof L Birkholtz |
| Ms M Robbertse | BSc Honours, Biochemistry | Research report | Localization of putative amino acid transporters in using selection linked integration | Dr J Niemand, Prof L Birkholtz |
| Ms M Ryder | BSc Honours, Biochemistry | Research report | Targeted gene disruption of a putative ORC3 cell cycle modulator | Dr J Niemand, Prof L Birkholtz |
| Ms F Shingange | BSc Honours, Biochemistry | Research report | Validation of drug affinity responsive target stability assay in the identification of antimalarial drugs biological targets in asexual blood stage | Prof L Birkholtz, Dr P Moyo |
| Student | Degree | Type | Title | Supervisors |
| Ms H Izadi | PhD, Chemical Engineering | Thesis | A novel pseudo-azeotrope mosquito repellent mixture | Prof WW Focke, Dr J Pretorius |
| Mr P Moyo | PhD, Biochemistry | Thesis | Natural products as potent and pan-reactive antimalarial agents: Discovery, isolation and biochemical characterisation | Prof L Birkholtz, Prof AI Louw, Prof VJ Maharaj,Prof JN Eloff |
| Mr EW Besaans | MSc, Biochemistry | Dissertation | Synthesis, structure-activity relationships and biological evaluation of optimised terminally alkylated (bis) urea and (bis)thiourea polyamine analogues as anti-plasmodial agents | Prof L Birkholtz, Dr N October |
| Ms J Connacher | MSc, Biochemistry ( ) | Dissertation | Deconvoluting drug mode-of-action: Transcriptomic evaluation of novel clinical lead antimalarials | Prof L Birkholtz |
| Ms JS Duvenhage | MSc, Biochemistry ( ) | Dissertation | Radiolabeled antibodies as detection tool for malaria parasite ( 3D7) - infected human erythrocytes | Prof L Birkholtz, Prof JR Zeevaart |
| Ms DMA Matlebjane | MSc, Biochemistry | Dissertation | efficacy assessment of targeted antimalarial drugs synthesized following design | Prof AD Cromarty, Prof L Birkholtz, Dr BA Stander |
| Ms ME Dilebo | BSc Honours, Biochemistry | Research report | Determination of cellular oxidative stress generation because of artemisinins and methylene blue treatment in intracellular malaria parasites, | Prof L Birkholtz, Dr D Coertzen |
| Ms D Joubert | BSc Honours, Biochemistry | Research report | Investigating polyamine agents as novel transmission blocking antimalarial drugs on the gamete forms of the parasites | Prof L Birkholtz, Dr BK Brider |
| Ms T Khan | BSc Honours, Biochemistry | Research report | Development of an in-house phenotype microarray plate for analysis of asexual parasites | Dr J Niemand, Prof L Birkholtz |
| Ms SM Maboane | BSc Honours, Biochemistry | Research report | The phenotypic characterization of carbohydrate and amino acid metabolism use in the Dd2 strain of parasites | Dr J Niemand, Prof L Birkholtz |
| JJM Marais | BSc Honours, Biochemistry | Research report | Vector construction for targeted CRISPR-Cas9 mediated gene disruption of an epigenetic gene regular PfGCN5 in parasites | Prof L Birkholtz |
| Ms A van Heerden | BSc Honours, Biochemistry | Research report | Optimisation of high-throughput screening platform for antiplasmodial drug activity determination against asexual stages | Prof L Birkholtz, Dr J Niemand |
| Student | Degree | Type | Title | Supervisors |
| Mr A Adeola | PhD, Geography | Thesis | Application of remotely sensed environmental variables for predicting malaria cases in Nkomazi municipality, South Africa | Dr OJ Botai, Dr JM Olwoch |
| Ms J Bapela | PhD, Plant and Soil Science | Thesis | NMR-based metabolomic study of medicinal plants used against malaria and the isolation of bioactive alkaloids | Prof M Meyer |
| Mr DK Komen | PhD, Geography | Thesis | Impact of climate on health: A specific focus on Malaria in South Africa’s Limpopo Province | Dr JM Olwoch, Dr OJ Botai |
| Mr SM Patrick | PhD, Environmental Health | Thesis | Effects of , lactational- and direct exposure to selected endocrine disrupting chemicals on the rat male reproductive system | Prof C de Jager, Prof MS Bornman, Prof A Joubert |
| Ms C Pillay | MSc, Pharmacology | Dissertation | Characterisation of proteins expressed on infected red blood cell surfaces as potential drug targets for severe malaria therapy | Prof AD Cromarty |
| Ms JS Duvenhage | BSc Honours, Biochemistry | Mini-dissertation | cultivation of and gametocyte producing strains of | Prof L Birkholtz |
| Ms C Abrie | BSc Honours, Biochemistry | Research report | Investigating antiplasmodial potential of (bis) urea and (bis)thiourea polyamine analogues on the gamete form of parasites | Prof L Birkholtz, Dr J Niemand |
| Mr C Breedt | BSc Honours, Biochemistry | Research report | An evaluation of the gametocytocidal activity of natural product compound against the parasite | Prof L Birkholtz, Dr J Niemand |
| Ms D Henn | BSc Honours, Biochemistry | Research report | Drug target deconvolution of antiplasmodial compounds using drug affinity responsive target stability on the sexual and asexual stages of parasites | Prof L Birkholtz, Dr J Niemand |
| Ms T Makhanthisa | BSc Honours, Biochemistry | Research report | Evaluation of the prevalence of CYP 2D6 mutation prevalence in the population of Vhembe District, Limpopo Province | Prof L Birkholtz, Dr J Niemand |
| Ms N Mmekwa | BSc Honours, Biochemistry | Research report | Evaluation of the effect of epigenetic modifying drugs during gametocyto-genesis of the parasite | Prof L Birkholtz, Dr J Niemand |
| Ms G Weidemann | BSc Honours, Biochemistry | Research report | Investigation of differential drug responses in different parasite life cycle stages | Prof L Birkholtz, Dr J Niemand |
| Student | Degree | Type | Title | Supervisors |
| Ms C Coertzen | PhD, Biochemistry | Thesis | Structural and functional characterisation of AdoMetDC in | Prof L Birkholtz, Prof AI Louw |
| Ms I Roussouw | PhD, Genetics | Thesis | Transcriptional response to the herbicide-derived compound A51B1C1_1 in the zoonotic parasite - s | Dr C Maritz-Olivier, Prof L Birkholtz |
| Mr M Sibanda | PhD, Chemical Engineering | Thesis | Polyolefin copolymers as controlled release devices for insecticides and repellents | Prof WW Focke |
| Mr MU Akhtar | MSc, Chemical Engineering | Dissertation | Towards controlled release of a natural mosquito repellent from polymer matrices | Prof WW Focke |
| Ms B Barnard | MSc, Biochemistry ( ) | Dissertation | Biochemical analysis of lysine-specific demethylases in malaria parasites | Prof L Birkholtz |
| Ms K Baron | MSc, Pharmacology ( ) | Dissertation | Enzymatic and chemical modifications of erythrocyte surface antigens to identify merozoite binding sites | Prof AD Cromarty |
| Ms N Coetzee | MSc, Biochemistry ( ) | Dissertation | Detailed and comprehensive description of the histone code in asexual and gametocyte stages through mass spectrometry based proteomics | Prof L Birkholtz |
| Ms C Pillay | MSc, Pharmacology | Dissertation | Characterisation of proteins expressed on infected red blood cell surfaces as potential drug targets for severe malaria therapy | Prof AD Cromarty |
| Ms D Steyn | MSc, Pharmacology | Dissertation | Mechanisms of potential novel antimalarials and proteomics of resistance | Prof AD Cromarty |
| Ms R van Biljon | MSc, Biochemistry ( ) | Dissertation | Probing the unusual cell cycle of using DFMO, a cytostatic life cycle stage-specific inhibitor | Prof L Birkholtz, Dr J Niemand |
| Mr S Kneitz | BSc Honours, Biochemistry ( ) | Research report | Identification of the biochemical target/s of novel antimalarial compounds with DARTS technology | Prof L Birkholtz |
| A Marevati | BSc Honours, Biochemistry | Research report | Characterisation of a novel S-adenosylmethionine decarboxylase mutant for structure based drug discovery in the malaria parasite, | Prof L Birkholtz |
| Ms A Ramjan | BSc Honours, Biochemistry ( ) | Research report | Validation of histone deacetylase inhibitors as novel antiplasmodial agents | Prof L Birkholtz, Dr J Niemand |
| Student | Degree | Type | Title | Supervisors |
| Ms BK Verlinden | PhD, Biochemistry | Thesis | Polyamine analogues exhibit an antimalarial effect through targeting cell cycle | Prof L Birkholtz |
| Ms M de Beer | MSc, Biochemistry | Dissertation | In vitro antiplasmodial activity of an optimised series of alkylated (bis)urea and (bis)thiourea polyamine analogs | Prof L Birkholtz |
| Ms N Ismail | MSc, Biochemistry | Dissertation | Inhibition of the histone deacetylase family as a drug target in the human malaria parasite, | Prof L Birkholtz |
| Mr M Szolkiewicz | MSc, Biochemistry | Dissertation | Homology-based in silico identification of putative protein-ligand interactions in the malaria parasite | Prof F Joubert |
| Ms TB Bawuba | MPH, Public Health | Mini-dissertation | Assessing the impact of indoor residual spraying (IRS) on Malaria Morbidity in Northern Uganda | Prof A Beke |
| Ms N Ramalwa | MPH, Public Health | Mini-dissertation | Insecticide susceptibility analysis of complex in Vlakbult, Mphumalanga 2012/13 | Prof C de Jager |
| Mr E Besaans | BSc Honours, Biochemistry | Research report | Novel polyamine trimer analogues as potential transport inhibitors in | Prof L Birkholtz |
| Ms JS Duvenhage | BSc Honours, Biochemistry | Research report | In vitro cultivation of and gametocyte producing strains of | Prof L Birkholtz |
| Mr Y Mbous | BSc Honours, Biochemistry | Research report | Characterising the link between polyamine metabolism and redox regulation in , BSc Hons mini-dissertation | Prof L Birkholtz, Prof AI Louw |
| VE Nkuna | Diploma in Public Health Medicine | Research report | Community knowledge, attitudes and perceptions about malaria prevention in rural area in Mopani District of Limpopo Province, South Africa | Prof C de Jager |
| Ms RM Ramathole | Diploma in Public Health | Research report | Integrated Vector Management for Malaria | Prof C de Jager |
| Ms H Roos | Diploma in Occupational Medicine and Health | Research report | HRA of a chemical Malaria vector control programme implemented at a large temporary construction camp site in Palma, Mozambique | Prof C de Jager |
| Student | Degree | Type | Title | Supervisors |
| Ms K Clarke | PhD, Biochemistry | Thesis | The effect of polyamine depletion on the transcriptome of | Prof L Birkholtz, Prof AI Louw |
| Ms C Rossouw | PhD, Biochemistry | Thesis | Investigations of liver stage functional genomics of | Prof L Birkholtz, D Mancama, H Hoppe, S Moolman |
| Ms C Griffiths | MSc, Biochemistry ( ) | Dissertation | The construction of high-resolution probability maps for the study of gene expression and regulation in | Prof L Birkholtz, M Mhlanga |
| Mr S Reekstring | MSc, Biochemistry | Dissertation | Structure-based drug discovery for malarial vit B6 and polyamine drug targets and evaluation thereof by functional genomics | Prof L Birkholtz, Prof AI Louw, C. Wrenger |
| Mr J Reynolds | MSc, Biochemistry | Dissertation | Structure-functional elucidations of ODC as antimalarial drug target | Prof L Birkholtz, Prof AI Louw |
| Ms N Coetzee | BSc Honours, Biochemistry ( ) | Research report | Novel antimalarial agents targeting spermidine synthase by way of rational-based drug design | Prof L Birkholtz, T van Brummelen |
| Mr de la Rey | BSc Honours, Biochemistry | Research report | Identifying lead compounds for S-Adenosyl methionine Decarboxylase | Prof L Birkholtz |
| Ms N Gouws | BSc Honours, Biochemistry | Research report | Investigating the effect of quinoline antimalarials on polyamine metabolism | Prof L Birkholtz |
| Ms R van Biljon | BSc Honours, Biochemistry ( ) | Research report | Investigations of the inhibitory effect of 1,4-diamino-2-butanone of the parasite | Prof L Birkholtz |
Postal Address: University of Pretoria Private Bag x 20 Hatfield 0028 South Africa -->
Location: GIBS | Groenkloof | Hatfield Hillcrest | Mamelodi | Onderstepoort | Prinshof
Student Service Centre (for Contact students): Contact Centre - Telephone: 012 420 3111 Contact Centre - Email: [email protected]
UPOnline Call Centre (for Online Students): Call Centre - Email: [email protected]
Get Social With Us
Download the UP Mobile App

Copyright © University of Pretoria 2024. All rights reserved.
Careers@UP | Tenders@UP | Ethics Hotline | PAIA Manual | Privacy Notices | Website Privacy Notice | Disclaimer | Terms of use
- Increase Text
- Decrease Text
- Links Underline
- Reader View

Challenges to Malaria Control in the Democratic Republic of Congo and Beyond
Add to collection, downloadable content.
- March 21, 2019
- Affiliation: College of Arts and Sciences, Department of Geography
- Roughly 40% of the world's population lives in areas where they are at risk of malaria infection. In the last 15 years, the global health community has made considerable progress in reducing transmission. Despite this progress, a number of challenges to further reductions remain. This dissertation addresses three such challenges. First, I focus on the ecology that serves as a backdrop to transmission, and focus on the role agriculture may play. In doing so, I attempt to understand how agriculture affects both mosquito behavior, as well as malaria risk in under-5 children in the Democratic Republic of Congo (DRC), a country with one of the world's highest malaria burdens. My findings from this work suggest that increasing exposure to agriculture is associated with increased indoor biting among Anopheles gambiae mosquitoes, which may be the mechanism driving the observed association between agriculture and increased malaria risk. Second, I turn to address insecticide resistance, which may undermine the contributions that bed nets have in reducing transmission. One challenge in monitoring insecticide resistance is the difficulty in obtaining representative samples of mosquitoes. I make some progress in overcoming this limitation using population-based survey data collected from 2009-2016 in 21 countries across sub-Saharan Africa, and find that the effects of bed nets treated with different insecticides vary considerably, and that certain countries need to transition away from using certain insecticides. Finally, I attempt to understand how malaria spreads. To do so, I leverage genetic data on the Plasmodium falciaprum malaria parasite from 28 neutral microsatellite markers drawn from malaria-infected children living in the DRC. I consider different population genetics tools to identify whether or not the malaria parasite population can be classied into smaller subpopulations, whether or not there is evidence of isoloation-by-distance, and if there appears to be gene flow between geographically and economically proximate regions. My results indicate that the malaria parasite population in DRC is best characterized as single population with weak evidence of isolation-by-distance, with no strong evidence of gene flow or barriers to it. However, outliers were observed along DRC's border.
- August 2017
- Biostatistics
- Public health
- Hierarchical modeling
- Bayesian statistics
- https://doi.org/10.17615/vp4n-1753
- Dissertation
- In Copyright
- Song, Conghe
- Reich, Brian
- Emch, Michael
- Meshnick, Steven R.
- Moody, Aaron
- Doctor of Philosophy
- University of North Carolina at Chapel Hill
This work has no parents.
| Thumbnail | Title | Date Uploaded | Visibility | Actions |
|---|---|---|---|---|
| 2019-04-11 | Public |
Select type of work
Master's papers.
Deposit your masters paper, project or other capstone work. Theses will be sent to the CDR automatically via ProQuest and do not need to be deposited.
Scholarly Articles and Book Chapters
Deposit a peer-reviewed article or book chapter. If you would like to deposit a poster, presentation, conference paper or white paper, use the “Scholarly Works” deposit form.
Undergraduate Honors Theses
Deposit your senior honors thesis.
Scholarly Journal, Newsletter or Book
Deposit a complete issue of a scholarly journal, newsletter or book. If you would like to deposit an article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.
Deposit your dataset. Datasets may be associated with an article or deposited separately.
Deposit your 3D objects, audio, images or video.
Poster, Presentation, Protocol or Paper
Deposit scholarly works such as posters, presentations, research protocols, conference papers or white papers. If you would like to deposit a peer-reviewed article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PLoS Negl Trop Dis
- v.8(10); 2014 Oct

P. vivax Malaria and Dengue Fever Co-infection: A Cross-Sectional Study in the Brazilian Amazon
Belisa m. l. magalhães.
1 Universidade do Estado do Amazonas, Manaus, Brazil
2 Fundação de Medicina Tropical Dr. Heitor Vieira Dourado, Manaus, Brazil
André M. Siqueira
Márcia a. a. alexandre, marcela s. souza, joão b. gimaque, michele s. bastos, regina m. p. figueiredo, gisely c. melo, marcus v. g. lacerda, maria p. g. mourão.
Conceived and designed the experiments: BMLM AMS MAAA MVGL MPGM. Performed the experiments: BMLM AMS MAAA MSS. Analyzed the data: BMLM AMS. Contributed reagents/materials/analysis tools: MSS JBG MSB RMPF GCM. Wrote the paper: BMLM AMS MVGL MPGM.
Associated Data
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Malaria and dengue are the most prevalent vector-borne diseases worldwide and represent major public health problems. Both are endemic in tropical regions, propitiating co-infection. Only few co-infection cases have been reported around the world, with insufficient data so far to enhance the understanding of the effects of co-infection in the clinical presentation and severity.
Methodology/Principal Findings
A cross-sectional study was conducted (2009 to 2011) in hospitalized patients with acute febrile syndrome in the Brazilian Amazon. All patients were submitted to thick blood smear and PCR for Plasmodium sp. detection, ELISA, PCR and NS1 tests for dengue, viral hepatitis, HIV and leptospirosis. In total, 1,578 patients were recruited. Among them, 176 (11.1%) presented P. vivax malaria mono-infection, 584 (37%) dengue fever mono-infection, and 44 (2.8%) were co-infected. Co-infected patients had a higher chance of presenting severe disease (vs. dengue mono-infected), deep bleeding (vs. P. vivax mono-infected), hepatomegaly, and jaundice (vs. dengue mono-infected).
Conclusions/Significance
In endemic areas for dengue and malaria, jaundice (in dengue patients) and spontaneous bleeding (in malaria patients) should raise the suspicion of co-infection. Besides, whenever co-infection is confirmed, we recommend careful monitoring for bleeding and hepatic complications, which may result in a higher chance of severity, despite of the fact that no increased fatality rate was seen in this group.
Author Summary
Malaria and dengue fever are typical diseases in tropical regions of developing countries; such as the Brazilian Amazon. They become serious problems in public health as they mostly affect vulnerable populations. Both diseases are mosquito-borne. These diseases present similar signs and symptoms. Brazil registers most of the malaria cases in the Amazon. The four dengue serotypes also circulate in this region. Similar to malaria, there are records of dengue outbreaks during the first months of the year, and isolated cases in the remaining months. Official records of malaria and dengue co-infection are infrequent in Brazil; however, we believe that this event is more frequent than usually reported. Our study detected high prevalence of the co-infection in the hospitalized patients infected with malaria or dengue in a tertiary health care unit, reference in the treatment of tropical and infectious diseases in Manaus, Amazonas, Brazil. We highlight the high likelihood of co-infected patients to present clinical complications. Besides, we observed that the presence of jaundice in dengue patients, and bleeding in malaria patients, are possible indications of co-infection. Therefore, this paper is useful to physicians working in the tropics, enabling the clinical suspicion of a not so rare condition.
Introduction
Malaria and dengue fever are the most prevalent vector-borne diseases worldwide and represent major public health problems. Dengue epidemics have been reported in several countries; 500,000 people with severe dengue require hospitalization each year, and 2.5% of those affected die. Similarly, malaria is a life-threatening disease which was responsible for 627,000 deaths in 2012 [1] , [2] . However, the occurrence of dengue and malaria co-infected patients is not well reported.
The dengue virus (DENV) is the major arbovirus responsible for human disease in Brazil. The four serotypes cause a variety of clinical presentation in humans, ranging from acute self-limited febrile illness to severe and fatal forms [3] , [4] . Regarding malaria, the Brazilian Amazon reports 50% of episodes in the Americas [5] . In 2012, 241,806 cases were reported, with 86.9% of them due to P. vivax [6] .
Malaria and dengue are endemic in similar tropical regions, and therefore, may result in the possibility of co-infection. Urban demographic expansion, deforestation and agricultural settlements in peri-urban areas, are known causes of the increase in the probability of concurrent infection of these two diseases [7] .
Considering the endemicity of dengue and malaria in the Amazon [8] , it is reasonable to envisage that the occurrence of concurrent infections would not be rare [9] , [10] . However, due to non-systematic investigation of both diseases, only a few cases of malaria and dengue co-infection have been reported [11] , [12] . In Brazil, for instance, a study performed in 2009 with 132 patients with vivax malaria found 11 co-infection episodes, all confirmed by molecular tests. These patients demonstrated severe manifestations, in particular hepatic injury [10] . The objective of the present study was to understand the interplay of both infections in a higher sample, and the impact on the clinical severity.
Ethical Statement
The study was approved by the Ethics Review Board of Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (FMT-HVD, 2009/15243), Manaus, Brazil. All participants signed an informed consent.
Study Design and Site
The study design was a cross-sectional study of patients hospitalized with acute febrile syndrome (history of fever in the past 10 days) from 2009 to 2011. The study was carried out in FMT-HVD, Manaus, capital of Amazonas State, Northern Brazil, where all four dengue serotypes co-circulate since 2008 and 95% of malaria cases result from P. vivax infection. FMT-HVD is a tertiary health care facility and a teaching and research center, which is the reference for infectious diseases in the region. Around 30% of all malaria cases reported in Manaus are assisted in this institution. During the study period, 14,884 cases of malaria and 6,302 cases of dengue fever were diagnosed in the hospital, from which 505 and 1,127, respectively, were hospitalized. In 2011, a dengue outbreak resulted in 5,400 cases reported at the FMT-HVD (∼10% of all reported cases in the state, based on data of surveillance system).
Patients and Data Collection
During the study period, all hospitalized patients with acute febrile syndrome were considered eligible. If they signed the informed consent, they were included and submitted to malaria and dengue investigation. They were also searched for hepatitis A, B and C, HIV and leptospirosis. Abdominal ultrasound and chest X-rays were also performed when indicated. Other tests were requested at physicians' discretion.
Patients with P. vivax infection with primaquine-induced hemolysis (hemoglobin <10 g/dL and reticulocytes >1.5% or increased indirect bilirubin after starting primaquine) were also excluded from the analysis.
The diagnosis of vivax malaria was confirmed by real-time PCR. The diagnosis of dengue was made either by a positive serology (IgM) or a positive NS1 protein or a positive molecular test (RT-PCR), considering that every patient was tested by all the three methods. The Group C was defined as patients co-infected with both dengue and P. vivax . They were compared to two different groups: malaria mono-infection (Group A), and dengue mono-infection (Group B). Severity was classified and managed according to the World Health Organization (WHO) guidelines for dengue and malaria [2] , [13] .
Laboratory Testing
Automatized blood biochemistry and whole blood count were performed systematically in all patients. The continuous variables used for analysis were the most altered throughout hospitalization. Walker's technique was used for thick blood smear [14] . The number of asexual parasites was counted in high magnification fields per 500 leukocytes and expressed as parasites per mm 3 . Real time polymerase chain reaction (qPCR) was performed to confirm P. vivax mono-infection. In brief, the extraction of total DNA from whole blood was performed using the QIAamp DNA Blood Mini Kit (Qiagen, USA), according to the manufacturer's protocol. The DNA was amplified in an Applied Biosystems 7500 Fast System (Applied Biosystems, USA) using primers and TaqMan fluorescence labeled probes for RT-PCR [15] .
The DENV diagnosis was based on three methods: a) IgM antibodies (MAC-ELISA) detection [16] ; b) detection of NS1 protein by Platelia Dengue NS1 Ag kit (Bio-Rad, France), and c) molecular diagnostics with the identification of viral serotype from the RT-PCR [17] . For extraction of viral RNA, mini kit QIAamp viral RNA (Qiagen, USA) was used, following the manufacturer's instructions. For the production of complementary DNA copy (cDNA) from RNA, AccessQuick kit RT-PCR System (Promega, USA) was used, according to the manufacturer's recommendations. The genomic region of dengue virus (DENV) was amplified by semi-nested PCR included genes C/prM.
Serological tests for leptospirosis (IgM) [18] , HIV-1/HIV-2 [19] , hepatitis A (anti-HAV IgM), hepatitis B (HBsAg), hepatitis C (anti-HCV), and hepatitis D (total anti-HDV), were based on commercial kits from Diasorin (Italy) and Bioeasy (Korea), following the manufacturers' instructions.
Statistical Analysis
Demographics, clinical and laboratorial characteristics from the group of patients co-infected with dengue and malaria vivax were compared to the group of patients mono-infected with dengue and the group of patients mono-infected with malaria vivax. The association between categorical variables and the risk of co-infection (as the outcome variable) was performed by means of univariable logistic regression with the presentation of the odds ratios and 95% confidence intervals. The 95% confidence intervals (95% CI) are presented. Means and standard deviation (SD) of continuous variables with normal distributions were compared using the Student's T test; those variables with non-normal distribution (as assessed by the Kolmogorov-Smirnov test) were described using median and interquartile range (IQR) and compared using the Kruskal-Wallis test. All the analyses were performed using Stata v.11 (College Station, Texas, USA) [20] .
From 2009 to 2011, 1,578 patients with acute febrile syndrome were hospitalized at the FMT-HVD. Among them, 176 (11.1%, 95% CI 9.6–12.7%) had vivax malaria mono-infection (Group A), 584 (37%, 95% CI 34.6–39.4%) had dengue fever mono-infection (Group B) and 44 (2.8%, 95% CI 2.0–3.6%) were co-infected with malaria and dengue (Group C). The prevalence of co-infected patients was 20% among patients with malaria and 7% among those with dengue ( Figure 1 ).

Gray boxes represent patients included in the analyses; Dashed boxes represent patients excluded.
As shown in Table 1 , the characteristics across the groups are homogenous. It is important to highlight though that fewer children and pregnant women were included in the co-infected group.
| Variables | (A) | Dengue fever (B) | Co-infection (C) | A×C | B×C | ||
| N = 176 (%) | N = 584 (%) | N = 44 (%) | |||||
| 92 (52.3) | 277 (47.4) | 22 (50) | 0.91 (0.47–1.76) | 0.787 | 1.1 (0.6–2.0) | 0.742 | |
| 29 (16.4) | 60 (10.4) | 3 (6.9) | 1 | 0.078 | 1 | 0.086 | |
| 105 (59.6) | 355 (61.5) | 22 (51.1) | 2.02 (0.56–7.24) | 1.23 (0.35–4.26) | |||
| 26 (14.7) | 126 (21.8) | 12 (27.9) | 4.46 (1.13–17.58) | 1.9 (0.51–7.0) | |||
| 16 (9.1) | 36 (6.2) | 6 (13.9) | 3.62 (0.79–16.48) | 3.33 (0.78–14.15) | |||
| 14 (18.1) | 8 (3.8) | 4 (19.0) | 1.05 (0.30–3.63) | 0.928 | 11.4 (3.12–41.65) | <0.001 | |
| 46 (26.4) | 98 (24.8) | 12 (27.2) | 1.04 (0.49–2.19) | 0.911 | 1.14 (0.56–2.29) | 0.714 | |
| 67 (38.1) | - | 15 (38.0) | 0.84 (0.42–1.68) | 0.626 | - | - | |
Tables 2 and and3 3 compare clinical and laboratorial data between Group C and Groups A and B. Patients with the co-infection had a higher chance of presenting severe disease (OR 4.71, 95% CI: 2.37–9.34) according to WHO's criteria than those mono-infected with dengue ( Table 2 ). Conversely, those with malaria mono-infection had less frequently severe disease than co-infected patients, but this was not statistically significant.
| Variables | (A) | Dengue fever (B) | Co-infection (C) | A×C | B×C | ||
| N = 176 (%) | N = 584 (%) | N = 44 (%) | |||||
| 54 (30.7) | 306 (52.4) | 24 (54.6) | 2.7 (1.4–5.3) | 0.004 | 1.1 (0.6–2) | 0.783 | |
| 51 (29) | 293 (50.2) | 14 (31.8) | 1.2 (0.6–2.4) | 0.712 | 0.5 (0.2–0.9) | 0.021 | |
| 7 (4.0) | 76 (13.0) | 15 (34.1) | 12.5 (4.7–33.3) | <0.001 | 3.5 (1.8–6.8) | <0.001 | |
| 3 (8.3) | 67 (16.9) | 6 (31.5) | 5.07 (1.10–23.38) | 0.037 | 2.3 (0.83–6.2) | 0.110 | |
| 8 (8.5) | 119 (30.1) | 1 (5.6) | 0.63 (0.74–5.39) | 0.675 | 0.13 (0.17–1.03) | 0.054 | |
| 60 (34.1) | 49 (22.5) | 14 (31.8) | 0.90 (0.44–1.82) | 0.775 | 1.60 (0.79–3.27) | 0.189 | |
| 52 (29.5) | 118 (29.4) | 16 (36.3) | 1.36 (0.68–2.72) | 0.382 | 1.37 (0.71–2.62) | 0.342 | |
| 139 (78.9) | 198 (49.9) | 31 (70.4) | 0.63 (0.30–1.33) | 0.230 | 2.39 (1.21–4.71) | 0.011 | |
| 162 (92) | 332 (84.1) | 41 (93.2) | 1.18 (0.32–4.30) | 0.801 | 2.59 (0.77–8.63) | 0.120 | |
| 118 (67) | 203 (51.2) | 31 (70.4) | 1.17 (0.57–2.40) | 0.665 | 2.26 (1.15–4.46) | 0.018 | |
| 67 (38.3) | 59 (27.1) | 24 (54.6) | 1.9 (0.99–3.8) | 0.053 | 3.23 (1.66–6.28) | <0.001 | |
| 142 (81.6) | 341 (86.3) | 37 (84.1) | 1.19 (0.48–2.91) | 0.701 | 0.83 (0.35–1.97) | 0.684 | |
| 91 (51.7) | 3 (1.4) | 29 (65.9) | 1.80 (0.90–3.60) | 0.093 | 138.5 (37.8–507.7) | <0.001 | |
| 77 (44) | 7 (3.9) | 26 (59.1) | 1.83 (0.93–3.59) | 0.075 | 35.28 (13.4–92.6) | <0.001 | |
| 7.4 (8.1) | 4.2 (2.8) | 7.59 (6.4) | 0.98 (0.95–1.02) | 0.540 | 1.32 (1.19–1.47) | <0.001 | |
| 45 (25.6) | - | 7 (15.9) | 0.6 (0.2–1.3) | 0.182 | - | 0.139 | |
| - | 211 (36.1) | 32 (72.7) | - | - | 4.71 (2.37–9.34) | <0.001 | |
| Variables | (A) | Dengue fever (B) | Co-infection (C) | A×C | B×C |
| N = 176 | N = 584 | N = 44 | |||
| 2.843 (1974–4094) | - | 4363 (2133–8924) | 0.155 | - | |
| 30.8 (8.8) | 38.0 (14.2) | 31.01 (8.5) | 0.473 | 0.002 | |
| 7.801 (5.9) | 5.700 (4.2) | 7.197 (4.7) | 0.457 | 0.810 | |
| 115,114 (136,920) | 41,824 (37,865) | 69.772 (71,486) | 0.055 | <0.001 | |
| 3.5 (0.6) | 3.0 (1.6) | 3.38 (0.6) | 0.677 | 0.154 | |
| 1.21 (1.4) | 1.0 (0.3) | 1.02 (0.4) | 0.214 | 0.951 | |
| 73.1 (98.3) | 189 (543.0) | 90.9 (173.6) | 0.263 | 0.007 | |
| 73.6 (83.5) | 134 (186.0) | 99.7 (192.9) | 0.328 | 0.251 | |
| 3.7 (5.7) | 0.7 (1.0) | 8.3 (13.0) | 0.008 | <0.001 | |
| 1.9 (3.8) | 0.4 (0.7) | 3.5 (3.4) | 0.033 | <0.001 |
Compared to P. vivax mono-infected patients, the increased odds of deep bleeding in co-infected patients (OR 12.5, 95% CI: 4.7–33.3) was statistically significant (p<0.001), although platelet count was not different ( Tables 2 and and3). 3 ). When compared with dengue mono-infected patients, co-infected patients had a higher chance of presenting deep bleeding (OR 3.5, 95% CI: 1.8–6.8). Conversely, superficial bleeding was more frequent among dengue mono-infected patients. The overall bleeding, however, was more frequent on co-infected patients, despite significant reduction in platelet counts ( Tables 2 and and3 3 ).
Regarding hepatic injury, co-infected patients had a higher chance of having hepatomegaly and clinical jaundice compared to those with malaria mono-infection, although this was not statistically significant ( Table 2 ), despite significant increase in bilirubin levels ( Table 3 ). When compared to dengue mono-infected patients, co-infected patients had a higher chance of presenting hepatomegaly (OR 35.28, 95% CI: 13.4–92.6) and jaundice (OR 138.5, 95% CI: 37.8–507.7), which was paralleled by significantly increased in bilirubin and AST levels ( Tables 2 and and3 3 ).
Co-infected patients also had prolonged fever when compared to dengue mono-infected patients. Finally, other dengue warning signs [2] , such as abdominal pain and vomiting, as well as dyspnea, were significantly more frequent among co-infected patients. Noteworthy, all four co-infected pregnant women had severe disease.
The predominant dengue serotypes in the co-infected group were DENV 2 and DENV 4, both with nine patients (33.3%). These serotypes were the most common among the dengue mono-infection group, 127 (49.6%) and 80 (31.2%), respectively.
No patient required hospitalization in the intensive care unit, and fatality rate was zero in our casuistic.
In an endemic area of dengue fever and vivax malaria, we found a high prevalence of the co-infection, mainly among those with malaria. In Brazil, a prospective study performed in 2009 on 132 patients with vivax malaria found 11 co-infected and the prevalence was 8.3% [10] . During a dengue outbreak in India, the prevalence of co-infection was 5.8% among all cases of fever (77 of 546) [12] . In the French Guiana, the prevalence of co-infection was 7.1% (17 of 238) among patients with dengue [11] , which is similar to our results. In Pakistan, however, the prevalence found was as high as 23.2% [21] . Thus, the prevalence of co-infection may fluctuate, depending on local endemicity. In these studies, the prevalence was estimated on hospitalized patients, therefore it could not be extrapolated to the community-based level.
In our study, being co-infected resulted in a much higher chance of presenting deep bleeding as compared to both groups of mono-infected patients, suggesting a possible synergistic pathogenic mechanism, which could be related to both capillary fragility and coagulation disorders, but not the low platelet count. Bleeding is reported as an infrequent finding in malaria, despite common platelet depletion [22] , [23] . Conversely, bleeding is the most feared complication of dengue fever, where in addition to platelet depletion, virus-induced endothelial and liver injury concur to the risk of coagulopathy [24] , [25] , [26] . In our casuistic, although bleeding was more frequent among co-infected patients, it was also frequent among mono-infected patients in both groups.
Hepatic injury was also a concern in the co-infected group, which, together with bleeding, resulted in a higher chance of dengue severity according to WHO criteria. Jaundice in malaria is mostly a result of cholestasis or intravascular hemolysis [27] , while in dengue fever it has been associated with fulminant liver failure [28] , [29] . Interestingly, like bleeding, jaundice is no longer considered to be a malaria severity criteria according to WHO [13] . A prospective study performed during a dengue outbreak in India, reported more frequent bleeding on co-infected patients, as well as thrombocytopenia and hepatic injury [12] . On the other hand, in the French Guiana, although co-infected patients presented more hematologic complications and hepatic injury, bleeding was uncommon [11] .
A warning sign commonly used to describe severe dengue is hemoconcentration (increase in the basal hematocrit ≥20%) [30] . However, even with more severe dengue cases, our co-infected patients presented a low mean of hematocrit. An explanation for this fact can be attributed to malaria-induced anemia, a common complication in vivax malaria [31] . For this reason, the malaria clinical manifestation may be a confounder for health care professionals during the interpretation and application of dengue severity criteria, in areas where both diseases occur. The proper clinical management of co-infected patients may be compromised due to diagnostic delays or misinterpretation, and inappropriate treatment may result in fatal complications [32] , [33] .
Dengue warning signs, such as vomiting, abdominal pain and hepatomegaly, were very frequent in the co-infection cases. The cautious detection of these signs is of extreme importance as they characterize potential dengue severity [2] . Our findings were similar to the results reported by the study performed in the French Guiana [11] , although they did not use the dengue severity criteria from WHO [2] ; in both cases, the co-infected patients presented a higher frequency of warning signs and the sample had more severe cases.
In addition to classical warning signs and symptoms, dyspnea was also frequent in all groups, particularly in co-infected patients. Dyspnea is an early clinical feature of plasma leakage and, in dengue, may be the evidence of fluid accumulation of in the pleural cavity [2] , [34] . In malaria, dyspnea may be an evidence of acute lung edema [35] , which is one of the severity criteria for falciparum malaria [13] . In a study conducted in Timor East, one patient co-infected with falciparum malaria and dengue presented respiratory distress with radiographic findings compatible with the presentation of acute lung edema [33] . The clinical management of these cases may be difficult, as the inadequate fluid therapy for dengue treatment may induce fluid overload and large fluid effusion to the lungs.
The pregnant women had a more complicated presentation, although we could not follow up them until the end of their pregnancy. In a case series of co-infected patients from the Amazon region, pregnant women (2 of 11) presented severe acute lung edema and anemia [10] . Dengue is known to cause obstetric complications and to increase the risk of dengue severity among pregnant women [36] . In malaria, on the other hand, this association is not clear, because reported studies on the impact of P. vivax on pregnancy are scarce [37] .
Co-infected patients presented similar days of fever as compared to malaria patients. That means that a patient with the diagnosis of dengue presenting with prolonged evolution should raise the suspicion of malaria co-infection. Our findings corroborate the results of a long case series in Pakistan, which presented longer disease duration on patients co-infected with vivax malaria and dengue [21] .
No specific dengue serotype was associated to the co-infected patients, however the number of cases was not big enough to test that hypothesis.
Our study has some limitations. It was not possible to confirm dengue infection by PCR in all patients due to the time of the disease presentation and possible non-viremic periods. On the other hand, a positive IgM in patients with malaria could also reflect recent dengue infection or recent yellow fever vaccination. In addition, our results are not extendable to other healthcare settings or to community basis, since we only included hospitalized patients.
On the other hand, this study has also some strengths. This is one of few studies addressing malaria and dengue co-infection, with a considerable amount of cases diagnosed by molecular tests. Besides, this work has been conducted by the same health care team, who applied consistent selection and severity criteria throughout the duration of the study. Furthermore, the majority of the existing works are case series reports and retrospective studies, which may produce low evidence level.
Malaria and dengue co-infection is a relatively common event. Being malaria the disease with easier and faster diagnosis, in areas with known endemicity, it is recommended the systematic testing for Plasmodium sp. on cases with acute febrile syndrome. At last, the patients with parasitological malaria diagnosis which present spontaneous bleeding must be systematically investigated for dengue, and likewise, in suspected and confirmed dengue patients presenting jaundice, Plasmodium sp. investigation must be performed. Besides, whenever co-infection is confirmed, we recommend a carefully monitoring for bleeding and hepatic complications, which may result in a higher chance of severity, regardless of WHO criteria.
Supporting Information
Checklist s1.
STROBE checklist.
Acknowledgments
As part of her PhD thesis, BMLM dedicates this manuscript to her son, Eduardo Magalhães Valentin. The authors would like to thank the staff of Fundação de Medicina Tropical Doutor Heitor Vieira Dourado ; the Universidade do Estado do Amazonas ; Mônica Costa, for contributing with malaria diagnosis; Márcia Castilho, for contributing with dengue diagnosis; Marcelo Cordeiro, Anette Trajman and Eduardo Valentin for reviewing the text. We are also thankful to Carlos Morel, coordinator of the National Institute of Science and Technology on Neglected Diseases Innovation, and to Cláudio Tadeu Daniel-Ribeiro, coordinator of the Laveran & Deane Seminar on Malaria.
Funding Statement
The authors received no specific funding for this work.
Data Availability
Malaria diagnosis based on a machine learning system
Downloadable content.
- Maduako, Chidinma
- Petra Rohrbach (Supervisor2)
- Timothy Geary (Supervisor1)
- SUMMARYIntroduction: The latest World Malaria Report released in November 2017 estimated that 219 million cases of malaria occurred and deaths due to malaria reached 435,000 in 2017(1). The WHO considers microscopy to be the gold standard for clinical diagnosis of malaria due to its ready availability. However, microscopy has many shortcomings, including inter-user variability and inconsistency, due to the fact that many microscopy technicians do not assess the standard number of high-power fields, are not adequately trained on recognizing all forms of malaria and the high disparity associated with the quality of manual Giemsa slide production (4) To remedy the mis-use of empiric (symptom-guided) treatment, malaria testing is required by many governmental health organizations before commencing antimalarial drug therapy, thereby resulting in increased demand for up to 500 million malaria tests in 2012 (10). Understanding the diagnostic expertise necessary and representing it by specifically tailored image processing, analysis and pattern recognition algorithms can help in designing an automated diagnosis system. Although it is not yet a widespread research topic, automated diagnosis of malaria directly addresses several current gaps (11). My research aims to develop a machine learning system that can identify the stage and number of Plasmodium falciparum in cultured erythrocytes based on their morphology using thin film slides, the second objective is to develop a machine learning system that can identify the number of ring-stage parasites in samples of cultured erythrocytes diluted with fresh whole blood.Methods: Giemsa stained thin blood smears were made from synchronized cultures of the 3D7 strain of plasmodium falciparum stored in an incubator with shaking at 37oC, 5% CO2, 3 % O2, 92 % N2. Thin blood smears were viewed with an EVOS microscope and digital images were acquired, saved as tiff format and stored in a memory stick. The images were transferred as files to a computer, then the images further pre-processed, segmented, and the parasites and the stages of the life cycle detected. The algorithm formulated with the MATLAB programme was trained using 109 images. For the first objective, 397 images were used and for the second objective 163 images were used.The Otsu algorithm was used for this study, gray level images were reduced to binary images. The algorithm assumes that the images contain foreground and background pixels. Results: This study showed a relatively strong, positive linear association/ correlation between automated count and manual counts. The correlation between the manual count and the automated count was 0.85. The Pearson correlation between the automated and manual count was 0.7. The diagnostic tool showed a sensitivity of 94.6% for rings, 96.5% for trophozoites and 98.2% for schizont. Moreso, it showed a specificity of 96.5% for rings, 88.9% for trophozoites and 81.8% for schizonts. The R and G channels of the RGB color scheme had clear features which were used to identify objects containing chromatin in Giemsa-stained blood films. The input images transformed to grayscale highlighted parasites containing chromatin.Conclusion: This study developed an automated system that could enhance the diagnosis and therefore treatment of malaria. The automated method detected more trophozoites and schizonts than the ring stage parasites as seen with a correlation value of 0.83, 0.86 and 0.94 for the ring, trophozoite and ring stages respectively
- SOMMAIREIntroduction : Le dernier Rapport sur la Malaria dans le Monde, publié en novembre 2017, estimait à 219 millions le nombre de cas de Malaria et à 435 000 le nombre de décès dus à celle-ci pour l'année 2017 (1). L'OMS considère que la microscopie est la référence en matière de diagnostic clinique de la Malaria en raison de sa disponibilité immédiate. Cependant, la microscopie présente de nombreuses lacunes, notamment la variabilité et l'incohérence entre les utilisateurs, dû au fait que de nombreux techniciens en microscopie n'analysent pas le nombre standard de champs à forte puissance (HPF), ne sont pas formés de manière adéquate afin de pouvoir reconnaitre toutes les variétés de Malaria et à la grande disparité associée avec la qualité de la production manuelle de lames Giemsa (4) Afin de remédier à la mauvaise utilisation d'un traitement empirique (guidé par les symptômes), de nombreux organismes de santé gouvernementaux exigent un test de dépistage de la malaria avant de commencer un traitement antipaludique, ce qui a entraîné une demande accrue de 500 millions de tests de dépistage de la malaria en 2012 (10). Comprendre le savoir-faire nécessaire en matière de diagnostic et le représenter au moyen de traitement d'image, d'analyse et de reconnaissance d'algorithmes modèles spécialement adaptés peut aider afin de concevoir un système de diagnostic automatisé. Bien que le sujet de la recherche ne soit pas encore très répandu, le diagnostic automatisé de la malaria traite directement plusieurs lacunes actuelles (11). Ma recherche vise à développer un système d'apprentissage automatique capable d'identifier le stade et le nombre de Plasmodium falciparum dans des érythrocytes en culture, sur la base de leur morphologie en utilisant des lames minces. Le second objectif est de développer un système d'apprentissage automatique permettant d'identifier le nombre de stades anneau parasites dans des érythrocytes en culture dilués avec du sang total frais.Méthode : Des frottis sanguins minces colorés au Giemsa ont été réalisés à partir de cultures synchronisées de la souche 3D7 de plasmodium falciparum conservée dans un incubateur sous agitation à 37 °C, 5% de CO2, 3 % de O2, 92 % de N2. Des frottis sanguins minces ont été observés à l'aide d'un microscope EVOS et des images numériques ont été acquises, enregistrées au format tiff et stockées sur une clé USB. Les images ont été transférées en tant que fichiers sur un ordinateur, prétraitées et segmentées puis les parasites et les diverses étapes du cycle de vie y ont été détectés. L'algorithme formulé à l'aide du programme MATLAB a été formé en utilisant 109 images. 397 images ont été utilisées pour le premier objectif et 163 pour le deuxième objectif. L'algorithme Otsu a été utilisé pour cette étude, les images en niveaux de gris ont été réduites à des images binaires. Résultats : Cette étude a montré une association/corrélation linéaire positive relativement forte entre le comptage automatisé et le comptage manuel. La corrélation entre le nombre manuel et le nombre automatisé était de 0,85. La corrélation de Pearson entre le comptage automatisé et manuel était de 0,7. L'outil de diagnostic a montré une sensibilité de 94,6% pour les anneaux, de 96,5% pour les trophozoïtes et de 98,2% pour les schizontes. De plus, une spécificité de 96,5% pour les anneaux, de 88,9% pour les trophozoïtes et 81,8% pour les schizontes a été démontrée. Les images d'entrée transformées en niveaux de gris ont mis en évidence des parasites contenant de la chromatine.Conclusion : Cette étude a développé un système automatisé pouvant améliorer le diagnostic et donc le traitement de la malaria. La méthode automatisée a détecté plus de trophozoïtes et de schizontes que de stades anneau parasites, avec une valeur de corrélation de 0,83, 0,86 et 0,94 respectivement pour l'anneau, le trophozoïte et les stades anneau
- Parasitology
- McGill University
- https://escholarship.mcgill.ca/concern/theses/8g84mr79f
- All items in eScholarship@McGill are protected by copyright with all rights reserved unless otherwise indicated.
- Institute of Parasitology
- Master of Science
- Theses & Dissertations
| Thumbnail | Title | Date Uploaded | Visibility | Actions |
|---|---|---|---|---|
| 2020-03-23 | Public |

- < Previous
Home > Public Health > SPH_DISS > 42
Public Health Dissertations
Epidemiology of malaria and other diseases of public health importance and implications for interventions in high transmission settings in sub-saharan africa.
Leah Moriarty Follow
Date of Award
Degree type.
Dissertation
Degree Name
Doctor of Philosophy (PhD)
Public Health
First Advisor
Gerardo Chowell
Second Advisor
Richard Rothenberg
Third Advisor
Mateusz Plucinski
Infectious diseases remain a major cause of disability of death in low-resource settings. Malaria alone was responsible for an estimated 405,000 deaths globally in 2018, with the 94% of these deaths occurring in sub-Saharan Africa. In Mozambique and Democratic Republic of the Congo (DRC), communicable diseases, including malaria, lower respiratory infections, and neonatal disorders are among the top causes of disability and death. Understanding malaria and co-endemic diseases in these two countries can aid the planning, evaluation, and targeting of public health interventions. Additionally, studying the efficacy of the drugs used to treat malaria will preserve the ability for malaria cases to be treated successfully.
The three studies in this dissertation describe the epidemiology of malaria and co-endemic diseases of public health importance in Mozambique and evaluate the efficacy of medicines used to treat malaria in DRC. The first study will describe the spatial epidemiology of malaria in two high-burden districts in northern Mozambique to explore the utility of exploration of local spatial heterogeneity in high-transmission settings. The second study will investigate patterns in antibody responses to several infectious pathogens of public health importance in Mozambique, providing an opportunity to understand common predictors of infectious diseases endemic in this region. The third study will examine the efficacy of three artemisinin-based combination therapies used to treat uncomplicated malaria and molecular markers of antimalarial resistance in five sites in DRC.
Collectively, the three studies in this dissertation describe factors that have implications for intervention planning and disease surveillance in areas with high malaria and other tropical disease burden and limited health resources. Careful consideration of transmission setting can support more efficient and higher quality data collection and may allow for intervention design tailored to the local realities that can target multiple diseases of public health importance.

Recommended Citation
Moriarty, Leah, "Epidemiology of Malaria and Other Diseases of Public Health Importance and Implications for Interventions in High Transmission Settings in Sub-Saharan Africa." Dissertation, Georgia State University, 2021. doi: https://doi.org/10.57709/20221191
https://doi.org/10.57709/20221191
File Upload Confirmation
Since November 19, 2020
Advanced Search
- Notify me via email or RSS
- Collections
- Disciplines
- Submit ETD (Thesis/Dissertation)
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
- Skip to main content
- Accessibility information

- Enlighten Enlighten
Enlighten Theses
- Latest Additions
- Browse by Year
- Browse by Subject
- Browse by College/School
- Browse by Author
- Browse by Funder
- Login (Library staff only)
In this section
The ecology and behaviour of insecticide resistant malaria vectors and implications for control in Burkina Faso
Sanou, Antoine (2020) The ecology and behaviour of insecticide resistant malaria vectors and implications for control in Burkina Faso. PhD thesis, University of Glasgow.
| |
Long-Lasting Insecticide-Treated Nets (LLINs) and Indoor Residual Spraying (IRS) are the most common and successful methods for malaria vector control in Africa. There is growing evidence of shifts in mosquito vector biting and resting behaviours in several African settings where high LLIN coverage has been achieved. These changes, combined with growing insecticide resistance, may reduce intervention success by decreasing the contact between vectors and insecticide-treated surfaces. While insecticide resistance in malaria vectors has been widely investigated, less is known about the implications of mosquito behavioural changes to malaria control. In recent years, LLIN programmes appear to have a reducing impact in a small number of high burden African countries including Burkina Faso. This reducing effectiveness is hypothesized to be the result of insecticide resistance, but the potential additional contribution of mosquito behavioural avoidance strategies has not yet been investigated in Burkina Faso. The aim of this PhD was to investigate the contribution of insecticide resistance and mosquito behaviours to the persistence of malaria transmission in southwestern Burkina Faso following a national LLIN-distribution campaign. Specific objectives were to (i) evaluate the performance of a new mosquito sampling method, the Mosquito Electrocuting Trap (MET) to measure spatial and temporal variation in human exposure to malaria vectors; and characterize the spatial, seasonal and longer-term trends in (ii) vector ecology and behaviours, (iii) insecticide resistance within Anopheles gambiae sensu lato (s.l.) and (iv) malaria vector survival and transmission potential in rural Burkina Faso. A two-year programme of longitudinal mosquito vector surveillance was initiated within 12 villages of south-western Burkina Faso in 2016, shortly after completion of a mass LLIN distribution. Host seeking malaria vectors were sampled monthly using Human Landing Catches (HLC) and METs conducted inside houses and in the surrounding outdoor area (911 households in total). Resting bucket traps (RBTs) were used to sample indoor and outdoor resting vectors. In an initial study (Chapter 2), I evaluated the performance of the MET relative to the HLC for sampling host-seeking malaria vectors over 15 months in 12 villages. Overall, the MET caught proportionately fewer An. gambiae s.l. than the HLC (mean estimated number of 0.78 versus 1.82 indoors, and 1.05 versus 2.04 outdoors). However provided a consistent representation of vector species composition, seasonal and spatial dynamics, biting behaviour (e.g. location and time) and malaria infection rates relative. The MET slightly underestimated the proportion of bites that could be prevented by LLINs relative to the HLC (5%). However, given the major advantage of the MET of reducing human infection risk during sampling, I conclude these limitations are acceptable and that the MET presents a promising and safer alternative for monitoring human exposure to malaria vectors in outdoor environments. Vector sampling was extended (using HLCs and RBTs) to investigate longer-term temporal changes in vector ecology and behaviour (Chapter 3). Analysis of a subset (20%) of the An. gambiae s.l. (N= 7852) indicated that An. coluzzii (53.82%) and An. gambiae (45.9%) were the main vector species. There was substantial variation in vector abundance between sites and seasons, with a predicted ~23% reduction in An. gambiae s.l. biting density from start to end of study. A higher proportion of outdoor biting (~54%) was detected than expected from previous studies; but there was no evidence of spatial, seasonal or longer-term changes in exophagy. Species level analyses indicated that revealed moderate but statistically significant different in the exophagy and biting time between An. coluzzii and An. gambiae. Combining information on biting times and location (indoors versus outdoors), I estimated that ~85% of exposure could be prevented using good quality and effective LLINs during standard sleeping hours (10 pm – 5 am). Bioassays were conducted on the An. gambiae s.l. population at 9 out of the original 12 study villages to estimate spatial, seasonal and longer-term variation in insecticide resistance (IR) over the study period. Overall, only 23% of An. gambiae s.l. exposed to a diagnostic dose of deltamethrin were killed within 24 hours; indicating that all surveyed populations are resistant. Furthermore, IR increased over the study period, with significant reduction in mortality after exposure to deltamethrin in bioassays. There was no evidence of variation in IR between An. gambiae and An. coluzzii. Finally, the transmission potential of An. gambiae s.l. in this area was investigated through assessment of mosquito parity rates (a proxy of survival), malaria infection rates and estimation of annual Entomological Inoculation Rates (EIR; Chapter 5). The daily survival rate of malaria vectors in this area was > 90%), but with variation between villages and seasons. After controlling for this spatial and seasonal variation, there was evidence of a longer-term increase in vector survival over the study period. In contrast, both mosquito vector biting densities and their malaria infection rates declined over the study period. This resulted in a drop in the predicted EIR from 320 to 105 infective bites per person/year respectively in year 1 and 2. Considering the proportion of exposure estimated to be preventable by effective LLIN use (~85%, Chapter 2 &3), I estimated that residents in this area are still exposed to ~32 infective bites per person per year even when this intervention is used. This confirms that even with 100% coverage and usage of highly effective LLINs, high levels of transmission will persist in this setting. Taking the case of Burkina Faso as an example, results obtained here confirm that both IR and outdoor biting by malaria vectors are contributing to the persistence of transmission in high burden African countries. Consequently, a successful vector control programme in this context need a clear insecticide resistance management plan and supplementary tools that target vectors feeding and resting outdoors.
| Item Type: | Thesis (PhD) |
|---|---|
| Qualification Level: | Doctoral |
| Keywords: | Anopheles gambiae s.l., ecology, biting and resting, behaviours, Mosquito Electrocuting Trap, insecticide resistance, Malaria transmission potentials, The Cascades Region, Burkina Faso. |
| Colleges/Schools: | > |
| Funder's Name: | |
| Supervisor's Name: | Ferguson, Professor M. Heather and Matthiopoulos, Professor Jason |
| Date of Award: | 2020 |
| Depositing User: | |
| Unique ID: | glathesis:2020-81392 |
| Copyright: | Copyright of this thesis is held by the author. |
| Date Deposited: | 22 Jun 2020 05:54 |
| Last Modified: | 15 Sep 2022 14:23 |
| Thesis DOI: | |
| URI: |
Actions (login required)
| View Item |
Downloads per month over past year
View more statistics

The University of Glasgow is a registered Scottish charity: Registration Number SC004401
- Open access
- Published: 23 August 2024
A review of selective indoor residual spraying for malaria control
- Seth R. Irish 1 , 2 , 3 ,
- Derric Nimmo 4 ,
- Jameel Bharmel 4 ,
- Frederic Tripet 1 , 2 ,
- Pie Müller 1 , 2 ,
- Pablo Manrique-Saide 5 &
- Sarah J. Moore 1 , 2 , 3 , 6
Malaria Journal volume 23 , Article number: 252 ( 2024 ) Cite this article
73 Accesses
Metrics details
Indoor residual spraying (IRS) is one of the most effective malaria control tools. However, its application has become limited to specific contexts due to the increased costs of IRS products and implementation programmes. Selective spraying—selective spray targeted to particular areas/surfaces of dwellings—has been proposed to maintain the malaria control and resistance-management benefits of IRS while decreasing the costs of the intervention.
A literature search was conducted to find (1) studies that assessed the resting behaviour of Anopheles mosquitoes and (2) studies that evaluated the impact of selective spraying on entomological and malaria outcomes. Additional articles were identified through hand searches of all references cited in articles identified through the initial search. A cost model was developed from PMI VectorLink IRS country programmes, and comparative cost analysis reports to describe the overall cost benefits of selective IRS.
In some studies, there appeared to be a clear resting preference for certain Anopheles species in terms of the height at which they rested. However, for other species, and particularly the major African malaria vectors, a clear resting pattern was not detected. Furthermore, resting behaviour was not measured in a standardized way.
For the selective spray studies that were assessed, there was a wide range of spray configurations, which complicates the comparison of methods. Many of these spray techniques were effective and resulted in reported 25–68% cost savings and reduced use of insecticide. The reported cost savings in the literature do not always consider all of the IRS implementation costs. Using the IRS cost model, these savings ranged from 17 to 29% for programs that targeted Anopheles spp. and 18–41% for programmes that targeted Aedes aegypti .
Conclusions
Resting behaviour is generally measured in a simplistic way; noting the resting spot of mosquitoes in the morning. This is likely an oversimplification, and there is a need for better monitoring of resting mosquitoes. This may improve the target surface for selective spray techniques, which could reduce the cost of IRS while maintaining its effectiveness. Reporting of cost savings should be calculated considering the entire implementation costs, and a cost model was provided for future calculations.
Malaria continues to cause high levels of morbidity and mortality, particularly in Africa, where the majority of malaria cases occur [ 1 ]. In 2022, malaria cases increased to an estimated 249 million cases, resulting in an estimated 608,000 deaths [ 1 ]. To decrease the number of cases, it is important to invest in effective testing and treatment of malaria, as well as undertaking strategies that prevent malaria transmission. Vector control is the most effective current malaria prevention strategy, and the main techniques employed are the distribution of insecticide-treated nets (ITNs) and indoor residual spraying (IRS). In recent years, there has been the development of highly effective nets with different active ingredients (e.g. [ 2 , 3 ]). This has resulted in some countries stopping their IRS programs, partly due to cost considerations, even though IRS remains highly cost effective [ 4 , 5 ]. However, IRS has several advantages which might be useful if the costs of IRS could be reduced. These advantages include the possibility for insecticide rotation as part of a resistance management plan [ 6 ], less necessity for active utilization (as compared to ITNs, which must be put in place by homeowners each night) [ 7 ], and, similar to ITNs, IRS can have a community protection effect when coverage is high [ 8 ].
One way to decrease the cost of IRS is through selective indoor spraying of some of the surfaces in houses. It should be noted that selective spraying is sometimes termed “targeted IRS” [ 9 ] or “partial IRS” [ 10 ] that should be distinguished from the targeted application of IRS to areas where there is evidence of recent malaria transmission rather than blanket application to all houses [ 11 ]. Conventional IRS recommended by the WHO for malaria control [ 12 ] involves the full spraying of all indoor walls and often the ceilings of houses.
Optimally, the selective indoor spray is applied where mosquitoes are most likely to rest [ 12 ]. Selectively applying residual insecticides, e.g., for Aedes aegypti on exposed lower sections of walls (< 1.5 m), under furniture, and on dark surfaces throughout houses provides an entomological impact similar to spraying entire walls (as performed in classic IRS), but in a fraction of the time (< 18%) and insecticide volume (< 30%) compared to classic IRS [ 9 ]. Other studies have shown important impacts using selective spraying [ 13 , 14 ]. This selective spraying approach is endorsed by the Pan American Health Organization for IRS spraying for control of Aedes aegypti in urban settings [ 15 ]. While numerous studies have been done to evaluate selective IRS for malaria control, this work has not provided conclusive findings required to change current policies. This narrative review summarizes previous research on the use of selective spraying for vector-borne disease control and the cost-saving implications to see whether there might be justification for the use of selective spraying for malaria control, and to determine what avenues of research might be the most impactful to maximize its efficacy.
Selection criteria
Studies were included if they considered the two key questions of this review: resting behaviour of Anopheles mosquitoes or efficacy of selective indoor residual spraying.
Search strategy
An initial search was conducted on PubMed in July 2022, without language or date limits to find (1) studies that assessed the resting behaviour of Anopheles mosquitoes and (2) studies that evaluated the impact of selective spraying on entomological and malaria outcomes. Search terms included “partial indoor residual spraying” and “targeted indoor residual spraying”. Additional articles were identified through hand searches of all references cited in articles identified through the initial search. This process continued until no further related articles were found.
Data extraction
Data from the selected papers were extracted to determine the resting heights and behaviours of Anopheles mosquitoes. Additionally, data was extracted from articles that discussed the impact of selective spraying, and the impact and cost savings of these studies were summarized.
Cost analysis
A cost model was constructed from the PMI VectorLink IRS country programs comparative cost analysis reports. The model is based mainly on the 2018 data across the 14 countries where PMI VectorLink performed IRS [ 16 ]. A comparison to the cost analysis data from 2019 to 2022 shows that the relative cost breakdown for each area has not changed significantly. The spray campaign costs were broken down further using the following data and assumptions. Training costs were calculated from the average percentage spray campaign costs used for Malawi, Rwanda and Uganda for training of trainers and SOP and team leader training (data provided by PMI VectorLink). Spray campaign personnel costs were calculated from the total campaign days and the daily wages minus the training costs. The rest of the spray campaign costs were assigned to transportation of spray personnel (mainly vehicle hire, drivers and fuel).
The main results from this review were separated into two categories, (1) description of the resting sites of mosquitoes inside houses and (2) reports of experiments or operational pilots of selective spraying. Seventeen studies were found reporting the resting sites of mosquitoes in houses, and nine were found reporting on experiments or pilot studies of selective spraying.
Resting sites of mosquitoes in houses
Resting height.
The results collated from the reviewed publications showed clear evidence that the resting sites and behaviour of the mosquitoes vary. These variations were observed both between and occasionally within species. In many of the publications, the height (distance above the floor) at which mosquitoes were collected was reported.
Based on these data, it was determined that Anopheles darlingi , Anopheles aquasalis, Anopheles ludlowi, Anopheles hyrcanus, Anopheles fluviatilis, Anopheles leucosphyrus, Anopheles aconitus, Anopheles kochi, Anopheles subpictus, Anopheles indefinitus, Anopheles marajoara , Anopheles punctimacula, Anopheles nuneztovari , and Anopheles flavirostris tended to rest primarily on the lower half of walls [ 17 , 18 , 19 , 20 , 21 , 22 , 23 ].
In contrast, Anopheles barbirostris , Anopheles oswaldi , and Anopheles rangeli were found to rest above 1.5 m above the floor, and often higher [ 21 , 22 ]. Sahu et al. [ 24 ] found 99% of Anopheles minimus and Anopheles fluviatilis to rest on walls (as opposed to eaves, hanging objects, and the roof), with most of these mosquitoes resting between 90 and 125 cm from the ground.
It is important to note that most of these studies were conducted outside of Africa. Despite this, a few key studies based in Africa have investigated the resting behaviour of Anopheles gambiae sensu lato ( s.l. ) and Anopheles funestus vectors . These studies can largely be grouped into monitoring the height of the resting site on the wall or roof, additional observations about the substrate on which mosquitoes rest, and their resting behaviour conducted within experimental huts were also noted.
In his first study looking at the resting height of malarial vectors, Smith [ 25 ]investigated the distribution of An. gambiae and An. funestus vectors in cone huts on Ukara Island (a Tanzanian island in Lake Victoria, near Mwanza). These cone huts measured 6.4 m high and 6.9 m wide at their bases, and typically housed both humans and cattle. The huts were searched until all observable mosquitoes had been collected and their location of collection was recorded. From the trial it was shown that the vast majority of female mosquitoes (80% of An. gambiae and 79% of An. funestus ) were found to be resting below 2.1 m (from the floor) in the huts during the rainy season. The majority of these rested on the human-habited side of the huts; nevertheless, considerable numbers were also found on the cattle-habited side of the huts. The same trend was found during the dry season. Later, Smith [ 26 ] collected mosquitoes from houses of three different types ( tembe , msonge , and banda ) in Tanzania. Initial catches were conducted between 0800 and 1200 with additional complementary catches between 1100 and 1500 being conducted three days later. During the collection period, the proportion of An. gambiae mosquitoes resting on the roof ranged from 42 to 74%. There were no large differences between the proportions resting on the roof during the night and day, but there were differences in roof-resting between the different types of huts. Mathis et al. [ 27 ] reported 94.6% of An. gambiae and An. funestus were collected on the ceilings in monitored huts. On the contrary, Mutinga et al. [ 28 ] noted An. gambiae mosquitoes resting primarily on the lower parts of walls and the darker parts of the room. Osae [ 29 ] found large proportions of all three species resting above 2 m ( An. gambiae : 76%, Anopheles coluzzii 58%, An. funestus 74%), and preferably on dark materials in cool, humid areas. Sande et al. [ 30 ] found the highest proportion of An. funestus and An. gambiae on the roof (although considerable numbers were found on walls, with fewer mosquitoes collected on furniture. When only wall surfaces were considered, the majority were collected below 1 m (44% of An. funestus , 64% of An. gambiae s.l. ). Msugupakulya et al. [ 31 ] evaluated the resting sites of An. gambiae and An. funestus in different types of houses. They found that the highest numbers of mosquitoes rested on the roof in houses with thatched roofs (with the exception of An. funestus in brick houses), and in houses with metal roofs, the highest numbers of mosquitoes rested on surfaces other than walls or roofs. It is worth noting that in all types of houses, mosquitoes were found resting on walls, roofs, and other surfaces (Table 1 ).
Resting substrate
Other studies have looked at the effect of resting substrate or other factors on the resting behaviour of African malaria vectors. Smith [ 26 ] evaluated the impact of different factors within experimental huts to evaluate their impact on mosquito resting behaviour. He found that neither building a partition wall in the hut, modifying the hut entry site, adding a ceiling, modifying the surface of the roof, nor the abdominal status (or source of blood meal) appeared to change the resting behaviour of An. gambiae in terms of resting on the roof or walls. However, modifying the substrate of the walls (from smooth mud to rough mud) resulted in greater resting on rough mud walls. Similarly, making a fire inside the huts resulted in decreased resting on the roof and increased resting on walls. Beds were not a major resting site for mosquitoes in experimental huts, with only nine percent of mosquitoes collected from beds. Mutinga et al. [ 28 ] found that An. gambiae preferred to rest on fabric attached to the walls. Osae found differences in resting sites between An. gambiae , An. coluzzii , and An. funestus in Ghana [ 29 ]. He found the main resting sites to be roofing beams for An. gambiae (28%), on netting or frames of windows for An. coluzzii (20%), and for An. funestus, it was the roof. He also looked at the materials that mosquitoes were resting, with An. gambiae and An. funestus resting primarily on wood surfaces, and An. coluzzii resting on nylon.
Resting sites of mosquitoes in experimental huts
Finally, some studies taking place in experimental huts have monitored the resting behaviour prior to introducing interventions such as wall spraying. Smith [ 26 ] found higher proportions of An. gambiae resting on the roof in experimental huts than in other types of structures, with 94–97% of mosquitoes resting on roofs, compared with 42–74% in local houses. Coleman et al. [ 10 ] monitored the resting sites of An. gambiae s.l. collected in West African experimental huts in Ghana. The majority of An. gambiae s.l. were collected from the ceiling and the top half of the veranda. In a follow up study, Chabi et al. [ 32 ] found 43% of An. gambiae s.l. resting on the lower half of walls, 24% of mosquitoes resting on the top half of walls, and 33% of mosquitoes resting on ceiling.
Evaluation of selective spraying
In the first year of the “Sardinian Project” an attempt to eliminate Anopheles labranchiae from Sardinia, selective spraying was conducted with spraying of walls below 1.5 m in the first campaign (1946–1947), but in successive campaigns “full spraying” was conducted [ 33 ]. Malaria cases declined from 74,641 in the first year (1946) to 39,303 in the second [ 34 ], although the impact of selective spraying with DDT cannot be disentangled from the impact of large-scale aerial adulticide/larvicide application and source reduction that was carried out in parallel. This highlights the previous/historical use of selective IRS, however, no further details on impact of the intervention were provided in this source.
Another method of selective spraying was evaluated in Lebanon [ 35 ], where “band spraying” was attempted, spraying horizontal swaths of DDT of 30 cm width separated by an equal distance of unsprayed areas (all 1 m above the ground). The impact of this type of spraying was measured in areas where Anopheles sacharovi and Anopheles superpictus were the main vectors both by looking at malaria rates, and collection of Anopheles in houses in areas where full spraying or selective spraying had been conducted (relative to control areas). While no impact on parasite rates was found, due to a drop in cases in both control and treatment areas, there was a reduction in Anopheles in the full and selectively sprayed houses. The authors estimated the cost savings that might be found with selective spraying was approximately 31.3% (including the costs of DDT, labour, transport, and storage (Table 2 ).
Pletsch and Demos [ 36 ] reported “selective spraying” in Taiwan against Anopheles minimus . Full spraying was conducted by spraying walls, roofs, ceilings, and undersides of furniture with DDT (2 g/m 2 ). The inner walls and undersides of roofs of all outbuildings were also sprayed except for the first 50 cm of the wall in pig pens. “Selective spraying” was done in several ways; on the walls of bedrooms and storerooms, the underside of the roof in bedrooms, the ceilings in bedrooms and storerooms (which were quite rare), the undersides of furniture and window recesses in bedrooms, storerooms, sitting rooms, and kitchens (only inside and under the food cabinet), and the underside of the bed or bed platform in bedrooms. Any room in which people slept was considered a bedroom. The results from two rounds of both spray types were positive, reducing malaria rates from over 20% to less than 1% in Chi-Shan, and reducing them from about 2% to 0% in an additional study in central Taiwan. Both entomological investigations supported the finding of effective control and reduction of the numbers of mosquitoes collected in bedrooms to zero with both techniques. The cost savings were generated from spraying 38.4% less surface area in the selective spraying treatment, and the overall costs were reduced by 25.6%. However, some disadvantages of selective spraying were noted, specifically, the detection of An. minimus mosquitoes in cattle sheds (a possible harborage that could result in the build-up of resistance), the detection of Anopheles sinensis in cattle sheds that bothered the farmers’ water buffalo, and hesitation from homeowners and sprayers about receiving less than full coverage.
Gandahusada et al. [ 37 ] built on the knowledge about Anopheles aconitus resting sites to evaluate full and selective spraying in Java, Indonesia, using fenitrothion as An. aconitus populations were becoming resistant to DDT. They designed three areas for the study, one for full spraying, one for selective spraying (between 10 and 85 cm on the wall, in addition to full spraying of cattle shelters), and one for the control. Cholinesterase levels were monitored in the sprayers to prevent negative health effects from exposure to the insecticide. More sprayers in the full spray arm had > 50% reduction in cholinesterase than those in the selective spray arm, indicating less exposure for those conducting the selective spray. The full spray arm reduced malaria slide-positive rates from 6.5% to 0.4%, while selective spray reduced the rate from 1.9% to 0.3%. However, there was a more substantial decrease in the Plasmodium falciparum index (proportion of cases caused by P. falciparum ) in the full coverage area than in the selective spray area.
Asinas et al. [ 23 ] observed resting heights of Anopheles flavirostris in a site outside of Manila, Philippines. They found the vast majority resting below 1 m on the walls and evaluated the impact of selective spraying (the lower 70 cm of the wall, as well as 10 cm around windows and interior and exterior eaves) in experimental huts for 6 months. They found similar results for full spraying and selective spraying, with never more than an 8% difference in mosquito mortality between the two.
Arredondo Jiménez et al. [ 38 ] evaluated full spraying and selective spraying (a horizontal swath on the wall between 0.75 and 1.75 m from the floor, as well as a 1 m swath of the roof from where it met the wall) with bendiocarb in Mexico. They followed the community for two years (over four spray rounds) and measured the entomological impact. They did not note substantial differences between the fully sprayed and selectively sprayed areas in terms of residual activity of the insecticide, resting behaviour or mortality of An. albimanus mosquitoes, or human landing collections. They found a 50% savings in spraying time in the selective spray area and 40% overall cost savings.
Coleman et al. [ 10 ] conducted an experimental hut study coupled with a village-level study to evaluate selective spraying. The experimental hut study evaluated half walls (lower and upper) in combination with the ceiling with full spraying. There was no significant difference in mortality of An. gambiae s.l. found between full spraying and either of the selective spraying treatments. The inclusion of the ceiling appeared to be important, as the mortality was more than 20% higher when the ceiling was included in the treatment arms. There was also no significant difference between human biting rates between full and selectively sprayed communities (upper half + ceiling), and both were significantly lower than in unsprayed communities.
Chabi et al. [ 32 ] conducted an experimental hut trial in Côte d’Ivoire, in an area of intense pyrethroid resistance. Three IRS insecticides (pirimiphos methyl 1 g/m 2 (Actellic), clothianidin 300 mg/m 2 (SumiShield) and clothianidin 200 mg/m 2 + deltamethrin 25 mg/m 2 (Fludora Fusion)) were evaluated with four treatments (unsprayed, fully sprayed, bottom half of the wall + ceiling, upper half of wall + ceiling). For all three insecticides, there was slightly higher mortality with the bottom half of the wall + ceiling than the upper half of the wall + ceiling. The differences in mortality between full spray and the two selective spray treatments were not statistically significant except for clothianidin, where the top half + ceiling spray resulted in less mortality than the other two treatments.
Snetselaar et al. (pers. commun.) evaluated selective spraying and uneven spraying in release-recapture and experimental hut studies. In the release-recapture study, Anopheles gambiae Kisumu (susceptible to all insecticides tested) was released in huts with clothianidin 200 mg/m2 + deltamethrin 25 mg/m2 (Fludora Fusion) sprayed using a selective, checkerboard spray (50% of walls sprayed), uneven spray (some areas sprayed at 10%, others at 100%, and others at 190%), full spray (manual or with a track sprayer), as well as full spraying of pirimithos-methyl 1 g/m 2 (Actellic). Mortality (24 h) was not significantly different between any of the treatments. For the experimental hut trial with Anopheles arabiensis , the highest 24 h mortality was found with the track sprayer full spray, and the mortality was not significantly different between the other treatments.
IRS program cost analysis
The percentage break down of the PMI VectorLink IRS program costs are shown in Fig. 1 .

Percentage breakdown of the average PMI VectorLink IRS spray campaign programme costs (2018–2021)
For all of the publications where cost savings of IRS are reported, most authors have shown the data for reduction in insecticide use and spray team costs (mainly staff costs) (Table 2 ). Coleman et al. [ 10 ] have also extrapolated that a reduction in the spray time (due to decreased spraying and non-removal of items from the house) would also reduce the transportation costs by 26%, as the team could spray more houses in a day, requiring less travel to complete the same number of houses.
Using these assumptions, the IRS cost model was used to show the overall savings that could be achieved when the entire programme costs are included, such as administration, monitoring, entomology, and community engagement. An example of the inputs and outputs from the IRS cost model are shown in Fig. 2 for the results reported by Coleman et al. [ 10 ]. The overall savings when selective IRS was used ranged from 15.5 to 28.6% for programmes which targeted Anopheles mosquitoes and 17.5–41.1% for programs that targeted Ae. aegypti .

An example of the cost model inputs and outputs
Selective spraying has been repeatedly proposed as a solution to optimize the cost effectiveness and minimize the logistical challenges of IRS. Observations of patterns in the resting behaviour of mosquitoes have led to the conclusion that if preferred resting places are sprayed, then a comparable impact can be achieved with less (but more targeted) spraying. As several authors have noted, this depends on using a non-irritant insecticide to ensure that mosquitoes do not avoid sprayed areas [ 23 , 39 ].
From some of the operational pilots and experimental hut studies, it appears that selective spraying can result in comparable results at a reduced cost. Some studies noted epidemiological impacts at reduced costs [ 36 , 37 , 40 ], whereas other studies noted important entomological impacts [ 10 , 23 , 32 , 35 , 38 ]. In some cases, there appeared to be a slightly reduced effect or other disadvantages such as possible selection of resistance, slower rates of decrease in malaria rates, and reluctance from homeowners [ 36 , 37 ], whereas in other cases, there appeared to be advantages other than reduced costs, i.e. reduced insecticide exposure [ 37 ].
An essential part of selective spraying is the determination of what parts of houses should be sprayed and what parts of houses should not be sprayed. While in some cases, this decision has been informed by previous work, in other cases, the choice seems to be somewhat arbitrary. The two main factors that could inform selective spraying are logistical (i.e. making spraying houses easier and faster) or behavioural (using the behaviour of the mosquito to target the key resting spaces).
Aspects of spraying that would reduce the amount of spraying and logistical costs could include:
Spraying that can be done from outside houses (including eaves, animal shelters)
Spraying that does not require the movement of furniture (upper halves of walls, ceilings, undersides of furniture), which may additionally benefit from increased user uptake
Targeted spraying of houses (i.e., only spraying houses at the edges of a village, near breeding sites or houses with children under five years of age)
Selective spraying might be improved through improved monitoring of the resting behaviour of mosquitoes through careful recording of:
Rooms in which mosquitoes are resting (bedrooms, kitchens, bathrooms, animal shelters)
The height of resting sites on the wall
The type of building in which mosquitoes are resting
The amount of light (lux) present in resting site
Temperature and humidity of resting sites
Air movements
The substrate on which mosquitoes are resting (wood, mud, clothes, furniture)(see Table 3 in [ 41 ])
The interaction between an insecticide and a mosquito (toxicity and irritancy)
Resting behaviour related to seasonality [ 25 ]
Types of houses (wall substrate, roof material) [ 31 ]
Orientation (north, east, west, south) with respect to sun, climatic conditions
Resting behaviour of mosquitoes infected with Plasmodium parasites.
As seen above, the behaviour of mosquitoes (in combination with an understanding of logistical issues) is essential for understanding the optimal design of a selective spray programme. One of the challenges for understanding the resting behaviour of mosquitoes is the fact that mosquitoes may move around the inside of houses over the course of the night, but the collection of mosquitoes at dawn may only capture one aspect of this movement. Indeed, when mosquitoes have been collected at different times or monitored through observation, it has been shown that they are moving inside houses to some degree [ 26 , 42 ]. It is likely that mosquitoes balance the need for homeostasis (optimal temperature and humidity) [ 43 ] with a choice of colours and low light to be the least visible. Better methods for monitoring mosquitoes (video recording, motion sensing, collections at multiple times) may allow for better targeting of insecticides. Furthermore, when multiple vector species are present in the same location, the behaviour of both must be considered when targeting insecticide spray.
This improved monitoring of resting site behaviour would seem especially important for the major African malaria vectors, An. gambiae s.l. and An. funestus , as there appear to be contradictory findings in the literature. The earliest recording of resting heights found most An. gambiae and An. funestus to be resting on walls below 2.1 m; however, this was in “cone huts” that reached 6.4 m in height [ 25 ]. Mathis et al. [ 27 ] reported that 94.6% of An. gambiae and An. funestus collected in houses were resting on the ceiling. Mutinga et al. [ 28 ] stated that An. gambiae rested primarily on the lower parts of walls, on fabric, and on the dark side of the room. Osae [ 29 ] reported a number of resting sites for An. gambiae , An. coluzzii , and An. funestus . He stated that most of the An. gambiae (56%) and An. funestus (59%) were resting on roofs, roofing beams, and ceilings between 6:00 and 10:00, whereas only 25% of An. coluzzii were found there. Msugupakulya et al. [ 31 ] found very low numbers of An. funestus (16–20%) and An. arabiensis (8–30%) resting under metal roofs, although higher numbers of the two species when roofs were thatched ( An. funestus (33–55%), An. arabiensis (43–50%)). Importantly, they noted that considerable proportions of mosquitoes in all houses were resting on “other surfaces” than walls and roofs, presenting challenges for spraying (although the movement in houses is not to be forgotten). The two most recent experimental hut studies found different results in their pre-spray collections, with the majority of An. gambiae s.s. remaining in huts in northern Ghana being found on the ceiling (followed by the top half of the wall), whereas the An. coluzzii in Côte d’Ivoire were primarily resting on the bottom half of the wall (followed by the ceiling). The apparent difference in behaviour might explain why in Côte d’Ivoire, in huts treated with clothianidin, the bottom half + ceiling treatment was more effective than the top half + ceiling treatment. However, there is much to be learned about the behaviour of mosquitoes inside houses, and what to do when there are multiple vector species. A better understanding of this behaviour will allow the development of better selective spray methods.
The potential cost savings of selective IRS could be substantial; and reported savings in the literature range from 38 to 85% for insecticide use and 25.7–82% for spray team costs (wages and food). The level of cost reduction depends on the type of selective spraying employed. In some cases, the selective spraying was limited to a single band in houses [ 37 ], whereas in other studies, it was only half of the wall that was excluded [ 10 , 32 ]. Reductions in costs can come from reduced insecticide and reduced time required to treat houses, especially if furniture does not have to be removed, including spray pump refilling time and water collection. These time savings should be monitored in future studies.
However, these reported cost savings do not consider other costs typically associated with an IRS control programme, such as surveillance and monitoring, administrative staff, chemical storage, environmental assessment, equipment etc. To understand the impact of selective IRS on the total cost of an IRS programme, an IRS cost model was developed from cost analysis reports of PMI VectorLink country programmes. When considering other IRS programme costs and accounting for savings in transport costs not reported in some publications, the overall cost savings ranged from 15.5 to 28.6% for programmes targeting Anopheles mosquitoes .
These percentage cost savings could reduce the cost per person per year of a PMI VectorLink IRS programme from USD 7.46 (average for 2020–2022) to between USD 5.33 to USD 6.19. These represent substantial cost savings of between 17 and 29%. However, the cost of IRS programmes has substantially increased over the past five years, from USD 5.36 per person per year in 2018 to USD 7.69 in 2022, and therefore the impact is substantially reduced due to rising costs [ 44 ].
Not all IRS programmes are run as comprehensively as PMI VectorLink programmes, and they may not have all the additional costs besides transport, staff for spraying, and insecticide, which may significantly increase the relative cost advantage of selective IRS. There may be other control programmes with lower costs, but these were not identified in the current review.
A clear understanding of mosquito resting behaviour is key to the effectiveness of indoor residual spraying, one of the major malaria control interventions. Currently, indoor residual spraying is conducted by spraying all sprayable interior surfaces of a house to maximize the likelihood of a mosquito coming in contact with the insecticide. However, this may not be necessary if mosquitoes preferentially rest on certain surfaces of the house. This review aimed to assess the resting behaviour of Anopheles mosquitoes. There were no clear patterns for African malaria vectors, and standardized methods for monitoring resting behaviour are necessary before a spray campaign is implemented. The existing data on selective spraying indicate that this may be a promising way of controlling malaria, but further work is necessary. The overall impact of selective IRS on control programme costs could be substantial, reducing the total programme costs by up to 30–40%, which could help mitigate some of the increased programme costs incurred over the past few years and help maintain IRS coverage and impact. However, these cost reductions must also be carefully considered against the total cost of an IRS programme, not just the spraying operations and insecticide costs.
IRS is being phased out from an increasing number of countries due to its cost despite clear evidence of effectiveness for malaria control and insecticide resistance management. Several operational studies have indicated substantial decreases in malaria prevalence using selective spraying at a fraction of the cost of full spraying. Studies that evaluate the entomological and epidemiological impact of selective spraying with existing IRS compounds are urgently required to enable this method to be fully validated and, if successful, pass on these cost savings to help maintain this important vector control tool.
Availability of data and materials
There is no new data presented here, and all can be found in published articles. Excel files for the cost model are available upon request.
Abbreviations
Dichlorodiphenyltrichloroethane
- Indoor residual spraying
Insecticide-treated Net
Pan American health organization
World health organization
Presidents malaria initiative
WHO. World malaria report 2023. Geneva: World Health Organization; 2023.
Google Scholar
Mosha JF, Matowo NS, Kulkarni MA, Messenger LA, Lukole E, Mallya E, et al. Effectiveness of long-lasting insecticidal nets with pyriproxyfen-pyrethroid, chlorfenapyr-pyrethroid, or piperonyl butoxide-pyrethroid versus pyrethroid only against malaria in Tanzania: final-year results of a four-arm, single-blind, cluster-randomised trial. Lancet Infect Dis. 2024;24:87–97.
Article CAS PubMed Google Scholar
Protopopoff N, Mosha JF, Lukole E, Charlwood JD, Wright A, Mwalimu CD, et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomized controlled, two-by-two factorial design trial. Lancet. 2018;391:1577–88.
Article CAS PubMed PubMed Central Google Scholar
Oxborough RM. Trends in US President’s Malaria Initiative-funded indoor residual spray coverage and insecticide choice in sub-Saharan Africa (2008–2015): urgent need for affordable, long-lasting insecticides. Malar J. 2016;15:146.
Article PubMed PubMed Central Google Scholar
Yukich J, Digre P, Scates S, Boydens L, Obi E, Moran N, et al. Incremental cost and cost-effectiveness of the addition of indoor residual spraying with pirimiphos-methyl in sub-Saharan Africa versus standard malaria control: results of data collection and analysis in the Next Generation Indoor Residual Sprays (NgenIRS) project, an economic-evaluation. Malar J. 2022;21:185.
WHO. Global plan for insecticide resistance management in malaria vectors WHO/HTM/GMP/2012.5. Geneva: World Health Organization; 2012.
Monroe A, Moore S, Olapeju B, Merritt AP, Okumu F. Unlocking the human factor to increase effectiveness and sustainability of malaria vector control. Malar J. 2021;20:404.
Rehman AM, Coleman M, Schwabe C, Baltazar G, Matias A, Gomes IR, et al. How much does malaria control quality matter: the epidemiological impact of holed nets and inadequate indoor residual spraying. PLoS ONE. 2011;6: e19205.
Manrique-Saide P, Dean NE, Halloran ME, Longini IM, Collins MH, Waller LA, et al. The TIRS trial: protocol for a cluster randomized controlled trial assessing the efficacy of preventive targeted indoor residual spraying to reduce Aedes -borne viral illnesses in Merida. Mexico Trials. 2021;21:839.
Article Google Scholar
Coleman S, Yihdego Y, Sherrard-Smith E, Churcher TS, Dengela D, Oxborough RM, et al. Partial indoor residual spraying with pirimiphos-methyl as an effective and cost-saving measure for the control of Anopheles gambiae s.l. in northern Ghana. Sci Rep. 2021;11:18055.
Bath D, Cook J, Govere J, Mathebula P, Morris N, Hlongwana K, et al. Effectiveness and cost-effectiveness of reactive, targeted indoor residual spraying for malaria control in low-transmission settings: a cluster-randomised, non-inferiority trial in South Africa. Lancet. 2021;397:816–27.
WHO. Operational manual on indoor residual spraying. control of vectors of malaria, Aedes-borne diseases, chagas disease, leishmaniases and lymphatic filariasis. Geneva: World Health Organization; 2023.
Vazquez-Prokopec GM, Che-Mendoza A, Kirstein OD, Bibiano-Marin W, González-Olvera G, Medina-Barreiro A, et al. Preventive residual insecticide applications successfully controlled Aedes aegypti in Yucatan. Mexico Sci Rep. 2022;12:21998.
Kirstein OD, Culquichicon C, Che-Mendoza N-CJ, Wang J, Bibiano-Marin W, et al. Targeted indoor residual insecticide applications shift Aedes aegypti age structure and arbovirus transmission potential. Sci Rep. 2023;13:21271.
Pan American Health Organization. Manual for indoor residual spraying in urban areas for Aedes aegypti control. Washington DC. 2019 60pp.
Johns B, Sitruk AC. PMI IRS country programs: 2018 comparative cost analysis. Rockville, MD: PMI VectorLink Project, Abt Associates Inc; 2019.
Deane LM, Damasceno RG. Altura de pouso das fêmeas de Anopheles darlingi e de Anopheles aquasalis nas paredes internas das casas. Mem Inst Evandro Chagas : Parasitol. 1948;2:317–23.
Chow CY, Liang KC, Pletsch DJ. Observations on anopheline populations in human dwellings in southern Taiwan (Formosa). Indian J Malariol. 1951;5:569–77.
CAS PubMed Google Scholar
Russell PF, West LS, Manwell RD, MacDonald G. Practical malariology. 2nd ed. Oxford University Press: London; 1963.
Elliott R. The influence of vector behavior on malaria transmission. Am J Trop Med Hyg. 1972;21:755–63.
Damar T, Fleming GA, Gandahusada S, Bang YH. Nocturnal indoor resting heights of the malaria vector Anopheles aconitus and other anophelines (Diptera: Culicidae) in central java. Indonesia J Med Entomol. 1981;18:362–5.
Quiñones ML, Suarez MF. Indoor resting heights of some anophelines in Colombia. J Am Mosq Control Assoc. 1990;6:602–4.
PubMed Google Scholar
Asinas CY, Hugo CT, Boase CJ, Evans RG. Evaluation of selective spraying of bendiocarb (Ficam VC®) for the control of Anopheles flavirostris in the Philippines. J Am Mosq Control Assoc. 1994;10:496–500.
Sahu SS, Gunasekaran P, Vanamail P, Jambulingam P. Seasonal prevalence & resting behaviour of Anopheles minimus Theobald & An. fluviatilis James (Diptera: Culicidae) in east-central India. Indian J Med Res. 2011;133:655–61.
CAS PubMed PubMed Central Google Scholar
Smith A. The distribution and host choice of resting A. gambiae Giles and A. funestus Giles in cone huts on Ukara Island, Tanganyika. East Afr Med J. 1955;32:7–13.
Smith A. Studies on domestic habits of A. gambiae that affect its vulnerability to insecticides. Part I. resting places in huts. East Afr Med J. 1962;39:15–24.
Mathis W, Cloud A, Eyraud M, Miller S, Hamon J. Initial field studies in Upper Volta with dichlorvos residual fumigant as a malaria eradication technique. 2. entomological evaluation. Bull World Health Organ. 1963;29:237–41.
Mutinga MJ, Odhiambo TR, Kamau CC, Odulaja A, Amimo FA, Wachira DW. Choice of resting sites by Anopheles gambiae (Diptera: Culici) in Mwea rice irrigation scheme, Kirinyaga District. Kenya East Afr Med J. 1995;72:170–5.
Osae MY. Resting behaviour of endophilic anopheline vectors in three ecological zones of southern Ghana and its implications for the use of entomopathogenic fungi. PhD thesis. University of Ghana. 2014.
Sande S, Zimbe M, Chinwada P, Masendu HT, Makuwaza A. Insights into resting behavior of malaria vector mosquitoes in mutare and mutasa districts of Manicaland Province. Zimbabwe J Med Entomol. 2016;53:866–72.
Msugupakulya BJ, Kaindoa EW, Ngowo HS, Kahamba NF, Msaky DS, Matoke-Muhia D, et al. Preferred resting surfaces of dominant malaria vectors inside different house types in rural south-eastern Tanzania. Malar J. 2020;19:22.
Chabi J, Seyoum A, Edi C, Kouassi BL, Yihdego Y, Oxborough R, et al. Efficacy of partial spraying of SumiShield, Fludora Fusion and actellic against wild populations of Anopheles gambiae s.l. in experimental huts in Tiassale Côte d’Ivoire. Sci Rep. 2023;13:11364.
Logan JA. The sardinian project: an experiment in the eradication of an indigenous malarious vector. Baltimore: The Johns Hopkins Press; 1953.
Tognotti E. Program to eradicate malaria in Sardinia, 1946–1950. Emerg Infect Dis. 2009;15:1460–6.
Gramiccia G, Garrett-Jones C, El Din SG. Band spraying: an experiment on cheaper residual spraying in malaria control. J Med Liban. 1953;6:245–56.
Pletsch DJ, Demos EA. Selective spraying of premises in the control of minimus-transmitted malaria in Taiwan. Geneva: World Health Organization; 1954.
Gandahusada S, Fleming GA, Sukamto DT, Suwarto SN, et al. Malaria control with residual fenitrothion in Central Java, Indonesia: an operational-scale trial using both full and selective coverage treatments. Bull World Health Organ. 1984;62:783–94.
Arredondo-Jiménez JI, Bown DN, Rodríguez MH, Loyola EG. Control of Anopheles albimanus mosquitos in southern Mexico by spraying their preferred indoor resting sites. Bull World Health Organ. 1995;73:329–37.
PubMed PubMed Central Google Scholar
Byford RL, Lockwood JA, Smith SM, Sparks TC, Luther DG. Insecticide mixtures as an approach to the management of pyrethroid-resistance horn flies. J Econ Entomol. 1987;80:111–6.
Lassen K, Liu SY, Lizarzaburu C, Ríos R. Preliminary report on the effect of selective application of propoxur on indoor surfaces in El Salvador. Am J Trop Med Hyg. 1972;21:813–8.
Urbino CM, Digma F, Abinoja B. Behavior pattern of adults of Anopheles minimus flavirostris Ludlow. Philippine J Sci. 1961;90:371–6.
Bown DN, Rios JR, del Angel CG, Guerrero JC, Méndez JF. Evaluation of chlorphoxim used against Anopheles albimanus on the south coast of Mexico: 1. results of indoor chlorphoxim applications and assessment of the methodology employed. Bull Pan Am Health Organ. 1984;18:379–88.
Verhulst NO, Brendle A, Blanckenhorn WU, Mathis A. Thermal preferences of subtropical Aedes aegypti and temperate Ae. japonicus mosquitoes. J Therm Biol. 2020;91:102637.
Article PubMed Google Scholar
Aghajanyan A, Riley M, Tesso E, Won N, Dawadi S, Longman B. PMI IRS Country Programs: 2022 Comparative Cost Analysis. Rockville, MD. PMI VectorLink Project, Abt Associates Inc.
Dunbar MW, Correa-Morales F, Dzul-Manzanilla F, Medina-Barreiro A, Bibiano-Marín W, Morales-Ríos E, et al. Efficacy of novel indoor residual spraying method targeting pyrethroid-resistant Aedes aegypti within experimental houses. PLoS Negl Trop Dis. 2019;13: e0007203.
Download references
Acknowledgements
Natalie Lissenden (IVCC) is kindly thanked for her review of the manuscript.
This publication is based on research funded by IVCC, which receives the generous support of the American people through the United States Agency for International Development (USAID), the Bill & Melinda Gates Foundation, the Swiss Agency for Development and Cooperation (SDC) and UK International Development funds from the UK government. The contents, findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of USAID, the Bill & Melinda Gates Foundation, the United States Government, the UK Government, nor SDC.
Author information
Authors and affiliations.
Swiss Tropical and Public Health Institute, Kreuzstrasse 2, 4123, Allschwil, Switzerland
Seth R. Irish, Frederic Tripet, Pie Müller & Sarah J. Moore
University of Basel, Petersplatz 1, 4001, Basel, Switzerland
Vector Control Product Testing Unit, Ifakara Health Institute, P.O. Box 74, Bagamoyo, Tanzania
Seth R. Irish & Sarah J. Moore
IVCC, Liverpool School of Tropical Medicine, Pembroke Place, Liverpool, L3 5QA, UK
Derric Nimmo & Jameel Bharmel
Unidad Colaborativa para Bioensayos Entomológicos, Campus de Ciencias Biológicas y Agropecuarias, Universidad Autónoma de Yucatán, Mérida, Yucatán, México
Pablo Manrique-Saide
Nelson Mandela African Institute of Science and Technology (NM-AIST), P.O. Box 447, Arusha, Tanzania
Sarah J. Moore
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: SRI, SJM. Methodology: SRI, SJM, DN (cost model). Literature review: SRI. Analysis: SRI. Writing—original draft: SRI. Development of cost model and writing: DN. Writing—review and editing: SRI, DN, JB, FT, PM, SJM. All authors read and reviewed the final version.
Corresponding authors
Correspondence to Seth R. Irish or Derric Nimmo .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Irish, S.R., Nimmo, D., Bharmel, J. et al. A review of selective indoor residual spraying for malaria control. Malar J 23 , 252 (2024). https://doi.org/10.1186/s12936-024-05053-3
Download citation
Received : 07 April 2024
Accepted : 20 July 2024
Published : 23 August 2024
DOI : https://doi.org/10.1186/s12936-024-05053-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Selective spraying
- Partial spraying
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
World Mosquito Day 2024—The Metabolic Mysteries of Mosquito Metabolism
NIAID Now | August 20, 2024

An Aedes mosquito, similar to those studied by Dr. Patricia Scaraffia.
Mosquitoes are considered one of the most dangerous animals on earth because of their broad distribution and the many pathogens they transmit to humans. Some of the most important human diseases in tropical and temperate regions of the planet are caused by mosquito-borne pathogens. Malaria, dengue, and filariasis, among other mosquito-borne diseases, kill or sicken millions of people worldwide every year.
Mosquito-borne pathogens are transmitted to the vertebrate host, such as a human, when the mosquito bites the host in search of blood. The proteins found in blood are essential for female mosquitoes: without it, they lack the resources to create eggs. Greater knowledge of the biological processes involved in the mosquito life cycle could lead to new or improved strategies to control mosquito populations.
Dr. Patricia Scaraffia, Associate Professor at the Tulane University School of Public Health and Tropical Medicine, has dedicated her career to understanding the metabolism of the mosquito Aedes aegypti that carries the pathogens responsible for dengue, Zika, chikungunya, and yellow fever to humans. NIAID reached out to Dr. Scaraffia about her team’s research.
What got you interested in studying mosquito metabolism?
I have studied the metabolism of insects that are vectors of pathogens causing human diseases since I was a graduate student at the Universidad Nacional de Cordoba, in Argentina. My Ph.D. dissertation was focused on the energy metabolism in Triatomine insects, vectors of Trypanosoma cruzi , the etiological agent of Chagas´ disease. After my dissertation, I participated as a speaker in a two-week course for PhD students entitled Biochemistry and molecular biology of insects of importance for public health . During the course, Argentinian professors encouraged me to contact the late Dr. Michael A. Wells, a leader in insect metabolism, and apply for a postdoctoral training in his lab. Soon after, I joined Dr. Wells´s lab at the University of Arizona as a research associate and opened a new line of investigation in his lab. Since then, I have never stopped working on A. aegypti mosquito metabolism. I am passionate and curious about the tremendous complexity of mosquito metabolism. It is a fascinating puzzle to work on. It constantly challenges me and my research team to think outside the box when trying to decipher the unknowns related to mosquito metabolism.

Dr. Patricia Scaraffia's work focuses on the secrets of mosquito metabolism.
What are the metabolic challenges faced by mosquitoes after feeding on blood?
Female mosquitoes are a very captivating biological system. It is during blood feeding that female mosquitoes can transmit dangerous, and sometimes lethal, pathogens to humans. Interestingly, the blood that the females take could be twice their body weight, which is impressive. Female mosquitoes have evolved efficient mechanisms to digest blood meals, eliminate excess water, absorb and transport nutrients, synthesize new molecules, metabolize excess nitrogen, remove nitrogen waste, and successfully lay eggs within 72 hours! Despite significant progress in understanding how females overcome these metabolic challenges, we have not yet fully elucidated the intricate metabolic pathways, networks, and signaling cascades, nor the molecular and biochemical bases underlying the multiple regulatory mechanisms that may exist in blood-fed female mosquitoes.
What are the greatest potential benefits of understanding mosquito metabolism?
Metabolism is a complicated process that involves the entire set of chemical transformations present in an organism. A metabolic challenge faced by mosquitoes is how to break down ammonia that results from digesting a blood meal and is toxic to the mosquito. With NIAID support, we found that in the absence of a functional metabolic cycle to detoxify ammonia, A. aegypti mosquitoes use specific metabolic pathways that were believed to be non-existent in insects. This discovery has opened a new field of study.
A better understanding of mosquito metabolism and its mechanisms of regulation in A. aegypti and other mosquito species could lead us to the discovery of common and novel metabolic targets and/or metabolic regulators. It would also provide a strong foundation for the development and implementation of more effective biological, chemical and/or genetic strategies to control mosquito populations around the world.
What are the biggest challenges to studying mosquito metabolism?
We have often observed that genetic silencing or knockdown—a technique to prevent or reduce gene expression—of one or more genes encoding specific proteins involved in mosquito nitrogen metabolism results in a variety of unpredictable phenotypes based on our knowledge of vertebrate nitrogen metabolism. Notably, female mosquitoes get control of the deficiency of certain key proteins by downregulating or upregulating one or multiple metabolic pathways simultaneously and at a very high speed. This highlights the tremendous adaptive capacity of blood-fed mosquitoes to avoid deleterious effects and survive.
We have been collaborating closely with scientists that work at the University of Texas MD Anderson Cancer Center Metabolomics Core Facility, and more recently, with bioanalytical chemists that work in the Microbiome Center’s Metabolomics and Proteomics Mass Spectrometry Laboratory in Texas Children’s Hospital in Houston. Our projects are not turn-key type of projects with quick turn-round times. We have to invest considerable time and effort to successfully develop and/or optimize methods before analyzing mosquito samples. Despite these challenges, our research work keeps motivating us to unlock the metabolic mysteries that female mosquitoes hold.
Your research has focused on Aedes aegypti , the main vector of dengue, Zika, etc. Why did you choose to study this mosquito species rather than others that are also important vectors of malaria and other diseases?
My research has focused on Aedes aegypti not only because it is a vector of pathogens that pose public health threats, but also because it is genetically one of the best-characterized insect species. The availability of the Aedes aegypti genome is a great resource for a wide range of investigations. In addition, Aedes aegypti is relatively simple to rear and maintain in the lab. In my lab, we are interested in expanding our metabolic studies to other mosquito species by working in collaboration with scientists with expertise in the biology of different vectors.
What important questions remain unanswered about mosquito metabolism?
Many important questions remain unanswered about mosquito metabolism. I’d like to highlight a few of them that may help us enhance our knowledge of the mosquito as a whole organism rather than as a linear sum of its parts. For example, what are the genetic and biochemical mechanisms that drive metabolic fluxes in mosquitoes in response to internal or external alterations? How do key proteins interact with each other, and how are they post-translationally regulated to maintain mosquito metabolism? How are the metabolic networks regulated in noninfected and pathogen-infected mosquitoes? What are the critical regulatory points within the mosquito metabolism and the vector-host-pathogen interface?
While basic science will continue to be crucial in answering these questions, to successfully fight against mosquitoes, we must work together as part of a multidisciplinary team of scientists to tightly coordinate our efforts and close the gap between basic and applied science.
Contact Information
Contact the NIAID Media Team.
301-402-1663 [email protected]
Search NIAID Blog
Stay connected.
- Subscribe to NIAID Now email updates

COMMENTS
cases and 438,000 deaths due to malaria, with 88% of the cases and 90% of the deaths occurring in sub-Saharan Africa [1]. The vast majority of deaths are among children 0-5 years of age, and these deaths are predominantly caused by P. falciparum malaria parasites [1]. It is estimated that
ii Dissertation Abstract Background: Recently, malaria has become a major global health priority.As a result there has been renewed interest in malaria control, elimination, and eradication. Zambia is one of the Elimination 8 countries and one of the President's Malaria Initiative focus
Mr AB Mapossa. PhD, Chemical Engineering. Thesis. Slow-release of mosquito repellents from microporous polyolefin strands. Prof WW Focke. Mr M Mpofu. PhD, Environmental Health. Thesis. Effectiveness of community larval source management (LSM) as an additional vector control intervention for malaria elimination.
Public health challenges facing malaria elimination in developing countries: a review of expert opinions Simon Manana HEL-3950 Master's thesis in Public Health August 2016 Supervisor: Ranjan Parajuli, PhD . iii Acknowledgement Let me convey my deepest gratitude to my Advisor, Dr.Ranjan Parajuli; for his inspiration,
Abstract and Figures. SUPERVISOR BY: Dr. HAMZE ALI ABDILLAHI. .5 your occupations. 13 IS malaria a common disease in your community. 19 shows the 52% answer to yes ,33.8% n0 and 13.8%l don't know ...
iv | P a g e malaria vectors over 15 months in 12 villages. Overall, the MET caught proportionately fewer An. gambiae s.l. than the HLC (mean estimated number of 0.78 versus 1.82 indoors, and 1.05 versus 2.04 outdoors). However provided a consistent representation of vector species composition, seasonal
PhD dissertation - Barriers to malaria prevention and chemoprophylaxis use among travelers who visit friends and relatives in Sub-Saharan Africa: A cross-sectional, multi-setting survey addressing ...
inexpensive way to potentially increase the sensitivity of the malaria RDT for diagnosing asymptomatic, subclinical malaria that solely involves the simple addition of the amino acid histidine. _____ Thesis Advisory Committee _____ Dr. David Sullivan Professor & Thesis Advisor Molecular Microbiology and Immunology Dr. William Moss
This dissertation addresses three such challenges. First, I focus on the ecology that serves as a backdrop to transmission, and focus on the role agriculture may play. In doing so, I attempt to understand how agriculture affects both mosquito behavior, as well as malaria risk in under-5 children in the Democratic Republic of Congo (DRC), a ...
during initial scale-up efforts of malaria control interventions which started around early 2000. This is also in line with the country's vision of achieving 'a malaria-free Zambia by 2030' (Ministry of Health [MOH], 2013). Data from 2001 to 2008 shows that there have been significant reductions in the malaria
climate parameters and the occurrence of malaria using both mathemati- cal and computational methods. In this respect, we develop new climate- based models using mathematical, agent-based and data-driven modelling techniques. A malaria model is developed using mathematical modelling to investigate the impact of temperature-dependent delays.
Resistance to antimalarial drugs inevitably follows their deployment in malaria endemic parts of the world. For instance, current malaria control efforts which significantly rely on artemisinin combination therapies (ACTs) are being threatened by the emergence of resistance to artemisinins and ACTs. ... PhD thesis, University of Glasgow. Full ...
iron adequacy beyond some threshold may increase malaria risk. Our results also suggest that the concurrent assessment of malaria, in addition to inflammation, may enhance the interpretation of retinol, ferritin and sTfR in endemic regions. DISSERTATION COMMITTEE ADVISOR: Christian L. Coles, PhD Assistant Professor, International Health READERS:
Malaria and dengue co-infection is a relatively common event. ... As part of her PhD thesis, BMLM dedicates this manuscript to her son, Eduardo Magalhães Valentin. The authors would like to thank the staff of Fundação de Medicina Tropical Doutor Heitor Vieira Dourado; ...
DOI. Identification and characterization of microRNAs expresse d in the African malaria ve ctor Anopheles funestus life stages using high throughput se quencing Author. Allam, Mushal, Spillings, Belinda L, Abdalla, Hiba, Mapiye, Darlington, Koekemoer, Lizette L, Christoffels, Alan. Published. 2016.
1. Introduction. Malaria remains a leading cause of death in the sub-Saharan Africa despite efforts to control it at vectoral and parasitic levels (World Health Organization [WHO], Citation 2016).The problems have been attributed to insecticide and drug resistance genes in the vector and in the parasite, which have proved very difficult to tackle over the years.
Timothy Geary (Supervisor1) Abstract. English. SUMMARYIntroduction: The latest World Malaria Report released in November 2017 estimated that 219 million cases of malaria occurred and deaths due to malaria reached 435,000 in 2017 (1). The WHO considers microscopy to be the gold standard for clinical diagnosis of malaria due to its ready ...
Additionally, studying the efficacy of the drugs used to treat malaria will preserve the ability for malaria cases to be treated successfully. The three studies in this dissertation describe the epidemiology of malaria and co-endemic diseases of public health importance in Mozambique and evaluate the efficacy of medicines used to treat malaria ...
Malaria is an acute febrile illness caused by the Plasmodium parasites, transmitted from person to person by the bites of infected female Anopheles mosquitoes. (World Health Organization [WHO], 2016). Five parasite species are responsible for malaria infection in humans, with two of these (P. falciparum and P. vivax) posing the biggest threat.
The symptoms of malaria include periodic bouts of fever, chills, sweating and rigors, which occur every 2 to 3 days depending on the Plasmodium species. The classic malaria triad is fever, splenomegaly and anemia. Patients often have constitutional symptoms of headaches, nausea, body aches and weakness.
Malaria research is of a great contribution to the socio-economic improvement of Africa since the groups most vulnerable to this disease are of great economic importance to the development of Africa. 1.1.2 Malaria transmission. Malaria is caused by protozoan parasite belonging to genus Plasmodium and transmitted by female Anopheles mosquito [4].
While insecticide resistance in malaria vectors has been widely investigated, less is known about the implications of mosquito behavioural changes to malaria control. In recent years, LLIN programmes appear to have a reducing impact in a small number of high burden African countries including Burkina Faso. ... PhD thesis, University of Glasgow ...
This Dissertation is brought to you for free and open access by the Walden Dissertations and Doctoral Studies Collection at ScholarWorks. It has been ... Malaria is one of the leading causes of death for children and women in Sierra Leone. ... aside for Walden PhD graduates. A big thanks to Walden University for encouraging
Indoor residual spraying (IRS) is one of the most effective malaria control tools. However, its application has become limited to specific contexts due to the increased costs of IRS products and implementation programmes. Selective spraying—selective spray targeted to particular areas/surfaces of dwellings—has been proposed to maintain the malaria control and resistance-management benefits ...
Malaria, dengue, and filariasis, among other mosquito-borne diseases, kill or sicken millions of people worldwide every year. ... After my dissertation, I participated as a speaker in a two-week course for PhD students entitled Biochemistry and molecular biology of insects of importance for public health. During the course, Argentinian ...