Case Study: A Systematic Approach to Early Recognition and Treatment of Sepsis
Submitted by Madeleine Augier RN BSN
Tags: assessment Case Study emergency department guidelines mortality prevention risk factors sepsis standard of care treatment
Share Article:
Sepsis is a serious medical condition that affects 30 million people annually, with a mortality rate of approximately 16 percent worldwide (Reinhart, 2017). The severity of this disease process is not well known to the public or health care workers. Often, health care providers find sepsis difficult to diagnose with certainty. Deaths related to sepsis can be prevented with accurate assessments and timely treatment. Sepsis must be considered an immediate life-threatening condition and needs to be treated as a true emergency.

Relevance and Significance
Sepsis is defined as “the life-threatening organ dysfunction resulting from a dysregulated host response to infection” (Kleinpell, Schorr, & Balk, 2016, p. 459). Jones (2017) study of managing sepsis affirms that the presence of sepsis requires a suspected source of infection plus two or more of the following: hyperthermia (>38.1 degrees Celsius) or hypothermia (<36 degrees Celsius), tachycardia (>91 beats per minute), leukocytosis or leukopenia, altered mental status, tachypnea (>21 breaths per minute), or no urine output for 12 hours. If the infection persists, acute organ dysfunction or failure occurs from widespread inflammation, eventually leading to septic shock (Palleschi, Sirianni, O’Connor, Dunn, & Hasenau, 2013). Palleschi et al. (2013) states that during septic shock, “the cardiovascular system fails, resulting in hypotension, depriving vitals organs of an adequate supply of oxygenated blood” (p. 23). Ultimately the body can go into multiple organ dysfunction syndrome (MODS), leading to death if there is inaccurate assessment and inadequate treatment.
The purpose of this case study is to make the nurse practitioner aware of the severity sepsis, and how to accurately diagnose and treat using evidence-based data. Sepsis can affect everyone, despite his or her age or comorbidity. Center for Medicare and Medicaid Services (CMS) has diagnosed this problem as a priority and uses sepsis management in determining payment to providers (Tedesco, Whiteman, Heuston, Swanson-Biearman, & Stephens, 2017). This medical diagnosis is unpredictable and presents a challenge to nurse practitioners worldwide. Early recognition and treatment of sepsis by the nurse practitioner is critical to decrease morbidity and mortality.
After completing this case study, the reader should be able to:
- Identify the risk factors of sepsis
- Identify the signs and symptoms of sepsis
- Identify the treatment course of sepsis
Case Presentation
A 65-year-old Asian female presented to the emergency department accompanied by her husband with a chief complaint of altered mental status. Upon assessment, the patient was lethargic, and alert and oriented to person only. The patient’s heart rate was 136, blood pressure 104/50, oral temperature 99 degrees Fahrenheit, oxygen saturation 97% on 4 liters nasal cannula, and respirations 26 per minute. The patient’s blood glucose was obtained with a result 454.
Further orders, such as labs and imaging were made by the provider to rule out potential diagnoses. A rectal temperature was obtained revealing a fever of 103.7 degrees Fahrenheit. The patient remained restless on the stretcher. After one hour in the emergency department, her heart rate spiked to 203 beats per minute, respirations became more rapid and shallow, and she became more lethargic. The patient’s altered mental status, increasing heart rate and respirations caused the providers to act rapidly.
Medical History
The patient’s husband reports that she is a type one diabetic, he denies any other medical conditions. In addition, the patient’s husband states that she has not been exposed to any sick individuals in the past few weeks. The husband reports a family history of diabetes, other wise no significant familial history. No history of smoking, drinking, or illicit drug use was to be noted.
Physical Assessment Findings
The patient appeared lethargic and confused with a Glasgow Coma Scale of 12. She appeared tachypnic, with shallow respirations, and a rate of 28 breaths per minute. Upon auscultation, breath sounds were coarse. Her abdomen was soft and non-tender, no nausea or vomiting noted. The patient appeared diaphoretic, and her legs were mottled.
Laboratory and Diagnostic Testing and Results
During the initial assessment, a complete blood count (CBC), basic metabolic panel (BMP), and lactic acid level were ordered for blood work. A STAT electrocardiogram (EKG), urinalysis, and a chest X-ray were ordered to differentiate possible diagnoses. The CBC revealed leukocytosis with a white blood cell count of 23,000 and an increased lactic acid level of 4.3. The anion gap and potassium level remained within a normal limit, ruling out the possibility of diabetic ketoacidosis (DKA). The patient’s EKG showed supraventricular tachycardia (SVT). The chest X-ray revealed infiltrates to the right lung. The urinalysis was free from leukocytes or nitrites. Blood cultures were ordered to confirm their hypothesized diagnosis, septicemia.
Pharmacology
The provider initiated intravenous (IV) fluid treatment with Lactated Ringers at a bolus of 30 mL/kg. Because the patient’s heart rate was elevated, 6 mg of adenosine was ordered to combat the SVT. Additionally, broad-spectrum IV antibiotics were initiated. One gram of vancomycin and 3.375 grams of piperacillin-tazobactam were the preferred antibiotics of choice.
Final Diagnosis
Upon arrival, the providers were ruling out DKA and sepsis, given the patient’s history.
The patient’s elevated white blood cell counts, temperature, lactic acid level, heart/respiratory rate, and altered mental status were all clinical indicators of sepsis. The chest X-ray revealed a right lung infiltrate, persuading the providers to diagnose the patient with sepsis secondary to pneumonia.
Patient Management
After sepsis was ruled as the patient’s diagnosis, rapid antibiotic administration and IV fluid treatment became priority after the patient’s heart rate was controlled. A cooling blanket and a temperature sensing urinary catheter was placed to continuously monitor and control the patient’s fever. Later, the patient was transferred to a critical care unit for further treatment. Shortly after being transferred, the patient went into respiratory failure and was placed on a ventilator. After two days in the ICU, the patient remained in septic shock, and died from multisystem organ failure.
When the patient initially presented to the emergency department, accurate and rapid diagnosis of sepsis was critical in order to stabilize the patient and prevent mortality. A challenge was presented to the provider regarding a rapid diagnosis due to the patient’s history and her presenting signs and symptoms. Increased awareness and interprofessional education regarding sepsis and its’ treatment is vital to decrease mortality. Health care providers need to be competent in recognizing and accurately treating sepsis in a rapid manner.
Research shows that outcomes in sepsis are improved with timely recognition and early resuscitation (Javed et al., 2017). It is important for the provider to identify certain risk factors and symptoms to easily diagnose sepsis. A research study by Henriksen et al. (2015) proved that age, and comorbidities including psychotic disorders, immunosuppression, diabetes, and alcohol abuse served as top risk factors for sepsis.
Once the diagnosis of sepsis is determined, rapid treatment must be initiated. The golden standard of treatment consists of a bundle of care that includes blood cultures, broad-spectrum antibiotic agents, and lactate measurement completed within 3 hours as described by Henriksen et al. (2015). A study by Seymour et al. (2017) showed that the more rapid administration of the bundle of care is correlated with a decreased mortality rate. In addition, The Survival of Sepsis Campaign formed a guideline to sepsis treatment; Rhodes et al. (2016) suggests giving a 30 mL/kg of IV crystalloid fluid for hypoperfusion. If hypotension persists (mean arterial pressure <65), vasopressors, preferably norepinephrine, should be initiated (Rhodes et al., 2016). Prompt recognition of sepsis and implementation of the bundle of care can help reduce avoidable deaths.
To increase awareness, interprofessional education regarding sepsis and its’ common signs and symptoms needs to be established. Evidence-based protocols should be utilized in hospital care settings that provide nurse practitioners with a guideline to follow to ensure rapid and accurate treatment is given. Increased awareness and education helps providers and other healthcare workers to properly identify and accurately treat sepsis.
The public and health care providers must become more aware and educated on the severity of sepsis. It is crucial to be able to recognize signs and symptoms of sepsis to prevent further complications such as septic shock and multi-organ failure. Increased awareness, interprofessional education, accurate assessment, and rapid treatment can help reduce incidence and mortality. Sepsis management must focus upon early goal-directed therapy (antibiotic administration, fluid resuscitation, blood cultures, lactate level) and individualized management pertaining to the patient’s history and assessment (Head & Coopersmith, 2016). Misdiagnosis and delay in emergency treatment can result in missed opportunities to save lives.
- Head, L. W., & Coopersmith, C. M. (2016). Evolution of sepsis management:from early goal-directed therapy personalized care. Advances in Surgery, 50 (1), 221-234. doi:10.1016/j.yasu.2016.04.002
- Henriksen, D. P., Pottegard, A., Laursen, C. B., Jensen, T. G., Hallas, J., Pedersen, C., & Lassen, A. T. (2015). Risk factors for hospitalization due to community-acquired sepsis-a population-based case-control study. PLOS ONE, 10 (4), 1-12. doi:10.1371/journal.pone.0124838
- Javed, A., Guirgis, F. W., Sterling, S. A., Puskarich, M. A., Bowman, J., Robinson, T., & Jones, A. E. (2017). Clinical predictors of early death from sepsis. Journal of Critical Care, 42 , 30-34. doi:10.1016/j.jcrc.2017.06.024
- Jones, J. (2017). Managing sepsis effectively with national early warning scores and screening tools. British Journal of Community Nursing, 22 (6), 278-281. doi:10.12968/bjcn.2017.22.6.278
- Kleinpell, R. M., Schorr, C. A., & Balk, R. A. (2016). The new sepsis definitions: Implications for critical care. American Journal of Critical Care, 25 (5), 457-464. doi:10.4037/ajcc2016574
- Palleschi, M. T., Sirianni, S., O'Connor, N., Dunn, D., & Hasenau, S. M. (2013). An interprofessioal process to improve early identification and treatment for sepsis. Journal for Healthcare quality, 36 (4), 23-31. doi:10.1111/jhq.12006
- Reinhart, K., Daniels, R., Kissoon, N., Machado, F. R., Schachter, R. D., & Finfer, S. (2017). Recognizing sepsis as a global health priority-A WHO resolution. The New England Journal of Medicine, 377 (5), 414-417. doi:10.1056/NEJMp1707170
- Rhodes, A., Evans, L. E., Alhazzani, W., Levy, M. M., Anotnelli, M., Ferrer, R.,...Beale, R. (2017). Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Medicine, 43 (3), 304-377. doi:10.1007/s00134-017-4683-6
- Seymour, C. W., Gesten, F., Prescott, H. C., Friedrich, M. E., Iwashyna, T. J., Phillips, G. S.,...Levy, M. M. (2017). Time to treatment and mortality during mandated emergency care for sepsis. The New England Journal of Medicine, 376 (23), 2235-2244. doi:10.1056/NEJMoal1703058
- Tedesco, E. R., Whiteman, K., Heuston, M., Swanson-Biearman, B., & Stephens, K. (2017). Interprofessional collaboration to improve sepsis care and survival within a tertiary care emergency department. Journal of Emergency Nursing, 43 (6), 532-538. doi:10.1016/j.jen.2017.04.014
Career Opportunities

More Like This

Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest Content
- BMJ Journals
You are here
- Volume 6, Issue 1
- Early identification of severe community-acquired pneumonia: a retrospective observational study
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-0952-0163 Frances S Grudzinska 1 ,
- Kerrie Aldridge 1 ,
- Sian Hughes 2 ,
- Peter Nightingale 2 ,
- Dhruv Parekh 3 ,
- Mansoor Bangash 2 ,
- Rachel Dancer 3 ,
- Jaimin Patel 1 ,
- Elizabeth Sapey 1 ,
- David R Thickett 1 and
- Davinder P Dosanjh 1
- 1 Institute of Inflammation and Ageing , University of Birmingham College of Medical and Dental Sciences , Birmingham , UK
- 2 Queen Elizabeth Hospital Birmingham , Birmingham , UK
- 3 Institute of Inflammation and Ageing , University of Birmingham , Birmingham , UK
- Correspondence to Dr Davinder P Dosanjh; d.dosanjh{at}bham.ac.uk
Background Community-acquired pneumonia (CAP) is a leading cause of sepsis worldwide. Prompt identification of those at high risk of adverse outcomes improves survival by enabling early escalation of care. There are multiple severity assessment tools recommended for risk stratification; however, there is no consensus as to which tool should be used for those with CAP. We sought to assess whether pneumonia-specific, generic sepsis or early warning scores were most accurate at predicting adverse outcomes.
Methods We performed a retrospective analysis of all cases of CAP admitted to a large, adult tertiary hospital in the UK between October 2014 and January 2016. All cases of CAP were eligible for inclusion and were reviewed by a senior respiratory physician to confirm the diagnosis. The association between the CURB65, Lac-CURB-65, quick Sequential (Sepsis-related) Organ Failure Assessment tool (qSOFA) score and National Early Warning Score (NEWS) at the time of admission and outcome measures including intensive care admission, length of hospital stay, in-hospital, 30-day, 90-day and 365-day all-cause mortality was assessed.
Results 1545 cases were included with 30-day mortality of 19%. Increasing score was significantly associated with increased risk of poor outcomes for all four tools. Overall accuracy assessed by receiver operating characteristic curve analysis was significantly greater for the CURB65 and Lac-CURB-65 scores than qSOFA. At admission, a CURB65 ≥2, Lac-CURB-65 ≥moderate, qSOFA ≥2 and NEWS ≥medium identified 85.0%, 96.4%, 40.3% and 79.0% of those who died within 30 days, respectively. A Lac-CURB-65 ≥moderate had the highest negative predictive value: 95.6%.
Conclusion All four scoring systems can stratify according to increasing risk in CAP; however, when a confident diagnosis of pneumonia can be made, these data support the use of pneumonia-specific tools rather than generic sepsis or early warning scores.
- community acquired pneumonia
- lac-curb-65
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/ .
https://doi.org/10.1136/bmjresp-2019-000438
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Key messages
What is the key question.
What risk stratification tool should you use in community-acquired pneumonia?
What is the bottom line?
Pneumonia-specific tools provide better discrimination of patients at high risk of adverse outcome than generic sepsis tools.
Why read on?
This paper assesses commonly used risk stratification tools in a pragmatic patient population comparing newer tools such as quick Sequential (Sepsis-related) Organ Failure Assessment tool with established scores.
Introduction
Community-acquired pneumonia (CAP) is the fourth leading cause of death worldwide when combined with lower respiratory tract infections. 1 It is associated with significant mortality 2 and frequently leads to sepsis 3 4 with mortality rates rising to 30%. 5 Early identification of patients with severe CAP enables modification of management strategies and improves outcomes for patients. 6–8
To identify those at risk of poor outcomes, guidelines for management of CAP and sepsis suggest risk stratification tools should be used 9–11 ; however there is no consensus as to which tool should be used. 11–16
Severity assessment tools have been developed specifically for identifying patients at risk of deterioration due to sepsis. The quick Sequential (Sepsis-related) Organ Failure Assessment tool (qSOFA) 15 is the recommended tool to screen patients with suspected infection outside the intensive care unit (ICU) 11 17–19 (one point for each of altered mentation, respiratory rate (RR) ≥22 and systolic blood pressure (SBP) ≤100 mm Hg, with a score ≥2 suggesting high risk for deterioration). 15 More generic tools designed to predict deterioration regardless of aetiology have also been designed, such as the National Early Warning Score (NEWS), widely used in the English National Health Service. 16 NEWS is a composite score assessing level of alertness, RR, blood pressure (BP), heart rate, oxygen saturation and temperature with increasing values for more abnormal measurements (see online supplementary eTable 1 for a full description). A score of ≥3 in any category or score ≥5 overall triggers urgent patient review.
Supplemental material
Disease-specific tools, such as CURB65, are recommended by respiratory societies worldwide. 9 10 20 Each of altered mentation, blood urea >7.0, RR ≥30, SBP <90 or diastolic BP ≤60 and age ≥65 scores one point, with scores ≥2 considered moderate–severe. Original validation of this tool, however, excluded patients from long-term care facilities as well as those with common comorbidities. 12
In addition, attempts have been made to refine previously well-described scores by using biomarkers such as lactate. Lactate is a strong independent predictor of mortality in both pneumonia and sepsis, 13 21 and work by other groups has shown that addition of lactate ≥1.8 mmol/L improves the ability of CURB65 to predict mortality. 7 13
Existing evidence supports early intervention and consideration of ICU for appropriate patients 8 12 22 using severity assessment tools to aid decision-making; however, the evidence to support one tool over another is lacking in patients with pneumonia. We compared the performance of four commonly used severity assessment tools (CURB65, Lac-CURB-65, NEWS and qSOFA) in a CAP-specific population to identify those at risk of adverse outcomes. We selected these four scores as they are commonly used in clinical practice and most widely recommended by sepsis and respiratory societies. We hypothesise that pneumonia-specific tools will more accurately predict patients at high risk of adverse outcomes.
Study institution and subjects
All adults admitted to the Queen Elizabeth Hospital Birmingham, UK with CAP between October 2014 and January 2016 were eligible for inclusion.
CAP cases were identified using the hospital coding system retrospectively. CAP was defined using British Thoracic Society (BTS) guidelines. 9 Senior respiratory physicians confirmed the diagnosis of CAP using admission documents, radiology and electronic patient records. Cases were excluded if there were no new infiltrates in relevant radiological investigations. We identified patients who would have been previously identified as healthcare-associated pneumonia (HCAP). Patients with hospital-acquired pneumonia (HAP) were excluded. HAP and HCAP were defined using the 2005 American Thoracic Society (ATS) and Infectious Diseases Society of America guidelines. 10 Ethics was deemed not to be required based on the Health Research Authority decision tool. 23 This was confirmed by our institution’s research team and local approval was granted.
In addition to the first set of physiological observations recorded on admission to hospital (level of alertness, respiratory rate, temperature, oxygen saturations, BP and heart rate), the first set of biochemical and haematological laboratory results were also collected from the electronic patient record.
CURB65, 12 Lac-CURB-65, 13 NEWS 16 and qSOFA 15 scores were calculated as previously described.
To assess for confusion in qSOFA, CURB65 and Lac-CURB-65, we reviewed the admission document and scored for confusion if any of the following were documented: abnormal AVPU score (alert, response to voice, pain or unresponsive), Glasgow coma scale ≤13, abnormal mental state examination, or documentation of confusion or delirium.
Lac-CURB-65 score and NEWS were grouped into predefined ‘Low’, ‘Moderate’ and ‘High’ risk categories. Lac-CURB-65 cut-offs: low—CURB-65 ≤1 and/or lactate <2.0 mmol/L; moderate—CURB65=2 and/or lactate 2.0–4.0 mmol/L; high—CURB65 ≥3 and/or lactate >4.0 mmol/L. 13
NEWS cut-offs: low—aggregate score 1–4; medium—aggregate score 5–6 or a score of ≥3 in a single category; high—aggregate score ≥7 as previously defined. 16
Outcome measures included admission to ICU, length of stay and in-hospital, 30-day, 90-day and 365-day mortality; data were collected from the electronic patient record.
Patient and public involvement
Patient and the public were not involved in the development of this research. This study was undertaken in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for cohort studies.
Statistical analysis
Comparison of proportions was performed using the χ 2 test for trend; trends in median length of stay were assessed using the Jonckheere-Terpstra test. Sensitivity, specificity, receiver operating characteristic (ROC) curve analysis, positive and negative predictive values, and likelihood ratios were calculated for each scoring system. Cases with missing data were excluded from analysis on a score-by-score basis. To assess the effect of missing data, analyses were repeated using multiple imputation and assumption of normal values where data points were absent. These analyses and detailed explanation of methods are presented in the online supplement . Statistical analysis was carried out using IBM SPSS Statistics for Windows V.24.0 and R (V.3.4.4, Vienna, Austria).
Participant demographics
A total of 2895 patients were coded as having CAP and 1545 were included in the final analysis ( figure 1 ). Due to missing data, there were variable numbers of cases included in the analysis for each score ( figure 1 ). For a detailed comparison of missing and included cases, see the online supplementary eTables 3–7 .
- Download figure
- Open in new tab
- Download powerpoint
Modified CONSORT diagram demonstrating patient inclusion and exclusion pathways. *Reasons for exclusion of patients for each severity assessment tool (number of cases excluded). Some cases were excluded due to more than one missing data point: CURB65: Confusion (230), urea (5), respiratory rate (4), blood pressure (4), age >65 years (0). Lac-CURB-65: as for CURB65 plus lactate (227). qSOFA: mentation (230), respiratory rate (4), blood pressure (4). NEWS: temperature (9), oxygen saturations (5), level of consciousness (4), respiratory rate (4), blood pressure (4), heart rate (4). CAP, community-acquired pneumonia; HAP, hospital-acquired pneumonia; NEWS, National Early Warning Score; qSOFA, quick Sequential (Sepsis-related) Organ Failure Assessment.
The median age of patients included was 76 (IQR 63–85). Of all cases, 50.8% (785) were men; 29.0% (449) of cases fulfilled the criteria for what was previously defined as HCAP. Eighty-nine per cent of cases had at least one comorbidity ( online supplementary eTable 2 ). Overall 30-day mortality was 19.0%; in-hospital mortality was 15.4% with an ICU admission rate of 6.4%. Full demographic and outcome information is available in the online supplement ( online supplementary eTable 2 ).
Validation of CURB65 for patients previously defined as HCAP
In 2005, HCAP was defined as a separate entity to CAP in order to describe a population of patients in long-term care or receiving home-based or hospital-based intravenous therapy or dialysis who had increased mortality 24 and high prevalence of antibiotic-resistant pathogens. 25 The concept of HCAP has more recently been rejected; however, the original validation of the CURB65 score excluded those that were labelled as HCAP. This has led to widespread use of CURB65 in a patient population it was not originally validated in. We analysed the non-HCAP and HCAP groups separately for CURB65 to ensure that there was no significant difference in risk stratification between the two groups.
CURB65 scoring was possible for 1311 (84.9%) of all cases, with complete data available for 83.5% (375) of patients with HCAP and 85.4% (936) of patients without HCAP ( table 1 ).
- View inline
CURB65 as a prognostic tool for different outcome measures stratified by CAP aetiology
CURB65 score was able to stratify according to increasing risk of in-hospital mortality as well as 30-day, 90-day and 365-day mortality ( table 1 ). Increasing CURB65 score was not associated with increased likelihood of ICU admission ( table 1 ). Increasing length of stay was significantly associated with increased CURB65 score in the whole cohort as well as the non-HCAP group, but not in the HCAP-alone group.
Ability of different severity scoring systems to risk stratify
Increasing NEWS and qSOFA scores were significantly associated with increased risk of ICU admission during admission ( table 2 ). Increasing severity score was significantly associated with increased risk of mortality for all four scoring systems. Increasing scores were also associated with increased length of stay for all scoring tools.
Ability of severity assessment tools to risk stratify for outcome measures in CAP
Overall accuracy of the different scoring systems to predict 30-day mortality
Overall accuracy of the scoring systems to identify those at risk of death within 30 days of presentation to hospital was calculated using ROC curve analysis ( figure 2 ). The area under the ROC curve (AUROC) for CURB65, Lac-CURB-65, NEWS and qSOFA were 0.69, 0.68, 0.63 and 0.62, respectively. AUROC values were significantly greater for CURB65 and Lac-CURB-65 scoring systems when compared with those generated using the qSOFA criteria (CURB65 vs qSOFA p<0.0001, Lac-CURB-65 vs qSOFA p=0.0024) ( table 3 ).
Comparison of overall accuracy of severity assessment tools to predict 30-day mortality at admission
Receiver operating characteristic (ROC) curves to assess overall accuracy of severity assessment tools using 30-day mortality as the standard. Area under the ROC curve for CURB65, Lac-CURB-65, NEWS and qSOFA were 0.69, 0.68, 0.63 and 0.62, respectively. NEWS, National Early Warning Score; qSOFA, quick Sequential (Sepsis-related) Organ Failure Assessment.
Performance characteristics of severity assessment tools
With 30-day mortality as the outcome measure, we calculated the performance characteristics of each of the scoring systems using previously defined cut-off points. 13 15 Lac-CURB-65, using ‘moderate’ as the cut-off, had the greatest sensitivity and negative predictive value (NPV), 96.4% and 95.6%, respectively. This was closely followed by CURB65 with a cut-off of ≥2 giving a sensitivity of 85.0% and NPV of 91.5%. qSOFA had the poorest sensitivity at 40.3%, but relatively high specificity of 79.9% ( table 4 ).
Performance characteristics of the severity scoring systems using 30-day mortality as the outcome measure
Assessment of the impact of missing values on the analysis
To assess the impact of missing data, patient characteristics and outcome measure data were compared between those with complete data and those without for each severity assessment tool ( online supplementary eTables 3–7 ). The complete analysis was repeated having replaced the absent data with either normal values or by using multiple imputation. The full results of these analyses can be reviewed in the online supplement (see online supplementary eTables 8–14 ). Both the assumed normal and multiple imputation analyses resulted in little significant change in the results.
This study describes a large cohort of hospitalised CAP and confirms that CURB65, Lac-CURB-65, NEWS and qSOFA scores at the time of hospital admission can stratify according to increasing risk of mortality in all patients with CAP. These data also suggest that using a ‘moderate’ Lac-CURB-65 score as a threshold for identifying those at increased risk of 30-day mortality may have utility as a ‘rule-out’ when assessing patients that may need escalation of care.
A key strength of this study was the use of a pragmatic approach to patient inclusion, which has led to the validation of these assessment tools in patients often excluded from other studies but among which the severity assessment scores are commonly used. Patients excluded from the original validation of the CURB65 score included those with bronchiectasis, malignancy, prior hospital admission within 14 days, immunocompromise, nursing home residents or where pneumonia was an expected terminal event. 12 The generalisability of our findings to real-life patient populations has been increased by including these patients.
A previous study has demonstrated that CURB65 had greater predictive ability for adverse outcomes in CAP than systemic inflammatory response syndrome criteria or early warning scores 26 ; however, we have used additional, comparatively novel scoring systems and applied them to a larger cohort of patients with more pragmatic inclusion criteria and measured long-term mortality outcomes. 26
The diagnosis of pneumonia has been verified by the review of radiological and clinical findings. A key finding of the UK-wide BTS pneumonia audit was that using clinical coding alone led to misdiagnosis in approximately a third of cases due to lack of clinicoradiographic features of pneumonia, 27 a finding borne out by this study.
Increasing NEWS and qSOFA scores were associated with increased rate of admission to ICU. It should be noted that during the study period, all scores were being used in clinical practice, except for qSOFA, and this may have had an impact on the decision-making process when a patient was admitted to ICU. Our ICU admission rate is lower than that seen in studies performed outside the UK 7 15 28 ; however, it is in keeping with the BTS pneumonia audit. 27 This is likely to be due to inclusion of patients with treatment limitations; we choose to include patients with treatment limitations to enable application of these scores to all patients admitted. Prediction of adverse outcome remains important for all patients, even if they would be unlikely to benefit from ICU admission as it informs decision-making regarding appropriate interventions that can be implemented, as well as informing decisions regarding withdrawal of care in cases where further treatment may be futile.
The qSOFA tool was designed as a quick and easy screening tool, to allow repeated and widespread use to identify deteriorating patients. 15 It was interesting to note that the sensitivity of the qSOFA score to predict 30-day mortality, when performed at the time of admission, was low in this CAP population, an observation that has been made in previous studies. 28 29 qSOFA was more accurate at predicting ICU admission in this study and previous work. 30 This suggests that though serial scoring may have use in identifying those that are deteriorating, in this cohort of patients with CAP, there was little use of the score as an indicator of 30-day mortality at the time of admission. The validation study for qSOFA defined adverse outcome as in-hospital mortality or ICU admission for greater than 3 days 15 ; our different definition of adverse outcome may also affect interpretation of these data.
A raised lactate has been consistently demonstrated to be an independent predictor of mortality in sepsis 21 and pneumonia. 7 Frenzen et al found that addition of lactate ≥1.8 mmol/L significantly improved the ability of CURB65 to predict a combined endpoint of ICU admission and inpatient mortality, 7 similarly confirmed by Chen and Li. 13 However, this effect was not observed in our cohort for ICU admission or 30-day mortality. This is likely to be due to key differences in study design and populations. For example, Frenzen et al excluded any patients with treatment limitations and had a high ICU admission rate (22%) with very low mortality (7%). Our mortality rates were in keeping with those from the BTS pneumonia audit 27 and large European cohorts. 2 Thirty-day mortality was higher than in-hospital mortality, and this is likely to reflect the increased long-term mortality 31 and high rates of re-admission seen after CAP 27 ; this is especially true for older people as seen in this study.
Increasing age is well recognised to be an independent risk factor for mortality in CAP 32 and is represented in CURB65. Greater than two-thirds of participants in this cohort were ≥65 years of age, meaning they score highly when using CURB65, whereas NEWS and qSOFA do not account for age. In the future, it would be pertinent to assess for impact of age on scoring systems to see if dichotomising by age criteria improves predictive ability.
To compare the overall accuracy of the scores to predict 30-day mortality, ROC curves were calculated. Though CURB65 and Lac-CURB-65 resulted in a significantly greater AUROC compared with qSOFA, the clinical significance of this difference is difficult to define, and none of the scores provided excellent discrimination of patients at high risk of adverse outcomes. The use of different inclusion criteria and management strategies, combined with different outcome measures used in previous studies, makes direct comparison with our findings challenging. The AUROC for the CURB65 has been reported as ranging from 0.71 7 to 0.829 8 28 33 in CAP populations (with patients with HCAP excluded), to 0.65 34 in a cohort which included patients with HCAP, similar to findings presented here. The use of CURB65 in the HCAP population has been validated previously by Ewig et al. 35
Goulden et al 18 used NEWS and qSOFA to predict mortality in a group of emergency admissions with sepsis and also found similar AUROCs to those presented here (0.65 and 0.62, respectively). A meta-analysis of qSOFA in predicting mortality identified an AUROC of 0.67; however, the sensitivity of qSOFA was very low. 29 Brabrand and Henriksen found that CURB65 was not superior to NEWS in predicting 30-day mortality. 36 A large CAP cohort study using the CAPNETZ 37 database found that qSOFA plus age ≥65 years was as good at predicting 30-day mortality as CRB65. 38 Data presented here for patients with CAP support other data in the literature and may suggest that the qSOFA score may not perform as well in a CAP-specific population when compared with a mixed sepsis population. The low AUROCs seen for these scores and by other groups demonstrate the weaknesses of these severity scoring systems in common clinical practice and highlight the need for better tools.
This study has limitations, including the retrospective single-centre study design and missing data. The most common missing data was documentation of the patient’s mental state. This may have introduced bias when comparing the different scoring systems. To account for this, we have presented analyses using multiple imputation and assumption of normal values. It is reassuring to note that there were no significant changes in the results when these alternative analysis methods were employed. With regards to prediction of ICU admission, we did not exclude patients with treatment limitations and this may have impacted on accuracy of these scores to predict ICU admission. Prospective multicentre studies to ensure collection of complete data sets and ensure generalisability are needed. In addition, further studies are warranted to examine the role of serial scoring to predict deterioration during an admission, rather than assessing risk at the time of admission. NEWS and qSOFA have already demonstrated validity for serial scoring 16 17 ; however, this has not been assessed for the pneumonia-specific scores.
We recognise that other severity scoring tools exist for pneumonia and are more widely used outside the UK 39 ; however, we opted for commonly used and simple scores that could be calculated at the point of admission rather than complex tools such as pneumonia severity index.
None of the commonly used existing tools provide excellent discrimination of patients at high risk of adverse outcomes, and more sophisticated scoring systems exist such as SOFA for sepsis or ATS minor criteria which provide better discrimination. However, refinement of existing simple tools or investigation of novel markers for poor prognosis in CAP would be beneficial. Furthermore, these data do not assist with the risk stratification of patients with HAP, and further studies are needed in this patient population. The development of an accurate risk stratification tool for CAP and HAP could lead to earlier identification of patients who would benefit from early escalation and targeted treatment. 12 22
These data suggest that four commonly used severity assessment tools are able to stratify patients according to increasing risk of mortality. Furthermore, a ‘low’ Lac-CURB-65 score appears to indicate that a poor outcome is unlikely. Tools specifically designed for sepsis and early recognition of patients at increased risk of ICU admission did not perform as well as the CAP-specific tools, particularly when compared with previous studies of all-cause sepsis, suggesting that organ-specific severity assessment tools may have greater use in early recognition of patients who are at risk of adverse outcomes.
- GBD 2016 Causes of Death Collaborators
- Marshall DC ,
- Goodson RJ ,
- Xu Y , et al
- Ranzani OT ,
- Alberti C ,
- Brun-Buisson C ,
- Chevret S , et al
- National Institute of Clinical Excellence
- Mukhopadhyay A , et al
- Frenzen FS ,
- Kutschan U ,
- Meiswinkel N , et al
- Kolditz M ,
- Klapdor B , et al
- Baudouin SV ,
- George RC , et al
- American Thoracic Society
- Infectious Diseases Society of America
- Deutschman CS ,
- Seymour CW , et al
- van der Eerden MM ,
- Laing R , et al
- Cerra F , et al
- Seymour CW ,
- Iwashyna TJ , et al
- Royal College of Physicans
- Lemachatti N ,
- Krastinova E , et al
- Goulden R ,
- Hoyle M-C ,
- Monis J , et al
- Aluisio AR , et al
- Woodhead M ,
- Ewig S , et al
- Mikkelsen ME ,
- Miltiades AN ,
- Gaieski DF , et al
- Rivers EP ,
- Central Office for Research Ethics Committees
- Venditti M ,
- Falcone M ,
- Corrao S , et al
- Dascomb K ,
- Stenehjem E , et al
- Nathwani D ,
- Daniel PBT ,
- Menéndez R , et al
- Jin Y , et al
- Guignard V ,
- Schefold JC , et al
- Johnstone J ,
- Eurich DT ,
- Majumdar SR , et al
- Cillóniz C ,
- Rodríguez-Hurtado D ,
- Torres A , et al
- Lan T , et al
- Noguchi S ,
- Kawanami T , et al
- Birkner N ,
- Strauss R , et al
- Brabrand M ,
- Henriksen DP
- Suttorp N ,
- Scherag A ,
- Rohde G , et al
- Niruban A , et al
Contributors FSG and DPD performed data acquisition, analysis and interpretation of data for the work. DP, RD and JP helped with data interpretation. SH and KA performed data acquisition. PN assisted with the statistical analysis of the data. ES, MB, DRT and DPD designed the study. All authors were involved with drafting and revising the work and approved the final submission.
Funding FSG is funded by NIHR, DPD and DP are funded by NIHR West Midlands Comprehensive Research Network, DRT is funded by the MRC and BLF, and ES is funded by NIHR, Wellcome Trust and Alpha 1 Foundation.
Disclaimer None of the funding bodies played any role in the design of the study and collection, analysis and interpretation of data, or in writing the manuscript.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available on reasonable request.
Read the full text or download the PDF:
- Subscriptions
- Advanced search

Advanced Search
Respiratory viral sepsis: epidemiology, pathophysiology, diagnosis and treatment
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- For correspondence: [email protected]
- Info & Metrics
According to the Third International Consensus Definition for Sepsis and Septic Shock, sepsis is a life-threatening organ dysfunction resulting from dysregulated host responses to infection. Epidemiological data about sepsis from the 2017 Global Burden of Diseases, Injuries and Risk Factor Study showed that the global burden of sepsis was greater than previously estimated. Bacteria have been shown to be the predominant pathogen of sepsis among patients with pathogens detected, while sepsis caused by viruses is underdiagnosed worldwide. The coronavirus disease that emerged in 2019 in China and now in many other countries has brought viral sepsis back into the vision of physicians and researchers worldwide. Although the current understanding of the pathophysiology of sepsis has improved, the differences between viral and bacterial sepsis at the level of pathophysiology are not well understood. Diagnosis methods that can broadly differentiate between bacterial and viral sepsis at the initial stage after the development of sepsis are limited. New treatments that can be applied at clinics for sepsis are scarce and this situation is not consistent with the growing understanding of pathophysiology. This review aims to give a brief summary of current knowledge of the epidemiology, pathophysiology, diagnosis and treatment of viral sepsis.
The disease burden of sepsis from viruses is great. Although current understanding of sepsis has improved, the differences between viral and bacterial sepsis were not well understood. It is urgent to pay more attention to respiratory viral sepsis. https://bit.ly/2Vujpfl
- Introduction
Sepsis is a complex syndrome that results from infection. Recognising sepsis as not just an inflammatory disorder was one of the key reasons to revise previous criteria of sepsis, which had the limitation of low specificity of systemic inflammatory response syndrome as one of the criteria for sepsis [ 1 – 3 ]. According to the Third International Consensus Definition for Sepsis and Septic Shock (Sepsis-3) in 2016, sepsis was defined as a life-threatening organ dysfunction resulting from dysregulated host responses to infection, with the Sequential Organ Failure Assessment (SOFA) score evaluating the degree of organ dysfunction [ 4 , 5 ]. The definition of sepsis in Sepsis-3 is similar to the previous definition of severe sepsis in Sepsis-2. A meta-analysis incorporating 27 studies from seven high-income countries showed that the incidence rate and case fatality ratio of severe sepsis were 270 per 100 000 person-years and 26%, respectively (Sepsis-2 definition) [ 6 ]. The latest data from the 2017 Global Burden of Diseases, Injuries and Risk Factor Study showed that 48.9 million incident cases of sepsis and 11.0 million sepsis-related deaths were reported globally in 2017, with the highest age-standardised incidence and mortality of sepsis occurring in areas with the lowest socio-demographic index [ 7 ]. The global burden of sepsis is larger than previously estimated and may continue to be great because of a prolonged life expectancy and an ageing population [ 8 – 11 ]. In 2017, the World Health Organization and World Health Assembly recognised sepsis as a global health priority, and adopted a resolution to improve the prevention, diagnosis and management of sepsis [ 12 ].
Bacteria have been shown to be the predominant pathogens of sepsis caused by infection [ 9 ]. The reported proportions of gram-positive and gram-negative organisms among adult septic patients were both around 40%, while the reported proportions of viruses were very low [ 13 , 14 ]. However, the proportion of negative cultures was up to 42% among patient with sepsis, for whom the possible cause could be virus [ 15 ]. Recent studies showed that respiratory viral infections were underdiagnosed in patients with sepsis or septic shock [ 16 , 17 ]. In both these studies, conducted in three middle-income countries from Southeast Asia and in a rural area of a high-income country (Sweden), viruses were detected in around one-third of adult patients with sepsis. The viruses, which can cause severe disease, included influenza A and B, respiratory syncytial virus, coronavirus, human metapneumovirus, parainfluenza virus types 1–3, adenovirus, enteroviruses, and rhinovirus [ 18 – 20 ]. Our CAP-China study, which was conducted in 34 hospitals from 10 provinces of mainland China, showed that the proportions of patients with community-acquired pneumonia (CAP) who developed sepsis during hospitals were 40.1 and 39.6% among those with influenza and non-influenza viral infections, respectively [ 18 ]. As well as for commonly detected viruses, emerging novel virus infections can also result in sepsis and have raised global health concerns [ 20 ], these include: severe acute respiratory syndrome-coronavirus (SARS-CoV) [ 21 ]; Middle East Respiratory Syndrome-coronavirus (MERS-CoV) [ 22 ]; and SARS-CoV-2 which caused the recent outbreak of the coronavirus disease 2019 (COVID-19) in China and in many other countries all over the world.
According to the Surviving Sepsis Campaign, intravenous antibiotics within 1 h after recognition of both sepsis and septic shock is strongly recommended [ 23 ]. This recommendation was based on previous findings that a delay in first antibiotic administration was associated with an increased in-hospital mortality [ 24 , 25 ]. Apart from the benefit of empirical antibiotic use for patients with sepsis, a more precise prescription of antimicrobial therapy, including antiviral therapy for patients without bacterial infection, should be further explored [ 26 ]. It is urgent to pay more attention to the role a virus plays in sepsis. The most common sites of infection among patients with sepsis are the respiratory tract (64–68%), followed by the abdominal tract, bloodstream, and renal and urinary tract [ 14 , 15 , 27 ]. In this review, we mainly focus on respiratory viral infection that could result in sepsis. Viral sepsis has been defined as life-threatening organ dysfunction due to a dysregulated host response to viral infection [ 28 ].
- Epidemiology of respiratory viral sepsis
Pneumonia was found to be the most common cause of sepsis and septic shock [ 14 , 29 ]. A recent retrospective cohort study that included hospitalised patients diagnosed as viral CAP without bacterial co-infection showed viral sepsis was present in 61% of these patients [ 30 ]. According to previously published data, 100 million cases of viral CAP occur every year in adults globally [ 31 ], so we can speculate the disease burden of viral sepsis is huge. The most common virus detected among patient with viral sepsis was influenza A virus, followed by rhinovirus, parainfluenza virus types 1–3, respiratory syncytial virus, adenovirus, influenza B virus and coronavirus [ 30 ]. A positive pathogen result for a virus is not sufficient for the diagnosis of viral sepsis. Whether the virus detected caused sepsis or not, such as being a coinfection of an unknown pathogen, leading to secondary infection of the other pathogen, or being just a false-positive result, needs to be decided by physicians according to clinical features and both laboratory and radiographic results of the patient [ 28 , 32 , 33 ].
Influenza virus related sepsis
Influenza viruses, including influenza A and B, can cause both seasonal epidemics and out-of-season sporadic cases and outbreaks [ 34 ]. The annual attack rate of influenza was estimated to be around 10% among adults [ 34 , 35 ]. Most people have self-limited upper respiratory tract symptoms, while some people develop severe illness. Seasonal influenza epidemics were estimated to account for about 291 243–645 832 respiratory deaths annually, with the highest mortality rate in sub-Saharan Africa and southeast Asia [ 36 ]. A retrospective cohort study using hospitalisation data and influenza surveillance data from the USA found that the incidence rate of influenza-associated severe-sepsis hospitalisation was 8.8 per 100 000 person-years (95% CI 3.9–16.5) [ 37 ]. Severe sepsis was present in 73% of influenza-associated critical illness hospitalisations defined as any hospitalisations with acute respiratory failure, severe sepsis, or in-hospital death.
A recent study showed that the most commonly detected virus among viral CAP patients who developed viral sepsis without bacterial co-infection was the influenza A virus, with a detection rate around seven times that of influenza B (52% versus 7%) [ 30 ]. This may be due to different virulences of different strains of influenza virus and the specific host response to them [ 38 , 39 ]. Previous studies also provide epidemiological data for sepsis related to different subtypes of influenza A virus [ 40 – 43 ]. Data from 26 patients infected with influenza A (H7N9) virus from one province in China showed that 10 (38.5%) developed septic shock quickly after the onset of illness, which was independently associated with mortality after multivariable adjustment [ 40 ]. For hospitalised patients infected with the 2009 pandemic influenza A(H1N1) (H1N1pdm09), the relative risk of sepsis and septic shock was 1.70 (95% CI 1.46–1.97) compared with hospitalised patients infected with seasonal influenza [ 41 ]. Furthermore, one study conducted among hospitalised patients with H1N1pdm09 showed that the proportion of sepsis was higher among patients with pneumonia than those without (18% versus 3%) [ 42 ]. The study results listed above not only provide epidemiological characteristics of influenza but also highlight that patients diagnosed with pneumonia during the pandemic season of influenza should be paid more attention by physicians because they are more likely to develop sepsis.
Coronavirus-related sepsis
The emerging novel coronavirus outbreak in China and many other countries worldwide has brought coronavirus back into our vision. To date, seven types of coronavirus are known to cause human disease, with four of them causing mild infections, while the other three betacoronaviruses, including SARS-CoV, MERS-CoV, and the recently isolated SARS-CoV-2, cause fatal cases [ 20 , 44 , 45 ]. SARS-CoV and MERS-CoV have caused 10 590 cases together, with 1632 being fatal cases. As of 10 June 2020, 84 652 cases of SARS-CoV-2 infection were confirmed in China, of which 4645 were fatal [ 46 ]. Another 1797 imported cases from overseas were reported. In other countries, territories or areas outside China, as of 10 June 2020, more than seven million COVID-19 cases were also confirmed, of which 403 380 were fatal [ 47 ].
D rosten et al . [ 22 ] reported that a 73-year-old man infected with MERS-CoV developed renal insufficiency and required dialysis on day 14 after onset of symptoms. With haemolysis and acute coagulation disorder, this patient died on day 18 due to septic shock. Infections with SARS-CoV were also reported to result in sepsis [ 21 ]. According to data from the first 41 cases infected with SARS-CoV-2, platelet counts were decreased while bilirubin and creatinine were elevated in several patients, which were signs of coagulation disorder, liver and renal dysfunction, respectively [ 20 ]. These patients can be diagnosed as having sepsis with the updated Sepsis-3 definition. Our recent study included 191 laboratory-confirmed COVID-19 patients in Wuhan who were discharged or died as of 31 January 2020. No bacterial pathogen was detected among these patients on admission. The results showed that the proportions of patients with sepsis and septic shock were 59% and 20%, respectively. All patients who died and 42% of patients who were discharged developed viral sepsis during hospitalisation. The median (interquartile) time from illness onset to sepsis was 9.0 (7.0–13.0) days [ 48 ].
Other respiratory virus-related sepsis
Among susceptible populations with sepsis, almost any virus could be detected, including respiratory viruses ( e.g. rhinovirus, parainfluenza virus types 1–3, respiratory syncytial virus, adenovirus, coronavirus and cytomegalovirus) and other viruses ( e.g. dengue viruses, hantaviruses, rotavirus and bocavirus) [ 17 , 22 , 30 , 48 – 55 ]. We mainly focus on respiratory viruses related to sepsis. Our study results from CAP-China found the risk of sepsis during hospitalisation between CAP patients with influenza and those with non-influenza respiratory virus infection were not statistically different (OR 1.00 (95% CI) 0.63–1.58) [ 18 ]. Previous studies showed diverse positive rates of non-influenza virus for patients with different clinical characteristics. Among viral CAP patients without bacterial co-infection and severe immunosuppression who developed viral sepsis, the most frequently detected non-influenza respiratory virus was rhinovirus (14%), followed by parainfluenza virus types 1–3 (11%), respiratory syncytial virus (10%), adenovirus (8%), and coronavirus (1%) [ 30 ]. Cytomegalovirus is one of the most common viral pathogens detected in immunocompromised patients. In the study conducted among Ugandan adults with sepsis of whom 84% were infected with HIV, the most common respiratory virus detected was cytomegalovirus, with a positive rate of 41% [ 56 ]. After multivariable adjustment, cytomegalovirus was associated with in-hospital mortality (OR 3.2; 95% CI, 2.1–10.0). A previous meta-analysis that included studies conducted among immunocompetent patients also suggested that cytomegalovirus was more likely to be detected among patients with severe sepsis or septic shock than mixed patients with or without severe sepsis (32% versus 15%) [ 57 ]. These results indicate that the specific pathogen spectrum among different populations may be attributable to, but not limited to, the clinical characteristics of study populations, such as immune status.
The aetiological and causal relevance of both influenza and non-influenza respiratory viruses with sepsis is still challenging and needs to be further identified among other prospective cohort studies. Studies conducted in both immunocompetent and immunocompromised hosts that can characterise host responses to viral infections are also needed to better determine causality.
- Pathophysiology of respiratory viral sepsis
Respiratory viral sepsis is a highly heterogeneous and multifaceted syndrome characterised by an overwhelming and systemic dysregulated host immune response to respiratory viral infection, with organ dysfunction including, but not limited to, the lung. Previous studies provided evidence for extrapulmonary organ dysfunction caused by respiratory viral infection: e.g. acute kidney injury and cardiac injury among cases with influenza infection; acute kidney injury and thrombocytopenia reported for MERS-CoV infection; high viral loads in the gut and liver and moderate viral loads in the kidney among fatal cases with SARS-CoV infection; and liver dysfunction reported for respiratory syncytial virus infection [ 58 – 62 ]. The recent findings from our study showed that around half of the COVID-19 fatalities developed acute kidney injury, heart failure or coagulopathy [ 48 ]. In another recent study that included 183 COVID-19 patients, disseminated intravascular coagulation was observed in 71.4% of fatal cases, and in 0.6% of non-fatal cases [ 63 ]. The multi-organ dysfunction determines that viral sepsis is a more complicated clinical status than severe viral pneumonia, with inflammation in the lung which is the primary and specific target organ of the respiratory virus. The type of infection and host response to the specific pathogen are determinants of sepsis and closely related to prognosis after the development of sepsis. The pathophysiology of sepsis includes that the immune response initiated by an invading pathogen fails to return to homeostasis, and thus culminating in a pathological syndrome that is characterised by sustained excessive inflammation and immune suppression [ 64 ].
The initial sensing of the host innate system after infection is to recognise pathogen-associated molecular patterns mediated by innate pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), retinoic acid-inducible gene-1 like receptors, NOD-like receptors, and C-type lectin receptors [ 65 , 66 ]. For most of the infections, the host innate immune system can eliminate the pathogen through pro-inflammatory responses, including the release of cytokines and chemokines (tumour necrosis factor (TNF), interleukin (IL)-1β, IL-12 and IL-18), the recruitment of phagocytes, and the local activation of the complement and coagulation systems [ 64 , 66 , 67 ]. Among patients with sepsis, pathogens cannot be eliminated by the host immune system and the homeostasis of the host immune system is disturbed, resulting in both an excessive inflammation and immune suppression.
The excessive inflammation of sepsis is mediated through the release of pro-inflammatory mediators by leukocytes and parenchymal cells, endothelium and platelets [ 68 – 71 ]. Leukocyte and parenchymal cell injury results in the release of damage-associated molecular patterns, further disrupting the host response by activating many of the PRRs [ 72 , 73 ]. These PRRs can also recognise pathogen-associated molecular patterns, leading to a vicious cycle that also involves organ damage and dysfunction. The coagulation system, complement system, neutrophils and vascular endothelium are also activated in this stage [ 74 – 77 ]. In the immune suppression stage, both adaptive and innate immune systems are involved. This stage is characterised by the apoptosis of T-cells, B-cells and dendritic cells, the exhaustion of T-cells, and the expansion of regulatory T-cell and myeloid-derived suppressor cell populations [ 78 – 80 ]. Patients with sepsis have increased numbers of myeloid-derived suppressor cells, which are immature myeloid cells that can impede immune responses, particularly T-cell function. Reprogramming of antigen-presenting cells leads to a reduced HLA-DR expression and a diminished capacity to produce pro-inflammatory cytokines [ 81 ]. The most important findings in sepsis are the delayed apoptosis of neutrophils and the appearance of immature band-like neutrophils in peripheral blood that have deficits in antimicrobial effector functions [ 64 ].
Host immune response to respiratory virus
The causes and characteristics of sepsis can be highly heterogeneous [ 64 ]. However, few studies provide evidence as to whether the pathophysiology of viral sepsis is different from that of bacterial sepsis, mainly because of the limited studies conducted that focus on viral sepsis. Current knowledge of the pathophysiology of respiratory viral sepsis is limited to the specific immune responses to viral infection.
For the influenza virus, haemagglutinins of different strains determine attachment to the epithelial of which specific part of the airway, and the viral polymerase complex is associated with different levels of viral replication and cytokine production in the infected epithelial cells [ 39 , 82 , 83 ]. Seasonal influenza, such as H3N2 and H1N1, targets preferential epithelium in the large airways (trachea, bronchi and bronchioles) by binding to α2,6-sialylated glycans, while H1N1pdm09 and H5N1 tend to infect both large airways and alveoli by binding to α2,3-sialylated glycans of pneumocytes [ 84 – 88 ]. Furthermore, mutation in the haemagglutinins of influenza leads to alteration of the cell tropism. The mutation of haemagglutinins of H5N1 results in the ability to bind not only α2,3- but also α2,6-sialylated glycans of pneumocytes [ 39 , 88 , 89 ]. Mutation in the viral RNA polymerase complex could result in better viral replication or increased secretion of pro-inflammatory cytokines [ 39 ].
Viral infections, such as the influenza virus, can also trigger initial sensing of the host innate system and recruitment of leukocytes through PRRs [ 90 ]. These PRRs include TLR-3, TLR-4, TLR-7, and RIG-I, the polymorphisms of which are associated with the susceptibility and severity of influenza virus infection in different individuals [ 91 – 96 ]. Different to bacteria, an influenza virus invades the alveolar epithelial cells first but not alveolar endothelial cells [ 82 , 97 ]. Pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IL-8 are produced by the infected epithelial cells and can damage the epithelial–endothelial barrier [ 82 ]. Endothelial cells can also be damaged through remodelling of the cellular cytoskeleton, loss of intercellular junctional integrity and cellular apoptosis. These processes lead to pulmonary oedema and respiratory insufficiency, which could further develop into severe pneumonia, acute respiratory distress syndrome and sepsis [ 98 ].
Data from the first 41 cases with confirmed SARS-CoV-2 infection showed that cytokines and chemokines, including IL-1β and TNFα, were higher in both intensive care unit (ICU) patients and non-ICU patients than healthy controls [ 20 ]. These indicators suggest that the host innate immune system initiated eliminating SARS-CoV-2 through a pro-inflammatory response, which is the same as the early stages of sepsis. Furthermore, a higher level of IL-10 was also observed among infected patients compared with healthy controls. The increased secretion of T-helper-2 cytokines that suppress inflammation suggested that immune suppression may present at an early stage after infection of SARS-CoV-2, which is different from sepsis as the immune-suppressive phase often presents after a pro-inflammatory and excessive inflammatory response. Recent pathological findings show that viral inclusions were found in some alveolar epithelial cells and macrophages of COVID-19 patients [ 99 ]. Inflammatory infiltrates dominated by monocytes and macrophages were seen in the lungs. The counts of CD4 and CD8 T-cells were substantially reduced in the spleen and lymphonodus. Furthermore, hepatic stestosis was observed in liver, and interstitial monocyte and lymphocyte inflammatory infiltrates were observed in the heart tissue. These findings need to be further investigated as more biopsy specimens become available.
Viral reactivation may also play a role in the prognosis of sepsis. Immune exhaustion during sepsis provides the probability for some latent infections to escape immunological control, and replicate under this more forgiving environment [ 28 ]. Some reactivated viral infections, such as the Epstein–Barr virus, were reported to be associated with clinical outcomes among patients with sepsis [ 28 , 100 ]. The potential mechanism is that reactivated viral microRNAs might be involved in sepsis by functional mimicry mechanisms with cellular microRNAs produced by the human genome, sharing the regulation of the same signalling pathways and regulating the same spectrum of microRNAs. For cytomegalovirus, the proportion of its reactivation in immunocompetent patients was around 30% and has become an area of increasing interest in recent years [ 101 ]. Previous studies provided the possibility for the causal relationship between cytomegalovirus reactivation and clinical outcomes among immunocompetent patients with sepsis [ 102 ]. However, definite evidence and a mechanism for this relationship are still not clear, e.g. no significant association between cytomegalovirus reactivation and host response biomarkers, including IL-6, IL-10, interferon-gamma-induced protein-10 or IL-1 receptor antagonist, was found among patients with sepsis [ 103 ]. Whether cytomegalovirus reactivation plays a role for disease progression or is only a marker of immune suppression in patients with sepsis needs to be further demonstrated.
Interaction of bacterial and viral infection for sepsis
As mentioned previously, immune suppression characterised by a decreased function in both innate and adaptive immunity following excessive inflammation in almost all forms of sepsis gives way to viral reactivation, which refers to the process of a latent virus switching to a lytic phase of replication. Immune suppression can also increase the probability for secondary bacterial infections, which is associated with increased mortality [ 38 , 64 ]. Previous studies showed the interaction between viral and bacterial infection. The interaction of influenza with Streptococcus pneumoniae and Staphylococcus aureus (including methicillin-resistant S. aureus ) was observed and can be reflected by an increased bacterial coinfection during seasonal epidemic and pandemic outbreaks of influenza. A previous study showed that bacterial coinfection was identified in approximately one-third of fatal H1N1pdm09 cases, with S. pneumoniae identified in 45.5% of cases and S. aureus in 31.8% of cases (including 71.4% of the S. aureus as methicillin-resistant S. aureus ) with bacterial coinfection [ 104 ]. Not only for influenza, respiratory syncytial virus was also reported to increases the virulence of S. pneumoniae [ 105 ]. Several mechanisms can provide clues to the predisposition of bacterial infection after respiratory viral infections. Respiratory viruses can damage the respiratory epithelium and the basement membrane of the epithelium is exposed, to which bacteria can adhere [ 106 ]. Platelet-activating factor receptor upregulated by released pro-inflammatory cytokines can provide a receptor for pneumococcal adherence and invasion [ 106 , 107 ]. In addition, antibacterial defence mechanisms can be impaired by influenza through increasing neutrophil apoptosis, and neutrophil and monocyte dysfunction [ 108 , 109 ]. This epidemiological evidence and the possible mechanisms provide indirect or direct evidence for bacterial reactivation at the stage of immune suppression among patients with viral sepsis.
- Diagnosis of respiratory viral sepsis
The diagnosis of respiratory viral sepsis depends on two steps: one step is the diagnosis of sepsis using the SOFA score, and the other important and challenging step is identifying the cause of the sepsis as a respiratory virus. The differentiation between bacterial and viral sepsis, especially at the initial stage after the development of sepsis, is important for the treatment of sepsis and prevention of mortality from sepsis. However, no golden standard was identified to broadly and efficiently determine and differentiate the presence and type of infection.
Pathogen detection is the most important step of differential diagnosis between respiratory viral and bacterial sepsis. Point-of-care testing and next-generation sequencing provide the possibility for a quick and accurate identification of the potential pathogen that is causing the sepsis. Next-generation sequencing is especially important for confirmation of infection by novel viruses. The role next-generation sequencing played in the laboratory confirmation of SARS-CoV-2 infection is important. Testing multiple pathogens in one test and saving time are the advantages of point-of-care testing, which are especially important for sepsis [ 110 ]. Furthermore, the use of point-of-care testing for sepsis was not limited to pathogen detection, but was also used for blood plasma protein quantification ( e.g. C-reactive protein and procalcitonin) and leukocyte monitoring (through antibody capture or intrinsic property characterisation) [ 111 ]. Clinical characteristics, blood biomarkers including C-reactive protein and procalcitonin, were not fully demonstrated to clearly discern viral and bacterial infection among patients with pneumonia, while the discrimination ability among patients with sepsis needs to be further demonstrated [ 112 ].
To distinguish infection compared to inflammation in the absence of infection and viral infection as compared to bacterial infection, several transcriptomics studies have been conducted to determine the presence of infection as compared to inflammation without infection [ 113 ], as well as to distinguish between the presence of bacterial and/or viral infection [ 114 – 117 ]. Some of these studies derived and validated models focusing on gene sets that can distinguish between viral and bacterial infection [ 117 , 118 ]. However, the many genes required for these models enlarged the difficulty to translate them into practical clinical tools. Sweeney and co-workers [ 113 , 114 ] derived and validated the “Sepsis MetaScore” based on 11 differentially expressed genes and the “Bacterial/viral MetaScore” based on seven differentially expressed genes which can profile the host gene response to build an integrated antibiotics decision model for sepsis. The sensitivities of this model for detecting bacterial infection and the specificity for viral infection were high (94.0% and 90.6%, respectively), but the specificity of this model for detecting bacterial infection and sensitivity for viral infection were not satisfying (59.8% and 53.0%, respectively). These results show the possibility to quickly discriminate between viral and bacterial sepsis and inform future research to identify biomarkers that can be translated to the clinical setting. Stratifying sepsis patients into more homogeneous subgroups should be the key points for future biomarker research, which can be realised with more consideration for pathophysiology of biomarkers.
- Treatment of respiratory viral sepsis
Timely intervention is the key to effective treatment among patients with sepsis. These include an initial fluid resuscitation and antibiotic therapy within the first hour [ 119 – 121 ]. In patients with haemodynamic instability after the initial fluid resuscitation, further haemodynamic stabilisation and assessment of fluid responsiveness should be continued [ 119 , 122 , 123 ]. During the disease progression of COVID-19, some patients with viral sepsis have clinical features including cold extremities, weak peripheral pulses and severe metabolic acidosis, while the blood pressure levels remain normal. These clinical features indicate the continuing internal environmental disorders and microcirculation dysfunction among these patients. Thus, haemodynamic stabilisation is necessary and important throughout the progress of treatment for patients with viral sepsis. The recommendation of antibiotic therapy is for all patients with sepsis. As mentioned above, a previous study showed that the proportion of sepsis cases with a negative culture was around 42% [ 15 ]. Future studies to evaluate effectiveness of antibiotic use and potential antibiotic resistance among these patients are needed, as inappropriate prescription can increase antibiotic resistance.
Pathogen-directed therapy should be the emphasis during treatment for patients with sepsis. For patients with suspected or confirmed respiratory viral sepsis, the early initiation of antiviral drugs with inhibiting viral replication and decreasing viral load is the most important step. Around 90 antiviral drugs have been formally approved for the treatment of human infectious diseases over the past 50 years, covering viruses that could cause viral sepsis, such as the influenza virus, human cytomegalovirus and respiratory syncytial virus [ 124 ]. However, studies with these antiviral drugs were rarely conducted to evaluate the effectiveness for respiratory viral sepsis, which should be the focus of future research. Current findings indicate the potential effect of baloxavir, oseltamivir, peramivir and zanamivir for influenza infections and cidofovir for adenovirus infections in immunocompromised patients [ 125 ]. Furthermore, the broad-spectrum antiviral drug ribavirin for the treatment of immunosuppressed patients with rhinovirus and respiratory syncytial virus infections, and arbidol for rhinovirus, respiratory syncytial virus, adenovirus and parainfluenza virus infections were also suggested. The broad-spectrum antiviral drugs, which refer to antivirals targeting viral entry and replication or modulating cellular defence systems, should be distinguished from broad-spectrum antibiotics which act against both gram-positive and gram-negative bacteria. To our knowledge, the potential effectiveness of ribavirin for rhinovirus infection and cidofovir for adenovirus infection were only indicated by several case reports, and need to be further demonstrated. Faced with the great challenge brought by SARS-CoV-2, our research group initiated two randomised controlled trials to evaluate the effectiveness and safety of remdesivir among COVID-19 patients, with one conducted among severe patients ( clinicaltrials.gov identifier NCT04257656 ) and the other one among mild and moderate patients ( clinicaltrials.gov identifier NCT04252664 ) [ 126 , 127 ]. Another trial evaluating the combined use of lopinavir/ritonavir in patients with SARS-CoV-2 infection has been completed (ChiCTR identifier ChiCTR2000029308). Looking back at the history of this infectious disease outbreak, antiviral treatment is the most important and powerful weapon to fight against the emerging and re-emerging viral pathogen, which should be a continuous focus of future research.
The potentially beneficial effects from the early initiation of antiviral treatment and optimal duration of antiviral drugs use among septic patients are not clear, with current study findings limited to severe or critical patients with virus infection. Findings from ICU patients with the H1N1pdm09 virus infection showed the initiation of antiviral treatment within 6 h of admission was associated with shorter lengths of hospital stay [ 128 ]. This indicates that antiviral treatment for critically ill patients with suspected pandemic influenza virus infection should be initiated as early as possible without waiting for the pathogen results. The duration of antiviral drug use is also not yet ascertained for the prolonged viral shedding among patients with critical illness [ 129 ]. Previous studies recommended the usage of antiviral drugs for at least 5 days and to repeat pathogen testing among patients at high risk of severe and life-threatening disease [ 130 ]. Our future understanding of viral sepsis and the effect of the early use of antiviral medicines will provide evidence to guide the use of antiviral drugs among patients with sepsis. As well as the potential role of antiviral drugs for viruses that causes sepsis, they were indicated in some studies to play a role in the treatment of viral reactivation, which can occur in the stage of host immune suppression. Future studies are still needed to evaluate the treatment effects of antiviral drugs for viral reactivation, with potential applications for all types of sepsis [ 131 ].
In recent years, an area of great interest to clinicians and the research of sepsis is immunomodulatory therapy for treating the host immune response. Excessive inflammatory responses can be inhibited by immunomodulatory therapy through altering or counteracting host inflammatory mediators, such as TNF and IL-1, or using broad-spectrum anti-inflammatory molecules with non-selective suppression of inflammation [ 132 , 133 ]. However, previous clinical trials to assess the effect of the inhibition of the excessive inflammatory response of septic patients did not show an improvement in the outcome [ 134 , 135 ]. Several clinical trials to evaluate the effect of anti-inflammatory agents, such as humanised C5a-specific monoclonal antibody and soluble recombinant human thrombomodulin, are still ongoing and cannot provide available data now [ 136 , 137 ]. Due to the failure to generate new treatments, immunomodulatory therapy with inhibition of excessive inflammatory response has become less popular, whereas immune stimulants have been advocated to be given to patients with sepsis for the potential effect to reverse immune suppression among sepsis cases [ 138 ]. The immune stimulatory system is intended to restore immune functions and promote the rapid clearance of pathogens, and thus reduce the incidence of secondary infections and late sepsis mortality. The use of some immune-stimulating cytokines, such as type II interferon-γ, IL-7, IL-15 and granulocyte-macrophage colony-stimulating factor, has been demonstrated to reverse immunosuppression in animal models or clinical trials [ 139 , 140 ]. As the immune stimulatory aims to restore immune function and reduce mortality related to secondary infections of sepsis, the application of this treatment should be specific to patients who may benefit from this therapy. The biomarkers, such as the reduced expression of monocyte HLA-DR and increased expression of monocyte programmed death ligand-1, may be considered for selecting patients who can benefit from immune stimulation [ 79 , 141 ]. Current knowledge of the potential benefit of immune stimulants therapy for sepsis provides clues for the generation of new treatments in the future. However, due to the limited studies of sepsis treatment and the limited attention from researchers and physicians about viral sepsis, there is a long way to go to evaluate the potential use of these relatively novel therapies from bench to bedside. The subtypes of sepsis also need to be fully considered in the studies conducted in the future.
For other therapies, which include glycaemic control and nutritional support for the treatment of sepsis, controversies also exist. The current consensus for the control of glycaemia is to maintain the glycaemic level at <180 mg·dL −1 [ 23 ], but to avoid tight glycaemic control because of the potential harm from hypoglycaemic episodes [ 142 ]. Although optimal nutritional support is important in critically ill patients, the timing, dose, duration and route of nutritional support are not clear. Previous studies did not find a superiority of the enteral compared with the parenteral route for association with mortality, but found a greater risk of digestive complications among those who received enteral nutrition [ 143 , 144 ]. Animal studies showed that anorexia was protective but nutritional supplementation was detrimental in bacterial sepsis, with glucose necessary and sufficient for these effects [ 145 ]. In contrast, nutritional supplementation protected against mortality from influenza infection and viral sepsis, while blocking glucose utilisation was lethal. Whether these results can be translated to a clinical setting needs to be further validated among patients with viral or bacterial sepsis.
- Conclusions
Sepsis is a heterogeneous syndrome identified as a life-threatening organ dysfunction that results from dysregulated host responses to infection. As pathogens of sepsis, viruses have not been received enough attention by physicians and researchers, which should be altered given the huge burden. Although our current understanding of the pathophysiology of sepsis has improved, the development of new treatments for sepsis seems not to be consistent with the understanding of the pathophysiology. Future studies should not only focus on understanding the host immune response in the development of sepsis, especially viral sepsis, but also explore how to stratify patients into more homogeneous subgroups on the basis of their pathophysiology. The identification of biomarkers that can differentiate who might benefit from a specific intervention can help the application of new treatments into clinics with monitoring the effects of future therapies. The development of antiviral drugs, immunomodulatory and immune stimulants therapy, and vaccine development, given the important role influenza vaccines play, should be the most important perspectives of future research to fight against the epidemic, emerging and re-emerging viral pathogens that can cause respiratory viral sepsis. Furthermore, with the growing knowledge of sepsis and more patients surviving sepsis in the future, the long-term sequelae of sepsis will be another problem for physicians and researchers to consider.
Provenance: Submitted article peer-reviewed.
Conflict of interest: X. Gu has nothing to disclose.
Conflict of interest: F. Zhou has nothing to disclose.
Conflict of interest: Y. Wang has nothing to disclose.
Conflict of interest: G. Fan has nothing to disclose.
Conflict of interest: B. Cao has nothing to disclose.
Support statement: This work is funded by the Special Project for Emergency of the Ministry of Science and Technology (2020YFC0841300), Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS 2018-I2M-1-003), National Science Grant for Distinguished Young Scholars (81425001/H0104), the National Key Research and Development Program of China (2018YFC1200102), and The Beijing Science and Technology Project (Z19110700660000). Funding information for this article has been deposited with the Crossref Funder Registry .
- Received February 6, 2020.
- Accepted April 4, 2020.
- Copyright ©ERS 2020.
This article is open access and distributed under the terms of the Creative Commons Attribution Non-Commercial Licence 4.0.
- Marshall JC , et al.
- Cerra FB , et al.
- Cecconi M ,
- Levy M , et al.
- Deutschman CS ,
- Seymour CW , et al.
- Vincent JL ,
- Takala J , et al.
- Fleischmann C ,
- Scherag A ,
- Adhikari NK , et al.
- Johnson SC ,
- Agesa KM , et al.
- Gaieski DF ,
- Edwards JM ,
- Kallan MJ , et al.
- Martin GS ,
- Mannino DM ,
- Eaton S , et al.
- Kaukonen KM ,
- Suzuki S , et al.
- Hodgin KE ,
- Reinhart K ,
- Daniels R ,
- Kissoon N , et al.
- Timsit JF ,
- Garrouste-Orgeas M , et al.
- Sprung CL , et al.
- See K , et al.
- Ljungstrom LR ,
- Jacobsson G ,
- Claesson BEB , et al.
- Southeast Asia Infectious Disease Clinical Research Network
- Liu Y , et al.
- Cilloniz C ,
- Polverino E , et al.
- Li X , et al.
- Drosten C ,
- Seilmaier M ,
- Corman VM , et al.
- Martin-Loeches I ,
- Phillips G , et al.
- Roberts D ,
- Wood KE , et al.
- Marshall J , et al.
- McGinley JP ,
- Drysdale SB , et al.
- Walden AP ,
- Clarke GM ,
- McKechnie S , et al.
- Dominedo C ,
- Magdaleno D , et al.
- Ruuskanen O ,
- Jennings LC , et al.
- Dawood FS ,
- Finelli L , et al.
- Fan J , et al.
- ↵ Vaccines against influenza WHO position paper – November 2012 . Wkly Epidemiol Rec 2012 ; 87 : 461 – 476 . OpenUrl PubMed
- Tokars JI ,
- Iuliano AD ,
- Roguski KM ,
- Chang HH , et al.
- Neuzil KM ,
- Shay DK , et al.
- Florescu DF ,
- Fukuyama S ,
- Zhao W , et al.
- Chaves SS ,
- Perez A , et al.
- Benoit SR ,
- Skarbinski J , et al.
- An X , et al.
- World Health Organization
- China National Health Commission
- Du R , et al.
- Modhiran N ,
- Watterson D ,
- Muller DA , et al.
- Zahariadis G ,
- Jerome KR ,
- Martinez E ,
- de Diego A ,
- Paradis A , et al.
- Lastere S ,
- Jean-Baptiste S
- Schepers K ,
- Cassadou S , et al.
- Kadambari S ,
- Harvala H ,
- Simmonds P , et al.
- Jennings L ,
- Banura P , et al.
- Florescu DF
- Balkhy HH , et al.
- Farcas GA ,
- Poutanen SM ,
- Mazzulli T , et al.
- Lorente JA ,
- Soto L , et al.
- Gu X , et al.
- Wang X , et al.
- van der Poll T ,
- van de Veerdonk FL ,
- Scicluna BP , et al.
- Russell JA ,
- Takeuchi O ,
- Iwasaki A ,
- Medzhitov R
- van der Poll T
- Tressel SL ,
- Kaneider NC ,
- Kasuda S , et al.
- Claushuis TA ,
- van Vught LA ,
- de Stoppelaar SF ,
- van ’t Veer C ,
- Oppenheim JJ , et al.
- Halbwachs-Mecarelli L , et al.
- Silasi-Mansat R ,
- Popescu NI , et al.
- Abraham E ,
- Opal S , et al.
- Boomer JS ,
- Chang KC , et al.
- Hotchkiss RS ,
- Monneret G ,
- Tinsley KW ,
- Swanson PE , et al.
- El Gazzar M , et al.
- Fouchier RAM , et al.
- Yamada S , et al.
- Perez-Padilla R ,
- de la Rosa-Zamboni D ,
- Ponce de Leon S , et al.
- Guarner J ,
- Falcon-Escobedo R
- Uiprasertkul M ,
- Kitphati R ,
- Puthavathana P , et al.
- Childs RA ,
- Matrosovich T , et al.
- Suzuki T , et al.
- Teijaro JR ,
- Cahalan S , et al.
- Esposito S ,
- Molteni CG ,
- Giliani S , et al.
- Guillot L ,
- Le Goffic R ,
- Bloch S , et al.
- Neely GG , et al.
- Diebold SS ,
- Hemmi H , et al.
- Pichlmair A ,
- Tan CP , et al.
- Matthay MA ,
- Steinberg BE ,
- Goldenberg NM ,
- Fuentes-Mattei E ,
- Bullock MD , et al.
- Marandu T ,
- Heininger A ,
- Haeberle H ,
- Fischer I , et al.
- van de Groep K ,
- Nierkens S ,
- Cremer OL , et al.
- Centers for Disease Control and Prevention
- Sandrini S ,
- Datta S , et al.
- McCullers JA ,
- Hament JM ,
- Kimpen JL ,
- Fleer A , et al.
- Colamussi ML ,
- Crouch E , et al.
- Nickerson CL ,
- Burnham-Marusich AR
- Oeschger T ,
- McCloskey D ,
- Kopparthy V , et al.
- Sweeney TE ,
- Shidham A ,
- Wong HR , et al.
- Chen M , et al.
- Suarez NM ,
- Falsey AR , et al.
- Tsalik EL ,
- Nichols M , et al.
- Andres-Terre M ,
- McGuire HM ,
- Pouliot Y , et al.
- Havstad S , et al.
- Seymour CW ,
- Prescott HC , et al.
- Cavallaro F ,
- Sandroni C ,
- Antonelli M
- De Clercq E ,
- Walter JM ,
- Wunderink RG
- ClinicalTrials.gov
- Alvarez-Lerma F ,
- Marin-Corral J ,
- Vila C , et al.
- Li IW , et al.
- de Jong MD ,
- Gilligan KJ , et al.
- Razonable RR
- Bellissant E ,
- Bollaert PE , et al.
- Marshall JC
- Venkatesh B ,
- Cohen J , et al.
- -ClinicalTrials.gov
- Leentjens J ,
- van der Hoeven JG , et al.
- Koch RM , et al.
- Kasten KR ,
- Prakash PS ,
- Unsinger J , et al.
- Colston E ,
- Yende S , et al.
- Chittock DR ,
- Su SY , et al.
- Reignier J ,
- Boisrame-Helms J ,
- Brisard L , et al.
- Harvey SE ,
- Parrott F ,
- Harrison DA , et al.
- Luan HH , et al.

- Table of Contents
- Index by author
Thank you for your interest in spreading the word on European Respiratory Society .
NOTE: We only request your email address so that the person you are recommending the page to knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Respiratory infections and tuberculosis
- Tweet Widget
- Facebook Like
- Google Plus One
More in this TOC Section
- Reversion of Mycobacterium tuberculosis immunoreactivity
- Diaphragmatic palsy following lung transplantation
- CXCR1 and CXCR2 and their role in acute respiratory distress syndrome
Related Articles

CritCases 2 – Is this Septic Shock with Pneumonia?
Welcome to EM Cases’ CritCases blog, a collaboration between Mike Betzner , the STARS air ambulance service and EM Cases’ Michael Misch and Anton Helman! These are educational cases with multiple decision points where there is no strong evidence to guide us. Various strategies and opinions from providers around the world are coalesced and presented to you in an engaging format. Enjoy!
Written by Michael Misch, edited by Anton Helman, March 2016
A 44 year-old male presents to Janus General, a small town rural emergency department with the chief complain of feeling unwell for 3 weeks. He endorses general malaise, cough, shortness of breath and chest pressure. In addition, he complains of chills, myalgias and loose stools during this time. He denies any travel or sick contacts.
Past medical history is remarkable for hypertension for which he is no longer taking medication. He has a 20 pack-year history of smoking cigarettes and regularly uses crack cocaine and methamphetamine. He also admits to heavy alcohol use. He denies IV drug use or history of HIV or Hepatitis.
On exam, he appears quite anxious and has increased work of breathing. Vitals are: BP 100/43, HR 140-170, RR 36-40, , temperature 36.5, oxygen saturation 95% room air. Heart sounds are normal. JVP is flat. There is reduced air entry bilaterally but the lungs are clear. Abdomen is soft, ENT exam unremarkable. No signs of IV drug use.
Initial Investigations are obtained:
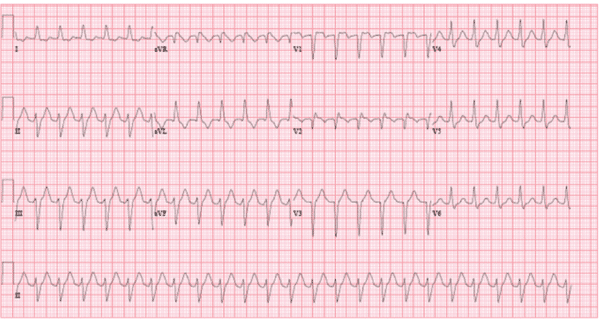
Initial blood work including venous blood gas
| Na | 125 | pH | 7.16 |
| K | 4.3 | pCO2 | 42 |
| Cl | 90 | HCO3 | 15 |
| HCO3 | 15 | pO2 | 20 |
| Glc | 10.6 | Lactate | 8.8 |
| Base Excess | – 13 | ||
Initial CXR

POCUS shows a hyperdynamic heart with a normal right ventricle and no pericardial effusion. IVC suggests hypovolemia. There are no B-lines on lung POCUS.
What is your differential diagnosis?
There is a huge differential in this patient. One useful framework is based on the type of shock:
- Septic Shock : Even though this patient is afebrile, sepsis should certainly be a consideration. With the respiratory symptoms, pneumonia would be a likely source. CXR suggests possible bilateral infiltrates.
- Obstructive Shock : Massive pulmonary embolism should be considered, however one would expect the patient to be more hypoxic if the PE is large enough to account for this degree of tachycardia.
- Cardiogenic Shock : The patient has cardiac risk factors for ACS. Clinically the patient did not appear volume overloaded as his JVP was flat. His CXR also does not suggest pulmonary edema. Compensated cardiogenic shock + wide complex tachycardia is a possibility.
Specific Differential: 1) Myocarditis – Consistent with the insidious onset, the low grade chills and overall SOB and chest pain findings; would account for the tachycardia (although I would expect him to be hypotensive to get a lactate of 9). 2) Septic Shock with pneumonia – Common things are common. Dehydrated and little to see initially on the CXR but may reveal itself with fluid resuscitation. Would account for the lactate and the hyponatremia due to SIADH. 3) Pancreatitis – Chronic pancreatitis could certainly give him weight loss and malabsorption diarrheal stools. Pain and local complications could account for many of the findings, however soft abdominal exam makes this unlikely. 4) PE – unlikely with that oxygen saturation and that severe a tachycardia 5) ACS – I think unlikely given the duration of the symptoms >1 week 6) Pulmonary hypertension from crack use – should have an elevated JVP and RV enlargement on POCUS 7) Boerhaaves – No history of vomiting just nausea 8) Cocaine cardiomyopathy 9) Myasthenia gravis new onset with muscle weakness (respiratory included) triggered by drugs or infection -Dr. Marc N. Francis MD, FRCPC
My question is whether this guy has somehow gotten into some metformin and this is a metformin lactic acidosis? -Rob Abernethy MD FRCPC
Toxic alcohol ingestion and thyroid storm are other considerations in the differential.
What are your immediate interventions?
1) IV bolus 250cc NS with low threshold to give more if tolerating same and assess for any change to the rate – would be careful NOT to hammer fluids into this guy. 2) I personally would not cardiovert this rhythm at this time. I am unconvinced that it is VT and the patient has reasonable vital signs in spite of the tachycardia. If he is a myocarditis there is the possibility of this degrading further with cardioversion. I am not saying I would not consider cardioversion at some point, but I would likely not go there right out of the gate. 3) If my fluids did nothing to the rate and I was certain it was not sinus tachycardia than I would try gentle IV B-blockers (low dose 1-2mg metoprolol or IV esmolol). 4) Broad spectrum antibiotics – Ceftriaxone and Vancomycin 5) Echogardiogram -Marc N. Francis MD, FRCPC Consider steroids (query Addisonian crisis given hypovolemia-like state and hypoNa) if not responding well to initial measures. – Chris Hall MD FRCPc
What is your interpretation of the above EKG? What steps will you take to further elicit the rhythm?
This patient is in a monomorphic wide complex tachycardia with a rate of 145. The QRS is 126 ms. There are no obvious p waves or flutter waves on the ECG.
Differential: Sinus tachycardia, AVNRT, atrial flutter with aberrancy or ventricular tachycardia. Variation in the rate would make SVT less likely.
I’m gonna have to go with atrial flutter as the most likely rhythm. I think I see flutter waves in lead l. I wouldn’t bet the farm on it, however, and as this patient is quite sick, cardioversion would not be a bad way to go (even though it would be James (Jamie) Fox, MD, FRCPC
The initial rhythm on the monitor does not look that wide to me. Even on the later EKG it is not “super-wide”. It certainly could be a sinus tachycardia with aberrancy at the rates seen. Could also be SVT and I would entertain atrial flutter with a 2:1 block + aberrancy. -Rob Abernethy MD FRCPC
Lewis Leads: Differentiating Atrial from Ventricular Tachydysrhythmias
While not done in this case, the use of Lewis Leads may help to bring out atrial activity and help to determine the underlying rhythm. To use this technique:
- Place the right arm lead to the right of the sternum at the 2 nd intercostal space.
- Place the left arm lead to the right of the sternum at the 4 th intercostal space
- Inspect the ECG for atrial activity in lead l
Consider the use of Lewis Leads when you have a monomorphic wide complex tachycardia and atrial activity is not clearly demonstrated on a standard 12-lead ECG.
For a more in-depth explanation of the use of Lewis leads to differentiate SVT with aberancy from VT see this great FOAMed Paramedic blog : My Variables Only Have 6 Letters
What is your interpretation of the venous blood gas?
The VBG is interesting – aside from the obvious lactate and the acidosis it appears that he is not appropriately compensating from a respiratory point of view to his metabolic acidosis. The rate of 36-40 seems adequate but the PCO2 is normal? Superimposed respiratory acidosis? Opiates on board? -Rob Abernethy MD FRCPC
Use adenosine for regular monomorphic rhythms only
The use of adenosine may be considered in regular monomorphic rhythms in an attempt to slow AV conduction and bring out the atrial activity. The use of adenosine should only be considered in regular monomorphic rhythms. Patients presenting in atrial fibrillation with an accessory pathway will present in an irregularly irregular wide complex rhythm. The refractory period of the AV node prevents ventricular rates in excess of 150-170 beats per minute. The use of adenosine in this scenario will block conduction down the AV node and encourage conduction down the accessory pathway, which can precipitate conduction at excessive rates into the ventricle and potentially cause ventricular fibrillation. While adenosine has been traditionally taught to only convert atrial rhythms such as SVT, there is a well-described subset of electrophysiologically-proven ventricular tachycardia that will cardiovert with adenosine; so called “adenosine-responsive ventricular tachycardia”. Thus, conversion to normal sinus rhythm using adenosine should not be used as a diagnostic tool to differentiate SVT with aberancy from Vtach.
Case Continued…
Clinically, this man’s respiratory distress does not improve following initial interventions. His heart rate is climbing to persistently above 150. Air transport is requested.
Vitals post 4.5 L crystalloid T 36.9, HR 160, BP 104/55, RR 40 SaO2 95% 4L NP
Further investigations at this point are resulted:
| Na | 122 | Hb | 141 | Billi | 16 | Acetaminophen | < 66 |
| K | 4.4 | Lk | 31.1 | ALP | 102 | Ethanol | 12 |
| Cl | 86 | Plt | 133 | LDH | 546 | Salicylates | < 0.07 |
| Cr | 148 | GGT | 54 | ||||
| Glc | 10.3 | Lipase | 17 | ||||
| Trop | |||||||
| Urea | 12.5 | ||||||
Repeat VBG 90 Mins later:
| Na | 125 | pH | 7.25 |
| K | 4.4 | pCO2 | 28 |
| Cl | 90 | HCO3 | 12 |
| HCO3 | 15 | pO2 | 29 |
| Glc | 10.1 | Lactate | 7.1 |
| Base Excess | – 14 |

Based on the investigations above, what is your differential diagnosis now? What are your next steps for this patient?
The very high white count might further support infectious cause. The markedly elevated troponin should prompt consideration for myocarditis, especially in context of significant tachycardia. However, POCUS showed grossly normal systolic function. PE should still be considered but one would expect hypoxia with the degree of tachycardia and hypotension caused by the clinical presentation. The repeat CXR more strongly suggests bilateral infiltrates, which in a drug-abuser should prompt consideration of Pneumocystis Jerovicii pneumonia (especially with the elevated LDH). Finally, these infiltrates could represent septic emboli from an endocardititis. As per Dr Carr’s Best Case ever on endocarditis , if endocarditis is considered, vancomycin and ceftriaxone should be given following at least 3 sets of blood cultures.
The patient’s hemodynamics fail to improve following a significant fluid challenge. The rhythm is not clear at this time. While en route to the tertiary care centre, the heart rate rises to 160-170 with a BP of 90/40.
Would you electrically cardiovert this patient with the information you have at this point?
Given the significant tachycardia and hemodynamic instability without a clear underlying cause, synchronized cardioversion is attempted . The patient is given boluses of 10-20 mg of propofol in rapid succession until sedation is achieved. Synchronized cardioversion with 100 J, 150J, and then 200 J is attempted but is not successful, making the diagnosis of sinus tachycardia more likely. He is subsequently started on a norepinephrine infusion. Upon arrival at the tertiary centre, cardiology and ICU are consulted. Cardiology is similarly unable to definitely determine the presenting rhythm although given failed cardioversion, it is presumed to be sinus tachycardia.
What is the underlying diagnosis in this patient in septic shock?
This 44 year-old man is admitted to the ICU with piperacillin-tazobactam added to the antimicrobial regimen. CT chest confirms the presence of bilateral lung infiltrates. Streptococcus Pneumoniae grows in his blood after 6 hours. An echocardiogram reveals vegetations on a bicuspid aortic valve. Additionally, there is severe aortic regurgitation as well as an aortoatrial fistula tracking from an area beyond the aortic valve back to the left atrium. The final diagnosis is endocarditis with probable pneumonia complicated by an aortoatrial fistula.
How does endocarditis with acute aortic regurgitation account for this presentation?
This man presented in mixed septic and cardiogenic shock from endocarditis complicated by acute aortic regurgitation (AR).
In patients with native aortic valves, acute AR is due to endocarditis or aortic dissection. With chronic AR, the left ventricle can dilate to accommodate for regurgitant flow, which maintains end diastolic pressure and cardiac output. In acute AR, however, the left ventricle is not able to dilate to accommodate for increased end diastolic pressure, which decreases stroke volume and cardiac output. Coronary ischemia results from decreased coronary blood flow during diastole, and is exacerbated by increased myocardial oxygen demand. The increased myocardial oxygen demand results from tachycardia and increased end diastolic pressures against which the left ventricle must contract. This would account for the markedly elevated troponin in this case.
His severe ongoing tachycardia was a result of very poor forward flow. His heart was beating the heck out of itself trying to get any forward flow at all. -M.J. Betzner MD FRCPc
Due to the pathophysiologic difference of acute and chronic AR, patients with acute AR may not present with classic signs of a holosystolic murmur and a decreased pulse pressure (which are typical of chronic AR). Instead, there is often only a soft decrescendo murmur, which may be easily missed when the patient is tachycardic and tachypneic. Pulse pressure is normal or may be decreased. Patients will present with dyspnea, hemodynamic instability and shock.
The presentation in this particular case is complicated by septic shock. S. Pneumoniae is an uncommon cause of endocarditis , accounting for 1-3% of cases. However it is particularly aggressive with a mortality rate approaching 25%. It is thought to occur most commonly in middle aged-men with a significant alcohol intake (as in this case). Two thirds of cases occur in patients without previous valvular pathology. S. Pneumoniae causes severe valve damage with one study finding a 20% rate of valve perforation and 13% rate of peri-valve abscess. In this same cohort, two thirds of patients required valve replacement. Rarely, S. Pneumoniae can cause the triad of endocarditis, meningitis and pneumonia known as Austrian (or Osler’s) Triad .
What is the definitive management for this patient with endocarditis and acute aortic regurgitation?
The definitive management of acute aortic regurgitation is surgical valve replacement. Medical management to bridge the gap to urgent surgery is two-fold:
1. Decrease afterload with a nitroprusside infusion
2. Increase contractility and cardiac output with dobutamine
While an intra-aortic balloon pump is sometimes used in cardiogenic shock, its use is contraindicated in AR because balloon inflation during diastole worsens regurgitant flow.
Case Resolution…
This man has acute endocarditis, of a bicuspid aortic valve, secondary to S. Pneumoniae as well as a probable pneumonia. Consistent with S. Pneumoniae endocarditis, there is significant valvular damage requiring emergent valve replacement.
Take Home Points
- Consider the use of Lewis Leads to accentuate atrial activity on ECG when faced with a tachydysrythmia of unknown origin.
- The use of adenosine should only be considered in regular monomorphic rhythms. A trial of adenosine with a regular monomorphic wide complex tachycardia may help to elicit p waves to different VT from SVT with abberancy. However, cardioversion with adenosine should not be used to diagnose SVT with aberrancy because a subset of electrophysiologically-proven VT will cardiovert with adenosine.
- Acute AR may present as cardiogenic shock. The classic murmur and pulse pressure of chronic AR may be absent. These patients require urgent valve replacement or repair.
- S. Pneumoniae is a relatively rare but devastating cause of endocarditis, which requires aggressive pneumococcal antibiotic coverage and often, urgent valve replacement.
For David Carr’s Top Ten Pearls and Pitfalls on Endocarditis see his Best Case Ever
Dr. Helman, Dr. Misch and Dr. Betzner have no conflicts of interest to declare
Bakker AL, Nijkerk G, Groenemeijer BE, et al. The Lewis lead: making recognition of P waves easy during wide QRS complex tachycardia. Circulation. 2009;119(24):e592-3. Full Text
Kanakadandi V, Annapureddy N, Agarwal SK, et al. The Austrian syndrome: a case report and review of the literature. Infection. 2013;41(3):695-700.
Lefort A, Mainardi JL, Selton-suty C, Casassus P, Guillevin L, Lortholary O. Streptococcus pneumoniae endocarditis in adults. A multicenter study in France in the era of penicillin resistance (1991-1998). The Pneumococcal Endocarditis Study Group. Medicine (Baltimore). 2000;79(5):327-37.
Wilber DJ, Baerman J, Olshansky B, Kall J, Kopp D. Adenosine-sensitive ventricular tachycardia. Clinical characteristics and response to catheter ablation. Circulation. 1993;87(1):126-34. Abstract
Additional FOAMed Resources
Salim Rezaie reviews differentiating SVT with aberrancy vs VT on R.E.B.E.L. EM
David Carr reviews management of endocarditis on EMCrit
About the Author: Anton Helman
Recent Posts

EM Quick Hits 58 – HIV PEP and PrEP, PREOXI Trial, Blast Crisis, Nitrous Oxide Poisoning, Vasopressors in Trauma

Compassionate Care to Improve Patient Outcomes and Your Career from EMU 2024

EM Cases Summit ’24 Registration Now Open!
Easy to say in retrospect however AR significant enough to contribute to his clinical presentation should be obvious with color flow doppler at his initial echo.
To my knowledge, aortic reurgitation is associated with widened pulse pressure, not decreased. please correct me if I am wrong.

Leave A Comment Cancel reply
Case Write-Up: 8-Year-Old Male with Sepsis and Pneumonia Resuscitated with LifeFlow
- Patient Stories
An 8-year old male with no significant past medical history presented to our Emergency Department (ED) with cough, fever, and shortness of breath. Symptoms began earlier that day and the parents elected to send him to the hospital due to breathing difficulty.
Upon arrival to the ED, the patient was pallor, dehydrated, hypotensive and tachypneic, qualifying him for sepsis protocol. During the initial assessment he was also noted to have labored substernal, intercostal, and accessory retractions with a pulse ox of 86%. Lung sounds were coarse and crackly and he was unable to speak more than one word at a time. Capillary refill on exam was delayed ~4 seconds.
In accordance with the hospital’s pediatric sepsis guidelines, an IV was placed and a rapid fluid bolus administered. In this case, the LifeFlow ® infuser was used due to its speed and control. In order to titrate the fluid resuscitation to the patient, careful patient assessments were performed in between each 20mL/kg bolus to evaluate patient response. After each bolus, the patient’s blood pressure increased. Following completion of the third LifeFlow fluid bolus the patient’s pallor reversed, heart rate decreased, retractions decreased, capillary refill time decreased to 3 seconds, and blood pressure increased to 110/60. Patient also became more alert, interactive and able to speak in full sentences. The parents commented on the quick transformation following the fluid boluses.
Once the patient was stabilized, he was admitted to the PICU for treatment of pneumonia. The ability to provide multiple rapid, controlled fluid boluses allowed our team to quickly stabilize this young patient who may have otherwise rapidly deteriorated and spent a longer time in the ED, potentially consuming more resources and time.
Pediatric Fluid Resuscitation: Nurse Perceptions and Practices
Pediatric septic shock – the impact of ultrasound-guided fluid resuscitation.
- OB Hemorrhage
- Hypotension
- Infection Control
- Pre-hospital
- Rapid Fluid Bolus
Subscribe to Blog & Updates
Please leave this field empty.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Thanks for subscribing!
Have questions about LifeFlow?
Want to get in touch?
Schedule a LifeFlow demo now with one of our Clinical Specialists
- ALiEM Library
- ALiEMU: Be Free To Learn
- Bridge to EM: Senior Medical Student Curriculum (2nd Edition, Updated 2024)
- Chief Resident Incubator: Legacy Edition
- EM Bound: Medical student newsletter
- EM Match Advice Series
- Faculty Incubator
- GroundED in EM: A Third-Year Student Curriculum
- How I Educate Series
- IDEA Series
- The Leader’s Library
- MEdIC series
- How I Podcast Smarter Series
- How I Stay Healthy Series
- How I Work Smarter Series
- Social Media & Tech
- 60-Second Soapbox
- EM Pharm Pearls
- Lab It Up Library
- SAEM Clinical Images
- SplintER Series
- Tricks of the Trade
- Ultrasound for the Win
- Meet the Team
- Culture Book
- Disclaimer / Privacy / Copyright
EM ReSCu Peds 13: Pneumonia and Septic Shock
Presentation, brief narrative description of case.
This scenario occurs in a community Emergency Department (ED) that is not a pediatric referral center. This patient with a complex medical history including tracheostomy and cerebral palsy has pneumonia and is brought to the ED due to respiratory distress and hypoxia. The patient acutely worsens due to a trach plug. The trach needs to be replaced in order for the clinical condition to improve. The child’s parents should also be kept updated on the patient’s care. This episode is very upsetting to the child’s parents as they were getting ready to have the trach removed.
Download the Case Summary
- What are case summaries? [PDF]
- Quick reference guide for the case [PDF]
Primary Learning Objectives
At the end of this simulation, participants should be able to:
- Describe signs/symptoms of septic shock in a pediatric patient (knowledge)
Demonstrate early evaluation of a critically ill, medically complex pediatric patient (application)
Identify the signs/symptoms of impending respiratory failure in a medically complex pediatric patient (application), construct and implement initial medical management of septic shock in a medically complex pediatric patient (application).
- Demonstrate airway management of a sick child using appropriate adjuncts and bag mask ventilation (BMV) (application)
- Develop a plan to troubleshoot a tracheostomy device (evaluation)
Demonstrate tracheostomy tube replacement (application)
Demonstrate focused history taking from a caregiver (application), explain diagnosis and management to caregivers (synthesis), demonstrate teamwork and closed loop communication (application), critical actions.
- Assign/assume team roles
- Obtain history from parent
- Perform primary assessment
- Place patient on continuous cardiac monitor
- Establish vascular access
- Perform focused physical exam
- Recognize severe respiratory distress in a child with a complex medical history
- Prevent hypoxia with supplemental oxygen
- Treat respiratory failure with BMV
- Troubleshoot tracheostomy
- Keep family updated on patient’s care
Case Creators
- Pavan Zaveri, MD, MEd, CHSE
- Michael Hrdy, MD
- Muhammed Waseem, MD, MS, FACEP, FAAP, CHSE-A
- Rebekah Burns, MD
- Sylvia Garcia, MD
- Justin Koch, DO, FACEP
Updated May 31, 2023
Chief complaint: Respiratory distress Patient age: 10 years old Weight: 20 kg
Recommended Supplies
- Manikin: Child-sized (5-8 year old) simulation manikin
- Moulage: None
- Resources: PALS cards and/or color-coded length-based resuscitation tape
- Manikin set up: IV lines x1 in place with drainage bag, neck piece with tracheostomy tube in place
- Simple face-mask
- Non-rebreather oxygen mask
- Nasal cannula
- Oxygen tubing
- Suction equipment
- Bag mask ventilation
- Intubation equipment (cuffed ETT, Miller 1 blade, stylet, ET CO2 monitoring)
- Block the end of the trach to mimic a large plug. Super glue works well for this.
- IV fluid bag, lines, pumps, poles, and angiocatheters
- Code medications: Epinephrine, calcium gluconate, norepinephrine, D25W
- Intubation medications: Succinylcholine, rocuronium, etomidate, ketamine, midazolam
- Antibiotics: Ampicillin, ceftriaxone, azithromycin, vancomycin
Supporting Files
- Chest x-ray (AP and lateral): Multifocal pneumonia
- Point of care lab tests
- RUSH images: Collapsible and non-collapsible IVC
Participants/Roles
- Team leader
- Airway manager
- Survey physician
- Medication giver
- Family liaison
- Standardized patient (actor) to play patient’s parent
Faculty or nurse can play a nurse or tech, if there are not enough learners to perform the above roles.
Prerequisite Knowledge
- PALS protocols
- General knowledge of emergency medicine
- Simulation implementation and debriefing experience
- Any stage of training (preferably PGY-2 or greater for team lead)
- Completed PALS certification
- Rapid sequence induction (RSI)
Case Alternatives
- A pneumothorax may develop if there is aggressive bagging during resuscitation of the child.
- For more advanced learners, the hypotension and peripheral perfusion might not resolve until one or more vasoactive medications are administered (e.g., epinephrine, dopamine).
- The child can develop anaphylaxis to an antibiotic requiring appropriate therapy with IM epinephrine and a second-line antibiotic medication.
Virtual Resus Room
This simulation case can be run virtually using Google Slides and Zoom from the Virtual Resus Room (Peds Sepsis & Trach Change) page.
PC1. Emergency Stabilization PC2. Performance of Focused History & Physical Exam PC3. Diagnostic Studies PC7. Disposition PC10. Airway Management ICS1. Patient Centered Communication ICS2. Team Management
- Ambroggio L, Mangeot C, Murtagh Kuroski E, et al. AAP Guideline Adoption for Community-Acquired Pneumonia in the Outpatient Setting. Pediatrics. 2018;142:e20180331. PMID 30254038
- Gopal P. Troubleshooting the crashing patient with a tracheostomy. Academic Life in Emergency Medicine. 2018.
- Nickson C. Respiratory distress in tracheostomy patient. Life in the Fast Lane. 2019.
- Weiss SL, Peters MJ, Alhazzani W, et al. Executive Summary: Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr Crit Care Med. 2020 Feb;21(2):186-195.
- Greenwood JC, Winters ME (2019). Tracheostomy Care. In Roberts and Hedges’ Clinical Procedures in Emergency Medicine and Acute Care (7th ed., pp. 142- 159.e2). Elsevier.
- Hess DR, Altobelli NP. Tracheostomy tubes. Respir Care. 2014;59(6):956-973. PMID 24891201
- Horeczko T, Enriquez B, McGrath NE, Gausche-Hill M, Lewis RJ. The Pediatric Assessment Triangle: accuracy of its application by nurses in the triage of children. J Emerg Nurs. 2013; 39(2):182-189. PMID 22831826
- White AC, Kher S, O’Connor HH. When to change a tracheostomy tube. Respir Care. 2010; 55(8):1069-1075. PMID 20667154
| ITEM | FINDING |
|---|---|
| Overall Appearance | A 10-year-old boy lying in bed, eyes closed, tracheostomy tube in place, in respiratory distress with tachypnea and retractions. |
| HPI | Child was brought by his parents. “He’s been sick for about a week. After 4 days, the cough wasn’t getting better and his fever got worse so we took him to his regular doctor. She started him on some antibiotics. Last night he was coughing a lot and the night nurse suctioned the trach several times and ended up replacing the tube. Since I started taking care of him, we haven’t been able to get his sats above 85% so I wanted him checked.” |
| Past Medical/Surgical History | Ex 24-week preemie, chronic lung disease, intellectual disability, cerebral palsy, pneumonia x3, last admitted 1 year ago, seizures. Tracheostomy (weaned off home oxygen 6 months ago) with no ventilator support. G-tube. Immunizations up-to-date. Prior to this episode he had been doing well and they were discussing having the tracheostomy removed. |
| Medications | Baclofen, oxcarbazepine, azithromycin for the past 4 days |
| Allergies | No known drug allergies |
| Family History | Father has asthma |
| Social History |
Initial Presentation
Start of evaluation through trach replacement
- Team leader assigns tasks
- Obtain relevant history from parent
- Perform primary survey and identify serious conditions
- Provide supplemental oxygen and basic airway maneuvers
- Perform BMV through tracheostomy
- Suction tracheostomy tube
- Replace tracheostomy tube
- Obtain point-of-care rapid glucose level
- Discuss progress and plan of care with the parent (and involve them in shared decision-making)
Physical Exam
| ITEM | FINDING |
|---|---|
| Vital Signs | : 39.2 C, 140, 95/45, 35, : 82% on room air |
| General | Boy lying in bed, eyes closed in respiratory distress with tachypnea and retractions |
| HEENT | Microcephalic, 4 mm pupils are equally round and reactive |
| Neck | Tracheostomy in place. Nontender. No step-offs. No crepitus. |
| Lungs | No crepitus. RR=35. Shallow, rapid respirations. Crackles bilaterally, no wheezing. |
| Cardiovascular | HR=140. Regular rhythm. Normal heart sounds. Weak pulses. 4-second capillary refill. |
| Abdomen | Normal bowel sounds. Soft, nontender. G-tube in place clean, dry, intact. No guarding/rebound. No distention. No mass. |
| Neurological | GCS = 7 Hypertonic in extremities |
| Skin | Cool, pale, diaphoretic |
| Back | Non-tender. No step-offs. No crepitus. |
| Extremities | Minimal muscle mass; no swelling or deformities |
Instructor Notes: Changes and Case Branch Points
| INTERVENTION / TIME POINT | CHANGE IN CASE | ADDITIONAL INFORMATION |
|---|---|---|
| One minute without assisted ventilation | decreases to 75% | |
| Non-rebreather placed w/o BMV | rises to 89% | The child is much more tachypneic with worsened subcostal retractions. |
| BMV performed via trach | decrease to 78% | This is because trach is plugged. |
| Trach is suctioned | decreases to 78% | Unable to pass suction catheter. Plugging the end of the tracheostomy tube with super glue can represent a plugged trach effectively. |
| Trach is replaced | . | The old trach has a thick mucus plug over the tip. The airway obstruction has been addressed but the patient is still in shock with significant pneumonia. |
| Bedside labs obtained | Point of care labs (other labs pending) VBG 75 mmHg 45 mmHg 12 mEq/L |
Management of Septic Shock
Trach change completed through fluid resuscitation and antibiotics
- Recognize and provide prompt management for shock and pneumonia with IV fluids and antibiotics
- Discuss progress and develop plan of care with the parent (and involve them in decision-making)
- OPTIONAL: Perform RUSH POCUS exam if unable to determine the cause of hypotension
| ITEM | FINDING |
|---|---|
| Vital Signs | : 39.2 C, 150, 85/40, 25, : 96% on non-rebreather mask placed over the trach or humidified trach collar at minimum 40% Fio |
| Exam Changes | Capillary refill 5 seconds |
| INTERVENTION / TIME POINT | CHANGE IN CASE | ADDITIONAL INFORMATION |
|---|---|---|
| Parent(s) not updated about trach change | Parent(s) become upset, demanding an update “What’s going on? What are you doing?” | The parents should not escalate to the point where security could be called. |
| Fluid resuscitation | After first 400 mL (20 mL/ kg): After second 400 mL: | |
| Antibiotics given | No change in exam | |
| If the two critical actions above are completed | Proceed to . |
Case Conclusion
Completion of fluid resuscitation/antibiotics through agreement for transfer to pediatric ICU
- Plan for transfer to pediatric ICU (sign out patient to accepting facility/ service)
- Ensure family is updated on plan of care and explain why tracheostomy tube was not functioning
NOTE: The patient should have received appropriate airway management and antibiotics by this final stage.
| ITEM | FINDING |
|---|---|
| Vital Signs | 123, 105/60, 20, : 96% on non-rebreather mask placed over the trach or humidified trach collar at minimum 40% Fio |
| Exam Changes |
Describe the signs/symptoms of septic shock in a pediatric patient (application)
Septic shock: Shock is a condition where the body’s ability to provide oxygen to the tissues is not adequate to the needs of the tissues. Septic shock is a complex pathophysiological state of distributive shock when shock is due to the inflammatory response to a systemic infection.
Shock can be recognized as:
- Symptoms include increased capillary refill time, tachycardia, a new flow murmur, diaphoresis, and fatigue.
- Symptoms include decreased blood pressure, altered mental status, and cardiac arrest.
In general, the evaluation of a medically complex child does not greatly differ from that of a previously healthy child as both populations require quick assessment of ABC’s with emphasis on overall clinical status.
Pediatric assessment triangle:
- Appearance (Mental status): Note abnormal tone, level of arousal, and changes in speech/cry
- Work of Breathing : Note presence of abnormal breath sounds, retractions, nasal flaring, grunting, apnea etc.
- Circulation to Skin : Note presence of pallor, delayed capillary refill, mottling, cyanosis etc.
Primary survey:
- Airway: Does the patient have a patent airway?
- Breathing: Auscultate for bilateral breath sounds
- Circulation: Assess for presence/absence of pulses and degree of peripheral perfusion
- Disability: Report Glasgow Coma Scale, examine pupils • Exposure: Allow for adequate visualization of the patient
However, the evaluation of the medically complex, technology- dependent child may require special vigilance compared to the previously healthy such as:
- Baseline status: What might be considered abnormal for a previously healthy child may be a baseline attribute of a more medically complex child (e.g., in this case, the patient is nonverbal and therefore the Glasgow Coma Score does not appropriately quantify the change in this patient’s mental status). Caregivers are the best resource for this information. Trust them!
- Medical equipment: To provide effective care for a medically complex child, providers should be familiar with the equipment they present with. This can range from common equipment such as a gastrostomy tube to more complex machinery, such as vagal nerve stimulators and baclofen pumps.
Respiratory failure is a condition where the body’s respiratory system is not able to meet the rest of the body’s demands for oxygenation and/or ventilation (elimination of carbon dioxide). Signs of respiratory distress in a medically complex patient are often similar to those of otherwise healthy children (e.g. tachypnea, nasal flaring, retractions, grunting), but depending on the patient’s medical history, signs of respiratory distress may be different. For example, a patient with hypotonia might not be able to sit up and tripod or even generate the muscular effort that produces retractions despite being significantly hypoxic.
In 2020, the Surviving Sepsis group released revisions to the updated guidelines for the management of pediatric septic shock.(Weiss et al. Intensive Care Med 2020). The guidelines are worth reviewing in detail but below is a modified summary:
- Early recognition of sepsis is essential to improve outcomes
- Place the child on supplemental O2 (non-rebreather mask or high flow nasal cannula)
- Establish IV/IO access x2 as quickly as possible. If traditional IV access is difficult (>2 attempts or trying for >90 seconds), consider an ultrasound-guided IV or IO access, whichever is quickly possible. IO access is appropriate even in awake patients in septic shock.
- Administer 20 mL/kg balance/buffered crystalloids (e.g., LR), up to 60 mL/kg.
- After each bolus, reassess vital signs and capillary refill.
- Goals include an improvement in heart rate, capillary refill time <2 seconds, and normalization of blood pressure.
- Keep in mind that a medically complex child may not have the same baseline vital signs expected for age. Previous records and caregivers can be helpful to establish each patient’s baseline vital signs.
- Reassess for crackles/rales and/or hepatomegaly. Stop fluids if any of these signs develop.
- Correct hypoglycemia if present (a point of care test can be helpful to get results back more rapidly).
- Do not use in patients <4 weeks of age. In this population, ampicillin + gentamicin or ampicillin + cefotaxime are appropriate. Dosing may change with the infant’s gestational and post-natal age.
- Some centers prefer cefepime over ceftriaxone in medically complex patients with septic shock.
- Vancomycin 15-20 mg/kg
- Blood cultures should ideally be obtained prior to administration of antibiotics; however, obtaining sterile access for a culture should not delay IV fluids administration.
- It is ok to initiate vasopressors peripherally but convert to centrally when possible. (Note: Intraosseous access is considered central access.)
- Options are epinephrine or norepinephrine at 0.05 mcg/kg/min and titrate to response
- Rough estimate: infants 25 mg, children 50-100 mg
Demonstrate airway management of a sick child using appropriate adjuncts and BMV (application)
In general, children have higher oxygen demand for body weight than adults. This means children may require a higher frequency of bagging, but a common mistake in acute situations is to hyperventilate due to too rapid a rate of bagging the patient. End- tidal carbon dioxide (EtCO2) monitoring attached to the bag can help identify the appropriate rate of bagging.
The size of the bag used for ventilation should be appropriate for the size of the child.
- Infants and small children can use a 450 mL bag
- Older children may benefit from a 1,000 mL bag
A useful mnemonic for pediatric airway management with bag mask ventilation comes from the Textbook of Neonatal Resuscitation (Weiner & Zaichkin, 2016): MR SOPA
- M: Mask adjustment. Attempt to obtain a full seal around the mouth and nose. This may require changing the size of the mask to better suit the patient. One size does not fit all!
- R: Reposition airway. Given the more prominent occiput in children relative to adults, a shoulder roll can be more effective than a neck roll. Try to place the patient in a “sniffing” position with the chin and nose tilted up. This can be accomplished with a head tilt-jaw thrust maneuver.
- S: Suction. Respiratory infection is a common cause for pediatric respiratory distress and providing suction can remove secretions that are obstructing the airway.
- O: Open mouth. Similarly, the nose may be obstructed despite suctioning and opening the mouth can be an effective way of improving gas exchange. In an obtunded patient with no gag reflex, this may be accomplished with an oropharyngeal airway.
- P: Pressure increase. Particularly in medically complex children, lung compliance may be decreased due to chronic lung disease or frequent pulmonary infections. Higher peak inspiratory pressures may be needed to adequately ventilate and oxygenate. However, caution should be taken to avoid barotrauma and a pneumothorax.
- A: Alternate airway. If the above steps are not effective in addressing the child’s respiratory distress then progression to more advanced airways are indicated. Besides endotracheal intubation, consider a properly sized laryngeal mask airway.
Propose a plan to troubleshoot a tracheostomy device (evaluation)
If the patient is in respiratory distress, consider that the patient may not have a stable airway. This may be due to obstruction, dislodgement, or the creation of a false passage.
- Obstruction may be due to mucus/secretions, blood or granulation tissue. Sometimes these obstructions may create a one-way valve allowing for inspiration but not expiration. Suctioning may not be sufficient to clear the obstruction.
- Dislodgement (accidental decannulation) may occur during patient movement, trach manipulation, or connecting/ disconnecting the trach from a ventilator.
- A false passage may be created during a difficult trach change that places the internal end of the tube into the soft tissues and not into the trachea. If this is recognized a stable airway must be established immediately.
In general, if a tracheostomy is more than 7 days old and the patient is in respiratory distress and there is concern that the tracheostomy tube is the source, the tracheostomy tube should be exchanged. If the tracheostomy tube exchange is unsuccessful or does not lead to improvement in the patient’s respiratory distress, consider alternate etiologies of respiratory distress such as pneumonia and pneumothorax.
- If the tracheostomy tube is still believed to be the problem, then you can attempt to provide bag-mask ventilation orally; making sure to occlude the tracheostomy stoma.
- If intubating orally, be sure to pass the cuff past the tracheostomy stoma.
- If intubating through the stoma then use the same size cuffed endotracheal tube as the patient’s tracheostomy tube.
If a tracheostomy is less than 7 days old, there is a high risk for complications and tracheostomy tube exchange in the Emergency Department should only rarely be considered appropriate.
The steps for a tracheostomy tube exchange:
- Before beginning, gather appropriately sized airway supplies including 2-3 tracheostomy tubes with the patient’s current tube size and 1-2 smaller tubes in case of difficulty.
- Check equipment for lack of defects and ensure the cuff inflates properly.
- Preoxygenate the patient for at least one minute with 100% FiO2.
- Deflate the old tube’s cuff and remove the tube.
- Place the new tube (with solid obturator in place) into the stoma.
- Once in place, remove the obturator, insert the hollow inner cannula, and inflate the cuff.
- Check placement with end tidal CO2 monitoring.
In acute settings, a useful mnemonic for taking a focused but appropriate history is AMPLE.
- Medications. This may be a long list in a medically complex child. Sometimes caregivers have a list or an app on their phones that list medications. It may be necessary to delegate a member of the medical team to obtain a full medication list from a caregiver. Often caregivers record medication doses in milliliters rather than milligrams. Most medications have standard concentrations from which doses can be calculated.
- Past medical history . Asking about immunizations and birth history can often be very helpful. In a medically complex patient be sure to ask about prior surgeries and device placement as well as common comorbidities such as seizures, chronic lung disease, and metabolic disorders.
- Last food/drink intake. Medically complex children may be fed orally, through a gastrostomy tube, or a gastro-jejunostomy tube. Be sure to ask open-ended questions about any intake rather than asking about meals.
- Events leading to presentation. Ask about changes from the patient’s baseline over the past few days with pointed questions attempting to identify critical neurologic, cardiac, and respiratory pathologies.
While the caregivers of a medically complex child may seem very knowledgeable and savvy (and may use advanced medical terminology), it is important in an acute setting to continue to use simple, patient-centered language. Caregivers are often very anxious and they may require repetition to fully understand what you are trying to convey, particularly if it is bad news. If time allows, using a teach-back method allows you to make sure that the caregiver understands what you are trying to communicate and allows for misunderstandings to be addressed.
Teams may use different frameworks to improve team dynamics and communication. Below are a few definitions that may be helpful to discuss, adapted from the AHRQ TeamSTEPPS Pocket Guide .
- Brief : Short session prior to start of encounter to share the plan, discuss team formation, assign roles and responsibilities, establish expectations and climate, anticipate outcomes and likely contingencies
- Huddle : Ad hoc team discussion to re-establish Situation Awareness; designed to reinforce plans already in place and assess the need to adjust the plan
- Callout: A strategy used to communicate critical information during an emergent event. Helps the team prepare for vital next steps in patient care. (Example: Leader- “Airway status?”; Surveying provider- “Airway clear”; Leader- “Breath sounds?”; Surveying provider- “Breath sounds decreased on right”)
- Check-back: A closed-loop communication strategy that requires a verification of information ensuring that information conveyed by the sender is understood by the receiver as intended. The sender initiates the message; the receiver accepts it and restates the message. In return, the sender verifies that the re-statement of the original message is correct or amends if not. (Example: Leader- “Give diphenhydramine 25 mg IV push”; Med Prep- “Diphenhydramine 25 mg IV push”; Leader- “That’s correct”)
- S = Situation (What is going on with the patient?)
- B = Background (What is the clinical background or context?)
- A = Assessment (What do I think the problem is?)
- R = Recommendation (What would I do to correct it?)
- Situation monitoring: The process of continually scanning and assessing a situation to gain and maintain an understanding of what is going on around you.
- Situation awareness: The state of “knowing what’s going on around you.”
- Shared mental model: Result of each team member maintaining situation awareness and ensures that all team members are “on the same page.” An organizing knowledge structure of relevant facts and relationships about a task or situation that are commonly held by team members.
- STEP: A tool for monitoring situations during complex situations. A systematic method to review S tatus of patient, T eam members’ performance and status, E nvironment, and P rogress towards goal.
- Cross-monitoring: A harm error reduction strategy that involves 1. Monitoring actions of other team members 2. Providing a safety net within the team. 3. Ensuring that mistakes or oversights are caught quickly and easily. 4. “Watching each other’s back.”
- CUS: Signal phrases that denote “I am C oncerned,” “I am U ncomfortable,” and “This is a S afety Issue.” When spoken, all team members should understand clearly not only the issue but also the magnitude of the issue.
File 1: Chest X-Ray

Chest X-ray: AP View

Chest X-ray – Lateral View
File 2: Laboratory Results

File 3: RUSH Images

Download Case 13 supporting files
- File 1: Chest X-ray Interpretation – The CXR shows a multifocal pneumonia. Images courtesy of the Division of Diagnostic Imaging and Radiology, Children’s National Hospital, Washington, DC
- File 3a. RUSH image before fluid resuscitation showing a collapsible IVC
- File 3b. RUSH image after fluid resuscitation showing a non-collapsible IVC
For the embedded participant playing the patient’s parent
Case Background Information
Your son has multiple medical problems due to being born prematurely, including a breathing tube in his neck (tracheostomy). Your son has been sick for about a week and is getting worse now with worse trouble breathing. So, you came to the hospital for him to be evaluated. He hasn’t been this sick in a year but when he looked like he does today it meant he had to be hospitalized.
Who are the Learners?
Emergency medicine interns: They are in their first year of specialty training and may have experience in gathering information from patients and families but are less familiar with medical treatments and procedures.
Emergency medicine residents: They are in their second to fourth year of specialty training and are growing more comfortable with gathering information, developing a plan and then performing medical treatments and procedures.
Standardized Patient Information
- Narrative: He’s been sick for about a week. After four days the cough wasn’t getting better and his fevers got worse so we took him to his regular doctor. She started him on some antibiotics. Last night he was coughing a lot and the night nurse suctioned the trach several times and ended up replacing the tube. Since I started taking care of him we haven’t been able to get his sats above 85% so I wanted him checked.
- Motivation: You are worried that your son may have to be hospitalized again. This usually means a long hospital stay, sometimes in the intensive care unit and this is frightening.
- Demeanor: Initially you are concerned that his breathing is very fast and that his oxygen number “saturation” is much lower than normal. Over time (especially if you do not feel that you have been kept informed about what is going on), it is ok to become more anxious. He has not been this sick for a long time and his physicians were talking about taking out his trach so this illness is clearly a setback.
- Communication Guidelines: While it is ok to ask questions, please DO NOT interrupt the learners when they are thinking out loud as one of their objectives is to verbalize their thoughts. Another goal of the session is to have the learners learn how to talk to families so please do not become so upset/obstructive that the learners feel justified having you removed from the room.
Patient Information
(Please remember not to offer any of this information, but when asked please respond while remaining in character.)
- CHIEF COMPLAINT (your response to open-ended questions such as “what’s going on?” or “what can we do for you? Or “what happened?”): “His breathing has gotten worse over the past few hours, and I can’t get his oxygen saturation above 85%.”
- AGE: 10 years old
- ADDITIONAL HISTORY: “He’s been sick for about a week. After 4 days, the cough wasn’t getting better and his fever got worse so we took him to his regular doctor. She started him on some antibiotics. Last night he was coughing a lot, and the night nurse suctioned the trach several times and ended up replacing the tube. Since I started taking care of him, we haven’t been able to get his saturation above 85% so I wanted him checked.”
- PAST MEDICAL HISTORY: Born premature at 24 weeks, chronic lung disease, intellectual disability, cerebral palsy, seizure disorder, admitted to the hospital for pneumonia x3 in his life, last admitted 1 year ago. The trach was weaned off of home oxygen 6 months ago, and he is not on a ventilator. He has a G-tube for feeding at night.P rior to this episode he had been doing well and his physicians were discussing having the tracheostomy removed.
- SOCIAL HISTORY: Lives at home with both parents and a younger brother (brother has a mild cold that started a week ago).
- FAMILY HISTORY : Father with asthma
- PAST SURGICAL HISTORY : Tracheostomy placement when he was a baby, G-tube placement around the same time
- MEDICATIONS: Baclofen, oxcarbazepine, azithromycin for the past 4 days (started by primary care doctor). The full medication list is with the other parent who is arriving by car “soon”.
- ALLERGIES: No known drug allergies
- IMMUNIZATIONS: Up-to-date
- FEEDINGS : 2 cans of Pediasure overnight by the G-tube. These feedings have been going normally while he’s been sick.
- BIRTH HISTORY: Born at 24 weeks premature. Prolonged NICU stay. The main complications of his prematurity are cerebral palsy and chronic lung disease.
Potential Dialogue
IMPORTANT: Do not offer unsolicited information. Please allow the learners to ask questions. Do not offer information unless they ask you.
Things you could say without being asked:
- “The doctors said he was getting better. Why is he so sick today?”
Things you might say triggered by events in the scenario:
| EVENT | YOUR POTENTIAL RESPONSE |
|---|---|
| After the tracheostomy (breathing tube in the neck) is suctioned | “We tried that at home and it didn’t make any difference” |
| If they mention the word “intubate” or talk about putting a “breathing tube in his mouth” | “Why are you doing that? He got the tube in his neck so he wouldn’t need a tube in his mouth anymore. His ENT doctor said that it would be really hard to get another tube down his mouth anyway.” |
| If you are not updated with a plan before the tracheostomy tube is removed | “What are you doing? It’s not time to change the tube and why would it do that if he’s already having trouble breathing?” It’s ok to gently press the issue until you feel you have been updated with a plan. |
| After the tracheostomy tube has been replaced | “He’s breathing better but he still doesn’t look right.” |
| If you are not told what the next steps are | “Are we spending the night here?” |
The learners enter the room to find a medically complex child in respiratory distress. They immediately place the child on bedside monitors and recognize that the patient is hypoxic and hypotensive with altered mental status. Supplemental oxygen is provided over the trach and IV access is established to start a fluid bolus. After completing a physical examination and obtaining an appropriate history, the providers note that the child’s respiratory status has not improved with supplemental oxygen (or BMV through the tracheostomy tube) and the trach should be investigated. The trach cannot be suctioned due to an obstructive plug and it must be changed. Once the trach is changed the patient’s respiratory status improves. At this point it should be recognized that the patient is still in septic shock. RUSH POCUS (if performed) at this point identifies a collapsible IVC consistent with hypovolemia. Appropriate management requires additional IV fluid boluses and antibiotics. Repeat POCUS (if performed) reveals non-collapsible IVC. The family should be updated throughout the course of this scenario. Once the patient has been stabilized, arrangements must be made for transfer to a facility with a pediatric intensive care unit. The chest x-ray (if ordered) reveals a multi-lobar pneumonia.
Anticipated Management Mistakes
- Not keeping the family updated: One of the goals of this case is to have learners balance direct patient care with keeping the family of the patient aware of the plan. We find it helpful to have the parent(s) become increasingly vocal, though not reaching the level of disruption where having the parent removed from the room becomes reasonable.
- Trach is repeatedly suctioned/lack of recognition of trach plug: Sometimes learners get stuck on suctioning the trach as the solution and will repeatedly insert the suction catheter despite the end of the trach being blocked. You can cue them by saying that they are not getting anything out when they suction.
- Attempting to intubate from above: Some groups of learners decide to intubate from above rather than change the trach. If attempts are made to intubate, the parents are instructed to object and point out that the patient’s ENT specialist said he would be a difficult airway. If the learners persist and attempt to intubate without removing the trach, continue to keep the patient in respiratory distress (they will likely notice the trach tube obstructing their attempts to pass the ETT). If the trach is removed and the tube is placed past the stoma then progress the case like the trach was successfully changed.
- Failure to recognize septic shock: At times, once the respiratory distress was addressed with a trach change, some learners feel like the case is over and do not recognize the persistently poor vital signs signifying underlying septic shock. If this is the case it may be helpful to continue to increase the heart rate and decrease the blood pressure until shock is recognized and treated.
- Lack of disposition plan: If the learners stabilize the patient but do not have a disposition plan (e.g., transfer to a facility with a pediatric ICU), the parents have been prompted to ask where they are going next.
Return to the EM ReSCu Peds curriculum home page
Please complete our brief survey describing your experience.
Learner (scan or click on QR code)

Facilitator (click or scan QR code)

- Latest Posts

Michelle Lin, MD
Latest posts by michelle lin, md ( see all ).
- EM Match Advice 44: Approaching your EM sub-internship clerkship – “Just gotta roll with it” - June 25, 2024
- From Collision to Clarity: PECARN cervical spine injury prediction rule for injured children - June 10, 2024
- Hot off the press: Bridge to EM curriculum (2nd edition) released - May 6, 2024
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The role of gut microbiota and the gut-lung axis in sepsis: A case study of a pregnant woman with severe rickettsial pneumonia and septic shock complicated by MODS
Affiliation.
- 1 Department of Critical Care Medicine The First People's Hospital of Yulin Yulin China.
- PMID: 38840756
- PMCID: PMC11150134
- DOI: 10.1002/ccr3.8815
Key clinical message: In this case report, we describe the successful management of severe scrub typhus with pneumonia, sepsis, and multiple organ dysfunction in a pregnant woman. Despite initial challenges, the patient responded favorably to fecal microbiota transplantation and oral fecal microbiota capsule therapy.
Abstract: Scrub typhus, caused by Orientia tsutsugamushi , can lead to severe multiorgan dysfunction and carries a mortality rate of up to 70% if not treated properly. In this report, we present the case of a 27-year-old pregnant woman at 18 + 6 weeks gestation whose symptoms worsened 15 days after onset and progressed to severe pneumonia with sepsis and multiple organ dysfunction syndrome. After the pathogen was confirmed by next-generation sequencing analysis of bronchoalveolar-lavage fluid and blood samples, the patient's treatment was switched to antiinfective chloramphenicol. The patient also underwent uterine evacuation due to a miscarriage. Extracorporeal membrane oxygenation was discontinued once the pulmonary infection significantly improved. Subsequently, the patient had recurrent diarrhea, abdominal distension, and difficulty eating. The antibiotic regimen was adjusted according to the drug sensitivity, but the diarrhea and abdominal distension still did not improve. Following a comprehensive multidisciplinary risk assessment, we initiated fecal microbiota transplantation and oral fecal microbiota capsule therapy. As a result, the patient's condition was effectively managed, and they were gradually discharged. Fecal microbiota transplantation may be a safe and effective treatment for severe pneumonia and shock in pregnant women. This has significant implications for maternal health. However, further clinical cases are required to observe its long-term effectiveness.
Keywords: fecal microbiota transplantation; multiple organ dysfunction syndrome; rickettsia tsutsugamushi; sepsis; severe pneumonia.
© 2024 The Author(s). Clinical Case Reports published by John Wiley & Sons Ltd.
PubMed Disclaimer
Conflict of interest statement
The authors have no conflicting interests to declare.
Lung ultrasound imaging prior to…
Lung ultrasound imaging prior to initiation of venovenous extracorporeal membrane oxygenation (VV‐ECMO).
Pulmonary computed tomography (CT) prior…
Pulmonary computed tomography (CT) prior to initiation of VV‐ECMO. On July 24th, two…
Pulmonary CT on the day…
Pulmonary CT on the day of ECMO removal. On July 31, the two…
CT scan of the lungs…
CT scan of the lungs 26 days post ECMO removal. On August 25th,…
Abdominal CT of the patient.…
Abdominal CT of the patient. The CT on August 5th revealed intestinal obstruction,…
Patient treatment timeline. This timeline…
Patient treatment timeline. This timeline illustrates the principal clinical events throughout the patient's…
Oxygenation index and blood lactic…
Oxygenation index and blood lactic acid during treatment. The oxygenation index progressively increased…
Similar articles
- Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Wei Y, Yang J, Wang J, Yang Y, Huang J, Gong H, Cui H, Chen D. Wei Y, et al. Crit Care. 2016 Oct 18;20(1):332. doi: 10.1186/s13054-016-1491-2. Crit Care. 2016. PMID: 27751177 Free PMC article.
- Successful treatment of severe sepsis and diarrhea after vagotomy utilizing fecal microbiota transplantation: a case report. Li Q, Wang C, Tang C, He Q, Zhao X, Li N, Li J. Li Q, et al. Crit Care. 2015 Feb 9;19(1):37. doi: 10.1186/s13054-015-0738-7. Crit Care. 2015. PMID: 25881250 Free PMC article.
- Successful diagnosis and treatment of scrub typhus associated with haemophagocytic lymphohistiocytosis and multiple organ dysfunction syndrome: A case report and literature review. Wu H, Xiong X, Zhu M, Zhuo K, Deng Y, Cheng D. Wu H, et al. Heliyon. 2022 Nov 3;8(11):e11356. doi: 10.1016/j.heliyon.2022.e11356. eCollection 2022 Nov. Heliyon. 2022. PMID: 36411909 Free PMC article.
- A Case Report and Literature Review of Scrub Typhus With Acute Abdomen and Septic Shock in a Child-The Role of Leukocytoclastic Vasculitis and Granulysin. Chang PH, Cheng YP, Chang PS, Lo CW, Lin LH, Lu CF, Chung WH. Chang PH, et al. Am J Dermatopathol. 2018 Oct;40(10):767-771. doi: 10.1097/DAD.0000000000001167. Am J Dermatopathol. 2018. PMID: 29697421 Review.
- Venous-arterial extracorporeal membrane oxygenation for psittacosis pneumonia complicated with cardiogenic shock: case report and literature review. Zhang Y, Hu H, Xu Y, Chen Y, Liu B, Chen J, Nie W, Zhong S, Ma J, Liu C. Zhang Y, et al. BMC Cardiovasc Disord. 2024 Jan 2;24(1):6. doi: 10.1186/s12872-023-03669-y. BMC Cardiovasc Disord. 2024. PMID: 38166547 Free PMC article. Review.
- Kelly DJ, Fuerst PA, Ching WM, Richards AL. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. 2009;48(Suppl 3):S203‐S230. - PubMed
- Kala D, Gupta S, Nagraik R, Verma V, Thakur A, Kaushal A. Diagnosis of scrub typhus: recent advancements and challenges. Biotech. 2020;3:10. - PMC - PubMed
- Bhandari M, Singh RK, Laishevtcev A, et al. Revisiting scrub typhus: a neglected tropical disease. Comp Immunol Microbiol Infect Dis. 2022;90‐91:101888. - PubMed
- Jayasimha K, Varma M, Kamath A, et al. Risk factors of acute respiratory distress syndrome in scrub typhus. Int J Res Med Sci. 2017;5:3912‐3915.
- Kullberg R, Wiersinga WJ, Haak BW. Gut microbiota and sepsis: from pathogenesis to novel treatments. Curr Opin Gastroenterol. 2021;37:578‐585. - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- PubMed Central
Research Materials
- NCI CPTC Antibody Characterization Program

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- World J Clin Cases
- v.3(8); 2015 Aug 16
When sepsis affects the heart: A case report and literature review
Correspondence to: Antonino Tuttolomondo, MD, U.O.C. di Medicina Interna e Cardioangiologia, University of Palermo, Pzza delle Cliniche, n.2, 90127 Palermo, Italy. [email protected]
Telephone: +39-91-6552128 Fax: +39-91-6552142
A 59-year-old nursing home patient with Down syndrome was brought to the internal medicine department of our hospital due to fever, cough without expectorate, and dyspnea. A thoracic computed tomography revealed the presence of bilateral basal parenchymal opacities. Her condition deteriorated after admission and troponin reached a peak serum concentration of 16.9 ng/mL. The patient was in cardiogenic shock. In addition to fluid resuscitation, vaso-active amine infusion was administered to achieve hemodynamic stabilization. The differential diagnosis investigated possible pulmonary embolism, myocardial infarction, and myocarditis. Furthermore, a second transthoracic echocardiogram suggested Tako-Tsubo syndrome. This is a septic patient. The purpose of this manuscript is to review studies which formerly examined the possible association between high levels of troponin and mortality to see if it can be considered a positive predictive factor of fatal prognosis as the case of thrombocytopenia, already a positive independent predictive factor of multiple organ failure syndrome, and generally to characterize risk profile in a septic patient.
Core tip: The importance of cardiac involvement during sepsis, when occurs, worsens prognosis. However, as myocardial dysfunction is reversible, an early diagnosis and treatment to improve the survival. The awareness of risk profile to develop a severe myocardial dysfunction in a septic patient would be suitable in order to enforce careful resources in this subset of patients. Moreover, other research are needful to perform the best therapeutic strategy of haemodynamic stay which, sometimes, ( e.g ., when Tako-Tsubo syndrome occurs) can call for intra-aortic balloon pump counter pulsation.
INTRODUCTION
Sepsis is a syndrome caused by the inefficiency of the mechanisms of control and containment of the infection. It is characterized by symptoms and signs of systemic inflammatory reaction to infection and manifestations of organ dysfunction resulting from alterations in the microcirculation.
It is the second most common cause of death in non-coronary intensive units, and the tenth in high-income countries, with a mortality rate between 15% and 50%. Approximately 150000 deaths per year are caused by sepsis in Europe. The number of cases is expected to increase at a rate of 1.5% per year from the current prevalence of 3 cases for every 1000 inhabitants[ 1 ].
The most common pathogenic Gram positives (whose incidence is progressively increasing) are Staphylococcus aureus and Streptococcus pneumonia e, whereas among the most frequent Gram negatives it is possible to include Escherichia coli , Klebsiella spp., and Pseudomonas aeruginosa [ 2 ].
In a smaller percentage of cases, sepsis can be caused by mycobacteria, mycetes, protozoa ( Plasmodium Falciparum ) and viruses[ 1 ].
CASE REPORT
A 59-year-old nursing home patient with Down syndrome had high fever unresponsive to paracetamol, and unproductive cough for 4 d. Accordingly, cefriaxone was administered with some improvement (defervescence and reduction of cough). After the reappearance of fever associated with dyspnea, acrocyanosis, and her deteriorating condition, she was brought to the emergency room, and on initial evaluation she was admitted to the internal medicine department of our hospital. She had a ventricular (pacing), ventricular (sensing), inhibition (response) (and) rate-adaptive holder pacemaker because of a third degree atrioventricular block. Furthermore, her past medical history included chronic cerebrovascular disease due to previous ischemic strokes complicated by vascular dementia and epilepsy. The latter was possibly due to Alzheimer-like disease as is often seen in down syndrome patients.
On arrival in the internal medicine department the patient was drowsy, tachypneic, tachycardic, low blood pressure (80/50 mmHg) and hypoxemic (PaO 2 57 mmHg). The thoracic computed tomography (CT) revealed the presence of bilateral basal parenchymal opacities. The patient was treated empirically with piperacillin/tazobactam, levofloxacin, and vancomycin according to protocol for health care-associated pneumonia. An initial bed-side echocardiogram evaluation revealed severe left ventricular dysfunction with an ejection fraction of 36%, and dilatation of the right ventricle with medium-apical akinesis. In addition to fluid resuscitation, dopamine, dobutamin, and norepinephrine infusion were administred. At times simultaneous administration of two vaso-active amines was necessary to achieve hemodynamic stabilization and adequate diuresis. The first electrocardiogram showed regular activation of the pacemaker and subsequent evaluations revealed repolarization abnormalities of probable hypoxic nature in the inferior wall only (Figure (Figure1 1 ).

Electrocardiogram at the third day from presentation (showed repolarization abnormalities of probable hypoxic nature in the inferior wall).
The results of blood tests are shown in Table Table1, 1 , reporting thrombocytopenia (80000 10 3 /μL) and the peak serum concentration of troponin I (16.9 ng/mL - reference range < 0.012 ng/mL). Blood and urine cultures showed no growth.
Results of blood tests on admission and discharge
| Aspartate aminotrasferase (U/L) | 94 | 17 | < 37 |
| Alanine aminotrasferase (U/L) | 66 | 37 | < 41 |
| Calcaemia (mg/dL) | 7.5 | 7.4 | 8.4-10.2 |
| Gamma-glutamyltranspeptidase (U/L) | 155 | 171 | 8-61 |
| C-reactive protein (mg/dL) | 14.6 | 3.2 | 0-0.5 |
| Alkaline phosphatase (U/L) | 163 | 47 | 40-129 |
| Lactate dehydrogenase (UI/L) | 755 | 511 | 240-480 |
| Ferritin (ng/mL) | 2440 | 15-150 | |
| D-dimer (ng/mL) | 478 | 338 | 10-250 |
| RB count (× 10 /μL) | 3.72 | 3.53 | 4.5-5.5 |
| Hemoglobin (g/dL) | 12.5 | 11.7 | 12-18 |
| Platelet count (× 10 /μL) | 92 | 217 | 150-450 |
| Myoglobin (ng/mL) | 1031 | 99 | 0-62 |
| Troponin I (ng/mL) | 6.43 | 1.36 | 0-0.034 |
A second transthoracic echocardiogram showed akinesis of medium-apical segments of both ventricles with moderate systolic dysfunction (E.F. 45%). This evidence does not rule out an acute ischemic event, but could be seen as suggesting Tako-Tsubo syndrome.
The differential diagnosis also concerned pulmonary embolism, myocardial infarction and myocarditis. The former was excluded through execution of CT angiography. In relation to myocardial infarction and myocarditis, it was not possible to perform coronary angiography or a myocardial biopsy. However, the absence of persistent regional abnormalities ruled out acute coronary syndrome. The third transthoracic echocardiogram showed complete remission of the regional abnormalities (E.F. 50%). The patient was discharged after gradual weaning from vaso-active amines in adequate clinical condition. Therefore, our patient had survived, in spite of severe cardiac involvement and possible Tako-Tsubo syndrome.
In our case there was a significant cardiac involvement associated with sepsis due to pneumonia, up to hearth failure which presented itself as an out-and-out cardiogenic shock. The “fluid resuscitation”, the administration of vasoactive amines and early antibiotic therapy were needed to restore the hemodynamic stability, until the complete recovery of cardiac function, as indeed typically happens in Tako-Tsubo syndrome. The latter, in our patient, was induced by septic injury and characterized initially by hypokinesia of intermediate and apical segments of left ventricle and at a later stage by akinesis of the same with hyperkinesis of basal segment that typically characterizes the disease.
Nevertheless, the absence of head trauma, cerebral hemorrhage, pheochromocytoma, hypertrophic cardiomyopathy made the diagnosis of Tako-Tsubo syndrome plausible. Instead, it was ruled out obstructive atherosclerosis of coronary epicardial artery, since coronary angiography has not been carried out within 48 h, as suggested by Mayo Clinic’s diagnostic criteria[ 3 ]. However, the disappearance of the alterations of the segmental kinesis at echocardiographic final assessment, allowed us to exclude this diagnosis. Endomyocardial biopsy would have been necessary for ruling out a myocarditis, in which the predominant involvement of right ventricle is quite, as it has been at any rate[ 4 ] initially in our case. The clinical presentation, at last, was not suggestive of Guillain-Barrè syndrome[ 5 ] nor electrocardiographic monitoring of recurrent ventricular tachycardia[ 6 ], conditions in which cases of reversible left ventricular dysfunction have been observed[ 7 ]. Therefore, differential diagnosis about Tako-Tsubo syndrome has been ruled out after analyzing anamnesis and clinical presentation. The latter (hypotension, tachycardia, hypoxiemia) also was suggestive of pulmonary embolism, excluded by CT angiography.
The principal cardiovascular manifestation of severe sepsis and septic shock is hypotension and myocardial dysfunction is often associated with them. Myocardial dysfunction does not seem to be caused by myocardial hypoperfusion[ 8 , 9 ] (coronary circulation is maintained or even intensified, although to observe disfunctions in the microcirculation is probable)[ 10 ] but rather by the action of depressant factors such as alpha tumor necrosis factor and beta interleukin 1 and does persist despite fluid resuscitation, as Court et al[ 11 ] have already shown. In addition to the effects of host’s immuno-inflammatory responses ( e.g ., cytokines and mechanisms related to nitric oxide)[ 12 ] circulating substances released by pathogens ( e.g ., endotoxins) also seem to play an important role in provoking myocardial depression. In this sense, the first-line therapy is causal and consists of antibiotic therapy associate with the possible surgical excision of the infectious focus[ 13 ]. However, the restoration of hemodynamic stability is an important goal for the survival of the patient. Fluids remain a first-step therapy in clinical management of the cardiovascular failure in sepsis but it is arguable which of them would be the gold standard. Recent results indicate that albumin also might be used with advantage in some specific subgroups of patients pending for the results of the ongoing trials on new generation starches[ 14 ]. Furthermore, thanks to its electrostatics properties, albumin reduces the endothelial permeability (sealing effect)[ 15 - 20 ]. Its efficacy is still now matter of debate. In patients with severe sepsis, treated with albumin and crystalloids compared with ones treated with crystalloids only, an increase in survival to 28 and 90 d was not observed[ 21 ]. As regards the methods of liquids’ administration, according to Surviving Sepsis Campaign 2012, an initial fluid challenge in patients with tissue hypoperfusion and suspected hypovolemia, up to achieve ≥ 30 mL of crystalloids per kilogram of body weight. It would be needed to continue with the fluid-challenge technique until an actual hemodynamic improvement. Yet, a particular attention in balancing the fluids is necessary, inasmuch a positive fluid balance and elevated central venous pressure are associated with increased mortality[ 22 , 23 ].
With ongoing sepsis, advantageous effects, especially as for cardiac output, could be gained with administration of hypertonic saline solutions, as Oliveira et al[ 24 ] already suggested in their review. For the first time, this kind of therapy was employed in the treatment of hemorrhagic and traumatic shock, with quick restoration of central and peripheral blood flow[ 25 ]. Intravenous infusion of hypertonic saline solution summons fluids into vascular compartment and determines a redistribution of blood flow which, as for that matter our team has shown, in refractory heart failure enhances myocardial performance[ 26 ]. The proposed mechanism to explain these effects suggests a direct action on myocardial functionality and a decreased sympathetic tone[ 27 ]. Hence, infusion of hypertonic saline could be an alternative to early volume resuscitation of a patient with sepsis[ 28 ].
Furthermore, in a multicentric trial conducted in a tertiary care setting, protocol-based resuscitation of patients with septic shock diagnosed in the emergency department, does not improve the outcomes[ 29 ].
Even more complex is the pathogenesis of heart failure that could occur during sepsis and which can provoke a significant increase of troponin.
The increase of troponin in sepsis is an event to be rationally expected. Its dosage, therefore, should not be taken for granted. Considering the heart’s fundamental cardiovascular adaptation role in sepsis, a significant metabolic-inflammatory impairment can occur with high levels of troponin, associated with severe myocardial dysfunction. Moreover, a meta-analysis in “Intensive Care Medicine” a year ago evaluated the prognostic role of troponin in sepsis, showing that its elevated serum concentration was associated with a subset of patients at higher risk of death. Nonetheless, further studies are needed to determine an optimal troponin cut-off value[ 30 ]. B-type natriuretic peptides could also have a role in alerting clinicians to myocardial dysfunction. Their low serum values could exclude severe myocardial impairment. Yet echocardiography is the gold standard method to reveal cardiac dysfunction. Heart rate has also been proposed in the prognostic evaluation of septic patients. A rate of < 106 bpm on presentation suggested a favorable prognosis[ 31 ]. Concerning the latter, it is still debatable whether the use of β-blockers in septic tachycardial patients improves the survival. It has been observed that patients being in chronic treatment with β-blockers and later developed sepsis, and were admitted to the intensive care unit (ICU), could have advantages in terms of survival. However, physicians’ doubts about using β-blockers in early stages of sepsis are licit[ 32 ]. Among other things, it is not still clear enough if in septic shock the increased cardiac rate is pathological or simply an expression of sympathetic hyperactivation. Instead, tachycardia is associated with a worse prognosis. In a prospective observational study in an ICU, esmolol’s titrated administration for 24 h, maintaining a cardiac rate between 80 and 94 bpm in selected adult patients in septic shock after 24 h of hemodynamic stabilization, was able to maintain the microvascular blood flow and reduced the demand for epinephrine. However, patients with severe myocardial disfunction had been excluded from the study[ 33 ]. Recent results suggest that β-blockers’ effects on metabolism, glucidic homeostasis, inflammatory feedback and cardiac function might be advantageous for septic patients[ 34 , 35 ]. In regard to the anti-inflammatory, antioxidant, immunomodulatory and anti-apoptotic actions, statins also might fall within preventing and treating patients with severe sepsis and septic shock[ 36 , 37 ].
However, beyond the value of troponin, B-type natriuretic peptides, and heart rate, the presence of myocardial dysfunction in sepsis is associated with higher mortality. It has been shown that cardiovascular disablement increased mortality from 70% to 90%, compared to 20% in septic patients without myocardial impairment[ 38 ].
Therefore, cardiac dysfunction in sepsis has prognostic value and coincides with its severity. Hence it’s mandatory to know the pathophysiology of cardiovascular disease in sepsis. Microcirculatory disfunctions and mitochondrial derangement occurring in septic shock reduce the cellular energetic production[ 39 ]. Septic injury triggers a reaction in the cardiovascular setting that aims to increase the peripheral availability of oxygen and reduce the cellular effects of oxygen deficiency[ 40 ]. The cellular deficiency of oxygen and reduction in systemic vascular resistance give rise to hyperdynamic syndrome (increased stroke volume, heart rate, usage of oxygen)[ 41 ] (Figure (Figure2). 2 ). Alteration of cellular energetic production following to mitochondrial imbalances might be of great relevance in determining tissue injury and sepsis-associated multi organ failure. Future studies should focus on mitochondrial disfunction in order to comprehend the pathophysiological mechanisms of apoptosis and cellular protection to achieve a increasingly accurate treatment[ 42 ].

Synopsis of heart failure pathogenesis sepsis.
Such a hyperdynamic reaction, favoring adrenergic stimulation, can be hidden by hypovolemia due to insufficient fluid contribution or a mechanism of myocardial impairment[ 43 ] (Figure (Figure3). 3 ). The most frequent occurrence is heart failure with high cardiac index, which is in a phase of unbalance, but it is insufficient to increase metabolic requirements. Hyperdynamic syndrome endows an organism with the possibility to reduce septic injury and survival derives largely from that[ 44 ]. Hence the usefulness of administering inotropic positive drugs and to correct hypovolemia because a possible condition of circulatory failure, in the presence of increased oxygen requirements, is linked to a fatal prognosis[ 45 ].

Possible mechanisms of myocardial impairment[ 43 ].
The management of myocardial dysfunction sepsis-induced encompasses fluids’s administration until the optimization of preload and, among positive inotropic agents, norepinephrine is the first choice[ 39 ]. The administration of dopamine should be reserved to carefully selected patients (those with a low risk of arrhythmias and either known grave left ventricular systolic dysfunction or low heart rate). The Surviving Sepsis Campaign guidelines 2012 promote either norepinephrine or dopamine as the first-choice vasopressor agent to maintain adequate perfusion tissue in septic shock[ 46 , 47 ]. Dobutamine also can be used in the early stages of sepsis in order to increase cardiac output. It has a certain selectivity for β 1 -receptors[ 48 ]. Anyway, β1-agonists can be less effective in case of septic shock. It has been shown that its infusion improves the left ventricular ejection fraction more than 10% in 35% of patients affected by septic shock[ 49 ]. Its use requires careful clinical and instrumental monitoring for risk of tachycardia or arrhythmias and hypotension through beta 2 -adrenergic receptors activation[ 50 ]. However, dobutamine is endorsed as the care’s fundamental element of sepsis-related cardiovascular failure in international guidelines. Furthermore, it has been demonstrated that dobutamine enhances liver function and hepatic perfusion after experimental hemorrhagic shock[ 51 ]. Since one of the mechanisms of sepsis-induced myocardial dysfunction is the alteration of intracellular transport of calcium, a possibility of therapy might be represented by levosimendan[ 52 ], inotrope and peripheral vasodilator which is employed in acute congestive heart failure[ 53 ]. Levosimendan, acting with a mechanism of calcium-sensibilization in randomized studies comparing it with dobutamine in patients with severe heart failure with low cardiac output, has been observed as emodynamically more effective than dobutamine[ 54 - 56 ].
Another kind of heart failure associated with sepsis is Tako-Tsubo syndrome. For this reason it has been supposed that sepsis-induced systemic inflammation could have a role in starting the pathogenesis of the syndrome[ 57 - 59 ]. Myocardial dysfunction in sepsis could be a consequence of the direct action of different mediators of flogosis (cathecolamines responsible for hyperdynamic syndrome included) and of products of microbial derivation[ 13 ]. On the other hand, another pathogenic hypothesis for Tako-Tsubo syndrome is cardiac cathecolamine toxicity, as it could occur in sepsis, which would constitute the trigger[ 60 ].
Our case shows that exogenous support of vasoactive amines can be essential in facilitating hyperdynamic syndrome which characterizes sepsis in the pre-clinical phase. As for Tako-Tsubo syndrome, even though β-agonist agents have often been used, the results are conflicting[ 7 ], so intra-aortic balloon pump counter pulsation remains the first-line treatment if, after medical therapy (dopamine) and volume resuscitation, hypotension endures[ 61 ]. However, the disappearance of segmental kinesis’s alterations and complete resolution of myocardial dysfunction, as in our case, if Tako-Tsubo syndrome is actually diagnosed, offer new perspectives that could improve our understanding of the physiopathology of this illness. Randomized clinical trials could demonstrate the possible efficacy of the treatment.
Case characteristics
A 59-year-old nursing home patient with down syndrome presented fever, cough and dyspnea.
Clinical diagnosis
Main clinical findings were tachypnea, tachycardia and hypotension.
Differential diagnosis
Computed tomography (CT) angiography, thoracic CT, transthoracic echocardiogram were executed and differential diagnosis concerned pulmonary embolism, myocardial infarction, myocarditis, Tako-Tsubo syndrome and sepsi with severe myocardial involvement.
Laboratory diagnosis
The results of blood tests showed alterations of liver function (aspartate aminotrasferase: 94 U/L; alanine aminotrasferase: 66 U/L; gamma-glutamyltranspeptidase: 155 U/L; alkaline phosphatase: 163 U/L); ferritin: 2240 ng/mL; myoglobin: 1031 ng/mL; C-reactive proteinmg: 14.6 mg/dL; lactate dehydrogenase: 755 UI/L; calcaemia: 75 mg/dL; D-Dimer: 478 ng/mL; thrombocytopenia (92000 × 10 3 /μL); myoglobin: 1031 ng/mL; troponin: I 643 with peak serum concentration of 169 ng/mL.
Imaging diagnosis
Echocardiogram revealed severe left ventricular dysfunction with an ejection fraction of 36% and dilatation of the right ventricle with medium-apical akinesis.
Pathological diagnosis
The thoracic CT showed the presence of bilateral basal parenchymal opacities but blood cultures showed no growth.
The patient was treated with piperacillin/tazobactam, levofloxacin, and vancomycin according to protocol for health care-associated pneumonia in add to fluid resuscitation and infusion of dopamine, dobutamin and norepinephrine.
Related reports
The sepsis-induced systemic inflammatory response syndrome can produce myocardial dysfunction that sometimes defines Tako-Tsubo syndrome.
Term explanation
The dosage of troponin and B-type natriuretic peptides, the monitoring cardiac rate can be helpful to identify setting risk of myocardial dysfunction during sepsis.
Experiences and lessons
This article points out the importance of early haemodinamic support with fluid resuscitation, vaso-active amine and catecholamines in sepsis-induced myocardial dysfunction, trying at the same time to define a risk profile of a septic patient with cardiac involvement whose mortality is high.
Peer-review
In spite of richness of literature about the cardiac involvment during sepsis and management of sepsis-induced myocardial dysfunction, other research to identify the more suitable therapeutic strategy is necessary.
P- Reviewer: Najafi M, Weber V S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
Informed consent statement: Patients provided informed consent.
Conflict-of-interest statement: None.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 25, 2014
First decision: November 27, 2014
Article in press: June 16, 2015

IMAGES
VIDEO
COMMENTS
An 81-year-old man presented with fever, cough, and shortness of breath. Within a few hours after presentation, chest pain and respiratory distress developed. A chest radiograph showed bilateral pa...
We report a case of septic shock syndrome caused by Streptococcus pneumoniae in a patient who had undergone splenectomy due to an autoimmune ... A recent study of the "National Reference Center for Pneumococci" at the Austrian Agency for Health ... 82 Sepsis- (0.98/100,000) and 118 pneumonia/bacteremia-diseases (1.42/100,000) were ...
Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017;45:486-552. Crossref
A 44-year-old woman presented with cough, dyspnea, and chest pain. On examination, she had tachycardia and hypotension. Evaluation revealed SARS-CoV-2 RNA in a nasopharyngeal swab, as well as eleva...
This case study discusses a female who presents to the emergency department with sepsis secondary to pneumonia. Over the course of three days, the patient's health quickly deteriorates, demonstrating the rapid progression of sepsis. Clinical findings, such as vitals signs, lab abnormalities, and symptoms of sepsis are discussed.
Pneumonia with sepsis was persistently suspected. The patient immediately received cefoperazone/sulbactam (dose, 3 g; administrated every 8 h) and moxifloxacin (dose, 0.4 g; administrated every 24 h) alongside non-invasive ventilation. ... The present study was the first to report a case within China of severe allergic shock attributed to HCTZ ...
Score Performance and Clinical Utility for In-Hospital Mortality in 6,024 Patients with Community-acquired Pneumonia (Complete-Case Analysis) Score Discrimination: AUROC (95% CI) Calibration Overall Performance ... Our results are in line with those of the pivotal Sepsis-3 clinical criteria study (5, 8), which showed better discrimination for ...
Sepsis, a life-threatening condition, is the main complication of severe community-acquired pneumonia (CAP) observed in approximately one in three patients with severe CAP.1 The complexity and heterogeneity of the biological mechanisms underlying sepsis illustrate the need to identify clearly defined phenotypes based on differential host immune responses recognizable in a clinical setting.2 ...
Case Study: An Unusual Cause of Sepsis Grace I. Chen, MD* and Alia Tuqan, MD** * Division of Geriatrics, Department of Medicine, UCLA David Geffen School of Medicine ** Department of Medicine, University of Nevada School of Medicine A 98-year-old male with multiple chronic medical problems, including ischemic cardiomyopathy, was brought to the
Background Community-acquired pneumonia (CAP) is a leading cause of sepsis worldwide. Prompt identification of those at high risk of adverse outcomes improves survival by enabling early escalation of care. There are multiple severity assessment tools recommended for risk stratification; however, there is no consensus as to which tool should be used for those with CAP. We sought to assess ...
Epidemiology of respiratory viral sepsis. Pneumonia was found to be the most common cause of sepsis and septic shock [14, 29].A recent retrospective cohort study that included hospitalised patients diagnosed as viral CAP without bacterial co-infection showed viral sepsis was present in 61% of these patients [].According to previously published data, 100 million cases of viral CAP occur every ...
Background: Little is known about risk and prognostic factors in very old patients developing sepsis secondary to community-acquired pneumonia (CAP).Methods: We conducted a retrospective observational study of data prospectively collected at the Hospital Clinic of Barcelona over a 13-year period.Consecutive patients hospitalized with CAP were included if they were very old (≥80 years) and ...
Key Clinical Message In this case report, we describe the successful management of severe scrub typhus with pneumonia, sepsis, and multiple organ dysfunction in a pregnant woman. ... The role of gut microbiota and the gut-lung axis in sepsis: A case study of a pregnant woman with severe rickettsial pneumonia and septic shock complicated by ...
Patients will present with dyspnea, hemodynamic instability and shock. The presentation in this particular case is complicated by septic shock. S. Pneumoniae is an uncommon cause of endocarditis, accounting for 1-3% of cases. However it is particularly aggressive with a mortality rate approaching 25%.
In accordance with the hospital's pediatric sepsis guidelines, an IV was placed and a rapid fluid bolus administered. In this case, the LifeFlow® infuser was used due to its speed and control. In order to titrate the fluid resuscitation to the patient, careful patient assessments were performed in between each 20mL/kg bolus to evaluate ...
In 2020, the Surviving Sepsis group released revisions to the updated guidelines for the management of pediatric septic shock.(Weiss et al. Intensive Care Med 2020). The guidelines are worth reviewing in detail but below is a modified summary: Early recognition of sepsis is essential to improve outcomes; When sepsis is recognized (minute zero):
CASE STUDY: Failure to Identify Sepsis and Initiate Treatment Leads to Patient Death. Jeanne E. Mapes, JD, CPCU, CPHRM. ... The patient had a difficult course; she developed pneumonia and suffered a massive stroke approximately a week after the third ED visit. Further complications developed, and the patient died several days after having the ...
current issue. current issue; browse recently published; browse full issue index; learning/cme
This condition can be defined as pneumonia requiring mechanical ventilation in the ICU and/or presenting with sepsis and organ failure due to pneumonia. The disease process is characterized by inflammation of the lung parenchyma, initiated by a combination of pathogens and lowered local defenses. ... and case studies have shown promising ...
Key clinical message: In this case report, we describe the successful management of severe scrub typhus with pneumonia, sepsis, and multiple organ dysfunction in a pregnant woman. Despite initial challenges, the patient responded favorably to fecal microbiota transplantation and oral fecal microbiota capsule therapy.
Core tip: The importance of cardiac involvement during sepsis, when occurs, worsens prognosis. However, as myocardial dysfunction is reversible, an early diagnosis and treatment to improve the survival. The awareness of risk profile to develop a severe myocardial dysfunction in a septic patient would be suitable in order to enforce careful resources in this subset of patients.