

Essay on Chemistry Importance In Daily Life
Students are often asked to write an essay on Chemistry Importance In Daily Life in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Chemistry Importance In Daily Life
What is chemistry.
Chemistry is like a secret recipe that makes everything around us. It is the study of matter, which means anything that takes up space and has weight. From the air we breathe to the food we eat, chemistry is a big part of our everyday lives.
Chemistry in Cooking
When we cook, we use chemistry. Mixing ingredients causes reactions that make our food taste good. For example, baking a cake changes liquid batter into a fluffy solid because of heat causing a chemical change.
Medicine and Health
Chemistry keeps us healthy. Doctors use medicines to fight sickness, and these medicines are made using chemistry. Even our bodies use chemistry to turn food into energy, which keeps us going every day.
Cleaning Our Homes
Cleaning products are full of chemicals that help us get rid of dirt and germs. Soaps and detergents break down grease and stains, making our clothes and homes clean thanks to chemistry.
Environment and Recycling
250 words essay on chemistry importance in daily life.
Chemistry is like a secret recipe that explains everything around us. It is the science that tells us what things are made of and how they work together. Every time we cook, clean, or even breathe, we are part of a big chemistry experiment.
When we cook food, chemistry is at play. For example, when bread rises, it’s because of a chemical reaction. Heat changes the food, making it taste different and easier to digest. Without chemistry, we wouldn’t have bread, cheese, or even yummy chocolate.
Cleaning products are full of chemicals. Soap helps wash away dirt because it can stick to both water and grease. It’s like a magnet that pulls the dirt off our clothes and dishes. Chemistry helps us keep our homes and ourselves clean and healthy.
Medicines and Health
Medicines are chemicals that help our bodies fight sickness. When we are hurt or ill, the medicine makes the pain less or helps us get better. Chemistry is behind the vitamins we take to stay strong and the vaccines that protect us from diseases.
Every Breath We Take
Every time we take a breath, we are living chemistry. Air is a mix of gases, and breathing is a chemical process that gives our body the oxygen it needs. Plants use chemistry to turn sunlight into food, which is a process called photosynthesis. This is how plants help make the air clean for us to breathe.
500 Words Essay on Chemistry Importance In Daily Life
Chemistry is like a secret language of everything we see, touch, and feel. It’s a part of science that tells us what stuff is made of and how different things work together. Imagine being a detective, but instead of solving mysteries, you’re figuring out the secrets of the world around you. That’s what chemists do. They study substances and how they change when they mix.
Chemistry in the Kitchen
Every day, we use chemistry when we cook. Have you ever baked a cake? The ingredients like flour, sugar, and eggs are mixed and heated to make something delicious. This is because of chemical reactions, which are like tiny events where the ingredients change and become a cake. When you cook eggs, they change from liquid to solid, and that’s chemistry at work too!
Cleaning Made Easy
Chemistry is super important for keeping us healthy. Medicines are chemicals that can fix or prevent health problems. For example, if you have a headache, you might take a painkiller. This medicine is made from chemicals that stop the pain signals in your body. Vaccines, which protect us from diseases, are also made thanks to chemistry.
Breathing and Living
Did you know that even breathing is a chemical process? When we breathe in, we take in oxygen, and when we breathe out, we release carbon dioxide. Plants do the opposite; they take in carbon dioxide and give out oxygen. This exchange is all because of chemical reactions that are essential for life on our planet.
Clothes and Materials
Technology and gadgets.
Our phones, computers, and TVs all work because of chemistry. The batteries that power them have chemicals that store electricity. The screens use chemicals to light up and show us pictures and videos. Without chemistry, none of these gadgets would work!
So, you see, chemistry is everywhere! It’s not just something that scientists think about in labs. It’s part of our everyday lives, helping us cook, clean, stay healthy, breathe, dress, and even use technology. Understanding chemistry helps us know more about the world and how to make our lives better. Next time you see something happening, like a cake rising in the oven or a bubble popping, remember, it’s all chemistry!
That’s it! I hope the essay helped you.
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Chemistry Application in Daily Life Essay
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
Introduction
The application of chemistry in life, the dangers of chemistry to life, works cited.
Chemistry involves more than the mere fact that that it entails the making of new combinations of chemical elements. It makes new combinations of industries and brings together different countries besides the chemical elements. It brings international competition, which results into the development of international cooperation.
Chemistry improves everyday life of millions of people around the globe. It helps in the protection of the environment, development of new live saving, medicine, design new materials for cars, electronics as well as medical implants. It has also played a pivotal role in the development of greener and more sustainable sources of energy.
Besides its usefulness, it has generated certain problems not only to the people but also to the environment. This paper focuses on the application of chemistry in life as well as some of the hazards that it has presented to life.
As the world’s population grows, some chemists are embarking on finding new ways of feeding the populations i.e. producing food. The senior vice president for chemical and physical sciences at DuPont Merck Pharmaceutical Company asserts that genetic engineering could lead to the development of saltwater-tolerant plants that will grow food in most saline places (Zare 7).
Genetic engineering has also been applied in the production of drought resistant crops that have helped in increasing food security especially in the developing and third world countries. Other than genetic engineering, chemists have employed other means of increasing the supply of food to many nations.
Hydrolyzing wood pulp has played a pivotal role in transforming it into a fermentable substance that is used in the production of alcohol. For many years, the production of alcohol has been the main goal for the process. Recent research has led to the realization of more useful food elements. The action of highly concentrated hydrochloric acid transforms wood pulp into soluble carbohydrates and finally into wood pulp. Based on laboratory results, the process yields 75 parts of crude foodstuffs that contain 85% of pure carbohydrates (Slosson 324).
This means the process can extract 60% of the carbohydrates in dry wood. The product proved to be high in nutritive value to not only animal feed but also to food. Furthermore, chemistry has played a pivotal role in the development of fertilizers that enhance productivity if crops thus help in achieving food security in the world. Moreover, for food security to be attained there must be a method that prolongs the shelf life of the food products. Food preservatives enhance a prolonged shelf life of most agricultural products.
The knowledge of chemistry has enabled researchers to come up with drugs for addressing not only human but also animal diseases. The medicine that patients receive form health care institutions is all products of chemistry. It is noteworthy that drugs that are more efficient are continually being invented. China’s research and development in biotechnology and biopharmaceutical has prioritized genetic breeding of high-yield and high-quality crops, transgenic technology and animal cloning (Chen et al. 950).
Additionally, they have embarked on gene- and protein-engineered vaccines and drugs, gene therapy and drug discovery and development. Through the advanced technological advancements, there has been the development of new drugs that enable medical practitioners to treat certain diseases that have been challenging in history. Some of the advancements include the therapeutic hepatitis B vaccine, gene-engineered HBV antigen-antibody complex as well as artificial blood substitutes.
Chemical products that are available in the market such as disinfectants help in fighting disease-causing agents. They also help keep water secure. The water treatment process that makes water safe for consumption is mainly a series of chemical reactions (Hoffman 141). Chemistry is also essential in the textile industry e.g. during the bleaching processes. This also applies in the paper industry.
It also helps in establishing international relations through trade of chemical substances and technologies. It has led to the breakdown of natural monopoly while promoting national independence. For instance, approximately two decades ago one could say that Chile had a natural monopoly of the world’s supply of nitrates. Different nations have tapped into new ways of meeting their need of nitrates. One of the main ways that nations have achieved this is by the utilization of nitrogen from the nitrogen of the air through fixation.
According to Wilson and Schwarzman (1203), in the last five decades, synthetic chemicals have become integrated into nearly all industrial processes and the commercial products and they constitute the material base of the contemporary society. There has been an enormous growth in the production of synthetic chemicals.
For instance, statistics show that the U.S. reported having produced approximately 15 trillion pounds of chemical substances in 2002. In 2005, there was a high record of chemicals with chemical manufacturers having produced nearly 27 trillion pounds off chemicals. There were more than six thousand types of chemicals reported.
All the chemicals that are produced are directly or indirectly. Some of them are used in industrial processes as well as in the production of products for human consumption. Research has shown that “global chemical production is projected to continue growing-about 3% per year with a doubling rate of 24 years, rapidly outpacing the rate of global population growth” (Wilson and Schwarzman, 1203). The global increasing trend in the production of chemicals will lead to a similar increase in the hazards associated with such chemicals.
Almost everyone in the world has an encounter with many chemicals in everyday life due to the wide distribution of chemicals throughout the economy and the environment. This occurs in different scenarios such as in the work place, in homes, through the air, food, water as well as waste streams.
Bio-monitoring experts have reported that there has been an increase in the exposure of industrial chemicals as well as pollutants. The Center for Disease Control has confirmed the presence of certain pollutants as well as synthetic chemicals in the body of over 140 U.S civilians (a representative sample of the U.S population). Such bio-accumulative chemicals pose a great threat to the lives of the victims since most of them have proven to be carcinogenic.
Some individuals are victims of early life exposure of some of the hazardous chemicals. For some individuals it occurs via the placenta. Once the chemicals get into the fetus’ body system, they tend to accumulate and this has detrimental effects not only to the mother but also to the fetus. Medical practitioners have argued that such accumulation of chemical in the fetus interfere with the normal and healthy development of the baby (Woodhouse and Breyman, 220).
Additionally, such early exposure to chemicals during the developmental stages is associated with some cases of cancers, asthma as well as developmental disorders. According to Wilson and Schwarzman, the prenatal exposure of synthetic chemicals as well as their accumulation is the cause of some of the common abnormalities of the male reproductive system (1204).
Most industrial workers are usually exposed to the risk of having certain occupational diseases. As compared to individuals whose line of work is not within the chemical industry, the industrial workers have a higher probability of being exposed to dangerous or rather hazardous chemicals.
Over 40,000 cases of asthma (on an annual basis) in the European continent are associated with exposure of the victims to workplace chemicals. This is also true for dermatitis cases. The prevalence of such diseases can be reduced by improving the safety of work place chemicals. It would also reduce 4,300 cases of cancer per year.
Technological advancements in chemistry have also exposed humanity to hazardous waste. For instance in 2007 in California, there were cases of underground water contamination/pollution (Wilson and Schwarzman 1204). It was caused by the direct leakage of hazardous chemical wastes into the underground water sources.
This poses a threat-what the Department of Toxic Substances Control calls a major threat to human health or the environment. Most of the hazardous chemicals at the disposal sites are known dermatogens, neurotoxicants and carcinogens. Such incidences pose not only health but also environmental concerns to the communities that live in such neighborhoods.
The paper has discussed the application of chemistry in life as well as some of the hazards that it has presented to life. It aids the provision of solutions to some of the challenges of the 21 st century. They include improving human health, finding sustainable sources of energy as well as protecting the environment.
Additionally, it has helped in addressing some of the problems that come with the advancement of technology in almost all the sectors of the economy. However, it has some life threatening effects to people besides being potentially disastrous to the environment. For instance, most of the synthetic chemicals are harmful to human health e.g. by being carcinogenic.
Chen, Zhu. Et al. “Life Sciences and Biotechnology in China.” Philosophical Transactions: Biological Sciences 362. 1482 (2007): 947-957. Print.
Hofmann, Mary. “Science of Everyday Things: Real Life Chemistry/ Science of Everyday Things: Real Life Physics.” School Library Journal 48.8 (2002): 141-143. Print.
Slosson, Edwin. “Chemistry Alters International Relations.” The Science-Newsletter 14. 398(1988): 323-324. Print.
Wilson, Michael, and Schwarzman, Megan. “Health Policy: Toward A New U.S Chemicals Policy: Rebuilding the Foundation to Advance New Science, Green Chemistry, and Environmental Health.” Environmental Health Perspectives 117.8(2009): 1202-1209. Print.
Woodhouse, Edward, and Breyman, Steve. “Green Chemistry as Social Movement?” Science, Technology, & Human Values 30.2 (2005): 199-222. Print.
Zare, Richard. “A Golden Age for Chemistry.” The Futurist 32.6 (1998): 7-9. Print.
- The Pollution Within: Foreign Substances in the Human Body
- How is Aluminium Ore Converted to Aluminium Metal?
- Chemistry: The Importance of the Discipline
- Biomimetics in Dentistry: Key Issues
- Determining the Strongest Predictors of Kappa Number in Kraft Pulp
- The Effect of Inhibitors and Temperature on Enzyme Reactions
- Gold's Production and Processing
- Chemical Spills in Forensic Setting
- Chemical Engineering and Influence on Economics
- Chemical and Physical Properties of Ethane
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2018, November 20). Chemistry Application in Daily Life. https://ivypanda.com/essays/chemistry-in-life/
"Chemistry Application in Daily Life." IvyPanda , 20 Nov. 2018, ivypanda.com/essays/chemistry-in-life/.
IvyPanda . (2018) 'Chemistry Application in Daily Life'. 20 November.
IvyPanda . 2018. "Chemistry Application in Daily Life." November 20, 2018. https://ivypanda.com/essays/chemistry-in-life/.
1. IvyPanda . "Chemistry Application in Daily Life." November 20, 2018. https://ivypanda.com/essays/chemistry-in-life/.
Bibliography
IvyPanda . "Chemistry Application in Daily Life." November 20, 2018. https://ivypanda.com/essays/chemistry-in-life/.
- Chemistry Class 11 Notes
- Physical Chemistry
- Organic Chemistry
- Inorganic Chemistry
- Analytical Chemistry
- Biochemistry
- Chemical Elements
- Chemical Compounds
- Chemical Formula
- Real life Application of Chemistry
- Chemistry Class 8 Notes
- Chemistry Class 9 Notes
- Chemistry Class 10 Notes
- Chemistry Class 12 Notes
Importance of Chemistry in Everyday Life
Importance of Chemistry in Everyday Life: The scientific study of matter’s properties and behavior is known as chemistry . It is a natural science that studies the elements that makeup matter, as well as the compounds, made up of atoms, molecules, and ions: their composition, structure, qualities, and behavior, as well as the changes that occur when they mix with other things.
In this article, we have provided the importance of chemistry in everyday life, including the uses and applications of various compounds of chemistry and how Chemistry affects our daily life.

Table of Content
What is Chemistry?
How chemistry is important in everyday life, importance of chemistry in food, importance of chemistry in medicines, importance of chemistry in cosmetics, importance of green chemistry, importance of chemistry in soaps and detergents, importance of chemistry in textiles, importance of chemistry in building and construction, importance of chemistry in fuel, importance of chemistry in battery, importance of chemistry in agriculture, importance of chemistry in wars, articles on importance of chemistry in everyday life, cbse class 12 – chemistry in everyday life important articles.
Chemistry is a discipline that falls somewhere between physics and biology in terms of scope. It is also referred to as the “core science” since it provides a fundamental framework for understanding both basic and applied scientific disciplines.
Chemistry, for example, explains aspects of plant chemistry (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties of lunar soil (cosmochemistry), how medications work (pharmacology), and how to collect DNA evidence at a crime scene (criminology) (forensics).
Chemistry is a discipline of science concerned with the study of matter, including its structure, composition, and changes that occur when it is exposed to various situations. As a result, chemistry not only investigates the qualities of matter, but also how and why it changes.
Chemistry is like our everyday lives unseen behind-the-scenes wizard, quietly influencing everything around us in a myriad of ways. It is found in the air we breathe, a mixture of gases that chemists comprehend and control for a variety of uses, including carbon dioxide for photosynthesis in plants and oxygen for human survival.
Every time you prepare food, chemistry is at work, changing the taste, texture, and appearance of raw components to create delectable dishes.
Below are the importances of chemistry in various fields:
Importance of Chemistry in Food Importance of Chemistry in Medicines Importance of Chemistry in Cosmetics Importance of Green Chemistry Importance of Chemistry in Soaps and Detergents Importance of Chemistry in Textiles Importance of Chemistry in Building and Construction Importance of Chemistry in Fuel Importance of Chemistry in Battery Importance of Chemistry in Agriculture Importance of Chemistry in Wars
Chemicals are the fundamental components of everything. Chemical molecules make up all food, including carbs , vitamins , lipids , proteins, and fiber, which are all safe and often desirable.

Food Additives
Chemicals have an important part in the manufacturing and preservation of food. Food preservation chemicals have tremendously aided in the preservation of food for a longer duration. Cans of food additives, flavourings, and nutritional supplements can all contribute to improve the quality and quantity of meals.
Chemistry has provided the globe with vital f ertilizers, herbicides, insecticides, and fungicides to aid in the production of healthy and nutritious crops, fruits, and vegetables. Urea, calcium superphosphates, ammonium sulphate, and sodium nitrate are all significant fertilise rs.
Medicines or pharmaceuticals are chemical substances that are used to treat diseases and relieve pain. Chemistry has made significant contributions to health care. Chemistry, for example, aids in the manufacture and application of surgical materials (sutures, artificial skin, and sterile materials).
For analysis, clinical laboratory tests employ a wide range of chemical procedures and substances.
Some important drugs in chemistry-
- Analgesics are pain relievers that are used to treat a variety of ailments.
- Tranquilizers are medications that are used to treat mental illnesses. Take, for instance, tension.
- Antiseptics are used to destroy or prevent the growth of microorganisms on the skin, wounds, and cuts.
- Disinfectants- These are chemicals that destroy microorganisms but are dangerous to humans.
- Antibiotics- Antibiotics are chemical substances produced by some microorganisms that can be used to kill bacteria that cause infections.
- Antacids- These are the compounds that are used to eliminate excess acid from the stomach and increase the pH to a healthy level.
In our daily lives, we use lotions, fragrances, talcum powder, and a variety of other cosmetic goods. All of these items are developed in laboratories using chemicals for our health and skin. All cosmetic items, from babies to adults, are made up of chemical components.
As a result, chemistry is important in maintaining the pH of our skin, keeping it healthy, and removing any marks.
Green chemistry contributes to environmental protection by monitoring, protecting, and enhancing the conditions in which we live, such as air, water, and soil. Many methods and strategies have been created to ensure that all types of pollution in the environment are measured and prevented from depleting.
To make the air cleaner, many non-polluting fuels and compounds that can easily absorb contaminants from the air are being researched and tested. The replacement of CFC in refrigerators is one such example.
For interpreting health impacts, controlling emissions, and creating pollution-reduction devices, chemistry gives a complete understanding of contaminants.
Chemists test the water and soil for contamination on a regular basis and offer pollution results as well as long-term prevention for ecological balance and human health.
Soaps are sodium and potassium salts of fatty acids with greater molecular weights, such as stearic acid, palmitic acid, and oleic acid. Sodium salts of long-chain alkyl hydrogen sulphates or sodium salts of long-chain alkyl benzene sulphonic acids are commonly used as detergents.
Detergents and soaps are used for washing, cleaning, and bathing, among other things. The saponification method is used to make them in chemical companies. As a result, chemistry plays a significant role in the development of molecules, chemicals, and procedures for the production of soaps and detergents.
Wool, silk, jute, cotton, flax, glass fiber, polyester, acrylic, nylon, and other raw materials are used in the textile industry to create usable items such as clothing, bags, carpets, furniture, towels, nets, and so on.
Raw materials go through a number of chemical procedures during which cleaning and smoothing reagents are employed to clean and smooth the fabric.
Other chemical processes including dyeing, bleaching, scouring, printing, and finishing are also involved. In addition, chemists seek to increase a product’s quality.
Chemical items such as bricks, cement, pipelines, and other building materials all play an essential part in the quality of construction. Floor and wall tiles are constructed of heat-resistant polymers that also add strength to the structure.
Ceiling and roof materials are also designed to be heat resistant and give cooling to the structure. All of the pipes and switches are composed of polymers, which are both heat and stress-resistant.
As a result, chemistry enabled the use of all of these goods for the development of structures and people’s lives.
Fuels are the sole thing that allows us to travel by land, sea, and air nowadays. Petrol, diesel, LPG, CNG, kerosene, oils, and other fuels are all obtained through sophisticated refining procedures from harsh oil found beneath the Earth’s crust.
Petrochemistry is a discipline of chemistry concerned with the study of petrochemical processes and how to use fuels in a way that is both pollution-free and long-term.
Batteries power our automobiles, electronic devices such as watches, laptops, mobile phones, and a variety of other power storage applications.
The electrochemistry concept governs the operation of batteries. Chemical energy is stored inside a battery and is transformed to electric energy through electrochemical processes.
- Chemistry enhances agricultural productivity and sustainability by enabling innovative solutions to feed a growing population while minimizing environmental impact.
- Soil health optimization is achievable through chemistry, analyzing and adjusting nutrient levels to ensure crops receive necessary nourishment.
- Pesticides and herbicides, formulated with chemical principles, safeguard crops from pests and weeds, reducing crop loss.
- Chemistry contributes to the development of fertilizers that boost crop yields, ensuring a consistent food supply.
- Sustainable farming practices are developed with chemistry’s aid, reducing agriculture’s environmental footprint through responsible chemical use.
- Overall, chemistry is indispensable in modern agriculture, driving increased food production, resource efficiency, and environmental stewardship.
TNT, RDX, HMX, gun powders used in bullets, and other explosives used in conflicts are all chemical compounds. It was the chemistry that allowed these chemicals to be used during the war. Nuclear weapons, which have become more well-known in recent years, are also chemical chemicals.
The important articles on uses & applications of chemistry in everyday life are provided in the table below:
In the CBSE Class 12 Chemistry curriculum, the “Chemistry in Everyday Life” unit is designed to highlight the connections between chemical principles and their applications in daily life. This unit covers various topics that explain how chemistry affects our health, environment, and various industries.
| Chemistry in Everyday Life: Important Articles |
|---|
Conclusion of Importance of Chemistry in Everyday Life
Chemistry quietly shapes our world, delving into what things are made of and how they transform under different circumstances. It’s integral to our food’s nutrition and preservation, our clothing, medicines, and cosmetics. It engineers materials for homes, fuels vehicles, and enhances farming for healthier crops while mitigating pollution. Chemistry’s influence is pervasive, enriching our lives with improved quality, safety, and comfort every day.
Importance of Chemistry in Everyday Life – FAQs
What is the importance of chemistry in engineering.
Chemistry impacts engineering across production, fuel research, and materials for construction. Understanding compound and element properties aids in comprehending mechanisms, fostering innovation for the future.
What is the Importance of Chemistry in Society?
Everything in our environment is formed of matter. Chemistry is significant in our civilization because it affects our basic needs for food, clothing, shelter, health, energy, and clean air, water, and soil, among other things.
What is the Importance of Chemistry in Agriculture?
Chemistry boosts agriculture with vital fertilizers, herbicides, insecticides, and fungicides, promoting healthy crop growth. Key fertilizers like urea, calcium superphosphates, ammonium sulfate, and sodium nitrate are instrumental in this process.
What is the Importance of Chemistry in Medicine?
Chemistry is vital in medicine, producing pharmaceuticals that treat diseases and alleviate pain. It facilitates the creation of surgical materials like sutures and artificial skin. Clinical lab tests rely on chemical procedures for analysis.
What are Some Examples of Chemistry in Daily Life?
Toothpaste, lotions, facewash, the food we eat, pharmaceuticals, batteries in watches, mobile phones, cars, laptops, and other electronic devices, and fuel in our vehicles are all instances of chemistry in our daily lives.
Why is Chemistry Important in Food?
Chemicals are essential components of all food, from nutrients like carbs, vitamins, and proteins to additives and flavorings that preserve quality and prolong shelf life

Please Login to comment...
Similar reads.
- School Chemistry
- School Learning
- Chemistry-Class-11
Improve your Coding Skills with Practice
What kind of Experience do you want to share?
- IIT JEE Study Material
Chemistry In Everyday Life

If you are studying chemistry, then you must have wondered about the importance of chemistry in everyday life . Chemistry is the branch of science which deals with the investigation of the properties and changes of matter. From the way how our body exchanges oxygen to how our universe was created, all have a part of chemistry associated with it.
Download Complete Chapter Notes of Chemistry in Everyday Life Download Now
Importance of Chemistry in Everyday Life
- Analgesics Types
- Antibiotics Classification
- Milk of Magnesia
- Slaked Lime
Chemicals of Food in Everyday Life
The following chemicals are widely used in food materials.
- Colouring agents
- Artificial preservatives
- Flow stabilisers
- Binding substance
- Artificial sweetness
- Antioxidants
These substances do not have nutritional value except vitamins.
Also Read: Important Questions on Chemistry in Everyday Life
Artificial Preservatives: They prevent spoilage of food by stopping the growth of microorganisms. For example, sodium benzoate and sodium meta bisulphate.
Artificial Sweetness: They do not impart any calories to the body since these substances are excreted through urine. For example,
- Aspartame: It is used in cool drinks and ice creams.
- Alitame: It is 2000 times sweeter than sucrose.
Antioxidants: They prevent the spoilage of food by preventing the oxidation of food. For example,
- Butylated hydroxyl tolerance (BHT)
- Butylated hydroxyl anisole (BHA)
Dyes are coloured organic compounds that are used to impart colour to various substrates, including paper, leather, fur, hair, drugs and cosmetics. Dyes are classified into natural dyes and s ynthetic dyes.
Chemistry of Cleansing Agents in Everyday Life
What are soap and detergents.
Soaps are sodium or potassium salt of higher carboxylic acid such as stearic acid, palmitic acid and oleic acid, whereas detergents contain a long chain of alkyl groups. Detergents, in comparison to soaps , can also function in hard water.
Saponification: Alkaline hydrolysis of triesters of glycerol to form soap is known as saponification. Soap does not function in hard water since they precipitate in it.
How do soaps work?
Soaps are generally sodium or potassium salts of long-chain fatty acids. Soap molecules have a hydrophobic as well as a hydrophilic part. While the hydrophilic part clings to the water when washing, the hydrophobic end clings to the dirt particles. Thus, when we pour away the water, the dirt particles wash away with the soap molecules.
Also Read: Cleansing Action of Soaps and Detergents
Types of Soaps
- Toilet Soaps: Potassium soaps are softer than sodium soaps.
- Floating Soaps: They can be prepared by beating soap bubbles.
- Transparent Soaps: They contain soap dissolved in excess of alcohol, and it is evaporated.
- Medicated Soaps: They contain soaps by adding little amounts of Dettol, Savlon, etc.
- Laundry Soaps: They mainly contain sodium rosinate and borax.
Types of Detergents
Anionic Detergent: In this type, anions act as detergents. For example, sodium lauryl sulphate
Cationic Detergent: In this type, cations act as detergents. For example, cetyl trimethyl ammonium bromide.
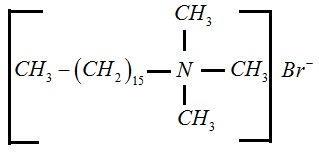
Non-ionic Detergent: They are neutral. The whole molecule acts as a detergent. For example, polyethylene glycol stearate.
Chemistry of Cosmetics in Everyday Life
Cosmetics contain the following categories of chemicals:
- Emulsifiers: They increase the stability of the emulsion . For example, potassium cetyl sulfate.
- Preservatives: They are added to cosmetics to increase their shelf life. For example, benzyl alcohol and salicylic acid.
- Thickeners: They give an appealing consistency. For example, cetyl alcohol and stearic acid.
- Emollients: They soften the skin by preventing water loss. For example, glycerine and zinc oxide .
- Glimmer and Shiners: For example, mica, bismuth oxychloride.
Other Examples of Chemistry in Everyday Life
Let us now discuss some common examples of chemistry in everyday life which most of us never knew about.
The Expiration Date on Bottled Drinking Water
Have you ever wondered why there is an expiration date on a bottle of drinking water? After all, it is just water, isn’t it? Well, most of us haven’t even noticed that there is, in fact, an expiration date on the bottle. The idea behind instilling an expiration on bottled drinking water is to standardise its packaging quality.
What the actual expiration date signifies is if the expiration date is up, the taste of the water will be different as there is a chance that the chemicals in the packaging material may ruin the quality of the water.
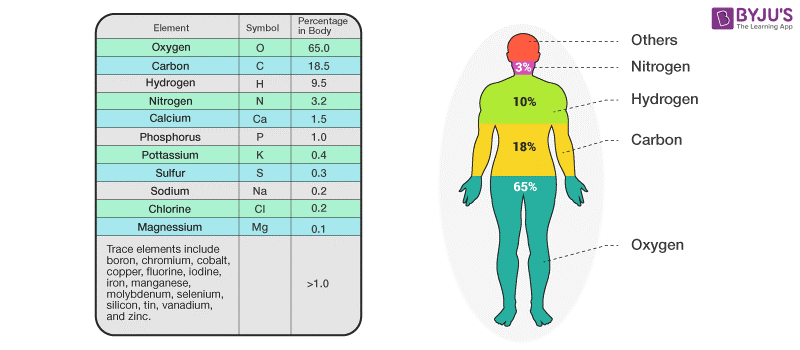
Elements in the Human Body
We all know that our body is about 60% water, but then what composes the rest of it? Carbon, Hydrogen, Nitrogen and Oxygen. These elements compose 96% of the human body. Whereas the rest 4% is composed of about 60 elements. Some of these elements include calcium, phosphorus, potassium and sulphur.
Sunblock and Sunscreen
There are two kinds of rays from the sun which are particularly bad for us, UV-A and UV-B. A sunscreen’s action, as the name suggests, functions as a screen and offers protection from sunburn which is caused by UV-B. Whereas, a sunblock has more of reflective nature and blocks both UV-A and UV-B radiations.
Related Topics
- NCERT Exemplar Questions on Chemistry in Everyday Life
- Revision Notes on Chemistry in Everyday Life
Frequently Asked Questions (FAQs)
Which artificial sweetener is used in cool drinks and ice creams, give an example of an anionic detergent., which chemical is added to increase the shelf life of cosmetics, give an example of cationic detergent., which rays do sunscreens block.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all JEE related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Register with Aakash BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
Students & Educators —Menu
- Educational Resources
- Educators & Faculty
- Standards & Guidelines
- Periodic Table
- Adventures in Chemistry
- Landmarks Directory
- Frontiers of Knowledge
- Medical Miracles
- Industrial Advances
- Consumer Products
- Cradles of Chemistry
- Nomination Process
- Science Outreach
- Publications
- ACS Student Communities
- LEADS Conference
- ChemMatters
- You are here:
- American Chemical Society
- Students & Educators
- Explore Chemistry
Chemistry Is Everywhere
Everything you hear, see, smell, taste, and touch involves chemistry and chemicals (matter). And hearing, seeing, tasting, and touching all involve intricate series of chemical reactions and interactions in your body. With such an enormous range of topics, it is essential to know about chemistry at some level to understand the world around us.
In more formal terms chemistry is the study of matter and the changes it can undergo. Chemists sometimes refer to matter as ‘stuff’, and indeed so it is. Matter is anything that has mass and occupies space. Which is to say, anything you can touch or hold. Common usage might have us believe that ‘chemicals’ are just those substances in laboratories or something that is not a natural substance. Far from it, chemists believe that everything is made of chemicals.
Although there are countless types of matter all around us, this complexity is composed of various combinations of some 100 chemical elements. The names of some of these elements will be familiar to almost everyone. Elements such as hydrogen, chlorine, silver, and copper are part of our everyday knowledge. Far fewer people have heard of selenium or rubidium or hassium.
Nevertheless, all matter is composed of various combinations of these basic elements. The wonder of chemistry is that when these basic particles are combined, they make something new and unique. Consider the element sodium. It is a soft, silvery metal. It reacts violently with water, giving off hydrogen gas and enough heat to make the hydrogen explode. Nasty ‘stuff’. Also consider chlorine, a green gas when at room temperature. It is very caustic and choking, and is nasty enough that it was used as a horrible chemical gas weapon in the last century. So what kind of horrible mess is produced when sodium and chlorine are combined? Nothing more than sodium chloride, common table salt. Table salt does not explode in water or choke us; rather, it is a common additive for foods we eat everyday.
And so it is with chemistry, understanding the basic properties of matter and learning how to predict and explain how they change when they react to form new substances is what chemistry and chemists are all about.
Chemistry is not limited to beakers and laboratories. It is all around us, and the better we know chemistry, the better we know our world.
Accept & Close The ACS takes your privacy seriously as it relates to cookies. We use cookies to remember users, better understand ways to serve them, improve our value proposition, and optimize their experience. Learn more about managing your cookies at Cookies Policy .
1155 Sixteenth Street, NW, Washington, DC 20036, USA | service@acs.org | 1-800-333-9511 (US and Canada) | 614-447-3776 (outside North America)
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Examples of Chemical Reactions in Everyday Life

Chemical reactions occur everywhere in the world around you, not just in a chemistry lab. Here are 20 examples of chemical reactions in everyday life and a closer look at what’s happening on a molecular level.
How to Recognize a Chemical Reaction
The first step to recognizing chemical reactions in the world around you is identifying when a reaction is taking place. Chemical reactions cause chemical changes . In other words, substances interact and form new products . Not every change in matter is a chemical reaction . For example, melting ice, tearing a sheet of paper into strips, and dissolving sugar in water are physical changes that don’t change the chemical identity of matter .
Here are some signs of a chemical reaction. If more than one sign is present, it’s likely a reaction has occurred:
- Temperature change
- Color change
- Bubbling or gas production
- Formation of a solid called a precipitate when liquids are mixed
20 Examples of Chemical Reactions in Everyday Life
Here are some broad examples of chemical reactions in daily life:
Photosynthesis
- Aerobic cellular respiration
- Anaerobic respiration (including fermentation )
- Oxidation (including rust)
- Metathesis reactions (such as baking soda and vinegar)
- Electrochemistry (including chemical batteries)
- Soap and detergent reactions
- Acid-base reactions
- Rotting of food
- Electroplating metals
- Disinfecting surfaces and contact lenses
- Leaves changing color with seasons
- Salt keeping ice off roads and helping to freeze ice cream

Examples of Organic Compounds
Some chemicals are inorganic, while those with carbon and hydrogen are organic. Here are examples in everyday life.
A Closer Look at Chemical Reactions in Daily Life
Here is a closer look at some everyday reactions, along with some chemical equations.
You experience combustion reactions when you strike a match, burn a candle, start a campfire, or light a grill. In a combustion reaction, a fuel reacts with oxygen from air to produce water and carbon dioxide. Here is the reaction for the combustion of propane, a fuel used in gas grills and some fireplaces: C 3 H 8 + 5O 2 → 4H 2 O + 3CO 2 + energy
Plants use a chemical reaction called photosynthesis to convert carbon dioxide and water into food (glucose) and oxygen. It’s a key reaction because it generates oxygen and yields food for plants and animals. The overall chemical reaction for photosynthesis is: 6 CO 2 + 6 H 2 O + light → C 6 H 12 O 6 + 6 O 2
Aerobic Cellular Respiration
Animals use the oxygen provided by plants to perform essentially the reverse reaction of photosynthesis to get energy for cells. Aerobic respiration reacts glucose and oxygen to form water and chemical energy in the form of adenosine triphosphate ( ATP ). Here is the overall equation for aerobic cellular respiration: C 6 H 12 O 6 + 6O 2 → 6CO 2 + 6H 2 O + energy (36 ATP)
Anaerobic Cellular Respiration
Organisms also have ways of getting energy without oxygen. Humans use anaerobic respiration during intense or prolonged exercise to get enough energy to muscle cells. Yeast and bacteria use anerobic respiration in the form of fermentation to make everyday products, such as wine, vinegar, yogurt, bread, cheese, and beer. The equation for one form of anerobic respiration is: C 6 H 12 O 6 → 2C 2 H 5 OH + 2CO 2 + energy
Rust, verdigris, and tarnish are all examples of common oxidation reactions. When iron rusts, it changes color and texture to form a flake coating called rust. The reaction also releases heat, but it usually occurs too slowly for this to be noticeable. Here is the chemical equation for the rusting of iron: Fe + O 2 + H 2 O → Fe 2 O 3 . XH 2 O
Electrochemistry
Electrochemical reactions are redox (oxidation and reduction) reactions that convert chemical energy into electrical energy. The type of reaction depends on the battery. Spontaneous reactions occur in galvanic cells, while nonspontaneous reactions take place in electrolytic cells.
Digestion is a complex process that involves thousands of chemical reactions. When you put food in your mouth, water and the enzyme amylase breaks down sugar and other carbohydrates into simpler molecules. Hydrochloric acid and enzymes break down proteins in your stomach. Sodium bicarbonate released into the small intestine neutralizes the acid and protects the digestive tract from dissolving itself.
Soap and Detergent Reactions
Washing your hands with water isn’t a chemical reaction because you’re just mechanically rinsing away grime. But, when you add soap or detergent, chemical reactions occur that emulsify grease and lower surface tension so you can remove oily grime. Even more reactions occur in laundry detergent, which may contain enzymes to break apart proteins and whiteners to prevent clothes from looking dingy.
Just mixing dry ingredients usually doesn’t result in a chemical reaction. But, adding a liquid ingredient often results in a reaction. Cooking with heat also causes reactions. Mixing flour, sugar, and salt is not a chemical reaction. Neither is mixing oil and vinegar. Cooking an egg is a chemical reaction because heat polymerizes proteins in egg white, while the hydrogen and sulfur in the yolk can react to form hydrogen sulfide gas. When you heat sugar, a reaction called caramelization occurs. When you heat meat, it browns due to the Maillard reaction . Baked goods rise due to carbon dioxide bubbles formed by the reaction between baking powder or soda and liquid ingredients.
Acid-Base Reactions
Acid-base reactions occur anytime you mix an acid (e.g., lemon juice, vinegar, muriatic acid, battery acid, carbonic acid from carbonated beverages) with a base (e.g., baking soda, ammonia, lye). A good example of an acid-base reaction is the reaction between baking soda and vinegar to form sodium acetate, water, and carbon dioxide gas: NaHCO 3 + HC 2 H 3 O 2 → NaC 2 H 3 O 2 + H 2 O + CO 2 In general, a reaction between an acid and a base produces a salt and water. For example, if you react muriatic acid (HCl) and lye (NaOH), you get table salt (NaCl) and water (H 2 O): HCl + NaOH → NaCl + H 2 O In this reaction, two clear liquids form another clear liquid, but you can tell a reaction occurs because it releases a lot of heat.
Related Posts
Home / Essay Samples / Science / Chemical Reaction / Everyday Chemistry: How Science Shapes Our Lives
Everyday Chemistry: How Science Shapes Our Lives
- Category: Science , Life , Education
- Topic: Chemical Reaction , Knowledge , Personal Statement
Pages: 1 (450 words)
- Downloads: -->
--> ⚠️ Remember: This essay was written and uploaded by an--> click here.
Found a great essay sample but want a unique one?
are ready to help you with your essay
You won’t be charged yet!
Online Classes Essays
Importance of Education Essays
Library Essays
Homework Essays
Illiteracy Essays
Related Essays
We are glad that you like it, but you cannot copy from our website. Just insert your email and this sample will be sent to you.
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Your essay sample has been sent.
In fact, there is a way to get an original essay! Turn to our writers and order a plagiarism-free paper.
samplius.com uses cookies to offer you the best service possible.By continuing we’ll assume you board with our cookie policy .--> -->