Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms
- Department of Health and Human Services
- National Institutes of Health


COVID-19 Research Studies
More information, about clinical center, clinical trials and you, participate in a study, referring a patient.
About Clinical Research
Research participants are partners in discovery at the NIH Clinical Center, the largest research hospital in America. Clinical research is medical research involving people The Clinical Center provides hope through pioneering clinical research to improve human health. We rapidly translate scientific observations and laboratory discoveries into new ways to diagnose, treat and prevent disease. More than 500,000 people from around the world have participated in clinical research since the hospital opened in 1953. We do not charge patients for participation and treatment in clinical studies at NIH. In certain emergency circumstances, you may qualify for help with travel and other expenses Read more , to see if clinical studies are for you.
Medical Information Disclaimer
Emailed inquires/requests.
Email sent to the National Institutes of Health Clinical Center may be forwarded to appropriate NIH or outside experts for response. We do not collect your name and e-mail address for any purpose other than to respond to your query. Nevertheless, email is not necessarily secure against interception. This statement applies to NIH Clinical Center Studies website. For additional inquiries regarding studies at the National Institutes of Health, please call the Office of Patient Recruitment at 1-800-411-1222

Find NIH Clinical Center Trials
The National Institutes of Health (NIH) Clinical Center Search the Studies site is a registry of publicly supported clinical studies conducted mostly in Bethesda, MD.

- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you, finding a clinical trial, around the nation and worldwide.

NIH conducts clinical research trials for many diseases and conditions, including cancer , Alzheimer’s disease , allergy and infectious diseases , and neurological disorders . To search for other diseases and conditions, you can visit ClinicalTrials.gov.
ClinicalTrials.gov [ How to Use Search ] This is a searchable registry and results database of federally and privately supported clinical trials conducted in the United States and around the world. ClinicalTrials.gov gives you information about a trial's purpose, who may participate, locations, and phone numbers for more details. This information should be used in conjunction with advice from health care professionals.
Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read the disclaimer on ClinicalTrials.gov for details.
Before participating in a study, talk to your health care provider and learn about the risks and potential benefits.
At the NIH Clinical Center in Bethesda, Maryland

Search NIH Clinical Research Studies The NIH maintains an online database of clinical research studies taking place at its Clinical Center, which is located on the NIH campus in Bethesda, Maryland. Studies are conducted by most of the institutes and centers across the NIH. The Clinical Center hosts a wide range of studies from rare diseases to chronic health conditions, as well as studies for healthy volunteers. Visitors can search by diagnosis, sign, symptom or other key words.
Join a National Registry of Research Volunteers

ResearchMatch This is an NIH-funded initiative to connect 1) people who are trying to find research studies, and 2) researchers seeking people to participate in their studies. It is a free, secure registry to make it easier for the public to volunteer and to become involved in clinical research studies that contribute to improved health in the future.
This page last reviewed on November 6, 2018
Connect with Us
- More Social Media from NIH
Librarians/Admins
- EBSCOhost Collection Manager
- EBSCO Experience Manager
- EBSCO Connect
- Start your research
- EBSCO Mobile App
Clinical Decisions Users
- DynaMed Decisions
- Dynamic Health
- Waiting Rooms
- NoveList Blog
Free Databases
EBSCO provides free research databases covering a variety of subjects for students, researchers and librarians.
Exploring Race in Society
This free research database offers essential content covering important issues related to race in society today. Essays, articles, reports and other reliable sources provide an in-depth look at the history of race and provide critical context for learning more about topics associated with race, ethnicity, diversity and inclusiveness.
EBSCO Open Dissertations
EBSCO Open Dissertations is a collaboration between EBSCO and BiblioLabs to increase traffic and discoverability of ETD research. You can join the movement and add your theses and dissertations to the database, making them freely available to researchers everywhere.
GreenFILE is a free research database covering all aspects of human impact to the environment. Its collection of scholarly, government and general-interest titles includes content on global warming, green building, pollution, sustainable agriculture, renewable energy, recycling, and more.
Library, Information Science and Technology Abstracts
Library, Information Science & Technology Abstracts (LISTA) is a free research database for library and information science studies. LISTA provides indexing and abstracting for hundreds of key journals, books, research reports. It is EBSCO's intention to provide access to this resource on a continual basis.
Teacher Reference Center
A complimentary research database for teachers, Teacher Reference Center (TRC) provides indexing and abstracts for more than 230 peer-reviewed journals.
European Views of the Americas: 1493 to 1750
European Views of the Americas: 1493 to 1750 is a free archive of indexed publications related to the Americas and written in Europe before 1750. It includes thousands of valuable primary source records covering the history of European exploration as well as portrayals of Native American peoples.
Recommended Reading
- All Solutions

Expertly curated abstract & citation database
Your brilliance, connected.
Scopus uniquely combines a comprehensive, expertly curated abstract and citation database with enriched data and linked scholarly literature across a wide variety of disciplines.
Scopus quickly finds relevant and authoritative research, identifies experts and provides access to reliable data, metrics and analytical tools. Be confident in progressing research, teaching or research direction and priorities — all from one database and with one subscription.
Speak with us about your organization's needs
Scopus indexes content from more than 25,000 active titles and 7,000 publishers—all rigorously vetted and selected by an independent review board.
Learn more about Scopus content coverage

Download the fact sheet

2021 CiteScore metrics are available on our free layer of Scopus.com .
Learn more about CiteScore metrics
Academic Institutions
Scopus is designed to serve the information needs of researchers, educators, students, administrators and librarians across the entire academic community.
Government & Funding Agencies
Agencies and government bodies can rely on Scopus to inform their overall strategic direction, identify funding resources, measure researcher performance and more.
Research & Development
Scopus helps industry researchers track market innovations, identify key contributors and collaborators, and develop competitive benchmarking.
How Scopus works
Scopus indexes content that is rigorously vetted and selected by an independent review board of experts in their fields. The rich metadata architecture on which Scopus is built connects people, published ideas and institutions.
Using sophisticated tools and analytics, Scopus generates precise citation results, detailed researcher profiles, and insights that drive better decisions, actions and outcomes.
Discover how Scopus works
What our customers say
Many of today’s research questions have to do with public health, particularly with public policy and informatics. This creates an overlap between computer science, informatics and health sciences ... having a database as inclusive and interdisciplinary as Scopus is invaluable to us.
— Bruce Abbott, Health Sciences Librarian, University of California, Davis Health Care (USA)
Read the full customer story
Scopus really plays a vital role in helping our researchers, particularly early investigators and people who are getting ready to become early-career postgraduates, better understand the scholarly communications landscape.
— Emily Glenn, Associate Dean, University of Nebraska Medical Center Library (USA) Read the full customer story
When it comes to measuring success, you can’t compare other products to Scopus — no other output metrics offer the same kind of depth and coverage ... faculty, department chairs, college deans, they are always amazed when they discover what’s possible.
— Hector R. Perez-Gilbe, Research Librarian for the Health Sciences, University of California, Irvine (USA) Read the full customer story
Why choose Scopus
Scopus brings together superior data quality and coverage, sophisticated analytics and advanced technology in one solution that is ready to combat predatory publishing, optimize analytic powers and researcher workflows, and empower better decision making.
See why you should choose Scopus
Other helpful resources
To learn more about using and administering Scopus, how to contact us and to request corrections to Scopus profiles and content, please visit our support center .
To find Scopus fact sheets, case studies, user guides, title lists and more, please visit our resource center .
Elsevier.com visitor survey
We are always looking for ways to improve customer experience on Elsevier.com. We would like to ask you for a moment of your time to fill in a short questionnaire, at the end of your visit . If you decide to participate, a new browser tab will open so you can complete the survey after you have completed your visit to this website. Thanks in advance for your time.

Explore millions of high-quality primary sources and images from around the world, including artworks, maps, photographs, and more.
Explore migration issues through a variety of media types
- Part of Street Art Graphics
- Part of The Journal of Economic Perspectives, Vol. 34, No. 1 (Winter 2020)
- Part of Cato Institute (Aug. 3, 2021)
- Part of University of California Press
- Part of Open: Smithsonian National Museum of African American History & Culture
- Part of Indiana Journal of Global Legal Studies, Vol. 19, No. 1 (Winter 2012)
- Part of R Street Institute (Nov. 1, 2020)
- Part of Leuven University Press
- Part of UN Secretary-General Papers: Ban Ki-moon (2007-2016)
- Part of Perspectives on Terrorism, Vol. 12, No. 4 (August 2018)
- Part of Leveraging Lives: Serbia and Illegal Tunisian Migration to Europe, Carnegie Endowment for International Peace (Mar. 1, 2023)
- Part of UCL Press
Harness the power of visual materials—explore more than 3 million images now on JSTOR.
Enhance your scholarly research with underground newspapers, magazines, and journals.
Explore collections in the arts, sciences, and literature from the world’s leading museums, archives, and scholars.
Reference management. Clean and simple.
The top list of research databases for medicine and healthcare
3. Cochrane Library
4. pubmed central (pmc), 5. uptodate, frequently asked questions about research databases for medicine and healthcare, related articles.
Web of Science and Scopus are interdisciplinary research databases and have a broad scope. For biomedical research, medicine, and healthcare there are a couple of outstanding academic databases that provide true value in your daily research.
Scholarly databases can help you find scientific articles, research papers , conference proceedings, reviews and much more. We have compiled a list of the top 5 research databases with a special focus on healthcare and medicine.
PubMed is the number one source for medical and healthcare research. It is hosted by the National Institutes of Health (NIH) and provides bibliographic information including abstracts and links to the full text publisher websites for more than 28 million articles.
- Coverage: around 35 million items
- Abstracts: ✔
- Related articles: ✔
- References: ✘
- Cited by: ✘
- Links to full text: ✔
- Export formats: XML, NBIB
Pro tip: Use a reference manager like Paperpile to keep track of all your sources. Paperpile integrates with PubMed and many popular databases. You can save references and PDFs directly to your library using the Paperpile buttons and later cite them in thousands of citation styles:
EMBASE (Excerpta Medica Database) is a proprietary research database that also includes PubMed. It can also be accessed by other database providers such as Ovid .
- Coverage: 38 million articles
- References: ✔
- Cited by: ✔
- Full text: ✔ (requires institutional subscription to EMBASE and individual publishers)
- Export formats: RIS
The Cochrane Library is best know for its systematic reviews. There are 53 review groups around the world that ensure that the published reviews are of high-quality and evidence based. Articles are updated over time to reflect new research.
- Coverage: several thousand high quality reviews
- Full text: ✔
- Export formats: RIS, BibTeX
PubMed Central is the free, open access branch of PubMed. It includes full-text versions for all indexed papers. You might also want to check out its sister site Europe PMC .
- Coverage: more than 8 million articles
- Export formats: APA, MLA, AMA, RIS, NBIB
Like the Cochrane Library, UpToDate provides detailed reviews for clinical topics. Reviews are constantly updated to provide an up-to-date view.
- Coverage: several thousand articles from over 420 peer-reviewed journals
- Related articles: ✘
- Full text: ✔ (requires institutional subscription)
- Export formats: ✘
PubMed is the number one source for medical and healthcare research. It is hosted at the National Institutes of Health (NIH) and provides bibliographic information including abstracts and links to the full text publisher websites for more than 35 million items.
EMBASE (Excerpta Medica Database) is a proprietary research database that also includes in its corpus PubMed. It can also be accessed by other database providers such as Ovid.

APA PsycInfo ®
The premier abstracting and indexing database covering the behavioral and social sciences from the authority in psychology.
Support research goals
Institutional access to APA PsycInfo provides a single source of vetted, authoritative research for users across the behavioral and social sciences. Students and researchers enjoy seamless access to cutting-edge and historical content with citations in APA Style ® .
The newest features available within APA PsycInfo leverage artificial intelligence and machine learning to equip users with a personalized research assistant that helps monitor trends, explore content analytics, and gain one-click access to full text within a centralized, essential source of credible psychology research.
Celebrating 55 years
For over 55 years, APA PsycInfo has been the most trusted index of psychological science in the world. With more than 5,000,000 interdisciplinary bibliographic records, our database delivers targeted discovery of credible and comprehensive research across the full spectrum of behavioral and social sciences. This indispensable resource continues to enhance the discovery and usage of essential psychological research to support students, scientists, and educators. Explore the past, present, and future of psychology research .
APA PsycInfo at a glance
- Over 5,000,000 peer-reviewed records
- 144 million cited references
- Spanning 600 years of content
- Updated twice-weekly
- Research in 30 languages from 50 countries
- Records from 2,400 journals
- Content from journal articles, book chapters, and dissertations
- AI and machine learning-powered research assistance
Support your campus community beyond psychology with APA PsycInfo’s broad subject coverage:
- Artificial Intelligence
- Linguistics
- Neuroscience
- Pharmacology
- Political science
- Social work
Institutional trial
Evaluate this resource free for 30 days to determine if it meets your library’s research needs.
Access options
Select from individual subscriptions or institutional licenses on your platform of choice.
Find webinars, tutorials, and guides to help promote your library’s subscription.
Key benefits of APA PsycInfo

Learn what’s new

AI-powered research tools
Join a webinar on new features courtesy of your access to APA PsycInfo

APA PsycInfo webinars
Help users search smarter this semester from APA training experts
Browse APA Databases and electronic products by publication type or subscriber type.
View Product Guide
More about APA PsycInfo
- APA PsycInfo FAQs
- Sample records
- Coverage List
- Full-Text Options
- APA PsycInfo Publishers
APA PsycInfo research services
Simplify the research process for your users with this personalized research assistant, a free tool courtesy of your institution's subscription to APA PsycInfo. This service leverages AI and machine learning to ease access to full text, content analytics, and discovery of the latest behavioral sciences research.
Get Started

Find full-text articles, book chapters, and more using APA PsycNet ® , the only search platform designed specially to deliver APA content.
MORE ABOUT APA PSYCNET
APA Publishing Blog
The blog is your source for training materials and information about APA’s databases and electronic resources. Stay up-to-date on training sessions, new features and content, and resources to support your subscription.
Follow blog
Stay connected

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

MEDLINE is the National Library of Medicine's (NLM) premier bibliographic database that contains references to journal articles in life sciences, with a concentration on biomedicine. See the MEDLINE Overview page for more information about MEDLINE.
MEDLINE content is searchable via PubMed and constitutes the primary component of PubMed, a literature database developed and maintained by the NLM National Center for Biotechnology Information (NCBI).
Last Reviewed: February 5, 2024
Database Search
What is Database Search?
Harvard Library licenses hundreds of online databases, giving you access to academic and news articles, books, journals, primary sources, streaming media, and much more.
The contents of these databases are only partially included in HOLLIS. To make sure you're really seeing everything, you need to search in multiple places. Use Database Search to identify and connect to the best databases for your topic.
In addition to digital content, you will find specialized search engines used in specific scholarly domains.
Related Services & Tools
Research and clinical trial databases
This non-exhaustive collection of research databases allows you to locate multiple study datasets as well as information on clinical trials free of charge., research databases.
- Array Express ArrayExpress is a database of functional genomics experiments that can be queried and the data downloaded.
- Cancer Cell Line Encyclopedia (CCLE) The CCLE provides public access to genomic data, analysis and visualization for about 1,000 cell lines.
- European-Genome-phenome Archive (EGA) EGA allows you to explore datasets from genomic studies, provided by a range of data providers.
- Gene Expression Omnibus (GEO) GEO is a public functional genomics data repository supporting MIAME-compliant data submissions.
- International Cancer Genomics Consortium (ICGC) The ICGC Data Portal provides tools for visualizing, querying and downloading the data released quarterly by the consortium's member projects.
- The Cancer Genome Atlas (TCGA) The TCGA Data Portal provides a platform for researchers to search, download, and analyze data sets generated by TCGA.
Clinical Trial Databases
- ClinicalTrials.gov ClinicalTrials.gov is a U.S. registry and results database of publicly and privately supported clinical studies of human participants conducted around the world.
- EORTC Clinical Trials Database The EORTC Clinical Trials Database contains information about EORTC clinical trials and clinical trials from other organisations with EORTC participation.
- EORTC Investigator's Area This section gives access to the clinical trials applications designed for the investigators.
- EU Clinical Trials Register (EU CTR) The EU CTR contains information on interventional clinical trials on medicines conducted in the European Union (EU), or the European Economic Area (EEA) which started after 1 May 2004. The Register enables you to search for information in the EudraCT database.
- WHO International Clinical Trials Registry Platform The mission of the WHO International Clinical Trials Registry Platform is to ensure that a complete view of research is accessible to all those involved in health care decision making.
This site uses cookies. Some of these cookies are essential, while others help us improve your experience by providing insights into how the site is being used.
For more detailed information on the cookies we use, please check our Privacy Policy .
Necessary cookies enable core functionality. The website cannot function properly without these cookies, and you can only disable them by changing your browser preferences.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
National Center for Biotechnology Information
Welcome to ncbi.
The National Center for Biotechnology Information advances science and health by providing access to biomedical and genomic information.
- About the NCBI |
- Organization |
- NCBI News & Blog
Deposit data or manuscripts into NCBI databases
Transfer NCBI data to your computer
Find help documents, attend a class or watch a tutorial
Use NCBI APIs and code libraries to build applications
Identify an NCBI tool for your data analysis task
Explore NCBI research and collaborative projects
- Resource List (A-Z)
- All Resources
- Chemicals & Bioassays
- Data & Software
- DNA & RNA
- Domains & Structures
- Genes & Expression
- Genetics & Medicine
- Genomes & Maps
- Sequence Analysis
- Training & Tutorials
Popular Resources
- PubMed Central
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Jump to navigation
- Bahasa Indonesia
- Bahasa Malaysia

Reevaluating statin trials
Latest news and events.

EMBL-EBI’s open data resources for biodiversity and climate research

More Technology and innovation

The EMBL-EBI File Replication Archive hits 100 petabytes 5 June 2024

AlphaMissense data integrated into Ensembl, UniProt, DECIPHER and AlphaFold DB 21 May 2024
AI annotations increase patent data in SureCheMBL 13 March 2024
ProteomicsML: empowering proteomics research with machine learning 26 January 2024
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Data-Driven Hypothesis Generation in Clinical Research: What We Learned from a Human Subject Study?
Affiliations.
- 1 Department of Public Health Sciences, College of Behavioral, Social and Health Sciences, Clemson University, Clemson, SC.
- 2 Informatics Institute, School of Medicine, University of Alabama, Birmingham, Birmingham, AL.
- 3 Cognitive Studies in Medicine and Public Health, The New York Academy of Medicine, New York City, NY.
- 4 Department of Educational Studies, Patton College of Education, Ohio University, Athens, OH.
- 5 Department of Clinical Sciences and Community Health, Touro University California College of Osteopathic Medicine, Vallejo, CA.
- 6 Department of Electrical Engineering and Computer Science, Russ College of Engineering and Technology, Ohio University, Athens, OH.
- 7 Department of Health Science, California State University Channel Islands, Camarillo, CA.
- PMID: 39211055
- PMCID: PMC11361316
- DOI: 10.18103/mra.v12i2.5132
Hypothesis generation is an early and critical step in any hypothesis-driven clinical research project. Because it is not yet a well-understood cognitive process, the need to improve the process goes unrecognized. Without an impactful hypothesis, the significance of any research project can be questionable, regardless of the rigor or diligence applied in other steps of the study, e.g., study design, data collection, and result analysis. In this perspective article, the authors provide a literature review on the following topics first: scientific thinking, reasoning, medical reasoning, literature-based discovery, and a field study to explore scientific thinking and discovery. Over the years, scientific thinking has shown excellent progress in cognitive science and its applied areas: education, medicine, and biomedical research. However, a review of the literature reveals the lack of original studies on hypothesis generation in clinical research. The authors then summarize their first human participant study exploring data-driven hypothesis generation by clinical researchers in a simulated setting. The results indicate that a secondary data analytical tool, VIADS-a visual interactive analytic tool for filtering, summarizing, and visualizing large health data sets coded with hierarchical terminologies, can shorten the time participants need, on average, to generate a hypothesis and also requires fewer cognitive events to generate each hypothesis. As a counterpoint, this exploration also indicates that the quality ratings of the hypotheses thus generated carry significantly lower ratings for feasibility when applying VIADS. Despite its small scale, the study confirmed the feasibility of conducting a human participant study directly to explore the hypothesis generation process in clinical research. This study provides supporting evidence to conduct a larger-scale study with a specifically designed tool to facilitate the hypothesis-generation process among inexperienced clinical researchers. A larger study could provide generalizable evidence, which in turn can potentially improve clinical research productivity and overall clinical research enterprise.
Keywords: Clinical research; data-driven hypothesis generation; medical informatics; scientific hypothesis generation; translational research; visualization.
PubMed Disclaimer
Conceptual model showing the relationships…
Conceptual model showing the relationships among scientific thinking, hypothesis generation, and their contributing…
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- StudySkills@Sheffield
- Research skills
- Research methods
How to manage your research data
When undertaking a dissertation or final project you may collect material to help you explore your research questions and which will inform your conclusions. This is your research data. Learn how to collect, store and manage this data in a secure and appropriate way.
Research Data Management
Research data is any material used to inform or support your research conclusions. It can take many different forms including recordings, transcripts, questionnaires, photographs and code. While a taught course research project may be relatively limited in time and scope, it is important to collect, store and manage data in a secure and appropriate way.
Research Data Management for postgraduate taught course students
- How to identify your research methods
- How to plan a dissertation or final year project
- Searching for information

Use your mySkills portfolio to discover your skillset, reflect on your development, and record your progress.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 12 September 2024
An open-source framework for end-to-end analysis of electronic health record data
- Lukas Heumos 1 , 2 , 3 ,
- Philipp Ehmele 1 ,
- Tim Treis 1 , 3 ,
- Julius Upmeier zu Belzen ORCID: orcid.org/0000-0002-0966-4458 4 ,
- Eljas Roellin 1 , 5 ,
- Lilly May 1 , 5 ,
- Altana Namsaraeva 1 , 6 ,
- Nastassya Horlava 1 , 3 ,
- Vladimir A. Shitov ORCID: orcid.org/0000-0002-1960-8812 1 , 3 ,
- Xinyue Zhang ORCID: orcid.org/0000-0003-4806-4049 1 ,
- Luke Zappia ORCID: orcid.org/0000-0001-7744-8565 1 , 5 ,
- Rainer Knoll 7 ,
- Niklas J. Lang 2 ,
- Leon Hetzel 1 , 5 ,
- Isaac Virshup 1 ,
- Lisa Sikkema ORCID: orcid.org/0000-0001-9686-6295 1 , 3 ,
- Fabiola Curion 1 , 5 ,
- Roland Eils 4 , 8 ,
- Herbert B. Schiller 2 , 9 ,
- Anne Hilgendorff 2 , 10 &
- Fabian J. Theis ORCID: orcid.org/0000-0002-2419-1943 1 , 3 , 5
Nature Medicine ( 2024 ) Cite this article
86 Altmetric
Metrics details
- Epidemiology
- Translational research
With progressive digitalization of healthcare systems worldwide, large-scale collection of electronic health records (EHRs) has become commonplace. However, an extensible framework for comprehensive exploratory analysis that accounts for data heterogeneity is missing. Here we introduce ehrapy, a modular open-source Python framework designed for exploratory analysis of heterogeneous epidemiology and EHR data. ehrapy incorporates a series of analytical steps, from data extraction and quality control to the generation of low-dimensional representations. Complemented by rich statistical modules, ehrapy facilitates associating patients with disease states, differential comparison between patient clusters, survival analysis, trajectory inference, causal inference and more. Leveraging ontologies, ehrapy further enables data sharing and training EHR deep learning models, paving the way for foundational models in biomedical research. We demonstrate ehrapy’s features in six distinct examples. We applied ehrapy to stratify patients affected by unspecified pneumonia into finer-grained phenotypes. Furthermore, we reveal biomarkers for significant differences in survival among these groups. Additionally, we quantify medication-class effects of pneumonia medications on length of stay. We further leveraged ehrapy to analyze cardiovascular risks across different data modalities. We reconstructed disease state trajectories in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) based on imaging data. Finally, we conducted a case study to demonstrate how ehrapy can detect and mitigate biases in EHR data. ehrapy, thus, provides a framework that we envision will standardize analysis pipelines on EHR data and serve as a cornerstone for the community.
Similar content being viewed by others

Data-driven identification of heart failure disease states and progression pathways using electronic health records
EHR foundation models improve robustness in the presence of temporal distribution shift
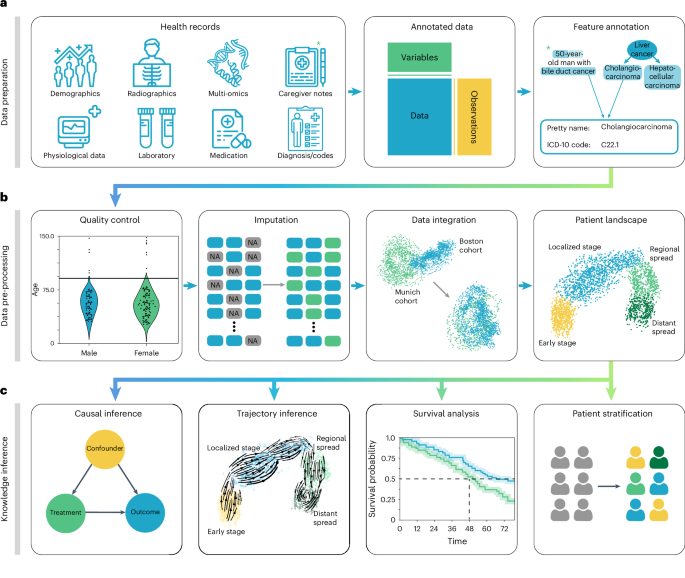
Harnessing EHR data for health research
Electronic health records (EHRs) are becoming increasingly common due to standardized data collection 1 and digitalization in healthcare institutions. EHRs collected at medical care sites serve as efficient storage and sharing units of health information 2 , enabling the informed treatment of individuals using the patient’s complete history 3 . Routinely collected EHR data are approaching genomic-scale size and complexity 4 , posing challenges in extracting information without quantitative analysis methods. The application of such approaches to EHR databases 1 , 5 , 6 , 7 , 8 , 9 has enabled the prediction and classification of diseases 10 , 11 , study of population health 12 , determination of optimal treatment policies 13 , 14 , simulation of clinical trials 15 and stratification of patients 16 .
However, current EHR datasets suffer from serious limitations, such as data collection issues, inconsistencies and lack of data diversity. EHR data collection and sharing problems often arise due to non-standardized formats, with disparate systems using exchange protocols, such as Health Level Seven International (HL7) and Fast Healthcare Interoperability Resources (FHIR) 17 . In addition, EHR data are stored in various on-disk formats, including, but not limited to, relational databases and CSV, XML and JSON formats. These variations pose challenges with respect to data retrieval, scalability, interoperability and data sharing.
Beyond format variability, inherent biases of the collected data can compromise the validity of findings. Selection bias stemming from non-representative sample composition can lead to skewed inferences about disease prevalence or treatment efficacy 18 , 19 . Filtering bias arises through inconsistent criteria for data inclusion, obscuring true variable relationships 20 . Surveillance bias exaggerates associations between exposure and outcomes due to differential monitoring frequencies 21 . EHR data are further prone to missing data 22 , 23 , which can be broadly classified into three categories: missing completely at random (MCAR), where missingness is unrelated to the data; missing at random (MAR), where missingness depends on observed data; and missing not at random (MNAR), where missingness depends on unobserved data 22 , 23 . Information and coding biases, related to inaccuracies in data recording or coding inconsistencies, respectively, can lead to misclassification and unreliable research conclusions 24 , 25 . Data may even contradict itself, such as when measurements were reported for deceased patients 26 , 27 . Technical variation and differing data collection standards lead to distribution differences and inconsistencies in representation and semantics across EHR datasets 28 , 29 . Attrition and confounding biases, resulting from differential patient dropout rates or unaccounted external variable effects, can significantly skew study outcomes 30 , 31 , 32 . The diversity of EHR data that comprise demographics, laboratory results, vital signs, diagnoses, medications, x-rays, written notes and even omics measurements amplifies all the aforementioned issues.
Addressing these challenges requires rigorous study design, careful data pre-processing and continuous bias evaluation through exploratory data analysis. Several EHR data pre-processing and analysis workflows were previously developed 4 , 33 , 34 , 35 , 36 , 37 , but none of them enables the analysis of heterogeneous data, provides in-depth documentation, is available as a software package or allows for exploratory visual analysis. Current EHR analysis pipelines, therefore, differ considerably in their approaches and are often commercial, vendor-specific solutions 38 . This is in contrast to strategies using community standards for the analysis of omics data, such as Bioconductor 39 or scverse 40 . As a result, EHR data frequently remain underexplored and are commonly investigated only for a particular research question 41 . Even in such cases, EHR data are then frequently input into machine learning models with serious data quality issues that greatly impact prediction performance and generalizability 42 .
To address this lack of analysis tooling, we developed the EHR Analysis in Python framework, ehrapy, which enables exploratory analysis of diverse EHR datasets. The ehrapy package is purpose-built to organize, analyze, visualize and statistically compare complex EHR data. ehrapy can be applied to datasets of different data types, sizes, diseases and origins. To demonstrate this versatility, we applied ehrapy to datasets obtained from EHR and population-based studies. Using the Pediatric Intensive Care (PIC) EHR database 43 , we stratified patients diagnosed with ‘unspecified pneumonia’ into distinct clinically relevant groups, extracted clinical indicators of pneumonia through statistical analysis and quantified medication-class effects on length of stay (LOS) with causal inference. Using the UK Biobank 44 (UKB), a population-scale cohort comprising over 500,000 participants from the United Kingdom, we employed ehrapy to explore cardiovascular risk factors using clinical predictors, metabolomics, genomics and retinal imaging-derived features. Additionally, we performed image analysis to project disease progression through fate mapping in patients affected by coronavirus disease 2019 (COVID-19) using chest x-rays. Finally, we demonstrate how exploratory analysis with ehrapy unveils and mitigates biases in over 100,000 visits by patients with diabetes across 130 US hospitals. We provide online links to additional use cases that demonstrate ehrapy’s usage with further datasets, including MIMIC-II (ref. 45 ), and for various medical conditions, such as patients subject to indwelling arterial catheter usage. ehrapy is compatible with any EHR dataset that can be transformed into vectors and is accessible as a user-friendly open-source software package hosted at https://github.com/theislab/ehrapy and installable from PyPI. It comes with comprehensive documentation, tutorials and further examples, all available at https://ehrapy.readthedocs.io .
ehrapy: a framework for exploratory EHR data analysis
The foundation of ehrapy is a robust and scalable data storage backend that is combined with a series of pre-processing and analysis modules. In ehrapy, EHR data are organized as a data matrix where observations are individual patient visits (or patients, in the absence of follow-up visits), and variables represent all measured quantities ( Methods ). These data matrices are stored together with metadata of observations and variables. By leveraging the AnnData (annotated data) data structure that implements this design, ehrapy builds upon established standards and is compatible with analysis and visualization functions provided by the omics scverse 40 ecosystem. Readers are also available in R, Julia and Javascript 46 . We additionally provide a dataset module with more than 20 public loadable EHR datasets in AnnData format to kickstart analysis and development with ehrapy.
For standardized analysis of EHR data, it is crucial that these data are encoded and stored in consistent, reusable formats. Thus, ehrapy requires that input data are organized in structured vectors. Readers for common formats, such as CSV, OMOP 47 or SQL databases, are available in ehrapy. Data loaded into AnnData objects can be mapped against several hierarchical ontologies 48 , 49 , 50 , 51 ( Methods ). Clinical keywords of free text notes can be automatically extracted ( Methods ).
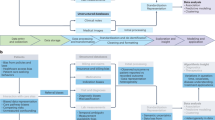
Powered by scanpy, which scales to millions of observations 52 ( Methods and Supplementary Table 1 ) and the machine learning library scikit-learn 53 , ehrapy provides more than 100 composable analysis functions organized in modules from which custom analysis pipelines can be built. Each function directly interacts with the AnnData object and adds all intermediate results for simple access and reuse of information to it. To facilitate setting up these pipelines, ehrapy guides analysts through a general analysis pipeline (Fig. 1 ). At any step of an analysis pipeline, community software packages can be integrated without any vendor lock-in. Because ehrapy is built on open standards, it can be purposefully extended to solve new challenges, such as the development of foundational models ( Methods ).
a , Heterogeneous health data are first loaded into memory as an AnnData object with patient visits as observational rows and variables as columns. Next, the data can be mapped against ontologies, and key terms are extracted from free text notes. b , The EHR data are subject to quality control where low-quality or spurious measurements are removed or imputed. Subsequently, numerical data are normalized, and categorical data are encoded. Data from different sources with data distribution shifts are integrated, embedded, clustered and annotated in a patient landscape. c , Further downstream analyses depend on the question of interest and can include the inference of causal effects and trajectories, survival analysis or patient stratification.
In the ehrapy analysis pipeline, EHR data are initially inspected for quality issues by analyzing feature distributions that may skew results and by detecting visits and features with high missing rates that ehrapy can then impute ( Methods ). ehrapy tracks all filtering steps while keeping track of population dynamics to highlight potential selection and filtering biases ( Methods ). Subsequently, ehrapy’s normalization and encoding functions ( Methods ) are applied to achieve a uniform numerical representation that facilitates data integration and corrects for dataset shift effects ( Methods ). Calculated lower-dimensional representations can subsequently be visualized, clustered and annotated to obtain a patient landscape ( Methods ). Such annotated groups of patients can be used for statistical comparisons to find differences in features among them to ultimately learn markers of patient states.
As analysis goals can differ between users and datasets, the ehrapy analysis pipeline is customizable during the final knowledge inference step. ehrapy provides statistical methods for group comparison and extensive support for survival analysis ( Methods ), enabling the discovery of biomarkers. Furthermore, ehrapy offers functions for causal inference to go from statistically determined associations to causal relations ( Methods ). Moreover, patient visits in aggregated EHR data can be regarded as snapshots where individual measurements taken at specific timepoints might not adequately reflect the underlying progression of disease and result from unrelated variation due to, for example, day-to-day differences 54 , 55 , 56 . Therefore, disease progression models should rely on analysis of the underlying clinical data, as disease progression in an individual patient may not be monotonous in time. ehrapy allows for the use of advanced trajectory inference methods to overcome sparse measurements 57 , 58 , 59 . We show that this approach can order snapshots to calculate a pseudotime that can adequately reflect the progression of the underlying clinical process. Given a sufficient number of snapshots, ehrapy increases the potential to understand disease progression, which is likely not robustly captured within a single EHR but, rather, across several.
ehrapy enables patient stratification in pneumonia cases
To demonstrate ehrapy’s capability to analyze heterogeneous datasets from a broad patient set across multiple care units, we applied our exploratory strategy to the PIC 43 database. The PIC database is a single-center database hosting information on children admitted to critical care units at the Children’s Hospital of Zhejiang University School of Medicine in China. It contains 13,499 distinct hospital admissions of 12,881 individual pediatric patients admitted between 2010 and 2018 for whom demographics, diagnoses, doctors’ notes, vital signs, laboratory and microbiology tests, medications, fluid balances and more were collected (Extended Data Figs. 1 and 2a and Methods ). After missing data imputation and subsequent pre-processing (Extended Data Figs. 2b,c and 3 and Methods ), we generated a uniform manifold approximation and projection (UMAP) embedding to visualize variation across all patients using ehrapy (Fig. 2a ). This visualization of the low-dimensional patient manifold shows the heterogeneity of the collected data in the PIC database, with malformations, perinatal and respiratory being the most abundant International Classification of Diseases (ICD) chapters (Fig. 2b ). The most common respiratory disease categories (Fig. 2c ) were labeled pneumonia and influenza ( n = 984). We focused on pneumonia to apply ehrapy to a challenging, broad-spectrum disease that affects all age groups. Pneumonia is a prevalent respiratory infection that poses a substantial burden on public health 60 and is characterized by inflammation of the alveoli and distal airways 60 . Individuals with pre-existing chronic conditions are particularly vulnerable, as are children under the age of 5 (ref. 61 ). Pneumonia can be caused by a range of microorganisms, encompassing bacteria, respiratory viruses and fungi.
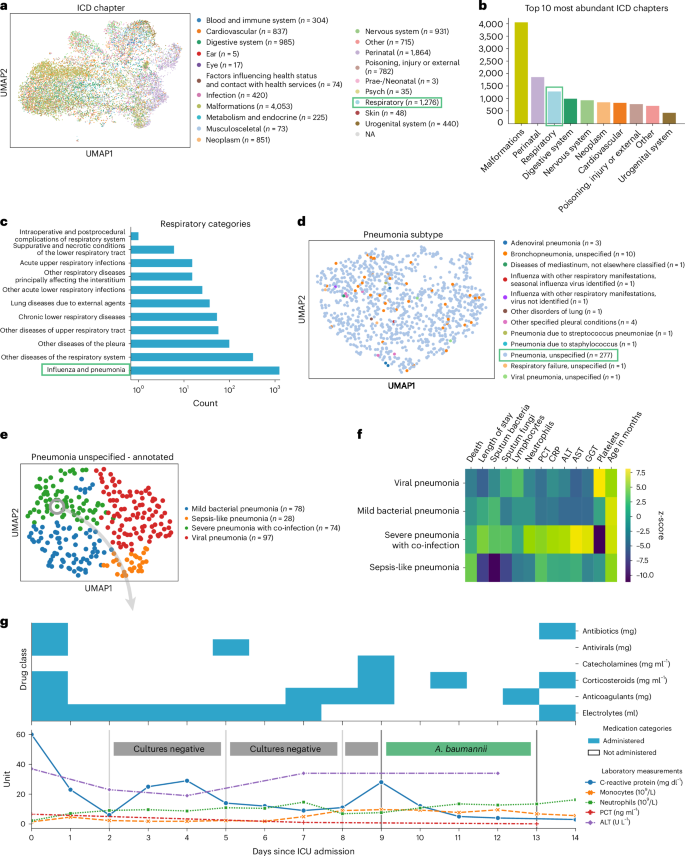
a , UMAP of all patient visits in the ICU with primary discharge diagnosis grouped by ICD chapter. b , The prevalence of respiratory diseases prompted us to investigate them further. c , Respiratory categories show the abundance of influenza and pneumonia diagnoses that we investigated more closely. d , We observed the ‘unspecified pneumonia’ subgroup, which led us to investigate and annotate it in more detail. e , The previously ‘unspecified pneumonia’-labeled patients were annotated using several clinical features (Extended Data Fig. 5 ), of which the most important ones are shown in the heatmap ( f ). g , Example disease progression of an individual child with pneumonia illustrating pharmacotherapy over time until positive A. baumannii swab.
We selected the age group ‘youths’ (13 months to 18 years of age) for further analysis, addressing a total of 265 patients who dominated the pneumonia cases and were diagnosed with ‘unspecified pneumonia’ (Fig. 2d and Extended Data Fig. 4 ). Neonates (0–28 d old) and infants (29 d to 12 months old) were excluded from the analysis as the disease context is significantly different in these age groups due to distinct anatomical and physical conditions. Patients were 61% male, had a total of 277 admissions, had a mean age at admission of 54 months (median, 38 months) and had an average LOS of 15 d (median, 7 d). Of these, 152 patients were admitted to the pediatric intensive care unit (PICU), 118 to the general ICU (GICU), four to the surgical ICU (SICU) and three to the cardiac ICU (CICU). Laboratory measurements typically had 12–14% missing data, except for serum procalcitonin (PCT), a marker for bacterial infections, with 24.5% missing, and C-reactive protein (CRP), a marker of inflammation, with 16.8% missing. Measurements assigned as ‘vital signs’ contained between 44% and 54% missing values. Stratifying patients with unspecified pneumonia further enables a more nuanced understanding of the disease, potentially facilitating tailored approaches to treatment.
To deepen clinical phenotyping for the disease group ‘unspecified pneumonia’, we calculated a k -nearest neighbor graph to cluster patients into groups and visualize these in UMAP space ( Methods ). Leiden clustering 62 identified four patient groupings with distinct clinical features that we annotated (Fig. 2e ). To identify the laboratory values, medications and pathogens that were most characteristic for these four groups (Fig. 2f ), we applied t -tests for numerical data and g -tests for categorical data between the identified groups using ehrapy (Extended Data Fig. 5 and Methods ). Based on this analysis, we identified patient groups with ‘sepsis-like, ‘severe pneumonia with co-infection’, ‘viral pneumonia’ and ‘mild pneumonia’ phenotypes. The ‘sepsis-like’ group of patients ( n = 28) was characterized by rapid disease progression as exemplified by an increased number of deaths (adjusted P ≤ 5.04 × 10 −3 , 43% ( n = 28), 95% confidence interval (CI): 23%, 62%); indication of multiple organ failure, such as elevated creatinine (adjusted P ≤ 0.01, 52.74 ± 23.71 μmol L −1 ) or reduced albumin levels (adjusted P ≤ 2.89 × 10 −4 , 33.40 ± 6.78 g L −1 ); and increased expression levels and peaks of inflammation markers, including PCT (adjusted P ≤ 3.01 × 10 −2 , 1.42 ± 2.03 ng ml −1 ), whole blood cell count, neutrophils, lymphocytes, monocytes and lower platelet counts (adjusted P ≤ 6.3 × 10 −2 , 159.30 ± 142.00 × 10 9 per liter) and changes in electrolyte levels—that is, lower potassium levels (adjusted P ≤ 0.09 × 10 −2 , 3.14 ± 0.54 mmol L −1 ). Patients whom we associated with the term ‘severe pneumonia with co-infection’ ( n = 74) were characterized by prolonged ICU stays (adjusted P ≤ 3.59 × 10 −4 , 15.01 ± 29.24 d); organ affection, such as higher levels of creatinine (adjusted P ≤ 1.10 × 10 −4 , 52.74 ± 23.71 μmol L −1 ) and lower platelet count (adjusted P ≤ 5.40 × 10 −23 , 159.30 ± 142.00 × 10 9 per liter); increased inflammation markers, such as peaks of PCT (adjusted P ≤ 5.06 × 10 −5 , 1.42 ± 2.03 ng ml −1 ), CRP (adjusted P ≤ 1.40 × 10 −6 , 50.60 ± 37.58 mg L −1 ) and neutrophils (adjusted P ≤ 8.51 × 10 −6 , 13.01 ± 6.98 × 10 9 per liter); detection of bacteria in combination with additional pathogen fungals in sputum samples (adjusted P ≤ 1.67 × 10 −2 , 26% ( n = 74), 95% CI: 16%, 36%); and increased application of medication, including antifungals (adjusted P ≤ 1.30 × 10 −4 , 15% ( n = 74), 95% CI: 7%, 23%) and catecholamines (adjusted P ≤ 2.0 × 10 −2 , 45% ( n = 74), 95% CI: 33%, 56%). Patients in the ‘mild pneumonia’ group were characterized by positive sputum cultures in the presence of relatively lower inflammation markers, such as PCT (adjusted P ≤ 1.63 × 10 −3 , 1.42 ± 2.03 ng ml −1 ) and CRP (adjusted P ≤ 0.03 × 10 −1 , 50.60 ± 37.58 mg L −1 ), while receiving antibiotics more frequently (adjusted P ≤ 1.00 × 10 −5 , 80% ( n = 78), 95% CI: 70%, 89%) and additional medications (electrolytes, blood thinners and circulation-supporting medications) (adjusted P ≤ 1.00 × 10 −5 , 82% ( n = 78), 95% CI: 73%, 91%). Finally, patients in the ‘viral pneumonia’ group were characterized by shorter LOSs (adjusted P ≤ 8.00 × 10 −6 , 15.01 ± 29.24 d), a lack of non-viral pathogen detection in combination with higher lymphocyte counts (adjusted P ≤ 0.01, 4.11 ± 2.49 × 10 9 per liter), lower levels of PCT (adjusted P ≤ 0.03 × 10 −2 , 1.42 ± 2.03 ng ml −1 ) and reduced application of catecholamines (adjusted P ≤ 5.96 × 10 −7 , 15% (n = 97), 95% CI: 8%, 23%), antibiotics (adjusted P ≤ 8.53 × 10 −6 , 41% ( n = 97), 95% CI: 31%, 51%) and antifungals (adjusted P ≤ 5.96 × 10 −7 , 0% ( n = 97), 95% CI: 0%, 0%).
To demonstrate the ability of ehrapy to examine EHR data from different levels of resolution, we additionally reconstructed a case from the ‘severe pneumonia with co-infection’ group (Fig. 2g ). In this case, the analysis revealed that CRP levels remained elevated despite broad-spectrum antibiotic treatment until a positive Acinetobacter baumannii result led to a change in medication and a subsequent decrease in CRP and monocyte levels.
ehrapy facilitates extraction of pneumonia indicators
ehrapy’s survival analysis module allowed us to identify clinical indicators of disease stages that could be used as biomarkers through Kaplan–Meier analysis. We found strong variance in overall aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT) and bilirubin levels (Fig. 3a ), including changes over time (Extended Data Fig. 6a,b ), in all four ‘unspecified pneumonia’ groups. Routinely used to assess liver function, studies provide evidence that AST, ALT and GGT levels are elevated during respiratory infections 63 , including severe pneumonia 64 , and can guide diagnosis and management of pneumonia in children 63 . We confirmed reduced survival in more severely affected children (‘sepsis-like pneumonia’ and ‘severe pneumonia with co-infection’) using Kaplan–Meier curves and a multivariate log-rank test (Fig. 3b ; P ≤ 1.09 × 10 −18 ) through ehrapy. To verify the association of this trajectory with altered AST, ALT and GGT expression levels, we further grouped all patients based on liver enzyme reference ranges ( Methods and Supplementary Table 2 ). By Kaplan–Meier survival analysis, cases with peaks of GGT ( P ≤ 1.4 × 10 −2 , 58.01 ± 2.03 U L −1 ), ALT ( P ≤ 2.9 × 10 −2 , 43.59 ± 38.02 U L −1 ) and AST ( P ≤ 4.8 × 10 −4 , 78.69 ± 60.03 U L −1 ) in ‘outside the norm’ were found to correlate with lower survival in all groups (Fig. 3c and Extended Data Fig. 6 ), in line with previous studies 63 , 65 . Bilirubin was not found to significantly affect survival ( P ≤ 2.1 × 10 −1 , 12.57 ± 21.22 mg dl −1 ).
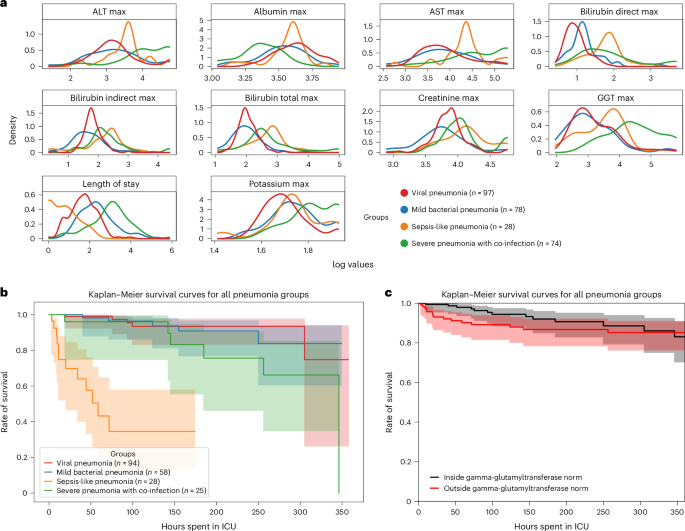
a , Line plots of major hepatic system laboratory measurements per group show variance in the measurements per pneumonia group. b , Kaplan–Meier survival curves demonstrate lower survival for ‘sepsis-like’ and ‘severe pneumonia with co-infection’ groups. c , Kaplan–Meier survival curves for children with GGT measurements outside the norm range display lower survival.
ehrapy quantifies medication class effect on LOS
Pneumonia requires case-specific medications due to its diverse causes. To demonstrate the potential of ehrapy’s causal inference module, we quantified the effect of medication on ICU LOS to evaluate case-specific administration of medication. In contrast to causal discovery that attempts to find a causal graph reflecting the causal relationships, causal inference is a statistical process used to investigate possible effects when altering a provided system, as represented by a causal graph and observational data (Fig. 4a ) 66 . This approach allows identifying and quantifying the impact of specific interventions or treatments on outcome measures, thereby providing insight for evidence-based decision-making in healthcare. Causal inference relies on datasets incorporating interventions to accurately quantify effects.
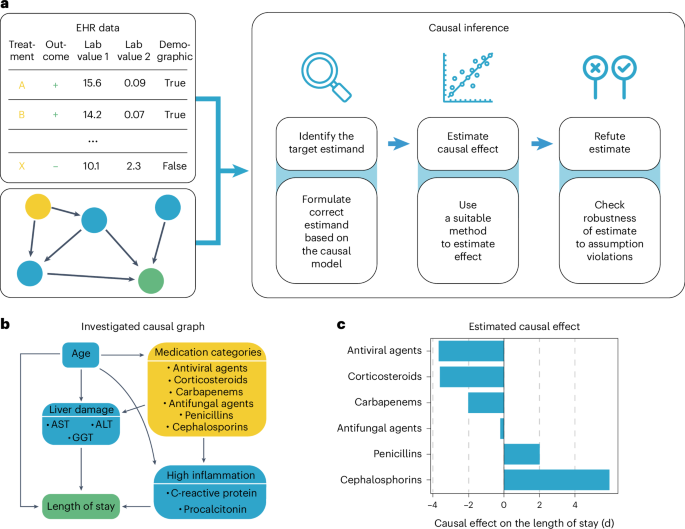
a , ehrapy’s causal module is based on the strategy of the tool ‘dowhy’. Here, EHR data containing treatment, outcome and measurements and a causal graph serve as input for causal effect quantification. The process includes the identification of the target estimand based on the causal graph, the estimation of causal effects using various models and, finally, refutation where sensitivity analyses and refutation tests are performed to assess the robustness of the results and assumptions. b , Curated causal graph using age, liver damage and inflammation markers as disease progression proxies together with medications as interventions to assess the causal effect on length of ICU stay. c , Determined causal effect strength on LOS in days of administered medication categories.
We manually constructed a minimal causal graph with ehrapy (Fig. 4b ) on records of treatment with corticosteroids, carbapenems, penicillins, cephalosporins and antifungal and antiviral medications as interventions (Extended Data Fig. 7 and Methods ). We assumed that the medications affect disease progression proxies, such as inflammation markers and markers of organ function. The selection of ‘interventions’ is consistent with current treatment standards for bacterial pneumonia and respiratory distress 67 , 68 . Based on the approach of the tool ‘dowhy’ 69 (Fig. 4a ), ehrapy’s causal module identified the application of corticosteroids, antivirals and carbapenems to be associated with shorter LOSs, in line with current evidence 61 , 70 , 71 , 72 . In contrast, penicillins and cephalosporins were associated with longer LOSs, whereas antifungal medication did not strongly influence LOS (Fig. 4c ).
ehrapy enables deriving population-scale risk factors
To illustrate the advantages of using a unified data management and quality control framework, such as ehrapy, we modeled myocardial infarction risk using Cox proportional hazards models on UKB 44 data. Large population cohort studies, such as the UKB, enable the investigation of common diseases across a wide range of modalities, including genomics, metabolomics, proteomics, imaging data and common clinical variables (Fig. 5a,b ). From these, we used a publicly available polygenic risk score for coronary heart disease 73 comprising 6.6 million variants, 80 nuclear magnetic resonance (NMR) spectroscopy-based metabolomics 74 features, 81 features derived from retinal optical coherence tomography 75 , 76 and the Framingham Risk Score 77 feature set, which includes known clinical predictors, such as age, sex, body mass index, blood pressure, smoking behavior and cholesterol levels. We excluded features with more than 10% missingness and imputed the remaining missing values ( Methods ). Furthermore, individuals with events up to 1 year after the sampling time were excluded from the analyses, ultimately selecting 29,216 individuals for whom all mentioned data types were available (Extended Data Figs. 8 and 9 and Methods ). Myocardial infarction, as defined by our mapping to the phecode nomenclature 51 , was defined as the endpoint (Fig. 5c ). We modeled the risk for myocardial infarction 1 year after either the metabolomic sample was obtained or imaging was performed.
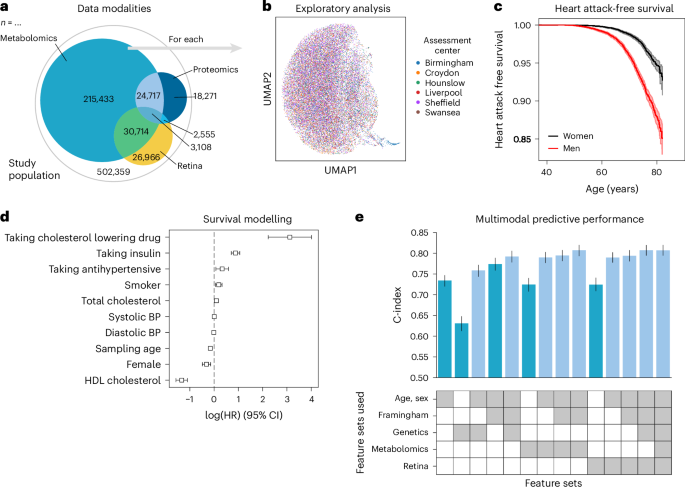
a , The UKB includes 502,359 participants from 22 assessment centers. Most participants have genetic data (97%) and physical measurement data (93%), but fewer have data for complex measures, such as metabolomics, retinal imaging or proteomics. b , We found a distinct cluster of individuals (bottom right) from the Birmingham assessment center in the retinal imaging data, which is an artifact of the image acquisition process and was, thus, excluded. c , Myocardial infarctions are recorded for 15% of the male and 7% of the female study population. Kaplan–Meier estimators with 95% CIs are shown. d , For every modality combination, a linear Cox proportional hazards model was fit to determine the prognostic potential of these for myocardial infarction. Cardiovascular risk factors show expected positive log hazard ratios (log (HRs)) for increased blood pressure or total cholesterol and negative ones for sampling age and systolic blood pressure (BP). log (HRs) with 95% CIs are shown. e , Combining all features yields a C-index of 0.81. c – e , Error bars indicate 95% CIs ( n = 29,216).
Predictive performance for each modality was assessed by fitting Cox proportional hazards (Fig. 5c ) models on each of the feature sets using ehrapy (Fig. 5d ). The age of the first occurrence served as the time to event; alternatively, date of death or date of the last record in the EHR served as censoring times. Models were evaluated using the concordance index (C-index) ( Methods ). The combination of multiple modalities successfully improved the predictive performance for coronary heart disease by increasing the C-index from 0.63 (genetic) to 0.76 (genetics, age and sex) and to 0.77 (clinical predictors) with 0.81 (imaging and clinical predictors) for combinations of feature sets (Fig. 5e ). Our finding is in line with previous observations of complementary effects between different modalities, where a broader ‘major adverse cardiac event’ phenotype was modeled in the UKB achieving a C-index of 0.72 (ref. 78 ). Adding genetic data improves predictive potential, as it is independent of sampling age and has limited prediction of other modalities 79 . The addition of metabolomic data did not improve predictive power (Fig. 5e ).
Imaging-based disease severity projection via fate mapping
To demonstrate ehrapy’s ability to handle diverse image data and recover disease stages, we embedded pulmonary imaging data obtained from patients with COVID-19 into a lower-dimensional space and computationally inferred disease progression trajectories using pseudotemporal ordering. This describes a continuous trajectory or ordering of individual points based on feature similarity 80 . Continuous trajectories enable mapping the fate of new patients onto precise states to potentially predict their future condition.
In COVID-19, a highly contagious respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), symptoms range from mild flu-like symptoms to severe respiratory distress. Chest x-rays typically show opacities (bilateral patchy, ground glass) associated with disease severity 81 .
We used COVID-19 chest x-ray images from the BrixIA 82 dataset consisting of 192 images (Fig. 6a ) with expert annotations of disease severity. We used the BrixIA database scores, which are based on six regions annotated by radiologists, to classify disease severity ( Methods ). We embedded raw image features using a pre-trained DenseNet model ( Methods ) and further processed this embedding into a nearest-neighbors-based UMAP space using ehrapy (Fig. 6b and Methods ). Fate mapping based on imaging information ( Methods ) determined a severity ordering from mild to critical cases (Fig. 6b–d ). Images labeled as ‘normal’ are projected to stay within the healthy group, illustrating the robustness of our approach. Images of diseased patients were ordered by disease severity, highlighting clear trajectories from ‘normal’ to ‘critical’ states despite the heterogeneity of the x-ray images stemming from, for example, different zoom levels (Fig. 6a ).
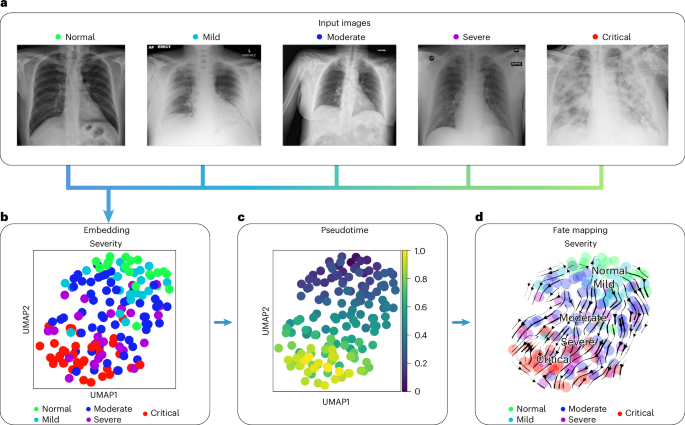
a , Randomly selected chest x-ray images from the BrixIA dataset demonstrate its variance. b , UMAP visualization of the BrixIA dataset embedding shows a separation of disease severity classes. c , Calculated pseudotime for all images increases with distance to the ‘normal’ images. d , Stream projection of fate mapping in UMAP space showcases disease severity trajectory of the COVID-19 chest x-ray images.
Detecting and mitigating biases in EHR data with ehrapy
To showcase how exploratory analysis using ehrapy can reveal and mitigate biases, we analyzed the Fairlearn 83 version of the Diabetes 130-US Hospitals 84 dataset. The dataset covers 10 years (1999–2008) of clinical records from 130 US hospitals, detailing 47 features of diabetes diagnoses, laboratory tests, medications and additional data from up to 14 d of inpatient care of 101,766 diagnosed patient visits ( Methods ). It was originally collected to explore the link between the measurement of hemoglobin A1c (HbA1c) and early readmission.
The cohort primarily consists of White and African American individuals, with only a minority of cases from Asian or Hispanic backgrounds (Extended Data Fig. 10a ). ehrapy’s cohort tracker unveiled selection and surveillance biases when filtering for Medicare recipients for further analysis, resulting in a shift of age distribution toward an age of over 60 years in addition to an increasing ratio of White participants. Using ehrapy’s visualization modules, our analysis showed that HbA1c was measured in only 18.4% of inpatients, with a higher frequency in emergency admissions compared to referral cases (Extended Data Fig. 10b ). Normalization biases can skew data relationships when standardization techniques ignore subgroup variability or assume incorrect distributions. The choice of normalization strategy must be carefully considered to avoid obscuring important factors. When normalizing the number of applied medications individually, differences in distributions between age groups remained. However, when normalizing both distributions jointly with age group as an additional group variable, differences between age groups were masked (Extended Data Fig. 10c ). To investigate missing data and imputation biases, we introduced missingness for the number of applied medications according to an MCAR mechanism, which we verified using ehrapy’s Little’s test ( P ≤ 0.01 × 10 −2 ), and an MAR mechanism ( Methods ). Whereas imputing the mean in the MCAR case did not affect the overall location of the distribution, it led to an underestimation of the variance, with the standard deviation dropping from 8.1 in the original data to 6.8 in the imputed data (Extended Data Fig. 10d ). Mean imputation in the MAR case skewed both location and variance of the mean from 16.02 to 14.66, with a standard deviation of only 5.72 (Extended Data Fig. 10d ). Using ehrapy’s multiple imputation based MissForest 85 imputation on the MAR data resulted in a mean of 16.04 and a standard deviation of 6.45. To predict patient readmission in fewer than 30 d, we merged the three smallest race groups, ‘Asian’, ‘Hispanic’ and ‘Other’. Furthermore, we dropped the gender group ‘Unknown/Invalid’ owing to the small sample size making meaningful assessment impossible, and we performed balanced random undersampling, resulting in 5,677 cases from each condition. We observed an overall balanced accuracy of 0.59 using a logistic regression model. However, the false-negative rate was highest for the races ‘Other’ and ‘Unknown’, whereas their selection rate was lowest, and this model was, therefore, biased (Extended Data Fig. 10e ). Using ehrapy’s compatibility with existing machine learning packages, we used Fairlearn’s ThresholdOptimizer ( Methods ), which improved the selection rates for ‘Other’ from 0.32 to 0.38 and for ‘Unknown’ from 0.23 to 0.42 and the false-negative rates for ‘Other’ from 0.48 to 0.42 and for ‘Unknown’ from 0.61 to 0.45 (Extended Data Fig. 10e ).
Clustering offers a hypothesis-free alternative to supervised classification when clear hypotheses or labels are missing. It has enabled the identification of heart failure subtypes 86 and progression pathways 87 and COVID-19 severity states 88 . This concept, which is central to ehrapy, further allowed us to identify fine-grained groups of ‘unspecified pneumonia’ cases in the PIC dataset while discovering biomarkers and quantifying effects of medications on LOS. Such retroactive characterization showcases ehrapy’s ability to put complex evidence into context. This approach supports feedback loops to improve diagnostic and therapeutic strategies, leading to more efficiently allocated resources in healthcare.
ehrapy’s flexible data structures enabled us to integrate the heterogeneous UKB data for predictive performance in myocardial infarction. The different data types and distributions posed a challenge for predictive models that were overcome with ehrapy’s pre-processing modules. Our analysis underscores the potential of combining phenotypic and health data at population scale through ehrapy to enhance risk prediction.
By adapting pseudotime approaches that are commonly used in other omics domains, we successfully recovered disease trajectories from raw imaging data with ehrapy. The determined pseudotime, however, only orders data but does not necessarily provide a future projection per patient. Understanding the driver features for fate mapping in image-based datasets is challenging. The incorporation of image segmentation approaches could mitigate this issue and provide a deeper insight into the spatial and temporal dynamics of disease-related processes.
Limitations of our analyses include the lack of control for informative missingness where the absence of information represents information in itself 89 . Translation from Chinese to English in the PIC database can cause information loss and inaccuracies because the Chinese ICD-10 codes are seven characters long compared to the five-character English codes. Incompleteness of databases, such as the lack of radiology images in the PIC database, low sample sizes, underrepresentation of non-White ancestries and participant self-selection, cannot be accounted for and limit generalizability. This restricts deeper phenotyping of, for example, all ‘unspecified pneumonia’ cases with respect to their survival, which could be overcome by the use of multiple databases. Our causal inference use case is limited by unrecorded variables, such as Sequential Organ Failure Assessment (SOFA) scores, and pneumonia-related pathogens that are missing in the causal graph due to dataset constraints, such as high sparsity and substantial missing data, which risk overfitting and can lead to overinterpretation. We counterbalanced this by employing several refutation methods that statistically reject the causal hypothesis, such as a placebo treatment, a random common cause or an unobserved common cause. The longer hospital stays associated with penicillins and cephalosporins may be dataset specific and stem from higher antibiotic resistance, their use as first-line treatments, more severe initial cases, comorbidities and hospital-specific protocols.
Most analysis steps can introduce algorithmic biases where results are misleading or unfavorably affect specific groups. This is particularly relevant in the context of missing data 22 where determining the type of missing data is necessary to handle it correctly. ehrapy includes an implementation of Little’s test 90 , which tests whether data are distributed MCAR to discern missing data types. For MCAR data single-imputation approaches, such as mean, median or mode, imputation can suffice, but these methods are known to reduce variability 91 , 92 . Multiple imputation strategies, such as Multiple Imputation by Chained Equations (MICE) 93 and MissForest 85 , as implemented in ehrapy, are effective for both MCAR and MAR data 22 , 94 , 95 . MNAR data require pattern-mixture or shared-parameter models that explicitly incorporate the mechanism by which data are missing 96 . Because MNAR involves unobserved data, the assumptions about the missingness mechanism cannot be directly verified, making sensitivity analysis crucial 21 . ehrapy’s wide range of normalization functions and grouping functionality enables to account for intrinsic variability within subgroups, and its compatibility with Fairlearn 83 can potentially mitigate predictor biases. Generally, we recommend to assess all pre-processing in an iterative manner with respect to downstream applications, such as patient stratification. Moreover, sensitivity analysis can help verify the robustness of all inferred knowledge 97 .
These diverse use cases illustrate ehrapy’s potential to sufficiently address the need for a computationally efficient, extendable, reproducible and easy-to-use framework. ehrapy is compatible with major standards, such as Observational Medical Outcomes Partnership (OMOP), Common Data Model (CDM) 47 , HL7, FHIR or openEHR, with flexible support for common tabular data formats. Once loaded into an AnnData object, subsequent sharing of analysis results is made easy because AnnData objects can be stored and read platform independently. ehrapy’s rich documentation of the application programming interface (API) and extensive hands-on tutorials make EHR analysis accessible to both novices and experienced analysts.
As ehrapy remains under active development, users can expect ehrapy to continuously evolve. We are improving support for the joint analysis of EHR, genetics and molecular data where ehrapy serves as a bridge between the EHR and the omics communities. We further anticipate the generation of EHR-specific reference datasets, so-called atlases 98 , to enable query-to-reference mapping where new datasets get contextualized by transferring annotations from the reference to the new dataset. To promote the sharing and collective analysis of EHR data, we envision adapted versions of interactive single-cell data explorers, such as CELLxGENE 99 or the UCSC Cell Browser 100 , for EHR data. Such web interfaces would also include disparity dashboards 20 to unveil trends of preferential outcomes for distinct patient groups. Additional modules specifically for high-frequency time-series data, natural language processing and other data types are currently under development. With the widespread availability of code-generating large language models, frameworks such as ehrapy are becoming accessible to medical professionals without coding expertise who can leverage its analytical power directly. Therefore, ehrapy, together with a lively ecosystem of packages, has the potential to enhance the scientific discovery pipeline to shape the era of EHR analysis.
All datasets that were used during the development of ehrapy and the use cases were used according to their terms of use as indicated by each provider.
Design and implementation of ehrapy
A unified pipeline as provided by our ehrapy framework streamlines the analysis of EHR data by providing an efficient, standardized approach, which reduces the complexity and variability in data pre-processing and analysis. This consistency ensures reproducibility of results and facilitates collaboration and sharing within the research community. Additionally, the modular structure allows for easy extension and customization, enabling researchers to adapt the pipeline to their specific needs while building on a solid foundational framework.
ehrapy was designed from the ground up as an open-source effort with community support. The package, as well as all associated tutorials and dataset preparation scripts, are open source. Development takes place publicly on GitHub where the developers discuss feature requests and issues directly with users. This tight interaction between both groups ensures that we implement the most pressing needs to cater the most important use cases and can guide users when difficulties arise. The open-source nature, extensive documentation and modular structure of ehrapy are designed for other developers to build upon and extend ehrapy’s functionality where necessary. This allows us to focus ehrapy on the most important features to keep the number of dependencies to a minimum.
ehrapy was implemented in the Python programming language and builds upon numerous existing numerical and scientific open-source libraries, specifically matplotlib 101 , seaborn 102 , NumPy 103 , numba 104 , Scipy 105 , scikit-learn 53 and Pandas 106 . Although taking considerable advantage of all packages implemented, ehrapy also shares the limitations of these libraries, such as a lack of GPU support or small performance losses due to the translation layer cost for operations between the Python interpreter and the lower-level C language for matrix operations. However, by building on very widely used open-source software, we ensure seamless integration and compatibility with a broad range of tools and platforms to promote community contributions. Additionally, by doing so, we enhance security by allowing a larger pool of developers to identify and address vulnerabilities 107 . All functions are grouped into task-specific modules whose implementation is complemented with additional dependencies.

Data preparation
Dataloaders.
ehrapy is compatible with any type of vectorized data, where vectorized refers to the data being stored in structured tables in either on-disk or database form. The input and output module of ehrapy provides readers for common formats, such as OMOP, CSV tables or SQL databases through Pandas. When reading in such datasets, the data are stored in the appropriate slots in a new AnnData 46 object. ehrapy’s data module provides access to more than 20 public EHR datasets that feature diseases, including, but not limited to, Parkinson’s disease, breast cancer, chronic kidney disease and more. All dataloaders return AnnData objects to allow for immediate analysis.
AnnData for EHR data
Our framework required a versatile data structure capable of handling various matrix formats, including Numpy 103 for general use cases and interoperability, Scipy 105 sparse matrices for efficient storage, Dask 108 matrices for larger-than-memory analysis and Awkward array 109 for irregular time-series data. We needed a single data structure that not only stores data but also includes comprehensive annotations for thorough contextual analysis. It was essential for this structure to be widely used and supported, which ensures robustness and continual updates. Interoperability with other analytical packages was a key criterion to facilitate seamless integration within existing tools and workflows. Finally, the data structure had to support both in-memory operations and on-disk storage using formats such as HDF5 (ref. 110 ) and Zarr 111 , ensuring efficient handling and accessibility of large datasets and the ability to easily share them with collaborators.
All of these requirements are fulfilled by the AnnData format, which is a popular data structure in single-cell genomics. At its core, an AnnData object encapsulates diverse components, providing a holistic representation of data and metadata that are always aligned in dimensions and easily accessible. A data matrix (commonly referred to as ‘ X ’) stands as the foundational element, embodying the measured data. This matrix can be dense (as Numpy array), sparse (as Scipy sparse matrix) or ragged (as Awkward array) where dimensions do not align within the data matrix. The AnnData object can feature several such data matrices stored in ‘layers’. Examples of such layers can be unnormalized or unencoded data. These data matrices are complemented by an observations (commonly referred to as ‘obs’) segment where annotations on the level of patients or visits are stored. Patients’ age or sex, for instance, are often used as such annotations. The variables (commonly referred to as ‘var’) section complements the observations, offering supplementary details about the features in the dataset, such as missing data rates. The observation-specific matrices (commonly referred to as ‘obsm’) section extends the capabilities of the AnnData structure by allowing the incorporation of observation-specific matrices. These matrices can represent various types of information at the individual cell level, such as principal component analysis (PCA) results, t-distributed stochastic neighbor embedding (t-SNE) coordinates or other dimensionality reduction outputs. Analogously, AnnData features a variables-specific variables (commonly referred to as ‘varm’) component. The observation-specific pairwise relationships (commonly referred to as ‘obsp’) segment complements the ‘obsm’ section by accommodating observation-specific pairwise relationships. This can include connectivity matrices, indicating relationships between patients. The inclusion of an unstructured annotations (commonly referred to as ‘uns’) component further enhances flexibility. This segment accommodates unstructured annotations or arbitrary data that might not conform to the structured observations or variables categories. Any AnnData object can be stored on disk in h5ad or Zarr format to facilitate data exchange.
ehrapy natively interfaces with the scientific Python ecosystem via Pandas 112 and Numpy 103 . The development of deep learning models for EHR data 113 is further accelerated through compatibility with pathml 114 , a unified framework for whole-slide image analysis in pathology, and scvi-tools 115 , which provides data loaders for loading tensors from AnnData objects into PyTorch 116 or Jax arrays 117 to facilitate the development of generalizing foundational models for medical artificial intelligence 118 .
Feature annotation
After AnnData creation, any metadata can be mapped against ontologies using Bionty ( https://github.com/laminlabs/bionty-base ). Bionty provides access to the Human Phenotype, Phecodes, Phenotype and Trait, Drug, Mondo and Human Disease ontologies.
Key medical terms stored in an AnnData object in free text can be extracted using the Medical Concept Annotation Toolkit (MedCAT) 119 .
Data processing
Cohort tracking.
ehrapy provides a CohortTracker tool that traces all filtering steps applied to an associated AnnData object. To calculate cohort summary statistics, the implementation makes use of tableone 120 and can subsequently be plotted as bar charts together with flow diagrams 121 that visualize the order and reasoning of filtering operations.
Basic pre-processing and quality control
ehrapy encompasses a suite of functionalities for fundamental data processing that are adopted from scanpy 52 but adapted to EHR data:
Regress out: To address unwanted sources of variation, a regression procedure is integrated, enhancing the dataset’s robustness.
Subsample: Selects a specified fraction of observations.
Balanced sample: Balances groups in the dataset by random oversampling or undersampling.
Highly variable features: The identification and annotation of highly variable features following the ‘highly variable genes’ function of scanpy is seamlessly incorporated, providing users with insights into pivotal elements influencing the dataset.
To identify and minimize quality issues, ehrapy provides several quality control functions:
Basic quality control: Determines the relative and absolute number of missing values per feature and per patient.
Winsorization: For data refinement, ehrapy implements a winsorization process, creating a version of the input array less susceptible to extreme values.
Feature clipping: Imposes limits on features to enhance dataset reliability.
Detect biases: Computes pairwise correlations between features, standardized mean differences for numeric features between groups of sensitive features, categorical feature value count differences between groups of sensitive features and feature importances when predicting a target variable.
Little’s MCAR test: Applies Little’s MCAR test whose null hypothesis is that data are MCAR. Rejecting the null hypothesis may not always mean that data are not MCAR, nor is accepting the null hypothesis a guarantee that data are MCAR. For more details, see Schouten et al. 122 .
Summarize features: Calculates statistical indicators per feature, including minimum, maximum and average values. This can be especially useful to reduce complex data with multiple measurements per feature per patient into sets of columns with single values.
Imputation is crucial in data analysis to address missing values, ensuring the completeness of datasets that can be required for specific algorithms. The ‘ehrapy’ pre-processing module offers a range of imputation techniques:
Explicit Impute: Replaces missing values, in either all columns or a user-specified subset, with a designated replacement value.
Simple Impute: Imputes missing values in numerical data using mean, median or the most frequent value, contributing to a more complete dataset.
KNN Impute: Uses k -nearest neighbor imputation to fill in missing values in the input AnnData object, preserving local data patterns.
MissForest Impute: Implements the MissForest strategy for imputing missing data, providing a robust approach for handling complex datasets.
MICE Impute: Applies the MICE algorithm for imputing data. This implementation is based on the miceforest ( https://github.com/AnotherSamWilson/miceforest ) package.
Data encoding can be required if categoricals are a part of the dataset to obtain numerical values only. Most algorithms in ehrapy are compatible only with numerical values. ehrapy offers two encoding algorithms based on scikit-learn 53 :
One-Hot Encoding: Transforms categorical variables into binary vectors, creating a binary feature for each category and capturing the presence or absence of each category in a concise representation.
Label Encoding: Assigns a unique numerical label to each category, facilitating the representation of categorical data as ordinal values and supporting algorithms that require numerical input.
To ensure that the distributions of the heterogeneous data are aligned, ehrapy offers several normalization procedures:
Log Normalization: Applies the natural logarithm function to the data, useful for handling skewed distributions and reducing the impact of outliers.
Max-Abs Normalization: Scales each feature by its maximum absolute value, ensuring that the maximum absolute value for each feature is 1.
Min-Max Normalization: Transforms the data to a specific range (commonly (0, 1)) by scaling each feature based on its minimum and maximum values.
Power Transformation Normalization: Applies a power transformation to make the data more Gaussian like, often useful for stabilizing variance and improving the performance of models sensitive to distributional assumptions.
Quantile Normalization: Aligns the distributions of multiple variables, ensuring that their quantiles match, which can be beneficial for comparing datasets or removing batch effects.
Robust Scaling Normalization: Scales data using the interquartile range, making it robust to outliers and suitable for datasets with extreme values.
Scaling Normalization: Standardizes data by subtracting the mean and dividing by the standard deviation, creating a distribution with a mean of 0 and a standard deviation of 1.
Offset to Positive Values: Shifts all values by a constant offset to make all values non-negative, with the lowest negative value becoming 0.
Dataset shifts can be corrected using the scanpy implementation of the ComBat 123 algorithm, which employs a parametric and non-parametric empirical Bayes framework for adjusting data for batch effects that is robust to outliers.
Finally, a neighbors graph can be efficiently computed using scanpy’s implementation.
To obtain meaningful lower-dimensional embeddings that can subsequently be visualized and reused for downstream algorithms, ehrapy provides the following algorithms based on scanpy’s implementation:
t-SNE: Uses a probabilistic approach to embed high-dimensional data into a lower-dimensional space, emphasizing the preservation of local similarities and revealing clusters in the data.
UMAP: Embeds data points by modeling their local neighborhood relationships, offering an efficient and scalable technique that captures both global and local structures in high-dimensional data.
Force-Directed Graph Drawing: Uses a physical simulation to position nodes in a graph, with edges representing pairwise relationships, creating a visually meaningful representation that emphasizes connectedness and clustering in the data.
Diffusion Maps: Applies spectral methods to capture the intrinsic geometry of high-dimensional data by modeling diffusion processes, providing a way to uncover underlying structures and patterns.
Density Calculation in Embedding: Quantifies the density of observations within an embedding, considering conditions or groups, offering insights into the concentration of data points in different regions and aiding in the identification of densely populated areas.
ehrapy further provides algorithms for clustering and trajectory inference based on scanpy:
Leiden Clustering: Uses the Leiden algorithm to cluster observations into groups, revealing distinct communities within the dataset with an emphasis on intra-cluster cohesion.
Hierarchical Clustering Dendrogram: Constructs a dendrogram through hierarchical clustering based on specified group by categories, illustrating the hierarchical relationships among observations and facilitating the exploration of structured patterns.
Feature ranking
ehrapy provides two ways of ranking feature contributions to clusters and target variables:
Statistical tests: To compare any obtained clusters to obtain marker features that are significantly different between the groups, ehrapy extends scanpy’s ‘rank genes groups’. The original implementation, which features a t -test for numerical data, is complemented by a g -test for categorical data.
Feature importance: Calculates feature rankings for a target variable using linear regression, support vector machine or random forest models from scikit-learn. ehrapy evaluates the relative importance of each predictor by fitting the model and extracting model-specific metrics, such as coefficients or feature importances.
Dataset integration
Based on scanpy’s ‘ingest’ function, ehrapy facilitates the integration of labels and embeddings from a well-annotated reference dataset into a new dataset, enabling the mapping of cluster annotations and spatial relationships for consistent comparative analysis. This process ensures harmonized clinical interpretations across datasets, especially useful when dealing with multiple experimental diseases or batches.
Knowledge inference
Survival analysis.
ehrapy’s implementation of survival analysis algorithms is based on lifelines 124 :
Ordinary Least Squares (OLS) Model: Creates a linear regression model using OLS from a specified formula and an AnnData object, allowing for the analysis of relationships between variables and observations.
Generalized Linear Model (GLM): Constructs a GLM from a given formula, distribution and AnnData, providing a versatile framework for modeling relationships with nonlinear data structures.
Kaplan–Meier: Fits the Kaplan–Meier curve to generate survival curves, offering a visual representation of the probability of survival over time in a dataset.
Cox Hazard Model: Constructs a Cox proportional hazards model using a specified formula and an AnnData object, enabling the analysis of survival data by modeling the hazard rates and their relationship to predictor variables.
Log-Rank Test: Calculates the P value for the log-rank test, comparing the survival functions of two groups, providing statistical significance for differences in survival distributions.
GLM Comparison: Given two fit GLMs, where the larger encompasses the parameter space of the smaller, this function returns the P value, indicating the significance of the larger model and adding explanatory power beyond the smaller model.
Trajectory inference
Trajectory inference is a computational approach that reconstructs and models the developmental paths and transitions within heterogeneous clinical data, providing insights into the temporal progression underlying complex systems. ehrapy offers several inbuilt algorithms for trajectory inference based on scanpy:
Diffusion Pseudotime: Infers the progression of observations by measuring geodesic distance along the graph, providing a pseudotime metric that represents the developmental trajectory within the dataset.
Partition-based Graph Abstraction (PAGA): Maps out the coarse-grained connectivity structures of complex manifolds using a partition-based approach, offering a comprehensive visualization of relationships in high-dimensional data and aiding in the identification of macroscopic connectivity patterns.
Because ehrapy is compatible with scverse, further trajectory inference-based algorithms, such as CellRank, can be seamlessly applied.
Causal inference
ehrapy’s causal inference module is based on ‘dowhy’ 69 . It is based on four key steps that are all implemented in ehrapy:
Graphical Model Specification: Define a causal graphical model representing relationships between variables and potential causal effects.
Causal Effect Identification: Automatically identify whether a causal effect can be inferred from the given data, addressing confounding and selection bias.
Causal Effect Estimation: Employ automated tools to estimate causal effects, using methods such as matching, instrumental variables or regression.
Sensitivity Analysis and Testing: Perform sensitivity analysis to assess the robustness of causal inferences and conduct statistical testing to determine the significance of the estimated causal effects.
Patient stratification
ehrapy’s complete pipeline from pre-processing to the generation of lower-dimensional embeddings, clustering, statistical comparison between determined groups and more facilitates the stratification of patients.
Visualization
ehrapy features an extensive visualization pipeline that is customizable and yet offers reasonable defaults. Almost every analysis function is matched with at least one visualization function that often shares the name but is available through the plotting module. For example, after importing ehrapy as ‘ep’, ‘ep.tl.umap(adata)’ runs the UMAP algorithm on an AnnData object, and ‘ep.pl.umap(adata)’ would then plot a scatter plot of the UMAP embedding.
ehrapy further offers a suite of more generally usable and modifiable plots:
Scatter Plot: Visualizes data points along observation or variable axes, offering insights into the distribution and relationships between individual data points.
Heatmap: Represents feature values in a grid, providing a comprehensive overview of the data’s structure and patterns.
Dot Plot: Displays count values of specified variables as dots, offering a clear depiction of the distribution of counts for each variable.
Filled Line Plot: Illustrates trends in data with filled lines, emphasizing variations in values over a specified axis.
Violin Plot: Presents the distribution of data through mirrored density plots, offering a concise view of the data’s spread.
Stacked Violin Plot: Combines multiple violin plots, stacked to allow for visual comparison of distributions across categories.
Group Mean Heatmap: Creates a heatmap displaying the mean count per group for each specified variable, providing insights into group-wise trends.
Hierarchically Clustered Heatmap: Uses hierarchical clustering to arrange data in a heatmap, revealing relationships and patterns among variables and observations.
Rankings Plot: Visualizes rankings within the data, offering a clear representation of the order and magnitude of values.
Dendrogram Plot: Plots a dendrogram of categories defined in a group by operation, illustrating hierarchical relationships within the dataset.
Benchmarking ehrapy
We generated a subset of the UKB data selecting 261 features and 488,170 patient visits. We removed all features with missingness rates greater than 70%. To demonstrate speed and memory consumption for various scenarios, we subsampled the data to 20%, 30% and 50%. We ran a minimal ehrapy analysis pipeline on each of those subsets and the full data, including the calculation of quality control metrics, filtering of variables by a missingness threshold, nearest neighbor imputation, normalization, dimensionality reduction and clustering (Supplementary Table 1 ). We conducted our benchmark on a single CPU with eight threads and 60 GB of maximum memory.
ehrapy further provides out-of-core implementations using Dask 108 for many algorithms in ehrapy, such as our normalization functions or our PCA implementation. Out-of-core computation refers to techniques that process data that do not fit entirely in memory, using disk storage to manage data overflow. This approach is crucial for handling large datasets without being constrained by system memory limits. Because the principal components get reused for other computationally expensive algorithms, such as the neighbors graph calculation, it effectively enables the analysis of very large datasets. We are currently working on supporting out-of-core computation for all computationally expensive algorithms in ehrapy.
We demonstrate the memory benefits in a hosted tutorial where the in-memory pipeline for 50,000 patients with 1,000 features required about 2 GB of memory, and the corresponding out-of-core implementation required less than 200 MB of memory.
The code for benchmarking is available at https://github.com/theislab/ehrapy-reproducibility . The implementation of ehrapy is accessible at https://github.com/theislab/ehrapy together with extensive API documentation and tutorials at https://ehrapy.readthedocs.io .
PIC database analysis
Study design.
We collected clinical data from the PIC 43 version 1.1.0 database. PIC is a single-center, bilingual (English and Chinese) database hosting information of children admitted to critical care units at the Children’s Hospital of Zhejiang University School of Medicine in China. The requirement for individual patient consent was waived because the study did not impact clinical care, and all protected health information was de-identified. The database contains 13,499 distinct hospital admissions of 12,881 distinct pediatric patients. These patients were admitted to five ICU units with 119 total critical care beds—GICU, PICU, SICU, CICU and NICU—between 2010 and 2018. The mean age of the patients was 2.5 years, of whom 42.5% were female. The in-hospital mortality was 7.1%; the mean hospital stay was 17.6 d; the mean ICU stay was 9.3 d; and 468 (3.6%) patients were admitted multiple times. Demographics, diagnoses, doctors’ notes, laboratory and microbiology tests, prescriptions, fluid balances, vital signs and radiographics reports were collected from all patients. For more details, see the original publication of Zeng et al. 43 .
Study participants
Individuals older than 18 years were excluded from the study. We grouped the data into three distinct groups: ‘neonates’ (0–28 d of age; 2,968 patients), ‘infants’ (1–12 months of age; 4,876 patients) and ‘youths’ (13 months to 18 years of age; 6,097 patients). We primarily analyzed the ‘youths’ group with the discharge diagnosis ‘unspecified pneumonia’ (277 patients).
Data collection
The collected clinical data included demographics, laboratory and vital sign measurements, diagnoses, microbiology and medication information and mortality outcomes. The five-character English ICD-10 codes were used, whose values are based on the seven-character Chinese ICD-10 codes.
Dataset extraction and analysis
We downloaded the PIC database of version 1.1.0 from Physionet 1 to obtain 17 CSV tables. Using Pandas, we selected all information with more than 50% coverage rate, including demographics and laboratory and vital sign measurements (Fig. 2 ). To reduce the amount of noise, we calculated and added only the minimum, maximum and average of all measurements that had multiple values per patient. Examination reports were removed because they describe only diagnostics and not detailed findings. All further diagnoses and microbiology and medication information were included into the observations slot to ensure that the data were not used for the calculation of embeddings but were still available for the analysis. This ensured that any calculated embedding would not be divided into treated and untreated groups but, rather, solely based on phenotypic features. We imputed all missing data through k -nearest neighbors imputation ( k = 20) using the knn_impute function of ehrapy. Next, we log normalized the data with ehrapy using the log_norm function. Afterwards, we winsorized the data using ehrapy’s winsorize function to obtain 277 ICU visits ( n = 265 patients) with 572 features. Of those 572 features, 254 were stored in the matrix X and the remaining 318 in the ‘obs’ slot in the AnnData object. For clustering and visualization purposes, we calculated 50 principal components using ehrapy’s pca function. The obtained principal component representation was then used to calculate a nearest neighbors graph using the neighbors function of ehrapy. The nearest neighbors graph then served as the basis for a UMAP embedding calculation using ehrapy’s umap function.
We applied the community detection algorithm Leiden with resolution 0.6 on the nearest neighbor graph using ehrapy’s leiden function. The four obtained clusters served as input for two-sided t -tests for all numerical values and two-sided g -tests for all categorical values for all four clusters against the union of all three other clusters, respectively. This was conducted using ehrapy’s rank_feature_groups function, which also corrects P values for multiple testing with the Benjamini–Hochberg method 125 . We presented the four groups and the statistically significantly different features between the groups to two pediatricians who annotated the groups with labels.
Our determined groups can be confidently labeled owing to their distinct clinical profiles. Nevertheless, we could only take into account clinical features that were measured. Insightful features, such as lung function tests, are missing. Moreover, the feature representation of the time-series data is simplified, which can hide some nuances between the groups. Generally, deciding on a clustering resolution is difficult. However, more fine-grained clusters obtained via higher clustering resolutions may become too specific and not generalize well enough.
Kaplan–Meier survival analysis
We selected patients with up to 360 h of total stay for Kaplan–Meier survival analysis to ensure a sufficiently high number of participants. We proceeded with the AnnData object prepared as described in the ‘Patient stratification’ subsection to conduct Kaplan–Meier analysis among all four determined pneumonia groups using ehrapy’s kmf function. Significance was tested through ehrapy’s test_kmf_logrank function, which tests whether two Kaplan–Meier series are statistically significant, employing a chi-squared test statistic under the null hypothesis. Let h i (t) be the hazard ratio of group i at time t and c a constant that represents a proportional change in the hazard ratio between the two groups, then:
This implicitly uses the log-rank weights. An additional Kaplan–Meier analysis was conducted for all children jointly concerning the liver markers AST, ALT and GGT. To determine whether measurements were inside or outside the norm range, we used reference ranges (Supplementary Table 2 ). P values less than 0.05 were labeled significant.
Our Kaplan–Meier curve analysis depends on the groups being well defined and shares the same limitations as the patient stratification. Additionally, the analysis is sensitive to the reference table where we selected limits that generalize well for the age ranges, but, due to children of different ages being examined, they may not necessarily be perfectly accurate for all children.
Causal effect of mechanism of action on LOS
Although the dataset was not initially intended for investigating causal effects of interventions, we adapted it for this purpose by focusing on the LOS in the ICU, measured in months, as the outcome variable. This choice aligns with the clinical aim of stabilizing patients sufficiently for ICU discharge. We constructed a causal graph to explore how different drug administrations could potentially reduce the LOS. Based on consultations with clinicians, we included several biomarkers of liver damage (AST, ALT and GGT) and inflammation (CRP and PCT) in our model. Patient age was also considered a relevant variable.
Because several different medications act by the same mechanisms, we grouped specific medications by their drug classes This grouping was achieved by cross-referencing the drugs listed in the dataset with DrugBank release 5.1 (ref. 126 ), using Levenshtein distances for partial string matching. After manual verification, we extracted the corresponding DrugBank categories, counted the number of features per category and compiled a list of commonly prescribed medications, as advised by clinicians. This approach facilitated the modeling of the causal graph depicted in Fig. 4 , where an intervention is defined as the administration of at least one drug from a specified category.
Causal inference was then conducted with ehrapy’s ‘dowhy’ 69 -based causal inference module using the expert-curated causal graph. Medication groups were designated as causal interventions, and the LOS was the outcome of interest. Linear regression served as the estimation method for analyzing these causal effects. We excluded four patients from the analysis owing to their notably long hospital stays exceeding 90 d, which were deemed outliers. To validate the robustness of our causal estimates, we incorporated several refutation methods:
Placebo Treatment Refuter: This method involved replacing the treatment assignment with a placebo to test the effect of the treatment variable being null.
Random Common Cause: A randomly generated variable was added to the data to assess the sensitivity of the causal estimate to the inclusion of potential unmeasured confounders.
Data Subset Refuter: The stability of the causal estimate was tested across various random subsets of the data to ensure that the observed effects were not dependent on a specific subset.
Add Unobserved Common Cause: This approach tested the effect of an omitted variable by adding a theoretically relevant unobserved confounder to the model, evaluating how much an unmeasured variable could influence the causal relationship.
Dummy Outcome: Replaces the true outcome variable with a random variable. If the causal effect nullifies, it supports the validity of the original causal relationship, indicating that the outcome is not driven by random factors.
Bootstrap Validation: Employs bootstrapping to generate multiple samples from the dataset, testing the consistency of the causal effect across these samples.
The selection of these refuters addresses a broad spectrum of potential biases and model sensitivities, including unobserved confounders and data dependencies. This comprehensive approach ensures robust verification of the causal analysis. Each refuter provides an orthogonal perspective, targeting specific vulnerabilities in causal analysis, which strengthens the overall credibility of the findings.
UKB analysis
Study population.
We used information from the UKB cohort, which includes 502,164 study participants from the general UK population without enrichment for specific diseases. The study involved the enrollment of individuals between 2006 and 2010 across 22 different assessment centers throughout the United Kingdom. The tracking of participants is still ongoing. Within the UKB dataset, metabolomics, proteomics and retinal optical coherence tomography data are available for a subset of individuals without any enrichment for specific diseases. Additionally, EHRs, questionnaire responses and other physical measures are available for almost everyone in the study. Furthermore, a variety of genotype information is available for nearly the entire cohort, including whole-genome sequencing, whole-exome sequencing, genotyping array data as well as imputed genotypes from the genotyping array 44 . Because only the latter two are available for download, and are sufficient for polygenic risk score calculation as performed here, we used the imputed genotypes in the present study. Participants visited the assessment center up to four times for additional and repeat measurements and completed additional online follow-up questionnaires.
In the present study, we restricted the analyses to data obtained from the initial assessment, including the blood draw, for obtaining the metabolomics data and the retinal imaging as well as physical measures. This restricts the study population to 33,521 individuals for whom all of these modalities are available. We have a clear study start point for each individual with the date of their initial assessment center visit. The study population has a mean age of 57 years, is 54% female and is censored at age 69 years on average; 4.7% experienced an incident myocardial infarction; and 8.1% have prevalent type 2 diabetes. The study population comes from six of the 22 assessment centers due to the retinal imaging being performed only at those.
For the myocardial infarction endpoint definition, we relied on the first occurrence data available in the UKB, which compiles the first date that each diagnosis was recorded for a participant in a hospital in ICD-10 nomenclature. Subsequently, we mapped these data to phecodes and focused on phecode 404.1 for myocardial infarction.
The Framingham Risk Score was developed on data from 8,491 participants in the Framingham Heart Study to assess general cardiovascular risk 77 . It includes easily obtainable predictors and is, therefore, easily applicable in clinical practice, although newer and more specific risk scores exist and might be used more frequently. It includes age, sex, smoking behavior, blood pressure, total and low-density lipoprotein cholesterol as well as information on insulin, antihypertensive and cholesterol-lowering medications, all of which are routinely collected in the UKB and used in this study as the Framingham feature set.
The metabolomics data used in this study were obtained using proton NMR spectroscopy, a low-cost method with relatively low batch effects. It covers established clinical predictors, such as albumin and cholesterol, as well as a range of lipids, amino acids and carbohydrate-related metabolites.
The retinal optical coherence tomography–derived features were returned by researchers to the UKB 75 , 76 . They used the available scans and determined the macular volume, macular thickness, retinal pigment epithelium thickness, disc diameter, cup-to-disk ratio across different regions as well as the thickness between the inner nuclear layer and external limiting membrane, inner and outer photoreceptor segments and the retinal pigment epithelium across different regions. Furthermore, they determined a wide range of quality metrics for each scan, including the image quality score, minimum motion correlation and inner limiting membrane (ILM) indicator.
Data analysis
After exporting the data from the UKB, all timepoints were transformed into participant age entries. Only participants without prevalent myocardial infarction (relative to the first assessment center visit at which all data were collected) were included.
The data were pre-processed for retinal imaging and metabolomics subsets separately, to enable a clear analysis of missing data and allow for the k -nearest neighbors–based imputation ( k = 20) of missing values when less than 10% were missing for a given participant. Otherwise, participants were dropped from the analyses. The imputed genotypes and Framingham analyses were available for almost every participant and, therefore, not imputed. Individuals without them were, instead, dropped from the analyses. Because genetic risk modeling poses entirely different methodological and computational challenges, we applied a published polygenic risk score for coronary heart disease using 6.6 million variants 73 . This was computed using the plink2 score option on the imputed genotypes available in the UKB.
UMAP embeddings were computed using default parameters on the full feature sets with ehrapy’s umap function. For all analyses, the same time-to-event and event-indicator columns were used. The event indicator is a Boolean variable indicating whether a myocardial infarction was observed for a study participant. The time to event is defined as the timespan between the start of the study, in this case the date of the first assessment center visit. Otherwise, it is the timespan from the start of the study to the start of censoring; in this case, this is set to the last date for which EHRs were available, unless a participant died, in which case the date of death is the start of censoring. Kaplan–Meier curves and Cox proportional hazards models were fit using ehrapy’s survival analysis module and the lifelines 124 package’s Cox-PHFitter function with default parameters. For Cox proportional hazards models with multiple feature sets, individually imputed and quality-controlled feature sets were concatenated, and the model was fit on the resulting matrix. Models were evaluated using the C-index 127 as a metric. It can be seen as an extension of the common area under the receiver operator characteristic score to time-to-event datasets, in which events are not observed for every sample and which ranges from 0.0 (entirely false) over 0.5 (random) to 1.0 (entirely correct). CIs for the C-index were computed based on bootstrapping by sampling 1,000 times with replacement from all computed partial hazards and computing the C-index over each of these samples. The percentiles at 2.5% and 97.5% then give the upper and lower confidence bound for the 95% CIs.
In all UKB analyses, the unit of study for a statistical test or predictive model is always an individual study participant.
The generalizability of the analysis is limited as the UK Biobank cohort may not represent the general population, with potential selection biases and underrepresentation of the different demographic groups. Additionally, by restricting analysis to initial assessment data and censoring based on the last available EHR or date of death, our analysis does not account for longitudinal changes and can introduce follow-up bias, especially if participants lost to follow-up have different risk profiles.
In-depth quality control of retina-derived features
A UMAP plot of the retina-derived features indicating the assessment centers shows a cluster of samples that lie somewhat outside the general population and mostly attended the Birmingham assessment center (Fig. 5b ). To further investigate this, we performed Leiden clustering of resolution 0.3 (Extended Data Fig. 9a ) and isolated this group in cluster 5. When comparing cluster 5 to the rest of the population in the retina-derived feature space, we noticed that many individuals in cluster 5 showed overall retinal pigment epithelium (RPE) thickness measures substantially elevated over the rest of the population in both eyes (Extended Data Fig. 9b ), which is mostly a feature of this cluster (Extended Data Fig. 9c ). To investigate potential confounding, we computed ratios between cluster 5 and the rest of the population over the ‘obs’ DataFrame containing the Framingham features, diabetes-related phecodes and genetic principal components. Out of the top and bottom five highest ratios observed, six are in genetic principal components, which are commonly used to represent genetic ancestry in a continuous space (Extended Data Fig. 9d ). Additionally, diagnoses for type 1 and type 2 diabetes and antihypertensive use are enriched in cluster 5. Further investigating the ancestry, we computed log ratios for self-reported ancestries and absolute counts, which showed no robust enrichment and depletion effects.
A closer look at three quality control measures of the imaging pipeline revealed that cluster 5 was an outlier in terms of either image quality (Extended Data Fig. 9e ) or minimum motion correlation (Extended Data Fig. 9f ) and the ILM indicator (Extended Data Fig. 9g ), all of which can be indicative of artifacts in image acquisition and downstream processing 128 . Subsequently, we excluded 301 individuals from cluster 5 from all analyses.
COVID-19 chest-x-ray fate determination
Dataset overview.
We used the public BrixIA COVID-19 dataset, which contains 192 chest x-ray images annotated with BrixIA scores 82 . Hereby, six regions were annotated by a senior radiologist with more than 20 years of experience and a junior radiologist with a disease severity score ranging from 0 to 3. A global score was determined as the sum of all of these regions and, therefore, ranges from 0 to 18 (S-Global). S-Global scores of 0 were classified as normal. Images that only had severity values up to 1 in all six regions were classified as mild. Images with severity values greater than or equal to 2, but a S-Global score of less than 7, were classified as moderate. All images that contained at least one 3 in any of the six regions with a S-Global score between 7 and 10 were classified as severe, and all remaining images with S-Global scores greater than 10 with at least one 3 were labeled critical. The dataset and instructions to download the images can be found at https://github.com/ieee8023/covid-chestxray-dataset .
We first resized all images to 224 × 224. Afterwards, the images underwent a random affine transformation that involved rotation, translation and scaling. The rotation angle was randomly selected from a range of −45° to 45°. The images were also subject to horizontal and vertical translation, with the maximum translation being 15% of the image size in either direction. Additionally, the images were scaled by a factor ranging from 0.85 to 1.15. The purpose of applying these transformations was to enhance the dataset and introduce variations, ultimately improving the robustness and generalization of the model.
To generate embeddings, we used a pre-trained DenseNet model with weights densenet121-res224-all of TorchXRayVision 129 . A DenseNet is a convolutional neural network that makes use of dense connections between layers (Dense Blocks) where all layers (with matching feature map sizes) directly connect with each other. To maintain a feed-forward nature, every layer in the DenseNet architecture receives supplementary inputs from all preceding layers and transmits its own feature maps to all subsequent layers. The model was trained on the nih-pc- chex-mimic_ch-google-openi-rsna dataset 130 .
Next, we calculated 50 principal components on the feature representation of the DenseNet model of all images using ehrapy’s pca function. The principal component representation served as input for a nearest neighbors graph calculation using ehrapy’s neighbors function. This graph served as the basis for the calculation of a UMAP embedding with three components that was finally visualized using ehrapy.
We randomly picked a root in the group of images that was labeled ‘Normal’. First, we calculated so-called pseudotime by fitting a trajectory through the calculated UMAP space using diffusion maps as implemented in ehrapy’s dpt function 57 . Each image’s pseudotime value represents its estimated position along this trajectory, serving as a proxy for its severity stage relative to others in the dataset. To determine fates, we employed CellRank 58 , 59 with the PseudotimeKernel . This kernel computes transition probabilities for patient visits based on the connectivity of the k -nearest neighbors graph and the pseudotime values of patient visits, which resembles their progression through a process. Directionality is infused in the nearest neighbors graph in this process where the kernel either removes or downweights edges in the graph that contradict the directional flow of increasing pseudotime, thereby refining the graph to better reflect the developmental trajectory. We computed the transition matrix with a soft threshold scheme (Parameter of the PseudotimeKernel ), which downweights edges that point against the direction of increasing pseudotime. Finally, we calculated a projection on top of the UMAP embedding with CellRank using the plot_projection function of the PseudotimeKernel that we subsequently plotted.
This analysis is limited by the small dataset of 192 chest x-ray images, which may affect the model’s generalizability and robustness. Annotation subjectivity from radiologists can further introduce variability in severity scores. Additionally, the random selection of a root from ‘Normal’ images can introduce bias in pseudotime calculations and subsequent analyses.
Diabetes 130-US hospitals analysis
We used data from the Diabetes 130-US hospitals dataset that were collected between 1999 and 2008. It contains clinical care information at 130 hospitals and integrated delivery networks. The extracted database information pertains to hospital admissions specifically for patients diagnosed with diabetes. These encounters required a hospital stay ranging from 1 d to 14 d, during which both laboratory tests and medications were administered. The selection criteria focused exclusively on inpatient encounters with these defined characteristics. More specifically, we used a version that was curated by the Fairlearn team where the target variable ‘readmitted’ was binarized and a few features renamed or binned ( https://fairlearn.org/main/user_guide/datasets/diabetes_hospital_data.html ). The dataset contains 101,877 patient visits and 25 features. The dataset predominantly consists of White patients (74.8%), followed by African Americans (18.9%), with other racial groups, such as Hispanic, Asian and Unknown categories, comprising smaller percentages. Females make up a slight majority in the data at 53.8%, with males accounting for 46.2% and a negligible number of entries listed as unknown or invalid. A substantial majority of the patients are over 60 years of age (67.4%), whereas those aged 30–60 years represent 30.2%, and those 30 years or younger constitute just 2.5%.
All of the following descriptions start by loading the Fairlearn version of the Diabetes 130-US hospitals dataset using ehrapy’s dataloader as an AnnData object.
Selection and filtering bias
An overview of sensitive variables was generated using tableone. Subsequently, ehrapy’s CohortTracker was used to track the age, gender and race variables. The cohort was filtered for all Medicare recipients and subsequently plotted.
Surveillance bias
We plotted the HbA1c measurement ratios using ehrapy’s catplot .
Missing data and imputation bias
MCAR-type missing data for the number of medications variable (‘num_medications‘) were introduced by randomly setting 30% of the variables to be missing using Numpy’s choice function. We tested that the data are MCAR by applying ehrapy’s implementation of Little’s MCAR test, which returned a non-significant P value of 0.71. MAR data for the number of medications variable (‘num_medications‘) were introduced by scaling the ‘time_in_hospital’ variable to have a mean of 0 and a standard deviation of 1, adjusting these values by multiplying by 1.2 and subtracting 0.6 to influence overall missingness rate, and then using these values to generate MAR data in the ‘num_medications’ variable via a logistic transformation and binomial sampling. We verified that the newly introduced missing values are not MCAR with respect to the ‘time_in_hospital’ variable by applying ehrapy’s implementation of Little’s test, which was significant (0.01 × 10 −2 ). The missing data were imputed using ehrapy’s mean imputation and MissForest implementation.
Algorithmic bias
Variables ‘race’, ‘gender’, ‘age’, ‘readmitted’, ‘readmit_binary’ and ‘discharge_disposition_id’ were moved to the ‘obs’ slot of the AnnData object to ensure that they were not used for model training. We built a binary label ‘readmit_30_days’ indicating whether a patient had been readmitted in fewer than 30 d. Next, we combined the ‘Asian’ and ‘Hispanic’ categories into a single ‘Other’ category within the ‘race’ column of our AnnData object and then filtered out and discarded any samples labeled as ‘Unknown/Invalid’ under the ‘gender‘ column and subsequently moved the ‘gender’ data to the variable matrix X of the AnnData object. All categorical variables got encoded. The data were split into train and test groups with a test size of 50%. The data were scaled, and a logistic regression model was trained using scikit-learn, which was also used to determine the balanced accuracy score. Fairlearn’s MetricFrame function was used to inspect the target model performance against the sensitive variable ‘race’. We subsequently fit Fairlearn’s ThresholdOptimizer using the logistic regression estimator with balanced_accuracy_score as the target object. The algorithmic demonstration of Fairlearn’s abilities on this dataset is shown here: https://github.com/fairlearn/talks/tree/main/2021_scipy_tutorial .
Normalization bias
We one-hot encoded all categorical variables with ehrapy using the encode function. We applied ehrapy’s implementation of scaling normalization with and without the ‘Age group’ variable as group key to scale the data jointly and separately using ehrapy’s scale_norm function.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Physionet provides access to the PIC database 43 at https://physionet.org/content/picdb/1.1.0 for credentialed users. The BrixIA images 82 are available at https://github.com/BrixIA/Brixia-score-COVID-19 . The data used in this study were obtained from the UK Biobank 44 ( https://www.ukbiobank.ac.uk/ ). Access to the UK Biobank resource was granted under application number 49966. The data are available to researchers upon application to the UK Biobank in accordance with their data access policies and procedures. The Diabetes 130-US Hospitals dataset is available at https://archive.ics.uci.edu/dataset/296/diabetes+130-us+hospitals+for+years+1999-2008 .
Code availability
The ehrapy source code is available at https://github.com/theislab/ehrapy under an Apache 2.0 license. Further documentation, tutorials and examples are available at https://ehrapy.readthedocs.io . We are actively developing the software and invite contributions from the community.
Jupyter notebooks to reproduce our analysis and figures, including Conda environments that specify all versions, are available at https://github.com/theislab/ehrapy-reproducibility .
Goldberger, A. L. et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation 101 , E215–E220 (2000).
Article CAS PubMed Google Scholar
Atasoy, H., Greenwood, B. N. & McCullough, J. S. The digitization of patient care: a review of the effects of electronic health records on health care quality and utilization. Annu. Rev. Public Health 40 , 487–500 (2019).
Article PubMed Google Scholar
Jamoom, E. W., Patel, V., Furukawa, M. F. & King, J. EHR adopters vs. non-adopters: impacts of, barriers to, and federal initiatives for EHR adoption. Health (Amst.) 2 , 33–39 (2014).
Google Scholar
Rajkomar, A. et al. Scalable and accurate deep learning with electronic health records. NPJ Digit. Med. 1 , 18 (2018).
Article PubMed PubMed Central Google Scholar
Wolf, A. et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Aurum. Int. J. Epidemiol. 48 , 1740–1740g (2019).
Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12 , e1001779 (2015).
Pollard, T. J. et al. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci. Data 5 , 180178 (2018).
Johnson, A. E. W. et al. MIMIC-III, a freely accessible critical care database. Sci. Data 3 , 160035 (2016).
Article CAS PubMed PubMed Central Google Scholar
Hyland, S. L. et al. Early prediction of circulatory failure in the intensive care unit using machine learning. Nat. Med. 26 , 364–373 (2020).
Rasmy, L. et al. Recurrent neural network models (CovRNN) for predicting outcomes of patients with COVID-19 on admission to hospital: model development and validation using electronic health record data. Lancet Digit. Health 4 , e415–e425 (2022).
Marcus, J. L. et al. Use of electronic health record data and machine learning to identify candidates for HIV pre-exposure prophylaxis: a modelling study. Lancet HIV 6 , e688–e695 (2019).
Kruse, C. S., Stein, A., Thomas, H. & Kaur, H. The use of electronic health records to support population health: a systematic review of the literature. J. Med. Syst. 42 , 214 (2018).
Sheikh, A., Jha, A., Cresswell, K., Greaves, F. & Bates, D. W. Adoption of electronic health records in UK hospitals: lessons from the USA. Lancet 384 , 8–9 (2014).
Sheikh, A. et al. Health information technology and digital innovation for national learning health and care systems. Lancet Digit. Health 3 , e383–e396 (2021).
Cord, K. A. M., Mc Cord, K. A. & Hemkens, L. G. Using electronic health records for clinical trials: where do we stand and where can we go? Can. Med. Assoc. J. 191 , E128–E133 (2019).
Article Google Scholar
Landi, I. et al. Deep representation learning of electronic health records to unlock patient stratification at scale. NPJ Digit. Med. 3 , 96 (2020).
Ayaz, M., Pasha, M. F., Alzahrani, M. Y., Budiarto, R. & Stiawan, D. The Fast Health Interoperability Resources (FHIR) standard: systematic literature review of implementations, applications, challenges and opportunities. JMIR Med. Inform. 9 , e21929 (2021).
Peskoe, S. B. et al. Adjusting for selection bias due to missing data in electronic health records-based research. Stat. Methods Med. Res. 30 , 2221–2238 (2021).
Haneuse, S. & Daniels, M. A general framework for considering selection bias in EHR-based studies: what data are observed and why? EGEMS (Wash. DC) 4 , 1203 (2016).
PubMed Google Scholar
Gallifant, J. et al. Disparity dashboards: an evaluation of the literature and framework for health equity improvement. Lancet Digit. Health 5 , e831–e839 (2023).
Sauer, C. M. et al. Leveraging electronic health records for data science: common pitfalls and how to avoid them. Lancet Digit. Health 4 , e893–e898 (2022).
Li, J. et al. Imputation of missing values for electronic health record laboratory data. NPJ Digit. Med. 4 , 147 (2021).
Rubin, D. B. Inference and missing data. Biometrika 63 , 581 (1976).
Scheid, L. M., Brown, L. S., Clark, C. & Rosenfeld, C. R. Data electronically extracted from the electronic health record require validation. J. Perinatol. 39 , 468–474 (2019).
Phelan, M., Bhavsar, N. A. & Goldstein, B. A. Illustrating informed presence bias in electronic health records data: how patient interactions with a health system can impact inference. EGEMS (Wash. DC). 5 , 22 (2017).
PubMed PubMed Central Google Scholar
Secondary Analysis of Electronic Health Records (ed MIT Critical Data) (Springer, 2016).
Jetley, G. & Zhang, H. Electronic health records in IS research: quality issues, essential thresholds and remedial actions. Decis. Support Syst. 126 , 113137 (2019).
McCormack, J. P. & Holmes, D. T. Your results may vary: the imprecision of medical measurements. BMJ 368 , m149 (2020).
Hobbs, F. D. et al. Is the international normalised ratio (INR) reliable? A trial of comparative measurements in hospital laboratory and primary care settings. J. Clin. Pathol. 52 , 494–497 (1999).
Huguet, N. et al. Using electronic health records in longitudinal studies: estimating patient attrition. Med. Care 58 Suppl 6 Suppl 1 , S46–S52 (2020).
Zeng, J., Gensheimer, M. F., Rubin, D. L., Athey, S. & Shachter, R. D. Uncovering interpretable potential confounders in electronic medical records. Nat. Commun. 13 , 1014 (2022).
Getzen, E., Ungar, L., Mowery, D., Jiang, X. & Long, Q. Mining for equitable health: assessing the impact of missing data in electronic health records. J. Biomed. Inform. 139 , 104269 (2023).
Tang, S. et al. Democratizing EHR analyses with FIDDLE: a flexible data-driven preprocessing pipeline for structured clinical data. J. Am. Med. Inform. Assoc. 27 , 1921–1934 (2020).
Dagliati, A. et al. A process mining pipeline to characterize COVID-19 patients’ trajectories and identify relevant temporal phenotypes from EHR data. Front. Public Health 10 , 815674 (2022).
Sun, Y. & Zhou, Y.-H. A machine learning pipeline for mortality prediction in the ICU. Int. J. Digit. Health 2 , 3 (2022).
Article CAS Google Scholar
Mandyam, A., Yoo, E. C., Soules, J., Laudanski, K. & Engelhardt, B. E. COP-E-CAT: cleaning and organization pipeline for EHR computational and analytic tasks. In Proc. of the 12th ACM Conference on Bioinformatics, Computational Biology, and Health Informatics. https://doi.org/10.1145/3459930.3469536 (Association for Computing Machinery, 2021).
Gao, C. A. et al. A machine learning approach identifies unresolving secondary pneumonia as a contributor to mortality in patients with severe pneumonia, including COVID-19. J. Clin. Invest. 133 , e170682 (2023).
Makam, A. N. et al. The good, the bad and the early adopters: providers’ attitudes about a common, commercial EHR. J. Eval. Clin. Pract. 20 , 36–42 (2014).
Amezquita, R. A. et al. Orchestrating single-cell analysis with Bioconductor. Nat. Methods 17 , 137–145 (2020).
Virshup, I. et al. The scverse project provides a computational ecosystem for single-cell omics data analysis. Nat. Biotechnol. 41 , 604–606 (2023).
Zou, Q. et al. Predicting diabetes mellitus with machine learning techniques. Front. Genet. 9 , 515 (2018).
Cios, K. J. & William Moore, G. Uniqueness of medical data mining. Artif. Intell. Med. 26 , 1–24 (2002).
Zeng, X. et al. PIC, a paediatric-specific intensive care database. Sci. Data 7 , 14 (2020).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562 , 203–209 (2018).
Lee, J. et al. Open-access MIMIC-II database for intensive care research. Annu. Int. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011 , 8315–8318 (2011).
Virshup, I., Rybakov, S., Theis, F. J., Angerer, P. & Alexander Wolf, F. anndata: annotated data. Preprint at bioRxiv https://doi.org/10.1101/2021.12.16.473007 (2021).
Voss, E. A. et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J. Am. Med. Inform. Assoc. 22 , 553–564 (2015).
Vasilevsky, N. A. et al. Mondo: unifying diseases for the world, by the world. Preprint at medRxiv https://doi.org/10.1101/2022.04.13.22273750 (2022).
Harrison, J. E., Weber, S., Jakob, R. & Chute, C. G. ICD-11: an international classification of diseases for the twenty-first century. BMC Med. Inform. Decis. Mak. 21 , 206 (2021).
Köhler, S. et al. Expansion of the Human Phenotype Ontology (HPO) knowledge base and resources. Nucleic Acids Res. 47 , D1018–D1027 (2019).
Wu, P. et al. Mapping ICD-10 and ICD-10-CM codes to phecodes: workflow development and initial evaluation. JMIR Med. Inform. 7 , e14325 (2019).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19 , 15 (2018).
Pedregosa, F. et al. Scikit-learn: machine learning in Python. J. Mach. Learn. Res . 12 , 2825–2830 (2011).
de Haan-Rietdijk, S., de Haan-Rietdijk, S., Kuppens, P. & Hamaker, E. L. What’s in a day? A guide to decomposing the variance in intensive longitudinal data. Front. Psychol. 7 , 891 (2016).
Pedersen, E. S. L., Danquah, I. H., Petersen, C. B. & Tolstrup, J. S. Intra-individual variability in day-to-day and month-to-month measurements of physical activity and sedentary behaviour at work and in leisure-time among Danish adults. BMC Public Health 16 , 1222 (2016).
Roffey, D. M., Byrne, N. M. & Hills, A. P. Day-to-day variance in measurement of resting metabolic rate using ventilated-hood and mouthpiece & nose-clip indirect calorimetry systems. JPEN J. Parenter. Enter. Nutr. 30 , 426–432 (2006).
Haghverdi, L., Büttner, M., Wolf, F. A., Buettner, F. & Theis, F. J. Diffusion pseudotime robustly reconstructs lineage branching. Nat. Methods 13 , 845–848 (2016).
Lange, M. et al. CellRank for directed single-cell fate mapping. Nat. Methods 19 , 159–170 (2022).
Weiler, P., Lange, M., Klein, M., Pe'er, D. & Theis, F. CellRank 2: unified fate mapping in multiview single-cell data. Nat. Methods 21 , 1196–1205 (2024).
Zhang, S. et al. Cost of management of severe pneumonia in young children: systematic analysis. J. Glob. Health 6 , 010408 (2016).
Torres, A. et al. Pneumonia. Nat. Rev. Dis. Prim. 7 , 25 (2021).
Traag, V. A., Waltman, L. & van Eck, N. J. From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep. 9 , 5233 (2019).
Kamin, W. et al. Liver involvement in acute respiratory infections in children and adolescents—results of a non-interventional study. Front. Pediatr. 10 , 840008 (2022).
Shi, T. et al. Risk factors for mortality from severe community-acquired pneumonia in hospitalized children transferred to the pediatric intensive care unit. Pediatr. Neonatol. 61 , 577–583 (2020).
Dudnyk, V. & Pasik, V. Liver dysfunction in children with community-acquired pneumonia: the role of infectious and inflammatory markers. J. Educ. Health Sport 11 , 169–181 (2021).
Charpignon, M.-L. et al. Causal inference in medical records and complementary systems pharmacology for metformin drug repurposing towards dementia. Nat. Commun. 13 , 7652 (2022).
Grief, S. N. & Loza, J. K. Guidelines for the evaluation and treatment of pneumonia. Prim. Care 45 , 485–503 (2018).
Paul, M. Corticosteroids for pneumonia. Cochrane Database Syst. Rev. 12 , CD007720 (2017).
Sharma, A. & Kiciman, E. DoWhy: an end-to-end library for causal inference. Preprint at arXiv https://doi.org/10.48550/ARXIV.2011.04216 (2020).
Khilnani, G. C. et al. Guidelines for antibiotic prescription in intensive care unit. Indian J. Crit. Care Med. 23 , S1–S63 (2019).
Harris, L. K. & Crannage, A. J. Corticosteroids in community-acquired pneumonia: a review of current literature. J. Pharm. Technol. 37 , 152–160 (2021).
Dou, L. et al. Decreased hospital length of stay with early administration of oseltamivir in patients hospitalized with influenza. Mayo Clin. Proc. Innov. Qual. Outcomes 4 , 176–182 (2020).
Khera, A. V. et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 50 , 1219–1224 (2018).
Julkunen, H. et al. Atlas of plasma NMR biomarkers for health and disease in 118,461 individuals from the UK Biobank. Nat. Commun. 14 , 604 (2023).
Ko, F. et al. Associations with retinal pigment epithelium thickness measures in a large cohort: results from the UK Biobank. Ophthalmology 124 , 105–117 (2017).
Patel, P. J. et al. Spectral-domain optical coherence tomography imaging in 67 321 adults: associations with macular thickness in the UK Biobank study. Ophthalmology 123 , 829–840 (2016).
D’Agostino Sr, R. B. et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 117 , 743–753 (2008).
Buergel, T. et al. Metabolomic profiles predict individual multidisease outcomes. Nat. Med. 28 , 2309–2320 (2022).
Xu, Y. et al. An atlas of genetic scores to predict multi-omic traits. Nature 616 , 123–131 (2023).
Saelens, W., Cannoodt, R., Todorov, H. & Saeys, Y. A comparison of single-cell trajectory inference methods. Nat. Biotechnol. 37 , 547–554 (2019).
Rousan, L. A., Elobeid, E., Karrar, M. & Khader, Y. Chest x-ray findings and temporal lung changes in patients with COVID-19 pneumonia. BMC Pulm. Med. 20 , 245 (2020).
Signoroni, A. et al. BS-Net: learning COVID-19 pneumonia severity on a large chest X-ray dataset. Med. Image Anal. 71 , 102046 (2021).
Bird, S. et al. Fairlearn: a toolkit for assessing and improving fairness in AI. https://www.microsoft.com/en-us/research/publication/fairlearn-a-toolkit-for-assessing-and-improving-fairness-in-ai/ (2020).
Strack, B. et al. Impact of HbA1c measurement on hospital readmission rates: analysis of 70,000 clinical database patient records. BioMed. Res. Int. 2014 , 781670 (2014).
Stekhoven, D. J. & Bühlmann, P. MissForest—non-parametric missing value imputation for mixed-type data. Bioinformatics 28 , 112–118 (2012).
Banerjee, A. et al. Identifying subtypes of heart failure from three electronic health record sources with machine learning: an external, prognostic, and genetic validation study. Lancet Digit. Health 5 , e370–e379 (2023).
Nagamine, T. et al. Data-driven identification of heart failure disease states and progression pathways using electronic health records. Sci. Rep. 12 , 17871 (2022).
Da Silva Filho, J. et al. Disease trajectories in hospitalized COVID-19 patients are predicted by clinical and peripheral blood signatures representing distinct lung pathologies. Preprint at bioRxiv https://doi.org/10.1101/2023.09.08.23295024 (2023).
Haneuse, S., Arterburn, D. & Daniels, M. J. Assessing missing data assumptions in EHR-based studies: a complex and underappreciated task. JAMA Netw. Open 4 , e210184 (2021).
Little, R. J. A. A test of missing completely at random for multivariate data with missing values. J. Am. Stat. Assoc. 83 , 1198–1202 (1988).
Jakobsen, J. C., Gluud, C., Wetterslev, J. & Winkel, P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—a practical guide with flowcharts. BMC Med. Res. Methodol. 17 , 162 (2017).
Dziura, J. D., Post, L. A., Zhao, Q., Fu, Z. & Peduzzi, P. Strategies for dealing with missing data in clinical trials: from design to analysis. Yale J. Biol. Med. 86 , 343–358 (2013).
White, I. R., Royston, P. & Wood, A. M. Multiple imputation using chained equations: issues and guidance for practice. Stat. Med. 30 , 377–399 (2011).
Jäger, S., Allhorn, A. & Bießmann, F. A benchmark for data imputation methods. Front. Big Data 4 , 693674 (2021).
Waljee, A. K. et al. Comparison of imputation methods for missing laboratory data in medicine. BMJ Open 3 , e002847 (2013).
Ibrahim, J. G. & Molenberghs, G. Missing data methods in longitudinal studies: a review. Test (Madr.) 18 , 1–43 (2009).
Li, C., Alsheikh, A. M., Robinson, K. A. & Lehmann, H. P. Use of recommended real-world methods for electronic health record data analysis has not improved over 10 years. Preprint at bioRxiv https://doi.org/10.1101/2023.06.21.23291706 (2023).
Regev, A. et al. The Human Cell Atlas. eLife 6 , e27041 (2017).
Megill, C. et al. cellxgene: a performant, scalable exploration platform for high dimensional sparse matrices. Preprint at bioRxiv https://doi.org/10.1101/2021.04.05.438318 (2021).
Speir, M. L. et al. UCSC Cell Browser: visualize your single-cell data. Bioinformatics 37 , 4578–4580 (2021).
Hunter, J. D. Matplotlib: a 2D graphics environment. Comput. Sci. Eng. 9 , 90–95 (2007).
Waskom, M. seaborn: statistical data visualization. J. Open Source Softw. 6 , 3021 (2021).
Harris, C. R. et al. Array programming with NumPy. Nature 585 , 357–362 (2020).
Lam, S. K., Pitrou, A. & Seibert, S. Numba: a LLVM-based Python JIT compiler. In Proc. of the Second Workshop on the LLVM Compiler Infrastructure in HPC. https://doi.org/10.1145/2833157.2833162 (Association for Computing Machinery, 2015).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17 , 261–272 (2020).
McKinney, W. Data structures for statistical computing in Python. In Proc. of the 9th Python in Science Conference (eds van der Walt, S. & Millman, J.). https://doi.org/10.25080/majora-92bf1922-00a (SciPy, 2010).
Boulanger, A. Open-source versus proprietary software: is one more reliable and secure than the other? IBM Syst. J. 44 , 239–248 (2005).
Rocklin, M. Dask: parallel computation with blocked algorithms and task scheduling. In Proc. of the 14th Python in Science Conference. https://doi.org/10.25080/majora-7b98e3ed-013 (SciPy, 2015).
Pivarski, J. et al. Awkward Array. https://doi.org/10.5281/ZENODO.4341376
Collette, A. Python and HDF5: Unlocking Scientific Data (‘O’Reilly Media, Inc., 2013).
Miles, A. et al. zarr-developers/zarr-python: v2.13.6. https://doi.org/10.5281/zenodo.7541518 (2023).
The pandas development team. pandas-dev/pandas: Pandas. https://doi.org/10.5281/ZENODO.3509134 (2024).
Weberpals, J. et al. Deep learning-based propensity scores for confounding control in comparative effectiveness research: a large-scale, real-world data study. Epidemiology 32 , 378–388 (2021).
Rosenthal, J. et al. Building tools for machine learning and artificial intelligence in cancer research: best practices and a case study with the PathML toolkit for computational pathology. Mol. Cancer Res. 20 , 202–206 (2022).
Gayoso, A. et al. A Python library for probabilistic analysis of single-cell omics data. Nat. Biotechnol. 40 , 163–166 (2022).
Paszke, A. et al. PyTorch: an imperative style, high-performance deep learning library. In Advances in Neural Information Processing Systems 32 (eds Wallach, H. et al.). 8024–8035 (Curran Associates, 2019).
Frostig, R., Johnson, M. & Leary, C. Compiling machine learning programs via high-level tracing. https://cs.stanford.edu/~rfrostig/pubs/jax-mlsys2018.pdf (2018).
Moor, M. et al. Foundation models for generalist medical artificial intelligence. Nature 616 , 259–265 (2023).
Kraljevic, Z. et al. Multi-domain clinical natural language processing with MedCAT: the Medical Concept Annotation Toolkit. Artif. Intell. Med. 117 , 102083 (2021).
Pollard, T. J., Johnson, A. E. W., Raffa, J. D. & Mark, R. G. An open source Python package for producing summary statistics for research papers. JAMIA Open 1 , 26–31 (2018).
Ellen, J. G. et al. Participant flow diagrams for health equity in AI. J. Biomed. Inform. 152 , 104631 (2024).
Schouten, R. M. & Vink, G. The dance of the mechanisms: how observed information influences the validity of missingness assumptions. Sociol. Methods Res. 50 , 1243–1258 (2021).
Johnson, W. E., Li, C. & Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8 , 118–127 (2007).
Davidson-Pilon, C. lifelines: survival analysis in Python. J. Open Source Softw. 4 , 1317 (2019).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 57 , 289–300 (1995).
Wishart, D. S. et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 34 , D668–D672 (2006).
Harrell, F. E. Jr, Califf, R. M., Pryor, D. B., Lee, K. L. & Rosati, R. A. Evaluating the yield of medical tests. JAMA 247 , 2543–2546 (1982).
Currant, H. et al. Genetic variation affects morphological retinal phenotypes extracted from UK Biobank optical coherence tomography images. PLoS Genet. 17 , e1009497 (2021).
Cohen, J. P. et al. TorchXRayVision: a library of chest X-ray datasets and models. In Proc. of the 5th International Conference on Medical Imaging with Deep Learning (eds Konukoglu, E. et al.). 172 , 231–249 (PMLR, 2022).
Cohen, J.P., Hashir, M., Brooks, R. & Bertrand, H. On the limits of cross-domain generalization in automated X-ray prediction. In Proceedings of Machine Learning Research , Vol. 121 (eds Arbel, T. et al.) 136–155 (PMLR, 2020).
Download references
Acknowledgements
We thank M. Ansari who designed the ehrapy logo. The authors thank F. A. Wolf, M. Lücken, J. Steinfeldt, B. Wild, G. Rätsch and D. Shung for feedback on the project. We further thank L. Halle, Y. Ji, M. Lücken and R. K. Rubens for constructive comments on the paper. We thank F. Hashemi for her help in implementing the survival analysis module. This research was conducted using data from the UK Biobank, a major biomedical database ( https://www.ukbiobank.ac.uk ), under application number 49966. This work was supported by the German Center for Lung Research (DZL), the Helmholtz Association and the CRC/TRR 359 Perinatal Development of Immune Cell Topology (PILOT). N.H. and F.J.T. acknowledge support from the German Federal Ministry of Education and Research (BMBF) (LODE, 031L0210A), co-funded by the European Union (ERC, DeepCell, 101054957). A.N. is supported by the Konrad Zuse School of Excellence in Learning and Intelligent Systems (ELIZA) through the DAAD program Konrad Zuse Schools of Excellence in Artificial Intelligence, sponsored by the Federal Ministry of Education and Research. This work was also supported by the Chan Zuckerberg Initiative (CZIF2022-007488; Human Cell Atlas Data Ecosystem).
Open access funding provided by Helmholtz Zentrum München - Deutsches Forschungszentrum für Gesundheit und Umwelt (GmbH).
Author information
Authors and affiliations.
Institute of Computational Biology, Helmholtz Munich, Munich, Germany
Lukas Heumos, Philipp Ehmele, Tim Treis, Eljas Roellin, Lilly May, Altana Namsaraeva, Nastassya Horlava, Vladimir A. Shitov, Xinyue Zhang, Luke Zappia, Leon Hetzel, Isaac Virshup, Lisa Sikkema, Fabiola Curion & Fabian J. Theis
Institute of Lung Health and Immunity and Comprehensive Pneumology Center with the CPC-M bioArchive; Helmholtz Zentrum Munich; member of the German Center for Lung Research (DZL), Munich, Germany
Lukas Heumos, Niklas J. Lang, Herbert B. Schiller & Anne Hilgendorff
TUM School of Life Sciences Weihenstephan, Technical University of Munich, Munich, Germany
Lukas Heumos, Tim Treis, Nastassya Horlava, Vladimir A. Shitov, Lisa Sikkema & Fabian J. Theis
Health Data Science Unit, Heidelberg University and BioQuant, Heidelberg, Germany
Julius Upmeier zu Belzen & Roland Eils
Department of Mathematics, School of Computation, Information and Technology, Technical University of Munich, Munich, Germany
Eljas Roellin, Lilly May, Luke Zappia, Leon Hetzel, Fabiola Curion & Fabian J. Theis
Konrad Zuse School of Excellence in Learning and Intelligent Systems (ELIZA), Darmstadt, Germany
Altana Namsaraeva
Systems Medicine, Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE), Bonn, Germany
Rainer Knoll
Center for Digital Health, Berlin Institute of Health (BIH) at Charité – Universitätsmedizin Berlin, Berlin, Germany
Roland Eils
Research Unit, Precision Regenerative Medicine (PRM), Helmholtz Munich, Munich, Germany
Herbert B. Schiller
Center for Comprehensive Developmental Care (CDeCLMU) at the Social Pediatric Center, Dr. von Hauner Children’s Hospital, LMU Hospital, Ludwig Maximilian University, Munich, Germany
Anne Hilgendorff
You can also search for this author in PubMed Google Scholar
Contributions
L. Heumos and F.J.T. conceived the study. L. Heumos, P.E., X.Z., E.R., L.M., A.N., L.Z., V.S., T.T., L. Hetzel, N.H., R.K. and I.V. implemented ehrapy. L. Heumos, P.E., N.L., L.S., T.T. and A.H. analyzed the PIC database. J.U.z.B. and L. Heumos analyzed the UK Biobank database. X.Z. and L. Heumos analyzed the COVID-19 chest x-ray dataset. L. Heumos, P.E. and J.U.z.B. wrote the paper. F.J.T., A.H., H.B.S. and R.E. supervised the work. All authors read, corrected and approved the final paper.
Corresponding author
Correspondence to Fabian J. Theis .
Ethics declarations
Competing interests.
L. Heumos is an employee of LaminLabs. F.J.T. consults for Immunai Inc., Singularity Bio B.V., CytoReason Ltd. and Omniscope Ltd. and has ownership interest in Dermagnostix GmbH and Cellarity. The remaining authors declare no competing interests.
Peer review
Peer review information.
Nature Medicine thanks Leo Anthony Celi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Lorenzo Righetto, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 overview of the paediatric intensive care database (pic)..
The database consists of several tables corresponding to several data modalities and measurement types. All tables colored in green were selected for analysis and all tables in blue were discarded based on coverage rate. Despite the high coverage rate, we discarded the ‘OR_EXAM_REPORTS’ table because of the lack of detail in the exam reports.
Extended Data Fig. 2 Preprocessing of the Paediatric Intensive Care (PIC) dataset with ehrapy.
( a ) Heterogeneous data of the PIC database was stored in ‘data’ (matrix that is used for computations) and ‘observations’ (metadata per patient visit). During quality control, further annotations are added to the ‘variables’ (metadata per feature) slot. ( b ) Preprocessing steps of the PIC dataset. ( c ) Example of the function calls in the data analysis pipeline that resembles the preprocessing steps in (B) using ehrapy.
Extended Data Fig. 3 Missing data distribution for the ‘youths’ group of the PIC dataset.
The x-axis represents the percentage of missing values in each feature. The y-axis reflects the number of features in each bin with text labels representing the names of the individual features.
Extended Data Fig. 4 Patient selection during analysis of the PIC dataset.
Filtering for the pneumonia cohort of the youths filters out care units except for the general intensive care unit and the pediatric intensive care unit.
Extended Data Fig. 5 Feature rankings of stratified patient groups.
Scores reflect the z-score underlying the p-value per measurement for each group. Higher scores (above 0) reflect overrepresentation of the measurement compared to all other groups and vice versa. ( a ) By clinical chemistry. ( b ) By liver markers. ( c ) By medication type. ( d ) By infection markers.
Extended Data Fig. 6 Liver marker value progression for the ‘youths’ group and Kaplan-Meier curves.
( a ) Viral and severe pneumonia with co-infection groups display enriched gamma-glutamyl transferase levels in blood serum. ( b ) Aspartate transferase (AST) and Alanine transaminase (ALT) levels are enriched for severe pneumonia with co-infection during early ICU stay. ( c ) and ( d ) Kaplan-Meier curves for ALT and AST demonstrate lower survivability for children with measurements outside the norm.
Extended Data Fig. 7 Overview of medication categories used for causal inference.
( a ) Feature engineering process to group administered medications into medication categories using drugbank. ( b ) Number of medications per medication category. ( c ) Number of patients that received (dark blue) and did not receive specific medication categories (light blue).
Extended Data Fig. 8 UK-Biobank data overview and quality control across modalities.
( a ) UMAP plot of the metabolomics data demonstrating a clear gradient with respect to age at sampling, and ( b ) type 2 diabetes prevalence. ( c ) Analogously, the features derived from retinal imaging show a less pronounced age gradient, and ( d ) type 2 diabetes prevalence gradient. ( e ) Stratifying myocardial infarction risk by the type 2 diabetes comorbidity confirms vastly increased risk with a prior type 2 (T2D) diabetes diagnosis. Kaplan-Meier estimators with 95 % confidence intervals are shown. ( f ) Similarly, the polygenic risk score for coronary heart disease used in this work substantially enriches myocardial infarction risk in its top 5% percentile. Kaplan-Meier estimators with 95 % confidence intervals are shown. ( g ) UMAP visualization of the metabolomics features colored by the assessment center shows no discernable biases. (A-G) n = 29,216.
Extended Data Fig. 9 UK-Biobank retina derived feature quality control.
( a ) Leiden Clustering of retina derived feature space. ( b ) Comparison of ‘overall retinal pigment epithelium (RPE) thickness’ values between cluster 5 (n = 301) and the rest of the population (n = 28,915). ( c ) RPE thickness in the right eye outliers on the UMAP largely corresponds to cluster 5. ( d ) Log ratio of top and bottom 5 fields in obs dataframe between cluster 5 and the rest of the population. ( e ) Image Quality of the optical coherence tomography scan as reported in the UKB. ( f ) Minimum motion correlation quality control indicator. ( g ) Inner limiting membrane (ILM) quality control indicator. (D-G) Data are shown for the right eye only, comparable results for the left eye are omitted. (A-G) n = 29,216.
Extended Data Fig. 10 Bias detection and mitigation study on the Diabetes 130-US hospitals dataset (n = 101,766 hospital visits, one patient can have multiple visits).
( a ) Filtering to the visits of Medicare recipients results in an increase of Caucasians. ( b ) Proportion of visits where Hb1Ac measurements are recorded, stratified by admission type. Adjusted P values were calculated with Chi squared tests and Bonferroni correction (Adjusted P values: Emergency vs Referral 3.3E-131, Emergency vs Other 1.4E-101, Referral vs Other 1.6E-4.) ( c ) Normalizing feature distributions jointly vs. separately can mask distribution differences. ( d ) Imputing the number of medications for visits. Onto the complete data (blue), MCAR (30% missing data) and MAR (38% missing data) were introduced (orange), with the MAR mechanism depending on the time in hospital. Mean imputation (green) can reduce the variance of the distribution under MCAR and MAR mechanisms, and bias the center of the distribution under an MAR mechanism. Multiple imputation, such as MissForest imputation can impute meaningfully even in MAR cases, when having access to variables involved in the MAR mechanism. Each boxplot represents the IQR of the data, with the horizontal line inside the box indicating the median value. The left and right bounds of the box represent the first and third quartiles, respectively. The ‘whiskers’ extend to the minimum and maximum values within 1.5 times the IQR from the lower and upper quartiles, respectively. ( e ) Predicting the early readmission within 30 days after release on a per-stay level. Balanced accuracy can mask differences in selection and false negative rate between sensitive groups.
Supplementary information
Supplementary tables 1 and 2, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Heumos, L., Ehmele, P., Treis, T. et al. An open-source framework for end-to-end analysis of electronic health record data. Nat Med (2024). https://doi.org/10.1038/s41591-024-03214-0
Download citation
Received : 11 December 2023
Accepted : 25 July 2024
Published : 12 September 2024
DOI : https://doi.org/10.1038/s41591-024-03214-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- Department of Health & Social Care
Management information on recruitment to clinical research studies
Published 12 September 2024

© Crown copyright 2024
This publication is licensed under the terms of the Open Government Licence v3.0 except where otherwise stated. To view this licence, visit nationalarchives.gov.uk/doc/open-government-licence/version/3 or write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or email: [email protected] .
Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned.
This publication is available at https://www.gov.uk/government/publications/management-information-on-recruitment-to-clinical-research-studies/management-information-on-recruitment-to-clinical-research-studies
Data on the recruitment to clinical research studies, reported to the Department of Health and Social Care ( DHSC ). This release is published as management information, in accordance with the Code of Practice for Statistics , to improve transparency and support publication of the Darzi review on 12 September 2024. The Darzi review is the independent investigation of the National Health Service in England, led by Lord Darzi.
Clinical research studies are important for advancing medical knowledge and enhancing patient care. These investigations are designed to evaluate the safety, efficacy and effectiveness of new treatments, medications, devices, or interventions. Clinical research includes various types of studies, such as clinical trials and observational studies.
Clinical trials, also known as interventional studies, test new medical approaches, including drugs, vaccines and surgical procedures. They are carried out in phases to assess safety, dosing and effectiveness. Observational studies monitor and analyse the effects of specific variables on health outcomes without intervening.
By systematically investigating in this way, clinical research plays a vital role in discovering new treatments and improving healthcare practices.
Monitoring clinical research delivery
One of the ways clinical research delivery in the UK is monitored is through the UK Clinical Research Delivery Key Performance Indicators Report . This report, previously known as the Research Status Report, brings together data from the National Institute for Health and Care Research ( NIHR ) and the Medicines and Healthcare products Regulatory Agency. It monitors progress on delivering parts of the government’s vision for UK clinical research delivery. The performance indicators measure trends in:
- the speed and predictability of regulatory and study set-up timelines
- the delivery of research to time and target
- overall recruitment levels
The report is published monthly by DHSC and is being used to support the development of policies to encourage clinical research delivery.
This publication focuses specifically on clinical research recruitment data from the UK Clinical Research Delivery Key Performance Indicators Report from April 2014 onwards. It is a supplementary management information release, showing detailed tables behind the recruitment charts published in June 2024’s UK Clinical Research Delivery Key Performance Indicators Report.
Definition of a research study
Research is defined in the UK Policy Framework for Health and Social Care Research as the attempt to derive generalisable or transferable new knowledge to answer or refine relevant questions with scientifically sound methods. This excludes: audit, needs assessments, quality improvement and other local service evaluations. It also excludes routine banking of biological samples or data except where this activity is integral to a self-contained research project designed to test a clear hypothesis.
This definition applies to all studies for which NIHR Clinical Research Network support is sought regardless of the study type or research funder.
Table 1 shows the number of participants recruited into studies since April 2014, held on the NIHR Clinical Research Network’s central portfolio management system.
The central portfolio management system consists of all studies held on the Clinical Research Network portfolio, as well as some studies held on the network portfolios of Northern Ireland, Scotland and Wales.
The data includes recruitment to both interventional and observational studies. The data represents the total number of participants recruited for a given month, based on the month and year the recruitment notification was received.
Table 1: recruitment to clinical research studies
| Recruitment month and year | Number of participants |
|---|---|
| April 2014 | 53,127 |
| May 2014 | 81,491 |
| June 2014 | 60,363 |
| July 2014 | 58,498 |
| August 2014 | 52,321 |
| September 2014 | 56,211 |
| October 2014 | 62,436 |
| November 2014 | 55,059 |
| December 2014 | 46,203 |
| January 2015 | 58,324 |
| February 2015 | 63,841 |
| March 2015 | 79,245 |
| April 2015 | 55,841 |
| May 2015 | 54,132 |
| June 2015 | 63,624 |
| July 2015 | 61,753 |
| August 2015 | 73,360 |
| September 2015 | 63,296 |
| October 2015 | 61,527 |
| November 2015 | 66,477 |
| December 2015 | 43,906 |
| January 2016 | 51,217 |
| February 2016 | 54,898 |
| March 2016 | 56,727 |
| April 2016 | 55,838 |
| May 2016 | 61,268 |
| June 2016 | 70,329 |
| July 2016 | 54,405 |
| August 2016 | 57,022 |
| September 2016 | 59,833 |
| October 2016 | 56,395 |
| November 2016 | 64,126 |
| December 2016 | 50,328 |
| January 2017 | 61,254 |
| February 2017 | 60,311 |
| March 2017 | 110,535 |
| April 2017 | 57,058 |
| May 2017 | 63,939 |
| June 2017 | 74,999 |
| July 2017 | 65,602 |
| August 2017 | 63,852 |
| September 2017 | 66,551 |
| October 2017 | 74,006 |
| November 2017 | 76,660 |
| December 2017 | 50,561 |
| January 2018 | 66,972 |
| February 2018 | 77,654 |
| March 2018 | 70,763 |
| April 2018 | 71,401 |
| May 2018 | 79,562 |
| June 2018 | 101,583 |
| July 2018 | 78,111 |
| August 2018 | 69,173 |
| September 2018 | 79,669 |
| October 2018 | 102,418 |
| November 2018 | 80,351 |
| December 2018 | 52,956 |
| January 2019 | 77,046 |
| February 2019 | 85,854 |
| March 2019 | 95,115 |
| April 2019 | 76,893 |
| May 2019 | 75,525 |
| June 2019 | 69,448 |
| July 2019 | 70,086 |
| August 2019 | 57,268 |
| September 2019 | 63,686 |
| October 2019 | 81,183 |
| November 2019 | 71,557 |
| December 2019 | 54,106 |
| January 2020 | 74,426 |
| February 2020 | 71,698 |
| March 2020 | 69,371 |
| April 2020 | 118,865 |
| May 2020 | 164,358 |
| June 2020 | 235,733 |
| July 2020 | 166,467 |
| August 2020 | 152,595 |
| September 2020 | 187,815 |
| October 2020 | 193,757 |
| November 2020 | 219,025 |
| December 2020 | 219,465 |
| January 2021 | 235,100 |
| February 2021 | 198,865 |
| March 2021 | 200,579 |
| April 2021 | 174,143 |
| May 2021 | 162,090 |
| June 2021 | 171,082 |
| July 2021 | 173,561 |
| August 2021 | 104,394 |
| September 2021 | 116,278 |
| October 2021 | 86,524 |
| November 2021 | 93,404 |
| December 2021 | 92,956 |
| January 2022 | 95,821 |
| February 2022 | 91,643 |
| March 2022 | 108,758 |
| April 2022 | 103,638 |
| May 2022 | 83,983 |
| June 2022 | 94,282 |
| July 2022 | 78,845 |
| August 2022 | 81,659 |
| September 2022 | 86,075 |
| October 2022 | 89,064 |
| November 2022 | 112,070 |
| December 2022 | 77,792 |
| January 2023 | 82,087 |
| February 2023 | 80,451 |
| March 2023 | 100,019 |
| April 2023 | 82,115 |
| May 2023 | 89,414 |
| June 2023 | 104,076 |
| July 2023 | 93,247 |
| August 2023 | 104,386 |
| September 2023 | 88,273 |
| October 2023 | 94,272 |
| November 2023 | 100,281 |
| December 2023 | 81,339 |
| January 2024 | 91,821 |
| February 2024 | 94,866 |
| March 2024 | 94,019 |
Source: NIHR Clinical Research Network, central portfolio management system
Table 2 shows the number of participants recruited into studies since April 2014, held on the NIHR Clinical Research Network’s central portfolio management system.
Table 2 includes recruitment to 3 study types:
- commercial contract studies. These are studies sponsored and fully funded by the life sciences industry
- commercial collaborative studies. These are studies typically funded, wholly or in part, by the life sciences industry and sponsored by a combination of life sciences industry and non-commercial organisations. This category has previously been included in non-commercial figures but it is now being presented separately to better represent the breadth of commercial studies. Commercial collaborative studies are supported in the same way as other non-commercial studies
- non-commercial studies. These are studies sponsored and wholly funded by one or more non-commercial organisations, including medical research charities, universities and public funders such as NIHR and UK Research and Innovation
The data includes recruitment to both interventional and observational studies.
Table 2: recruitment to clinical research studies broken down by study type, 2014 to 2024
| Recruitment month and year | Non-commercial | Commercial collaborative | Commercial contract |
|---|---|---|---|
| April 2014 | 43,173 | 5,578 | 4,376 |
| May 2014 | 66,388 | 12,326 | 2,777 |
| June 2014 | 50,265 | 6,927 | 3,171 |
| July 2014 | 48,828 | 6,187 | 3,483 |
| August 2014 | 43,815 | 5,009 | 3,497 |
| September 2014 | 47,757 | 5,369 | 3,085 |
| October 2014 | 50,973 | 7,761 | 3,702 |
| November 2014 | 45,728 | 5,678 | 3,653 |
| December 2014 | 38,317 | 5,003 | 2,883 |
| January 2015 | 48,852 | 5,983 | 3,489 |
| February 2015 | 54,665 | 5,675 | 3,501 |
| March 2015 | 67,630 | 6,463 | 5,152 |
| April 2015 | 46,398 | 5,491 | 3,952 |
| May 2015 | 45,736 | 5,128 | 3,268 |
| June 2015 | 52,262 | 6,359 | 5,003 |
| July 2015 | 52,094 | 6,341 | 3,318 |
| August 2015 | 65,660 | 4,992 | 2,708 |
| September 2015 | 53,527 | 6,263 | 3,506 |
| October 2015 | 51,673 | 6,508 | 3,346 |
| November 2015 | 54,737 | 7,400 | 4,340 |
| December 2015 | 35,944 | 5,501 | 2,461 |
| January 2016 | 41,691 | 6,574 | 2,952 |
| February 2016 | 44,330 | 7,153 | 3,415 |
| March 2016 | 46,049 | 7,676 | 3,002 |
| April 2016 | 46,580 | 6,286 | 2,972 |
| May 2016 | 51,508 | 6,409 | 3,351 |
| June 2016 | 61,073 | 6,254 | 3,002 |
| July 2016 | 46,045 | 5,596 | 2,764 |
| August 2016 | 48,837 | 5,245 | 2,940 |
| September 2016 | 50,791 | 5,029 | 4,013 |
| October 2016 | 48,241 | 5,313 | 2,841 |
| November 2016 | 54,962 | 5,560 | 3,604 |
| December 2016 | 43,086 | 4,334 | 2,908 |
| January 2017 | 52,886 | 5,674 | 2,694 |
| February 2017 | 51,752 | 5,208 | 3,351 |
| March 2017 | 99,600 | 6,468 | 4,467 |
| April 2017 | 48,363 | 4,920 | 3,775 |
| May 2017 | 54,453 | 5,861 | 3,625 |
| June 2017 | 65,652 | 5,645 | 3,702 |
| July 2017 | 56,161 | 5,809 | 3,632 |
| August 2017 | 53,720 | 6,230 | 3,902 |
| September 2017 | 56,392 | 6,642 | 3,517 |
| October 2017 | 58,803 | 7,150 | 8,053 |
| November 2017 | 59,785 | 6,976 | 9,899 |
| December 2017 | 41,825 | 4,981 | 3,755 |
| January 2018 | 56,295 | 6,907 | 3,770 |
| February 2018 | 67,389 | 7,026 | 3,239 |
| March 2018 | 59,613 | 7,229 | 3,921 |
| April 2018 | 58,101 | 9,893 | 3,407 |
| May 2018 | 65,842 | 10,130 | 3,590 |
| June 2018 | 90,507 | 7,226 | 3,850 |
| July 2018 | 65,253 | 8,034 | 4,824 |
| August 2018 | 56,754 | 7,628 | 4,791 |
| September 2018 | 64,719 | 9,524 | 5,426 |
| October 2018 | 81,222 | 15,107 | 6,089 |
| November 2018 | 64,477 | 9,960 | 5,914 |
| December 2018 | 43,264 | 6,072 | 3,620 |
| January 2019 | 64,392 | 7,900 | 4,754 |
| February 2019 | 73,602 | 8,027 | 4,225 |
| March 2019 | 78,586 | 12,842 | 3,687 |
| April 2019 | 65,227 | 8,619 | 3,047 |
| May 2019 | 64,321 | 8,270 | 2,934 |
| June 2019 | 58,436 | 7,384 | 3,628 |
| July 2019 | 57,487 | 8,988 | 3,611 |
| August 2019 | 46,164 | 8,027 | 3,077 |
| September 2019 | 51,515 | 9,676 | 2,495 |
| October 2019 | 65,035 | 13,081 | 3,067 |
| November 2019 | 58,227 | 9,737 | 3,593 |
| December 2019 | 43,547 | 8,062 | 2,497 |
| January 2020 | 60,631 | 10,273 | 3,522 |
| February 2020 | 59,870 | 9,134 | 2,694 |
| March 2020 | 61,025 | 6,653 | 1,693 |
| April 2020 | 115,845 | 2,424 | 596 |
| May 2020 | 161,882 | 2,093 | 383 |
| June 2020 | 231,322 | 3,031 | 1,380 |
| July 2020 | 158,874 | 5,974 | 1,619 |
| August 2020 | 146,518 | 4,112 | 1,965 |
| September 2020 | 180,769 | 5,629 | 1,417 |
| October 2020 | 175,345 | 10,031 | 8,381 |
| November 2020 | 197,086 | 10,666 | 11,273 |
| December 2020 | 205,579 | 9,453 | 4,433 |
| January 2021 | 223,132 | 6,580 | 5,388 |
| February 2021 | 190,285 | 6,573 | 2,007 |
| March 2021 | 188,380 | 10,219 | 1,980 |
| April 2021 | 159,679 | 12,641 | 1,823 |
| May 2021 | 144,032 | 11,711 | 6,347 |
| June 2021 | 158,606 | 9,939 | 2,537 |
| July 2021 | 162,320 | 8,037 | 3,204 |
| August 2021 | 93,479 | 8,438 | 2,477 |
| September 2021 | 104,652 | 9,317 | 2,309 |
| October 2021 | 73,910 | 10,407 | 2,207 |
| November 2021 | 79,500 | 11,252 | 2,652 |
| December 2021 | 84,682 | 6,125 | 2,149 |
| January 2022 | 86,960 | 6,681 | 2,180 |
| February 2022 | 82,369 | 6,405 | 2,869 |
| March 2022 | 97,714 | 8,093 | 2,951 |
| April 2022 | 91,085 | 8,537 | 4,016 |
| May 2022 | 70,674 | 10,166 | 3,143 |
| June 2022 | 82,982 | 9,150 | 2,150 |
| July 2022 | 67,393 | 9,077 | 2,375 |
| August 2022 | 68,433 | 10,222 | 3,004 |
| September 2022 | 72,358 | 10,821 | 2,896 |
| October 2022 | 74,041 | 11,959 | 3,064 |
| November 2022 | 93,771 | 13,873 | 4,426 |
| December 2022 | 65,879 | 8,793 | 3,120 |
| January 2023 | 67,678 | 10,949 | 3,460 |
| February 2023 | 64,479 | 12,045 | 3,927 |
| March 2023 | 82,690 | 10,892 | 6,437 |
| April 2023 | 69,130 | 8,885 | 4,100 |
| May 2023 | 75,632 | 9,370 | 4,412 |
| June 2023 | 82,690 | 8,525 | 12,861 |
| July 2023 | 75,273 | 8,535 | 9,439 |
| August 2023 | 82,591 | 9,036 | 12,759 |
| September 2023 | 71,893 | 8,170 | 8,210 |
| October 2023 | 68,843 | 10,785 | 14,644 |
| November 2023 | 72,188 | 11,216 | 16,877 |
| December 2023 | 49,048 | 7,820 | 24,471 |
| January 2024 | 68,659 | 10,785 | 12,377 |
| February 2024 | 71,830 | 10,266 | 12,770 |
| March 2024 | 67,475 | 11,090 | 15,454 |
Methodology and quality note
Inclusion and exclusion criteria.
Recruitment is only recorded in the central portfolio management system if it meets the definitions of recruitment as outlined in the NIHR Clinical Research Network Recruitment Policy Document . For example, recruitment data is not collected for studies classified as non-consenting. These are exceptional circumstances where no form of consent can be obtained.
Only research activity with a status of confirmed and provisional is included. Research activity which is indicated as inaccurate (queried) is excluded from figures.
Confirmed status includes manually uploaded data or data from the local portfolio management system that has been confirmed as accurate by the Chief Investigator, their representative or a representative of the commercial sponsor or contract research organisation.
Provisional status is given to data from the local portfolio management system that has yet to be confirmed or that requires reconfirmation following queries.
Data source and coverage
The data has been sourced from the central portfolio management system. The central portfolio management system consists of all studies held on the Clinical Research Network portfolio, as well as some studies held on the network portfolios of Northern Ireland, Scotland and Wales.
The data is recorded monthly from April 2014 to March 2024. For the purpose of the UK Clinical Research Delivery Key Performance Indicators Report, monthly snapshots are taken according to a data cut schedule. The snapshot used in this publication was taken on 21 June 2024.
Data caveats
The data does not show recruitment to the whole clinical research system, only recruitment to studies on the central portfolio management system. Therefore, this data will be an underestimate of total clinical research recruitment in the UK.
The data is not exclusive to NHS sites and includes recruitment to non- NHS sites.
While data covers the whole of the UK, data relating to studies led by devolved administrations may be incomplete. This is because they are only included in the central portfolio management system when added by the devolved administrations.
The quality of the data is dependent on the accuracy and timeliness of recruitment data being recorded in the system.
There is often a lag between activity taking place at a study site and data being recorded in the system. This means that previous months’ data may be updated retrospectively. Changes in recruitment for individual recent months should not be taken as an indication of overall trend in recruitment.
From the 2023 to 2024 financial year onwards, the data for the commercial collaborative category will be more robust. This is due to data quality improvements linked to reporting this category separately, instead of classifying activities as purely commercial or non-commercial. Not all studies will have been reviewed retrospectively and re-classified where necessary, particularly studies that had already closed.
Using the data
The data cannot be used for:
- measuring overall UK study recruitment as the data is only on studies on the central portfolio management system (not activity of the whole research environment). It is unknown what proportion of studies in the UK at that particular point in time are contributing to the figures
- comparing (portfolio) recruitment over the time. The network as an organisation and its remit has changed significantly over time. Caution should be taken with time comparisons as portfolio and associated data collection may have changed
- UK-wide data within the central portfolio management system across years. It has only been in recent years that UK-wide data for commercial (for example) has been collected into the central portfolio management system
The portfolio balance between observational studies and interventional ones will influence the numbers. For example, if at a particular time, the portfolio has some large sample size observational studies, this will affect recruitment numbers and make it difficult to compare to other years.
Recruitment from private sites may not be collected and so may not be included in the data.
If you have any questions in relation to these statistics, please contact [email protected] .
Is this page useful?
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. Please fill in this survey (opens in a new tab) .
Cookies on GOV.UK
We use cookies to collect information about how you use data.gov.uk. We use this information to make the website work as well as possible.
You can change your cookie settings at any time.
- 1987 Field Studies Council (Oil Pollution Research Unit), Christchurch Harbour, Dorset, Intertidal coring and qualitative recording and subtidal pipe-dredge survey
Survey run by Field Studies Council (Oil Pollution Research Unit) for the Nature Conservancy Council (NCC). All these records relate to "Dixon (1988) Surveys of Harbours, Rias and Estuaries in Southern Britain: Christchurch Harbour". A copy of the report is held at English Nature (Dorset Team). This report is from the series 'Surveys of Harbours, Rias and Estuaries in Southern Britain' commissioned by the Nature Conservancy Council between 1985-1988. The study aims to review existing information and undertake further fieldwork, where required. The first volume of each study provides a report of the field surveys. The aim of the fieldwork was to collect information on the abundance of macrofauna and flora (at sites selected to include a wide range of different shore and seabed types, areas of known or likely nature conservation importance, or where rare or unusual species might be present). The combined intertidal and subtidal survey took place during 13th -18th June 1987 and a total of 32 sites were sampled. Sites 1 to 23 were sited on the extensive intertidal sediments. At each intertidal site, the habitats present were recorded and infauna sampled by a combination of coring and qualitative recording. Subtidal surveying was undertaken at 6 sites along the main channel using a pipe-dredge, and at 3 sites divers recorded habitat type and conspicuous species.
More from this publisher
Related datasets.
- MNCR Area Summaries - Inlets in the Bristol Channel and approaches
- 1982 - 1985 Nature Conservancy Council (NCC), Poole Harbour, Subtidal dredge and grab sampling survey
This data hasn’t been released by the publisher.
Contact the publisher for more information.
Additional information
Contact Marine Environmental Data & Information Network regarding this dataset
Licence information
Data is freely available for research or commercial use providing that the originators are acknowledged in any publications produced.

IMAGES
VIDEO
COMMENTS
A structured online system, such as the ClinicalTrials.gov results database, that provides the public with access to registration and summary results information for completed or terminated clinical studies. A study with results available on ClinicalTrials.gov is described as having the results "posted."
Google Scholar provides a simple way to broadly search for scholarly literature. Search across a wide variety of disciplines and sources: articles, theses, books, abstracts and court opinions.
The Clinical Center provides hope through pioneering clinical research to improve human health. We rapidly translate scientific observations and laboratory discoveries into new ways to diagnose, treat and prevent disease. More than 500,000 people from around the world have participated in clinical research since the hospital opened in 1953.
Organize your papers in one place. Try Paperpile. 1. Scopus. Scopus is one of the two big commercial, bibliographic databases that cover scholarly literature from almost any discipline. Besides searching for research articles, Scopus also provides academic journal rankings, author profiles, and an h-index calculator. 2.
The NIH maintains an online database of clinical research studies taking place at its Clinical Center, which is located on the NIH campus in Bethesda, Maryland. Studies are conducted by most of the institutes and centers across the NIH. The Clinical Center hosts a wide range of studies from rare diseases to chronic health conditions, as well as ...
Library, Information Science & Technology Abstracts (LISTA) is a free research database for library and information science studies. LISTA provides indexing and abstracting for hundreds of key journals, books, research reports. It is EBSCO's intention to provide access to this resource on a continual basis. Access now.
Find the research you need | With 160+ million publication pages, 1+ million questions, and 25+ million researchers, this is where everyone can access science
Access 160+ million publications and connect with 25+ million researchers. Join for free and gain visibility by uploading your research.
Search all biomedical databases provided by the National Center for Biotechnology Information (NCBI), an agency of the U.S. National Library of Medicine at the NIH ... Gene sequences and annotations used as references for the study of orthologs structure, expression, and evolution. Gene. Collected information about gene loci. ... Research news ...
Your brilliance, connected. Scopus uniquely combines a comprehensive, expertly curated abstract and citation database with enriched data and linked scholarly literature across a wide variety of disciplines. Scopus quickly finds relevant and authoritative research, identifies experts and provides access to reliable data, metrics and analytical ...
Broaden your research with images and primary sources. Harness the power of visual materials—explore more than 3 million images now on JSTOR. Enhance your scholarly research with underground newspapers, magazines, and journals. Take your research further with Artstor's 3+ million images. Explore collections in the arts, sciences, and ...
Advanced. Journal List. PubMed Central ® (PMC) is a free full-text archive of biomedical and life sciences journal literature at the U.S. National Institutes of Health's National Library of Medicine (NIH/NLM)
1. PubMed. PubMed is the number one source for medical and healthcare research. It is hosted by the National Institutes of Health (NIH) and provides bibliographic information including abstracts and links to the full text publisher websites for more than 28 million articles. Coverage: around 35 million items.
For over 55 years, APA PsycInfo has been the most trusted index of psychological science in the world. With more than 5,000,000 interdisciplinary bibliographic records, our database delivers targeted discovery of credible and comprehensive research across the full spectrum of behavioral and social sciences. This indispensable resource continues ...
MEDLINE is the National Library of Medicine's (NLM) premier bibliographic database that contains references to journal articles in life sciences, with a concentration on biomedicine. See the MEDLINE Overview page for more information about MEDLINE.. MEDLINE content is searchable via PubMed and constitutes the primary component of PubMed, a literature database developed and maintained by the ...
PubMed® comprises more than 37 million citations for biomedical literature from MEDLINE, life science journals, and online books. Citations may include links to full text content from PubMed Central and publisher web sites.
What is Database Search? Harvard Library licenses hundreds of online databases, giving you access to academic and news articles, books, journals, primary sources, streaming media, and much more. The contents of these databases are only partially included in HOLLIS. To make sure you're really seeing everything, you need to search in multiple places.
3.3 million articles on ScienceDirect are open access. Articles published open access are peer-reviewed and made freely available for everyone to read, download and reuse in line with the user license displayed on the article. ScienceDirect is the world's leading source for scientific, technical, and medical research.
Stand on the shoulders of giants. Google Scholar provides a simple way to broadly search for scholarly literature. Search across a wide variety of disciplines and sources: articles, theses, books, abstracts and court opinions.
This non-exhaustive collection of research databases allows you to locate multiple study datasets as well as information on clinical trials free of charge. Research Databases. Array Express ArrayExpress is a database of functional genomics experiments that can be queried and the data downloaded. Cancer Cell Line Encyclopedia (CCLE)
APA PsycNet is the leading database for psychology research, offering access to journals, books, and trials. Search by keyword or browse by topic.
The National Center for Biotechnology Information advances science and health by providing access to biomedical and genomic information. About the NCBI |. Mission |. Organization |. NCBI News & Blog. Submit. Deposit data or manuscripts into NCBI databases. Submit Icon.
Cochrane Hong Kong: Advancing evidence-based healthcare in Asia. 19 July 2024. News. World Evidence-Based Healthcare Day: Cochrane and global evidence leaders unite for world health and beyond. 17 July 2024. News. Cochrane is a global independent network of researchers, professionals, patients, carers and people interested in health.
EMBL-EBI data resources help advance biodiversity and climate change research by enabling scientists to study species interactions, evolutionary processes, ecosystem health, and more. News ; All EMBL-EBI news ; Technology and innovation Victoria Hatch. 13 September 2024.
Despite its small scale, the study confirmed the feasibility of conducting a human participant study directly to explore the hypothesis generation process in clinical research. This study provides supporting evidence to conduct a larger-scale study with a specifically designed tool to facilitate the hypothesis-generation process among ...
Research data is any material used to inform or support your research conclusions. It can take many different forms including recordings, transcripts, questionnaires, photographs and code. While a taught course research project may be relatively limited in time and scope, it is important to collect, store and manage data in a secure and ...
PIC database analysis Study design. We collected clinical data from the PIC 43 version 1.1.0 database. PIC is a single-center, bilingual (English and Chinese) database hosting information of ...
Data on the recruitment to clinical research studies, reported to the Department of Health and Social Care (DHSC).This release is published as management information, in accordance with the Code ...
Introduction. Smart home is an attractive vision of future 6G network, providing intelligent services to humans such as intruder detection and health detection [1, 2].The integrated sensing and communication (ISAC) technology with terahertz band (0.1-10 THz) is poised to meet the sensing demands of centimetre-precision and millisecond-latency, which has a superior prospect in indoor scenario [].
This report is from the series 'Surveys of Harbours, Rias and Estuaries in Southern Britain' commissioned by the Nature Conservancy Council between 1985-1988. The study aims to review existing information and undertake further fieldwork, where required. The first volume of each study provides a report of the field surveys.