Suggestions or feedback?

MIT News | Massachusetts Institute of Technology
- Machine learning
- Sustainability
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
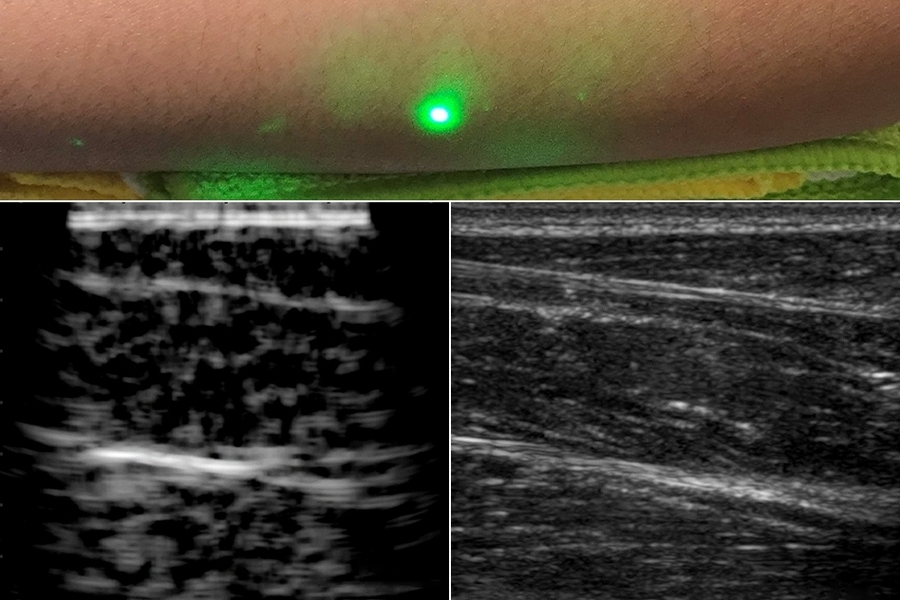
Researchers produce first laser ultrasound images of humans
Press contact :.
Previous image Next image
For most people, getting an ultrasound is a relatively easy procedure: As a technician gently presses a probe against a patient’s skin, sound waves generated by the probe travel through the skin, bouncing off muscle, fat, and other soft tissues before reflecting back to the probe, which detects and translates the waves into an image of what lies beneath.
Conventional ultrasound doesn’t expose patients to harmful radiation as X-ray and CT scanners do, and it’s generally noninvasive. But it does require contact with a patient’s body, and as such, may be limiting in situations where clinicians might want to image patients who don’t tolerate the probe well, such as babies, burn victims, or other patients with sensitive skin. Furthermore, ultrasound probe contact induces significant image variability, which is a major challenge in modern ultrasound imaging.
Now, MIT engineers have come up with an alternative to conventional ultrasound that doesn’t require contact with the body to see inside a patient. The new laser ultrasound technique leverages an eye- and skin-safe laser system to remotely image the inside of a person. When trained on a patient’s skin, one laser remotely generates sound waves that bounce through the body. A second laser remotely detects the reflected waves, which researchers then translate into an image similar to conventional ultrasound.
In a paper published today by Nature in the journal Light: Science and Applications , the team reports generating the first laser ultrasound images in humans. The researchers scanned the forearms of several volunteers and observed common tissue features such as muscle, fat, and bone, down to about 6 centimeters below the skin. These images, comparable to conventional ultrasound, were produced using remote lasers focused on a volunteer from half a meter away.
“We’re at the beginning of what we could do with laser ultrasound,” says Brian W. Anthony, a principal research scientist in MIT’s Department of Mechanical Engineering and Institute for Medical Engineering and Science (IMES), a senior author on the paper. “Imagine we get to a point where we can do everything ultrasound can do now, but at a distance. This gives you a whole new way of seeing organs inside the body and determining properties of deep tissue, without making contact with the patient.”
Early concepts for noncontact laser ultrasound for medical imaging originated from a Lincoln Laboratory program established by Rob Haupt of the Active Optical Systems Group and Chuck Wynn of the Advanced Capabilities and Technologies Group, who are co-authors on the new paper along with Matthew Johnson. From there, the research grew via collaboration with Anthony and his students, Xiang (Shawn) Zhang, who is now an MIT postdoc and is the paper’s first author, and recent doctoral graduate Jonathan Fincke, who is also a co-author. The project combined the Lincoln Laboratory researchers’ expertise in laser and optical systems with the Anthony group's experience with advanced ultrasound systems and medical image reconstruction.
Yelling into a canyon — with a flashlight
In recent years, researchers have explored laser-based methods in ultrasound excitation in a field known as photoacoustics. Instead of directly sending sound waves into the body, the idea is to send in light, in the form of a pulsed laser tuned at a particular wavelength, that penetrates the skin and is absorbed by blood vessels.
The blood vessels rapidly expand and relax — instantly heated by a laser pulse then rapidly cooled by the body back to their original size — only to be struck again by another light pulse. The resulting mechanical vibrations generate sound waves that travel back up, where they can be detected by transducers placed on the skin and translated into a photoacoustic image.
While photoacoustics uses lasers to remotely probe internal structures, the technique still requires a detector in direct contact with the body in order to pick up the sound waves. What’s more, light can only travel a short distance into the skin before fading away. As a result, other researchers have used photoacoustics to image blood vessels just beneath the skin, but not much deeper.
Since sound waves travel further into the body than light, Zhang, Anthony, and their colleagues looked for a way to convert a laser beam’s light into sound waves at the surface of the skin, in order to image deeper in the body.
Based on their research, the team selected 1,550-nanometer lasers, a wavelength which is highly absorbed by water (and is eye- and skin-safe with a large safety margin). As skin is essentially composed of water, the team reasoned that it should efficiently absorb this light, and heat up and expand in response. As it oscillates back to its normal state, the skin itself should produce sound waves that propagate through the body.
The researchers tested this idea with a laser setup, using one pulsed laser set at 1,550 nanometers to generate sound waves, and a second continuous laser, tuned to the same wavelength, to remotely detect reflected sound waves. This second laser is a sensitive motion detector that measures vibrations on the skin surface caused by the sound waves bouncing off muscle, fat, and other tissues. Skin surface motion, generated by the reflected sound waves, causes a change in the laser’s frequency, which can be measured. By mechanically scanning the lasers over the body, scientists can acquire data at different locations and generate an image of the region.
“It’s like we’re constantly yelling into the Grand Canyon while walking along the wall and listening at different locations,” Anthony says. “That then gives you enough data to figure out the geometry of all the things inside that the waves bounced against — and the yelling is done with a flashlight.”
In-home imaging
The researchers first used the new setup to image metal objects embedded in a gelatin mold roughly resembling skin’s water content. They imaged the same gelatin using a commercial ultrasound probe and found both images were encouragingly similar. They moved on to image excised animal tissue — in this case, pig skin — where they found laser ultrasound could distinguish subtler features, such as the boundary between muscle, fat, and bone.
Finally, the team carried out the first laser ultrasound experiments in humans, using a protocol that was approved by the MIT Committee on the Use of Humans as Experimental Subjects. After scanning the forearms of several healthy volunteers, the researchers produced the first fully noncontact laser ultrasound images of a human. The fat, muscle, and tissue boundaries are clearly visible and comparable to images generated using commercial, contact-based ultrasound probes.
The researchers plan to improve their technique, and they are looking for ways to boost the system’s performance to resolve fine features in the tissue. They are also looking to hone the detection laser’s capabilities. Further down the road, they hope to miniaturize the laser setup, so that laser ultrasound might one day be deployed as a portable device.
“I can imagine a scenario where you’re able to do this in the home,” Anthony says. “When I get up in the morning, I can get an image of my thyroid or arteries, and can have in-home physiological imaging inside of my body. You could imagine deploying this in the ambient environment to get an understanding of your internal state.”
This research was supported in part by the MIT Lincoln Laboratory Biomedical Line Program for the United States Air Force and by the U.S. Army Medical Research and Material Command's Military Operational Medicine Research Program.
Share this news article on:
Press mentions.
MIT researchers have created a “new laser ultrasound technique [that] utilizes an eye and skin safe laser system to image the inside of a person remotely,” reports Jennifer Kite-Powell for Forbes .
MIT researchers have developed a new non-invasive, hands-off medical imaging technique, reports Andrew Liszewski for Gizmodo . “Using lasers, they can peer beneath the surface of the skin without any physical contact required, improving upon the limitations of equipment like ultrasound machines,” Liszewski explains.
Previous item Next item
Related Links
- Paper: "Full noncontact laser ultrasound: first human data"
- Brian W. Anthony
- Institute for Medical Engineering and Science
- Department of Mechanical Engineering
Related Topics
- Institute for Medical Engineering and Science (IMES)
- Mechanical engineering
- Health sciences and technology
- Medical devices
Related Articles

3 Questions: Why sensing, why now, what next?

Fast-tracking medical device development
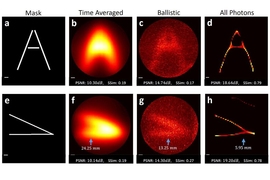
Toward visible-light-based imaging for medical devices, autonomous vehicles
Giving everyone a window into the human body
More mit news.

Pursuing the secrets of a stealthy parasite
Read full story →

Study of disordered rock salts leads to battery breakthrough
Toward a code-breaking quantum computer

Uphill battles: Across the country in 75 days

3 Questions: From the bench to the battlefield

Duane Boning named vice provost for international activities
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih research matters.
August 16, 2022
Ultrasound imaging gets small and wearable
At a glance.
- Scientists created a wearable ultrasound device—about the size of a postage stamp—that can continuously image inside the body for at least two days.
- With further development, wearable ultrasound devices can provide long-term visuals of internal tissues in a variety of settings.

Ultrasound is a noninvasive technique that lets clinicians peer inside the body to monitor health or diagnose disease. Imaging sessions are generally brief because ultrasound often requires the expertise of trained technicians working in medical settings.
Several research groups have been seeking more versatile approaches that would allow longer-term ultrasound monitoring in a variety of settings via wearable devices. To date, most of these efforts have provided relatively low-resolution images or are unable to visualize deep tissues or organs.
Now, an NIH-funded research team led by Dr. Xuanhe Zhao at the Massachusetts Institute of Technology has developed a new type of wearable ultrasound patch that overcomes many of the limitations of earlier approaches. This multi-layered device is about the size of a thick postage stamp, and it adheres to skin in both wet and dry environments. The device was described in Science on July 29, 2022.
Ultrasound works by first placing a probe, or transducer, on the body. The transducer emits high-frequency sound waves that enter the body and bounce off internal tissues, creating echoes that are captured and transmitted to instruments that translate the data into pictures or videos. A soft gel applied between the skin and probe helps to enhance soundwave transmission.
The patch created by Zhao’s team used several advanced techniques to combine all of these ultrasound components in a miniature package. A thin, rigid array of ultrasound probes sits atop a tough but flexible hydrogel layer. An elastomer membrane protects the hydrogel from drying out, and a bioadhesive binds the probe strongly to skin. The combination of a rigid probe array and flexible hydrogel-elastomer layers enables more stable and higher-resolution imaging than other wearable ultrasound devices that are thin and stretchy.
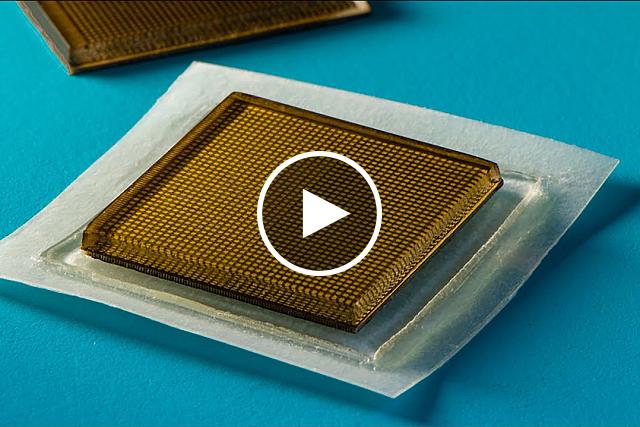
New stamp-sized ultrasound adhesives produce clear images of heart, lungs, and other internal organs. MIT
The researchers tested the patch on 15 human volunteers. They showed that the device could be comfortably worn for at least 48 hours. Depending on placement, the patch could provide continuous imaging of blood vessels, heart, muscle, diaphragm, stomach, or lung. The heart or lungs could be stably and continuously imaged even while volunteers were jogging or cycling.
Despite the patch’s potential for on-the-fly mobile imaging, the device currently must be hooked to computer systems for intensive data processing. But Zhao and his team foresee future possibilities:
“We envision a few patches adhered to different locations on the body, and the patches would communicate with your cellphone, where AI algorithms would analyze the images on demand,” Zhao says. “We believe this represents a breakthrough in wearable devices and medical imaging.”
—by Vicki Contie
Related Links
- Wearable Ultrasound Patch Tracks Blood Pressure
- System Reveals 3D Details of Living Tissues
- Monitoring Bacteria in the Body with Ultrasound
- Medical Scans Explained
References: Bioadhesive ultrasound for long-term continuous imaging of diverse organs . Wang C, Chen X, Wang L, Makihata M, Liu H-C, Zhou T, Zhao X. Science . 2022 Jul 29;377(6605):517-523. doi: 10.1126/science.abo2542. Epub 2022 Jul 28. PMID: 35901155.
Funding: NIH’s National Heart, Lung, and Blood Institute (NHLBI); Defense Advanced Research Projects Agency; National Science Foundation; US Army Research Office.
Connect with Us
- More Social Media from NIH
Maintenance work is planned from 21:00 BST on Tuesday 20th August 2024 to 21:00 BST on Wednesday 21st August 2024, and on Thursday 29th August 2024 from 11:00 to 12:00 BST.
During this time the performance of our website may be affected - searches may run slowly, some pages may be temporarily unavailable, and you may be unable to log in or to access content. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.

Materials Advances
Recent trends of contrast agents in ultrasound imaging: a review of the classifications and applications.

* Corresponding authors
a Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences, Tabriz, Iran E-mail: [email protected] Tel: +98 41 33367914
b Department of Medical Physics and Biomedical Engineering, Tehran University of Medical Sciences, Tehran, Iran E-mail: [email protected]
c Research Center for Molecular and Cellular Imaging, Advanced Medical Technologies and Equipment Institute, Tehran University of Medical Sciences, Tehran, Iran
d Department of Anesthesiology, University at Buffalo, Jacobs School of Medicine and Biomedical Sciences, Buffalo, New York, USA
Ultrasound (US) imaging, due to its capabilities of real-time imaging, portability, low cost and favorable safety, is frequently used as a diagnostic modality for the visualization of different diseases. US imaging is currently the first step in estimating the severity of oncological diseases, cardiovascular conditions, and for accurate assessment and diagnosis. Novel contrast agents have propelled US imaging into a new realm in the cellular and molecular fields and improved its sensitivity and specificity for detecting earlier stages of diseases. Selecting nanoparticles with appropriate structure and performance and a promising feature of binding to the target is a powerful strategy for the targeted imaging and early detection of disease. Here, we update the classification of the most attractive ultrasound contrast agents (USCAs), especially with regards to their advantages and disadvantages for application in US imaging. We also discuss how various technical detection modes of ultrasound imaging and quantitative analysis are affected by disease diagnosis. The clinical translations of US diagnostic strategies have prompted us to explore nanoparticle-based USCAs against various diseases. We also looked into the applications of USCAs in the diagnosis of cardiovascular disorders and oncological diseases based on anatomical section classification.

- This article is part of the themed collection: Recent Review Articles
Article information
Download Citation
Permissions.
A. Tarighatnia, M. R. Fouladi, N. D. Nader, A. Aghanejad and H. Ghadiri, Mater. Adv. , 2022, 3 , 3726 DOI: 10.1039/D1MA00969A
This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence . You can use material from this article in other publications, without requesting further permission from the RSC, provided that the correct acknowledgement is given and it is not used for commercial purposes.
To request permission to reproduce material from this article in a commercial publication , please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party commercial publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author, advertisements.
A wearable ultrasound scanner could detect breast cancer earlier
Canan Dagdeviren
via MIT News
July 28, 2023
- #civic technology
- #technology
- #healthcare
- #biomedical imaging
- Canan Dagdeviren Associate Professor of Media Arts and Sciences; LG Career Development Professor of Media Arts and Sciences
- Wenya Du Research Assistant
- Lin Zhang Former Research Scientist
- Conformable Ultrasound Breast Patch (cUSBr-Patch) for Deep Tissue Scanning and Imaging
- Media Lab Research Theme: Life with AI
- Media Lab Research Theme: Connected Mind + Body
Share this article
By Anne Trafton
When breast cancer is diagnosed in the earliest stages, the survival rate is nearly 100 percent. However, for tumors detected in later stages, that rate drops to around 25 percent.
In hopes of improving the overall survival rate for breast cancer patients, MIT researchers have designed a wearable ultrasound device that could allow people to detect tumors when they are still in early stages. In particular, it could be valuable for patients at high risk of developing breast cancer in between routine mammograms.
The device is a flexible patch that can be attached to a bra, allowing the wearer to move an ultrasound tracker along the patch and image the breast tissue from different angles. In the new study, the researchers showed that they could obtain ultrasound images with resolution comparable to that of the ultrasound probes used in medical imaging centers.
“We changed the form factor of the ultrasound technology so that it can be used in your home. It’s portable and easy to use, and provides real-time, user-friendly monitoring of breast tissue,” says Canan Dagdeviren, an associate professor in MIT’s Media Lab and the senior author of the study.
MIT graduate student Wenya Du, Research Scientist Lin Zhang, Emma Suh ’23, and Dabin Lin, a professor at Xi’an Technological University, are the lead authors of the paper , which appears today in Science Advances.
A wearable diagnostic
For this project, Dagdeviren drew inspiration from her late aunt, Fatma Caliskanoglu, who was diagnosed with late-stage breast cancer at age 49, despite having regular cancer screens, and passed away six months later. At her aunt’s bedside, Dagdeviren, then a postdoc at MIT, drew up a rough schematic of a diagnostic device that could be incorporated into a bra and would allow for more frequent screening of individuals at high risk for breast cancer.
Breast tumors that develop in between regularly scheduled mammograms — known as interval cancers — account for 20 to 30 percent of all breast cancer cases, and these tumors tend to be more aggressive than those found during routine scans.
“My goal is to target the people who are most likely to develop interval cancer,” says Dagdeviren, whose research group specializes in developing wearable electronic devices that conform to the body. “With more frequent screening, our goal to increase the survival rate to up to 98 percent.”
To make her vision of a diagnostic bra a reality, Dagdeviren designed a miniaturized ultrasound scanner that could allow the user to perform imaging at any time. This scanner is based on the same kind of ultrasound technology used in medical imaging centers, but incorporates a novel piezoelectric material that allowed the researchers to miniaturize the ultrasound scanner.
To make the device wearable, the researchers designed a flexible, 3D-printed patch, which has honeycomb-like openings. Using magnets, this patch can be attached to a bra that has openings that allow the ultrasound scanner to contact the skin. The ultrasound scanner fits inside a small tracker that can be moved to six different positions, allowing the entire breast to be imaged. The scanner can also be rotated to take images from different angles, and does not require any special expertise to operate.
“This technology provides a fundamental capability in the detection and early diagnosis of breast cancer, which is key to a positive outcome,” says Anantha Chandrakasan, dean of MIT’s School of Engineering, the Vannevar Bush Professor of Electrical Engineering and Computer Science, and one of the authors of the study. “This work will significantly advance ultrasound research and medical device designs, leveraging advances in materials, low-power circuits, AI algorithms, and biomedical systems.”
Early detection
Working with the MIT Center for Clinical and Translational Research, the researchers tested their device on one human subject, a 71-year-old woman with a history of breast cysts. Using the new device, the researchers were able to detect the cysts, which were as small as 0.3 centimeters in diameter — the size of early-stage tumors. They also showed that the device achieved resolution comparable to that of traditional ultrasound, and tissue can be imaged at a depth up to 8 centimeters.
“Access to quality and affordable health care is essential for early detection and diagnosis. As a nurse I have witnessed the negative outcomes of a delayed diagnosis. This technology holds the promise of breaking down the many barriers for early breast cancer detection by providing a more reliable, comfortable, and less intimidating diagnostic,” says Catherine Ricciardi, nurse director at MIT’s Center for Clinical and Translational Research and an author of the study.
To see the ultrasound images, the researchers currently have to connect their scanner to the same kind of ultrasound machine used in imaging centers. However, they are now working on a miniaturized version of the imaging system that would be about the size of a smartphone.
The wearable ultrasound patch can be used over and over, and the researchers envision that it could be used at home by people who are at high risk for breast cancer and could benefit from frequent screening. It could also help diagnose cancer in people who don’t have regular access to screening.
“Breast cancer is the most common cancer among women, and it is treatable when detected early,” says Tolga Ozmen, a breast cancer surgeon at Massachusetts General Hospital who is also an author of the study. “One of the main obstacles in imaging and early detection is the commute that the women have to make to an imaging center. This conformable ultrasound patch is a highly promising technology as it eliminates the need for women to travel to an imaging center.”
The researchers hope to develop a workflow so that once data are gathered from a subject, artificial intelligence can be used to analyze how the images change over time, which could offer more accurate diagnostics than relying on the assessment of a radiologist comparing images taken years apart. They also plan to explore adapting the ultrasound technology to scan other parts of the body.
The research was funded, in part, by the National Science Foundation, a 3M Non-Tenured Faculty Award, the Sagol Weizmann-MIT Bridge Program, and MIT Media Lab Consortium Funding.

A new ultrasound patch can measure how full your bladder is
The wearable device, designed to monitor bladder and kidney health, could be adapted for earlier diagnosis of cancers deep within the body.
Wearable ultrasound that could detect breast cancer developed by MIT
Prof. Canan Dagdeviren talks to CBS News about a wearable ultrasound device that could allow users to detect early changes in breast tissue.
MIT-developed device could be a lifesaving gamechanger in diagnosing breast cancer
Prof. Canan Dagdeviren talks to Boston 25 News about a wearable ultrasound device that could help detect early-stage breast cancer.
New portable breast cancer scanner can fit in a bra
To help combat "interval" cancers, the Conformable Decoders group led by Prof. Dagdeviren, has developed a wearable ultrasound scanner.
CME Tracker
At UltraCon 2024, the Latest Breakthroughs in Ultrasound Technology Take Center Stage
Futuristic. Transformative. Groundbreaking.
These are three of the words used to describe the upcoming medical ultrasound event in Austin, Texas. The American Institute of Ultrasound in Medicine (AIUM) has announced UltraCon 2024, a gathering that promises to bring together the brightest minds in ultrasound technology. From April 6–10, the Hilton Austin will serve as the focal point for comprehensively exploring the advancements shaping the field's future.
UltraCon 2024 emerges not merely as a conference but as a pivotal forum for debate, discovery, and dialogue. It is designed to cater to a broad audience, from seasoned practitioners to those at the pioneering edge of industry innovation and individuals just beginning to navigate the complexities of medical imaging. This convergence of experience and expertise underscores the dynamic nature of ultrasound technology, highlighting its critical role in enhancing patient outcomes.
The conference agenda is curated to reflect the breadth of the field, offering insights into the latest technological advancements, clinical applications, and the impact of artificial intelligence on diagnostic practices. UltraCon 2024 stands as a testament to the spirit of collaboration and innovation that drives the medical ultrasound community forward.
Networking opportunities will provide a unique platform for professionals to connect with peers and luminaries alike. Such interactions are essential for fostering the exchange of ideas and catalyzing the development of new technologies and methodologies in ultrasound. As an attendee, you can also meet up with committee members who work on clinical and technical standards to improve your professional work.
"The essence of UltraCon 2024 lies in its ability to bridge the gap between current practices and future possibilities," notes Dr. Richard Hoppmann, MD, FACP, FAIUM, President of the AIUM. "It's an immersive experience that offers participants a comprehensive view of the cutting-edge developments within the field, guided by its most esteemed figures."
Adding a tangible dimension to the discussions, UltraCon 2024 will also feature an exhibition space where leading companies, such as GE HealthCare and Siemens, will showcase the latest in ultrasound technology and solutions. This segment of the conference provides attendees with a hands-on look at the innovations poised to redefine patient care in the coming years.
As the event draws near, the anticipation among the medical ultrasound community is unmistakable. UltraCon 2024 represents a critical juncture in the ongoing dialogue about the future of ultrasound technology, offering a rare opportunity for participants to engage directly with the ideas and individuals shaping the field's trajectory.
For those committed to advancing medical imaging, UltraCon 2024 is a must-attend engagement. It offers a window into the future of ultrasound technology, surrounded by the field's leading thinkers and innovators. This April in Austin, the dialogue about ultrasound's future is not just ongoing—it's evolving.
For registration and additional information about UltraCon 2024, please visit https://ultracon2024.eventscribe.net/ . For a list of all UltraCon 2024 exhibitors, please visit this link .
The American Institute of Ultrasound in Medicine is a multidisciplinary medical association of more than 9,000 physicians, sonographers, scientists, students, and other healthcare professionals. Established in the early 1950s, AIUM is dedicated to empowering and cultivating a global multidisciplinary community engaged in the use of medical ultrasound through raising awareness, education, sharing information, and research.
LATEST NEWS
- Aug 6, 2024 The American Institute of Ultrasound in Medicine Welcomes Steven R. Meyers, PhD, as Chief Executive Officer Steven R. Meyers, PhD, joins the medical ultrasound association with more than a decade of transformative leadership and association experience.
- Apr 24, 2024 AIUM Recognizes Leaders in Ultrasound Medicine at UltraCon 2024 The AIUM closed its UltraCon2024 event in Austin, TX, by honoring individuals who advance the field of medical imaging and ultrasound and improve patient outcomes across the globe.
- Mar 26, 2024 Music, Advanced Medicine, and Robotics Merge in Groundbreaking UltraCon 2024 Ultrasound Presentations Dr. Omar Ishrak and Dr. Gil Weinberg to Headline Medical Tech Conference
- Mar 5, 2024 At UltraCon 2024, the Latest Breakthroughs in Ultrasound Technology Take Center Stage The American Institute of Ultrasound in Medicine (AIUM) has announced UltraCon 2024, a gathering that promises to bring together the brightest minds in ultrasound technology.
- Aug 3, 2023 Pulsenmore Wins the AIUM's Shark Tank Competition at UltraCon, Showcasing Revolutionary Patient-Centered Home Ultrasound Solution Pulsenmore, a leading innovator in connected patient-driven home ultrasound, emerged victorious in the American Institute of Ultrasound in Medicine’s first-ever Shark Tank competition held this past March at UltraCon this year in Orlando, Florida.
Partner with Us | Press | Privacy Policy | Contact Us
14750 Sweitzer Lane, Suite 100, Laurel, MD 20707 301-498-4100 © American Institute of Ultrasound in Medicine, a 501(c)(3) nonprofit educational organization. All Rights Reserved.
Web Design & Development by Matrix Group International, Inc .

Office of Medical Communications
New study in radiology shows benefit of ultrasound screening for some women with dense breasts.

Brian Sprague, PhD
More than 40% of women undergoing mammography screening have normal breast tissue that is radiologically dense and may obscure the presence of breast cancer on a mammogram. Laws require that women with dense breast tissue be informed about the limitations of mammography, but there is no consensus regarding whether women with dense breast tissue should undergo additional breast cancer screening tests. Supplemental screening with whole breast ultrasound is one option, but prior studies have indicated a high rate of false positive exams that has limited enthusiasm. Now, new work by UVM Cancer Center investigators Brian Sprague, Sally Herschorn, Hannah Perry, and Donald Weaver, published in the journal Radiology , finds that supplemental ultrasound screening has favorable outcomes among women with dense breast tissue who also have other breast cancer risk factors.
Women at high risk of invasive or advanced breast cancer according to established risk prediction models had high cancer detection rates on ultrasound screening after a negative mammogram, with an acceptably low rate of false positive exams. In contrast, women with dense breasts who were at low or average risk of breast cancer had low cancer detection on ultrasound screening after a negative mammogram and a higher fraction of false positive exams. The collaborative study used data on over 30,000 ultrasound screening exams from three regional breast imaging registries (Vermont, San Francisco, Chicago) of the Breast Cancer Surveillance Consortium .
The results of this study demonstrate the importance of determining breast cancer risk while counseling women with dense breasts regarding supplemental screening options. According to Dr. Sprague, “These findings can help clinicians identify women with dense breasts who are good candidates for supplemental ultrasound screening. Approximately 20% of women with dense breasts have high invasive or advanced breast cancer risk according to these risk models. These women are most likely to benefit from supplemental ultrasound screening.”
The breast cancer risk models used in the study were developed by Dr. Sprague and colleagues within the Breast Cancer Surveillance Consortium and are publicly available on the web and as iPhone and Android apps ( https://www.bcsc-research.org/tools ). The risk models were developed using data from over 1 million women undergoing mammography screening at healthcare facilities participating in the Breast Cancer Surveillance Consortium, including data from over 200,000 women in the state of Vermont collected by the Vermont Breast Cancer Surveillance System led by Drs. Sprague, Herschorn, Perry, and Weaver.
Future work will evaluate long-term outcomes for women undergoing supplemental ultrasound screening, including computer simulation modeling of breast cancer deaths averted.
This study was conducted with collaborators at the University of California-San Francisco, the University of Illinois, the Fred Hutchinson Cancer Center, University of California-Davis, Harvard Medical School, and Kaiser Permanente Washington Health Research Institute. The study was funded by the National Cancer Institute after initial pilot grant support from the UVM Larner College of Medicine and the Department of Surgery.
Read full study in the journal of Radiology : https://pubs.rsna.org/doi/10.1148/radiol.232380

Guest Column | June 15, 2022
Emerging trends in ultrasound imaging.
By Karen Koblan, Ultrasound Solutions Corp.
We are close to a new era in ultrasound technology. From helping healthcare specialists detect several diseases such as cancerous cells to showing real-time images inside the mother’s womb, ultrasound technology is a go-to way for various specialists to deal with a wide range of diseases and tasks.
Let us take a closer look at how the emerging technologies of ultra-compact ultrasound, 3D and 4D ultrasound, artificial intelligence (AI), tissue harmonic imaging, and volumetric ultrasound are impacting the future of ultrasound imaging.
1. Ultra-Compact Ultrasound
Ultra-compact or portable ultrasound machines have taken imaging technology by storm. Previously, medical professionals had to use large, bulky, and complicated ultrasound machines for treating patients. Now, healthcare and clinical laboratories are opting for these ultra-compact imaging machines due to their portability and ease of use.
Portable ultrasound machines are also being used by healthcare specialists. In particular, portable ultrasound machines are useful in detecting UTIs. These machines offer a number of advantages over standard methods of UTI detection, such as computed tomography (CT) or magnetic resonance imaging (MRI). Portable ultrasound machines provide superior imaging quality and allow for real-time image guidance. These machines are perfect for primary care settings.
2. 3D And 4D Real-Time Ultrasound Imaging
The use of three-dimensional (3D) real-time imaging ultrasound technology is being driven by the demand for more accurate diagnostic images. This type of imaging provides a clear picture of the internal organs and can be used to detect abnormalities such as tumors. It is becoming more popular because it gives a better view of what is going on inside the body.
3D real-time imaging is becoming more popular for fetal ultrasound. This technology gives a more detailed view of the baby. This technology is new, and there is no standard protocol for its use yet. However, more hospitals are expected to start using this technology in the near future.
Further, this technology helps determine diseases with ease. It helps to achieve better visuals of human body organs. Compared to 2D imaging technology, 3D ultrasound imaging technology also takes less time.
Four-dimensional (4D) ultrasound imaging technology is even more convenient for healthcare specialists such as gynecologists. Compared to 3D ultrasound technology, 4D ultrasound shows live motion with the help of several images. With this technology, gynecologists can observe the live movement of the baby in the mother’s womb.
4D real-time ultrasound imaging provides a lot of benefits that traditional two-dimensional imaging does not, especially to gynecologists. This type of imaging gives a more complete view of the fetus, as well as showing how the fetus is developing over time. This technology can also be used to monitor multiple fetuses simultaneously. This is beneficial for high-risk pregnancies.
3. Artificial Intelligence
AI or artificial intelligence has a lot of potential in the medtech and imaging industry, including in the area of ultrasound technology. Ultrasound waves are used to create images of the inside of the body. This technology has been used for diagnostic purposes for many years. However, interpreting these images can be tricky, even for experienced radiologists. This is where AI comes in to help.
AI-enabled ultrasound machines can quickly and accurately interpret images. This can help doctors diagnose and treat patients faster. Additionally, AI can help identify patterns that human observers may miss. For example, AI can help identify early signs of several serious diseases, including cancer, heart disease, and stroke. This technology has the potential to save lives by providing earlier diagnosis and treatment.
4. Tissue Harmonic Imaging
Tissue harmonic imaging (THI) is another new emerging technology that's rapidly changing the use of standard ultrasound techniques. THI is an advanced technology that produces images with greater clarity than standard ultrasound. This means that clinicians can make more accurate diagnoses using THI, and this makes it particularly well-suited for use in cardiac imaging.
In addition, THI technology requires less power and can be performed more quickly, making it more convenient for both patients and clinicians. Further, THI is less likely than standard ultrasound to produce artifacts, which can often lead to inaccurate diagnoses.
5. Volumetric Ultrasound
In general, volumetric imaging creates images of objects in space by combining multiple 2D images taken from different angles. This allows for a more complete view of an object than would be possible with just a single image. Now, the same concept is being used for medical diagnosis purposes.
Volumetric ultrasound provides 3D images of the body by steering a 2D array transducer in a scan format, using sound waves and computer algorithms to create images of the inside of the body. This imaging modality can be helpful in identifying cancer cells, tumor cells, and other abnormalities, as well as diagnosing various conditions, such as various heart diseases. Further, volumetric ultrasound is often used to help guide procedures such as biopsies and needle injections.
It is often used to image the fetus during pregnancy. It can also be used to image other organs and structures, as well as to assess relationships between different structures of human body organs. It is a great way to see small structures and is very clear.
Final Thought
Ultra-compact ultrasound, 3D and 4D ultrasound, artificial intelligence (AI), tissue harmonic imaging, and volumetric ultrasound are impacting – and will continue to impact -- the future of ultrasound technology, benefiting both healthcare providers and patients.
For any questions, you may contact the author via the Ultrasound Solutions Corp. website .
Like what you are reading?
Sign up for our free newsletter, newsletter signup.

- Open access
- Published: 19 August 2024
Deep learning based uterine fibroid detection in ultrasound images
- Haibin Xi 1 &
- Wenjing Wang 1
BMC Medical Imaging volume 24 , Article number: 218 ( 2024 ) Cite this article
140 Accesses
Metrics details
Uterine fibroids are common benign tumors originating from the uterus’s smooth muscle layer, often leading to symptoms such as pelvic pain, and reproductive issues. Early detection is crucial to prevent complications such as infertility or the need for invasive treatments like hysterectomy. One of the main challenges in diagnosing uterine fibroids is the lack of specific symptoms, which can mimic other gynecological conditions. This often leads to under-diagnosis or misdiagnosis, delaying appropriate management. In this research, an attention based fine-tuned EfficientNetB0 model is proposed for the classification of uterine fibroids from ultrasound images. Attention mechanisms, permit the model to focus on particular parts of an image and move forward the model’s execution by empowering it to specifically go to imperative highlights whereas overlooking irrelevant ones. The proposed approach has used a total of 1990 images divided into two classes: Non-uterine fibroid and uterine fibroid. The data augmentation methods have been connected to improve generalization and strength by exposing it to a wider range of varieties within the training data. The proposed model has obtained the value of accuracy as 0.99. Future research should focus on improving the accuracy and efficiency of diagnostic techniques, as well as evaluating their effectiveness in diverse populations with higher sensitivity and specificity for the detection of uterine fibroids, as well as biomarkers to aid in diagnosis.
Peer Review reports
Introduction
Fibroids are non-cancerous/benign growths that occur in the muscle wall of the uterus of the woman. These Uterine Fibroids (UF) are commonly found in middle-aged and elderly women with an occurrence rate of 20–25% in women over 30 years old. Pelvic pain, infertility and heavy menstrual bleeding are the common symptoms of UF [ 1 ]. However, hormonal imbalances and genetics may be one of the reasons but the accurate cause of UF is unknown. UF can have a substantial impact on women’s reproductive health and quality of life as UFs are a major cause of hysterectomies worldwide, losing their uterus each year due to fibroids [ 2 ]. Ultrasound (US) imaging is a non-invasive and commonly used to diagnose and monitor UF. Initial diagnosis of UF typically involves ultrasound images but treatment depend on the type, size, and location, as well as the symptoms and the reproductive goals. Generally, medications are suggested to reduce fibroid size and to control the symptoms [ 3 , 4 ]. However, manual identification of UFs in the US can be challenging for small or obscured lesions. Therefore, deep learning offers a promising method for automatic classification of UFs in ultrasound images. As per the literature review, it has been observed that deep learning models can achieve high accuracy in classifying non-fibroids and fibroids by surpassing human performance in certain cases. Moreover, it helped in removing human bias and subjectivity from the classification process leading to more consistent results. Automated classification can potentially lead to earlier detection of UFs, allowing for timely intervention and improved patient outcomes by saving radiologists and sonographers valuable time. Therefore, in this proposed work, attention mechanism is combined with EfficientNetB0 model for the uterine fibroids classification from ultrasound images. The chief offerings of the study are as follows:
An attention based fine-tuned EfficientNetB0 model is proposed for the classification of uterine fibroids from ultrasound images. Attention mechanisms, permit the model to focus on detailed parts of an image and improve the model’s performance by enabling it to selectively attend to important features while ignoring irrelevant ones.
The data augmentation techniques have been applied to improve model generalization and robustness by revealing it to a wider range of disparities in the training data.
The rest of the research is shown as: Sect. 2 shows the literature review, followed by dataset description in Sect. 3 , methodology in Sect. 4 followed by results in Sect. 5 , conclusion and future scope in Sect. 6.
Literature review
The researchers had performed work on the classification of UF. They had worked using 3D CNN using a dataset of 3D ultrasound images and had obtained the value of accuracy as 91.3% [ 5 ]. The researchers commended the 3D CNN points of interest over conventional 2D in identifying UF due to its upgraded capacity to seizure spatial information. Authors [ 6 ] attained 98.8% accuracy by employing a pre-trained ResNet50 CNN calibrated on their dataset of 2D ultrasound images. A study [ 7 ] accomplished 96.4% accuracy utilizing the VGG16 model, which was prepared on their ultrasound images dataset. The proposed model extracted highlights from US images employing a grouping of convolutional layers. The proposed model had an accuracy of 97.5%, illustrating the adequacy of DCNNs inside the assurance of UF [ 8 ]. In a study [ 9 ], examiners proposed a cross breed DL illustrated to recognize UF. The ultrasound images were fed into the show, and highlights were removed utilizing a gathering of CNN and dreary neural frameworks. The revelations of the think about show up that the hybrid DL show has the potential for utilize in helpful picture dealing with since the proposed demonstrate accomplished an accuracy of 96.8%. For the location of UF from ultrasound images, another thinks about [ 10 ] proposed a DCNN plan. The proposed shows extricated characteristics from ultrasound images utilizing a combination of convolutional and pooling layers. The proposed demonstration showed up that DCNN can be profitable for recognizing UF with an accuracy of 96.7%. A DL-based system was proposed for programmed UF location from ultrasound pictures in think about [ 11 ] to extricate qualities from the ultrasounds.
Dataset description
Input dataset.
The dataset comprises 1990 images divided into two classes: Non-uterine fibroid (NUF) and uterine fibroid (UF) as shown in Fig. 1 [ 12 ]. The data is split into an 80 − 20 ratio for training and testing, respectively. for the test set, the total NUF is 223 and UF is 173, whereas for the train set, the total NUF is 892 and the total UF is 702. Each image is resized to a uniform size of 224 × 224 pixels. This dataset is crucial for the development and evaluation of deep learning models aimed at the automated detection and classification of uterine fibroids. The class imbalance between the two classes presents a challenge that must be addressed to ensure the model’s robustness and effectiveness. The utilization of such a dataset enables the exploration and implementation of various deep learning architectures and algorithms for improved diagnosis and treatment planning in the context of uterine fibroids.

( a ) Non-Uterine Fibroid (NUF): The red arrow points to the fibroid mass located outside the uterus. ( b ) Uterine Fibroid (UF): The blue arrow points to the fibroid mass within the uterus. The dataset
Data augmentation
Information increase could be a vital strategy in machine learning for misleadingly growing a dataset by making altered adaptations of images [ 13 ]. This process makes a difference move forward demonstrate generalization and robustness by uncovering it to a wider range of varieties within the training data, such as rotations, translations, flips, and changes in brightness or contrast. By augmenting the dataset, the model learns to recognize objects in various positions, orientations, and lighting conditions, making it more effective when applied to real-world data.
Random Rotation: Randomly rotates the image by a factor of up to 0.15, introducing variations to the orientation of the images.
Random Translation: Randomly translates the image horizontally and vertically by up to 10% of the image height and width, respectively, adding positional variance.
Random Flip: Randomly flips the image horizontally or vertically, augmenting the dataset with mirror images.
Random Contrast: Randomly adjusts the contrast of the image by a factor of up to 0.1, modifying the intensity of pixel values.
These augmentation methods offer assistance to avoid overfitting and move forward the model’s generalization by exposing it to a more extensive extend of varieties inside the dataset, eventually upgrading the model’s execution on unseen information. After the application of information augmentation procedures presently the image count expanded to 10,000. Out of which 8000 images are taken for training and 2000 images are taken for testing reasons.
Methodology
The Fig. 2 has outlined the process of developing a deep learning model for ultrasound image classification. The specific task here is to distinguish between uterine fibroids (UF) and non-uterine fibroids (NUF) in ultrasound images. The process started with a data source of ultrasound images. Subsequently, data augmentation has been applied to enhance the robustness of the model by including some operations like random rotations, translations, flips and contrast adjustments. Following the augmentation, the data has been fed into a pre-trained EfficientNetB0 model, which is a kind of convolutional neural network (CNN) architecture that has been specifically a kind of artificial neural network suggested to analyse visual images. EfficientNetB0 model has acted as a feature extractor by learning patterns from the data. Furthermore, an attention mechanism has been incorporated to the EfficientNetB0 model which has allowed the proposed model to focus on relevant aspects of the input data that are most relevant to the task. Afterward, the global average pooling layer was employed to sum up the features that have been extracted by the EfficientNetB0 model [ 14 ], which were afterward followed by a dropout layer that randomly dropped out a specified percentage of neurons during the training to mitigate the overfitting. Henceforth, the data has been served to a dense layer with a softmax activation function. The softmax function has generated a probability distribution over two set of classes that is Uterine Fibroid (UF) or Non-Uterine Fibroid (NUF).
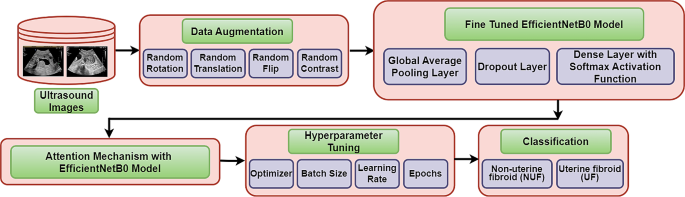
Proposed methodology
Lastly, the hyperparameters such as learning rate, optimizer, and batch size of the model have been tuned to manage the learning process of the model. Tuning of these hyper-parameters has involved setting of their values to accomplish optimal model performance. This optimization process is normally concerned the evaluation of the model’s performance on a validation set with diverse combinations of hyperparameter values. The objective is to determine the set of hyperparameters that has obtained the best balance between efficiency and accuracy.
EfficientNetB0 model
EfficientNetB0 is a CNN architecture that has been proposed to obtain the balance between the state-of-the-art performance and computational efficiency. Scaling approach has been employed that allowed the EfficientNet model to get better performance as compared to existing models while being more efficient in terms of computational resources in terms of width, depth and resolution. EfficientNetB0 has achieved better performance by scaling these dimensions in a organized way than the other models that only scale one or two dimensions while claiming computational efficiency. EfficientNetB0 has appeared to realize state-of-the-art execution on different picture classification assignments while being more proficient in terms of show measure and computational assets compared to other models. EfficientNetB0 has scaled the width by increasing the number of channels in each layer, the depth of the network by increasing the number of layers and the resolution by increasing the input image size. This multi-dimensional scaling approach has contributed to the model’s superior performance. It is efficient on resource-constrained gadgets such as portable phones or implanted frameworks, where computational assets are constrained [ 15 ]. In this work, the EfficientNetB0 model has been optimized through fine-tuning which entailed freezing the weights of the pre-trained base model (base_model) while adding new layers (GlobalAveragePooling2D, Dropout, Dense) on top of it for a new classification task. The frozen base model has acted as a feature extractor by capturing general features from the input images. The new added layers are then trained to learn task-specific features from these extracted features to enhance the model’s performance.
Fine-tuning is performed because pre-trained models like EfficientNetB0 have already learned rich representations from a large dataset (e.g., ImageNet) and can generalize well to new tasks with less data. Instead of training a model entirely from scratch, the power of fine-tuning has been employed. This process has taken a pre-trained model, that is EfficientNetB0, which has already learned valuable features from a massive dataset. These learned features have been adapted to the specific task by fine-tuning for the classification of uterine fibroids. This scheme has offered several benefits. Fine-tuning has led to faster progress as the model does not need to re-learn the elementary image recognition skills which permitted it to focus on the unique characteristics of fibroids in ultrasound images resulting in quicker training times. While dealing with the limited data, fine-tuning has improved the performance. The pre-trained model has acted as a strong basis and fine-tuning has aided it in adapting to the specific patterns in the fibroid classification dataset. This is very helpful when the dataset is fairly small as there is less information for the model to learn from scratch. Finally, fine-tuning has aided in avoiding the overfitting. The pre-trained weights were performed as a method of control which prevented the model from learning specific details in the training data that might not generalize well to unseen images. Moreover, fine-tuning has permitted the model to transfer knowledge acquired from the pre-trained model’s substantial dataset, even if the unfamiliar fibroid dataset is lesser.
Proposed attention mechanism based EfficientNetB0 method
In this research work, a new model named as Attention based EfficientNetB0 has been developed where EfficientB0 is a CNN architecture known for its performance and efficiency which has been improved by the addition of attention mechanisms. The attention mechanism has allowed the model to prioritize the precise areas of an image that are most appropriate for the desired task. This has improved the performance of the model by allowing it to select the significant features while ignoring the inappropriate ones. In the context of EfficientNetB0, attention mechanisms have been typically applied in the form of attention layers, which are added at the several phases of the network. These layers have enhanced the capability of model to capture fine-grained details and long-range dependencies within an image which lead to better generalization and improve feature representation.
Therefore, attention mechanisms have been incorporated to further enhance the capabilities of the model. The integration of attention mechanisms has significantly enhanced the performance of EfficientNetB0 by capturing crucial dependencies and contextual information from images by mimic the human visual focus that allowed the model to highlight specific image regions which are most relevant to the task. There are two main types of attention mechanisms that can be beneficial: Self-Attention has enabled the EfficientNetB0 to capture long-range dependencies and contextual information within the image. It has essentially allowed different regions of the image to communicate with each other that lead to more comprehensive understanding of the content. By employing self-attention, EfficientNetB0 has effectively learned the global dependencies within an image. This attention mechanism has aided in identifying relationships between distant image elements for improving performance in various tasks. Another Spatial Attention mechanism has focused on specific locations within the image. It has focused on specific image regions highlighting the areas of interest while suppressing non-relevant ones in tasks where spatial information is essential, such as object detection and segmentation. Integrating attention mechanisms into EfficientNetB0’s architecture has involved the addition of attention layers. A prevalent approach is to integrate the self-attention through the transformer mechanism which has enhanced the model’s ability to extract relevant information from images by boosting its performance in computer vision tasks.
Hyperparameter tuning
In this work, proposed model has been trained using four distinct hyperparameters named as batch size, optimizer, learning rate and epochs.
It determined the number of training examples used in a single update of the model’s internal parameters during gradient descent. It could be a vital hyperparameter in deep learning models that influences both the training speed and the quality of the model. Choosing a suitable batch estimate depends on the particular dataset size, model complexity, and available resources. Batch size is regularly tuned along with other hyperparameters to optimize the execution of the demonstrate. Batch size is related to the training set size (N) and the number of iterations per epoch (M) by the given formula.
Where N is the total number of training examples. M is the number of iterations per epoch.
Nadam Optimizer is an optimization algorithm in place of the Adam optimizer that has combined the benefits of Nesterov accelerated gradient (NAG) descent and Adam. By integration of the NAG technique, Nadam is the modification over Adam by enabling more precise and stable convergence. It has integrated the NAG technique to adjust the update direction based on momentum with adaptive learning rates for each parameter. This integration has permitted Nadam to offer steadier and more effective optimization in comparison to the other optimizers. It is helpful in providing training to deep neural networks where fast convergence and robustness to noisy data are crucial. Nadam’s adaptive learning rate method has assisted in navigating complex loss settings which makes it a widespread selection for various deep learning tasks.
An epoch is represented as a single training cycle where the entire dataset is fed through the model once. During each epoch, the model has renewed its internal parameters (weights and biases) based on the errors (loss) it has faced in the training. Training for more epochs has permitted the model to learn from the data multiple times to improve its performance. However, it may lead to overfitting. Overfitting occurs when a model has memorized the training data too well, losing its ability to generalize to unseen data. It’s a balancing act: train for too few epochs and the model might underfit (fail to learn the patterns in the data), train for too many and you risk overfitting. Epochs are related to the batch size ( B ), the total number of training examples ( N ), and the number of iterations per epoch ( M ) by the formula:
Learning rate
The learning rate is a hyperparameter in deep learning that controls the step size during optimization. The learning rate is typically set before training and can be fixed or adjusted dynamically during training using techniques like learning rate schedules or adaptive learning rate methods. In this work, the learning rate is set to 0.00005. Lower learning rates often result in a more stable optimization process, as the updates to the parameters are smaller and less likely to lead to divergence. With a lower learning rate, the optimization algorithm takes smaller steps toward the minimum point, potentially allowing it to find a more precise solution.
Accuracy and loss analysis
The Fig. 3 represents the accuracy metrics of a model trained over multiple epochs. Each row corresponds to an epoch number, and the columns indicate the accuracy achieved on the training data (Accuracy) and the validation data (Val_Accuracy) at that epoch. The accuracy values show a clear trend of improvement over epochs for both the training and validation sets. Initially, at epoch 1, the model started with a relatively low training accuracy of 56.11% on the training data and an even lower 34.85% validation accuracy, indicating that the model is not performing well and likely underfitting. However, as the training progresses, the model’s performance has been improved significantly. By epoch 4, the model has achieved a high training accuracy of 96.70% on the training data and 86.11% validation accuracy on the validation data, indicating that the model is learning the underlying patterns in the data well. Towards the later epochs, the model’s performance has continued to improve, with accuracy values nearing 100% on both the training and validation sets. From these experiments, it has been observed that the model has learned the dataset’s features effectively and is performing very well, likely indicating that it has reached a point of overfitting, especially as the validation accuracy started to plateau.
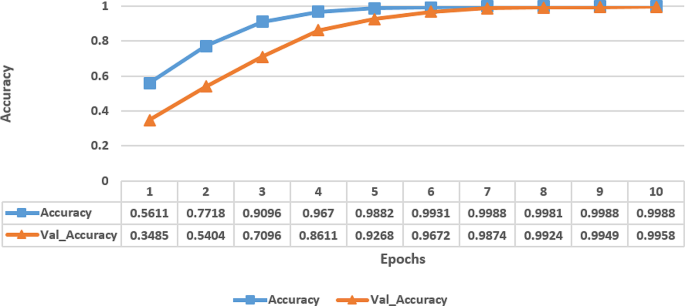
Proposed Attention based fine-tuned EfficientNetB0 model’s accuracy
Figure 4 displays the loss metrics of a proposed model trained over ten epochs, showing both training loss (Loss) and the validation loss (Val_Loss) at each epoch. At initial state, during epoch 1, both the training and validation losses are relatively high, which indicated that the model has not performed well and likely has high error rates.
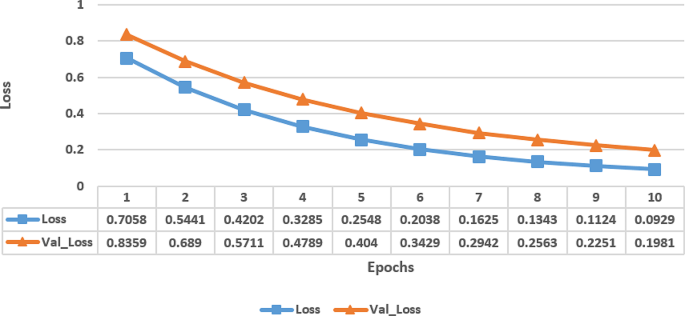
Loss of Attention based fine-tuned EfficientNetB0 model
However, as the training progressed, the losses steadily has decreased which indicated that the model has improved its performance by learning the dataset’s patterns. By epoch 4, the losses have reduced significantly, indicating that the model has become more accurate by making fewer errors. This trend continued with the losses decreasing further in each subsequent epoch. Towards the later epochs, the model’s performance has continued to improve, with the losses approaching very low values. This suggested that the model has learned the dataset well and has performed well at a high level of accuracy, especially as the validation loss closely tracked the training loss, indicating that the model did not have any overfitting issue.
State-of-art comparative analysis
The table has listed a comparative analysis of various techniques and their corresponding accuracy rates. Table 1 , it has shown that Dilna et al. [ 11 ] have achieved an accuracy of 95.1% in classifying ultrasound scanned uterus images indicating the effectiveness of their classification method. Furthermore, Behboodi et al. [ 10 ] have utilized UNet-based networks for US diagnostic imaging and have achieved an accuracy of 86.2% with the consideration of the utility of this architecture for medical image analysis. Besides, Li et al. [ 16 ] have used deep learning on the ChEMBL dataset by achieving an accuracy of 85% which shows the potential of deep learning for pharmaceutical research. Moreover, Tang et al. [ 17 ] has introduced the AR-Unet Network and has achieved an impressive accuracy of 94.56% on the AR-Unet dataset which indicated the robustness of approach. Then, Yang et al. [ 18 ] utilized neural networks on an ultrasound image and achieved an accuracy of 88.5% which demonstrated the effectiveness of deep learning for medical image analysis. Girija et al. [ 19 ] has employed various data mining techniques on 450 patients with an accuracy of 89.54% which has illustrated the importance of data mining in healthcare research. Additionally, Huo et al. [ 20 ] have used a Deep learning-based method on a dataset of 3870 ultrasound images with an accuracy of 87.45%. Overall, the accuracies attained by these studies have displayed the efficiency of different methods and procedures in healthcare with each approach showcasing its strengths in diverse perspectives.
Conclusion and future scope
Fibroids in the uterine are a gynaecological disorder that can substantially influence the health of women and their quality of life. Fibroids can impede the implantation of a fertilized egg or interrupt the blood flow to the uterus which may lead to recurrent miscarriages or infertility. Thus, early detection of uterine fibroids (UF) is essential for maintaining the fertility. By early treatment of fibroids, these risks can be reduced, and women can have a better chance of conceiving and carrying a pregnancy. Though, all fibroids don’t cause any symptoms or require any form of treatment, therefore, detection of those that are likely to cause problems can help in providing treatment plans to individual needs. This can lessen the use of unnecessary treatments and minimize the impact of fibroids. Another challenge is the lack of reliable diagnostic tests for uterine fibroids. Therefore, in this work, for the early detection of fibroids in the uterine, an attention mechanism based fine-tuned EfficientNetB0 model has been proposed for the classification of uterine fibroids and non-fibroids from ultrasound images.
Future research directions include the use of biomarkers that can specify the occurrence of fibroids at an early stage or predict their growth and progression at diverse stages. Biomarkers such as specific proteins or genetic markers could help to improve the accuracy of diagnosis and guide in treatment decisions. This will help to ensure that new diagnostic techniques are applicable and accessible to a wide range of women, regardless of their age, ethnicity, or socioeconomic status.
Data availability
Dataset of Fibroid is publicly available at https://data.mendeley.com/datasets/n2zcmcypgb/2 .
Dolmans MM, Petraglia F, Catherino WH, Donnez J. Pathogenesis of uterine fibroids: current understanding and future directions. Fertility and Sterility; 2024.
Srinivas T, Lulseged B, Attari MMA, Borahay M, Weiss CR. 2024. Patient characteristics Associated with embolization vs Hysterectomy for Uterine fibroids: a systematic review and Meta-analysis. J Am Coll Radiol.
Anand V, Gupta S, Nayak SR, Koundal D, Prakash D, Verma KD. An automated deep learning models for classification of skin disease using dermoscopy images: a comprehensive study. Multimedia Tools Appl. 2022;81(26):37379–401.
Article Google Scholar
Ahmadzade M, Rouientan H, Golzarian J, Akhlaghpoor S. An evaluation of Ultrasound-guided percutaneous microwave ablation for the treatment of symptomatic uterine fibroids. J Vasc Interv Radiol. 2024;35(1):45–50.
Article PubMed Google Scholar
Sadullaeva SA, Sadullaeva UA, Artikova MA, Radjabova MR. Analysis of detection and segmentation of uterine fibroids between Uzbek women. NeuroQuantology. 2022;20(10):83.
Google Scholar
Stoelinga B, Hehenkamp WJK, Brölmann HAM, Huirne JAF. Real-time elastography for assessment of uterine disorders. Ultrasound Obstet Gynecol. 2014;43(2):218–26.
Article CAS PubMed Google Scholar
Manek AS, Mishra P. 2021, March. UFMDRA: Uterine Fibroid Medicinal Drugs Review Analysis. In IOP Conference Series: Materials Science and Engineering (Vol. 1110, No. 1, p. 012006). IOP Publishing.
Raimondo D, Raffone A, Aru AC, Giorgi M, Giaquinto I, Spagnolo E, Travaglino A, Galatolo FA, Cimino MGCA, Lenzi J, Centini G. 2023. Application of deep learning model in the sonographic diagnosis of uterine adenomyosis. International Journal of Environmental Research and Public Health , 20 (3), p.1724.
Liu J, Wang Z. 2022. Advances in the preoperative identification of uterine sarcoma. Cancers , 14 (14), p.3517.
Behboodi B, Rivaz H, Lalondrelle S, Harris E. 2021, September. Automatic 3D ultrasound segmentation of uterus using deep learning. In 2021 IEEE international ultrasonics symposium (IUS) (pp. 1–4). IEEE.
Dilna KT, Hemanth DJ. Detection of uterus fibroids in ultrasound images: a survey. Int J Pure Appl Math. 2018;118:139–59.
Yang T. Uterine fibroid ultrasound images. Mendeley Data. 2023;V2. https://doi.org/10.17632/n2zcmcypgb.2
Sulaiman A, Anand V, Gupta S, Asiri Y, Elmagzoub MA, Reshan MSA, Shaikh A. A convolutional neural network architecture for segmentation of lung diseases using chest X-ray images. Diagnostics. 2023;13(9):1651.
Article PubMed PubMed Central Google Scholar
Anand V, Gupta S, Koundal D, Mahajan S, Pandit AK, Zaguia A. Deep learning based automated diagnosis of skin diseases using dermoscopy. Computers Mater Continua. 2022;71(2):3145–60.
Anand V, Gupta S, Koundal D, Nayak SR, Nayak J, Vimal S. 2022. Multi-class skin disease classification using transfer learning model. International Journal on Artificial Intelligence Tools, 31(02), p.2250029.
Li S, Ke S, Yang C, Chen J, Xiong Y, Zheng LA. Ligand-and-structure dual-driven Deep Learning Method for the Discovery of highly potent GnRH1R antagonist to treat Uterine diseases. arXiv preprint 2022, arXiv:2207.11547.
Tang CM, Liu D, Yu XMRI. Image Segmentation System of Uterine fibroids based on AR-Unet Network. Am Sci Res J Eng Technol Sci. 2020;71:1–10.
Yang T, Yuan L, Li P, Liu P. Real-time automatic assisted detection of uterine fibroid in Ultrasound images using a deep learning detector. Ultrasound Med Bio. 2023;49:1616–26. [CrossRef] [PubMed.
Girija DK, Varshney M. Proposed model to detect uterine fibroid by using Data Mining techniques. J Posit Sch Psychol. 2022;6:2062–5.
Huo T, Chen X, Wang Z. Artificial intelligence-aided method to detect uterine fibroids in ultrasound images: a retrospective study. Sci Rep. 2022;13:3714. [CrossRef] [PubMed]].
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
Department of Obstetrics and Gynecology, The Second Hospital of Shanxi Medical University, No. 382, Wuyi Road, Xinghualing District, Taiyuan City, 030001, Shanxi Province, China
Haibin Xi & Wenjing Wang
You can also search for this author in PubMed Google Scholar
Contributions
Haibin Xi and Wenjing Wang participated in the design of this study, and Haibin Xi performed the statistical analysis. Haibin Xi and Wenjing Wang carried out the study and collected background information. Haibin Xi drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Haibin Xi .
Ethics declarations
Ethics statement.
The study was approved by the ethics committee of The Second Hospital of Shanxi Medical University.
Consent for publication
Conflict of interest.
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Xi, H., Wang, W. Deep learning based uterine fibroid detection in ultrasound images. BMC Med Imaging 24 , 218 (2024). https://doi.org/10.1186/s12880-024-01389-z
Download citation
Received : 28 May 2024
Accepted : 01 August 2024
Published : 19 August 2024
DOI : https://doi.org/10.1186/s12880-024-01389-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Attention mechanism
- EfficientNetB0
- Classification

BMC Medical Imaging
ISSN: 1471-2342
- General enquiries: [email protected]

Ultrasound for the future
New NIH-funded program emphasizes ultrasound research commercialization
This article was originally published on July 26, 2024 by NC State Engineering Communications and can be read here .

Ultrasound is one of the best-known medical imaging devices. It is safe, cost-efficient and relatively portable, making it one of the most accessible diagnostic and therapeutic medical technologies.
And its potential goes far beyond what it is being used for today.
“I think ultrasound is going to impact public health by providing a lot more information to a lot more people a lot more easily,” said Caterina Gallippi, professor of biomedical engineering. “There’s a term that’s sometimes thrown around, and that’s called the democratization of ultrasound. Meaning that everyone has equal access to it, or at least ready access to it.”
Innovations in ultrasound like handheld devices, wearable sensors and the use of artificial intelligence (AI) are already helping improve access to it. Smaller, handheld ultrasound systems are less expensive, costing as little as $2,000 compared to the $100,000 for a traditional system. More advancements in the field are coming, and to ensure these developments are effectively contributing to the democratization of ultrasound, it’s important for researchers to be factoring in the needs of physicians and patients.
To help prepare biomedical engineers to be better trained in conducting their research and pursuing innovation with end users in mind, Gallippi applied for T-32 funding from the National Institutes of Health — which requires that a program provides unique experiences to students and prepares them to meet critical health care needs — to create a training program focused on ultrasound and entrepreneurship.
The Unified Medical Ultrasound Technology Development (UNMUTED) Predoctoral Training Program teaches students entrepreneurship skills that they can apply to their doctoral research and beyond. The earlier students start thinking about how their research might translate to a commercial space, the more prepared they will be to commercialize their research and make a broader impact.

The program is the first of its kind, and the Joint Department of Biomedical Engineering at North Carolina State University and the University of North Carolina at Chapel Hill is the perfect home for it due to ready access to clinical settings and to medical imaging companies in Research Triangle Park.
Selected students do not have to be biomedical engineers, but they do have to be working in a lab focused on ultrasound research. UNMUTED fellows are part of the program for two years. They take two graduate-level courses on technology commercialization and startups, go through several entrepreneurship trainings, shadow physicians in clinical settings to see how they are using ultrasound and complete a summer internship to learn more about industry.
“When [applying for the grant], I had to really think hard about what we are going to offer the trainees in this program that’s different or unique, because really any Ph.D. student who’s studying ultrasound in the Triangle has access to a large pool of expertise, resources and collaborations,” Gallippi said. “And I started to think about not only the environment in terms of expertise in ultrasound, but also in terms of the potential for commercially translating technology.”
Knowing your users
One of the most common reasons a startup fails is because its founders never determined if there was a customer for the technology it created.
That’s a lesson the first two UNMUTED fellows, Ph.D. students Roshni Gandhi and Shureed Deepro Qazi, took away from the National Science Foundation (NSF) Innovation Corps (I-Corps) program.
The immersive I-Corps program focuses on training participants to perform customer discovery, with the goal of commercializing research projects to broaden their societal and economic benefits. Gandhi, Qazi and Gallippi completed the training through Kickstart Venture Services, an Innovate Carolina department that provides entrepreneurial and commercialization resources to research-based startups and the UNC-Chapel Hill community. UNC-Chapel Hill and NC State are both part of the NSF Mid-Atlantic I-Corps Hub.
The UNMUTED team performed customer discovery for a university invention that detects blood viability using ultrasound. They came up with a hypothesis on how the product would help hospitals and blood banks. Rather than pitching their product, they interviewed 20 potential users, asking them unbiased questions about their processes for determining blood viability.
“We learned after interviewing lots of different people, like emergency medical technicians and directors of blood banks, that our product actually didn’t necessarily have a space in that market,” Gandhi said.
Most blood banks and hospitals use an expiration date system to determine blood viability. The researchers found in their interviews that there was little blood being wasted, and that the main problem is a shortage in blood donations.
But just because their product didn’t have a critical need in one market doesn’t mean it won’t be useful somewhere.
“There’s never a negative result [in the I-Corps program],” said Mireya McKee, director of KickStart. “You learn from that. Pivoting is a vital skill that all of us in research and in startups need to learn how to do. Just because something doesn’t work out the way that you’re trying to do it doesn’t mean that there’s not another market out there.”
Making waves in ultrasound entrepreneurship
Emphasizing entrepreneurship is not new to Joint BME. Several faculty members and students have launched startups from their research. Coming up with a way to immerse students in commercialization and entrepreneurship training early in their academic years has been a natural progression.
“The companies that have spun out from BME, the faculty, innovators, the trainees that support the development of the intellectual property, they’re very committed to commercialization,” said Judy Prasad, associate director of KickStart. “They show a strong interest in entrepreneurship.”
As they move forward with their research, both Qazi and Gandhi recognize the value of their early forays into entrepreneurship-based experiences.
“I wanted to get some of the knowledge that it takes to commercialize these technologies,” Qazi said. “For the first couple years of the Ph.D., you’re focused on finishing your classes. And you don’t feel too deep into your research yet, so I thought it’d be cool if I could learn about commercializing.”
The companies that have spun out from BME, the faculty, innovators, the trainees that support the development of the intellectual property, they’re very committed to commercialization…” – Judy Prasad
Qazi, who is part of Gallippi’s lab, is interested in the diagnostic applications of ultrasound and in using machine learning to help decipher imaging results. He is also working on a project on clutter filtering to improve 3-D Doppler ultrasound imaging, which is used to measure blood flow.
Gandhi is a member of BME Chair Paul Dayton’s lab, which has launched several startups. She is currently focused on nanodroplet sonothrombolysis, a therapeutic ultrasound-based technique used to break up blood clots.
“Microbubble sonothrombolysis has been well-studied in the past, and I’m focusing on nanodroplet sonothrombolysis, and that’s more novel,” she said. “There’s a lot more to be done in that field and more that I can discover.”
Ultrasound is only going to become more accessible and easier to use, while its applications continue to grow in two extremes: more advanced computer architecture that enables high-resolution imaging, and more portable, handheld and wearable devices that can be brought anywhere.
UNMUTED will help students have a leg up as they discover their own research breakthroughs that contribute to ultrasound innovation.
“We hope that our students are trained to look at their science with that sort of critical eye,” Gallippi said. “And that they proactively take steps to support the development of that technology toward commercial impact.”
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
August 23, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New imaging device improves ear disease diagnosis
In the realm of ear health, accurate diagnosis is crucial for effective treatment, especially when dealing with conditions that can lead to hearing loss. Traditionally, otolaryngologists have relied on the otoscope, a device that provides a limited view of the eardrum's surface. This conventional tool, while useful, has its limitations, particularly when the tympanic membrane (TM) is opaque due to disease.
Enter a groundbreaking advancement from the University of Southern California's Caruso Department of Otolaryngology: a portable OCT otoscope that integrates optical coherence tomography (OCT) with the traditional otoscope, to improve diagnostic capabilities in hearing clinics. As reported in Journal of Biomedical Optics , the integrated device allows clinicians to obtain detailed views of both the surface and the deeper structures of the eardrum and middle ear, enabling a more comprehensive picture of ear health and improving diagnostic accuracy.
Traditional otoscopes only allow for a superficial examination of the TM, often missing deeper pathologies. In contrast, the OCT otoscope combines the familiar otoscopic view with high-resolution imaging of the inner structures of the TM and middle ear (ME), offering a clearer and more comprehensive view, which can help in diagnosing conditions that were previously missed.
This state-of-the-art device features a 7.4 mm field of view and impressive lateral and axial resolutions of 38 micrometers and 33.4 micrometers, respectively. It also integrates advanced algorithms to enhance image clarity and correct distortions, ensuring precise and reliable results.
During a clinical study at USC Keck Hospital, the researchers tested the OCT otoscope on over 100 patients. These tests demonstrate the new device's ability to reveal pathological features that were previously invisible using standard otoscopy.
Notably, the article showcases a few clinical applications including monitoring myringitis, tympanic membrane perforation healing, retraction pockets, and subsurface scarring / air pockets; the new imaging system identified several critical conditions that were not apparent through traditional methods, offering valuable insights for more effective management and treatment of ear diseases.
The OCT otoscope's design allows for seamless integration into existing clinical workflows, with an easy-to-use interface controlled by a foot pedal for image acquisition. This user-friendly approach ensures that the device can be readily adopted by clinicians, providing them with a powerful new tool for diagnosing and managing TM and ME disorders.
Overall, this advancement marks a significant step forward in otolaryngology, enhancing the precision of ear examinations and potentially leading to better outcomes for patients suffering from hearing loss due to ear pathologies. As this technology becomes more widely available, it promises to transform the way ear health is assessed and treated, offering hope for more accurate diagnoses and improved patient care.
Explore further
Feedback to editors

Self-deployable, biodegradable electrode offers minimally invasive brain signal monitoring
14 hours ago

Study identifies metabolic switch essential for generation of memory T cells and anti-tumor immunity
Aug 24, 2024

Multiple sclerosis appears to protect against Alzheimer's disease
Aug 23, 2024

Good sleep habits important for overweight adults, study suggests

Mediterranean diet supplement can affect epigenetics associated with healthy aging

New method for quantifying boredom in the body during temporary stress

Cancer researchers develop new method that uses internal clock inside tumor cells to optimize therapies

Strength training activates cellular waste disposal, interdisciplinary research reveals

Being a 'weekend warrior' could be as good for brain health as exercising throughout the week
Simple blood test for Alzheimer's disease could change how the disease is detected and diagnosed
Related stories.

New study shows promising diagnosis of multiple sclerosis from images of the eye
Jul 17, 2024

Updates from the 2022 WHO classification of kidney epithelial tumors
Jun 18, 2024

Deep learning approach enhances HER2 scoring in breast cancer

New system offers more reliable, cost-effective solution for continuous glucose monitoring
Aug 14, 2024

Applications of AI in medicine
Jul 2, 2024

Cutting-edge 3D-printed microneedle technology revolutionizes remote health care
Aug 7, 2024
Recommended for you
Spike mutations that help SARS-CoV-2 infect the brain discovered

Chlamydia can settle in the intestine, organoid experiments reveal
Men infected with high-risk types of HPV could struggle with fertility

Macrophage mix helps determine rate and fate of fatty liver disease, study finds
Aug 22, 2024

A new culprit in Huntington's: Brain organoid model implicates gene in disease progression
Universal flu vaccine candidate protects against infection in mice
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 26 October 2020
Applications of focused ultrasound in the brain: from thermoablation to drug delivery
- Ying Meng ORCID: orcid.org/0000-0003-3180-2404 1 , 2 , 3 ,
- Kullervo Hynynen 4 , 5 &
- Nir Lipsman ORCID: orcid.org/0000-0002-4820-3056 1 , 2 , 3
Nature Reviews Neurology volume 17 , pages 7–22 ( 2021 ) Cite this article
12k Accesses
228 Citations
108 Altmetric
Metrics details
- Neurological disorders
- Therapeutics
Focused ultrasound (FUS) is a disruptive medical technology, and its implementation in the clinic represents the culmination of decades of research. Lying at the convergence of physics, engineering, imaging, biology and neuroscience, FUS offers the ability to non-invasively and precisely intervene in key circuits that drive common and challenging brain conditions. The actions of FUS in the brain take many forms, ranging from transient blood–brain barrier opening and neuromodulation to permanent thermoablation. Over the past 5 years, we have seen a dramatic expansion of indications for and experience with FUS in humans, with a resultant exponential increase in academic and public interest in the technology. Applications now span the clinical spectrum in neurological and psychiatric diseases, with insights still emerging from preclinical models and human trials. In this Review, we provide a comprehensive overview of therapeutic ultrasound and its current and emerging indications in the brain. We examine the potential impact of FUS on the landscape of brain therapies as well as the challenges facing further advancement and broader adoption of this promising minimally invasive therapeutic alternative.
Recent advances have led to a surge of interest in focused ultrasound (FUS) as a non-invasive, potentially disruptive tool for the most intractable neurological conditions.
Magnetic resonance-guided FUS thermoablation has been approved for the treatment of essential tremor and tremor-dominant Parkinson disease and is being investigated in psychiatric applications as well as in chronic pain and epilepsy.
Transient opening of the blood–brain barrier for drug delivery is a burgeoning field, with early human studies demonstrating a favourable safety profile as well as versatility across and scalability within a range of clinical indications.
Future studies will investigate the delivery of established pharmaceuticals and novel therapies in combination with FUS blood–brain barrier opening.
Emerging applications are also harnessing the myriad of ways in which FUS can interact with the CNS, including immune modulation and neuromodulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
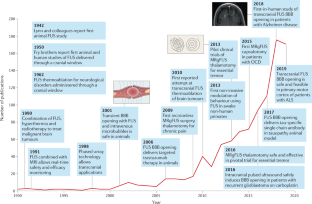
Similar content being viewed by others
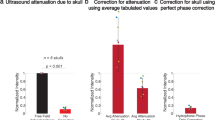
Controlled noninvasive modulation of deep brain regions in humans

Guiding and monitoring focused ultrasound mediated blood–brain barrier opening in rats using power Doppler imaging and passive acoustic mapping
Use of transcranial low-intensity focused ultrasound for targeted delivery of stem cell-derived exosomes to the brain
Aubry, J.-F. et al. The road to clinical use of high-intensity focused ultrasound for liver cancer: technical and clinical consensus. J. Ther. Ultrasound 1 , 13 (2013).
PubMed PubMed Central Google Scholar
Tempany, C. M. C., McDannold, N. J., Hynynen, K. & Jolesz, F. A. Focused ultrasound surgery in oncology: overview and principles. Radiology 259 , 39–56 (2011).
El-Hayek, Y. H. et al. Tip of the iceberg: assessing the global socioeconomic costs of Alzheimer’s disease and related dementias and strategic implications for stakeholders. J. Alzheimers Dis. 70 , 323–341 (2019).
Aldape, K. et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 16 , 509–520 (2019).
CAS PubMed PubMed Central Google Scholar
Makin, S. The amyloid hypothesis on trial. Nature 559 , S4–S7 (2018).
CAS PubMed Google Scholar
Lozano, A. M. et al. A phase II study of fornix deep brain stimulation in mild Alzheimer’s disease. J. Alzheimers Dis. 54 , 777–787 (2016).
Elias, W. J. et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med. 375 , 730–739 (2016). This pivotal study led to regulatory approval of the first approved indication for MRgFUS thermoablation in the treatment of essential tremor .
PubMed Google Scholar
Bond, A. E. et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease: a randomized clinical trial. JAMA Neurol. 74 , 1412–1418 (2017). This pivotal study led to regulatory approval of the second — and, to date, only other — approved clinical indication for MRgFUS thermoablation in the treatment of TDPD .
Leinenga, G., Langton, C., Nisbet, R. & Götz, J. Ultrasound treatment of neurological diseases — current and emerging applications. Nat. Rev. Neurol. 12 , 161–174 (2016).
Gandaglia, G. et al. Effect of minimally invasive surgery on the risk for surgical site infections: results from the National Surgical Quality Improvement Program (NSQIP) Database. JAMA Surg. 149 , 1039–1044 (2014).
Hynynen, K. & Jones, R. M. Image-guided ultrasound phased arrays are a disruptive technology for non-invasive therapy. Phys. Med. Biol. 61 , R206–R248 (2016).
Raymond, S. B. & Hynynen, K. Acoustic transmission losses and field alterations due to human scalp hair. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 52 , 1415–1419 (2005).
Meyers, R. et al. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. J. Neurosurg. 16 , 32–54 (1959).
Nelson, E., Lindstrom, P. A. & Haymaker, W. Pathological effects of ultrasound on the human brain: a study of 25 cases in which ultrasonic irradiation was used as a lobotomy procedure. J. Neuropathol. Exp. Neurol. 18 , 489–508 (1959).
Leksell, L. Echo-encephalography. I. Detection of intracranial complications following head injury. Acta Chir. Scand. 110 , 301–315 (1956).
Jagannathan, J. et al. High-intensity focused ultrasound surgery of the brain: part 1 — a historical perspective with modern applications. Neurosurgery 64 , 201–210 (2009).
Guthkelch, A. N. et al. Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: results of a phase I trial. J. Neurooncol. 10 , 271–284 (1991).
Ram, Z. et al. Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery 59 , 949–955 (2006).
Hynynen, K. et al. Pre-clinical testing of a phased array ultrasound system for MRI-guided noninvasive surgery of the brain — a primate study. Eur. J. Radiol. 59 , 149–156 (2006).
Clement, G. T. & Hynynen, K. A non-invasive method for focusing ultrasound through the human skull. Phys. Med. Biol. 47 , 1219–1236 (2002).
Aubry, J.-F. & Tanter, M. MR-guided transcranial focused ultrasound. Adv. Exp. Med. Biol. 880 , 97–111 (2016).
Haworth, K. J., Fowlkes, J. B., Carson, P. L. & Kripfgans, O. D. Towards aberration correction of transcranial ultrasound using acoustic droplet vaporization. Ultrasound Med. Biol. 34 , 435–445 (2008).
Hynynen, K., Darkazanli, A., Unger, E. & Schenck, J. F. MRI-guided noninvasive ultrasound surgery. Med. Phys. 20 , 107–115 (1993).
Jeanmonod, D. et al. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg. Focus 32 , E1 (2012).
Carpentier, A. et al. Clinical trial of blood–brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 8 , 343re2 (2016).
Maimbourg, G., Houdouin, A., Deffieux, T., Tanter, M. & Aubry, J.-F. 3D-printed adaptive acoustic lens as a disruptive technology for transcranial ultrasound therapy using single-element transducers. Phys. Med. Biol. 63 , 025026 (2018).
Haar, G. T. & Coussios, C. High intensity focused ultrasound: physical principles and devices. Int. J. Hyperth. 23 , 89–104 (2007).
Google Scholar
Mouratidis, P. X. E., Rivens, I., Civale, J., Symonds-Tayler, R. & Ter Haar, G. ‘Relationship between thermal dose and cell death for “rapid” ablative and “slow” hyperthermic heating’. Int. J. Hyperth. 36 , 228–242 (2019).
Hynynen, K., McDannold, N., Vykhodtseva, N. & Jolesz, F. A. Noninvasive MR imaging-guided focal opening of the blood–brain barrier in rabbits. Radiology 220 , 640–646 (2001).
Sukovich, J. R. et al. In vivo histotripsy brain treatment. J. Neurosurg. 131 , 1331–1338 (2019).
Lozano, A. M. et al. Deep brain stimulation: current challenges and future directions. Nat. Rev. Neurol. 15 , 148–160 (2019).
Deuschl, G. et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 355 , 896–908 (2006).
McDannold, N., Clement, G. T., Black, P., Jolesz, F. & Hynynen, K. Transcranial magnetic resonance imaging-guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery 66 , 323–332 (2010).
Coluccia, D. et al. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J. Ther. Ultrasound 2 , 17 (2014).
Jung, N. Y. et al. Factors related to successful energy transmission of focused ultrasound through a skull: a study in human cadavers and its comparison with clinical experiences. J. Korean Neurosurg. Soc. 62 , 712–722 (2019).
Benito-León, J. & Louis, E. D. Essential tremor: emerging views of a common disorder. Nat. Rev. Neurol. 2 , 666–678 (2006).
Elble, R. J. The essential tremor syndromes. Curr. Opin. Neurol. 29 , 507–512 (2016).
Elble, R. J. Mechanisms of deep brain stimulation for essential tremor. Brain 137 , 4–6 (2014).
Sharifi, S., Nederveen, A. J., Booij, J. & van Rootselaar, A.-F. Neuroimaging essentials in essential tremor: a systematic review. Neuroimage Clin. 5 , 217–231 (2014).
Dallapiazza, R. F. et al. Outcomes from stereotactic surgery for essential tremor. J. Neurol. Neurosurg. Psychiatry 90 , 474–482 (2019).
Lipsman, N. et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol. 12 , 462–468 (2013).
Elias, W. J. et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med. 369 , 640–648 (2013).
Scantlebury, N. et al. Change in some quality of life domains mimics change in tremor severity after ultrasound thalamotomy. Mov. Disord. 34 , 1400–1401 (2019).
Chang, J. W. et al. A prospective trial of magnetic resonance guided focused ultrasound thalamotomy for essential tremor: results at the 2-year follow-up. Ann. Neurol. 83 , 107–114 (2017).
Meng, Y. et al. Magnetic resonance-guided focused ultrasound thalamotomy for treatment of essential tremor: a 2-year outcome study: MRgFUS thalamotomy for ET: 2-year outcome. Mov. Disord. 33 , 1647–1650 (2018).
Park, Y.-S., Jung, N. Y., Na, Y. C. & Chang, J. W. Four-year follow-up results of magnetic resonance-guided focused ultrasound thalamotomy for essential tremor. Mov. Disord. 34 , 727–734 (2019).
Weidman, E. K., Kaplitt, M. G., Strybing, K. & Chazen, J. L. Repeat magnetic resonance imaging-guided focused ultrasound thalamotomy for recurrent essential tremor: case report and review of MRI findings. J. Neurosurg. 132 , 211–216 (2020).
Fishman, P. S. et al. Neurological adverse event profile of magnetic resonance imaging-guided focused ultrasound thalamotomy for essential tremor. Mov. Disord. 33 , 843–847 (2018).
Boutet, A. et al. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain 141 , 3405–3414 (2018).
Pineda-Pardo, J. A. et al. Transcranial magnetic resonance-guided focused ultrasound thalamotomy in essential tremor: a comprehensive lesion characterization. Neurosurgery 87 , 256–265 (2019).
Wintermark, M. et al. Thalamic connectivity in patients with essential tremor treated with MR imaging-guided focused ultrasound: in vivo fiber tracking by using diffusion-tensor MR imaging. Radiology 272 , 202–209 (2014).
Pineda-Pardo, J. A. et al. Microstructural changes of the dentato-rubro-thalamic tract after transcranial MR guided focused ultrasound ablation of the posteroventral VIM in essential tremor. Hum. Brain Mapp. 40 , 2933–2942 (2019).
Pouratian, N., Baltuch, G., Elias, W. J. & Gross, R. American Society for Stereotactic and Functional Neurosurgery position statement on magnetic resonance-guided focused ultrasound for the management of essential tremor. Neurosurgery 87 , E126–E129 (2020).
Ravikumar, V. K. et al. Cost-effectiveness of focused ultrasound, radiosurgery, and DBS for essential tremor. Mov. Disord. 32 , 1165–1173 (2017).
Li, C. et al. Cost-effectiveness of magnetic resonance-guided focused ultrasound for essential tremor. Mov. Disord. 34 , 735–743 (2019).
Horisawa, S. et al. A single case of MRI-guided focused ultrasound ventro-oral thalamotomy for musician’s dystonia. J. Neurosurg. 131 , 384–386 (2018).
Meng, Y., Suppiah, S., Scantlebury, N., Lipsman, N. & Schwartz, M. L. Treatment of a patient with task-specific writing tremor using magnetic resonance-guided focused ultrasound. Can. J. Neurol. Sci. 45 , 474–477 (2018).
Fasano, A. et al. MRI-guided focused ultrasound thalamotomy in non-ET tremor syndromes. Neurology 89 , 771–775 (2017).
Poewe, W. et al. Parkinson disease. Nat. Rev. Dis. Primers 3 , 17013 (2017).
Kalia, S. K., Sankar, T. & Lozano, A. M. Deep brain stimulation for Parkinson’s disease and other movement disorders. Curr. Opin. Neurol. 26 , 374–380 (2013).
Prasad, S. et al. Spinal cord stimulation for very advanced Parkinson’s disease: a 1-year prospective trial. Mov. Disord. 35 , 1082–1083 (2020).
Stefani, A. et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain 130 , 1596–1607 (2007).
López-Azcárate, J. et al. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. J. Neurosci. 30 , 6667–6677 (2010).
Martínez-Fernández, R. et al. Focused ultrasound subthalamotomy in patients with asymmetric Parkinson’s disease: a pilot study. Lancet Neurol. 17 , 54–63 (2018). This small open-label trial showed that unilateral MRgFUS subthalamotomy was technically feasible, effective and associated with a relatively low risk of hemichorea–ballism .
Jung, N. Y. et al. The efficacy and limits of magnetic resonance-guided focused ultrasound pallidotomy for Parkinson’s disease: a phase I clinical trial. J. Neurosurg. 130 , 1853–1861 (2018).
Gallay, M. N. et al. MRgFUS pallidothalamic tractotomy for chronic therapy-resistant Parkinson’s disease in 51 consecutive patients: single center experience. Front. Surg. 6 , 76 (2020).
Alvarez, L. Bilateral subthalamotomy in Parkinson’s disease: initial and long-term response. Brain 128 , 570–583 (2005).
Meng, Y. et al. Cost-effectiveness analysis of MR-guided focused ultrasound thalamotomy for tremor-dominant Parkinson’s disease. J. Neurosurg. https://doi.org/10.3171/2020.5.JNS20692 (2020).
Article PubMed Google Scholar
Stein, D. J. et al. Obsessive–compulsive disorder. Nat. Rev. Dis. Primers 5 , 52 (2019).
Garnaat, S. L. et al. Who qualifies for deep brain stimulation for OCD? Data from a naturalistic clinical sample. J. Neuropsychiatry Clin. Neurosci. 26 , 81–86 (2014).
Pauls, D. L., Abramovitch, A., Rauch, S. L. & Geller, D. A. Obsessive–compulsive disorder: an integrative genetic and neurobiological perspective. Nat. Rev. Neurosci. 15 , 410–424 (2014).
Whiteside, S. P., Port, J. D. & Abramowitz, J. S. A meta-analysis of functional neuroimaging in obsessive–compulsive disorder. Psychiatry Res. 132 , 69–79 (2004).
Hamani, C. et al. Deep brain stimulation for obsessive–compulsive disorder. Neurosurgery 75 , 327–333 (2014).
Mallet, L. et al. Subthalamic nucleus stimulation in severe obsessive–compulsive disorder. N. Engl. J. Med. 359 , 2121–2134 (2008).
Denys, D. et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive–compulsive disorder. Arch. Gen. Psychiatry 67 , 1061–1068 (2010).
Rück, C. et al. Capsulotomy for obsessive–compulsive disorder: long-term follow-up of 25 patients. Arch. Gen. Psychiatry 65 , 914–921 (2008).
Alonso, P. et al. Deep brain stimulation for obsessive–compulsive disorder: a meta-analysis of treatment outcome and predictors of response. PLoS ONE 10 , e0133591 (2015).
Jung, H. H. et al. Bilateral thermal capsulotomy with MR-guided focused ultrasound for patients with treatment-refractory obsessive–compulsive disorder: a proof-of-concept study. Mol. Psychiatry 20 , 1205–1211 (2015). The first use of MRgFUS thermoablation for bilateral anterior capsulotomy to treat a psychiatric disorder .
Kim, S. J. et al. A study of novel bilateral thermal capsulotomy with focused ultrasound for treatment-refractory obsessive–compulsive disorder: 2-year follow-up. J. Psychiatry Neurosci. 43 , 170188 (2018).
Brown, L. T. et al. Dorsal anterior cingulotomy and anterior capsulotomy for severe, refractory obsessive–compulsive disorder: a systematic review of observational studies. J. Neurosurg. 124 , 77–89 (2016).
Davidson, B. et al. Magnetic resonance-guided focused ultrasound capsulotomy for refractory obsessive compulsive disorder and major depressive disorder: clinical and imaging results from two phase I trials. Mol. Psychiatry 25 , 1946–1957 (2020).
Otte, C. et al. Major depressive disorder. Nat. Rev. Dis. Primers 2 , 16065 (2016).
Kim, M., Kim, C.-H., Jung, H. H., Kim, S. J. & Chang, J. W. Treatment of major depressive disorder via magnetic resonance-guided focused ultrasound surgery. Biol. Psychiatry 83 , e17–e18 (2018).
Treede, R.-D. et al. A classification of chronic pain for ICD-11. Pain 156 , 1003–1007 (2015).
The Lancet Neurology. Novel ways to manage chronic pain are needed. Lancet Neurol. 17 , 829 (2018).
Burchiel, K. J. & Raslan, A. M. Contemporary concepts of pain surgery. J. Neurosurg. 130 , 1039–1049 (2019).
Martin, E., Jeanmonod, D., Morel, A., Zadicario, E. & Werner, B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann. Neurol. 66 , 858–861 (2009). The first report of incisionless surgery using MRgFUS thermoablation in humans, undertaken in patients with chronic pain .
Clary, A., Tyler, W. J. & Wetmore, D. Z. Abstract #45: ultrasound neuromodulation for the treatment of peripheral nerve compression syndromes. Brain Stimul. 12 , e16 (2019).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14 , 133–150 (2018).
Obermeier, B., Verma, A. & Ransohoff, R. M. The blood–brain barrier. Handb. Clin. Neurol. 133 , 39–59 (2016).
Galea, I., Bechmann, I. & Perry, V. H. What is immune privilege (not)? Trends Immunol. 28 , 12–18 (2007).
van Tellingen, O. et al. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 19 , 1–12 (2015).
Arvanitis, C. D., Ferraro, G. B. & Jain, R. K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 20 , 26–41 (2020).
Garbuzova-Davis, S., Thomson, A., Kurien, C., Shytle, R. D. & Sanberg, P. R. Potential new complication in drug therapy development for amyotrophic lateral sclerosis. Expert Rev. Neurother. 16 , 1397–1405 (2016).
Pardridge, W. M. The blood–brain barrier: bottleneck in brain drug development. NeuroRX 2 , 3–14 (2005).
Jablonski, M. R. et al. Inhibiting drug efflux transporters improves efficacy of ALS therapeutics. Ann. Clin. Transl. Neurol. 1 , 996–1005 (2014).
Meng, Y. et al. Safety and efficacy of focused ultrasound induced blood–brain barrier opening, an integrative review of animal and human studies. J. Control. Release 309 , 25–36 (2019).
O’Reilly, M. A., Waspe, A. C., Chopra, R. & Hynynen, K. MRI-guided disruption of the blood–brain barrier using transcranial focused ultrasound in a rat model. J. Vis. Exp. 61 , 3555 (2012).
Pelekanos, M. et al. Establishing sheep as an experimental species to validate ultrasound-mediated blood–brain barrier opening for potential therapeutic interventions. Theranostics 8 , 2583–2602 (2018).
Kovacs, Z. I. et al. Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl Acad. Sci. USA 114 , E75–E84 (2017).
Poon, C. T. et al. Time course of focused ultrasound effects on β-amyloid plaque pathology in the TgCRND8 mouse model of Alzheimer’s disease. Sci. Rep. 8 , 14061 (2018).
Jordão, J. F. et al. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp. Neurol. 248 , 16–29 (2013).
McMahon, D., Bendayan, R. & Hynynen, K. Acute effects of focused ultrasound-induced increases in blood–brain barrier permeability on rat microvascular transcriptome. Sci. Rep. 7 , 45657 (2017).
McMahon, D. & Hynynen, K. Acute inflammatory response following increased blood–brain barrier permeability induced by focused ultrasound is dependent on microbubble dose. Theranostics 7 , 3989–4000 (2017).
Olumolade, O. O., Wang, S., Samiotaki, G. & Konofagou, E. E. Longitudinal motor and behavioral assessment of blood–brain barrier opening with transcranial focused ultrasound. Ultrasound Med. Biol. 42 , 2270–2282 (2016).
Horodyckid, C. et al. Safe long-term repeated disruption of the blood–brain barrier using an implantable ultrasound device: a multiparametric study in a primate model. J. Neurosurg. 126 , 1351–1361 (2017).
Kinoshita, M., McDannold, N., Jolesz, F. A. & Hynynen, K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Proc. Natl Acad. Sci. USA 103 , 11719–11723 (2006).
Wu, S.-K. et al. Characterization of different microbubbles in assisting focused ultrasound-induced blood–brain barrier opening. Sci. Rep. 7 , 46689 (2017).
McDannold, N., Vykhodtseva, N. & Hynynen, K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood–brain barrier disruption. Ultrasound Med. Biol. 34 , 930–937 (2008).
Chen, H. & Konofagou, E. E. The size of blood–brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure. J. Cereb. Blood Flow Metab. 34 , 1197–1204 (2014).
Jordão, J. F. et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer’s disease. PLoS ONE 5 , e10549 (2010).
Kobus, T., Zervantonakis, I. K., Zhang, Y. & McDannold, N. J. Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated blood–brain barrier disruption. J. Control. Release 238 , 281–288 (2016).
Liu, H.-L. et al. Focused ultrasound enhances central nervous system delivery of bevacizumab for malignant glioma treatment. Radiology 281 , 99–108 (2016).
Alecou, T., Giannakou, M. & Damianou, C. Amyloid β plaque reduction with antibodies crossing the blood–brain barrier, which was opened in 3 sessions of focused ultrasound in a rabbit model. J. Ultrasound Med. 36 , 2257–2270 (2017).
Alli, S. et al. Brainstem blood brain barrier disruption using focused ultrasound: a demonstration of feasibility and enhanced doxorubicin delivery. J. Control. Release 281 , 29–41 (2018).
Coluccia, D. et al. Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomedicine 14 , 1137–1148 (2018).
Thévenot, E. et al. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum. Gene Ther. 23 , 1144–1155 (2012).
Burgess, A. et al. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood–brain barrier. PLoS ONE 6 , e27877 (2011).
Alkins, R. et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 73 , 1892–1899 (2013).
Alkins, R., Burgess, A., Kerbel, R., Wels, W. S. & Hynynen, K. Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro Oncol. 18 , 974–981 (2016).
Noroozian, Z. et al. MRI-guided focused ultrasound for targeted delivery of rAAV to the brain. Methods Mol. Biol. 1950 , 177–197 (2019).
Lipsman, N. et al. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 9 , 2336 (2018). The first report that transcranial FUS BBBO is safe in people with mild-to-moderate AD .
Pouliopoulos, A. N. et al. A clinical system for non-invasive blood–brain barrier opening using a neuronavigation-guided single-element focused ultrasound transducer. Ultrasound Med. Biol. 46 , 73–89 (2020).
Asquier, N. et al. Blood–brain barrier disruption in humans using an implantable ultrasound device: quantification with MR images and correlation with local acoustic pressure. J. Neurosurg. 132 , 875–883 (2019).
Beccaria, K. et al. Blood–brain barrier disruption with low-intensity pulsed ultrasound for the treatment of pediatric brain tumors: a review and perspectives. Neurosurg. Focus. 48 , E10 (2020).
Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. Alzheimers Dement. 13 , 325–373 (2017).
Masters, C. L. et al. Alzheimer’s disease. Nat. Rev. Dis. Primers 1 , 15056 (2015).
Greenberg, S. M. et al. Cerebral amyloid angiopathy and Alzheimer disease — one peptide, two pathways. Nat. Rev. Neurol. 16 , 30–42 (2020).
Nisbet, R. M. et al. Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. Brain 140 , 1220–1230 (2017).
Xhima, K. et al. Focused ultrasound delivery of a selective TrkA agonist rescues cholinergic function in a mouse model of Alzheimer’s disease. Sci. Adv. 6 , eaax6646 (2020).
Burgess, A. et al. Alzheimer disease in a mouse model: MR imaging-guided focused ultrasound targeted to the hippocampus opens the blood–brain barrier and improves pathologic abnormalities and behavior. Radiology 273 , 736–745 (2014).
Leinenga, G. & Götz, J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer’s disease mouse model. Sci. Transl. Med. 7 , 278ra33 (2015).
Leinenga, G. & Götz, J. Safety and efficacy of scanning ultrasound treatment of aged APP23 mice. Front. Neurosci. 12 , 55 (2018).
Scarcelli, T. et al. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul. 7 , 304–307 (2014).
Mooney, S. J. et al. Focused ultrasound-induced neurogenesis requires an increase in blood–brain barrier permeability. PLoS ONE 11 , e0159892 (2016).
Nation, D. A. et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 25 , 270–276 (2019).
Rezai, A. R. et al. Noninvasive hippocampal blood−brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc. Natl Acad. Sci. USA 117 , 9180–9182 (2020).
Panza, F., Lozupone, M., Logroscino, G. & Imbimbo, B. P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 15 , 73–88 (2019).
Hardiman, O. et al. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 3 , 17085 (2017).
Hobson, E. V. & McDermott, C. J. Supportive and symptomatic management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 12 , 526–538 (2016).
Hetz, C. & Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 13 , 477–491 (2017).
Lacomblez, L., Bensimon, G., Meininger, V., Leigh, P. N. & Guillet, P. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Lancet 347 , 1425–1431 (1996).
Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 16 , 505–512 (2017).
Geevasinga, N., Menon, P., Özdinler, P. H., Kiernan, M. C. & Vucic, S. Pathophysiological and diagnostic implications of cortical dysfunction in ALS. Nat. Rev. Neurol. 12 , 651–661 (2016).
Thomsen, G. M. et al. Delayed disease onset and extended survival in the SOD1G93A rat model of amyotrophic lateral sclerosis after suppression of mutant SOD1 in the motor cortex. J. Neurosci. 34 , 15587–15600 (2014).
Thomsen, G. M. et al. Transplantation of neural progenitor cells expressing glial cell line-derived neurotrophic factor into the motor cortex as a strategy to treat amyotrophic lateral sclerosis. Stem Cell 36 , 1122–1131 (2018).
CAS Google Scholar
Abrahao, A. et al. First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat. Commun. 10 , 4373 (2019). The first study of transcranial FUS BBBO in eloquent cortex in people with ALS, showing that the procedure was safe and technically successful .
Axelsen, T. M. & Woldbye, D. P. D. Gene therapy for Parkinson’s disease, an update. J. Parkinsons Dis. 8 , 195–215 (2018).
Xhima, K., Nabbouh, F., Hynynen, K., Aubert, I. & Tandon, A. Noninvasive delivery of an α-synuclein gene silencing vector with magnetic resonance-guided focused ultrasound. Mov. Disord. 33 , 1567–1579 (2018).
Fan, C.-H. et al. Noninvasive, targeted, and non-viral ultrasound-mediated GDNF-plasmid delivery for treatment of Parkinson’s disease. Sci. Rep. 6 , 19579 (2016).
Fan, C.-H., Lin, C.-Y., Liu, H.-L. & Yeh, C.-K. Ultrasound targeted CNS gene delivery for Parkinson’s disease treatment. J. Control. Release 261 , 246–262 (2017).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352 , 987–996 (2005).
Mainprize, T. et al. Blood–brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci. Rep. 9 , 321 (2019).
Idbaih, A. et al. Safety and feasibility of repeated and transient blood–brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin. Cancer Res. 25 , 3793–3801 (2019). This study indicated that the use of an implanted ultrasound device to deliver repeated BBBO during carboplatin administration for recurrent glioblastoma is safe and potentially enhances progression-free survival .
Razavi, S.-M. et al. Immune evasion strategies of glioblastoma. Front. Surg. 3 , 11 (2016).
Chen, P.-Y. et al. Focused ultrasound-induced blood–brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study. J. Transl. Med. 13 , 93 (2015).
Sheybani, N. D., Witter, A. R., Stevens, A. D., Bullock, T. N. & Price, R. J. Focused ultrasound ablation as an immunomodulatory strategy for metastatic breast cancer therapy. J. Immunol. 200 , 178.39 (2018).
Sperling, R. A. et al. Amyloid related imaging abnormalities (ARIA) in amyloid modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 7 , 367–385 (2011).
Schneider, S., Potthast, S., Komminoth, P., Schwegler, G. & Böhm, S. PD-1 checkpoint inhibitor associated autoimmune encephalitis. Case Rep. Oncol. 10 , 473–478 (2017).
Meng, Y. et al. Glymphatics visualization after focused ultrasound-induced blood–brain barrier opening in humans. Ann. Neurol. 86 , 975–980 (2019). Localized and transient BBB opening using FUS allowed the first in vivo visualization of the glymphatic system in humans .
Engelhardt, B., Vajkoczy, P. & Weller, R. O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 18 , 123–131 (2017).
Park, E.-J., Zhang, Y.-Z., Vykhodtseva, N. & McDannold, N. Ultrasound-mediated blood–brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J. Control. Release 163 , 277–284 (2012).
Park, S. H. et al. Safety and feasibility of multiple blood–brain barrier disruptions for the treatment of glioblastoma in patients undergoing standard adjuvant chemotherapy. J. Neurosurg . https://doi.org/10.3171/2019.10.JNS192206 (2020).
O’Reilly, M. A. et al. Preliminary investigation of focused ultrasound-facilitated drug delivery for the treatment of leptomeningeal metastases. Sci. Rep. 8 , 9013 (2018).
Blumberger, D. M. et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet 391 , 1683–1692 (2018).
Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 18 , 1213–1225 (2015).
Dallapiazza, R. F. et al. Noninvasive neuromodulation and thalamic mapping with low-intensity focused ultrasound. J. Neurosurg. 128 , 875–884 (2018).
Folloni, D. et al. Manipulation of subcortical and deep cortical activity in the primate brain using transcranial focused ultrasound stimulation. Neuron 101 , 1109–1116.e5 (2019).
Nicodemus, N. E. et al. Focused transcranial ultrasound for treatment of neurodegenerative dementia. Alzheimers Dement. 5 , 374–381 (2019).
Legon, W. et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat. Neurosci. 17 , 322–329 (2014).
Verhagen, L. et al. Offline impact of transcranial focused ultrasound on cortical activation in primates. eLife 8 , e40541 (2019). Demonstration that the neuromodulatory effect of FUS extends beyond the immediate period of sonication, which increases the translational potential of this technology .
Khalighinejad, N. et al. A basal forebrain–cingulate circuit in macaques decides it is time to act. Neuron 105 , 370–384.e8 (2020).
Wattiez, N. et al. Transcranial ultrasonic stimulation modulates single-neuron discharge in macaques performing an antisaccade task. Brain Stimul. 10 , 1024–1031 (2017).
Younan, Y. et al. Influence of the pressure field distribution in transcranial ultrasonic neurostimulation. Med. Phys. 40 , 082902 (2013).
Sato, T., Shapiro, M. G. & Tsao, D. Y. Ultrasonic neuromodulation causes widespread cortical activation via an indirect auditory mechanism. Neuron 98 , 1031–1041.e5 (2018).
Guo, H. et al. Ultrasound produces extensive brain activation via a cochlear pathway. Neuron 98 , 1020–1030.e4 (2018).
Constans, C., Mateo, P., Tanter, M. & Aubry, J.-F. Potential impact of thermal effects during ultrasonic neurostimulation: retrospective numerical estimation of temperature elevation in seven rodent setups. Phys. Med. Biol. 63 , 025003 (2018).
Deffieux, T. et al. Low-intensity focused ultrasound modulates monkey visuomotor behavior. Curr. Biol. 23 , 2430–2433 (2013).
Yoon, K. et al. Effects of sonication parameters on transcranial focused ultrasound brain stimulation in an ovine model. PLoS ONE 14 , e0224311 (2019).
Darrow, D. P., O’Brien, P., Richner, T. J., Netoff, T. I. & Ebbini, E. S. Reversible neuroinhibition by focused ultrasound is mediated by a thermal mechanism. Brain Stimul. 12 , 1439–1447 (2019).
Oh, S.-J. et al. Ultrasonic neuromodulation via astrocytic TRPA1. Curr. Biol. 29 , 3386–3401.e8 (2019).
Chen, S.-G. et al. Transcranial focused ultrasound pulsation suppresses pentylenetetrazol induced epilepsy in vivo. Brain Stimul. 13 , 35–46 (2020).
Cui, Z. et al. Enhanced neuronal activity in mouse motor cortex with microbubbles’ oscillations by transcranial focused ultrasound stimulation. Ultrason. Sonochem. 59 , 104745 (2019).
Cho, H. et al. Localized down-regulation of P-glycoprotein by focused ultrasound and microbubbles induced blood–brain barrier disruption in rat brain. Sci. Rep. 6 , 31201 (2016).
Meng, Y. et al. Resting state functional connectivity changes after MR-guided focused ultrasound mediated blood–brain barrier opening in patients with Alzheimer’s disease. Neuroimage 200 , 275–280 (2019).
Todd, N., Zhang, Y., Livingstone, M., Borsook, D. & McDannold, N. The neurovascular response is attenuated by focused ultrasound-mediated disruption of the blood–brain barrier. Neuroimage 201 , 116010 (2019).
Wang, Z., Yan, J., Wang, X., Yuan, Y. & Li, X. Transcranial ultrasound stimulation directly influences the cortical excitability of the motor cortex in Parkinsonian mice. Mov. Disord. 35 , 693–698 (2020).
Lee, W. et al. Transcranial focused ultrasound stimulation of human primary visual cortex. Sci. Rep. 6 , 34026 (2016).
Salminen-Vaparanta, N., Noreika, V., Revonsuo, A., Koivisto, M. & Vanni, S. Is selective primary visual cortex stimulation achievable with TMS? Hum. Brain Mapp. 33 , 652–665 (2012).
Beisteiner, R. et al. Transcranial pulse stimulation with ultrasound in Alzheimer’s disease — a new navigated focal brain therapy. Adv. Sci. 7 , 1902583 (2020).
Cotero, V. et al. Noninvasive sub-organ ultrasound stimulation for targeted neuromodulation. Nat. Commun. 10 , 952 (2019).
Lea-Banks, H., O’Reilly, M. A., Hamani, C. & Hynynen, K. Localized anesthesia of a specific brain region using ultrasound-responsive barbiturate nanodroplets. Theranostics 10 , 2849–2858 (2020).
Todd, N. et al. Modulation of brain function by targeted delivery of GABA through the disrupted blood–brain barrier. Neuroimage 189 , 267–275 (2019).
Wu, X. et al. Sono-optogenetics facilitated by a circulation-delivered rechargeable light source for minimally invasive optogenetics. Proc. Natl Acad. Sci. USA 116 , 26332–26342 (2019).
Wang, S. et al. Non-invasive, focused ultrasound-facilitated gene delivery for optogenetics. Sci. Rep. 7 , 39955 (2017).
Wang, J. B., Aryal, M., Zhong, Q., Vyas, D. B. & Airan, R. D. Noninvasive ultrasonic drug uncaging maps whole-brain functional networks. Neuron 100 , 728–738.e7 (2018).
Szablowski, J. O., Lee-Gosselin, A., Lue, B., Malounda, D. & Shapiro, M. G. Acoustically targeted chemogenetics for the non-invasive control of neural circuits. Nat. Biomed. Eng. 2 , 475–484 (2018).
Constans, C. et al. Non-invasive ultrasonic modulation of visual evoked response by GABA delivery through the blood brain barrier. J. Control. Release 318 , 223–231 (2020).
Frey, B. et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int. J. Hyperth. 28 , 528–542 (2012).
Cohen-Inbar, O., Xu, Z. & Sheehan, J. P. Focused ultrasound-aided immunomodulation in glioblastoma multiforme: a therapeutic concept. J. Ther. Ultrasound 4 , 2 (2016).
Man, J. et al. Hyperthermia sensitizes glioma stem-like cells to radiation by inhibiting AKT signaling. Cancer Res. 75 , 1760–1769 (2015).
Zhu, L. et al. Ultrasound hyperthermia technology for radiosensitization. Ultrasound Med. Biol. 45 , 1025–1043 (2019).
Yoshida, M. et al. Sonodynamic therapy for malignant glioma using 220-kHz transcranial magnetic resonance imaging-guided focused ultrasound and 5-aminolevulinic acid. Ultrasound Med. Biol. 45 , 526–538 (2019).
Ricci, S. et al. Sonothrombolysis for acute ischaemic stroke. Cochrane Database Syst. Rev. 10 , CD008348 (2012).
Alexandrov, A. V. et al. Safety and efficacy of sonothrombolysis for acute ischaemic stroke: a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Neurol. 18 , 338–347 (2019). The addition of an operator-independent ultrasound device to alteplase was safe but did not improve functional outcomes at 90 days after ischaemic stroke .
Alexandrov, A. V. et al. Endovascular equipoise shift in a phase III randomized clinical trial of sonothrombolysis for acute ischemic stroke. Ther. Adv. Neurol. Disord. 12 , 1756286419860652 (2019).
Gerhardson, T. et al. Histotripsy clot liquefaction in a porcine intracerebral hemorrhage model. Neurosurgery 86 , 429–436 (2020).
Burgess, A. et al. High-intensity focused ultrasound (HIFU) for dissolution of clots in a rabbit model of embolic stroke. PLoS ONE 7 , e42311 (2012).
Chang, W. S. et al. Factors associated with successful magnetic resonance-guided focused ultrasound treatment: efficiency of acoustic energy delivery through the skull. J. Neurosurg. 124 , 411–416 (2016).
D’Souza, M. et al. Impact of skull density ratio on efficacy and safety of magnetic resonance-guided focused ultrasound treatment of essential tremor. J. Neurosurg. 132 , 1392–1397 (2019).
Schwartz, M. L. et al. Skull bone marrow injury caused by MR-guided focused ultrasound for cerebral functional procedures. J. Neurosurg. 130 , 758–762 (2019).
Hughes, A. & Hynynen, K. Design of patient-specific focused ultrasound arrays for non-invasive brain therapy with increased trans-skull transmission and steering range. Phys. Med. Biol. 62 , L9–L19 (2017).
Arvanitis, C. D., Vykhodtseva, N., Jolesz, F., Livingstone, M. & McDannold, N. Cavitation-enhanced nonthermal ablation in deep brain targets: feasibility in a large animal model. J. Neurosurg. 124 , 1450–1459 (2015).
Macklin, R. The ethical problems with sham surgery in clinical research. N. Engl. J. Med. 341 , 992–996 (1999).
Whone, A. et al. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain 142 , 512–525 (2019).
Alonso, A. et al. Focal delivery of AAV2/1-transgenes into the rat brain by localized ultrasound-induced BBB opening. Mol. Ther. Nucleic Acids 2 , e73 (2013).
Download references
Acknowledgements
We acknowledge Hang Yu Lin for her artistic contribution to the figures in this article. N. L. acknowledges and is grateful for the generous philanthropic gifts to the Sunnybrook Foundation, Sunnybrook Research Institute and the Harquail Centre for Neuromodulation as well as the support of the Focused Ultrasound Foundation.
Author information
Authors and affiliations.
Division of Neurosurgery, Sunnybrook Health Sciences Centre, Toronto, ON, Canada
Ying Meng & Nir Lipsman
Sunnybrook Research Institute, Hurvitz Brain Sciences Program, Harquail Centre for Neuromodulation, Toronto, ON, Canada
Institute of Medical Sciences, Faculty of Medicine, University of Toronto, Toronto, ON, Canada
Sunnybrook Research Institute, Toronto, ON, Canada
Kullervo Hynynen
Department of Medical Biophysics and Institute of Biomaterials & Biomedical Engineering (IBBME), University of Toronto, Toronto, ON, Canada
You can also search for this author in PubMed Google Scholar
Contributions
Y.M. researched data for the article. Y.M. and N.L. wrote the article. All authors made substantial contributions to discussions of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Correspondence to Nir Lipsman .
Ethics declarations
Competing interests.
K.H. is an inventor on intellectual property owned by Brigham and Women’s Hospital in Boston, MA, USA, and Sunnybrook Research Institute in Toronto, Canada, related to intracranial focused ultrasound technology. N.L. has received an honorarium from the Focused Ultrasound Foundation, a not-for-profit funding agency, for serving on an expert steering committee on focused ultrasound in Alzheimer disease. Y.M. declares no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
(BBB). A structural and functional border along the capillaries in the brain that tightly regulates paracellular and transcellular transport.
A surgical procedure in which a piece of the skull is removed and the overlying skin flap is replaced to create a window for ultrasound propagation.
The active delivery of ultrasound. Currently, each typical sonication lasts ~0.5 min for thermoablation and ~1 min for blood–brain barrier (BBB) opening. A rest period allows scalp cooling in thermoablation and systemic clearance of microbubbles in BBB opening.
The change of a liquid to a gas state when subjected to reduced pressures and/or interactions of ultrasound with gas bubbles.
The spatial extent of the brain regions where the desired biological effect (for example, thermoablation) can be successfully achieved with FUS.
Sustained beta frequency oscillations (12.5–30 Hz) in the cortex and subthalamic nucleus are a characteristic of Parkinson disease and related motor impairments.
A member of the ATP-binding cassette transporter B subfamily that pumps a wide range of foreign substances out of cells and is important in multidrug resistance.
A stereotactic frame is fixed to the head to provide a reference for precise targeting. Common examples include the Leksell (polar coordinate) and Cosman–Roberts–Wells (Cartesian coordinate) frames.
The Common Terminology Criteria for Adverse Events allows the standardized classification of adverse events with condition-specific severity designations. Generally, grade 1 denotes a mild adverse event and grade 5 denotes death.
A neuromodulation technology that uses specific wavelengths of light to excite or inhibit neurons through light-sensitive ion channels, which can be introduced through viral transfection.
(SDR). The ratio of cancellous to cortical bone density in the skull.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Meng, Y., Hynynen, K. & Lipsman, N. Applications of focused ultrasound in the brain: from thermoablation to drug delivery. Nat Rev Neurol 17 , 7–22 (2021). https://doi.org/10.1038/s41582-020-00418-z
Download citation
Accepted : 16 September 2020
Published : 26 October 2020
Issue Date : January 2021
DOI : https://doi.org/10.1038/s41582-020-00418-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Ultrasound and antibodies — a potentially powerful combination for alzheimer disease therapy.
- Jürgen Götz
- Pranesh Padmanabhan
Nature Reviews Neurology (2024)
Focused ultrasound enables selective actuation and Newton-level force output of untethered soft robots
Nature Communications (2024)
Focused ultrasound brain therapy is a new tool in the box
- Raúl Martínez-Fernández
Scanning ultrasound-mediated memory and functional improvements do not require amyloid-β reduction
- Gerhard Leinenga
- Xuan Vinh To
Molecular Psychiatry (2024)
The future of brain circuit-targeted therapeutics
- Shan H. Siddiqi
- Sanaz Khosravani
- Michael D. Fox
Neuropsychopharmacology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Ultrasound Imaging
Ultrasound imaging is considered by many to be a mature imaging technology, but the review by Wang et al 1 in this issue of Investigational Radiology highlights the myriad advancements that prove it is anything but a plateaued technique. The inception of ultrasound as an imaging modality harkens back to the post World War II era when a diverse cadre of investigators involved in sonar and radar research began to explore the technology's diagnostic potential. Indeed, the concept that pulse echo, which was fundamental to its military applications, was adaptable to the medical field began in the 1950s. Pioneering work in ultrasound medical applications reported in the 1950s and 1960s was very crude by today's standards. 2 , 3 The early medical devices were large and awkward, but continual refinement occurred at a relatively slow pace. In the 1970s, the mapping of the nonlinear echo amplitudes as grayscale images was likely the first inflection point for this emergent technology, which led to widespread acceptance by the medical community. 4 Although the origins of ultrasound imaging are traced to radiology and continue in that medical discipline, experimentation of ultrasound for cardiac imaging, coined echocardiography, perhaps propelled the technique into the pivotal patient management technology it has become. 2 In one single hospital, Barnes Jewish Hospital in St Louis, MO, approximately 26,000 echocardiography studies are performed annually. This example offers some insight into why the global market for ultrasound in 2018, US $6.3 billion, is expected to increase to US $8.3 billion by 2023 for technologies available today ( https://www.marketsandmarkets.com/Market-Reports/ultrasound-market ). But as Wang et al 1 discuss, the future of ultrasound imaging, driven by advancements in material sciences, electronics miniaturization, and computing power, may be far more rapid and amazing than the collective progress achieved over the past 70 years.
EVOLVING TRANSDUCER TECHNOLOGIES
In classic “pulse mode,” transducers generate and transmit ultrasonic pulses from electrical pulses and in “receive mode” convert returning ultrasonic pressure waves into electrical voltages. Piezoelectric ceramics, predominantly lead zirconate titanate, have been used to balance optimal electrical characteristics with nonoptimal acoustic performance since the 1970s. 5 Over time, engineers have achieved −6 dB fractional bandwidths in the 75% to 80% range by designing transducers with “sandwiches” of multiple matching ceramic and appropriate backing layers. 6 However, the demand for bandwidths exceeding 100% to accommodate nonlinear harmonic imaging, particularly for microbubble-related applications, has spawned innovative predominantly lead zirconate titanate/polymer composite materials that address the need. 7 Modern transducers incorporating these unique materials include dual-layer designs, 8 1-dimensional and 2-dimensional arrays, 9 and high-frequency applications.
Transducer elements have also improved with the development of single crystal piezoelectric materials formulated for high Curie temperatures and high permittivity, which equated to lower electrical impedance for practical medical imaging. 10 New device machining techniques, such deep reactive ion etching, produced high aspect ratio elements with extremely fine interpositioning kerfs to minimize transducer size and better isolate neighboring elements from interbeam interference. 11
Wang et al 1 discuss a revolutionary departure from classic piezoelectric ceramic to cMUTs (capacitive ultrasound micromachined transducers). 12 cMUTs are composed of an electrode micromachined into a thin membrane and suspended under tension above a conducting silicon wafer-based substrate. This overall design continues to evolve, but in general, the low acoustic impedance of the thin membrane affords a very high intrinsic signal bandwidth for medical applications. cMUTs have a significant advantage over high density transducer element designs for 2-dimensional arrays and high-frequency applications, including ultrasound catheters. 12 Another important benefit is the close placement of front-end electronics near the transducer elements. Although the cMUT technology may be transformative, its widespread implementation has been dampened by the potential for image artifacts secondary to interelement crosstalk and very high microfabrication costs.
ULTRAFAST IMAGING
Conventional ultrasound imaging utilized sequential insonification of narrowly focused transmit beams that were reconstructed line by line. In contradistinction, ultrafast imaging achieves very high frame rates, typically in kilohertz range using wide beam transmission and parallel receive beamforming. 13 Ultrafast imaging came to the fore in the late 1970s when conventional sequential processing was superseded by parallel approaches. 14 , 15 An entire image frame could be generated from a single ultrasound pulse. Continuous increases in computing and image processing power initially facilitated phased array imaging 16 and, more recently, volumetric (3-dimensional) imaging. 17
Wang et al 1 expand the discussion of ultrafast imaging to include spatial compound sonography. 16 , 17 Spatial compound sonography combines acoustic information obtained from several different angles of insonification into a single image, whereas conventional B-mode images are processed from a single angle of insonification. Image artifacts inherent in conventional sonography are reduced through the multiangle interrogation technique, 18 , 19 which has been illustrated for breast, 20 thyroid, 21 and atherosclerotic plaque. 22
Ultrafast imaging capability has enabled sonoelastography, particularly for the evaluation of musculoskeletal disease. 23 Elastography is based on tissue elasticity or deformability, which reflects its structure and composition. There are 3 types of sonoelastography varying by the method of stress application and the test objectives: compression elastography, 24 shear-wave elastography, 25 and transient elastography. 26 As an example, shear-wave elastography applies an acoustic radiation force pulse sequence to generate shear waves that propagate perpendicular to the ultrasound beam to induce transient displacement. The distribution of shear-wave velocities at each pixel are correlated with Young's modulus, a measure of the tissue's elastic properties. Shear-wave images provide quantitative color elastograms that are coregistered with standard B-mode images to define anatomic specificity. From the musculoskeletal perspective, shear waves propagate faster through stiff contracted tissue or along the long axis of tendons and muscles.
Advances in ultrafast imaging are particularly relevant for transient elastography, which has gained favor for measuring shear-wave speed through the liver as an indicator of fibrosis. 27 A handheld probe emits a mild amplitude, low-frequency vibration pulse to induce the shearwave. The shear wave propagates through the liver while simultaneous pulse-echo ultrasonic acquisitions follow the shear wave and measure its velocity. Transient elastography provides an indication of liver stiffness and is expressed in kPa. The stiffer the liver, the greater the fibrosis, and the faster the shear wave, assuming that other causes of liver tissue density are negligible.
Another related ultrafast imaging concept is supersonic shear imaging. 28 Supersonic shear imaging affords real-time visualization of soft tissue viscoelastic properties by using ultrasonic focused beams to generate low-frequency mechanical vibration sources in tissues. However, supersonic shear imaging waves move at supersonic speed, producing shear waves that interfere constructively along a Mach cone as 2 intense plane shear waves. As the shear waves propagate, they become progressively distorted by tissue heterogeneities. Ultrafast scanners generate this supersonic source and image the propagation of shear waves at framing rates of 5 kHz. Supersonic shear imaging enables tissue elasticity mapping in less than 20 milliseconds. These elasticity estimates can be further enhanced by tilting shear waves in different directions or a technique referred to as shear compounding. 28
A related ultrafast imaging advancement is noninvasive cardiac electromechanical wave imaging (EWI). 29 Electromechanical wave imaging maps the electromechanical activation contraction sequence of the myocardium along various echocardiographic planes in vivo. The direction of propagation of this wave is determined by the pacing origin. By increasing the frame rate of standard echocardiography 20-fold, EWI maps the small, transient deformations caused by myocyte depolarization and is a direct, tissue-level metric of cardiac excitation-contraction coupling. 30 With cessation of the depolarization, myocyte relaxation occurs with reuptake of calcium into the sarcoplasmic reticulum, which reinitiates contraction in response to an action potential after an electromechanical delay. Electromechanical wave deformations are small (<0.25% interframe at 481 frames/s) and their propagation is fast (0.5–2 m/s). 29 They are undetectable with routine echocardiography. Electromechanical wave imaging relies on radiofrequency (RF)-based cross-correlation methods, which provide higher accuracy as the frame rate increases. 29 Ultrafast imaging capability could eventually allow EWI to be performed in the clinic or hospital to monitor therapeutic interventions such as cardiac resynchronization therapy or RF ablation.
A critical element of ultrasound studies is Doppler flow imaging, which is typically performed using line-by-line acquisitions to capture continuous wave (maximum velocities), pulsed wave Doppler (narrow sector velocities), and to color map brief acquisitions of flow over a larger sector area. The emergence of ultrafast Doppler imaging can provide in 1 or 2 cardiac cycles the pulsed wave Doppler data for an entire image frame, including both high velocity flow and low velocity tissue motion. 31
The limits of traditional Doppler ultrasound for assessing hemodynamics can be overcome by implementing high-speed, plane-wave imaging, 13 multiangle vector-flow techniques, 32 or vector Doppler Imaging. 33 The core concept is to rapidly transmit wide field-of-view plane waves into tissue by firing all of the elements of a linear array with appropriate interelement transmission delays to create a coherent wave front at a steered angle. The returning echoes are digitized on all of the array elements, and delay-and-sum beamforming is implemented to generate a full 2-dimensional image from each transmission. By summing the beamformed data acquired with steered transmit angles, the signal-to-noise ratio and resolution can be improved while preserving a high transmission rate. Importantly, multiangle, vector-flow methods yield axial and lateral velocity estimates at each pixel without assumptions regarding the angle of flow relative to the probe. This is highly differentiated from traditional clinical Doppler that only estimates axial velocity with the potential for significant error if the angle of insonification deviates from parallel. Moreover, clinical Doppler cannot resolve lateral flow nor provide insight into complicated flow patterns, which appear as turbulence. As an emerging application, ultrafast Doppler imaging with very high sensitivity has promise for functional brain imaging.
ULTRAFAST IMAGING WITH SOFTWARE-BASED PLATFORMS
Wang et al 1 discuss how conventional ultrasound scanners adopted multiline processing capabilities to increase frame rate but were typically hardware limited to between 2 and 32 parallel processing units. The emergence of software-based approaches theoretically allows computations of unlimited receive lines in parallel, constructing a full image in one insonification with framing rates reaching the kilohertz range. Indeed, the rapid growth of graphical processing unit-based platforms with high-speed data transfer and powerful parallel computing strength are now used by ultrasound manufacturers and are implemented in several upgraded clinical products.
PORTABLE ULTRASOUND DEVICES
As pointed out by Wang et al, 1 advances in transducers and fast imaging technologies combined with miniaturized computational and image processing power have ushered the emergence of portable ultrasound systems (PoUSs). 34 These devices do not replace the traditional thorough organ focused examination, but they have created a new category of devices intended for specific procedural and diagnostic purposes. The term portable describes battery-powered ultrasound devices that can range in size from laptop-like computers to handheld devices the size of a smartphone with attached transducer. Whether guiding needle placements, providing ultrasound stimulation therapy, or affording emergency medical triage data, these PoUSs have become available worldwide at a fraction of the cost of a conventional scanner. How these devices are integrated into medical use depends greatly on the country, the intended application, and the required ultrasound training. Minute in size compared with conventional radiological scanner systems, some portable ultrasound devices offer conventional 2-dimensional structural imaging and Doppler flow imaging, and a few add nonlinear harmonic microbubble contrast imaging.
Portable ultrasound systems are trending toward pocket-sized devices with low-power consumption that increasingly utilize artificial intelligence to overcome barriers. Beginning in 2012, early portable ultrasound system concepts utilized field programmable gate arrays. 35 A 16-channel device used a 10-inch LCD touch screen, but success was limited overall by the large size, poor spatial resolution, and use of a proprietary display. 35 Later in 2014, a tablet with a more commonly available display (11.6 inch) and 16 channels was described, 36 but it was too large and spatial resolution was inadequate to gain acceptance. Another PoUS device was reported, which offered both analog and digital front-ends, used a high-speed USB interface, possessed a 32-channel system, and operated in combination with a SmartPhone through a custom graphic user interface. 37 It was a convenient device with improved spatial resolution that utilized a lookup table-based pseudodynamic receive beamforming method implemented in an field programmable gate array to minimize hardware and computational complexity. Unfortunately, the instrument suffered from severe near-field image degradation. Advancements continued with Kim et al 38 incorporating a run-length encoding method in a lookup table–based beamformer to lower memory requirements while maintaining image quality with conventional dynamic receive beamforming. 39 Despite using a custom-made laboratory transducer, this PoUS prototype provided proof of principle with good real-time image quality. Since that report, the same group describes an upgraded system compatible with multiple ultrasound transducers and Wi-Fi interfaces. An accelerated USB communication protocol (USB 3.0) enabled the transfer of raw format data into the phone in real time, further reducing transducer probe design complexity. 37 This system supports Doppler, including color flow imaging, in addition to B-mode imaging. 37
A key unmet engineering need of portable, 3-dimensional, and ultrafast ultrasound imaging systems remains the reconstruction of high-quality images from limited RF data. 40 Typically, optimal spatial resolution requires power-hungry high-speed analog-to-digital converters for the receive transducer elements. To reduce power consumption in portable devices, a smaller number of receive elements with reduced aperture size can be considered, but that power-reduction technique comes at the expense of image degradation. An alternative path would reduce the number of transmit events, but this method leads to downsampling artifacts. Unlike magnetic resonance imaging, compressed sensing with ultrasound has been associated with performance deterioration. However, new AI deep-learning techniques reported to successfully overcome classification 41 - 43 and computer vision problems, 44 - 46 encouraged exploration of deep learning techniques for biomedical image reconstruction, achieving significant performance enhancement over compressed sensing approaches. 47 , 48 Initially, advanced machine learning neural networks were used to identify and remove reflection artifacts in photoacoustic data or to implement ultrasound beamforming tasks, but Yoon et al 40 introduced a deep learning algorithm that generates efficient image reconstruction from subsampled RF data. In other words, a deep neural network estimated the missing RF data from subsampled receive and/or transmit events without sacrificing image quality.
Artificial intelligence and deep learning neural networks are increasingly studied as solutions to PoUS device feasibility, but perhaps the greatest human advancement will be widespread use and enhancement of health care in third world countries by enabling individuals with minimal medical training to achieve robust results. One interesting example was a pilot study to triage breast cancer using PoUSs. Love et al 49 recognized that, in low- to middle-income countries, highly trained medical professionals were very limited, leaving only lower skilled medical workers as first-line caregivers for patients. They conducted a proof-of-principle study to use AI-based computer-assisted diagnosis to triage women with palpable lumps for possible malignancy, recognizing that the majority of masses are benign. The AI-based computer-assisted diagnosis approach was operated on a low-cost PoUS system in Jalisco, Mexico. Three nonradiologist medical workers screened 32 women and diagnosed 2 masses as suspicious of cancer and 30 as benign. The imaging results were in 100% agreement with the overreads performed by radiologists. This small pilot study points to a greater concept for world health: the use of PoUSs with AI to improve health care in low-income countries, even in remote territories.
SUPERRESOLUTION IMAGING
Wang et al 1 are highly recognized for microbubble research, and their manuscript provides an excellent review of microbubble contrast imaging with applications for perfusion imaging, drug delivery, molecular imaging, and blood pool imaging. In this review, they introduced the topic of superresolution ultrasound imaging, a concept that requires overcoming the diffraction of sound waves penetrating tissue, since 2 points are resolved only when separated by more than a half-wavelength. 50 , 51 In 2006, fluorescence photoactivated localization microscopy (PALM or FPALM) and stochastic optical reconstruction microscopy (STORM) were introduced, breaking the diffraction limit in optics by an order of magnitude. 52 - 54 Using photo-switchable fluorescent dyes and fast cameras, sequential images were acquired, each revealing a subset of the optical sources in the field of view at that instant. With knowledge of the system point-spread function, localization of isolated sources is estimated from their intensity map. After acquiring thousands of subwavelength localizations, an image with tens of nanometer resolution is obtained with a visible light microscope. For this unprecedented achievement, Eric Betzig, Stefan Hell, and William E. Moerner were awarded the 2014 Chemistry Nobel Prize. From a practical research perspective, optical superresolution imaging was recently used to follow the disposition of single dyes and drugs molecules trafficking through intracellular membranes after targeted nanoparticle drug delivery. 55
Actually, superresolution ultrasound imaging has been considered for over 40 years. 56 , 57 Both higher-frequency imaging 58 and near-field imaging 59 have been considered, but the former attenuates in the media too rapidly for practical use. For the latter, image resolution close to a probe is proportional to the distance of the object rather than the wavelength, 60 but clinically, organs of interest are imaged in the far-field. When precise characterization of the acoustic source is known, subwavelength imaging can be achieved, 61 but for routine B-mode imaging, acoustic scatterers from cells to organ scale variably comprise the image and cannot be precisely known.
In 2010, an ultrasonic version of FPALM, now called ultrasound localization microscopy (ULM), was proposed. 62 Ultrasound contrast agents replaced the optical beacons, and an ultrafast programmable ultrasonic scanner 63 replaced the cameras. Applying the same principles, the interference between different microbubbles was avoided by observing them sequentially to capture isolated signal sources in each image. With the point-spread function on the RF channel data, spatial localization with micrometer precision was obtained for each microbubble. Because microbubbles are inherently intravascularly constrained, the serial accumulation of these subwavelength spatial data yielded a superresolved map of the microvasculature.
The ULM approach was first illustrated in vitro for microbubbles flowing in a single microchannel. 64 In that same year, Siepmann et al 65 demonstrated improved maximum intensity projection images of dilute microbubbles based on centroid detection. In 2013, Viessman et al 66 showed that ULM can distinguish 2 vessels less than half a wavelength apart. These results were followed by proposals for two 3-dimensional superresolution techniques, one using a 1.5-dimensional array 67 and the other using a hemispherical array imaging through human skull bone. 68
Then in 2015, Errico et al 69 obtained ultrasound images of rat blood vessels infused with sparse gas-filled microbubbles. The images were acquired rapidly (500 Hz), and the locations were precisely determined. In 2.5 minutes, a composite superresolution image was assembled revealing the locations of thousands of microbubbles. Organ microvasculature is impacted by many diseases, particularly diabetes. To date, the effects on the microvasculature are inferred as an ensemble effect, but now ultrasound superresolution imaging offers a technique for direct dynamic interrogation. This new realm of research could lead to alternative diagnostic and therapeutic options to better manage microvascular disorders whether in the heart, the kidney, a tumor, or the nervous system.
CONCLUSIONS
In this review, Wang et al 1 provide much needed insight into the future of ultrasound. They show that the prospects empowered by advances in transducers, image acquisition, and processing, and by artificial intelligence are enabling a myriad of new opportunities for very high-tech applications in the laboratory and clinic. Portable devices offer not only convenient enhancement for focused ultrasound applications but may further address fundamental health care problems and disparities. The prospects for ultrasound in the near future are remarkable. What will evolve from these early advances over the next decades cannot be imagined by most of us today, but one thing is assured, ultrasound is far from being a mature technology.
Acknowledgments
G.M.L. is a cofounder of Capella Imaging, Inc (nuclear imaging) and BGJ Pharma, LLC (nanomedicine). G.M.L. serves on the Scientific Advisory Board for KaloCyte, Inc. (artificial blood). G.M.L. enjoys cMRI research equipment and technical support from United Imaging in Healthcare America as part of an institutional collaboration with Washington University Medical School. All financial funding is derived from NIH grants: R01 CA216840, R01 HL122471, and U54 CA199092.
Conflicts of interest and sources of funding: none declared.
- University of Wisconsin-Madison

Focus on new faculty: Aarushi Bhargava expands ultrasound’s impact
Departments:, focus areas:.
When you hear the term ultrasound, you likely think of pregnancy and fuzzy images of developing babies. And the technology, which relies upon reflected soundwaves to generate images, is indeed frequently used to monitor fetal growth or to examine other parts of the body to inform diagnoses.
But high-intensity ultrasound can also destroy unwanted tissue, such as tumor cells or uterine fibroids—and researchers like Aarushi Bhargava are exploring its other possibilities.
Bhargava joined the University of Wisconsin-Madison as an assistant professor of biomedical engineering in summer 2024, bringing her interdisciplinary research on the mechanics of soft materials and acoustics for developing biomedical technologies.
“A major obstacle to successfully bringing a technology to market is poor communication between the different experts involved in its development. For instance, researchers with expertise in mechanics investigate how materials react to mechanical stimuli like sound. On the other hand, clinicians focus on the induced bioeffects of ultrasound in materials like tissues,” says Bhargava, who studied mechanical engineering and biology at the Birla Institute of Technology and Science in India. “As new ultrasound-based technologies move to the clinical evaluation phase, a disconnect often occurs between these two domain experts. This disconnect prevents the optimization of the technology’s performance. The goal is, therefore, to bridge this gap across different disciplines—in this case, material experts and clinicians—throughout an ultrasound technology’s development.”
While earning her PhD at Virginia Tech and completing postdoctoral research stints at the University of Chicago and the Max Planck Institute for Intelligent Systems in Stuttgart, Germany, Bhargava has used ultrasound for drug delivery, to break up blood clots, and to activate medical microrobots. And she says those are just a few examples of how ultrasound can augment or replace existing treatment strategies.
“It’s a huge range,” says Bhargava, who’s originally from Jaipur in western India. “Ultrasound traditionally has been used to heat cells and tissues. And now we are using it to mechanically manipulate a material, opening up a wide variety of applications. You can induce drug delivery, perform neuromodulation, disintegrate a tissue—all through the mechanical force of sound, and it just depends on few ultrasound parameters such as its intensity and frequency, and then, obviously, on the material.”
Bhargava sees herself as a natural fit in the Department of Biomedical Engineering , where nearly all research spans departmental and college boundaries and requires collaborative expertise in biology, materials and technology.
“Many faculty members in BME are working in complementary fields ” she says. “ For instance, researchers like Melissa Skala and Kevin Eliceiri focus on immune cells and extracellular matrix behaviors, while I specialize in how ultrasound interacts with various soft materials. This synergy brings exciting opportunities to develop new treatment technologies. Additionally, researchers like Chris Brace are using other non-invasive methods, especially microwaves, to interact with tissues in ways similar to ultrasound. These aligned interests create a vibrant and collaborative environment for innovating hybrid therapeutic strategies.”
In the classroom, Bhargava will teach biomechanics courses, which will allow her to share her research with undergraduate students.
“One thing about teaching I like the most is that it’s a two-way transfer of knowledge,” she says. “A classroom makes you examine a concept you are teaching from different perspectives, not just your own, and that in my opinion is a very comprehensive way to increase your knowledge about that topic. You see students getting excited about things that you’re also excited about. It’s a very nice and satisfying feeling.”
Top photo by Joel Hallberg
- Clinical Research
- Interventional
- Radiation Oncology
- Women's
- Artificial Intelligence
- Enterprise Imaging
- Imaging Informatics
- Informatics
- Compensation
- Education & Training
- Patient Care
- Policy & Regulations
- Practice Management
- Professional Associations
- Experience Stories
- Webinars & Videos
Search form
New ir procedure could be a viable alternative to surgery for cubital tunnel syndrome.
Courtesy of EJR
A new paper in the European Journal of Radiology details the potential of a newer minimally invasive ultrasound procedure as a viable alternative to surgery for cubital tunnel syndrome (CuTS).
Over time, constant compression of the ulnar nerve can cause chronic pain, numbness, weakness and even deformities in parts of the hand and wrist. Conservative treatments for the condition include physical therapy, over-the-counter anti-inflammatory medications and behavioral changes. However, if symptoms do not resolve and interfere with an individual’s day-to-day functioning, providers often turn to more invasive options, such as surgery or corticosteroid injections in the joint.
A newly developed ultrasound-guided thread technique (first described in Guo et al. and Kang et al.) could provide patients relief without the need for more invasive surgeries that are often accompanied by unpleasant side effects and prolonged downtime. The method involves guiding a thread around Osborne’s ligament under ultrasound guidance and then using small sawing motions to cut it. It takes around 20 minutes to perform.
“Due to the more invasive nature of surgical procedures, they are associated with greater intraoperative damage to surrounding tissue, extended rehabilitation periods, larger scars, and a heightened risk of recompression, in contrast to the minimally invasive ultrasound-guided thread release approach,” corresponding author Suren Jengojan, from the Department of Biomedical Imaging and Image-guided Therapy at the Medical University of Vienna, in Austria, and colleagues noted.
Researchers sought to delve deeper into the efficacy and safety of the procedure. To do this, they performed the ultrasound-guided release technique on 10 softly embalmed anatomic specimens. After the procedure, the team did a thorough examination of the anatomy surrounding Osborne’s ligament to check for any signs of damage it may have caused.
Encouragingly, there were no signs of damage in the area. The surrounding nerves, blood vessels, tendons and muscles all remained intact and unharmed, while the ligament was successfully cut in each cadaveric arm.
Although each procedure was successful, the group noted that the ultrasound quality varied, even with experienced radiologists completing the procedures.
“While proficient radiologists successfully executed the ligament transection without harm, it could prove more challenging for junior radiologists with limited ultrasound experience. Thus, a solid grasp of anatomical knowledge and adept handling of ultrasound, especially in an off-plane view, are essential prerequisites for the safe conduct of the procedure, with a requisite amount of practice,” the authors suggested.
Although all studies on the technique have been completed on cadavers thus far, the team maintained that it could still have a role as “an efficient and secure alternative to existing procedures.” They suggested that future studies assess the safety and efficacy in living patients and focus on any subsequent side effects that may emerge, including post-release instability, as those cannot be monitored in cadavers.
Learn more about the procedure here .
Related IR content:
Ablation therapy versus partial nephrectomy for small renal masses, simple ir procedure could prevent women from having hysterectomies, but most have never heard of it, radiology's role in colorectal cancer care set to grow following 'groundbreaking' trial results.

In addition to her background in journalism, Hannah also has patient-facing experience in clinical settings, having spent more than 12 years working as a registered rad tech. She joined Innovate Healthcare in 2021 and has since put her unique expertise to use in her editorial role with Health Imaging.
Related Content
Popular Searches
- Master’s of AI Engineering
- Engineering Magazine
- graduate programs
- Manufacturing Futures Institute
- student organizations
- Rethink the Rink
Social Media
- @CMUEngineering
- CMUEngineering
- College of Engineering
Breakthrough approach enables bidirectional BCI functionality
by Sara Pecchia
To strengthen the case for noninvasive BCIs, Carnegie Mellon researchers have demonstrated that through precision neuromodulation using focused ultrasound, the performance of a BCI could be improved for communication.
- Refined AI approach improves noninvasive BCI performance
- Advancing dynamic brain imaging with AI
- Noninvasive technology steps ahead to help epilepsy patients
Brain-computer interfaces, or BCIs, hold immense potential for individuals with a wide range of neurological conditions, but the road to implementation is long and nuanced for both the invasive and noninvasive versions of the technology. Bin He of Carnegie Mellon University is highly driven to improve noninvasive BCIs, and his lab uses an innovative electroencephalogram (EEG) wearable to push the boundaries of what’s possible. For the first time on record, the group successfully integrated a novel focused ultrasound stimulation to realize bidirectional BCI that both encodes and decodes brain waves using machine learning in a study with 25 human subjects. This work opens up a new avenue to significantly enhance not only the signal quality, but also, overall nonivasive BCI performance by stimulating targeted neural circuits.
Noninvasive BCI is lauded for its merits of being cheap, safe, and virtually applicable to everyone, but because signals are recorded over the scalp versus inside the brain, low signal quality presents some limitations. The He group is exploring ways to improve the effectiveness of noninvasive BCIs and, over time, has used deep learning approaches to decode what an individual was thinking and then facilitate control of a cursor or robotic arm.
In their latest research, published in Nature Communications , the He group demonstrated that through precision noninvasive neuromodulation using focused ultrasound, the performance of a BCI could be improved for communication.
“This paper reports a breakthrough in noninvasive BCIs by integrating a novel focused ultrasound stimulation to realize bidirectional BCI functionality,” explained Bin He, professor of biomedical engineering at Carnegie Mellon University. “Using a communication prosthetic, 25 human subjects spelled out phrases like ‘Carnegie Mellon’ using a BCI speller. Our findings showed that the addition of focused ultrasound neuromodulation significantly boosted the performance of EEG-based BCI. It also elevated theta neural oscillation that enhanced attention and led to enhanced BCI performance.”
This video shows the BCI speller progress used by study subjects to spell “Carnegie Mellon.”
For context, a BCI speller is a 6x6 visual motion aide containing the entire alpabet that is commonly used by nonspeakers to communicate. In He’s study, subjects donned an EEG cap and just by looking at the letters, were able to generate EEG signals to spell the desired words. When a focused ultrasound beam was applied externally to the V5 area (part of the visual cortex) of the brain, the performance of the noninvasive BCI greatly improved among subjects. The neuromodulation-integrated BCI actively altered the engagement of neural circuits to maximize the BCI performance, compared to previous uses, which consisted of pure processing and decoding recorded signals.
“The BRAIN Initiative has supported more than 60 ultrasound projects since its inception. This unique application of noninvasive recording and modulation technologies expands the toolkit, with a potentially scalable impact on assisting people living with communication disabilities,” said Dr. Grace Hwang, program director at the Brain Research Through Advancing Innovative Neurotechnologies ® initiative ( The BRAIN Initiative ® ) at the National Institutes of Health (NIH).
The BRAIN Initiative has supported more than 60 ultrasound projects since its inception. This unique application of noninvasive recording and modulation technologies expands the toolkit, with a potentially scalable impact on assisting people living with communication disabilities. Grace Hwang , program director , National Institutes of Health
Following this discovery, the He lab is further investigating the merits and applications of focused ultrasound neuromodulation to the brain, beyond the visual system, to enhance noninvasive BCIs. They also aim to develop more compact-focused ultrasound neuromodulation device for better integration with EEG-based BCIs, and to integrate AI to continue to enhance the overall system performance.
“This is my lifelong interest, and I will never give up,” emphasized He. “Working to improve noninvasive technology is difficult, but I strongly believe that if we can find a way to make it work, everyone will benefit. I will keep working, and someday, noninvasive lifesaving technology will be available for every household.
This work was supported by NIH’s The BRAIN Initiative and the Helping to End Addiction Long-term ® Initiative ( NIH HEAL Initiative ® ), the National Institute of Neurological Disorders and Stroke, the National Institute of Biomedical Imaging and Bioengineering, the National Center for Complementary and Integrative Health, and by the National Science Foundation.
Other collaborators on the Nature Communications paper include the first author Joshua Kosnoff, BME Ph.D. student, Kai Yu, BME research scientist; and Chang Liu, former BME master’s student.

IMAGES
COMMENTS
Ultrasound is a non-invasive imaging technique that uses the differential reflectance of acoustic waves at ultrasonic frequencies to detect objects and measure distances. ... Latest Research and ...
Pushing the Boundaries of Ultrasound Imaging: Breaking New Ground With Ultrafast Technology Jan. 17, 2024 — Researchers have achieved a successful contrast agent-free imaging of complex ...
Introduction. Making waves at the frontiers of medicine, the rise of clinical ultrasound in today's 21 st century highlights its many robust applications in medicine and guiding patient-centered management for patients at the bedside. Illuminated through photography and long exposure light technique, "Riding the waves" captures the serenity of the sea at sunrise (Figure 1); the waves and ...
A wearable ultrasonic device to image cardiac function. Researchers have engineered a wearable device that adheres to the skin and uses ultrasound imaging and a deep learning model to produce a ...
Results. The novel sonographic software techniques can be divided into algorithms that deal with conventional B-scan optimization and new programs that extend the scope of sonographic examination. The latter include elastography, contrast-enhanced sonography, and image fusion in combination with other cross-sectional imaging modalities.
Civilian applications include imaging in the intensive care unit. With NCLUS, emergency medical technicians, paramedics, and medical staff without specialized sonography training might be able to perform ultrasound imaging outside of a hospital — in a doctor's office, at home, or in a remote battlefield setting.
In the new study, the researchers showed that they could obtain ultrasound images with resolution comparable to that of the ultrasound probes used in medical imaging centers. ... and one of the authors of the study. "This work will significantly advance ultrasound research and medical device designs, leveraging advances in materials, low ...
MIT researchers have produced the first "laser ultrasound" images in humans, using eye- and skin-safe lasers. The new technique enables noncontact ultrasound imaging, which may help clinicians to remotely assess patients, infants, and contact-sensitive subjects such as burn victims.
Wearable ultrasound technology opens up a new dimension for deep-tissue sensing 3 - 5. The technology could be extended to image various deep tissues and central organs, not just the heart.
The laboratory has made major advances in vascular imaging over the last 10 years, with the development of ultrasensitive Doppler imaging (uDoppler) and then ultrasound localization microscopy ...
In order to expand these new technologies of ultrasound imaging in 3D, 2D array transducer is required, ... Although it is not mentioned above, there are additional ultrasound related research areas such as photoacoustics and ultrasound neuromodulation. Photoacoustic imaging (PAI) uses a pulsed laser excitation to generate sound in body and ...
Researchers from MIT Lincoln Laboratory and their collaborators at the Massachusetts General Hospital (MGH) Center for Ultrasound Research and Translation (CURT) have developed a new medical ...
Pushing the boundaries of ultrasound imaging: Breaking new ground with ultrafast technology. ScienceDaily . Retrieved August 24, 2024 from www.sciencedaily.com / releases / 2024 / 01 ...
This editorial offers one individual's experience and perspective on a proposed high-level overview of the foundational pillars of advanced ultrasound imaging architecture that should be integrated into future sonography educational endeavors. The content of this symposia article is expounded in greater depth in a separate white paper by the ...
New stamp-sized ultrasound adhesives produce clear images of heart, lungs, and other internal organs. MIT. The researchers tested the patch on 15 human volunteers. They showed that the device could be comfortably worn for at least 48 hours. Depending on placement, the patch could provide continuous imaging of blood vessels, heart, muscle ...
Ultrasound (US) imaging, due to its capabilities of real-time imaging, portability, low cost and favorable safety, is frequently used as a diagnostic modality for the visualization of different diseases. ... c Research Center for Molecular and Cellular Imaging, ... Novel contrast agents have propelled US imaging into a new realm in the cellular ...
DOI: 10.1056/NEJMra1916062. VOL. 385 NO. 17. Point-of-care ultrasonography (POCUS) is defined as the acquisition, interpretation, and immediate clinical integration of ultrasonographic imaging ...
To make her vision of a diagnostic bra a reality, Dagdeviren designed a miniaturized ultrasound scanner that could allow the user to perform imaging at any time. This scanner is based on the same kind of ultrasound technology used in medical imaging centers, but incorporates a novel piezoelectric material that allowed the researchers to ...
UltraCon 2024 represents a critical juncture in the ongoing dialogue about the future of ultrasound technology, offering a rare opportunity for participants to engage directly with the ideas and individuals shaping the field's trajectory. For those committed to advancing medical imaging, UltraCon 2024 is a must-attend engagement.
Now, new work by UVM Cancer Center investigators Brian Sprague, Sally Herschorn, Hannah Perry, and Donald Weaver, published in the journal Radiology, finds that supplemental ultrasound screening has favorable outcomes among women with dense breast tissue who also have other breast cancer risk factors.
<p data-pm-slice="1 1 []">We are close to a new era in ultrasound technology. From helping healthcare specialists detect diseases such as cancerous cells to showing real-time images inside the mother’s womb, this technology is a go-to way for specialists to deal with a range of diseases and tasks. Let's take a closer look at the 5 emerging technologies impacting this field's ...
Ultrasound (US) imaging is a non-invasive and commonly used to diagnose and monitor UF. Initial diagnosis of UF typically involves ultrasound images but treatment depend on the type, size, and location, as well as the symptoms and the reproductive goals. ... In this research work, a new model named as Attention based EfficientNetB0 has been ...
New NIH-funded program emphasizes ultrasound research commercialization This article was originally published on July 26, 2024 by NC State Engineering Communications and can be read here. Ultrasound is one of the best-known medical imaging devices. It is safe, cost-efficient and … Read more
Citation: New imaging device improves ear disease diagnosis (2024, August 23 ... Daily science news on research developments and the latest scientific innovations.
Focused ultrasound (FUS) is a disruptive medical technology, and its implementation in the clinic represents the culmination of decades of research. Lying at the convergence of physics ...
Ultrasound therapy has become widely used in recent decades. 1 The production of ultrasound waves, through the absorption of mechanical energy, generates heat and induces therapeutic effects on tissues. For example, the application of heat relaxes muscles, enhances local blood flow, reduces inflammation, and promotes tissue regeneration. 2 In cases of chronic pain, the continuous mode is ...
Ultrasound imaging is considered by many to be a mature imaging technology, but the review by Wang et al 1 in this issue of Investigational Radiology highlights the myriad advancements that prove it is anything but a plateaued technique. The inception of ultrasound as an imaging modality harkens back to the post World War II era when a diverse cadre of investigators involved in sonar and radar ...
When you hear the term ultrasound, you likely think of pregnancy and fuzzy images of developing babies. And the technology, which relies upon reflected soundwaves to generate images, is indeed frequently used to monitor fetal growth or to examine other parts of the body to inform diagnoses. ... bringing her interdisciplinary research on the ...
A new paper in the European Journal of Radiology details the potential of a newer minimally invasive ultrasound procedure as a viable alternative to surgery for cubital tunnel syndrome (CuTS). Over time, constant compression of the ulnar nerve can cause chronic pain, numbness, weakness and even deformities in parts of the hand and wrist.
In their latest research, published in Nature Communications, the He group demonstrated that through precision noninvasive neuromodulation using focused ultrasound, the performance of a BCI could be improved for communication. "This paper reports a breakthrough in noninvasive BCIs by integrating a novel focused ultrasound stimulation to realize bidirectional BCI functionality," explained ...