Advertisement


Introduction
Combination therapy and cancer heterogeneity, historical clinical evidence for bet-hedging by combinations of cancer therapies, assessing the role of synergy, additivity, and independent action in the lab and the clinic, independent action explains the clinical activity of many drug combinations, the role of drug additivity in curative combination therapies, sequential, concurrent, and biomarker-guided combination therapy to address interpatient heterogeneity in advanced cancers, is identifying the mechanisms underlying successful combination therapy important, common misconceptions and limitations in the analysis of combination therapy, moving beyond independent drug action: replacing bet-hedging with precision, prioritizing heterogeneity in the preclinical setting, making the data from clinical trials accessible to promote mechanistic studies, authors’ disclosures, acknowledgments, independent drug action in combination therapy: implications for precision oncology.
D. Plana and A.C. Palmer contributed equally to this article.
- Funder(s): NIH
- Award Id(s): U54-CA225088
- Funder(s): NIGMS grant
- Award Id(s): T32-GM007753
- Funder(s): NCI grant
- Award Id(s): F30-CA260780

- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
- Version of Record March 1 2022
- Proof January 25 2022
- Accepted Manuscript January 4 2022
Deborah Plana , Adam C. Palmer , Peter K. Sorger; Independent Drug Action in Combination Therapy: Implications for Precision Oncology. Cancer Discov 1 March 2022; 12 (3): 606–624. https://doi.org/10.1158/2159-8290.CD-21-0212
Download citation file:
- Ris (Zotero)
- Reference Manager
Combination therapies are superior to monotherapy for many cancers. This advantage was historically ascribed to the ability of combinations to address tumor heterogeneity, but synergistic interaction is now a common explanation as well as a design criterion for new combinations. We review evidence that independent drug action, described in 1961, explains the efficacy of many practice-changing combination therapies: it provides populations of patients with heterogeneous drug sensitivities multiple chances of benefit from at least one drug. Understanding response heterogeneity could reveal predictive or pharmacodynamic biomarkers for more precise use of existing drugs and realize the benefits of additivity or synergy.
The model of independent drug action represents an effective means to predict the magnitude of benefit likely to be observed in new clinical trials for combination therapies. The “bet-hedging” strategy implicit in independent action suggests that individual patients often benefit from only a subset—sometimes one—of the drugs in a combination. Personalized, targeted combination therapy, consisting of agents likely to be active in a particular patient, will increase, perhaps substantially, the magnitude of therapeutic benefit. Precision approaches of this type will require a better understanding of variability in drug response and new biomarkers, which will entail preclinical research on diverse panels of cancer models rather than studying drug synergy in unusually sensitive models.
The introduction of targeted anticancer drugs and the use of these drugs in various combinations have broadly and substantially improved rates and durability of response to therapy; like blood cancers, many advanced solid cancers are now treated with multidrug combinations (in addition to surgery and radiation where applicable; refs. 1–3 ). For optimal use of existing combination therapies and discovery of new ones, it is important to understand precisely why combining some but not all drugs is successful. Contemporary clinical trial reports make few if any claims about mechanism of action, and most scientific understanding of combination cancer therapy derives from preclinical studies (in cell culture or mouse models)—often performed after a drug or combination is evaluated clinically. The theoretical rationale for combination therapy—as historically understood—derives from the heterogeneity of cancer ( 4 ). The explanation more commonly advanced today is drug synergy ( 1 ), and screening for synergy is the focus of many ongoing research programs ( 5–17 ). However, whereas pharmacologic synergy is well defined in the case of preclinical experiments, particularly in cell lines, it has no precise definition in the context of survival data collected in cancer clinical trials.
In this review, we discuss historical and contemporary perspectives on combination cancer therapy, particularly the hypothesis that combinations can be highly effective in patient populations in the absence of drug interaction (either additivity or synergy). The underlying mechanism in this case is independent drug action. We review evidence from trials run by the founders of combination therapy, including the Acute Leukemia Group B (ALGB) and its chairs Emil Frei III and James Holland ( 18 ), that led to this hypothesis. We then review contemporary evidence collected from solid tumors that provides further support for this independent action in the context of targeted therapies and solid tumors. We close by describing an alternative framework for thinking about combination therapies and discuss its impact on future trials and preclinical mechanism-of-action studies.
This perspective does not call into question the results of any published randomized clinical trial (RCT), and it is not intended to guide clinical practice. It therefore differs from systematic reviews and meta-analyses ( 19, 20 ), the primary formats in which multiple clinical trials are compared retrospectively. The goal of a systematic review or meta-analyses is to guide clinical practice using scientific evidence, whereas this perspective primarily aims to inform the design of future trials and preclinical studies.
The proteins targeted by anticancer drugs are commonly part of multicomponent mitogenic networks and drug resistance pathways (acquired, adaptive, and innate; refs. 21–23 ). By analogy with synthetic lethality in genetic screens ( 24 ), the existence of parallel and converging molecular mechanisms in oncogenic and drug resistance networks provides a molecular rationale for the use of combination therapy ( 25–27 ). These arguments also have historical antecedents in cytotoxic chemotherapies that target multiple metabolic pathways ( 28 ). Identifying examples of synergy has now become an explicit goal for preclinical development of combination therapies ( 5, 6, 29–32 ). Clinically successful combination therapies are, in turn, often described as arising from synergy and explained in terms of activity against overlapping response and resistance networks ( 33–35 ).
Intra- and intertumor heterogeneity is evident at the level of genetics, histology, and disease progression, and single-cell sequencing has revealed rapid and diverse cancer genome evolution in response to therapy ( 36, 37 ). Moreover, heterogeneity and evolvability, via genetic or nongenetic mechanisms, are two of the greatest obstacles to the successful treatment of cancers. Variability in drug response is observed in clinical trials of even highly successful precision therapies that target mutant or amplified oncogenes (the drugs that are ideal Ehrlich “magic bullets”; ref. 38 ). For example, fewer than half of biomarker-positive patients respond initially to trastuzumab (Herceptin) in HER2-overexpressing breast cancers or to MEK/BRAF inhibitors in BRAF V600E/K -mutant melanomas ( 39, 40 ). However, such responses can be of sufficient magnitude in the patients in whom they occur to make trastuzumab and MEK/BRAF inhibitors practice-changing ( 41, 42 ). Studies in genetically homogeneous mouse models of cancer suggest that, were all cancers biologically similar (and tumor evolution relatively limited), a single therapeutic vulnerability might be sufficient for tumor eradication. However, heterogeneity among cells within a single human cancer commonly promotes drug resistance and disease progression, and heterogeneity between cancers limits clinically meaningful responses (with rare exception) to a subset of patients. The molecular origins of variability could include patient-to-patient (or tumor-to-tumor) differences in cancer cell genetics, drug pharmacokinetics, or patient immune function ( 43 ). Among these possibilities, patient-specific differences in pharmacokinetics remain the least studied or understood.
Since some of the earliest preclinical (mouse model; ref. 44 ) and clinical ( 45 ) studies of chemotherapies, combination therapy has been understood as a way of addressing intra- and intertumor heterogeneity, whatever its origins. Whereas a single drug might not be effective in killing every cancer cell in a heterogeneous tumor, drug combinations have the potential to kill different subsets of cells, improving the likelihood and durability of response ( 46 ). Indeed, this logic applies to antibacterial and antiviral drug combinations ( 47–50 ), and the ability of combination therapy to cure tuberculosis inspired early studies on combination cancer therapy ( 51 ). The same reasoning also applies to interpatient heterogeneity: any single therapy will not be effective in every patient, but combination therapies provide patients with several opportunities for a clinically meaningful response.
Here, we focus on the role that interpatient heterogeneity has on the effectiveness of combination therapies. We revisit historical theories in light of contemporary clinical trial data and a molecular understanding of cancer and discuss the implications for the modern concept of precision medicine. In particular, we review evidence that many combination therapies used for solid tumors, including all combination therapies with immune checkpoint inhibitors reported up to early 2020 ( 52 ), provide a benefit to a patient population equal to that expected from independent drug action. In this case, different patients benefit from the independent activities of different drugs (exhibiting “highest single-agent” response), without enhanced drug activity from the other constituents of the combination (either additive or synergistic pharmacologic interaction). Because patients exhibit differences in response, some patients benefit from one drug and others from a different drug, increasing response rates in the population as a whole. We describe how such a benefit can be quantified and discuss how independent drug action can be used to predict the likely benefit of new combinations ( 53 ). We also discuss why independent action is not sufficient to explain curative regimens for lymphoma, leukemia, and germ-cell tumors; in these cases, drugs exhibit additivity ( 54 ). We close by discussing how increasing the precision of cancer therapy may allow us to realize the substantial benefits of pharmacologic interactions.
Sidney Farber's 1948 report that aminopterin induced temporary remissions in a fraction of children with acute lymphocytic leukemia (ALL; ref. 55 ) was the first demonstration of a successful cancer chemotherapy. Achieving any degree of cancer control in these patients was remarkable at the time, but two limitations were immediately evident, each of which arose from heterogeneity in drug response. First, only a subset of patients with disease responded to therapy. Second, among responders, remissions were temporary, with a duration of around three months, consistent with the hypothesis that some cancer cells survived therapy and caused recurrent disease. Fortunately, childhood ALL was subsequently found to be responsive to multiple chemotherapies having different mechanisms of action and lacking cross-resistance, which allowed the ALGB [today Cancer and Leukemia Group B (CALGB); ref. 18 ] to develop an increasingly effective series of multidrug regimens.
In a 1961 clinical trial on ALL by the ALGB, Frei and colleagues ( 45 ) compared sequential monotherapy with combination therapy and identified pharmacologic principles that remain relevant today. ALGB “Protocol 2” randomized patients to either 6-mercaptopurine (6MP) combined with methotrexate (MTX) or sequential monotherapy with the same agents—switching from one agent to the other when the first failed. Sequential treatment made it possible to compare each of 74 individual patient responses to the first therapy (6MP or MTX) with the responses of the same patients to the second (MTX or 6MP, respectively; Fig. 1 ). It was found that responses of patients (partial or complete remission) to the first and second stages of therapy were not correlated, and remission rates were not significantly different between the first and second phases of treatment. Frei and colleagues ( 45 ) concluded that MTX and 6MP exhibited neither “cross-resistance” nor “collateral sensitivity.”

The earliest clinical trials of combination cancer therapy found that therapeutic benefit was due to independent drug action. Clinical trials of drug combinations for ALL repeatedly observed that a model of independent drug action could accurately explain the superior remission rates achieved by drug combinations ( 45, 56 ). In this example from 1961, trial arms evaluating sequential monotherapy found that MTX and 6MP were not cross-resistant (no correlation in response), and that the proportion of patients experiencing a complete remission was identical whether the drugs were given sequentially or concurrently in a combination. Furthermore, the complete remission rate of the combination therapy was consistent with that expected from independent drug action.
In addition, no difference in survival was observed among the sequential and combination therapy arms (42% or 44% complete remission with sequential therapy as compared with 44% with simultaneous combination therapy). Thus, patients treated with combination therapy effectively had two unbiased chances of responding to one of the two agents ( Fig. 1 ). The probability of responding to either of two agents present in a combination individually having probabilities P A and P B was calculated by Frei and colleagues as:

That is, the probability of responding to the combination, P AB is the sum of the probability of response from drug A ( P A ) and probability of response from drug B in patients who did not respond to drug A ((1 – P A ) × P B ). This calculation was found to be accurate to within 1% of observed response rates, which excluded the possibility that therapies each became more effective when used in combination (that is, that P A , P B became larger, as expected for pharmacologic interaction; ref. 45 ). A scenario in which individual drug efficacies ( P A , P B ) do not change when combined was described by Frei and colleagues ( 45 ) as independent drug action (Box 1), and was subsequently observed for multiple drug pairs for ALL ( 56 ).
The ALGB's insights into independent action in ALL came from a comparison of sequential monotherapy with combination therapy, but such trials are uncommon in solid cancers. A notable exception is the 2003 trial (E1193; ref. 57 ) by the Eastern Cooperative Oncology Group (ECOG), which randomized patients with metastatic breast cancer to either doxorubicin plus paclitaxel, or sequential monotherapy with the same agents. Patients treated with the combination experienced a higher rate of response (complete or partial reduction in lesion size; ref. 58 ) than those treated with a single drug, but no differences in overall survival were observed between arms in which the drugs were given sequentially or simultaneously. Echoing Emil Frei's conclusion 40 years prior, Sledge and colleagues interpreted E1193 as showing that different patients respond best to different drugs and the “composite response rate” of two therapies given sequentially (calculated as 49%) approximated that of the simultaneous combination (47%; ref. 57 ). As a result of this trial, sequential mono-therapy remains the internationally recognized approach to chemotherapy for metastatic breast cancer. Sequential therapy can, in principle, deliver the same therapeutic benefit as a combination with less toxicity and higher quality of life, provided that patients are able to receive the second therapy and the therapies are not cross-resistant ( 59–61 ). Sequential therapy is not appropriate when there is an urgent need for tumor control or for cancers where progressing patients may be too ill for a second line: use of up-front combination therapy allows the benefits of multiple drugs to be realized immediately ( 59 ).
What general lessons emerge from these studies? First, trials with drugs administered sequentially clearly show that different patients respond best to different drugs. Second, because of patient-to-patient variability, combinations of individually active therapies can increase the number of positive responses overall simply by providing several opportunities for benefit from monotherapies. Because it was not possible then, nor is it possible now, to predict which drug will be superior at the level of an individual patient, using combination therapy provides a therapeutic advantage in the form of “bet-hedging.” Third, when it is observed in a clinical trial that a population of patients responds better to combination therapy A + B as compared with monotherapies A or B given individually, it is not necessarily true that any individual patient will experience a superior response to the two drugs given together. As discussed below, data from a limited number of sequential human trials as well as patient-derived xenograft (PDX) studies involving panels of mice carrying PDXs make it possible to compare drug responses at the level of individual patients. These data confirm that drug combinations can improve outcomes in solid tumors without any pharmacologic interaction. Thus, the clinical superiority of a combination therapy does not demonstrate (or require) synergistic or additive interaction among the constituent drugs.
Drug “additivity” and “synergy” have rigorous definitions for preclinical experiments used to inform—or at least rationalize—which combinations proceed to clinical trials (Box 1; ref. 62 ). Pharmacologic interaction among two drugs was evaluated using isobologram analysis as early as 1872 ( 63 ) and defined mathematically in 1928 ( 64 ). As described by Loewe, two (equipotent) drugs are additive if combining a half-dose of each drug is as effective as a full dose of one drug; the drugs are synergistic if the effect is greater than additivity (this is often quantified by the combination index). Synergy is therefore assessed against a null hypothesis of additivity. In “Bliss Independence,” the expected effect of noninteracting drugs or toxins (the null model) is computed by assuming that each drug has a statistically independent chance of killing a target cell or organism; for example, if each of two drugs kills 90% of cancer cells alone, Bliss Independence predicts that the combination will kill 99% of cancer cells (that is, 10% of 10% survive). Synergy is demonstrated when this level of killing is exceeded. For a more thorough discussion of drug interactions in preclinical studies, see reviews by Foucquier and Guedj ( 65 ) and Twarog and colleagues ( 66 ) as well as extensions to pharmacodynamic ( 67 ) and toxicologic ( 68 ) interactions. Meyer and colleagues ( 69 ) have recently proposed an elegant reconciliation of different definitions of additivity and synergy.
Drug additivity, synergy, and antagonism are evaluated using measures of potency (e.g., IC 50 ) and efficacy (e.g., fractional cell kill) from dose–response measurements that are common only in preclinical settings (Box 1). As yet, no rigorous definition exists for drug synergy using survival or other data commonly obtained from patients in oncology trials. This reflects the fact that sufficiently resolved dose–response data are rarely available from human trials. It is also noteworthy that assessing pharmacologic interaction using laboratory data is more complex than the definitions in Box 1 imply, in part due to experimental noise and in part because dose–response data vary in parameters other than IC 50 (e.g., maximum effect and slope of the dose–response curve; ref. 70 ).
“Bliss Independence” is a null model for efficacy based on the statistically independent probability of a drug-induced death; if a combination exceeds the expected level of cytotoxicity, the drugs are judged to be synergistic.
“Loewe Additivity” is a null model for potency based on a principle of “dose equivalence.” For example, drugs are additive if combining half the IC 50 of drug A and half the IC 50 of drug B also achieves a 50% effect; this principle applies to any threshold of effect, e.g., IC 90 . A drug combination is synergistic if it is more potent than the null model of additivity.
Independent drug action—“Frei Independence”—is a null model for progression-free survival (PFS) in clinical trials. It assumes that PFS for each patient is equal to the longer of the two possible PFS times conferred by one or the other drug (see Box 2 for details). Combinations whose activity equals that expected from independent action are neither additive nor synergistic: the effect is the “highest single agent” per patient. Because of interpatient variability in drug response, combination therapy can confer substantial benefit, relative to monotherapy, via independent drug action and “bet-hedging.”
Regardless of which null model is used, a drug combination exhibiting synergy allows the same level of efficacy to be achieved at lower drug doses (or superior efficacy to be achieved with the same doses) as compared with an additive combination, and is therefore desirable ( 2 ). In principle, an efficacious drug combination exhibiting strong synergy could be achieved using individual agents that are inactive individually. Retrospective analysis has shown, however, that high single-agent activity is usually associated with good activity in a combination ( 71 ). Moreover, if two drugs are each highly effective, a combination can be weaker than additive and still provide a substantial level of benefit ( Fig. 2A ). This is particularly true when patients vary in their responses to the agents individually. This observation motivates a definition of drug interaction first explored by Gaddum in 1940, developed by Frei, and now known as “independent drug action” (or “Frei Independence” to distinguish it from Bliss Independence; Box 1).

The basis for combinatorial efficacy is difficult to discern in clinical trial data because of patient heterogeneity in single-drug response. A, Clinical benefit from combination therapy can be mediated both by high single-agent efficacy (enabling many log-kills of tumor cells) and by combined effects that could be affected by positive or negative pharmacologic interactions (additive, synergistic, or antagonistic). The observation that a combination response in a single patient is superior to the population median single-drug response could therefore have multiple possible explanations. B, Patient responses to a single therapy are heterogeneous across a population. Variation in drug response among patients makes it challenging to understand the precise nature of drug interaction across a population. It is therefore necessary to formulate appropriate null models and then determine whether the data in aggregate exceed the predictions of the null model. Note that this scenario is purely illustrative because current technology does not make it possible to observe single-drug effects in individual patients who are treated with combination therapy.
Independent drug action is the benefit provided by a combination when the effect is equal to the stronger of the effects of the two drugs considered individually ( 72 ). In this case, neither additivity nor synergy is present at the level of individual tumor (or patient in the case of survival data), and responses are scored as though the less effective agent is neither present nor active ( Fig. 2A ). The key insight is that the most effective single agent typically varies across patients, and thus drug independence can be sufficient for a combination to provide substantial clinical benefit: some patients benefit from one drug and others from a different drug in the combination (because we do not know this information a priori , this is a bet-hedging approach). The level of efficacy observed for a drug combination exhibiting independent action is therefore less than the null model for either Loewe additivity or Bliss Independence. Independent action is of interest only when response is measured in a heterogeneous population of samples, as in a human clinical trial or a panel of PDXs ( 73, 74 ).
Drug synergy evaluated by either Loewe or Bliss criteria is most commonly assessed in cell lines and sometimes in animal models. However, translating simple mathematical formulations of additivity and synergy into reliable experimental methodologies to detect these mechanisms is not straightforward even in cell lines, and a series of papers dating back to at least 1977 ( 65 ) has raised concerns about the widespread misuse of experiments and definitions associated with this terminology. All too often, “synergy” is used as a substitute for “more active” in cell line studies, and there is reason to approach the concept with caution even in preclinical studies ( 75 ).
Measuring Loewe additivity or Bliss Independence in patients is generally impossible because dose–response measurements comparing monotherapy and combination therapy are required ( 76 ). Were it possible to dose a population of patients with varying levels of a drug (as in a phase I trial, for example), differences in pharmacokinetics would mean that the effective drug concentration in each patient's tumor would be unknown. In a population of patients, interpreting responses to combination therapy is even more challenging, as each patient is likely to experience different magnitudes of antitumor efficacy from different therapies ( Fig. 2A and B ). Thus, any single patient's response to a combination therapy will have competing explanations in terms of best single-agent response, additivity, or synergy ( Fig. 2A ).
In contrast to a Loewe or Bliss null, evaluating the null model of independent drug action is straightforward using data from RCTs. When this model is exceeded, either additivity or synergy is present, and these cannot currently be distinguished. Estimating the magnitude of independent action in the precomputing era of 1960s oncology, as undertaken by Frei and others ( 56 ), involved rates of response but did not consider time-series survival data or correlation ( ρ ) in the probabilities of response [Box 2; Eq. (B)]; such correlations can arise from shared mechanism, cross-resistance, or prognostic factors. Today, independent action is evaluated using a simple algorithm whose inputs are the clinically observed distributions of responses to each of two treatments given individually, commonly progression-free survival (PFS) from Kaplan–Meier survival curves and an estimate of correlation in response [Box 2; Eqs. (C) and (D)]. Computing this null model is most conveniently implemented as the computational procedure shown in Supplementary Fig. S1. This procedure provides another way of thinking about independence: for each of a series of simulated patients, a response to therapy A and to therapy B is chosen at random from a joint distribution of PFS values, constructed from P A ( t ), P B ( t ), and the correlation in response ρ . Then, the better of the two responses for each simulated patient to either drug A or B is selected under the assumption that the less effective drug does not enhance response to the more effective drug. Because empirical survival data are used to compute the magnitude of independent action, the affect of prognostic factors, tumor heterogeneity, and time-dependent treatment effects is accounted for to the same extent as in monotherapy data from the original phase III trials.
The null model for independent drug action is applicable to a population of patients, or potentially to animal models (e.g., panels of diverse PDX tumors; refs. 73, 74 ), when individuals differ in response. If all individuals had the same drug sensitivities, independent drug action would predict no benefit relative to monotherapy. Benefit from independent action also requires that drugs be active as monotherapies, at least in some patients. A complication in determining if a particular drug combination exhibits benefit consistent with or exceeding drug independence is that efficacy data must be available for the constituent monotherapies at the same dosage, ideally from the same trial or one with closely matched patient characteristics.
In the simple case in which responses to drugs A and B are not correlated, the expected PFS at time t from A + B combination therapy is calculated similarly to Eq. (A):

Eq. (B) shows that the probability of tumor control, or PFS, from the combination ( P AB ( t )) is the sum of the probability of tumor control by drug A ( P A ( t )) and the probability of tumor control by drug B if drug A fails ( P B ( t ) × (1 – P A ( t ))). Here, “independence” means that the activity of drug A ( P A ( t )) does not change the activity of drug B ( P B ( t )) or vice versa.
Drug responses are often partially correlated (partially cross-resistant; ref. 74 ). To account for this, Eq. (B) is extended with a correlation coefficient ρ :

where A is the more effective of the two drugs. When ρ = 0 (no cross-resistance), Eqs. (B) and (C) are the same, and the benefit of independence is maximized. When ρ = 1, the drugs are completely cross-resistant, and the less active drug provides no additional benefit.
Chen and colleagues ( 53 ) recently derived a version of this equation that is symmetric for drugs A and B :

The correlation in drug responses [ ρ in Eqs. (C) and (D)] is the only parameter in these calculations. Clinical trials of sequential therapy and studies with PDX panels provide an opportunity to estimate this value. For example, Fig. 3A and B show data from a study by Gao and colleagues ( 73 ) of approximately 1,000 PDXs from 277 patients and six different types of tumors exposed to one of 62 different monotherapies or combination therapies ( 73, 74 ). For each type of therapy, 30 to 40 different human tumors were propagated in mice and exposed to drugs individually or in combination, and response duration was measured. The data show that only a subset of tumors responds strongly to a single drug and even fewer respond strongly to both drugs. However, tumors responding poorly to one drug (e.g., the green bars at the top of each graph) frequently exhibited strong responses to the other drug (depicted in magenta), so that animals receiving both drugs are predicted to benefit from a combination simply because they have two chances at a substantial monotherapy response; this is the essence of independent action. Figure 3C shows that the same reasoning can be applied to human patients receiving sequential therapy with pemetrexed and crizotinib for ALK -positive non–small cell lung cancer ( 77 ). Correlation values used to compute the magnitude of independent action can be estimated from these data and vary from Spearman ρ of 0.1 to 0.5 for mechanistically dissimilar drugs (e.g., cytotoxic agents vs. oncogene inhibitors), up to ≈ 0.7 for drugs with related targets (e.g., inhibitors of signaling kinases). Data from sequential human clinical trials are consistent with low correlations between dissimilar drugs, and ranges for benefits attributable to independent action have been found to lie within the range of ρ = 0.3 ± 0.2 ( 52, 74 ). Because responses are correlated to this degree, as opposed to truly independent in a statistical sense, the term “independent action” refers to pharmacologic, not statistical, independence ( 72 ).

Different cancer drugs benefit different patients in a population. Responses to different single drugs can be measured in the same tumor (as duration of PFS) through PDXs ( A and B ; ref. 73 ) or human patients ( C ) treated with sequential monotherapy ( 77 ). None of these drug pairs have a statistically significant correlation in response duration (as determined by a Spearman rank test). Patients and xenografts are sorted based on response to the single agent depicted in green.
It is currently possible to evaluate the magnitude of independent action using PFS data for a total of 21 published phase III clinical trial combination results (the great majority of which led to FDA approvals), including 13 recent trials involving immune checkpoint inhibitors (ICI), which in aggregate involve 11 types of advanced solid cancers. The key requirement for such an evaluation is the availability of sufficient data on responsiveness to monotherapies. Roughly three quarters of approved combinations analyzed, including all combinations involving ICIs, exhibit clinical activity very close to that predicted by independent action ( Fig. 4A and B ; Pearson r = 0.98, P < 10 –8 , n = 21 comparisons; data from 13,689 patients in 38 clinical trials; refs. 52, 74 ). These conclusions are supported in biomarker-stratified and nonstratified RCTs across a variety of advanced cancers (cancers of the lung, breast, skin, head and neck, ovary, pancreas, stomach, and kidney) and treatment modalities (including chemotherapies, molecularly targeted therapies, and ICIs).

Independent drug action explains the survival benefit of many drug combinations. A, Combination therapies with efficacy equal to or greater than independent action simulations, based on analysis of clinical trial PFS data ( 52, 74 ). Parentheses denote combinations tested as a single arm of a clinical trial. GEJ, gastroesophageal junction; NSCLC, non–small cell lung cancer; TNBC, triple-negative breast cancer. B, Observed combination PFS at 12 months (mostly from phase III clinical trials) correlates with 12-month PFS estimated by independent action simulations ( n = 21 combinations). Note that differences from the independent action predictions (black or purple color) were calculated using longitudinal data over the total trial length. C, Observed combination ORR (mostly from phase I and II trials) correlates with ORR estimated by independent action ( n = 100 combinations; ref. 78 ).
Similar approaches have recently been used to estimate the magnitude of independent action using overall response rate (ORR) data from 98 early trials (primarily phase I and II) performed by Merck & Co. on combination therapies involving ICIs ( 78, 79 ). The Merck group found that independent drug action was also sufficient to explain response rates observed for the majority of drug combinations in this data set ( Fig. 4C ; Pearson r = 0.84, P < 10 –26 , n = 100 comparisons). Unfortunately, for some standard-of-care combination therapies, it is not currently possible to assess the magnitude of either drug independence or synergy: most commonly, the efficacy of at least one drug has not been measured in the appropriate patient population at the appropriate dose for a formal comparison with the combination. It is also unfortunate that there exist relatively few phase III trials involving multiple targeted agents for which preclinical research has provided molecular hypotheses about expected mechanistic interactions. However, the fact that the independent action model can only rarely be rejected when analysis of suitable RCTs is possible implies that it is not reasonable to presume synergy whenever data are lacking.
The most effective drug combinations in oncology have historically been those that combine active single agents ( 71 ), particularly those having nonoverlapping mechanisms of drug resistance ( 28, 80 ). In these cases, calculation of independence provides a quantitively accurate explanation for disease-specific differences in observed activity. For example, in advanced non–small cell lung cancer, ICIs and chemotherapy ( 81, 82 ) are active as single agents and are superior in a combination. Similarly, ICIs and multitargeted receptor tyrosine kinase inhibitors are effective in advanced renal cancer, and a combination of the two is superior to either one alone ( 83, 84 ). Benefit from independent drug action is possible only when both agents in a combination are active. The magnitude of this benefit falls as cross-resistance (correlation) between drugs increases (Box 2). In this regard, it is important to note that “no single-agent activity” has two different meanings in the clinical setting. The first meaning is that an agent truly has no measurable ability to shrink tumors or delay progression, and the second is that a drug has some antitumor activity, but it is insufficient for approval as a single agent (typically this means the new drug is inferior to standard of care). Very few approved combination therapies contain drugs in the first category, whereas many successful combination therapies contain a drug in the second category, which is expected according to independent drug action. Ambiguity about the meaning of “no single-agent activity” has contributed to the erroneous perception that synergy is commonly observed in clinical trial data.
Conversely, among trials analyzed to date, independent action is most commonly exceeded when at least one drug is relatively inactive on its own; this is the case in the metastatic setting for combinations involving bevacizumab, which targets VEGF and appears to enhance the effects of chemotherapies by affecting tumor vasculature (ref. 85 ; Fig. 4 ).
Two notable cases of coinhibiting one pathway exhibit synergistic (supra-additive) interaction, because at least one agent is devoid of single-agent activity in the given disease but improves the activity of a combination. The first is fluorouracil plus leucovorin ( 86 ), which is approved in many indications, and the second is EGFR plus BRAF inhibition for BRAF -mutant colorectal cancer (ref. 87 ; these effects are not quantified in Fig. 4 due to lack of monotherapy data). These examples demonstrate that synergy does not ensure high response rates, and analysis of 18 approved combinations involving one agent with limited activity (~40% being VEGF inhibitors; ref. 71 ) has found that median overall survival increased only by ~1.6 months.
Whether coinhibition of one pathway or even a single target (e.g., a small-molecule and biological drug against the same protein) is more likely to result in supra-additive efficacy than drugs inhibiting multiple pathways is not yet known. Regardless, synergy is not guaranteed: combinations of antibacterial drugs targeting a single protein complex, the ribosome, have been shown to exhibit synergy, no interaction (independence), and even antagonism ( 88 ). Pathway-specific models are needed to understand such effects, and additional mechanistic analysis could help us understand what principles distinguish independence from interaction in these scenarios. Higher-order drug combinations can also provide benefit using a mixture of effects, including independent action against different targets and pharmacologic interaction for one target or pathway. For example, the triplet combination of trastuzumab, pertuzumab, and docetaxel (two HER2 antibodies and a microtubule inhibitor) may confer benefit by both “bet-hedging” and pharmacologic interaction ( 74 ). Whether interacting or independent, it is nevertheless the case that combinations of inactive agents rarely succeed in the clinic, which is cause for caution in the development of agents that lack single-agent antitumor effect.
Some combination therapies that have been tested in RCTs exhibit activity less than predicted by independence ( 52 ). This could arise because drugs are strongly cross-resistant, such that patients nonresponsive to therapy A almost never benefit from therapy B . Alternatively, the drugs could have a strong antagonistic interaction, which can render a combination less active than monotherapy ( 89 ). A third possibility is that the combination induces adverse side effects that either worsen patient survival directly or require dose reductions or interruptions. In the latter case, the use of lower doses of each drug results in a weaker antitumor effect than full-dose monotherapy. This appears to explain the underperformance of dabrafenib plus trametinib plus pembrolizumab in the treatment of metastatic BRAF -mutant melanoma; the management of adverse effects necessitated dose reduction or interruption in nearly all patients ( 90, 91 ).
In summary, the available evidence provides strong support for independent action as the mechanism underlying the majority of approved combination therapies for advanced cancers as well as many combinations in current development. “Bet-hedging” is therefore an effective strategy for increasing ORRs in a heterogeneous population of patients, precisely as the ALGB first observed in 1961 ( 45 ). Merck investigators have recently suggested that the ability of independent action to estimate the outcomes of future combination trials will be particularly valuable for ICIs ( 78 ), because more than 3,000 ICI trials are currently under way, and historical experience suggests that many will fail (based on data in ClinicalTrials.gov).
In multiple hematologic malignancies, it is possible to administer combination therapies that achieve long-lasting responses and even cures. Independent drug action is inadequate as an explanation for such therapies. The classic “fractional kill hypothesis,” as well as recent preclinical experiments ( 54 ), suggests that drug additivity but not synergy is involved in cures. For example, the five-drug R-CHOP regimen cures most patients with diffuse large B-cell lymphoma (DLBCL) and is strictly additive (or even slightly antagonistic) in cell lines by both Loewe and Bliss criteria, as demonstrated by clone-tracing experiments that measure a 10 6 -fold range of tumor cell killing ( 54 ); a similar conclusion is supported by murine models of B-cell lymphoma ( 25 ). The mechanistic basis for curative therapies for advanced cancers, which remain largely restricted to chemosensitive liquid tumors and a small number of solid tumors, involves a high degree of cell killing through the use of multiple drugs, each potent on its own in the majority of patients and having low cross-resistance. Intratumor heterogeneity and tumor evolution are a still a challenge in these therapies, and it appears that many tumors are initially resistant to one or more drugs that make up the combination ( Fig. 5A ). Resistance is also acquired in the course of therapy. However, when cross-resistance is low, the likelihood that a single resistance mechanism will cause multiple drugs to be ineffective initially is correspondingly low. When the components of a combination are each highly effective (e.g., >99% tumor cell killing each), additivity results in many-orders-of-magnitude improvements in fractional cell kill ( Fig. 5B ). Hematologic oncologists have long been taught ( 4 ) that drug additivity of this type is responsible for the efficacy of curative combination therapies, and recent evidence supports this interpretation ( 54 ). The fractional kill of individual therapies (illustrated as arrow length in Fig. 5 ) will depend on the chemosensitivity of a given cancer type, and may explain why some curative regimens involve five to eight therapies, whereas germ-cell tumors can often be cured with fewer therapies [e.g., the bleomycin, etoposide, and cisplatin (BEP) combination for testicular cancer; ref. 92 ].

Clinical context affects the prevalence of benefit from drug additivity or from bet-hedging via independent action. A, In chemosensitive cancers treated with high-order combinations (e.g., DLBCL), multiple agents are typically active per patient, although to varying extents. B, Additive interactions among many highly active therapies are sufficient for strong tumor response and cure. C, In many solid tumors, single therapies have low response rates and heterogeneous effects. Low single-drug response rates imply that few patients are highly responsive to multiple therapies. D, Bet-hedging confers substantial advantage in solid tumors without the need for drug interaction. Additivity or synergy may arise in some patients but are statistically expected to be uncommon, or small in effect because one therapy is usually the principal contributor to tumor response (e.g., patient 6).
Many advanced solid malignancies are more difficult to treat than blood cancers, and response rates to single drugs are typically lower than in hematologic malignancies. Low response rates alone are a reason to expect that drug additivity will be less often observed in solid tumors, because few patients will experience strong responses to multiple agents ( Fig. 5C ). These are the scenarios in which independent drug action is expected to provide the greatest benefit relative to monotherapy: even if additivity is possible with these drugs, it would be limited to too few “doubly responsive” patients to substantially affect survival in a trial arm ( Fig. 5D ).
There are two main therapeutic strategies for exploiting independently acting combinations in the clinic ( Fig. 6A ): (i) concurrent combination therapy (commonly used for new drug approvals, for example, Checkmate 067 for ipilimumab and nivolumab in advanced melanoma, ref. 93 ; and the combinations shown in Fig. 4B and C ), and (ii) sequential administration of different single agents, switching on a predetermined schedule or on evidence of disease progression (e.g., ECOG 1193 showing benefit of sequential chemotherapy in metastatic breast cancer, ref. 57 ; a strategy described in detail below). Because benefit consistent with independence assumes that each patient benefits from the best monotherapy, the efficacy conferred by concurrent or sequentially administered combinations could in principle be matched by a third strategy: treating each patient with the therapies most likely to be active in that specific patient ( Fig. 6B ). This strategy could use biomarkers predictive of therapeutic response, such that combination therapy A + B is restricted to patients predicted to benefit from both drugs (standard therapy A alone would be assigned to patients whose biomarker status predicts no benefit from B , as in KEYNOTE-355 with pembrolizumab and chemotherapy combined for PD-L1–positive advanced triple-negative breast cancer; ref. 94 ). A variant of this approach is to start all patients on A + B and then use pharmacodynamic biomarkers to measure response at a molecular level; the inactive agent could then be withdrawn and the dose of the active agent increased or a new drug introduced ( Fig. 6B ). These approaches have a critical limitation: we currently lack the biomarkers to make accurate treatment assignments at the level of individual patients. A corollary of independent action is that, were response biomarkers available, benefit could be increased using existing drugs. On the basis of data from PDX panels in which drug additivity or synergy is occasionally demonstrable ( 73, 95 ), the magnitude of improvement relative to independent action could be dramatic and might even extend to cures (as in Fig. 5B ).

Possible strategies for combining multiple cancer drugs. Patient heterogeneity in drug response presents a fundamental challenge in designing combination cancer therapies. A, Existing, imprecise strategies for use of multiple drugs include first-line combination therapies for all patients and sequential therapy. B, Precision-based approaches can use pretreatment predictive biomarkers to select one or more agents likely to be active in a given patient (patients labeled 1a, 1b, and 1c), or use on-treatment pharmacodynamic biomarkers (patient 2) to identify which agents are active or inactive in a patient to stop use of an inactive agent and increase dose of an active agent (particularly if combination therapy required dose reduction). Here, treatment “arrows” are to compare strategies and are not intended to evaluate response duration.
The distinction between independent action and additivity or synergy is not simply semantic and has profound implications for improving patient care with existing drug combinations and for developing new and improved combination therapies. In the immediate term, clinical research on drug combinations would benefit from better predicting the efficacy expected in new clinical trials by simulating the likely benefits conferred by independent action ( 53, 79 ). In the longer term, the focus of preclinical and translational cancer pharmacology needs to include understanding, measuring, and predicting variability in drug response and in developing better response biomarkers, including pharmacodynamic biomarkers ( 96, 97 ).
The Benefits Conferred by New Drug Combinations Are Largely Predictable
One of the great advantages of independent drug action is that it provides a simple methodology for predicting the likely benefits of a drug combination based on knowledge of responses to monotherapy in similar patient populations. Critically, accurate prediction does not require making any assumptions about targets or mechanisms of action. Criteria for combinations likely to provide substantial benefit via independent action include drugs with high single-agent activity and uncorrelated responses (correlation is lowest when mechanisms of action differ). As discussed below, this does not mean that drugs can be combined at random. One substantial limitation with respect to the design of new combinations is that we cannot currently predict treatment-related adverse events from drugs individually or in combination; we do not view this as a limitation of the model of independent action per se , but as a reflection of generally poor understanding of drug toxicity. With further empirical data, adverse effects could be scored for independence, additivity, synergy, or antagonism, and their relationships to therapeutic effects could be ascertained. This may be particularly important in the case of immune-related adverse events (irAE), which appear to be closely related in mechanism to the therapeutic effects of ICIs ( 98 ).
Sequential Therapy May Be as Good as a Combination in Some Cancers
Intensive combination therapy is vital to most treatments with curative intent, and coadministration is necessary for drug synergy to manifest ( Fig. 6 ). However, among noncurative combination therapies exhibiting independent action, drugs need not be given at the same time to benefit patients. This implies that combinations in current clinical practice could be modified to involve sequential monotherapy, as currently practiced for metastatic breast cancer, where patients switch to a different chemotherapy when the first one fails ( 60 ). This approach has the potential to reduce drug toxicities while delivering equivalent duration of tumor control (and possibly better control if dose escalation of the monotherapy is possible; ref. 59 ). Of course, sequential therapy is possible only when a disease does not progress so rapidly that it is necessary to maximize the probability of response up front. Sequential therapy requires that patients are healthy enough to switch therapy upon progression. First-line combination therapy will also remain optimal for cancer types, or specific patients, where there is a sizable risk that patients will be unable to receive a second line of therapy, for example, in many cases of advanced non–small cell lung cancer ( 99 ).
Prioritize Rather Than Penalize Single-Agent Efficacy in a Preclinical Setting
One of the practical problems in identifying drug combinations involving synergy by either Loewe Additivity or Bliss Independence criteria in preclinical studies is that the greatest deviation from additivity occurs over a relatively narrow range of drug doses typically centered on the midpoint (IC 50 ) of a sigmoidal dose–response curve. At these concentrations, target coverage is incomplete, and responses are suboptimal by definition. In contrast, the goal with many oncology drugs, particularly biologics, is to achieve a dose that results in substantially more than 50% of target engagement ( 100 ). Screens for synergistic drug combinations therefore penalize highly active drugs and the dose ranges at which target coverage is optimal. Furthermore, drug combinations with meager activity can score as “synergistic” if the constituent drugs are inactive alone ( 12 ) and therefore much less likely to be useful clinically ( 71 ). In contrast, efficacy via independent action (as well as the additivity required for successful curative therapy) benefits from maximal tolerable dosing and high single-agent activities—the hallmarks of most clinically useful combinations. Matching preclinical to clinical drug concentration ranges has also been shown to facilitate accurate prediction of combination drug efficacy from in vitro data ( 101 ). Preclinical studies should therefore shift to studying drugs at dose ranges that provide target coverage similar to that achieved in patients (we note that these might not be the same drug concentrations in vivo and in vitro due to pharmacokinetic effects). Under these conditions, additivity should be considered a very promising finding and could be sufficient for the design of curative therapies; for example, clinical and experimental data show that rituximab is additive with the CHOP regimen for DLBCL ( 54 ).
Deprioritize Screening for Synergy and Emphasize Screening to Avoid Cross-Resistance
Data from curative drug combinations suggest that preclinical studies in cell lines should also shift away from searching for synergy to screening for single-agent activity, drug additivity, and identification of drugs having nonoverlapping resistance mechanisms. As mentioned above, pharmacologic interaction is scored near drug IC 50 values, but the primary obstacle to cure in most settings is thought to be acquired drug resistance caused by outgrowth of rare resistant cells. Systematic analysis of cross-resistance is challenging using conventional cell culture techniques, but clone tracing and genome-wide CRISPR screening make it feasible to empirically measure cross-resistance for any combination therapy. We propose that screening for drug cross-resistance be performed alongside screens for collateral drug sensitivities ( 102 ). Resistance screens are best performed one drug at a time, with cross-resistance identified in a subsequent computational comparison, making it possible to build systematic resources relevant to many therapeutic approaches ( 54, 103 ).
Given the long history of the drug independence model ( 45 ), we have been surprised by resistance to the idea that it likely explains the benefits conferred by many approved combination therapies. We believe that this resistance arises from several misunderstandings. Most importantly, in saying that a particular drug combination does not involve synergy (or pharmacologic interaction more generally) we are not saying that the combination is not clinically superior to monotherapy. Independent action can explain practice-changing improvements in patient outcomes without the need to invoke any specific (and possibly poorly understood) molecular mechanism. In contrast, concluding that drugs interact synergistically is a statement about a specific mechanism of action; it is not a measure of the quality or importance of a combination therapy. As noted above, primary articles that report clinical trial results rarely make specific claims about drug interactions. These claims typically arise in reviews and preclinical studies. By equating clinical benefit with synergistic drug interaction, these claims make it difficult to determine how outcomes might best be improved in the clinic and where preclinical studies are likely to be most informative.
Another common misconception is that the model of independent action implies that any two (or more) drugs can be combined to improve outcomes. This is not true. Instead, benefit by independent action occurs only when both drugs are individually sufficiently active and their responses weakly correlated. In an analysis of all possible drug pairs in PDX data from Gao and colleagues ( 73 ), fewer than 5% of combinations were predicted to improve PFS when compared with the best observed monotherapy for that tumor type ( 74 ). This low rate of success is consistent with evidence that drug combinations superior to standard monotherapies are challenging to identify and develop clinically.
The model of independent drug action, like that of Loewe Additivity or Bliss Independence, represents a null model against which evidence of additivity or synergy is scored. If the null model cannot be rejected by an appropriate statistical test, then it is considered the most likely explanation. By analogy, if we cannot reject the null model that survival is no greater in the presence of therapy than a placebo, as evaluated by Cox proportional hazards regression, we conclude that a therapy provides no benefit ( 104 ). However, this is not the same as saying that drugs in a combination cannot interact. On the contrary, there is ample evidence from preclinical models and clinical samples that cells exposed to multiple therapies adopt states distinct from monotherapy-treated cells ( 29, 105 ). An additive or synergistic response to therapy might also be attributable to different tumor cell subpopulations responding to different drugs in a combination ( 106 ). However, if survival distributions are consistent with independent action, then any pharmacologic interactions that do occur must not measurably extend response duration across a patient cohort.
The simplest way to reconcile preclinical and clinical data on drug interactions is to note that variability of response in patients is so large that it is statistically unlikely that a patient will experience a similar magnitude of response to two agents, and one agent therefore has the dominant effect ( Figs. 5 and 6 ). Large-scale preclinical experiments ( 107, 108 ) have shown that synergy at the level of cytostasis or cytotoxicity is relatively uncommon when drugs are combined and tested across panels of cell lines, and in many cases, additivity is not observed either ( 101 ). Moreover, computational methods attempting to predict synergy in cancer cells have found that the magnitude of drug interaction varies across cell line identity ( 17 ). Finally, it is not true that most approved combination therapies have been demonstrated to exhibit better-than-additive efficacy in cell lines or animal models using Bliss or Loewe criteria ( 109 ).
Patient heterogeneity in drug response is a fundamental challenge in oncology that has often been addressed with concurrent or sequential use of multiple therapies to maximize chance of response (bet-hedging; Fig. 6A ). Because only a small minority of solid tumors (10%–15% in PDX studies or in sequential human trials) actually respond to both drugs, it is uncommon for favorable pharmacologic interaction by drug combinations to have a significant impact. Were it routinely achievable, positive pharmacologic interaction between drugs in a combination (additivity or synergy) by definition provides a magnitude of benefit that exceeds independent action. How might this be achieved? The key is likely to be greater precision in the use of drugs individually and in combination ( Fig. 6B ). However, combination therapies generally cannot be used in a personalized manner, because for many individual therapies, biomarkers are presently not available to identify responsive patients. To generate such biomarkers, we need to measure and then understand determinants of drug response at the level of individual patients, including the possibility that differences in response involve patient-to-patient variability in pharmacokinetics and optimal dosing, which are not areas of current emphasis in publicly funded translational cancer research. At a molecular level, the development of sensitive methods for transcriptional profiling ( 110, 111 ) and multiplexed imaging ( 112–114 ) of biopsies, along with increasing acceptance of sequential biopsies as a means to monitor patients, may provide an opportunity to develop sensitive new pharmacodynamic assays. Patients could potentially be started on a combination followed by use of a biopsy to see if their tumor is responding at a molecular level to both drugs; if not, then the inactive drug might be withdrawn (in advance of radiologic evidence of tumor progression) and a new combination tried, or the active agent dose escalated. In the intermediate term, drug responses might be measured in patient-derived primary tumor cells using BH3 profiling ( 115 ), microfluidic technologies ( 116 ), or image-based assays ( 117 ), or assessed in situ using implantable drug-delivery microdevices ( 118 ). Some of these approaches are already being used to guide therapy in programs such as the SMMART trial initiative at Oregon Health and Science University (Portland, OR; ref. 119 ).
Improving rates of response outside of academic medical centers, where multiple biopsies are unlikely to be feasible, will ultimately require diagnostics predictive of drug response (or lack of resistance) based on genetic, histologic, and transcriptional features of tumors pretreatment ( 95, 120–124 ). The I-PREDICT trial recently demonstrated proof of concept for personalized combination therapies in advanced metastatic cancers using pretreatment genomic and histologic profiling ( 125 ), and such approaches should improve with advances in biomarker discovery. In cases in which an evaluation is possible (e.g., BRAF -mutant melanomas), existing biomarkers significantly improve average response, as expected, but they do not appear to reduce variability in response as measured by the coefficient of variation in PFS distributions ( 74 ). This is most easily understood as reflecting the presence of multiple additional and unknown drug response determinants. Identifying these unknown determinants must become a priority for preclinical and translational research, even in the case of successful therapies. Conversely, it will also be important to understand why a small minority of univariate biomarkers are so effective at predicting some drug responses, for example, NTRK fusions and sensitivity to entrectinib and larotrectinib in multiple cancer types ( 126 ).
Molecular oncology has been well served by a focus in preclinical studies on outliers in response that reveal the presence of genes and mutations conferring high drug sensitivity or strong acquired resistance ( 127, 128 ). However, the resulting emphasis on drug-sensitive cell lines and murine models is the likely cause of consistent overestimation of drug efficacy (whether of individual agents or combinations) in the preclinical setting as compared with observed benefit in human trials. We do not yet fully understand the molecular origins of diversity in drug response even in panels of cancer lines carrying a known driving oncogene, such as BRAF V600E in cutaneous melanoma or EGFR L858R in non–small cell lung cancer; therefore, it is not surprising that we do not understand the phenomenon in human patients. The problem becomes more acute as the models get more complex; PDX panels such as those used by Gao and colleagues ( 73 ) show promise, but in-depth molecular analysis is required as a complement to what is most commonly a phenomenologic study of tumor volume. In the case of preclinical study of immunotherapies, genetically engineered mice and syngeneic models have been valuable, but the current focus on a relatively small number of responsive models should be complemented by a mechanistic study of variability in response in a set of models having different drug sensitivities. Relatively simple experiments have the potential to be informative in the context of a panel of heterogenous preclinical models. We might then ask: if a panel of cell lines or tumor explants ( 129 ) is exposed to a drug over a concentration range, is target coverage similar in all cases (as measured using a pharmacodynamic assay)? If the target is equally covered (e.g., inhibited) in multiple lines, are the phenotypic consequences the same ( 130, 131 )? Drug–response studies using large panels of cell lines and PDX explants have become increasingly common ( 70, 129, 132, 133 ), and what is now needed is a molecular understanding of observed differences in response. Bringing the full power of preclinical cancer biology to bear on these questions, first in cell lines and then in PDX studies, would almost certainly yield information useful in the more challenging problem of understanding variability in drug response in patients. In humans, variability in drug pharmacokinetics also needs more investigation.
The analysis of clinical trial data reviewed here, and any assessment of pharmacologic mechanism in clinical trials, would greatly benefit from access to the individual participant data (IPD) used to create the Kaplan–Meier curves and compute survival functions. Analysis of IPD is widely regarded as the gold standard for formal meta-analysis ( 134, 135 ), and the International Committee of Medical Journal Editors (ICMJE) in fact required a data-sharing statement from papers reporting clinical trials effective July 1, 2018; as of April 2020, only two of 487 relevant articles in the Journal of the American Medical Association , the Lancet , and the New England Journal of Medicine had actually made IPD publicly accessible ( 136 ). Figures showing Kaplan–Meier estimators are universally part of oncology clinical trial reports, and the absence of numerical data has little to do with patient privacy or intellectual property and more with the continuing view of computational biologists as data parasites ( 137 ). As a result, much of the analysis described above comes from papers in which image processing was used to extract data from published figures ( 52, 74 ). One positive development is the depiction of individual patients’ tumor responses in “waterfall” and “swimmer” plots in phase I and II trials ( 138 ). Such data are rarely reported in phase III trials, but their publication could provide rich insights into interpatient heterogeneity. It may nonetheless be necessary to amend the requirements for data deposition on ClinicalTrials.gov (per U.S. Public Law 110-85: Food and Drug Administration Amendments Act of 2007, Title VIII, Section 801) so that trial reporting includes IPD and not just summary statistics. Journals should also enforce the ICMJE standards that already exist.
The (re)realization that many approved and effective combination therapies perform in a manner consistent with independent drug action and therefore represent a necessary and beneficial form of bet-hedging, in the face of continued ignorance about many of the determinants of drug response and resistance, should be viewed as a positive development. Not only does it provide a realistic way to design and predict the probable benefit conferred by new drug combinations, it suggests that a renewed focus on precision medicine will yield very substantial benefits because it will unlock the potential for pharmacologic interaction. In the immediate future, assays that reduce the number of ineffective therapies received by each patient could also reduce toxicity and cost without compromising effective tumor control. Moreover, data from combinations used in liquid tumors suggest that additivity among active agents (not necessarily synergy) will be sufficient to elicit the degree of curative control over solid cancers currently possible in some hematologic malignancies.
A.C. Palmer reports personal fees from Merck and grants from Prelude Therapeutics outside the submitted work. P.K. Sorger reports grants from NCI during the conduct of this work, as well as other support from Glencoe Inc. and personal fees from Applied Biomath, RareCyte, NanoString, Flagship Pioneering, and Merck outside the submitted work. No disclosures were reported by the other authors.
This work was supported by NIH/NCI grant U54-CA225088. D. Plana is also supported by NIGMS grant T32-GM007753 and NCI grant F30-CA260780. We thank Emmett Schmidt and David Weinstock for discussions.
Note: Supplementary data for this article are available at Cancer Discovery Online (http://cancerdiscovery.aacrjournals.org/).
Supplementary data
Citing articles via, email alerts.
- Online First
- Online ISSN 2159-8290
- Print ISSN 2159-8274
AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Information on Advertising & Reprints
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Open access
- Published: 04 January 2024
The promise and challenges of combination therapies with antibody-drug conjugates in solid tumors
- Qing Wei 1 , 2 , 3 na1 na2 ,
- Peijing Li 2 , 4 na1 na2 ,
- Teng Yang 2 , 5 ,
- Jiayu Zhu 6 ,
- Lu Sun 2 , 7 ,
- Ziwen Zhang 1 , 2 ,
- Lu Wang 8 ,
- Xuefei Tian 2 , 9 , 10 ,
- Jiahui Chen 2 , 3 , 11 ,
- Can Hu 2 , 3 , 11 ,
- Junli Xue 12 ,
- Letao Ma 2 , 5 ,
- Takaya Shimura 13 ,
- Jianmin Fang 14 ,
- Jieer Ying 1 , 2 , 3 na1 ,
- Peng Guo 2 , 3 na1 &
- Xiangdong Cheng 2 , 3 , 11 na1
Journal of Hematology & Oncology volume 17 , Article number: 1 ( 2024 ) Cite this article
11k Accesses
18 Citations
1 Altmetric
Metrics details
Antibody-drug conjugates (ADCs) represent an important class of cancer therapies that have revolutionized the treatment paradigm of solid tumors. To date, many ongoing studies of ADC combinations with a variety of anticancer drugs, encompassing chemotherapy, molecularly targeted agents, and immunotherapy, are being rigorously conducted in both preclinical studies and clinical trial settings. Nevertheless, combination therapy does not always guarantee a synergistic or additive effect and may entail overlapping toxicity risks. Therefore, understanding the current status and underlying mechanisms of ADC combination therapy is urgently required. This comprehensive review analyzes existing evidence concerning the additive or synergistic effect of ADCs with other classes of oncology medicines. Here, we discuss the biological mechanisms of different ADC combination therapy strategies, provide prominent examples, and assess their benefits and challenges. Finally, we discuss future opportunities for ADC combination therapy in clinical practice.
In the past decade, antibody-drug conjugates (ADCs) have emerged as a transformative treatment modality for a broad spectrum of solid and hematological malignancies [ 1 , 2 ]. ADCs are antibody-based macromolecular complexes comprising three main constituents: antibodies, linkers, and payloads. Their mechanism of action can be summarized as follows: when the antibody binds to the antigen on the surface of a target cell, the ADC is internalized, releasing the payload and exerting cytotoxicity [ 3 ] (Fig. 1 ). Following the initial approval of ADCs for solid tumors in 2013 [ 4 ], interest in this field has increased, and numerous such conjugates have been evaluated across various tumor categories.
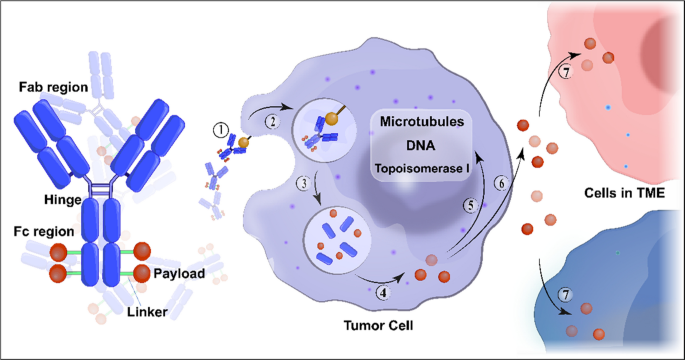
Structure and mechanism of action of conventional ADCs. ADCs consist of three essential components: a monoclonal antibody that binds to an antigen primarily expressed on the surface of tumor cells, providing specificity in targeting tumor cells; a linker that prevents premature release of the payload in the bloodstream but instead releases it in the tumor cells; and a cytotoxic payload that triggers tumor cell death by targeting critical components such as DNA, microtubules, and topoisomerase. ADC cytotoxicity involves a series of sequential stages: ① binding of the antibody to the antigen, ② internalization of the ADC-antigen complex, ③ degradation of the ADC in the lysosomes, ④ release of the payload in the cytoplasm, ⑤ its interaction with the target; ⑥ possible discharge of a fraction of the payload into the extracellular milieu, ⑦ subsequent occurrence of the bystander effect where it is internalized by neighboring cells in the tumor microenvironment. Abbreviation: TME, tumor microenvironment
Several ADCs have shown potent anti-tumor activities against treatment-refractory cancers. To date, eight ADCs have been approved for solid tumors with different indications (Table 1 ). Nevertheless, even for target-positive tumor types, most patients do not achieve long-lasting disease control and develop resistance to ADCs. Thus, for many tumor types, a single treatment is insufficient and many ADCs are undergoing clinical trials with more responsive regimens.
In the realm of cancer treatment, it is widely acknowledged that the likelihood of achieving complete remission and cure is often heightened by combining therapeutic agents that operate through diverse mechanisms of action, particularly when dealing with the complexities of tumor heterogeneity [ 5 ]. The primary approach for addressing resistance and/or enhancing ADC therapies involves the integration of ADCs with different therapeutic strategies. Synergy is commonly defined as the effect of two or more agents working in combination that is greater than the expected additive effect. An additive effect is generally considered as the baseline effect for synergy detection methods. Consequently, active research is exploring the combination of ADCs with various other types of anticancer medications, such as chemotherapy, radiotherapy, endocrine therapy, targeted molecular agents, and immunotherapy, both in preclinical models and clinical trials. There is an interest in developing rational combinations that could prolong survival compared to monotherapies.
In this review, we discuss the mechanisms of different ADC combination therapies and review the ongoing clinical trials for their selection and evaluation. Finally, we outline and examine key translational, statistical, and regulatory considerations from a combination perspective, highlighting the current progress and significant challenges yet to be addressed.
ADCs combined with chemotherapy
Integrating different forms of chemotherapy with ADC has proven to be a well-accepted strategy for overcoming drug resistance and achieving favorable treatment outcomes in preclinical and clinical studies [ 6 ]. Exploring the most effective combination regimen requires a comprehensive understanding of how ADC antibodies and payloads work synergistically with chemotherapy drugs to affect the cell cycle and alter the presence of surface antigens. However, to date, many ADCs have been added to commonly used chemotherapeutic regimens merely as carriers for the delivery of toxic payloads without considering their synergistic effects, leading to mixed results in both preclinical and clinical research. This highlights the significant and unmet need for continued efforts in designing clinical trials for ADCs combined with chemotherapy. Table 2 presents a list of such trials.
Mechanism of ADCs combined with chemotherapy
According to reported findings, chemotherapy and ADCs act synergistically in ways that include targeting different phases of the cell cycle or modulating tumor cell surface antigen expression.
Cell cycle phase blockers
Many chemotherapeutic drugs are DNA-damaging agents, such as antimetabolites, platinum-based compounds, and topoisomerase inhibitors that target the S phase of the cell cycle and induce G2/M arrest, which can be effectively combined with ADC containing microtubule-disrupting payloads that target the G2/M phase of the cell cycle. This concept has been illustrated through the effective combination of carboplatin with mirvetuximab soravtansine (targeting folate receptor α with DM4), anetumab ravtansine (targeting mesothelin with DM4), or luveltamab tazevibulin (targeting folate receptor α with SC239) in ovarian cancer preclinically [ 7 , 8 , 9 ]. During early phase trials investigating the synergistic effects of ravtansine-based ADCs in combination with carboplatin or doxorubicin, positive treatment responses were observed in both platinum-sensitive and -resistant patients with ovarian cancer [ 10 , 11 , 12 , 13 , 14 ].
Improved surface-antigen expression
The choice of chemotherapeutic companion may affect the levels of surface antigens targeted by ADCs. For instance, gemcitabine can upregulate HER2 expression on pancreatic adenocarcinoma cells by 14.81 folds, predominantly within the G2/M phase. Thus, the effect of gemcitabine on DNA synthesis renders it effective against G1 and early S phase cells, whereas G2/M phase cells are more resistant. The enhanced HER2 expression in G2/M cells implies a greater likelihood of gemcitabine effectively binding with trastuzumab emtansine (T-DM1, HER2 targeted with DM1 payload), which contributes to the improved efficacy of the combination on pancreatic ductal adenocarcinoma cells [ 15 ]. Thus, gemcitabine generate synergistic effects in combination with T-DM1 through their ability to enhance antigen availability. However, it remains uncertain whether this observation holds true for other ADC-chemotherapy combinations with different targets, and whether the increased antigen expression levels are directly related to the actual available antigenic epitopes for ADC binding or even to the efficacy of ADCs.
Coordination of different drugs
The timing of administration is a significant factor to consider when designing ADC combinations, as most conjugates must be internalized by tumor cells to be effective, which involves systemic transport and cell entry processes. For example, induction of G2/M phase arrest by DNA damage requires at least 15 h for microtubule disruptors to act [ 16 ]. Wahl et al. elegantly demonstrated this concept in preclinical models of colon, lung, and breast cancers. They observed that sequential management of SGN-15 (a construct targeting the Lewis Y antigen with a doxorubicin payload) followed by paclitaxel resulted in greater DNA fragmentation than simultaneous treatment [ 17 ].This observation suggests that the sequence of drug administration may be taken into consideration when combined chemotherapy with ADCs. However, these concepts await clinical trial assessment and should be explored in light of the recognized rates of ADC internalization and cell cycle progression in individual tumor types.
Safety profile of the ADC–chemotherapy combination
Notably, the combination of ADCs and chemotherapy presents challenges related to overlapping toxicities. Substantial insights in this regard have been gained from clinical trials. For example, a study evaluating T-DM1 in combination with docetaxel (with or without pertuzumab) for HER2-positive breast cancer demonstrated dose-limiting toxicities (DLTs) and grade ≥ 3 adverse events in approximately 80% patients with metastatic breast cancer [ 18 ]. These adverse effects included neutropenia, fatigue, epistaxis, stomatitis, nausea, and diarrhea. Similarly, the combination of T-DM1 with capecitabine resulted in increased discontinuation rates without a significant improvement in response rates [ 19 ]. The combination of trastuzumab deruxtecan (T-DXd) with 5-fluorouracil (5-FU) or capecitabine resulted in notable toxicities in patients with metastatic HER2-positive gastric cancer, with dose-limiting stomatitis and a high incidence of grade ≥ 3 adverse events in DESTINYGastric03 trial [ 14 ].
Datopotamab deruxtecan (Dato-DXd) in combination with platinum-based chemotherapy and pembrolizumab resulted in substantial grade ≥ 3 toxicities in a significant proportion of patients, characterized by the common occurrence of nausea, anemia, fatigue, and stomatitis [ 13 ]. In addition, the combination of mirvetuximab soravtansine with carboplatin in a Phase Ib trial resulted in notable toxicities including nausea, vomiting, diarrhea, eye problems, fatigue, and cytopenia [ 10 ].
In summary, the results of these studies suggested a notable increase in toxicity when ADCs were combined with conventional chemotherapy. This is likely due to the overlap of toxicities resulting from the off-target and off-tumor effects of the ADC payloads.
ADCs combined with endocrine therapy
Endocrine therapy is a widely used therapeutic approach for hormone-sensitive cancers (e.g., breast and prostate cancers). It works either by blocking hormone synthesis or interfering with hormones that stimulate the growth of tumor cells. Both ADCs and endocrine therapy drugs can induce cellular effects that jointly impede tumor cell survival and proliferation. Combination therapy reduces the likelihood of tumor cells developing resistance by employing multiple drugs with distinct mechanisms of action. There have been some clinical trials related to ADC combined with endocrine therapy (Table 3 ), while basic research to explore the mechanism of combined action is lacking.
Safety profile of the ADC–endocrine therapy combination
The side effects of endocrine therapy are minimal [ 29 ]. This gives clinicians more confidence in adding ADC drugs to endocrine therapy, as demonstrated in the KATHERINE phase III clinical trial. In this trial, the adjuvant utilization of T-DM1 was compared with that of trastuzumab in patients with HER2-positive breast cancer with residual disease who had undergone neoadjuvant HER2-targeted therapy. Both treatment arms were permitted to include concurrent adjuvant endocrine therapy. Patients administered T-DM1 with or without endocrine therapy exhibited comparable toxicity rates of any grade. Similarly, no significant differences between the two groups were observed in terms of grade ≥ 3 adverse events (26.0% versus 24.9%), serious adverse events (12.9% versus 12.2%), and events that resulted in a T-DM1 dosage reduction (11.0% versus 15.0%) [ 30 ].
The possibility of combining endocrine therapy with T-DXd has been investigated in patients with HER2-low breast cancer at both early and advanced stages. The TALENT study is a randomized phase II trial evaluating the administration of neoadjuvant T-DXd, with or without anastrozole, in patients with early breast cancer and low HER2 expression. Interestingly, this study showed that both treatment arms exhibited similar toxicity profiles, highlighting the feasibility of this combination approach [ 31 ]. Similarly, the Phase Ib study, DESTINY-Breast08, revealed that adding anastrozole or fulvestrant to T-DXd did not result in any DLTs. This combination approach was observed to maintain a toxicity profile akin to that of the solitary administration of T-DXd in individuals diagnosed with metastatic HER2 low breast cancer.
In general, the co-administration of ADCs and endocrine therapy does not appear to result in increased toxicity. This observation is consistent with the distinct patterns of adverse effects exhibited by each agent when administered independently. This is also consistent with the favorable safety profiles of most endocrine therapies compared to other systemic cancer treatments.
ADCs combined with radiotherapy
The combination of radiotherapy and ADC includes external radiation therapy combined with ADC and radionuclide antibody conjugates (RACs). RACs, also known as radioimmunoconjugates, radioimmunotherapy, or targeted radiotherapy, are a type of medical treatment that uses specific monoclonal antibodies labelled with radioactive isotopes (radionuclides, generally beta emitters), as described in a review by Mattes [ 32 ]. Therefore, RAC is not discussed in the present review. Based on the timing of radiotherapy and ADC administration, the combination is either concomitant or sequential. Concomitant radiotherapy involves simultaneous administration of ADC and radiotherapy. However, the definition of sequential ADC administration varies across studies: the temporal span ranges from 77 to 131 days when ADC is administered before radiotherapy and 420 to 1426 days when administered after radiotherapy [ 33 ]. The fractionation regimens include conventional fractionated radiotherapy and stereotactic radiosurgery (SRS)/stereotactic body radiotherapy (SBRT). The clinical studies on ADCs combined with radiotherapy are shown in Table 4 .
Mechanism of ADCs combined with radiotherapy
The synergistic mechanisms of the combined application of radiotherapy and ADC include the regulation of surface antigen expression in tumor cells by radiotherapy, an increase in radiation sensitivity of tumor cells by ADC, and other potential mechanisms, such as affecting the tumor microenvironment (TME) and vascular permeability.
Radiation induces generation of (neo)antigens
Ionizing radiation (IR) induces morphological and functional alterations in tissues [ 44 ]. Tumor cells are more prone to survival and propagation when “stress-regulated proteins” are highly upregulated by external stimuli. (Neo-) antigens expressed on the surface of cancer cells after IR exposure provide opportunities to develop cancer-targeted therapeutics. Cell adhesion molecules were the first IR-inducible proteins identified [ 45 , 46 ]. However, these inducible proteins are expressed on microvascular endothelial cells rather than on tumor cells, and some are shed from the cell surface. Glucose-regulated protein 78 (GRP78) is a radiation-induced endoplasmic reticulum stress response protein that plays an important role in radioresistance, enhancement of tumor cell proliferation, protection against apoptosis, and promotion of tumor angiogenesis [ 47 , 48 ]. The effectiveness of combining GRP78 antibodies with radiotherapy has been studied previously [ 49 , 50 ]. The researchers discovered that antibodies targeting the functional domain of GRP78 disrupt its interactions with its binding partners. Consequently, this reduces tumor cell viability and enhances radiosensitization. Tax interacting protein 1 (TIP-1), another radiation-induced tumor-specific target, translocates to the cell plasma membrane after exposure to IR [ 51 ]. Based on this finding, Lewis et al. conjugated a high-affinity anti-TIP-1 antibody (7H5) to a payload such as monomethyl auristatin E (MMAE) with a valine-citrulline (Vc) linker to form a radiosensitizer (7H5-VcMMAE) [ 52 ]. The use of 7H5-Vc-MMAE in combination with radiotherapy resulted in a prolonged delay in tumor growth and improved the survival of A549 and H1299 non-small cell lung cancer (NSCLC) animal models. To date, the targeting of radiation-inducible antigens using ADCs combined with radiotherapy has not been explored in clinical trials. The new clinical paradigm of using IR to guide drug delivery merits further investigation in preclinical and clinical trials involving patients with radiation-resistant cancers.
ADCs increase the sensitivity of tumor cells to radiotherapy
The cell cycle phase plays an important role in determining the relative radiosensitivity of cells [ 53 ]. Cells are most sensitive to IR during the G2/M phase, display intermediate sensitivity in the G1 phase, and exhibit the lowest sensitivity in the later stages of the S phase. Based on these findings, many radiosensitizing drugs have been developed to increase the anti-tumor activity and optimize patient outcomes. However, in practice, the clinical utility of radiosensitizing drugs is substantially curtailed due to unintended off-target side effects. To address this critical challenge, radiation-sensitizing payloads (e.g., MMAE, MMAF, and DM1) have been conjugated to antibodies to selectively radiosensitize tumors based on antigen overexpression. These radiosensitizer-ADCs are capable of increasing radiosensitizer delivery to tumors, enhancing radiation-induced cytotoxicity, and improving tumor control [ 54 , 55 ]. Furthermore, in combination with radiotherapy, radiosensitizer-ADCs have been shown to enhance the effectiveness of radiation and improve survival in preclinical tumor models of the lung, head and neck, oesophageal, breast, and pancreatic cancers [ 52 , 54 , 55 , 56 , 57 , 58 ].
Other potential mechanisms
Radiotherapy not only directly acts on tumor cells but also affects the TME in a complex and dynamic manner. Several radiation-induced molecules within tumor blood vessels, including ICAM-1, E-selectin, P-selectin, and β3 integrin, reportedly have the potential to serve as therapeutic targets [ 59 , 60 ]. Moreover, there is mounting evidence that the interplay between radiotherapy and the TME could be utilized to enhance the accumulation and intratumoral distribution of nanoparticles and liposome formulations mediated by changes in the vasculature and stroma, with secondary effects on hypoxia, interstitial fluid pressure, solid tissue pressure, and the recruitment and activation of bone marrow-derived myeloid cells [ 61 , 62 , 63 ]. A previous study found that vascular permeability in tumors significantly increased 24 h after irradiation at doses higher than 400 cGy, resulting in increased antibody uptake following radiation [ 64 ]. In addition to affecting the tumor blood vessels, radiotherapy also has a direct impact on blood–brain barrier permeability. Nakata et al. found that a single large dose of 20–40 Gy promoted the extravasation of serum albumin in the rat brain tissue preclinically [ 65 ]. Nevertheless, whether conventional fractionated radiotherapy, routinely used in clinics, can promote ADC penetration of the blood–brain barrier remains unknown and the underlying mechanism remains elusive. Finally, the effect of radiotherapy on the distribution of antibodies and the payload of linker-cleavable ADCs in tumors is yet to be investigated.
In summary, multiple potential synergistic benefits and underlying mechanisms of the combination of radiotherapy and ADCs deserve further exploration at both the preclinical and clinical stages.
Safety profile of the ADC–radiotherapy combination
The clinical applications of radiosensitizer-ADCs have also been evaluated. For non-CNS tumors, there is insufficient robust evidence regarding the safety profile and efficacy of ADCs in combination with different radiotherapy segmentations. Available data mainly focus on breast cancer treatments [ 33 , 66 ]. The KATHERINE trial evaluated the effectiveness of adjuvant T-DM1 in patients with HER2-positive breast cancer and residual disease after neoadjuvant chemotherapy with anti-HER2 therapy [ 35 ]. The results demonstrated that adjuvant T-DM1 treatment reduces the risk of disease recurrence and death (50%). In this clinical trial, patients who underwent breast-conserving surgery and those who had locally advanced disease following mastectomy (clinical T 3 N + /T 4 N x /T x N 2-3 disease) were administered radiation within 60 days of surgery. However, a subgroup analysis specific to patients undergoing radiation was not conducted to assess the safety profile of the combined treatment. An increase in ≥ 3 grade toxicities was noted among the irradiation group in comparison to the non-irradiated group (27.4% vs. 16.2%). Comparable incidences of radiation-related cutaneous complications were observed, affecting 25.4% and 27.6% patients in the T-DM1 and trastuzumab groups, respectively. Patients who were administered T-DM1 showed a modest increase in the occurrence of radiation-induced pneumonitis and pulmonary radiation injury rates (1.5% and 0.1%, respectively), in contrast to those administered trastuzumab (0.7% and 0%) [ 30 ]. Considering the potential risk of cardiotoxicity associated with trastuzumab, a study was conducted to investigate the cardiac safety and feasibility of radiotherapy in combination with T-DM1 in a group of 116 patients ( n concurrent = 39, n sequential = 77) [ 34 ]. Roughly 95% patients receiving T-DM1 plus radiotherapy successfully adhered to ≥ 95% of the planned radiotherapy dosage with a delay of ≤ 5 days. No protocol-prespecified cardiac side effects or instances of heart failure were reported following T-DM1. However, Zolcsak et al. presented the initial safety profile associated with the concurrent use of T-DM1 and radiotherapy in a group of 14 patients diagnosed with residual invasive HER2-positive breast cancer. A dosage of 50 Gy delivered in 25 fractions was administered for adjuvant irradiation of the breast or chest wall. A reversible grade 2 decrease in left ventricular ejection fraction (LVEF) was observed in two patients [ 42 ]. Considering the mechanism of T-DM1’s action involving radiosensitization through microtubule inhibitors and in the absence of solid safety data, the delivery of concurrent radiation should be approached with caution. Recent clinical studies have revealed that T-DXd improved both progression-free survival (PFS) and overall survival (OS) compared to T-DM1, but information concerning the combined use of T-DXd and radiotherapy is scarce. T-DXd exhibited an increased incidence of drug-related interstitial lung disease (ILD) or pneumonitis (10.5% vs. 1.9%), and gastrointestinal toxicities were more frequently reported with T-DXd treatment. The combination of T-Dxd with thoracic or abdominal radiotherapy requires extreme caution. In a metastatic breast cancer setting, a case-series study assessed the toxicity of concurrent palliative radiotherapy and T-DM1 in three patients with bone metastases [ 39 ]. The radiotherapy field involved the thoracic vertebrae, sacrum, and shoulder, with a prescribed dose of 15 Gy delivered in five fractions or 8 Gy delivered in one fraction. All patients experienced substantial pain relief, and no documented adverse reactions associated with the concomitant use of radiotherapy and T-DM1 were reported. Furthermore, approximately 50% of HER2-positive metastatic breast cancer cases are associated with brain metastases [ 67 ]. In terms of CNS metastatic tumors, high-level data on the effectiveness and tolerance of concurrent administration of ADCs and brain radiation therapy are insufficient. Evidence gleaned from case reports or a small series of patients indicates that the combination of T-DM1 and concomitant whole-brain radiation therapy is manageable, without severe side effects or any increase in clinically significant toxicity [ 40 ]. However, prudence is needed when considering concurrent or sequential SRS as several cases of complications have been documented in the literature [ 36 , 41 , 43 , 68 , 69 ]. The case series presented by Carlson et al. showed that four out of seven (57.1%) patients who underwent SRS after T-DM1 administration developed radiation brain necrosis [ 36 ]. This elevated rate of clinical radiation brain necrosis is clearly unacceptable. In another study involving 45 patients diagnosed with CNS metastases from breast cancer, Stumpf et al. observed a 13.5-fold rise in the risk of radiation necrosis when T-DM1 was administered in conjunction with SRS [ 68 ]. The DEBBRAH phase II study demonstrated the feasibility of combining intracranial treatment with T-Dxd and radiation, showing manageable toxicity in patients with HER2-positive and HER2-low breast cancer who underwent whole-brain radiation therapy and/or SRS. However, the authors did not mention the timing between irradiation and the sequential administration of T-DXd [ 70 ]. Notably, significant heterogeneity was observed among these studies. Regarding primary CNS tumors, there are already some data on the effectiveness and safety outcomes of the combination of ADC and radiation treatment. An anti-EGFR antibody conjugated to MMAF, Depatuxizumab-mafodotin (Depatux-M), was administered to patients with glioblastoma (GBM) receiving standard treatment with radiotherapy plus temozolomide [ 37 ]. The safety profile of Depatux-M combined with radiotherapy and temozolomide for the treatment of newly diagnosed GBM is acceptable. However, interim analysis revealed that Depatux-M did not yield an OS benefit for the treatment of newly diagnosed EGFR-amplification GBM, notwithstanding the longer PFS. The study was terminated at the early stage. The potential reasons for these negative results are as follows: 1. Depatux-M may be ineffective in treating GBM; 2. there is a probability that Depatux-M effectively eliminated EGFR-amplification (and particularly EGFRvIII-mutant) tumor cells, improving PFS; however, resistant clones emerged and voided any OS benefit, a hypothesis supported by results from patient-derived xenografts [ 71 ]; 3. heterogeneous delivery across the blood–brain barrier limits the efficacy of Depatux-M in CNS tumors [ 71 ], and non-cleavable linkers are detrimental to drug diffusion within the tumors.
In summary, radiosensitizer-ADCs combined with radiation are a promising treatment strategy; however, there is an urgent need for high-level evidence regarding their safety.
ADCs combined with molecular targeted cancer therapy
Targeted therapies, including monoclonal antibodies, tyrosine kinase inhibitors (TKIs), and anti-angiogenic agents, have been used in clinical practice for decades to treat tumors with specific mutations, overexpression, and amplification, with clinically proven safety and efficacy. However, the efficacy of these treatments in combination with ADC remains poorly understood. In this section, we focus on the treatment strategies that combine ADCs with targeted therapeutics and discuss their potential synergistic effects and safety profiles. The corresponding clinical trials are shown in Table 5 .
Mechanism of ADCs combined with targeted therapy
The combined effects of molecular-targeted drugs (including antibodies) and ADCs synergistically involve multiple mechanisms, such as improving intratumoral drug delivery by targeting tumor blood vessels, regulating tumor cell surface antigen expression, overcoming intratumoral heterogeneity and tumor drug resistance, and synthetic lethality.
Enhanced cellular uptake and anti-tumor activity
The macromolecular size of ADCs limits extravasation and leads to impaired distribution of ADCs in tumor tissues, ultimately resulting in unsatisfactory efficacy [ 88 ]. Two types of barriers affecting ADC delivery have been identified: the blood-tumor barrier, a physical barrier, and the binding-site barrier (BSB), a biological one. Within the blood-tumor barrier microenvironment, blood vessels present in solid tumors are composed of many immature and disorganized vessels, resulting in poor blood flow and hypoxia [ 89 ]. Meanwhile, high interstitial oncotic pressure collapses the tumor blood vessels, thus limiting the convective transport of ADCs from the blood into the tumor interstitial fluid [ 90 ]. The primary method for altering the tumor vasculature involves modulating angiogenesis and vessel porosity using agents such as anti-vascular endothelial growth factor antibodies such as bevacizumab. Jose F Ponte et al. showed that co-treatment with mirvetuximab soravtansine (an FRα-targeting ADC) and bevacizumab induced swift disruption of tumor microvasculature and extensive necrosis, and improved the efficacy in platinum-resistant epithelial ovarian cancer (EOC) models, emphasizing the superior bioactivity profile of the combination [ 9 ]. Another preclinical study showed that the combination of anetumab ravtansine (a mesothelin-targeting ADC) with bevacizumab improved the anti-tumor activity in ST081 and OVCAR-3 human ovarian cancer models [ 8 ]. Two studies that investigated the safety and efficacy of mirvetuximab soravtansine in combination with bevacizumab in treating advanced ovarian cancer showed that co-treatment with mirvetuximab soravtansine (6 mg/kg adjusted ideal body weight) and bevacizumab (15 mg/kg), administered intravenously once every three weeks, was well tolerated and presented encouraging efficacy in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancers [ 11 , 91 ]. However, there is not just one opinion when it comes to ADC combined with anti-angiogenesis therapy [ 92 , 93 , 94 , 95 ]. Arjaans et al. reported that the normalization of tumor blood vessels triggered by bevacizumab hampers antibody uptake [ 92 ]. The timeframe spanning from normalization to excessive pruning is dependent on both the dose of the anti-angiogenic agent and the duration following administration, which has proven challenging [ 93 ]. Importantly, grade 1–2 pneumonitis was detected in six patients (9%) when bevacizumab was introduced alongside mirvetuximab soravtansine, whereas no instances of pneumonitis were observed with the single use of mirvetuximab soravtansine. Therefore, there is an urgent need to rigorously design preclinical and clinical trials to explore the underlying mechanisms of this combination therapy-induced pneumonitis. Co-treatment with ADCs and bevacizumab in a non-clinical trial setting must be performed with caution because of a possible reduction in tumoral accumulation of ADCs that may be caused by bevacizumab.
The BSB is a biological barrier in tumor vasculature regions, which constrains the efficacy of high-affinity antibodies because of the successful binding of antibodies to cellular antigens at the point of extravasation, resulting in antibody sequestration and suboptimal tumor exposure [ 96 ]. Many factors, including elevated antigen expression and rapid antigen internalization combined with sluggish tumor uptake and slow interstitial diffusion of therapeutic antibodies, result in poor antibody penetration into the tumor. Transient competitive inhibition, which improves antibody distribution in solid tumors, is one strategy for overcoming BSB. The combined utilization of T-DM1 and pertuzumab showed synergistic activity in cell culture models and had an acceptable safety profile in phase Ib and II studies [ 97 , 98 ]. Bordeau et al. [ 99 ] found that the co-administration of an anti-trastuzumab single domain antibody (1HE) with trastuzumab significantly increased both the penetration of trastuzumab from the vasculature and the percentage of tumor area that stained positive for trastuzumab. 1HE co-administered with a single dose of T-DM1 to NCI-N87 xenograft-bearing mice significantly enhanced T-DM1 efficacy and increased the median survival. However, results from multiple phase III clinical trials (MARIANNE, KRISTINE, and KAITLIN) have shown that the T-DM1 combined with pertuzumab (T-DM1 + P) regimen reduced grade ≥ 3 adverse events and ensured a better quality of life and the this regimen resulted in a higher chance of event-free survival and invasive disease-free survival than regimens of chemotherapy combined with trastuzumab and pertuzumab [ 72 , 100 , 101 , 102 ]. However, Although monoclonal antibody combined with ADCs could overcome BSB, improve the distribution and anti-tumor efficacy of ADCs in tumor cells, and reduce toxicity, ADC is still not a replacement for standard chemotherapy.
In summary, the dose and time window of ADCs combined with anti-angiogenesis therapy should be further explored in future studies. The establishment of a reasonable model is crucial. Current data show that naked antibodies combined with ADC can overcome BSB to increase tumor penetration and anti-tumor tumor effects; however, they are still unable to replace traditional chemotherapy in clinical settings.
Upregulation of surface antigens and overcoming intratumor heterogeneity and drug resistance
Intratumor heterogeneity is a key factor contributing to therapeutic failure. Furthermore, the appearance of compensatory pathways in tumor therapy is one of the mechanisms of drug resistance, which is frequently accompanied by the downregulation of surface antigens [ 103 ]. Tumor heterogeneity and drug resistance are major challenges in cancer treatment and research. The different sites of action of monoclonal antibodies and TKI make combination therapy a potential strategy for overcoming these difficulties. The addition of a TKI to a combinational target blockade may provide greater selectivity, with a potentially improved therapeutic index.
Data on TKI that can overcome ADC resistance are scarce. Recently, a preclinical study on T-DM1 resistance was reported. PLK1, a key cell cycle regulator, was upregulated in both acquired and primary T-DM1 resistance models. And inhibition of PLK1 using volasertib led to T-DM1 re-sensitization both in vitro and in vivo [ 104 ]. ADCs may also be effective companions for modulating resistance mechanisms of targeted drugs [ 105 , 106 , 107 ]. Patients with NSCLC frequently develop acquired drug resistance to EGFR TKIs [ 108 ]. HER3 is a unique pseudokinase member of the ERBB family. It dimerizes with other ERBB family members (EGFR and HER2) and is frequently overexpressed in EGFR-mutant NSCLC [ 109 ]. Haikala et al. reported that EGFR inhibition by osimertinib leads to increased HER3 membrane expression and promotes HER3-DXd ADC internalization and efficacy, supporting the clinical development of an EGFR inhibitor/HER3-DXd combination in EGFR-mutant lung cancer preclinically [ 106 ]. Another example is the co-administration of the EGFR-TKI osimertinib and T-DM1, which contributed to an synergistic anti-tumor effect, where T-DM1 was able to delay or overcome osimertinib resistance in EGFR-mutant NSCLC models [ 105 ]. In melanomas, AXL-high cells are resistant to MAPK pathway inhibitors, whereas AXL-low cells are sensitive to them. Heterogeneous tumors show partial therapeutic responses, allowing for the emergence of drug-resistant clones that often express high levels of the receptor tyrosine kinase AXL [ 110 ]. Boshuizen et al. [ 111 ] found that AXL-107-MMAE and MAPK pathway inhibitors cooperatively inhibited tumor growth by eliminating distinct populations in heterogeneous melanoma cell pools in a preclinical study. Furthermore, the BRAF/MEK inhibitors potentiated the efficacy of AXL-107-MMAE by inducing AXL transcription [ 111 ]. In acute myeloid leukemia, a preclinical study found a promising and potent anti-leukemic strategy involving the co-administration of midostaurin (a TKI that inhibits the FLT3 pathway) and a novel FLT3-targeting ADC [ 112 ]. The mechanisms behind TKI inducing drug resistance and regulating surface antibody expression are complicated and vary with different drugs. HER2-targeting TKIs (lapatinib, neratinib, tucatinib, and poziotinib) have been shown to increase the efficacy of T-DM1. However, while lapatinib enhances HER2 abundance via robust transcriptional upregulation and reduced ubiquitination, neratinib downregulates surface HER2 abundance by stimulating internalization and endocytosis. The effectiveness of tucatinib on cell surface HER2 is still intricate, and poziotinib upregulates the exon 20 mutant, but not wild-type HER2, suggesting synergistic mechanisms independent of HER2 surface density [ 21 , 113 , 114 , 115 , 116 , 117 , 118 , 119 ].
Several clinical trials have explored the use of TKI in combination with ADCs. The TEAL study showed that employing a combination of T-DM1, lapatinib, and nab-paclitaxel for the neoadjuvant treatment of patients with HER2-positive breast cancer yielded improved responses compared to the standard paclitaxel, trastuzumab, and pertuzumab combination, which was accentuated in the traditionally challenging hormone receptor-positive subset [ 21 ]. The combination regimen of T-DM1/T-DXd and tucatinib for advanced breast cancer progression with prior taxane and trastuzumab showed acceptable toxicity and preliminary anti-tumor activity in patients with ERBB2/HER2-positive metastatic breast cancer with and without brain metastases [ 74 , 79 , 120 ]. Moreover, phase III trials testing T-DM1 or T-DXd with tucatinib (HER2CLIMB-02 and HER2CLIMB-04) are ongoing [ 79 , 120 ], and we look forward to their deterministic results.
Synthetic lethality and combined targeting
Synthetic lethality is a promising and clinically effective therapeutic strategy for tumors with defects in DNA homologous recombination repair pathways. Given the recent focus on DNA damage response (DDR) pathways in cancer therapy, several DDR proteins, including ATR, ATM, DNA-PK, CHK1, CHK2, Wee1, and PARP, have been extensively explored as promising synthetic lethality targets for anticancer drug development [ 121 , 122 , 123 ]. While PARP inhibitors first achieved clinical approval in 2014, inhibitors targeting other DDR proteins are currently under intense clinical investigation.
A subset of ADCs incorporates topoisomerase I (TOPO 1) inhibitors as payloads. For instance, TOPO 1, an enzyme, initiates the cleavage of one strand of double-stranded DNA, resulting in partial unwinding and subsequent reannealing of the strand to relieve tension. Camptothecin and its derivatives bind to the TOPO 1/DNA complex and prevent proper reannealing. This disruption can lead to cell death owing to the accumulation of partially cleaved DNA. SN38, a semi-synthetic derivative of camptothecin, is the active component of irinotecan and has been used in sacituzumab govitecan, a clinically approved TROP2-targeting ADC [ 124 ]. Another camptothecin derivative, DXd, is a derivative of exatecan that is approximately 10 times more potent than SN38, with an IC50 value of 310 nM. This potent compound is used as a payload in HER2-targeting (DS-8201a) and TROP2-targeting (DS1062) ADCs.
PARP-1, the most abundant member of the PARP protein family, has been observed to co-localizes with Topo I throughout the cell cycle. However, when DNA damage occurs, PARP-1 dissociates from Topo I, leading to decreased enzymatic activity [ 125 ]. Combining a TOPO I inhibitor with a PARP inhibitor (PARPi) results in the accumulation of double-stranded DNA breaks (DSBs) by retarding the homologous recombination repair pathways that effectively and precisely repair DNA damage. This DSB accumulation ultimately triggers apoptosis and cell death. In addition, in cells lacking functional BRCA1/2 genes or those deficient in the homologous recombination repair mechanism, an alternative but less precise DNA damage repair pathway known as non-homologous end-joining emerges. This more error-prone pathway further compromises cells toward irreparable DNA damage and apoptosis [ 126 ]. Recent research has also shown that combining CPT-11 (the prodrug of SN-38) and PARPi achieved synergistic inhibition in both BRCA1 wild-type and BRCA1 mutant triple-negative breast cancer (TNBC) cell lines in vitro [ 127 ]. Concurrently, sacituzumab govitecan combined with olaparib, rucaparib, or talazoparib also synergistically inhibited tumor cell growth and increased DSBs in HCC1806 TNBC tumors harboring mutations in the BRCA1/2 genes, as well as in those with wild-type counterparts [ 128 ].
Safety profile of ADCs combined with targeted therapies
Among the 15 types of ADC currently approved, the combination of T-DM1 and targeted agents has the greatest evidence of efficacy and safety. A phase Ib trial evaluated the combination of T-DM1 and the HER2 TKI, tucatinib. Although this combination is well-tolerated, it is associated with frequent gastrointestinal and hepatic toxicities. In particular, 37% of the patients experienced at least one clinically significant adverse event, and 56% required discontinuation of tucatinib [ 74 ]. More information is expected from the ongoing phase III HER2CLIMB-02 trial evaluating T-DM1 with tucatinib. Compared with T-DM1 alone, these results may provide more insight into the additional toxicity associated with this combination. Another Ib study evaluated the combination of intermittent inhibition of T-DM1 and CDK4/6 using riboflavin; however, no DLTs were observed. However, ribociclib dose reductions were required in 58% of the patients due to thrombocytopenia or neutropenia, and one patient experienced grade 2 QTcF prolongation [ 129 ]. In a phase Ib study involving patients with metastatic TNBC, sacituzumab govitecan was administered in combination with talazoparib, a PARP inhibitor. This study demonstrated several DLTs primarily caused by severe myelosuppression, as described in the initial study results. In particular, the majority of enrolled patients experienced febrile neutropenia [ 83 ]. Finally, a phase Ib study evaluated the effects of adding bevacizumab to mirvetuximab soravtansine. This study included patients with platinum-resistant ovarian cancer [ 11 ]. The combination resulted in a toxicity profile similar to that of ADC alone [ 130 ]. However, it is worth noting that grade 1–2 pneumonitis was observed in six patients (9%) with the addition of bevacizumab. This was in contrast to the absence of pneumonitis when mirvetuximab soravtansine was administered alone.
In summary, although we observed promising results from combining TKIs and ADCs in preclinical models and clinical trials, the underlying molecular interplay is still far from being completely understood. A better mechanistic understanding is helpful for the selection of drug combinations and the management of potential side effects.
ADCs combined with immunotherapy
Accumulating evidence suggests that ADCs are sensitive to the effectiveness of immunotherapeutic agents [ 131 ]. Combining immunotherapy with ADCs is a current trend in clinical practice, with a number of preclinical studies and initial findings from early-stage clinical trials showing improved anti-tumor effects [ 131 ]. The clinical trials are listed in Table 6 . We still await the outcomes of large-cohort randomized phase III clinical trials to demonstrate the determinant evidence of this combination’s efficacy compared with that of conventional treatments.
Mechanism of ADCs combined with immunotherapy
The underlying mechanisms are diverse and encompass Fc-mediated effector functions, initiation of immunogenic cell death (ICD), maturation of dendritic cells (DCs), enhancement of T cell infiltration, reinforcement of immunological memory, and expression of immunomodulatory proteins such as programmed death ligand 1 (PD-L1) and major histocompatibility complex (MHC) [ 132 , 133 , 134 , 135 , 136 ]. Multiple studies have revealed that ADCs exert a stronger effect in immunocompetent animal models than in immunodeficient models, indicating their significant immunomodulatory capabilities [ 137 , 138 ]. These findings provide a basis for devising clinical trials that incorporate low doses of ADCs as immunostimulants to improve the efficacy of immunotherapy without causing adverse effects.
Fc-mediated effector functions
In the design of an ADC, the antibody component plays a multifaceted role, instead of solely delivering cytotoxic agents to cancer cells. Its unique Y-shaped structure has many additional functions, including regulation of innate immune responses. While the antigen-binding fragments of an antibody are responsible for recognizing the target antigen and determining its specificity, the crystallizable fragment (Fc) interacts with immune cells and regulates the duration of the antibody’s circulation in the bloodstream. The Fc region, in fact, plays key roles in several vital functions, including antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cell-mediated phagocytosis, and complement-dependent cytotoxicity. The effectiveness of the initial two actions relies on Fcγ receptors (FcγRs), which are present on natural killer cells, macrophages, and various other immune cells. Conversely, complement-dependent cytotoxicity is triggered by C1q protein.
The IgG antibody family consists of IgG1, IgG2, IgG3, and IgG4 subclasses, each of which exert different effects on factors such as antibody solubility, half-life, interaction with the C1q protein, and binding strength to FcγRs. The IgG1 subclass of antibodies is most commonly used in the 15 clinically approved types of ADCs [ 166 ]. The reason for selecting IgG1 as the backbone of ADC is because it has a long half-life of approximately 21 days, similar to IgG2 and IgG4, but is characterized by its enhanced ability to activate the complement system and bind to FcγRs. In contrast, IgG2 and IgG4 have limited efficacy in triggering effector functions, and are used strategically in antibody design when eliciting an immune response is not the primary goal [ 167 ]. In contrast, IgG3 is the most immunogenic subclass capable of eliciting an immune response. However, these antibodies are typically bypassed in the design of ADCs because of their short half-lives (approximately seven days).
In preclinical models, both T-DXd and T-DM1 exhibit the ability to maintain functions inherent to unconjugated trastuzumab, including triggering ADCC associated with the IgG1 isotype [ 137 , 168 ]. In addition to these therapeutic benefits, Fc-mediated effector functions give rise to undesirable side effects. For instance, T-DM1 is internalized by megakaryocytes through its interaction with FcγRIIA, which could potentially lead to the development of thrombocytopenia, a known side effect of this agent [ 169 ].
Modulating the ability of an ADC to engage the immune system may involve engineering the Fc region. One approach is to produce afucosylated IgGs, which enhance ADCC by increasing the binding affinity for FcγRIIIa [ 170 , 171 ]. Conversely, the Fc region can be modified by introducing mutations that impact effector functions, yielding what is known as "Fc silent antibodies" [ 138 ]. MEDI4276, for instance, employs this strategy with three mutations in its Fc domain to curtail FcγR binding, aiming to minimize thrombocytopenia as observed with T-DM1 [ 172 ].
Another approach that involves the interaction between Fc and FcγRs takes place in the TME, in which the tumor-associated macrophages (TAMs) constitute a significant proportion. In preclinical models, non-targeted ADCs have been shown to be effectively engulfed by TAMs. Through the engagement of FcγRs, TAMs internalize and process these ADCs, resulting in the release of the cytotoxic payloads within the TME. This results in the killing of neighboring tumor cells which is called “bystander effect”. This mechanism may enhance the efficacy of ADCs against tumors that exhibit heterogeneity or low levels of target antigens [ 173 ].
However, it is important to recognize that this mechanism of antigen-independent ADC uptake into non-malignant cells within the TME could exacerbate the toxicity of ADCs. For instance, it could potentially lead to more rapid clearance of ADCs and reduced overall efficacy. Another theoretical concern related to the release of cytotoxic payload within the TME and the subsequent bystander effect is the potential destruction of local T cells, which can negatively affect the efficacy of immune checkpoint inhibitors (ICIs).
In summary, while the interaction of Fc and FcγRs between ADCs and the TME represents a potential avenue for enhancing ADC activity against tumors with challenging characteristics, careful consideration must be given to balance efficacy with potential drawbacks such as increased toxicity and interference with immune checkpoint inhibition.
Immunogenic cell death
Based on the initial stimulus, cancer cell death can either activate the immune system (immunogenic) or go unnoticed by it (non-immunogenic). ICD is a regulatory process characterized by the induction of stress within the endoplasmic reticulum and cellular structures. This process is accompanied by changes in the cell surface composition and the subsequent release of soluble mediators, which follow a precise spatiotemporal sequence and ultimately lead to cell death [ 174 , 175 ].
The ability of a drug to induce ICD and establish an immunological memory is often predicted by its ability to induce damage-associated molecular patterns (DAMPs) in vitro [ 176 , 177 ]. A wide array of anticancer therapeutics, including traditional chemotherapy, radiotherapy, and targeted anticancer agents, has demonstrated the potential to induce DAMPs [ 178 , 179 , 180 ]. Only a small fraction (< 10%) of all chemotherapeutic agents, such as anthracyclines [ 181 , 182 ] and oxaliplatin [ 183 ], are classified as ICD-inducing drugs. The majority of cytotoxic payloads employed in ADCs exhibit the ability to activate immune cells both in the laboratory and in living organisms, which not only improves their anti-tumor tumor efficacy, but also synergistically enhances the effect of ICIs in preclinical models [ 184 ].
In mouse models, ADCs with payloads such as maytansine, pyrrolobenzodiazepine, and tubulysin have shown the ability to induce ICD [ 135 ] trigger immune modulation, and establish immune memory. These ADCs not only exhibit potent cytotoxicity but also synergize with various ICIs. Notably, these ADCs exhibited significantly greater anti-tumor activity in immunocompetent mice than in immunocompromised mice, highlighting the role of the immune system in their efficacy. Similarly, a newly developed anti-HER2 ADC containing a potent anthracycline derivative payload (T-PNU) increased DAMP expression and enhanced efficacy when combined with an anti-PD-1 drug in a breast cancer model that developed resistance to other HER2-targeted therapies. Notably, the efficacy of T-PNU was significantly reduced when CD8 + T cells were depleted, confirming the critical role of the adaptive immune system in regulating the anticancer activity of T-PNU. In addition, T-PNU appeared to promote the formation of an immunological memory in tumor-bearing animals, resulting in protection against tumor rechallenge [ 132 ]. Such an ICD-induced intrinsic inflammatory response has also been observed with brentuximab vedotin [ 185 ], ladiratuzumab vedotin [ 186 ], and enapotamab vedotin [ 136 ], which are three ADCs that share the same MMAE payload. This unique property further enhances the efficacy of ICIs.
Direct activation and maturation of dendritic cells
Mature DCs play a pivotal role in tumor immunity by acting as antigen-presenting cells capable of activating anti-tumor T cell responses through the MHC class II complex [ 187 ]. However, cancer cells often develop immunosuppression by inhibiting DC maturation or causing them to become dysfunctional, ultimately resulting in immune evasion [ 188 ]. Overcoming these barriers is essential for improving immunotherapy outcomes in clinical practice.
Previous research has shown that compounds that destabilize microtubules are capable of inducing the phenotypic and functional maturation of DCs, which was not observed with microtubule-stabilizing compounds such as taxanes [ 189 ]. This phenomenon appears to be a common feature of this class of compounds, indicating their potential use as "immunostimulatory" agents. This immunostimulatory effect was first reported in vinblastine [ 190 ] and subsequently in many other microtubule-disrupting agents, such as maytansinoids (e.g., ansamitocin P3 and its synthetic derivative DM1) and dolastatins (from which auristatins are derived), which are frequently used as payloads in ADCs [ 133 , 191 ]. In preclinical models, these payloads have been demonstrated to directly trigger DC activation and maturation. Importantly, these potent immunoregulatory effects were observed even without cancer cell death, indicating an independent binary mode of action. The complete therapeutic efficacy of these ADCs includes payload cytotoxicity and immunoregulatory functions; the latter strongly depends on an intact host immune system, which is significantly diminished in immunocompromised mouse models.
Preclinical studies combining tubule inhibitor-based ADCs with ICIs have confirmed that these two types of treatment modalities can work synergistically to increase therapeutic efficacy rather than have additive effects [ 134 , 191 ]. In cases where tumors responded completely to the combination treatment, mice showed protection upon rechallenge with the same tumor, indicating the successful establishment of immunological memory. Notably, an analysis of paired samples from 28 patients with breast cancer who underwent short-term preoperative treatment with T-DM1 as part of the WSG-ADAPT protocol sub-trial revealed significant increases in the number and density of tumor-infiltrating T cells [ 168 ]. There is evidence that topoisomerase I inhibitors can also act as immunomodulators by activating DCs [ 192 ], as exemplified by T-DXd, an HER2-targeted ADC that carries the exatecan derivative DXd, a topoisomerase I inhibitor, as payload. T-DXd has been found to significantly increase the presence of tumor-infiltrating DCs and the expression of markers indicative of maturation and activation, leading to an increase in tumor-infiltrating CD8 + T cells, along with increased expression of PD-L1 and MHC class I on tumor cells. Notably, in a CT26-HER2 tumor model, the combination of T-DXd with an anti-PD-1 agent proved to be more effective than either treatment alone, possibly because of the immunomodulatory changes induced by T-DXd [ 134 ]. It is worth noting that the T-DXd payload possesses a tenfold more potent topoisomerase I inhibitor activity compared to SN-38, which may contribute to a more robust immunologic effect compared to other agents in the same class [ 168 ].
Combining ADCs and ICIs
Currently, HER2-targeted ADCs are under intense clinical investigation for their synergistic effects in combination with ICIs; however, many trials are still ongoing, and determinant evidence is very limited [ 137 , 193 , 194 ]. The only published randomized trial evaluating the combination of an ADC and ICI is the KATE2 trial. This study evaluated the efficacy of T-DM1 plus atezolizumab and compared it with T-DM1 plus placebo. The study was conducted in patients who had previously been treated for HER2-positive breast cancer and, disappointingly, the combination therapy did not result in a statistically significant improvement in PFS in the overall patient population, with a median PFS of 8.2 months in the combination arm and 6.2 months in the control arm ( P = 0.33). However, a trend suggesting potential benefit in a specific subset of patients with positive PD-L1 expression was observed, where median PFS was 8.5 months for the combination arm and 4.1 months for the control arm ( P = 0.099). This indicated that adding an ICI to HER2-targeted treatment for HER2 + breast cancer may be particularly beneficial in the PD-L1-positive subset [ 77 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 ]. Most cancer patients enrolled in published studies had not previously received treatment with an ICI. Thus, we are uncertain about the synergistic benefits that ADC may provide when combined with ICIs in tumor types that are known to respond well to immunotherapy. However, currently available clinical data suggest significantly improved response rates, as shown by a comparison with the historical efficacy results achieved with standalone immunotherapy in these specific tumor types [ 132 , 133 , 135 , 136 , 184 , 185 , 186 , 187 , 188 , 189 , 190 ].
After witnessing remarkable improvements in efficacy in specific cancer contexts, certain combinations will likely be adopted as new standards of care, potentially replacing traditional cytotoxic treatment approaches. To illustrate this theory, the combination of enfortumab vedotin, an ADC targeting nectin 4, and pembrolizumab has been evaluated as a first-line treatment option for individuals diagnosed with locally advanced or metastatic urothelial cancer (as demonstrated in the EV-103/KEYNOTE-869 study; ClinicalTrials.gov identifier: NCT03288545) [ 184 ]. In this patient population, the combination achieved an impressive objective response rate of 73% and extended the PFS to 12.3 months. This result led to a breakthrough designation granted by the US FDA, specifically for patients ineligible for cisplatin-based therapy. Moreover, other cancers, including cervical cancer, triple-negative breast cancer, Hodgkin's lymphoma, and primary mediastinal large B cell lymphoma, have shown encouraging results when treated with a combination of anti-PD-1 antibodies and ADCs targeting specific markers, such as tissue factor, TROP2, and CD30 [ 205 ]. This novel strategy of combination therapy holds great promise, particularly for older and frail patients who are at an increased risk of experiencing severe side effects from traditional chemotherapy regimens [ 187 , 188 , 190 , 206 , 207 , 208 ]. In the coming months and years, we expect to see more trial results that employ various immunotherapeutic agents to enhance ADC activity.
Safety profile of ADCs combined with immunotherapy
In the phase III KATE2 trial, a significant increase in adverse events, including one treatment-related death, was observed in the combination arm that combined atezolizumab and T-DM1 in patients with previously treated HER2-positive metastatic breast cancer. The frequencies of clinically significant adverse events (33% vs. 19%) and most adverse reactions, particularly fever (35% vs. 16%, including several hospitalizations), increased after the introduction of atezolizumab [ 140 ]. Similarly, randomized data are now available for enfortumab vedotin, with and without pembrolizumab, in 149 patients with advanced-stage urothelial carcinoma. The introduction of pembrolizumab resulted in a higher occurrence of clinically significant (23.7% vs. 15.1%) and fatal (3.9% vs. 2.7%) adverse events, and an overall increase in the incidence of all adverse events. Notably, an increase in serious skin reactions was observed [ 209 ]. Nevertheless, the combination received accelerated approval from the FDA in April 2023 for patients with locally advanced or metastatic urothelial carcinoma, particularly those who could not receive cisplatin-based chemotherapy. This approval was based on the efficacy of the combination. However, the lack of a randomized design in most other studies on ADC and ICI combination therapies makes it difficult to reach definitive conclusions regarding the many unanswered questions. To date, there have been no alarming signs of increased toxicity induced by ICIs in combination with T-DXd [ 77 , 210 , 211 ], Dato-DXd [ 204 ], or sacituzumab-govitecan [ 212 ], all of which have additive toxicity profiles. These safety profiles include similar rates and intensities of ILD, which frequently occurs with DXd-based ADC treatments. Interestingly, the addition of ICIs does not appear to increase the incidence of ILD associated with ADCs [ 141 , 175 , 177 , 213 ]. Ongoing randomized phase III trials (NCT05629585, NCT05382286, and NCT05633654) are expected to offer additional insights into this domain. These trials may further clarify the toxicity patterns of treatment approaches that combine ICIs with ADCs beyond T-DM1.
Conclusions
Monotherapy with ADCs has exhibited transformative anti-tumor efficacy across a broad spectrum of solid and hematological malignancies. The present landscape is characterized by substantial efforts from both the academic and industrial sectors focused on advancing the understanding of ADC combination therapy, which entails the progress of next-generation ADCs by identifying novel tumor targets and clarifying their pharmacological properties.
Notably, the combination of ADCs with chemotherapy or chemoimmunotherapy regimens, excluding ICIs, has yielded demonstrable survival advantages over the established standard regimens in randomized investigations of hematological malignancies. Although the combination of ADCs with ICIs has exhibited encouraging outcomes, as exemplified by the FDA breakthrough designations of enfortumab vedotin and pembrolizumab for cisplatin-ineligible urothelial cancer, the envisioned survival improvements and underlying biological mechanisms for solid tumors remain elusive within the context of randomized controlled trials.
Furthermore, ADC combinations with targeted agents, particularly inhibitors targeting the HER2 and DDR pathways, hold substantial promise, although their potential is contingent upon validation through more mature datasets. The constrained success witnessed thus far in combination therapy with first-generation ADCs (e.g. T-DM1) can be attributed to many factors that encompass the indiscriminate expression of target molecules, resulting in off-tumor side effects on normal tissues, overlapping toxicities, limited efficacy, and unclear procedures conferring resistance. The landscape of ADC-based combination therapies remains dynamic, with current challenges underscoring the complexities of target expression, tumor heterogeneity, and the intricate interplay of therapeutic modalities.
In addition to understanding the pharmacological properties (e.g. DAR and bystander effect) to improve ADC efficacy, there is also a strong need to stratify patients with high response rates and detect relevant predictive biomarker profiles. Exploring preclinical experiments in carefully characterized patient-derived xenograft models and conducting clinical trials in window-of-opportunity contexts could facilitate the identification of promising ADC-based combinations in clinical practice. More strategic methodologies are required to effectively identify suitable ADC-based combination approaches for selected patient cohorts and tumor types. This will not only capitalize on the refinement of ADC design and properties, but also leverage well-informed patient selection strategies to optimize therapeutic outcomes.
Availability of data and materials
Not applicable.
DeVita VT, Rosenberg SA. Two hundred years of cancer research. N Engl J Med. 2012;366:2207–14.
Article CAS PubMed PubMed Central Google Scholar
Criscitiello C, Morganti S, Curigliano G. Antibody-drug conjugates in solid tumors: a look into novel targets. J Hematol Oncol. 2021;14.
Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021;18:327–44.
Article PubMed PubMed Central Google Scholar
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91.
Pomeroy AE, Schmidt EV, Sorger PK, Palmer AC. Drug independence and the curability of cancer by combination chemotherapy. Trends Cancer. 2022;8:915–29.
Yardley DA. Drug resistance and the role of combination chemotherapy in improving patient outcomes. Int J Breast Cancer. 2013;2013:1–15.
Article Google Scholar
Abrahams C, Krimm S, Li X, Zhou S, Hanson J, Masikat MR, et al. Abstract NT-090: Preclinical activity and safety of STRO-002, a novel ADC targeting folate receptor alpha for ovarian and endometrial cancer. Clinical Cancer Res. 2019;25:NT-090. https://doi.org/10.1158/1557-3265.OVCASYMP18-NT-090
Quanz M, Hagemann UB, Zitzmann-Kolbe S, Stelte-Ludwig B, Golfier S, Elbi C, et al. Anetumab ravtansine inhibits tumor growth and shows additive effect in combination with targeted agents and chemotherapy in mesothelin-expressing human ovarian cancer models. Oncotarget. 2018;9:34103–21.
Ponte JF, Ab O, Lanieri L, Lee J, Coccia J, Bartle LM, et al. Mirvetuximab Soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, potentiates the activity of standard of care therapeutics in ovarian cancer models. Neoplasia. 2016;18:775–84.
Moore KN, O’Malley DM, Vergote I, Martin LP, Gonzalez-Martin A, Malek K, et al. Safety and activity findings from a phase 1b escalation study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with carboplatin in patients with platinum-sensitive ovarian cancer. Gynecol Oncol. 2018;151:46–52.
Article CAS PubMed Google Scholar
O’Malley DM, Matulonis UA, Birrer MJ, Castro CM, Gilbert L, Vergote I, et al. Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol Oncol. 2020;157:379–85.
Article PubMed Google Scholar
Bulat I, Moore KN, Haceatrean A, Chung JW, Rajagopalan P, Xia C, et al. Abstract 5571: Phase Ib study of anti-mesothelin antibody drug conjugate anetumab ravtansine in combination with pegylated liposomal doxorubicin in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer. 2018;36:5571–5571.
Levy B, Paz-Ares L, Rixe O, Su W-C, Yang T-Y, Tolcher A, et al. MA13.07 TROPION-Lung02: initial results for datopotamab deruxtecan plus pembrolizumab and platinum chemotherapy in advanced NSCLC. J Thoracic Oncol 2022;17:S91.
Janjigian YY, Oh D-Y, Rha SY, Lee KW, Steeghs N, Chao Y, et al. Abstract 295: Dose-escalation and dose-expansion study of trastuzumab deruxtecan (T-DXd) monotherapy and combinations in patients (pts) with advanced/metastatic HER2+ gastric cancer (GC)/gastroesophageal junction adenocarcinoma (GEJA): DESTINY-Gastric03. 2022;40:295–295.
Kan S, Koido S, Okamoto M, Hayashi K, Ito M, Kamata Y, et al. Up-regulation of HER2 by gemcitabine enhances the antitumor effect of combined gemcitabine and trastuzumab emtansine treatment on pancreatic ductal adenocarcinoma cells. BMC Cancer. 2015;15.
Tseng CJ, Wang YJ, Liang YC, Jeng JH, Lee W Sen, Lin JK, et al. Microtubule damaging agents induce apoptosis in HL 60 cells and G2/M cell cycle arrest in HT 29 cells. Toxicology. 2002;175:123–42.
Wahl AF, Donaldson KL, Mixan BJ, Trail PA, Siegall CB. Selective tumor sensitization to taxanes with the mAb-drug conjugate cBR96-doxorubicin. Int J Cancer. 2001;93:590–600.
Martin M, Fumoleau P, Dewar JA, Albanell J, Limentani SA, Campone M, et al. Trastuzumab emtansine (T-DM1) plus docetaxel with or without pertuzumab in patients with HER2-positive locally advanced or metastatic breast cancer: results from a phase Ib/IIa study. Ann Oncol. 2016;27:1249–56.
Cortés J, Diéras V, Lorenzen S, Montemurro F, Riera-Knorrenschild J, Thuss-Patience P, et al. Efficacy and safety of trastuzumab emtansine plus capecitabine vs trastuzumab emtansine alone in patients with previously treated ERBB2 (HER2)-positive metastatic breast cancer: a phase 1 and randomized phase 2 trial. JAMA Oncol. 2020;6:1203–9.
Patel TA, Ensor J, Rodriguez AA, Belcheva A, Darcourt JG, Niravath PA, et al. Phase ib study of trastuzumab emtansine (TDM1) in combination with lapatinib and nab-paclitaxel in metastatic HER2-neu overexpressed breast cancer patients: Stela results. J Clin Oncol. 2018;36:1035–1035.
Patel TA, Ensor JE, Creamer SL, Boone T, Rodriguez AA, Niravath PA, et al. A randomized, controlled phase II trial of neoadjuvant ado-trastuzumab emtansine, lapatinib, and nab-paclitaxel versus trastuzumab, pertuzumab, and paclitaxel in HER2-positive breast cancer (TEAL study). Breast Cancer Res. 2019;21.
López-Miranda E, Pérez-García JM, Di Cosimo S, Brain E, Ravnik M, Escrivá-De-romaní S, et al. Trastuzumab emtansine plus non-pegylated liposomal doxorubicin in HER2-positive metastatic breast cancer (thelma): a single-arm, multicenter, phase Ib trial. Cancers (Basel). 2020;12:1–14.
Zimmer AS, Steinberg SM, Smart DD, Gilbert MR, Armstrong TS, Burton E, et al. Temozolomide in secondary prevention of HER2-positive breast cancer brain metastases. Future Oncol. 2020;16:899–909.
Planchard D, Brahmer JR, Yang JC, Chen Y-M, Lee K-Y, Suksombooncharoen T, et al. Abstract CT572: Phase 1b dose-escalation and dose-expansion study evaluating trastuzumab deruxtecan (T-DXd) in combination with durvalumab and cisplatin, carboplatin, or pemetrexed in advanced or metastatic, HER2-overexpressing, nonsquamous non-small cell lung cancer (NSCLC): DESTINY-Lung03. Cancer Res. 2022;82:CT572–CT572. https://doi.org/10.1158/1538-7445.AM2022-CT572
O’Donnell PH, Milowsky MI, Petrylak DP, Hoimes CJ, Flaig TW, Mar N, et al. Enfortumab vedotin with or without pembrolizumab in cisplatin-ineligible patients with previously untreated locally advanced or metastatic urothelial cancer. J Clin Oncol. 2023.
Le X, Carneiro BA, Hong DS, Birnbaum AE, Taylor MH, Patel SA, et al. innovaTV 207: New combination dosing cohorts in the open label phase 2 study of tisotumab vedotin in solid tumors. 2022;40:TPS6100–TPS6100.
Lassman AB, Van Den Bent MJ, Gan HK, Reardon DA, Kumthekar P, Butowski N, et al. Safety and efficacy of depatuxizumab mafodotin + temozolomide in patients with EGFR-amplified, recurrent glioblastoma: results from an international phase I multicenter trial. Neuro Oncol. 2019;21:106–14.
O’Malley DM, Oaknin A, Matulonis UA, Mantia-Smaldone G, Lim PC, Castro CM, et al. Abstract 5504: Mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients (pts) with platinum-agnostic ovarian cancer: final analysis. 2021;19:8–10.
Risbridger GP, Davis ID, Birrell SN, Tilley WD. Breast and prostate cancer: more similar than different. Nat Rev Cancer. 2010;10:205–12.
Mamounas EP, Untch M, Mano MS, Huang CS, Geyer CE, von Minckwitz G, et al. Adjuvant T-DM1 versus trastuzumab in patients with residual invasive disease after neoadjuvant therapy for HER2-positive breast cancer: subgroup analyses from KATHERINE. Ann Oncol. 2021;32:1005–14.
Hurvitz SA, Wang LS, Chan D, Phan V, Lomis T, McAndrew NP, et al. TRIO-US B-12 TALENT: Phase II neoadjuvant trial evaluating trastuzumab deruxtecan with or without anastrozole for HER2-low, HR+ early-stage breast cancer. 2022;40:TPS623–TPS623.
Mattes MJ. Radionuclide-antibody conjugates for single-cell cytotoxicity. Cancer. John Wiley and Sons Inc.; 2002. p. 1215–23.
Salvestrini V, Kim K, Caini S, Alkner S, Ekholm M, Skyttä T, et al. Safety profile of trastuzumab-emtansine (T-DM1) with concurrent radiation therapy: a systematic review and meta-analysis. Radiother Oncol. 2023;186: 109805.
Krop IE, Suter TM, Dang CT, Dirix L, Romieu G, Zamagni C, et al. Feasibility and cardiac safety of trastuzumab emtansine after anthracycline-based chemotherapy as (neo)adjuvant therapy for human epidermal growth factor receptor 2-positive early-stage breast cancer. J Clin Oncol. 2015;33:1136–42.
von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–28. https://doi.org/10.1056/NEJMoa1814017 .
Carlson JA, Nooruddin Z, Rusthoven C, Elias A, Borges VF, Diamond JR, et al. Trastuzumab emtansine and stereotactic radiosurgery: an unexpected increase in clinically significant brain edema. Neuro Oncol. 2014;16:1006–9. https://doi.org/10.1093/neuonc/not329 .
Lassman AB, Pugh SL, Wang TJC, Aldape K, Gan HK, Preusser M, et al. Depatuxizumab-mafodotin in EGFR-amplified newly diagnosed glioblastoma: a phase III randomized clinical trial. Neuro Oncol. 2022;
Narita Y, Muragaki Y, Kagawa N, Asai K, Nagane M, Matsuda M, et al. Safety and efficacy of depatuxizumab mafodotin in Japanese patients with malignant glioma: a nonrandomized, phase 1/2 trial. Cancer Sci. 2021;112:5020–33.
Géraud A, Xu HP, Beuzeboc P, Kirova YM. Preliminary results of the concurrent use of radiotherapy for bone metastases and trastuzumab emtansine in patients with HER2-positive metastatic breast cancer. Cancer/Radiothérapie. 2016;20:312–3.
Jacot W, Pons E, Frenel JS, Guiu S, Levy C, Heudel PE, et al. Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res Treat. 2016;157:307–18. https://doi.org/10.1007/s10549-016-3828-6 .
Geraud A, Xu HP, Beuzeboc P, Kirova YM. Preliminary experience of the concurrent use of radiosurgery and T-DM1 for brain metastases in HER2-positive metastatic breast cancer. J Neurooncol. 2017;131:69–72. https://doi.org/10.1007/s11060-016-2265-z .
Zolcsák Z, Loirat D, Fourquet A, Kirova YM. Adjuvant trastuzumab emtansine (T-DM1) and concurrent radiotherapy for residual invasive HER2-positive breast cancer: single-center preliminary results. Am J Clin Oncol Cancer Clin Trials. 2020;43:895–901.
Google Scholar
Mills MN, Walker C, Thawani C, Naz A, Figura NB, Kushchayev S, et al. Trastuzumab emtansine (T-DM1) and stereotactic radiation in the management of HER2+ breast cancer brain metastases. BMC Cancer. 2021;21:1–8. https://doi.org/10.1186/s12885-021-07971-w .
Article CAS Google Scholar
Bennardo L, Passante M, Cameli N, Cristaudo A, Patruno C, Nisticò SP, et al. Skin manifestations after ionizing radiation exposure: a systematic review. Bioengineering 2021;8:153.
Hariri G, Zhang Y, Fu A, Han Z, Brechbiel M, Tantawy MN, et al. Radiation-guided P-selectin antibody targeted to lung cancer. Ann Biomed Eng. 2008;36:821–30. https://doi.org/10.1007/s10439-008-9444-9 .
Shamay Y, Elkabets M, Li H, Shah J, Brook S, Wang F, et al. P-selectin is a nanotherapeutic delivery target in the tumor microenvironment. Sci Transl Med. 2016. https://doi.org/10.1126/scitranslmed.aaf7374 .
Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68:498–505. https://doi.org/10.1158/0008-5472.CAN-07-2950 .
Lin TY, Chang JTC, Wang HM, Chan SH, Chiu CC, Lin CY, et al. Proteomics of the radioresistant phenotype in head-and-neck cancer: GP96 as a novel prediction marker and sensitizing target for radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:246–56.
Dadey DYA, Kapoor V, Hoye K, Khudanyan A, Collins A, Thotala D, et al. Antibody targeting GRP78 enhances the efficacy of radiation therapy in human glioblastoma and non-small cell lung cancer cell lines and tumor models. Clin Cancer Res. 2017;23:2556–64.
Leo R, Emmanuelle M, Valentina D, Eline M, Karin V, Constantin L, et al. A GRP78-Directed monoclonal antibody recaptures response in refractory multiple myeloma with extramedullary involvement. Clin Cancer Res. 2016;22:4341–9. https://doi.org/10.1158/1078-0432.CCR-15-3111 .
Wang H, Yan H, Fu A, Han M, Hallahan D, Han Z. TIP-1 Translocation onto the cell plasma membrane is a molecular biomarker of tumor response to ionizing radiation. PLoS ONE. 2010;5:e12051. https://doi.org/10.1371/journal.pone.0012051 .
Lewis CD, Singh AK, Hsu FF, Thotala D, Hallahan DE, Kapoor V. Targeting a radiosensitizing antibody-drug conjugate to a radiation-inducible antigen. Clin Cancer Res. 2021;27:3224–33.
Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:928–42.
Buckel L, Savariar EN, Crisp JL, Jones KA, Hicks AM, Scanderbeg DJ, et al. Tumor radiosensitization by monomethyl auristatin E: mechanism of action and targeted delivery. Cancer Res. 2015;75:1376–87.
Hingorani DV, Doan MK, Camargo MF, Aguilera J, Song SM, Pizzo D, et al. Precision chemoradiotherapy for HER2 tumors using antibody conjugates of an auristatin derivative with reduced cell permeability. Mol Cancer Ther. 2020;19:157–67.
Norbury CJ, Hickson ID. Cellular responses to DNA damage. Annu Rev Pharmacol Toxicol. 2001;41:367–401. https://doi.org/10.1146/annurev.pharmtox.41.1.367 .
Bourillon L, Bourgier C, Gaborit N, Garambois V, Llès E, Zampieri A, et al. An auristatin-based antibody-drug conjugate targeting HER3 enhances the radiation response in pancreatic cancer. Int J Cancer. 2019;145:1838–51. https://doi.org/10.1002/ijc.32273 .
Adams SR, Yang HC, Savariar EN, Aguilera J, Crisp JL, Jones KA, et al. Anti-tubulin drugs conjugated to anti-ErbB antibodies selectively radiosensitize. Nature Communications 2016 7:1. 2016;7:1–11.
Stacy DR, Lu B, Hallahan DE. Radiation-guided drug delivery systems. Expert Rev Anticancer Ther. 2004;4:283–8.
Hallahan DE, Geng L, Cmelak AJ, Chakravarthy AB, Martin W, Scarfone C, et al. Targeting drug delivery to radiation-induced neoantigens in tumor microvasculature. J Control Release. 2001;74:183–91.
Stapleton S, Jaffray D, Milosevic M. Radiation effects on the tumor microenvironment: implications for nanomedicine delivery. Adv Drug Deliv Rev. 2017;109:119–30.
Stolarz AJ, Chhetri BP, Borrelli MJ, Jenkins S V., Jamshidi-Parsian A, Phillips JH, et al. Liposome formulation for tumor-targeted drug delivery using radiation therapy. Int J Mol Sci. 2022;23.
Geng L, Osusky K, Konjeti S, Fu A, Hallahan D. Radiation-guided drug delivery to tumor blood vessels results in improved tumor growth delay. J Control Release. 2004;99:369–81.
Kalofonos H, Rowlinson G, Epenetos2 AA. Enhancement of monoclonal antibody uptake in human colon tumor xenografts following irradiation. Cancer Res. 1990;50:159–63.
Nakata H, Yoshimine T, Murasawa A, Kumura E, Harada K, Ushio Y, et al. Early blood-brain barrier disruption after high-dose single-fraction irradiation in rats. Acta Neurochir (Wien). 1995;136:82–7.
Debbi K, Grellier N, Loganadane G, Boukhobza C, Mahé M, Cherif MA, et al. Interaction between radiation therapy and targeted therapies in HER2-positive breast cancer: literature review, levels of evidence for safety and recommendations for optimal treatment sequence. Cancers 2023;15:2278.
Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17:4834–43. https://doi.org/10.1158/1078-0432.CCR-10-2962 .
Stumpf PK, Cittelly DiM, Robin TP, Carlson JA, Stuhr KA, Contreras-Zarate MJ, et al. Combination of trastuzumab emtansine and stereotactic radiosurgery results in high rates of clinically significant radionecrosis and dysregulation of aquaporin-4. Clinical Cancer Res. 2019;25:3946–53. https://doi.org/10.1158/1078-0432.CCR-18-2851
Id Said B, Chen H, Jerzak KJ, Warner E, Myrehaug S, Tseng CL, et al. Trastuzumab emtansine increases the risk of stereotactic radiosurgery-induced radionecrosis in HER2 + breast cancer. J Neurooncol. 2022;159:177–83. https://doi.org/10.1007/s11060-022-04055-y .
Pérez-García JM, Vaz Batista M, Cortez P, Ruiz-Borrego M, Cejalvo JM, de la Haba-Rodriguez J, et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: the DEBBRAH trial. Neuro Oncol. 2023;25:157–66. https://doi.org/10.1093/neuonc/noac144 .
Marin B, Porath KA, Jain S, Kim M, Conage-pough JE, Oh J, et al. Heterogeneous delivery across the blood-brain barrier limits the efficacy of an EGFR-targeting antibody drug conjugate in glioblastoma. Neuro Oncol. 2021;23:2042–53.
Perez EA, Barrios C, Eiermann W, Toi M, Im YH, Conte P, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol. 2017;35:141–8.
Sartore-Bianchi A, Lonardi S, Martino C, Fenocchio E, Tosi F, Ghezzi S, et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: the phase II HERACLES-B trial. ESMO Open. 2020;5.
Borges VF, Ferrario C, Aucoin N, Falkson C, Khan Q, Krop I, et al. Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a Phase 1b clinical trial. JAMA Oncol. 2018;4:1214–20.
Jain S, Shah AN, Santa-Maria CA, Siziopikou K, Rademaker A, Helenowski I, et al. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res Treat. 2018;171:371–81.
Goel S, Pernas S, Tan-Wasielewski Z, Barry WT, Bardia A, Rees R, et al. Ribociclib plus trastuzumab in advanced HER2-positive breast cancer: results of a phase 1b/2 trial. Clin Breast Cancer. 2019;19:399–404.
Galsky MD, Conte G Del, Foti S, Yu EY, Machiels J-PH, Doger B, et al. Primary analysis from DS8201-A-U105: A phase 1b, two-part, open-label study of trastuzumab deruxtecan (T-DXd) with nivolumab (nivo) in patients (pts) with HER2-expressing urothelial carcinoma (UC). 2022;40:438–438.
Borghaei H, Besse B, Bardia A, Mazieres J, Popat S, Augustine B, et al. Abstract TPS1100: Trastuzumab deruxtecan (T-DXd; DS-8201) in combination with pembrolizumab in patients with advanced/metastatic breast or non-small cell lung cancer (NSCLC): A phase Ib, multicenter, study. 2020;38:TPS1100–TPS1100.
Hurvitz S, Vahdat L, Harbeck N, Wolff AC, Tolaney SM, Loi S, et al. Abstract OT-28–01: HER2CLIMB-02: A randomized, double-blind, phase 3 study of tucatinib or placebo with T-DM1 for unresectable locally-advanced or metastatic HER2+ breast cancer. Cancer Res. 2021;81:OT-28–01. https://doi.org/10.1158/1538-7445.SABCS20-OT-28-01
Zhou L, Xu H, Li S, Yan X, Li J, Wu X, et al. Study RC48-C014: Preliminary results of RC48-ADC combined with toripalimab in patients with locally advanced or metastatic urothelial carcinoma. J Clin Oncol. 2022;40:515–515.
Andre F, Hamilton EP, Loi S, Schmid P, Anders CK, Yu T, et al. Abstract TPS1096: Trastuzumab deruxtecan (T-DXd) combinations in patients with HER2-positive advanced or metastatic breast cancer: a phase 1b/2, open-label, multicenter, dose-finding and dose-expansion study (DESTINY-Breast07). 2021;39:TPS1096–TPS1096.
Carey LA, Krop I, Ramos J, Feng W, Hamilton E. 331TiP HER2CLIMB-04: phase II trial of tucatinib + trastuzumab deruxtecan in patients with HER2+ locally advanced or metastatic breast cancer with and without brain metastases. Ann Oncol. 2021;32:S510–1.
Bardia A, Coates JT, Spring L, Sun S, Juric D, Thimmiah N, et al. Abstract 2638: Sacituzumab Govitecan, combination with PARP inhibitor, Talazoparib, in metastatic triple-negative breast cancer (TNBC): Translational investigation. Cancer Res. 2022;82:2638–2638. https://doi.org/10.1158/1538-7445.AM2022-2638 .
McGregor BA, Kwak L, Mantia C, Ravi A, Berchuck JE, Ravi P, et al. Sacituzumab govitecan (SG) plus enfortumab vedotin (EV) for metastatic urothelial carcinoma (UC) progressing on platinum-based chemotherapy and PD1/L1 inhibitors (ICB): Double antibody drug conjugate (DAD) phase I trial. J Clin Oncol 2022;40:TPS588–TPS588.
Naumann RW, Martin LP, Oaknin A, Spira AI, Hamilton EP, Schilder RJ, et al. STRO-002-GM2: A phase 1, open-label, safety, pharmacokinetic, and preliminary efficacy study of STRO-002, an anti-folate receptor alpha (FolRα) antibody-drug conjugate (ADC), in combination with bevacizumab in patients with advanced epithelial ovarian cancer (EOC, including fallopian tube or primary peritoneal cancers). 2022;40:TPS5622–TPS5622.
Camidge DR, Barlesi F, Goldman JW, Morgensztern D, Heist R, Vokes E, et al. A Phase 1b study of telisotuzumab vedotin in combination with nivolumab in patients with NSCLC. JTO Clin Res Rep. 2021;3.
Beckwith HC, Medgyesy DC, Abraham J, Nanda R, Tkaczuk KHR, Krop IE, et al. SGNLVA-001: A phase I open-label dose escalation and expansion study of SGN-LIV1A administered weekly in breast cancer. J Clin Oncol 2020;38:TPS1104–TPS1104.
Cilliers C, Guo H, Liao J, Christodolu N, Thurber GM. Multiscale modeling of antibody-drug conjugates: connecting tissue and cellular distribution to whole animal pharmacokinetics and potential implications for efficacy. AAPS J. 2016;18:1117–30.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62.
Heldin CH, Rubin K, Pietras K, Östman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–13.
Gilbert L, Oaknin A, Matulonis UA, Mantia-Smaldone GM, Lim PC, Castro CM, et al. Safety and efficacy of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol Oncol. 2023;170:241–7.
Arjaans M, Munnink THO, Oosting SF, Van Scheltinga AGTT, Gietema JA, Garbacik ET, et al. Bevacizumab-induced normalization of blood vessels in tumors hampers antibody uptake. Cancer Res. 2013;73:3347–55.
Huang Y, Stylianopoulos T, Duda DG, Fukumura D, Jain RK. Benefits of vascular normalization are dose- and time-dependent. Cancer Res. 2013;73:7144–6.
Arjaans M, Oosting SF, Schröder CP, De Vries EGE. Bevacizumab-induced vessel normalization hampers tumor uptake of antibodies–response. Cancer Res. 2013;73:7147–8.
Oosting SF, Brouwers AH, Van Es SC, Nagengast WB, Munnink THO, Lub-De Hooge MN, et al. 89Zr-bevacizumab PET visualizes heterogeneous tracer accumulation in tumor lesions of renal cell carcinoma patients and differential effects of antiangiogenic treatment. J Nucl Med. 2015;56:63–9.
Fujimori K, Covell DG, Fletcher JE, Weinstein JN. A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier. J Nucl Med. 1990;31:1191–8.
CAS PubMed Google Scholar
Phillips GDL, Fields CT, Li G, Dowbenko D, Schaefer G, Miller K, et al. Dual targeting of HER2-positive cancer with trastuzumab emtansine and pertuzumab: critical role for neuregulin blockade in antitumor response to combination therapy. Clin Cancer Res. 2014;20:456–68.
Miller KD, Diéras V, Harbeck N, Andre F, Mahtani RL, Gianni L, et al. Phase IIa trial of trastuzumab emtansine with pertuzumab for patients with human epidermal growth factor receptor 2-positive, locally advanced, or metastatic breast cancer. J Clin Oncol. 2014;32:1437–44.
Bordeau BM, Yang Y, Balthasar JP. Transient competitive inhibition bypasses the binding site barrier to improve tumor penetration of trastuzumab and enhance T-DM1 efficacy. Cancer Res. 2021;81:4145–54.
Krop IE, Im SA, Barrios C, Bonnefoi H, Gralow J, Toi M, et al. Trastuzumab emtansine plus pertuzumab versus taxane plus trastuzumab plus pertuzumab after anthracycline for high-risk human epidermal growth factor receptor 2-positive early breast cancer: the phase III KAITLIN study. J Clin Oncol. 2022;40:438–48.
Hurvitz SA, Martin M, Symmans WF, Jung KH, Huang CS, Thompson AM, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19:115–26.
Hurvitz SA, Martin M, Jung KH, Huang CS, Harbeck N, Valero V, et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2-positive breast cancer: three-year outcomes from the phase III KRISTINE study. J Clin Oncol. 2019;37:2206–16.
Hunter FW, Barker HR, Lipert B, Rothé F, Gebhart G, Piccart-Gebhart MJ, et al. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br J Cancer. 2020;122:603–12.
Saatci Ö, Borgoni S, Akbulut Ö, Durmuş S, Raza U, Eyüpoǧlu E, et al. Targeting PLK1 overcomes T-DM1 resistance via CDK1-dependent phosphorylation and inactivation of Bcl-2/xL in HER2-positive breast cancer. Oncogene. 2018;37:2251–69.
La Monica S, Cretella D, Bonelli M, Fumarola C, Cavazzoni A, Digiacomo G, et al. Trastuzumab emtansine delays and overcomes resistance to the third-generation EGFR-TKI osimertinib in NSCLC EGFR mutated cell lines. J Exp Clin Cancer Res. 2017;36.
Haikala HM, Lopez T, Köhler J, Eser PO, Xu M, Zeng Q, et al. EGFR inhibition enhances the cellular uptake and antitumor-activity of the HER3 antibody-drug conjugate HER3-DXd. Cancer Res. 2022;82:130–41.
Kayatani H, Ohashi K, Ninomiya K, Makimoto G, Nishii K, Higo H, et al. Beneficial effect of erlotinib and trastuzumab emtansine combination in lung tumors harboring EGFR mutations. Biochem Biophys Res Commun. 2020;532:341–6.
Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17.
Johnson M, Garassino MC, Mok T, Mitsudomi T. Treatment strategies and outcomes for patients with EGFR-mutant non-small cell lung cancer resistant to EGFR tyrosine kinase inhibitors: focus on novel therapies. Lung Cancer. 2022;170:41–51.
Konieczkowski DJ, Johannessen CM, Abudayyeh O, Kim JW, Cooper ZA, Piris A, et al. A melanoma cell state distinction influences sensitivity to MAPK pathway inhibitors. Cancer Discov. 2014;4:816–27.
Boshuizen J, Koopman LA, Krijgsman O, Shahrabi A, Van Den Heuvel EG, Ligtenberg MA, et al. Cooperative targeting of melanoma heterogeneity with an AXL antibody-drug conjugate and BRAF/MEK inhibitors. Nat Med. 2018;24:203–12.
Roas M, Vick B, Kasper MA, Able M, Polzer H, Gerlach M, et al. Targeting FLT3 with a new-generation antibody-drug conjugate in combination with kinase inhibitors for treatment of AML. Blood. 2023;141:1023–35.
Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–14.
Collins DM, Gately K, Hughes C, Edwards C, Davies A, Madden SF, et al. Tyrosine kinase inhibitors as modulators of trastuzumab-mediated antibody-dependent cell-mediated cytotoxicity in breast cancer cell lines. Cell Immunol. 2017;319:35–42.
O’Brien NA, Huang HKT, McDermott MSJ, Madrid AM, Luo T, Ayala R, et al. Tucatinib has selective activity in HER2-positive cancers and significant combined activity with approved and novel breast cancer-targeted therapies. Mol Cancer Ther. 2022;21:751–61.
Abraham J, Montero AJ, Jankowitz RC, Salkeni MA, Beumer JH, Kiesel BF, et al. Safety and efficacy of T-DM1 plus neratinib in patients with metastatic HER2-positive breast cancer: NSABP foundation trial FB-10. J Clin Oncol. 2019;37:2601–9.
Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382:597–609.
Robichaux JP, Elamin YY, Vijayan RSK, Nilsson MB, Hu L, He J, et al. Pan-cancer landscape and analysis of ERBB2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T-DM1 activity. Cancer Cell. 2019;36:444-457.e7.
Kulukian A, Taylor J, Jain N, Olson D, Zaval M, Thurman R, et al. Abstract PS10–08: Tucatinib potentiates the activity of the antibody-drug conjugate T-DM1 in preclinical models of HER2-positive breast cancer. Cancer Res. 2021;81:PS10–08. https://doi.org/10.1158/1538-7445.SABCS20-PS10-08
Krop IE, Ramos J, Zhang C, Hamilton EP. HER2CLIMB-04: Phase 2 open label trial of tucatinib plus trastuzumab deruxtecan in patients with HER2+ unresectable locally advanced or metastatic breast cancer with and without brain metastases (trial in progress). 2021;39:TPS1097–TPS1097.
Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–8.
Carrassa L, Damia G. DNA damage response inhibitors: Mechanisms and potential applications in cancer therapy. Cancer Treat Rev. 2017;60:139–51.
Brown JS, O’Carrigan B, Jackson SP, Yap TA. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 2017;7:20–37.
Zhu S, Wu Y, Song B, Yi M, Yan Y, Mei Q, et al. Recent advances in targeted strategies for triple-negative breast cancer. J Hematol Oncol. 2023;16.
Yung TMC, Sato S, Satoh MS. Poly(ADP-ribosyl)ation as a DNA damage-induced post-translational modification regulating poly(ADP-ribose) polymerase-1-topoisomerase I interaction. J Biol Chem. 2004;279:39686–96.
Plummer R. Perspective on the pipeline of drugs being developed with modulation of DNA damage as a target. Clin Cancer Res. 2010;16:4527–31.
Boerner JL, Nechiporchik N, Mueller KL, Polin L, Heilbrun L, Boerner SA, et al. Protein expression of DNA damage repair proteins dictates response to topoisomerase and PARP inhibitors in triple-negative breast cancer. PLoS ONE. 2015;10.
Cardillo TM, Sharkey RM, Rossi DL, Arrojo R, Mostafa AA, Goldenberg DM. Synthetic lethality exploitation by an anti-trop-2-SN-38 antibody-drug conjugate, IMMU-132, plus PARP inhibitors in BRCA1/2-wild-type triple-negative breast cancer. Clin Cancer Res. 2017;23:3405–15.
Spring LM, Clark SL, Li T, Goel S, Tayob N, Viscosi E, et al. Phase 1b clinical trial of ado-trastuzumab emtansine and ribociclib for HER2-positive metastatic breast cancer. NPJ Breast Cancer. 2021;7.
Matulonis UA, Lorusso D, Oaknin A, Pignata S, Dean A, Denys H, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41:2436–45.
Nicolò E, Giugliano F, Ascione L, Tarantino P, Corti C, Tolaney SM, et al. Combining antibody-drug conjugates with immunotherapy in solid tumors: current landscape and future perspectives. Cancer Treat Rev. 2022;106.
D’Amico L, Menzel U, Prummer M, Müller P, Buchi M, Kashyap A, et al. A novel anti-HER2 anthracycline-based antibody-drug conjugate induces adaptive anti-tumor immunity and potentiates PD-1 blockade in breast cancer. J Immunother Cancer. 2019;7.
Müller P, Martin K, Theurich S, Schreiner J, Savic S, Terszowski G, et al. Microtubule-depolymerizing agents used in antibody-drug conjugates induce antitumor immunity by stimulation of dendritic cells. Cancer Immunol Res. 2014;2:741–55.
Iwata TN, Ishii C, Ishida S, Ogitani Y, Wada T, Agatsuma T. A HER2-targeting antibody-drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol Cancer Ther. 2018;17:1494–503.
Rios-Doria J, Harper J, Rothstein R, Wetzel L, Chesebrough J, Marrero A, et al. Antibody-drug conjugates bearing pyrrolobenzodiazepine or tubulysin payloads are immunomodulatory and synergize with multiple immunotherapies. Cancer Res. 2017;77:2686–98.
Boshuizen J, Pencheva N, Krijgsman O, DanielaD’Empaire Altimari, Castro PG, De Bruijn B, et al. Cooperative targeting of immunotherapy-resistant melanoma and lung cancer by an AXL-targeting antibody-drug conjugate and immune checkpoint blockade. Cancer Res. 2021;81:1775–87.
Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. 2011;128:347–56.
Vafa O, Gilliland GL, Brezski RJ, Strake B, Wilkinson T, Lacy ER, et al. An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods. 2014;65:114–26.
Hamilton EP, Kaklamani V, Falkson C, Vidal GA, Ward PJ, Patre M, et al. Abstract PD1–05: Atezolizumab in combination with trastuzumab emtansine or with trastuzumab and pertuzumab in patients with HER2-positive breast cancer and atezolizumab with doxorubicin and cyclophosphamide in HER2-negative breast cancer: safety and biomarker outcomes from a multi-cohort Phase Ib study. Cancer Res. 2020;80:PD1–05. https://doi.org/10.1158/1538-7445.SABCS19-PD1-05
Emens LA, Esteva FJ, Beresford M, Saura C, De Laurentiis M, Kim SB, et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020;21:1283–95.
Waks AG, Keenan TE, Li T, Tayob N, Wulf GM, Richardson ET, et al. Phase Ib study of pembrolizumab in combination with trastuzumab emtansine for metastatic HER2-positive breast cancer. J Immunother Cancer. 2022;10.
Janjigian YY, Viglianti N, Liu F, Mendoza-Naranjo A, Croydon L. A phase Ib/II, multicenter, open-label, dose-escalation, and dose-expansion study evaluating trastuzumab deruxtecan (T-DXd, DS-8201) monotherapy and combinations in patients with HER2-overexpressing gastric cancer (DESTINY-Gastric03). J Clin Oncol. 2021;39:TPS261–TPS261.
Peters S, Dafni U, Perol M, Felip E, Polito L, Pal N, et al. VP2-2021: effectiveness of PD-(L)1 inhibitors alone or in combination with platinum-doublet chemotherapy in first-line (1L) non-squamous non-small cell lung cancer (Nsq-NSCLC) with high PD-L1 expression using real-world data. Ann Oncol. 2021;32:687–8.
Powles T, Yu EY, Iyer G, Campbell MT, Loriot Y, Santis M De, et al. Phase 2 clinical study evaluating the efficacy and safety of disitamab vedotin with or without pembrolizumab in patients with HER2-expressing urothelial carcinoma (RC48G001). J Clin Oncol. 2023;41:TPS594–TPS594.
Rogerson S, Turner H, Bilkhu A, Monturus E, Knott A, Restuccia E, et al. Diversity, inclusion, and patient (pt)-centricity in the randomized, double-blind, phase III ASTEFANIA study of ado-trastuzumab emtansine (T-DM1) ± atezolizumab in pts with HER2-positive early breast cancer (EBC) with residual invasive disease after preoperative chemotherapy and anti-HER2 therapy. J Clin Oncol. 2022;40:e12504–e12504.
Loi S, Schneeweiss A, Song E, Harries M, De Laurentiis M, Li Y, et al. 329TiP KATE3: A phase III study of trastuzumab emtansine (T-DM1) in combination with atezolizumab or placebo in patients with previously treated HER2-positive and PD-L1–positive locally advanced or metastatic breast cancer. Ann Oncol. 2021;32:S509.
Schmid P, Im S-A, Armstrong A, Park YH, Chung W-P, Nowecki Z, et al. BEGONIA: Phase 1b/2 study of durvalumab (D) combinations in locally advanced/metastatic triple-negative breast cancer (TNBC)—Initial results from arm 1, d+paclitaxel (P), and arm 6, d+trastuzumab deruxtecan (T-DXd). J Clin Oncol. 2021;39:1023–1023.
Johnson ML, Solomon BJ, Awad MM, Cho BC, Gainor JF, Goldberg SB, et al. MORPHEUS: A phase Ib/II multi-trial platform evaluating the safety and efficacy of cancer immunotherapy (CIT)-based combinations in patients (pts) with non-small cell lung cancer (NSCLC). J Clin Oncol. 2018;36:TPS9105–TPS9105.
Drakaki A, Kalebasty AR, Lee J-L, Martin-Liberal J, Kim M, Shin SJ, et al. Phase Ib/II umbrella trial to evaluate the safety and efficacy of multiple 2L cancer immunotherapy (CIT) combinations in advanced/metastatic urothelial carcinoma (mUC): MORPHEUS-mUC. J Clin Oncol. 2020;38:TPS591–TPS591.
Mittendorf E, Tolaney S, Wileyto P, DeMeo M, Rugo H, Nanda R, et al. Abstract OT-33–01: Combination ipatasertib and atezolizumab to prevent recurrence in triple negative breast cancer(TNBC): A phase II single arm trial. Cancer Res. 2021;81:OT-33–01. https://doi.org/10.1158/1538-7445.SABCS20-OT-33-01
Garrido-Castro AC, Keenan TE, Li T, Lange P, Callahan C, Guerriero J, et al. Saci-IO TNBC: Randomized phase II trial of sacituzumab govitecan (SG) +/− pembrolizumab in PD-L1—metastatic triple-negative breast cancer (mTNBC). J Clin Oncol. 2021;39:TPS1106–TPS1106.
Garrido-Castro AC, Keenan TE, Li T, Lange P, Callahan C, Guerriero J, et al. Saci-IO HR+: Randomized phase II trial of sacituzumab govitecan (SG) +/− pembrolizumab in PD-L1+ hormone receptor-positive (HR+) / HER2- metastatic breast cancer (MBC). J Clin Oncol. 2021;39:TPS1102–TPS1102.
Subudhi SK, Bendell JC, Carducci MA, Kopp LM, Scott J, Grady MM, et al. ARC-6: A phase 1b/2, open-label, randomized platform study to evaluate efficacy and safety of etrumadenant (AB928)-based treatment combinations in patients with metastatic castrate-resistant prostate cancer (mCRPC). J Clin Oncol. 2021;39:5039–5039.
Jain RK, Yang Y, Chadha J, Chatwal MS, Kish JA, Raymond S, et al. Phase I/II study of ipilimumab plus nivolumab combined with sacituzumab govitecan in patients with metastatic cisplatin-ineligible urothelial carcinoma. J Clin Oncol. 2023;41:521–521.
Garon EB, Liu S V., Owen SP, Reck M, Neal JW, Vicente D, et al. EVOKE-02: A phase 2 study of sacituzumab govitecan (SG) plus pembrolizumab (pembro) with or without platinum chemotherapy in first-line metastatic non–small cell lung cancer (NSCLC). J Clin Oncol. 2022;40:TPS9146–TPS9146.
Hoffman-Censits J, Grivas P, Powles T, Martincic D, Hawley J, Tyroller K, et al. 665 JAVELIN Bladder Medley: a phase 2 trial of avelumab in combination with other antitumor drugs as first-line maintenance therapy for advanced urothelial carcinoma. J Immunother Cancer. 2022;10:A694–A694.
Meric-Bernstam F, Janjigian Y, Lang J, Ciombor KK, Ray-Coquard I, Oza A, et al. Abstract CT058: TROPION-PanTumor03: Phase 2, multicenter study of datopotamab deruxtecan (Dato-DXd) as monotherapy and in combination with anticancer agents in patients (pts) with advanced/metastatic solid tumors. Cancer Res. 2023;83:CT058–CT058. https://doi.org/10.1158/1538-7445.AM2023-CT058
Goto Y, Su W-C, Levy BP, Rixe O, Yang T-Y, Tolcher AW, et al. TROPION-Lung02: Datopotamab deruxtecan (Dato-DXd) plus pembrolizumab (pembro) with or without platinum chemotherapy (Pt-CT) in advanced non-small cell lung cancer (aNSCLC). J Clin Oncol. 2023;41:9004–9004.
Borghaei H, Waqar SN, Bruno DS, Kitazono S, Wakuda K, Spira AI, et al. TROPION-Lung04: Phase 1b, multicenter study of datopotamab deruxtecan (Dato-DXd) in combination with immunotherapy ± carboplatin in advanced/metastatic non-small cell lung cancer (mNSCLC). J Clin Oncol. 2023;41:TPS3158–TPS3158.
Necchi A, Bedke J, Galsky MD, Shore ND, Plimack ER, Xylinas E, et al. Phase 3 KEYNOTE-905/EV-303: Perioperative pembrolizumab (pembro) or pembro + enfortumab vedotin (EV) for muscle-invasive bladder cancer (MIBC). J Clin Oncol. 2023;41:TPS585–TPS585.
Hoimes CJ, Flaig TW, Milowsky MI, Friedlander TW, Bilen MA, Gupta S, et al. Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J Clin Oncol. 2023;41:22–31.
Powles T, Drakaki A, Teoh JY-C, Grande E, Fontes-Sousa M, Porta C, et al. A phase 3, randomized, open-label, multicenter, global study of the efficacy and safety of durvalumab (D) + tremelimumab (T) + enfortumab vedotin (EV) or D + EV for neoadjuvant treatment in cisplatin-ineligible muscle-invasive bladder cancer (MIBC) (VOLGA). J Clin Oncol. 2022;40:TPS579–TPS579.
Hoimes CJ, Bedke J, Loriot Y, Nishiyama H, Fang X, Kataria RS, et al. KEYNOTE-B15/EV-304: Randomized phase 3 study of perioperative enfortumab vedotin plus pembrolizumab versus chemotherapy in cisplatin-eligible patients with muscle-invasive bladder cancer (MIBC). J Clin Oncol. 2021;39:TPS4587–TPS4587.
Porter R, Tayob N, Polak M, Sawyer H, Gardner J, Campos S, et al. TP015/#1566 A phase 2, two-stage, study of mirvetuximab soravtansine (IMGN853) in combination with pembrolizumab in patients with microsatellite stable (MSS) recurrent or persistent endometrial cancer (EC). Int J Gynecol Cancer. 2022;32:A229.3-A230.
Ahnert JR, Taylor MH, O’Reilly EM, Zhang J, Doebele RC, Ben Y, et al. A phase 1/2 dose-escalation and expansion study of a conditionally active anti-AXL humanized monoclonal antibody (BA3011) in patients with advanced solid tumors. J Clin Oncol. 2018;36:TPS12126–TPS12126.
Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5.
Hoffmann RM, Coumbe BGT, Josephs DH, Mele S, Ilieva KM, Cheung A, et al. Antibody structure and engineering considerations for the design and function of Antibody Drug Conjugates (ADCs). Oncoimmunology. 2017;7.
Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, A novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22:5097–108.
Uppal H, Doudement E, Mahapatra K, Darbonne WC, Bumbaca D, Shen BQ, et al. Potential mechanisms for thrombocytopenia development with trastuzumab emtansine (T-DM1). Clin Cancer Res. 2015;21:123–33.
Tai YT, Mayes PA, Acharya C, Zhong MY, Cea M, Cagnetta A, et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood. 2014;123:3128–38.
Beck A, Reichert JM. Marketing approval of mogamulizumab: a triumph for glyco-engineering. MAbs. 2012;4:419–25.
Pegram MD, Hamilton EP, Tan AR, Storniolo AM, Balic K, Rosenbaum AI, et al. First-in-human, phase 1 dose-escalation study of biparatopic anti-HER2 antibody-drug conjugate MEDI4276 in patients with HER2-positive advanced breast or gastric cancer. Mol Cancer Ther. 2021;20:1442–54.
Li F, Ulrich M, Jonas M, Stone IJ, Linares G, Zhang X, et al. Tumor-associated macrophages can contribute to antitumor activity through FcγR-mediated processing of antibody-drug conjugates. Mol Cancer Ther. 2017;16:1347–54.
Garg AD, Galluzzi L, Apetoh L, Baert T, Birge RB, Bravo-San Pedro JM, et al. Molecular and translational classifications of DAMPs in immunogenic cell death. Front Immunol. 2015;6.
Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72.
Kepp O, Tartour E, Vitale I, Vacchelli E, Adjemian S, Agostinis P, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3.
Sukkurwala AQ, Adjemian S, Senovilla L, Michaud M, Spaggiari S, Vacchelli E, et al. Screening of novel immunogenic cell death inducers within the NCI Mechanistic Diversity Set. Oncoimmunology. 2014;3.
Rios-Doria J, Durham N, Wetzel L, Rothstein R, Chesebrough J, Holoweckyj N, et al. Doxil synergizes with cancer immunotherapies to enhance antitumor responses in syngeneic mouse models. Neoplasia. 2015;17:661–70.
Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–33.
Liu L, Mayes PA, Eastman S, Shi H, Yadavilli S, Zhang T, et al. The BRAF and MEK inhibitors dabrafenib and trametinib: effects on immune function and in combination with immunomodulatory antibodies targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res. 2015;21:1639–51.
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61.
Voorwerk L, Slagter M, Horlings HM, Sikorska K, van de Vijver KK, de Maaker M, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25:920–8.
Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–91.
Bauzon M, Drake PM, Barfield RM, Cornali BM, Rupniewski I, Rabuka D. Maytansine-bearing antibody-drug conjugates induce in vitro hallmarks of immunogenic cell death selectively in antigen-positive target cells. Oncoimmunology. 2019;8.
Cao AT, Law C-L, Gardai SJ, Heiser RA. Abstract 5588: Brentuximab vedotin-driven immunogenic cell death enhances antitumor immune responses, and is potentiated by PD1 inhibition in vivo. Cancer Res. 2017;77:5588–5588. https://doi.org/10.1158/1538-7445.AM2017-5588 .
Cao AT, Higgins S, Stevens N, Gardai SJ, Sussman D. Abstract 2742: additional mechanisms of action of ladiratuzumab vedotin contribute to increased immune cell activation within the tumor. Cancer Res. 2018;78:2742–2742. https://doi.org/10.1158/1538-7445.AM2018-2742 .
Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20:7–24.
Hargadon KM. Tumor-altered dendritic cell function: implications for anti-tumor immunity. Front Immunol. 2013;4.
Martin K, Müller P, Schreiner J, Prince SS, Lardinois D, Heinzelmann-Schwarz VA, et al. The microtubule-depolymerizing agent ansamitocin P3 programs dendritic cells toward enhanced anti-tumor immunity. Cancer Immunol Immunother. 2014;63:925–38.
Tanaka H, Matsushima H, Nishibu A, Clausen BE, Takashima A. Dual therapeutic efficacy of vinblastine as a unique chemotherapeutic agent capable of inducing dendritic cell maturation. Cancer Res. 2009;69:6987–94.
Müller P, Kreuzaler M, Khan T, Thommen DS, Martin K, Glatz K, et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med. 2015;7.
Tanaka H, Matsushima H, Mizumoto N, Takashima A. Classification of chemotherapeutic agents based on their differential in vitro effects on dendritic cells. Cancer Res. 2009;69:6978–86.
Huang L, Wang R, Xie K, Zhang J, Tao F, Pi C, et al. A HER2 target antibody drug conjugate combined with anti-PD-(L)1 treatment eliminates hHER2+ tumors in hPD-1 transgenic mouse model and contributes immune memory formation. Breast Cancer Res Treat. 2022;191:51–61.
Xia L, Wen L, Qin Y, Dobson HE, Zhang T, Comer FI, et al. HER2-targeted antibody-drug conjugate induces host immunity against cancer stem cells. Cell Chem Biol. 2021;28:610-624.e5.
Friedlander TW, Milowsky MI, Bilen MA, Srinivas S, McKay RR, Flaig TW, et al. Study EV-103: update on durability results and long term outcome of enfortumab vedotin + pembrolizumab in first line locally advanced or metastatic urothelial carcinoma (la/mUC). 2021;39:4528–4528.
Sheng X, Zhou L, He Z, Guo H, Yan X, Li S, et al. Abstract 4581: Preliminary results of a phase Ib/II combination study of RC48-ADC, a novel humanized anti-HER2 antibody-drug conjugate (ADC) with toripalimab, a humanized IgG4 mAb against programmed death-1 (PD-1) in patients with locally advanced or metastatic urothelial carcinoma. 2022;40:4518–4518.
Calvo E, Spira A, Miguel M de, Kondo S, Gazzah A, Millward M, et al. Safety, pharmacokinetics, and efficacy of budigalimab with rovalpituzumab tesirine in patients with small cell lung cancer. Cancer Treat Res Commun. 2021;28.
Malhotra J, Nikolinakos P, Leal T, Lehman J, Morgensztern D, Patel JD, et al. A phase 1–2 study of rovalpituzumab tesirine in combination with nivolumab plus or minus ipilimumab in patients with previously treated extensive-stage SCLC. J Thorac Oncol. 2021;16:1559–69.
O’Malley DM, Oaknin A, Matulonis UA, Mantia-Smaldone G, Lim PC, Castro CM, et al. Abstract5504: Mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients (pts) with platinum-agnostic ovarian cancer: final analysis. 2021;19:8–10.
Lorusso D, Vergote I, O’Cearbhaill RE, Westermann AM, Banerjee SN, Nieuwenhuysen E Van, et al. Abstract 5507: Tisotumab vedotin (TV) + pembrolizumab (pembro) in first-line (1L) recurrent or metastatic cervical cancer (r/mCC): Interim results of ENGOT Cx8/GOG 3024/innovaTV 205. 2022;40:5507–5507.
Schmid P, Nunes AT, Dry H, Dougherty R, Karwe V, Scheuring UJ. BEGONIA: Phase 1b/2, open-label, platform study of the safety and efficacy of durvalumab (D) ± paclitaxel (P) with novel oncology therapies for first-line metastatic triple-negative breast cancer (mTNBC): Addition of arm 7, D + datopotamab deruxtecan (Dato-DXd; DS-1062). 2021;39:TPS1105–TPS1105.
Boni V, Alemany C, Meisel JL, Sinha R, Sterrenberg D, Tkaczuk KHR, et al. SGNLVA-002: Single arm, open label, phase Ib/II study of ladiratuzumab vedotin (LV) in combination with pembrolizumab for first-line treatment of patients with unresectable locally-advanced or metastatic triple-negative breast cancer. Annals of Oncology. 2019;30:iii63.
Cheson BD, Bartlett NL, LaPlant B, Lee HJ, Advani RJ, Christian B, et al. Brentuximab vedotin plus nivolumab as first-line therapy in older or chemotherapy-ineligible patients with Hodgkin lymphoma (ACCRU): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2020;7:e808–15.
Schmid P, Jung KH, Wysocki PJ, Jassem J, Ma CX, Fernandes R, et al. 166MO Datopotamab deruxtecan (Dato-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): Initial results from BEGONIA, a phase Ib/II study. Ann Oncol. 2022;33:S199.
Fuentes-Antrás J, Genta S, Vijenthira A, Siu LL. Antibody-drug conjugates: in search of partners of choice. Trends Cancer. 2023;9:339–54.
Vergote IB, Monk BJ, O’Cearbhaill RE, Westermann AM, Banerjee S, Collins DC, et al. 723MO Tisotumab vedotin (TV) + carboplatin (Carbo) in first-line (1L) or + pembrolizumab (Pembro) in previously treated (2L/3L) recurrent or metastatic cervical cancer (r/mCC): Interim results of ENGOT-Cx8/GOG-3024/innovaTV 205 study. Ann Oncol. 2021;32:S726–7.
Zinzani PL, Santoro A, Gritti G, Brice P, Barr PM, Kuruvilla J, et al. Nivolumab combined with brentuximab vedotin for relapsed/refractory primary mediastinal large B-cell lymphoma: efficacy and safety from the phase II checkmate 436 study. J Clin Oncol. 2019;37:3081–9.
Advani RH, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, et al. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood. 2021;138:427–38.
Rosenberg JE, Milowsky M, Ramamurthy C, Mar N, McKay RR, Friedlander T, et al. LBA73 Study EV-103 Cohort K: Antitumor activity of enfortumab vedotin (EV) monotherapy or in combination with pembrolizumab (P) in previously untreated cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (la/mUC). Ann Oncol. 2022;33:S1441.
Schmid P, Im S-A, Armstrong A, Park YH, Chung W-P, Nowecki Z, et al. BEGONIA: Phase 1b/2 study of durvalumab (D) combinations in locally advanced/metastatic triple-negative breast cancer (TNBC)—Initial results from arm 1, d+paclitaxel (P), and arm 6, d+trastuzumab deruxtecan (T-DXd). 2021;39:1023–1023.
Hamilton E, Shapiro CL, Petrylak D, Boni V, Martin M, Conte G Del, et al. Abstract PD3–07: Trastuzumab deruxtecan (T-DXd; DS-8201) with nivolumab in patients with HER2-expressing, advanced breast cancer: A 2-part, phase 1b, multicenter, open-label study. Cancer Res. 2021;81:PD3–07. https://doi.org/10.1158/1538-7445.SABCS20-PD3-07
Grivas P, Pouessel D, Park CH, Barthélémy P, Bupathi M, Petrylak DP, et al. TROPHY-U-01 Cohort 3: Sacituzumab govitecan (SG) in combination with pembrolizumab (Pembro) in patients (pts) with metastatic urothelial cancer (mUC) who progressed after platinum (PLT)-based regimens. 2022;40:434–434.
Schmid P, Wysocki P, Park YH, Jassem J, Jung KH, Lord S, et al. Abstract PD11–08: PD11–08 Trastuzumab deruxtecan (T-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic hormone receptor-negative (HR−), HER2-low breast cancer: updated results from BEGONIA, a phase 1b/2 study. Cancer Res. 2023;83:PD11–08. https://doi.org/10.1158/1538-7445.SABCS22-PD11-08
Download references
Acknowledgements
National Natural Science Foundation of China (NSFC; 82172598, 82074245, 82373782, 82303963). The National Key R&D Program of China (2021YFA0910100). The Natural Science Foundation of Zhejiang Province, China (LZ22H310001, LQ21H160005). The 551 Health Talent Training Project of the Health Commission of Zhejiang Province. The Agricultural and Social Development Research Project of Hangzhou Municipal Science and Technology Bureau (2022ZDSJ0474). The Zhejiang Leading Innovation and Entrepreneurship Team (2022R01006). The Key Laboratory of Prevention, Diagnosis and Therapy of Upper Gastrointestinal Cancer of Zhejiang Province (2022E10021). Medical Science and Technology Project of Zhejiang Province (WKJ-ZJ-2104). Zhejiang Provincial Research Center for Upper Gastrointestinal Track Center (JBZX-202006). Program of Zhejiang Provincial TCM Sci-Tech Plan (GZY-ZJ-KJ-230003).
Author information
Jieer Ying, Peng Guo and Xiangdong Cheng: Co corresponding authors.
Qing Wei and Peijing Li: Co first authors.
Authors and Affiliations
Department of Medical Oncology, Zhejiang Cancer Hospital, Hangzhou, China
Qing Wei, Ziwen Zhang & Jieer Ying
Hangzhou Institute of Medicine (HIM), Chinese Academy of Sciences, Hangzhou, China
Qing Wei, Peijing Li, Teng Yang, Lu Sun, Ziwen Zhang, Xuefei Tian, Jiahui Chen, Can Hu, Letao Ma, Jieer Ying, Peng Guo & Xiangdong Cheng
Key Laboratory of Prevention, Diagnosis and Therapy of Upper Gastrointestinal Cancer of Zhejiang Province, Hangzhou, China
Qing Wei, Jiahui Chen, Can Hu, Jieer Ying, Peng Guo & Xiangdong Cheng
Department of Radiation Oncology, Zhejiang Cancer Hospital, Key Laboratory of Head and Neck Cancer Translational Research of Zhejiang Province, Hangzhou, China
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, China
Teng Yang & Letao Ma
Zhejiang Chinese Medical University, Hangzhou, China
Department of Gynecologic Oncology, Zhejiang Cancer Hospital, Hangzhou, China
Department of Radiation Oncology, Second Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang University, Hangzhou, China
Shanghai Institute of Materia Medica, University of Chinese Academy of Sciences, Shanghai, China
Xuefei Tian
College of Molecular Medicine, Hangzhou Institute for Advanced Study (HIAS), University of Chinese Academy of Sciences, Hangzhou, China
Department of Gastric Surgery, Zhejiang Cancer Hospital, Hangzhou, China
Jiahui Chen, Can Hu & Xiangdong Cheng
Department of Oncology, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China
Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan
Takaya Shimura
School of Life Science and Technology, Tongji University, Shanghai, China
Jianmin Fang
You can also search for this author in PubMed Google Scholar
Contributions
P.G., X.C. and J.Y. conceived and designed the review. T.Y. and J.Z. performed analyses of ADC combined with endocrine therapy and chemotherapy. L.S., Z.Z., L.W. and X.T. performed analyses of ADC combined with targeted therapy. J.C., C.H, J.X. and L.M. performed analyses of ADC combined with immunotherapy. T.S. and J.F. interpreted and discussed the results. Q.W. and P.L. wrote the manuscript. All authors approved this manuscript for publication.
Corresponding authors
Correspondence to Jieer Ying , Peng Guo or Xiangdong Cheng .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
QW, TY, ZWZ, XDC and PG are co-inventors of a patent application based on this study. The other authors have declared no conflicts of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wei, Q., Li, P., Yang, T. et al. The promise and challenges of combination therapies with antibody-drug conjugates in solid tumors. J Hematol Oncol 17 , 1 (2024). https://doi.org/10.1186/s13045-023-01509-2
Download citation
Received : 06 September 2023
Accepted : 06 November 2023
Published : 04 January 2024
DOI : https://doi.org/10.1186/s13045-023-01509-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Antibody-drug conjugate
- Solid tumor
- Combination therapy
- Immunotherapy
- Targeted therapy
Journal of Hematology & Oncology
ISSN: 1756-8722
- General enquiries: [email protected]
- Introduction
- Conclusions
- Article Information
Weighted according to random-effects analysis.
Efficacy was measured as standardized mean difference (SMD) and weighted according to random-effects analysis.
eFigure 1. Forest Plot 3: Primary Outcome – Subgroup Analysis: First-line Studies Only
eFigure 2. Forest Plot 4: Primary Outcome – Subgroup Analysis: Nonresponder Studies Only
eFigure 3. Forest Plot 5: Primary Outcome – Subgroup Analysis: Bupropion Combinations
eFigure 4. Funnel Plot of Primary Outcome Analysis
eReferences
eAppendix. Explicit Search Entry
- Antidepressant Combinations Compared With Monotherapy JAMA News From the JAMA Network May 3, 2022 Anita Slomski
- Combination Antidepressant Therapy vs Monotherapy—Further Considerations—Reply JAMA Psychiatry Comment & Response August 1, 2022 Jonathan Henssler, MD; Tom Bschor, MD; Christopher Baethge, MD
- Combination Antidepressant Therapy vs Monotherapy—Further Considerations JAMA Psychiatry Comment & Response August 1, 2022 Philip J. Cowen, MD
- Combination Antidepressant Therapy vs Monotherapy—Further Considerations JAMA Psychiatry Comment & Response August 1, 2022 Qi Wang, MMed; Liang Yao, MMed; Yaolong Chen, PhD
- Combination Antidepressant Therapy vs Monotherapy—Further Considerations JAMA Psychiatry Comment & Response August 1, 2022 Klaus Munkholm, MD, DMSc; Asger Sand Paludan-Müller, MSc
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Henssler J , Alexander D , Schwarzer G , Bschor T , Baethge C. Combining Antidepressants vs Antidepressant Monotherapy for Treatment of Patients With Acute Depression : A Systematic Review and Meta-analysis . JAMA Psychiatry. 2022;79(4):300–312. doi:10.1001/jamapsychiatry.2021.4313
Manage citations:
© 2024
- Permissions

Combining Antidepressants vs Antidepressant Monotherapy for Treatment of Patients With Acute Depression : A Systematic Review and Meta-analysis
- 1 Department of Psychiatry and Psychotherapy, University of Cologne Medical School, Cologne, Germany
- 2 Charité University Medicine, St Hedwig-Krankenhaus, Clinic for Psychiatry and Psychotherapy, Berlin, Germany
- 3 Institute of Medical Biometry and Statistics, Faculty of Medicine and Medical Center, University of Freiburg, Freiburg, Germany
- 4 Department of Psychiatry and Psychotherapy, University Hospital of Dresden, Dresden, Germany
- News From the JAMA Network Antidepressant Combinations Compared With Monotherapy Anita Slomski JAMA
- Comment & Response Combination Antidepressant Therapy vs Monotherapy—Further Considerations—Reply Jonathan Henssler, MD; Tom Bschor, MD; Christopher Baethge, MD JAMA Psychiatry
- Comment & Response Combination Antidepressant Therapy vs Monotherapy—Further Considerations Philip J. Cowen, MD JAMA Psychiatry
- Comment & Response Combination Antidepressant Therapy vs Monotherapy—Further Considerations Qi Wang, MMed; Liang Yao, MMed; Yaolong Chen, PhD JAMA Psychiatry
- Comment & Response Combination Antidepressant Therapy vs Monotherapy—Further Considerations Klaus Munkholm, MD, DMSc; Asger Sand Paludan-Müller, MSc JAMA Psychiatry
Question What is the treatment efficacy and tolerability of antidepressant combination therapy compared with monotherapy in the treatment of acute depression, and are specific combinations preferable to others?
Findings This meta-analysis of 39 trials comprising 6751 patients found that combination treatment using a reuptake inhibitor with an antagonist of presynaptic α2-autoreceptors (mianserin, mirtazapine, trazodone) was associated with significantly superior treatment outcomes compared with monotherapy, both as first-line treatment and for nonresponder populations. The dropout numbers did not differ between treatments.
Meaning Combination therapy using an antagonist of presynaptic α2-autoreceptors may be an effective and safe antidepressant treatment option for patients who are nonresponders to monotherapy and as a potential first-line treatment in severe cases of depression.
Importance Combining antidepressants is frequently done in the treatment of acute depression, but studies have yielded conflicting results.
Objective To conduct a systematic review and meta-analysis assessing efficacy and tolerability of combination therapy. Combinations using presynaptic α2-autoreceptor antagonists or bupropion were investigated separately.
Data Sources MEDLINE, Embase, PsycINFO, and the Cochrane Central Register of Controlled Trials were systematically searched from each database inception through January 2020.
Study Selection Randomized clinical trials (RCTs) comparing combinations of antidepressants with antidepressant monotherapy in adult patients with acute depression were included.
Data Extraction and Synthesis Following guidelines from Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and recommendations from the Cochrane Handbook, 2 reviewers independently performed a literature search, study selection, data extraction, and evaluation of risk of bias. Data were pooled in random-effects analyses.
Main Outcomes and Measures Primary outcome was efficacy measured as standardized mean difference (SMD); secondary outcomes were response, remission, change from baseline in rating scale scores, number of dropouts, and number of dropouts due to adverse events.
Results Thirty-nine RCTs including 6751 patients were eligible. Combination treatment was statistically significantly associated with superior treatment outcomes relative to monotherapy (SMD = 0.31; 95% CI, 0.19-0.44). Combining a reuptake inhibitor with an antagonist of presynaptic α2-autoreceptors was superior to other combinations (SMD = 0.37; 95% CI, 0.19-0.55). Bupropion combinations were not superior to monotherapy (SMD = 0.10; 95% CI, −0.07 to 0.27). Numbers of dropouts and dropouts due to adverse events did not differ between treatments. Studies were heterogeneous, and there was indication of publication bias (Egger test result was positive; P = .007, df = 36), but results remained robust across prespecified secondary outcomes and sensitivity and subgroup analyses, including analyses restricted to studies with low risk of bias.
Conclusions and Relevance In this meta-analysis of RCTs comparing combinations of antidepressants with antidepressant monotherapy, combining antidepressants was associated with superior treatment outcomes but not with more patients dropping out of treatment. Combinations using an antagonist of presynaptic α2-autoreceptors may be preferable and may be applied as a first-line treatment in severe cases of depression and for patients considered nonresponders.
Guidelines by the National Institute for Health and Care Excellence, 1 American Psychological Association, 2 and American Psychiatric Association, 3 as well as the German National Clinical Practice Guideline 4 recommend use of a single, non–monoamine oxidase inhibitor antidepressant as initial treatment in severe depression. Despite a host of antidepressant agents, response rates to initial antidepressant monotherapy hover at 60%, and remissions occur in only up to 40% of patients, even after 12 to 24 weeks of treatment. 5
Guidelines advocate a number of second-step treatments for patients considered nonresponders, most prominently switching to a different monotherapy, dose escalation, augmentation (eg, with lithium or second-generation antipsychotics), or combining 2 antidepressants. 1 , 2 , 6 Combining 2 antidepressants is a common next step, particularly in primary care settings, 7 , 8 based on the assumption that combining 2 antidepressants with different modes of action increases clinical efficacy.
In a previous meta-analysis, 9 we showed that, compared with monotherapy, combination therapy is more effective and comparably tolerable as a treatment for acute depression, most notably when applied as a first-line treatment. We also found that this was particularly the case for combinations that include monoamine reuptake inhibitors (selective serotonin reuptake inhibitor, serotonin-norepinephrine reuptake inhibitor, or tricyclic antidepressant) and antagonists of presynaptic α2-autoreceptors (mianserin, mirtazapine, trazodone). In the meantime, several important studies have been published, presenting partly contradictory results. 10 - 13 Based on complementary mechanisms of action, combining mirtazapine or bupropion with reuptake inhibitors has been viewed as particularly promising, with regard to both efficacy and tolerability. 9 , 14 In light of these recent developments, an updated synopsis of the evidence is warranted.
This systematic review and meta-analysis of randomized clinical trials (RCTs) comparing combinations of 2 antidepressants with antidepressant monotherapy in adults with acute depression addresses a number of questions. What is the efficacy of combination therapy, relative to monotherapy, both as first-line treatment and as treatment for nonresponders? Are combination treatments that include mirtazapine or bupropion particularly effective? What is the comparative tolerability of combination therapies?
The protocol of this study has been published on PROSPERO ( CRD42020167739 ). We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses ( PRISMA ) reporting guidelines for systematic reviews 15 and closely adhered to recommendations from the Cochrane Collaboration. 16 The methods are described in detail in the eMethods and eAppendix in the Supplement . In brief, we searched MEDLINE, PsycINFO, Embase, and the Cochrane Central Register of Controlled Trials and selected RCTs meeting the following criteria: an intervention using a combination of 2 antidepressants, irrespective of dosage; a control group of patients taking antidepressant monotherapy; inclusion of participants 18 years or older; and depressive disorder diagnosed according to standard operationalized criteria. Comorbid medical conditions and concomitant diagnoses of other psychiatric disorders were not exclusion criteria. Studies solely focusing on bipolar depression were excluded. We also excluded trials of maintenance therapy. Trials of first-line treatment and trials with patients who had resistance to previous antidepressive treatments were eligible, including both initial combination therapy and adjunctive administration of a second antidepressant. In first-line studies, after randomization, monotherapy control groups received antidepressant monotherapy. In studies including patients resistant to previous antidepressive treatment, monotherapy control-group patients received either ongoing monotherapy with the same antidepressant (the same dose or an increased dose) or monotherapy with a different (switched) antidepressant.
Literature search, study selection, data extraction, and evaluation of risk of bias all were carried out independently by 2 reviewers (J.H. and D.A.) and followed the Cochrane Collaboration Handbook. 16 The included studies were added to the trials retrieved by our previous systematic search, 9 and all analyses were based on the combined set of studies, thus covering all available evidence from the inception of each database to January 1, 2020.
The primary outcome criterion was treatment efficacy measured as the standardized mean difference (SMD) between combination and monotherapy, on an intention-to-treat basis, if possible. Secondary outcome criteria were remission (score below predetermined thresholds, eg, ≤7 on the 17-item Hamilton Depression Rating Scale [HDRS]) and response (eg, ≥50% decrease on the 17-item HDRS or the Montgomery-Asberg Depression Rating Scale [MADRS]) as defined by the study authors, change from baseline on a rating scale score, and numbers of dropouts and dropouts due to adverse events.
Prespecified subgroup analyses included studies with nonresponders to previous treatment trials and with patients new to treatment, combinations including antagonists of presynaptic α2-autoreceptors and combinations including bupropion, and RCTs with low risk of bias. Following the Cochrane Handbook, 16 RCTs were evaluated according to the Cochrane risk-of-bias tool, taking into account random sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective reporting; sponsorship; and other potential sources of bias. An overall assessment of risk of bias (low or unknown/high) was added. Summary SMDs and odds ratios (with 95% CI) were calculated in random-effects meta-analyses because included studies differed methodologically, eg, in regard to blinding or diagnostic criteria and the assessment scales used. Meta-regression analyses carried out post hoc investigated a possible association of baseline depression severity with effect size. Statistical significance was set at α = .05 (2-sided) for the primary, hypothesis-testing outcome. For all secondary outcomes and for all subgroup analyses, P values are presented, but not as a marker of statistical significance. Data analyses were carried out with Comprehensive Meta-analysis software (Version 3, Professional version; Biostat).
During screening of titles and abstracts, most articles were excluded because they did not report on combination treatment, RCTs, or clinical depression.
Our database search retrieved 4244 different articles. During screening of titles and abstracts, most articles were excluded because they did not report on combination treatment, RCTs, or clinical depression. The full texts of 146 articles were read and 7 new studies included. In addition to the previously retrieved set of trials, this amounted to a final set of 39 studies as a basis for the analyses ( Figure 1 ).
In total, trials included 6751 patients. Publication dates ranged from 1977 to 2020. Articles were published in English, Chinese (1 article), and Korean (1 article). Twenty-three studies (59%) were double-blind, 5 studies single-blind, and 11 studies open-label. Twenty-one trials (54%) recruited nonresponders to initial antidepressant treatment. ( Table 1 lists study groups, trial size, and initial antidepressant pharmacotherapy in nonresponder studies.) According to the published reports, only 1 of the studies 17 included patients previously exposed to antidepressant combination treatment.
Of 39 studies included, 38 trial reports provided data on the primary outcome. The SMD was 0.31 (95% CI, 0.19-0.44) in favor of combination treatment ( P < .001). Thirty-one of 38 studies (82%) suggested superior efficacy of combination treatments. Between-study heterogeneity was I 2 = 77.5% and τ was 0.296 ( Figure 2 ).
Combination therapy was associated with superior outcomes when analyses were restricted to studies of low risk of bias (SMD = 0.29; 95% CI, 0.15-0.42), among nonresponder populations (SMD = 0.18; 95% CI, 0.04-0.33), and when applied as a first-line treatment (SMD = 0.52; 95% CI, 0.24-0.79) (eFigure 1 and eFigure 2 in the Supplement ).
Results for sensitivity and subgroup analyses are presented in Table 2 .
Combination of a monoamine reuptake inhibitor with an antagonist of presynaptic α2-autoreceptors (RI+α2) was associated with superior outcomes relative to monotherapy: among all 18 RCTs (SMD = 0.37; 95% CI, 0.19-0.55) ( Figure 3 ), among nonresponder populations (SMD = 0.24; 95% CI, 0.03-0.45), and in particular when applied as a first-line treatment (SMD = 0.64; 95% CI, 0.12-1.15).
Combination therapy that included bupropion was not associated with superior outcomes compared with monotherapy. This applied to analyses among all 7 RCTs (SMD = 0.10; 95% CI, −0.07 to 0.27) (eFigure 3 in the Supplement ), and to its application as first-line treatment (SMD = 0.04; 95% CI, −0.20 to 0.29). Among nonresponder populations, bupropion combinations were superior to monotherapy, with an SMD of 0.17 (95% CI, 0.02 to 0.31).
To avoid undue reliance on single studies, we removed each of the 38 studies in our primary outcome analysis 1 at a time from the calculation of the summary effect. None of the 38 rounds resulted in a substantial change of point estimate or significance for the primary outcome analysis of all RCTs. Effect sizes varied between 0.2 (after elimination of Xu et al 50 ) and 0.34 (when Navarro et al 11 was removed).
For RI+α2 analyses of RCTs, effect sizes varied between 0.32 (after elimination of Blier et al 18 ) and 0.43 (when Kato et al 10 was removed).
For bupropion combination analyses of RCTs, effect sizes varied between 0.06 (after elimination of Gulrez et al 29 ) and 0.15 (when Leuchter et al 33 , 34 was removed).
In meta-regression, baseline HDRS scores were not associated with the SMD between combination treatment and mono-therapy (coefficient: 1.1; 95% CI, −1.3 to 3.5; P = .37; n = 26 studies). In a sensitivity analysis of 18 studies reporting outcome data that were based on follow-up examinations, such as remission rates, meta-regression returned similar results (coefficient: −2.6; 95% CI, −8.3 to 3.1; P = .37). Meta-regression based on MADRS baseline scores was not calculated as planned because the number of studies providing data was too small.
Primary and secondary outcomes as well as subgroup analyses are presented in Table 2 .
Secondary outcome analyses of efficacy, based on remission and response rates as well as continuous data (change from baseline in rating scale scores), produced results that were generally in line with our primary outcome results ( Table 2 ).
With respect both to patients dropping out of treatment for any reason and to dropouts due to adverse events, data for combination and monotherapy were similar (odds ratio = 0.99; 95% CI, 0.86-1.14; and odds ratio = 1.17; 95% CI, 0.79-1.75, respectively). Heterogeneity in these analyses was low ( I 2 = 3.66% and I 2 = 20.87%, respectively).
Fifteen of the 39 included studies (38%) were considered to be of higher methodological rigor (“low” risk of bias). Summary ratings confirmed our primary outcome analysis and are displayed in Table 1 (also Figure 2 and Figure 3 ).
I 2 statistics indicated substantial between-study heterogeneity in most of the primary outcome analyses, but significantly less so in most of the subgroup analyses, especially in analyses of response and dropouts ( Table 2 ). Heterogeneity as measured by τ was substantially lower in sensitivity analyses (restricted to studies with low risk of bias), and τ indicated that the standard deviation of the weighted SMD estimate was approximately equal to or lower than the effect size.
The funnel plot of studies included in the primary outcome analysis indicated small study effects (eFigure 4 in the Supplement ). An Egger test result was positive ( P = .007, df = 36). A trim-and-fill procedure (Duval and Tweedie) with 10 studies trimmed to the left of the mean resulted in a reduced effect size that was still statistically significant (0.13; 95% CI, 0.001-0.26). Twenty-two studies with an effect size of 0 would be necessary to reduce the overall effect to 0.1 (Orwin fail-safe N).
For RI+α2 analyses, an Egger test result was positive ( P = .02, df = 16). A trim-and-fill procedure (Duval and Tweedie) with 6 studies trimmed to the left of the mean resulted in a reduced effect size that was still statistically significant (0.19; 95% CI, 0.01-0.36).
This study yielded 2 main results. First, combination treatment as a general principle seems to be more effective than monotherapy without being associated with higher numbers of patients dropping out. Second, the combination of monoamine reuptake inhibitors (selective serotonin reuptake inhibitor, serotonin-norepinephrine reuptake inhibitor, or tricyclic antidepressant) and α2-adrenergic receptor antagonists (RI+α2) seems to be the most effective and preferable antidepressant combination.
Combination therapy may primarily be applied as a second-step treatment after insufficient response to initial monotherapy. Our findings suggest that using an RI+α2 combination is more effective in these cases compared with monotherapy. On the other hand, in a recent meta-analysis, switching antidepressant monotherapy for patients considered nonresponders was not more effective than sticking to the initial antidepressant. 54 In the same vein, after nonresponse to a standard dose of selective serotonin reuptake inhibitor, a dose increase did not result in superior efficacy compared with continuation of the initial dose. 55
Combination therapy was not associated with more dropouts or adverse events leading to discontinuation. It may thus be a safe treatment alternative when compared with other second-step strategies in treatment-resistant depression, such as augmenting monotherapy with lithium or atypical antipsychotics. 56 , 57 Our analysis of the RI+α2 combination in nonresponders resulted in statistically significant but small effect sizes (SMD = 0.2). Still, patients who are resistant to treatment present a particular challenge, and effect sizes resulted from comparisons with active treatment (ongoing monotherapy, increasing the dose, or switching antidepressants). Such comparisons are likely to result in lower estimates of efficacy than contrasting combination and monotherapy in first-line treatment trials. Here, the RI+α2 combination seems to be particularly effective, with an effect size of SMD = 0.64. Antidepressant monotherapy itself has effect sizes of no more than about 0.3 compared with placebo. 58 , 59 Of note, trials in our analysis also included populations with difficult-to-treat chronic depression. 32 , 45
We have previously shown that the favorable treatment outcomes of combination therapy in comparison with monotherapy are not a dosage effect only. 9 Also, some of the included trials found superior effects with subtherapeutic doses of a second antidepressant in RI+α2 combinations. 32 , 36 - 38 Therefore, pharmacodynamic and clinical synergisms seem likely. For example, sedating α2-adrenergic receptor antagonists may counteract the restlessness, agitation, and sexual dysfunction associated with monoamine reuptake inhibitors. Reuptake inhibitors in monotherapy are likely to stimulate presynaptic α2-receptors by enhancing the intrasynaptic concentrations of serotonin and norepinephrine. However, combinations with blockers of presynaptic α2-receptors are supposed to prevent the negative feedback effect on neurotransmission induced by a stimulation of α2-receptors.
The relative tolerability of combination therapy and the modest response rates with initial antidepressant monotherapy also suggest considering RI+α2 combination therapy as a first-line treatment, at least in severe cases of depression.
On the whole, in our analysis, treatment effects of antidepressant combinations were not associated with baseline severity. According to these results, combination treatment is effective regardless of initial illness severity. Nevertheless, this finding must be viewed as preliminary because it rests on a subset of studies and only on outcomes ascertained by HDRS.
While the addition of bupropion has previously been shown to alleviate antidepressant-induced sexual dysfunction, 14 and its addition to antidepressant monotherapy can be clinically sensible, our findings indicate that bupropion combinations in general are not associated with substantial enhancement of antidepressive efficacy compared with monotherapy. This result is counterintuitive because bupropion, with its dopaminergic properties, has a mechanism of action that may complement classical antidepressant pathways. Note that in nonresponder populations, the summary results for bupropion combinations remain inconclusive rather than negative, mainly because of the small number of methodologically sound studies existing to date: the CI spans a negative as well as a sizable positive effect.
First, I 2 values indicated substantial heterogeneity of effects. However, heterogeneity is known to increase with accumulating numbers. Additional τ statistics were calculated, indicating a spread of data not unfamiliar in medical studies: the standard deviation was lower than or had the same order of magnitude as the effect size. Nevertheless, as in most meta-analyses, included studies were not homogenous in their design, eg, with differences in blinding status or in the definition of nonresponse to previous antidepressant treatment. As a consequence, we applied random-effects models and showed that results remained robust after each study was left out. Further, dichotomizing criteria of treatment success in subgroup analyses, as in remission and response, supported the main results and explained large parts of the between-study heterogeneity. In the same vein, sensitivity analyses among studies of high methodological rigor (low risk of bias) and among double-blind studies (data not shown) also backed our main findings.
Second, funnel plot asymmetry indicated possible reporting bias. However, in combination treatment studies, reporting bias might not be as important as it is in placebo trials of antidepressant monotherapies because there is no negative result in the strict sense, and thus no disincentive to publish. Nevertheless, even when fully adjusting for possible publication bias, a reduced but still positive and statistically significant effect remained (for RI+α2 combination: SMD = 0.19; 95% CI, 0.01-0.36). Also, the observed funnel plot asymmetry may be caused by plot distortion associated with transforming a variety of outcomes into SMD, as has recently been emphasized. 60 Reassuringly, therefore, sensitivity analyses using raw mean differences resulted in a substantially reduced funnel plot asymmetry (data not shown).
There also is considerable indication that the funnel plot asymmetry may represent a true asymmetry of observable effects. For example, many of the studies on bupropion combinations were recent and had large sample sizes but yielded only small effects. Low effect size and small-variance studies may distort funnel plots to the upper left quadrant. Besides, we observed funnel plot asymmetry only among studies of treatment-resistant depression and not among studies using a combination as a first-line treatment.
Third, true between-study heterogeneity may result not only from different study populations and combination treatments but also from different control groups. This particularly applies to studies of treatment-resistant depression, where active comparators were continuation, increased dose, or switching antidepressant. And yet, regardless of the kind of comparator, combination treatment was associated with higher efficacy (data not shown).
It is conceivable that antidepressant discontinuation syndromes may have interacted with outcomes. However, it has been shown that when the switch is between antidepressants, discontinuation syndromes rarely pose clinical problems. 61
For clinical practice, physicians should be aware that combinations of reuptake inhibitors (selective serotonin reuptake inhibitor, serotonin-norepinephrine reuptake inhibitor, or tricyclic antidepressant) with α2-autoreceptor antagonists are a potent treatment option, associated with superior outcomes relative to monotherapy. Clinicians can inform patients that on average this advantage does not come at the cost of lower tolerability and that there is reason to believe in a synergistic therapeutic effect. While we did not find an association of outcome and severity of depression, we believe combination treatment particularly suggests itself in severe cases of depression and for patients resistant to standard treatment. Research should focus on the dearth of methodologically rigorous data on bupropion combinations for nonresponder populations.
Accepted for Publication: December 8, 2021.
Published Online: February 16, 2022. doi:10.1001/jamapsychiatry.2021.4313
Corresponding Author: Christopher Baethge, MD ( [email protected] ), and Jonathan Henssler, MD ( [email protected] ), Klinik für Psychiatrie und Psychotherapie, Universität zu Köln; Kerpener Straße 62, 50937 Köln, Germany.
Author Contributions: Dr Henssler had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Henssler, Bschor, Baethge.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Henssler, Alexander, Baethge.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Henssler, Schwarzer, Bschor, Baethge.
Administrative, technical, or material support: Henssler, Alexander, Bschor.
Supervision: Henssler, Bschor, Baethge.
Conflict of Interest Disclosures: Outside of this work, Dr Henssler received a research grant from the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) (grant No. 01KG1808). Dr Schwarzer reported personal fees from Roche Pharma as an external statistical consultant outside the submitted work. No other disclosures were reported.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Long-term outcomes of combination biologic therapy in uncontrolled severe asthma: a case study
Affiliations.
- 1 Department of Health Sciences, Respiratory Unit, ASST Santi Paolo e Carlo, San Paolo Hospital, University of Milan, Milan, Italy.
- 2 Department of Internal Medicine, Asthma and Allergy, Norbert Barlicki Memorial Teaching Hospital No. 1; Medical University of Lodz, Lodz, Poland.
- PMID: 35913268
- DOI: 10.1080/02770903.2022.2109162
Introduction: Treatment with biologics has significantly reduced the social and economic burden of severe asthma. However, some patients may still feature a suboptimal control of their symptoms while on therapy. In this subset of asthmatic patients, a benefit from a dual biologic therapy has sporadically been reported in literature. Our aim is to add our experience to the limited body of evidence supporting combination biologic therapies.
Case study: Here we present the case of a 68-year-old nonsmoker female, with an allergic and eosinophilic corticosteroid-dependent severe asthma. She displayed well controlled comorbidities and good adherence to the inhaled therapy. Omalizumab was started in 2008 with an initial remarkable clinical improvement. After nine years of biologic therapy, she reported a gradual worsening of her symptoms and exacerbations. Mepolizumab was then added in 2019.
Results: The addition of Mepolizumab resulted in a meaningful amelioration of her quality of life, asthma control, number of exacerbations and 6-minute-walking-distance at 3-year follow-up. The average Prednisone dosage was tapered from 25 mg to 20 mg daily. No adverse events were observed since the introduction of the second biologic.
Conclusion: Our experience indicates that Mepolizumab may be beneficial and safe as an add-on biologic in a patient whose allergic and eosinophilic asthma remains uncontrolled despite treatment with an anti-IgE strategy. Further studies on a larger number of patients are required to demonstrate whether the positive outcomes published so far are replicable on a larger scale.
Keywords: Severe asthma; allergic asthma; biologic therapy; eosinophilic asthma; uncontrolled asthma.
PubMed Disclaimer
Similar articles
- Switching from omalizumab to mepolizumab: real-life experience from Southern Italy. Carpagnano GE, Pelaia C, D'Amato M, Crimi N, Scichilone N, Scioscia G, Resta O, Calabrese C, Pelaia G, Quarato CMI, Foschino Barbaro MP. Carpagnano GE, et al. Ther Adv Respir Dis. 2020 Jan-Dec;14:1753466620929231. doi: 10.1177/1753466620929231. Ther Adv Respir Dis. 2020. PMID: 32482128 Free PMC article.
- Clinical and economic burden of severe asthma among US patients treated with biologic therapies. Reibman J, Tan L, Ambrose C, Chung Y, Desai P, Llanos JP, Moynihan M, Tkacz J. Reibman J, et al. Ann Allergy Asthma Immunol. 2021 Sep;127(3):318-325.e2. doi: 10.1016/j.anai.2021.03.015. Epub 2021 Mar 26. Ann Allergy Asthma Immunol. 2021. PMID: 33775904
- A tailored approach to refractory severe Mepolizumab-associated headache: a case study. Salerni C, Baccelli A, Parazzini EM, Rinaldo R, Centanni S. Salerni C, et al. J Asthma. 2024 Jun;61(6):649-652. doi: 10.1080/02770903.2023.2294913. Epub 2023 Dec 21. J Asthma. 2024. PMID: 38088891
- Omalizumab as alternative to chronic use of oral corticosteroids in severe asthma. Katsaounou P, Buhl R, Brusselle G, Pfister P, Martínez R, Wahn U, Bousquet J. Katsaounou P, et al. Respir Med. 2019 Apr;150:51-62. doi: 10.1016/j.rmed.2019.02.003. Epub 2019 Feb 7. Respir Med. 2019. PMID: 30961951 Review.
- Omalizumab for the treatment of severe persistent allergic asthma: a systematic review and economic evaluation. Norman G, Faria R, Paton F, Llewellyn A, Fox D, Palmer S, Clifton I, Paton J, Woolacott N, McKenna C. Norman G, et al. Health Technol Assess. 2013 Nov;17(52):1-342. doi: 10.3310/hta17520. Health Technol Assess. 2013. PMID: 24267198 Free PMC article. Review.
- Combination of Biological Therapy in Severe Asthma: Where We Are? Carriera L, Fantò M, Martini A, D'Abramo A, Puzio G, Scaramozzino MU, Coppola A. Carriera L, et al. J Pers Med. 2023 Nov 10;13(11):1594. doi: 10.3390/jpm13111594. J Pers Med. 2023. PMID: 38003909 Free PMC article. Review.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Taylor & Francis
- Genetic Alliance
- MedlinePlus Consumer Health Information
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Help & FAQ
Immunogenicity of BNT162b2 Vaccine Booster Dose in Patients With Inflammatory Bowel Disease Receiving Infliximab Combination Therapy: A Prospective Observational Study
- Kuwait University
- Dasman Diabetes Institute
Research output : Contribution to journal › Article › peer-review
Introduction: Few data exist regarding the immunogenicity of the third dose of BNT162b2 relative to the second dose in patients with inflammatory bowel disease (IBD) on different immunosuppressive therapies. We investigated the immunogenicity of BNT162b2 vaccine booster dose in patients with IBD on infliximab combination therapy. Method: This is a prospective single-center observational study conducted from January 1, 2022 to February 28, 2022. Patients were recruited at the time of attendance at the infusion center. Eligibility criteria included patients with a confirmed diagnosis of IBD who are receiving infliximab with azathioprine or 6-mercaptopurine. Patients who received two doses of BNT162b2 vaccine (second dose group) were compared to patients who had received three doses of BNT162b2 vaccine [third dose (booster) group]. Patients were excluded if they were infected or had symptoms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) previously since the start of the pandemic or received other vaccines than the BNT162b2. Our primary outcome was the concentrations of SARS-CoV-2 antibodies Immunoglobulin G (IgG) and neutralizing antibodies 40–45 weeks from the first dose of BNT162b2 vaccine in patients with IBD receiving infliximab combination therapy. Medians with interquartile range (IQR) were calculated. Results: In total, 162 patients with IBD and receiving infliximab combination therapy were recruited, and the number of patients in both the second dose group and third dose (booster) group was 81. Mean age was 35 years old in both groups. Median (IQR) SARS-CoV-2 IgG levels were significantly lower after the second dose [125 BAU/ml (43, 192)] compared to patients who received the third booster dose [207 BAU/ml (181, 234)] (P = 0.003). Neutralizing antibody levels were also lower after the second dose [80% (21, 95)] compared to patients who received the third booster dose [96% (93, 99)] (P ≤ 0.001). The percentage of patients who achieved positive SARS-CoV-2 IgG levels in the third (booster) dose group was 96.3%, whereas it was 86.4% in the second dose group. The percentage of participants who received the third (booster) dose and achieved a positive SARS-CoV-2-neutralizing antibody level was 100%, whereas it was 88.9% in the participants who received the second dose only. Conclusion: Most patients with IBD on infliximab combination therapy had positive SARS-CoV-2 IgG and neutralizing antibody concentrations 40–45 weeks post BNT162b2 vaccination. However, SARS-CoV-2 IgG and neutralizing antibody concentrations were lower in patients who received two doses only compared to patients who received a third dose. A longer follow-up study is needed to evaluate decay in antibodies over time.
| Original language | English |
|---|---|
| Article number | 933996 |
| Journal | |
| Volume | 9 |
| DOIs | |
| State | Published - 4 Jul 2022 |
- immunogenicity
This output contributes to the following UN Sustainable Development Goals (SDGs)
Access to Document
- 10.3389/fmed.2022.933996
Other files and links
- Link to publication in Scopus
Fingerprint
- Vaccine Efficacy Immunology and Microbiology 100%
- Immunogenicity Immunology and Microbiology 100%
- Booster Dose Immunology and Microbiology 100%
- Infliximab Immunology and Microbiology 100%
- Inflammatory Bowel Disease Immunology and Microbiology 100%
- Severe Acute Respiratory Syndrome Coronavirus 2 Immunology and Microbiology 87%
- Neutralizing Antibody Immunology and Microbiology 62%
- Immunoglobulin G Antibody Immunology and Microbiology 37%
T1 - Immunogenicity of BNT162b2 Vaccine Booster Dose in Patients With Inflammatory Bowel Disease Receiving Infliximab Combination Therapy
T2 - A Prospective Observational Study
AU - Shehab, Mohammad
AU - Alrashed, Fatema
AU - Alfadhli, Ahmad
AU - Alsayegh, Abdulwahab
AU - Aldallal, Usama
AU - Alsayegh, Mariam
AU - Cherian, Preethi
AU - Alkhair, Irina
AU - Thanaraj, Thangavel Alphonse
AU - Channanath, Arshad
AU - Dashti, Ali A.
AU - Albanaw, Anwar
AU - Ali, Hamad
AU - Abu-Farha, Mohamed
AU - Abubaker, Jehad
AU - Al-Mulla, Fahd
N1 - Funding Information: This study was funded by the Kuwait Foundation for the Advancement of Sciences (KFAS) grant (RA HM-2021-008). Publisher Copyright: Copyright © 2022 Shehab, Alrashed, Alfadhli, Alsayegh, Aldallal, Alsayegh, Cherian, Alkhair, Thanaraj, Channanath, Dashti, Albanaw, Ali, Abu-Farha, Abubaker and Al-Mulla.
PY - 2022/7/4
Y1 - 2022/7/4
N2 - Introduction: Few data exist regarding the immunogenicity of the third dose of BNT162b2 relative to the second dose in patients with inflammatory bowel disease (IBD) on different immunosuppressive therapies. We investigated the immunogenicity of BNT162b2 vaccine booster dose in patients with IBD on infliximab combination therapy. Method: This is a prospective single-center observational study conducted from January 1, 2022 to February 28, 2022. Patients were recruited at the time of attendance at the infusion center. Eligibility criteria included patients with a confirmed diagnosis of IBD who are receiving infliximab with azathioprine or 6-mercaptopurine. Patients who received two doses of BNT162b2 vaccine (second dose group) were compared to patients who had received three doses of BNT162b2 vaccine [third dose (booster) group]. Patients were excluded if they were infected or had symptoms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) previously since the start of the pandemic or received other vaccines than the BNT162b2. Our primary outcome was the concentrations of SARS-CoV-2 antibodies Immunoglobulin G (IgG) and neutralizing antibodies 40–45 weeks from the first dose of BNT162b2 vaccine in patients with IBD receiving infliximab combination therapy. Medians with interquartile range (IQR) were calculated. Results: In total, 162 patients with IBD and receiving infliximab combination therapy were recruited, and the number of patients in both the second dose group and third dose (booster) group was 81. Mean age was 35 years old in both groups. Median (IQR) SARS-CoV-2 IgG levels were significantly lower after the second dose [125 BAU/ml (43, 192)] compared to patients who received the third booster dose [207 BAU/ml (181, 234)] (P = 0.003). Neutralizing antibody levels were also lower after the second dose [80% (21, 95)] compared to patients who received the third booster dose [96% (93, 99)] (P ≤ 0.001). The percentage of patients who achieved positive SARS-CoV-2 IgG levels in the third (booster) dose group was 96.3%, whereas it was 86.4% in the second dose group. The percentage of participants who received the third (booster) dose and achieved a positive SARS-CoV-2-neutralizing antibody level was 100%, whereas it was 88.9% in the participants who received the second dose only. Conclusion: Most patients with IBD on infliximab combination therapy had positive SARS-CoV-2 IgG and neutralizing antibody concentrations 40–45 weeks post BNT162b2 vaccination. However, SARS-CoV-2 IgG and neutralizing antibody concentrations were lower in patients who received two doses only compared to patients who received a third dose. A longer follow-up study is needed to evaluate decay in antibodies over time.
AB - Introduction: Few data exist regarding the immunogenicity of the third dose of BNT162b2 relative to the second dose in patients with inflammatory bowel disease (IBD) on different immunosuppressive therapies. We investigated the immunogenicity of BNT162b2 vaccine booster dose in patients with IBD on infliximab combination therapy. Method: This is a prospective single-center observational study conducted from January 1, 2022 to February 28, 2022. Patients were recruited at the time of attendance at the infusion center. Eligibility criteria included patients with a confirmed diagnosis of IBD who are receiving infliximab with azathioprine or 6-mercaptopurine. Patients who received two doses of BNT162b2 vaccine (second dose group) were compared to patients who had received three doses of BNT162b2 vaccine [third dose (booster) group]. Patients were excluded if they were infected or had symptoms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) previously since the start of the pandemic or received other vaccines than the BNT162b2. Our primary outcome was the concentrations of SARS-CoV-2 antibodies Immunoglobulin G (IgG) and neutralizing antibodies 40–45 weeks from the first dose of BNT162b2 vaccine in patients with IBD receiving infliximab combination therapy. Medians with interquartile range (IQR) were calculated. Results: In total, 162 patients with IBD and receiving infliximab combination therapy were recruited, and the number of patients in both the second dose group and third dose (booster) group was 81. Mean age was 35 years old in both groups. Median (IQR) SARS-CoV-2 IgG levels were significantly lower after the second dose [125 BAU/ml (43, 192)] compared to patients who received the third booster dose [207 BAU/ml (181, 234)] (P = 0.003). Neutralizing antibody levels were also lower after the second dose [80% (21, 95)] compared to patients who received the third booster dose [96% (93, 99)] (P ≤ 0.001). The percentage of patients who achieved positive SARS-CoV-2 IgG levels in the third (booster) dose group was 96.3%, whereas it was 86.4% in the second dose group. The percentage of participants who received the third (booster) dose and achieved a positive SARS-CoV-2-neutralizing antibody level was 100%, whereas it was 88.9% in the participants who received the second dose only. Conclusion: Most patients with IBD on infliximab combination therapy had positive SARS-CoV-2 IgG and neutralizing antibody concentrations 40–45 weeks post BNT162b2 vaccination. However, SARS-CoV-2 IgG and neutralizing antibody concentrations were lower in patients who received two doses only compared to patients who received a third dose. A longer follow-up study is needed to evaluate decay in antibodies over time.
KW - COVID-19
KW - booster
KW - immunogenicity
KW - infliximab
KW - vaccine
UR - http://www.scopus.com/inward/record.url?scp=85134412277&partnerID=8YFLogxK
U2 - 10.3389/fmed.2022.933996
DO - 10.3389/fmed.2022.933996
M3 - Article
AN - SCOPUS:85134412277
SN - 2296-858X
JO - Frontiers in Medicine
JF - Frontiers in Medicine
M1 - 933996
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 08 December 2021
Combination therapy patents: a new front in evergreening
- Garth W. Strohbehn ORCID: orcid.org/0000-0003-2973-3040 1 , 2 na1 nAff7 ,
- Alec J. Kacew 1 ,
- Daniel A. Goldstein 2 , 3 , 4 , 5 ,
- Robin C. Feldman ORCID: orcid.org/0000-0002-3453-134X 6 na1 &
- Mark J. Ratain 1 , 2
Nature Biotechnology volume 39 , pages 1504–1510 ( 2021 ) Cite this article
12k Accesses
8 Citations
172 Altmetric
Metrics details
- Intellectual-property rights
- Randomized controlled trials
As pharmaceutical companies seek patent protection for combinations of cancer therapeutics, it is worthwhile to assess what constitutes an ‘unexpected result’ for the purpose of an appropriate patent and whether randomized, controlled trials of drug combinations have the ability to generate them.
The patent system is designed so that inventors who bear the burden of innovation risks and are successful in their efforts have the potential to reap substantial rewards. From a constitutional perspective, however, the ultimate goal is not the benefit of individual inventors but the benefit to society as a whole 1 . The government provides particular citizens the right to exclude others from certain products or activities for a limited time, in the hope that doing so will lead to benefits for everyone 2 .
Pharmaceutical companies have become adept at legal and business strategies aimed at extending the period of protection, frequently through minor modifications to a drug’s dosage, formulation or delivery system 3 , 4 , 5 . Commonly known as ‘evergreening’, these strategies allow innovators to increase the period of time during which their successful drug can generate revenue against limited competition, while imposing financial and patient care costs on society 3 , 4 . Here, we review the legal concepts of obviousness and unexpected results — terms with which both physicians and regulators (for example, the US Food and Drug Administration (FDA)) should be familiar in light of the emergence of pharmaceutical combination patents — and examine their interaction with current clinical research. In the highly profitable oncology sector, we evaluated whether pharma companies are employing a new variant of these strategies by combining ‘backbone’ drugs (widely accepted standards of care) with other drugs likely to be used to treat a given disease state 3 , 4 . Specifically, we focused our efforts on a subset of combination therapies developed in cancer as a case study. Using publicly available information, we assess the current patent and clinical trial landscapes for this combination. We demonstrate that this new front in evergreening not only lacks the inventive nature typically justifying the 20-year patent reward but also leads to the design and development of clinical trials that lack even the potential to yield unexpected results characteristic of a patentable invention — a finding similar to the strategic behaviors of drug- and device-makers in generating and studying drug–device combinations 6 . Given the implications of these emerging strategic behaviors, we close by offering a proposal for clinical trial elements that are necessary to provide a thoughtful evaluation of the non-obviousness of combination therapy patents, as well as suggesting ways in which inter-agency collaboration at the federal level could help both industry and researchers focus on achieving optimal innovation in the public interest.
A brief review of patentability
Five canonical elements are required of an innovation for it to be patentable under US law: the invention must be of proper subject matter, useful, novel, non-obvious and the application must include proper disclosure 7 . Of particular relevance to combination therapy patents, the inventor must demonstrate that the invention would not have been obvious to a person having ordinary skill in the art (POSITA), which is defined as a person who has the capability to understand the scientific and engineering principles applicable to the relevant art 8 , 9 . Precise requirements vary according to the case and the invention, but in most patent cases involving drug development, the POSITA has skills commensurate with an advanced degree (for example, PhD or MD) along with experience in the research or treatment of the specific disease state(s) 10 . As the US Supreme Court has noted, a POSITA is expected to be a person of ordinary creativity, one who is able to fit the teachings of multiple patents together like pieces of a puzzle 8 . Some recent courts have found that a POSITA would also be supported by the insights of a multi-disciplinary drug discovery and development team 10 . If an invention would be obvious to this creative POSITA who is backed by a team, then the court should reject the patent.
Importantly, the term ‘invention’ goes beyond the notion of tinkering. A related concept, ‘obvious to try’, holds that an invention is unpatentable when it is comprised of a set of re-combined elements, the various permutations of which would be predictable to try 8 . Absolute predictability is not required, nor does it matter whether the trials of the re-combined elements require extensive time, money and effort to test 11 . Rather, the invention is unpatentable if there is a finite number of identified solutions — that is, a set of things to try — and a POSITA would have a reasonable expectation of success if he or she were to try each of these solutions in turn 8 , 11 . Multiple courts have explicitly stated that it is normal for scientists to optimize each of the variables in a known process to improve on what is generally known already — but that acts of optimization are not themselves patentable 11 , 12 .
A claimant can potentially overcome obviousness rejections by demonstrating what are known as objective indicia of an invention’s non-obviousness. These may include achievement of commercial success, fulfillment of a long felt but unsolved need, prior unsuccessful attempts by other POSITAs to solve the problem, evidence that the field’s conventional wisdom ‘teaches away’ from the inventor’s solution, or unexpected results that a POSITA would not anticipate 8 . Among the objective indicia, generation of unexpected results is the most common avenue for successfully demonstrating non-obviousness, particularly for pharmaceutical inventions 13 , 14 . As courts explain, the principle behind the doctrine of unexpected results is straightforward: an invention that exhibits a superior characteristic or advantage in a way that surprises even a skilled artisan immersed in the field is clearly not obvious 15 . Courts have determined that for a result to be unexpected, it must differ from the POSITA’s expected results in kind rather than degree 16 . In the context of a combination of two drugs that are each effective when given sequentially, the patent claimant must demonstrate that the properties of the combination are specifically the result of having combined the drugs, rather than reflecting properties of one or both of the drugs, given alone or sequentially. Consider, for example, two modestly effective drugs that each improves survival by two months when given in sequence. Prolongation of survival by, say, not two months but two years and an observed decrease in side effects by administering the two drugs as a combination would be an unexpected result.
Combination therapy in medicine
The prior art.
Combining two active therapies into a single regimen is a common practice in medicine: angiotensin-converting enzyme inhibitors and diuretics in hypertension, long-acting beta agonists and inhaled corticosteroids in chronic obstructive pulmonary disease, and combinations of antiretrovirals and protease inhibitors in HIV are but three widely prescribed examples. Oncology is particularly fertile ground, as chemotherapy combinations have been used to treat cancer for well over a half-century, leading to the cures of select malignancies in the 1960s and 1970s 17 , 18 , 19 , 20 , 21 . Consequently, combining two or more drugs has become a standard approach in the treatment of the vast majority of cancers 22 . Testing whether patients who receive the combination of therapies A and B (A + B) have improved outcomes, relative to those who receive either A or B alone, became a standard approach in oncology drug development 23
With decades of accumulated experience, key principles of oncology clinical trial design have been derived and are distilled in Box 1 . These precepts are understood not only by the sophisticated multi-disciplinary drug discovery and development teams of the modern age but, at the very least, are intuited by clinical practitioners. Combining active anticancer drugs in the search for more efficacious regimens has been employed for decades. Consequently, the resulting patent claims may be entirely unpatentable, as the combination approach has long existed in the prior art and was obvious to try. In light of these obviousness concerns, the question becomes whether a clinical trial — in any subspecialty of medicine — might produce results so surprising that they would meet the threshold of ‘unexpected’.
Box 1 Key principles of oncology clinical trial design
Rather than hard and fast ‘laws’, the following generally accepted precepts in oncology clinical trials and oncology clinical practice are known to both practicing and researching oncologists alike 23 . Consequently, these precepts help to inform oncology’s prior art.
Precept 1: combination and progression-free survival
Begin with two hypothetical drugs, A and B, with different mechanisms of action, each of which independently increases the amount of time that a tumor does not grow, or progression-free survival (PFS), in a disease state. Combining A and B would be expected to extend PFS (as well as, possibly, the amount of time that the patient lives, called overall survival (OS), more than either of the individual drugs alone.
If PFS A > 0 and PFS B > 0, and OS A > 0 and OS B > 0, then none of the following are unexpected results:
Precept 2: sequential administration and PFS
Sequentially administering individually active chemotherapy agents is a standard approach taken by practicing oncologists in treating an incurable malignancy. The goal is to extract maximal benefit from a single drug before the tumor achieves resistance to that drug. Administering up front and in combination two drugs that may otherwise be administered sequentially may increase PFS without impacting OS.
If PFS A > 0 and PFS B > 0, then neither of the following are unexpected results:
Precept 3: structurally similar drugs behave similarly
When used in the same disease state, drugs with the same mechanism of action are likely to confer similar PFS and OS benefit.
If A ≅ C or B ≅ D, PFS A > 0 and PFS B > 0, and OS A > 0 and OS B > 0, then none of the following are unexpected results:
Precept 4: the straw man
Many drugs, administered as single agents with palliative intent in later lines of therapy, appear to prolong PFS and OS by clinically relevant amounts of time, but only when compared to the counterfactuals of administering a poorly performing drug or no therapy at all. Combining two active drugs with different mechanisms of action will likely confer statistically and clinically significant PFS and OS benefit when compared to the third drug.
If PFS A > 0 and PFS B > 0, OS A > 0 and OS B > 0, and both PFS E and OS E → 0, then neither of the following are unexpected results:
Discovering the unexpected
From the clinical trial precepts in Box 1 flow features necessary of a clinical trial of an oncology combination therapy (A + B) to demonstrate an unexpected result. First, clinical trial participants ought to be randomly assigned to either the intervention or the comparator arm(s) — they must be randomized controlled trials (RCTs). Randomization helps ensure that the observed outcome of the clinical trial is due to differences in the treatments rather than biased treatment arm assignment. Without randomization, differences in outcomes can be driven by baseline patient characteristics, rather than differences in the treatments.
Second, the most appropriate comparator arm(s) is a sequence (or sequences) of the two drugs that make up the combination (for example, A → B and/or B → A). If A + B confers 12 months of survival while A → B confers 6 months of survival, then combining A and B is clearly advantageous. Alternatively, if the comparator arm is, say, A followed by something else, then claims about the benefit act of combining cannot be made. Clinical trials that allow crossover from comparator to intervention arm at progression are unable to make strong conclusions about overall survival (OS — the amount of time between randomization and the patient’s death) without assuming that subsequent treatments are similar in their effects on outcomes 24 . Only utilization of pre-determined sequences of therapy allow for meaningful comparison, albeit with limitation of freedom of choice at first progression.
Finally, the primary outcome measure of the RCT ought to be OS. This is contrasted with progression-free survival (PFS), a surrogate outcome that measures the time between randomization and the patient’s disease getting worse (progressing) that has controversially been relied on for recent regulatory decisions 25 , 26 . As demonstrated in Box 1 , due to the nature of combining individually active therapies, none of the possible patentable PFS results would be unexpected: given the individual activity in the disease state of both A and B, the POSITA would expect A + B to have equal or greater PFS benefit to A → B or B → A, and if A + B has lower PFS benefit, then the point is moot.
Armed with a basic understanding of patentability, oncology’s prior art, and principles of clinical trials that ought to be present to enable identification of truly unexpected results, we can now turn our attention to real-world applications. Let us examine an emerging combination therapy in oncology as a case example.
Evergreening combinations
The results of analyses in numerous clinical trials suggesting the exceptional benefit of combining vascular endothelial growth factor (VEGF) inhibitors with immune checkpoint blockade (also referred to as immunotherapy (IO)) were presented at the European Society of Molecular Oncology 2020 Annual Meeting 27 , 28 , 29 , 30 . VEGF and IO have individually demonstrated efficacy in a variety of cancers, including advanced and metastatic renal cell carcinoma (mRCC) 31 , 32 . VEGF inhibitors reduce a tumor’s growth by blocking its ability to recruit and build a blood supply, while IO acts by inhibiting the signals that a cancer uses to evade the body’s immune system 33 . VEGF inhibitors may also increase intratumoral T-cell infiltration, an immunologic change that likely enhances IO’s anticancer efficacy 34 .
Given the decades-long approach in oncology of combining drugs with different mechanisms of action, it would be obvious for a POSITA to consider combining these two therapies. In fact, VEGF–IO combinations have been hypothesized since 2006 (ref. 34 ), trialed in a variety of cancers 33 , and have since become, arguably, standard of care for the first-line treatment of advanced or mRCC 35 . All of this occurred before the 2020 trial result announcements.
The VEGF–IO patent landscape
Given the ongoing clinical investigation surrounding VEGF–IO, it would be consistent with the self-interested behavior of a corporation to seek out patent protection for this combination. We therefore set out to determine whether substantial patent activity — evidence beyond anecdote — is occurring for combinations of drugs ordinarily known by relevant oncology practitioners. Given oncology’s prior knowledge, these combinations are likely to constitute obvious (or obvious to try) formulations 8 . We searched TotalPatent One (LexisNexis) during July and August 2020 for 54 combinations of drugs, containing one each of FDA-approved VEGF or multi-tyrosine kinase inhibitors — axitinib, cabozantinib, lenvatinib, pazopanib, ponatinib, regorafenib, sorafenib, sunitinib, and vandetanib — and FDA-approved programmed cell death protein 1 (PD-1)-axis-inhibiting IO monoclonal antibodies — pembrolizumab, nivolumab, atezolizumab, durvalumab, avelumab and cemiplimab.
Our database search yielded 19,509 patents and patent applications (Fig. 1 ). Among these, we considered only patents filed by the owner company of the VEGF drug or IO monoclonal antibody in question, given that such patents are the most likely to suggest that a company is attempting to evergreen the protections on an existing drug therapy 3 . To verify that at least some of the claims within the patents were specifically drafted to the drug combination in question, and to eliminate any patents that specified additional components beyond the combination therapy, such as other antibodies or proteins, we examined in detail those patents determined to be the most relevant for a given drug combination. Selection of the most relevant patent is best illustrated with an example: if two similar patent applications were identified for the same drug combination, one mentioning the drug combination in the specifications and the other mentioning it in both the specifications and the claims, we evaluated the latter. Once an example of patent activity meeting these criteria for one of the 54 drug combinations was identified, we proceeded to the subsequent drug combination.
TotalPatentOne (LexisNexis), a repository of patents and patent applications, was searched for patents and patent applications covering the combinations of VEGF-inhibiting small molecules and IO drugs listed. *Note: for each of the 15 combinations where initial analysis did not yield any patents or patent applications meeting our criteria for inclusion, it remains possible that, within the thousands of claims in these patents or others, there exist ones that could be asserted for the purpose of evergreening the combination therapy.
In this manner, we identified patent activity pertaining to 39 of the 54 drug combinations searched, presented in Supplementary Table 1 . The results demonstrate that, across a variety of specific formulations, those companies likely to have incentive to engage in evergreening behavior have been, and continue to be, engaged in substantial patent activity for drug combinations known to those in the field. Given the discussion above and the potential implications of evergreening, this area of patenting and drug development merited critical evaluation.
Teaching away
A patent claimant can overcome obviousness concerns by demonstrating objective indicia of non-obviousness, such as when the prior art teaches away from an invention. That is, was there evidence in the prior art telling inventors not to combine VEGF and IO? Early phase 1 clinical trial work conducted between 2012 and 2014 and publicly disclosed in 2014 evaluated two VEGF–IO combinations in mRCC. In these clinical trials, sunitinib–nivolumab and pazopanib–nivolumab demonstrated approximately 70% and 60%, respectively, grade 3/4 adverse event rates 36 , 37 . A subsequent phase 1/2 study of pazopanib–pembrolizumab in mRCC conducted between 2013 and 2017 and publicly disclosed in 2017 demonstrated grade 3/4 adverse events of >80% and at the time concluded that certain VEGF–IO combinations were unsuitable for RCTs 38 , 39 . At first glance, these studies — having raised the possibility of a VEGF–IO combination conferring prohibitive toxicity — may have taught away from VEGF–IO combination therapy. To the POSITA with experience in drug discovery and development, though, the observed toxicities were ‘off-target’ effects of the particular VEGF drugs tested that would not be expected to generalize to all VEGF inhibitors, as demonstrated by the first-in-class axitinib–pembrolizumab combination 40 .
VEGF–IO clinical trials
As the most commonly used objective indicium for overcoming obviousness concerns is demonstration of an unexpected result, we asked whether the RCTs in the VEGF–IO development space have the capacity to demonstrate an unexpected result, if one were to exist. To examine this landscape, we searched the National Clinical Trials Registry (ClinicalTrials.gov) on 20 August 2020 for each of the 54 combinations of one of the above VEGF inhibitors and one of the above IO drugs. Building on two of the key clinical trial principles described above — (1) randomization; and (2) evaluation of OS as a primary or co-primary endpoint — we excluded any clinical trials that were not randomized; did not contemporaneously administer one of the above combinations; assessed a primary endpoint(s) other than OS, PFS or recurrence-free survival; or indexed in ClinicalTrials.gov as having a status other than ‘active, not recruiting’ or ‘completed’ (Fig. 2 ).
Starting with search results of 187 clinical trials, 129 distinct clinical trials were identified. Out of these, 105 trials were excluded from further analysis for non-randomized design, failure to utilize contemporaneous combination or withdrawal/suspension of the study. The remaining 24 RCTs were included in the final analysis. TKI, tyrosine kinase inhibitor; IO, immunotherapy.
We identified 24 eligible RCTs, summarized in Supplementary Table 2 , with total anticipated enrollment of 14,614 patient volunteers. Twelve (50%) trials evaluate lenvatinib–pembrolizumab and cabozantinib–nivolumab and cabozantinib–atezolizumab are each evaluated by four (17%). Twenty-one (88%) are phase 3 trials, and the remaining three (13%) are randomized phase 2 trials. Nineteen (79%) are industry-funded trials and five (21%) are government-funded. These trials used five dominant designs:
Type 1: A + B versus A;
Type 2: A + B versus B;
Type 3: A + B versus C;
Type 4: A + B versus D;
Type 5: A + B versus E;
in which A is one of the VEGF inhibitors listed above, B is one of the IO agents listed above, C is an established and related VEGF inhibitor (for example, sunitinib), D is an established but unrelated standard of care (for example, chemotherapy, physician’s choice) and E is a non-active comparator (placebo or otherwise).
Importantly, none of the 24 eligible RCTs possess the three criteria needed to demonstrate an unexpected result that were derived above: randomization, sequential treatment with A and B as a comparator, and OS as the primary or co-primary endpoint. The absence of sequential treatments is clear. Thirteen of the 24 eligible RCTs employ comparator arms that include at least one drug from the VEGF–IO combination. None incorporate A → B or B → A as a comparator, and there is no pre-specified OS or PFS analysis of A + B compared to A → B or B → A subpopulations. Consequently, these RCTs cannot demonstrate an unexpected result attributable to the act of combining 6 . Nine of the 13 trials that include either A or B as a component of the comparator arm employ OS as the primary or co-primary endpoint (Supplementary Table 2 ). Representative RCTs are discussed in Box 2 .
Importantly, five (21%) type-3 trials were identified. Four of these trials were conducted in mRCC and compared VEGF–IO combinations to sunitinib; all have reported results. First, axitinib–avelumab, in the JAVELIN Renal-101 study reported on 21 March 2019, failed to demonstrate benefit of the combination 41 . Second, axitinib–pembrolizumab, in the KEYNOTE-426 study also reported on 21 March 2019, demonstrated an OS benefit 35 . The remaining two RCTs, evaluating cabozantinib–nivolumab and lenvatinib–pembrolizumab, continued to randomize patients to sunitinib control arms after the announcement of KEYNOTE-426 results 42 , 43 . Nearly 24 months after KEYNOTE-426 publication, in March 2021, the CheckMate 9ER trial of cabozantinib–nivolumab was published, demonstrating modest PFS and OS benefits 44 ; FDA approval had occurred 6 weeks earlier 45 . One month later, in April 2021, the CLEAR trial evaluating lenvatinib–pembrolizumab in mRCC failed to demonstrate any more than a modest benefit in OS 46 , yet FDA approval followed 4 months later 47 . Complicating interpretation of the mRCC VEGF–IO trials still further, nearly all enrolled a substantial number of patients from countries where access to IO only occurs through clinical trials, increasing the likelihood that patients randomized to the sunitinib arms of these studies would never receive standard-of-care, second-line IO in the event of disease progression, and thereby overestimating the OS benefit of VEGF–IO 48 .
Box 2 Representative randomized controlled trials
The most common combination
Twelve of the eligible RCTs evaluate the lenvatinib–pembrolizumab combination, but as presently designed none have the design features needed to demonstrate an unexpected result 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 . Seven of the lenvatinib–pembrolizumab RCTs incorporate pembrolizumab or lenvatinib monotherapy as a comparator arm 51 , 52 , 53 , 54 , 55 , 56 , 57 . Only two of the lenvatinib–pembrolizumab RCTs fail to utilize OS as a co-primary endpoint 58 , 59 . Critically, none of the lenvatinib–pembrolizumab trials require crossover to lenvatinib (in the case of pembrolizumab) or pembrolizumab (in the case of lenvatinib) at the time of disease progression.
None of the trials utilize OS as the sole primary endpoint. After having learned that the FDA will award approvals to VEGF–IO combination therapies on the basis of PFS benefit 63 , trial sponsors lack the incentive to wait for an OS endpoint to read out before seeking regulatory approval. All but one of the clinical trials involving the combination of lenvatinib and pembrolizumab are industry-sponsored and expect to enroll 7,038 patients; the one trial sponsored by a public entity will enroll 192 patients 59 .
How trials can be skewed
The LEAP-008 RCT evaluates lenvatinib–pembrolizumab against docetaxel or lenvatinib monotherapy as second-line treatment for metastatic non-small cell lung cancer that had progressed despite prior PD-1-axis-inhibiting therapy. It serves as an example for the myriad ways in which clinical trial design can be ‘gamed’ to achieve a desired outcome. First, despite the publication of evidence demonstrating modest OS benefit with the addition of ramucirumab to docetaxel four years before its opening 64 , LEAP-008 fails to incorporate VEGF-inhibiting therapy in the comparator arm. Second, although lenvatinib is one of the comparator arms of the study, no crossover to pembrolizumab monotherapy after progression on lenvatinib is mandated. Third, given the long half-life and target engagement of IO 65 , it is possible that lenvatinib administered in short succession following progression through pembrolizumab may ‘rescue’ durable IO response. LEAP-008, however, incorporates a 4-week washout period, reducing the probability of observing this phenomenon in a sequential administration paradigm. Fourth, LEAP-008 employs an unusual randomization schema of 4:4:1 to lenvatinib–pembrolizumab to docetaxel to lenvatinib. Despite stratification on whether IO was a remote therapy or the immediate prior therapy, LEAP-008’s ability to disentangle lenvatinib’s relative contribution to the efficacy of lenvatinib–pembrolizumab is likely to be limited due to only 45 patients receiving lenvatinib monotherapy. Due to these issues, LEAP-008 cannot demonstrate an unexpected result.
How do government-funded RCTs do?
Lest we think these design flaws only occur in industry-sponsored clinical trials, NCT03595124 is a National Cancer Institute-funded RCT opened in July 2018 comparing axitinib–nivolumab (A + B) to axitinib (A) and nivolumab (B) monotherapy arms in transcription factor E family translocation-positive RCC 66 . Crossover in the comparator arms at the time of progression from axitinib to nivolumab (A → B) or nivolumab to axitinib (B → A) is not pre-specified, however, and the study’s primary endpoint is PFS. Despite reasonable first choices for comparator arms, NCT03595124 cannot demonstrate an unexpected result.
Recommendations
Reviewing the available evidence, the RCTs of VEGF–IO combinations appear designed to achieve FDA approval, rather than identify truly unexpected or scientifically novel results that would overcome obviousness concerns. While such approvals will yield significant financial rewards for the drug sponsor(s), they should not result in extension of exclusivity in the absence of a demonstration of non-obviousness through the discovery of unexpected results.
In truth, encouraging combination patents and RCTs that do not aim to identify unexpected results carries societal costs. First, the limited resources that can be dedicated to research and development efforts are directed away from the truly innovative approaches that represent the constitutional goal of the patent system and toward the commercialization of combinations that are, in the context of oncology’s prior art, non-innovative. Second, even if VEGF–IO patents were to be invalidated in court, the time and expense required to challenge them often deters competitors from entering the market, interfering with the natural competitive forces expected to discipline high prices 4 . In short, this new frontier in evergreening raises serious societal concerns and requires coordinated action from all involved parties — from the end users who have demand for these products and the government-supported entities that enable patent-seeking research rather than true innovation, through to the executive branch entities responsible for conferring patents.
Physicians, as end users of combination therapies, bear a societal responsibility to be thoughtful producers and consumers of biomedical research — industry-sponsored or not. As with any therapy, physicians should reflect on the designs of the relevant combination therapy clinical trials. In the context of cancer, oncologists need to disabuse themselves of the notion that ‘more therapy is better and in the best interests of the patient’. Learning health systems would be wise to assess the real-world outcomes of patients treated with combination therapies, especially as the gap between performance as observed in clinical trials versus real life yawns. Finally, disclosure of the shortcomings of clinical trial design is an absolutely necessary component in the desired goal of clinical care — shared clinical decision-making.
Clinical trialists and institutions have critical roles in the current pharmaceutical research and development landscape; it is this infrastructure that enables both innovative and non-innovative research. Realizing that the potential benefits of a clinical trial redound to patients other than the ones for which they care, clinical trialists must ask themselves, “Is the trial on which I am proposing to enroll patients something new or is it an incremental step?” It is unlikely physicians can do this alone, but demanding more from the research that we and others design and enact is a good first step, along with thoughtful skepticism toward certain types of patent claims. Similarly, the academic research infrastructure, as well as the cachet, on which many non-innovative clinical trials rest is supported in no small part by federal grants. Within the context of oncology, the National Cancer Institute designated 53 Comprehensive Cancer Centers in the United States, with federal grant support (P30) exceeding US$265 million in fiscal year 2020 (ref. 49 ). Incorporating review of the relative amounts of industry- versus non-industry-sponsored research activity occurring at a given cancer center in the NCI P30 grant renewal process may help to promote truly innovative research. The concept of taxpayers ‘paying twice’ for drug development — once through government-funded research and again in the form of high drug prices — has gained traction during the COVID-19 pandemic and should be seriously considered.
As the guardian of the patent and drug-making processes, coordinated federal action on the part of the US Patent and Trademark Office (USPTO) and FDA is important. As the prior arts of medicine’s subspecialties become increasingly complex, the case for inter-agency collaboration between the USPTO and FDA becomes stronger. The FDA has the capacity to provide information about all registered clinical trials, fully informing the prior art and context that the USPTO requires to make its determinations. The FDA also has a supply of experts who can provide additional perspective for USPTO examiners, who are rarely (if ever) clinicians (for example, physicians, pharmacists) or pharmaceutical scientists — FDA experts would be, to a first approximation, the government’s best proxy for a well-informed POSITA. In addition, the USPTO is optimally positioned to communicate to researchers and industry the requirements for patentability and to issue guidance on what constitutes unexpected results (positive or negative) in clinical trials.
The challenge in designing interagency cooperation is to ensure that the process is meaningful, rather than pro forma or simply burdensome. Fortunately, the USPTO has a model in place to better inform prior art determinations. In 2019, USPTO director Andrei Iancu reported to the Senate Judiciary Subcommittee on Intellectual Property that the USPTO was piloting projects to help examiners better identify prior art by collaboration between multiple USTPO examiners, as well as examiners from foreign patent offices 50 . This project could be expanded to include expertise closer to home — specifically at the FDA. Such guidance for the USPTO is in the interests of both drug developers (by providing a measure of certainty) and the public (by enhancing the risk–benefit calculus of biomedical research). Similarly, any regulatory standards that emerge at the USPTO could be incorporated by the FDA into its oversight of clinical trials and more fully inform its safety and efficacy determinations. This would allow both agencies to better carry out their mandates in serving the public interest.
Woodbridge v. U.S . 263 50 (Supreme Court, 1923).
Feldman, R. Colum. Sci. Tech. L. Rev. 17 , 30–89 (2016).
Google Scholar
Feldman, R. J. Law Biosci. 5 , 590–647 (2018).
Article Google Scholar
Gaudry, K. S. Nat. Biotechnol. 29 , 876–878 (2011).
Article CAS Google Scholar
Gowda, V., Beall, R. F., Kesselheim, A. S. & Sarpatwari, A. Nat. Biotechnol. 39 , 414–417 (2021).
Beall, R. F. & Kesselheim, A. S. Nat. Biotechnol. 36 , 142–145 (2018).
Conditions for Patentability; Non-Obvious Subject Matter 35 USC§103 (US Congress, 2011).
KSR Int’l Co. v. Teleflex Inc . 550 US 398 (Justia, 2007).
Manual of Patent Examining Procedure (MPEP) Sec. 2141.03 (USPTO, 2020).
Novartis Pharms. Corp. v. Accord Healthcare, Inc . (Lexis, 2020).
Pfizer v. Apotex . 480 1348 F.3d (Federal Circuit, 2007).
In re: Peterson . 315 1325 F.3d (Federal Circuit, 2003).
Thomas, N. A. NYU L. Rev. 86 , 2070–2112 (2011).
Pitlick, H. A. J. Pat. Trademark Off. Soc. 86 , 169–182 (2004).
In re: Soni . 54 746 F. 3d (Federal Circuit, 1995).
Galderma Labs., LP. v. Tolmar, Incl . 737 731 F.3d (Federal Circuit, 2013).
DeVita, V. T. Jr. Br. J. Haematol. 122 , 718–727 (2003).
Frei, E. III et al. Blood 13 , 1126–1148 (1958).
Frei, E. III Cancer 18 , 1580–1584 (1965).
Einhorn, E. H. Clin. Cancer Res. 3 , 2630–2632 (1997).
CAS PubMed Google Scholar
Bagley, C. M. Jr, Young, R. C., Canellos, G. P. & DeVita, V. T. N. Engl. J. Med. 287 , 856–862 (1972).
National Comprehensive Cancer Network. https://www.nccn.org/guidelines/category_1 (2020).
Gyawali, B. & Prasad, V. Nat. Rev. Clin. Oncol. 14 , 521–522 (2017).
Prasad, V. & Grady, C. Contemp. Clin. Trials 37 , 167–169 (2014).
Kim, C. & Prasad, V. JAMA Intern. Med. 175 , 1992–1994 (2015).
Kovic, B. et al. JAMA Intern. Med. 178 , 1586–1596 (2018).
Choueri, T. K. et al. Ann. Oncol. https://oncologypro.esmo.org/meeting-resources/esmo-virtual-congress-2020/nivolumab-cabozantinib-vs-sunitinib-in-first-line-treatment-for-advanced-renal-cell-carcinoma-first-results-from-the-randomized-phase-iii-checkmate-9er-trial (2020).
Arance Fernandez, A. M. et al. Ann. Oncol. https://oncologypro.esmo.org/meeting-resources/esmo-virtual-congress-2020/lenvatinib-len-plus-pembrolizumab-pembro-for-advanced-melanoma-mel-that-progressed-on-a-pd-1-or-pd-l1-inhibitor-initial-results-of-leap-004 (2020).
Lwin, Z. et al. Ann. Oncol. https://oncologypro.esmo.org/meeting-resources/esmo-virtual-congress-2020/leap-005-phase-ii-study-of-lenvatinib-len-plus-pembrolizumab-pembro-in-patients-pts-with-previously-treated-advanced-solid-tumours (2020).
Pal, S. Ann. Oncol. https://oncologypro.esmo.org/meeting-resources/esmo-virtual-congress-2020/cabozantinib-c-in-combination-with-atezolizumab-a-as-first-line-therapy-for-advanced-clear-cell-renal-cell-carcinoma-ccrcc-results-from-the (2020).
Motzer, R. J. et al. J. Clin. Oncol. 27 , 3584–3590 (2009).
Sternberg, C. N. et al. J. Clin. Oncol. 28 , 1061–1068 (2010).
Ciciola, P., Cascetta, P., Bianco, C., Formisano, L. & Bianco, R. J. Clin. Med. 9 , 675 (2020).
Dirkx, A. E. et al. FASEB J. 20 , 621–630 (2006).
Rini, B. I. et al. N. Engl. J. Med. 380 , 1116–1127 (2019).
Amin, A. et al. Am. Soc. Clin. Oncol. https://doi.org/10.1200/jco.2014.32.15_suppl.5010 (2014).
Amin, A. et al. J. Immunother. Cancer 6 , 109 (2019).
Chowdhury, S. et al. Am. Soc. Clin. Oncol. https://doi.org/10.1200/JCO.2017.35.15_suppl.4506 (2017).
Chowdhury, S. et al. Clin. Genitourin. Cancer 19 , 434–446 (2021).
Atkins, M. B. et al. Lancet Oncol. 19 , 405–415 (2018).
Motzer, R. J. et al. N. Engl. J. Med. 380 , 1103–1115 (2019).
ClinicalTrials.gov (National Library of Medicine, accessed 1 November 2021); https://clinicaltrials.gov/ct2/history/NCT03141177?A=38&B=39&C=Side-by-Side#StudyPageTop
ClinicalTrials.gov (National Library of Medicine, accessed 1 November 2021); https://clinicaltrials.gov/ct2/history/NCT02811861?A=20&B=21&C=Side-by-Side#StudyPageTop
Choueiri, T. K. et al. N. Engl. J. Med. 384 , 829–841 (2021).
US Food and Drug Administration. FDA approves nivolumab plus cabozantinib for advanced renal cell carcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-plus-cabozantinib-advanced-renal-cell-carcinoma (2021).
Motzer, R. et al. N. Engl. J. Med. 384 , 1289–1300 (2021).
US Food and Drug Administration. FDA approves lenvatinib plus pembrolizumab for advanced renal cell carcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenvatinib-plus-pembrolizumab-advanced-renal-cell-carcinoma (2021).
Strohbehn, G. W. & Goldstein, D. A. Nat. Rev. Clin. Oncol. 18 , 395–396 (2021).
National Cancer Institute. Vol. 2021 (2021) 2019 NCI Budget Fact Book: Cancer Centers. https://www.cancer.gov/about-nci/budget/fact-book/extramural-programs/cancer-centers (2019).
US Patent and Trademark Office. Statement by Director Iancu before the United States Senate Subcommittee on Intellectual Property, Committee on the Judiciary. https://www.uspto.gov/about-us/news-updates/statement-director-iancu-united-states-senate-subcommittee-intellectual (2019).
A study of pembrolizumab with or without lenvatinib as first line (1L) intervention in a programmed cell death-ligand 1 (PD-L1) selected population with recurrent or metastatic head and neck squamous cell carcinoma (LEAP-010). National Clinical Trials Registry (accessed 1 November 2021); https://clinicaltrials.gov/ct2/show/NCT04199104
Efficacy and safety of pembrolizumab with lenvatinib vs. docetaxel in participants with metastatic non-small cell lung cancer (NSCLC) and progressive disease after platinum doublet chemotherapy and immunotherapy (LEAP-008). National Clinical Trials Registry (accessed 1 November 2021); https://clinicaltrials.gov/ct2/show/NCT03976375
Efficacy and safety study of pembrolizumab with or without lenvatinib in adults with programmed cell death-ligand 1 (PD-L1)-positive treatment-naive non-small cell lung cancer (LEAP-007). National Clinical Trials Registry (accessed 1 November 2021); https://clinicaltrials.gov/ct2/show/NCT03829332
Safety and efficacy of lenvatinib in combination with pembrolizumab versus lenvatinib as first-line therapy in participants with advanced hepatocellular carcinoma (LEAP-002). National Clinical Trials Registry (accessed 1 November 2021); https://clinicaltrials.gov/ct2/show/NCT03713593
Safety and efficacy study of pembrolizumab combined with lenvatinib as first-line intervention in adults with advance melanoma (LEAP-003). National Clinical Trials Registry (accessed 1 November 2021); https://clinicaltrials.gov/ct2/show/NCT03820986
Safety and efficacy study of pemetrexed + platinum chemotherapy + pembrolizumab with or without lenvatinib as first-line intervention in adults with metastatic nonsquamous non-small cell lung cancer (LEAP-006). National Clinical Trials Registry (accessed 1 November 2021); https://clinicaltrials.gov/ct2/show/NCT03829319
Study of first-line pembrolizumab with lenvatinib in urothelial carcinoma cisplatin-ineligible participants whose tumors express programmed cell death-ligand 1 and in participants ineligible for platinum-containing chemotherapy (LEAP-011). National Clinical Trials Registry (accessed 1 November 2021); https://clinicaltrials.gov/ct2/show/NCT03898180
Lenvatinib/everolimus or lenvatinib/pembrolizumab versus sunitinib alone as treatment of advanced renal cell carcinoma (CLEAR). National Clinical Trials Registry (accessed 1 November 2021); https://clinicaltrials.gov/ct2/show/NCT02811861
Pembrolizumab and lenvatinib in participants with hepatocellular carcinoma (HCC) before liver transplant (PLENTY202001). National Clinical Trials Registry (accessed 1 November 2021); https://clinicaltrials.gov/ct2/show/NCT04425226
Lenvatinib in combination with pembrolizumab versus treatment of physician’s choice in participants with advanced endometrial cancer (KEYNOTE-775). National Clinical Trials Registry (accessed 1 November 2021); https://clinicaltrials.gov/ct2/show/NCT03517449
Pembrolizumab plus lenvatinib versus chemotherapy for endometrial carcinoma (LEAP-001). National Clinical Trials Registry (accessed 1 November 2021); https://clinicaltrials.gov/ct2/show/NCT03884101
Safety and efficacy of lenvatinib with pembrolizumab in combination with transarterial chemoembolization in participants with incurable/non-metastatic hepatocellular carcinoma (LEAP-012). National Clinical Trials Registry (accessed 1 November 2021); https://clinicaltrials.gov/ct2/show/NCT04246177
FDA. FDA approves avelumab plus axitinib for renal cell carcinoma. (15 May 2019); https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-avelumab-plus-axitinib-renal-cell-carcinoma
Garon, E. B. et al. Lancet 384 , 665–673 (2014).
Peer, C. J. et al. Br. J. Clin. Pharmacol. 86 , 1769–1777 (2020).
A study to compare treatments for a type of kidney cancer called TFE/translocation renal cell carcinoma (tRCC). National Clinical Trials Registry (accessed 1 November 2021); https://clinicaltrials.gov/ct2/show/NCT03595124
Download references
Acknowledgements
The authors wish to thank L. Yang for assistance with patent research, as well as N. Brown and M. Dorji for assistance with legal research. G.W.S. is an employee of the US Federal Government; the views expressed in this manuscript do not reflect the views of the US Federal Government and are his personal views.
Author information
- Garth W. Strohbehn
Present address: Veterans Affairs Center for Clinical Management and Research, Ann Arbor, MI, USA
These authors contributed equally: Garth W. Strohbehn, Robin Feldman.
Authors and Affiliations
Section of Hematology/Oncology, Department of Medicine, University of Chicago, Chicago, IL, USA
Garth W. Strohbehn, Alec J. Kacew & Mark J. Ratain
Optimal Cancer Care Alliance, Ann Arbor, MI, USA
Garth W. Strohbehn, Daniel A. Goldstein & Mark J. Ratain
Tel Aviv University, Tel Aviv, Israel
Daniel A. Goldstein
Davidoff Cancer Center, Rabin Medical Center, Petah Tikva, Israel
Department of Health Policy and Management, Gillings School of Public Health, University of North Carolina, Chapel Hill, NC, USA
Center for Innovation, University of California Hastings College of the Law, San Francisco, CA, USA
Robin C. Feldman
You can also search for this author in PubMed Google Scholar
Contributions
All authors meet International Committee of Medical Journal Editors criteria for authorship in the presented work.
Corresponding authors
Correspondence to Robin C. Feldman or Mark J. Ratain .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Supplementary information
Supplementary information.
Supplementary Tables 1 and 2
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Strohbehn, G.W., Kacew, A.J., Goldstein, D.A. et al. Combination therapy patents: a new front in evergreening. Nat Biotechnol 39 , 1504–1510 (2021). https://doi.org/10.1038/s41587-021-01137-6
Download citation
Published : 08 December 2021
Issue Date : December 2021
DOI : https://doi.org/10.1038/s41587-021-01137-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
‘contribution of component’ and the perioperative immune-checkpoint inhibitor precedent.
- Bishal Gyawali
Nature Reviews Clinical Oncology (2024)
Improving combination drug trials using ‘definitive screening designs’
- Michael Dodds
- James Roberts
- Brian Finrow
Nature Biotechnology (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Curr Health Sci J
- v.46(2); Apr-Jun 2020
Combination Therapy for Treatment of Spasticity in Stroke Patients: A Case Study
Dimitrios athanasiadis.
1 Physiotherapy Practice "Dimitrios Athanasiadis", Kavala, Greece
ELEFTHERIOS STEFAS
2 Department of Physical Medicine and Rehabilitation, Rehabilitation Center EVEXIA, Chalkidiki, Greece
ATHINA KAPSOKOULOU
3 School of Medicine, University of Patras, Patras, Greece
JANNIS PAPATHANASIOU
4 Department of Kinesitherapy, Faculty of Public Health, Medical University of Sofia, Sofia, Bulgaria
YANNIS DIONYSSIOTIS
5 1 st Physical Medicine and Rehabilitation Department, National Rehabilitation Center EKA, Athens, Greece
Background and purpose: Spasticity is a disorder of sensory-motor control that appears as an effect of a lesion in the upper motor neuron and demonstrates sustained or intermittent unintentional muscle activation. Many treatment interventions exist to treat spasticity, and in this study, three of them were combined: vibration, static positioning and transcutaneous nerve stimulation (TENS). Evidence exists regarding the application of each intervention per se, but not in combination. Hence, the aim of the study is to present an innovative treatment approach for spasticity and show the effects it produced on one patient. Methods: The study was a case report. The subject was a 31-year-old male patient who had ischemic stroke a year ago. He demonstrated severe plantar flexion of the left foot due to spasticity. There was a baseline assessment and measurement, one on the end of the intervention (10 th week) and a follow-up 8 months after it. Assessment and measurement tools: a dynamic gait analysis on the treadmill by Zebris FDM-T system, electromyography testing (F-wave parameter and stretch reflex activity), the Modified Ashworth Scale (MAS), a standard goniometer, the Motricity index (MI) leg score and a pain dichotomous when stretching and while at rest. Intervention: The intervention lasted 10 weeks, 5 days per week for 30 minutes. The patient was standing on a 30-degree-inclination wedge. The wedge was positioned on a whole-body vibrator set to vibrate with 91Hz of frequency and 1.0mm amplitude. TENS was offered through surface electrodes which were placed on the tibialis anterior and triceps surae muscles, along the sural nerve (impulse frequency: 100Hz, pulse width: 250μs, intensity: 30mA). Results: The range of motion and the MI was increased and the swing-phase of the right foot as well as the standing-phase of the left foot were increased an hour after the intervention. The results were slightly diminished a day and a week after the intervention but a statistically significant improvement still remained. Conclusion: Combination therapy intervention could offer an alternative for treating spasticity. Further studies are needed to establish a treatment protocol and maybe combine other spasticity-centered treatment modalities in order to produce new interventions.
Introduction
Stroke is one of the most lethal diseases worldwide [ 1 ].
Stroke is a disease in cerebral vasculature where oxygen fails to be supplied to the brain cells, leading them to death and can be classified as either ischemic or hemorrhagic [ 2 ].
Many physical disabilities could result from a stroke accident among which spasticity is one with long term effects on the patient’s live [ 3 ].
Spasticity is most holistically defined as “disordered sensory-motor control, resulting from an upper motor neuron lesion, presenting as intermittent or sustained involuntary activation of muscles” by Pandyan et al.’s [ 4 ].
However, another term is that of McCrea et al.’s [ 5 ] where spasticity is suggested to be used as an umbrella term for every positive, active symptom of the upper motor neurone syndrome that a therapist might confront in daily clinical practice. Patients suffering from spasticity could be disabled by a mixture of soft tissue contracture, muscle overactivity and paresis [ 6 ].
According to a Cochrane review performed by Monaghan et al.’s [ 7 ], there are 25 different interventions employed to treat spasticity in the literature, among which: vibration, static positioning and transcutaneous nerve stimulation (TENS).
In this case report, there was a combination of the last three interventions.
According to Yetley et al.’s [ 8 ] case studies are in the lower middle part of the hierarchy of evidence pyramid, with the higher risk of bias and the lower quality of evidence.
However, it was considered best to use this research method, as it allows a detail exploratory examination of data, within a particular context [ 9 ].
Hence, the primary objective of this study is to present an innovative treatment intervention and establish the proof-of-safety of the intervention.
The secondary objective was to present the effect that combination therapy had on a stroke patient, discuss the results and make some future recommendations.
Scientific Background
Given that spasticity is deeply affected by a muscle’s length, the position of the patient’s limb affects the result when assessing spasticity, especially when focusing on bi-articular muscles [ 10 ].
It has been demonstrated on the Root mean square (RMS) values that several positions affect the stretch reflex activity of muscles with spasticity [ 11 ].
That is presumably due to alterations of the inner muscle characteristics [ 7 ].
Notably, the stretch reflex activity is higher in an elongated muscle than in a shortened muscle [ 10 ].
In this case report, the static positioning was offered to the patient in the form of standing which has beneficial effects due to activity promotion from the anti-gravity muscles of the lower limbs and the trunk, neural alteration of spasticity triggered by changed sensory input and prolonged stretch, amelioration or preservation of the flexibility of joints or soft tissue, spasms decline on the lower limbs and, lastly, psychological effects [ 12 , 13 , 14 ].
Regarding vibration, it is considered to reduce the gamma-afferent fibers’ activity through a decrease of impulses from the muscle spindles in a “nervous-system response” [ 15 ].
Additionally, vibratory stimulation elicit relaxation and strengthening of soft tissues and muscular tissues [ 15 ].
The aforementioned findings could interpret the alterations observed on the F-wave parameters of the excitability of the motor neuron which are being modified when applying external vibratory stimulation [ 7 ].
With regards to TENS, there is evidence in support of the application of TENS for inducing inhibitory effects, for a short period of time after the stimulation, on stretch reflex activity which is unnaturally increased due to spasticity that originates in the cerebrum; for reducing the spastic antagonists’ co-contraction, and; for disinhibiting the voluntary motor commands of paretic muscles [ 16 , 17 ].
Noteworthy, the use of TENS in treating spasticity is supported by a plethora of studies [ 18 , 19 , 20 ].
Generally, it is supported that TENS application may have positive effect on treating spasticity [ 21 ].
Case Presentation
Although eight out of ten stroke patients belong to the ischemic stroke category [ 22 ], the subject of the current case report belongs to the hemorrhagic category as the patient suffered a saccular aneurysm [ 2 ] at the basilar artery on the September of 2016.
The subject is a male, 31-year-old ex-smoker, and his dominant side was the right one. Apart from the stroke accident, other inclusion criteria involved that the patient was treated early after stroke, was aged between 21 and 85 years old and that the patient presented lower-limb spasticity of at least 3 according to the Modified Ashworth Scale (MAS). Exclusion criteria were a Mini Mental Exam Status ≤24, 2, significant neglect, and spatiotemporal or visual or vestibular deficits. As this patient was the first one to receive Combination Therapy in a monitored and regular manner, the sample had to be of convenience.
Moreover, the patient showed history of hyperlipidemia and hypertension.
Pharmaceutical treatment included: Lopresor, Salospir, Malortil, Ivor, Ovepin, Efient in Blister (blist 2X10), Escitalo, Χanax and Proteins. Furthermore, the patient received two botulinum toxin injections in the 6 th and 9 th month after starting his treatment at the rehabilitation center. Given that the effects of botulinum toxin are known to fade away about 8 to 12 weeks after applying it to the patient [ 23 ], it was considered that the medicine had no effect over the effect of the treatment which started precisely 12 months after the first day of the patient’s treatment at the rehabilitation clinic.
Additionally, the subject’s spastic movement pattern involved a genu recurvatum type of gait which he tried to alter by activating muscles from the hip and back. The subject, also, presented foot drop with the ankle being impossible to dorsiflex from neutral and the toes being able to dorsiflex over 5° from neutral and only for the big toe. The subject had no sensory loss, no spatial or language impairment, was not overweight and had recovered most of his muscle strength and cardiovascular ability within his first year of rehabilitation. The patient gave a written informed consent to participate in the study. The baseline characteristics are shown on Τable 1 .
Patient's baseline features
|
|
|
| Age | 31 |
| Days Since Stroke | 3 |
| Hemiplegic Side | Right |
| Dominant Side | Right |
| Pain/No Pain when stretching and while at rest | No pain |
| Modified Asworth Scale | 3 |
| Motricity Index Leg Score | 81 |
| Maximum voluntary dorsiflexion range of ankle and big toe | 0° and 5° from the neutral position |
As it is already mentioned, the intervention was offered one year after the first day of the subject’s rehabilitation program. Interventions employed separately before applying Combination Therapy included: manual stretching, electrotherapy, mat activities, splinting and vibration. The aforementioned program was offered for 1 hour daily, 5 times per week. Additionally, the patient was instructed to perform a stretching program at home.
The intervention lasted 30 minutes, 5 days per week for 10 weeks. Measurements and assessments were conducted thrice: once before the intervention, a second time on the end of the intervention (end of 10 th week) and a third in a follow-up of 8 months after the end of the intervention (week 42). A physiotherapist was recruited to be the independent assessor. The assessor was blinded towards the intervention. On the contrary, no blinding was possible for the patient.
Measurement tools included: a dynamic gait analysis on the treadmill by Zebris FDM-T system in order to analyze gait and stance, the Modified Asworth Scale (MAS) to assess spasticity, a standard goniometer to measure range of motion (ROM) and the Motricity index (MI) leg score to assess muscle strength. To assess pain, VAS was overly criticized when used on stroke patients [ 24 ].
As a result, a pain dichotomous was preferred (pain versus no pain), a choice driven by older studies conducted on stroke patients [ 25 ].
This pain dichotomous was used when stretching the ankle and toes in dorsiflexion and while at rest. Regarding the measurement of range of motion, three measurements were conducted by a standard goniometer and the clinicians based their record according to the lowest of the three. The study’s design abides by the CARE guidelines [ 26 ] for case reports.
To perform the intervention, the patient was asked to step on a solid plastic wedge of 30° angle which was placed over a whole-body vibration plate. As seen in the attached video of the intervention, the whole-body vibration platform had support handles by each side.
While vibrating the body, the therapist was behind the patient in case of emergency.
Before every intervention, the patient’s blood pressure and heart rate was checked. Recess was given whenever the patient wanted. A picture that most accurately depicts the intervention is given at Figure Figure1 1 .

Vibration+TENS+positioning
Focusing on the parameters of the intervention, the vibration was offered in a frequency of 91Hz and 1.0mm amplitude. The TENS was offered cutaneously through surface electrodes, which were placed along the sural nerve of the affected limb. The TENS was offered on an impulse frequency of 100Hz, pulse width of 0,25ms and intensity of 30mA. Apart from Combination Therapy, the patient also received classical physiotherapy which included stretching and mat activities for 30 minutes daily. Along with the Combination Therapy intervention, the patient was educated to perform a home-based stretching program. During the intervention period, no other intervention was offered. Lastly, it is important to notice that the patient was making use of ankle-foot orthosis since day 1 of his rehabilitation in the clinic and henceforward.
The patient was informed about the purpose and content of the project and gave written informed consent to participate in the study, which conformed to the Declaration of Helsinki and was approved by the Local Ethical Committee.
Statistical methods applied in the experiment were determined before planning the experiment. Due to the inadequacy of the number of samples, the data's characteristics to be obtained by the experiment were determined and the statistical method to be used was decided. The Kruskal Wallis Analysis of Variance (p>0.05) was implemented. Normality was tested by using the K-S test from the SPSS and the Mann-Whitney U Test was applied to compare mean scores. Power analysis was performed which produced results above 80%.
The results showed no pain increase due to the intervention either at calm or when stretching at neither week 10 nor follow-up measurement. Spasticity was reduced by 40%, while the Motricity Index Leg score showed an increase by 11%, both compared to baseline. The aforementioned scores were maintained at the follow-up. The range of motion was increased by 13° for the ankle and 19° for the big toe compared to baseline and these scores were marginally lessened by 2° for the ankle and 3° for the big toe on week 42. A summary of the results of the assessment is presented on Table Table2 2 .
Scores at Baseline, Week 10 and Week 42
| Outcome Measures/Timing of the Intervention | Baseline | Week 10 | Week 42 |
| Pain/No Pain when stretching and while at rest | No pain | No pain | No pain |
| Modified Ashworth Scale | 3 | 1 | 1 |
| Motricity Index Leg Score | 81 | 92 | 92 |
| Maximum voluntary dorsiflexion range of ankle and big toe | 0° and 5° from neutral position | 13° and 24° from neutral position | 11° and 21° from neutral position |
Regarding the Zebris FDM-T measurements, Figure Figure2 2 depicts the foot prints produced while walking on the treadmill at baseline, week 10 and week 42. This illustrates how pressure was allocated progressively throughout the experiment, as the patient was being able to make use of the whole area of the plantar surface of the foot.

Foot prints pressure while walking in baseline, week 10 and week 42
The following Figures Figures3, 3 , ,4 4 and and5 5 illustrate the progression of force distribution changes in the static posture of the patient. Interestingly, the patient started the experiment with 40.9% of his pressure being laid on the left foot and 59.1% on the right foot, progressed to 45.1%-54.9% respectively on week 10 and the follow-up on week 42 showed 41.1%-58.9% respectively.

Stance analysis on baseline

Stance analysis on week 10

Stance analysis on week 42
Most of the pressure was laid on the foreword of the plantar surface of the foot than backwards in all three measurements.
Moving deeper on the gait analysis, the results on baseline, week 10 and week 42 are thoroughly displayed on Figures Figures6, 6 , ,7 7 and and8 8 .

Gait analysis parameters on baseline

Gait analysis parameters on week 10

Gait analysis parameters on week 42
Almost 40% reduction on step width was revealed between the first two measurements which was maintained until week 42.
Moreover, the stride length was increased by 47% on week 10 compared to baseline and almost doubled on week 42.
Total double support was lessened by 16% and 33% on week 10 and week 42 respectively, compared to baseline.
About 31% was the decrease in stride time by the end of the experiment and it was further lowered in the follow-up when it reached 43% compared to baseline. Regarding the affected limb, step length, single support during stance phase and the swing phase were significantly increased (44%, 16% and 41% respectively) and did not deteriorate on follow-up compared to baseline (66%, 43% and 64% respectively).
Noteworthy, the affected limb’s step time, stance phase, pre-swing phase during stance phase and swing phase was decreased (37%, 9% and 31% respectively) and did not deteriorate on follow-up compared to baseline (49%, 13% and 46% respectively).
These results present an improvement on the stability the patient produced while walking, but also an amelioration in the movement patterns generated by the affected side and the whole body in general.
Lastly, some postural changes and movement variability throughout the experiment, can be seen on the butterfly parameters depicted on Figures Figures9, 9 , ,10 10 and and11 11 .

Butterfly parameters on baseline

Butterfly parameters on week 10

Butterfly parameters on week 42
The butterfly parameters showed that the gait line length, the anterior/posterior variability, the lateral symmetry and variability have all improved between the baseline and week 10.
Thereafter, for most of the aforementioned, the effects remained or further ameliorated, except from the lateral symmetry and the gait line length for the left foot, both of which were reduced back to the baseline.
The current piece of evidence is the first study on Combination Therapy for the treatment of spasticity in stroke patients. It verifies proof that the intervention might be safe to use.
Additionally, it suggests that Combination Therapy might have a positive effect on alleviating spasticity of the lower extremity in chronic stroke patients.
Reducing levels of spasticity implies an improvement in functional recovery with regards to gait pattern and dynamic stability.
This piece of evidence comes to be connected with previous studies that support the separate use of each of the combined treatment modalities for the purpose of decreasing spasticity [ 7 ].
Since this is the first study to combine different interventions, the study also ignites interest about what other combinations of treatment modalities could be recruited in order to decrease the levels of spasticity in either stroke or other patients.
It should be reminded to the reader that there are at least 25 different evidence-based interventions that have been applied to treat spasticity [ 7 ].
Regarding the measurement tools, the Modified Asworth Scale [ 27 ] was used which a highly-reliable assessment tool, supported by evidence in several aspects of its psychometrics regarding stroke patients [ 28 , 29 , 30 , 31 , 32 , 33 , 34 ].
However, it should be noted that it is a very ambiguous assessment tool because resistance to passive motion could be intercepted by several other factors [ 35 ] and this intermingles with other issues that interfere with the quantification of spasticity [ 36 , 37 ].
With regards to the assessment of motor function, the Motricity Index [ 38 ] was utilized which is a reliable [ 39 , 40 , 41 , 42 ], valid [ 39 , 40 , 43 ], sensitive [ 39 ] and highly-responsive [ 44 ] assessment tool for stroke patients.
However, it is supported to have poor ceiling effects when used for the lower extremity [ 45 ] and small sensitivity for over 3 months after stroke [ 46 ].
Especially on the lower extremity, the Motricity Index produced small sensitivity in the acute phase [ 47 ].
A standard goniometer was used to measure range of motion which had some disadvantages. Firstly, a joint’s range of motion differentiates depending on gender, with males producing less ROM, and age, with older populations presenting less ROM [ 48 ].
Secondly, the study of Gajdosik & Bohannon’s [ 49 ] showed that the use of goniometer raises validity and reliability concerns.
Goniometer reliability depends on the time intervals between each measurement, with the longer being the least accurate, the different joints, with lower extremity to have lower inter-rater reliability than the upper, the procedure’s standardization and the condition of each patient’s problem [ 49 ].
All the above, combined with the size and weight of the patient’s extremity and the position of the hip and knee, affect the measurements’ validity [ 49 ].
In conclusion, a standard goniometer generates results regarding the ROM, but a potential error underlies every measurement, undermining the final outcome.
Besides the positive effects, this clinical trial gathers proof-of-safety for the intervention.
Pain when stretching and when at rest was absent before the intervention and remained absent even 42 weeks after.
This may further imply that the pain will not feel any pain in the future as this phenomenon is mostly prevalent at six months after stroke [ 50 ].
Moreover, no negative side-effect was apparent with regards to the increase of spasticity or decrease in muscle strength or range of motion.
Likewise, this patient may not have further worsening regarding spasticity given that spasticity could appear at the second week post-stroke the earliest [ 51 ] and its prevalence increases at the 3 rd week [ 52 ] and the 6 th week [ 53 ] post-stroke.
To further support, stroke patients with low sensory or motor deficits probably will not be further affected by spasticity [ 53 , 54 ].
Hence, this case study could support that Combination Therapy is a safe treatment approach towards chronic stroke patients.
Conclusions
In conclusion, the presented data create evidence that could possibly support the use of Combination
Therapy for the treatment of spasticity and, thus, functional improvement in chronic stroke patients.
Additionally, evidence for the proof-of-safety of the intervention was partially granted, especially for younger populations of chronic stroke.
As this was the first study to research the specific area of interested, it is mandated to conduct more studies, with more subjects and more rigid and standardized procedures, in order to generalize the conclusions and validate the responsiveness of the effects to the greater stroke population.
Combination Therapy is a safe treatment option to be used in order to cope with spasticity in stroke patients.
However, caution should be taken as further studies are necessary to generalize the conclusions of the study to the entire stroke population.
Acknowledgements
No funding was received for this work.
Conflict of interests
None to declare.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
“curious is as curious does”: fostering question-asking in a sino-foreign engineering school—a case study.

Share and Cite
Rakedzon, T.; Van Horne, C. “Curious Is as Curious Does”: Fostering Question-Asking in a Sino-Foreign Engineering School—A Case Study. Sustainability 2024 , 16 , 7308. https://doi.org/10.3390/su16177308
Rakedzon T, Van Horne C. “Curious Is as Curious Does”: Fostering Question-Asking in a Sino-Foreign Engineering School—A Case Study. Sustainability . 2024; 16(17):7308. https://doi.org/10.3390/su16177308
Rakedzon, Tzipora, and Constance Van Horne. 2024. "“Curious Is as Curious Does”: Fostering Question-Asking in a Sino-Foreign Engineering School—A Case Study" Sustainability 16, no. 17: 7308. https://doi.org/10.3390/su16177308
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

COMMENTS
Combination therapy, a treatment modality that combines two or more therapeutic agents, is a cornerstone of cancer therapy. The amalgamation of anti-cancer drugs enhances efficacy compared to the mono-therapy approach because it targets key pathways in a characteristically synergistic or an additive manner. ... In another study, the combination ...
Fourth, although combination therapy in clinical practice has been administered for up to 5-6 months at a time (Brown 2004; Pohl et al. 2009), most studies assessed combination therapy for only 2-6 weeks (Carlson et al. 2007; Quintana et al. 2007; Agarwal and Sitholey 2008; Hammerness et al. 2009; Wilens et al. 2009) and did not assess ...
The success of combination therapy for the treatment of ... An interesting case of intrinsic resistance concerns the use of the EGFR blocking antibody cetuximab for the treatment of advanced CRC ...
Using the ezetimibe/simvastatin combination therapy as a case study, a comprehensive overview of the successful discovery and development of the single-pill combination, Vytorin, is presented in this review. A cursory introduction to combination therapy and the rationale for the ezetimibe/simvastatin combination for the treatment of ...
Personalized, targeted combination therapy, consisting of agents likely to be active in a particular patient, will increase, perhaps substantially, the magnitude of therapeutic benefit. ... In the case of preclinical study of immunotherapies, genetically engineered mice and syngeneic models have been valuable, but the current focus on a ...
For each approved combination, a hazard ratio was calculated for the expected combination therapy PFS versus the trial's control arm (Cox proportional hazards model; ɑ = 0.05).
Case study: combination therapy for acute myeloid leukaemia. Acute Myeloid Leukaemia (AML) is a blood cancer characterised by the transformation of haematopoietic stem cells into leukaemic blast cells, primarily in the bone marrow (Crowell, Mac Lean, Stumpf, 2016, Mughal, Goldman, Mughal, 2010).
Abstract. Combination treatment, i.e., the use of two or more drugs for the same condition, is frequent in medicine if monotherapy yields an insufficient therapeutic response. We review and challenge clinical study designs and formats of reporting outcomes for the evaluation of the benefit/risk ratio of combination treatment over monotherapy.
In our study, EDP plus mitotane administered as first-line therapy in patients with advanced adrenocortical carcinoma resulted in a higher rate of objective tumor response than did streptozocin ...
Step 4. For the j th patient, escalate or deescalate to the dose combination \ ( (a_j, b_j)\). Simulate and record toxicity response \ (y_j\) and add \ ( (a_j, b_j, y_j)\) to the list of all ...
The study was conducted in patients who had previously been treated for HER2-positive breast cancer and, disappointingly, the combination therapy did not result in a statistically significant improvement in PFS in the overall patient population, with a median PFS of 8.2 months in the combination arm and 6.2 months in the control arm (P = 0.33).
Recent studies show increased expression of human epidermal growth factor receptor 2 (HER2) in cervical cancer, but the efficacy of anti-HER2 therapy remains under-researched. Here, we present a case of recurrent HER2-positive serous carcinoma, presumably arising in the cervix, diagnosed by comprehensive genomic profiling, which responded to ...
Combination treatment, i.e., the use of two or more drugs for the same condition, is frequent in medicine if monotherapy yields an insufficient therapeutic response. We review and challenge clinical study designs and formats of reporting outcomes for the evaluation of the benefit/risk ratio of combination treatment over monotherapy. We demonstrate that benefits of combination treatment at the ...
ability of combination therapy to cure tuberculosis inspired early studies on combination cancer therapy (51). The same reasoning also applies to interpatient heterogeneity: any sin-gle therapy will not be effective in every patient, but combina-tion therapies provide patients with several opportunities for a clinically meaningful response.
Case study: combination therapy for acute myeloid leukaemia. Acute Myeloid Leukaemia (AML) is a blood cancer characterised by the transformation of haematopoietic stem cells into leukaemic blast cells, primarily in the bone marrow (Crowell, Mac Lean, Stumpf, 2016, Mughal, Goldman, Mughal, 2010). The presence of leukaemic cells in the bone ...
Importance Combining antidepressants is frequently done in the treatment of acute depression, but studies have yielded conflicting results.. Objective To conduct a systematic review and meta-analysis assessing efficacy and tolerability of combination therapy. Combinations using presynaptic α2-autoreceptor antagonists or bupropion were investigated separately.
Our aim is to add our experience to the limited body of evidence supporting combination biologic therapies. Case study. Here we present the case of a 68-year-old nonsmoker female, with an allergic and eosinophilic corticosteroid-dependent severe asthma. She displayed well controlled comorbidities and good adherence to the inhaled therapy.
based studies [14]. We see a strong case for using large-scale data-collection systems to help address combination therapy access issues. In the UK, the Systemic Anti-Cancer Therapy dataset is a comprehensive database allowing an understand-ing of cancer treatment patterns on a national scale [15].
a combination therapy based on a single-arm trial These case studies were intended to frame the issue of combination therapy development and highlight real-world examples of the use of external data sources in the combination develop-ment and approval processes. 5 US Food and Drug Administration. FDA approves Darzalex for patients with ...
An early mega-analysis of studies that compared combining interpersonal therapy with medication versus psychotherapy (interpersonal therapy or CBT) alone found that duration of episode did not predict the benefit of combination treatment, but severity did: remission rates among patients with mild to moderate depression were nonsignificantly ...
We present a case where a patient with COVID-19 complicated by CRAB infection was successfully treated with a combination therapy including carrimycin, offering clinical insights and experience. ... This study was approved by Ruijin Hospital Institutional Review Board (No. 2018CR004) and has been performed in accordance with the ethical ...
The cornerstones of WM therapy are rituximab-based chemoimmunotherapy (CIT) and covalent BTK inhibitors (cBTKi), ... Nevertheless, this study has shown that the combination is feasible, and the addition of pembrolizumab to rituximab adds minimal toxicity. It is also possible that modulation of the PD-1 axis may improve response to further CIT ...
Studies that have evaluated LT4/LT3 combination therapy used variable doses of both LT4 and LT3 . Therefore, if a decision is made to start combined LT4/LT3 therapy, it must be taken into account that the daily production of T3 for a 70 kg person is approximately 30 µg, of which 5 to 6 µg is secreted by the thyroid, and the rest (~25 µg/day ...
Combination pharmacotherapy yields superior efficacy in acute depression. This 2022 systematic review and meta-analysis (39 randomized clinical trials [RCTs]; N = 6751) compared the efficacy and tolerability of monotherapy to combination therapy in the treatment of patients with acute depression. 1 The study also aimed to address which specific combination therapies were superior.
Immunogenicity of BNT162b2 Vaccine Booster Dose in Patients With Inflammatory Bowel Disease Receiving Infliximab Combination Therapy: A Prospective Observational Study. / Shehab, Mohammad; Alrashed, Fatema; Alfadhli, Ahmad et al. In: Frontiers in Medicine, Vol. 9, 933996, 04.07.2022. Research output: Contribution to journal › Article › peer ...
Specifically, we focused our efforts on a subset of combination therapies developed in cancer as a case study. Using publicly available information, we assess the current patent and clinical trial ...
Intratumoural oncolytic virotherapy may have promise as a means to debulk and downstage inoperable tumours in preparation for successful surgery. Here, we describe the unique case of a 50-year-old self-experimenting female virologist with locally recurrent muscle-invasive breast cancer who was able to proceed to simple, non-invasive tumour resection after receiving multiple intratumoural ...
Conclusion: Combination therapy intervention could offer an alternative for treating spasticity. Further studies are needed to establish a treatment protocol and maybe combine other spasticity-centered treatment modalities in order to produce new interventions. ... Hence, this case study could support that Combination Therapy is a safe ...
Curiosity and question-asking are at the heart of science and engineering education. However, question-asking can be difficult for students due to several factors, including fear, language barriers, and cultural norms. This is especially true among Chinese students, who represent a growing number of upcoming engineers. To address this, in this case study from a university teaching reform ...