Exercise-induced asthma
On this page, alternative medicine, preparing for your appointment.
To diagnose exercise-induced bronchoconstriction, your health care provider first takes a medical history and does a physical exam. You may have tests to check your lung function and rule out other conditions.

Test of current lung function
Your provider will likely perform a spirometry (spy-ROM-uh-tree) test. This exam shows how well your lungs function when you aren't exercising. A spirometer measures how much air you inhale, how much you exhale and how quickly you exhale.
Your provider might have you repeat the test after you take an inhaled medicine to open your lungs. This medicine is known as a bronchodilator. Your provider compares the results of the two measurements to see whether the bronchodilator improved your airflow. This initial lung function test is important for ruling out chronic asthma as the cause of symptoms.

A spirometer is a diagnostic device that measures the amount of air you're able to breathe in and out. It also tracks the time it takes you to exhale completely after you take a deep breath.
Exercise challenge tests
During an exercise challenge test, you run on a treadmill or use other stationary exercise equipment to increase your breathing rate.
The exercise needs to be intense enough to trigger your symptoms. If needed, you might be asked to perform a real-life exercise challenge, such as climbing stairs. Spirometry tests before and after the challenge can provide evidence of exercise-induced bronchoconstriction.
Methacholine challenge breathing test
This test involves inhaling an agent, often methacholine, that narrows the airways in some people with exercise-induced bronchoconstriction. Afterward, a spirometry test checks lung function. This test mimics the conditions likely to trigger exercise-induced bronchoconstriction.
Your health care provider might prescribe medicines to take shortly before exercise or to take daily for long-term control.
Preexercise medicines
If your provider prescribes a medicine to take before exercising, ask how much time you need between taking the medicine and starting the activity.
- Short-acting beta agonists (SABAs) are the most commonly prescribed medicines to take before exercising. These medicines include albuterol (ProAir HFA, Proventil-HFA, Ventolin HFA) and levalbuterol (Xopenex HFA). short-acting beta2 agonists (SABAs) are inhaled medicines that help open airways. Do not use these medicines every day because it can make them less effective.
- Ipratropium (Atrovent HFA) is an inhaled medicine that relaxes the airways and may be effective for some people. A generic version of ipratropium also can be taken with a device called a nebulizer.
Long-term control medicines
Your provider may prescribe a long-term control medicine to manage underlying asthma or to control symptoms when preexercise treatment alone doesn't work. These medicines are usually taken daily. They include:
- Inhaled corticosteroids, which help calm inflammation in your airways. You take these medicines by breathing them in. You might need to use this treatment for up to four weeks before it will have maximum benefit.
- Combination inhalers, which contain a corticosteroid and a long-acting beta agonist (LABA), a medicine that relaxes airways. These inhalers are prescribed for long-term control, but your provider may recommend using it before you exercise.
Leukotriene modifiers, which are medicines that block inflammatory activity for some people. These medicines are taken by mouth. They can be used daily or before exercise if taken at least two hours in advance.
Possible side effects of leukotriene modifiers include behavior and mood changes and suicidal thoughts. Talk to your provider if you have these symptoms.
Don't rely only on quick-relief medicines
You also can use preexercise medicines as a quick-relief treatment for symptoms. However, you shouldn't need to use your preexercise inhaler more often than recommended.
Keep a record of:
- How many puffs you use each week.
- How often you use your preexercise inhaler for prevention.
- How often you use it to treat symptoms.
If you use your inhaler daily or you frequently use it for symptom relief, your provider might adjust your long-term control medication.
Exercise is an important part of a healthy lifestyle for everyone, including most people with exercise-induced bronchoconstriction. Besides taking your medicine, you can take these steps to prevent or reduce symptoms:
- Do about 15 minutes of warmup that varies in intensity before you begin regular exercise.
- Breathe through your nose to warm and humidify air before it enters your lungs.
- Wear a face mask or scarf when exercising, especially in cold, dry weather.
- If you have allergies, avoid triggers. For example, don't exercise outside when pollen counts are high.
- Try to avoid areas with high levels of air pollution, such as roads with heavy traffic.
If your child has exercise-induced bronchoconstriction, talk to your health care provider about providing an action plan. This document provides step-by-step instructions for teachers, nurses and coaches that explain:
- What treatments your child needs.
- When treatments should be given.
- What to do if your child has symptoms.
There is limited clinical evidence that any alternative treatments benefit people with exercise-induced bronchoconstriction. For example, it's been suggested that fish oil, vitamin C or vitamin C supplements can help prevent exercise-induced bronchoconstriction, but there isn't enough evidence to show if they're useful.
You're likely to start by seeing your primary health care provider. Your provider may refer you to someone who specializes in asthma, such as an allergist-immunologist or a pulmonologist.
Be prepared to answer the following questions:
- What symptoms have you had?
- Do they start immediately when you start exercising, sometime during a workout or after?
- How long do the symptoms last?
- Do you have breathing difficulties when you're not exercising?
- What are your typical workouts or recreational activities?
- Have you recently made changes to your exercise routine?
- Do the symptoms occur every time you exercise or only in certain environments?
- Have you been diagnosed with allergies or asthma?
- What other medical conditions do you have?
- What medications do you take? What is the dosage of each medication?
- What dietary supplements or herbal medications do you take?
Dec 07, 2022
- Exercise-induced bronchoconstriction (EIB). American College of Allergy, Asthma & Immunology. https://acaai.org/asthma/types-of-asthma/exercise-induced-bronchoconstriction-eib/. Accessed Oct. 21, 2022.
- Klain A, et al. Exercise-induced bronchoconstriction in children. Frontiers in Medicine. 2022; doi:10.3389/fmed..
- Malewska-Kaczmarek K, et al. Adolescent athletes at risk of exercise-induced bronchoconstriction: A result of training or pre-existing asthma? International Journal of Environmental Research and Public Health. 2022; doi:10.3390/ijerph19159119.
- Pigakis KM, et al. Exercise-induced bronchospasm in elite athletes. Cureus. 2022; doi:10.7759/cureus.20898.
- Asthma and physical activity in the school. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/resources/asthma-and-physical-activity-school. Accessed Oct. 27, 2022.
- Broaddus VC, et al., eds. Exercise testing. In: Murray and Nadel's Textbook of Respiratory Medicine. 7th ed. Elsevier; 2022. https://www.clinicalkey.com. Accessed Oct. 27, 2022.
- Burks AW, et al. Asthma pathogenesis. In: Middleton's Allergy: Principles and Practice. 9th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Oct. 27, 2022.
- O'Byrne PM. Exercise induced bronchoconstriction. https://www.uptodate.com/contents/search. Accessed Oct. 27, 2022.
- FDA requires Boxed Warning about serious mental health side effects for asthma and allergy drug montelukast (Singulair); advises restricting use for allergic rhinitis. Food & Drug Administration. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-boxed-warning-about-serious-mental-health-side-effects-asthma-and-allergy-drug. Accessed Oct. 27, 2022.
- Li JT (expert opinion). Mayo Clinic. Oct. 31, 2022.
- Symptoms & causes
- Doctors & departments
- Diseases & Conditions
- Exercise-induced asthma diagnosis & treatment
Products & Services
- A Book: Mayo Clinic Guide to Home Remedies
CON-XXXXXXXX
5X Challenge
Thanks to generous benefactors, your gift today can have 5X the impact to advance AI innovation at Mayo Clinic.
Exercise-Induced Bronchoconstriction (EIB)
Many professional athletes have asthma, but with the right treatment, they can still breathe easy during exercise. Do you have symptoms of asthma only during exercise?

On this page
Exercise-induced bronchoconstriction, or EIB, is the preferred term for what was known for years as exercise-induced asthma . Symptoms develop when airways narrow as a result of physical activity. As many as 90 percent of people with asthma also have EIB, but not everyone with EIB has asthma . Many elite and world-class athletes have EIB – including Olympic medal winners in sports like cross country skiing, figure skating and ice hockey. EIB didn’t hold them back, and it shouldn’t hold you back either. An allergist will customize a treatment plan that allows you to get back to the exercise you love, and feel better while doing it.
Find expert care.
Don’t let allergies or asthma hold you back from the things you love.
EIB is caused by the loss of heat, water or both from the airways during exercise when quickly breathing in air that is drier than what is already in the body. Symptoms typically appear within a few minutes after you start exercising and may continue for 10 to 15 minutes after you finish your workout. Anyone can experience these symptoms (especially someone who is out of shape), but with EIB, they are more severe. Wheezing in children after physical activity is often the first symptom of asthma.
Common symptoms of EIB include:
- Shortness of breath or wheezing
- Decreased endurance
- Tightness in the chest
- Upset stomach
- Sore throat
EIB triggers include airborne irritants related to specific sports. Examples are:
- Chorine when swimming
- Pollution while running or cycling
- Cold, dry air while ice skating or playing hockey
- Air temperature during hot yoga.
When you are working out or competing in a gym, perfume, cleaners, paint, and new equipment or carpet could also be triggers.
While it was thought for years that breathing cold air makes EIB worse, more recent studies indicate that the dryness of the air, rather than the temperature, is more likely the trigger. Cold air typically contains less moisture than warm air, and quickly breathing dry air dehydrates the bronchial tubes, causing them to narrow and restrict airflow.
The sports and activities that are most likely to cause EIB symptoms require constant activity or are done in cold weather. These include soccer, basketball, long-distance running, ice hockey, ice skating and cross-country skiing.
The activities that are least likely to cause EIB symptoms include walking, hiking and recreational biking, or sports requiring only short bursts of activity. These include volleyball, gymnastics, baseball, wrestling, golf, swimming, football, and short-distance track and field sports. Some swimming events can demand constant activity, but the warmth and humidity from the water make it easier for people with EIB to breathe.
Do you have EIB? Sometimes this can be difficult for athletes to know. Everyone has had trouble completing a workout at times, and athletes don’t often think of EIB or asthma as the cause. An allergist can determine whether your symptoms are exercise-induced alone, are a reaction to allergens or irritants in the air, or are an indication of underlying asthma.
As part of an examination, your allergist will take a history (including asking for information about any relatives with asthma or other breathing difficulties). You also may be asked for specific details about your physical activity, including where and how often you exercise. Your allergist will also consider other conditions, such as upper-airway problems, that might play a role in your difficulties with exercise.
To check how exercise affects your breathing, your allergist may measure your breathing before, during and after you run on a treadmill or ride an exercise bike. During the test you will breathe into a tube that connects to a spirometer, a device that measures the volume of air being inhaled and exhaled.
In some cases, environmental factors may contribute to EIB. Skaters and hockey players can be affected by a combination of cold, dry air in ice rinks and pollutants from ice-resurfacing machines. EIB in distance runners has been linked to exercising in high-allergen and high-ozone environments. In addition, indoor air with high levels of trichloramine, a chemical used in pool chlorination, has been linked to asthma and EIB in swimmers.
Treatment and Management
Two types of medicines used to treat asthma are also used to prevent and treat EIB symptoms. They are usually taken through an inhaler, though some are available in tablet form:
- Short-acting inhaled beta2-agonists (bronchodilators) stop symptoms right away. They may be taken 15 to 30 minutes before vigorous exercise and generally prevent symptoms for two to four hours. These medications are extremely effective in treating or preventing EIB symptoms, so if symptoms do not improve, let your allergist know.
- Long-term control asthma medicines are taken daily to prevent symptoms and attacks.
- Inhaled corticosteroids. These are the most commonly prescribed long-term asthma medications. They help to relieve narrowing and inflammation of the bronchial tubes. It may take two to four weeks before these drugs reach their maximum effect.
- Long-acting inhaled beta2-agonists (bronchodilators). Taken 30 to 60 minutes before exercise, these medications help prevent symptoms for 10 to 12 hours. They should be used only once within a 12-hour period, and they should be taken only in combination with an inhaled corticosteroid.
- Montelukast, a leukotriene receptor inhibitor, is also approved for the treatment of exercise-induced asthma symptoms. Taken once daily, this medication can help prevent symptoms that accompany exercise.
Elite athletes should check with the governing bodies of their sport about the medicines they are allowed to take to relieve their EIB or asthma symptoms. Another resource is the Prohibited List, published by the World Anti-Doping Agency. Some medications (including beta2-agonists) are considered performance-enhancing drugs and cannot be used by athletes in competition unless a Therapeutic Use Exemption is granted for medical need. Your allergist can help you answer questions about your medications.
Other suggestions for relieving symptoms of EIB include:
- Warm up with gentle exercises for about 15 minutes before you start more intense physical activity.
- Cover your mouth and nose with a scarf or face mask when you exercise in cold weather.
- Try to breathe through your nose while you exercise. This helps warm the air that goes into your lungs.
- Avoid triggers by making changes to your exercise routine.
- See an allergist to discuss prescription medications, which may be more effective than over-the-counter treatments.
If you continue to experience symptoms when you exercise, talk to your allergist . Together, you can work to adjust your personal treatment plan to make sure you’re feeling and performing your best.
Privacy Overview
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 14 August 2018
Exercise-induced bronchoconstriction: prevalence, pathophysiology, patient impact, diagnosis and management
- Bhumika Aggarwal 1 ,
- Aruni Mulgirigama 2 &
- Norbert Berend 3 , 4
npj Primary Care Respiratory Medicine volume 28 , Article number: 31 ( 2018 ) Cite this article
14k Accesses
78 Citations
56 Altmetric
Metrics details
- Respiratory signs and symptoms
Exercise-induced bronchoconstriction (EIB) can occur in individuals with and without asthma, and is prevalent among athletes of all levels. In patients with asthma, symptoms of EIB significantly increase the proportion reporting feelings of fearfulness, frustration, isolation, depression and embarrassment compared with those without symptoms. EIB can also prevent patients with asthma from participating in exercise and negatively impact their quality of life. Diagnosis of EIB is based on symptoms and spirometry or bronchial provocation tests; owing to low awareness of EIB and lack of simple, standardised diagnostic methods, under-diagnosis and mis-diagnosis of EIB are common. To improve the rates of diagnosis of EIB in primary care, validated and widely accepted symptom-based questionnaires are needed that can accurately replicate the current diagnostic standards (forced expiratory volume in 1 s reductions observed following exercise or bronchoprovocation challenge) in patients with and without asthma. In patients without asthma, EIB can be managed by various non-pharmacological methods and the use of pre-exercise short-acting β 2 -agonists (SABAs). In patients with asthma, EIB is often associated with poor asthma control but can also occur in individuals who have good control when not exercising. Inhaled corticosteroids are recommended when asthma control is suboptimal; however, pre-exercise SABAs are also widely used and are recommended as the first-line therapy. This review describes the burden, key features, diagnosis and current treatment approaches for EIB in patients with and without asthma and serves as a call to action for family physicians to be aware of EIB and consider it as a potential diagnosis.
Similar content being viewed by others
Targeting exertional breathlessness to improve physical activity: the role of primary care
Effects of physical therapy on lung function in children with asthma: a systematic review and meta-analysis

Handgrip strength associates with effort-dependent lung function measures among adolescents with and without asthma
Introduction.
Exercise-induced bronchoconstriction (EIB) was first recognised as a condition in the 1960s, when it was noted that the forced expiratory volume in 1 s (FEV 1 ) in some patients with asthma fell below the resting level during and after exercise compared with other patients with asthma, whose FEV 1 returned to normal 10–15 min post exercise. 1 This phenomena was first given the term of exercise-induced asthma (EIA), 2 subsequently exercise-induced bronchospasm 3 and finally EIB in 1970. 4 The introduction of lung function tests, performed before and repeatedly after exercise, helped to identify EIB. 5 , 6 , 7 Cut-off points were introduced for FEV 1 (13% reduction) to reduce the likelihood of misclassifying children without EIB. 8 These methodologies led to the discovery that EIB was affected by environmental factors, such as air temperature and humidity. EIB symptoms were improved by inhaling humid air at ambient temperatures and were completely prevented by inhaling fully saturated air, warmed to body temperature. These experiments formed the basis of the heat vs osmotic hypothesis to describe EIB pathophysiology. 9 Today, updated international guidelines provide a summary of standard approaches to the diagnosis and management of EIB. 10 , 11 , 12
EIB mostly presents in patients with asthma, but can also be experienced by individuals without asthma, including athletes. 11 , 13 , 14 , 15 , 16 The number of patients with EIB is likely to be underestimated, due to the limited number of studies investigating the prevalence of EIB in patients both with and without asthma. This has contributed to a lack of awareness among physicians and the general population. 13 Access to effective diagnostic methods is limited, resulting in under- or mis-diagnosis. 13 In addition, there is a risk that physicians will misdiagnose EIB as asthma, and subsequently over- or undertreat the disease. Because EIB can restrict a patients’ ability to exercise and can negatively impact their quality of life (QoL), 14 , 17 there is a growing consensus that the management of EIB needs to be improved so that patients with the condition can continue to lead a physically active lifestyle. This review aims to increase awareness of EIB by providing an update on its burden, key features, diagnosis and current treatment approaches.
Definition and prevalence
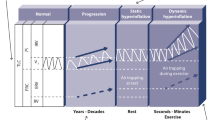
EIB is defined as acute airway narrowing (which is transient and reversible) that occurs during or after exercise and can be observed in both patients who have and those who do not have chronic asthma. 11 , 18 Typical symptoms include dyspnoea, wheezing, cough, chest tightness, excessive mucus production or the feeling of a lack of fitness when the patient is in good physical condition. 12 , 13 EIB reportedly usually occurs within 2−5 min after exercise, peaks after 10 min and resolves in approximately 60 min.
Prevalence of EIB in the general population
The prevalence of EIB in the general population is approximately 5−20%. 19 , 20 , 21 , 22 , 23 However, because few epidemiological studies differentiate people with asthma from the general population, the true prevalence of EIB within the non-asthmatic general population is poorly understood. 12
The prevalence of EIB is greater in high-performance athletes than in the general population owing to prolonged inhalation of cold, dry air and airborne pollutants. 18 Studies have reported a prevalence of EIB among elite or Olympic-level athletes of 30–70%, 15 , 19 but reports are variable depending upon the environment in which the sport is performed, the type of sport and the maximum intensity achieved. 12
In children, the prevalence of EIB is also higher than in the general population, ranging from 3 to 35% (children ≤16 years old) (Fig. 1 ). 20 , 21 , 22 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 There is large variation in the prevalence of EIB in children worldwide, with studies conducted in Nigeria, 25 Brazil 30 and Poland 29 reporting higher rates of EIB than Ghana, 24 India 40 and Greece 26 (Fig. 1 ). The impact of ethnicity on the prevalence of EIB is unclear, as only one study has directly compared prevalence between different ethnic groups in Scottish and English children. 41 Children from an Asian background were 3.6 times more likely to experience EIB compared with Caucasian inner-city children. 41 The prevalence of EIB was 12.3% in children with Asian ethnicity compared with 9.1% in Afro-Caribbean children and 4.5% in Caucasian inner-city children. 41 These results should be interpreted with care because studies of ethnicity are invariably confounded by non-genetic factors.
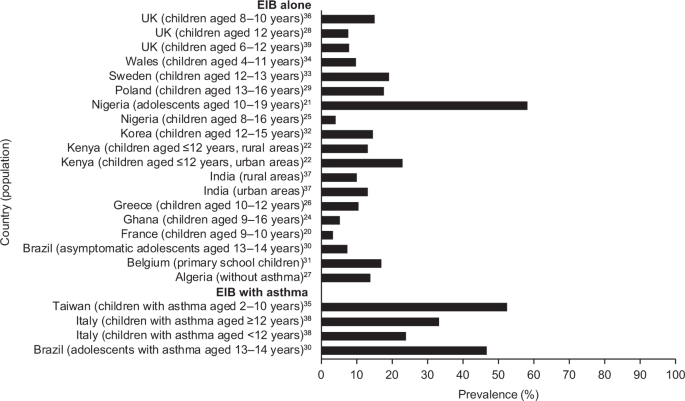
Country-specific prevalence* of EIB in children (general population). 20 , 21 , 22 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 *Owing to differences in study methodology, comparisons between studies should be treated with caution. EIB exercise-induced bronchoconstriction
Children living in urban environments are 1.6 times more likely to experience EIB compared with those living in rural areas, based on a study conducted in Kenya. 22 The higher rates observed in urban areas were partially explained by an increased family history of asthma symptoms or increased exposure to environmental factors in urban areas, such as vehicle fumes, crowdedness and household animals. 22 Similar urban–rural difference were observed in India. In addition, children with a low or middle socio-economic status were 8–10% more likely to experience EIB than those with a high socio-economic status. 25 However, this finding is not universal; a study conducted in Nigeria demonstrated that EIB was not related to socio-economic class.
Prevalence of EIB in patients with asthma
Asthma is the main co-morbid factor associated with EIB, and EIB is estimated to occur in approximately 90% of patients with asthma. 12 , 19 Patients with poorly controlled or severe asthma are more likely to manifest with EIB than patients with well-controlled or milder disease. 12 , 19 Consequently, between-country differences in the prevalence of EIB should be considered in the context country-specific asthma control levels. 42 In children and adolescents with asthma, the prevalence of EIB is estimated to be approximately 20–90%, 29 , 30 , 35 , 38 with one study reporting that 46.7% of children with asthma display symptoms of EIB compared with 7.4% of those who do not have asthma. 30 The majority of patients with chronic asthma will likely experience a transient increase in symptoms following an appropriate exercise challenge. EIB is known to hinder children’s participation in vigorous activities. Other risk factors contributing to the prevalence of EIB include allergic rhinitis, a personal history of allergies, history of asthma in a close relative or history of wheeze. 20 , 21 , 30 , 35
Challenges of determining EIB prevalence and future work
It remains challenging to understand the extent of EIB within the general non-asthmatic population and among patients with asthma when such substantial variability in the prevalence of EIB is reported. This variability is likely due to differences in geographical regions and population characteristics (age, background, diagnosis of asthma) and differences in study design. The prevalence of EIB may be affected by the type of exercise test used to induce symptoms (treadmill, cycling, free running) or the diagnostic method used to define EIB (FEV 1 , peak expiratory flow (PEF), direct/indirect bronchial provocation tests or self-reported). 19 , 20 , 22 Moreover, the lung function index used (time of pre- and post-exercise measurements), temperature, seasons and humidity are also factors that may have affected prevalence data. 19 , 20 , 22 The influence of these factors highlights the need for standardised diagnostic measures to more accurately assess the prevalence of EIB. In addition, there is a pressing need for more epidemiological studies to assess the prevalence of EIB in the general population, excluding patients with asthma, to allow the prevalence of EIB without asthma to be better understood.
Pathophysiology
At present, the osmotic theory is widely accepted as the established underlying mechanism of EIB. The osmotic theory suggests that increased ventilation in the airways during periods of exercise leads to water loss from the airway surfaces by evaporation, thus dehydrating the airway surfaces and initiating the events that lead to the contraction of bronchial smooth muscle. 43 During exercise-related hyperventilation, transient osmotic change at the airway surface occurs owing to reductions in epithelium liquid volume, which in turn triggers mast cell degranulation. 43 Consequently, there is mast cell-mediated release of prostaglandins (prostaglandin D2), leukotrienes, histamine and tryptase. These signalling molecules are known to mediate airway smooth muscle contraction and increase mucus production and microvascular permeability and sensory nerve activation, and their release is thought to be the main stimulus for bronchoconstriction and airway oedema. 43
Precipitating factors for EIB
In patients with EIB and chronic asthma, the pathophysiological mechanisms described above simply represent a trigger of underlying airway hyperactivity associated with poorly controlled asthma. 44
On the other hand, in patients with EIB who do not have asthma, the mechanisms described by the osmotic theory are believed to be directly responsible for causing bronchoconstriction and associated symptoms. Intense ventilation of cold air can further increase dehydration of the airway surfaces and cause changes in bronchial blood flow, explaining why athletes performing in cold weather (e.g., ice hockey, Nordic skiing) demonstrate the highest rates of EIB. 10 , 45 Epithelial injury that is caused by the inhalation of air pollutants and poorly conditioned air during exercise has also been hypothesised to be a contributing factor for the development of EIB in patients without asthma. 46 , 47 This hypothesis likely explains why reported prevalence rates for EIB in competitive swimmers approach 50%, with exposure to chloramines from the pool water considered the probable cause of epithelial injury. 45 Supporting this theory, a family or personal history of atopy to environmental factors has been identified as a known risk for EIB. 45
Impact of EIB on patients
EIB is associated with both a physical and an emotional burden. From our review of the literature, we found that a limited number of studies have investigated the emotional burden associated with EIB. A large-scale, survey-based study of more than 30,000 children aged 6–14 years in Japan revealed that children self-reporting symptoms of EIB with or without asthma had significantly lower QoL scores than children without EIB ( p < 0.001). 48 For children with asthma, the presence of EIB had a significant negative association with QoL regardless of the severity of asthma symptoms. 48 In the United States, adolescent athletes with or without asthma who reported dyspnoea during exercise ( n = 32) showed significantly lower scores for health-related QoL (HRQoL), including sub-scores for physical functioning, general well-being and emotional functioning, than those without exercise-associated dyspnoea ( n = 128). 49 However, adolescents with spirometry-defined EIB compared with non-spirometry-defined EIB in this study did not show significant reductions in HRQoL, possibly owing to the low number of patients included ( n = 18). 49 A similar Swedish study of adolescents with or without asthma ( n = 140) demonstrated a significant association between spirometry-defined EIB and reduced HRQoL. 50 Interestingly, this effect was revealed to be primarily driven by reduced total HRQoL and physical function in girls with EIB, with no significant difference evident between boys with or without EIB. 50 Girls with EIB also exhibited significantly higher scores for anxiety, but not depression, compared with girls without EIB. 50 A telephone-based survey, the Exercise-Induced Bronchospasm Landmark National Survey in the United States, provided comprehensive information relating to exercise-induced respiratory symptoms from the perspective of both the general population ( n = 1085) and adults with EIB and asthma (defined as those who reported taking asthma medication in the previous year; n = 1001). 14 The survey found a significant burden of disease associated with EIB, including emotional burden. 14 Patients with asthma who reported ≥1 symptom of EIB reported feeling more fearful (10.9 vs 27.7%; p < 0.001), isolated (6.0 vs 15.1%; p < 0.01), depressed (9.1 vs 23.4%; p < 0.001), frustrated (22.9 vs 54.5%; p < 0.001) and embarrassed (4.2 vs 20.0%; p < 0.001) compared with those not reporting EIB symptoms. 14 While current evidence indicates a significant functional and emotional impairment among patients with EIB and asthma, there is a need for more studies to assess the burden of disease and HRQoL among patients with objectively measured EIB and underlying asthma, as well as among patients with EIB alone.
Almost half of patients (45.6%) with asthma reported impact on both their participation and performance in sports, and a similar number (42.7%) reported they could not keep pace with peers during physical activities. 14 A systematic review of studies assessing the impact of EIB on athletic performance failed to show a significant effect but did highlight the need for more well-designed, sport-specific studies on the physiological impact of EIB. 51
Impact of effective EIB management
Given the well-known health benefits of exercise in both the general population and individuals with asthma, 52 the need to manage EIB effectively is clear. Exercise, in particular swimming, 53 has been shown to improve lung function and asthma symptoms and outcomes, including QoL in patients with asthma. 54 An analysis of the impact of an aerobic training programme ( N = 101) on asthma-specific health-related QoL, asthma symptoms, anxiety and depression scores in patients with moderate or severe persistent asthma found that aerobic training had an important role in the clinical management of persistent asthma. 54 Significant ( p < 0.001) reductions in physical limitation and symptom frequency (Fig. 2 ) were reported in the training group compared with the control group. Moreover, only patients from the training group reported reductions in anxiety and depression levels ( p < 0.001). 54

Impact of aerobic training on symptomatic burden in patients with moderate or severe persistent asthma. 54 Patients were 20–50 years old with moderate or severe persistent asthma. Patients were under medical treatment for 6 months and considered clinically stable; * p < 0.05 compared with baseline; † p < 0.05 compared with baseline and control group (two-way repeated-measure analysis of variance). Control group, n = 45; aerobic training group n = 44. **Time points are 0 days (1 month before treatment), 30 days (first month of treatment), 60 days (second month of treatment) and 90 days (third month of treatment) 54
Many patients stop exercising because of their EIB symptoms. In the 2011 EIB Landmark Survey, 22.2% of children with asthma aged 4−12 years and 31.8% of those aged 13−17 years avoided sports activities as a result of their EIB. As EIB affects up to 90% of patients with asthma, 12 the potential impact on aerobic exercise participation is substantial. Arguably, patients with asthma and EIB are at greater disadvantage than those with asthma and no EIB, for symptom precipitation during exercise often leads to avoidance of regular exercise and reduced QoL. It is important to raise awareness in primary care settings that EIB restricts exercise in patients with asthma, given the clinical and psychosocial benefits associated with physical activity.
The diagnosis of EIB in patients with and without asthma is multifactorial, leading to the condition often being either under- or over-diagnosed. 13 A recent systematic review found insufficient evidence to support the widespread adoption of any existing EIB screening tools, and highlighted that there exists a substantial unmet need for a validated questionnaire. 13 Here we will discuss a number of diagnostic methods that are currently used for diagnosing EIB in both patients with underlying asthma and in those with EIB alone.
EIB should be considered when patients report respiratory symptoms that are induced by exercise. One potential approach for family physicians is to ask the patient to measure his/her PEF after the typical exercise that usually provokes symptoms. 55 , 56 If peak flow results are reduced compared with the patient’s baseline readings, formal investigation is required. Diagnosis of EIB is confirmed based on specific changes in lung function provoked by exercise, rather than on the basis of symptoms. 11 , 18 Such testing can involve the use of both spirometric and bronchoprovocation techniques (Fig. 3 ; see refs. 11 , 18 , 44 , 47 , 57 ). 43
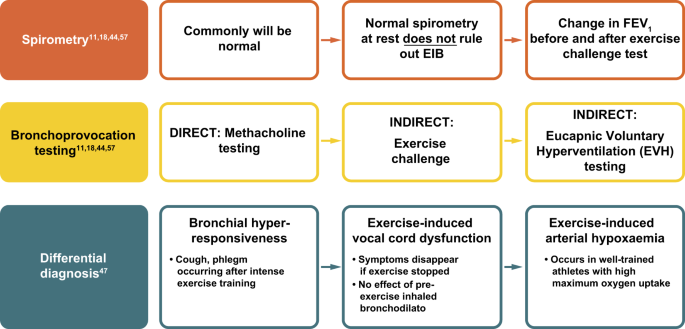
Algorithm for diagnosis of EIB. 11 , 18 , 44 , 47 , 57 EIB exercise-induced bronchoconstriction, FEV 1 forced expiratory volume in 1 s
The American Thoracic Society (ATS) Clinical Practice Guidelines outline a decline in FEV 1 of ≥10% from baseline after exercise or hyperpnoea challenge as confirmation of a positive EIB diagnosis. 11 A minimum of two reproducible FEV 1 measurements are taken in series post-exercise challenge, with the highest acceptable value being recorded at each interval (usually 5, 10, 15 and 30 min after exercise). The lowest percentage decline in FEV 1 within 30 min post exercise from the pre-exercise level can then be used to determine the severity of EIB (mild, 10– < 25%; moderate, 25– < 50%; severe ≥50%). 11
Bronchoprovocation testing
Many protocols recommend breathing dry air (10 mg H 2 O/L) with a nose clip in place while completing an exercise challenge. Several surrogates for exercise testing in the form of bronchoprovocation tests are available which, depending upon available resources, may be more suitable than a dry air exercise challenge. 11 The widely used methacholine challenge is a direct bronchoprovocation test; two versions of the methacholine challenge are used, a standard protocol recommended in ATS guidelines, and a second, more rapid protocol. 58 , 59 Alternatively, there are a number of indirect bronchoprovocation tests. The Eucapnic Voluntary Hyperventilation (EVH) test was developed specifically for identifying EIB. 60 Dry air (containing 5% carbon dioxide) is hyperventilated at room temperature for 6 min at a target ventilation of 30 times the subject’s FEV 1 , with a reduction of ≥10% of the pre-test value being diagnostic of EIB. 60 EVH testing is considered a reproducible, well-standardised test that is both quick and easy to administer; however, it is laboratory dependent and thus not widely available. 13 Other indirect bronchoprovocation tests include the hypertonic saline challenge and the mannitol test. 61 The latter was developed to improve the availability and standardisation of osmotic challenge testing; 62 , 63 however, the sensitivity and specificity of the mannitol challenge has yet to be well established. 11 , 13
While none of these bronchoprovocation tests are sensitive or specific to EIB, they all complement clinical history to identify airway hyperresponsiveness consistent with a diagnosis of EIB. 11 In addition, although these tests may be used for diagnosis of EIB in patients with and without underlying asthma, it has been suggested that indirect bronchoprovocation tests better reproduce the effects of exercise and may therefore be more accurate in diagnosing EIB in patients without asthma. 44
Distinguishing EIB from asthma
A key consideration for physicians when a patient presents with symptoms of wheeze and shortness of breath triggered by exercise is whether a diagnosis of asthma with EIB or EIB alone is appropriate. The management of EIB in patients without asthma is very different from the management of patients who experience EIB in association with poorly controlled asthma. As such, it is crucial to avoid over-diagnosis of asthma and subsequent over- or under-treatment.
The Global Initiative for Asthma (GINA) Guidelines outline several symptoms that increase or decrease the probability of a patient having asthma. 52 Most notably, symptoms that often worsen at night or in the early morning, that vary over time and in intensity, and that are triggered by exercise, viral infections, irritants and allergens increase the probability of asthma. Conversely, exercise-induced dyspnoea with noisy inspiration decreases the probability of asthma. 52 The guidelines also highlight the importance of determining if the patient’s symptoms occur only during or after exercise, and if the patient has any other risk factors for exacerbations. If symptoms are solely related to exercise, and there is no additional risk of exacerbation, a diagnosis of EIB rather than asthma should be considered. 52
Differential diagnosis
In the absence of airway hyperresponsiveness to challenge, differential diagnoses must be considered, particularly in adolescent athletes. Consideration must be given to the following conditions: bronchial hyperresponsiveness (the occurrence of cough or phlegm after intense exercise); exercise-induced vocal cord dysfunction (symptoms disappear when exercise is stopped and there is no observed effect of pre-exercise inhaled bronchodilator); and exercise-induced arterial hypoxaemia (occurring typically in well-trained athletes with high maximum oxygen uptake).
A Joint Task Force for defining practice parameters for the management of EIB (2016) suggested physicians can also consider cardiopulmonary exercise testing to determine if symptoms are resulting from exercise-induced dyspnoea and hyperventilation, particularly in children and adolescents. 43 Shortness of breath during exercise can also be associated with underlying conditions such as chronic obstructive pulmonary disease or restrictive lung conditions (e.g., obesity). 43 A history of shortness of breath alongside other systemic symptoms (e.g., pruritus, urticaria and hypotension) may rarely be indicative of exercise-induced anaphylaxis. 43 Finally, if EIB has been ruled out, referral to a specialist should be considered for patients who present as breathless when exercising (with or without chest pain) and for whom heart disease or other conditions are suspected. 43
Under-diagnosis and under-treatment
There is growing evidence that objectively confirmed EIB is more prevalent than would be assumed from using self-reported symptoms alone, 15 , 64 possibly because a decline in lung function post exercise (the criterion for EIB) may occur in the absence of symptoms. 23
A prospective study of varsity-level college athletes in the United States found that the use of symptoms to diagnose EIB is not predictive of whether athletes have objectively documented EIB. Of the 107 athletes included in the study, 42 (39%) recorded EVH results considered positive for EIB. 15 Of these athletes, 86% (36/42) reported no previous history of asthma. The EVH-confirmed prevalence of EIB was 36% in athletes without EIB symptoms compared with 35% in those with EIB symptoms. As such, the authors concluded that the empiric diagnosis and treatment of EIB following self-reported symptoms alone may result in an increase in inaccurate diagnoses and ultimately increased morbidity. 15 These results are corroborated by a study of elite British athletes, which showed that the majority (73%) with EVH-confirmed EIB were previously undiagnosed. 16
Failure to adequately diagnose EIB is also likely to result in under-treatment of symptoms. A survey conducted solely among patients with asthma found that although 83% of participants with asthma experienced at least one exercise-related respiratory symptom (shortness of breath, wheezing, coughing, difficulty taking a deep breath, noisy breathing or chest tightness during or immediately after exercising), only 30.6% reported a diagnosis of EIB. Importantly, despite these impairments, few respondents adhered to treatment guidelines relating to prophylactic medication prior to exercise. 14
Overall, current estimates reveal that approximately 70% of patients with asthma and EIB are diagnosed based on history and symptoms alone, and only 18% following exercise, medication or lung function testing (Table 1 65 ). A survey indicates that family physicians, in particular, are significantly less likely than pulmonologists to utilise objective testing for EIB. 66 This is likely to be due, at least in part, to access issues. Among family practitioners in England, 85% reported that they had no access to bronchoprovocation testing; 11% had access to laboratory-based exercise testing; and 4% had access to EVH, methacholine or mannitol provocation testing. 67
Treatment of EIB
Treatment of eib in patients without asthma.
For patients without underlying asthma, management of EIB should focus on relief of bronchoconstriction, and the reduction in risk (or prevention entirely) of the occurrence of bronchoconstriction, to allow the patient to continue to engage in physical exercise with minimal respiratory symptoms. There are many non-pharmacological approaches recommended to reduce the risk of bronchoconstriction, which include warm-up before exercise to induce a refractory period; interventions that pre-warm and humidify inhaled air during exercise (e.g., breathing through a face mask or scarf) and avoiding high exposure to air pollutants and allergens. 11 , 44 Some athletes use a physical warm-up of 10–15 min of moderately vigorous exercise before the planned period of exercise or competition to induce a so-called 'refractory period', during which EIB symptoms may be reduced. 43 If EIB symptoms continue despite these non-pharmacological approaches, use of pharmacological methods such as short-acting β 2 -agonists (SABAs) 15 min before exercise, leukotriene receptor antagonists (LTRAs) or chromones should be considered as alternative pre-exercise treatments in accordance with guidelines recommendations. 52
Treatment of EIB in patients with asthma
EIB in patients with asthma can be a sign of poor asthma control. In these cases, management of EIB should focus on following global treatment guidelines to ensure the underlying asthma is controlled. 52 Those patients who achieve good overall asthma control but retain EIB will require additional treatment. In addition to the non-pharmacological approaches described above, 11 guidelines recommend various pharmacological therapies to help prevent EIB in patients with chronic asthma.
Currently, patient understanding of EIB treatment may be characterised as inadequate. Only 22.2% of individuals experiencing exercise-related symptoms reported taking quick-relief medications prior to exercise ‘always’ or ‘most of the time’ (with this proportion increasing to just 38% in cases of diagnosed EIB).
The authors of the EIB Landmark Survey concluded that their findings highlighted an urgent need for better asthma education, with almost one-third of people with asthma reporting that they take rescue medication ≥3–6 times per week for uncontrolled asthma symptoms. 14 They suggest that exercise-related symptoms in this population reflect inadequate management of the underlying disease. Notably, 37% of patients with asthma were unaware that exercise-related symptoms indicate poor asthma control. 14 This finding highlights the need to confirm or refute a diagnosis of asthma as the first step in EIB management.
The ATS guidelines
The ATS guidelines 11 acknowledge that EIB may be present in both patients with and without asthma, and as such do not make specific recommendations based on the presence of asthma.
In patients diagnosed with EIB and asthma, the use of an inhaled SABA, typically 15 min before exercise, is strongly recommended 11 However, daily use of SABAs has been shown to lead to tolerance, and therefore should be used to prevent EIB on an intermittent basis only (i.e., less than daily on average). 11
Although not licensed specifically for EIB, the ATS recommends daily use of inhaled corticosteroids (ICS) for these patients, though it recognises that maximal improvement may require 2–4 weeks of treatment. The main benefit of ICS is as maintenance therapy to address underlying suboptimal control of asthma symptoms. The ATS recommendation against the use of a single dose of ICS immediately before exercise reflects this understanding. For patients who continue to have symptoms despite using an inhaled SABA before exercise, or who require an inhaled SABA daily or more frequently, daily use of long-acting β 2 -agonist (LABA) as a single therapy is not recommended due to known associations with acute exacerbations. 68 , 69 When EIB is unresponsive to SABA therapy, daily use of an LTRA taken at least 2 h before exercise or pre-exercise use of a mast cell stabiliser are recommended.
Guidelines on EIB by the Joint Task Force on Practice Parameters
This practice parameter summary is a 2016 update of contemporary practice guidelines first published in 2010 and based on a systematic literature review. 12 , 43 The updated guidelines recommend the use of SABAs for protection against EIB in both patients with and without asthma, and for accelerating recovery of pulmonary function. The Task Force recommends caution regarding the daily use of SABA alone or in combination with ICS for the management of EIB owing to the potential for tolerance (leading to a reduced duration/magnitude of effect). ICSs in combination with other preventive therapies are considered a good treatment option because of their ability to decrease the frequency and severity of EIB, although they do not necessarily eliminate it in patients with asthma. However, the guidelines do note that the use of ICS in the prevention of EIB in patients without asthma is controversial owing to a current lack of support from ad hoc designed clinical trials and impaired responses in patients with underlying neutrophilic inflammation. Consistent with the ATS guidelines, the use of daily LABAs with ICS therapy is not recommended for EIB unless this approach is needed to treat underlying moderate to severe persistent asthma.
Both LTRAs and mast cell-stabilising agents are considered suitable pre-exercise treatment options. 43 Inhaled ipratropium bromide should be considered for patients who have not responded to other agents; however, its ability to attenuate EIB is considered inconsistent. 43
Recommended treatment options: the evidence
Short-acting β 2 -agonists.
SABAs are the single most effective therapeutic agents for the acute prevention of intermittent EIB 43 (Fig. 4 ). SABAs stimulate β 2 -receptors on the surface of the airway smooth muscle, causing relaxation and bronchodilation, as well as possibly preventing mast cell degranulation. 11 In patients' asthma and EIB, SABAs have been shown to be effective in preventing a fall in FEV 1 (Fig. 4 ). 70 Evidence shows that when combined with pre-exercise warm-ups, SABAs still provide an additive protective effect in patients with asthma and EIB 11 and confer a greater protective effect against developing EIB than either warm-up or SABA alone. 71
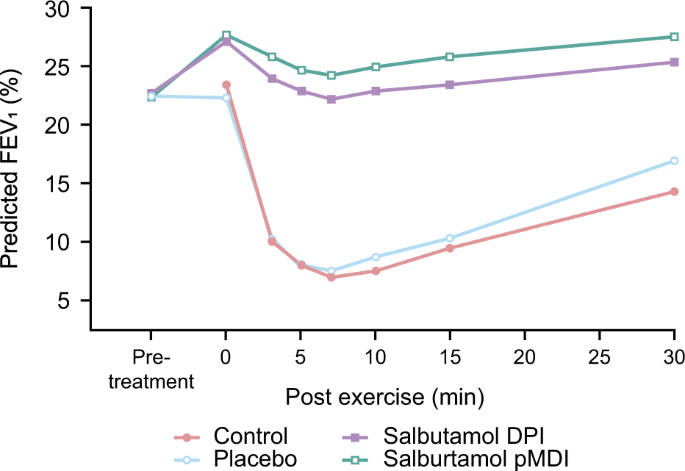
Mean values for forced expiratory volume in 1 s in patients with exercise-induced asthma treated with a short-acting β 2 -agonist. 70 Data are expressed as a percentage of the predicted normal value, measured before and 30 min after each treatment and for 15 min after exercise. Crossover study conducted in 27 patients. Reproduced from Anderson et al. (2001) with permission from Wolters Kluwer Health, Inc. DPI dry powder inhaler, FEV 1 forced expiratory volume in 1 s, pDMI pressurised metered dose
Inhaled corticosteroids
A Cochrane review of results from eight randomised controlled trials involving 162 participants found that ICS taken for 4 weeks pre-exercise can reduce post-exercise declines in FEV 1 in both children and adults. 72 ICS is licensed only for patients with asthma and may not be as effective against EIB alone. One study noted that EIB symptoms were unchanged in the majority (67%) of patients with EIB alone following a mean of 22 weeks of ICS therapy. 48
Long-acting β 2 -agonists
The LABA formoterol has also been shown to provide improvements in EIB and but daily use of LABAs with ICS therapy is not recommended for EIB unless to treat underlying moderate to severe persistent asthma. 12 , 43 , 11 Comparisons of SABAs and LABAs (salmeterol) in patients ( N = 12) with mild-to-moderate stable asthma showed that both treatments reduced mean declines in FEV 1 following exercise, with the SABA (3.8 ± 5.5%) and LABA (0.83 ± 6.2%) showing large effects 1 h post challenge compared with placebo (27.1 ± 7.3%). 73 However, a meta-analysis has demonstrated that the bronchoprotective effect of salmeterol at 9 h post treatment is reduced after 4 weeks. 74
Leukotriene receptor antagonists
Finally, LTRAs have also been shown to be efficacious for EIB in patients with and without asthma; LTRAs are also specifically indicated for prophylaxis in patients with asthma and EIB. 75 Clinical data have shown that once-daily treatment with montelukast (5 or 10 mg tablet) can improve a number of post-exercise deficits in lung function within 3 days in some patients. 76 In a pooled analysis of seven trials, patients with asthma and EIB had a mean maximum decline in post-exercise FEV1 that was 10.7% less with LTRAs compared with placebo. 11
Treatment of EIB in athletes
The treatment of EIB in elite athletes is a topic of particular interest and one that falls outside the scope of this review. Diagnosis and treatment of EIB in elite athletes has been extensively covered by a Joint Task Force Report prepared by the European Respiratory Society, the European Academy of Allergy and Clinical Immunology and GALEN, 10 as well as the World-Anti-Doping Agency. 77 Notably, the International Olympic Committee recommend that treatment follows international guidelines as described above; ICS and some inhaled SABAs can be used in accordance with the Therapeutic Use Exemption Standard. 78 In addition, athletes should be warned of the diminishing therapeutic effects of inhaled SABAs when used frequently, and offered education in order to develop self-management skills and ensure appropriate use of medication.
Conclusions
EIB can occur in both patients with and without asthma, with the prevalence in patients with asthma estimated at approximately 90%. 12 EIB may lead to a substantial emotional burden on patients, and restrict exercise and sports participation. This potentially leads to long-term QoL and physical health consequences in patients with EIB, with or without asthma. Increased awareness among patients and physicians of the symptoms and risk factors for EIB and increased use of objective diagnostic tests is key to the holistic management of patients with EIB. As such, there is a pressing need for more research into EIB in patients with and without asthma, and the development of validated and widely acceptable screening methods and/or accurate diagnostic methods, which can be made accessible to family physicians.
For patients with and without asthma, pre-exercise SABAs are recommended as the first-line option for pharmacological treatment of EIB. 11 , 43 The primary focus should be to increase awareness of EIB and educate patients to recognise symptoms and risk factors of EIB. Improved diagnosis and patient education further helps to optimise symptom control. Furthermore, increasing the accuracy of EIB diagnoses and providing education in how the patient can use SABA to prevent symptoms are needed.
Jones, R. S., Wharton, M. J. & Buston, M. H. The place of physical exercise and bronchodilator drugs in the assessment of the asthmatic child. Arch. Dis. Child. 38 , 539–545 (1963).
Article PubMed PubMed Central CAS Google Scholar
McNeill, R. S., Nairn, J. R., Millar, J. S. & Ingram, C. G. Exercise-induced asthma. Q. J. Med. 35 , 55–67 (1966).
PubMed CAS Google Scholar
Sly, R. M., Heimlich, E. M., Busser, R. J. & Strick, L. Exercise-induced bronchospasm: effect of adrenergic or cholinergic blockade. J. Allergy 40 , 93–99 (1967).
Article PubMed CAS Google Scholar
Fisher, H. K., Holton, P., Buxton, R. S., & Nadel, J. A. Resistance to breathing during exercise-induced asthma attacks. Am. Rev. Respir. Dis. 101 , 885–896 (1970).
Bierman, E. W., Kawabori, I. & Pierson, W. E. Incidence of exercise-induced asthma in children. Pediatrics 56 , 847–850 (1975).
Cropp, G. J. Relative sensitivity of different pulmonary function tests in the evaluation of exercise-induced asthma. Pediatrics 56 , 860–867 (1975).
Cropp, G. J. The exercise bronchoprovocation test: standardization of procedures and evaluation of response. J. Allergy Clin. Immunol. 64 , 627–633 (1979).
Godfrey, S., Springer, C., Bar-Yishay, E. & Avital, A. Cut-off points defining normal and asthmatic bronchial reactivity to exercise and inhalation challenges in children and young adults. Eur. Respir. J. 14 , 659–668 (1999).
Anderson, S. D. Is there a unifying hypothesis for exercise-induced asthma? J. Allergy Clin. Immunol. 73 , 660–665 (1984).
Carlsen, K. H. et al. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: epidemiology, mechanisms and diagnosis: part I of the report from the Joint Task Force of the European Respiratory Society (ERS) and the European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA2LEN. Allergy 63 , 387–403 (2008).
Parsons, J. P. et al. An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. Am. J. Respir. Crit. Care Med. 187 , 1016–1027 (2013).
Weiler, J. M. et al. Pathogenesis, prevalence, diagnosis, and management of exercise-induced bronchoconstriction: a practice parameter. Annals Allergy Asthma Immunol. 105 , S1–S47 (2010).
Article Google Scholar
Weiler, J. M. et al. Improving screening and diagnosis of exercise-induced bronchoconstriction: a call to action. J. Allergy Clin. Immunol. Pract. 2 , 275–280.e277 (2014).
Article PubMed Google Scholar
Parsons, J. P. et al. Impact of exercise-related respiratory symptoms in adults with asthma: Exercise-Induced Bronchospasm Landmark National Survey. Allergy Asthma Proc. 32 , 431–437 (2011).
Parsons, J. P. et al. Prevalence of exercise-induced bronchospasm in a cohort of varsity college athletes. Med. Sci. Sports Exerc. 39 , 1487–1492 (2007).
Dickinson, J., McConnell, A. & Whyte, G. Diagnosis of exercise-induced bronchoconstriction: eucapnic voluntary hyperpnoea challenges identify previously undiagnosed elite athletes with exercise-induced bronchoconstriction. Br. J. Sports Med. 45 , 1126–1131 (2011).
Khan, D. A. Exercise-induced bronchoconstriction: burden and prevalence. Allergy Asthma Proc. 33 , 1–6 (2012).
Krafczyk, M. A. & Asplund, C. A. Exercise-induced bronchoconstriction: diagnosis and management. Am. Fam. Physician 84 , 427–434 (2011).
PubMed Google Scholar
Weiler, J. M. et al. American Academy of Allergy, Asthma & Immunology Work Group report: exercise-induced asthma. J. Allergy Clin. Immunol. 119 , 1349–1358 (2007).
Caillaud, D. et al. Exercise-induced bronchospasm related to different phenotypes of rhinitis without asthma in primary schoolchildren: the French Six Cities Study. Clin. Exp. Allergy.: J. Br. Soc. Allergy. Clin. Immunol. 44 , 858–866 (2014).
Article CAS Google Scholar
Kuti, B. P. et al. Prevalence and factors associated with exercise-induced bronchospasm among rural school children in Ilesa, Nigeria. Niger. Postgrad. Med. J. 24 , 107–113 (2017).
Ng’ang’a, L. W. et al. Prevalence of exercise induced bronchospasm in Kenyan school children: an urban-rural comparison. Thorax 53 , 919–926 (1998).
Article PubMed PubMed Central Google Scholar
Rundell, K. W. et al. Self-reported symptoms and exercise-induced asthma in the elite athlete. Med. Sci. Sports Exerc. 33 , 208–213 (2001).
Addo-Yobo, E. O. et al. Exercise-induced bronchospasm and atopy in Ghana: two surveys ten years apart. PLoS Med. 4 , e70 (2007).
Adewumi, A. A. et al. Association between exercise-induced asthma and parental socio-economic status among school-aged adolescents in a semiurban community in Nigeria. J. Exerc. Rehabil. 13 , 292–299 (2017).
Anthracopoulos, M. B. et al. Physical activity and exercise-induced bronchoconstriction in Greek schoolchildren. Pediatr. Pulmonol. 47 , 1080–1087 (2012).
Benarab-Boucherit, Y. et al. Prevalence rate of exercise-induced bronchoconstriction in Annaba (Algeria) schoolchildren. J. Asthma 48 , 511–516 (2011).
Burr, M. L., Wat, D., Evans, C., Dunstan, F. D. & Doull, I. J. Asthma prevalence in 1973, 1988 and 2003. Thorax 61 , 296–299 (2006).
Cichalewski, L. et al. Prevalence of exercise-induced cough in schoolchildren: a pilot study. Allergy Asthma Proc. 36 , 65–69 (2015).
Correia Junior, M. A. V., Costa, E. C., Sarinho, S. W., Rizzo, J. A., & Sarinho, E. S. C. Exercise-induced bronchospasm in a hot and dry region: study of asthmatic, rhinitistic and asymptomatic adolescents. Expert Rev. Respir. Med. 11 , 1013–1019 (2017).
De Baets, F. et al. Exercise-induced respiratory symptoms are poor predictors of bronchoconstriction. Pediatr. Pulmonol. 39 , 301–305 (2005).
Hong, S. J. et al. Self-reported prevalence and risk factors of asthma among Korean adolescents: 5-year follow-up study, 1995-2000. Clin. Exp. Allergy 34 , 1556–1562 (2004).
Johansson, H. et al. Prevalence of exercise-induced bronchoconstriction and exercise-induced laryngeal obstruction in a general adolescent population. Thorax 70 , 57–63 (2015).
Jones, A. Asymptomatic bronchial hyperreactivity and the development of asthma and other respiratory tract illnesses in children. Thorax 49 , 757–761 (1994).
Lin, L. L. et al. Exercise-induced bronchoconstriction in children with asthma: an observational cohort study. J. Microbiol. Immunol. Infect. pii: S1684-118 , 30196-2 (2017).
Google Scholar
Powell, C. V., White, R. D. & Primhak, R. A. Longitudinal study of free running exercise challenge: reproducibility. Arch. Dis. Child. 74 , 108–114 (1996).
Sudhir, P. & Prasad, C. E. Prevalence of exercise-induced bronchospasm in schoolchildren: an urban-rural comparison. J. Trop. Pediatr. 49 , 104–108 (2003).
Tripodi, S. et al. Asthma control test and bronchial challenge with exercise in pediatric asthma. Front. Pediatr. 4 , 16 (2016).
Tsanakas, J. N., Milner, R. D., Bannister, O. M. & Boon, A. W. Free running asthma screening test. Arch. Dis. Child. 63 , 261–265 (1988).
Chhabra, S. K., Gupta, C. K., Chhabra, P., & Rajpal, S. Risk factors for development of bronchial asthma in children in Delhi. Ann Allergy Asthma Immunol 83 , 385–390 (1999).
Jones, C. O., Qureshi, S., Rona, R. J. & Chinn, S. Exercise-induced bronchoconstriction by ethnicity and presence of asthma in British nine year olds. Thorax 51 , 1134–1136 (1996).
Rabe, K. F. et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J. Allergy Clin. Immunol. 114 , 40–47 (2004).
Weiler, J. M. et al. Exercise-induced bronchoconstriction update-2016. J. Allergy Clin. Immunol. 138 , 1292–1295.e36 (2016).
Bonini, M. & Palange, P. Exercise-induced bronchoconstriction: new evidence in pathogenesis, diagnosis and treatment. Asthma Res. Pract. 1 , 2 (2015).
Molis, M. A. & Molis, W. E. Exercise-induced bronchospasm. Sports Health 2 , 311–317 (2010).
Anderson, S. D., & Kippelen, P. Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. J. Allergy Clin. Immunol. 122 , 225–235 (2008).quiz 236-227.
Carlsen, K. H., Hem, E., & Stensrud, T. Asthma in adolescent athletes. Br. J. Sports Med. 45 , 1266–1271 (2011).
Kojima, N. et al. Exercise-induced asthma is associated with impaired quality of life among children with asthma in Japan. Allergol. Int. 58 , 187–192 (2009).
Hallstrand, T. S., Curtis, J. R., Aitken, M. L. & Sullivan, S. D. Quality of life in adolescents with mild asthma. Pediatr. Pulmonol. 36 , 536–543 (2003).
Johansson, H. et al. The relationship between exercise induced bronchial obstruction and health related quality of life in female and male adolescents from a general population. BMC Pulm. Med. 16 , 63 (2016).
Price, O. J., Hull, J. H., Backer, V., Hostrup, M. & Ansley, L. The impact of exercise-induced bronchoconstriction on athletic performance: a systematic review. Sports Med. 44 , 1749–1761 (2014).
GINA. Global Strategy for Asthma Management and Prevention. http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/ (2017).
Beggs, S. et al. Swimming training for asthma in children and adolescents aged 18 years and under. Cochrane Database Syst. Rev. 4 , CD009607 (2013).
Mendes, F. A. et al. Effects of aerobic training on psychosocial morbidity and symptoms in patients with asthma: a randomized clinical trial. Chest 138 , 331–337 (2010).
Kirkby, R. E. & Ker, J. A. Exercise-induced asthma in a group of South African schoolchildren during physical education classes. South Afr. Med. J. 88 , 136–138 (1998).
Silva, L. S. P. et al. Evaluation of exercise-induced bronchospasm assessed by peak flow meter in obese adolescents. Rev. Bras. Med. Esporte 17 , 6 (2011).
Smoliga, J. M., Weiss, P. & Rundell, K. W. Exercise induced bronchoconstriction in adults: evidence based diagnosis and management. BMJ 352 , h6951 (2016).
Yan, K., Salome, C. & Woolcock, A. J. Rapid method for measurement of bronchial responsiveness. Thorax 38 , 760–765 (1983).
Crapo, R. O. et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am. J. Respir. Crit. Care Med. 161 , 309–329 (2000).
Anderson, S. D., Argyros, G. J., Magnussen, H. & Holzer, K. Provocation by eucapnic voluntary hyperpnoea to identify exercise induced bronchoconstriction. Br. J. Sports Med. 35 , 344–347 (2001).
Caggiano, S., Cutrera, R., Di Marco, A., & Turchetta, A. Exercise-induced bronchospasm and allergy. Front. Pediatr. 5 , 131 (2017).
Brannan, J. D. et al. The safety and efficacy of inhaled dry powder mannitol as a bronchial provocation test for airway hyperresponsiveness: a phase 3 comparison study with hypertonic (4.5%) saline. Respir. Res. 6 , 144 (2005).
Anderson, S. D. et al. A new method for bronchial-provocation testing in asthmatic subjects using a dry powder of mannitol. Am. J. Respir. Crit. Care Med. 156 , 758–765 (1997).
Simpson, A. J., Romer, L. M. & Kippelen, P. Self-reported symptoms after induced and inhibited bronchoconstriction in athletes. Med. Sci. Sports Exerc. 47 , 2005–2013 (2015).
Teva Respiratory, L. EIB: A Landmark Survey. https://www.eiblandmarksurvey.com accessed July 2017 (2016).
Parsons, J. P., O’Brien, J. M., Lucarelli, M. R. & Mastronarde, J. G. Differences in the evaluation and management of exercise-induced bronchospasm between family physicians and pulmonologists. J. Asthma. 43 , 379–384 (2006).
Hull, J. H., Hull, P. J., Parsons, J. P., Dickinson, J. W. & Ansley, L. Approach to the diagnosis and management of suspected exercise-induced bronchoconstriction by primary care physicians. BMC Pulm. Med. 9 , 29 (2009).
Castle, W., Fuller, R., Hall, J., & Palmer, J. Serevent nationwide surveillance study: comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment. BMJ 306 , 1034–1037 (1993).
Nelson, H. S., Weiss, S. T., Bleecker, E. R., Yancey, S. W. & Dorinsky, P. M. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 129 , 15–26 (2006).
Anderson, S. et al. Laboratory protocol for exercise asthma to evaluate salbutamol given by two devices. Med. Sci. Sports Exerc. 33 , 893 (2001).
Mickleborough, T. D. L., M., R. & Turner, L. A. Comparative effects of a high-intensity interval warm-up and salbutamol on the bronchoconstrictor response to exercise in asthmatic athletes. Int. J. Sports Med. 28 , 456–462 (2007).
Koh, M. S., Tee, A., LassersonT. J. & Irving, L. B. Inhaled corticosteroids compared to placebo for prevention of exercise induced bronchoconstriction. Cochrane Database Syst. Rev. 3 , CD002739 (2007).
Newnham, D. M., Ingram, C. G., Earnshaw, J., Palmer, J. B. & Dhillon, D. P. Salmeterol provides prolonged protection against exercise-induced bronchoconstriction in a majority of subjects with mild, stable asthma. Respir. Med. 87 , 439–444 (1993).
Bonini, M. Beta-2 agonists for exercise-induced bronchoconstriction in children. Paediatr. Respir. Rev. 15 , 42–44 (2014).
M. S. D. Limited. Summary of Product Characteristics . http://www.medicines.org.uk/emc/medicine/17718/SPC (2015).
Colice, G., & Calhoun, W. J. Section 2. Exercise-induced bronchospasm: albuterol versus montelukast: highlights of the Asthma Summit 2009: beyond the guidelines. World Allergy Organ. J. 3 , 23–30 (2010).
WADA. World Anti-Doping Agency. https://www.wada-ama.org/ (2018).
IOC. International Olympic Committee Consensus Statement on Asthma in Elite Athletes . https://stillmed.olympic.org/media/Document%20Library/OlympicOrg/IOC/Who-We-Are/Commissions/Medical-and-Scientific-Commission/EN-IOC-Consensus-Statement-on-Asthma-in-Elite-Athletes.pdf (2008).
Download references
Acknowledgements
Each author received non-financial support from GSK in the form of editorial support for the preparation of this manuscript. Medical writing assistance (in the form of assistance with developing the initial draft of the manuscript, collating author comments, copyediting and compiling figures and tables) was provided by Matthew Robinson, Fishawack Indicia Ltd, UK, and was funded by GSK. Funding for this manuscript was provided by GSK.
Author information
Authors and affiliations.
Respiratory, Global Classic & Established Products, GSK, Singapore, Singapore
Bhumika Aggarwal
Respiratory, Global Classic & Established Products, GSK, Middlesex, London, UK
Aruni Mulgirigama
Global Respiratory Franchise, GSK, Middlesex, London, UK
Norbert Berend
George Institute for Global Health, Newtown, NSW, Australia
You can also search for this author in PubMed Google Scholar
Contributions
B.A., A.M. and N.B. all contributed to the concept development, literature review, content development, writing and reviewing of this manuscript.
Corresponding author
Correspondence to Bhumika Aggarwal .
Ethics declarations
Competing interests.
B.A., A.M. and N.B. are employees of GSK and hold stocks or shares in the company.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Aggarwal, B., Mulgirigama, A. & Berend, N. Exercise-induced bronchoconstriction: prevalence, pathophysiology, patient impact, diagnosis and management. npj Prim Care Resp Med 28 , 31 (2018). https://doi.org/10.1038/s41533-018-0098-2
Download citation
Received : 21 December 2017
Revised : 05 July 2018
Accepted : 11 July 2018
Published : 14 August 2018
DOI : https://doi.org/10.1038/s41533-018-0098-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Exercise and asthma: an overview
Affiliations.
- 1 Department of Medical Sciences "M. Aresu", University of Cagliari, Cagliari, Italy; [email protected].
- 2 Department of Medical Sciences "M. Aresu", University of Cagliari, Cagliari, Italy.
- 3 Department of Respiratory Medicine and Allergology, Lund University, Lund, Sweden.
- 4 Oslo University Hospital, Oslo, Norway.
- PMID: 26672959
- PMCID: PMC4653278
- DOI: 10.3402/ecrj.v2.27984
The terms 'exercise-induced asthma' (EIA) and 'exercise-induced bronchoconstriction' (EIB) are often used interchangeably to describe symptoms of asthma such as cough, wheeze, or dyspnoea provoked by vigorous physical activity. In this review, we refer to EIB as the bronchoconstrictive response and to EIA when bronchoconstriction is associated with asthma symptoms. EIB is a common occurrence for most of the asthmatic patients, but it also affects more than 10% of otherwise healthy individuals as shown by epidemiological studies. EIA and EIB have a high prevalence also in elite athletes, especially within endurance type of sports, and an athlete's asthma phenotype has been described. However, the occurrence in elite athletes shows that EIA/EIB, if correctly managed, may not impair physical activity and top sports performance. The pathogenic mechanisms of EIA/EIB classically involve both osmolar and vascular changes in the airways in addition to cooling of the airways with parasympathetic stimulation. Airways inflammation plays a fundamental role in EIA/EIB. Diagnosis and pharmacological management must be carefully performed, with particular consideration of current anti-doping regulations, when caring for athletes. Based on the demonstration that the inhaled asthma drugs do not improve performance in healthy athletes, the doping regulations are presently much less strict than previously. Some sports are at a higher asthma risk than others, probably due to a high environmental exposure while performing the sport, with swimming and chlorine exposure during swimming as one example. It is considered very important for the asthmatic child and adolescent to master EIA/EIB to be able to participate in physical activity on an equal level with their peers, and a precise early diagnosis with optimal treatment follow-up is vital in this aspect. In addition, surprising recent preliminary evidences offer new perspectives for moderate exercise as a potential therapeutic tool for asthmatics.
Keywords: EIA; EIB; allergy; exercise-induced asthma; exercise-induced bronchoconstriction; sports.
PubMed Disclaimer
Simplified flow-chart for EIA treatment.
Suggested dose–response relationship between physical…
Suggested dose–response relationship between physical activity and asthma risk. (Courtesy of A. Moreira…
Similar articles
- Exercise-Induced Asthma: Managing Respiratory Issues in Athletes. Ora J, De Marco P, Gabriele M, Cazzola M, Rogliani P. Ora J, et al. J Funct Morphol Kinesiol. 2024 Jan 3;9(1):15. doi: 10.3390/jfmk9010015. J Funct Morphol Kinesiol. 2024. PMID: 38249092 Free PMC article. Review.
- Exercise-induced bronchoconstriction in asthmatic children: a comparative systematic review of the available treatment options. Grzelewski T, Stelmach I. Grzelewski T, et al. Drugs. 2009 Aug 20;69(12):1533-53. doi: 10.2165/11316720-000000000-00000. Drugs. 2009. PMID: 19678711 Review.
- Asthma and exercise-induced bronchoconstriction in athletes: Diagnosis, treatment, and anti-doping challenges. Hostrup M, Hansen ESH, Rasmussen SM, Jessen S, Backer V. Hostrup M, et al. Scand J Med Sci Sports. 2024 Jan;34(1):e14358. doi: 10.1111/sms.14358. Epub 2023 Jul 20. Scand J Med Sci Sports. 2024. PMID: 36965010 Review.
- Special considerations for adolescent athletic and asthmatic patients. Wuestenfeld JC, Wolfarth B. Wuestenfeld JC, et al. Open Access J Sports Med. 2013 Jan 10;4:1-7. doi: 10.2147/OAJSM.S23438. Open Access J Sports Med. 2013. PMID: 24379703 Free PMC article. Review.
- Exercise-induced bronchoconstriction, allergy and sports in children. Klain A, Giovannini M, Pecoraro L, Barni S, Mori F, Liotti L, Mastrorilli C, Saretta F, Castagnoli R, Arasi S, Caminiti L, Gelsomino M, Indolfi C, Del Giudice MM, Novembre E. Klain A, et al. Ital J Pediatr. 2024 Mar 13;50(1):47. doi: 10.1186/s13052-024-01594-0. Ital J Pediatr. 2024. PMID: 38475842 Free PMC article. Review.
- Exercise-Induced Bronchoconstriction in Children: State of the Art from Diagnosis to Treatment. Grandinetti R, Mussi N, Rossi A, Zambelli G, Masetti M, Giudice A, Pilloni S, Deolmi M, Caffarelli C, Esposito S, Fainardi V. Grandinetti R, et al. J Clin Med. 2024 Aug 5;13(15):4558. doi: 10.3390/jcm13154558. J Clin Med. 2024. PMID: 39124824 Free PMC article. Review.
- The impact of exercise on gene regulation in association with complex trait genetics. Vetr NG, Gay NR; MoTrPAC Study Group; Montgomery SB. Vetr NG, et al. Nat Commun. 2024 May 1;15(1):3346. doi: 10.1038/s41467-024-45966-w. Nat Commun. 2024. PMID: 38693125 Free PMC article.
- Effect of exposure to disinfection by-products during swimming exercise on asthma-related immune responses. Lee BA. Lee BA. J Water Health. 2024 Apr;22(4):735-745. doi: 10.2166/wh.2024.390. Epub 2024 Feb 26. J Water Health. 2024. PMID: 38678426
- Physical exercise in asthma adolescents: a concept review. Privitera A, Privitera S. Privitera A, et al. Multidiscip Respir Med. 2023 Sep 1;18(1):924. doi: 10.4081/mrm.2023.924. eCollection 2023 Jan 17. Multidiscip Respir Med. 2023. PMID: 37753201 Free PMC article.
- A Sociodemographic Analysis of the Impact of COVID-19-Related Schools' Closure on the Diet and Physical Activity of Children and Adolescents in Qatar. Abed Alah M, Abdeen S, Selim N, Tayar E, Al-Dahshan A, Kehyayan V, AlDahnaim L, Bougmiza I. Abed Alah M, et al. J Epidemiol Glob Health. 2023 Jun;13(2):248-265. doi: 10.1007/s44197-023-00101-8. Epub 2023 May 4. J Epidemiol Glob Health. 2023. PMID: 37140850 Free PMC article.
- Jones RS, Buston MH, Wharton MJ. The effect of exercise on ventilatory function in the child with asthma. Br J Dis Chest. 1962;56:78–86. - PubMed
- Weiler JM, Anderson SD, Randolph C, Bonini S, Craig TJ, Pearlman DS, et al. Pathogenesis, prevalence, diagnosis, and management of exercise-induced bronchoconstriction: a practice parameter. Ann Allergy Asthma Immunol. 2010;105(6 Suppl):S1–47. - PubMed
- Carlsen KH, Anderson SD, Bjermer L, Bonini S, Brusasco V, Canonica W, et al. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: epidemiology, mechanisms and diagnosis: part I of the report from the Joint Task Force of the European Respiratory Society (ERS) and the European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA2LEN. Allergy. 2008;63:387–403. - PubMed
- Crimi E, Bartalucci C, Brusasco V. Asthma, exercise and the immune system. Exerc Immunol Rev. 1996;2:45–64.
- Ulrik CS, Backer V. Increased bronchial responsiveness to exercise as a risk factor for symptomatic asthma: findings from a longitudinal population study of children and adolescents. Eur Respir J. 1996;9:1696–700. - PubMed
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- PubMed Central
Other Literature Sources
- The Lens - Patent Citations
- scite Smart Citations
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Causes & Prevention
- Symptoms & Types
- Tests & Diagnosis
- Asthma Diet Tips
- Exercising With Asthma
- Living with Asthma: Stress, Anxiety & Depression
- Support & Resources
- View Full Guide
Exercise-Induced Asthma

What Is Exercise-Induced Asthma?
Like it sounds, exercise -induced asthma is asthma that is triggered by vigorous or prolonged exercise or physical exertion. Most people with chronic asthma experience symptoms of asthma during exercise . However, there are many people without chronic asthma who develop symptoms only during exercise.
Why Does Exercise Trigger Asthma?
During normal breathing, the air we take in is first warmed and moistened by the nasal passages. Because people tend to breathe through their mouths when they exercise, they are inhaling colder and drier air.
In exercise-induced asthma, the muscle bands around the airways are sensitive to these changes in temperature and humidity and react by contracting, which narrows the airway. This results in symptoms of exercise-induced asthma, which include:
- Coughing with asthma
- Tightening of the chest
- Unusual fatigue while exercising
- Shortness of breath when exercising
The symptoms of exercise-induced asthma generally begin within 5 to 20 minutes after the start of exercise, or 5 to 10 minutes after brief exercise has stopped. If you are experiencing any of these symptoms with exercise, inform your doctor.
If I Have Asthma, Should I Avoid Exercise?
No. You shouldn't avoid physical activity because of exercise-induced asthma. There are steps you can take for prevention of asthma symptoms that will allow you to maintain normal physical activity. In fact, many athletes -- even Olympic athletes -- compete with asthma.
Can My Exercise-Induced Asthma Be Prevented?
Yes. Asthma inhalers or bronchodilators used before exercise can control and prevent exercise-induced asthma symptoms . The preferred asthma medications are short-acting beta-2 agonists such as albuterol . Taken 10 minutes before exercise, these medications can prevent the airways from contracting and help control exercise-induced asthma.
Another asthma treatment that may be useful when taken before exercise is inhaled ipratropium , which helps the airways to relax.
Having good control of asthma in general will also help prevent exercise-induced symptoms. Medications that may be part of routine asthma management include inhaled corticosteroids such as beclomethasone dipropionate (Qvar) or budesonide ( Pulmicort ). Your doctor can also prescribe an inhaled long-acting beta-2 agonist combined with a corticosteroid (like Advair or Symbicort), or with both a corticosteroid and an anticholinergic drug ( Trelegy Ellipta ). Tiotropium bromide ( Spiriva Respimat) is a long-acting anticholinergic medication you may use along with your regular maintenance medication.
In addition to taking medications, warming up before exercising and cooling down after can help prevent asthma. For those with allergies and asthma , exercise should be limited during high- pollen days or when temperatures are extremely low and air pollution levels are high. Infections ( colds , flu , sinusitis ) can cause asthma and increase asthma symptoms , so it's best to restrict your exercise when you're sick.
What Are the Best Exercises for Someone With Asthma?
For people with exercise-induced asthma, some activities are better than others. Activities that involve short, intermittent periods of exertion, such as volleyball, gymnastics, baseball, walking, and wrestling, are generally well tolerated by people with exercise-induced asthma.
Activities that involve long periods of exertion, like soccer, distance running, basketball, and field hockey, may be less well tolerated, as are cold weather sports like ice hockey, cross-country skiing, and ice skating. However, many people with asthma are able to fully participate in these activities.
Swimming , which is a strong endurance sport, is generally better tolerated by those with asthma because it is usually performed in a warm, moist air environment.
Maintaining an active lifestyle, even exercising with asthma, is important for both physical and mental health . You should be able to actively participate in sports and activities.
How Do You Treat Exercise-Induced Asthma?
Call 911 right away if the person:
- Is struggling to breathe
- Has blue lips
- Can’t walk or talk
- Shows other signs of a severe attack
1. Stop the activity.
- Have the person sit down and rest.
2. Follow the person’s asthma plan, if possible.
- Find out if the person has an individualized asthma action plan from a doctor.
- If so, follow its directions.
3. Give asthma first aid.
- For an adult, follow directions for first aid and using an inhaler in Acute Asthma Attack Treatment for Adults.
- For a child, follow directions for first aid and using an inhaler in Acute Asthma Attack Treatment for Children.
4. Resume activity when it’s safe.
- Wait until the person can breathe easily and is symptom-free before resuming exercise .
- If symptoms return when person starts exercise again, repeat treatment and stop exercise for rest of day.
5. Follow up.
- If symptoms do not improve with treatment, call the person's doctor for advice.
If an attack happens at school:
- Notify a school nurse or other designated staff member if the child does not have asthma medication or symptoms do not go away within 5 to 10 minutes after using an inhaler.
- Notify the child’s parents.
- Do not let the child leave the gym or play area alone.
Are There Tips to Prevent and Treat Exercise-Induced Asthma?
- Always use your pre-exercise inhaled drugs.
- Do warm-up exercises and have a cool-down period after exercise.
- If the weather is cold, exercise indoors or wear a mask or scarf over your nose and mouth .
- Avoid exercising outdoors when pollen counts are high (if you have allergies ), and avoid exercising outdoors when there is high air pollution.
- Limit exercise when you have a viral infection .
- Exercise at a level that is appropriate for you.
Again, asthma should not be used as an excuse to avoid exercise. With proper diagnosis and treatment of asthma, you should be able to enjoy the benefits of an exercise program without asthma symptoms.

Top doctors in ,
Find more top doctors on, related links.
- Asthma News
- Asthma Reference
- Asthma Slideshows
- Asthma Quizzes
- Asthma Blogs
- Asthma Videos
- Asthma Community
- Asthma Medications
- Find an Asthma Specialist
- Allergy Relief Advisor
- Cold, Cough & Flu
- Quit-Smoking Assessment
- Lung Disease
- Smoking Cessation
- More Related Topics

MICHAEL A. KRAFCZYK, MD, AND CHAD A. ASPLUND, MD
Am Fam Physician. 2011;84(4):427-434
Related letter: Vitamin C for Preventing Exercise-Induced Asthma
Patient information: See related handout on exercise-induced wheezing , written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
Exercise-induced bronchoconstriction describes the narrowing of the airway that occurs with exercise. More than 10 percent of the general population and up to 90 percent of persons previously diagnosed with asthma have exercise-induced bronchoconstriction. Common symptoms include coughing, wheezing, and chest tightness with exercise; however, many athletes will present with nonspecific symptoms, such as fatigue and impaired performance. Spirometry should be performed initially to evaluate for underlying chronic asthma, although results are often normal. An empiric trial of short-acting beta 2 agonists or additional bronchial provocation testing may be necessary to confirm the diagnosis. Nonpharmacologic treatment options include avoiding known triggers, choosing sports with low minute ventilation, warming up before exercising, and wearing a heat exchange mask in cold weather. Short-acting beta 2 agonists are recommended first-line agents for pharmacologic treatment, although leukotriene receptor antagonists or inhaled corticosteroids with or without long-acting beta 2 agonists may be needed in refractory cases. If symptoms persist despite treatment, alternative diagnoses such as cardiac or other pulmonary etiologies, vocal cord dysfunction, or anxiety should be considered.
| Self-reported symptoms alone should not be used to diagnose EIB. | C | – | — |
| The exercise challenge test can accurately diagnose EIB. | C | Varying results because of differences in how the test is performed | |
| Warming up before exercise may reduce the degree of EIB. | B | , | — |
| Wearing a heat exchange mask over the mouth and nose during cold-weather exercise may reduce symptoms of EIB. | B | , | — |
| Inhaled short-acting beta agonist use before exercise can attenuate symptoms of EIB. | A | , , | — |
| Using inhaled corticosteroids as controller therapy is an effective management strategy for EIB in patients with underlying asthma. | A | , , | — |
| Leukotriene receptor antagonist therapy can effectively manage EIB. | A | , , | — |
EIB = exercise-induced bronchoconstriction .
A = consistent, good-quality patient-oriented evidence; B = inconsistent or limited-quality patient-oriented evidence; C = consensus, disease-oriented evidence, usual practice, expert opinion, or case series. For information about the SORT evidence rating system, go to https://www.aafp.org/afpsort.xml .
Epidemiology and Etiology
EIB is more common in persons who participate in endurance sports and sports that require high minute ventilation. More than 10 percent of the general population and up to 90 percent of persons previously diagnosed with asthma have EIB. 3 The prevalence of EIB in athletes ranges from 11 to 50 percent, although it approaches 90 percent in athletes with asthma. 3 EIB also commonly occurs in cold-weather athletes and has been found to be present in approximately 50 percent of Olympic cross-country skiers. 4
In persons without asthma, the rapid breathing of cold, dry air over a prolonged period is an ideal setting for EIB. When a person finishes exercising, the airway responds with vasodilation to warm the airway, resulting in water loss and engorgement of the airways. This process causes bronchoconstriction and the release of proinflammatory mediators. Another possible etiology may be environmental irritants, such as chlorine gas in a swimming pool or gases from ice-resurfacing equipment. Persons with EIB and underlying asthma usually experience exacerbation of underlying inflammation and airway hyperactivity caused by any of these mechanisms or by poorly controlled chronic asthma. 5
Typical symptoms of EIB include wheezing, shortness of breath, dyspnea, cough, or chest tightness during or after exercise. These symptoms usually occur during strenuous exercise and peak about five to 10 minutes after exercise. Atypical symptoms include fatigue, feeling out of shape, feeling unable to keep up with peers, and abdominal discomfort. Self-reported symptoms have been shown to be poor predictors of EIB because other conditions, such as vocal cord dysfunction, can cause similar symptoms. 6 – 8 Therefore, symptoms alone should not be used to diagnose EIB.
The physical examination in patients with EIB is often unremarkable. If patients are evaluated when symptomatic, the most common findings are tachypnea and wheezing during end expiration.
In patients with possible EIB, spirometry should be performed to rule out underlying asthma 2 ( Figure 1 ) . Normal resting spirometry results are common in patients with EIB. If spirometry reveals an obstruction, additional testing before and after albuterol use is recommended. Obstruction with reversibility is indicative of underlying chronic persistent asthma.
If the patient is not an elite athlete, the next step is to prescribe an empiric trial of a short-acting beta 2 agonist. Only athletes who participate in high-level competition will need documentation of objective testing to use banned asthma medication. Follow-up, usually after one to two weeks, is necessary to determine whether treatment is successful. If the patient's response to treatment is inadequate, additional testing is warranted.
For elite athletes or for persons with EIB that does not respond to a trial of a short-acting beta 2 agonist, bronchial provocation tests can be used to identify provoked decreases in forced expiratory volume in one second (FEV 1 ). There are two main types of bronchial provocation tests: direct and indirect. Most pulmonary function laboratories can perform direct challenges, such as methacholine challenge. Some are also equipped to perform indirect challenges, such as an exercise challenge test, which can accurately diagnose EIB. 9 In addition, indirect challenges can be performed with a handheld spirometer at the location in which the athlete develops symptoms. Direct challenges have a lower sensitivity than indirect challenges 10 ; therefore, indirect challenge is the preferred diagnostic test for EIB. Examples of indirect testing are listed in Table 1 . 9 If bronchial provocation testing results are normal, other diagnoses should be considered ( Table 2 11 ) .
| Eucapnic voluntary hyperpnea test | Patient hyperventilates a mixture of cold, dry air | ||
| Spirometry performed before and after hyperventilation | |||
| Field-based exercise challenge | Usually performed in the environment that causes symptoms | ||
| Spirometry performed before and after exercise | |||
| Hypertonic saline test | Patient is given nebulized hypertonic saline | ||
| Spirometry performed before and after nebulization | |||
| Laboratory-based exercise challenge | Usually performed on a treadmill or stationary bike | ||
| Spirometry performed before and after exercise |
| Anxiety |
| Cardiac abnormalities (e.g., congestive heart failure, coronary artery disease, dysrhythmias, hypertrophic cardiomyopathy, valvular abnormalities) |
| Deconditioning |
| Hyperventilation syndrome |
| Myopathies |
| Obesity |
| Pulmonary arteriovenous malformations |
| Pulmonary disease (e.g., chronic asthma, chronic obstructive pulmonary disease, cystic fibrosis, interstitial lung disease, pectus excavatum, scoliosis, tracheobronchial malacia) |
| Vocal cord dysfunction |
EIB can affect many aspects of a patient's life, regardless of the severity of symptoms. The main goal of treatment is to allow patients to exercise safely. Secondary goals should include keeping athletes of all levels active and helping competitive athletes maximize performance. Asthma symptoms in association with exercise have been shown to reduce health-related quality of life scores in adolescents. 12 One report found that among asthma-related deaths during exercise, many athletes had only mild asthma. 13
NONPHARMACOLOGIC TREATMENT
Several nonpharmacologic options exist for managing EIB. Basic measures include avoiding known triggers (allergen and environmental) and choosing sports with low minute ventilation (short bursts of exercise), such as football, baseball, wrestling, or sprinting. Although nonpharmacologic treatment options can be effective, all athletes with EIB need to have a short-acting beta 2 agonist available.
Preexercise Warm-up . There is some evidence that a preexercise interval warm-up may attenuate the bronchoconstriction associated with EIB by inducing a refractory period. 2 , 14 However, this has not been shown to be helpful in elite cold-weather athletes. 15
Heat Exchange Mask . Heat exchange masks are designed to limit cold air exposure during exercise in athletes with EIB. They typically can be found in running stores or online. Using a mask has not been shown to be as effective as pretreatment albuterol in the prevention of bronchoconstriction. 2 , 16 One of the main limitations of a heat exchange mask is that it may not be practical during competition.
Nutrition . A review of the literature suggests that restricting dietary sodium intake for one to two weeks may reduce bronchoconstriction after exercise in patients with asthma and EIB; however, long-term studies are lacking. 17 Additionally, a small, double-blind crossover study showed that high-dose omega-3 fish oil supplementation for three weeks reduced the use of bronchodilators in the treatment group. The limitations of this study included the small size and no mention of adverse effects from the high dosage of omega-3 fish oil. 18
PHARMACOLOGIC TREATMENT
Medication is the mainstay of treatment for persons with EIB ( Table 3 19 , 20 ) . Although pharmacologic treatment has been well studied, more research is needed to differentiate between optimal treatment of persons who have EIB with underlying asthma and those who have EIB without asthma.
| Albuterol | 90 mcg per spray; two puffs 15 minutes before exercise and as needed | Five to seven minutes | Three to six hours | First-line prevention of acute asthma | 90 mcg per actuation, 17-g inhaler: $29 to $58 (NA) | |
| Levalbuterol (Xopenex HFA) | 45 mcg per spray; two puffs 15 minutes before exercise and as needed | Five to 10 minutes | Three to six hours | First-line prevention of acute asthma; similar to albuterol in safety and effectiveness, but more expensive | Box of 24 | |
| 1.25 mg per 3 mL vials: NA ($123) | ||||||
| 0.63 mg per 3 mL vials: NA ($136) | ||||||
| 0.31 mg per 3 mL vials: NA ($121) | ||||||
| Pirbuterol (Maxair) | 200 mcg per spray; one or two puffs 15 minutes before exercise and as needed | Five minutes | Five hours | First-line prevention of acute asthma; not available as generic; more expensive than albuterol | 200 mcg per actuation, 14-g inhaler: NA ($146) | |
| Formoterol (Foradil Aerolizer); combination budesonide/formoterol (Symbicort) | Aerolizer: 12 mcg per capsule; one spray twice per day | One to three minutes | 12 hours | Second-line prevention of chronic asthma in conjunction with inhaled corticosteroid | Aerosol: 60 12-mcg capsules: NA ($176) | |
| Metered-dose inhaler: 4.5 mcg per spray; two puffs twice per day | Inhaler | |||||
| 80/4.5 mcg per actuation aerosol, 10.2-g inhaler: NA ($196) | ||||||
| 160/4.5 mcg per actuation aerosol, 10.2-g inhaler: NA ($230) | ||||||
| Salmeterol (Serevent Diskus); combination fluticasone/salmeterol (Advair Diskus or HFA) | Diskus: 50 mcg per blister; one puff twice per day | 30 to 48 minutes | 12 hours | Second-line prevention of chronic asthma in conjunction with inhaled corticosteroid | Serevent Diskus (60 doses) | |
| 50 mcg per dose powder inhaler: NA ($178) | ||||||
| Advair Diskus (60 doses) | ||||||
| 100/50 mcg per dose aerosol: NA ($186) | ||||||
| 250/50 mcg per dose aerosol: NA ($216) | ||||||
| 500/50 mcg per dose aerosol: NA ($286) | ||||||
| HFA: 21 mcg per puff; two puffs twice per day | Advair HFA | |||||
| 45/21 mcg per actuation aerosol, 12-g inhaler: NA ($198) | ||||||
| 115/21 mcg per actuation aerosol, 12-g inhaler: NA ($231) | ||||||
| 230/21 mcg per actuation aerosol, 12-g inhaler: NA ($296) | ||||||
| Cromolyn (nebulizer solution) | 20 mg per 2 mL; 20 mg taken 10 to 60 minutes before exercise or 20 mg four times per day | Less than one week | Unknown | Second-line prevention of chronic asthma | 120 vials: 20 mg per 2 mL: $190 (NA) | |
| Beclomethasone (QVAR) | 40 or 80 mcg per spray; one to four puffs twice per day | One week | Variable | Second-line prevention of chronic asthma | 40 mcg per actuation, 8.7-g inhaler: NA ($110) | |
| 80 mcg per actuation, 8.7-g inhaler: NA ($132) | ||||||
| Budesonide (Pulmicort Flexhaler) | 90 or 180 mcg per spray; two puffs twice per day | One week | Variable | Second-line prevention of chronic asthma | 90 mcg per actuation aerosol inhaler: NA ($122) | |
| 180 mcg per actuation aerosol inhaler: NA ($160) | ||||||
| Ciclesonide (Alvesco) | 80 or 160 mcg per spray; one or two puffs twice per day | One week | Variable | Second-line prevention of chronic asthma | 80 mcg per actuation aerosol, 6.1-g inhaler: NA ($166) | |
| 160 mcg per actuation aerosol, 6.1-g inhaler: NA ($167) | ||||||
| Flunisolide | 250 mcg per spray; one or two puffs twice per day | One week | Variable | Second-line prevention of chronic asthma | 250 mcg per actuation aerosol, 7-g inhaler: NA ($98) | |
| Fluticasone (Flovent Diskus or HFA) | Diskus: 50, 100, or 250 mcg per blister; one or two puffs twice per day | One week | Variable | Second-line prevention of chronic asthma | Diskus (60 doses) | |
| 50 mcg per blister aerosol inhaler: NA ($115) | ||||||
| 100 mcg per blister aerosol inhaler: NA ($116) | ||||||
| 250 mcg per blister aerosol inhaler: NA ($155) | ||||||
| HFA: 44, 110, or 220 mcg per spray; two puffs twice per day | HFA | |||||
| 44 mcg per actuation aerosol, 10.6-g inhaler: NA ($120) | ||||||
| 110 mcg per actuation aerosol, 12-g inhaler: NA ($153) | ||||||
| 220 mcg per actuation aerosol, 12-g inhaler: NA ($247) | ||||||
| Mometasone (Asmanex) | 110 or 220 mcg per spray; one or two puffs, divided, once or twice per day | One week | Variable | Second-line prevention of chronic asthma | 60 metered doses | |
| 220 mcg per inhalation aerosol, 0.24-g inhaler: NA ($168) | ||||||
| Montelukast (Singulair) | Six to 14 years of age: 5 mg per day or two hours before exercise 15 years and older: 10 mg per day or two hours before exercise | Two hours | 24 hours | Second-line prevention of chronic asthma and allergic rhinitis | 30 5-mg tablets: NA ($157) | |
| 30 10-mg tablets: NA ($152) | ||||||
| Zafirlukast (Accolate) | Five to 11 years of age: 10 mg twice per day, one hour before or two hours after meal 12 years and older: 20 mg twice per day, one hour before or two hours after meal | Unknown | 12 hours | Second-line prevention of chronic asthma | 60 10-mg tablets: NA ($90) | |
| 60 20-mg tablets: NA ($90) | ||||||
| Zileuton, extended-release (Zyflo CR) | Older than 12 years: 1,200 mg twice per day | Two hours | 12 hours | Second-line prevention of chronic asthma | 120 600-mg tablets: NA ($610) | |
| Ipratropium (Atrovent HFA) | 17 mcg per spray: two to four puffs, 15 to 30 minutes before exercise and as needed | 15 minutes | Two to four hours | Third-line prevention of acute asthma | 17 mcg per actuation aerosol, 12.9-g inhaler: NA ($172) | |
Beta 2 Agonists . There are two types of inhaled beta 2 agonists: short-acting and long-acting. Short-acting beta 2 agonists are recommended first-line treatment in the management of EIB, preventively and for acute symptoms. 2 , 14 , 21 They should be used 15 minutes before exercise, typically have a peak action of 15 to 60 minutes, and last approximately three hours. There is growing concern about the development of tachyphylaxis with daily use of short-acting beta 2 agonists; therefore, they should be used only before more strenuous workouts or before competition. 5 Although long-acting beta 2 agonists have been shown to be effective in persons with EIB, 2 the U.S. Food and Drug Administration has recommended that they not be used in persons with asthma unless there is concomitant use of a controller medication, such as inhaled corticosteroids. 22 Concurrent use of inhaled corticosteroids and long-acting beta 2 agonists has been shown to be effective and superior to use of inhaled corticosteroids alone in managing EIB. 23
Mast Cell Stabilizers . Mast cell stabilizers have been shown to be more effective than anticholinergics but less effective than short-acting beta 2 agonists for managing EIB. 24 Mast cell stabilizers should be used 15 to 20 minutes before exercise. Metered-dose inhalers have been discontinued because they are difficult to manufacture without chlorofluorocarbon propellants; however, cromolyn is still available as a nebulized solution.
Inhaled Corticosteroids . Inhaled corticosteroids are considered controller medications and are the mainstay of treatment in patients with persistent asthma. 2 , 14 A meta-analysis showed that use of inhaled corticosteroids for four weeks or more reduced the percentage decrease in FEV 1 after exercise. 25 There is a paucity of studies comparing inhaled corticosteroids with other treatments for EIB.
Leukotriene Receptor Antagonists . Leukotriene receptor antagonists have been shown to have a persistent benefit against EIB. 14 , 26 Montelukast (Singulair) has an onset of action within two hours and continued EIB preventive benefit up to 24 hours after a single oral dose. 27 Compared with salmeterol (Serevent), montelukast is equally effective at preventing EIB at two hours and at eight and one-half hours, but montelukast is more effective at 24 hours. 21 Short-acting beta 2 agonists have been shown to be more effective than montelukast in the prevention of EIB. 28 Use of montelukast has not been shown to cause tachyphylaxis. 29
Other Agents . Ipratropium (Atrovent) is an anticholinergic that provides some protection against EIB but is not as effective as short-acting beta 2 agonists or leukotriene receptor antagonists. 14 Inhaled heparin 30 and furosemide 31 (Lasix) have been shown to be effective for treating EIB, but only small sample sizes have been used in studies.
A Practical Approach to the Patient
| Anticholinergics | Not prohibited | Not prohibited |
| Inhaled beta agonists | Permitted only by prescription | Salmeterol (Serevent) and albuterol: athletes must declare use |
| All other inhaled beta agonists: therapeutic use exemption is needed | ||
| Inhaled corticosteroids | Not prohibited | Declaration of use required in competition |
| Leukotriene receptor antagonists | Not prohibited | Not prohibited |
| Mast cell stabilizers | Not prohibited | Not prohibited |
NCAA = National Collegiate Athletic Association; USOC = United States Olympic Committee .
Information from references 32 and 33 .
Data Sources : A search was performed using the Agency for Healthcare Research and Quality, Cochrane Database of Systematic Reviews, the U.S. Preventive Services Task Force, UpToDate, and the National Guideline Clearinghouse. Search date: May 3, 2010. A PubMed search was performed on May 7 and May 17, 2010. For each source, the following keywords were used: exercise-induced asthma, exercise-induced bronchoconstriction, exercise-induced bronchospasm, exercise-induced, and asthma.
Randolph C. An update on exercise-induced bronchoconstriction with and without asthma. Curr Allergy Asthma Rep. 2009;9(6):433-438.
Managing asthma long term—special situations. In: National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert panel report 3: guidelines for the diagnosis and management of asthma. NIH publication no. 07-4051. Bethesda, Md.: National Heart, Lung, and Blood Institute; 2007:363–372. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf . Accessed May 3, 2010.
Parsons JP, Mastronarde JG. Exercise-induced bronchoconstriction in athletes. Chest. 2005;128(6):3966-3974.
Wilber RL, Rundell KW, Szmedra L, Jenkinson DM, Im J, Drake SD. Incidence of exercise-induced bronchospasm in Olympic winter sport athletes. Med Sci Sports Exerc. 2000;32(4):732-737.
Weiler JM, Bonini S, Coifman R, et al. Ad Hoc Committee of Sports Medicine Committee of American Academy of Allergy, Asthma & Immunology. American Academy of Allergy, Asthma & Immunology Work Group report: exercise-induced asthma. J Allergy Clin Immunol. 2007;119(6):1349-1358.
Parsons JP, Kaeding C, Phillips G, Jarjoura D, Wadley G, Mastronarde JG. Prevalence of exercise-induced bronchospasm in a cohort of varsity college athletes. Med Sci Sports Exerc. 2007;39(9):1487-1492.
De Baets F, Bodart E, Dramaix-Wilmet M, et al. Exercise-induced respiratory symptoms are poor predictors of bronchoconstriction. Pediatr Pulmonol. 2005;39(4):301-305.
Rundell KW, Im J, Mayers LB, Wilber RL, Szmedra L, Schmitz HR. Self-reported symptoms and exercise-induced asthma in the elite athlete. Med Sci Sports Exerc. 2001;33(2):208-213.
Rundell KW, Slee JB. Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J Allergy Clin Immunol. 2008;122(2):238-246.
Holzer K, Anderson SD, Douglass J. Exercise in elite summer athletes: Challenges for diagnosis. J Allergy Clin Immunol. 2002;110(3):374-380.
Weiss P, Rundell KW. Imitators of exercise-induced bronchoconstriction. Allergy Asthma Clin Immunol. 2009;5(1):7.
Hallstrand TS, Curtis JR, Aitken ML, Sullivan SD. Quality of life in adolescents with mild asthma. Pediatr Pulmonol. 2003;36(6):536-543.
Becker JM, Rogers J, Rossini G, et al. Asthma deaths during sports: report of a 7-year experience. J Allergy Clin Immunol. 2004;113(2):264-267.
Dryden DM, Spooner CH, Stickland MK, et al. Exercise-induced bronchoconstriction and asthma. Evidence Reports/Technology Assessments, no. 189. AHRQ publication no. 10-E001. Rockville, Md.: Agency for Healthcare Research and Quality; January 2010.
Rundell KW, Wilber RL, Szmedra L, Jenkinson DM, Mayers LB, Im J. Exercise-induced asthma screening of elite athletes: field versus laboratory exercise challenge. Med Sci Sports Exerc. 2000;32(2):309-316.
Beuther DA, Martin RJ. Efficacy of a heat exchanger mask in cold exercise-induced asthma. Chest. 2006;129(5):1188-1193.
Mickleborough TD. Salt intake, asthma, and exercise-induced bronchoconstriction: a review. Phys Sportsmed. 2010;38(1):118-131.
Mickleborough TD, Lindley MR, Ionescu AA, Fly AD. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006;129(1):39-49.
Physicians' Desk Reference . 64th ed. Montvale, N.J.: Physicians' Desk Reference, Inc.; 2010.
Sinha T, David AK. Recognition and management of exercise-induced bronchospasm. Am Fam Physician. 2003;67(4):769-774.
Medications. In: National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert panel report 3: guidelines for the diagnosis and management of asthma. NIH publication no. 07-4051. Bethesda, Md.: National Heart, Lung, and Blood Institute; 2007:213–276. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf . Accessed May 3, 2010.
U.S. Food and Drug Administration. FDA announces new safety controls for long-acting beta agonists, medications used to treat asthma. February 18, 2010. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm200931.htm . Accessed May 3, 2010.
Pearlman D, Qaqundah P, Matz J, Yancey SW, Stempel DA, Ortega HG. Fluticasone propionate/salmeterol and exercise-induced asthma in children with persistent asthma. Pediatr Pulmonol. 2009;44(5):429-435.
Spooner CH, Spooner GR, Rowe BH. Mast-cell stabilising agents to prevent exercise-induced bronchoconstriction. Cochrane Database Syst Rev. 2003;4:CD002307.
Koh MS, Tee A, Lasserson TJ, Irving LB. Inhaled corticosteroids compared to placebo for prevention of exercise induced bronchoconstriction. Cochrane Database Syst Rev. 2007;3:CD002739.
Coreno A, et al. Comparative effects of long-acting beta 2 -agonists, leukotriene receptor antagonists, and a 5-lipoxygenase inhibitor on exercise-induced asthma. J Allergy Clin Immunol. 2000;106(3):500-506.
Philip G, Pearlman DS, Villarán C, et al. Single-dose montelukast or salmeterol as protection against exercise-induced bronchoconstriction. Chest. 2007;132(3):875-883.
Raissy HH, Harkins M, Kelly F, Kelly HW. Pretreatment with albuterol versus montelukast for exercise-induced bronchospasm in children. Pharmacotherapy. 2008;28(3):287-294.
Edelman JM, Turpin JA, Bronsky EA, et al. Oral montelukast compared with inhaled salmeterol to prevent exercise-induced bronchoconstriction. A randomized, double-blind trial. Exercise Study Group. Ann Intern Med. 2000;132(2):97-104.
Ahmed T, Gonzalez BJ, Danta I. Prevention of exercise-induced bronchoconstriction by inhaled low-molecular-weight heparin. Am J Respir Crit Care Med. 1999;160(2):576-581.
Melo RE, Solé D, Naspitz CK. Comparative efficacy of inhaled furosemide and disodium cromoglycate in the treatment of exercise-induced asthma in children. J Allergy Clin Immunol. 1997;99(2):204-209.
National Collegiate Athletic Association. NCAA banned drug list. http://www.ncaa.org/wps/wcm/connect/public/NCAA/Student-Athlete+Experience/NCAA+banned+drugs+list . Accessed May 3, 2010.
World Anti-Doping Agency. The World Anti-Doping Code: The 2010 Prohibited List: International Standard. http://www.wada-ama.org/Documents/World_Anti-Doping_Program/WADP-Prohibited-list/WADA_Prohibited_List_2010_EN.pdf . Accessed May 3, 2010.
Continue Reading


More in AFP
More in pubmed.
Copyright © 2011 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Eur Clin Respir J
Exercise and asthma: an overview
Stefano r. del giacco.
1 Department of Medical Sciences “M. Aresu”, University of Cagliari, Cagliari, Italy
Davide Firinu
Leif bjermer.
2 Department of Respiratory Medicine and Allergology, Lund University, Lund, Sweden
Kai-Håkon Carlsen
3 Oslo University Hospital, Oslo, Norway
The terms ‘exercise-induced asthma’ (EIA) and ‘exercise-induced bronchoconstriction’ (EIB) are often used interchangeably to describe symptoms of asthma such as cough, wheeze, or dyspnoea provoked by vigorous physical activity. In this review, we refer to EIB as the bronchoconstrictive response and to EIA when bronchoconstriction is associated with asthma symptoms. EIB is a common occurrence for most of the asthmatic patients, but it also affects more than 10% of otherwise healthy individuals as shown by epidemiological studies. EIA and EIB have a high prevalence also in elite athletes, especially within endurance type of sports, and an athlete's asthma phenotype has been described. However, the occurrence in elite athletes shows that EIA/EIB, if correctly managed, may not impair physical activity and top sports performance. The pathogenic mechanisms of EIA/EIB classically involve both osmolar and vascular changes in the airways in addition to cooling of the airways with parasympathetic stimulation. Airways inflammation plays a fundamental role in EIA/EIB. Diagnosis and pharmacological management must be carefully performed, with particular consideration of current anti-doping regulations, when caring for athletes. Based on the demonstration that the inhaled asthma drugs do not improve performance in healthy athletes, the doping regulations are presently much less strict than previously. Some sports are at a higher asthma risk than others, probably due to a high environmental exposure while performing the sport, with swimming and chlorine exposure during swimming as one example. It is considered very important for the asthmatic child and adolescent to master EIA/EIB to be able to participate in physical activity on an equal level with their peers, and a precise early diagnosis with optimal treatment follow-up is vital in this aspect. In addition, surprising recent preliminary evidences offer new perspectives for moderate exercise as a potential therapeutic tool for asthmatics.
Exercise-induced respiratory symptoms were first described by Araeteus the Cappadocian in the 1st century A.D. ( ‘if from running, gymnastics, or any other work, breathing becomes difficult, it is called “Asthma”’ ). In the ‘modern’ era, Jones et al. ( 1 ) firstly described in 1962 the effects of exercise on ventilatory function in children, together with systematic exercise tests.
The terms ‘exercise-induced asthma’ (EIA) and ‘exercise-induced bronchoconstriction’ (EIB) are often used interchangeably. A consensus between the American Academy of Allergy, Asthma and Immunology (AAAAI), the American College of Allergy, Asthma and Immunology (ACAAI), and the Joint Council of Allergy, Asthma and Immunology (JCAAI) used the term ‘EIB with asthma’ for exercise-induced bronchoconstriction (EIB) with clinical symptoms of asthma and ‘EIB without asthma’ for an acute airflow obstruction without asthma symptoms ( 2 ). A joint Task force of European Academy of Allergy and Clinical Immunology and European Respiratory Society defined EIA as symptoms of asthma occurring after heavy exercise, whereas EIB denoted the reduction in lung function occurring after exercise, as seen in a standardized exercise test ( 3 ). In this review, we will refer to EIB as the bronchoconstrictive response while EIA also includes EIB + asthma symptoms. The two conditions will be discussed separately when the populations are specifically identified; otherwise, they are collectively referred to as EIB/EIA.
Aim of this review is to provide the reader with the current knowledge on this topic, focusing in particular on the athlete's asthma phenotype, together with a paragraph on new perspectives for exercise as a therapeutic tool for asthmatics.
It has been claimed that up to 75–80% of asthmatic subjects without anti-inflammatory treatment may experience an asthma attack provoked by exercise ( 4 ), but also individuals without a diagnosis of asthma may experience a significant reduction in lung function after heavy exercise, sometimes representing a risk factor for the development of asthma ( 5 ). Whereas the physiologic response to exercise usually result in slight bronchodilation, in population-based studies individuals without an asthma diagnosis may also suffer from EIB ( 6 ).
A minimum of 5–8 min continuous high-intensity effort is required to develop an exercise-induced bronchoconstrictive response. EIB is usually observed 2–10 min after heavy exercise, and not during maximum exercise intensity. However, in a study from Van Leeuwen et al., EIB occurred in children during sub-maximal exercise, not after ( 7 ).
Children and adolescents are more frequently affected than adults ( 8 ) and in the Oslo birth cohort study ‘Environment Childhood Study’ 36.7% of 10-year-old children with a diagnosis of asthma showed EIB, with a positive exercise test, while 8.6% had a positive EIB test in the entire population-based birth cohort ( 6 ).
Elite athletes also have an increased risk for EIA/EIB, especially those that participate in endurance sports such as swimming, running, and cycling and in winter sports ( 9 ).
Despite this increased prevalence, it is reassuring that many asthmatic elite athletes with optimal asthma treatment are able to participate on an equal level with their peers in the Olympic Games and in other top level international competitions. Fitch even described that asthmatic athletes even succeeded to win more medals than other athletes ( 10 ).
On the other hand, it is well known that asthmatic children suffering from EIA will become passive and participate at a low level in physical activity and play. Strunk reported that physical fitness was related to self-perception and psychological functioning in asthmatic children ( 11 ). However, a more recent study showed that although mild-to-moderate asthma had a moderate impact upon daily life quality in children, it did not seem to influence psychological functioning of the child or the family ( 12 ). Thus, optimal treatment of EIA becomes as important in the child with asthma as in the elite athlete with asthma, and in the common international asthma treatment guidelines, mastering EIA during childhood is one of the main aims of treating asthmatic children ( 13 , 14 ).
EIA and EIB: pathophysiological background
Pathogenic mechanisms of EIA/EIB probably differ in the athlete compared to children, adolescent, or adult with asthma ( 15 ).
Exercise is a quantifiable and reproducible stressor that can be modified experimentally and can be considered as a model of stress ( 16 ). It has an effect on the endocrine activity and the nervous and the immune systems, thereby activating several complex interacting mechanisms within the psycho-neuro-immune-endocrine pathways ( 17 ).
Classical mechanisms behind EIA and EIB include the so-called osmolar (or airway drying) and vascular (or ‘thermal’) hypothesis. Both hypotheses are based on the marked increased ventilation during physical activity, leading to increased water and heat loss through respiration. Increased water loss increases the osmolality of the extracellular fluid lining the bronchial mucosa, causing water to move extracellularly possible through the water channels, aquaporins, and bronchial epithelial cells to ‘shrink’, with an increase of intracellular ion concentration ( 18 ) and release of inflammatory mediators from mast cells, eosinophils, neutrophils, and other inflammatory cells including newly formed eicosanoids ( 19 , 20 ). The epithelium may serve as a key regulator of the balance of eicosanoids in the airways by activating the release of bronchoconstrictive eicosanoids in inflammatory cells in close contact and by alterations that reduce the synthesis of the protective PGE2 ( 21 ).
The ‘ Vascular’ or ‘ Thermal ’ hypothesis involves airway rewarming after cooling of the airways as the initiating mechanism. During normal tidal breathing, the nose functions like a rebreathing organ with warming up (up to 37°C) and humidifying the inspired air. The respiratory heat loss increases with increasing exercise intensity due to the increased ventilation. If the inhaled air is cold, the respiratory heat loss with the resulting cooling of the airways is further enhanced ( 15 , 22 , 23 ). The cooling of the airways results in reflex parasympathetic nerve stimulation causing bronchoconstriction through the vagal nerve ( 24 ). At first, it is notable that a reflex vasoconstriction of bronchial venules to conserve heat occurs, but when exercise ceases, the increased ventilation ceases, as does the cooling stimulus, causing a rebound vasodilatation of the peribronchial venules. The resulting smooth muscle constriction due to nerve stimulation and mucosal oedema due to vasodilatation in susceptible individuals reduces the size of the bronchial lumen with increased airways resistance ( 25 ). The main factor is now thought to be the inflammation induced by changes in airway osmolarity, and both mechanisms (osmolar and thermal) may work together under conditions of significant heat loss ( 3 ).
Also, exercise, with its repeated hyperventilation challenges, may cause bronchial epithelial damage with eosinophil and neutrophil influx and increased peptidoleukotriene concentrations in broncho-alveolar lavage fluid. In cultured human bronchial epithelial cells, experimental exposure to a hyperosmolar medium or the cooling–rewarming process is capable of triggering an inflammatory cascade by increasing the expression of various chemokines and cytokines such as IL-8 and RANTES (Regulated on Activation, Normal T cell Expressed and Secreted). This may suggest a possible mechanism for exercise-induced leukocyte migration into the airways ( 26 , 27 ). Athletes of different sports show an increased number of inflammatory cells in induced sputum, when measured at rest. Furthermore, pro-inflammatory cytokines are increased after prolonged, strenuous exercise ( 28 ). This interplay between hyperventilation, hyperosmolarity, and immune changes seems capable of causing a multi-factorial bronchial inflammatory response, involving common pathways of allergic and asthmatic inflammation.
Finally, the impact of rhinitis on asthma must also be considered ( 29 ). Epidemiological studies indicate that asthma and rhinitis frequently coexist, even in the absence of atopy ( 30 ). A total of 80–90% of asthma patients may report rhinitis symptoms and 19–38% of patients with allergic rhinitis report symptoms of asthma. Rhinitis is also very frequently found in athletes ( 31 ), with a variable prevalence depending on the criteria used for diagnosis in different studies. In a Swiss study, athletes with hay fever had significantly more frequent exercise-related airway symptoms, but received inadequate treatment ( 32 ).
Why do elite athletes develop asthma?
The prevalence of EIA is higher among elite athletes than in the general population ( 33 ), with a reported prevalence of up to 22.8% in summer sports and even higher (up to 54.8%) in winter sports. Such variability may depend on a lack of uniformity in the study methods (e.g. self-reported questionnaires, anti-doping applications for anti-asthmatic drug use, spirometry with broncho-reversibility test, etc.). However, those undertaking endurance sports seem to be at particular risk. Studies performed in US Olympic athletes show an increasing trend of the disease with 9.7% in 1976, 16.7% in 1996 ( 34 ), and 21.9% both at Nagano Winter Games ( 35 ) and at the Sydney 2000 Games ( 36 ) being reported. Between 4.2 and 7.7% of Olympic Athletes had a confirmed diagnosis of asthma with a positive bronchodilator or a bronchoprovocation test in 2006, 2008, and 2010 Olympic Games ( 37 ). In certain groups of athletes, such as swimmers and skiers, prevalence is even higher compared to the athlete population in general.
In addition to the type of sport with focus on endurance training, environmental factors are also of importance. This includes cold air for cross-country skiers and organic chlorine products for swimmers ( 38 ). The exposure to the environmental agents is further increased for these athletes due to their heavily increased ventilation during their daily repeated training and competitions ( 9 , 39 ).
In elite endurance athletes, the respiratory epithelial damage and reduced repair seem to be central in the development of inflammation in the athlete's asthma phenotype ( 15 , 40 ). Epithelial damage is the final result of repeated courses of intensive training sessions and competitions at the intensity of hyperpnoea necessitated by exercise at an elite level. This mechanism is partially reversible at the end of career and is also supported by evidence that transient airway hyperresponsiveness (AHR) can be associated with periods of intensive training ( 41 – 43 ). In addition, when other stimuli contribute to this process by causing increased epithelial damage and inflammation, such as respiratory virus infections, AHR develops and asthma symptoms may appear for the first time in previously asymptomatic athletes, as demonstrated by increased AHR for prolonged periods of time after respiratory virus infections ( 39 ).
The level of exercise load is fundamental, and its relevance was already confirmed by Carlsen et al. in 1989, with the first findings that AHR correlated with the increase in exercise load (increase in blood lactate) in both asthmatic and healthy swimmers ( 44 ). A kind of ‘biological gradient’ exists, and can be evaluated by examining exposure time in a sport or cumulative hours of training and how this modifies the risk of airway dysfunction ( 41 ). Heir and Larsen ( 39 ) reported that airway sensitivity to methacholine in cross-country skiers was negatively correlated with changes in the volume of exercise performed. In addition, Stensrud et al. ( 45 ) observed increased AHR to methacholine in elite athletes with increasing age and training volume. Other authors ( 46 , 47 ) reported a correlation between training load and sputum neutrophilia. Furthermore, in young competitive rowers, the cellularity of induced sputum obtained shortly after ‘all-out’ tests correlates directly with minute ventilation during the bout ( 48 ). Recently, the finding of an increased number of basophils in the induced sputum of athletes with and without asthma, but not in controls, may suggest the role of basophils as new possible players in the peculiar features of the athlete's asthma ( 49 ).
Data in humans have been confirmed also in exercising animals, where inflammatory changes in the airways have been found. In particular, epithelial damage has been found in exercising mice ( 50 ), sledge dogs ( 51 ), and in experimental studies ( 52 ), suggesting this to be the primary lesion in asthma and EIB.
Signs of lung epithelial stress and subsequent inflammation after exercise or bronchoprovocation tests can be found in some interesting experimental studies. Increased number of bronchial epithelial cells with apoptosis in induced sputum after repeated half-marathon races, in addition to increased serum levels of Clara Cell protein 16 (CC16) and increased supernatant interleukin 8 levels in induced sputum have been found in endurance runners ( 53 ). Inhaled air humidity and temperature can influence CC16 urinary levels that have been found increased in another study, but the levels were reduced when the study subjects inhaled warm, humid air during running ( 54 ). Eucapnic voluntary hyperpnoea (EVH) test with dry air also increased urinary CC16 levels irrespective of the athletes’ positive or negative bronchoprovocation result ( 55 ). Similar findings have been found in athletes that underwent a bronchoprovocation test with mannitol ( 56 ). The increased number of columnar epithelial cells in induced sputum of asthmatic patients with EIB, compared with asthmatic patients without EIB ( 57 ), related to degree of EIB, and associated with the supernatant sputum levels of cysteinyl leukotrienes, histamine, and increased expression of MUC5AC ( 20 ).
The link between respiratory epithelial damage and increased airways inflammation is further strengthened by the role of the ‘inflammasome’ IL-1-Th17 response in asthma ( 58 ). The extracellular water movement across cell membranes is important in the mechanism of EIA. Aquaporin (Aqp) is a channel for osmotic aqueous water transport, expressed in respiratory sub-epithelial glandular cells and alveolar type 1 cells of the lungs, and mice lacking the gene for Aqp five show methacholine-induced bronchiolar hyperresponsiveness compared with normal mice ( 59 , 60 ). A relationship between methacholine bronchial responsiveness and diminished pilocarpine-induced sweat secretion, tearing rate, and salivary flow rate in healthy athletes indicating an autonomic dysfunction with involvement of the parasympathetic nervous system has also been demonstrated ( 59 ).
The increased parasympathetic activity has also been demonstrated in endurance runners by Filipe et al. through pupillometry ( 61 ), by Knopfli et al. in two studies reporting higher parasympathetic nervous activity in top cross-country skiers ( 62 , 63 ) and indirectly by Deal et al. through the blocking effect of atropine inhalation on EIB induced by cold air inhalation, already in 1978 ( 64 ).
Which sport for the asthmatic?
One dilemma that physicians caring for athletic adolescents are faced with is what kind of recommendation should be given to those suffering from asthma. Athletes who participate in endurance and winter sports as well as swimming are at higher risk for EIA/EIB. Long-duration exercise and very low air temperature easily expose these athletes to the osmolar and vascular changes in the airway, fundamental in the EIA/EIB pathophysiology. Types of training and atopy are independent risk factors for EIA/EIB: combining the two factors in a logistic regression model, atopic speed, and power athletes have a 25-fold increased risk of EIA/EIB compared to non-atopic subjects, long-distance runners a 42-fold and swimmers a 92-fold increased risk ( 65 ). In addition, for the asthmatic athlete it is also important to avoid strenuous exercise during temporarily increased exposure to ‘biological stress’. This can be increased aeroallergen load, extreme cold air environment, or strenuous exercise too close to a recent viral respiratory tract infection. With an early and precise diagnosis, insightful precaution protecting the airways from extreme biological stress and an early start of anti-inflammatory treatment, the progression of bronchial hyperresponsiveness and asthma in these athletes may usually be well controlled. Data from the recent Olympic Games show that asthmatic athletes have won more medals during recent Olympic Games than athletes without asthma ( 10 ), demonstrating beyond doubt that the asthmatic athletes may compete on an equal level with their peers.
Sports with low risk for the development of asthma and bronchial hyperresponsiveness are the ones in which the physical effort is of short duration and in which high ventilatory levels are not reached. Medium-risk sports are team sports in general, in which the alternation of aerobic and anaerobic phases, as well as the relatively brief periods of continuous high-intensity exercise (in any case usually lower than 5–8 min) result in a lower risk of bronchial hyperreactivity. High-risk sports, as already stated, are endurance and winter sports in general ( Table 1 ).
Examples of sports and their potential risk of EIA/EIB
| Low-risk sports | Medium-risk sports | High-risk sports |
| All sports in which the athlete performs a<5–8 min effort | Team sports in general, in which the continuous effort rarely lasts more than 5–8 min | All sports in which the athlete performs a >5–8 min effort and/or in a dry/cold air environment |
| Track and field: Tennis Fencing Gymnastics Downhill skiing Boxing Golf Body building Weightlifting Martial arts | Soccer Rugby American football Basketball Volleyball Handball Baseball Cricket Field hockey | Track and field: Cycling Cross-country skiing Ice hockey Ice skating Biathlon |
| Swimming, water polo | ||
The swimming issue: is swimming beneficial or detrimental for asthma?
Swimming has been considered for many years as a safe and healthy sport activity for children with asthma, due to the humid air inhaled during swimming thus reducing the risk of EIA, and suggested to have beneficial effects on disease severity ( 66 ). However, in recent years, several studies, especially from the group of Bernard, reported a potential risk of asthma with an increased swimming pool attendance in children ( 67 ). Other studies have demonstrated the association between the availability of chlorinated swimming pools and the prevalence of childhood asthma ( 68 ), independently of climate, altitude, and the socio-economic status of the country. The findings according to the ‘pool chlorine hypothesis’ postulate that the rise of childhood asthma may partly result from increased exposure of children to chlorine-based irritants, especially swimming pool disinfection by-products, such as trichloramine. Bernard et al. recently reported that asthma development during adolescents was clearly associated with cumulative pool attendance before the age 7 ( 69 ). This hypothesis is further supported by studies on occupational asthma in swimming pool workers and lifeguards ( 70 , 71 ) and by studies comparing exposures to non-chlorinated pools (‘copper–silver pools’) ( 72 ) vs. chlorinated pools ( 73 ), in which attendance to the latter exerts a strong adjuvant effect to asthma and allergic rhinitis. A thorough evaluation of swimming and competing in chlorinated pools was recently published ( 74 ). Furthermore, very recent studies on mouse models of allergy showed hypochlorite-induced airway hyperreactivity, without evidence for allergic sensitization ( 75 ). All these studies are partly contradicted by a recent, large birth cohort study (the Avon Longitudinal Study of Parents and Children birth cohort) ( 76 ), in which British children from birth to the age of 10 did not increase their asthma risk with swimming pool attendance, and improved their lung function with a decreased risk of asthma symptoms.
However, although this question is unclear for development of asthma throughout childhood, competitive swimmers show an increase in asthma prevalence, with a mixed eosinophilic–neutrophilic airways inflammation ( 77 , 78 ), epithelial damage ( 46 ), and very frequent bronchial hyperresponsiveness ( 79 ). Further, increased levels of leukotriene B4 have been reported in elite swimmers ( 77 ), supporting the hypothesis that repeated hyperventilation challenges ( 80 ) together with exposure to chlorine derivatives can contribute to a peculiar inflammation mechanism that may support the theory of a phenotype of its own for the ‘competitive swimmers’ asthma’, a syndrome that may be potentially reversible when the athlete quits the competitive activity ( 65 , 81 ).
Environmental issues
Various environmental factors may influence performance in different types of sports. Due to the almost daily repeated periods of high minute ventilation during the intense physical activity of training and competitions, typical for top athletes, they will have a higher exposure to possible pollutants and allergens in the environmental air. Different environmental factors may be important for different types of sports. For cold weather types of sports like cross-country skiing and biathlon, cold air may be the harmful environmental factor ( 15 ). For other types of sports, other environmental factors or pollutants may be important. For water sports taking place in swimming pools, organic chlorine products originating from chlorine used in the disinfection of the water in the pools are probably harmful ( 82 ), and, as already stated, bronchial hyperresponsiveness is frequently found in competitive swimmers ( 79 ) as well as airways inflammation ( 46 , 77 , 78 ). High levels of CO 2 , NO 2 , and ultrafine particles are often present in indoor ice rinks where propane- or gasoline-powered ice resurfacers and edgers are used ( 83 , 84 ). Several studies demonstrated high prevalence of respiratory symptoms in ice-hockey players ( 85 , 86 ), figure skaters ( 87 ), and speed skaters ( 88 ). Electric resurfacers, increased ventilation, and emission control systems have been recommended to reduce the exposure ( 89 ).
Outdoor sports may expose the athlete to environmental pollutants, and furthermore pollen and moulds may influence performances and the presence of EIB in allergic athletes ( 90 ). Pollution from traffic may influence air quality in athletic fields ( 91 ).
Diagnosis: current guidelines
EIA should be suspected when cough, wheezing, and phlegm occur together with expiratory dyspnoea and audible rhonchi and sibilating rhonchi on lung auscultation after intense exercise of at least 5 min duration. Specific validated questionnaires can help to screen allergic athletes ( 92 ). Differential diagnoses must be considered ( Table 2 ), bearing in mind that intense physical exercise may produce increased amounts of respiratory secretions that may mimic asthmatic symptoms ( 93 ). Thus, it is important to confirm the clinical suspicion by objective measurements in order to provide the optimal treatment for the athlete with respiratory problems.
Exercise-induced asthma: differential diagnosis
| Diagnosis | Relevant for: | Clinical presentation | Verification of diagnosis |
|---|---|---|---|
| EIA | Symptoms occur shortly after (sometimes during) physical exercise. The dyspnoea is of expiratory type. By auscultation: rhonchi and sibilating rhonchi. Respiratory retractions. Gradual improvement either spontaneously or after inhaled bronchodilator. | Exercise test with sub-maximal exercise load (95% load). Spirometry before and after exercise. | |
| Exercise-induced laryngeal obstruction (EILO) | Asthmatics and individuals active in sports | Symptoms occur during maximum exertion. Symptoms disappear when exercise is stopped unless the patient continues to hyperventilate. The dyspnoea is of inspiratory type. There are audible inspiratory sounds from the laryngeal area and no signs of bronchial obstruction. No effect of pre-treatment with inhaled bronchodilator. | Exercise test with maximal exercise load, 6–8 min duration. Direct laryngoscopy during exercise test. |
| Exercise-induced hyperventilation | Individuals active in sports, general population | Hyperventilation with respiratory dyspnoea and decreased end-tidal CO . | Case history, observation during dyspnoea. |
| Exercise-induced arterial hypoxemia (EIAH) | Individuals active in sports | Occurs in well-trained athletes with high maximum oxygen uptake. Thought to be due to diffusion limitations and ventilation–perfusion inequality. Incomplete diffusion in the healthy lung may be due to a rapid red blood cell transit time through the pulmonary capillaries. | Exercise test, sub-maximal to maximal level. |
| Swimming-induced pulmonary oedema (SIPE) | Individuals active in sports | May occur after heavy swimming exercises with symptoms of haemoptysis, cough, and respiratory distress. Reduced diffusion capacity (TLCO) for up to weeks afterwards. | Case history, clinical examination, and lung function measurements during an active episode. |
| Other chronic lung diseases | Individuals with chronic lung disease | Reduced baseline lung function may reduce physical performance due to limitations in airflow and lung volumes. | Exercise test with measurement of tidal flow volume loops during exercise. |
| Other general disease | Individuals with chronic illnesses – cardiovascular disorders | Chronic heart diseases and others general disorders. | General diagnostic workout. |
| Poor physical fitness including obesity | General population | Related to expectations. High heart rate after low-grade exercise load. | Exercise test: assessment of physical fitness by determination of O max or maximal exercise load. |
Modified with permission from ( 93 ).
Objective tests were necessary to obtain approval from the World Anti-Doping Association (WADA) or International Olympic Committee for the use of inhaled corticosteroids (ICSs) and inhaled β 2 -agonists in international competitive sports, but from 1st of January 2012 ICSs and the inhaled β 2 -agonists salbutamol, salmeterol and formoterol have been taken off the list of prohibited drugs and now do not have any restrictions for use in sports.
It has been claimed that a field exercise test is most likely to reproduce symptoms of the real-life exercise ( 94 ), but this has not been confirmed by other studies ( 45 , 95 ). Furthermore, the field exercise test may be inconvenient both for logistical and standardization issues.
A standardized exercise test or other tests for bronchial hyperresponsiveness performed in a laboratory can be performed in a standardized way, including both environmental issues and exercise load by objective measures. Exercise tests can be performed by means of a motorized treadmill or a cycle ergometer, in a temperature- and humidity-controlled environment: the absolute water content should be below 10 mg H 2 O·L −1 ; otherwise, the test should be postponed until conditions become suitable ( 96 ). A very high workload is necessary to induce EIB which athletes can sometimes find difficult to reach in a lab environment. A key requirement is the ventilation reached and sustained with a target workload of 60–80% of the predicted Maximum Voluntary Ventilation that must be sustained for the last 4 min of an 8-min test ( 97 ). Heart rate recorded electronically or by electrocardiogram is also frequently used as a measure of exercise load. In a 6-min treadmill test, the speed of the treadmill is increased over the first 2 min to reach a level of 90–95% of maximum heart rate, which is sustained for the 4 min.
The objective tests of bronchial responsiveness are divided into ‘direct tests’ (methacholine, histamine) and ‘indirect tests’ (exercise, mannitol, adenosine 5′-monophosphate [AMP], non-isotonic aerosols, and hyperpnoea [EVH]). The methacholine (MCH) test is widely used. MCH acts as an analogue of acetylcholine, directly stimulating the cholinergic receptors in the airways’ smooth muscle. It has a high sensitivity but a low specificity for active asthma ( 45 , 98 ), and a low sensitivity to identify EIB ( 99 ).
Mannitol is an osmotic agent that mimics the ‘osmolar’ mechanism of EIA/EIB, compares to the exercise test, and shows a similar sensitivity and specificity with MCH for the diagnosis of EIA/EIB ( 99 – 101 ).
The EVH test requires the subject to ventilate dry air containing ~5% CO 2 for 6 min through a low-resistance circuit at a rate higher than that usually achieved on maximum exercise ( 102 ). Test is positive when a ≥10% sustained reduction in FEV1 is achieved. Very recent data, however, showed poor clinical reproducibility for the diagnosis of EIB in a cohort of recreational athletes ( 103 ), supporting the recommendations of a recent review in which bronchial provocation using both a direct and an indirect test is useful in some patients to confirm or exclude a diagnosis of asthma with certainty ( 104 ). A very basic exercise test that may help family doctors and paediatricians to suspect a primary diagnosis of EIA/EIB and refer the patient to a specialised centre is the ‘free-running asthma screening test’ (FRAST). FRAST measures peak expiratory flow rate before and at 1, 5, and 10 min after maximum voluntary running for at least 5 min, and it represents an acceptable, feasible, and cost-effective screening test ( 105 ), even if not in line with the most recent guidelines. In a recent study on elite swimmers it was suggested that parameters other than the direct drop in FEV compared to baseline should also be considered in the evaluation. Some subjects improve FEV during exercise with a subsequent drop after exercise providing a variability in FEV exceeding 15% (variability). Some subjects, previously without confirmed reversibility may respond to β 2 with marked improved FEV compared to maximum drop after exercise (reversibility). Thus, when both direct EIB, significant variability or reversibility were considered, a closer association between symptoms and disease activity was found ( 106 ).
Treatment: current guidelines
The treatment of EIA and of asthma in athletes should follow the same international guidelines as for the individual with general asthmatic symptoms. In all international guidelines for treating asthma in children and adults, a major aim of the treatment is to master EIA, as physical activity is seen as very important for the development and growth of children and for the self-perception. Fitness was found to correlate with psychological functioning in children with asthma ( 11 ). Considering that inflammation is the final result of the osmolar and vascular modifications described, anti-inflammatory treatment through inhaled steroids is often effective and sufficient to achieve a good EIA/EIB control ( 93 ). It should be noted that ICSs are the only anti-inflammatory drugs that improve respiratory epithelial healing ( 107 ). ICSs reduce the damage induced by repeated training and competitions, as we have seen for the phenotype of the ‘athlete's asthma’, enabling the athletes to master their sports and improving the long-term prognosis ( 10 ). However, a study in cross-country skiers showed no benefit from budesonide 800 µg/day during 3 months of treatment ( 108 ).
Inhaled short-acting β 2 -agonists are frequently needed and strongly suggested as pre-treatment before competition. If insufficient, long-acting β 2 -agonists (LABAs) and leukotriene antagonists may be added. Ipratropium bromide can be tried in addition to other treatments and after individual assessment (see flow chart in Fig. 1 ) ( 109 ). Based on the finding of increased parasympathetic tone in endurance athletes ( 110 ), the contribution of increased parasympathetic activity in the development of asthma in athletes would suggest a speculation for the role of inhaled ipratropium bromide or tiotropium in the treatment of asthma in athletes (15).

Simplified flow-chart for EIA treatment.
It is important to underline some concerns raised about tolerance of regular use of β 2 -agonists in EIA/EIB. First, there is a significant minority (15–20%) of asthmatics whose EIA is not prevented by β 2 -agonists, even when ICSs are used concomitantly; second, β 2 -agonists long-term regular use induces tolerance, with a decline in duration of the protective effect with their daily use, and lacks of sufficient safety data ( 111 , 112 ). In addition, a recent report raised attention on a potential loss of bronchoprotection for athletes using LABAs, independent from the Arg16Gly polymorphisms that may affect the efficacy of these medications ( 113 ). Non-pharmacological measures are also of importance: nasal breathing and pre-exercise warm-ups (15–30 sec exertions alternate with 60–90 sec rest) followed by a warm-down segment are suggested ( 114 ), together with anti-cold masks for cold environments.
Anti-doping: current regulations
For many years, the WADA issued strict regulations for the use of asthma drugs in sports. Initially, one feared that these drugs might improve performance, but after several studies on maximum performance in healthy subjects after inhaled β 2 -agonists, both short- and long-acting, it is generally accepted that inhaled steroids and inhaled β 2 -agonists do not improve performance. In a recent study combining three β 2 -agonists (salbutamol, formoterol, and salmeterol) all in WADA permitted doses, small but significant improvements could be seen in isometric quadriceps contraction and swim ergometric sprint performance. However, swim performance in an exhaustive race of 110 m did not improve ( 115 ). Since 1 January 2012, all ICSs have not been on the prohibited list, as well as the inhaled β 2 -agonists salbutamol, salmeterol, and formoterol. At present, there are no restrictions for the use of inhaled steroids; inhaled ipratropium bromide; leukotriene antagonists; and the inhaled β 2 -agonists salbutamol, salmeterol, and formoterol. Still, inhaled terbutaline is restricted in competitive sports, and objective measurements of AHR, EIB, or bronchodilator reversibility must be documented for approval of its use. Oral corticosteroids and oral or intravenous β 2 -agonists are prohibited. The list of prohibited drugs is usually updated every year and can be found on the WADA website ( www.wada-ama.org ).
Exercise to improve exercise-induced symptoms: from animal models to field
At present, it is clear that strenuous exercise increases the risk for asthma development assuming a dose–response relationship between physical activity and EIA/EIB risk (see Fig. 2 ), with a ‘U’-shaped curve showing that moderate exercise training carries a lower risk of asthma in comparison to high-intensity exercise training especially endurance training and interval training. These observations are confirmed by a growing number of studies on the murine model of allergic asthma: low-to-moderate intensity aerobic exercise decreases eosinophilic and lymphocytic inflammation in mice exercising for 4 weeks, 5 days a week, at 50% exercise capacity ( 116 ). Aerobic exercise seems to reduce airway remodelling, with reduced airway smooth muscle hypertrophy and hyperplasia ( 116 ), a reduction in leukocyte infiltration, pro-inflammatory cytokine production, adhesion molecules expression ( 117 ), and enhanced regulatory T cell (Treg) responses ( 118 ). Aerobic exercise also shows an anti-inflammatory effect in mice exposed to air pollution ( 119 ). Furthermore, a single session of moderate aerobic exercise can decrease airway inflammation (but not bronchial responsiveness) in mice, with a down-regulation of inflammatory mediators’ genes expression and Th-2 derived cytokines production ( 120 ). Similar findings are also being demonstrated in humans, where a reduction in neutrophils count in patients with chronic inflammatory conditions has been observed ( 121 ). Another recent study on humans ( 122 ) showed that asthmatic patients subjected to aerobic exercise training have a reduction in the number of eosinophils in induced sputum and lower levels of FeNO. In addition, Moreira et al. also demonstrated, in children with persistent allergic asthma, that a physical training program did not increase airways inflammation but decreased their total and allergen-specific IgE levels ( 123 ). Finally, preliminary data show that regular exercise reduces IL-2 production, meaning that lymphocytes are probably less responsive to exogenous stimuli, and IL-4 producing lymphocytes are also reduced, suggesting a better clinical condition for allergic people that exercise regularly ( 124 ).

Suggested dose–response relationship between physical activity and asthma risk. (Courtesy of A. Moreira and L. Delgado, University of Porto, Portugal.)
Therefore, it is apparent that aerobic, moderate-intensity exercise training (e.g. running or cycling) can be beneficial for allergic inflammation: these data open a new door on the possibility for ‘exercise therapy’ for asthmatics, in which exercise, in general a potential trigger for EIA/EIB, is instead a comprehensive part of the prevention and therapy strategies for asthmatics. However, on the other hand it has been shown that physical training programs in asthmatics improve cardiovascular fitness, but do not improve baseline lung function or bronchial hyperresponsiveness ( 125 ).
EIA/EIB are highly common, and their prevalence is markedly increased in competitive athletes, especially within endurance sports. The role of swimming as an ‘asthmogenic’ or ‘non-asthmogenic’ sport in childhood is still debated, but for competitive swimmers sufficient evidence exists for the increased prevalence of asthma and bronchial hyperresponsiveness in young competitive swimmers. There are now convincing data implicating immune-mediated airway inflammation and epithelial damage in EIA/EIB pathogenesis together with an increased parasympathetic activity, and this improved understanding of the underlying mechanisms may lead to new treatments in terms of new drugs and different strategies focused on different therapeutic approaches based on different phenotypes and endotypes ( 126 ). Furthermore, murine models and preliminary studies on humans have demonstrated that exercise, despite being the cause of EIA/EIB, can also be a new tool for its treatment, and exercise prescriptions should be included in the treatment guidelines for EIA/EIB.
Acknowledgements
The authors thank Mr. Barry Mark Wheaton for his linguistic assistance, Mr. Gian Paolo Carta of the University of Cagliari, Italy, for his library services, Dr. André Moreira and Prof. Luis Delgado of the University of Porto, Portugal, for allowing the use of Fig. 2 .
† These authors equally contributed to this article.
Authors' contributions
SDG and KHC equally contributed to this article and all the authors read and approved the final version of this manuscript.
Conflict of interest and funding
The authors have no conflict of interests to declare in connection with this article.
Exercise and Asthma
- First Online: 28 April 2020
Cite this chapter

- Shengguang Ding 6 &
- Chongjun Zhong 6
Part of the book series: Advances in Experimental Medicine and Biology ((AEMB,volume 1228))
13k Accesses
13 Citations
1 Altmetric
Asthma is a chronic lower respiratory disease that is very common worldwide, and its incidence is increasing year by year. Since the 1970s, asthma has become widespread, with approximately 300 million people affected worldwide and about 250,000 people have lost their lives. Asthma seriously affects people’s physical and mental health, resulting in reduced learning efficiency, limited physical activities, and decreased quality of life. Therefore, raising awareness of the risk of asthma and how to effectively treat asthma have become important targets for the prevention and management of asthma in recent years. For patients with asthma, exercise training is a widely accepted adjunct to drug-based and non-pharmacological treatment. It has been recommended abroad that exercise prescriptions are an important part of asthma management.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others
Regular exercise improves asthma control in adults: A randomized controlled trial

A 12-month, moderate-intensity exercise training program improves fitness and quality of life in adults with asthma: a controlled trial
Mauad T, Silva LF, Santos MA, Grinberg L, Bernardi FD, Martins MA, Saldiva PH, Dolhnikoff M (2004) Abnormal alveolar attachments with decreased elastic fiber content in distal lung in fatal asthma. Am J Respir Crit Care Med 170(8):857–862
PubMed Google Scholar
Patadia MO, Murrill LL, Corey J (2014) Asthma: symptoms and presentation. Otolaryngol Clin North Am 47(1):23–32
Bonnelykke K, Ober C (2016) Leveraging gene-environment interactions and endotypes for asthma gene discovery. J Allergy Clin Immunol 137(3):667–679
PubMed PubMed Central Google Scholar
Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, Lemanske RF Jr, Wardlaw AJ, Wenzel SE, Greenberger PA (2011) Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol 127(2):355–360
Pepe C, Foley S, Shannon J, Lemiere C, Olivenstein R, Ernst P, Ludwig MS, Martin JG, Hamid Q (2005) Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol 116(3):544–549
Sapienza S, Du T, Eidelman DH, Wang NS, Martin JG (1991) Structural changes in the airways of sensitized brown Norway rats after antigen challenge. Am Rev Respir Dis 144(2):423–427
CAS PubMed Google Scholar
Nystad W, Stigum H, Carlsen KH (2001) Increased level of bronchial responsiveness in inactive children with asthma. Respir Med 95(10):806–810
Nguyen TT, Ward JP, Hirst SJ (2005) beta1-Integrins mediate enhancement of airway smooth muscle proliferation by collagen and fibronectin. Am J Respir Crit Care Med 171(3):217–223
Xia M, Dai Y, Xu H (2014) [New perspective on Th2 and non-Th2 asthma phenotype]. Zhonghua Jie He He Hu Xi Za Zhi 37(12):931–933
Google Scholar
Tran TN, Zeiger RS, Peters SP, Colice G, Newbold P, Goldman M, Chipps BE (2016) Overlap of atopic, eosinophilic, and TH2-high asthma phenotypes in a general population with current asthma. Ann Allergy Asthma Immunol 116(1):37–42
Kuo CS, Pavlidis S, Loza M, Baribaud F, Rowe A, Pandis I, Sousa A, Corfield J, Djukanovic R, Lutter R, Sterk PJ, Auffray C, Guo Y, Adcock IM, Chung KF, Group UBS (2017) T-helper cell type 2 (Th2) and non-Th2 molecular phenotypes of asthma using sputum transcriptomics in U-BIOPRED. Eur Respir J 49(2):pii: 1602135
Abramson MJ, Perret JL, Dharmage SC, McDonald VM, McDonald CF (2014) Distinguishing adult-onset asthma from COPD: a review and a new approach. Int J Chron Obstruct Pulmon Dis 9:945–962
Singh N, Singh V (2014) Clinical presentations and investigations in asthma. J Assoc Phys India 62(3 Suppl):7–11
Jeffery PK (2001) Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med 164(10 Pt 2):S28–S38
Vadasz I, Sznajder JI (2017) Gas exchange disturbances regulate alveolar fluid clearance during acute lung injury. Front Immunol 8:757
Rohrer V, Schmidt-Trucksass A (2014) [Impact of exercise, sport and rehabilitation therapy in asthma and COPD]. Ther Umsch 71 (5):295–300
Evaristo KB, Saccomani MG, Martins MA, Cukier A, Stelmach R, Rodrigues MR, Santaella DF, Carvalho CR (2014) Comparison between breathing and aerobic exercise on clinical control in patients with moderate-to-severe asthma: protocol of a randomized trial. BMC Pulm Med 14:160
Cox L, Platts-Mills TA, Finegold I, Schwartz LB, Simons FE, Wallace DV, American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology (2007) American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology Joint Task Force Report on omalizumab-associated anaphylaxis. J Allergy Clin Immunol 120(6):1373–1377
Green RL (2013) Comment on terminology, close-calls, and bracketology for allergy, asthma, and immunology. Ann Allergy Asthma Immunol 111(1):79
Liang Z, Liu L, Zhao H, Xia Y, Zhang W, Ye Y, Jiang M, Cai S (2016) A systemic inflammatory endotype of asthma with more severe disease identified by unbiased clustering of the serum cytokine profile. Medicine (Baltimore) 95(25):e3774
Pavord ID, Afzalnia S, Menzies-Gow A, Heaney LG (2017) The current and future role of biomarkers in type 2 cytokine-mediated asthma management. Clin Exp Allergy 47(2):148–160
Prussin C, Metcalfe DD (1995) Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods 188(1):117–128
Ichinose M (2017) Asthma prevention and management guideline 2015. Arerugi 66(2):77–81
Eaton M, Granata C, Barry J, Safdar A, Bishop D, Little JP (2018) Impact of a single bout of high-intensity interval exercise and short-term interval training on interleukin-6, FNDC5, and METRNL mRNA expression in human skeletal muscle. J Sport Health Sci 7(2):191–196
Fleg JL (2017) Exercise therapy for older heart failure patients. Heart Fail Clin 13(3):607–617
Westergren T, Berntsen S, Lodrup Carlsen KC, Mowinckel P, Haland G, Fegran L, Carlsen KH (2017) Perceived exercise limitation in asthma: the role of disease severity, overweight, and physical activity in children. Pediatr Allergy Immunol 28(1):86–92
Salmon M, Walsh DA, Huang TJ, Barnes PJ, Leonard TB, Hay DW, Chung KF (1999) Involvement of cysteinyl leukotrienes in airway smooth muscle cell DNA synthesis after repeated allergen exposure in sensitized Brown Norway rats. Br J Pharmacol 127(5):1151–1158
CAS PubMed PubMed Central Google Scholar
Yang XX, Powell WS, Hojo M, Martin JG (1997) Hyperpnea-induced bronchoconstriction is dependent on tachykinin-induced cysteinyl leukotriene synthesis. J Appl Physiol (1985) 82(2):538–544
Boyd A, Yang CT, Estell K, Ms CT, Gerald LB, Dransfield M, Bamman M, Bonner J, Atkinson TP, Schwiebert LM (2012) Feasibility of exercising adults with asthma: a randomized pilot study. Allergy Asthma Clin Immunol 8(1):13
Cheng B, Huang Y (2014) [Role of exercise in asthma management in children]. Nan Fang Yi Ke Da Xue Xue Bao J South Med Univ 34(1):75–78
Lambert RK, Wiggs BR, Kuwano K, Hogg JC, Pare PD (1993) Functional significance of increased airway smooth muscle in asthma and COPD. J Appl Physiol (1985) 74(6):2771–2781
Avallone KM, McLeish AC (2013) Asthma and aerobic exercise: a review of the empirical literature. J Asthma 50(2):109–116
Lang DM, Butz AM, Duggan AK, Serwint JR (2004) Physical activity in urban school-aged children with asthma. Pediatrics 113(4):e341–e346
Heikkinen SA, Quansah R, Jaakkola JJ, Jaakkola MS (2012) Effects of regular exercise on adult asthma. Eur J Epidemiol 27(6):397–407
Anderson HR, Gupta R, Strachan DP, Limb ES (2007) 50 years of asthma: UK trends from 1955 to 2004. Thorax 62(1):85–90
Boulet LP, Chakir J, Dube J, Laprise C, Boutet M, Laviolette M (1998) Airway inflammation and structural changes in airway hyper-responsiveness and asthma: an overview. Can Respir J 5(1):16–21
O’Byrne PM, Gauvreau GM, Wood LJ (1999) Interaction between haemopoietic regulation and airway inflammation. Clin Exp Allergy 29(Suppl 2):27–32
Warner JO (1998) Bronchial hyperresponsiveness, atopy, airway inflammation, and asthma. Pediatr Allergy Immunol 9(2):56–60
Barnes PJ (1990) Reactive oxygen species and airway inflammation. Free Radic Biol Med 9(3):235–243
Munakata M, Ukita H, Masaki Y, Doi I, Ohtsuka Y, Ohe M, Amishima M, Chen H, Nasuhara Y, Taguchi H et al (1993) [Bronchial asthma—airway inflammation and asthma—role of airway epithelium]. Nihon Kyobu Shikkan Gakkai Zasshi 31 Suppl:154–158
Crimi E, Spanevello A, Neri M, Ind PW, Rossi GA, Brusasco V (1998) Dissociation between airway inflammation and airway hyperresponsiveness in allergic asthma. Am J Respir Crit Care Med 157(1):4–9
Shepherd M (1998) The risks of polypharmacy. Nurs Times 94(32):60–62
Welsh L, Kemp JG, Roberts RG (2005) Effects of physical conditioning on children and adolescents with asthma. Sports Med 35(2):127–141
Chetta A, Zanini A, Foresi A, D’Ippolito R, Tipa A, Castagnaro A, Baraldo S, Neri M, Saetta M, Olivieri D (2005) Vascular endothelial growth factor up-regulation and bronchial wall remodelling in asthma. Clin Exp Allergy 35(11):1437–1442
Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, Burgess JK, Black JL (2001) Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med 164(3):474–477
Duijts L (2012) Fetal and infant origins of asthma. Eur J Epidemiol 27(1):5–14
Serrano RL, Yu W, Graham RM, Bryan RL, Terkeltaub R (2019) A vascular smooth muscle cell X-box binding protein 1 and transglutaminase 2 regulatory circuit limits neointimal hyperplasia. PLoS One 14(4):e0212235
Vucevic D, Radosavljevic T, Mladenovic D, Todorovic V (2011) [The role of psychic factors in the pathogenesis of bronchial asthma]. Srp Arh Celok Lek 139(3–4):209–215
Boulet LP (2018) Airway remodeling in asthma: update on mechanisms and therapeutic approaches. Curr Opin Pulm Med 24(1):56–62
Burioka N (2013) [Chronotherapy of bronchial asthma]. Nihon Rinsho Jpn J Clin Med 71(12):2146–2152
Baumeister H, Korinthenberg K, Bengel J, Harter M (2005) [Bronchial asthma and mental disorders—a systematic review of empirical studies]. Psychother Psychosom Med Psychol 55(5):247–255
Bouabdallaoui N, Arlet JB, Hagege AA (2015) Cardiogenic shock, asthma, and hypereosinophilia. Am J Emerg Med 33(2):309 e301–309 e302
Courand PY, Croisille P, Khouatra C, Cottin V, Kirkorian G, Bonnefoy E (2012) Churg-Strauss syndrome presenting with acute myocarditis and cardiogenic shock. Heart Lung Circ 21(3):178–181
Li X, Wilson JW (1997) Increased vascularity of the bronchial mucosa in mild asthma, Am J Respir Crit Care Med. 156(1):229–233
Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Rodriguez M, Lee SY, McElwain K, McElwain S, Raz E, Broide DH (2004) Immunostimulatory DNA inhibits transforming growth factor-beta expression and airway remodeling. Am J Respir Cell Mol Biol 30(5):651–661
Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG (1989) Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med 320(5):271–277
Canitez Y, Cekic S, Celik U, Kocak A, Sapan N (2016) Health-care conditions in elementary schools and teachers’ knowledge of childhood asthma. Paediatr Int Child Health 36(1):64–71
Padrid P, Snook S, Finucane T, Shiue P, Cozzi P, Solway J, Leff AR (1995) Persistent airway hyperresponsiveness and histologic alterations after chronic antigen challenge in cats. Am J Respir Crit Care Med 151(1):184–193
Kuyper LM, Pare PD, Hogg JC, Lambert RK, Ionescu D, Woods R, Bai TR (2003) Characterization of airway plugging in fatal asthma. Am J Med 115(1):6–11
Chu HW, Rino JG, Wexler RB, Campbell K, Harbeck RJ, Martin RJ (2005) Mycoplasma pneumoniae infection increases airway collagen deposition in a murine model of allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol 289(1):L125–L133
Hashimoto S, Gon Y, Takeshita I, Matsumoto K, Maruoka S, Horie T (2001) Transforming growth Factor-beta1 induces phenotypic modulation of human lung fibroblasts to myofibroblast through a c-Jun-NH2-terminal kinase-dependent pathway. Am J Respir Crit Care Med 163(1):152–157
Pearce N (2012) Classification of epidemiological study designs. Int J Epidemiol 41(2):393–397
Lee DA, Winslow NR, Speight AN, Hey EN (1983) Prevalence and spectrum of asthma in childhood. Br Med J (Clin Res Ed) 286(6373):1256–1258
Lemanske RF Jr, Busse WW (1997) Asthma. JAMA 278(22):1855–1873
Shoji S, Ertl RF, Linder J, Romberger DJ, Rennard SI (1990) Bronchial epithelial cells produce chemotactic activity for bronchial epithelial cells. Possible role for fibronectin in airway repair. Am Rev Respir Dis 141(1):218–225
King TE Jr (1999) A new look at the pathophysiology of asthma. J Natl Med Assoc 91(8 Suppl):9S–15S
Lim TK (1999) Asthma management: evidence based studies and their implications for cost-efficacy. Asian Pac J Allergy Immunol 17(3):195–202
Burney PG, Chinn S, Rona RJ (1990) Has the prevalence of asthma increased in children? Evidence from the national study of health and growth 1973-86. Br Med J 300(6735):1306–1310
CAS Google Scholar
Cohn L, Elias JA, Chupp GL (2004) Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol 22:789–815
Miller M, Cho JY, McElwain K, McElwain S, Shim JY, Manni M, Baek JS, Broide DH (2006) Corticosteroids prevent myofibroblast accumulation and airway remodeling in mice. Am J Physiol Lung Cell Mol Physiol 290(1):L162–L169
Vermeer PD, Harson R, Einwalter LA, Moninger T, Zabner J (2003) Interleukin-9 induces goblet cell hyperplasia during repair of human airway epithelia. Am J Respir Cell Mol Biol 28(3):286–295
Panettieri RA Jr (2004) Effects of corticosteroids on structural cells in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 1(3):231–234
Nicolacakis K, Skowronski ME, Coreno AJ, West E, Nader NZ, Smith RL, McFadden ER Jr (2008) Observations on the physiological interactions between obesity and asthma. J Appl Physiol (1985) 105(5):1533–1541
Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA (1999) IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol 162(10):6233–6237
Yang IA, Savarimuthu S, Kim ST, Holloway JW, Bell SC, Fong KM (2007) Gene-environmental interaction in asthma. Curr Opin Allergy Clin Immunol 7(1):75–82
O’Byrne PM, FitzGerald JM, Zhong N, Bateman E, Barnes PJ, Keen C, Almqvist G, Pemberton K, Jorup C, Ivanov S, Reddel HK (2017) The SYGMA programme of phase 3 trials to evaluate the efficacy and safety of budesonide/formoterol given ‘as needed’ in mild asthma: study protocols for two randomised controlled trials. Trials 18(1):12
Busse WW, Casale TB, Dykewicz MS, Meltzer EO, Bird SR, Hustad CM, Grant E, Zeldin RK, Edelman JM (2006) Efficacy of montelukast during the allergy season in patients with chronic asthma and seasonal aeroallergen sensitivity. Ann Allergy Asthma Immunol 96(1):60–68
Vaghi A, Berg E, Liljedahl S, Svensson JO (2005) In vitro comparison of nebulised budesonide (Pulmicort Respules) and beclomethasone dipropionate (Clenil per Aerosol). Pulm Pharmacol Ther 18(2):151–153
Wanrooij VH, Willeboordse M, Dompeling E, van de Kant KD (2014) Exercise training in children with asthma: a systematic review. Br J Sports Med 48(13):1024–1031
Cho JY, Miller M, McElwain K, McElwain S, Broide DH (2005) Combination of corticosteroid therapy and allergen avoidance reverses allergen-induced airway remodeling in mice. J Allergy Clin Immunol 116(5):1116–1122
Milanese M, Miraglia Del Giudice E, Peroni DG (2019) Asthma, exercise and metabolic dysregulation in paediatrics. Allergol Immunopathol 47(3):289–294
Holgate ST, Holloway J, Wilson S, Bucchieri F, Puddicombe S, Davies DE (2004) Epithelial-mesenchymal communication in the pathogenesis of chronic asthma. Proc Am Thorac Soc 1(2):93–98
Leung E, Xue A, Wang Y, Rougerie P, Sharma VP, Eddy R, Cox D, Condeelis J (2017) Blood vessel endothelium-directed tumor cell streaming in breast tumors requires the HGF/C-Met signaling pathway. Oncogene 36(19):2680–2692
Huovinen E, Kaprio J, Koskenvuo M (2003) Factors associated to lifestyle and risk of adult onset asthma. Respir Med 97(3):273–280
Guo F, Yu X, Chen W, Yang Q, Pan B, Lin L, Jiang H (2019) Preliminary analysis on characteristics of rib cartilage calcification in patients with congenital microtia. J Craniofac Surg 30(1):e28–e32
Ebina M, Takahashi T, Chiba T, Motomiya M (1993) Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma. A 3-D morphometric study. Am Rev Respir Dis 148(3):720–726
Yang T, Xiao Y, Zhang Z, Liang Y, Li G, Zhang M, Li S, Wong TW, Wang Y, Li T, Huang Z (2018) A soft artificial muscle driven robot with reinforcement learning. Sci Rep 8(1):14518
Lochte L, Nielsen KG, Petersen PE, Platts-Mills TA (2016) Childhood asthma and physical activity: a systematic review with meta-analysis and Graphic Appraisal Tool for Epidemiology assessment. BMC Pediatr 16:50
Cochrane LM, Clark CJ (1990) Benefits and problems of a physical training programme for asthmatic patients. Thorax 45(5):345–351
Wang H, Shi C, Kong M, Mu C, Wei H, Wang C (2018) Cloning and expression of a transcription factor activator protein-1 member identified from the swimming crab Portunus trituberculatus. Cell Stress Chaperones 23(6):1275–1282
Dornowski M, Makar P, Sawicki P, Wilczynska D, Vereshchaka I, Ossowski Z (2019) Effects of low- vs high-volume swimming training on pelvic floor muscle activity in women. Biol Sport 36(1):95–99
Bicer M, Baltaci SB, Mogulkoc R, Baltaci AK, Avunduk MC (2019) Effect of resveratrol administration on muscle glycogen levels in rats subjected to acute swimming exercise. Cell Mol Biol (Noisy-le-Grand) 65(2):28–31
Matsumoto I, Araki H, Tsuda K, Odajima H, Nishima S, Higaki Y, Tanaka H, Tanaka M, Shindo M (1999) Effects of swimming training on aerobic capacity and exercise induced bronchoconstriction in children with bronchial asthma. Thorax 54(3):196–201
Ramos MRF, Junior Y, de Souza LAB (2019) Swimming as treatment of scapular dyskinesis. J Orthop 2019:5607970
Gouvea AL, Martinez CG, Kurtenbach E (2018) Determining maximal muscle strength in mice: validity and reliability of an adapted swimming incremental overload test. J Strength Cond Res. https://doi.org/10.1519/JSC.0000000000002777
Beggs S, Foong YC, Le HC, Noor D, Wood-Baker R, Walters JA (2013) Swimming training for asthma in children and adolescents aged 18 years and under. Cochrane Database Syst Rev 4:CD009607
Bacque-Cazenave J, Courtand G, Beraneck M, Lambert FM, Combes D (2018) Temporal relationship of ocular and tail segmental movements underlying locomotor-induced gaze stabilization during undulatory swimming in larval Xenopus. Front Neural Circuits 12:95
Bovard JM, Welch JF, Houghton KM, McKenzie DC, Potts JE, Sheel AW (2018) Does competitive swimming affect lung growth? Physiol Rep 6(15):e13816
Tuesta M, Alvear M, Carbonell T, Garcia C, Guzman-Venegas R, Araneda OF (2016) Effect of exercise duration on pro-oxidants and pH in exhaled breath condensate in humans. J Physiol Biochem 72(2):353–360
Aljawarneh YM, Wardell DW, Wood GL, Rozmus CL (2019) A systematic review of physical activity and exercise on physiological and biochemical outcomes in children and adolescents with type 1 diabetes. J Nurs Scholarsh 51(3):337–345
Ferreira MS, Aride PHR, Val AL (2018) Could resistance to lactate accumulation contribute to the better swimming performance of Brycon amazonicus when compared to Colossoma macropomum? PeerJ 6:e5719
Vogelberg C, Hirsch T, Radon K, Dressel H, Windstetter D, Weinmayr G, Weiland SK, von Mutius E, Nowak D, Leupold W (2007) Leisure time activity and new onset of wheezing during adolescence. Eur Respir J 30(4):672–676
Boskovic S, Marin-Juez R, Jasnic J, Reischauer S, El Sammak H, Kojic A, Faulkner G, Radojkovic D, Stainier DYR, Kojic S (2018) Characterization of zebrafish (Danio rerio) muscle ankyrin repeat proteins reveals their conserved response to endurance exercise. PLoS One 13(9):e0204312
Langdeau JB, Turcotte H, Bowie DM, Jobin J, Desgagne P, Boulet LP (2000) Airway hyperresponsiveness in elite athletes. Am J Respir Crit Care Med 161(5):1479–1484
Lang JE (2012) Obesity, nutrition, and asthma in children. Pediatr Allergy Immunol Pulmonol 25(2):64–75
Mathur R, Chua KS, Mamelak M, Morales W, Barlow GM, Thomas R, Stefanovski D, Weitsman S, Marsh Z, Bergman RN, Pimentel M (2016) Metabolic effects of eradicating breath methane using antibiotics in prediabetic subjects with obesity. Obesity (Silver Spring) 24(3):576–582
Koziol-White CJ, Panettieri RA Jr (2019) Modulation of bronchomotor tone pathways in airway smooth muscle function and bronchomotor tone in asthma. Clin Chest Med 40(1):51–57
van Veldhoven NH, Vermeer A, Bogaard JM, Hessels MG, Wijnroks L, Colland VT, van Essen-Zandvliet EE (2001) Children with asthma and physical exercise: effects of an exercise programme. Clin Rehabil 15(4):360–370
Brennan FH Jr, Alent J, Ross MJ (2018) Evaluating the athlete with suspected exercise-induced asthma or bronchospasm. Curr Sports Med Rep 17(3):85–89
Mancuso CA, Sayles W, Robbins L, Phillips EG, Ravenell K, Duffy C, Wenderoth S, Charlson ME (2006) Barriers and facilitators to healthy physical activity in asthma patients. J Asthma 43(2):137–143
Buchvald F, Phillipsen LD, Hjuler T, Nielsen KG (2016) Exercise-induced inspiratory symptoms in school children. Pediatr Pulmonol 51(11):1200–1205
Cai Y, Xie KL, Zheng F, Liu SX (2018) Aerobic exercise prevents insulin resistance through the regulation of miR-492/resistin axis in aortic endothelium. J Cardiovasc Transl Res 11(6):450–458
Ballester R, Huertas F, Molina E, Sanabria D (2018) Sport participation and vigilance in children: influence of different sport expertise. J Sport Health Sci 7(4):497–504
McClafferty H (2014) An overview of integrative therapies in asthma treatment. Curr Allergy Asthma Rep 14(10):464
Hammami A, Randers MB, Kasmi S, Razgallah M, Tabka Z, Chamari K, Bouhlel E (2018) Effects of soccer training on health-related physical fitness measures in male adolescents. J Sport Health Sci 7(2):169–175
Del Giacco SR, Cappai A, Gambula L, Cabras S, Perra S, Manconi PE, Carpiniello B, Pinna F (2016) The asthma-anxiety connection. Respir Med 120:44–53
Meyer A, Gunther S, Volmer T, Taube K, Baumann HJ (2015) A 12-month, moderate-intensity exercise training program improves fitness and quality of life in adults with asthma: a controlled trial. BMC Pulm Med 15:56
Okolo FC, Zhang G, Rhodes J, Potoka DA (2018) Intra-amniotic sildenafil treatment modulates vascular smooth muscle cell phenotype in the nitrofen model of congenital diaphragmatic hernia. Sci Rep 8(1):17668
Gounni AS (2006) The high-affinity IgE receptor (FcepsilonRI): a critical regulator of airway smooth muscle cells? Am J Physiol Lung Cell Mol Physiol 291(3):L312–L321
Lin J, Wang W, Chen P, Zhou X, Wan H, Yin K, Ma L, Wu C, Li J, Liu C, Su N, Liu G, Xie H, Tang W, Huang M, Chen Y, Liu Y, Song L, Chen X, Zhang Y, Li W, Sun L (2018) Prevalence and risk factors of asthma in mainland China: the CARE study. Respir Med 137:48–54
Mathew A, MacLean JA, DeHaan E, Tager AM, Green FH, Luster AD (2001) Signal transducer and activator of transcription 6 controls chemokine production and T helper cell type 2 cell trafficking in allergic pulmonary inflammation. J Exp Med 193(9):1087–1096
Hassall D, Brealey N, Wright W, Hughes S, West A, Ravindranath R, Warren F, Daley-Yates P (2018) Hair analysis to monitor adherence to prescribed chronic inhaler drug therapy in patients with asthma or COPD. Pulm Pharmacol Ther 51:59–64
Boulet LP, Turmel J (2019) Cough in exercise and athletes. Pulm Pharmacol Ther 55:67–74
Batacan RB Jr, Duncan MJ, Dalbo VJ, Buitrago GL, Fenning AS (2018) Effect of different intensities of physical activity on cardiometabolic markers and vascular and cardiac function in adult rats fed with a high-fat high-carbohydrate diet. J Sport Health Sci 7(1):109–119
Wang WQ, Lin JT, Zhou X, Wang CZ, Huang M, Cai SX, Chen P, Lin QC, Zhou JY, Gu YH, Yuan YD, Sun DJ, Yang XH, Yang L, Huo JM, Chen ZC, Jiang P, Zhang J, Ye XW, Liu HG, Tang HP, Liu RY, Liu CT, Zhang W, Hu CP, Chen YQ, Liu XJ, Dai LM, Zhou W, Huang YJ, Xu JY (2018) [Evaluation of asthma management from the surveys in 30 provinces of China in 2015-2016]. Zhonghua Nei Ke Za Zhi 57(1):15–20
Babwah T, Roopchan V, Baptiste B, Ali A, Dwarika K (2018) Exercise prescriptions given by GPs to sedentary patients attending chronic disease clinics in health centres—the effect of a very brief intervention to change exercise behavior. Fam Med Prim Care 7(6):1446–1451
Download references
Author information
Authors and affiliations.
Department of Thoracic and Cardiovascular Surgery, The Second Affiliated Hospital of Nantong University, Nantong, China
Shengguang Ding & Chongjun Zhong
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Cardiac Regeneration and Ageing Lab Institute of Cardiovascular Sciences School of Life Science, Shanghai University, Shanghai, China
Junjie Xiao
Ethics declarations
The authors declare no competing financial interests.
Rights and permissions
Reprints and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Ding, S., Zhong, C. (2020). Exercise and Asthma. In: Xiao, J. (eds) Physical Exercise for Human Health. Advances in Experimental Medicine and Biology, vol 1228. Springer, Singapore. https://doi.org/10.1007/978-981-15-1792-1_25
Download citation
DOI : https://doi.org/10.1007/978-981-15-1792-1_25
Published : 28 April 2020
Publisher Name : Springer, Singapore
Print ISBN : 978-981-15-1791-4
Online ISBN : 978-981-15-1792-1
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Help & Support
- Lung Health & Diseases
- Lung Disease Lookup
- Managing Asthma
- Exercise & Asthma
Being Active with Asthma
Benefits of exercise when you have asthma.
Exercise is important for overall health as well as lung health , and there are many benefits of physical activity for people living with asthma. Daily exercise helps to improve your lungs capacity, in other words, the maximum amount of oxygen your body can use. Also, exercise increases blood flow to your lungs, promoting blood flow to the heart which pumps oxygen throughout your body. For example, people who exercise have more ability to pull oxygen from the lungs and into the blood that feeds the muscles that keep us going.
Exercise-Induced Asthma
Some people with asthma only have symptoms (e.g., shortness of breath, chest tightness, wheezing or coughing) during exercise or when doing physically demanding tasks. This is called exercise-induced asthma or exercise-induced bronchospasm. But you don't have to let asthma hold you back from being active. In fact, many Olympians and professional athletes have asthma. As long as you manage your symptoms, you can participate in any sport or activity.
If you have asthma symptoms during or shortly after you exercise, be sure to talk to your doctor about it. Are you just out of shape? Is it exercise-induced asthma or poorly controlled asthma? You will need to work with your doctor to find out what type of asthma you have . You may benefit from different treatment options, like adding a daily controller medicine to your asthma treatment plan.
Tips for Healthy Lungs
If asthma symptoms keep you from being physically active, ask your doctor:
- "Are there any activities that I should avoid?"
- "Are there any asthma medicines that I should take before I exercise?"
Generally, people with asthma can participate in all types of exercise. You may need to take medicine before you exercise. Some additional things that can help include:
- Start any exercise with a warm-up period.
- Cover your nose and mouth with a scarf when exercising outdoors in cold temperatures.
- Limit exercise or strenuous activities outdoors when the air quality is unhealthy (orange) and avoid outdoor activities when the air quality is red, purple or maroon.
- Remember to include a cool down period.
If you start to have pain or a tight feeling in your chest, have a cough or become short of breath during exercise, stop the activity right away. Take your quick-relief inhaler. Sit down and try to relax. Try a belly breathing exercise for relaxation. For kids, they should be sure to tell an adult as soon as symptoms start, take their medicine, and sit down and try to relax.
Being aware of your asthma signs and symptoms can help you take action before your breathing gets worse. Not everyone has the same symptoms, so be sure to learn yours.
Don't let your asthma hold you back from being active and healthy. Learn more about managing your asthma and download Staying Active with Lung Disease to share your doctor at your next visit.
Page last updated: June 7, 2024
A Breath of Fresh Air in Your Inbox
Want updates on the latest lung health news, including COVID-19, research, inspiring stories and health information?
You will now receive email updates from the American Lung Association.
Make a Donation
Your tax-deductible donation funds lung disease and lung cancer research, new treatments, lung health education, and more.
Become a Lung Health Insider
Join over 700,000 people who receive the latest news about lung health, including research, lung disease, air quality, quitting tobacco, inspiring stories and more!
Thank you! You will now receive email updates from the American Lung Association.
Select Your Location
Select your location to view local American Lung Association events and news near you.
Change Language
Lung helpline.
Talk to our lung health experts at the American Lung Association. Our service is free and we are here to help you.
1-800-LUNG-USA
(1-800-586-4872)

- Exercise-Induced Asthma
- Author: Joseph P Garry, MD, FACSM, FAAFP; Chief Editor: Craig C Young, MD more...
- Sections Exercise-Induced Asthma
- Practice Essentials
- Pathophysiology
- Epidemiology
- Patient Education
- Physical Examination
- Phases of EIA
- Complications
- Approach Considerations
- Allergy and Infection Evaluation
- Thyroid Function Evaluation
- Radiography
- Echocardiography
- Laryngoscopy
- Challenge Tests
- Nonpharmacologic Measures
- Return to Play
- Medication Summary
- Beta2-Adrenergic Agonists, Short-Acting
- Beta2-Adrenergic Agonists, Long-Acting
- Mast Cell Stabilizers
- Inhaled Corticosteroids
- Xanthine Derivatives
- Leukotriene Receptor Antagonist
- 5-lipoxygenase Inhibitor
- Adrenergic Agents
- Questions & Answers
Exercise-induced asthma is a condition of respiratory difficulty (bronchoconstriction) that is related to histamine release, [ 1 , 2 , 3 ] is triggered by aerobic exercise, and lasts several minutes. Causes include medical conditions, environmental factors, and medications. [ 4 ]
The image below illustrates the pathogenesis of asthma.
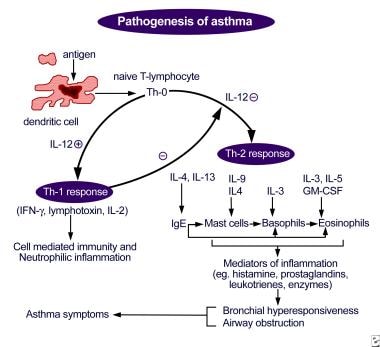
Signs and symptoms
Symptoms of exercise-induced asthma during or following exercise include the following [ 1 , 3 ] :
Chest tightness or pain
Cough, shortness of breath, wheezing
Underperformance or poor performance on the field of play
Fatigue, prolonged recovery time
Gastrointestinal discomfort
See Clinical Presentation for more detail.
The patient's physical examination is often unremarkable in the clinical setting but may have a higher yield on the field or after an exercise challenge. [ 5 ]
Examination should include the following areas:
Skin: Note any signs of atopic disease
Head, ears, eyes, nose, and throat: Note any evidence of acute infection, chronic infection, or allergic/atopic disease
Pharynx: Note any mucus, cobblestoning, and/or erythema
Nose: Note presence of enlarged turbinates, erythema, and/or congestion
Sinuses: Note presence of tenderness
Lungs: Note presence of rales, rhonchi, wheezes, and/or prolonged expiratory phase
Heart: Note presence of murmurs and/or an irregular rhythm
Laboratory tests
Exercise-induced asthma is generally a clinical diagnosis. Laboratory evaluation is usually reserved for equivocal cases, for treatment failures, and to narrow the differential diagnosis.
Laboratory studies used to assess for allergy and infection include the following:
Complete blood count: To determine likelihood of infection and to evaluate eosinophil counts (for allergy)
Immunoglobulin E levels, with/without nasal swab for eosinophils: To determine likelihood of allergic disease
Skin allergen testing/radioallergosorbent test: To help identify specific allergens
Erythrocyte sedimentation rate or C-reactive protein levels: To evaluate for inflammatory and infectious conditions
Sputum analysis and culture: To help identify presence of infection and treatment options for strains of resistant organisms
Thyroid function tests: To evaluate for thyroid dysfunction if anxiety is suspected of mimicking asthma symptoms
Challenge testing to formalize the diagnosis of exercise-induced asthma includes the following:
Treadmill exercise challenges with preexercise and postexercise pulmonary function levels
Informal exercise challenge: Substitutes for treadmill exercise challenge; heart rate not monitored, and level of work not reliable
Pulmonary function testing: To evaluate baseline pulmonary function or allergic asthma; to categorize pulmonary function as obstructive or restrictive disease
Bronchoprovocation testing: Positive results indicative of general asthma rather than specific for exercise-induced asthma
Eucapnic voluntary hyperventilation: Sensitive and accurate for diagnosis of exercise-induced asthma [ 6 , 7 ] ; can be applied in a laboratory setting and altered to mimic the environmental conditions of the patient’s specific sport
Imaging studies
Imaging studies are often not indicated in the evaluation of routine exercise-induced asthma. However, the following radiologic studies may be useful for assessing other possibilities in the differential diagnosis:
Chest radiography: To evaluate for signs of chronic lung disease (eg, hyperexpansion, scarring, fibrosis, hilar adenopathy), for congestive heart failure and/or valvular heart disease (eg, chamber enlargement, pulmonary edema, vascular or valvular calcification), and for a foreign body
Lateral neck radiography/soft-tissue penetration: To evaluate the upper airway for a foreign body or obstruction
Echocardiography: To evaluate for cardiac valvular abnormality or global contractile function, as well as dysrhythmia, cardiomegaly, or other heart disease that may manifest during exercise
Laryngoscopy can be performed to evaluate for foreign body or other obstruction in the upper airway. Postexercise laryngoscopy can be used to evaluate for vocal cord dysfunction, a condition often mistaken for exercise-induced asthma.
See Workup for more detail.
Treatment of the athlete who is experiencing an acute attack of exercise-induced asthma is the same as in any asthma attack situation and includes immediately removing the patient from competition or play.
The optimal treatment for exercise-induced asthma is to prevent symptomatic onset. After controlling the patient's underlying and contributing factors (eg, respiratory infection, allergy, allergic asthma), a combination of drugs can be used to prevent this condition. [ 1 ]
Pharmacotherapy
The basis of treatment for exercise-induced asthma is with preexercise short-acting beta2-agonist administration. [ 1 ] There is less of a role for traditional asthma medications (eg, corticosteroids, theophylline) in managing pure exercise-induced asthma.
The following medications are used in the treatment of exercise-induced asthma:
Short-acting beta2-adrenergic agonists (eg, albuterol, pirbuterol, levalbuterol)
Long-acting beta2-adrenergic agonists (eg, salmeterol, formoterol)
Mast cell stabilizers (eg, cromolyn sodium)
Inhaled corticosteroids (eg, flunisolide, beclomethasone dipropionate, ciclesonide, fluticasone, budesonide)
Xanthine derivatives (eg, theophylline)
Leukotriene receptor antagonists (eg, zafirlukast, montelukast)
5-Lipoxygenase inhibitors (eg, zileuton)
Adrenergic agents (eg, epinephrine)
Other approaches
Nonpharmacologic measures in the treatment of exercise-induced asthma include the following:
Sports selection
Altering breathing techniques (eg, predominant mouth breathing to nasal breathing)
Coordination and timing of warm-up techniques, medication, and competition
See Treatment and Medication for more detail.
Exercise-induced asthma (EIA) is a condition of respiratory difficulty that is related to histamine release, [ 1 , 2 , 3 ] triggered by aerobic exercise, and lasts several minutes (see Pathophysiology). Causes include medical conditions, environmental factors, and medications (see Etiology). [ 4 ]
Symptoms of EIA may resemble those of allergic asthma, or they may be much more vague and go unrecognized, resulting in probable underreporting of the disease (see Clinical Presentation). The optimal treatment for EIA is to prevent the onset of symptoms, and the basis of treatment is with preexercise short-acting β 2 -agonist administration. [ 1 ] Long-acting β 2 -agonists and mast cell stabilizers, as well as antileukotriene drugs have also been shown to be effective (see Treatment and Management). [ 8 , 9 ]
With proper interventions, the prognosis is excellent for athletes with asthma. Most symptoms can be prevented, and performance should not be limited by EIA with proper treatment (see Prognosis).
Exercise-induced urticaria, or exercise-induced anaphylaxis, is often presumed to be related to EIA, even though this condition is extremely rare and unrelated (see Diagnostic Considerations).
Go to Asthma , Pediatric Asthma , Exercise-Induced Anaphylaxis , Angioedema , and Urticaria for more information on these topics.
The problem with the functional anatomy in exercise-induced asthma (EIA) occurs distal to the glottis, in the lower airway. Bronchoconstriction is involved that is distinguishable from laryngospasm, which can occur in other exercise-related conditions. One such example is the condition known as vocal cord dysfunction in which there is paradoxical narrowing of the vocal cords during inspiration, resulting in stridor that is often misconstrued as audible wheezing. [ 10 , 11 ] Normally, the vocal cords open with inspiration. (Go to Vocal Cord Dysfunction for more information on this topic.)
EIA usually affects individuals who participate in sports that include an aerobic component. The condition can be seen in any sport, but EIA is much less common in predominantly anaerobic activities. This is likely due to the role of consistent and repetitive air movement through the airways (seen in aerobic sports), which affect airway humidity and temperature. EIA triggers an unknown biochemical and neurochemical pathway, resulting in the bronchospasm, which manifests as the symptoms of the disease.
Although the exact mechanism of EIA is unknown, there are 2 predominant theories as to how the symptom complex is triggered. One is the airway humidity theory, which suggests that air movement through the airway results in relative drying of the airway. This, in turn, is believed to trigger a cascade of events that results in airway edema secondary to hyperemia and increased perfusion in an attempt to combat the drying. The result is bronchospasm.
The other theory is based on airway cooling and assumes that the air movement in the bronchial tree results in a decreased temperature of the bronchi, which may also trigger a hyperemic response in an effort to heat the airway. Again, the result is a spasm in the bronchi.
Many authors believe there may be a combination of the above 2 theories that takes place, but the biochemical or physical pathways that mediate these responses are unclear. Evidence may even exist to support the idea that the resulting cascades are not the inflammatory pathways to which we attribute allergic asthma.
Likewise, certain sports and their environments predispose individuals with asthma to experience EIA. Sports played in cold and dry environments usually result in more symptom manifestation for athletes with this condition. On the other hand, when the environment is warm and humid, the incidence and severity of EIA decrease.
Also see the figure below.
The causes of EIA can be divided into the categories of medical, environmental, and drug related. Eliminating some causes can diminish—but may not eliminate—the athlete's symptoms. EIA may also exist without the presence of any of these causes.
Medical conditions
Poorly controlled asthma results in increased patient symptoms with exercise. Maximizing control of the patient's baseline asthma, when present, is critical in the treatment of EIA. [ 1 ] In addition, poorly controlled allergic rhinitis also results in increased patient symptoms with exercise, and secretions resulting from hay fever can aggravate both allergic asthma and EIA.
Viral, bacterial, and other forms of upper respiratory tract infections also aggravate the symptoms of EIA. Controlling the secretions of these illnesses, as with allergic rhinitis, can make the EIA symptoms much more tolerable.
Environmental factors
Excess of pollens or other allergens in the air can exacerbate the allergic and exercise-induced forms of asthma. Pollutants in the air are irritants to the airways and can lower the threshold for symptomatic bronchospasm.
The chemicals used in certain sports for environmental maintenance can predispose individuals to wheezing and worsen EIA symptoms. These chemicals include the following:
Chlorination in pools
Insecticides and pesticides used to maintain playing fields
Fertilizers and herbicides used to maintain playing fields
Paints and other decorative substances to enhance the appearance of playing fields
Asthmogenic agents
Certain medication classifications and specific drugs can provoke or exacerbate bronchial reactivity in EIA, such as the following:
Nonsteroidal anti-inflammatory drugs (NSAIDs)
EIA affects 12-15% of the population. Ninety percent of asthmatic individuals and 35-45% of people with allergic rhinitis experience EIA, but even when those with rhinitis and allergic asthma are excluded, a 3-10% incidence of EIA is seen in the general population. [ 3 , 12 ]
EIA seems to be more prevalent in some winter or cold-weather sports. [ 13 ] Some studies have demonstrated rates as high as 35% or even 50% in competitive-caliber figure skaters, ice hockey players, and cross-country skiers. [ 14 , 6 , 15 ] EIA is also more common among swimmers. [ 16 ]
An observational cohort study of 149 pediatric asthma patients found that exercise-induced bronchoconstriction was present in 52.5% of these children. [ 17 ]
The prognosis is excellent for athletes with asthma. With proper interventions, most symptoms can be prevented, and performance should not be limited by EIA if this condition is treated properly. Newly diagnosed young athletes need to be educated that this condition should not be perceived as an insurmountable disability. Using examples of the numerous elite athletes (eg, Jackie Joyner-Kersee [track and field Olympian]; Amy Van Dyken [Olympic swimmer]; Jerome Bettis [former running back for the Pittsburgh Steelers]) with this condition can help young impressionable athletes continue in their endeavors without fear of failure or medical distress.
Patient education is a critical part of the treatment of EIA. Once the diagnosis is made, athletes should be encouraged to continue in their activities with the reassurance that proper treatment can allow for an unhampered performance for most individuals.
In addition to reassurance, it is also important to teach individuals to recognize the signs of an impending attack. Once recognized, individuals should be taught to remove themselves from the aggravating activity and initiate treatment as necessary. This includes education about the proper choice of agents to abort an acute attack (ie, albuterol), but not cromolyn, salmeterol, or an inhaled steroid.
Teaching the proper mechanics of inhalant medication administration is also important, along with, if needed, teaching and demonstrating the proper use of a spacer device to the patient; without the proper mechanics in using such devices, the medication does not reach the area of pathology and does not benefit the athlete.
Education of the coaching staff is also crucial, because coaches need to know that shortness of breath in athletes does not always indicate poor conditioning and that the consequences of ignoring an asthma attack can be serious.
National Heart, Lung,and Blood Institute, National Asthma Education and Prevention Program. Expert Panel Report 3:Guidelines for the Diagnosis and Management of Asthma: Full Report 2007 . Bethesda, Md: NHLBI; August 2007. Publication no. 07-4051:
Anderson SD. How does exercise cause asthma attacks?. Curr Opin Allergy Clin Immunol . 2006 Feb. 6(1):37-42. [QxMD MEDLINE Link] .
Hough DO, Dec KL. Exercise-induced asthma and anaphylaxis. Sports Med . 1994 Sep. 18(3):162-72. [QxMD MEDLINE Link] .
Smith BW, MacKnight JM. Pulmonary. Safran MR, McKeag DB, Van Camp SP, eds. Manual of Sports Medicine . Philadelphia, Pa: Lippincott-Raven; 1998. Vol 1.: 244-9.
Kaplan TA. Exercise challenge for exercise-induced bronchospasm: confirming presence, evaluating control. Phys Sports Med . 1995. 23(8):47-57.
Dickinson JW, Whyte GP, McConnell AK, Harries MG. Screening elite winter athletes for exercise induced asthma: a comparison of three challenge methods. Br J Sports Med . 2006 Feb. 40(2):179-82; discussion 179-82. [QxMD MEDLINE Link] . [Full Text] .
Rundell KW, Anderson SD, Spiering BA, Judelson DA. Field exercise vs laboratory eucapnic voluntary hyperventilation to identify airway hyperresponsiveness in elite cold weather athletes. Chest . 2004 Mar. 125(3):909-15. [QxMD MEDLINE Link] .
Storms W. Update on montelukast and its role in the treatment of asthma, allergic rhinitis and exercise-induced bronchoconstriction. Expert Opin Pharmacother . 2007 Sep. 8(13):2173-87. [QxMD MEDLINE Link] .
Steinshamn S, Sandsund M, Sue-Chu M, Bjermer L. Effects of montelukast and salmeterol on physical performance and exercise economy in adult asthmatics with exercise-induced bronchoconstriction. Chest . 2004 Oct. 126(4):1154-60. [QxMD MEDLINE Link] .
Wilson JJ, Wilson EM. Practical management: vocal cord dysfunction in athletes. Clin J Sport Med . 2006 Jul. 16(4):357-60. [QxMD MEDLINE Link] .
Kenn K. [Vocal Cord Dysfunction--what do we really know? A review]. Pneumologie . 2007 Jul. 61(7):431-9. [QxMD MEDLINE Link] .
de Aguiar KB, Anzolin M, Zhang L. Global prevalence of exercise-induced bronchoconstriction in childhood: A meta-analysis. Pediatr Pulmonol . 2018 Apr. 53 (4):412-425. [QxMD MEDLINE Link] .
Stensrud T, Berntsen S, Carlsen KH. Exercise capacity and exercise-induced bronchoconstriction (EIB) in a cold environment. Respir Med . 2007 Jul. 101(7):1529-36. [QxMD MEDLINE Link] .
Butcher JD. Exercise-induced asthma in the competitive cold weather athlete. Curr Sports Med Rep . 2006 Dec. 5(6):284-8. [QxMD MEDLINE Link] .
Irewall T, Söderström L, Lindberg A, Stenfors N. High incidence rate of asthma among elite endurance athletes: a prospective 4-year survey. J Asthma . 2021 Jun. 58 (6):735-41. [QxMD MEDLINE Link] .
Hashim SH, Alenezi MI, Alenezi RM, et al. Exercise-Induced Bronchoconstriction Among Adolescent Athletes With Asthma: A Systematic Review. Cureus . 2023 Jun. 15 (6):e40643. [QxMD MEDLINE Link] . [Full Text] .
Lin LL, Huang SJ, Ou LS, Yao TC, Tsao KC, Yeh KW, et al. Exercise-induced bronchoconstriction in children with asthma: An observational cohort study. J Microbiol Immunol Infect . 2017 Sep 6. 1 (8583):483. [QxMD MEDLINE Link] .
Gould CF, Perzanowski MS, Evans D, Bruzzese JM. Association of exercise-induced wheeze and other asthma symptoms with emergency department visits and hospitalizations in a large cohort of urban adolescents. Respir Med . 2018 Feb. 135:42-50. [QxMD MEDLINE Link] .
Weiler JM, Hallstrand TS, Parsons JP, Randolph C, Silvers WS, Storms WW, et al. Improving screening and diagnosis of exercise-induced bronchoconstriction: a call to action. J Allergy Clin Immunol Pract . 2014 May-Jun. 2(3):275-80.e7. [QxMD MEDLINE Link] .
Beaudouin E, Renaudin JM, Morisset M, Codreanu F, Kanny G, Moneret-Vautrin DA. Food-dependent exercise-induced anaphylaxis--update and current data. Eur Ann Allergy Clin Immunol . 2006 Feb. 38(2):45-51. [QxMD MEDLINE Link] .
Sánchez-García S, Rodríguez del Río P, Escudero C, García-Fernández C, Ibáñez MD. Exercise-induced bronchospasm diagnosis in children. Utility of combined lung function tests. Pediatr Allergy Immunol . 2015 Feb. 26 (1):73-9. [QxMD MEDLINE Link] .
Weiler JM, Brannan JD, Randolph CC, Hallstrand TS, Parsons J, Silvers W, et al. Exercise-induced bronchoconstriction update-2016. J Allergy Clin Immunol . 2016 Nov. 138 (5):1292-1295.e36. [QxMD MEDLINE Link] .
He T, Song T. Exercise-induced bronchoconstriction in elite athletes: a narrative review. Phys Sportsmed . 2023 Dec. 51 (6):549-57. [QxMD MEDLINE Link] .
Jong M, Hanstock HG, Stenfors N, Ainegren M. Elite skiers' experiences of heat- and moisture-exchanging devices and training and competition in the cold: A qualitative survey. Health Sci Rep . 2023 Sep. 6 (9):e1511. [QxMD MEDLINE Link] . [Full Text] .
Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, et al. An Official American Thoracic Society Clinical Practice Guideline: Exercise-induced Bronchoconstriction. Am J Respir Crit Care Med . 2013 May 1. 187(9):1016-27. [QxMD MEDLINE Link] .
Stelmach I, Sztafiska A, Jerzyska J, Podlecka D, Majak P, Stelmach W. New insights into treatment of children with exercise-induced asthma symptoms. Allergy Asthma Proc . 2016 Nov. 37 (6):466-474. [QxMD MEDLINE Link] .
ProAir Digihaler (albuterol) [package insert]. Frazer, PA: Teva Respiratory, LLC. 12/2018. Available at [Full Text] .
- Pathogenesis of asthma. Antigen presentation by the dendritic cell with the lymphocyte and cytokine response leading to airway inflammation and asthma symptoms.

Contributor Information and Disclosures
Joseph P Garry, MD, FACSM, FAAFP Associate Professor, Department of Family Medicine and Community Health, University of Minnesota Medical School Joseph P Garry, MD, FACSM, FAAFP is a member of the following medical societies: American Academy of Family Physicians , American Medical Society for Sports Medicine , Minnesota Medical Association , American College of Sports Medicine Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference Disclosure: Received salary from Medscape for employment. for: Medscape.
Russell D White, MD Clinical Professor of Medicine, Clinical Professor of Orthopedic Surgery, Department of Community and Family Medicine, University of Missouri-Kansas City School of Medicine, Truman Medical Center-Lakewood Russell D White, MD is a member of the following medical societies: Alpha Omega Alpha , American Academy of Family Physicians , American Association of Clinical Endocrinology , American College of Sports Medicine , American Diabetes Association , American Medical Society for Sports Medicine Disclosure: Nothing to disclose.
Craig C Young, MD Professor, Departments of Orthopedic Surgery and Community and Family Medicine, Medical Director of Sports Medicine, Medical College of Wisconsin Craig C Young, MD is a member of the following medical societies: American Academy of Family Physicians , American College of Sports Medicine , American Medical Society for Sports Medicine , Phi Beta Kappa Disclosure: Nothing to disclose.
Anthony J Saglimbeni, MD President, South Bay Sports and Preventive Medicine Associates; Private Practice; Team Internist, San Francisco Giants; Team Internist, West Valley College; Team Physician, Bellarmine College Prep; Team Physician, Presentation High School; Team Physician, Santa Clara University; Consultant, University of San Francisco, Academy of Art University, Skyline College, Foothill College, De Anza College Anthony J Saglimbeni, MD is a member of the following medical societies: California Medical Association , Santa Clara County Medical Association, Monterey County Medical Society Disclosure: Received ownership interest from South Bay Sports and Preventive Medicine Associates, Inc for board membership.
What would you like to print?
- Print this section
- Print the entire contents of
- Print the entire contents of article

- Genetics of Asthma
- Asthma in Pregnancy
- Allergic and Environmental Asthma
- Pediatric Asthma
- Asthma in Older Adults
- Asthma: Reduce Reliance on the Blue Inhaler
- What Are Platanus Cough and Thunderstorm Asthma?
- Short-Term Wildfire Pollution Promotes Asthma Hospitalizations

- Drug Interaction Checker
- Pill Identifier
- Calculators

- 2002796274-overviewDiseases & Conditions Diseases & Conditions Asthma in Pregnancy

- 2002137501-overviewDiseases & Conditions Diseases & Conditions Allergic and Environmental Asthma
- Advanced search

Advanced Search
Experiences of exercise in patients with asthma: a qualitative analysis of discussions in a UK asthma online community
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Nadya L Ow
- ORCID record for Anna De Simoni
- For correspondence: [email protected]
- Figures & Data
Background Engagement with exercise in adults with asthma is suboptimal. Limited information is available regarding factors affecting engagement with exercise.
Aim To explore experiences of exercise and linked unmet needs in adults with asthma.
Design & setting Qualitative thematic analysis of posts in a UK asthma online community, written between 2015 and 2020.
Method Posts were identified using keywords searches. Posts in the ‘Exercise’ topic section were additionally included. Thematic analysis of posts was undertaken.
Results A total of 143 relevant posts were analysed. Ninety-two participants were identified through posts (11 male, 33 female, 48 sex not stated, aged 26–73 years). Emerging themes included the following: fear of experiencing asthma symptoms during exercise; lack of information about how to deal with symptoms; external barriers; emotional response; and involvement of healthcare providers. Environmental factors, concomitant life stressors, distrust of healthcare professionals, and embarrassment about displaying asthma symptoms during exercise were barriers to engagement. Facilitators included experiencing positive health outcomes following exercise and positive discussions regarding exercise with healthcare professionals. Strategies participants developed to enable exercise were warming up, increasing reliever and preventer inhalers when exercising, and finding exercises the individual felt were enjoyable.
Conclusion Future interventions to address fears of exercise-induced physical symptoms, and clear instructions on the use of inhalers when exercising are needed. Exploring patients’ attitudes to exercise in clinical consultations, especially in primary care, may be beneficial.
- primary health care
- How this fits in
Exercise in patients with asthma is not routinely discussed in consultations. Patients are unsure about how to exercise and use inhalers with exercise. Receiving positive reinforcement and support by healthcare professionals is a facilitator to exercise. Experiencing exercise-related asthma symptoms triggers emotional and embarrassment responses that may be underestimated and affect subsequent engagement with exercise. Guidelines for healthcare professionals do not currently hold issue-specific instructions on management of exercise in patients with asthma. Exploring patients' attitudes to exercise in clinical consultations, especially in primary care, may be beneficial. Novel interventions aimed at raising clinicians' awareness, as well as providing practical and emotional support to patients with asthma engaging with exercise, are warranted.
- Introduction
Asthma is one of the most common chronic conditions, 1 with 5.4 million people affected in the UK. 2
The relationship between exercise and asthma is complex, with exercise potentially aggravating an increase in airway resistance and resulting in physical symptoms that can be offputting for patients. Exercise-induced bronchoconstriction describes a temporary narrowing of the airway that occurs during exercise. 3 This occurs in 40%–90% of people with asthma (referred to as exercise-induced asthma) and 20% of those without asthma (simply referred to as exercise-induced bronchoconstriction). 4 , 5
Exercise has shown to be beneficial, although often overlooked, in the long-term management of asthma. 6 Previous studies assessing the impact of exercise in patients with asthma have reported positive effects on cardiopulmonary fitness, as well as improved emotional status and decreased levels of wheezing. 7 Both the American College of Sports Medicine 8 and American Thoracic Society 9 recommend exercise for patients with asthma. Exercise and physical activity are important in maintenance or achievement of healthy weight status, with exercise directly impacting metabolic rate and energy expenditure. 10 , 11 Obesity rates have risen substantially in the UK, with 28% of adults categorised as clinically obese based on their body mass index (BMI) in 2019. 12 Obesity is a risk factor for the development of asthma, 13 with the incidence of asthma increasing 2.0-fold in children and 2.3-fold in adults who are obese. 14 In patients already diagnosed with asthma, obesity can affect both asthma control and exacerbation severity, 15 resulting in poorer outcomes and reduced quality of life. 16
The British Thoracic Society (BTS) states that exercise-induced asthma may indicate poor asthma control and warrants regular treatment review. 17 Guidelines recommend the use of a short-acting beta-2 agonist (SABA) immediately before exercise as well as regular use of an inhaled corticosteroid. Where this is not sufficient to control symptoms caused by exercise, consideration of adding a leukotriene receptor antagonist, long-acting beta-2 agonist (LABA), chromones, or theophyllines is warranted. 17
Multiple studies have reported that patients with asthma are less likely to take part in exercise, with a recent systematic review reporting 11 studies where participants with asthma engaged in less exercise than controls, versus six studies reporting no difference. 18 Several barriers to exercise, such as fear and difficulty breathing, have been reported in the adolescent population with asthma. 19 Lack of time is more likely to be reported as a barrier in younger patients. 20 Fear of exacerbating symptoms is also a common theme among adolescents 19 and adults, 20 with patients with more severe disease more likely to view exercise as detrimental. Facilitators include the desire to be healthy and encouragement from a motivated companion or physician. In terms of intrinsic characteristics, patients with less asthma knowledge, lower self-efficacy, and more negative attitudes towards asthma are more likely to view exercise negatively. 20 Studies examining exercise-promoting interventions in adults with asthma have focused on the involvement of more structured exercise plans and tips specifically focused on patients with asthma. 21 Little published information was, however, found regarding strategies that patients with asthma came up with to help them exercise and the role of primary care.
Better understanding of the underlying factors that result in reduced engagement with exercise in this group of patients is needed. Online communities might include people who do not take part in traditional research studies, therefore offering perspectives from an unrepresented patient population. 22 Such data can provide new and insightful perspectives on engagement with exercise in patients with asthma, with the potential to inform healthcare interventions. 23 , 24
This study aimed to explore whether exercise was a topic of discussion in asthma online communities and to identify potential unmet needs, and barriers and facilitators to engagement with exercise.
A qualitative analysis of posts was conducted within the Asthma UK community. The Asthma UK community has more than 18 000 members and 22 000 posts. 25 The Asthma UK forum is used by patients with asthma to share their stories, and give and receive information and support. The online community was chosen following an initial Google search, which showed a wealth of information being exchanged on exercise and asthma, as well as ‘Exercise’ being one of the discussion topics listed within the community. The authors aimed to include posts made by adults about exercising with asthma, whether discussing their own experiences and stories or providing support to others.
Ethical issues
Ethics approval for this study was assessed by the Queen Mary’s University Research Ethics Committee and was exempt from full review. Permission was granted by both HealthUnlocked and Asthma UK before starting the study, as in previous investigations by the same authors. 26 – 28 The passive analysis approach used in this study is generally considered non-intrusive. 29 In order to protect the identity and intellectual property of forum participants, direct quotes have not been used, despite this being normal practice in qualitative research. Summative descriptions of quotes will instead be used throughout the article, as previously described. 23
Posts and participant identification
To identify relevant posts, the following terms were searched: 'exercise', 'fitness', 'physical activity', and 'weight', using the search facility on the Asthma UK website. Posts and threads included within the topic 'Exercise' were also included. All posts belonging to the threads were analysed, provided they were relevant to the research question. Participants were retrieved through the posts. Posts written between 2015 and December 2020 were exported into an Excel database in chronological order. To avoid third-party interpretation bias, posts written by family members or friends talking about patients with asthma were not included. Data on usernames, sex, age, asthma treatment, and asthma severity were retrieved within the posts, where available.
Posts were analysed using thematic analysis 30 using a data-driven approach. SSA read all posts to familiarise with the data and participants.
Initial codes produced looked specifically at barriers, facilitators, and strategies discussed in relation to exercising with asthma on the community. NLO independently coded 20% of the posts. Disagreement was identified between the coders on three out of 30 posts, and was resolved with discussion between SSA, NLO, and ADS. Following coding, main themes and sub-themes were identified, and were iteratively reviewed and refined throughout the analysis.
Microsoft Excel (version 16.63.1) was used for data collection, coding, and statistical measures (mean, standard deviation [SD]).
Exercising in asthma was a topic of discussion, with 149 posts retrieved, 143 of which (96%, 15 701 words in total, averaging >100 words per post) were considered relevant to the research question and included in the analysis. Most posts discussed concerns regarding exercising with asthma, use of inhalers when exercising, and how to safely exercise without exacerbating asthma symptoms. Posts were written evenly throughout the years from 2015–2020 (12.2% ±8), with the exception of 2016 (39% of posts written in the year).
Participants
A total of 92 participants were identified from 143 posts ( Table 1 ). Most participants did not reveal personal characteristics such as sex, ethnic group, or age. No information on ethnic background or geographical location was reported. Among participants who disclosed their sex, there were three times the number of females to males (33 versus 11).
- View inline
A range of themes relating to barriers, facilitators, and strategies to engage in exercise were highlighted in the context of exercising in asthma (see Table 2 ).
A relationship was observed between the themes organised as barriers and facilitators, in that some users were suggesting how barriers could be overcome (for example, naming facilitators or strategies). Strategies are reported within the facilitators section.
Fear of physical symptoms
It was common for participants to report unpleasant asthma symptoms when exercising such as breathlessness, discomfort, wheeze, and burning in the chest. Post-exercise symptoms included increased phlegm, cough, and chest pain. These were considered as barriers to exercise.
A participant described that she wanted to improve her fitness levels, but whenever she attempted exercise, she experienced severe shortness of breath, a tight chest, cough and an 'itchy ' feeling in her lungs. She described that the feeling of breathlessness scared her and put her off trying to push onwards with exercising. To overcome this, her strategy involved using salbutamol before exercise, which she found helped a bit. (Female, age not stated, participant N. 7)
Lack of information on exercising in asthma
How to exercise with asthma.
Participants looking for advice on how to safely start exercising with asthma wrote a number of posts. They recognised that they needed to lose weight and improve their fitness levels, and that this would help with their asthma symptoms, but they did not know how to do so without causing an asthma attack, showing a possible lack of knowledge when it comes to exercise.
A participant described that she was trying to lose weight and was very unfit but was struggling to take part in any meaningful exercise. She expressed worries that she wanted to exercise in a way that avoided her needing to take oral steroids but felt that this put her in a difficult situation. (Female, age not stated, participant N. 1) A participant asked for advice, explaining that they really wanted to start running to lose weight but that they didn’t know how they could begin this when they already struggled with walking. They mentioned specifically wanting to avoid having an asthma attack. (Age and sex not stated, participant N. 89)
How to use inhalers before, during, and after exercise
Participants described their use of inhalers while exercising and suggested strategies they developed to prevent physical symptoms triggered by exercise. A common suggestion was the use of a reliever inhaler before starting exercise.
A participant explained that she was given advice from a physiotherapist to take two puffs of her reliever inhaler 30 minutes before taking part in exercise and recommended this to others on the community. (Female, age not stated, participant N. 12) One participant described that they experienced increased phlegm while they were exercising and afterwards, and that using their Symbicort inhaler before exercise and a few times throughout the day prevented this symptom. (Age and sex not stated, participant N. 92)
External barriers
Environmental factors.
Cold weather and uphill conditions were considered to be barriers in many posts made by users of the community, with participants experiencing worse physical symptoms when these factors were present.
A participant described that exercise is very important to them and that they frequently run and swim, but when it is cold outside their strategy is to run indoors on a treadmill to avoid the cold air. They wrote that their asthma is worsened by the cold air and that they have to make sure they don’t run too vigorously in the spring or autumn so that they don’t have a bad asthma attack. (Male, age not stated, participant N. 73)
Stress from work and private life
Stressful life circumstances were also reported as a barrier to exercise and to affect ability to take part in exercise. The most common stressors mentioned were family worries and occupational concerns.
A participant described that he believed the biggest factor in the development of his asthma was stress from his work and his personal life. Before this he could run long distances a few times a week, but now required medication to be able to do so. He also described that he has young children, one with a severe disability, which has also been difficult for his family and him to cope with. (Male, age mid-40s, participant N. 6) Another participant described that she has had to reduce the amount of exercise she takes part in as she had experienced increasing asthma flare-ups in the last few years. She described that stress is a big trigger for her and she found this very hard to avoid as she has multiple children who have asthma and one child with a developmental disorder. (Female, age not stated, participant N. 21)
Emotional response
Fear of negative symptoms or outcomes.
Participants described fear of worsening their asthma with exercise and potentially experiencing negative physical symptoms, asthma attacks, or hospital admission. Negative emotional symptoms were also reported following exercise, including embarrassment and shame.
A participant wrote that they visited their asthma nurse who explained to them that stopping exercise would have a negative effect on their asthma, however they were too scared to continue with exercise after previously having to visit A&E following an asthma attack triggered by exercise. (Age and sex not reported, participant N. 28) A participant wrote that he used to run long distances a few times a week, but now could only do this following a course of steroid tablets and in warm weather conditions. He used to take part in kickboxing and tried to return to this recently, but really struggled at his class and felt ashamed of his fitness levels. He thought that his instructor looked very worried about him due to his breathlessness, and after his experience did not return to this class. (Male, age mid-40s, participant N. 6)
Facilitators
Positive effects of exercise.
A positive impact on asthma symptoms, fitness levels, and mood encouraged exercise uptake in asthma and persistence with exercise. This was also reported by older users of the community.
One participant wrote that following a hospital admission their peak flow was lower than previously. They began to run with their dogs three times a week and found that their peak flow had increased substantially. This made them feel positive about their next appointment for their asthma check-up. They described feeling a good improvement in their lungs since they started to exercise more and that they were in a better mood. (Male, age 68, participant N. 80) Another participant described that they felt better both physically and mentally when they take part in regular exercise. They explained that this made them feel that they had a level of control over their health and asthma, a feeling that they described was sometimes lost when their asthma symptoms were flaring. (Age and sex not reported, participant N. 60)
Involvement of healthcare professionals
Regular asthma medication monitoring.
Regular asthma medication monitoring was shown to have a positive impact on uptake of exercise in patients with asthma. Being able to discuss any asthma symptoms experienced in relation to exercise with a trusted healthcare professional allowed better adjustment of participants’ medications and encouraged them to continue exercising.
A participant described that she was struggling with soreness in her chest and that any form of exercise was causing her asthma symptoms to worsen. She wrote that she saw her GP and was advised to increase her Ventolin dose, with four puffs to be taken before exercise. Since increasing this, she had been able to do a lot more exercise and encouraged other posters to raise issues with their asthma nurse and GP. (Female, age not reported, participant N. 34)
Relationship with healthcare professional
The relationship between the participants and their healthcare providers had an impact on people’s engagement with exercise. Positive reinforcement and encouragement given by asthma nurses and doctors to continue with exercise was recognised as a facilitator to exercise in participants on the community, whereas a lack of trust in the medical advice acted as a barrier to engagement with exercise.
A participant wrote that they discussed their exercise with their asthma consultant who was happy with their exercise levels and encouraged them to persevere. The participant wrote that it took them longer to reach their exercise goals, but that they could now run long distances every few days and have to use their inhaler less frequently than they did before. They encouraged others on the community to keep going and explained that they felt it helped both their physical and mental health. (Age and sex not reported, participant N. 35) A participant wrote that she was worried about making an appointment with her asthma nurse or GP as she felt that they were both 'rubbish '. She described that she felt her asthma nurse had given her incorrect advice in relation to her peak flow and told her that her asthma could not be that bad if she was not wheezing. She felt that because her asthma symptoms were less obvious, she was dismissed by her nurse and GP who may think it was 'all in her head '. (Female, age not reported, participant N. 7) Another participant described that their doctor wanted them to begin a ' graded exercise programme' to rebuild strength and prevent relapses in their asthma. They described that the doctor encouraged them to do a bit more each day, and that they felt this was too vague and they didn’t know what to do. They wrote about being concerned that they would overdo the exercise and could become very unwell again. (Age and sex not reported — hidden username group).
Use of medications
Issues around asthma medications and exercise included participants being unsure about using their inhalers as much as they needed during exercise, or whether there was a limit to this in terms of safety. Participants also reported that they were worried that needing to increase their inhalers during exercise could indicate that they had exercised too hard and negatively affect their lungs. There was confusion as to whether they should increase their preventer or reliever inhalers as a precaution before exercise and which would be more beneficial. Some participants reported that they were unsure as to whether they needed to increase their inhaler use or change the type of asthma medication, or whether this was a normal thing to expect because of their asthma. Some participants described that they could not exercise without first taking a course of oral steroids because this helped with their exercise tolerance.
Engagement with exercise in asthma is a topic of discussion in online asthma communities, with participants revealing unmet needs, barriers, and facilitators. The data suggest that exercise in asthma is not routinely discussed in primary care consultations and patients are unsure about how to exercise and use inhalers with it. Receiving positive reinforcement and support from healthcare professionals was a facilitator to exercise. Encouragement to persist with exercise leads to better outcomes in terms of engagement with exercise, positive mental health benefits, and reduced long-term symptoms. Experiencing exercise-linked asthma symptoms also triggers an emotional response, which may affect subsequent engagement.
Strengths and limitations
A main strength of this work was the use of an established online community. 21 Results were based on participants’ agendas and allowed views to be identified that may not have been captured otherwise, 22 , 31 from a wide geographical location. Online discussions are self-initiated and people communicate with each other without time, length, or behavioural constraints, offering a window to understand patients' issues that bypasses reactivity and self-representation bias of traditional research approaches. Additionally, online communities might include people who do not take part in traditional research studies, therefore offering perspectives from an unrepresented patient population. 22 , 31 Despite the inability of the authors to ask clarification questions, users could read and reply to each other’s posts in an asynchronous way, contributing to the topic on an ad hoc basis in their own time in a single post. Participants’ insights on topics were offered without the need of facilitation and multiple interactions, unlike during face-to-face interviews or focus groups. As such, online discussions could even enable a more in-depth exploration of themes compared with interviews. 30
The main limitation was the lack of information about participant characteristics in terms of age, sex, asthma medication, and socioeconomic factors. This made it difficult to recognise patterns within groups of people and potential common features that may have played a part in their experiences with asthma and exercise.
There is an element of participant selection and sample bias, as only the views of those taking part in the Asthma UK online community were represented. The number of participants who wrote 7% of the posts could not be determined, as 10 participants used a ‘hidden’ username. The authors cannot be certain that each username represented a distinct participant, as members can create multiple accounts. Community participants were required to be aged >16 years, meaning that younger adolescents could not be included in this study who may have had different experiences with exercise than adults.
Comparison with existing literature
The results of this study align with the barriers to exercise seen in previous literature. 20 , 32 , 33 These studies report that despite patients with asthma recognising the importance of exercise, they may not participate owing to barriers such as cold weather, lack of motivation, and perceived symptom burden. 32 In one of these studies, it was found that those with more severe asthma were more likely to hold beliefs that exercise was not good for them, and that patients with worse attitudes and less knowledge about their asthma were more likely to have negative ideas about exercise. 20 Other barriers, such as fear of worsening asthma symptoms, feeling unsafe to exercise, and exacerbation of asthma, were also described in previous literature. 20 Facilitators to engagement with exercise were identified such as improved psychological wellbeing, asthma control, and encouragement to exercise from healthcare professionals. These mirror what has been previously described in literature. 20
There is limited research assessing the psychological barriers to exercise in asthma. The results of the present study found that the most common barriers included fear of breathlessness and unpleasant symptoms, embarrassment owing to lack of fitness, and anxiety about overall worsening of their asthma. These barriers represent key targets for future interventions seeking to improve engagement with exercise in asthma. However, there are only a small number of studies exploring intervention development, and, in many studies, the relationship between exercise and the emotional aspects patients experience is lacking. Indeed, while Clarke and Mansur 33 called for more research investigating the effect that fear of exercise has on asthma worsening, 33 there remain limited new data.
Few studies have explored the effect of exercise interventions on asthma improvement and uptake of exercise. Freitas et al 34 carried out a randomised controlled trial where one group was given a behavioural intervention consisting of education and physical activity counselling. 34 Participants in the intervention group had improved asthma control, physical activity levels, sleep quality, reduced sedentary time, and reduced anxiety symptoms compared with controls. 34 One of the main barriers was lack of information, despite the willingness to take part in exercise.
Within the present study, specific characteristics between the participants that may have impacted their experiences with exercise could not be determined. Previous studies have aimed to assess this and found that several factors including BMI and age of diagnosis may be significant. Those with higher BMI perceived higher barriers to exercise than those with lower BMI, similarly to those who were diagnosed with asthma at an older age compared with those diagnosed before age 5 years. 33
Implications for research and practice
Awareness of the significant barriers to exercise, which are most important and commonly experienced by patients, may lead to improved clinical consultations with better outcomes and compliance with exercise. National Institute for Health and Care Excellence (NICE) guidelines for healthcare professionals regarding engagement with exercise in other respiratory conditions, such as cystic fibrosis, 35 indicate that an individualised exercise programme that considers patients’ abilities and preferences should be formulated and reviewed regularly. 36 Similarly, in chronic obstructive pulmonary disorder (COPD), the guidelines recommend that pulmonary rehabilitation programmes should be available to all patients diagnosed with COPD. 37 Similar guidelines should be developed for clinicians treating patients with asthma. Specific instructions on the use of inhalers with exercise are required and should be discussed with patients, especially in primary care.
Discussion regarding exercise in clinical consultations is a facilitator to engagement with exercise. This allows identification of barriers such as fear of worsening asthma and physical symptoms, and addressing the lack of knowledge surrounding how to use asthma inhalers safely while exercising. The data suggest that this is not routinely happening and call for increasing clinicians’ awareness, exploration of issues with exercise, and addressing them during consultations. Formulating realistic plans and having open discussions regarding concerns is likely to be beneficial. Indeed, participants with little understanding, or who were given vague recommendations on exercise, were less likely to feel confident when taking part in exercise.
Attention to the emotional barriers to exercise (such as fear of or embarrassment when displaying asthma symptoms during exercise) is also warranted, as these are not currently addressed within guidelines. 17 There is a need to explore the impact of mood and interoception (the sense of the internal state of the body) on (fear of) symptoms of asthma, 36 with a specific focus on exercise.
Novel interventions aimed at raising clinicians’ awareness, as well as providing practical and emotional support to patients with asthma engaging with exercise, are warranted.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Anna De Simoni was partly funded by Barts Charity MGU0419. REAL - Health: REsearch Actionable Learning Health Systems Asthma programme. The views expressed are those of the author(s) and not necessarily those of the NHS, NIHR or Department of Health.
Ethical Approval
Ethics approval for this study was assessed by the Queen Mary’s University Research Ethics Committee and was exempt from full review (QMERC2020.060). Permission was granted by both HealthUnlocked and Asthma UK before starting the study.
Freely submitted; externally peer reviewed.
In order to protect the identity and intellectual property of forum participants, direct quotes have not been used in the article nor would it be appropriate for these to be made available by request to the authors.
Acknowledgements
The authors are grateful to Asthma UK and HealthUnlocked for granting permission to analyse the online community.
Competing interests
The authors declare that no competing interests exist.
The authors report no conflicts of interest in this work
- Received September 6, 2021.
- Revision received December 28, 2021.
- Accepted January 31, 2022.
- Copyright © 2022, The Authors
This article is Open Access: CC BY license ( https://creativecommons.org/licenses/by/4.0/ )
- Anandan C ,
- Simpson CR ,
- Asthma + Lung UK
- Parsons JP ,
- Hallstrand TS ,
- Mastronarde JG ,
- Jayasinghe H ,
- Kopsaftis Z ,
- Robinson SM ,
- Orenstein DM
- 9. ↵ ( 1999 ) Pulmonary rehabilitation—1999. American Thoracic Society . Am J Respir Crit Care Med 159 ( 5 Pt 1 ): 1666 – 1682 , doi: 10.1164/ajrccm.159.5.ats2-99 . OpenUrl CrossRef PubMed
- NHS Digital
- Rönmark E ,
- Andersson C ,
- Nyström L ,
- Sutherland ER ,
- Mohanan S ,
- McWilliams A ,
- British Thoracic Society, Scottish Intercollegiate Guidelines Network
- Cordova-Rivera L ,
- Gibson PG ,
- Gardiner PA ,
- McDonald VM
- Mackintosh KA ,
- Eddolls WTB ,
- Mancuso CA ,
- Robbins L ,
- Corbridge SJ ,
- Nyenhuis SM
- De Simoni A ,
- Balasooriya-Smeekens C ,
- Bateman A ,
- De Simoni A
- HealthUnlocked
- Fleming L ,
- Joglekar S ,
- Coulson NS ,
- Eysenbach G ,
- Jamison J ,
- Freeman AT ,
- Freitas PD ,
- Passos NFP ,
- Carvalho-Pinto RM ,
- National Institute for Health and Care Excellence (NICE)
- Ainsworth B ,
- Stanescu S ,
In this issue
- Table of Contents
- Index by author
Thank you for recommending BJGP Open.
NOTE: We only request your email address so that the person to whom you are recommending the page knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Tweet Widget
- Facebook Like
- Google Plus One
Jump to section
More in this toc section.
- Continuity of care and mortality in patients with type 2 diabetes
- Sex differences and trends in managing cardiovascular risk factors in primary care: a dynamic cohort study
- Translating in-person care to telehealth: analysis of GP consultations on musculoskeletal conditions
Related Articles
Cited by....

British Journal of General Practice
- Environmental Assessment
- Environmental Science
- Agricultural Science
Exercise and asthma: An overview
- November 2015
- European Clinical Respiratory Journal 2(27984 - http://dx.doi.org/10.3402/ecrj.v2.27984)
- 2(27984 - http://dx.doi.org/10.3402/ecrj.v2.27984)

- Università degli studi di Cagliari

- Lund University
- This person is not on ResearchGate, or hasn't claimed this research yet.
Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- Nicole Mussi
- Arianna Rossi

- ARQ BRAS CARDIOL

- Gabriella Ayuni Amir
- Rifaldi Saputra
- Nikolai G. Vetr
- Nicole R. Gay
- Joshua N Adkins
- Stephen B. Montgomery

- Nur Fatin Nabilah Md Zemberi
- Ruimiao Liang
- Matthew W. Wise
- Chelsea Cole
- Aaron Provance
- J Pediatr Health Care

- Yuanmin Jia
- Baosheng Zhao
- EQUINE VET J

- Louis-Philippe Boulet
- Robert J Hancox

- Julie Turmel
- Immunol Today
- L. Hoffman-Goetz
- B.K. Pedersen
- P. O'Byrne
- CLIN J SPORT MED
- Bruno H KnOpfli
- Oded Bar-Or
- EXERC IMMUNOL REV

- C. Bartalucci

- Moises A Calderon

- Edward T. Mannix
- ENVIRON RES

- Xavier Dumont
- Arch Pediatr Adolesc Med

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up

IMAGES
VIDEO
COMMENTS
A person may have asthma symptoms that become worse with exercise (more common) or may have only exercise-induced bronchoconstriction, without symptoms at other times. If a person's asthma is triggered only during vigorous exercise (exercise-induced bronchoconstriction), they are considered to have mild intermittent asthma.
Exercise-induced bronchoconstriction (EIB) occurs during physical exertion and involves a narrowing of the airway.[1][2] EIB occurs in 40% to 90% of people with asthma and up to 20% of those without asthma.[1][3][4] The benefits of regular exercise for all people are well established, and activity is an integral part of a healthy lifestyle. People suffering from EIB may avoid exertion due to ...
Exercise-induced asthma is when the airways narrow or squeeze during hard physical activity. It causes shortness of breath, wheezing, coughing, and other symptoms during or after exercise.
Asthma is a chronic respiratory disease that affects millions worldwide. Medication management is the current mainstay of treatment; however, there is evidence to suggest additional benefit with lifestyle changes, particularly with increased physical ...
The terms 'exercise-induced asthma' (EIA) and 'exercise-induced bronchoconstriction' (EIB) are often used interchangeably to describe symptoms of asthma such as cough, wheeze, or dyspnoea provoked by vigorous physical activity. In this review, we refer to EIB as the bronchoconstrictive response and to EIA when bronchoconstriction is ...
Diagnosis To diagnose exercise-induced bronchoconstriction, your health care provider first takes a medical history and does a physical exam. You may have tests to check your lung function and rule out other conditions. Test of current lung function Your provider will likely perform a spirometry (spy-ROM-uh-tree) test. This exam shows how well your lungs function when you aren't exercising. A ...
Exercise-induced bronchoconstriction, or EIB, is the preferred term for what was known for years as exercise-induced asthma. Symptoms develop when airways narrow as a result of physical activity. As many as 90 percent of people with asthma also have EIB, but not everyone with EIB has asthma. Many elite and world-class athletes have EIB ...
Abstract Exercise-induced bronchoconstriction (EIB) can occur in individuals with and without asthma, and is prevalent among athletes of all levels. In patients with asthma, symptoms of EIB ...
The terms 'exercise-induced asthma' (EIA) and 'exercise-induced bronchoconstriction' (EIB) are often used interchangeably to describe symptoms of asthma such as cough, wheeze, or dyspnoea provoked by vigorous physical activity. In this review, we refer to EIB as the bronchoconstrictive response and to EIA when bronchoconstriction is associated ...
The terms 'exercise-induced asthma' (EIA) and 'exercise-induced bronchoconstriction' (EIB) are often used interchangeably to describe symptoms of asthma such as cough, wheeze, or dyspnoea provoked by vigorous physical activity. In this review, we refer to EIB as the bronchoconstrictive response and to EIA when bron-choconstriction is associated with asthma symptoms. EIB is a common ...
Some forms of exercise are likelier than others to trigger asthma symptoms. Learn more from WebMD about preventing symptoms before, during, and after a workout.
Exercise-induced asthma is triggered by aerobic activity. Inhaling a lot of cold, dry air can cause swelling in the airways, making it difficult to breathe.
Exercise-induced bronchoconstriction describes the narrowing of the airway that occurs with exercise. More than 10 percent of the general population and up to 90 percent of persons previously ...
Exercise-induced bronchoconstriction (EIB) describes acute airway narrowing that occurs as a result of exercise. A substantial proportion of patients with asthma experience exercise-induced respiratory symptoms. EIB has also been shown to occur in subjects without a known diagnosis of asthma.
The terms 'exercise-induced asthma' (EIA) and 'exercise-induced bronchoconstriction' (EIB) are often used interchangeably to describe symptoms of asthma such as cough, wheeze, or dyspnoea provoked by vigorous physical activity. ...
Asthma induced by exercise is called exercise-induced asthma (EIA). During exercise, the patients contract bronchial smooth muscle due to increased breathing; in addition, loss of heat and moisture in the respiratory tract causes contraction of bronchial smooth muscle [ 59 ].
Exercise-Induced Asthma Some people with asthma only have symptoms (e.g., shortness of breath, chest tightness, wheezing or coughing) during exercise or when doing physically demanding tasks. This is called exercise-induced asthma or exercise-induced bronchospasm. But you don't have to let asthma hold you back from being active.
Exercise-induced asthma (EIA) is a condition of respiratory difficulty that is related to histamine release, triggered by aerobic exercise, and lasts several minutes (see Pathophysiology). Causes include medical conditions, environmental factors, and medications (see Etiology).
The relationship between exercise and asthma is complex, with exercise potentially aggravating an increase in airway resistance and resulting in physical symptoms that can be offputting for patients. Exercise-induced bronchoconstriction describes a temporary narrowing of the airway that occurs during exercise. 3 This occurs in 40%-90% of people with asthma (referred to as exercise-induced ...
Asthma or asthmalike conditions can limit the ability of athletes to perform. This article reviews the diagnosis and treatment of asthma and exercise-induced bronchoconstriction in athletes.
The terms 'exercise-induced asthma' (EIA) and 'exercise-induced bronchoconstriction' (EIB) are often used. interchangeably to describe symptoms of asthma such as cough, w heeze, or ...
Good Essays. 1857 Words. 8 Pages. Open Document. Exercise Induced Asthma. "Asthma is a pulmonary disease with the following characteristics: 1) airway obstruction that is reversible in most patients either spontaneously or with treatment; 2) airway inflammation; and 3) increased airway responsiveness to a variety of stimuli" (Enright, 1996, p ...