Scientists Have Reached a Key Milestone in Learning How to Reverse Aging
I t’s been 13 years in the making, but Dr. David Sinclair and his colleagues have finally answered the question of what drives aging. In a study published Jan. 12 in Cell , Sinclair, a professor of genetics and co-director of the Paul F. Glenn Center for Biology of Aging Research at Harvard Medical School, describes a groundbreaking aging clock that can speed up or reverse the aging of cells.
Scientists studying aging have debated what drives the process of senescence in cells—and primarily focused on mutations in DNA that can, over time, mess up a cell’s normal operations and trigger the process of cell death. But that theory wasn’t supported by the fact that older people’s cells often were not riddled with mutations, and that animals or people harboring a higher burden of mutated cells don’t seem to age prematurely .
Sinclair therefore focused on another part of the genome, called the epigenome. Since all cells have the same DNA blueprint, the epigenome is what makes skin cells turn into skin cells and brain cells into brain cells. It does this by providing different instructions to different cells for which genes to turn on, and which to keep silent. Epigenetics is similar to the instructions dressmakers rely on from patterns to create shirts, pants, or jackets. The starting fabric is the same, but the pattern determines what shape and function the final article of clothing takes. With cells, the epigenetic instructions lead to cells with different physical structures and functions in a process called differentiation.

More from TIME
In the Cell paper, Sinclair and his team report that not only can they age mice on an accelerated timeline, but they can also reverse the effects of that aging and restore some of the biological signs of youthfulness to the animals. That reversibility makes a strong case for the fact that the main drivers of aging aren’t mutations to the DNA, but miscues in the epigenetic instructions that somehow go awry. Sinclair has long proposed that aging is the result of losing critical instructions that cells need to continue functioning, in what he calls the Information Theory of Aging. “Underlying aging is information that is lost in cells, not just the accumulation of damage,” he says. “That’s a paradigm shift in how to think about aging. “
His latest results seem to support that theory. It’s similar to the way software programs operate off hardware, but sometimes become corrupt and need a reboot, says Sinclair. “If the cause of aging was because a cell became full of mutations, then age reversal would not be possible,” he says. “But by showing that we can reverse the aging process, that shows that the system is intact, that there is a backup copy and the software needs to be rebooted.”
In the mice, he and his team developed a way to reboot cells to restart the backup copy of epigenetic instructions, essentially erasing the corrupted signals that put the cells on the path toward aging. They mimicked the effects of aging on the epigenome by introducing breaks in the DNA of young mice. (Outside of the lab, epigenetic changes can be driven by a number of things, including smoking, exposure to pollution and chemicals.) Once “aged” in this way, within a matter of weeks Sinclair saw that the mice began to show signs of older age—including grey fur, lower body weight despite unaltered diet, reduced activity, and increased frailty.
Stay up-to-date on the latest health news, and get expert advice on living well in TIME’s Health Matters newsletter. Subscribe here.
The rebooting came in the form of a gene therapy involving three genes that instruct cells to reprogram themselves—in the case of the mice, the instructions guided the cells to restart the epigenetic changes that defined their identity as, for example, kidney and skin cells, two cell types that are prone to the effects of aging. These genes came from the suite of so-called Yamanaka stem cells factors—a set of four genes that Nobel scientist Shinya Yamanaka in 2006 discovered can turn back the clock on adult cells to their embryonic, stem cell state so they can start their development, or differentiation process, all over again. Sinclair didn’t want to completely erase the cells’ epigenetic history, just reboot it enough to reset the epigenetic instructions. Using three of the four factors turned back the clock about 57%, enough to make the mice youthful again.
“We’re not making stem cells, but turning back the clock so they can regain their identity,” says Sinclair. “I’ve been really surprised by how universally it works. We haven’t found a cell type yet that we can’t age forward and backward.”
Read More: The Best Anti-Aging Serums, Tested and Reviewed
Rejuvenating cells in mice is one thing, but will the process work in humans? That’s Sinclair’s next step, and his team is already testing the system in non-human primates. The researchers are attaching a biological switch that would allow them to turn the clock on and off by tying the activation of the reprogramming genes to an antibiotic, doxycycline. Giving the animals doxycycline would start reversing the clock, and stopping the drug would halt the process. Sinclair is currently lab-testing the system with human neurons, skin, and fibroblast cells, which contribute to connective tissue.
In 2020, Sinclair reported that in mice, the process restored vision in older animals; the current results show that the system can apply to not just one tissue or organ, but the entire animal. He anticipates eye diseases will be the first condition used to test this aging reversal in people, since the gene therapy can be injected directly into the eye area.
“We think of the processes behind aging, and diseases related to aging, as irreversible,” says Sinclair. “In the case of the eye, there is the misconception that you need to regrow new nerves. But in some cases the existing cells are just not functioning, so if you reboot them, they are fine. It’s a new way to think about medicine.”
That could mean that a host of diseases—including chronic conditions such as heart disease and even neurodegenerative disorders like Alzheimer’s —could be treated in large part by reversing the aging process that leads to them. Even before that happens, the process could be an important new tool for researchers studying these diseases. In most cases, scientists rely on young animals or tissues to model diseases of aging, which doesn’t always faithfully reproduce the condition of aging. The new system “makes the mice very old rapidly, so we can, for example, make human brain tissue the equivalent of what you would find in a 70 year old and use those in the mouse model to study Alzheimer’s disease that way,” Sinclair says.
Beyond that, the implications of being able to age and rejuvenate tissues, organs, or even entire animals or people are mind-bending. Sinclair has rejuvenated the eye nerves multiple times, which raises the more existential question for bioethicists and society of considering what it would mean to continually rewind the clock on aging.
This study is just the first step in redefining what it means to age, and Sinclair is the first to acknowledge that it raises more questions than answers. “We don’t understand how rejuvenation really works, but we know it works,” he says. “We can use it to rejuvenate parts of the body and hopefully make medicines that will be revolutionary. Now, when I see an older person, I don’t look at them as old, I just look at them as someone whose system needs to be rebooted. It’s no longer a question of if rejuvenation is possible, but a question of when.”
More Must-Reads from TIME
- Breaking Down the 2024 Election Calendar
- How Nayib Bukele’s ‘Iron Fist’ Has Transformed El Salvador
- What if Ultra-Processed Foods Aren’t as Bad as You Think?
- How Ukraine Beat Russia in the Battle of the Black Sea
- Long COVID Looks Different in Kids
- How Project 2025 Would Jeopardize Americans’ Health
- What a $129 Frying Pan Says About America’s Eating Habits
- The 32 Most Anticipated Books of Fall 2024
Write to Video by Andrew. D Johnson at [email protected]
share this!
July 13, 2023
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
Discovery of chemical means to reverse aging and restore cellular function
by Impact Journals LLC
In a groundbreaking study, researchers have unlocked a new frontier in the fight against aging and age-related diseases. The study, conducted by a team of scientists at Harvard Medical School, has published the first chemical approach to reprogram cells to a younger state. Previously, this was only achievable using a powerful gene therapy.
On July 12, 2023, researchers from Harvard Medical School, University of Maine and Massachusetts Institute of Technology (MIT) published a new research paper in Aging , titled, "Chemically induced reprogramming to reverse cellular aging."
The team's findings build upon the discovery that the expression of specific genes, called Yamanaka factors, could convert adult cells into induced pluripotent stem cells (iPSCs). This Nobel Prize-winning discovery raised the question of whether it might be possible to reverse cellular aging without causing cells to become too young and turn cancerous.
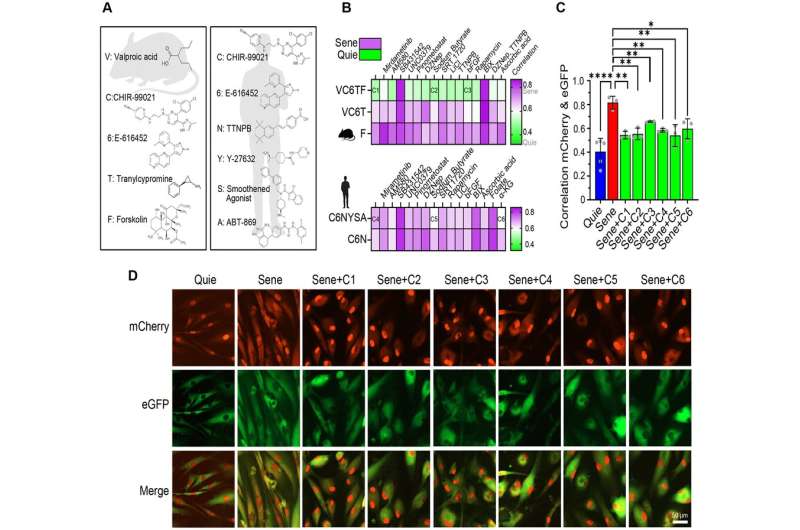
In this new study, the researchers screened for molecules that could, in combination, reverse cellular aging and rejuvenate human cells. They developed high-throughput cell-based assays to distinguish young cells from old and senescent cells, including transcription-based aging clocks and a real-time nucleocytoplasmic protein compartmentalization (NCC) assay. In an exciting discovery, the team has identified six chemical cocktails that restore NCC and genome-wide transcript profiles to youthful states and reverse transcriptomic age in less than a week.

The Harvard researchers previously demonstrated that it is indeed possible to reverse cellular aging without uncontrolled cell growth by virally-introducing specific Yamanaka genes into cells. Studies on the optic nerve , brain tissue , kidney, and muscle have shown promising results, with improved vision and extended lifespan observed in mice and, recently, a report of improved vision in monkeys.
The implications of this new discovery are far-reaching, opening avenues for regenerative medicine and, potentially, whole-body rejuvenation. By developing a chemical alternative to age reversal via gene therapy, this research could revolutionize the treatment of aging, injuries and age-related diseases and offers the potential for lower costs and shorter timelines in development. On the heels of positive results in reversing blindness in monkeys in April 2023, preparations for human clinical trials of the lab's age reversal gene therapy are in progress.
"Until recently, the best we could do was slow aging. New discoveries suggest we can now reverse it," said David A. Sinclair, A.O., Ph.D., Professor in the Department of Genetics and co-Director of the Paul F. Glenn Center for Biology of Aging Research at Harvard Medical School and lead scientist on the project. "This process has previously required gene therapy, limiting its widespread use."
The team at Harvard envisions a future where age-related diseases can be effectively treated, injuries can be repaired more efficiently, and the dream of whole-body rejuvenation becomes a reality. "This new discovery offers the potential to reverse aging with a single pill, with applications ranging from improving eyesight to effectively treating numerous age-related diseases ," Sinclair said.
Provided by Impact Journals LLC
Explore further
Feedback to editors

Data from space probes show that Alfvén waves drive the acceleration and heating of the solar wind
20 minutes ago

Saturday Citations: Corn sweat! Nanoplastics! Plus: Massive objects in your area are dragging spacetime
3 hours ago

How fruit flies use internal representations of head direction to support goal-directed navigation

Study finds RNA molecule controls butterfly wing coloration
8 hours ago

Doughnut-shaped region found inside Earth's core deepens understanding of planet's magnetic field
21 hours ago

Study combines data and molecular simulations to accelerate drug discovery

Biodiversity loss: Many students of environment-related subjects are partly unaware of the causes
22 hours ago

How stressed are you? Nanoparticles pave the way for home stress testing
23 hours ago

Researchers identify genes for low glycemic index and high protein in rice
Aug 30, 2024

New discoveries about how mosquitoes mate may help the fight against malaria
Relevant physicsforums posts, the predictive brain (stimulus-specific error prediction neurons).
6 hours ago
Will cryosleep ever be a reality?
Any suggestions to dampen the sounds of a colostomy bag.
Aug 28, 2024
Any stereo audio learning resources for other languages?
Aug 25, 2024
Cannot find a comfortable side-sleeping position
Therapeutic interfering particle.
Aug 24, 2024
More from Biology and Medical
Related Stories

Researchers identify a protein as a potential therapeutic target for age-related diseases
Jun 15, 2023

Researchers identify gene responsible for cellular aging
Nov 30, 2020
Cellular senescence involves gene repression through p53-p16/RB-E2F-DREAM complex
Jun 13, 2023

Scientists reverse age-related vision loss, eye damage from glaucoma in mice
Dec 2, 2020

Has first person to live to be 150 been born?
Jan 31, 2023

Research unveils dynamic human eye architecture: A breakthrough in ophthalmology
Jun 1, 2023
Recommended for you

Scientists discover molecular mechanism that plays key role in gene transcription and macrophage functional activation

Enhancing microbe memory to better upcycle excess CO₂

AI tool maps out cell metabolism with precision

Promising antibiotic candidates discovered in microbes deep in the Arctic Sea

Molecularly imprinted polymers help get the stink out of smoke-tainted wine
Aug 29, 2024

Tuberculosis under the sea: A marine sponge microbe provides insights into the bacterium's evolution
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Single molecule reverses signs of aging in muscles and brains, mouse study reveals
A new study in mice and human cells suggests that a small molecule can help reverse signs of aging by extending telomeres and modulating key genes.
A single, small molecule can restore muscle strength, fuel brain cell growth and reduce inflammation in old mice, new research shows.
So far, the anti-aging molecule has only been tested in rodents and in human cells in lab dishes. But the researchers say the results are compelling enough to move the compound toward human trials, potentially within a few years.
"Given the strength of the preclinical data, it is my view that there's justification for moving this forward," said senior study author Dr. Ronald DePinho , a professor and former president at The University of Texas MD Anderson Cancer Center.
"We have confidence that this mechanism would have beneficial effects with respect to things that impact health span," enabling people to live healthier lives into old age, DePinho told Live Science.
Related: 'Biological aging' speeds up in times of great stress, but it can be reversed during recovery
Reversing aging with one molecule
In the new study, published June 21 in the journal Cell , researchers looked to increase the amount of a protein that normally dwindles with age: telomerase reverse transcriptase (TERT).
TERT is a key cog in a cellular machine that extends the length of telomeres — protective caps that prevent fraying at the ends of chromosomes . The shortening of telomeres has been tied to aging and age-related diseases, such as cancer. This shortening happens partly because, with age, chemical tags build up on our chromosomes, causing what's known as " epigenetic " changes. Some of these changes switch off the gene for TERT, causing cells to make less of the protein.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
This threatens the integrity of telomeres and has wide-ranging effects on how much other genes are expressed. That's because TERT seems to be a master controller that helps regulate a suite of genes tied to aging, including genes involved in brain cell growth and senescence , a zombie-like state that more and more cells enter as the body ages. As these zombies grow in number, they trigger damaging inflammation in the body.
"Our lab was the first to show that aging is a reversible process," and that TERT can mediate that shift into reverse, DePinho said. In 2010 , DePinho and colleagues reported that, when they switched off the TERT gene in mice using experimental methods, the animals aged prematurely.
"When we flipped it back on, we were expecting just an arrest of the aging process," DePinho said. "But instead we saw rejuvenation."
This rejuvenation showed up in cells across the body. Subsequent work by the team showed that restoring "youthful" levels of TERT in a mouse model of Alzheimer's disease reversed signs of the illness, including the accumulation of abnormal proteins in the brain.
Given these results, in the new study the researchers wanted to uncover drug-like substances that could boost TERT to levels seen in healthy, young cells. They developed a screen using mouse cells tweaked to carry the human version of the TERT gene. They screened 653,000 compounds in total, landing on one that appeared most potent, which they dubbed TERT-activating compound (TAC).
Related: Extreme longevity: The secret to living longer may be hiding with nuns... and jellyfish

In lab dishes, the molecule increased the amount of TERT in healthy human cells and in cells derived from people with Werner syndrome , a rare condition that causes rapid, premature aging. These latter cells notably lengthened their telomeres when exposed to the molecule.
In mice injected with TAC, the molecule boosted TERT in tissues throughout the body, including the brain. This suggests the drug passes easily into the brain, DePinho said, which many molecules cannot.
In aged mice, the researchers looked at short-term treatments with TAC, lasting around one week, and chronic treatment lasting six months. The short-term treatment reversed signs of aging in blood cells; reduced a known driver of senescence in many tissues; and boosted a key molecule for brain cell growth. Long-term treatment increased brain cell growth in the hippocampus , a key memory center in the brain, and also seemed to improve the rodents' performance in memory tests. Additional tests showed it improved the mice's coordination and muscle strength, too.
TAC works by jump-starting a chain of events in cells that switches on a master gene regulator and ultimately unmutes the TERT gene. These effects are temporary, peaking within about eight hours and wearing off after 24 hours of injection, DePinho said.
Within that time window, the drug "restores physiological, youthful levels of TERT," he said.
— Pregnancy may speed up 'biological aging,' study suggests
— Exercise may reverse sign of aging by 'flushing' fat from muscle
— Skin cells made 30 years younger with new 'rejuvenation' technique
More work will be needed to bring TAC to human patients. The next steps will be to modify the drug to improve its potency as well as identify and weed out any harmful effects. (None were observed in these initial experiments.) The drug, or a derivative of it, will need to be tested further in animals before moving into trials with healthy human volunteers and then people with various age-related diseases, DePinho said.
In theory, the drug could be explored as a way to prevent age-related disease before it even sets in, but it would likely be approved for a specific disease, like Alzheimer's, first, DePinho said.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun ? Send us your questions about how the human body works to [email protected] with the subject line "Health Desk Q," and you may see your question answered on the website!
Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
World's oldest person, Maria Branyas Morera, dies at 117 years old
Human aging accelerates dramatically at age 44 and 60
Why does heat cause headaches?
Most Popular
- 2 Deadly 'triple E' kills New Hampshire man — what is eastern equine encephalitis?
- 3 Ancient submerged bridge in Spain reveals that humans inhabited Mediterranean island nearly 6,000 years ago
- 4 Colon-cancer risk in young people linked to one amino acid, small study finds
- 5 'Closer than people think': Woolly mammoth 'de-extinction' is nearing reality — and we have no idea what happens next
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

Weight-loss drug linked to fewer COVID deaths

Billions worldwide deficient in essential micronutrients

Smokers are less likely to develop Parkinson’s. Why?
Looking to rewind the aging clock.

Kira Sampson
BWH Communications
Harvard researchers create model that better measures biological age, distinguishes between harmful and adaptive changes during life
New “clocks” may provide answers to the question: What causes us to age?
Researchers at Harvard-affiliated Brigham and Women’s Hospital have developed a novel epigenetic clock, a machine-learning model designed to predict biological age from DNA structure. The clock can distinguish between genetic differences that slow and accelerate aging, predict biological age, and evaluate anti-aging interventions with increased accuracy. The results are published in Nature Aging.
“Previous clocks considered the relationship between methylation patterns and features we know are correlated with aging, but they don’t tell us which factors cause one’s body to age faster or slower,” said corresponding author said corresponding author Vadim Gladyshev , a principal investigator in the Division of Genetics at BWH. “Our clocks distinguish between changes that accelerate and counteract aging to predict biological age and assess the efficacy of aging interventions.”
Aging researchers have long acknowledged the influence that DNA methylation — alterations to our genetic structure that shape gene function — has on the aging process. Notably, specific regions of our DNA, known as CpG sites are strongly associated with aging. While lifestyle choices, such as smoking and diet, influence DNA methylation, so does our genetic inheritance, explaining why individuals with similar lifestyles may age at different rates.
“Our clocks distinguish between changes that accelerate and counteract aging to predict biological age and assess the efficacy of aging interventions.” Vadim Gladyshev, principal investigator
Existing epigenetic clocks predict biological age (the actual age of our cells rather than chronological) using DNA methylation patterns. However, until now, none has distinguished between methylation differences that cause biological aging and those simply correlated with the process.
Using a large genetic data set, first author Kejun (Albert) Ying, a graduate student in the Gladyshev lab, performed an epigenome-wide Mendelian Randomization (EWMR), a technique used to randomize data and establish causation between DNA structure and observable traits, on 20,509 CpG sites causal to eight aging-related characteristics.
The eight aging-related traits included lifespan, extreme longevity (defined as survival beyond the 90th percentile), health span (age at first incidence of major age-related disease), frailty index (a measure of one’s frailty based on the accumulation of health deficits during the lifespan), self-rated health, and three broad aging-related measurements incorporating family history, socioeconomic status, and other health factors.
With these traits and their associated DNA sites in mind, Ying created three models: CausAge, a general clock that predicts biological age based on causal DNA factors, and DamAge and AdaptAge, which include only damaging or protective changes. Investigators then analyzed blood samples from 7,036 individuals ages 18 to 93 years old from the Generation Scotland Cohort and ultimately trained their model on data from 2,664 individuals in the cohort.
With these data, researchers developed a map pinpointing human CpG sites that cause biological aging. This map allows researchers to identify biomarkers causative to aging and evaluate how different interventions promote longevity or accelerate aging.
Scientists tested their clocks’ validity on data collected from 4,651 individuals in the Framingham Heart Study and the Normative Aging Study. They found that DamAge correlated with adverse outcomes, including mortality, and AdaptAge correlated with longevity, suggesting that age-related damage contributes to the risk of death, while protective changes to DNA methylation may contribute to a longer lifespan.
Next, they tested the clocks’ ability to assess biological age by reprogramming stem cells — transforming specialized cells, like skin cells, back into a younger, less-defined state where they can develop into various types of cells in the body. When applying the clocks to the newly transformed cells, DamAge decreased, indicating a reduction in age-related damage during reprogramming, while AdaptAge did not show a particular pattern.
Finally, the team tested their clocks’ performance in biological samples from patients with various chronic conditions, including cancer and hypertension, as well as samples damaged by lifestyle choices like smoking cigarettes. DamAge consistently increased in conditions associated with age-related damage, while AdaptAge decreased, effectively capturing protective adaptations.
“Aging is a complex process, and we still do not know what interventions against it actually work,” said Gladyshev. “Our findings present a step forward for aging research, allowing us to more accurately quantify biological age and evaluate the ability of novel aging interventions to increase longevity.”
Disclosures: Kejun Ying and Vadim Gladyshev are inventors on a patent application related to the research reported.
This study is supported by the National Institute on Aging, Impetus grants and the Michael Antonov Foundation.
Share this article
You might like.
Large-scale study finds Wegovy reduces risk of heart attack, stroke

Inadequate levels carry risk of adverse pregnancy outcomes, blindness

Researchers test theory explaining medical mystery and identify potential new treatment
You want to be boss. You probably won’t be good at it.
Study pinpoints two measures that predict effective managers
Your kid can’t name three branches of government? He’s not alone.
Efforts launched to turn around plummeting student scores in U.S. history, civics, amid declining citizen engagement across nation
Good genes are nice, but joy is better
Harvard study, almost 80 years old, has proved that embracing community helps us live longer, and be happier
Is reverse aging already possible? Drugs that could treat aging might already be on the pharmacy shelves

At 67 years old, Dr. Nir Barzilai looks about the same as, if not younger than, he did 10 years ago. It’s apparent in side-by-side photographs, and it’s what most people who know him say. Barzilai lives a healthy lifestyle . He exercises every day, eats right, and practices intermittent fasting .
He’s also been taking the diabetes drug metformin off label for 10 years. He has never been diagnosed with diabetes or prediabetes—the conditions for which the drug is approved and prescribed—but takes it for a different off-label reason.
“We know that it targets aging,” said Barzilai, who is a professor of medicine and genetics at Albert Einstein College of Medicine and director of the American Federation for Aging Research.
“People on metformin have 30% lower rates of almost every kind of cancer. It delays cognitive decline . Even people with diabetes who are obese and have more disease to start with but are on metformin have lower mortality rates than people without diabetes who aren’t on the drug.”
What he says is born out in numerous studies. Overall, this safe, super-cheap, decades-old drug not only treats diabetes, but it also seems to delay and compress the years of chronic illness associated with the final stage of life and extend what geroscientists call the “healthspan.”
Metformin is just one of many medications, including other old ones and some brand new inventions, that academic researchers and biotech startups are exploring to slow, stop, or perhaps even reverse aging.
What is aging
All sorts of processes are happening in our bodies as we age. Some of which make us more vulnerable to the diseases most linked to old age: cancer, dementia, heart disease, stroke, macular degeneration and so forth.
Doctors and scientists in the longevity field are trying to determine which of those processes is the strongest hallmark of overall aging and declining health and how to target that process with a drug in the same way that current drugs target specific diseases.
“We can target aging,” Barzilai said. “We can delay it. And in several instances, we can stop and reverse it. At one point we had hope. Then we moved to promise. Now, we need to move to realize that promise. That’s where we are.”

Targeting undead cells
Dozens of biotechs want to be the first to realize that promise through drugs called senolytics. In certain diseases, these pharmaceuticals can clear out toxic, old, dysfunctional cells and leave only young, healthy, well functioning ones behind. Senolytic Dasatinib (Sprycel) is FDA-approved for certain types of chronic myelogenous leukemia.
Here’s why researchers think senolytics could do more than treat one specific disease .
Your cells constantly reproduce and divide throughout their lifespan to create new, healthy cells. When they stop doing this, they die. But with some cells, even though they’ve stopped reproducing, they don’t die off like they should. These undead cells, called senescent cells, stick around and give off toxic substances that can harm the healthy cells around them—like the one bad apple that spoils the whole bunch.
As you get older, your aging body becomes less efficient at clearing out senescent cells, so they accumulate, especially around the sites where chronic diseases develop, like macular degeneration.
Unity Biotechnology has a senolytic, dubbed UBX1325 , in phase 2 clinical trials for diabetic macular edema (DME) and age-related macular degeneration (AMD). In both conditions, damage to the retina can lead to vision loss.
Preliminary findings in DME patients in the trial suggest that the drug clears out problem-causing senescent cells in the eye and allows the remaining healthy cells to repair and regenerate the retina and bring lost vision back.
“It takes about 8 weeks to kick in, and at 24 weeks, you can see that the tissue has dramatically remodeled,” said Anirvan Ghosh, PhD, Unity CEO. “We had patients who had big gains in vision and big improvements in retinal structure.”
As incredible as these results are, vision restoration isn’t the endgame for Unity or most any company invested in senolytics development. The idea is to develop another drug that will have this same effect on another progressive disease and another and another until, ideally, researchers can figure out how to make a drug that would clear out all senescent cells, not just the one type behind a certain disease.
“In different tissues, it’s not always the same cell type that becomes senescent. I think next we will have tissue-specific senolytics for a specific disease or if you’re high-risk for that disease.” But to target aging, rather than just individual diseases, he says, “We’d have to have something that clears these cells from multiple tissue types.” That’s the long-term goal. And it may still be a long road to get there.
Understanding biological age
While zombie cells build up in the aging body, wreaking havoc as their numbers grow, critical changes are taking place on the surface of DNA, too. That is, in the epigenome , a landscape of proteins and chemicals that sits atop your genetic material.
These changes over time are the result of your environment, behaviors and exposures throughout your lifetime. Think: pollution, trauma, diet, exercise, and secondhand smoke. They don’t change your DNA, but they change the way your DNA acts. Genes that once functioned perfectly may at some point in life slow down, speed up, shut off, or just go generally haywire. Any dysregulation can cause disease or the signs and symptoms of old age.
Epigenetic changes are like scratches on a record: You can still hear the music, but it’s not what it used to be.
Led by Harvard Medical School professor and molecular geneticist David Sinclair, PhD, Tally Health is already bringing epigenetic approaches to aging directly to consumers. The company offers a cheek swab test that estimates customers’ biological age—how old they seem based on their epigenetics rather than their birth year.
“ Biological age is a much better representation of health status than birthday candles,” Sinclair says. “Birthday candles don’t tell you how well you’ve been living and they certainly don’t tell you how many years you’ve got left.”
Tally Health creates personalized recommendations based on customers’ biological age for how they might reduce that age because, as Sinclair points out, “Your biological clock is not unidirectional.”
For now, the means to turn back the clock are mostly lifestyle changes. But Sinclair, who has founded several biotechs, and others are researching and developing drugs that might slow or restart the clock so genes will act like they are young again.
Sinclair and his collaborators have shown that this is possible in the eyes of blind mice. In newborn mice, if the optic nerve – the nerve that carries messages from the retina to the brain—is damaged, it will recover. But in old mice, it can no longer heal.
In Sinclair’s lab, they crushed the optic nerves of mice to blind them. The serious injury to the eye caused epigenetic changes that resemble those that happen in old age. They then injected the nerves with genes that contained factors they expected would reprogram the genes to behave like they were young again.
The treatment reversed the age-related epigenetic changes in the eyes, rescued retinal cells, and led to regeneration of neurons. Since then , Sinclair and his colleagues have corrected similar age-related epigenetic changes in muscle and kidney tissue. Other researchers have successfully used the technique to extend the overall lifespan of mice. Sinclair expects to release results of his study which tests this concept in primates in a few months.
The holy grail, of course, is to erase the scratches that time puts on our own epigenetic records. Sinclair says that’s coming.
“Resetting the whole human body in this way is a different matter,” he says. “Are we one day going to be able to turn ourselves back 20 years? I don’t see any reason why that won’t be possible. It’s just a question of when.”
Drugs that treat aging
But some drugs that could lower risk for multiple age-related diseases at once and, perhaps, treat aging as a whole, might already be on the shelves of the pharmacy.
Rapamycin, an mTOR inhibitor, got FDA approval in 1991 as an immune suppressor that prevents organ recipients from rejecting a new organ. By shutting off the mTOR protein, it prevents immune system cells from proliferating to attack the donated organ.
“But in every species that’s been studied to date – yeast, worms, flies, mice – when they are given rapamycin, healthspan and lifespan are extended. No other therapeutic has that degree of validation,” says Joan Mannick, MD, CEO of Tornado Therapeutics.
Mannick and other mTOR researchers believe that, among the other deleterious processes of aging, mTOR proteins might start to malfunction, too. In a healthy, young person, mTOR, which supports cell growth, is active when we eat. That’s critical for growth, development and reproduction. When we fast, like during the night, mTOR is inactive, which allows for cell repair.
This, by the way, is probably one of the reasons intermittent fasting has so many health benefits, Mannick says, because it blocks mTOR and allows for more cell repair.
As we get older, mTOR may stay active all the time—opening the door to out-of-control cell growth that can lead to cancer and closing the door on cell repair. In older adults, low doses of rapamycin, an mTOR inhibitor, seem to set mTOR activity back to its youthful state: on when you need it, off when you don’t.
“When they get rapamycin, their immune systems, which have already been damaged by old age, start to function better,” Mannick says. “There’s research to suggest that when you rejuvenate the immune system, you make a lot of other organ systems function better. But we need well-powered, placebo-controlled clinical trials to find out the dose, what conditions it improves, and who responds best.”
To carry out that research, Tornado Therapeutics has acquired a portfolio of rapamycin derivatives, or “rapalogs,” from Novartis , which they are studying as treatments for aging. If it can increase the lifespan of every plant and animal that’s been exposed to it, Mannick and the company’s investors, such as Cambrian Bio, bet it might increase the human lifespan, too.
“I think in the next 5 to 10 years, FDA will have approved the first drug to target aging biology,” Mannick says. “It very well could be a new rapalog that is going to have benefits for an aging-related condition as our first step to a much broader aging medicine advance.”
Type 2 diabetes is an age-related condition. Numerous studies show that metformin, a drug that’s FDA-approved to treat it, may also lower risk for cancer, heart disease, stroke, dementia, or death for any other reason, including COVID-19.
The benefits, researchers suspect, are a result of metformin’s ability to control blood sugar, blunt the effects of a lifetime of oxidative stress on the body, and protect cardiovascular function. That is, it may meet a host of needs that grow greater as we age.
Of course, the overwhelming majority of data on metformin’s benefits comes from people with diabetes. Numerous clinical trials currently underway are looking at its effects on specific diseases in people who don’t have diabetes. But longevity researchers, like Barzilai, want to prove to the FDA that it doesn’t have to be taken for a specific disease. Older adults should simply take it for aging.
Barzilai’s TAME (Targeting Aging with Metformin) trial, which will last six years, aims to prove that anyone between the ages of 65 and 79 can extend their healthspan with metformin.
“We’re using metformin as a tool,” he says, “to show the FDA that aging itself can be targeted.”
Targeting the healthspan
No matter which approach becomes the first prescription drug for aging, researchers in the area tend to agree it’s coming soon. It would be expected to improve and extend life not only for average older adults, but also for those who currently tend to get chronic diseases and die sooner: childhood cancer survivors, people living with HIV, people living in poverty.
Researchers predict the implications will be huge when the healthy years of life last longer for everyone. Economist Andrew Scott calculated that a slowdown in aging that increases life expectancy by just one year would be worth $38 trillion. A ten-year increase would be worth $367 trillion.
“That’s because with this kind of increase in life expectancy, we are not spending that time in the hospital, we are not sick,” Barzilai says. “We’re shopping, we’re traveling, we are participating in society. We are living life. And that’s what we want.”
Latest in Aging Well

Staying fit as you age isn’t just about exercise. Experts say to prioritize these 4 habits

Warren Buffett turns 94 today. His secret to longevity? Coca-Cola, candy, and a life of joy

Why this year’s Medicare Annual Notice of Change will be vital reading for beneficiaries

4 ways women can make the most of their ‘longevity bonus’

Medicare costs significantly lowered on popular prescription drugs for cancer, diabetes, heart disease and arthritis

Medical care and prescription costs among top 10 worries of older adults before 2024 election
Most popular.

New COVID vaccines are here. What to know about the latest shots as summer surge continues

What you need to know about the deadly listeria outbreak linked to Boar’s Head deli meats

Parental stress is so debilitating, the surgeon general has declared it a public health issue

Rob Pitts of Netflix’s ‘Tex Mex Motors’ has died of stomach cancer. These are signs of the disease

Suspect who hit NHL’s Johnny Gaudreau and his little brother drank five or six beers before the crash: report
New Potential for Reversing Aging: Scientists Discover Changes in Aging Stem Cells

Scientists have developed a method to identify aging muscle stem cells.
The issue of aging and the fight against it has long been prevalent in both classic and contemporary literature throughout human history. From the ill-fated Qin Shi Huang’s expedition to the sea in pursuit of eternal life to the fame of Count Dracula in the West, aging has caught the world’s fascination for thousands of years and remains unsolved.
In an exciting breakthrough, scientists have developed a way to identify aged muscle stem cells (MuSCs) based on their chromatin signature. MuSCs play an important role in muscle repair. The research team was from the Hong Kong University of Science and Technology (HKUST) and was led by Professor Tom Cheung, an associate professor of life sciences.
In contrast to their younger counterparts, aging MuSCs have decreased stemness (the ability to become new stem cells or turn into specialized cells to replace damaged tissues). If the chromatin signature of an aged cell can be restored to that of a young cell, the process of cellular aging—and, in this example, skeletal muscle tissue aging—may be slowed or even reversed.
The findings were recently published in the journal iScience .
“The regulation of chromatin accessibility is critical for cell fate decisions,” said Professor Cheung. “Changes in the chromatin state can lead to dysregulation of gene expression. In our study, we were able to identify the chronically activated chromatin state as a hallmark of stem cell aging, which could be a target for developing anti-aging strategies.”
Chromatin, a complex of DNA that wraps around histones to maintain DNA in its proper architecture, undergoes rapid changes in its structure in response to the extrinsic environment. As a continuation of their previous study, the team pre-fixed muscle stem cells in the mouse to obtain quiescent cells (dormant cells that will activate to repair injured muscle) and obtained their gene and chromatin signatures, in which they then compared the chromatin accessibility over time.
“We showed that the chromatin environment of young muscle stem cells is very compact during quiescence, becomes highly accessible on early activation, and gradually re-establishes the compact state after long-term regeneration. However, aged muscle stem cells lose their ability to maintain such a compact chromatin environment during quiescence,” said Dr. Anqi Dong, first author of the study and a former member of Professor Cheung’s research group who is now a Postdoctoral Fellow at the Université libre de Bruxelles.
Many possibilities are waiting to be unearthed now that scientists have gained a better understanding of what happens to an aging cell, opening a variety of avenues for anti-aging strategies to be pursued further.
“Have we solved the mystery of aging? Yes, but not quite,” noted Professor Cheung. “If we can find chromatin-modifying regulators that are downregulated in aged stem cells, these will be potential targets to prevent aging by restoring their expression. As we are able to make a clear comparison between the chromatin states of young and old muscle stem cells, we have also identified target locations that are specifically accessible in young muscle stem cells. If the accessibility of those regions can be maintained during aging, we may be able to find ways to keep cells young and healthy longer.”
“Our current study describes the changes in chromatin accessibility during stem cell isolation and activation, but the journey has just begun,” said Professor Cheung. “We look forward to further investigating the mechanisms that alter the chromatin state during muscle stem cell isolation and activation, and it is important we conduct the same study in vivo for more insights.”
Reference: “Global chromatin accessibility profiling analysis reveals a chronic activation state in aged muscle stem cells” by Anqi Dong, Jing Liu, Kangning Lin, Wenshu Zeng, Wai-Kin So, Shenyuan Hu and Tom H. Cheung, 17 August 2022, iScience . DOI: 10.1016/j.isci.2022.104954
Related Articles
Is immortality in our reach unveiling sea anemone secrets, unlocking immortality: t cells as the new fountain of youth, unlocking healthy longevity: anti-aging function discovered in cell protein, turning back the clock: genetic engineers rewire cells for an 82% increase in lifespan, life-extending effects: how serotonin, dopamine, and the smell of food affect aging, microbes turn back the clock: new research discovers their potential to reverse aging in the brain, synergistic cellular pathways identified that extend lifespan by 500%, working to save infants, researchers transform stem cells into cells that form blood vessels, scientists create stem-cell-derived neurons from alzheimer’s disease.
Save my name, email, and website in this browser for the next time I comment.
Type above and press Enter to search. Press Esc to cancel.
- Subscribe to BBC Science Focus Magazine
- Previous Issues
- Future tech
- Everyday science
- Planet Earth
- Newsletters
10 breakthroughs that could soon slow or reverse your biological age
The biggest killers in the modern world, like dementia and cancer, are down to ageing. Is it possible to tackle the ageing process so we can live for longer and stay healthier too?
Andrew Steele
Ageing is the single largest cause of human suffering. It might sound counter-intuitive, but it makes sense when you think it through: all of the biggest killers in the modern world, from cancer to heart disease to dementia, affect older people far more often than younger ones.
We’ve even seen it with coronavirus , with the oldest patients hundreds of times more likely to die of the disease than children or young adults.
If you add it all up, of the 150,000 deaths that happen every day on Earth, over 100,000 of them are caused by ageing. Deaths from problems like heart disease are preceded by years of physical decline, loss of independence, and so on.
And then there’s the problems we don’t list as diseases: the frailty, the forgetfulness, the incontinence… Add all this suffering together, across billions of people, and nothing else comes close.
So, rather than tackle these individual problems one at a time, why not go after the real prize: the ageing process that causes them?
I think this is the most exciting idea in modern medicine – which is why I’ve written a book about it, called Ageless: The New Science Of Getting Older Without Getting Old . To whet your appetite, these are the top 10 breakthroughs that prove this idea isn’t science fiction – from the discoveries of the past, to the cutting-edge science of the present day.
1. Dietary restriction
For tens of thousands of years of human history, ageing seemed inevitable. However, this dogma was overturned by experiments in rats in the 1930s. Scientist Clive McCay found that rats fed substantially less than normal could live much longer than their compatriots whose food wasn’t being rationed. And, crucially, they weren’t having their time as rat geriatrics extended – they were doing so by staying young for longer, deferring the disease and frailty of old age.
Whether this phenomenon works in larger and longer-lived animals like, most pressingly, humans, remains somewhat uncertain. Plus, people who try dietary restriction report that the hunger is unrelenting, so I’m not sure whether even a few more healthy years are worth the trade-off! However, these long-lived hungry rats deserve a special place in the history of ageing research because they showed us that slowing ageing is possible.
And, as a bonus, researchers are working on ‘dietary restriction mimetic’ drugs, like rapamycin or metformin, which could mimic the effects of eating less, but without the constant hunger pangs.
2. Negligible senescence

Getting old might seem like a fact of life. It appears at first glance rather like a gradual process of wearing out, inevitable for both human-made machines and the biological machinery of living things. However, looking around the animal kingdom, we can see that there’s no law of biology mandating ageing.
Humans’ risk of death doubles every eight years. However, some animals possess ‘negligible senescence’ – senescence being the scientific term for ageing. Some kinds of tortoises, salamanders, fish , and a few other animals have a risk of death that doesn’t depend on how old they are. By this definition, they don’t age.
With the appropriate incentives, evolution can equip organisms with mechanisms to repair broken cells and molecules, and get rid of and replace the unfixable. There’s no reason to think that science couldn’t eventually make it possible for humans too.

3. The hallmarks of ageing

Dietary restriction and negligible senescence show us that slowing or even stopping ageing is possible in theory, but how can we do it in practice?
Enter the hallmarks of ageing. This framework was proposed in 2013, and set out a list of the biological underpinnings of the ageing process – the causes behind everything from wrinkles to cancer.
A framework might not sound like a breakthrough, but it really is. After decades of theories and counter-theories, there’s finally some scientific agreement about what causes ageing, and that means, if we can learn to slow, stop or reverse these hallmarks, we can do the same to the ageing process overall.
In fact, the rest of the breakthroughs on this list follow exactly this approach: slowing or stopping the progression of one of these hallmarks and, with it, ageing overall.
Read more about ageing:
- Why does time speed up when you get older?
- Epigenetic clock can predict the human brain’s biological age
4. Telomerase
Inside our cells, our DNA is split into 46 lengths known as chromosomes. At each of these chromosomes’ two ends is a protective region known as a telomere. Your telomeres get shorter over your lifetime, and people with shorter telomeres for their age are at increased risk of diseases of old age, and die sooner than people with longer ones.
Luckily, there’s an enzyme called ‘telomerase’ which can elongate your telomeres. In the late 1990s, there was a buzz around telomerase as a potential life-extending therapy – until scientists found that mice given more of the stuff had a substantially increased risk of cancer.
However, research in the last few years has shown us that as long as you turn on telomerase temporarily, you can top up telomeres seemingly without increasing cancer risk. Mice given this treatment live longer, and have higher bone density and better control of their blood sugar.
5. Rejuvenating the thymus
Just behind your breastbone and in front of your heart is – or, depending on the age you’re reading this at, was – a small organ called your thymus, responsible for the production of immune cells. The decline of the thymus is one of the reasons we get more susceptible to infection with age, as shown by older people dying more often from flu, and coronavirus.
The good news is that we have multiple ideas to reverse the decline of the thymus, from gene therapies and stem cells to hormones and drugs. One trial of a hormonal approach to thymic regrowth managed not only to increase its size and the number of new immune cells in participants. It also seemed to make them biologically younger overall as measured by their ‘epigenetic clock’ (which we’ll get to in a moment).
Often, a treatment’s effects on ageing overall are greater than the narrow hallmark it seeks to affect – but it’s particularly amazing that revitalising such a tiny organ seems to affect our whole biological clock.
6. Induced pluripotent stem cells

These could feature in a top 10 in many fields of medicine, but induced pluripotent stem cells (or iPSCs for short) have particular potential in the field of ageing biology.
These cells are made by taking normal body cells and using a cocktail of four different genes to allow them to turn into any kind of cell researchers can dream up – or, hopefully in the not-too-distant future, any kind of cell a doctor needs to replenish cells lost due to accident, injury, or the ageing process.
Probably the most advanced finding is turning iPSCs into fresh eye cells to replace those lost in a disease called age-related macular degeneration, but it might not be long before we’re using them against Parkinson’s, arthritis, thymus shrinkage (as we just mentioned) and even to make new teeth to replace those lost to decay over our lifetimes.
7. The Amish gene
In the mid-1980s, a girl in the Old Order Amish community in Indiana was rushed to hospital after a minor head injury wouldn’t stop bleeding. She survived, and started a chain of genetic detective work that eventually led to one of the most startling discoveries in the genetics of longevity.
She had a mutation in both copies of a gene called SERPINE1, which is needed for blood clotting. It turned out that many other members of this community, including both of her parents, had a single copy of this mutation.
Having just one mutated copy doesn’t seem to cause them any blood-clotting issues. However, on going back through the Old Order Amish family tree, the researchers discovered something remarkable: people with one copy of mutated SERPINE1 had better heart health, less diabetes, and lived a full 10 years longer than those without.
For decades, biologists had thought that ageing was too complicated a process for single genes to dramatically alter its course. This research proves that idea wrong, and provides hope that targeting single genes – including this one – could be a path to longer, healthier lives for the rest of us.
8. Epigenetic clocks

Epigenetics is the collective name for a set of chemical flags stuck to our DNA . This is a hot topic of research and has been studied for decades, but what came as a huge surprise to scientists was that observing how your epigenetics change can give us incredibly precise estimates of how old you are.
The first ‘epigenetic clock’ based on this idea could predict how old someone is to within a few years. More morbidly, if your ‘epigenetic age’ is higher than your chronological age, you can expect to get ill and die sooner than someone whose epigenetic age is lower than the number of candles on their birthday cake.
What’s exciting about this isn’t its morbid predictions – it’s that it will allow us to do experiments on all the anti-ageing treatments we’re discussing much more quickly.
Rather than giving trial participants a new drug and sending them away for a decade to see how many of them die, which takes a long time and is very expensive, we could just do a before-and-after epigenetic age measurement a few months later.
This would make testing these new treatments much quicker and cheaper, which, when we’ve got so many ideas for potential treatments that need testing, is going to massively accelerate our progress against the ageing process.
- Is age an illness?
- Middle aged? You’ll live longer, but not necessarily healthier
9. Intermittent reprogramming

An unexpected side-effect of iPSC research is that those same four genes that can allow a cell to turn into any other kind of cell also turn back its epigenetic clock. The process, known as cellular reprogramming, seems to make cells biologically younger.
It also works in whole animals, as long as it’s only done intermittently. Continuous reprogramming turns cells back to iPSCs, and this is bad news because iPSCs aren’t much use in themselves, only for what they can turn into. For example, an iPSC in your heart doesn’t have the skillset to pump blood, so replacing a large number of your cells with iPSCs would result in a hasty demise.
However, if you just do a little bit of reprogramming – enough to shave years off of cells’ biological clocks, but without turning them all the way back to being stem cells – you can rejuvenate the body as a whole.
Experiments in mice have shown that they can live longer and improve their health, and allow damaged optic nerve cells to regenerate, something which is normally only possible in the womb. If we can safely translate this idea into a therapy for humans, we could hope to restore our cells to a youthful state too.
10. Senolytic drugs

Probably the most exciting breakthrough in ageing biology is ‘senolytic’ drugs – drugs that kill aged ‘senescent’ cells. We all accumulate these cells throughout our lives: they’re cells that have divided too many times, accrued unacceptable levels of damage to their DNA, or are just under too high a level of stress. And so, to be on the safe side, these cells stop dividing.
Unfortunately, these cells don’t just sit there, benignly not dividing – they secrete molecules that basically conspire to accelerate the ageing process. And as we age, these cells increase in number in a vicious cycle of degeneration.
The good news is that we can get rid of them. Scientists have identified a number of drugs and other treatments that get rid of these errant cells in mice. They extend healthy life, defer cancer and heart problems, and even give these mice better fur.
Even more exciting, the first senolytics are beginning trials in humans. If everything goes to plan, it might only be a few years before the first senolytic treatments are approved for diseases from arthritis to cancer.
And, if those trials show that these drugs are safe and effective, it might not be many more years before we’re all taking senolytic drugs, to remove these cells before the problems they cause have arisen. This should be the endgame of medicines inspired by ageing biology: preventative medicine that, rather than fighting a disease we’ve already developed, stops us getting ill in the first place.
Barring a surprise entrant from left-field, senolytics are hotly tipped to win the race to be the first true anti-ageing medicine.
This article was originally published 18 March 2021.
Ageless: The New Science Of Getting Older Without Getting Old by Andrew Steele is available now (£20, Bloomsbury).
- Buy now from Amazon UK , Bookshop.org and Waterstones
Share this article

- Terms & Conditions
- Privacy policy
- Cookies policy
- Code of conduct
- Magazine subscriptions
- Manage preferences
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Technical Report
- Published: 29 August 2024
ImAge quantitates aging and rejuvenation
- Martin Alvarez-Kuglen ORCID: orcid.org/0009-0002-8197-4538 1 na1 ,
- Kenta Ninomiya ORCID: orcid.org/0000-0002-9566-4875 2 na1 ,
- Haodong Qin 3 na1 ,
- Delany Rodriguez 1 na1 ,
- Lorenzo Fiengo 1 ,
- Chen Farhy 1 ,
- Wei-Mien Hsu 4 ,
- Brian Kirk 1 ,
- Aaron Havas ORCID: orcid.org/0000-0003-4291-8015 1 ,
- Gen-Sheng Feng 5 ,
- Amanda J. Roberts 6 ,
- Rozalyn M. Anderson ORCID: orcid.org/0000-0002-0864-7998 7 , 8 ,
- Manuel Serrano ORCID: orcid.org/0000-0001-7177-9312 9 , 10 , 11 ,
- Peter D. Adams ORCID: orcid.org/0000-0002-0684-1770 1 ,
- Tatyana O. Sharpee 4 &
- Alexey V. Terskikh ORCID: orcid.org/0000-0003-4641-3997 2 , 12
Nature Aging ( 2024 ) Cite this article
10 Altmetric
Metrics details
- Fluorescence imaging
For efficient, cost-effective and personalized healthcare, biomarkers that capture aspects of functional, biological aging, thus predicting disease risk and lifespan more accurately and reliably than chronological age, are essential. We developed an imaging-based chromatin and epigenetic age (ImAge) that captures intrinsic age-related trajectories of the spatial organization of chromatin and epigenetic marks in single nuclei, in mice. We show that such trajectories readily emerge as principal changes in each individual dataset without regression on chronological age, and that ImAge can be computed using several epigenetic marks and DNA labeling. We find that interventions known to affect biological aging induce corresponding effects on ImAge, including increased ImAge upon chemotherapy treatment and decreased ImAge upon caloric restriction and partial reprogramming by transient OSKM expression in liver and skeletal muscle. Further, ImAge readouts from chronologically identical mice inversely correlated with their locomotor activity, suggesting that ImAge may capture elements of biological and functional age. In sum, we developed ImAge, an imaging-based biomarker of aging with single-cell resolution rooted in the analysis of spatial organization of epigenetic marks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Single-cell omics in ageing: a young and growing field

TIME-seq reduces time and cost of DNA methylation measurement for epigenetic clock construction
Profiling epigenetic age in single cells
Data availability.
The minimal dataset is provided on Open Science Framework ( https://osf.io/mkc9u/ ). All data supporting the findings of this study are available from the corresponding author upon request.
Code availability
The Python code is available on GitHub ( https://github.com/terskikh-lab/ImAge ).
Zampino, M. et al. Biomarkers of aging in real life: three questions on aging and the comprehensive geriatric assessment. GeroScience 44 , 2611–2622 (2022).
Article PubMed PubMed Central Google Scholar
Baker, G. T. & Sprott, R. L. Biomarkers of aging. Exp. Gerontol. 23 , 223–239 (1988).
Article PubMed Google Scholar
Wagner, K.-H., Cameron-Smith, D., Wessner, B. & Franzke, B. Biomarkers of aging: from function to molecular biology. Nutrients 8 , 338 (2016).
Moqri, M. et al. Biomarkers of aging for the identification and evaluation of longevity interventions. Cell 186 , 3758–3775 (2023).
Article CAS PubMed PubMed Central Google Scholar
Jylhävä, J., Pedersen, N. L. & Hägg, S. Biological age predictors. eBioMedicine 21 , 29–36 (2017).
Jones, J. A. B. et al. The AgeGuess database, an open online resource on chronological and perceived ages of people aged 5–100. Sci. Data 6 , 1–8 (2019).
Article CAS Google Scholar
Simm, A. et al. Potential biomarkers of ageing. Biol. Chem. 389 , 257–265 (2008).
Article CAS PubMed Google Scholar
Jazwinski, S. M. & Kim, S. Examination of the dimensions of biological age. Front. Genet. 10 , 263 (2019).
Rantanen, T. et al. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 55 , M168–M173 (2000).
Whitehead, J. C. et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 69 , 621–632 (2014).
Article Google Scholar
Kane, A. E., Keller, K. M., Heinze-Milne, S., Grandy, S. A. & Howlett, S. E. A murine frailty index based on clinical and laboratory measurements: links between frailty and pro-inflammatory cytokines differ in a sex-specific manner. J. Gerontol. Ser. A 74 , 275–282 (2019).
Schultz, M. B. et al. Age and life expectancy clocks based on machine learning analysis of mouse frailty. Nat. Commun. 11 , 4618 (2020).
Alpert, A. et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 25 , 487–495 (2019).
de Toda, I. M., Vida, C., San Miguel, L. S. & De la Fuente, M. When will my mouse die? Life span prediction based on immune function, redox and behavioural parameters in female mice at the adult age. Mech. Ageing Dev. 182 , 111125 (2019).
Mather, K. A., Jorm, A. F., Parslow, R. A. & Christensen, H. Is telomere length a biomarker of aging? A review. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 66 , 202–213 (2011).
Krištić, J. et al. Glycans are a novel biomarker of chronological and biological ages. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 69 , 779–789 (2014).
Wang, A. S. & Dreesen, O. Biomarkers of cellular senescence and skin aging. Front. Genet. 9 , 247 (2018).
Hannum, G. et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 49 , 359–367 (2013).
Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 14 , R115 (2013).
Lowe, R. et al. Ageing-associated DNA methylation dynamics are a molecular readout of lifespan variation among mammalian species. Genome Biol. 19 , 22 (2018).
Galow, A.-M. & Peleg, S. How to slow down the ticking clock: age-associated epigenetic alterations and related interventions to extend life span. Cells 11 , 468 (2022).
Lu, A. T. et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 11 , 303–327 (2019).
Weidner, C. I. et al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol. 15 , R24 (2014).
Levine, M. E. et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 10 , 573–591 (2018).
Belsky, D. W. et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife 11 , e73420 (2022).
Bell, C. G. et al. DNA methylation aging clocks: challenges and recommendations. Genome Biol. 20 , 249 (2019).
Lu, A. T. et al. Universal DNA methylation age across mammalian tissues. Nat. Aging https://doi.org/10.1038/s43587-023-00462-6 (2023).
Levine, M. E., Higgins-Chen, A., Thrush, K., Minteer, C. & Niimi, P. Clock work: deconstructing the epigenetic clock signals in aging, disease, and reprogramming. Preprint at bioRxiv https://doi.org/10.1101/2022.02.13.480245 (2022).
Liu, Z. et al. Underlying features of epigenetic aging clocks in vivo and in vitro. Aging Cell 19 , e13229 (2020).
Feridooni, H. A. et al. The impact of age and frailty on ventricular structure and function in C57BL/6J mice. J. Physiol. 595 , 3721–3742 (2017).
Rockwood, K. et al. A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci. Rep. 7 , 43068 (2017).
Kane, A. E. et al. Impact of longevity interventions on a validated mouse clinical frailty index. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 71 , 333–339 (2016).
Wang, T. et al. Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol. 18 , 57 (2017).
Field, A. E. et al. DNA methylation clocks in aging: categories, causes, and consequences. Mol. Cell 71 , 882–895 (2018).
Horvath, S. & Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 19 , 371–384 (2018).
Williams, T. D. Individual variation in endocrine systems: moving beyond the tyranny of the golden mean. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363 , 1687–1698 (2008).
Amundson, R. Against normal function. Stud. Hist. Philos. Biol. Biomed. Sci. 31 , 33–53 (2000).
Westneat, D. F., Wright, J. & Dingemanse, N. J. The biology hidden inside residual within-individual phenotypic variation. Biol. Rev. Camb. Philos. Soc. 90 , 729–743 (2015).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186 , 243–278 (2023).
Dang, W. et al. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature 459 , 802–807 (2009).
Ocampo, A. et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 167 , 1719–1733.e12 (2016).
Yang, J.-H. et al. Loss of epigenetic information as a cause of mammalian aging. Cell https://doi.org/10.1016/j.cell.2022.12.027 (2023).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126 , 663–676 (2006).
Chondronasiou, D. et al. Multi-omic rejuvenation of naturally aged tissues by a single cycle of transient reprogramming. Aging Cell 21 , e13578 (2022).
Ng, R. K. & Gurdon, J. B. Epigenetic inheritance of cell differentiation status. Cell Cycle 7 , 1173–1177 (2008).
Barrero, M. J., Boué, S. & Izpisúa Belmonte, J. C. Epigenetic mechanisms that regulate cell identity. Cell Stem Cell 7 , 565–570 (2010).
Whyte, W. A. et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153 , 307–319 (2013).
Balsalobre, A. & Drouin, J. Pioneer factors as master regulators of the epigenome and cell fate. Nat. Rev. Mol. Cell Biol. 23 , 449–464 (2022).
Hosokawa, H. & Rothenberg, E. V. How transcription factors drive choice of the T cell fate. Nat. Rev. Immunol. 21 , 162–176 (2021).
Ma, S. et al. Chromatin potential identified by shared single-cell profiling of RNA and chromatin. Cell 183 , 1103–1116.e20 (2020).
Adam, R. C. et al. Temporal layering of signaling effectors drives chromatin remodeling during hair follicle stem cell lineage progression. Cell Stem Cell 22 , 398–413.e7 (2018).
Song, M.-R. & Ghosh, A. FGF2-induced chromatin remodeling regulates CNTF-mediated gene expression and astrocyte differentiation. Nat. Neurosci. 7 , 229–235 (2004).
Meshorer, E. Chromatin in embryonic stem cell neuronal differentiation. Histol. Histopathol. 22 , 311–319 (2007).
CAS PubMed Google Scholar
Arnsdorf, E. J., Tummala, P., Castillo, A. B., Zhang, F. & Jacobs, C. R. The epigenetic mechanism of mechanically induced osteogenic differentiation. J. Biomech. 43 , 2881–2886 (2010).
Morales Berstein, F. et al. Assessing the causal role of epigenetic clocks in the development of multiple cancers: a Mendelian randomization study. eLife 11 , e75374 (2022).
Tsurumi, A. & Li, W. X. Global heterochromatin loss: a unifying theory of aging? Epigenetics 7 , 680–688 (2012).
Lee, J.-H., Kim, E. W., Croteau, D. L. & Bohr, V. A. Heterochromatin: an epigenetic point of view in aging. Exp. Mol. Med. 52 , 1466–1474 (2020).
Farhy, C. et al. Improving drug discovery using image-based multiparametric analysis of the epigenetic landscape. eLife 8 , e49683 (2019).
Villeponteau, B. The heterochromatin loss model of aging. Exp. Gerontol. 32 , 383–394 (1997).
Sen, P., Shah, P. P., Nativio, R. & Berger, S. L. Epigenetic mechanisms of longevity and aging. Cell 166 , 822–839 (2016).
Creyghton, M. P. et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl Acad. Sci. USA 107 , 21931–21936 (2010).
Ucar, D. et al. The chromatin accessibility signature of human immune aging stems from CD8(+) T cells. J. Exp. Med. 214 , 3123–3144 (2017).
Weigert, M., Schmidt, U., Haase, R., Sugawara, K. & Myers, G. Star-convex polyhedra for 3D object detection and segmentation in microscopy. In Proc. IEEE/CVF Winter Conference on Applications of Computer Vision 3666–3673 (2020).
Hamilton, N. A., Pantelic, R. S., Hanson, K. & Teasdale, R. D. Fast automated cell phenotype image classification. BMC Bioinform. 8 , 110 (2007).
Tahir, M., Jan, B., Hayat, M., Shah, S. U. & Amin, M. Efficient computational model for classification of protein localization images using extended threshold adjacency statistics and support vector machines. Comput. Methods Programs Biomed. 157 , 205–215 (2018).
Wagenaar, W. A. & Padmos, P. Quantitative interpretation of stress in kruskal’s multidimensional scaling technique. Br. J. Math. Stat. Psychol. 24 , 101–110 (1971).
Zhou, Y. & Sharpee, T. O. Hyperbolic geometry of gene expression. iScience 24 , 102225 (2021).
Zhou, Y., Smith, B. H. & Sharpee, T. O. Hyperbolic geometry of the olfactory space. Sci. Adv. 4 , eaaq1458 (2018).
Praturu, A. & Sharpee, T. A Bayesian approach to hyperbolic embeddings. Bull. Am. Phys. Soc. 19 , e1011084 (2022).
Google Scholar
Zhang, H., Rich, P. D., Lee, A. K. & Sharpee, T. O. Hippocampal spatial representations exhibit a hyperbolic geometry that expands with experience. Nat. Neurosci. 26 , 131–139 (2023).
Zhou, Y. & Sharpee, T. O. Using global t -SNE to preserve inter-cluster data structure. Neural Comput. https://doi.org/10.1162/neco_a_01504 (2022).
Praturu, A. & Sharpee, T. A Bayesian approach to hyperbolic multi-dimensional scaling. Preprint at bioRxiv https://doi.org/10.1101/2022.10.12.511940 (2022).
Meer, M. V., Podolskiy, D. I., Tyshkovskiy, A. & Gladyshev, V. N. A whole lifespan mouse multi-tissue DNA methylation clock. eLife 7 , e40675 (2018).
Piening, B. D., Lovejoy, J. & Earls, J. C. Ageotypes: distinct biomolecular trajectories in human aging. Trends Pharmacol. Sci. 41 , 299–301 (2020).
Ahadi, S. et al. Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nat. Med. 26 , 83–90 (2020).
Blayney, J. W. et al. Super-enhancers include classical enhancers and facilitators to fully activate gene expression. Cell 186 , 5826–5839.e18 (2023).
Madeo, F., Carmona-Gutierrez, D., Hofer, S. J. & Kroemer, G. Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab. 29 , 592–610 (2019).
Ma, S. & Gladyshev, V. N. Molecular signatures of longevity: Insights from cross-species comparative studies. Semin. Cell Dev. Biol. 70 , 190–203 (2017).
Balasubramanian, P., Howell, P. R. & Anderson, R. M. Aging and caloric restriction research: a biological perspective with translational potential. eBioMedicine 21 , 37–44 (2017).
Lacar, B. et al. Nuclear RNA-seq of single neurons reveals molecular signatures of activation. Nat. Commun. 7 , 11022 (2016).
Demaria, M. et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov . 7 , 165–176 (2017).
Baskin, K. K., Winders, B. R. & Olson, E. N. Muscle as a ‘mediator’ of systemic metabolism. Cell Metab. 21 , 237–248 (2015).
Petr, M. A. et al. A cross-sectional study of functional and metabolic changes during aging through the lifespan in male mice. eLife 10 , e62952 (2021).
Wang, C. et al. In vivo partial reprogramming of myofibers promotes muscle regeneration by remodeling the stem cell niche. Nat. Commun. 12 , 3094 (2021).
Browder, K. C. et al. In vivo partial reprogramming alters age-associated molecular changes during physiological aging in mice. Nat. Aging 2 , 243–253 (2022).
Finn, E. H. & Misteli, T. Molecular basis and biological function of variability in spatial genome organization. Science 365 , eaaw9498 (2019).
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. Cell 155 , 934–947 (2013).
Beacon, T. H. et al. The dynamic broad epigenetic (H3K4me3, H3K27ac) domain as a mark of essential genes. Clin. Epigenetics 13 , 138 (2021).
Khan, A. & Zhang, X. dbSUPER: a database of super-enhancers in mouse and human genome. Nucleic Acids Res. 44 , D164–D171 (2016).
Liu, X. et al. Resurrection of endogenous retroviruses during aging reinforces senescence. Cell https://doi.org/10.1016/j.cell.2022.12.017 (2023).
Simon, M. et al. LINE1 derepression in aged wild-type and SIRT6-deficient mice drives inflammation. Cell Metab. 29 , 871–885.e5 (2019).
Gorbunova, V. et al. The role of retrotransposable elements in ageing and age-associated diseases. Nature 596 , 43–53 (2021).
Hong, C. et al. Epigenetic age acceleration of stomach adenocarcinoma associated with tumor stemness features, immunoactivation, and favorable prognosis. Front. Genet. 12 , 563051 (2021).
Castle, J. R. et al. Estimating breast tissue-specific DNA methylation age using next-generation sequencing data. Clin. Epigenetics 12 , 45 (2020).
Hao, J., Liu, T., Xiu, Y., Yuan, H. & Xu, D. High DNA methylation age deceleration defines an aggressive phenotype with immunoexclusion environments in endometrial carcinoma. Front. Immunol. 14 , 1208223 (2023).
Abad, M. et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature 502 , 340–345 (2013).
Wang, K. et al. Epigenetic regulation of aging: implications for interventions of aging and diseases. Signal Transduct. Target Ther. 7 , 374 (2022).
Zhang, F., Icyuz, M., Bartke, A. & Sun, L. Y. The effects of early-life growth hormone intervention on tissue specific histone H3 modifications in long-lived Ames dwarf mice. Aging 13 , 1633–1648 (2020).
Tinsley, F. C., Taicher, G. Z. & Heiman, M. L. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes. Res. 12 , 150–160 (2004).
Taicher, G. Z., Tinsley, F. C., Reiderman, A. & Heiman, M. L. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal. Bioanal. Chem. 377 , 990–1002 (2003).
Champy, M., Argmann, C. A., Chambon, P. & Auwerx, J. in Standards of Mouse Model Phenotyping (eds Hrabe de Angelis, M., Chambon, P. & Brown, S.) 109–133 (Wiley, 2006).
Yang, G. et al. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 297 , E211–E224 (2009).
Chang, Y. et al. Ablation of NG2 proteoglycan leads to deficits in brown fat function and to adult onset obesity. PLoS ONE 7 , e30637 (2012).
Crawley, J. N. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 835 , 18–26 (1999).
Johnson, S. A., Fournier, N. M. & Kalynchuk, L. E. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav. Brain Res. 168 , 280–288 (2006).
Winters, B. D., Forwood, S. E., Cowell, R. A., Saksida, L. M. & Bussey, T. J. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J. Neurosci. 24 , 5901–5908 (2004).
Mumby, D. G., Tremblay, A., Lecluse, V. & Lehmann, H. Hippocampal damage and anterograde object‐recognition in rats after long retention intervals. Hippocampus 15 , 1050–1056 (2005).
Berlyne, D. E. Novelty and curiosity as determinants of exploratory behaviour. Br. J. Psychol. 41 , 68 (1950).
Ennaceur, A. & Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav. Brain Res. 31 , 47–59 (1988).
Heyser, C. J. & Chemero, A. Novel object exploration in mice: not all objects are created equal. Behav. Processes 89 , 232–238 (2012).
Crawley, J. N. & Paylor, R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm. Behav. 31 , 197–211 (1997).
Carter, R. J., Morton, J. & Dunnett, S. B. Motor coordination and balance in rodents. Curr. Protoc. Neurosci . https://doi.org/10.1002/0471142301.ns0812s15 (2001).
Holmes, A., Wrenn, C. C., Harris, A. P., Thayer, K. E. & Crawley, J. N. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav. 1 , 55–69 (2002).
Bach, M. E., Hawkins, R. D., Osman, M., Kandel, E. R. & Mayford, M. Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the θ frequency. Cell 81 , 905–915 (1995).
Barnes, C. A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 93 , 74–104 (1979).
Paylor, R., Zhao, Y., Libbey, M., Westphal, H. & Crawley, J. N. Learning impairments and motor dysfunctions in adult Lhx5-deficient mice displaying hippocampal disorganization. Physiol. Behav. 73 , 781–792 (2001).
Freitag, S., Schachner, M. & Morellini, F. Behavioral alterations in mice deficient for the extracellular matrix glycoprotein tenascin-R. Behav. Brain Res. 145 , 189–207 (2003).
Crawley, J. N. What’s Wrong With My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice (John Wiley & Sons, 2007).
Castro, B. & Kuang, S. Evaluation of muscle performance in mice by treadmill exhaustion test and whole-limb grip strength assay. Bio. Protoc. 7 , e2237 (2017).
Mager, S. R. et al. Standard operating procedure for the collection of fresh frozen tissue samples. Eur. J. Cancer 43 , 828–834 (2007).
Naber, S. P. Continuing role of a frozen-tissue bank in molecular pathology. Diagn. Mol. Pathol. 5 , 253–259 (1996).
Shabihkhani, M. et al. The procurement, storage, and quality assurance of frozen blood and tissue biospecimens in pathology, biorepository, and biobank settings. Clin. Biochem. 47 , 258–266 (2014).
Peng, T. et al. A BaSiC tool for background and shading correction of optical microscopy images. Nat. Commun. 8 , 14836 (2017).
Goldman, D. B. Vignette and exposure calibration and compensation. IEEE Trans. Pattern Anal. Mach. Intell. 32 , 2276–2288 (2010).
Meilă, M. in Learning Theory and Kernel Machines 173–187 (Springer, 2003).
Sharpee, T. O. An argument for hyperbolic geometry in neural circuits. Curr. Opin. Neurobiol. 58 , 101–104 (2019).
Verleysen, M. & François, D. The Curse of Dimensionality in Data Mining and Time Series Prediction (Springer, 2005).
Kuo, F. Y. & Sloan, I. H. Lifting the curse of dimensionality. Notices of the AMS 52 , 1320–1328 (2005).
Bellman, R. Dynamic Programming (Courier Corp., 2003).
Ganea, O., Becigneul, G. & Hofmann, T. Hyperbolic neural networks. Adv. Neural Information Processing Systems 31 (NeurIPS, 2018).
Kreuger, F. et al. FelixKreuger/TrimGalore: v0.6.10 - add default decompression path. Zenodo https://doi.org/10.5281/zenodo.7598955 (2023).
Krueger, F. & Andrews, S. R. Bismark: a flexible aligner and methylation caller for bisulfite-seq applications. Bioinformatics 27 , 1571–1572 (2011).
Download references
Acknowledgements
We greatly appreciate the invaluable help of the personnel and leadership of the Sanford Burnham Prebys animal and high-throughput imaging core facilities, in particular M. O’Rourke-Braxtan and S. Heynen-Genel (SBP NCI Cancer Centre Support grant P30 CA030199). We thank C. Kaufman for his help with preparing tissue samples. We thank all members of the Terskikh laboratory for the helpful discussion. This work was supported by National Institutes of Health grants R21 AG068913, R21 AG075483 and R21 AG083782 to A.V.T. and P01 AG031862 to P.D.A.
Author information
These authors contributed equally: Martin Alvarez-Kuglen, Kenta Ninomiya, Haodong Qin, Delany Rodriguez.
Authors and Affiliations
Sanford Burnham Prebys, La Jolla, CA, USA
Martin Alvarez-Kuglen, Delany Rodriguez, Lorenzo Fiengo, Chen Farhy, Brian Kirk, Aaron Havas & Peter D. Adams
Harry Perkins Institute of Medical Research, The University of Western Australia, Perth, Western Australia, Australia
Kenta Ninomiya & Alexey V. Terskikh
Department of Physics, University of California San Diego, La Jolla, CA, USA
Haodong Qin
Salk Institute for Biological Studies, La Jolla, CA, USA
Wei-Mien Hsu & Tatyana O. Sharpee
School of Medicine, Univerity of California San Diego, La Jolla, CA, USA
Gen-Sheng Feng
The Scripps Research Institute, La Jolla, CA, USA
Amanda J. Roberts
University of Wisconsin, Madison, WI, USA
Rozalyn M. Anderson
GRECC, William S Middleton Memorial Veterans Hospital, Madison, WI, USA
Institute for Research in Biomedicine (IRB Barcelona), Barcelona, Spain
Manuel Serrano
Barcelona Institute of Science and Technology (BIST), Barcelona, Spain
Altos Labs, Cambridge Institute of Science, Granta Park, UK
The Scintillon Research Institute, San Diego, CA, USA
Alexey V. Terskikh
You can also search for this author in PubMed Google Scholar
Contributions
A.V.T., R.M.A., M.S., P.D.A. and T.O.S. designed and supervised the study; D.R., L.F., C.F., A.H., A.J.R. and B.K. conducted the experiments; G.S.F., R.M.A. and M.S. provided critical reagents; M.A.K., H.Q., K.N., W.M.H., D.R., T.O.S. and A.V.T. analyzed the data and prepared the manuscript.
Corresponding author
Correspondence to Alexey V. Terskikh .
Ethics declarations
Competing interests.
L.F. is currently an employee of Bristol Myers Squibb; W.-M.H. is currently an employee of Ingram Micro; B.K. is currently an employee of Zafrens; M.S. is an employee of Altos Labs and shareholder of Altos Labs, Life Biosiences, Senolytic Therapeutics and Rejuveron Senescence Therapeutics; A.V.T. is a shareholder of Stemson Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information.
Nature Aging thanks Argyris Papantonis, Peter Tessarz, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 image accuracy measurements in blood..
a , b , Peripheral blood mononuclear cells (PBMCs) were isolated from mice aged 2, 5, 9, 15, 21, 23, 32 months (n = 2 for all groups), and immunolabeled for CD3 and H3K4me1 and colabelled with DAPI. Lineplots: Accuracy measurements versus bin size split by train and test data. 95% Confidence intervals are shown. Measurements were made for all models constructed: a centroid-based b , support vector machine SVM data.
Extended Data Fig. 2 ImAge versus orthogonal distance plots.
a , b , Peripheral blood mononuclear cells (PBMCs) were isolated from mice aged 2, 5, 9, 15, 21, 23, 32 months (n = 2 for all groups), and immunolabeled for CD3 and H3K4me1 and colabelled with DAPI. Scatter-plots show ImAge (normalized 0–1) versus orthogonal distance to the ImAge axis. ImAge was calculated using either a , centroid- or b , support vector machine (SVM)-based method. c , Performance comparison of Euclidean multidimensional scaling (EMDS) and hyperbolic multidimensional scaling (HMDS) within a 12-dimensional space. HMDS demonstrates significantly reduced distortion and uncertainty of distances (R² = 0.99) following the embedding process in hyperbolic space as compared to EMDS (R² = 0.67). This outcome supports the notion that our data exhibits an inherent hyperbolic structure.
Extended Data Fig. 3 Age-related ImAge progression in multiple solid organs.
ImAge readouts of the brain, cardiac muscle (heart), kidney, liver, and skeletal muscle (quadriceps) collected from three differentially aged cohorts of mice: 2 months (n = 5) 15 months (n = 4) and 27 months (n = 4). Two plates were analyzed, both immunolabeled with H3K27ac+DAPI and then with either H3K27me3 or H3k4me1. Data for overlapping channels (DAPI & H3K27ac) were combined for computations. Boxplots of the test set of bootstrapped data are shown with the normalized ImAge readouts from maximum-minimum to 0–1. Differences of means were calculated via Tukey’s HSD. Significance values for all tests shown represent: * = p < 0.05, ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001
Extended Data Fig. 4 ImAge accuracy measurements in multiple solid organs.
a–e , Nuclei isolated from solid organs of young (2 months, n = 4) and aged (27 months, n = 4) control mice were immunolabeled and imaged with two sets of antibodies: (H3K27ac & H3K4me1), (H3K27ac & H3K27me3), both colabelled with DAPI. Test accuracy versus bin size was shown with 95% Confidence intervals. Dashed or solid lines represent plate/antibody set of origin: (H3K27ac & H3K4me1), (H3K27ac & H3K27me3), respectively.
Extended Data Fig. 5 Separation between different cell types with ageing, including the Brain.
a , 2-dimensional Euclidean multidimensional scaling (EMDS) of young (2 months) and old (27 months) liver, kidney, quads, and heart and brain. The observed clustering pattern reveals that the brain tissue cluster maintains a distinct separation from the clusters representing other tissue types, such as Kidney, Liver, Skeletal Muscle, and Cardiac Muscle. b , c , Silhouette scores of 5 organs at indicated ages for individual marks ( b ) or their combination ( c ) using the information distance metric. The Silhouette scores do not indicate a decline in tissue differentiation with ageing in the presence of brain tissue, likely due to a slower progression of ageing in the brain.
Extended Data Fig. 6 ImAge accuracy metrics for the interventions in biological age.
a–c , Nuclei from young and aged control mice were immunolabeled for H3K27ac and either H3K9me3 ( a and b ), or H3K27me3 and H3k4me1 ( c ) colabelled with DAPI. Lineplots: Accuracy measurements versus bin size split by train and test data. 95% Confidence intervals are shown. a , Accuracy measurements in Caloric Restriction (CR) dataset for separating 3 month (n = 3) and 24 month (n = 3) old mice at various bin sizes. b , Accuracy measurements in Doxorubicin (DXR) dataset for separating 1 month (n = 4) and 27 month (n = 4) old mice at various bin sizes. c , Accuracy measurements in induced hepatocarcinoma (tumor) dataset for separating 2 month (n = 5) and 8 month (n = 6) old mice at various bin sizes. Dashed or solid lines represent plate/antibody set of origin: (H3K27ac & H3K4me1), (H3K27ac & H3K27me3).
Extended Data Fig. 7 Statistics of the behaviors and the ImAge.
a , Performance comparison of Euclidean Multidimensional Scaling (EMDS) and Hyperbolic Multidimensional Scaling (HMDS) within a 9-dimensional space. HMDS demonstrates significantly reduced distortion of distances (R² = 0.87) following the embedding process in hyperbolic space as compared to EMDS (R² = 0.35). b , The ImAge distribution along the ageing geodesic in hyperbolic space and the orthogonal distance distribution to the same ageing geodesic for skeletal muscle cells in chronologically identical mice (25 months old) utilized for behavior performance evaluation. The ImAge axis captures ~82% of the total variance (the orthogonal projections ~18% of the variance). c, d , Clustering of individual behaviors; Person correlation (left) and significance (right)., Orthogonal bases from individual behaviors were used to identify unique linear coefficients. The criterion for behaviors clustering together was a high (>0.7) and significant (p < 0.05) Pearson correlation between the behaviors. e , Correlation between ImAge and individual orthogonal clusters of behaviors. f , Histogram of the test statistics for the correlation between ImAge and the optimized linear combination of behaviors. g , Correlations between ImAge and the individual clusters of behaviors. h , Histogram of the test statistics for the correlation between ImAge and the linear combination of individual orthogonal clusters of behaviors (without optimization). i Scatter plot for the correlation between ImAge and the linear combination of individual orthogonal clusters of behaviors (without optimization).
Extended Data Fig. 8 ImAge detected partial reprogramming of multiple organs.
The chronological ages of young and aged mice are 3.2 and 13.8 months, respectively. a , A graphical representation of the experimental condition: aged mice were treated with doxycycline to overexpress OSKM factors to evaluate the degree of reprogramming on the liver, quadriceps and cardiac muscle (heart). b-d (left) , Distribution of ImAge obtained from 100 iterations of the test procedure using the linear support vector machine. Round-shaped symbols on the right side of violin plots for each age group represent the mean ImAge of each mouse sample. b-d (right) , Sample-wise comparison of ImAge. Each violin plot represents the distribution of the ImAge for each mouse sample with the numbers from one to five for young, Aged-OSKM and aged groups. In all panels, an asterisk indicates the significant decrease from the aged mouse showed the lowest ImAge in the aged group with respect to the median ImAge (Mann-Whitney U-test, significant threshold; p < 0.05).
Extended Data Fig. 9 The degree of partial reprogramming reported by ImAge varies depending on tissues and epigenetic marks.
The chronological ages of young and aged mice are 3.2 and 13.8 months, respectively. a , Each violin plot represents the distribution of the ImAge for each mouse sample with the numbers from one to five for young, Old-OSKM and aged groups. The Left and right columns are for the liver and muscle, respectively. In all panels, an asterisk indicates the significant decrease from the aged mouse showed the lowest ImAge in the aged group with respect to the median ImAge (Mann-Whitney U-test, p < 0.05). b , c , proportion of cells with young and aged signature ImAge in each age group in the liver (b) and muscle (c) (Mann-Whitney U-test, p < 0.05). Partial reprogramming after doxycycline-induced OSKM factors (i4F mice, old-OSKM) was observed in both HNF4a+ and HNF4- cell populations using indicated marks.
Extended Data Fig. 10 Overview of young/aged indicative cells revealed at the single-cell level in liver samples following partial reprogramming in vivo.
Uniform manifold approximation and projection (UMAP) of three-dimensional threshold adjacency statistic (TAS) features for single cells. The green and brown data points represent young and aged indicative cells, respectively. The gray points represent the intermediate single cells.
Supplementary information
Supplementary information.
Supplementary Figs. 1–5.
Reporting Summary
Supplementary table 1.
Statistics and significant differences of ImAge readouts between different age/conditions . Differences of means of all combination of age or perturbation conditions were calculated via Tukey’s HSD for all channels and their combinations tested.
Supplementary Table 2
Chronological age correlation with ImAge and PCA . Two-sided Pearson correlation and P values were calculated between chronological age and ImAge readouts, PC1, and PC2 of the texture feature space, per tissue per channel. All correlations calculated are shown.
Supplementary Table 3
Correlation between ImAge readouts and PCA . Two-sided Pearson correlation and P values were calculated between ImAge readouts and PC1 and PC2 of the texture feature space, per tissue per channel. The table displays all statistically significant correlations that were found based on the criteria P < 0.05.
Supplementary Table 4
Correlation between ImAge readouts from different tissues . Two-sided Pearson correlation and P values were calculated between ImAge readouts from different tissues within the same channel for all combinations of tissues. The table displays all statistically significant correlations that were found based on the criteria P < 0.05.
Supplementary Table 5
Correlation between ImAge readouts from different channels . Two-sided Pearson correlation and P values were calculated between ImAge readouts from different channels within the same tissue for all combinations of channels. The table displays all statistically significant correlations that were found based on the criteria P < 0.05.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Alvarez-Kuglen, M., Ninomiya, K., Qin, H. et al. ImAge quantitates aging and rejuvenation. Nat Aging (2024). https://doi.org/10.1038/s43587-024-00685-1
Download citation
Received : 23 October 2023
Accepted : 11 July 2024
Published : 29 August 2024
DOI : https://doi.org/10.1038/s43587-024-00685-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

- NEW STUDY: Discovery of Chemical Means …
- Editorial Board
- Editorial Policies
- Advance Publications
- Current Issue
- Special Collections
- Scientific Integrity
- Publication Ethics Statements
- Interviews with Outstanding Authors
- Sponsored Conferences
- Announcements
- Press Releases
- Altmetric Top 100
NEW STUDY: Discovery of Chemical Means to Reverse Aging and Restore Cellular Function
On July 12, 2023, a new priority research paper was published in Aging, titled, “Chemically induced reprogramming to reverse cellular aging.”
Listen to an audio version of this press release
BUFFALO, NY- July 12, 2023 – In a groundbreaking study, researchers have unlocked a new frontier in the fight against aging and age-related diseases. The study, conducted by a team of scientists at Harvard Medical School, has published the first chemical approach to reprogram cells to a younger state. Previously, this was only achievable using a powerful gene therapy.
On July 12, 2023, researchers Jae-Hyun Yang, Christopher A. Petty, Thomas Dixon-McDougall, Maria Vina Lopez, Alexander Tyshkovskiy, Sun Maybury-Lewis, Xiao Tian, Nabilah Ibrahim, Zhili Chen, Patrick T. Griffin, Matthew Arnold, Jien Li, Oswaldo A. Martinez, Alexander Behn, Ryan Rogers-Hammond, Suzanne Angeli, Vadim N. Gladyshev, and David A. Sinclair from Harvard Medical School , University of Maine and Massachusetts Institute of Technology (MIT) published a new priority research paper in Aging , titled, “ Chemically induced reprogramming to reverse cellular aging .”
The team's findings build upon the discovery that the expression of specific genes, called Yamanaka factors, could convert adult cells into induced pluripotent stem cells (iPSCs). This Nobel Prize-winning discovery raised the question of whether it might be possible to reverse cellular aging without causing cells to become too young and turn cancerous.
In this new study, the researchers screened for molecules that could, in combination, reverse cellular aging and rejuvenate human cells. They developed high-throughput cell-based assays to distinguish young cells from old and senescent cells, including transcription-based aging clocks and a real-time nucleocytoplasmic protein compartmentalization (NCC) assay. In an exciting discovery, the team has identified six chemical cocktails that restore NCC and genome-wide transcript profiles to youthful states and reverse transcriptomic age in less than a week.
The Harvard researchers previously demonstrated that it is indeed possible to reverse cellular aging without uncontrolled cell growth by virally-introducing specific Yamanaka genes into cells. Studies on the optic nerve, brain tissue, kidney, and muscle have shown promising results, with improved vision and extended lifespan observed in mice and, recently, a report of improved vision in monkeys.
The implications of this new discovery are far-reaching, opening avenues for regenerative medicine and, potentially, whole-body rejuvenation. By developing a chemical alternative to age reversal via gene therapy, this research could revolutionize the treatment of aging, injuries and age-related diseases and offers the potential for lower costs and shorter timelines in development. On the heels of positive results in reversing blindness in monkeys in April 2023, preparations for human clinical trials of the lab’s age reversal gene therapy are in progress.
“Until recently, the best we could do was slow aging. New discoveries suggest we can now reverse it,” said David A. Sinclair, A.O., Ph.D. , Professor in the Department of Genetics and co-Director of the Paul F. Glenn Center for Biology of Aging Research at Harvard Medical School and lead scientist on the project. “This process has previously required gene therapy, limiting its widespread use.”
The team at Harvard envisions a future where age-related diseases can be effectively treated, injuries can be repaired more efficiently, and the dream of whole-body rejuvenation becomes a reality. “This new discovery offers the potential to reverse aging with a single pill, with applications ranging from improving eyesight to effectively treating numerous age-related diseases,” Sinclair said.
Image 1. Mice in the Sinclair lab have been engineered to age rapidly to test the effectiveness of therapies to reverse the aging process. The mouse on the right has been aged to 150% that of its sibling on the left by disrupting its epigenome. Photo credit: D. Sinclair, Harvard Medical School.
Image 2. Rejuvenation and age reversal of senescent human skin cells by chemical means. Cells in the right two panels have restored compartmentalization of the red fluorescent protein in the nucleus, a marker of youth that was used to find the cocktails, before the scientists confirmed they were younger, based on how genes were expressed. Image credit: J. -H. Yang, Harvard Medical School.
Read the full study: DOI: https://doi.org/10.18632/aging.204896
Corresponding Author: David A. Sinclair
Corresponding Email: [email protected]
Keywords: reprogramming, rejuvenation medicine, information theory of aging, small molecules, epigenetics
Sign up for free Altmetric alerts about this article: https://aging.altmetric.com/details/email_updates?id=10.18632%2Faging.204896
About Aging-US :
Cancer and aging are two sides of age-related tumorigenesis.
The mission of the journal is to understand the mechanisms surrounding aging and age-related diseases, including cancer as the main cause of death in the modern aged population.
The journal aims to promote 1) treatment of age-related diseases by slowing down aging, 2) validation of anti-aging drugs by treating age-related diseases, and 3) prevention of cancer by inhibiting aging. (Cancer and COVID-19 are age-related diseases.)
Please visit our website at www.Aging-US.com and connect with us:
- Spotify , Apple , SoundCloud , and available wherever you listen to podcasts
For media inquiries, please contact [email protected] .
December 15, 2016
Aging Is Reversible—at Least in Human Cells and Live Mice
Changes to gene activity that occur with age can be turned back, a new study shows
By Karen Weintraub

Tim Flach Getty Images
New research suggests it is possible to slow or even reverse aging, at least in mice, by undoing changes in gene activity—the same kinds of changes that are caused by decades of life in humans.
By tweaking genes that turn adult cells back into embryoniclike ones, researchers at the Salk Institute for Biological Studies reversed the aging of mouse and human cells in vitro, extended the life of a mouse with an accelerated-aging condition and successfully promoted recovery from an injury in a middle-aged mouse, according to a study published Thursday in Cell .
The study adds weight to the scientific argument that aging is largely a process of so-called epigenetic changes, alterations that make genes more active or less so. Over the course of life cell-activity regulators get added to or removed from genes. In humans those changes can be caused by smoking, pollution or other environmental factors—which dial the genes’ activities up or down. As these changes accumulate, our muscles weaken, our minds slow down and we become more vulnerable to diseases.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The new study suggests the possibility of reversing at least some of these changes, a process researchers think they may eventually get to work in living humans. “Aging is something plastic that we can manipulate,” says Juan Carlos Izpisua Belmonte, the study’s senior author and an expert in gene expression at Salk. In their study Belmonte and his colleagues rejuvenated cells by turning on, for a short period of time, four genes that have the capacity to convert adult cells back into an embryoniclike state.
In living mice they activated the four genes (known as “Yamanaka factors,” for researcher Shinya Yamanaka, the Nobelist who discovered their combined potential in 2006). This approach rejuvenated damaged muscles and the pancreas in a middle-aged mouse, and extended by 30 percent the life span of a mouse with a genetic mutation responsible for Hutchinson–Gilford progeria syndrome, which causes rapid aging in children.
Because the Yamanaka factors reverse changes made to gene regulators, some scientists see the study as further evidence that aging is driven by epigenetic changes. “I do think that epigenetic reprogramming is the ultimate way to reverse aging,” says David Sinclair, a Harvard University geneticist and anti-aging researcher who was not involved in the study but is doing similar work. “My lab has a lot of evidence that the primary driver of what we call the hallmarks of aging is the epigenetic change.” Sinclair says his lab is preparing a paper explaining what causes these changes as we age.
The Salk study was conducted on middle-aged mice. But in theory, reprogramming epigenetics should work on mice and people at any age, says first author Alejandro Ocampo, adding that even cells from human centenarians could eventually be rejuvenated. He and Belmonte say they think they can improve the efficiency and results of the technique with more research—and that they can undo the epigenetic changes responsible for aging by using easier-to-handle chemicals instead of the Yamanaka factors, hopefully moving toward the possibility of treatment for people.
Matt Kaeberlein, a molecular biologist at the University of Washington who studies aging but was not part of the work, says other researchers have found that the Yamanaka factors can rejuvenate cells—so in some ways this study is not surprising. But Kaeberlein says no one else had yet shown that the factors can treat age-related diseases in an animal by making the same changes. “That’s the wow factor,” he explains.
Kaeberlein says the study suggests it may be possible not just to slow aging but to actually reverse it. “That’s really exciting—that means that even in elderly people it may be possible to restore youthful function,” he says. Plus, it is easier to imagine a treatment that makes changes to the epigenome than to consider going into every cell and changing its genes. He also notes that the results of the new study are very similar to those seen when senescent cells—those that have lost function due to aging—are removed from an organism. It is not yet clear, he says, whether “this is another way to shut down or maybe reprogram senescent cells.”
Manuel Serrano, an expert on senescence at the Spanish National Cancer Research Center in Madrid, was not associated with the new research but says he is impressed with the study and its results. “I fully agree with the conclusions. This work indicates that epigenetic shift is in part responsible for aging, and reprogramming can correct these epigenetics errors,” he wrote in an e-mail. “This will be the basis for future exciting developments.”
The study also showed how fine the line can be between benefit and harm. When the researchers treated mice continually, some developed tumors and died within a week. When the scientists cut the treatment to two days out of seven, however, the mice benefited significantly. Sinclair says this should be taken as a note of caution by anyone trying to increase the human life span. “We’ve all been playing with fire,” he says, adding that this fine line will make it challenging to get a drug approved by regulatory agencies. “This is going to be what we spend the next 10 years figuring out: how to reprogram cells to be young again without taking it too far so they become tumors.”
Both Sinclair and Kaeberlein say they wish Belmonte’s lab had shown that a normal mouse could live longer after the gene tinkering—instead of just reversing an aging-related illness.
Belmonte, like some other anti-aging researchers, says his initial goal is to increase the “health span”—the number of years that someone remains healthy. Extending life span, the number of years someone remains alive, will likely take longer to achieve. Most major killers, including heart disease, cancer and Alzheimer’s, are diseases of aging that become far more common past middle age. “This is not just a matter of how many years we can live but how well we can live the rest of our life,” Ocampo says.
Belmonte says his team is also trying to determine if aging is a process that occurs simultaneously throughout the body. Or, as he puts it, “Is there some tissue that regulates aging—and when that goes bad, the entire organism goes bad?” He says they currently think the brain’s hypothalamus—known as the seat of control for hormones, body temperature, mood, hunger and circadian rhythms—may also act as a regulator of aging.
Other approaches that have been discovered to have anti-aging benefits in animals include calorie restriction, the drug rapamycin and parabiosis—the practice of giving old mice a blood supply from younger ones. The fact that these diverse strategies all seem to work suggests there may be more than one way to age, and that multiple complementary therapies may be needed to significantly extend longevity, Kaeberlein says.
Some compounds such as resveratrol, a substance found in red wine that seems to have anti-aging properties in high concentrations, appear to delay epigenetic change and protect against damage from epigenetic deterioration, Sinclair says. These approaches can reverse some aspects of aging, such as muscle degeneration—but aging returns when the treatment stops, he adds. With an approach like the one Belmonte lays out in the new study, theoretically “you could have one treatment and go back 10 or 20 years,” he says. If aging starts to catch up to you again, you simply get another treatment.
“This work is the first glimmer that we could live for centuries,” Sinclair says, adding that he would happily do so himself: “Forty-seven years went by pretty quickly.”
What links aging and disease? A growing body of research says it’s a faulty metabolism
Aging is the most significant risk factor for many of society’s most common diseases. A key factor behind the onset of these health issues is the disruption of cellular and metabolic balance,
/cloudfront-us-east-1.images.arcpublishing.com/pmn/KHUBAGQVGZGP3LOFY5TJBBX7YQ.jpg)
Aging is a biological process that no one can avoid. Ideally, growing old should be a time to relax and enjoy the fruits of your labor. Aging also has a darker side, however, often linked to disease.
Every second, your cells perform billions of biochemical reactions that fuel essential functions for life, forming a highly interconnected metabolic network . This network enables cells to grow, proliferate, and repair themselves, and its disruption can drive the aging process .
But does aging cause metabolic decline, or does metabolic disruption accelerate aging? Or both?
To address this chicken-or-egg question, you first need to understand how metabolic processes break down during aging and disease. I am a scientist and researcher , and my lab focuses on exploring the complex relationship between metabolism, stress, and aging. Ultimately, we hope this work will provide strategies to promote healthier aging and more vibrant lives.
Link between metabolism and aging
Aging is the most significant risk factor for many of society’s most common diseases , including diabetes, cancer, cardiovascular disease, and neurodegenerative disorders. A key factor behind the onset of these health issues is the disruption of cellular and metabolic homeostasis, or balance . Disrupting homeostasis destabilizes the body’s internal environment, leading to imbalances that can trigger a cascade of health issues, including metabolic disorders, chronic diseases, and impaired cellular functions that contribute to aging and other serious conditions.
Disrupted metabolism is linked to many hallmarks of aging cells, such as telomere shortening , which is damage to the protective ends of chromosomes, and genomic instability , the tendency to form genetic mutations.
Metabolism can be divided into two broad processes: anabolism, or building up molecules, and catabolism, or breaking down molecules.
A dysfunctional metabolism is also linked to poorly functioning mitochondria ; cellular senescence , or when cells stop dividing; imbalances in gut microbes ; and cells’ reduced ability to detect and respond to different nutrients .
Neurological disorders, such as Alzheimer’s disease, are prime examples of age-related conditions with a strong link between dysregulated metabolism and functional decline. For example, my research team previously discovered that in aging mice, the ability of bone marrow cells to produce, store, and use energy is suppressed due to increased activity from a protein that modulates inflammation. This energy-deficient state leads to an increase in inflammation that’s worsened by these aging cells’ reliance on glucose as their main fuel source.
Experimentally inhibiting this protein in the bone marrow cells of aging mice, however, revitalizes the cells’ ability to produce energy, reduces inflammation, and improves plasticity of an area of the brain involved in memory. This finding suggests that some cognitive aging could be reversed by reprogramming the glucose metabolism of bone marrow cells to restore immune functions.
Repurposing drugs to treat Alzheimer’s
In our newly published research, my team and I discovered a new connection between disrupted glucose metabolism and neurodegenerative disease. This led us to identify a drug originally designed for cancer that could potentially be used to treat Alzheimer’s.
We focused on an enzyme called IDO1 that plays a critical role in the first step of breaking down amino acid tryptophan. This pathway produces a key compound called kynurenine, which fuels additional energy pathways and inflammatory responses. However, excessive kynurenine can have detrimental effects , including increasing the risk of developing Alzheimer’s.
We found that inhibiting IDO1 can recover memory and brain function in a range of preclinical models, including in cell cultures and mice. To understand why, we looked at the metabolism of brain cells. The brain is one of the most glucose-dependent tissues in the body. An inability to properly use glucose to fuel critical brain processes can lead to metabolic and cognitive decline.
High levels of IDO1 reduce glucose metabolism by producing excess kynurenine. So IDO1 inhibitors — originally designed to treat cancers such as melanoma, leukemia, and breast cancer — could be repurposed to reduce kynurenine and improve brain function.
Using a range of lab models, including mice and cells from Alzheimer’s patients, we also found that IDO1 inhibitors can restore glucose metabolism in brain cells . Furthermore, we were able to restore glucose metabolism in mice with both amyloid and tau accumulation — abnormal proteins involved in many neurodegenerative disorders — by blocking IDO1. We believe repurposing these inhibitors could be beneficial across various neurodegenerative disorders.
Promoting healthier cognitive aging
The effects of neurological disorders and metabolic decline weigh heavily on individuals and families, and even the economy.
While many scientists have focused on targeting the downstream effects of these diseases, such as managing symptoms and slowing progression, treating these diseases earlier can improve cognition with aging. Our findings suggest that targeting metabolism has the potential to not only slow neurological decline but also to reverse the progression of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and dementia.
Discovering new insights at the intersection of stress, metabolism, and aging can pave the way for healthier aging. More research can improve our understanding of how metabolism affects stress responses and cellular balance throughout life.
Melanie R. McReynolds is an assistant professor of biochemistry at Penn State University.
Republished from The Conversation .
Watch CBS News
Hormone therapy for women in menopause can slow aging and benefit health, study shows
By Sara Moniuszko
Edited By Allison Elyse Gualtieri
August 30, 2024 / 10:48 AM EDT / CBS News
Hormone therapy can benefit women's health during menopause , according to new research.
In the study, published Thursday in JAMA Network Open , researchers looked at more than 100,000 women in the U.K. and found being on hormone replacement therapy seems to slow biological aging.
"In this study, postmenopausal women with historical (hormone therapy) use were biologically younger than those not receiving (hormone therapy), with a more evident association observed in those with low (socioeconomic status)," the authors wrote. "Promoting (hormone therapy) in postmenopausal women could be important for healthy aging."
Dr. Céline Gounder, CBS News medical contributor and editor-at-large for public health at KFF Health News, said Friday on "CBS Mornings" the study is "really significant."
"It wasn't just about how you look, it was about your risk of death, in particular from cardiovascular disease," she said, adding the study looked at both chronological age (the age based on your date of birth) as well as biological age, which is "not just about how you look, but it's really about your health."
Gounder said the association might have been stronger with those of a lower socioeconomic status because aging is "also a reflection of your day-to-day stress ."
"When we talk about somebody having lived a hard life, it's not just a question of smoking or heavy drinking or using drugs. It's also the day-to-day stress of financial stress, worried about keeping a roof over your head, food on the table, your children's futures — and that stress also ages you," she said. "So of course, women of lower socioeconomic status are experiencing that more. Education is also a important factor here, being able to better access healthcare."
The study comes after longtime public misunderstanding around horomone therapy, stemming partly, Gounder said, from studies done about 20 years ago that have since been show to be problematic in terms of how they were designed.
"Unfortunately, a lot of women were discouraged from taking hormone replacement therapy as a result of those studies," she said.
Still, for some women, hormone therapy isn't an option, so it's important to talk to your doctor.
"There are some women who cannot take hormone replacement therapy. For example, if you have a history of breast cancer or blood clots," said Gounder. "And so this really depends on who we're talking about, the timing of when to start and how long to be on them."
- Women's Health
Sara Moniuszko is a health and lifestyle reporter at CBSNews.com. Previously, she wrote for USA Today, where she was selected to help launch the newspaper's wellness vertical. She now covers breaking and trending news for CBS News' HealthWatch.
More from CBS News

What is EEE? Mosquito-borne virus symptoms and prevention, explained

Catching up on sleep on weekends may lower risk of heart disease, study finds

2 die from West Nile virus in New Jersey, bringing reported deaths in U.S. to 5

Parental stress is an urgent public health issue, U.S. surgeon general says
- Aug 30 2024
UK researchers find Alzheimer’s-like brain changes in long COVID patients
New research from the University of Kentucky’s Sanders-Brown Center on Aging shows compelling evidence that the cognitive impairments observed in long COVID patients share striking similarities with those seen in Alzheimer’s disease and related dementias.
The study, published in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association , highlights a potential commonality in brain disorders across these conditions that could pave the way for new avenues in research and treatment.
The study was a global effort, funded by a multitude of grants from the U.S. National Institutes of Health, the Alzheimer’s Association and international organizations. The project also brought together experts from various fields of neuroscience.
Researchers at the UK College of Medicine led the study, including Yang Jiang, Ph.D., professor in the Department of Behavioral Science; Chris Norris, Ph.D., professor in the Department of Pharmacology and Nutritional Sciences; and Bob Sompol, Ph.D., assistant professor in the Department of Pharmacology and Nutritional Sciences. Their work focuses on electrophysiology, neuroinflammation, astrocytes and synaptic functions.
“This project benefited greatly from interdisciplinary collaboration,” Jiang said. “We had input from experts, associated with the Alzheimer’s Association International Society to Advance Alzheimer's Research and Treatment (ISTAART), across six countries, including the U.S., Turkey, Ireland, Italy, Argentina and Chile.”
Jiang and the collaborative team focused their work on understanding the “brain fog” that many COVID-19 survivors experience, even months after recovering from the virus. This fog includes memory problems, confusion and difficulty concentrating. According to Jiang, “the slowing and abnormality of intrinsic brain activity in COVID-19 patients resemble those seen in Alzheimer’s and related dementias.”
This research sheds light on the connection between the two conditions, suggesting that they may share underlying biological mechanisms. Both long COVID and Alzheimer’s disease involve neuroinflammation, the activation of brain support cells known as astrocytes and abnormal brain activity. These factors can lead to significant cognitive impairments, making it difficult for patients to think clearly or remember information.
The idea that COVID-19 could lead to Alzheimer’s-like brain changes is a significant development.
“People don’t usually connect COVID-19 with Alzheimer’s disease,” Jiang said, “but our review of emerging evidence suggests otherwise.”
The publication in Alzheimer’s & Dementia reveals that the cognitive issues caused by COVID-19 reflect similar underlying brain changes as those in dementia.
The study’s insights emphasize the importance of regular brain function check-ups for these populations, particularly through the use of affordable and accessible tools like electroencephalography (EEG).
The study not only highlights the shared traits between long COVID and Alzheimer’s, but also points to the importance of further research.
“The new insight opens avenues for future research and clinical practice, particularly in studying brain oscillations related to neural biomarkers of mild cognitive impairment in people with long COVID,” said Jiang.
One of the key findings is the role of astrocytes — support cells in the brain that have not been as thoroughly studied as neurons. The research suggests that damage or activation of these cells by COVID-19 can cause synaptic dysfunctions, leading to the abnormal brain activity observed in both conditions. This discovery is significant because it may help explain why EEG patterns in COVID-19 patients resemble those seen in the early stages of neurodegenerative diseases like Alzheimer’s.
Researchers believe this work could have a direct impact on patient care. They are advocating for routine EEG exams to detect early brain changes in both COVID-19 survivors and those at risk for cognitive decline.
“EEG patterns in COVID-19 patients resemble those seen in early neurodegenerative diseases,” said Norris.
“These similarities may be due to shared issues such as brain inflammation, astrocyte activity, low oxygen levels and blood vessel damage,” said Sompol.
By detecting these changes early, health care providers could potentially identify at-risk individuals sooner and implement interventions to prevent or slow the progression of cognitive decline.
As research continues, the team is particularly interested in how EEG monitoring can predict long-term outcomes in COVID-19 patients and assess the effectiveness of treatments aimed at preventing cognitive decline.
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Numbers P30AG072946, P01AG078116 and R56AG060608. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Words: Hillary Smith (Public Relations and Strategic Communications) Research illustration by Tom Dolan.
- Alzheimer's Disease
- Neuroscience
You might also like...

More from this series Research Priorities - Neuroscience

Environmental and social factors impact brain aging, study shows
- Download PDF Copy
Your brain ages at different paces according to social and physical environments in aging and dementia.
Countries with greater inequalities - whether economic, pollution or disease-based - exhibited older brain ages, according to a study published in Nature Science, involving the University of Surrey.
The pace at which the brain ages can vary significantly among individuals, leading to a gap between the estimated biological age of the brain and the chronological age (the actual number of years a person has lived). This difference may be affected by several things, such as environmental factors like pollution and social factors like income or health inequalities, especially in older people and those with dementia. Until now, it was unclear how these combined factors could either accelerate or delay brain ageing across diverse geographical populations.
In the study, a team of international researchers developed ways to measure brain ageing using advanced brain clocks based on deep learning of brain networks. This study involved a diverse dataset of 5,306 participants from 15 countries, including Latin American and Caribbean (LAC) nations and non-LAC countries. By analysing data from functional magnetic resonance imaging (fMRI) and electroencephalography (EEG), the researchers quantified brain age gaps in healthy individuals and those with neurodegenerative conditions such as mild cognitive impairment (MCI), Alzheimer's disease, and frontotemporal lobe degeneration (FTLD).
Our research shows that in countries where inequality is higher, people's brains tend to age faster, especially in areas of the brain most affected by ageing. We found that factors like socioeconomic inequality, air pollution, and the impact of diseases play a big role in this faster ageing process, particularly in poorer countries." Dr. Daniel Abasolo, co-author of the study and Head of the Centre for Biomedical Engineering at the University of Surrey
Related Stories
- How diet shapes gut microbiota and affects brain function
- Study reveals how different types of love activate the brain
- Fibrin fuels thromboinflammation and brain damage in COVID-19
Participants with a diagnosis of dementia, particularly Alzheimer's disease, exhibited the most critical brain age gaps. The research also highlighted sex differences in brain ageing, with women in LAC countries showing greater brain age gaps, particularly in those with Alzheimer's disease. These differences were linked to biological sex and gender disparities in health and social conditions. Variations in signal quality, demographics, or acquisition methods did not explain the results. These findings underscore the role of environmental and social factors in brain health disparities.
The findings of this study have profound implications for neuroscience and brain health, particularly in understanding the interaction between macro factors (exposome) and the mechanisms that underlie brain ageing across diverse populations in healthy ageing and dementia. The study's approach, which integrates multiple dimensions of diversity into brain health research, offers a new framework for personalized medicine. This framework could be crucial for identifying individuals at risk of neurodegenerative diseases and developing targeted interventions to mitigate these risks. Moreover, the study's results highlight the importance of considering the biological embedding of environmental and social factors in public health policies. Policymakers can reduce brain age gaps and promote healthier ageing across populations by addressing issues such as socioeconomic inequality and environmental pollution.
University of Surrey
Moguilner, S., et al. (2024). Brain clocks capture diversity and disparities in aging and dementia across geographically diverse populations. Nature Medicine . doi.org/10.1038/s41591-024-03209-x .
Posted in: Medical Science News | Medical Research News
Tags: Air Pollution , Alzheimer's Disease , Brain , Deep Learning , Dementia , Electroencephalography , Health Disparities , Imaging , Magnetic Resonance Imaging , Medicine , Neurodegenerative Diseases , Neuroscience , Pollution , Public Health , Research
Suggested Reading

Cancel reply to comment
- Trending Stories
- Latest Interviews
- Top Health Articles

Global and Local Efforts to Take Action Against Hepatitis
Lindsey Hiebert and James Amugsi
In this interview, we explore global and local efforts to combat viral hepatitis with Lindsey Hiebert, Deputy Director of the Coalition for Global Hepatitis Elimination (CGHE), and James Amugsi, a Mandela Washington Fellow and Physician Assistant at Sandema Hospital in Ghana. Together, they provide valuable insights into the challenges, successes, and the importance of partnerships in the fight against hepatitis.

Addressing Important Cardiac Biology Questions with Shotgun Top-Down Proteomics
In this interview conducted at Pittcon 2024, we spoke to Professor John Yates about capturing cardiomyocyte cell-to-cell heterogeneity via shotgun top-down proteomics.

A Discussion with Hologic’s Tim Simpson on the Future of Cervical Cancer Screening
Tim Simpson
Hologic’s Tim Simpson Discusses the Future of Cervical Cancer Screening.

Latest News

Newsletters you may be interested in
Your AI Powered Scientific Assistant
Hi, I'm Azthena, you can trust me to find commercial scientific answers from News-Medical.net.
A few things you need to know before we start. Please read and accept to continue.
- Use of “Azthena” is subject to the terms and conditions of use as set out by OpenAI .
- Content provided on any AZoNetwork sites are subject to the site Terms & Conditions and Privacy Policy .
- Large Language Models can make mistakes. Consider checking important information.
Great. Ask your question.
Azthena may occasionally provide inaccurate responses. Read the full terms .
While we only use edited and approved content for Azthena answers, it may on occasions provide incorrect responses. Please confirm any data provided with the related suppliers or authors. We do not provide medical advice, if you search for medical information you must always consult a medical professional before acting on any information provided.
Your questions, but not your email details will be shared with OpenAI and retained for 30 days in accordance with their privacy principles.
Please do not ask questions that use sensitive or confidential information.
Read the full Terms & Conditions .
Provide Feedback
COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK

Research Assistant
- Taub Inst Res Alzheimers Disease Aging Brain
- Columbia University Medical Center
- Opening on: Aug 29 2024
- Technical Grade 5
- Job Type: Support Staff - Union
- Bargaining Unit: SSA
- Regular/Temporary: Regular
- End Date if Temporary:
- Hours Per Week: 35
- Standard Work Schedule:
- Salary Range: $59,845.48 - $59,845.48 annually
Position Summary
The Research Assistant is an individual who will work at the Gertrude H. Sergievsky Center and the Taub Institute for Research on Alzheimer’s and the Aging Brain.
The Research Assistant (RA) will be supervised by the site co-investigators, Drs. Jennifer Manly and Stephanie Assuras, as well as the Research Coordinator. The RA will be trained and certified on standard administration and scoring procedures by observing other research staff and independently scoring assessments under supervision.
Responsibilities
The Research Assistant will work with the Principal Investigator and the Project Coordinator on the following:
- Planning and data collection of standardized cognitive assessment for nationally representative studies of older adults that are focused on how education and workforce experiences during early adulthood affects later life health.
- Under supervision from the Research Coordinator, will aid in the development, implementation, and oversight of test administration training.
- Help to train and certify individuals employed by a survey contractor on administration of tests of memory, language, and attention, survey questions about daily functioning and demographic background, and physical assessments (such as timed walk and chair stands).
- Scoring and double scoring protocols
- Lead or observe occasional Zoom conferences to coordinate the activities
- Supervise testers
- Maintain and revise operating manuals
- Organize, file, data cleaning, present data collected and data entry.
- Perform other related duties and responsibilities as assigned/requested.
Additional information: We will have a bi-weekly meeting with the PI/team to review the work being done as well as a weekly meeting(s) with the project coordinator to go over day-to-day activities.
Minimum Qualifications
- Requires a bachelor’s degree and at least 1.5 years of related experience, or an equivalent in education and experience.
Preferred Qualifications
- Previous experience in a research setting.
- Experience working with Excel and/or SPSS.
Equal Opportunity Employer / Disability / Veteran
Columbia University is committed to the hiring of qualified local residents.
Commitment to Diversity
Columbia university is dedicated to increasing diversity in its workforce, its student body, and its educational programs. achieving continued academic excellence and creating a vibrant university community require nothing less. in fulfilling its mission to advance diversity at the university, columbia seeks to hire, retain, and promote exceptionally talented individuals from diverse backgrounds. , share this job.
Thank you - we'll send an email shortly.
Other Recently Posted Jobs
Non-Student Short-Term Casual
Clinical research coordinator, technician b.
Refer someone to this job

- ©2022 Columbia University
- Accessibility
- Administrator Log in
Wait! Before you go, are you interested in a career at Columbia University? Sign up here!
Thank you, for sharing your information. A member of our team will reach out to you soon!

This website uses cookies as well as similar tools and technologies to understand visitors' experiences. By continuing to use this website, you consent to Columbia University's usage of cookies and similar technologies, in accordance with the Columbia University Website Cookie Notice .

IMAGES
VIDEO
COMMENTS
The team at Harvard envisions a future where age-related diseases can be effectively treated, injuries can be repaired more efficiently, and the dream of whole-body rejuvenation becomes a reality. "This new discovery offers the potential to reverse aging with a single pill, with applications ranging from improving eyesight to effectively ...
In a study published Jan. 12 in Cell, Sinclair, a professor of genetics and co-director of the Paul F. Glenn Center for Biology of Aging Research at Harvard Medical School, describes a ...
On July 12, 2023, researchers from Harvard Medical School, University of Maine and Massachusetts Institute of Technology (MIT) published a new research paper in Aging, titled, "Chemically induced ...
The quest to turn back the clock. Approaches to slow or reverse aging have gained momentum in recent years, but the field is still in its infancy with hurdles to overcome, as illustrated by ...
The world has seen an explosion in aging research in recent decades, with billions of dollars spent by government agencies and private companies. And the consumer market for products is forecast to hit $93 billion by 2027. As a result, false or exaggerated claims by companies promising longer life are currently on the rise, Ramakrishnan noted.
Harvard geneticist David Sinclair finds a group of "chemical cocktails" reversed aging in a one-week animal study, but experts say the results are preliminary. Harvard researchers found a ...
Reversing aging with one molecule. In the new study, published June 21 in the journal Cell, researchers looked to increase the amount of a protein that normally dwindles with age: telomerase ...
New discoveries suggest we can now reverse it," said David A. Sinclair, A.O., Ph.D., Professor in the Department of Genetics and co-Director of the Paul F. Glenn Center for Biology of Aging ...
The clock can distinguish between genetic differences that slow and accelerate aging, predict biological age, and evaluate anti-aging interventions with increased accuracy. The results are published in Nature Aging. "Previous clocks considered the relationship between methylation patterns and features we know are correlated with aging, but ...
In Boston labs, old, blind mice have regained their eyesight, developed smarter, younger brains and built healthier muscle and kidney tissue. On the flip side, young mice have prematurely aged ...
Analyses of the mice's muscles, kidneys, and retinas suggest the cocktail reversed some of the epigenetic changes induced by the DNA breaks. The findings point to a way to drive an animal's age "forwards and backwards at will," Sinclair says, and support the idea of epigenome-targeting treatments for aging in humans.
The Human Longevity Laboratory is part of the multi-center Potocsnak Longevity Institute, whose goal is to foster new discoveries and build on Northwestern's ongoing research in the rapidly advancing science of aging. The Institute is funded by a gift from Chicago industrialist John Potocsnak and family. "Aging is a primary risk factor for ...
While research on delaying and reversing aging is relatively new, experts say it concerns your epigenetics. Longevity experts have outlined the 12 hallmarks of aging, including epigenetic ...
The work shows that a breakdown in epigenetic information causes mice to age and that restoring the integrity of the epigenome reverses those signs of aging. Get more HMS news here. Findings are published online Jan. 12 in Cell. "We believe ours is the first study to show epigenetic change as a primary driver of aging in mammals," said the ...
Drugs that could treat aging might already be on the pharmacy shelves. At 67 years old, Dr. Nir Barzilai looks about the same as, if not younger than, he did 10 years ago. It's apparent in side ...
The research team was from the Hong Kong University of Science and Technology (HKUST) and was led by Professor Tom Cheung, an associate professor of life sciences. In contrast to their younger counterparts, aging MuSCs have decreased stemness (the ability to become new stem cells or turn into specialized cells to replace damaged tissues).
Discover the latest scientific advances that could help us live longer and healthier lives. From gene editing to senolytics, these are the 10 breakthroughs that will change ageing.
Scientists around the world are scurrying to reverse the hands of time. Here's a look at one lab's search for the fountain of youth, where old mice have grown young again.
The findings were published on 5 September in Aging ... Horvath — a pioneer in epigenetic-clock research — has developed some of the most accurate ones. The latest trial was designed mainly to ...
For efficient, cost-effective and personalized healthcare, biomarkers that capture aspects of functional, biological aging, thus predicting disease risk and lifespan more accurately and reliably ...
On July 12, 2023, a new priority research paper was published in Aging, titled, "Chemically induced reprogramming to reverse cellular aging.". Listen to an audio version of this press release. BUFFALO, NY- July 12, 2023 - In a groundbreaking study, researchers have unlocked a new frontier in the fight against aging and age-related diseases.
Changes to gene activity that occur with age can be turned back, a new study shows. New research suggests it is possible to slow or even reverse aging, at least in mice, by undoing changes in gene ...
Aging is the most significant risk factor for many of society's most common diseases, including diabetes, cancer, cardiovascular disease, and neurodegenerative disorders.A key factor behind the onset of these health issues is the disruption of cellular and metabolic homeostasis, or balance.Disrupting homeostasis destabilizes the body's internal environment, leading to imbalances that can ...
Hormone therapy can benefit women's health during menopause, according to new research. In the study, published Thursday in JAMA Network Open, researchers looked at more than 100,000 women in the ...
New research from the University of Kentucky's Sanders-Brown Center on Aging shows compelling evidence that the cognitive impairments observed in long COVID patients share striking similarities with those seen in Alzheimer's disease and related dementias.. The study, published in Alzheimer's & Dementia: The Journal of the Alzheimer's Association, highlights a potential commonality in ...
Volatile components were identified and then quantified by Equation (3) to depict natural aging and artificial aging. Fig. 2 a) shows the standardized, scaled (by column), and clustered results, where the color at the top indicates the category of compound. For more details, refer to Table S2, supplementary data.The relative amounts of various compounds in different samples can be compared ...
Participants with a diagnosis of dementia, particularly Alzheimer's disease, exhibited the most critical brain age gaps. The research also highlighted sex differences in brain ageing, with women ...
The Research Assistant is an individual who will work at the Gertrude H. Sergievsky Center and the Taub Institute for Research on Alzheimer's and the Aging Brain. The Research Assistant (RA) will be supervised by the site co-investigators, Drs. Jennifer Manly and Stephanie Assuras, as well as the Research Coordinator.