Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State

Pathophysiology and Clinical Presentation
Pathophysiology:
Type 1 Diabetes Mellitus is a syndrome characterized by hyperglycemia and insulin deficiency resulting from the loss of beta cells in pancreatic islets (Mapes & Faulds, 2014). Nonimmune (type 1B diabetes), occurs secondary to other diseases and is much less common than autoimmune (type 1A). The destruction of beta cells in Type 1A diabetes results from the interaction of both genetic and environmental factors. Although the genetic susceptibility is not well understood, type 1 diabetes is most strongly associated with major histocompatibility complex (MHC), specifically histocompatibility leukocyte antigen (HLA) class II alleles (HLA-DQ and HLA-DR) (McCance & Heuther, 2014). Type 1 diabetes is less hereditary than type 2 but 7-13% of patients also have a first degree relative with type 1 diabetes (Mapes & Faulds, 2014). Environmental factors include viral infections (especially enteroviruses), exposure to infectious microorganisms (such as Helicobacter pylori ), exposure to cow’s milk proteins and a lack of vitamin D (McCance & Heuther, 2014).
The destruction of insulin-producing beta cells in the pancreas starts with the formation of autoantigens. These autoantigens are ingested by antigen-presenting cells which activate T helper 1 (Th1) and T helper 2 (Th2) lmphocytes. Activated Th1 lymphocytes secrete interluekin-2 (IL-2) and interferon. IL-2 activates autoantigen-specific T cytotoxic lymphocytes which destroy islet cells through the secretion of toxic perforins and granzymes. Interferon activates macrophages and stimulates the release of inflammatory cytokines (including IL-1 and tumor necrosis factor [TNF]) which further destroy beta cells (McCance & Heuther, 2014). Activated Th2 lymphocytes produce IL-4 which stimulates B lymphocytes to proliferate and produce islet cell autoantibodies (ICAs) and anti-glutamic acid decarboxylase (antiGAD65) antibodies. AntiGAD65 is an enzyme that helps control the release of insulin from beta cells and can be used to determine the cause of diabetes (McCance & Heuther, 2014). Insulin autoantibodies [IAAs]) and zinc transporter 8 (Znt8) protein are also associated with type 1 diabetes mellitus. Despite it’s complicated pathophysiology, it is important to understand the destruction of beta cells in type 1 diabetes because it leads to a lack of insulin and amylin. Without insulin or amylin the body cannot promote glucose disappearance or limit glucose appearance from the bloodstream, respectively, resulting in hyperglycemia (Mapes & Faulds, 2014).
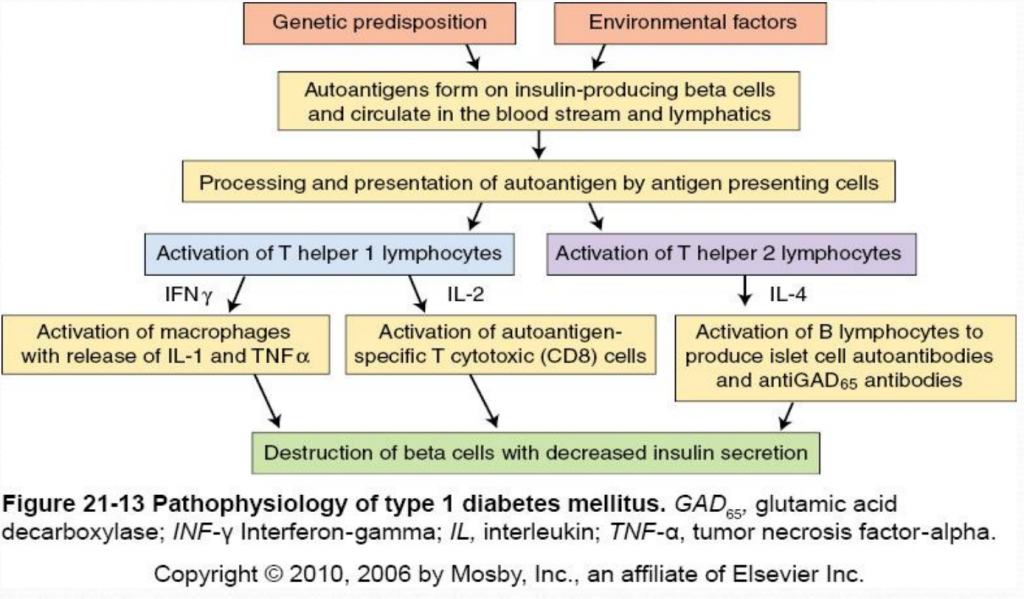
Clinical Presentation:
Type 1 diabetes does not present clinically until 80-90% of the beta cells have been destroyed (McCance & Heuther, 2014). Because insulin stimulates glucose uptake into tissues, stores glycose as glycogen, inhibits glucagon secretion and inhibits glucose production from the liver, the destruction of insulin-producing beta cells causes hyperglycemia (Mapes & Faulds, 2014). Type 1 diabetics may present with abrupt onset of diabetic ketoacidosis, polyuria, polyphagia, polydipsia, or rapid weight loss with marked hyperglycemia (Mapes & Faulds, 2014). To diagnose diabetes, patients must have an A1C level greater than 6.5% percent on two separate tests; the presence of ketones in the urine and/or autoantibodies in the blood can distinguish type 1 from type 2 diabetes (Mayo Clinic, 2014).

2 thoughts on “ Pathophysiology and Clinical Presentation ”
none for now
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

- Type 1 Diabetes Mellitus
- Author: Romesh Khardori, MD, PhD, FACP; Chief Editor: George T Griffing, MD more...
- Sections Type 1 Diabetes Mellitus
- Practice Essentials
- Pathophysiology
- Epidemiology
- Patient Education
- Physical Examination
- Complications
- Laboratory Studies
- Tests to Differentiate Type 1 from Type 2 Diabetes
- Approach Considerations
- Self-Monitoring of Glucose Levels
- Continuous Glucose Monitoring
- Insulin Therapy
- Management of Hypoglycemia
- Management of Hyperglycemia
- Management of Complications
- Glycemic Control During Serious Medical Illness and Surgery
- Glycemic Control During Pregnancy
- Consultations
- Medication Summary
- Antidiabetics, Insulins
- Antidiabetics, Amylinomimetics
- Hypoglycemia Antidotes
- Monoclonal Antibodies
- Allogeneic Islet Cells
- Questions & Answers
Type 1 diabetes is a chronic illness characterized by the body’s inability to produce insulin due to the autoimmune destruction of the beta cells in the pancreas. Although onset frequently occurs in childhood, the disease can also develop in adults. [ 1 ]
ICD-10 code
The International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code for type 1 diabetes without complications is E10.9.
Signs and symptoms
The classic symptoms of type 1 diabetes are as follows:
Unexplained weight loss
Other symptoms may include fatigue, nausea, and blurred vision.
The onset of symptomatic disease may be sudden. It is not unusual for patients with type 1 diabetes to present with diabetic ketoacidosis (DKA).
See Clinical Presentation for more detail.
Diagnostic criteria by the American Diabetes Association (ADA) include the following [ 2 ] :
A fasting plasma glucose (FPG) level ≥126 mg/dL (7.0 mmol/L), or
A 2-hour plasma glucose level ≥200 mg/dL (11.1 mmol/L) during a 75-g oral glucose tolerance test (OGTT), or
A random plasma glucose ≥200 mg/dL (11.1 mmol/L) in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis
Lab studies
A fingerstick glucose test is appropriate for virtually all patients with diabetes. All fingerstick capillary glucose levels must be confirmed in serum or plasma to make the diagnosis. All other laboratory studies should be selected or omitted on the basis of the individual clinical situation.
An international expert committee appointed by the ADA, the European Association for the Study of Diabetes (EASD), and the International Diabetes Association recommended the HbA 1c assay for diagnosing type 1 diabetes only when the condition is suspected but the classic symptoms are absent. [ 3 ]
Screening for type 1 diabetes in asymptomatic low-risk individuals is not recommended. [ 2 ] However, in patients at high risk (eg, those who have first-degree relatives with type 1 diabetes), it may be appropriate to perform annual screening for anti-islet antibodies before the age of 10 years, along with 1 additional screening during adolescence. [ 4 ]
See Workup for more detail.
Glycemic control
The ADA recommends using patient age as one consideration in the establishment of glycemic goals, with different targets for preprandial, bedtime/overnight, and hemoglobin A 1c (HbA 1c ) levels in patients aged 0-6, 6-12, and 13-19 years. [ 5 ] Benefits of tight glycemic control include not only continued reductions in the rates of microvascular complications but also significant differences in cardiovascular events and overall mortality.
Self-monitoring
Optimal diabetic control requires frequent self-monitoring of blood glucose levels, which allows rational adjustments in insulin doses. All patients with type 1 diabetes should learn how to self-monitor and record their blood glucose levels with home analyzers and adjust their insulin doses accordingly.
Real-time continuous monitoring of glucose—using continuous glucose monitors (CGMs)—can help patients improve glycemic control. [ 6 , 7 ] CGMs contain subcutaneous sensors that measure interstitial glucose levels every 1-5 minutes, providing alarms when glucose levels are too high or too low or are rapidly rising or falling.
Insulin therapy
Patients with type 1 diabetes require lifelong insulin therapy. Most require 2 or more injections of insulin daily, with doses adjusted on the basis of self-monitoring of blood glucose levels. Insulin replacement is accomplished by giving a basal insulin and a preprandial (premeal) insulin. The basal insulin is either long-acting (glargine or detemir) or intermediate-acting (NPH). The preprandial insulin is either rapid-acting (lispro, aspart, insulin inhaled, or glulisine) or short-acting (regular).
Common insulin regimens include the following:
Split or mixed: NPH with rapid-acting (eg, lispro, aspart, or glulisine) or regular insulin before breakfast and supper
Split or mixed variant: NPH with rapid-acting or regular insulin before breakfast, rapid-acting or regular insulin before supper, and NPH before bedtime (the idea is to reduce fasting hypoglycemia by giving the NPH later in the evening)
Multiple daily injections (MDI): A long-acting insulin (eg, glargine or detemir) once a day in the morning or evening (or twice a day in about 20% of patients) and a rapid-acting insulin before meals or snacks (with the dose adjusted according to the carbohydrate intake and the blood glucose level)
Continuous subcutaneous insulin infusion (CSII): Rapid-acting insulin infused continuously 24 hours a day through an insulin pump at 1 or more basal rates, with additional boluses given before each meal and correction doses administered if blood glucose levels exceed target levels
Diet and activity
All patients on insulin should have a comprehensive diet plan, created with the help of a professional dietitian, that includes the following:
A daily caloric intake prescription
Recommendations for amounts of dietary carbohydrate, fat, and protein
Instructions on how to divide calories between meals and snacks
Exercise is also an important aspect of diabetes management. Patients should be encouraged to exercise regularly.
See Treatment and Medication for more detail.
Type 1 diabetes mellitus (DM) is a multisystem disease with both biochemical and anatomic/structural consequences. It is a chronic disease of carbohydrate, fat, and protein metabolism caused by the lack of insulin, which results from the marked and progressive inability of the pancreas to secrete insulin because of autoimmune destruction of the beta cells. [ 1 ] (See Pathophysiology.) (See also Glucose Intolerance .)
Type 1 DM can occur at any age. Although it frequently arises in juveniles, it can also develop in adults. (See Epidemiology.)
Unlike people with type 2 DM , those with type 1 DM usually are not obese and usually present initially with diabetic ketoacidosis (DKA). The distinguishing characteristic of a patient with type 1 DM is that if his or her insulin is withdrawn, ketosis and eventually ketoacidosis develop. Therefore, these patients are dependent on exogenous insulin. (See Presentation.)
Treatment of type 1 DM requires lifelong insulin therapy. A multidisciplinary approach by the physician, nurse, and dietitian, with regular specialist consultation, is needed to control glycemia, as well as to limit the development of its devastating complications and manage such complications when they do occur. (See Treatmentand Medication.)
Despite the differences between type 1 and type 2 DM, the costs of the 2 conditions are often combined. In a study that focused on type 1 alone, Tao et al estimated that in the United States, type 1 DM is responsible for $14.4 billion in medical costs and lost income each year. [ 8 ]
Type 1 DM is the culmination of lymphocytic infiltration and destruction of insulin-secreting beta cells of the islets of Langerhans in the pancreas. As beta-cell mass declines, insulin secretion decreases until the available insulin no longer is adequate to maintain normal blood glucose levels. After 80-90% of the beta cells are destroyed, hyperglycemia develops and diabetes may be diagnosed. Patients need exogenous insulin to reverse this catabolic condition, prevent ketosis, decrease hyperglucagonemia, and normalize lipid and protein metabolism.
Currently, autoimmunity is considered the major factor in the pathophysiology of type 1 DM. In a genetically susceptible individual, viral infection may stimulate the production of antibodies against a viral protein that trigger an autoimmune response against antigenically similar beta cell molecules.
Approximately 85% of type 1 DM patients have circulating islet cell antibodies, and the majority also have detectable anti-insulin antibodies before receiving insulin therapy. The most commonly found islet cell antibodies are those directed against glutamic acid decarboxylase (GAD), an enzyme found within pancreatic beta cells.
The prevalence of type 1 DM is increased in patients with other autoimmune diseases, such as Graves disease, Hashimoto thyroiditis, and Addison disease. Pilia et al found a higher prevalence of islet cell antibodies (IA2) and anti-GAD antibodies in patients with autoimmune thyroiditis. [ 9 ]
A study by Philippe et al used computed tomography (CT) scans, glucagon stimulation test results, and fecal elastase-1 measurements to confirm reduced pancreatic volume in individuals with DM. [ 10 ] This finding, which was equally present in both type 1 and type 2 DM, may also explain the associated exocrine dysfunction that occurs in DM.
Polymorphisms of the class II human leukocyte antigen (HLA) genes that encode DR and DQ are the major genetic determinants of type 1 DM. Approximately 95% of patients with type 1 DM have either HLA-DR3 or HLA-DR4. Heterozygotes for those haplotypes are at significantly greater risk for DM than homozygotes. HLA-DQs are also considered specific markers of type 1 DM susceptibility. In contrast, some haplotypes (eg, HLA-DR2) confer strong protection against type 1 DM. [ 11 ]
Sensory and autonomic neuropathy
Sensory and autonomic neuropathy in people with diabetes are caused by axonal degeneration and segmental demyelination. Many factors are involved, including the accumulation of sorbitol in peripheral sensory nerves from sustained hyperglycemia. Motor neuropathy and cranial mononeuropathy result from vascular disease in blood vessels supplying nerves.
Using nailfold video capillaroscopy, Barchetta et al detected a high prevalence of capillary changes in patients with diabetes, particularly those with retinal damage. This reflects a generalized microvessel involvement in both type 1 and type 2 DM. [ 12 ]
Microvascular disease causes multiple pathologic complications in people with diabetes. Hyaline arteriosclerosis, a characteristic pattern of wall thickening of small arterioles and capillaries, is widespread and is responsible for ischemic changes in the kidney, retina, brain, and peripheral nerves.
Atherosclerosis of the main renal arteries and their intrarenal branches causes chronic nephron ischemia. It is a significant component of multiple renal lesions in diabetes.
Vitamin D deficiency is an important independent predictor of development of coronary artery calcification in individuals with type 1 DM. [ 13 ] Joergensen et al determined that vitamin D deficiency in type 1 diabetes may predict all causes of mortality but not development of microvascular complications. [ 14 ]
Nephropathy
In the kidneys, the characteristic wall thickening of small arterioles and capillaries leads to diabetic nephropathy, which is characterized by proteinuria, glomerular hyalinization (Kimmelstiel-Wilson), and chronic renal failure. Exacerbated expression of cytokines such as tumor growth factor beta 1 is part of the pathophysiology of glomerulosclerosis, which begins early in the course of diabetic nephropathy.
Genetic factors influence the development of diabetic nephropathy. Single-nucleotide polymorphisms affecting the factors involved in its pathogenesis appear to influence the risk for diabetic nephropathy in different people with type 1 DM. [ 15 ]
Double diabetes
In areas where rates of type 2 DM and obesity are high, individuals with type 1 DM may share genetic and environmental factors that lead to their exhibiting type 2 features such as reduced insulin sensitivity. This condition is termed double diabetes.
In a study that included 207 patients with type 1 DM, Epstein et al used the estimated glucose disposal rate (eGDR) to assess insulin resistance and found that mean eGDR was significantly lower (and, thus, insulin resistance was higher) in black patients (5.66 mg/kg/min) than in either Hispanic patients (6.70 mg/kg/min) or white patients (7.20 mg/kg/min). In addition, low eGDR was associated with an increased risk of vascular complications of diabetes (eg, cardiovascular disease, diabetic retinopathy, or severe chronic kidney disease). [ 16 , 17 ]
Type 1A DM results from autoimmune destruction of the beta cells of the pancreas and involves both genetic predisposition and an environmental component.
Genetic factors
Although the genetic aspect of type 1 DM is complex, with multiple genes involved, there is a high sibling relative risk. [ 18 ] Whereas dizygotic twins have a 5-6% concordance rate for type 1 DM, [ 19 ] monozygotic twins will share the diagnosis more than 50% of the time by the age of 40 years. [ 20 ]
For the child of a parent with type 1 DM, the risk varies according to whether the mother or the father has diabetes. Children whose mother has type 1 DM have a 2-3% risk of developing the disease, whereas those whose father has the disease have a 5-6% risk. When both parents are diabetic, the risk rises to almost 30%. In addition, the risk for children of parents with type 1 DM is slightly higher if onset of the disease occurred before age 11 years and slightly lower if the onset occurred after the parent’s 11th birthday.
The genetic contribution to type 1 DM is also reflected in the significant variance in the frequency of the disease among different ethnic populations. Type 1 DM is most prevalent in European populations, with people from northern Europe more often affected than those from Mediterranean regions. [ 21 ] The disease is least prevalent in East Asians. [ 22 ]
Genome-wide association studies have identified several loci that are associated with type 1 DM, but few causal relations have been established. The genomic region most strongly associated with other autoimmune diseases, the major histocompatibility complex (MHC), is the location of several susceptibility loci for type 1 DM—in particular, class II HLA DR and DQ haplotypes. [ 23 , 24 , 25 ]
A hierarchy of DR-DQ haplotypes associated with increased risk for type 1 DM has been established. The most susceptible haplotypes are as follows [ 26 ] :
DRB1*0301 - DQA1*0501 - DQB1*0201 (odds ratio [OR] 3.64)
DRB1*0405 - DQA1*0301 - DQB1*0302 (OR 11.37)
DRB1*0401 - DQA1*0301 - DQB*0302 (OR 8.39)
DRB1*0402 - DQA1*0301 - DQB1*0302 (OR 3.63)
DRB1*0404 - DQA1*0301 - DQB1*0302 (OR 1.59)
DRB1*0801 - DQB1*0401 - DQB1*0402 (OR 1.25)
Other haplotypes appear to offer protection against type 1 DM. These include the following [ 26 ] :
DRB1*1501 - DQA1*0102 - DQB1*0602 (OR 0.03)
DRB1*1401 - DQA1*0101 - DQB1*0503 (OR 0.02)
DRB1*0701 - DQA1*0201 - DQB1*0303 (OR 0.02)
From 90% to 95% of young children with type 1 DM carry HLA-DR3 DQB1*0201, HLA-DR4 DQB1*0302, or both. Carriage of both haplotypes (ie, DR3/4 heterozygotes) confers the highest susceptibility.
These high-risk haplotypes are found primarily in people of European descent; other ethnic groups are less well studied. In African Americans, the DRB1*07:01 - DQA1*03:01 -DQB1*02:01g haplotype is associated with increased risk (OR 3.96), whereas the DRB1*07:01-DQA1*02:01 - DQB1*02:01g haplotype appears to be protective (OR 0.34). [ 27 ]
The insulin gene ( INS ), which encodes for the pre-proinsulin peptide, is adjacent to a variable number of tandem repeats (VNTR) polymorphism at chromosome 11p15.5. [ 28 ] Different VNTR alleles may promote either resistance or susceptibility to type 1 DM through their effect on INS transcription in the thymus; for example, protective VNTRs are associated with higher INS expression, which may promote deletion of insulin-specific T cells. [ 29 ]
Other genes that have been reported to be involved in the mechanism of type 1 DM include CTLA4 (important in T-cell activation), PTPN22 (produces LYP, a negative regulator of T-cell kinase signaling), and IL2RA (encodes for CD25 which is involved with regulating T-cell function). UBASH3A (also known as STS2 ), may be involved in the increased risk not only of type 1 DM but also of other autoimmune disease and Down syndrome; it is located on locus chromosome 21q22.3. [ 30 ]
In addition, genome-wide association studies have implicated numerous other genes, including the following [ 31 ] :
Environmental factors
Extragenetic factors also may contribute. Potential triggers for immunologically mediated destruction of the beta cells include viruses (eg, enterovirus, [ 32 ] mumps, rubella, and coxsackievirus B4), toxic chemicals, exposure to cow’s milk in infancy, [ 33 ] and cytotoxins.
Combinations of factors may be involved. Lempainen et al found that signs of an enterovirus infection by 12 months of age were associated with the appearance of type 1 DM–related autoimmunity among children who were exposed to cow's milk before 3 months of age. These results suggest an interaction between the 2 factors and provide a possible explanation for the contradictory findings obtained in studies that examined these factors in isolation. [ 34 ]
One meta-analysis found a weak but significant linear increase in the risk of childhood type 1 DM with increasing maternal age. [ 35 ] However, little evidence supports any substantial increase in childhood type 1 DM risk after pregnancy complicated by preeclampsia. [ 36 ]
A study by Simpson et al found that neither vitamin D intake nor 25-hydroxyvitamin D levels throughout childhood were associated with islet autoimmunity or progression to type 1 DM. [ 37 ] This study was based in Denver, Colorado, and has been following children at increased risk of diabetes since 1993.
Early upper respiratory infection may also be a risk factor for type 1 diabetes. In an analysis of data on 148 children considered genetically at risk for diabetes, upper respiratory infections in the first year of life were associated with an increased risk for type 1 diabetes . [ 38 , 39 ] All children in the study who developed islet autoimmunity had at least 2 upper respiratory infections in the first year of life and at least 1 infection within 6 months before islet autoantibody seroconversion.
Children with respiratory infections in the first 6 months of life had the greatest increased hazard ratio (HR) for islet autoantibody seroconversion (HR = 2.27), and the risk was also increased in those with respiratory infections at ages 6 to almost 12 months (HR = 1.32). [ 38 , 39 ] The rate of islet autoantibody seroconversion was highest among children with more than 5 respiratory infections in the first year of year of life. Respiratory infections in the second year of life were not related to increased risk. [ 38 , 39 ]
Evidence exists that coronavirus disease 2019 (COVID-19) may actually lead to the development of type 1 and type 2 diabetes. One theory is that diabetes arises when severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, binds “to angiotensin-converting enzyme 2 (ACE2) receptors in key metabolic organs and tissues, including pancreatic beta cells and kidneys.” The CoviDiab registry was established by an international group of diabetes researchers to gather data on COVID-19–related diabetes. [ 40 ]
A report by Xie and Al-Aly found that among study patients who had survived the first 30 days of COVID-19, the risk for diabetes at 1 year was increased by about 40%. More specifically, the hazard ratios (HRs) for diabetes at 1 year among patients who, during the acute infection, were not hospitalized, were hospitalized, or were admitted to intensive care were 1.25, 2.73, and 3.76, respectively. The investigators stated that diabetes "should be considered as a facet of the multifaceted long COVID syndrome." [ 41 , 42 ]
A study by Tang et al detected SARS-CoV-2 antigen in pancreatic beta cells, as taken from autopsy samples from individuals who had had COVID-19. The research indicated that insulin expression decreases in SARS-CoV-2–infected beta cells, with these cells possibly undergoing transdifferentiation. [ 43 ] A study by Wu et al also indicated that infected beta cells secrete less insulin, with the investigators finding evidence that SARS-CoV-2 can induce beta-cell apoptosis. [ 44 ]
A study from the US Centers for Disease Control and Prevention (CDC) indicates that SARS-CoV-2 infection increases the likelihood of diabetes developing in children under age 18 years, more than 30 days post infection. The investigators, using two US health claims databases, reported that pediatric patients with COVID-19 in the HealthVerity database were 31% percent more likely than other youth to receive a new diabetes diagnosis, while those in the IQVIA database were 166% more likely. The study could not specify the type or types of diabetes specifically related to COVID-19, with the report saying that the disease could be causing both type 1 and type 2 diabetes but through differing mechanisms. The researchers suggested, however, that COVID-19 may induce diabetes by directly attacking pancreatic cells that express ACE2 receptors, that it may give rise to diabetes “through stress hyperglycemia resulting from the cytokine storm and alterations in glucose metabolism caused by infection,” or that COVID-19 may cause diabetes via the conversion of prediabetes to diabetes. Whether the diabetes is transient or chronic was also unknown. [ 45 , 46 ]
A study by Kendall et al found that compared with pediatric subjects with a non–SARS-CoV-2 respiratory infection, the proportion of children who were diagnosed with new-onset type 1 DM within 6 months after a SARS-CoV-2 infection was 72% greater. According to the investigators, who looked at patients aged 18 years or younger, the rate of new-onset type 1 DM among the two groups was 0.025% and 0.043%, respectively, at 6 months. [ 47 ]
However, a study by Cromer et al looked at adult patients with newly diagnosed diabetes mellitus at the time of hospital admission for COVID-19, finding that a number of them subsequently regressed to a state of normoglycemia or prediabetes. The investigators reported that out of 64 survivors in the study with newly diagnosed diabetes (62 of whom had type 2 diabetes), 26 (40.6%) were known to undergo such regression (median 323-day follow-up). [ 48 ]
United States statistics
A 2011 report from the US Centers for Disease Control and Prevention (CDC) estimated that approximately 1 million Americans have type 1 DM. [ 49 ] The CDC estimated that each year from 2002 to 2005, type 1 DM was newly diagnosed in 15,600 young people. Among children younger than 10 years, the annual rate of new cases was 19.7 per 100,000 population; among those 10 years or older, the rate was 18.6 per 100,000 population. [ 49 ]
Type 1 DM is the most common metabolic disease of childhood. About 1 in every 400-600 children and adolescents has type 1 DM. In adults, type 1 DM constitutes approximately 5% of all diagnosed cases of diabetes. [ 49 ]
A study by Mayer-Davis et al indicated that between 2002 and 2012, the incidence of type 1 and type 2 DM saw a significant rise among youths in the United States. According to the report, after the figures were adjusted for age, sex, and race or ethnic group, the incidence of type 1 (in patients aged 0-19 years) and type 2 DM (in patients aged 10-19 years) during this period underwent a relative annual increase of 1.8% and 4.8%, respectively. The greatest increases occurred among minority youths. [ 50 ]
International statistics
Internationally, rates of type 1 DM are increasing. In Europe, the Middle East, and Australia, rates of type 1 DM are increasing by 2-5% per year. [ 51 ] The prevalence of type 1 DM is highest in Scandinavia (ie, approximately 20% of the total number of people with DM) and lowest in China and Japan (ie, fewer than 1% of all people with diabetes). Some of these differences may relate to definitional issues and the completeness of reporting.
The 10th edition of the International Diabetes Federation Diabetes Atlas, published in December 2021, reported that worldwide, 1 in 10 adults has diabetes. The data predicted that there would be a global increase in the number of adults with diabetes from 537 million in 2021 to 786 million by 2045, a 46% rise. Although increases are expected throughout the world, Africa, the Middle East, and Southeast Asia are predicted to have the greatest expansion. [ 52 ]
Age-related demographics
Previously referred to as juvenile-onset diabetes, type 1 DM is typically diagnosed in childhood, adolescence, or early adulthood. Although the onset of type 1 DM often occurs early in life, 50% of patients with new-onset type 1 DM are older than 20 years of age.
Type 1 DM usually starts in children aged 4 years or older, appearing fairly abruptly, with the peak incidence of onset at age 11-13 years (ie, in early adolescence and puberty). There is also a relatively high incidence in people in their late 30s and early 40s, in whom the disease tends to present less aggressively (ie, with early hyperglycemia without ketoacidosis and gradual onset of ketosis). This slower-onset adult form of type 1 DM is referred to as latent autoimmune diabetes of the adult (LADA). [ 49 ]
A study by Thomas et al, using data from the UK Biobank, determined that in 42% of type 1 DM cases reviewed, disease onset occurred in patients aged 31 to 60 years. The report also found that because type 2 DM is far more common than type 1 in individuals in the 31- to 60-year age group, with type 1 DM making up only 4% of all diabetes cases in this population, identification of type 1 DM is difficult in patients over age 30 years. The presence of type 1 DM was identified in the study using a genetic risk score that employed 29 common genetic variants. [ 53 , 54 ]
The risk of development of antibodies (anti-islet) in relatives of patients with type 1 DM decreases with increasing age. This finding supports annual screening for antibodies in relatives younger than 10 years and 1 additional screening during adolescence. [ 4 ]
Sex- and race-related demographics
Type 1 DM is more common in males than in females. In populations of European origin, the male-to-female ratio is greater than 1.5:1.
Type 1 DM is most common among non-Hispanic whites, followed by African Americans and Hispanic Americans. It is comparatively uncommon among Asians.
Type 1 DM is associated with a high morbidity and premature mortality. More than 60% of patients with type 1 DM do not develop serious complications over the long term, but many of the rest experience blindness, end-stage renal disease (ESRD), and, in some cases, early death. The risk of ESRD and proliferative retinopathy is twice as high in men as in women when the onset of diabetes occurred before age 15 years. [ 55 ]
Patients with type 1 DM who survive the period 10-20 years after disease onset without fulminant complications have a high probability of maintaining reasonably good health. Other factors affecting long-term outcomes are the patient’s education, awareness, motivation, and intelligence level. The 2012 American Diabetes Association (ADA) standard of care emphasizes the importance of long-term, coordinated care management for improved outcomes and suggests structural changes to existing systems of long-term care delivery. [ 5 ]
The morbidity and mortality associated with diabetes are related to the short- and long-term complications. Such complications include the following:
Hypoglycemia from management errors
Increased risk of infections
Microvascular complications (eg, retinopathy and nephropathy)
Neuropathic complications
Macrovascular disease
These complications result in increased risk for ischemic heart disease, cerebral vascular disease, peripheral vascular disease with gangrene of lower limbs, chronic renal disease, reduced visual acuity and blindness, and autonomic and peripheral neuropathy. Diabetes is the major cause of blindness in adults aged 20-74 years, as well as the leading cause of nontraumatic lower-extremity amputation and ESRD.
In both diabetic and non-diabetic patients, coronary vasodilator dysfunction is a strong independent predictor of cardiac mortality. In diabetic patients without coronary artery disease, those with impaired coronary flow reserve have event rates similar to those with prior coronary artery disease, while patients with preserved coronary flow reserve have event rates similar to non-diabetic patients. [ 56 ]
A study by Bode et al indicated that among patients with coronavirus disease 2019 (COVID-19), the US in-hospital death rate for individuals living with diabetes, patients with an HbA 1c of 6.5% or higher, and those with hyperglycemia throughout their stay is 29%, a figure over four times greater than that for patients without diabetes or hyperglycemia. Moreover, the in-hospital death rate for patients with no evidence of preadmission diabetes who develop hyperglycemia while admitted was found to be seven times higher (42%). [ 57 , 58 ]
A whole-population study from the United Kingdom (UK) reported that the risk of in-hospital death for patients with COVID-19 was 2.0 times greater for those with type 2 diabetes and 3.5 times higher for individuals with type 1 diabetes. However, patients under age 40 years with either type of diabetes were at extremely low risk for death. [ 59 , 60 ]
A French study, by Wargny et al, indicated that among patients with diabetes who are hospitalized with COVID-19, approximately 20% will die within 28 days. Individuals particularly at risk for mortality over this 4-week period include patients of advanced age, as well as those with a history of microvascular complications (especially those who have had kidney or eye damage), who have dyspnea on admission or inflammatory markers (increased white blood cell [WBC] count, raised C-reactive protein, elevated aspartate transaminase), or who have undergone routine insulin and statin treatment. It should be kept in mind, however, that the data was gathered between March 10 and April 10, 2020, with a statement from Diabetes UK explaining that in people with diabetes, COVID-19–associated mortality has decreased over time as treatment has improved. [ 61 , 62 ]
Another study, by Barrera et al, looking at 65 observational reports (15,794 participants), found that among COVID-19 patients with diabetes, the unadjusted relative risk for admission to an intensive care unit (ICU) was 1.96, and for mortality, 2.78. [ 63 , 64 ]
Another study from the United Kingdom found that risk factors for mortality in COVID-19 patients with type 1 or type 2 diabetes include male sex, older age, renal impairment, non-White ethnicity, socioeconomic deprivation, and previous stroke and heart failure. Moreover, patients with type 1 or type 2 diabetes had a significantly greater mortality risk with an HbA 1c level of 86 mmol/mol or above, compared with persons with an HbA 1c level of 48-53 mmol/mol. In addition, an HbA 1c of 59 mmol/mol or higher in patients with type 2 diabetes increased the risk as well. The study also found that in both types of diabetes, body mass index (BMI) had a U-shaped relationship with death, the mortality risk being increased in lower BMI and higher BMI but being reduced between these (25.0-29.9 kg/m 2 ). [ 65 , 60 ]
A literature review by Schlesinger et al strengthened the association between severe diabetes and COVID-19–related mortality, finding that among study patients with diabetes, the likelihood of death from COVID-19 was 75% greater in chronic insulin users. The study also indicated that the chance of death from COVID-19 is 50% less in individuals undergoing metformin therapy than in other patients with diabetes. The investigators suggested that the medications themselves did not impact survival but were indicators of the severity of diabetes in each group, with the prognosis being poorer among those with more severe diabetes. [ 66 , 67 ]
However, a Belgian study, by Vangoitsenhoven et al, indicated that in most people, the presence of type 1 diabetes mellitus is not associated with a greater risk of hospitalization for COVID-19. The investigators found that during the first 3 months of the pandemic in Belgium, the COVID-19 hospitalization rate was similar between individuals with type 1 diabetes and those without (0.21% vs 0.17%, respectively). Among the patients with type 1 diabetes, older persons had a greater tendency toward COVID-19–related hospitalization, although glucose control, comorbidity profile, and angiotensin-converting enzyme (ACE) inhibitor/angiotensin II receptor blocker (ARB) therapy did not significantly differ between the hospitalized and non-hospitalized groups. This and other research suggest that in persons with type 1 diabetes, an increased risk of death from COVID-19 is found primarily in particularly vulnerable individuals instead of in such patients overall. [ 68 , 69 ]
A retrospective, multicenter study by Carrasco-Sánchez et al indicated that among noncritical patients with COVID-19, the presence of hyperglycemia on hospital admission independently predicts progression to critical status, as well as death, whether or not the patient has diabetes. The in-hospital mortality rate in persons with a blood glucose level of higher than 180 mg/dL was 41.1%, compared with 15.7% for those with a level below 140 mg/dL. Moreover, the need for ventilation and intensive care unit admission were also greater in the presence of hyperglycemia. The report involved over 11,000 patients with confirmed COVID-19, only about 19% of whom had diabetes. [ 70 , 71 ]
In contrast to the above study, a report by Klonoff et al on over 1500 US patients with COVID-19 found no association between hyperglycemia on hospital admission and mortality, in non-ICU patients. However, the in-hospital mortality rate was significantly greater in such patients if they had a blood glucose level above 13.88 mmol/L on the second or third hospital day, compared with those with a level below 7.77 mmol/L. Findings for patients admitted directly to the ICU differed from these, with the investigators determining that mortality was associated with the presence of hyperglycemia on admission but was not significantly linked with a high glucose level on the second hospital day. [ 72 , 73 ]
Type 1 diabetic patients also have a high prevalence of small-fiber neuropathy. [ 74 , 75 ] In a prospective study of 27 patients who had type 1 diabetes with a mean disease duration of 40 years, almost 60% of the subjects showed signs or symptoms of neuropathy, including sensory neuropathy symptoms (9 patients), pain (3 patients), and carpal-tunnel symptoms (5 patients). [ 74 , 75 ] Of the 27 patients, 22 were diagnosed with small-fiber dysfunction by means of quantitative sensory testing.
Abnormal results on intraepidermal nerve-fiber density measurement (IENFD) were seen in 19 patients. [ 75 ] IENFD was negatively correlated with HbA 1c , but this relation was no longer significant after adjustment for age, body mass index, and height. N-ε-(carboxymethyl) lysine (CML), which is linked to painful diabetic neuropathy, remained independently associated with IENFD even after adjustment for these variables. Large-fiber neuropathy was also common, being found in 16 patients.
Although ESRD is one of the most severe complications of type 1 DM, its incidence is relatively low: 2.2% at 20 years after diagnosis and 7.8% at 30 years after diagnosis. [ 76 ] A greater risk is that mild diabetic nephropathy in type 1 diabetic persons appears to be associated with an increased likelihood of cardiovascular disease. [ 77 ] Moreover, the long-term risk of an impaired glomerular filtration rate (GFR) is lower in persons treated with intense insulin therapy early in the course of disease than in those given conventional therapy. [ 78 ]
Although mortality from early-onset type 1 DM (onset age, 0-14 y) has declined, the same may not be true for late-onset type 1 DM (onset age, 15-29 y). One study suggest that women tend to fare worse in both cohorts and that alcohol and drug use account for more than one third of deaths. [ 79 ]
Control of blood glucose, hemoglobin A 1c (HbA 1c ), lipids, blood pressure, and weight significantly affects prognosis. Excess weight gain with intensified diabetes treatment is associated with hypertension, insulin resistance, dyslipidemia and extensive atherosclerotic cardiovascular disease. [ 80 ]
Patients with diabetes face a lifelong challenge to achieve and maintain blood glucose levels as close to the normal range as possible. With appropriate glycemic control, the risk of both microvascular and neuropathic complications is decreased markedly. In addition, aggressive treatment of hypertension and hyperlipidemia decreases the risk of macrovascular complications.
A study by Zheng et al indicated that HbA 1c levels in persons with diabetes are longitudinally associated with long-term cognitive decline, as found using a mean 4.9 cognitive assessments of diabetes patients over a mean 8.1-year follow-up period. The investigators saw a significant link between each 1 mmol/mol rise in HbA 1c and an increased rate of decline in z scores for global cognition, memory, and executive function. Patients in the study had a mean age of 65.6 years. The report cited a need for research into whether optimal glucose control in people with diabetes can affect their cognitive decline rate. [ 81 , 82 ]
A study indicated that children with type 1 DM who have an HbA 1c level of 9% or above are at greater risk of mortality, intubation, and sepsis due to COVID-19 than are children without type 1 DM. However, the report also found evidence that such risk is not greater in children with an HbA 1c level at or below 7%. The investigators found the COVID-19 mortality rates in children without type 1 DM, those with type 1 DM, and those with type 1 DM with an HbA 1c of 7% or lower to be 0.047%, 0.328%, and 0%, respectively. [ 83 ]
The benefits of glycemic control and control of comorbidities in type 1 DM must be weighed against the risk of hypoglycemia and the short-term costs of providing high-quality preventive care. However, studies have shown cost savings due to a reduction in acute diabetes-related complications within 1-3 years of starting effective preventive care.
Education is a vital aspect of diabetes management. Patients with new-onset type 1 DM require extensive education if they are to manage their disease safely and effectively and to minimize long-term complications. Such education is best coordinated by the patient’s long-term care providers.
At every encounter, the clinician should educate the patient—and, in the case of children, the parents—about the disease process, management, goals, and long-term complications. In particular, clinicians should do the following:
Make patients aware of the signs and symptoms of hypoglycemia and knowledgeable about ways to manage it
Help patients understand and acknowledge the course of diabetes (eg, by teaching patients that they have a chronic condition that requires lifestyle modification and that they are likely to have chronic complications if they do not take control of their disease)
Reassure patients about the prognosis in properly managed type 1 DM
ADA guidelines urge that attention be paid to older adolescent patients who may be leaving their home and their current health care providers. At the transition between pediatric and adult health care, older teens can become detached from the health care system, putting their medical care and their glycemic control at risk. [ 5 ] The guidelines identify the National Diabetes Education Program (NDEP) as a source of materials that can help smooth the transition to adult health care.
Education about an appropriate treatment plan and encouragement to follow the plan are especially important in patients with diabetes. Physicians must ensure that the care for each patient with diabetes includes all necessary laboratory tests, examinations (eg, foot and neurologic examinations), and referrals to specialists (eg, an ophthalmologist or podiatrist).
A dietitian should provide specific diet control education to the patient and family. A nurse should educate the patient about self–insulin injection and performing fingerstick tests for blood glucose level monitoring.
For patient education information, see the Diabetes Center , as well as Diabetes .
Aathira R, Jain V. Advances in management of type 1 diabetes mellitus. World J Diabetes . 2014 Oct 15. 5 (5):689-96. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Diagnosis and classification of diabetes mellitus. Diabetes Care . 2010 Jan. 33 Suppl 1:S62-9. [QxMD MEDLINE Link] . [Full Text] .
International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care . 2009 Jul. 32(7):1327-34. [QxMD MEDLINE Link] . [Full Text] .
Vehik K, Beam CA, Mahon JL, et al. Development of Autoantibodies in the TrialNet Natural History Study. Diabetes Care . 2011 Sep. 34(9):1897-1901. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care . 2011 Jan. 34 Suppl 1:S11-61. [QxMD MEDLINE Link] . [Full Text] .
Nainggolan L. Continuous Glucose Monitoring: Navigator Beats Rival Devices. Medscape Medical News. January 14, 2013. Available at https://www.medscape.com/viewarticle/777607 . Accessed: January 24, 2013.
Damiano ER, El-Khatib FH, Zheng H, Nathan DM, Russell SJ. A Comparative Effectiveness Analysis of Three Continuous Glucose Monitors. Diabetes Care . 2013 Jan 3. [QxMD MEDLINE Link] .
Tao B, Pietropaolo M, Atkinson M, Schatz D, Taylor D. Estimating the cost of type 1 diabetes in the U.S.: a propensity score matching method. PLoS One . 2010 Jul 9. 5(7):e11501. [QxMD MEDLINE Link] . [Full Text] .
Pilia S, Casini MR, Cambuli VM, et al. Prevalence of Type 1 diabetes autoantibodies (GAD and IA2) in Sardinian children and adolescents with autoimmune thyroiditis. Diabet Med . 2011 Aug. 28(8):896-9. [QxMD MEDLINE Link] .
Philippe MF, Benabadji S, Barbot-Trystram L, et al. Pancreatic volume and endocrine and exocrine functions in patients with diabetes. Pancreas . 2011 Apr. 40(3):359-63. [QxMD MEDLINE Link] .
Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Curr Diab Rep . 2011 Dec. 11(6):533-42. [QxMD MEDLINE Link] . [Full Text] .
Barchetta I, Riccieri V, Vasile M, et al. High prevalence of capillary abnormalities in patients with diabetes and association with retinopathy. Diabet Med . 2011 Sep. 28(9):1039-44. [QxMD MEDLINE Link] .
Young KA, Snell-Bergeon JK, Naik RG, Hokanson JE, Tarullo D, Gottlieb PA, et al. Vitamin D deficiency and coronary artery calcification in subjects with type 1 diabetes. Diabetes Care . 2011 Feb. 34(2):454-8. [QxMD MEDLINE Link] . [Full Text] .
Joergensen C, Hovind P, Schmedes A, Parving HH, Rossing P. Vitamin d levels, microvascular complications, and mortality in type 1 diabetes. Diabetes Care . 2011 May. 34(5):1081-5. [QxMD MEDLINE Link] .
Zhang D, Efendic S, Brismar K, Gu HF. Effects of MCF2L2, ADIPOQ and SOX2 genetic polymorphisms on the development of nephropathy in type 1 Diabetes Mellitus. BMC Med Genet . 2010 Jul 28. 11:116. [QxMD MEDLINE Link] . [Full Text] .
Busko M. Phenomenon of 'double diabetes' common among blacks. Medscape Medical News . April 25, 2013. [Full Text] .
Epstein EJ, Osman JL, Cohen HW, Rajpathak SN, Lewis O, Crandall JP. Use of the Estimated Glucose Disposal Rate (eGDR) as a Measure of Insulin Resistance in an Urban Multiethnic Population With Type 1 Diabetes. Diabetes Care . 2013 Apr 17. [QxMD MEDLINE Link] .
Davies JL, Kawaguchi Y, Bennett ST, Copeman JB, Cordell HJ, Pritchard LE, et al. A genome-wide search for human type 1 diabetes susceptibility genes. Nature . 1994 Sep 8. 371(6493):130-6. [QxMD MEDLINE Link] .
Steck AK, Barriga KJ, Emery LM, Fiallo-Scharer RV, Gottlieb PA, Rewers MJ. Secondary attack rate of type 1 diabetes in Colorado families. Diabetes Care . 2005 Feb. 28(2):296-300. [QxMD MEDLINE Link] .
Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med . 2008 Dec 25. 359(26):2849-50. [QxMD MEDLINE Link] .
Borchers AT, Uibo R, Gershwin ME. The geoepidemiology of type 1 diabetes. Autoimmun Rev . 2010 Mar. 9(5):A355-65. [QxMD MEDLINE Link] .
Diabetes Epidemiology Research International Group. Geographic patterns of childhood insulin-dependent diabetes mellitus. Diabetes Epidemiology Research International Group. Diabetes . 1988 Aug. 37(8):1113-9. [QxMD MEDLINE Link] .
Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes . 2008 Apr. 57(4):1084-92. [QxMD MEDLINE Link] .
Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature . 1987 Oct 15-21. 329(6140):599-604. [QxMD MEDLINE Link] .
Corper AL, Stratmann T, Apostolopoulos V, Scott CA, Garcia KC, Kang AS, et al. A structural framework for deciphering the link between I-Ag7 and autoimmune diabetes. Science . 2000 Apr 21. 288(5465):505-11. [QxMD MEDLINE Link] .
Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes . 2008 Apr. 57(4):1084-92. [QxMD MEDLINE Link] . [Full Text] .
Noble JA, Johnson J, Lane JA, Valdes AM. Race-specific type 1 diabetes risk of HLA-DR7 haplotypes. Tissue Antigens . 2011 Nov. 78(5):348-51. [QxMD MEDLINE Link] . [Full Text] .
Rotwein P, Yokoyama S, Didier DK, Chirgwin JM. Genetic analysis of the hypervariable region flanking the human insulin gene. Am J Hum Genet . 1986 Sep. 39(3):291-9. [QxMD MEDLINE Link] . [Full Text] .
Pugliese A, Zeller M, Fernandez A Jr, Zalcberg LJ, Bartlett RJ, Ricordi C, et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet . 1997 Mar. 15(3):293-7. [QxMD MEDLINE Link] .
Polychronakos C, Li Q. Understanding type 1 diabetes through genetics: advances and prospects. Nat Rev Genet . 2011 Oct 18. 12(11):781-92. [QxMD MEDLINE Link] .
Concannon P, Chen WM, Julier C, Morahan G, Akolkar B, Erlich HA, et al. Genome-wide scan for linkage to type 1 diabetes in 2,496 multiplex families from the Type 1 Diabetes Genetics Consortium. Diabetes . 2009 Apr. 58(4):1018-22. [QxMD MEDLINE Link] . [Full Text] .
Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ . 2011 Feb 3. 342:d35. [QxMD MEDLINE Link] . [Full Text] .
Paronen J, Knip M, Savilahti E, Virtanen SM, Ilonen J, Akerblom HK, et al. Effect of cow's milk exposure and maternal type 1 diabetes on cellular and humoral immunization to dietary insulin in infants at genetic risk for type 1 diabetes. Finnish Trial to Reduce IDDM in the Genetically at Risk Study Group. Diabetes . 2000 Oct. 49(10):1657-65. [QxMD MEDLINE Link] .
Lempainen J, Tauriainen S, Vaarala O, Mäkelä M, Honkanen H, Marttila J, et al. Interaction of enterovirus infection and cow's milk-based formula nutrition in type 1 diabetes-associated autoimmunity. Diabetes Metab Res Rev . 2012 Feb. 28(2):177-85. [QxMD MEDLINE Link] .
Cardwell CR, Stene LC, Joner G, Bulsara MK, Cinek O, Rosenbauer J, et al. Maternal age at birth and childhood type 1 diabetes: a pooled analysis of 30 observational studies. Diabetes . 2010 Feb. 59(2):486-94. [QxMD MEDLINE Link] . [Full Text] .
Henry EB, Patterson CC, Cardwell CR. A meta-analysis of the association between pre-eclampsia and childhood-onset Type 1 diabetes mellitus. Diabet Med . 2011 Aug. 28(8):900-5. [QxMD MEDLINE Link] .
Simpson M, Brady H, Yin X, et al. No association of vitamin D intake or 25-hydroxyvitamin D levels in childhood with risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia . 2011 Nov. 54(11):2779-88. [QxMD MEDLINE Link] .
Melville N. Early Upper-Respiratory Infections Linked to Type 1 Diabetes. Medscape Medical News. Available at https://www.medscape.com/viewarticle/807205 . Accessed: July 8, 2013.
Beyerlein A, Wehweck F, Ziegler AG, Pflueger M. Respiratory Infections in Early Life and the Development of Islet Autoimmunity in Children at Increased Type 1 Diabetes Risk: Evidence From the BABYDIET Study. JAMA Pediatr . 2013 Jul 1. [QxMD MEDLINE Link] .
Tucker ME. New Global Registry Investigates COVID-19 and New-Onset Diabetes. Medscape Medical News . 2020 Jun 13. [Full Text] .
Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol . 2022 Mar 21. [Full Text] .
Tucker ME. 'Profound Implications': COVID Ups Diabetes Risk 40% a Year Later. Medscape Medical News . 2022 Mar 23. [Full Text] .
Tang X, Uhl S, Zhang T, et al. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metab . 2021 May 19. [QxMD MEDLINE Link] . [Full Text] .
Wu CT, Lidsky PV, Xiao Y, et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab . 2021 May 18. [QxMD MEDLINE Link] . [Full Text] .
Barrett CE, Koyama AK, Alvarez P, et al. Risk for Newly Diagnosed Diabetes >30 Days After SARS-CoV-2 Infection Among Persons Aged MMWR Morb Mortal Wkly Rep</i>. 2022 Jan 7. 71: [Full Text] .
Tucker ME. COVID-19 Associated With Increased Diabetes Risk in Youth. Medscape Medical News . 2022 Jan 10. [Full Text] .
Kendall EK, Olaker VR, Kaelber DC, Xu R, Davis PB. Association of SARS-CoV-2 Infection With New-Onset Type 1 Diabetes Among Pediatric Patients From 2020 to 2021. JAMA Netw Open . 2022 Sep 1. 5 (9):e2233014. [QxMD MEDLINE Link] . [Full Text] .
Cromer SJ, Colling C, Schatoff D, et al. Newly diagnosed diabetes vs. pre-existing diabetes upon admission for COVID-19: Associated factors, short-term outcomes, and long-term glycemic phenotypes. J Diabetes Complications . 2022 Feb 4. 108145. [QxMD MEDLINE Link] . [Full Text] .
U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Available at https://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf . Accessed: January 28, 2011.
Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med . 2017 Apr 13. 376 (15):1419-29. [QxMD MEDLINE Link] .
Imkampe AK, Gulliford MC. Trends in Type 1 diabetes incidence in the UK in 0- to 14-year-olds and in 15- to 34-year-olds, 1991-2008. Diabet Med . 2011 Jul. 28(7):811-4. [QxMD MEDLINE Link] .
Tucker ME. IDF Atlas: 1 in 10 Adults Worldwide Now Has Diabetes. Medscape Medical News . 2021 Dec 7. [Full Text] .
Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol . 2017 Nov 30. [QxMD MEDLINE Link] . [Full Text] .
Tucker ME. Needle in a Haystack: Type 1 Diabetes Arises Equally in Adulthood. Medscape . 2017 Dec 4. [Full Text] .
Harjutsalo V, Maric C, Forsblom C, et al. Sex-related differences in the long-term risk of microvascular complications by age at onset of type 1 diabetes. Diabetologia . 2011 Aug. 54(8):1992-1999. [QxMD MEDLINE Link] .
Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, et al. Association Between Coronary Vascular Dysfunction and Cardiac Mortality in Patients with and without Diabetes Mellitus. Circulation . 2012 Aug 23. [QxMD MEDLINE Link] .
Bode B, Garrett V, Messler J, et al. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. J Diabetes Sci Technol . 2020. [Full Text] .
Tucker ME. Pay Attention to In-Hospital Glucose to Save Lives in COVID-19. Medscape Medical News . 2020 Apr 20. [Full Text] .
Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol . 2020 Aug 13. [QxMD MEDLINE Link] . [Full Text] .
Tucker ME. Newly Published Articles Inform on COVID-19 Risk by Diabetes Type. Medscape Medical News . 2020 Aug 17. [Full Text] .
Wargny M, Potier L, Gourdy P, et al. Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia . 2021 Feb 17. [QxMD MEDLINE Link] . [Full Text] .
Davenport L. 1 in 5 Diabetes Patients Hospitalized With COVID-19 Die in 28 Days. Medscape Medical News . 2021 Feb 18. [Full Text] .
Zoler ML. Cleaner data confirm severe COVID-19 link to diabetes, hypertension. MDedge Cardiology News . 2020 Jul 27. [Full Text] .
Barrera FJ, Shekhar S, Wurth R, et al. Prevalence of Diabetes and Hypertension and their Associated Risks for Poor Outcomes in Covid-19 Patients. J Endocr Soc . 2020 Jul 21. [Full Text] .
Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol . 2020 Aug 13. [QxMD MEDLINE Link] . [Full Text] .
Schlesinger S, Neuenschwander M, Lang A, et al. Risk phenotypes of diabetes and association with COVID-19 severity and death: a living systematic review and meta-analysis. Diabetologia . 2021 Apr 28. [QxMD MEDLINE Link] . [Full Text] .
Busko M. Older, Sicker Diabetes Patients Have Worse COVID-19 Prognosis. Medscape Medical News . 2021 Apr 28. [Full Text] .
Vangoitsenhoven R, Martens PJ, van Nes F, et al. No Evidence of Increased Hospitalization Rate for COVID-19 in Community-Dwelling Patients With Type 1 Diabetes. Diabetes Care . 2020 Oct. 43 (10):e118-9. [QxMD MEDLINE Link] . [Full Text] .
Tucker ME. Emerging Data on Type 1 Diabetes and COVID-19 Reassuring. Medscape Medical News . 2020 Oct 9. [Full Text] .
Carrasco-Sanchez FJ, Lopez-Carmona MD, Martinez-Marcos FJ, et al. Admission hyperglycaemia as a predictor of mortality in patients hospitalized with COVID-19 regardless of diabetes status: data from the Spanish SEMI-COVID-19 Registry. Ann Med . 2021 Dec. 53 (1):103-16. [QxMD MEDLINE Link] .
Tucker ME. Blood Glucose on Admission Predicts COVID-19 Severity in All. Medscape Medical News . 2020 Nov 30. [Full Text] .
Klonoff DC, Messler JC, Umpierrez GE, et al. Association Between Achieving Inpatient Glycemic Control and Clinical Outcomes in Hospitalized Patients With COVID-19: A Multicenter, Retrospective Hospital-Based Analysis. Diabetes Care . 2020 Dec 15. [QxMD MEDLINE Link] . [Full Text] .
Harding A. Glycemia in Early COVID-19 Hospitalization Linked to Mortality. Reuters Health Information . 2020 Dec 21. [Full Text] .
Tucker ME. Small-fiber neuropathy common at 40 years of type 1 diabetes. Medscape Medical News . September 18, 2013. [Full Text] .
Sveen KA, Karimé B, Jørum E, Mellgren SI, Fagerland MW, Monnier VM, et al. Small- and Large-Fiber Neuropathy After 40 Years of Type 1 Diabetes: Associations with glycemic control and advanced protein glycation: The Oslo Study. Diabetes Care . 2013 Sep 11. [QxMD MEDLINE Link] .
Finne P, Reunanen A, Stenman S, Groop PH, Grönhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA . 2005 Oct 12. 294(14):1782-7. [QxMD MEDLINE Link] .
Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med . 2005 Dec 22. 353(25):2643-53. [QxMD MEDLINE Link] . [Full Text] .
DCCT/EDIC Research Group, de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med . 2011 Dec 22. 365(25):2366-76. [QxMD MEDLINE Link] . [Full Text] .
Harjutsalo V, Forsblom C, Groop PH. Time trends in mortality in patients with type 1 diabetes: nationwide population based cohort study. BMJ . 2011 Sep 8. 343:d5364. [QxMD MEDLINE Link] . [Full Text] .
Purnell JQ, Hokanson JE, Cleary PA, Nathan DM, Lachin JM, Zinman B, et al. The Effect of Excess Weight Gain with Intensive Diabetes Treatment on Cardiovascular Disease Risk Factors and Atherosclerosis in Type 1 Diabetes: Results from the Diabetes Control and Complications Trial / Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) Study. Circulation . 2012 Dec 4. [QxMD MEDLINE Link] .
Zheng F, Yan L, Yang Z, Zhong B, Xie W. HbA 1c , diabetes and cognitive decline: the English Longitudinal Study of Ageing. Diabetologia . 2018 Jan 25. [QxMD MEDLINE Link] . [Full Text] .
Melville NA. HbA1c Levels in Diabetes Linked to Cognitive Decline. Medscape Medical News . 2018 Jan 30. [Full Text] .
Tucker ME. Type 1 Diabetes Raises COVID-19 Risk in Kids if A1c Is High. Medscape Medical News . 2021 Mar 22. [Full Text] .
Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med . 1999 Dec 16. 341(25):1906-12. [QxMD MEDLINE Link] .
Garg S, Kim L, Whitaker M, et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR . 2020 Apr 8. [Full Text] .
Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus Disease 2019 Case Surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep . 2020 Jun 15. [Full Text] .
Franki R. Comorbidities Increase COVID-19 Deaths by Factor of 12. Medscape Medical News . 2020 Jun 17. [Full Text] .
Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 Diabetes and COVID-19: Preliminary Findings From a Multicenter Surveillance Study in the U.S. Diabetes Care . 2020 Jun 5. [QxMD MEDLINE Link] . [Full Text] .
Tucker ME. Half of Those With Type 1 Diabetes and COVID-19 Manage at Home. Medscape Medical News . 2020 Jun 11. [Full Text] .
Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19): People of Any Age with Underlying Medical Conditions. CDC. Available at https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html . Updated June 25, 2020; Accessed: June 27, 2020.
Wong VH, Bui BV, Vingrys AJ. Clinical and experimental links between diabetes and glaucoma. Clin Exp Optom . 2011 Jan. 94(1):4-23. [QxMD MEDLINE Link] .
Gillespie KM. Type 1 diabetes: pathogenesis and prevention. CMAJ . 2006 Jul 18. 175(2):165-70. [QxMD MEDLINE Link] . [Full Text] .
Harris SS. Vitamin D in type 1 diabetes prevention. J Nutr . 2005 Feb. 135(2):323-5. [QxMD MEDLINE Link] .
Hammes HP, Kerner W, Hofer S, et al. Diabetic retinopathy in type 1 diabetes-a contemporary analysis of 8,784 patients. Diabetologia . 2011 Aug. 54(8):1977-1984. [QxMD MEDLINE Link] .
Julius MC, Schatz DA, Silverstein JH. The prevention of type I diabetes mellitus. Pediatr Ann . 1999 Sep. 28(9):585-8. [QxMD MEDLINE Link] .
Vinik AI, Mehrabyan A. Diabetic neuropathies. Med Clin North Am . 2004 Jul. 88(4):947-99, xi. [QxMD MEDLINE Link] .
Chou KL, Galetta SL, Liu GT, Volpe NJ, Bennett JL, Asbury AK, et al. Acute ocular motor mononeuropathies: prospective study of the roles of neuroimaging and clinical assessment. J Neurol Sci . 2004 Apr 15. 219(1-2):35-9. [QxMD MEDLINE Link] .
Gerstein HC, Islam S, Anand S, et al. Dysglycaemia and the risk of acute myocardial infarction in multiple ethnic groups: an analysis of 15,780 patients from the INTERHEART study. Diabetologia . 2010 Dec. 53(12):2509-17. [QxMD MEDLINE Link] .
Falcone C, Nespoli L, Geroldi D, Gazzaruso C, Buzzi MP, Auguadro C, et al. Silent myocardial ischemia in diabetic and nondiabetic patients with coronary artery disease. Int J Cardiol . 2003 Aug. 90(2-3):219-27. [QxMD MEDLINE Link] .
[Guideline] Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloomgarden ZT, Bray GA, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract . 2011 Mar-Apr. 17 Suppl 2:1-53. [QxMD MEDLINE Link] .
[Guideline] Hattersley A, Bruining J, Shield J, Njolstad P, Donaghue KC. The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes . 2009 Sep. 10 Suppl 12:33-42. [QxMD MEDLINE Link] .
Hemoglobin A1c and Mean Glucose in Patients With Type 1 Diabetes: Analysis of data from the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Care . 2011 Mar. 34(3):540-4. [QxMD MEDLINE Link] . [Full Text] .
Mianowska B, Fendler W, Szadkowska A, Baranowska A, Grzelak-Agaciak E, Sadon J, et al. HbA(1c) levels in schoolchildren with type 1 diabetes are seasonally variable and dependent on weather conditions. Diabetologia . 2011 Apr. 54(4):749-56. [QxMD MEDLINE Link] . [Full Text] .
Suzuki S, Koga M, Amamiya S, et al. Glycated albumin but not HbA(1c) reflects glycaemic control in patients with neonatal diabetes mellitus. Diabetologia . 2011 Sep. 54(9):2247-53. [QxMD MEDLINE Link] .
Brooks M. Hemoglobin A1c misses many cases of diabetes. Medscape . 2019 Mar 28. [Full Text] .
McDonald TJ, Colclough K, Brown R, et al. Islet autoantibodies can discriminate maturity-onset diabetes of the young (MODY) from Type 1 diabetes. Diabet Med . 2011 Sep. 28(9):1028-33. [QxMD MEDLINE Link] .
[Guideline] Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). ADA. Available at https://professional.diabetes.org/sites/professional.diabetes.org/files/media/draft_easdada_t1dm_adults_consensusreport_0.pdf . 2021; Accessed: July 19, 2021.
[Guideline] Tucker ME. ADA/EASD draft guidance aims to bring adults with type 1 diabetes out of shadows. MDedge . 2021 Jul 14. [Full Text] .
[Guideline] Tucker ME. 'Push the Bar Higher': New Statement on Type 1 Diabetes in Adults. Medscape Medical News . 2021 Oct 4. [Full Text] .
[Guideline] Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia . 2021 Sep 30. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 Diabetes Through the Life Span: A Position Statement of the American Diabetes Association. Diabetes Care . 2014 Jun 16. [QxMD MEDLINE Link] .
Tucker M. First-Ever ADA Guidance Specifically for Type 1 Diabetes. Medscape Medical news. Available at https://www.medscape.com/viewarticle/826854 . Accessed: June 20, 2014.
Kielgast U, Holst JJ, Madsbad S. Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual ß-cell function. Diabetes . 2011 May. 60(5):1599-607. [QxMD MEDLINE Link] .
Lantidra (donislecel) [package insert]. Chicago, IL: CellTrans Inc. June 2023. Available at [Full Text] .
US Food and Drug Administration. FDA Approves First Cellular Therapy to Treat Patients with Type 1 Diabetes. FDA. Available at https://www.fda.gov/news-events/press-announcements/fda-approves-first-cellular-therapy-treat-patients-type-1-diabetes . June 28, 2023; Accessed: July 3, 2023.
[Guideline] American Diabetes Association. Standards of Medical Care in Diabetes-2018 Abridged for Primary Care Providers. Clin Diabetes . 2018 Jan. 36 (1):14-37. [QxMD MEDLINE Link] . [Full Text] .
The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med . 1993 Sep 30. 329(14):977-86. [QxMD MEDLINE Link] .
Genuth S. Insights from the diabetes control and complications trial/epidemiology of diabetes interventions and complications study on the use of intensive glycemic treatment to reduce the risk of complications of type 1 diabetes. Endocr Pract . 2006 Jan-Feb. 12 Suppl 1:34-41. [QxMD MEDLINE Link] .
Lind M, Bounias I, Olsson M, et al. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet . 2011 Jul 9. 378(9786):140-6. [QxMD MEDLINE Link] .
Tomlin A, Dovey S, Tilyard M. Health outcomes for diabetes patients returning for three annual general practice checks. N Z Med J . 2007 Apr 13. 120(1252):U2493. [QxMD MEDLINE Link] .
Jacobson AM, Ryan CM, Cleary PA, Waberski BH, Weinger K, Musen G, et al. Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow-up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia . 2011 Feb. 54(2):245-55. [QxMD MEDLINE Link] .
Asvold BO, Sand T, Hestad K, Bjørgaas MR. Cognitive function in type 1 diabetic adults with early exposure to severe hypoglycemia: a 16-year follow-up study. Diabetes Care . 2010 Sep. 33(9):1945-7. [QxMD MEDLINE Link] . [Full Text] .
Sherwood JS, Russell SJ, Putman MS. New and Emerging Technologies in Type 1 Diabetes. Endocrinol Metab Clin North Am . 2020 Dec. 49 (4):667-78. [QxMD MEDLINE Link] . [Full Text] .
Garg SK, Voelmle MK, Beatson CR, et al. Use of Continuous Glucose Monitoring in Subjects With Type 1 Diabetes on Multiple Daily Injections Versus Continuous Subcutaneous Insulin Infusion Therapy: A prospective 6-month study. Diabetes Care . 2011 Mar. 34(3):574-9. [QxMD MEDLINE Link] . [Full Text] .
Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care . 2011 Apr. 34(4):795-800. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Klonoff DC, Buckingham B, Christiansen JS, et al. Continuous glucose monitoring: an endocrine society clinical practice guideline. J Clin Endocrinol Metab . 2011 Oct. 96(10):2968-79. [QxMD MEDLINE Link] .
[Guideline] Tucker ME. ADA 2018 Standards Address Diabetes Drugs With CV Benefit. Medscape . 2017 Dec 8. [Full Text] .
Medtronic, Inc. Medtronic gains approval of first artificial pancreas device system with threshold suspend automation [press release]. September 27, 2013. Available at https://newsroom.medtronic.com/phoenix.zhtml?c=251324&p=irol-newsArticle&ID=1859361&highlight . Accessed: October 7, 2013.
Tucker ME. FDA OKs insulin pump with low-glucose suspend feature. Medscape Medical News . September 27, 2013. [Full Text] .
Tucker ME. FDA Okays Use of Dexcom G5 CGM for Insulin Dosing. Medscape Medical News . 2016 Dec 20. [Full Text] .
Tucker ME. FDA Approves New 'Smart' Continuous Glucose Monitor for Diabetes. Medscape Medical News . 2018 Mar 13. [Full Text] .
Tucker ME. FDA Approves First Implantable Continuous Glucose Monitor. Medscape Medical News . 2018 Jun 21. [Full Text] .
FDA approves first continuous glucose monitoring system with a fully implantable glucose sensor and compatible mobile app for adults with diabetes. FDA. Available at https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm611454.htm . Jun 21, 2018; Accessed: Jun 25, 2018.
Nelson R, Tucker ME. FDA Approves FreeStyle Libre System for Patients. Medscape Medical News . 2017 Sep 27. [Full Text] .
What is the pancreas? What is an artificial pancreas device system?. US Food and Drug Administration. Available at https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/HomeHealthandConsumer/ConsumerProducts/ArtificialPancreas/ucm259548.htm . May 16, 2016; Accessed: July 6, 2016.
Tucker ME. Coming Soon: 'Artificial Pancreas' Options for Diabetes. Medscape Medical News . June 20, 2016. [Full Text] .
Boggs W. Round-the-Clock Closed-Loop Glucose Control Leads to Better Outcomes. Medscape . May 13, 2016. [Full Text] .
Renard E, Farret A, Kropff J, et al. Day-and-Night Closed-Loop Glucose Control in Patients With Type 1 Diabetes Under Free-Living Conditions: Results of a Single-Arm 1-Month Experience Compared With a Previously Reported Feasibility Study of Evening and Night at Home. Diabetes Care . 2016 Jul. 39 (7):1151-60. [QxMD MEDLINE Link] .
US Food and Drug Administration. FDA approves first automated insulin delivery device for type 1 diabetes. FDA. Available at https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm . Sep 28, 2016; Accessed: Sep 30, 2016.
Busko M. FDA Approves Artificial Pancreas for Children With Type 1 Diabetes. Medscape Medical News . 2018 Jun 22. [Full Text] .
US Food and Drug Administration. FDA Approves First-of-its-Kind Automated Insulin Delivery and Monitoring System for Use in Young Pediatric Patients. Available at https://www.fda.gov/news-events/press-announcements/fda-approves-first-its-kind-automated-insulin-delivery-and-monitoring-system-use-young-pediatric . August 31, 2020; Accessed: December 1, 2020.
Tucker M. FDA Approves Inhaled Insulin Afrezza for Diabetes. Medscape Medical News. Available at https://www.medscape.com/viewarticle/827539. . Accessed: July 14, 2014.
Afrezza (insulin inhaled) prescribing information [package insert]. Valencia CA, United States: MannKind Corporation. June, 2014. Available at [Full Text] .
US Food and Drug Administration. Mixups between Insulin U-100 and U-500 (April 2008). FDA Patient Safety News. Available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/psn/transcript.cfm?show=79 . Accessed: January 28, 2012.
de la Pena A, Riddle M, Morrow LA, et al. Pharmacokinetics and pharmacodynamics of high-dose human regular u-500 insulin versus human regular u-100 insulin in healthy obese subjects. Diabetes Care . 2011 Dec. 34(12):2496-501. [QxMD MEDLINE Link] . [Full Text] .
Garg S, Ampudia-Blasco FJ, Pfohl M. Rapid-acting insulin analogues in Basal-bolus regimens in type 1 diabetes mellitus. Endocr Pract . 2010 May-Jun. 16(3):486-505. [QxMD MEDLINE Link] .
Fiasp Product Information [package insert]. 800 Scudders Mill Road, Plainsboro, NJ 08536: Novo Nordisk Inc. September 2017. Available at [Full Text] .
Nainggolan L. FDA Approves New Fast-Acting Insulin, Fiasp, for Diabetes in Adults. Medscape Medical News . 2017 Sep 29. [Full Text] .
Blair HA, Keating GM. Insulin Glargine 300 U/mL: A Review in Diabetes Mellitus. Drugs . 2016 Mar. 76 (3):363-74. [QxMD MEDLINE Link] .
Toujeo. US Food and Drug Administration. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206538s006lbl.pdf . Accessed: 2018 25 Apr.
Birkeland KI, Home PD, Wendisch U, Ratner RE, Johansen T, Endahl LA, et al. Insulin Degludec in Type 1 Diabetes: A randomized controlled trial of a new-generation ultra-long-acting insulin compared with insulin glargine. Diabetes Care . 2011 Mar. 34(3):661-5. [QxMD MEDLINE Link] . [Full Text] .
Davies MJ, Gross JL, Ono Y, Sasaki T, Bantwal G, Gall MA, et al. Efficacy and safety of insulin degludec given as part of basal-bolus treatment with mealtime insulin aspart in type 1 diabetes: a 26-week randomized, open-label, treat-to-target non-inferiority trial. Diabetes Obes Metab . 2014 Oct. 16 (10):922-30. [QxMD MEDLINE Link] . [Full Text] .
Zinman B, DeVries JH, Bode B, Russell-Jones D, Leiter LA, Moses A, et al. Efficacy and safety of insulin degludec three times a week versus insulin glargine once a day in insulin-naive patients with type 2 diabetes: results of two phase 3, 26 week, randomised, open-label, treat-to-target, non-inferiority trials. Lancet Diabetes Endocrinol . 2013 Oct. 1 (2):123-31. [QxMD MEDLINE Link] .
Nainggolan L. First Launch for Fiasp : 'Ultrafast' Mealtime Insulin Aspart. Medscape . 2017 29 Mar. [Full Text] .
Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab . 2007 Sep. 9(5):648-59. [QxMD MEDLINE Link] .
Suissa S, Azoulay L, Dell'aniello S, et al. Long-term effects of insulin glargine on the risk of breast cancer. Diabetologia . 2011 Sep. 54(9):2254-62. [QxMD MEDLINE Link] .
Johnson JA, Bowker SL, Richardson K, Marra CA. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia . 2011 Sep. 54(9):2263-71. [QxMD MEDLINE Link] .
Bao J, Gilbertson HR, Gray R, et al. Improving the Estimation of Mealtime Insulin Dose in Adults With Type 1 Diabetes: The Normal Insulin Demand for Dose Adjustment (NIDDA) study. Diabetes Care . 2011 Oct. 34(10):2146-51. [QxMD MEDLINE Link] . [Full Text] .
Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med . 2010 Jul 22. 363(4):311-20. [QxMD MEDLINE Link] .
Busko M. Insulin pump therapy bests injection therapy in large study. Medscape Medical News . August 19, 2013. [Full Text] .
Johnson SR, Cooper MN, Jones TW, Davis EA. Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case-control study. Diabetologia . 2013 Aug 21. [QxMD MEDLINE Link] . [Full Text] .
King BR, Goss PW, Paterson MA, Crock PA, Anderson DG. Changes in Altitude Cause Unintended Insulin Delivery From Insulin Pumps: Mechanisms and implications. Diabetes Care . 2011 Sep. 34(9):1932-3. [QxMD MEDLINE Link] . [Full Text] .
Grunberger G, Abelseth JM, Bailey TS, Bode BW, Handelsman Y, Hellman R. Consensus statement by the american association of clinical endocrinologists/american college of endocrinology insulin pump management task force. Endocr Pract . 2014 May 1. 20(5):463-89. [QxMD MEDLINE Link] .
Babiker A, Datta V. Lipoatrophy with insulin analogues in type I diabetes. Arch Dis Child . 2011 Jan. 96(1):101-2. [QxMD MEDLINE Link] .
Giménez M, Gilabert R, Monteagudo J, Alonso A, Casamitjana R, Paré C, et al. Repeated episodes of hypoglycemia as a potential aggravating factor for preclinical atherosclerosis in subjects with type 1 diabetes. Diabetes Care . 2011 Jan. 34(1):198-203. [QxMD MEDLINE Link] . [Full Text] .
Asvold BO, Sand T, Hestad KA, Bjorgaas MR. Quantitative EEG in type 1 diabetic adults with childhood exposure to severe hypoglycaemia: a 16 year follow-up study. Diabetologia . 2011 Sep. 54(9):2404-8. [QxMD MEDLINE Link] .
Kacerovsky M, Jones J, Schmid AI, et al. Postprandial and fasting hepatic glucose fluxes in long-standing type 1 diabetes. Diabetes . 2011 Jun. 60(6):1752-8. [QxMD MEDLINE Link] . [Full Text] .
Ahmedani MY, Haque MS, Basit A, Fawwad A, Alvi SF. Ramadan Prospective Diabetes Study: the role of drug dosage and timing alteration, active glucose monitoring and patient education. Diabet Med . 2012 Jun. 29(6):709-15. [QxMD MEDLINE Link] .
Pannu N, Wiebe N, Tonelli M. Prophylaxis strategies for contrast-induced nephropathy. JAMA . 2006 Jun 21. 295(23):2765-79. [QxMD MEDLINE Link] .
Salardi S, Balsamo C, Zucchini S, Maltoni G, Scipione M, Rollo A, et al. High rate of regression from micro-macroalbuminuria to normoalbuminuria in children and adolescents with type 1 diabetes treated or not with enalapril: the influence of HDL cholesterol. Diabetes Care . 2011 Feb. 34(2):424-9. [QxMD MEDLINE Link] . [Full Text] .
de Boer IH, Rue TC, Cleary PA, et al. Long-term Renal Outcomes of Patients With Type 1 Diabetes Mellitus and Microalbuminuria: An Analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Cohort. Arch Intern Med . 2011 Mar 14. 171(5):412-420. [QxMD MEDLINE Link] . [Full Text] .
Strippoli GF, Bonifati C, Craig M, Navaneethan SD, Craig JC. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev . 2006 Oct 18. CD006257. [QxMD MEDLINE Link] .
Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation . 2007 Jan 23. 115(3):387-97. [QxMD MEDLINE Link] .
Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis . 2004 Oct 1. 39(7):885-910. [QxMD MEDLINE Link] .
Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA . 2005 Jan 12. 293(2):217-28. [QxMD MEDLINE Link] .
Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care . 2007 Jan. 30(1):162-72. [QxMD MEDLINE Link] .
Margeirsdottir HD, Stensaeth KH, Larsen JR, Brunborg C, Dahl-Jørgensen K. Early signs of atherosclerosis in diabetic children on intensive insulin treatment: a population-based study. Diabetes Care . 2010 Sep. 33(9):2043-8. [QxMD MEDLINE Link] . [Full Text] .
van Dieren S, Nöthlings U, van der Schouw YT, Spijkerman AM, Rutten GE, van der A DL, et al. Non-fasting lipids and risk of cardiovascular disease in patients with diabetes mellitus. Diabetologia . 2011 Jan. 54(1):73-7. [QxMD MEDLINE Link] . [Full Text] .
Lee SH, Kim JH, Kang MJ, et al. Implications of nocturnal hypertension in children and adolescents with type 1 diabetes. Diabetes Care . 2011 Oct. 34(10):2180-5. [QxMD MEDLINE Link] . [Full Text] .
Leiter LA, Lundman P, da Silva PM, et al. Persistent lipid abnormalities in statin-treated patients with diabetes mellitus in Europe and Canada: results of the Dyslipidaemia International Study. Diabet Med . 2011 Nov. 28(11):1343-1351. [QxMD MEDLINE Link] .
Lund SS, Tarnow L, Astrup AS, Hovind P, Jacobsen PK, Alibegovic AC, et al. Effect of adjunct metformin treatment on levels of plasma lipids in patients with type 1 diabetes. Diabetes Obes Metab . 2009 Oct. 11(10):966-77. [QxMD MEDLINE Link] .
Tucker ME. ACC/AHA statin guidelines, with caveats. WebMD. Available at https://www.medscape.com/viewarticle/837138 . Accessed: Dec 24, 2014.
[Guideline] ElSayed NA, Aleppo G, Aroda VR, et al. Introduction and Methodology: Standards of Care in Diabetes-2023. Diabetes Care . 2023 Jan 1. 46 (Supplement_1):S1-S4. [QxMD MEDLINE Link] . [Full Text] .
Tucker ME. ADA Advises New BP, Lipid Targets for People With Diabetes. Medscape Medical News . 2022 Dec 13. [Full Text] .
Marks JB. Perioperative management of diabetes. Am Fam Physician . 2003 Jan 1. 67(1):93-100. [QxMD MEDLINE Link] .
[Guideline] Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med . 2011 Feb 15. 154(4):260-7. [QxMD MEDLINE Link] .
Kansagara D, Fu R, Freeman M, Wolf F, Helfand M. Intensive insulin therapy in hospitalized patients: a systematic review. Ann Intern Med . 2011 Feb 15. 154(4):268-82. [QxMD MEDLINE Link] .
[Guideline] Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care . 2009 Jun. 32(6):1119-31. [QxMD MEDLINE Link] . [Full Text] .
Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control: what is the evidence?. Crit Care Med . 2007 Sep. 35(9 Suppl):S496-502. [QxMD MEDLINE Link] .
Murphy HR, Steel SA, Roland JM, et al. Obstetric and perinatal outcomes in pregnancies complicated by Type 1 and Type 2 diabetes: influences of glycaemic control, obesity and social disadvantage. Diabet Med . 2011 Sep. 28(9):1060-7. [QxMD MEDLINE Link] .
Diabetes Prevention Trial--Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med . 2002 May 30. 346(22):1685-91.
Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med . 2002 May 30. 346(22):1685-91. [QxMD MEDLINE Link] .
Gale EA, Bingley PJ, Emmett CL, Collier T. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet . 2004 Mar 20. 363(9413):925-31. [QxMD MEDLINE Link] .
Herold KC, Bundy BN, Long SA, and the, Type 1 Diabetes TrialNet Study Group. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med . 2019 Aug 15. 381 (7):603-13. [QxMD MEDLINE Link] . [Full Text] .
Sims EK, Bundy BN, Stier K, and the, Type 1 Diabetes TrialNet Study Group. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci Transl Med . 2021 Mar 3. 13 (583): [QxMD MEDLINE Link] . [Full Text] .
Wherrett DK, Bundy B, Becker DJ, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet . 2011 Jul 23. 378(9788):319-27. [QxMD MEDLINE Link] .
Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet . 2011 Jul 30. 378(9789):412-9. [QxMD MEDLINE Link] .
[Guideline] Tucker ME. ADA Issues New Guidance on Type 1 Diabetes in Youth. Medscape Medical News . 2018 Aug 10. [Full Text] .
[Guideline] Chiang JL, Maahs DM, Garvey KC, et al. Type 1 Diabetes in Children and Adolescents: A Position Statement by the American Diabetes Association. Diabetes Care . 2018 Sep. 41 (9):2026-44. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] American Diabetes Association. Introduction: Standards of Medical Care in Diabetes-2022. Diabetes Care . 2022 Jan 1. 45 (Supplement_1):S1-S2. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] American Diabetes Association Professional Practice Committee. Summary of Revisions: Standards of Care in Diabetes-2024. Diabetes Care . 2024 Jan 1. 47 (Supplement_1):S5-S10. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Jenkins K. ADA Updates Recommendations for Managing Hypertension in Diabetes. Medscape . 2017 Sep 4. [Full Text] .
[Guideline] de Boer IH, Bangalore S, Benetos A, et al. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care . 2017 Sep. 40 (9):1273-1284. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Donaghue KC, Marcovecchio ML, Wadwa RP, et al. ISPAD Clinical Practice Consensus Guidelines 2018: microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes . 2018 Oct. 19 Suppl 27:262-74. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] DiMeglio LA, Acerini CL, Codner E, et al. ISPAD Clinical Practice Consensus Guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes . 2018 Oct. 19 Suppl 27:105-14. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of Diabetes in Older Adults: An Endocrine Society* Clinical Practice Guideline. J Clin Endocrinol Metab . 2019 May 1. 104 (5):1520-74. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Tucker ME. New Endocrine Society Guidelines Address Diabetes in Older Adults. Medscape Medical News . 2019 Mar 23. [Full Text] .
[Guideline] Tucker ME. More Guidance on 'Vulnerable Subgroup' With Diabetes and COVID-19. Medscape Medical News . 2020 Apr 28. [Full Text] .
[Guideline] Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol . 2020 Apr 23. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Grunberger G, Sherr J, Allende M, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons With Diabetes Mellitus. Endocr Pract . 27 (2021):505-37. [Full Text] .
[Guideline] Tucker ME. 'A Better Picture': First AACE Guidelines on Diabetes Technology. Medscape Medical News . 2021 May 31. [Full Text] .
Lei J, Coronel MM, Yolcu ES, et al. FasL microgels induce immune acceptance of islet allografts in nonhuman primates. Sci Adv . 2022 May 13. 8 (19):eabm9881. [QxMD MEDLINE Link] . [Full Text] .
University of Missouri-Columbia. Harvard Scientists Have Developed a Revolutionary New Treatment for Diabetes. SciTechDaily. Available at https://scitechdaily.com/harvard-scientists-have-developed-a-revolutionary-new-treatment-for-diabetes/ . June 12, 2022; Accessed: June 13, 2022.
US Food and Drug Administration. FDA authorizes first interoperable, automated insulin dosing controller designed to allow more choices for patients looking to customize their individual diabetes management device system. Available at https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-interoperable-automated-insulin-dosing-controller-designed-allow-more-choices?fbclid=IwAR3TSBssEd4n6b9hR5oe9Bzwmz3su1yQny8bcQeHVi0WFSsvURBh3nPjR-Y . December 13, 2019; Accessed: December 1, 2020.
[Guideline] American Diabetes Association. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes . 2015 Apr. 33 (2):97-111. [QxMD MEDLINE Link] . [Full Text] .
Brooks M. FDA Clears Blood Test to Help Diagnose Type 1 Diabetes. Medscape Medical News . Aug 21 2014. [Full Text] .
Hsiao-Chuan L, et al. Enterovirus infection is associated with an increased risk of childhood type 1 diabetes in Taiwan: A nationwide population-based cohort study. Diabetologia . 2014. [Full Text] .
Leegaard A, Riis A, Kornum JB, et al. Diabetes, Glycemic Control, and Risk of Tuberculosis: A population-based case-control study. Diabetes Care . 2011 Dec. 34(12):2530-5. [QxMD MEDLINE Link] . [Full Text] .
Ludwig J, Sanbonmatsu L, Gennetian L, et al. Neighborhoods, obesity, and diabetes--a randomized social experiment. N Engl J Med . 2011 Oct 20. 365(16):1509-19. [QxMD MEDLINE Link] .
[Guideline] Peters A, Laffel L. Diabetes Care for Emerging Adults: Recommendations for Transition From Pediatric to Adult Diabetes Care Systems: A position statement of the American Diabetes Association, with representation by the American College of Osteopathic Family Physicians, the American Academy of Pediatrics, the American Association of Clinical Endocrinologists, the American Osteopathic Association, the Centers for Disease Control and Prevention, Children with Diabetes, The Endocrine Society, the International Socie... Diabetes Care . 2011 Nov. 34(11):2477-85. [QxMD MEDLINE Link] . [Full Text] .
US Food and Drug Administration. Early Communication About Safety of Lantus (Insulin Glargine). Available at https://www.fda.gov/Drugs/DrugSafety/ucm239376.htm . Accessed: May 22, 2012.

Contributor Information and Disclosures
Romesh Khardori, MD, PhD, FACP (Retired) Professor, Division of Endocrinology, Diabetes and Metabolism, Department of Internal Medicine, Eastern Virginia Medical School Romesh Khardori, MD, PhD, FACP is a member of the following medical societies: American Association of Clinical Endocrinology , American College of Physicians , American Diabetes Association , Endocrine Society Disclosure: Nothing to disclose.
George T Griffing, MD Professor Emeritus of Medicine, St Louis University School of Medicine George T Griffing, MD is a member of the following medical societies: American Association for Physician Leadership , American Association for the Advancement of Science , American College of Medical Practice Executives , American College of Physicians , American Diabetes Association , American Federation for Medical Research , American Heart Association , Central Society for Clinical and Translational Research , Endocrine Society , International Society for Clinical Densitometry , Southern Society for Clinical Investigation Disclosure: Nothing to disclose.
Howard A Bessen, MD Professor of Medicine, Department of Emergency Medicine, University of California, Los Angeles, David Geffen School of Medicine; Program Director, Harbor-UCLA Medical Center
Howard A Bessen, MD is a member of the following medical societies: American College of Emergency Physicians
Disclosure: Nothing to disclose.
Barry E Brenner, MD, PhD, FACEP Professor of Emergency Medicine, Professor of Internal Medicine, Program Director, Emergency Medicine, Case Medical Center, University Hospitals, Case Western Reserve University School of Medicine
Barry E Brenner, MD, PhD, FACEP is a member of the following medical societies: Alpha Omega Alpha , American Academy of Emergency Medicine , American College of Chest Physicians , American College of Emergency Physicians , American College of Physicians , American Heart Association , American Thoracic Society , Arkansas Medical Society , New York Academy of Medicine , New York Academy ofSciences ,and Society for Academic Emergency Medicine
Aneela Naureen Hussain, MD, FAAFM Assistant Professor, Department of Family Medicine, State University of New York Downstate Medical Center; Consulting Staff, Department of Family Medicine, University Hospital of Brooklyn
Aneela Naureen Hussain, MD, FAAFM is a member of the following medical societies: American Academy of Family Physicians , American Medical Association , American Medical Women's Association , Medical Society of the State of New York , and Society of Teachers of Family Medicine
Anne L Peters, MD, CDE Director of Clinical Diabetes Programs, Professor, Department of Medicine, University of Southern California, Keck School of Medicine, Los Angeles, California, Los Angeles County/University of Southern California Medical Center
Anne L Peters, MD, CDE is a member of the following medical societies: American College of Physicians and American Diabetes Association
Disclosure: Amylin Honoraria Speaking and teaching; AstraZeneca Consulting fee Consulting; Lilly Consulting fee Consulting; Takeda Consulting fee Consulting; Bristol Myers Squibb Honoraria Speaking and teaching; NovoNordisk Consulting fee Consulting; Medtronic Minimed Consulting fee Consulting; Dexcom Honoraria Speaking and teaching; Roche Honoraria Speaking and teaching
Don S Schalch, MD Professor Emeritus, Department of Internal Medicine, Division of Endocrinology, University of Wisconsin Hospitals and Clinics
Don S Schalch, MD is a member of the following medical societies: American Diabetes Association , American Federation for Medical Research , Central Society for Clinical Research , and Endocrine Society
Erik D Schraga, MD Staff Physician, Department of Emergency Medicine, Mills-Peninsula Emergency Medical Associates
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
Miriam T Vincent, MD, PhD Professor and Chair, Department of Family Practice, State University of New York Downstate Medical Center
Miriam T Vincent, MD, PhD is a member of the following medical societies: Alpha Omega Alpha , American Academy of Family Physicians , American Association for the Advancement of Science , Medical Society of the State of New York , North American Primary Care Research Group , Sigma Xi , and Society of Teachers of Family Medicine
Disclosure: Joslin Diabetes Group, Harvard Honoraria Speaking and teaching
Scott R Votey, MD Director of Emergency Medicine Residency, Ronald Reagan UCLA Medical Center; Professor of Medicine/Emergency Medicine, University of California, Los Angeles, David Geffen School of Medicine
Scott R Votey, MD is a member of the following medical societies: Society for Academic Emergency Medicine
Frederick H Ziel, MD Associate Professor of Medicine, University of California, Los Angeles, David Geffen School of Medicine; Physician-In-Charge, Endocrinology/Diabetes Center, Director of Medical Education, Kaiser Permanente Woodland Hills; Chair of Endocrinology, Co-Chair of Diabetes Complete Care Program, Southern California Permanente Medical Group
Frederick H Ziel, MD is a member of the following medical societies: American Association of Clinical Endocrinologists , American College of Endocrinology , American College of Physicians , American College of Physicians-American Society of Internal Medicine , American Diabetes Association , American Federation for Medical Research , American Medical Association , American Society for Bone and Mineral Research , California Medical Association , Endocrine Society , andInternational Society for Clinical Densitometry
What would you like to print?
- Print this section
- Print the entire contents of
- Print the entire contents of article

- Pediatric Type 1 Diabetes Mellitus
- Type 2 Diabetes Mellitus
- Fast Five Quiz: Atrial Fibrillation and Diabetes
- Pediatric Type 2 Diabetes Mellitus
- Perioperative Management of the Diabetic Patient
- Fast Five Quiz: How Much Do You Know About Diabetic Neuropathy?
- Diabetes Mellitus Type 1 News & Perspectives
- Are Immune Therapies for Type 1 Diabetes Worthwhile?
- 'No Excuses': Training for the Olympics with Type 1 Diabetes
- Don't Overlook Cardiovascular Risk in Type 1 Diabetes
- Preoperative HbA1c and Postoperative Outcomes in Spine Surgery
- Emergence of Melioidosis in Brazil: A Case Series
- Drug Interaction Checker
- Pill Identifier
- Calculators

- 2003/viewarticle/do-night-owls-have-higher-risk-type-2-diabetes-2023a1000qi1education Do Night Owls Have a Higher Risk for Type 2 Diabetes? 0.25 LOC / CME / MOC Credit / CE Credits education You are being redirected to Medscape Education Yes, take me there 0.25 LOC / CME / MOC Credit / CE Do Night Owls Have a Higher Risk for Type 2 Diabetes?
- 2002117739-overviewDiseases & Conditions Diseases & Conditions Type 1 Diabetes Mellitus
- 2010trulicity-dulaglutide-999965Drugs Drugs dulaglutide
If you have:
- A BMJ Best Practice user profile
- A personal subscription
- Registered for a free trial
If you are having difficulty logging in, please contact us
Do you have access through your institution?
If your hospital, university or other institution provides access to BMJ Best Practice, they will have selected one of the following access methods:
- Open Athens or Shibboleth
- Access code
- Your institution's website or intranet
- Your institution's wifi or network
Help us improve BMJ Best Practice
Please complete all fields.
I have some feedback on:
We will respond to all feedback.
For any urgent enquiries please contact our customer services team who are ready to help with any problems.
Phone: +44 (0) 207 111 1105
Email: [email protected]
Your feedback has been submitted successfully.
Website maintenance is scheduled for Saturday, August 17, and Sunday, August 18. Short disruptions may occur during these days.

PARITA PATEL, MD, AND ALLISON MACEROLLO, MD
Am Fam Physician. 2010;81(7):863-870
A more recent article on diabetes mellitus is available .
See related editorial on page 843 .
Author disclosure: Nothing to disclose.
Based on etiology, diabetes is classified as type 1 diabetes mellitus, type 2 diabetes mellitus, latent autoimmune diabetes, maturity-onset diabetes of youth, and miscellaneous causes. The diagnosis is based on measurement of A1C level, fasting or random blood glucose level, or oral glucose tolerance testing. Although there are conflicting guidelines, most agree that patients with hypertension or hyperlipidemia should be screened for diabetes. Diabetes risk calculators have a high negative predictive value and help define patients who are unlikely to have diabetes. Tests that may help establish the type of diabetes or the continued need for insulin include those reflective of beta cell function, such as C peptide levels, and markers of immune-mediated beta cell destruction (e.g., autoantibodies to islet cells, insulin, glutamic acid decarboxylase, tyrosine phosphatase [IA-2α and IA-2β]). Antibody testing is limited by availability, cost, and predictive value.
Prevention, timely diagnosis, and treatment are important in patients with diabetes mellitus. Many of the complications associated with diabetes, such as nephropathy, retinopathy, neuropathy, cardiovascular disease, stroke, and death, can be delayed or prevented with appropriate treatment of elevated blood pressure, lipids, and blood glucose. 1 – 4
In 1997, the American Diabetes Association (ADA) introduced an etiologically based classification system and diagnostic criteria for diabetes, 5 which were updated in 2010. 1 Type 2 diabetes accounts for approximately 90 to 95 percent of all persons with diabetes in the United States, and its prevalence is increasing in adults worldwide. 6 With the rise in childhood obesity, type 2 diabetes is increasingly being diagnosed in children and adolescents. 6
| Patients with a sustained blood pressure of greater than 135/80 mm Hg should be screened for diabetes. | A | , |
| Patients with hypertension or hyperlipidemia should be screened for diabetes. | B | |
| Risk calculators can be used to determine which patients do not need screening for diabetes. | C | |
| A1C value of greater than 6.5 percent on two separate occasions is diagnostic for diabetes. | C | |
| Patients at increased risk of diabetes should be counseled on effective strategies to lower their risk, such as weight loss and exercise. | C | , |
The risk of diabetes is increased in close relatives suggesting a genetic predisposition, although no direct genetic link has been identified. 7 Type 1 diabetes accounts for 5 to 10 percent of persons with diabetes 6 and is characterized by insulin deficiency that is typically an autoimmune-mediated condition.
Latent autoimmune diabetes in adults includes a heterogenous group of conditions that are phenotypically similar to type 2 diabetes, but patients have autoantibodies that are common with type 1 diabetes. Diagnostic criteria include age of 30 years or older; no insulin treatment for six months after diagnosis; and presence of autoantibodies to glutamic acid decarboxylase, islet cells, tyrosine phosphatase (IA-2α and IA-2β), or insulin.
Patients with maturity-onset diabetes of youth typically present before 25 years of age, have only impaired insulin secretion, and have a monogenetic defect that leads to an autosomal dominant inheritance pattern. These patients are placed in a subcategory of having genetic defects of beta cell. 8
The old terminology of prediabetes has now been replaced with “categories of increased risk for diabetes.” This includes persons with impaired fasting glucose, impaired glucose tolerance, or an A1C level of 5.7 to 6.4 percent. 1 , 9 , 10
Diagnostic Criteria and Testing
The 1997 ADA consensus guidelines lowered the blood glucose thresholds for the diagnosis of diabetes. 5 This increased the number of patients diagnosed at an earlier stage, although no studies have demonstrated a reduction in long-term complications. Data suggest that as many as 5.7 million persons in the United States have undiagnosed diabetes. 6 Table 1 compares specific diagnostic tests for diabetes. 11 – 14
| OGTT (two hour) | Reference standard | $19 | ||||
| Random blood glucose level | ||||||
| ≥ 140 mg per dL (7.8 mmol per L) | 55 | 92 | 30.5 | 97 | $6 | |
| ≥ 150 mg per dL (8.3 mmol per L) | 50 | 95 | 39.9 | 96.7 | ||
| ≥ 160 mg per dL (8.9 mmol per L) | 44 | 96 | 41.2 | 96.4 | ||
| ≥ 170 mg per dL (9.4 mmol per L) | 42 | 97 | 47.2 | 96.3 | ||
| ≥ 180 mg per dL (10.0 mmol per L) | 39 | 98 | 55.5 | 96 | ||
| A1C levels (%) | ||||||
| 6.1 | 63.2 | 97.4 | 60.8 | 97.6 | $14, serum test or point of-care test | |
| 6.5 | 42.8 | 99.6 | 87.2 | 96.5 | ||
| 7.0 | 28.3 | 99.9 | 94.7 | 95.6 | ||
| Diabetes Risk Calculator , | 78.2 to 88.2 | 66.8 to 74.9 | 6.3 to 13.6 | 99.2 to 99.3 | Free | |
TESTS TO DIAGNOSE DIABETES
Blood Glucose Measurements . The diagnosis of diabetes is based on one of three methods of blood glucose measurement ( Table 2 ) . 1 Diabetes can be diagnosed if the patient has a fasting blood glucose level of 126 mg per dL (7.0 mmol per L) or greater on two separate occasions. The limitations of this test include the need for an eight-hour fast before the blood draw, a 12 to 15 percent day-to-day variance in fasting blood glucose values, and a slightly lower sensitivity for predicting microvascular complications. 15 , 16
| Categories of increased risk (formerly prediabetes) | Fasting glucose test: 100 to 125 mg per dL (5.6 to 6.9 mmol per L) | — |
| Two-hour OGTT (75-g load): 140 to 199 mg per dL (7.8 to 11.0 mmol per L) | ||
| A1C measurement: 5.7 to 6.4 percent | ||
| Type 1, type 2, LADA, MODY | Fasting glucose test: ≥ 126 mg per dL (7.0 mmol per L) | |
| Two-hour OGTT (75-g load): ≥ 200 mg per dL (11.1 mmol per L) | ||
| Random glucose test: ≥ 200 mg per dL with symptoms | ||
| A1C measurement: ≥ 6.5 percent | ||
| Gestational diabetes | OGTT (100-g load): | One-hour Glucola OGTT (50-g load): |
| OGTT (75-g load): |
Diabetes can also be diagnosed with a random blood glucose level of 200 mg per dL (11.1 mmol per L) or greater if classic symptoms of diabetes (e.g., polyuria, polydipsia, weight loss, blurred vision, fatigue) are present. Lower random blood glucose values (140 to 180 mg per dL [7.8 to 10.0 mmol per L]) have a fairly high specificity of 92 to 98 percent; therefore, patients with these values should undergo more definitive testing. A low sensitivity of 39 to 55 percent limits the use of random blood glucose testing. 15
The oral glucose tolerance test is considered a first-line diagnostic test. Limitations include poor reproducibility and patient compliance because an eight-hour fast is needed before the 75-g glucose load, which is followed two hours later by a blood draw. 17 The criterion for diabetes is a serum blood glucose level of greater than 199 mg per dL (11.0 mmol per L).
In 2003, the ADA lowered the threshold for diagnosis of impaired fasting glucose to include a fasting glucose level between 100 and 125 mg per dL (5.6 and 6.9 mmol per L). Impaired glucose tolerance continues to be defined as a blood glucose level between 140 and 199 mg per dL (7.8 and 11.0 mmol per L) two hours after a 75-g load. Patients meeting either of these criteria are at significantly higher risk of progression to diabetes and should be counseled on effective strategies to lower their risk, such as weight loss and exercise. 1 , 9
A1C . A1C measurement has recently been endorsed by the ADA as a diagnostic and screening tool for diabetes. 1 One advantage of using A1C measurement is the ease of testing because it does not require fasting. An A1C level of greater than 6.5 percent on two separate occasions is considered diagnostic of diabetes. 18 Lack of standardization has historically deterred its use, but this test is now widely standardized in the United States. 19 A1C measurements for diagnosis of diabetes should be performed by a clinical laboratory because of the lack of standardization of point-of-care testing. Limitations of A1C testing include low sensitivity, possible racial disparities, and interference by anemia and some medications. 15
TESTS TO IDENTIFY TYPE OF DIABETES
Tests that can be used to establish the etiology of diabetes include those reflective of beta cell function (e.g., C peptide) and markers of immune-mediated beta cell destruction (e.g., insulin, islet cell, glutamic acid decarboxylase, IA-2α and IA-2β autoantibodies). Table 3 presents the characteristics of these tests. 20 – 27
| C peptide | < 1.51 ng per mL (0.5 nmol per L): PPV of 96 percent for diagnosis in adults and children | > 1.51 ng per mL: NPV of 96 percent for diagnosis in adults and children | Not available | $30 |
| GADA | 60 percent prevalence in adults and children | 7 to 34 percent prevalence in adults and children , | Presence: PPV of 92 percent for requiring insulin at three years in persons 15 to 34 years of age | $28 |
| 73 percent prevalence in children | NPV of 94 percent for requiring insulin at six years in adults | Absence: NPV of 49 percent for requiring insulin at three years in persons 15 to 34 years of age | ||
| IA-2α and IA-2β | 40 percent prevalence in adults and children | 2.2 percent prevalence in adults | PPV of 75 percent for requiring insulin at three years in persons 15 to 34 years of age | Cost not available |
| 86 percent prevalence in children | ||||
| ICA | 75 to 85 percent prevalence in adults and children | 4 to 21 percent prevalence in adults | PPV of 86 percent for requiring insulin at three years in persons 15 to 34 years of age | $28 |
| 84 percent prevalence in children | ||||
C peptide is linked to insulin to form proinsulin and reflects the amount of endogenous insulin. Patients with type 1 diabetes have low C peptide levels because of low levels of endogenous insulin and beta cell function. Patients with type 2 diabetes typically have normal to high levels of C peptide, reflecting higher amounts of insulin but relative insensitivity to it. In a Swedish study of patients with clinically well-defined type 1 or 2 diabetes, 96 percent of patients with type 2 diabetes had random C peptide levels greater than 1.51 ng per mL (0.50 nmol per L), whereas 90 percent of patients with type 1 diabetes had values less than 1.51 ng per mL. 20 In the clinically undefined population, which is the group in which the test is most often used, the predictive value is likely lower.
Antibody testing is limited by availability, cost, and predictive value, especially in black and Asian patients. Prevalence of any antibody in white patients with type 1 diabetes is 85 to 90 percent, 5 whereas the prevalence in similar black or Hispanic patients is lower (19 percent in both groups in one study). 28 In persons with type 2 diabetes, the prevalence of islet cell antibody is 4 to 21 percent; glutamic acid decarboxylase antibody, 7 to 34 percent; IA-2, 1 to 2 percent; and any antibody, 11.6 percent. 24 , 25 , 29 In healthy persons, the prevalence of any antibody marker is 1 to 2 percent 30 ; thus, overlap of the presence of antibodies in various types of diabetes and patients limits the utility of individual tests.
As with any condition, a rationale for screening should first be established. Diabetes is a common disease that is associated with significant morbidity and mortality. It has an asymptomatic stage that may be present for up to seven years before diagnosis. The disease is treatable, and testing is acceptable and accessible to patients. Early treatment of diabetes that was identified primarily by symptoms improves microvascular outcomes. 31 However, it is not clear whether universal screening reduces diabetes-associated morbidity and mortality. Table 4 presents screening guidelines from several organizations. 1 , 8 , 32 – 38
| AACE | All persons 30 years or older who are at risk of having or developing type 2 diabetes should be screened annually. | |
| ADA | Testing to detect type 2 diabetes should be considered in asymptomatic adults with a BMI of 25 kg per m or greater and one or more additional risk factors for diabetes. | |
| Additional risk factors include physical inactivity; hypertension; HDL cholesterol level of less than 35 mg per dL (0.91 mmol per L) or a triglyceride level of greater than 250 mg per dL (2.82 mmol per L); history of CV disease; A1C level of 5.7 percent or greater; IGT or IFG on previous testing; first-degree relative with diabetes; member of a high-risk ethnic group; in women, history of gestational diabetes or delivery of a baby greater than 4.05 kg (9 lb), or history of PCOS; other conditions associated with insulin resistance (e.g., severe obesity, acanthosis nigricans). | ||
| In persons without risk factors, testing should begin at 45 years of age. | ||
| If test results are normal, repeat testing should be performed at least every three years. | ||
| CTFPHC | There is fair evidence to recommend screening patients with hypertension or hyperlipidemia for type 2 diabetes to reduce the incidence of CV events and CV mortality. | |
| USPSTF | All adults with a sustained blood pressure of greater than 135/80 mm Hg should be screened for diabetes. | |
| Current evidence is insufficient to assess balance of benefits and harms of routine screening for type 2 diabetes in asymptomatic, normotensive patients. | ||
| AACE | In all pregnant women, fasting glucose should be measured at the first prenatal visit (no later than 20 weeks' gestation). | |
| A 75-g OGTT should be performed if the fasting glucose concentration is greater than 85 mg per dL (4.7 mmol per L). | ||
| ACOG , | All pregnant women should be screened through history, clinical risk factors, or laboratory testing. | |
| Women at low-risk may be excluded from glucose testing. | ||
| Low-risk criteria include age younger than 25 years, BMI of 25 kg per m or less, no history of abnormal OGTT result, no history of adverse obstetric outcomes usually associated with gestational diabetes, no first-degree relative with diabetes, not a member of a high-risk ethnic group. | ||
| Women with gestational diabetes should be screened six to 12 weeks postpartum and should receive subsequent screening for the development of diabetes. | ||
| ADA , | Risk assessment should be performed at the first prenatal visit. | |
| Women with clinical characteristics consistent with a high risk of gestational diabetes (e.g., marked obesity, personal history of gestational diabetes, glycosuria, strong family history of diabetes) should undergo glucose testing as soon as possible. If glucose test results are negative, retesting should be performed at 24 to 28 weeks' gestation. | ||
| Testing may be excluded in low-risk women (see ACOG criteria above). All other women should receive Glucola test or OGTT at 24 to 28 weeks' gestation. | ||
| Women with gestational diabetes should be screened for diabetes six to 12 weeks postpartum and should receive subsequent screening for the development of diabetes. | ||
| CTFPHC | There is poor evidence to recommend for or against screening using Glucola testing in the periodic health examination of pregnant women. | |
| USPSTF | Evidence is insufficient to assess the balance of benefits and harms of screening for gestational diabetes, either before or after 24 weeks' gestation. | |
| Physicians should discuss screening with patients and make case-by-case decisions. | ||
TYPE 1 DIABETES
Screening for type 1 diabetes is not recommended because there is no accepted treatment for patients who are diagnosed in the asymptomatic phase. The Diabetes Prevention Trial identified a group of high-risk patients based on family history and positivity to islet cell antibodies. However, treatment did not prevent progression to type 1 diabetes in these patients. 39
TYPE 2 DIABETES
Medications and lifestyle interventions may reduce the risk of diabetes, although 20 to 30 percent of patients with type 2 diabetes already have complications at the time of presentation. 40 Although a recent analysis suggests that screening for and treating impaired glucose tolerance in persons at risk of diabetes may be cost-effective, the data on screening for type 2 diabetes are less certain. 41 It is unclear whether the early diagnosis of type 2 diabetes through screening programs, with subsequent intensive interventions, provides an incremental benefit in final health outcomes compared with initiating treatment after clinical diagnosis.
Guidelines differ regarding who should be screened for type 2 diabetes. The U.S. Preventive Services Task Force (USPSTF) recommends limiting screening to adults with a sustained blood pressure of greater than 135/80 mm Hg. 34 , 42 The American Academy of Family Physicians concurs, but specifically includes treated and untreated patients. 43 The Canadian Task Force on Preventive Health Care recommends screening all patients with hypertension or hyperlipidemia. 33 The ADA recommends screening a much broader patient population based on risk. 1
There are several questionnaires to predict a patient's risk of diabetes. The Diabetes Risk Calculator was developed using data from the National Health and Nutrition Examination Survey III and incorporates age, height, weight, waist circumference, ethnicity, blood pressure, exercise, history of gestational diabetes, and family history. 13 , 14 For diagnosis of diabetes, it has a positive predictive value (PPV) of 14 percent and a negative predictive value (NPV) of 99.3 percent. The tool is most valuable in helping define which patients are very unlikely to have diabetes. 13
GESTATIONAL DIABETES
Whether patients should be screened for gestational diabetes is unclear. The USPSTF states that there is insufficient evidence to recommend for or against screening. 34 The ADA and the American College of Obstetricians and Gynecologists recommend risk-based testing, although most women require testing based on these inclusive guidelines. 36 The Glucola test is the most commonly used screening test for gestational diabetes and includes glucose testing one hour after a 50-g oral glucose load. An abnormal Glucola test result (i.e., blood glucose level of 140 mg per dL or greater) should be confirmed with a 75-g or 100-g oral glucose tolerance test. Whether screening and subsequent treatment of gestational diabetes alter clinically important perinatal outcomes is unclear. Untreated gestational diabetes is associated with a higher incidence of macrosomia and shoulder dystocia. 44 A randomized controlled trial found that treatment led to a reduction in serious perinatal complications, with a number needed to treat of 34. Treatment did not reduce risk of cesarean delivery or admission to the neonatal intensive care unit, however. 44
New-Onset Symptomatic Hyperglycemia
Patients may initially present with diabetic ketoacidosis or hyperglycemic hyperosmolar state ( Table 5 ) , 45 both of which are initially managed with insulin because they are essentially insulin deficiency states. Both groups of patients may present with polyuria, polydipsia, and signs of dehydration. Diagnostic criteria of diabetic ketoacidosis include a blood glucose level greater than 250 mg per dL (13.9 mmol per L), pH of 7.3 or less, serum bicarbonate level less than 18 mEq per L (18 mmol per L), and moderate ketonemia. However, significant ketosis has also been shown to occur in up to one third of patients with hyperglycemic hyperosmolar state. 46
| Plasma glucose | > 250 mg per dL (13.9 mmol per L) | > 250 mg per dL | > 250 mg per dL | > 600 mg per dL (33.3 mmol per L) |
| Arterial pH | 7.25 to 7.30 | 7.00 to 7.24 | < 7.00 | > 7.30 |
| Serum bicarbonate | 15 to 18 mEq per L (15 to 18 mmol per L) | 10 to 15 mEq per L (10 to 15 mmol per L) | < 10 mEq per L (10 mmol per L) | > 15 mEq per L (15 mmol per L) |
| Urine ketones | Positive | Positive | Positive | Small |
| Serum ketones | Positive | Positive | Positive | Small |
| Serum osmolality | Variable | Variable | Variable | > 320 mOsm per kg |
| Anion gap | > 10 mEq per L | > 12 mEq per L | > 12 mEq per L | < 12 mEq per L |
| Mental status | Alert | Alert/drowsy | Stupor/coma | Stupor/coma |
Although diabetic ketoacidosis typically occurs in persons with type 1 diabetes, more than one half of newly diagnosed black patients with unprovoked diabetic ketoacidosis are obese and many display classic features of type 2 diabetes—most importantly with a measurable insulin reserve. 47 Thus, the presentation does not definitively determine the type of diabetes a patient has. Presence of antibodies, particularly glutamic acid decarboxylase antibody, predicts a higher likelihood of lifelong insulin requirement. There is, however, an overlap of presence of antibodies in type 1 and type 2 diabetes, and among patients with type 2 diabetes who may not require insulin. 48
A Swedish population-based study showed that among the 9.3 percent of young adults with newly diagnosed diabetes that could not be classified as type 1 or type 2, the presence of glutamic acid decarboxylase antibody was associated with a need for insulin within three years (odds ratio = 18.8; 95% confidence interval, 1.8 to 191). 26 The PPV for insulin treatment was 92 percent in those with the antibody. It should be noted that among patients who were negative for antibodies, 51 percent also needed insulin within three years. In contrast, the United Kingdom Prospective Diabetes Study found that only 5.7 percent of patients without glutamic acid decarboxylase antibody eventually needed insulin therapy, giving the test an NPV of 94 percent. 25 With these conflicting data, clinical judgment using a patient's phenotype, history, presentation, and selective laboratory testing is the best way to manage patients with diabetes.
American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(suppl 1):S11-S61.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group [published correction appears in Lancet . 1999;354(9178):602]. Lancet. 1998;352(9131):837-853.
The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977-986.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589.
Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183-1197.
Centers for Disease Control and Prevention. 2007 national diabetes fact sheet. http://www.cdc.gov/diabetes/pubs/factsheet07.htm. Accessed July 8, 2009.
Tuomi T. Type 1 and type 2 diabetes: what do they have in common?. Diabetes. 2005;54(suppl 2):S40-S45.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62-S69.
Knowler WC, Barrett-Connor E, Fowler SE, et al.; for the Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403.
Tuomilehto J, Lindström J, Eriksson JG, et al.; for the Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343-1350.
Tabaei BP, Herman WH. A multivariate logistic regression equation to screen for diabetes: development and validation. Diabetes Care. 2002;25(11):1999-2003.
Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25(2):275-278.
Heikes KE, Eddy DM, Arondekar B, Schlessinger L. Diabetes Risk Calculator: a simple tool for detecting undiagnosed diabetes and pre-diabetes. Diabetes Care. 2008;31(5):1040-1045.
Mochan E, Ebell M. Risk-assessment tools for detecting undiagnosed diabetes. Am Fam Physician. 2009;80(2):175-178.
Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab. 2008;93(7):2447-2453.
Petersen PH, Jørgensen LG, Brandslund I, De Fine Olivarius N, Stahl M. Consequences of bias and imprecision in measurements of glucose and HbA1C for the diagnosis and prognosis of diabetes mellitus. Scand J Clin Lab Invest Suppl. 2005;240:51-60.
Ko GT, Chan JC, Woo J, et al. The reproducibility and usefulness of the oral glucose tolerance test in screening for diabetes and other cardiovascular risk factors. Ann Clin Biochem. 1998;35(pt 1):62-67.
International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327-1334.
Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE for the NGSP Steering Committee. The National Glycohemoglobin Standardization Program: a five-year progress report. Clin Chem. 2001;47(11):1985-1992.
Berger B, Stenström G, Sundkvist G. Random C-peptide in the classification of diabetes. Scand J Clin Lab Invest. 2000;60(8):687-693.
Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48(3):436-472.
Sabbah E, Savola K, Kulmala P, et al. Diabetes-associated autoanti-bodies in relation to clinical characteristics and natural course in children with newly diagnosed type 1 diabetes. The Childhood Diabetes in Finland Study Group. J Clin Endocrinol Metab. 1999;84(5):1534-1539.
Tuomi T, Carlsson A, Li H, et al. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes. 1999;48(1):150-157.
Turner R, Stratton I, Horton V, et al. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group [published correction appears in Lancet . 1998;351(9099):376]. Lancet. 1997;350(9087):1288-1293.
Bottazzo GF, Bosi E, Cull CA, et al. IA-2 antibody prevalence and risk assessment of early insulin requirement in subjects presenting with type 2 diabetes (UKPDS 71) [published correction appears in Diabetologia . 2005;48(6):1240]. Diabetologia. 2005;48(4):703-708.
Törn C, Landin-Olsson M, Ostman J, et al. Glutamic acid decarboxylase antibodies (GADA) is the most important factor for prediction of insulin therapy within 3 years in young adult diabetic patients not classified as type 1 diabetes on clinical grounds. Diabetes Metab Res Rev. 2000;16(6):442-447.
Savola K, Bonifacio E, Sabbah E, et al. IA-2 antibodies—a sensitive marker of IDDM with clinical onset in childhood and adolescence. Childhood Diabetes in Finland Study Group. Diabetologia. 1998;41(4):424-429.
Avilés-Santa L, Maclaren N, Raskin P. The relationship between immune-mediated type 1 diabetes mellitus and ethnicity. J Diabetes Complications. 2004;18(1):1-9.
Davis TM, Wright AD, Mehta ZM, et al. Islet autoantibodies in clinically diagnosed type 2 diabetes: prevalence and relationship with metabolic control (UKPDS 70). Diabetologia. 2005;48(4):695-702.
Maclaren N, Lan M, Coutant R, et al. Only multiple autoantibodies to islet cells (ICA), insulin, GAD65, IA-2 and IA-2beta predict immune-mediated (type 1) diabetes in relatives. J Autoimmun. 1999;12(4):279-287.
Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care. 1992;15(7):815-819.
American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the management of diabetes mellitus. http://www.aace.com/pub/pdf/guidelines/DMGuidelines2007.pdf .Accessed July 8, 2009.
Feig DS, Palda VA, Lipscombe L. Screening for type 2 diabetes mellitus to prevent vascular complications: updated recommendations from the Canadian Task Force on Preventive Health Care. CMAJ. 2005;172(2):177-180.
U.S. Preventive Services Task Force. Screening for type 2 diabetes mellitus in adults. Recommendation statement. June 2008. http://www.ahrq.gov/clinic/uspstf08/type2/type2rs.htm#clinical . Accessed July 8, 2009.
Committee on Obstetric Practice. ACOG Committee Opinion No. 435: postpartum screening for abnormal glucose tolerance in women who had gestational diabetes mellitus. Obstet Gynecol. 2009;113(6):1419-1421.
American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces technical bulletin number 200, December 1994). Gestational diabetes. Obstet Gynecol. 2001;98(3):525-538.
Canadian Task Force on Preventive Health Care. Summary table of recommendations. Sreening for gestational diabetes mellitus. http://www.ctfphc.org/Tables/Ch02tab.htm . Accessed January 18, 2010.
U.S. Preventive Services Task Force. Screening for gestational diabetes mellitus. Recommendation statement. May 2008. http://www.ahrq.gov/clinic/uspstf08/gestdiab/gdrs.htm . Accessed January 18, 2010.
Diabetes Prevention Trial—Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685-1691.
Glucose tolerance and mortality: comparison of WHO American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354(9179):617-621.
Gillies CL, Lambert PC, Abrams KR, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ. 2008;336(7654):1180-1185.
Screening for type 2 diabetes mellitus in adults: U.S. Preventive Services Task Force recommendation statement [published correction appears in Ann Intern Med . 2008;149(2):147]. Ann Intern Med. 2008;148(11):846-854.
American Academy of Family Physicians. Recommendations for clinical preventive services. https://www.aafp.org/patient-care/clinical-recommendations/a-z.html . Accessed July 8, 2009.
Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS for the Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477-2486.
Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome. Diabetes Spectrum. 2002;15(1):28-36.
Kitabchi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153.
Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes. 1995;44(7):790-795.
Palmer JP, Hampe CS, Chiu H, Goel A, Brooks-Worrell BM. Is latent auto-immune diabetes in adults distinct from type 1 diabetes or just type 1 diabetes at an older age?. Diabetes. 2005;54(suppl 2):S62-S67.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2010 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.

- Next Article
Introduction
Understanding adult-onset type 1 diabetes, article information, adult-onset type 1 diabetes: current understanding and challenges.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
R. David Leslie , Carmella Evans-Molina , Jacquelyn Freund-Brown , Raffaella Buzzetti , Dana Dabelea , Kathleen M. Gillespie , Robin Goland , Angus G. Jones , Mark Kacher , Lawrence S. Phillips , Olov Rolandsson , Jana L. Wardian , Jessica L. Dunne; Adult-Onset Type 1 Diabetes: Current Understanding and Challenges. Diabetes Care 1 November 2021; 44 (11): 2449–2456. https://doi.org/10.2337/dc21-0770
Download citation file:
- Ris (Zotero)
- Reference Manager
Recent epidemiological data have shown that more than half of all new cases of type 1 diabetes occur in adults. Key genetic, immune, and metabolic differences exist between adult- and childhood-onset type 1 diabetes, many of which are not well understood. A substantial risk of misclassification of diabetes type can result. Notably, some adults with type 1 diabetes may not require insulin at diagnosis, their clinical disease can masquerade as type 2 diabetes, and the consequent misclassification may result in inappropriate treatment. In response to this important issue, JDRF convened a workshop of international experts in November 2019. Here, we summarize the current understanding and unanswered questions in the field based on those discussions, highlighting epidemiology and immunogenetic and metabolic characteristics of adult-onset type 1 diabetes as well as disease-associated comorbidities and psychosocial challenges. In adult-onset, as compared with childhood-onset, type 1 diabetes, HLA-associated risk is lower, with more protective genotypes and lower genetic risk scores; multiple diabetes-associated autoantibodies are decreased, though GADA remains dominant. Before diagnosis, those with autoantibodies progress more slowly, and at diagnosis, serum C-peptide is higher in adults than children, with ketoacidosis being less frequent. Tools to distinguish types of diabetes are discussed, including body phenotype, clinical course, family history, autoantibodies, comorbidities, and C-peptide. By providing this perspective, we aim to improve the management of adults presenting with type 1 diabetes.
Clinically, it has been relatively easy to distinguish the acute, potentially lethal, childhood-onset diabetes from the less aggressive condition that affects adults. However, experience has taught us that not all children with diabetes are insulin dependent and not all adults are non–insulin dependent. Immune, genetic, and metabolic analysis of these two, apparently distinct, forms of diabetes revealed inconsistencies, such that insulin-dependent and immune-mediated diabetes was redefined as type 1 diabetes, while most other forms were relabeled as type 2 diabetes. Recent data suggest a further shift in our thinking, with the recognition that more than half of all new cases of type 1 diabetes occur in adults. However, many adults may not require insulin at diagnosis of type 1 diabetes and have a more gradual onset of hyperglycemia, often leading to misclassification and inappropriate care. Indeed, misdiagnosis occurs in nearly 40% of adults with new type 1 diabetes, with the risk of error increasing with age ( 1 , 2 ). To consider this important issue, JDRF convened a workshop of international experts in November 2019 in New York, NY. In this Perspective, based on that workshop, we outline the evidence for a new viewpoint, suggesting future directions of research and ways to alter disease management to help adults living with type 1 diabetes.
Incidence of Type 1 Diabetes Among Adults Worldwide
Adult-onset type 1 diabetes is more common than childhood-onset type 1 diabetes, as shown from epidemiological data from both high-risk areas such as Northern Europe and low-risk areas such as China ( 3 – 8 ). In southeastern Sweden, the disease incidence among individuals aged 0–19 years is similar to that among individuals 40–100 years of age (37.8 per 100,000 persons per year and 34.0/100,000/year, respectively) ( 3 ). Given that the comparable incidence spans only two decades in children, it follows that adult-onset type 1 diabetes is more prevalent. Similarly, analysis of U.S. data from commercially insured individuals demonstrated an overall lower incidence in individuals 20–64 years of age (18.6/100,000/year) than in youth aged 0–19 years (34.3/100,000/year), but the total number of new cases in adults over a 14-year period was 19,174 compared with 13,302 in youth ( 4 ). Despite the incidence of childhood-onset type 1 diabetes in China being among the lowest in the world, prevalence data show similar trends across the life span. From 2010–2013, the incidence was 1.93/100,000 among individuals aged 0–14 years and 1.28/100,000 among those 15–29 years of age versus 0.69/100,000 among older adults ( 5 ). In aggregate, adults comprised 65.3% of all clinically defined newly diagnosed type 1 diabetes cases in China, which is similar to estimates using genetically stratified data from the population-based UK Biobank using a childhood-onset polygenic genetic risk score (GRS) ( 6 ). It is important to note that the proportion would likely be higher if autoimmune cases not requiring insulin initially were classified as type 1 diabetes. For example, in a clinic-based European study, the proportion of adults with diabetes not initially requiring insulin yet with type 1 diabetes–associated autoantibodies was even higher than those started on insulin at diagnosis with a defined type 1 diabetes diagnosis ( 9 ). Moreover, in an adult population-based study in China, the fraction (8.6%) with diabetes not requiring insulin yet with type 1 diabetes–associated autoantibodies was similar to that in Europe, implying that there could be over 6 million Chinese with adult-onset type 1 diabetes ( 10 ). While there is a wide range in the incidence of type 1 diabetes across different ethnic groups, even using differing methods of case identification ( 7 ), these data support the notion that, worldwide, over half of all new-onset type 1 diabetes cases occur in adults.
Natural History Studies of Type 1 Diabetes
Our understanding of the natural history of type 1 diabetes has been informed by a number of longitudinal and cross-sectional studies. At one end of the spectrum are prospective birth cohort studies, such as the BABYDIAB study in Germany and The Environmental Determinants of Diabetes in the Young (TEDDY) study, which includes sites in Germany, Finland, Sweden, and the U.S. While these studies now have the potential to explore the pathogenesis of islet autoimmunity by being extended into adulthood, they have primarily focused on events occurring in childhood ( 11 ). Clinical centers in North America, Europe, and Australia collaborate within Type 1 Diabetes TrialNet, a study that identifies autoantibody-positive adults and children in a cross-sectional manner to examine the pathogenesis of type 1 diabetes and to perform clinical trials on those at high risk in order to preserve β-cell function ( 12 ). At the other end of the spectrum, the European Prospective Investigation into Cancer and Nutrition (EPIC)-InterAct study is a case-cohort study nested in the U.K. prospective adult population-based EPIC study ( 13 ), while the clinical, immunogenetic, and metabolic characteristics of autoimmune adult-onset type 1 diabetes have been extensively studied in large American, European, and Chinese studies, including UK Prospective Diabetes Study (UKPDS), Action LADA, Scandia, Non Insulin Requiring Autoimmune Diabetes (NIRAD), and LADA China ( 9 , 14 – 19 ). Based on these cross-sectional and prospective studies, considerable data have been generated to define differences within type 1 diabetes according to the age at onset. Here, we highlight key aspects of age-related genetic, immune, and metabolic heterogeneity in type 1 diabetes. Of note, the term latent autoimmune diabetes in adults (LADA) has been used to describe adults with slowly progressive autoimmunity, sometimes exhibiting features overlapping with those of type 2 diabetes ( 9 , 14 , 18 ). At the outset of the workshop and for the purposes of this Perspective, LADA was not considered a unique entity; rather, we considered the classification of type 1 diabetes to include all individuals with evidence of autoimmunity, regardless of the trajectory of disease development (i.e., rapid or slowly progressive) or other associated demographic and/or clinical features (e.g., obesity).
Age-Related Genetic Heterogeneity
Type 1 diabetes shows heterogeneity across a broad range of clinical, genetic, immune, histological, and metabolic features ( 20 ). Childhood-onset type 1 diabetes is most often attributed to susceptibility alleles in human leukocyte antigen (HLA), which contribute ∼50% of the disease heritability. Whereas ethnic differences exist, notably for specific HLA genotypes, several broad principles apply. Compared with childhood-onset disease, adult-onset type 1 diabetes cases show lower type 1 diabetes concordance rates in twins ( 21 ), less high-risk HLA heterozygosity ( 19 ), lower HLA class I ( 14 ), more protective genotypes ( 14 , 15 ), and lower GRS ( 6 , 22 ), which are calculated by summing the odds ratios (OR) for disease-risk alleles.
Diabetes-Associated Immune Changes
Adult-onset type 1 diabetes, like childhood-onset type 1 diabetes, is associated with the presence of serum autoantibodies against β-cell antigens. Serum glutamic acid decarboxylase (GADA) autoantibodies may be useful as a predictor of type 1 diabetes in adults, as adult-onset cases most often present with GADA positivity ( 9 , 10 , 15 , 17 , 18 , 20 , 22 ) and possess an HLA-DR3 genotype ( 9 , 14 , 15 , 20 , 21 , 23 ). In one prospective study of a general population, the hazard risk of incident diabetes in those with a high type 1 diabetes GRS and GADA positivity was 3.23 compared with all other individuals, suggesting that 1.8% of incident diabetes in adults was attributable to that combination of risk factors ( 13 ). In adult-onset type 1 diabetes, multiple diabetes-associated autoantibodies tend to be less prevalent with increasing age at diagnosis ( 1 , 8 ), yet GADA remains the dominant autoantibody irrespective of the need for insulin treatment at diagnosis and irrespective of ethnicity ( 9 , 17 , 18 , 24 , 25 ), even despite a paucity of HLA DR3, as in Japan and China ( 17 , 18 ). In contrast, childhood-onset type 1 diabetes cases often have insulin autoantibodies and an HLA-DR4 genotype, higher identical twin disease concordance, more HLA heterozygosity, and higher GRS ( 20 ). Taken together, these data indicate that type 1 diabetes is heterogeneous across the spectrum of diagnoses, suggesting that pathogenesis and optimal therapy are also diverse.
Data from the TrialNet Pathway to Prevention cohort demonstrated lower risk of progression to type 1 diabetes in adults than children, even when both show multiple autoantibodies on a single occasion and are monitored over 10 years ( 12 ). One recent analysis found that the 5-year rate of progression to diabetes in multiple autoantibody–positive adults was only ∼15%, with a number of them remaining diabetes-free for decades ( 26 ). A combined cohort study, known as the Slow or Nonprogressive Autoimmunity to the Islets of Langerhans (SNAIL) study, is following such “slow progressors” with multiple autoantibodies who have yet to progress to stage 3 type 1 diabetes (i.e., clinical diagnosis) over at least a 10-year period ( 27 ). Many of these slow progressors lose disease-associated autoantibodies over time, adding complexity to cross-sectional classification ( 28 ). Based on estimates from natural history studies, slow progressors, even if identified when young, cannot account for all autoimmune adult-onset diabetes, indicating that autoantibodies must develop at all ages ( 11 ). However, little is known about those who initially develop autoimmunity as adults, mostly due to the lack of longitudinal studies focusing on this population.
People with type 1 diabetes, in contrast to the majority of those with type 2 diabetes, have altered adaptive immunity (i.e., islet autoantibodies and T-cell activation), while innate immune changes, including cytokine changes, are common to both ( 29 ). Increased T-cell activation by islet proteins has also been found in a proportion of adults with initially non-insulin-requiring diabetes, even when they lack diabetes autoantibodies ( 30 ). However, there is a paucity of immune studies on adult-onset type 1 diabetes and few histologic studies. An analysis of tissues from the Network for Pancreatic Organ Donors with Diabetes (nPOD) showed no relationship between age at diabetes onset and the frequency of islet insulitis ( 31 ). The composition of islet insulitis differs in very young children compared with older individuals, with the former having an increased frequency of B cells in islet infiltrates ( 32 ). However, relating pancreatic histological changes to changes in peripheral blood remains a challenge.
Adults with new-onset type 1 diabetes are at increased risk of other autoimmune conditions. About 30% of individuals with adult-onset type 1 diabetes have thyroid autoimmunity ( 27 , 29 ). In addition, adults with type 1 diabetes who possess high-titer GADA and/or multiple islet autoantibodies are at increased risk of progression to hypothyroidism ( 24 , 33 ). In a large population-based Chinese study, the prevalence of adult-onset type 1 diabetes was 6% among initially non-insulin-requiring diabetes cases, and 16.3% of them had thyroid autoimmunity (OR 2.4) ( 10 ). Of note, those with islet antigen 2 autoantibodies had a high risk of tissue transglutaminase autoantibodies, a marker for celiac disease (OR 19.1) ( 10 ). Thus, in the clinical setting, there should be a high index of suspicion for other autoimmune conditions in individuals with adult-onset type diabetes, and associated autoimmunity should be screened where clinically indicated.
Metabolic Characteristics of Adult-Onset Type 1 Diabetes
Age-related differences in type 1 diabetes extend to metabolic parameters. C-peptide at diagnosis is higher in adults than children, driven in part by higher BMI ( 34 ). Analysis of U.K., TrialNet, and Chinese cohorts has identified two distinct phases of C-peptide decline in stage 3 disease: an initial exponential fall followed by a period of relative stability. Along with initial differences at the time of clinical diagnosis, the rate of decline over 2–4 years was inversely related to age at onset ( 10 , 34 – 36 ). Furthermore, the U.S. T1D Exchange Study found that glycemic control was better in adults with type 1 diabetes than in children and adolescents with type 1 diabetes ( 37 ). The American Diabetes Association (ADA) targets for glycemia are higher in children, so that in this same cohort, 17% of children, compared with 21% of adults, achieved the ADA hemoglobin A 1c (HbA 1c ) goal of <7.5% and <7.0%, respectively ( 37 ). Other factors confound this relationship between age at diagnosis and metabolic control. First, individuals with adult-onset type 1 diabetes are more likely to have residual insulin-producing β-cells and persistent measurable C-peptide in disease of long duration, the latter of which has been linked to improved glycemic control ( 38 , 39 ). Second, individuals with adult-onset type 1 diabetes, initially not on insulin therapy, tend to have worse metabolic control than people with type 2 diabetes, even when receiving insulin treatment ( 9 , 40 ). The sole exception is the LADA China study, where worse control was noted only among those with a high GAD titer ( 18 ). Metabolic differences between adults and children extend beyond C-peptide. Adults with autoantibody positivity who progressed to type 1 diabetes were less likely than very young children to exhibit elevated proinsulin/C-peptide ratios prior to stage 3 disease onset ( 41 ). In addition, in individuals with disease of long duration, those diagnosed at an older age had evidence of improved proinsulin processing and nutrient-induced proinsulin secretory capacity ( 42 ).
Diagnosis and Management of Adult-Onset Type 1 Diabetes
Correctly identifying diabetes etiology and type is difficult, and misclassification may occur in up to 40% of adults presenting with type 1 diabetes ( 1 , 2 ). Reasons underlying misclassification are multiple and include 1 ) lack of awareness that the onset of type 1 diabetes is not limited to children; 2 ) the overwhelming majority of people developing diabetes as older adults have type 2 diabetes, contributing to a confirmation bias ( 2 ); 3 ) typical clinical criteria, such as BMI and metabolic syndrome, can be poor discriminators, especially as rates of obesity in the overall population increase ( 9 , 43 ); 4 ) clinical characteristics of adult-onset type 1 diabetes can masquerade as type 2 diabetes, given their slow metabolic progression and risk of metabolic syndrome (which occurs in about 40%), so that the distinction between types of diabetes may be blurred ( 43 – 45 ); and 5 ) lack of awareness of and accessibility to biomarkers that may serve as tools to distinguish type 1 diabetes and type 2 diabetes.
Tools to distinguish type 1 and type 2 diabetes are under active development. For example, classification models integrating up to five prespecified predictor variables, including clinical features (age of diagnosis and BMI) and clinical biomarkers (autoantibodies and GRS) in a White European population, had high accuracy to identify adults with recently diagnosed diabetes with rapid insulin requirement despite using GRS derived from childhood-onset type 1 diabetes. While GRS have the potential to assist diagnosis of type 1 diabetes in uncertain cases, they are not yet widely available in clinical practice. Moreover, it is important to note that while the model was optimized with the inclusion of all five variables, the addition of GRS had only a modest effect on overall model performance ( 22 ).
Classification can be aided by the measurement of autoantibodies and C-peptide. Recommended autoantibodies to assay at the time of diagnosis include those to insulin (insulin autoantibody), glutamate decarboxylase isoform 65 (GAD65A), insulinoma antigen 2, and zinc transporter isoform 8 (Znt8A), with GAD65A being the most prevalent autoantibody among adults. High levels or the presence of more than one antibody increases the likelihood of type 1 diabetes. However, it is important to realize that islet autoantibodies are a continuous marker that can also occur in the population without diabetes. As with many other tests, an abnormal test is usually based on a threshold signal from control populations without diabetes, usually the 97.5th or the 99th centile. Therefore, false-positive results with these assays can occur and can be reduced by using higher-specificity assays or thresholds and targeting testing toward those with clinical features suggestive of type 1 diabetes ( 46 ). Finally, since antibody levels can wane over time in established type 1 diabetes, the absence of autoantibodies does not rule out the possibility of a diagnosis of type 1 diabetes.
Measurement of C-peptide, paired with a blood glucose in the same sample, provides an estimate of endogenous insulin production and has the most utility in disease of long duration when levels fall below 300 pmol/L ( 39 , 47 ). However, C-peptide levels are typically higher at presentation and may be difficult to distinguish from levels in type 2 diabetes, which are usually >600 pmol/L. Thus, thresholds of C-peptide that clearly delineate type 1 diabetes from type 2 diabetes at diagnosis cannot be categorically defined, and C-peptide must be interpreted within the context of other clinical and laboratory features. Measurement of a random nonfasting C-peptide is superior to fasting C-peptide in identifying type 1 diabetes ( 48 ) and is well correlated with stimulated C-peptide levels measured during a mixed-meal tolerance test, which is considered the gold standard assessment of insulin secretory function in established type 1 diabetes ( 49 ). A recent analysis found that concomitant blood glucose ≥144 mg/dL (8 mmol/L) increased the specificity of random C-peptide in predicting a stimulated C-peptide level <600pmol/L, suggesting this is a reasonable threshold of blood glucose to employ for C-peptide interpretation ( 49 ).
C-peptide also can be used to guide therapy ( 50 ). Individuals with a random C-peptide level ≤300 pmol/L should be managed mainly with insulin. For those with random C-peptide levels >300 pmol/L, insulin could be combined with other diabetes therapies, although evidence about safety and efficacy is limited. It is generally agreed that sulfonylureas should be avoided because of the potential to hasten β-cell failure ( 50 ). There is concern for increased risk of diabetic ketoacidosis (DKA) with sodium–glucose cotransporter 1 (SGLT1) and SGLT2 inhibitors when these agents are used in type 1 diabetes, especially in nonobese individuals who may need only low dosages of insulin ( 51 ). All other agents could be considered for therapy in those not requiring insulin initially. In individuals with random C-peptide levels exceeding 600 pmol/L, management can be much as recommended for type 2 diabetes, with the caveats outlined above ( 50 ). An important consideration is that loss of β-cell function may be rapid in autoimmune diabetes. As such, individuals treated without insulin should be closely monitored.
In the absence of prospectively validated decision support tools that have been tested in multiethnic populations, we suggest, as an approach to aid the practicing physician, assessment of age, autoimmunity, body habitus/BMI, background, control, and comorbidities, using the acronym AABBCC ( Table 2 ). This approach includes the clinical consideration of autoimmunity and other clinical features suggestive of type 1 diabetes, including age at diagnosis, low BMI, an unexplained or rapid worsening of clinical course manifesting as a lack of response or rising HbA 1c with type 2 diabetes medications, and a rapid requirement for insulin therapy, especially within 3 years of diagnosis. It should be emphasized that among these features, age at diagnosis (<40 years), low BMI (<25 kg/m 2 ), and rapid need for insulin therapy are the most discriminatory ( 43 ). We recommend measurement of islet antibodies and C-peptide be considered in all older people with clinical features that suggest type 1 diabetes, with islet autoantibodies being the initial test of choice in short-duration disease (<3 years) and C-peptide the test of choice at longer durations.
Diabetes-Associated Comorbidities and Complications
The U.S. SEARCH for Diabetes in Youth study reported that nearly 30% of youth with newly diagnosed type 1 diabetes age <20 years presented with DKA ( 52 ). The frequency of DKA among adults at diagnosis with type 1 diabetes is unknown but is believed to be lower given that they often have higher C-peptide levels at diagnosis and a slower decline in β-cell function over time, even in those requiring insulin initially ( 34 ). Among childhood-onset type 1 diabetes, most episodes of DKA beyond diagnosis are associated with insulin omission, pump failure, or treatment error ( 53 ). However, for adults with type 1 diabetes, the primary risk factors are noncompliance and infections ( 54 ), the former sometimes due to the cost of insulin ( 55 ). Thus, there is a need to further understand DKA in adults, not least because it is associated with long-term worsening glycemic control ( 56 ).
Hypoglycemia
Fear of hypoglycemia remains a major problem in the clinical management of adults with type 1 diabetes ( 57 ), influencing quality of life and glycemic control. The effect of diabetes duration or age at diagnosis on hypoglycemia risk is not consistent among different studies. However, α-cell responses to hypoglycemia and hypoglycemia risk are both lower in individuals with higher C-peptide levels ( 38 ). Because residual C-peptide is more likely to be observed in those with a later age of onset, hypoglycemia risk may be different between those with childhood- and adult-onset diabetes. While insulin pumps and continuous glucose monitors are associated with improved glycemic control and reduced hypoglycemia ( 37 ), adults may show reluctance or inertia in adopting newer technologies. In the T1D Exchange study population, 63% of adults used an insulin pump while only 30% used a continuous glucose monitor, and use of these technologies tended to be lower in adults than in children ( 37 ). Factors that dictate use of these technologies are multiple and may include reduced access to or acceptance of wearable technology, challenges with insurance coverage, especially in the context of past misclassification, and/or inadequate education about hypoglycemia risk ( 58 ). A better understanding of potential barriers to technology use in adult-onset type 1 diabetes is needed. Furthermore, little is known about changes in hypoglycemia risk across the life span of individuals with adult-onset disease, representing an important gap in knowledge.
Microvascular and Macrovascular Disease Complications
Despite the prevalence of adult-onset type 1 diabetes, there is a paucity of data on the burden of microvascular complications in this population. Current knowledge is largely based on small, cross-sectional studies. In aggregate, these studies suggest that the prevalence of nephropathy and retinopathy are lower in adult-onset type 1 than in type 2 diabetes, but this conclusion is potentially confounded by diabetes duration. For example, the prevalence of nephropathy and retinopathy was lower in Chinese individuals with adult-onset type 1 diabetes than in those with type 2, but only in those with a disease duration <5 years, while in the Botnia Study, retinopathy risk in adult-onset type 1 diabetes increased, as expected, with disease duration ( 59 ). Two substantial prospective studies recently reported that those adults with diabetes enrolled in the UKPDS who were also GADA positive (i.e., presumably with type 1 diabetes) compared with those who were GADA negative (with type 2 diabetes) showed a higher prevalence of retinopathy and lower prevalence of cardiovascular events ( 60 , 61 ). These results are consistent with people with adult-onset type 1 diabetes compared with those with type 2 diabetes, showing a general tendency to higher HbA 1c levels ( 40 , 44 , 60 , 61 ) as well as reduced traditional cardiovascular risk factors, including reduced adiposity (BMI and waist circumference), metabolic (lipid levels), and vascular (blood pressure) profiles ( 9 , 24 , 62 ). Nevertheless, all-cause mortality and cardiovascular mortality rates in such individuals with adult-onset type 1 diabetes ( 59 ) are still higher than those among individuals without diabetes. In addition, there are discrepancies across studies, likely related to differences in populations under study (i.e., age, race/ethnicity, and diabetes duration), lack of consistent case definitions (i.e., adult-onset type 1 diabetes or LADA cases), and different outcomes, as well as small sample sizes with insufficient events on which to base strong recommendations.
Psychosocial Challenges
Negative stressors, including pressure to achieve target HbA 1c levels, lifestyle considerations, and fear of complications, are factors leading to the increased frequency of mood disorders, attempted suicide, and psychiatric care in adults with diabetes ( 63 ). In individuals who have experienced misclassification, additional stress derives from conflicting messages about the nature of their diabetes. Among adults with type 1 diabetes, those with high psychological coping skills (e.g., self-efficacy, self-esteem, and optimism) and adaptive skills may buffer the negative effect of stress and should be cultivated ( 64 ). Relationship challenges, including sexual intimacy, starting a family, caring for children, and relational stress, are major stressors for adults with type 1 diabetes ( 65 ). In addition, there is the looming threat of complications, including blindness and amputations ( 65 ). Adults with type 1 diabetes describe a sense of powerlessness, fear of hypoglycemia, and the challenges of both self-management and appropriate food management ( 66 ). A common misunderstanding is that while they face the same life choices associated with type 2 diabetes (e.g., weight loss, exercise, and limiting intake of simple sugars), adults with type 1 diabetes may require different management skills ( 67 ). Moreover, there is a strong association in adults with type 1 diabetes between chronic, stressful life events and fluctuating HbA 1c , possibly due to indirect mechanisms, including adherence to diabetes management ( 68 ). Whether these risks differ between those diagnosed as children or as adults is unclear and requires additional study.
In this Perspective, we have summarized the current understanding of adult-onset type 1 diabetes while identifying many knowledge gaps ( Table 1 ). Epidemiological data from diverse ethnic groups show that adult-onset type 1 diabetes is often more prevalent than childhood-onset type 1 diabetes. However, our understanding of type 1 diabetes presenting in adults is limited. This striking shortfall in knowledge ( Table 1 ) results in frequent misclassification, which may negatively impact disease management. Here, we outline a roadmap for addressing these deficiencies ( Fig. 1 ). A cornerstone of this roadmap is a renewed emphasis on the careful consideration of the underlying etiology of diabetes in every adult presenting with diabetes.

Proposed roadmap to better understand, diagnose, and care for adults with type 1 diabetes (T1D). Created in BioRender ( BioRender.com ).
Knowledge gaps
| Area of focus . | Description . |
|---|---|
| Eliminating cultural bias in order to understand what impacts disease development | Most large-scale studies of adult type 1 diabetes have been done in Europe, North America, and China. There is a pressing need to extend these studies to other continents and to diverse racial and ethnic groups. Such studies could help us identify and understand the nature and implications of diversity, whether in terms of pathogenesis, cultural differences, or health care disparity. In addition, prospective childhood studies of high-risk birth cohorts could be extended into adulthood and new studies initiated to better understand mechanisms behind disease development and whether there is a differentiation in the disease process between young and adult type 1 diabetes. |
| Population screening | At present, universal childhood screening programs are being developed in many countries. Research will be needed to develop strategies for the follow-up of autoantibody-positive populations throughout adulthood. |
| Disease-modifying therapies in early-stage disease | Trials of disease-modifying therapies have generally shown better efficacy in children ( ). There are likely to be important differences in agent selection between adult and pediatric populations, and these differences require study. |
| Diagnosis and misclassification | There is a need to build a diagnostic decision tree to aid in diabetes classification. Tools are needed to estimate individual-level risk. |
| Adjunctive therapies | There is a need to better understand the benefits and risks of using therapies that are adjunctive to insulin in adult-onset type 1 diabetes. To this end, large-scale drug trials need to be performed, and therapeutic decision trees are required to help health care professionals and endocrinologists select such therapies. |
| Post-diagnosis education and support | Improving education and support post-diagnosis is vital and should include psychosocial support, health care provision, and analysis of long-term outcomes (including complications) in adult-onset type 1 diabetes. Current knowledge is limited with respect to complications, especially related to the complex mechanisms contributing to macrovascular disease in adult-onset type 1 diabetes. Surveillance efforts based on larger and representative cohorts of patients with clear and consistent case definitions are needed to better understand the burden and risk of diabetes-related chronic complications in this large population. |
| Area of focus . | Description . |
|---|---|
| Eliminating cultural bias in order to understand what impacts disease development | Most large-scale studies of adult type 1 diabetes have been done in Europe, North America, and China. There is a pressing need to extend these studies to other continents and to diverse racial and ethnic groups. Such studies could help us identify and understand the nature and implications of diversity, whether in terms of pathogenesis, cultural differences, or health care disparity. In addition, prospective childhood studies of high-risk birth cohorts could be extended into adulthood and new studies initiated to better understand mechanisms behind disease development and whether there is a differentiation in the disease process between young and adult type 1 diabetes. |
| Population screening | At present, universal childhood screening programs are being developed in many countries. Research will be needed to develop strategies for the follow-up of autoantibody-positive populations throughout adulthood. |
| Disease-modifying therapies in early-stage disease | Trials of disease-modifying therapies have generally shown better efficacy in children ( ). There are likely to be important differences in agent selection between adult and pediatric populations, and these differences require study. |
| Diagnosis and misclassification | There is a need to build a diagnostic decision tree to aid in diabetes classification. Tools are needed to estimate individual-level risk. |
| Adjunctive therapies | There is a need to better understand the benefits and risks of using therapies that are adjunctive to insulin in adult-onset type 1 diabetes. To this end, large-scale drug trials need to be performed, and therapeutic decision trees are required to help health care professionals and endocrinologists select such therapies. |
| Post-diagnosis education and support | Improving education and support post-diagnosis is vital and should include psychosocial support, health care provision, and analysis of long-term outcomes (including complications) in adult-onset type 1 diabetes. Current knowledge is limited with respect to complications, especially related to the complex mechanisms contributing to macrovascular disease in adult-onset type 1 diabetes. Surveillance efforts based on larger and representative cohorts of patients with clear and consistent case definitions are needed to better understand the burden and risk of diabetes-related chronic complications in this large population. |
In the absence of data-driven classification tools capable of estimating individual-level risk, we offer a simple set of questions, incorporating what we have termed the AABBCCs of diabetes classification and management ( Table 2 ). In parallel, we invite the research community to join together in addressing key gaps in knowledge through studies aimed at defining the genetic, immunologic, and metabolic phenotype of adult-onset type 1 diabetes with the goal of using this knowledge to develop improved approaches for disease management and prevention ( Fig. 1 ).
AABBCC approach to diabetes classification
| Parameter . | Description . |
|---|---|
| Age | Autoimmune diabetes is most prevalent in patients aged <50 years at diagnosis. Those aged <35 years at diagnosis should be considered for maturity-onset diabetes of the young as well as type 1 diabetes |
| Autoimmunity | Does this individual have islet autoantibodies or a history of autoimmunity (i.e., thyroid disease, celiac disease)? Is there a goiter or vitiligo on exam? |
| Body habitus/BMI | Is the body habitus or BMI inconsistent with a diagnosis of type 2 diabetes, especially if BMI <25 kg/m ? |
| Background | What is the patient’s background? Is there a family history of autoimmunity and/or type 1 diabetes? Are they from a high-risk ethnic group? |
| Control | Are diabetes control and HbA worsening on noninsulin therapies? Has there been an accelerated change in HbA ? Is the C-peptide low, that is, ≤300 pmol/L (especially <200 pmol/L), or is there clinical evidence that β-cell function is declining? Was there a need for insulin therapy within 3 years of diabetes diagnosis? |
| Comorbidities | Irrespective of immunogenetic background, coexistent cardiac or renal disease and their risk factors impact the approach to therapy and HbA targets. |
| Parameter . | Description . |
|---|---|
| Age | Autoimmune diabetes is most prevalent in patients aged <50 years at diagnosis. Those aged <35 years at diagnosis should be considered for maturity-onset diabetes of the young as well as type 1 diabetes |
| Autoimmunity | Does this individual have islet autoantibodies or a history of autoimmunity (i.e., thyroid disease, celiac disease)? Is there a goiter or vitiligo on exam? |
| Body habitus/BMI | Is the body habitus or BMI inconsistent with a diagnosis of type 2 diabetes, especially if BMI <25 kg/m ? |
| Background | What is the patient’s background? Is there a family history of autoimmunity and/or type 1 diabetes? Are they from a high-risk ethnic group? |
| Control | Are diabetes control and HbA worsening on noninsulin therapies? Has there been an accelerated change in HbA ? Is the C-peptide low, that is, ≤300 pmol/L (especially <200 pmol/L), or is there clinical evidence that β-cell function is declining? Was there a need for insulin therapy within 3 years of diabetes diagnosis? |
| Comorbidities | Irrespective of immunogenetic background, coexistent cardiac or renal disease and their risk factors impact the approach to therapy and HbA targets. |
Acknowledgments. Sharon Saydah, Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, attended the workshop and participated in subsequent discussions of the manuscript. Elizabeth Seaquist, Division of Diabetes, Endocrinology, and Metabolism at the University of Minnesota, participated in the workshop. The authors acknowledge Marilyn L. Wales for her assistance with formatting the manuscript.
Funding and Duality of Interest. This manuscript is the result of a one-day meeting held at JDRF headquarters in New York, NY. Financial support for the workshop was provided by JDRF and Janssen Research and Development, LLC. Financial support from Janssen Research and Development, LLC, for the workshop was in an unrestricted grant to JDRF. JDRF provided participants with transportation, lodging, and meals to attend the workshop. No additional support was provided for the writing of the manuscript. R.D.L. is supported by a grant from the European Union (contract no. QLGi-CT-2002-01886). C.E.-M. is supported by National Institute for Health Research grants R01 DK093954, R21DK11 9800, U01DK127786, R01DK127308, and P30DK 097512; VA Merit Award I01BX001733; JDRF grant 2-SRA-2019-834-S-B; and gifts from the Sigma Beta Sorority, the Ball Brothers Foundation, and the George and Frances Ball Foundation. R.B. is supported in part by the Italian Ministry of University and Research (project code 20175L9H7H). A.G.J. is funded by a National Institute for Health Research (NIHR) Clinician Scientist fellowship (CS-2015-15-018). L.S.P. is supported in part by U.S. Department of Veterans Affairs (VA) awards CSP #2008, I01 CX001899, I01 CX001737, and Health Services Research & Development IIR 07-138; National Institute for Health Research awards R21 DK099716, R18 DK066204, R03 AI133172, R21 AI156161, U01 DK091958, U01 DK098246, and UL1 TR002378; and Cystic Fibrosis Foundation award PHILLI12A0.
R.D.L. received unrestricted educational grants from Novo Nordisk, Sanofi, MSD, and AstraZeneca. C.E.-M. has participated in advisory boards for Dompé Pharmaceuticals, Provention Bio, MaiCell Technologies, and ISLA Technologies. C.E.M. is the recipient of in-kind research support from Nimbus Pharmaceuticals and Bristol Myers Squibb and an investigator-initiated research grant from Eli Lilly and Company. J.F.-B. and J.L.D. were employed by JDRF during the workshop and early stages of writing. J.F.-B. is currently an employee of Provention Bio, and J.L.D. is currently an employee of Janssen Research and Development, LLC. R.B. participated in advisory boards for Sanofi and Eli Lilly and received honoraria for speaker bureaus from Sanofi, Eli Lilly, AstraZeneca, Novo Nordisk, and Abbott. L.S.P. has served on scientific advisory boards for Janssen and has or had research support from Merck, Pfizer, Eli Lilly, Novo Nordisk, Sanofi, PhaseBio, Roche, AbbVie, Vascular Pharmaceuticals, Janssen, GlaxoSmithKline, and the Cystic Fibrosis Foundation. L.S.P. is also a cofounder and officer and board member and stockholder for a company, Diasyst, Inc., that markets software aimed to help improve diabetes management. No other potential conflicts of interest relevant to this article were reported.
The sponsors had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, and preparation, review, or approval of the manuscript. This work is not intended to reflect the official opinion of the VA or the U.S. Government.
Author Contributions. R.D.L., C.E.M., J.F.-B., and J.L.D. conceived of the article and wrote and edited the manuscript. All other authors were involved in the writing and editing of the manuscript. R.D.L. and C.E.-M. are guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Email alerts
- Online ISSN 1935-5548
- Print ISSN 0149-5992
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- New advances in type 1...
New advances in type 1 diabetes
- Related content
- Peer review
This article has a correction. Please see:
- New advances in type 1 diabetes - June 03, 2024
- Savitha Subramanian , professor of medicine ,
- Farah Khan , clinical associate professor of medicine ,
- Irl B Hirsch , professor of medicine
- University of Washington Diabetes Institute, Division of Metabolism, Endocrinology and Nutrition, University of Washington, Seattle, WA, USA
- Correspondence to: I B Hirsch ihirsch{at}uw.edu
Type 1 diabetes is an autoimmune condition resulting in insulin deficiency and eventual loss of pancreatic β cell function requiring lifelong insulin therapy. Since the discovery of insulin more than 100 years ago, vast advances in treatments have improved care for many people with type 1 diabetes. Ongoing research on the genetics and immunology of type 1 diabetes and on interventions to modify disease course and preserve β cell function have expanded our broad understanding of this condition. Biomarkers of type 1 diabetes are detectable months to years before development of overt disease, and three stages of diabetes are now recognized. The advent of continuous glucose monitoring and the newer automated insulin delivery systems have changed the landscape of type 1 diabetes management and are associated with improved glycated hemoglobin and decreased hypoglycemia. Adjunctive therapies such as sodium glucose cotransporter-1 inhibitors and glucagon-like peptide 1 receptor agonists may find use in management in the future. Despite these rapid advances in the field, people living in under-resourced parts of the world struggle to obtain necessities such as insulin, syringes, and blood glucose monitoring essential for managing this condition. This review covers recent developments in diagnosis and treatment and future directions in the broad field of type 1 diabetes.
Introduction
Type 1 diabetes is an autoimmune condition that occurs as a result of destruction of the insulin producing β cells of the pancreatic islets, usually leading to severe endogenous insulin deficiency. 1 Without treatment, diabetic ketoacidosis will develop and eventually death will follow; thus, lifelong insulin therapy is needed for survival. Type 1 diabetes represents 5-10% of all diabetes, and diagnosis classically occurs in children but can also occur in adulthood. The burden of type 1 diabetes is expansive; it can result in long term complications, decreased life expectancy, and reduced quality of life and can add significant financial burden. Despite vast improvements in insulin, insulin delivery, and glucose monitoring technology, a large proportion of people with type 1 diabetes do not achieve glycemic goals. The massive burden of type 1 diabetes for patients and their families needs to be appreciated. The calculation and timing of prandial insulin dosing, often from food with unknown carbohydrate content, appropriate food and insulin dosing when exercising, and cost of therapy are all major challenges. The psychological realities of both acute management and the prospect of chronic complications add to the burden. Education programs and consistent surveillance for “diabetes burnout” are ideally available to everyone with type 1 diabetes.
In this review, we discuss recent developments in the rapidly changing landscape of type 1 diabetes and highlight aspects of current epidemiology and advances in diagnosis, technology, and management. We do not cover the breadth of complications of diabetes or certain unique scenarios including psychosocial aspects of type 1 diabetes management, management aspects specific to older adults, and β cell replacement therapies. Our review is intended for the clinical reader, including general internists, family practitioners, and endocrinologists, but we acknowledge the critical role that people living with type 1 diabetes and their families play in the ongoing efforts to understand this lifelong condition.
Sources and selection criteria
We did individual searches for studies on PubMed by using terms relevant to the specific topics covered in this review pertaining to type 1 diabetes. Search terms used included “type 1 diabetes” and each individual topic—diagnosis, autoantibodies, adjuvant therapies, continuous glucose monitoring, automated insulin delivery, immunotherapies, diabetic ketoacidosis, hypoglycemia, and under-resourced settings. We considered all studies published in the English language between 1 January 2001 and 31 January 2023. We selected publications outside of this timeline on the basis of relevance to each topic. We also supplemented our search strategy by a hand search of the references of key articles. We prioritized studies on each highlighted topic according to the level of evidence (randomized controlled trials (RCTs), systematic reviews and meta-analyses, consensus statements, and high quality observational studies), study size (we prioritized studies with at least 50 participants when available), and time of publication (we prioritized studies published since 2003 except for the landmark Diabetes Control and Complications Trial and a historical paper by Tuomi on diabetes autoantibodies, both from 1993). For topics on which evidence from RCTs was unavailable, we included other study types of the highest level of evidence available. To cover all important clinical aspects of the broad array of topics covered in this review, we included additional publications such as clinical reviews as appropriate on the basis of clinical relevance to both patients and clinicians in our opinion.
Epidemiology
The incidence of type 1 diabetes is rising worldwide, possibly owing to epigenetic and environmental factors. Globally in 2020 an estimated 8.7 million people were living with type 1 diabetes, of whom approximately 1.5 million were under 20 years of age. 2 This number is expected to rise to more than 17 million by 2040 ( https://www.t1dindex.org/#global ). The International Diabetes Federation estimates the global prevalence of type 1 diabetes at 0.1%, and this is likely an underestimation as diagnoses of type 1 diabetes in adults are often not accounted for. The incidence of adult onset type 1 diabetes is higher in Europe, especially in Nordic countries, and lowest in Asian countries. 3 Adult onset type 1 diabetes is also more prevalent in men than in women. An increase in prevalence in people under 20 years of age has been observed in several western cohorts including the US, 4 5 Netherlands, 6 Canada, 7 Hungary, 8 and Germany. 9
Classically, type 1 diabetes presents over the course of days or weeks in children and adolescents with polyuria, polydipsia, and weight loss due to glycosuria. The diagnosis is usually straightforward, with profound hyperglycemia (often >300 mg/dL) usually with ketonuria with or without ketoacidemia. Usually, more than one autoantibody is present at diagnosis ( table 1 ). 10 The number of islet autoantibodies combined with parameters of glucose tolerance now forms the basis of risk prediction for type 1 diabetes, with stage 3 being clinical disease ( fig 1 ). 11 The originally discovered autoantibody, islet cell antibody, is no longer used clinically owing to variability of the assay despite standardisation. 12
Autoantibody characteristics associated with increased risk of type 1 diabetes 10
- View inline

Natural history of type 1 diabetes. Adapted with permission from Insel RA, et al. Diabetes Care 2015;38:1964-74 11
- Download figure
- Open in new tab
- Download powerpoint
Half of all new cases of type 1 diabetes are now recognized as occurring in adults. 13 Misclassification due to misdiagnosis (commonly as type 2 diabetes) occurs in nearly 40% of people. 14 As opposed to typical childhood onset type 1 diabetes, progression to severe insulin deficiency, and therefore its clinical presentation in adults, is variable. The term latent autoimmune diabetes of adults (LADA) was introduced 30 years ago to identify adults who developed immune mediated diabetes. 15 An international consensus defined the diagnostic criteria for LADA as age >30 years, lack of need for insulin use for at least six months, and presence of islet cell autoantibodies. 16 However, debate as to whether the term LADA should even be used as a diagnostic term persists. The American Diabetes Association (ADA) Standards of Care note that for the purpose of classification, all forms of diabetes mediated by autoimmune β cell destruction are included in the classification of type 1 diabetes. 17 Nevertheless, they note that use of the term LADA is acceptable owing to the practical effect of heightening awareness of adults likely to have progressive autoimmune β cell destruction and thereby accelerating insulin initiation by clinicians to prevent diabetic ketoacidosis.
The investigation of adults with suspected type 1 diabetes is not always straightforward ( fig 2 ). 18 Islet cell autoantibodies such as glutamic acid decarboxylase antibody (GADA), tyrosine phosphatase IA2 antibody, and zinc transporter isoform 8 autoantibody act as markers of immune activity and can be detected in the blood with standardized assays ( table 1 ). The presence of one or more antibodies in adults with diabetes could mark the progression to severe insulin deficiency; these individuals should be considered to have type 1 diabetes. 1 Autoantibodies, especially GADA, should be measured only in people with clinically suspected type 1 diabetes, as low concentrations of GADA can be seen in type 2 diabetes and thus false positive measurements are a concern. 19 That 5-10% of cases of type 1 diabetes may occur without diabetes autoantibodies is also now clear, 20 and that the diabetes autoantibodies disappear over time is also well appreciated. 21

Flowchart for investigation of suspected type 1 diabetes in adults, based on data from white European populations. No single clinical feature in isolation confirms type 1 diabetes. The most discriminative feature is younger age at diagnosis (<35 years), with lower body mass index (<25), unintentional weight loss, ketoacidosis, and glucose >360 mg/dL at presentation. Adapted with permission from Holt RIG, et al. Diabetes Care 2021;44:2589-625 1
Genetic risk scoring (GRS) for type 1 diabetes has received attention to differentiate people whose classification is unclear. 22 23 24 Developed in 2019, the T1D-GRS2 uses 67 single nucleotide polymorphisms from known autoimmune loci and can predict type 1 diabetes in children of European and African ancestry. Although GRS is not available for routine clinical use, it may allow prediction of future cases of type 1 diabetes to allow prevention strategies with immune intervention (see below).
A major change in the type 1 diabetes phenotype has occurred over the past few decades, with an increase in obesity; the reasons for this are complex. In the general population, including people with type 1 diabetes, an epidemic of sedentary lifestyles and the “westernized diet” consisting of increased processed foods, refined sugars, and saturated fat is occurring. In people with type 1 diabetes, the overall improvement in glycemic control since the report of the Diabetes Control and Complications Trial (DCCT) in 1993 (when one or two insulin injections a day was standard therapy) has resulted in less glycosuria so that the typical patient with lower body weight is uncommon in high income countries. In the US T1D Exchange, more than two thirds of the adult population were overweight or obese. 25
Similarly, obesity in young people with type 1 diabetes has also increased over the decades. 26 The combination of autoimmune insulin deficiency with obesity and insulin resistance has received several descriptive names over the years, with this phenotype being described as double diabetes and hybrid diabetes, among others, 26 27 but no formal nomenclature in the diabetes classification exists. Many of these patients have family members with type 2 diabetes, and some patients probably do have both types of diabetes. Clinically, minimal research has been done into how this specific population responds to certain antihyperglycemic oral agents, such as glucagon-like peptide 1 (GLP-1) receptor agonists, given the glycemic, weight loss, and cardiovascular benefits seen with these agents. 28 These patients are common in most adult diabetes practices, and weight management in the presence of insulin resistance and insulin deficiency remains unclear.
Advances in monitoring
The introduction of home blood glucose monitoring (BGM) more than 45 years ago was met with much skepticism until the report of the DCCT. 29 Since then, home BGM has improved in accuracy, precision, and ease of use. 30 Today, in many parts of the world, home BGM, a static measurement of blood glucose, has been replaced by continuous glucose monitoring (CGM), a dynamic view of glycemia. CGM is superior to home BGM for glycemic control, as confirmed in a meta-analysis of 21 studies and 2149 participants with type 1 diabetes in which CGM use significantly decreased glycated hemoglobin (HbA 1c ) concentrations compared with BGM (mean difference −0.23%, 95% confidence interval −3.83 to −1.08; P<0.001), with a greater benefit if baseline HbA 1c was >8% (mean difference −0.43%, −6.04 to −3.30; P<0.001). 31 This newer technology has also evolved into a critical component of automated insulin delivery. 32
CGM is the standard for glucose monitoring for most adults with type 1 diabetes. 1 This technology uses interstitial fluid glucose concentrations to estimate blood glucose. Two types of CGM are available. The first type, called “real time CGM”, provides a continuous stream of glucose data to a receiver, mobile application, smartwatch, or pump. The second type, “intermittently scanned CGM,” needs to be scanned by a reader device or smartphone. Both of these technologies have shown improvements in HbA 1c and amount of time spent in the hypoglycemic range compared with home BGM when used in conjunction with multiple daily injections or “open loop” insulin pump therapy. 33 34 Real time CGM has also been shown to reduce hypoglycemic burden in older adults with type 1 diabetes ( table 2 ). 36 Alerts that predict or alarm with both hypoglycemia and hyperglycemia can be customized for the patient’s situation (for example, a person with unawareness of hypoglycemia would have an alert at a higher glucose concentration). Family members can also remotely monitor glycemia and be alerted when appropriate. The accuracy of these devices has improved since their introduction in 2006, so that currently available sensors can be used without a confirmation glucose concentration to make a treatment decision with insulin. However, some situations require home BGM, especially when concerns exist that the CGM does not match symptoms of hypoglycemia.
Summary of trials for each topic covered
Analysis of CGM reports retrospectively can assist therapeutic decision making both for the provider and the patient. Importantly, assessing the retrospective reports and watching the CGM in real time together offer insight to the patient with regard to insulin dosing, food choices, and exercise. Patients should be encouraged to assess their data on a regular basis to better understand their diabetes self-management. Table 3 shows standard metrics and targets for CGM data. 52 Figure 3 shows an ambulatory glucose profile.
Standardized continuous glucose monitoring metrics for adults with diabetes 52

Example of ambulatory glucose profile of 52 year old woman with type 1 diabetes and fear of hypoglycemia. CGM=continuous glucose monitoring; GMI=glucose management indicator
Improvements in technology and evidence for CGM resulting in international recommendations for its widespread use have resulted in greater uptake by people with type 1 diabetes across the globe where available and accessible. Despite this, not everyone wishes to use it; some people find wearing any device too intrusive, and for many the cost is prohibitive. These people need at the very least before meal and bedtime home BGM.
A next generation implantable CGM device (Sensionics), with an improved calibration algorithm that lasts 180 days after insertion by a healthcare professional, is available in both the EU and US. Although fingerstick glucose calibration is needed, the accuracy is comparable to that of other available devices. 53
Advances in treatments
The discovery of insulin in 1921, resulting in a Nobel Prize, was considered one of the greatest scientific achievements of the 20th century. The development of purified animal insulins in the late 1970s, followed by human insulin in the early 1980s, resulted in dramatic reductions in allergic reactions and lipoatrophy. Introduction of the first generation of insulin analogs, insulin lispro in the mid-1990s followed by insulin glargine in the early 2000s, was an important advance for the treatment of type 1 diabetes. 54 We review the next generation of insulin analogs here. Table 4 provides details on available insulins.
Pharmacokinetics of commonly used insulin preparations
Ultra-long acting basal insulins
Insulin degludec was developed with the intention of improving the duration of action and achieving a flatter profile compared with the original long acting insulin analogs, insulin glargine and insulin detemir. Its duration of action of 42 hours at steady state means that the profile is generally flat without significant day-to-day variability, resulting in less hypoglycemia compared with U-100 glargine. 39 55
When U-100 insulin glargine is concentrated threefold, its action is prolonged. 56 U-300 glargine has a different kinetic profile and is delivered in one third of the volume of U-100 glargine, with longer and flatter effects. The smaller volume of U-300 glargine results in slower and more gradual release of insulin monomers owing to reduced surface area in the subcutaneous space. 57 U-300 glargine also results in lesser hypoglycemia compared with U-100 glargine. 58
Ultra-rapid acting prandial insulins
Rapid acting insulin analogs include insulin lispro, aspart, and glulisine. With availability of insulin lispro, the hope was for a prandial insulin that better matched food absorption. However, these newer insulins are too slow to control the glucose spike seen with ingestion of a high carbohydrate load, leading to the development of insulins with even faster onset of action.
The first available ultra-rapid prandial insulin was fast acting insulin aspart. This insulin has an onset of appearance approximately twice as fast (~5 min earlier) as insulin aspart, whereas dose-concentration and dose-response relations are comparable between the two insulins ( table 4 ). 59 In adults with type 1 diabetes, mealtime and post-meal fast acting aspart led to non-inferior glycemic control compared with mealtime aspart, in combination with basal insulin. 60 Mean HbA 1c was 7.3%, 7.3%, and 7.4% in the mealtime faster aspart, mealtime aspart, and post‐meal faster aspart arms, respectively (P<0.001 for non-inferiority).
Insulin lispro-aabc is the second ultra-rapid prandial insulin. In early kinetic studies, insulin lispro-aabc appeared in the serum five minutes faster with 6.4-fold greater exposure in the first 15 minutes compared with insulin lispro. 61 The duration of exposure of the insulin concentrations in this study was 51 minutes faster with lispro-aabc. Overall insulin exposure was similar between the two groups. Clinically, lispro-aabc is non-inferior to insulin lispro, but postprandial hyperglycemia is lower with the faster acting analog. 62 Lispro-aabc given at mealtime resulted in greater improvement in post-prandial glucose (two hour post-prandial glucose −31.1 mg/dL, 95% confidence interval −41.0 to −21.2; P<0.001).
Both ultra-rapid acting insulins can be used in insulin pumps. Lispro-aabc tends to have more insertion site reactions than insulin lispro. 63 A meta-analysis including nine studies and 1156 participants reported increased infusion set changes on rapid acting insulin analogs (odds ratio 1.60, 95% confidence interval 1.26 to 2.03). 64
Pulmonary inhaled insulin
The quickest acting insulin is pulmonary inhaled insulin, with an onset of action of 12 minutes and a duration of 1.5-3 hours. 65 When used with postprandial supplemental dosing, glucose control is improved without an increase in hypoglycemia. 66
Insulin delivery systems
Approved automated insulin delivery systems.
CGM systems and insulin pumps have shown improvement in glycemic control and decreased risk of severe hypoglycemia compared with use of self-monitoring of blood glucose and multiple daily insulin injections in type 1 diabetes. 67 68 69 Using CGM and insulin pump together (referred to as sensor augmented pump therapy) only modestly improves HbA 1c in patients who have high sensor wear time, 70 71 but the management burden of diabetes does not decrease as frequent user input is necessary. Thus emerged the concept of glucose responsive automated insulin delivery (AID), in which data from CGM can inform and allow adjustment of insulin delivery.
In the past decade, exponential improvements in CGM technologies and refined insulin dosing pump algorithms have led to the development of AID systems that allow for minimization of insulin delivery burden. The early AID systems reduced hypoglycemia risk by automatically suspending insulin delivery when glucose concentrations dropped to below a pre-specified threshold but did not account for high glucose concentrations. More complex algorithms adjusting insulin delivery up and down automatically in response to real time sensor glucose concentrations now allow close replication of normal endocrine pancreatic physiology.
AID systems (also called closed loop or artificial pancreas systems) include three components—an insulin pump that continuously delivers rapid acting insulin, a continuous glucose sensor that measures interstitial fluid glucose at frequent intervals, and a control algorithm that continuously adjusts insulin delivery that resides in the insulin pump or a smartphone application or handheld device ( fig 4 ). All AID systems that are available today are referred to as “hybrid” closed loop (HCL) systems, as users are required to manually enter prandial insulin boluses and signal exercise, but insulin delivery is automated at night time and between meals. AID systems, regardless of the type used, have shown benefit in glycemic control and cost effectiveness, improve quality of life by improving sleep quality, and decrease anxiety and diabetes burden in adults and children. 72 73 74 Limitations to today’s HCL systems are primarily related to pharmacokinetics and pharmacodynamics of available analog insulins and accuracy of CGM in extremes of blood glucose values. The iLet bionic pancreas, cleared by the US Food and Drug Administration (FDA) in May 2023, is an AID system that determines all therapeutic insulin doses for an individual on the basis of body weight, eliminating the need for calculation of basal rates, insulin to carbohydrate ratios, blood glucose corrections, and bolus dose. The control algorithms adapt continuously and autonomously to the individual’s insulin needs. 38 Table 5 lists available AID systems.

Schematic of closed loop insulin pump technology. The continuous glucose monitor senses interstitial glucose concentrations and sends the information via Bluetooth to a control algorithm hosted on an insulin pump (or smartphone). The algorithm calculates the amount of insulin required, and the insulin pump delivers rapid acting insulin subcutaneously
Comparison of commercially available hybrid closed loop systems 75
Unapproved systems
Do-it-yourself (DIY) closed loop systems—DIY open artificial pancreas systems—have been developed by people with type 1 diabetes with the goal of self-adjusting insulin by modifying their individually owned devices. 76 These systems are built by the individual using an open source code widely available to anyone with compatible medical devices who is willing and able to build their own system. DIY systems are used by several thousand people across the globe but are not approved by regulatory bodies; they are patient-driven and considered “off-label” use of technology with the patient assuming full responsibility for their use. Clinicians caring for these patients should ensure basic diabetes skills, including pump site maintenance, a knowledge of how the chosen system works, and knowing when to switch to “manual mode” for patients using an artificial pancreas system of any kind. 76 The small body of studies on DIY looping suggests improvement in HbA 1c , increased time in range, decreased hypoglycemia and glucose variability, improvement in night time blood glucose concentrations, and reduced mental burden of diabetes management. 77 78 79 Although actively prescribing or initiating these options is not recommended, these patients should be supported by clinical teams; insulin prescription should not be withheld, and, if initiated by the patient, unregulated DIY options should be openly discussed to ensure open and transparent relationships. 78
In January 2023, the US FDA cleared the Tidepool Loop app, a DIY AID system. This software will connect the CGM, insulin pump, and Loop algorithm, but no RCTs using this method are available.
β cell replacement therapies
For patients with type 1 diabetes who meet specific clinical criteria, β cell replacement therapy using whole pancreas or pancreatic islet transplantation can be considered. Benefits of transplantation include immediate cessation of insulin therapy, attainment of euglycemia, and avoidance of hypoglycemia. Additional benefits include improved quality of life and stabilization of complications. 80 Chronic immunosuppression is needed to prevent graft rejection after transplantation.
Pancreas transplantation
Whole pancreas transplantation, first performed in 1966, involves complex abdominal surgery and lifelong immunosuppressive therapy and is limited by organ donor availability. Today, pancreas transplants are usually performed simultaneously using two organs from the same donor (simultaneous pancreas-kidney transplant (SPKT)), sequentially if the candidate has a living donor for renal transplantation (pancreas after kidney transplant (PAKT)) or on its own (pancreas transplantation alone). Most whole pancreas transplants are performed with kidney transplantation for end stage diabetic kidney disease. Pancreas graft survival at five years after SPKT is 80% and is superior to that with pancreas transplants alone (62%) or PAKT (67%). 81 Studies from large centers where SPKT is performed show that recipients can expect metabolic improvements including amelioration of problematic hypoglycemia for at least five years. 81 The number of pancreas transplantations has steadily decreased in the past two decades.
Islet transplantation
Islet transplantation can be pursued in selected patients with type 1 diabetes marked by unawareness of hypoglycemia and severe hypoglycemic episodes, to help restore the α cell response critical for responding to hypoglycemia. 82 83 Islet transplantation involves donor pancreas procurement with subsequent steps to isolate, purify, culture, and infuse the islets. Multiple donors are needed to provide enough islet cells to overcome islet cell loss during transplantation. Survival of the islet grafts, limited donor supply, and lifelong need for immunosuppressant therapy remain some of the biggest challenges. 84 Islet transplantation remains experimental in the US and is offered in a few specialized centers in North America, some parts of Europe, and Australia. 85
Disease modifying treatments for β cell preservation
Therapies targeting T cells, B cells, and cytokines that find use in a variety of autoimmune diseases have also been applied to type 1 diabetes. The overarching goal of immune therapies in type 1 diabetes is to prevent or delay the loss of functional β cell mass. Studies thus far in early type 1 diabetes have not yet successfully shown reversal of loss of C peptide or maintenance of concentrations after diagnosis, although some have shown preservation or slowing of loss of β cells. This suggests that a critical time window of opportunity exists for starting treatment depending on the stage of type 1 diabetes ( fig 1 ).
Teplizumab is a humanized monoclonal antibody against the CD3 molecule on T cells; it is thought to modify CD8 positive T lymphocytes, key effector cells that mediate β cell death and preserves regulatory T cells. 86 Teplizumab, when administered to patients with new onset of type 1 diabetes, was unable to restore glycemia despite C peptide preservation. 87 However, in its phase II prevention study of early intervention in susceptible individuals (at least two positive autoantibodies and an abnormal oral glucose tolerance test at trial entry), a single course of teplizumab delayed progression to clinical type 1 diabetes by about two years ( table 2 ). 43 On the basis of these results, teplizumab received approval in the US for people at high risk of type 1 diabetes in November 2022. 88 A phase III trial (PROTECT; NCT03875729 ) to evaluate the efficacy and safety of teplizumab versus placebo in children and adolescents with new diagnosis of type 1 diabetes (within six weeks) is ongoing. 89
Thus far, targeting various components of the immune response has been attempted in early type 1 diabetes without any long term beneficial effects on C peptide preservation. Co-stimulation blockade using CTLA4-Ig abatacept, a fusion protein that interferes with co-stimulation needed in the early phases of T cell activation that occurs in type 1 diabetes, is being tested for efficacy in prevention of type 1 diabetes ( NCT01773707 ). 90 Similarly, several cytokine directed anti-inflammatory targets (interleukin 6 receptor, interleukin 1β, tumor necrosis factor ɑ) have not shown any benefit.
Non-immunomodulatory adjunctive therapies
Adjunctive therapies for type 1 diabetes have been long entertained owing to problems surrounding insulin delivery, adequacy of glycemic management, and side effects associated with insulin, especially weight gain and hypoglycemia. At least 50% of adults with type 1 diabetes are overweight or obese, presenting an unmet need for weight management in these people. Increased cardiovascular risk in these people despite good glycemic management presents additional challenges. Thus, use of adjuvant therapies may tackle these problems.
Metformin, by decreasing hepatic glucose production, could potentially decrease fasting glucose concentrations. 91 It has shown benefit in reducing insulin doses and possibly improving metabolic control in obese/overweight people with type 1 diabetes. A meta-analysis of 19 RCTs suggests short term improvement in HbA 1c that is not sustained after three months and is associated with higher incidence of gastrointestinal side effects. 92 No evidence shows that metformin decreases cardiovascular morbidity in type 1 diabetes. Therefore, owing to lack of conclusive benefit, addition of metformin to treatment regimens is not recommended in consensus guidelines.
Glucagon-like peptide receptor agonists
Endogenous GLP-1 is an incretin hormone secreted from intestinal L cells in response to nutrient ingestion and enhances glucose induced insulin secretion, suppresses glucagon secretion, delays gastric emptying, and induces satiety. 93 GLP-1 promotes β cell proliferation and inhibits apoptosis, leading to expansion of β cell mass. GLP-1 secretion in patients with type 1 diabetes is similar to that seen in people without diabetes. Early RCTs of liraglutide in type 1 diabetes resulted in weight loss and modest lowering of HbA 1c ( table 2 ). 49 50 Liraglutide 1.8 mg in people with type 1 diabetes and higher body mass index decreased HbA 1c , weight, and insulin requirements with no increased hypoglycemia risk. 94 However, on the basis of results from a study of weekly exenatide that showed similar results, these effects may not be sustained. 51 A meta-analysis of 24 studies including 3377 participants showed that the average HbA 1c decrease from GLP-1 receptor agonists compared with placebo was highest for liraglutide 1.8 mg daily (−0.28%, 95% confidence interval −0.38% to−0.19%) and exenatide (−0.17%, −0.28% to 0.02%). The estimated weight loss from GLP-1 receptor agonists compared with placebo was −4.89 (−5.33 to−4.45) kg for liraglutide 1.8 mg and −4.06 (−5.33 to−2.79) kg for exenatide. 95 No increase in severe hypoglycemia was seen (odds ratio 0.67, 0.43 to 1.04) but therapy was associated with higher levels of nausea. GLP-1 receptor agonist use may be beneficial for weight loss and reducing insulin doses in a subset of patients with type 1 diabetes. GLP-1 receptor agonists are not a recommended treatment option in type 1 diabetes. Semaglutide is being studied in type 1 diabetes in two clinical trials ( NCT05819138 ; NCT05822609 ).
Sodium-glucose cotransporter inhibitors
Sodium-glucose cotransporter 2 (SGLT-2), a protein expressed in the proximal convoluted tubule of the kidney, reabsorbs filtered glucose; its inhibition prevents glucose reabsorption in the tubule and increases glucose excretion by the kidney. Notably, the action of these agents is independent of insulin, so this class of drugs has potential as adjunctive therapy for type 1 diabetes. Clinical trials have shown significant benefit in cardiovascular and renal outcomes in type 2 diabetes; therefore, significant interest exists for use in type 1 diabetes. Several available SGLT-2 inhibitors have been studied in type 1 diabetes and have shown promising results with evidence of decreased total daily insulin dosage, improvement in HbA 1c , lower rates of hypoglycemia, and decrease in body weight; however, these effects do not seem to be sustained at one year in clinical trials and seem to wane with time. Despite beneficial effects, increased incidence of diabetic ketoacidosis has been observed in all trials, is a major concern, and is persistent despite educational efforts. 96 97 98 Low dose empagliflozin (2.5 mg) has shown lower rates of diabetic ketoacidosis in clinical trials ( table 2 ). 47 Favorable risk profiles have been noted in Japan, the only market where SGLT-2 inhibitors are approved for adjunctive use in type 1 diabetes. 99 In the US, SGLT-2 inhibitors are approved for use in type 2 diabetes only. In Europe, although dapagliflozin was approved for use as adjunct therapy to insulin in adults with type 1 diabetes, the manufacturer voluntarily withdrew the indication for the drug in 2021. 100 Sotagliflozin is a dual SGLT-1 and SGLT-2 inhibitor that decreases renal glucose reabsorption through systemic inhibition of SGLT-2 and decreases glucose absorption in the proximal intestine by SGLT-1 inhibition, blunting and delaying postprandial hyperglycemia. 101 Studies of sotagliflozin in type 1 diabetes have shown sustained HbA 1c reduction, weight loss, lower insulin requirements, lesser hypoglycemia, and more diabetic ketoacidosis relative to placebo. 102 103 104 The drug received authorization in the EU for use in type 1 diabetes, but it is not marketed there. Although SGLT inhibitors are efficacious in type 1 diabetes management, the risk of diabetic ketoacidosis is a major limitation to widespread use of these agents.
Updates in acute complications of type 1 diabetes
Diabetic ketoacidosis.
Diabetic ketoacidosis is a serious and potentially fatal hyperglycemic emergency accompanied by significant rates of mortality and morbidity as well as high financial burden for healthcare systems and societies. In the past decade, increasing rates of diabetic ketoacidosis in adults have been observed in the US and Europe. 105 106 This may be related to changes in the definition of diabetic ketoacidosis, use of medications associated with higher risk, and admission of patients at lower risk. 107 In a US report of hospital admissions with diabetic ketoacidosis, 53% of those admitted were between the ages of 18 and 44, with higher rates in men than in women. 108 Overall, although mortality from diabetic ketoacidosis in developed countries remains low, rates have risen in people aged >60 and in those with coexisting life threatening illnesses. 109 110 Recurrent diabetic ketoacidosis is associated with a substantial mortality rate. 111 Frequency of diabetic ketoacidosis increases with higher HbA 1c concentrations and with lower socioeconomic status. 112 Common precipitating factors include newly diagnosed type 1 diabetes, infection, poor adherence to insulin, and an acute cardiovascular event. 109
Euglycemic diabetic ketoacidosis refers to the clinical picture of an increased anion gap metabolic acidosis, ketonemia, or significant ketonuria in a person with diabetes without significant glucose elevation. This can be seen with concomitant use of SGLT-2 inhibitors (currently not indicated in type 1 diabetes), heavy alcohol use, cocaine use, pancreatitis, sepsis, and chronic liver disease and in pregnancy 113 Treatment is similar to that for hyperglycemic diabetic ketoacidosis but can require earlier use and greater concentrations of a dextrose containing fluid for the insulin infusion in addition to 0.9% normal saline resuscitation fluid. 114
The diagnosis of diabetic ketoacidosis has evolved from a gluco-centric diagnosis to one requiring hyperketonemia. By definition, independent of blood glucose, a β-hydroxybutyrate concentration >3 mmol/L is required for diagnosis. 115 However, the use of this ketone for assessment of the severity of the diabetic ketoacidosis is controversial. 116 Bedside β-hydroxybutyrate testing during treatment is standard of care in many parts of the world (such as the UK) but not others (such as the US). Concerns have been raised about accuracy of bedside β-hydroxybutyrate meters, but this is related to concentrations above the threshold for diabetic ketoacidosis. 116
Goals for management of diabetic ketoacidosis include restoration of circulatory volume, correction of electrolyte imbalances, and treatment of hyperglycemia. Intravenous regular insulin infusion is the standard of care for treatment worldwide owing to rapidity of onset of action and rapid resolution of ketonemia and hyperglycemia. As hypoglycemia and hypokalemia are more common during treatment, insulin doses are now recommended to be reduced from 0.1 u/kg/h to 0.05 u/kg/h when glucose concentrations drop below 250 mg/dL or 14 mM. 115 Subcutaneous rapid acting insulin protocols have emerged as alternative treatments for mild to moderate diabetic ketoacidosis. 117 Such regimens seem to be safe and have the advantages of not requiring admission to intensive care, having lower rates of complications related to intravenous therapy, and requiring fewer resources. 117 118 Ketonemia and acidosis resolve within 24 hours in most people. 115 To prevent rebound hyperglycemia, the transition off an intravenous insulin drip must overlap subcutaneous insulin by at least two to four hours. 115
Hypoglycemia
Hypoglycemia, a common occurrence in people with type 1 diabetes, is a well appreciated effect of insulin treatment and occurs when blood glucose falls below the normal range. Increased susceptibility to hypoglycemia from exogenous insulin use in people with type 1 diabetes results from multiple factors, including imperfect subcutaneous insulin delivery tools, loss of glucagon within a few years of diagnosis, progressive impairment of the sympatho-adrenal response with repeated hypoglycemic episodes, and eventual development of impaired awareness. In 2017 the International Hypoglycemia Study Group developed guidance for definitions of hypoglycemia; on the basis of this, a glucose concentration of 3.0-3.9 mmol/L (54-70 mg/dL) was designated as level 1 hypoglycemia, signifying impending development of level 2 hypoglycemia—a glucose concentration <3 mmol/L (54 mg/dL). 119 120 At approximately 54 mg/dL, neuroglycopenic hypoglycemia symptoms, including vision and behavior changes, seizures, and loss of consciousness, begin to occur as a result of glucose deprivation of neurons in the central nervous system. This can eventually lead to cerebral dysfunction at concentrations <50 mg/dL. 121 Severe hypoglycemia (level 3), denoting severe cognitive and/or physical impairment and needing external assistance for recovery, is a common reason for emergency department visits and is more likely to occur in people with lower socioeconomic status and with the longest duration of diabetes. 112 Prevalence of self-reported severe hypoglycemia is very high according to a global population study that included more than 8000 people with type 1 diabetes. 122 Severe hypoglycemia occurred commonly in younger people with suboptimal glycemia according to a large electronic health record database study in the US. 123 Self- reported severe hypoglycemia is associated with a 3.4-fold increase in mortality. 124 125
Acute consequences of hypoglycemia include impaired cognitive function, temporary focal deficits including stroke-like symptoms, and memory deficits. 126 Cardiovascular effects including tachycardia, arrhythmias, QT prolongation, and bradycardia can occur. 127 Hypoglycemia can impair many activities of daily living, including motor vehicle safety. 128 In a survey of adults with type 1 diabetes who drive a vehicle at least once a week, 72% of respondents reported having hypoglycemia while driving, with around 5% reporting a motor vehicle accident due to hypoglycemia in the previous two years. 129 This contributes to the stress and fear that many patients face while grappling with the difficulties of ongoing hypoglycemia. 130
Glucagon is highly efficacious for the primary treatment of severe hypoglycemia when a patient is unable to ingest carbohydrate safely, but it is unfortunately under-prescribed and underused. 131 132 Availability of nasal, ready to inject, and shelf-stable liquid glucagon formulations have superseded the need for reconstituting older injectable glucagon preparations before administration and are now preferred. 133 134 Real time CGM studies have shown a decreased hypoglycemic exposure in people with impaired awareness without a change in HbA 1c . 34 135 136 137 138 CGM has shown benefit in decreasing hypoglycemia across the lifespan, including in teens, young adults, and older people. 36 139 Although CGM reduces the burden of hypoglycemia including severe hypoglycemia, it does not eliminate it; overall, such severe level 3 hypoglycemia rates in clinical trials are very low and hard to decipher in the real world. HCL insulin delivery systems integrated with CGM have been shown to decrease hypoglycemia. Among available rapid acting insulins, ultra-rapid acting lispro (lispro-aabc) seems to be associated with less frequent hypoglycemia in type 1 diabetes. 140 141
As prevention of hypoglycemia is a crucial aspect of diabetes management, formal training programs to increase awareness and education on avoidance of hypoglycemia, such as the UK’s Dose Adjustment for Normal Eating (DAFNE), have been developed. 142 143 This program has shown fewer severe hypoglycemia (mean 1.7 (standard deviation 8.5) episodes per person per year before training to 0.6 (3.7) episodes one year after training) and restoration of recognition of hypoglycemia in 43% of people reporting unawareness. Clinically relevant anxiety and depression fell from 24.4% to 18.0% and from 20.9% to 15.5%, respectively. A structured education program with cognitive and psychotherapeutic aspects for changing hypoglycemia related behaviors, called the Hypoglycemia Awareness Restoration Program despite optimized self-care (HARPdoc), showed a positive effect on changing unhelpful beliefs around hypoglycemia and improved diabetes related and general distress and anxiety scores. 144
Management in under-resourced settings
According to a recent estimate from the International Diabetes Federation, 1.8 million people with type 1 diabetes live in low and middle income countries (LMICs). 2 In many LMICs, the actual burden of type 1 diabetes remains unknown and material resources needed to manage type 1 diabetes are lacking. 145 146 Health systems in these settings are underequipped to tackle the complex chronic disease that is type 1 diabetes. Few diabetes and endocrinology specialist physicians are available owing to lack of specific postgraduate training programs in many LMICs; general practitioners with little to no clinical experience in managing type 1 diabetes care for these patients. 146 This, along with poor availability and affordability of insulin and lack of access to technology, results in high mortality rates. 147 148 149 In developed nations, low socioeconomic status is associated with higher levels of mortality and morbidity for adults with type 1 diabetes despite access to a universal healthcare system. 150 Although global governments have committed to universal health coverage and therefore widespread availability of insulin, it remains very far from realization in most LMICs. 151
Access to technology is patchy and varies globally. In the UST1DX, CGM use was least in the lowest fifth of socioeconomic status. 152 Even where technology is available, successful engagement does not always occur. 153 In a US cohort, lower CGM use was seen in non-Hispanic Black children owing to lower rates of device initiation and higher rates of discontinuation. 154 In many LMICs, blood glucose testing strips are not readily available and cost more than insulin. 151 In resource limited settings, where even diagnosis, basic treatments including insulin, syringes, and diabetes education are limited, use of CGM adds additional burden to patients. Need for support services and the time/resources needed to download and interpret data are limiting factors from a clinician’s perspective. Current rates of CGM use in many LMICs are unknown.
Inequities in the availability of and access to certain insulin formulations continue to plague diabetes care. 155 In developed countries such as the US, rising costs have led to insulin rationing by around 25% of people with type 1 diabetes. 156 LMICs have similar trends while also remaining burdened by disproportionate mortality and complications from type 1 diabetes. 155 157 With the inclusion of long acting insulin analogs in the World Health Organization’s Model List of Essential Medicines in 2021, hope has arisen that these will be included as standard of care across the world. 158 In the past, the pricing of long acting analogs has limited their use in resource poor settings 159 ; however, their inclusion in WHO’s list was a major step in improving their affordability. 158 With the introduction of lower cost long acting insulin biosimilars, improved access to these worldwide in the future can be anticipated. 160
Making insulin available is not enough on its own to improve the prognosis for patients with diabetes in resource poor settings. 161 Improved healthcare infrastructure, better availability of diabetes supplies, and trained personnel are all critical to improving type 1 diabetes care in LMICs. 161 Despite awareness of limitations and barriers, a clear understanding of how to implement management strategies in these settings is still lacking. The Global Diabetes Compact was launched in 2021 with the goal of increasing access to treatment and improving outcomes for people with diabetes across the globe. 162
Emerging technologies and treatments
Monitoring systems.
The ability to measure urinary or more recently blood ketone concentrations is an integral part of self-management of type 1 diabetes, especially during acute illness, intermittent fasting, and religious fasts to prevent diabetic ketoacidosis. 163 Many people with type 1 diabetes do not adhere to urine or blood ketone testing, which likely results in unnecessary episodes of diabetic ketoacidosis. 164 Noting that blood and urine ketone testing is not widely available in all countries and settings is important. 1 Regular assessment of patients’ access to ketone testing (blood or urine) is critical for all clinicians. Euglycemic diabetic ketoacidosis in type 1 diabetes is a particular problem with concomitant use of SGLT-2 inhibitors; for this reason, these agents are not approved for use in these patients. For sick day management (and possibly for the future use of SGLT-2 inhibitors in people with type 1 diabetes), it is hoped that continuous ketone monitoring (CKM) can mitigate the risks of diabetic ketoacidosis. 165 Like CGM, the initial CKM device measures interstitial fluid β-hydroxybutyrate instead of glucose. CKM use becomes important in conjunction with a hybrid closed loop insulin pump system and added SGLT-2 inhibitor therapy, where insulin interruptions are common and hyperketonemia is frequent. 166
Perhaps the greatest technological challenge to date has been the development of non-invasive glucose monitoring. Numerous attempts have been made using strategies including optics, microwave, and electrochemistry. 167 Lack of success to date has resulted in healthy skepticism from the medical community. 168 However, active interest in the development of non-invasive technology with either interstitial or blood glucose remains.
Insulin and delivery systems
In the immediate future, two weekly basal insulins, insulin icodec and basal insulin Fc, may become available. 169 Studies of insulin icodec in type 1 diabetes are ongoing (ONWARDS 6; NCT04848480 ). How these insulins will be incorporated in management of type 1 diabetes is not yet clear.
Currently available AID systems use only a single hormone, insulin. Dual hormone AID systems incorporating glucagon are in development. 170 171 Barriers to the use of dual hormone systems include the need for a second chamber in the pump, a lack of stable glucagon formulations approved for long term subcutaneous delivery, lack of demonstrated long term safety, and gastrointestinal side effects from glucagon use. 74 Similarly, co-formulations of insulin and amylin (a hormone co-secreted with insulin and deficient in people with type 1 diabetes) are in development. 172
Immunotherapy for type 1 diabetes
As our understanding of the immunology of type 1 diabetes expands, development of the next generation of immunotherapies is under active pursuit. Antigen specific therapies, peptide immunotherapy, immune tolerance using DNA vaccination, and regulatory T cell based adoptive transfer targeting β cell senescence are all future opportunities for drug development. Combining immunotherapies with metabolic therapies such as GLP-1 receptor agonists to help to improve β cell mass is being actively investigated.
The quest for β cell replacement methods is ongoing. Transplantation of stem cell derived islets offers promise for personalized regenerative therapies as a potentially curative method that does away with the need for donor tissue. Since the first in vivo model of glucose responsive β cells derived from human embryonic stem cells, 173 different approaches have been attempted. Mesenchymal stromal cell treatment and autologous hematopoietic stem cells in newly diagnosed type 1 diabetes may preserve β cell function without any safety signals. 174 175 176 Stem cell transplantation for type 1 diabetes remains investigational. Encapsulation, in which β cells are protected using a physical barrier to prevent immune attack and avoid lifelong immunosuppression, and gene therapy techniques using CRISPR technology also remain in early stages of investigation.
Until recently, no specific guidelines for management of type 1 diabetes existed and management guidance was combined with consensus statements developed for type 2 diabetes. Table 6 summarizes available guidance and statements from various societies. A consensus report for management of type 1 diabetes in adults by the ADA and European Association for the Study of Diabetes became available in 2021; it covers several topics of diagnosis and management of type 1 diabetes, including glucose monitoring, insulin therapy, and acute complications. Similarly, the National Institute for Health and Care Excellence also offers guidance on management of various aspects of type 1 diabetes. Consensus statements for use of CGM, insulin pump, and AID systems are also available.
Guidelines in type 1 diabetes
Conclusions
Type 1 diabetes is a complex chronic condition with increasing worldwide prevalence affecting several million people. Several successes in management of type 1 diabetes have occurred over the years from the serendipitous discovery of insulin in 1921 to blood glucose monitoring, insulin pumps, transplantation, and immunomodulation. The past two decades have seen advancements in diagnosis, treatment, and technology including development of analog insulins, CGM, and advanced insulin delivery systems. Although we have gained a broad understanding on many important aspects of type 1 diabetes, gaps still exist. Pivotal research continues targeting immune targets to prevent or delay onset of type 1 diabetes. Although insulin is likely the oldest of existing modern drugs, no low priced generic supply of insulin exists anywhere in the world. Management of type 1 diabetes in under resourced areas continues to be a multifaceted problem with social, cultural, and political barriers.
Glossary of abbreviations
ADA—American Diabetes Association
AID—automated insulin delivery
BGM—blood glucose monitoring
CGM—continuous glucose monitoring
CKM—continuous ketone monitoring
DCCT—Diabetes Control and Complications Trial
DIY—do-it-yourself
FDA—Food and Drug Administration
GADA—glutamic acid decarboxylase antibody
GLP-1—glucagon-like peptide 1
GRS—genetic risk scoring
HbA1c—glycated hemoglobin
HCL—hybrid closed loop
LADA—latent autoimmune diabetes of adults
LMIC—low and middle income country
PAKT—pancreas after kidney transplant
RCT—randomized controlled trial
SGLT-2—sodium-glucose cotransporter 2
SPKT—simultaneous pancreas-kidney transplant
Questions for future research
What future new technologies can be helpful in management of type 1 diabetes?
How can newer insulin delivery methods benefit people with type 1 diabetes?
What is the role of disease modifying treatments in prevention and delay of type 1 diabetes?
Is there a role for sodium-glucose co-transporter inhibitors or glucagon-like peptide 1 receptor angonists in the management of type 1 diabetes?
As the population with type 1 diabetes ages, how should management of these people be tailored?
How can we better serve people with type 1 diabetes who live in under-resourced settings with limited access to medications and technology?
How patients were involved in the creation of this manuscript
A person with lived experience of type 1 diabetes reviewed a draft of the manuscript and offered input on important aspects of their experience that should be included. This person is involved in large scale education and activism around type 1 diabetes. They offered their views on various aspects of type 1 diabetes, especially the use of adjuvant therapies and the burden of living with diabetes. This person also raised the importance of education of general practitioners on the various stages of type 1 diabetes and the management aspects. On the basis of this feedback, we have highlighted the burden of living with diabetes on a daily basis.
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: SS and IBH contributed to the planning, drafting, and critical review of this manuscript. FNK contributed to the drafting of portions of the manuscript. All three authors are responsible for the overall content as guarantors.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: SS has received an honorarium from Abbott Diabetes Care; IBH has received honorariums from Abbott Diabetes Care, Lifescan, embecta, and Hagar and research support from Dexcom and Insulet.
Provenance and peer review: Commissioned; externally peer reviewed.
- DeVries JH ,
- Hess-Fischl A ,
- Gregory GA ,
- Robinson TIG ,
- Linklater SE ,
- International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group
- Harding JL ,
- Wander PL ,
- Dabelea D ,
- Mayer-Davis EJ ,
- SEARCH for Diabetes in Youth Study
- Lawrence JM ,
- SEARCH for Diabetes in Youth Study Group
- Fazeli Farsani S ,
- Souverein PC ,
- van der Vorst MM ,
- Sutherland J ,
- Rokszin G ,
- Stahl-Pehe A ,
- Kamrath C ,
- Atkinson MA ,
- Bingley PJ ,
- Bonifacio E ,
- Leslie RD ,
- Evans-Molina C ,
- Freund-Brown J ,
- Thomas NJ ,
- Zimmet PZ ,
- Rowley MJ ,
- Knowles W ,
- Fourlanos S ,
- Greenbaum CJ ,
- ElSayed NA ,
- American Diabetes Association
- Miller RG ,
- Secrest AM ,
- Sharma RK ,
- Songer TJ ,
- McDonald T ,
- Greenfield JR
- Tridgell DM ,
- Spiekerman C ,
- Greenbaum CJ
- Glessner J ,
- Foster NC ,
- Miller KM ,
- Becker DJ ,
- Khawandanah J
- Pedrosa MR ,
- Franco DR ,
- Gieremek HW ,
- Nathan DM ,
- Diabetes Control and Complications Trial Research Group
- Klonoff DC ,
- Parkes JL ,
- Kovatchev BP ,
- Raghinaru D ,
- iDCL Trial Research Group
- Riddlesworth T ,
- DIAMOND Study Group
- Leelarathna L ,
- Neupane S ,
- FLASH-UK Trial Study Group
- Polonsky W ,
- Hirsch IB ,
- Pratley RE ,
- Kanapka LG ,
- Rickels MR ,
- Wireless Innovation for Seniors With Diabetes Mellitus (WISDM) Study Group
- Tauschmann M ,
- APCam11 Consortium
- Russell SJ ,
- Damiano ER ,
- Bionic Pancreas Research Group
- Bailey TS ,
- Group Information ,
- Bergenstal RM ,
- Dellva MA ,
- Mathieu C ,
- Herold KC ,
- Type 1 Diabetes TrialNet Study Group
- Libman IM ,
- DiMeglio LA ,
- T1D Exchange Clinic Network Metformin RCT Study Group
- Petrie JR ,
- Chaturvedi N ,
- REMOVAL Study Group
- Dandona P ,
- Phillip M ,
- DEPICT-1 Investigators
- Rosenstock J ,
- Marquard J ,
- Laffel LM ,
- Hemmingsson JU ,
- ADJUNCT ONE Investigators
- Pieber TR ,
- ADJUNCT TWO Investigators
- Reynolds J ,
- Battelino T ,
- Liljenquist D ,
- Becker RH ,
- Bergmann K ,
- Lehmann A ,
- Cheng AYY ,
- Stender-Petersen K ,
- Hövelmann U ,
- Carlson AL ,
- Komatsu M ,
- Coutant DE ,
- Stamati A ,
- Karagiannis T ,
- Christoforidis A
- Heinemann L ,
- Baughman R ,
- Akturk HK ,
- Snell-Bergeon JK ,
- Prabhu JN ,
- Seyed Ahmadi S ,
- Westman K ,
- Pivodic A ,
- Hermann JM ,
- Freiberg C ,
- DPV Initiative
- Dicembrini I ,
- Cosentino C ,
- Mannucci E ,
- Abelseth J ,
- Karageorgiou V ,
- Papaioannou TG ,
- Weisman A ,
- Cardinez M ,
- Kramer CK ,
- Asarani NAM ,
- Reynolds AN ,
- Elbalshy M ,
- Jennings P ,
- Burnside MJ ,
- Williman JA ,
- Fridell JA ,
- Stratta RJ ,
- Gruessner AC
- Robertson RP
- Shapiro AM ,
- Pepper AR ,
- Shapiro AMJ
- Walker JT ,
- Saunders DC ,
- Brissova M ,
- Gitelman SE ,
- Bluestone JA
- Hagopian W ,
- Ferry RJ Jr . ,
- Protégé Trial Investigators
- von Scholten BJ ,
- Kreiner FF ,
- Gough SCL ,
- von Herrath M
- Type 1 Diabetes TrialNet Abatacept Study Group
- Hardie DG ,
- Dejgaard TF ,
- Christiansen E ,
- ADJUNCT ONE and ADJUNCT TWO Investigators
- Yunasan E ,
- Peters AL ,
- Palanca A ,
- van Nes F ,
- Ampudia Blasco FJ ,
- Dobbins RL ,
- Greenway FL ,
- Zambrowicz BP ,
- Brodovicz K ,
- Soleymanlou N ,
- Wissinger E ,
- Juhaeri J ,
- Mayer-Davis EJ
- Dhatariya KK ,
- Glaser NS ,
- Umpierrez GE
- Ramphul K ,
- Umpierrez G ,
- Korytkowski M
- Weinstock RS ,
- T1D Exchange Clinic Network
- Eshkoli T ,
- Brandstaetter E ,
- Jotkowitz A
- Dhatariya KK
- Joint British Diabetes Societies for Inpatient Care
- Kilpatrick ES ,
- Butler AE ,
- Ostlundh L ,
- Koufakis T ,
- Agiostratidou G ,
- International Hypoglycaemia Study Group
- van de Ven KC ,
- Heerschap A ,
- van der Graaf M ,
- de Galan BE
- Alsifri S ,
- Aronson R ,
- HAT Investigator Group
- Pettus JH ,
- Shepherd L ,
- Van Houten HK ,
- Ziegenfuss JY ,
- Wermers RA ,
- Schernthaner G ,
- Anderson J ,
- Saunders AL ,
- Snell-Bergeon J ,
- Forlenza GP ,
- Bispham J ,
- Gabbay RA ,
- Pontiroli AE ,
- Tagliabue E
- T1D Exchange Intranasal Glucagon Investigators
- Freckmann G ,
- Ehrmann D ,
- El Laboudi A ,
- Spanudakis E ,
- Anantharaja S ,
- Peleckis AJ ,
- Dalton-Bakes C ,
- van Beers CA ,
- Kleijer SJ ,
- CGM Intervention in Teens and Young Adults with T1D (CITY) Study Group ,
- Piras de Oliveira C ,
- Ribeiro A ,
- Chigutsa F ,
- Malecki MT ,
- Hopkins D ,
- Lawrence I ,
- Mansell P ,
- Stanton-Fay SH ,
- Hamilton K ,
- Chadwick PM ,
- DAFNEplus study group
- Goldsmith K ,
- Yudkin JS ,
- Buntinx F ,
- Mapatano MA ,
- De Clerck M ,
- Truyers C ,
- Chambers D ,
- O’Cathain A
- Klatman EL ,
- Auzanneau M ,
- Tanenbaum ML ,
- Iturralde E ,
- Lipman TH ,
- Bhutta ZA ,
- Pfiester E ,
- Thieffry A ,
- Ballhausen H ,
- Gajewska KA ,
- O’Donnell S
- Wareham NJ ,
- Joosse HJ ,
- Raposo JF ,
- de Courten M
- Hemmingsen B ,
- Abouhassan T ,
- Albanese-O’Neill A ,
- Jacobsen L ,
- Haller MJ ,
- Castorino K ,
- Lovblom LE ,
- Cardinez N ,
- Nguyen KT ,
- Andersen G ,
- Meiffren G ,
- Famulla S ,
- Castellanos LE ,
- Balliro CA ,
- Sherwood JS ,
- Tsoukas MA ,
- Bernier-Twardy S ,
- Martinson LA ,
- Carlsson PO ,
- Schwarcz E ,
- Korsgren O ,
- Oliveira MC ,
- Stracieri AB ,
- Buzzetti R ,
- Mauricio D ,
- McGibbon A ,
- Ingersoll K ,
- Tugwell B ,
- Diabetes Canada Clinical Practice Guidelines Expert Committee
- Fleming GA ,
- McCall AL ,
- Gianchandani R ,
This comprehensive slide deck of ADA's 2023 Standards of Care contains content created, reviewed, and approved by the American Diabetes Association. You are free to use the slides in presentations without further permission as long as the slide content is not altered in any way and appropriate attribution is made to the American Diabetes Association (the Association name and logo on the slides constitutes appropriate attribution).
Permission is required from the Association for any commercial use or for reproduction in any print materials (contact [email protected] ).
Using the links to the slide deck will download the deck. You may need to check the folder for downloaded documents on your device to see/open the file.
| |||||||||||||||||||||||||||||||||||||||||||||||












IMAGES
COMMENTS
Type 1 diabetes is a chronic illness characterized by the body's inability to produce insulin due to the autoimmune destruction of the beta cells in the pancreas. Onset most often occurs in childhood, but the disease can also develop in adults in their late 30s and early 40s. ... Symptoms at the time of the first clinical presentation can ...
This topic will review the clinical presentation, diagnosis, and initial evaluation of diabetes in nonpregnant adults. Screening for and prevention of diabetes, the etiologic classification of diabetes mellitus, the treatment of diabetes, as well as diabetes during pregnancy are discussed separately. (See "Screening for type 2 diabetes mellitus" .)
Clinical Presentation: Type 1 diabetes does not present clinically until 80-90% of the beta cells have been destroyed (McCance & Heuther, 2014). Because insulin stimulates glucose uptake into tissues, stores glycose as glycogen, inhibits glucagon secretion and inhibits glucose production from the liver, the destruction of insulin-producing beta ...
Type 1 diabetes mellitus (T1D) is an autoimmune disease that leads to the destruction of insulin-producing pancreatic beta cells. There is heterogeneity in the metabolic, genetic, and immunogenetic characteristics of T1D and age-related differences, requiring a personalized approach for each individual. Loss of insulin secretion can occur quickly or gradually.
Type 1 diabetes mellitus is a chronic medical condition that occurs when the pancreas, an organ in the abdomen, produces very little or no insulin . Insulin is a hormone that helps the body to use glucose for energy. ... Clinical presentation, diagnosis, and initial evaluation of diabetes mellitus in adults
The clinical presentation may differ, but the classical triad of thirst and polydipsia, polyuria, and weight loss are common symptoms of type 1 diabetes. Accurate classification of the type of diabetes has implications beyond the use of insulin treatment; education, insulin regimen, use of adjuvant therapies, access to newer technologies, need ...
Definition and Description. Type 1 diabetes (T1D) is a T-cell mediated autoimmune disease in which destruction of pancreatic β-cells causes insulin deficiency which leads to hyperglycemia and a tendency to ketoacidosis. 1 Excesses glucose levels must be managed by exogenous insulin injections several times a day. 2 Patients with T1D constitute ...
Type 1 diabetes, once known as juvenile diabetes or insulin-dependent diabetes, is a chronic condition. ... Ask your provider if you might be eligible for one of these clinical trials. It is important to carefully weigh the risks and benefits of any treatment available in a trial. ... presentation, and diagnosis of type 1 diabetes mellitus in ...
Type 1 diabetes is a chronic illness characterized by the body's inability to produce insulin due to the autoimmune destruction of the beta cells in the pancreas. Onset most often occurs in childhood, but the disease can also develop in adults in their late 30s and early 40s. ... Clinical Presentation References. Aathira R, Jain V. Advances ...
Clinical presentation. Type 1 diabetes can be diagnosed at any age, with a peak around 10 to 14 years. [13] In children and young people aged under 18 years of age, the signs of type 1 diabetes include: [35] Hyperglycaemia (random plasma glucose ≥11.1 mmol/L [≥200 mg/dL]) Polyuria. Polydipsia.
Treatment for type 1 diabetes includes: Taking insulin. Counting carbohydrates, fats and protein. Monitoring blood sugar often. Eating healthy foods. Exercising regularly and keeping a healthy weight. The goal is to keep the blood sugar level as close to normal as possible to delay or prevent complications.
Type 1 diabetes and type 2 diabetes are heterogeneous diseases in which clinical presentation and disease progression may vary considerably. Classification is important for determining therapy, but some individuals cannot be clearly classified as having type 1 or type 2 diabetes at the time of diagnosis.
Based on etiology, diabetes is classified as type 1 diabetes mellitus, type 2 diabetes mellitus, latent autoimmune diabetes, maturity-onset diabetes of youth, and miscellaneous causes. The ...
Adult-onset type 1 diabetes is more common than childhood-onset type 1 diabetes, as shown from epidemiological data from both high-risk areas such as Northern Europe and low-risk areas such as China (3-8).In southeastern Sweden, the disease incidence among individuals aged 0-19 years is similar to that among individuals 40-100 years of age (37.8 per 100,000 persons per year and 34.0 ...
The clinical determination of type 1 vs. 2 diabetes is made with consideration of factors including the patient's age, body composition, symptom progression and clinical presentation. Type 1 diabetes typically manifests in young patients, often before the age of 14, who frequently appear thin and have a sudden onset of symptoms, with diabetic ...
Type 1 diabetes doesn't develop only in children; There have been recent advances in type 1 diabetes screening and treatment; If you have a family history of type 1 diabetes, your health care provider may suggest screening for type 1 diabetes. They will order a blood test to measure your islet autoantibodies. The test results can go one of ...
Half of all new cases of type 1 diabetes are now recognized as occurring in adults.13 Misclassification due to misdiagnosis (commonly as type 2 diabetes) occurs in nearly 40% of people.14 As opposed to typical childhood onset type 1 diabetes, progression to severe insulin deficiency, and therefore its clinical presentation in adults, is variable.
This comprehensive slide deck of ADA's 2023 Standards of Care contains content created, reviewed, and approved by the American Diabetes Association. You are free to use the slides in presentations without further permission as long as the slide content is not altered in any way and appropriate attribution is made to the American Diabetes Association (the Association name and logo on the slides ...
Abstract. Abstract: Objective: To identify the presenting features of type 1 diabetes in a national incident cohort aged under 15 yr, the duration of symptoms, the occurrence of diabetic ketoacidosis (DKA) at presentation, and the frequency of a family history of diabetes. Methods: A prospective study was undertaken of incident cases of type 1 ...
Immune-mediated (auto-immune) Type 1 diabetes mellitus is not a homogenous entity, but nonetheless has distinctive characteristics. In children, it may present with classical insulin deficiency and ketoacidosis at disease onset, whereas autoimmune diabetes in adults may not always be insulin dependent. Indeed, as the adult-onset form of ...
Type 1 Diabetes: Cellular, Molecular & Clinical Immunology Edited by George Eisenbarth ... Clinical Trials for the Prevention of Type I Diabetes - Updated 7/09 H. Peter Chase, Peter Gottlieb, & George S. Eisenbarth: Chapter 12 Powerpoint slide set - Updated 9/09 Mark Pescovitz Immunotherapy Review 2008 Slideset (added 6/08) ...
The median duration of symptoms was highest in the youngest (under 2 yr) and oldest (10-14.99 yr) age categories. Presentation in moderate/severe DKA occurred in 25% overall and six of nine of those aged under 2 yr. A family history of type 1 diabetes in a first-degree relative was found in 10.2%. Conclusions: This study confirms the abrupt ...
Lead Investigator for Sernova's Clinical Trial With Cell Pouch for Type 1 Diabetes to Deliver Oral Presentation at the 2024 EASD Annual Meeting NewMediaWire Thu, Aug 15, 2024, 4:00 AM 4 min read
Diabetes technology, such as insulin pumps and continuous glucose monitoring devices, can help improve glucose control for people with type 1 diabetes (T1D), which keeps them at lower risk for diabetes complications, but many Latinx adolescents, who make up the largest marginalized ethnic group of youth with T1D in California, use these devices less often and have less optimal glucose control ...
Access up-to-date information on glucoregulatory hormones and GLP-1/GIP receptor agonists with clinical guidance at Healio.
The pattern of presentation of type 1 diabetes has changed as the incidence of DKA has decreased; unlike in previous studies, DKA was not the most common presenting symptom in this study. ... The recorded information included the age at onset, sex, nationality, consanguinity, clinical presentation, duration of symptoms before diagnosis, and ...
The impact of rosuvastatin versus atorvastatin on new-onset diabetes mellitus (NODM) among patients treated with high-intensity statin therapy for coronary artery disease (CAD) remains to be clarified. This study aimed to evaluate the risk of NODM in patients with CAD treated with rosuvastatin compared to atorvastatin in the randomized LODESTAR trial.
Key Points. Question Among adults with type 2 diabetes (T2D), what is the efficacy, safety, and tolerability of the novel, orally administered, small molecule glucagon-like peptide 1 receptor agonist danuglipron?. Findings In this phase 2 randomized clinical trial in 411 adults with T2D, danuglipron reduced glycated hemoglobin and fasting plasma glucose (at all doses) and body weight (at the ...
Exclusion criteria included: type 1 diabetes or gestational diabetes, < 18 years of age and a documented diagnosis of cognitive decline obtained from the participant's medical records. This diagnosis was based on comprehensive assessments of the individual's clinical presentation, medical history, and relevant test results conducted by ...
Adults with type 2 diabetes who are referred to a clinical pharmacist are more likely to meet their HbA1c goal and receive appropriate medication management of comorbidities than people receiving ...