
Alzheimer’s Breakthrough: New Drug Shows Promise in Reversing Memory Loss and Cognitive Decline

New research reveals a potential breakthrough in Alzheimer’s treatment, showing that the drug troriluzole can reverse memory loss and cognitive decline in mice.
The study found that troriluzole reduced harmful glutamate levels and improved cognitive functions, suggesting its potential to maintain healthy brain function and possibly slow the disease’s progression.
Novel Drug Rescues Memory Loss in Alzheimer’s Mouse Model
In a recent breakthrough in Alzheimer’s disease research, Auburn University scientists have studied a new drug, troriluzole, that can prevent brain changes leading to memory loss and cognitive decline in a mouse model of the disease. This study, recently published in the Journal of Neurochemistry , is the first to show how troriluzole can target early-stage alterations associated with Alzheimer’s, providing new hope for potential treatments.
Dr. Miranda Reed, a Professor in the department of Drug Discovery at Auburn University and Delivery and the studies main researcher, noted that “by examining how drug treatments can intervene early in the disease process, we aim to develop therapies that might prevent or even cure Alzheimer’s.”
“This study also highlights how scientific advancements can transform our understanding of complex diseases like Alzheimer’s,” said Dr. Michael Gramlich, an Assistant Professor of Biophysics and the study’s other main researcher.

Groundbreaking Effects on Alzheimer’s Disease
Alzheimer’s disease affects millions of people worldwide, causing progressive memory loss, confusion, and eventually the inability to perform basic tasks. Despite decades of research, a cure remains elusive. Alzheimer’s is characterized by the accumulation of amyloid plaques and tau tangles in the brain, which disrupt neural communication. In the early stages, excessive levels of the neurotransmitter glutamate cause damaging overactivity in synapses, the connections between nerve cells.
The study conducted by Auburn University researchers, led by Drs. Miranda Reed and Michael Gramlich, investigated how troriluzole, a novel drug, can maintain normal brain function in mice genetically modified to replicate early Alzheimer’s stages. The results are compelling: troriluzole not only reduced harmful glutamate levels but also improved memory and learning in the mice, suggesting a maintenance of healthy brain function.
“Our research demonstrates that by targeting synaptic activity early, we may be able to prevent or slow the progression of Alzheimer’s. This could revolutionize the way we approach treatment for this disease,” noted both researchers.
How Troriluzole Works
In the Auburn study, mice treated with troriluzole showed a significant reduction in synaptic glutamate levels and decreased brain hyperactivity. These molecular changes led to tangible improvements: the treated mice performed better in memory tests, such as navigating mazes, indicating that their cognitive functions were restored.
“These findings are promising because they suggest that troriluzole can protect the brain at a fundamental level, starting with molecular changes and resulting in improved cognitive abilities,” said Dr. Reed. “It’s like repairing an engine before it fails completely.”
Impact of Collaborative Research
This research was a collaborative effort involving Auburn University’s College of Science and Mathematics, the Harrison College of Pharmacy, and the Center for Neuroscience Initiative, along with private researchers and students. The team’s combined expertise in neuroscience and pharmacology was crucial to the study’s success.
“This collaboration blends basic science and pharmaceutical research to tackle one of the most challenging neurological issues of our time,” Dr. Gramlich emphasized. “Our work not only enhances scientific understanding of Alzheimer’s disease but also offers a potential new treatment that could improve the lives of millions worldwide.”
Looking Forward
While the results in mice are encouraging, the researchers emphasize the need for further studies to determine how troriluzole works at different stages of disease progression.
Reference: “Troriluzole rescues glutamatergic deficits, amyloid and tau pathology, and synaptic and memory impairments in 3xTg-AD mice” by Jeremiah Pfitzer, Priyanka D. Pinky, Savannah Perman, Emma Redmon, Luca Cmelak, Vishnu Suppiramaniam, Vladimir Coric, Irfan A. Qureshi, Michael W. Gramlich and Miranda N. Reed, 30 August 2024, Journal of Neurochemistry . DOI: 10.1111/jnc.16215
Related Articles
Neuroscientists discover promising way to restore cognitive function impaired by alzheimer’s disease, century of data shows covid-19 likely to impact the brain long-term, an amazonian tea containing dmt stimulates the formation of new brain neurons, significant link found between air pollution and neurological disorders in u.s., blocking hdac2 enzyme may reverse memory loss caused by alzheimer’s disease, lysosomes may contribute to alzheimer’s disease, study shows poor sleep linked to alzheimer’s disease, ucla researchers image abnormal brain proteins in retired nfl players, galactic cosmic radiation poses significant threat to astronauts, could accelerate the onset of alzheimer’s, 90 comments.
Rush human trials. This is a disease of desperation. I might participate.
I couldn’t agree with you more. So many people could benefit from this kind of research. I just hate that it takes forever to bring breakthrough discoveries to market. It’s a real shame, I feel like more than safety, it is unfortunately wrapped around corporate greed.
I’m so sorry to hear that you may be suffering from this condition. Hang in there! 🥺
Please let my wife join human trial. She is disappearing. We all need this new hope.
As I watch my sister who is in the final stages of Alzheimer’s disease, a family ailment that has taken our father and grandmother and now our sister will be next despite discovered hope in 2020 when I found my sister having cognitive difficulties. We both agreed to get tested for Alzheimer’s disease and we both were tested by Roskamp Institute in Sarasota Florida, where they determined that Sue was deep into Alzheimer’s and that I was five years behind her with the same diagnosis. During 2020 we had to move my sister to a very nice memory care facility in Sarasota and I stayed focus on a daily search for an altzheimer cure on the Internet. This search concerned my wife, a retired occupational therapist who felt my obsession to find a cure to be in itself to be a concern. When she agreed to let me show her how I was searching in December of 2020 when I entered the word altzheimer into YouTube which brought up a podcast of Dr Dhru of Broken Brain and Dr Dale Bredesen on his book The End of Alzheimer’s disease which I ordered, read and realized we might be able to save my sister’s life and in turn prevent me from the same fate. We set up Re-Code with Apollo and pre-code for myself, my wife, out two sons and my brother. Their diagnostic results identified that I, one of our sons, my sister and my brother have the APOE4 gene. When we approached the memory care facility to help us in changing Sue’s diet to the recommended KetoFLEX 12 3 protocol they accused me of following a “Witch Doctor” charlatan and they would prefer that I moved my sister to another memory care facility. A local private clinic that had submitted my and my sister’s blood labs to Dr Bredesen at Apollo and I learned that he had commented that it would be a miracle if they could help Sue, but that I was the money spot. I very much needed to find a way to create a miracle and while searching I Discovered a Seminar with Dr Bredesen at New College in Bradenton Florida which I attended with my wife and my sister. Though the seminar was informative I realized I could have given the seminar or at the very least answered the Q&A at the end, at which time I stood up and pleated for help for my sister as I had hit a stone wall. We were sitting in the front row and Dr Bredesen walked toward me and recommended we contact Marama in Vista California, which we did and they accepted Sue and though improvement started, Sue’s challenges over came any chance of success and Marama recommended that Sue return to be with family at this point of her life. Sue now is in palliative care at another incredible facility in Sarasota called Sunnyside Village which practices Montessori Adult Care. Sue will pass at any time now and I am no longer a candidate for Alzheimer’s, though I do have MCI, which I will overcome while following ketoFLEX-16-3 and under treatment with Grey Matter’s in Sarasota. Sue is presently 80 years old, I will be 82 in December and my brother will be 83 next month and was just diagnosed with Alzheimer’s disease. Why is Big Pharma a major block to this natural cure for one of the largest killers of humans in the world? Additionally why is the Altzheimer Society which solicit for contributions not providing funds for the obvious success of Bredesen, but rather fund Big Pharma and their search for another magic pill? Our lifestyles and diet is killing us early and must be reversed. I believe greed is a partner in killing us and practiced by Big Pharma.
We agree with you, Paul it’s a total shame and disgrace to the industry that greed seems to be the basis for everything that could be positive in the 21st century. My mother lived to 103 and had all of her faculties working perfectly physically, mentally, and emotionally.
I would definitely like to be one of the first test individuals for this new drug. I wish I knew who to contact to get on that list. I would do it today..
Peter, I wonder why there is no mention about a “Protein”, the lack of which is the problem. I know I’m throwing this out there, but that has been the results of studies coming out of the U S. of late
I am so sorry for all your suffering. Yes, greed and dishonesty has taken over. I wish you well.
It is so-o heartbreaking to have a soul partner struggle like this.
I have had major sleep disturbances for the past few years and major memory issues. I am 78 years old & have had no testing for Alzheimer’s. I am interested in helping with testing of this new treatment. Ellen C. Manson, dob 12/20/1945. RN,MSN, Psychiatric Nurse & therapist. Worked for 50 years before retiring.
My husband is in the early stages of Alzeimers, we are desperate for a cure. Please keep us posted
I feel this would be a signicant help to my already started Alzheimers and would welcome it with open arms and heart, Where in B.C. Canada can I go to get this help tight now?
Read everything you can. Find out the side effects. There are thousands of Peer Reviewed, Patented research with the company name in the research. It has saved my life in more ways than one. Does this cure or possibly make it better for a quality life? Is the rate high enough to go through trials that have side effects that’s you aren’t willing for? UsingPubMed, search for NRF 2 and Alz. NRF2 and oxidative stress. Doctors are discover tools that have helped, cured, lower medication, things that help disease including Alz. Look up HHV6_foundation and put in viruses and Alz. With correct care that is balancing the body, it’s amazing what possibly can happen. Just be an educated patient and I hope this is a good thing. I’m still going down rabbit holes before suggesting it. Many in my family have/had Alz. It’s very hard and I wish us all the best!!
Why is it always greed that’s why the world’s in such a mess. well done in your work it gives great hope.
Simply amazing search for a cure…and i have to add things are going to changing dramatically on account of AI which is nothing like traditional way of looking at things n i wish you n others at risk of this dreaded disease.
WHERE in Surrey. B.C., Canada can I get this much needed help now?
I don’t think it’s greed. If it was greed, they would go to market immediately and make the money. The research stage is all money losing… betting that one day you’ll make it back, at least on many of your research topics. The hold-up is safety and there’s a delicate balance between getting cures to people asap vs. putting out untested medicines with side effects that can potentially be worse than the original problem. With more devastating illnesses, people are more willing to take risks on less-thoroughly-tested drugs, but this article only says there was some sign of benefit in mice. That doesn’t mean the benefit will necessarily transfer to humans, though it may be likely.
I have family members suffering also and am extremely concerned about this disease. But I don’t think it is helpful to throw the very people researching the cures under the bus under the label of greed unfairly. But let’s go QUICKLY, PLEASE!
Great news long overdue for generations.. Ready for trials..
Please update us on the journey of yourself and your family. Your story is very interesting and im sure everyone would like to hear more on any success you have had with your diet or anything else you might have come across. Thank you and i hope you are well.
Where is Auburn University in the world?
Alabama USA
How can we be tested for alz. since my family has history of having it.
There is a new blood test for P-TAU 181A It was approved for Medicare payment this summer. Do not let your doctor tell you that you don’t need it because they do not see evidence of ALZ. If your IQ was already high, (148) you will still have relatively high IQ at 120. You will notice the difference, even though the doctor sees an above average person.
RD Hall is correct. I am an Advanced Registered Nurse Practitioner with a clinical concentration in gerontology.
I noticed changes in my cognitive functions, specifically word finding and spelling as well as decrease in concentration. Decrease in concentration causes the brain to NOT encode memory efficiently, thereby, resulting in the symptom of short term memory loss.
My mother had dementia. Most probably vascular type dementia (VD) as her high blood pressure had not been regulated appropriately. Her primary physician would not diagnose her with dementia. She kept saying it was “memory loss appropriate for age.” NO type of memory loss is appropriate for age. As the brain ages there is definitely a slowing of the brain’s ability to either maintain the appropriate amount of neurotransmitters and block degenerating neurotransmitters. BUT memory should not decline significantly. Individuals with high IQs or who have had extensive education should not have a great decrease in IQ to the extent of dementia just because of aging.
I explained my symptoms to both my primary and neurologist. At first, both stated I was showing signs of “memory loss appropriate for age.” Nope. I knew better. I went back to the neurologist and presented him, in writing, my symptoms and specific incidences. That caught his attention.
Finally my work up included detailed lab analysis, MRI, and cognitive testing via computer, The P-TAU 181A blood analysis indicated a slight increase in TAU tangles in my blood. Computer testing also indicated a decline in concentration and visual memory. So, yeah, my brain is not encoding as before and I definitely had noticed.
However, the two most important diagnostic tests, that are not available to everyone, are APOE4 gene testing and PET SCAN. I am hoping to be accepted for one of the longitudinal studies at the Memory Clinic at Mayo Hospital in Jacksonville, FL.
I encourage everyone with concerns of memory problems for themselves or family members to write down specifics and write down the appropriate diagnostic tests needed to appropriately diagnose cognitive decline. Give what you have written down to your doctor. Do not take “no” for an answer. Change doctors if needed. And, yes, begin with current pharmaceuticals available as soon as possible.
Cognitive impairment and decline is not covered in med school with any depth. After med school and internship, a doctor has to specialize in cognitive functions of the brain. General neurologists also do not have the specialized education to diagnose and manage cognitive functions to the detail needed to help patients and family members deal with dementia. And, yes, a full healthcare team including cognitive specialist, Social Worker, Rehab and occupational therapy, and psychiatrist are needed to truly help patients through this devastating disease process.
Do you know the name of the blood test for Alzheimer since my husband just passed a question and answer and was then told that he is in the beginning of this dreaded disease which is taking two of his sisters.
Wish to receiving latest discovery on alz
You are jumping the gun. “Mice genetically modified to replicate early Alzheimer’s stages” is not a good model for a chronic neurodegenerative human disease like Alzheimer’s. It also ignores all the cultural factors involved in this disease, including lifestyle. I would not want to be the guinea pig for these experiments.
Also, you need to ask if there is a conflict of interest here, since they are promoting a drug for prevention of Alzheimer’s, which would be an extremely lucrative market. If you check the reference at the bottom of the article above, it takes you to the study, where it says, “Vladimir Coric and Irfan A. Qureshi are employees and shareholders of Biohaven Pharmaceuticals.” The authors have a conflict of interest, and it was not mentioned in this article by SciTechDaily. Seems like STD has a conflict of interest, too, with this product/article.
Well spotted!
Wyeth Pharmaceutical who was bought by Pfizer had a drug that helped the body remove the plaque in the brain and actually cured people with Elzheimer’s disease. They were in the process of FDA approval it was in all the science journals and in the media. They shelved the drug because of cost of the final approval and they already held patents for drugs that just slow the progression down. They poison our food and then create drugs to keep us going. They will never market products that cure. There just isn’t any money in it.
I would just like a straight answer to the questions! Is there anything a person can do to prevent the onset of alzheimers or to atleast slow it down? Do vitamins help in the prevention or not? This issue effects so many families and the research needs to reflect the great need for help for this disease!
Please start human trials ASAP! I want to participate if at all possible, I feel like I’m sinking fast.
Yes I would participate as well.
put me down for human trials.
I have a family member who needs this how can she get into the trial
How do I get in on the trial?
My mother had Alzheimer’s and it was terrible to see her decline and eventual death. Anything that can be done is a blessing.
How not to get Alzheimer’s…eat everything organic, no fried food, no factory food, no restaurant food, no packaging food….detox you liver and kidneys …. buy land and grow you food. Eat raw organic plant base diet no dairy.
A little extreme, IMHO. However, That’s great advice for incorporating into anybody’s life.
I think the bigger problem with Alzheimer’s is we don’t really understand it fully, and there is, based on some research, a large percentage of this disease that is introduced from genetics. Medical breakthroughs are required to assist those of us unfortunate enough to not be able to follow such an intense regimen, if the genetics will just crush everything that you’re trying to do.
“A little extreme”? A mere hundred years ago it was called “normal lifestyle”.
It’s difficult living with Altz patient, my mom. She is perfectly healthly, just forgets, dates, reasons, daily events. She could easily live another 10 years. she is 80. My dad got cancer, lasted 4 months. This is cruel to say. I wish for the cancer, it is very hard watching a parent slowly disappaite.
This drug has already failed a human Alzheimer’s trial as well as trials for other conditions. Disgraceful that this wasn’t mentioned.
BS. The two people I know with Alzheimer’s are health nuts. All the organic food didn’t help them or slow down the disease one bit.
Hi… I completely understand. I am also taking care of both my parents who both have dementia. Watching the declined is unimaginable. They have become children. I am a single mom and raising my autistic son was easier than taking care of my parents. My mom is more advanced then my dad but he is catching up. She has been fighting this disease since 2009. She can still walk, talk and sometimes hold a conversation. But she can’t remember where to put her used toilet paper. I completely see the thoughts of how a modified Alzimer disease is NOT the same as the real article. Why can you not use the real Gene’s of dementia or Alzimers to get better results? Is that not possible? Maybe I should silly and uneducated in this field… All I know are people and families all over the world are suffering. I applaud the scientists and encourage them to continue their work because maybe by the time I am diagnosed there will be a cure.
Does this research other types of dementia. I have white matter disease. It affects my speech, my memory, my unsteadiness, etc. Alzheimer’s is grey after disease, but some of the same problems. So far haven’t located a new neurologist that knows about it and won’t take me as a patient. The neurologist that diagnosed and went through all my cat scans to show me my problem areas in my brain has long retired and moved.
If only it was that easy!
The mice are happy, when will it be our chance to try this drug on us?
A carnivore diet has been shown to reverse cognitive decline in the elderly. Many elderly care facilities have adopted this with great results. There are ongoing studies but why wait. There are several you tube videos chronicling the patients health before and after, If they haven’t been banned.The changes in these people are remarkable
That is not a guarantee by eating all organic,etc.etc Very extreme
If you start with avoiding sugar consumption you will make a great step towards avoiding alzheimer. So, in other words: no soda (coke, Pepsi, 7up, etc), no processed foods, nothing labeled as diet, no candies, no ice cream, no processed yogurt, etc. What a big compromise.
My gramma did this exactly and still got it..
Lol the majority of land is toxic already and why not enjoy what you eat. We all die sometimes.
It’s about how you die! You would have to be there and observe the process.
There was Alzheimer’s back when we did all that because we had no other option. Not saying that it isn’t a great idea. Just not a way to never get Alzheimer’s.
Your answer is so vague that it’s almost worthless. Limit carbohydrate grams to no more than 35 per meal, consume a minimum of 100 MG of phosphatidylserine daily. Maintain a healthy body weight, not overweight and not obesity, that eliminates 70% of the American population! Like I said your answer is pretty worthless, follow these 3 recommendations and you can achieve your goal of avoiding dementia and alzheimers. Good luck!
Nice thought , but your can’t avoid the air and water, that are highly toxic and polluted, just about everywhere in the world now. Humans have pretty much doomed the world to neurodegenerative disease and early death.
In other words, don’t go out to celebrate at restaurant’s or such , don’t eat comfort food, don’t drink , don’t smoke , don’t take drugs of any form , just eat boring food and stay at home being bored all hole life 🤔🤔
Oh also don’t ever get takeaway foods 😢
Why release headlines that get false hope up? Even if it works on humans, only an idiot would think Big Pharma won’t buy & bury this unless they can find a way to sell it to you monthly for the rest of your life. They want manageable conditions, not cures!
Look at Ozempic. It’s perfect. You stop taking it and you get hungry beyond imagination and gain all the weight back and then some! They’ve got you! The OWN you! And if you have a severe side effect like permanent stomach paralysis that leads to suicide (and will because you can NEVER eat again and have to be tube fed the rest of your life), you’re easily replaced because there’s an endless supply of fat people…ahem… customers.
Why would they bury it..they would make a fortune. It not like it’s a one and done pill. I never understood the logic of hiding drugs.
I am a medical anthropologist researcher and author, and have done sleep research which shows that the way you sleep can be reducing brain circulation. Circulate or deteriorate. To better circulation your brain at night, raise the head of your bed! You will feel the difference the next morning. This also helps with sleep apnea, migraines, glaucoma, stroke, ADHD, sinus congestion, ear infections, and more. See my article, Heads Up! The Way You are Sleeping May be Killing You! https://www.academia.edu/1483361/Heads_Up_The_Way_You_Are_Sleeping_May_Be_Killing_You_
Too late for old Joey B, tho… even if he had a brain worth saving.
As said by truckers: “10-4.”
Lets all ignore the big fat elephant in the room of terrible diet and lifestyle and instead make big pharma more money. I dont think the biggest criminals in the history of man kind have enough $$$. Keep poisening yourself with processed garbage and sugar and then spend 1200 bucks a month on a magic pill that manages symptoms while creating others. Yeah, that sounds like a great plan of action. 🤣🤣🤣
He has more brains than that numb skull you’re going to vote back in to run your country he’s nuts
Give it to Joe Biden
Joe needs this drug.
My moms like late stage 5 or 6 Dimentia..can this work on her? I’m desperate to save her life. I love her and dont wanna lose her.
Mark, I’m with you, I have recently read that a deep depression can mimic dementia. My Mother had a back surgery 10 years ago, which went wrong, and now she has lost the ability to walk. This woman was once amazing. Built 3 houses, ran companies, cared for grand kids, and so much activities in her life… I feel she has gave up, kids don’t call, don’t visit, I do all I can for her, I just don’t feel enough, but biggest heart breaker, we pay crazy high insurance, and it seems they deny everything… And it burns my rear raw, how we can give illegal aliens all this free medical, free whatever, and we people like my 92, I fight for everything. Her sister 99, probably still be here, she got pneumonia in 21, they deemed covid, it wasn’t she passed just shy weeks of 100. Grandparents 103- 108 Longevity is in our family, but this now with mom, dementia no help, and the Dr’s say, well.. What do you expect ? She is 92 and has had a great life??? So yes, I get it and I understand, it’s all about money… Do take from what I hear, 3x a day cold pressed, virgin olive oil in dark green glass, and eat 1/2 cup a day of blueberries… I cut out all her sweets… And give her a good salad every day for lunch, fish 3 X a week, chicken 2x a week.. We still fighting for each day be new.. Good Luck!
The great orange one who is the oldest candidate in history to run for president definitely needs help from this medication! Maybe that will help him to stop repeating the same stories at all of his rallies, Mixing up the names of current political figures with figures from 30 years ago, talking about his terror of sharks, etc.… LOL
Yes Trumpy and joe and Putin would benefit. Hopefully many more do
Give it to DT. He needs it as much as JB.
Na, ol’ DT’s too far gone 😆
Pharmaceutical greed is very sad.Money of there expectations is really also sad.We need more new laws to prevent the over burden of large pockets.
All well and good producing these drug’s but NICE in this country won’t release them to the NHS because they are too expensive. I stopped giving to Cancer for research because of this problem.
I’d participate. I know when I get really stress & also lack of sleep my memory fails. A big difference from a few years ago. Also having apnea & lack of oxygen can cause lots of ailments including but not limited to memory issues & onset of many diseases.
Got excited when I saw this for my hubby the doctors say he is in early stages and he was diagnosed at 60. We work daily to reverse it, I have advised him for years to keep the phone away from his head. We also increase his brain activity thought games, puzzles, we have changed his diet somewhat, but the decline is still there. It he taking Donezepril but it does a little. Not that impressed with the results. I trust and believe God that he is healed, in the meantime we continue to enjoy life together treasuring all we have.
How can I get help before it’s to late? I forget everything. I need help now.
Well JB was dropped like a cheap suit by his own party and now Commie CackKam ho is even worse than that demented JB
They need to test it on biden whether he likes it or not
I highly doubt this drug is capable of “reversing memory loss.” That claim is borderline science fiction. A drug like this isn’t going to magically make you remember things again. I could see preventing further cognitive decline, but reversing is a huge stretch.
Where and how to get it
My mother has Alzheimer please let me know more information.
Someone asked what they can do about oncoming Alzheimer’s. Memintine is one drug that can delay the onset of full Alzheimer’s. Far from perfect but better than nothing.
Love the story. Love the possibility. WHAT IS CONFUSING is an earlier very hopeful, different Alzheimer compound discovery from UCLA. I hope the on it’s face conflicting approaches resolve to understandable differences in what aspect of GABA is involved by Auburn’s team by ELEVATING the level of GABA to reduce glutamate vs the UCLA team of INHIBITING GABA to boost electrical oscillation: VERY ODDLY HOWEVER, A DIFFERENT RECENT ‘Alzheimer’s Breakthrough – Claims to Switch the Alzheimer’s Memory Back On by Focusing on INHIBITING GABA, and thereby BOOSTING ELECTRICAL OSCILLATION activity in the brain with a newly developed compound DDL-920. https://newatlas.com/health-wellbeing/memory-molecule-alzheimers/
Great discovery. Eager to hear more breakthroughs from this wonder drug.
Save my name, email, and website in this browser for the next time I comment.
Type above and press Enter to search. Press Esc to cancel.
New Alzheimer’s drug shows promise in slowing disease but may include significant risks, study shows
An experimental Alzheimer’s drug modestly slowed the brain disease’s inevitable worsening, researchers reported Tuesday — but it remains unclear how much difference that might make in people’s lives.
Japanese drugmaker Eisai and its U.S. partner Biogen had announced earlier this fall that the drug lecanemab appeared to work, a badly needed bright spot after repeated disappointments in the quest for better Alzheimer’s treatments.
Now the companies are providing full results of the study of nearly 1,800 people in the earliest stages of the mind-robbing disease. The data was presented at an Alzheimer’s meeting in San Francisco and published in The New England Journal of Medicine. U.S. regulators could approve the drug as soon as January.
Every two weeks for 18 months, study participants received intravenous lecanemab or a dummy infusion. Researchers tracked them using an 18-point scale that measures cognitive and functional ability.
Those given lecanemab declined more slowly — a difference of not quite half a point on that scale, concluded the research team led by Dr. Christopher van Dyck at Yale University.
That’s a hard-to-understand change, but measured a different way, lecanemab delayed patients’ worsening by about five months over the course of the study, Eisai’s Dr. Michael Irizarry told The Associated Press. Also, lecanemab recipients were 31% less likely to advance to the next stage of the disease during the study.
“That translates to more time in earlier stages” when people function better, Irizarry said.
But doctors are divided over how much difference those changes may make for patients and families.
“It is unlikely that the small difference reported in this trial will be noticeable by individual patients,” said Dr. Madhav Thambisetty of the National Institute on Aging, who noted he wasn’t speaking for the government agency.
He said many researchers believe a meaningful improvement would require at least a difference of a full point on that 18-point scale.
But Dr. Ron Petersen, an Alzheimer’s expert at the Mayo Clinic, said the drug’s effect was “a modest one but I think it’s clinically meaningful” — because even a few months’ delay in progression could give someone a little more time when they’re functioning independently.
The trial is important because it shows a drug that attacks a sticky protein called amyloid — considered one of several culprits behind Alzheimer’s — can delay disease progression, said Maria Carrillo, chief science officer for the Alzheimer’s Association.
“We all understand that this is not a cure and we’re all trying to really grasp what it means to slow Alzheimer’s, because this is a first,” Carrillo said.
But any delay in cognitive decline early on could be meaningful for “how much time we have with our loved ones in a stage of disease where we can still enjoy family and outings, vacations, bucket lists,” she said.
Amyloid-targeting drugs can cause side effects that include swelling and bleeding in the brain, and lecanemab did as well. One type of this swelling was seen in about 13% of recipients. Eisai said most were mild or asymptomatic.
Also, two deaths have been publicly reported among lecanemab users who also were taking blood-thinning medications for other health problems. Eisai said Tuesday the deaths can’t be attributed to the Alzheimer’s drug.
But Mayo’s Petersen said if lecanemab is approved for use in the U.S., he’d avoid prescribing it to people on blood thinners at least initially.
And Thambisetty said the death reports raise concern about how the drug may be tolerated outside of research studies “where patients are likely to be sicker and have multiple other medical conditions.”
The Food and Drug Administration is considering approving lecanemab under its fast-track program, with a decision expected in early January. If approved, it would be the second anti-amyloid drug on the market.
Nearly all treatments available for the 6 million Americans with Alzheimer’s — and millions more worldwide — only temporarily ease symptoms. Scientists don’t yet know exactly how Alzheimer’s forms but one theory is that gunky amyloid buildup plays a key role, although drug after drug that targets it has failed.
In a contentious move last year, the FDA approved the first amyloid-targeting drug, Biogen’s Aduhelm , despite a lack of evidence of better patient outcomes. Insurers and many doctors have hesitated to prescribe the pricey drug — another reason experts have anxiously awaited word of how well the newer lecanemab may work.
If the FDA approves lecanemab, patients and their families will need a voice in deciding whether it’s worth the hassle of IV infusions and the risk of side effects for the chance of at least some delay in progression, Petersen said.
“I don’t think we’re going to stop the disease in its tracks” with just amyloid-targeting drugs, he added, saying it will take a combination of medications that target additional Alzheimer’s culprits.
Researchers are preparing to test lecanemab with other experimental drugs, and how it works in high-risk people before they show the first signs of memory problems.
New class of Alzheimer's drugs showing promise in patients in early stage of disease
Topic: Alzheimer's and Dementia
Melbourne grandmother Jan Cody was not eligible for the new drug trial but now has hope. ( ABC News: Loretta Florance )
For decades scientists and families have been frustrated by the intractable nature of Alzheimer's disease.
Key points:
- A study showed donanemab could slow Alzheimer's disease progression by 35pc in patients in the earliest stages of the disease
- Geriatrician Michael Woodward says the medical community is excited by the results
- The donanemab study findings were similar to those of its predecessor lecanemab
As the population ages and more people develop the devastating condition, there have been no new treatments coming onto the market and for many, no hope in sight.
That was until two years ago.
In a short time, decades of research has started to come to fruition, with at least three new drugs demonstrating the first glimmers of promise.
The latest is called donanemab, with the findings of a global trial involving 1,700 patients presented at a major Alzheimer's conference in The Netherlands.
Sixteen Australians took part in the trial at eight sites in Victoria and New South Wales.
The drug, from pharmaceutical giant Eli Lilly, was able to slow Alzheimer's disease progression by 35 per cent in patients in the earliest stages of the disease.
Across the whole study, there was a 22 per cent slowdown in the disease's progress at the 18-month mark.
Sixteen Australians took part in the donanemab trial. ( Supplied: Eli Lilly )
Michael Woodward, a geriatrician who has been involved in Alzheimer's research for decades, was at the Alzheimer's conference and said the medical community was excited by the results.
"I would regard this as the end of the beginning in Alzheimer's therapies," he said.
"The word breakthrough is used perhaps a little too often, but this is a major breakthrough.
"We now have three drugs that have been shown that can critically slow down the decline."
How does the new drug work?
Donanemab is a monoclonal antibody designed to clear the brain of amyloid plaque, which experts believe plays a role in Alzheimer's disease.
Researchers have long been trying to work out whether a protein called beta-amyloid plaque (BAP) or another protein called tau is responsible for Alzheimer's, or a combination of the two.
Those in the study were all in the early stages of Alzheimer's and aged between 60 and 85.
At the 12-month mark, the researchers said 47 per cent had no evidence of amyloid plaques, compared with 29 per cent in the placebo group.
Associate Professor Stephen Macfarlane says donanemab has shown positive results in three of his patients. ( Supplied )
Patients also did not need indefinite treatment, with injections being able to reduce amyloid to non-existent levels where they would not re-accumulate for many years.
Stephen Macfarlane had three patients in the study through his work with The Dementia Centre at HammondCare in Victoria.
He said the medication was the equivalent of slowing the rate of the disease by seven and a half months compared to someone who was not taking it.
"These drugs slow the progression of the disease, they don't cause people to improve," Dr Macfarlane said.
He said it was the most promising drug in two decades for Alzheimer's research.
"It's the most effective, and the safety data seems to be on a par with similar drugs," he said.
The findings show there was a risk of brain bleeding and swelling in a subset of patients, including 1.6 per cent of participants who experienced serious forms, and three who died.
"Bearing in mind that Alzheimer's disease is a fatal and otherwise untreatable illness, some degree of risk is inherent in the process," Dr Macfarlane said.
Drug follows on heels of another, lecanemab
The donanemab study findings were similar to those of its predecessor lecanemab, sold under the brand name Leqembi.
It reduced cognitive decline by 27 per cent in patients with early Alzheimer's in a study published last year.
Lou Coenen is among the Australian patients in a lecanemab trial.
This drug from Japanese drug maker Eisa is being tested in four trials that include Australian sites across 18 locations.
The 72-year-old was diagnosed with Alzheimer's about five years ago and had a family history of the disease.
"You just start feeling your thinking doesn't work quite as fast," Mr Coenen said.
"You start to wonder why."
He decided to take part in a clinical trial of lecanemab through the KaRa Institute of Neurological Diseases to help give back to the health community.
He says he does notice a difference on the medication.
It is allowing him to spend more time with his wife and family and still participate in community activities such as The Men's Shed.
"I know compared to other people this is working," he said. "But I don't have a comparative of another me that says otherwise."
On June 30 Australia's Therapeutic Goods Administration (TGA) started work to consider approving lecanemab in Australia.
This drug has shown similar results to donanemab in patients with early Alzheimer's but also comes with risks of brain swelling and bleeding in a small subset of patients.
Melbourne grandmother Jan Cody had to give up work and other activities after realising she had Alzheimer's disease. ( ABC News: Loretta Florance )
How much will it cost?
New Alzheimer's drugs to the market are predicted to be hugely expensive for governments because of the significant time and cost they took to develop.
Leqembi is priced at about $US26,500 ($39,974) for a year's supply of infusions every two weeks but there is no potential price for donanemab yet, which will involve monthly injections.
"That's going to be a big challenge," Dr Woodward said.
"But we've got to look also at the savings. The total cost of care for Alzheimer's disease is probably closer to about $6-7 billion per year in Australia."
Dr Macfarlane said the drug would also mean Australia would need to revamp its Alzheimer's infrastructure so PET scans were more available for early diagnoses, regular hospital infusions were easier to access, and patients were diagnosed much sooner.
"We know in Australia that on average there's about a three-year delay between people first experiencing symptoms of memory loss and actually receiving a diagnosis," he said.
Biogen drug caused controversy
The drugs follow the groundbreaking but controversial release of Biogen's Aducanumab in 2021.
It is another monoclonal antibody that also works by removing the build-up of amyloid plaque proteins.
It was controversial because of the way the research was structured and the pharmaceutical company's relationship with US regulators.
In June this year the Therapeutic Goods Administration found the drug did not meet its safety and efficacy requirements for approval in Australia and Biogen withdrew its application.
Latest findings bring hope for patients
For Melbourne grandmother Jan Cody, the first sign she knew her memory was failing was when her three children met to discuss her declining mental state.
Victorian grandmother Jan Cody has noticed improvements since beginning treatment for Alzheimer's disease. ( Supplied )
The 75-year-old had to give up her work as a psychologist, as well as cooking and driving.
"My world just shrank. There's really no medication to take," she said.
She has been involved in some Alzheimer's trials but was not eligible for donanemab.
"The slowing it down takes a long time," she said. "So one really doesn't know whether you're going to last."
"But now I do have a glimmer of hope."
Alzheimer's Drug Shows Promise For Treating Another Type of Dementia
A new study suggests that a treatment for Alzheimer's , the most common type of dementia , can also treat the second most common type – dementia with Lewy bodies (DLB).
The treatment is a cholinesterase inhibitor (ChEI) : it's already been used with DLB patients, but up until now it was unclear how effective it was. This new research indicates it can indeed slow cognitive decline.
DLB is closely related to Alzheimer's and Parkinson's , but we know less about it. The team from the Karolinska Institutet in Sweden says their findings add some important depth to our understanding of the condition.
"Our results highlight the potential benefits of ChEIs for patients with DLB and support updating treatment guidelines," says neurobiologist Maria Eriksdotter.
The study involved 1,095 people with DLB, who were being treated with a ChEI or memantine (another Alzheimer's treatment), or not being given either of the medications. The study participants were followed for a period of up to 10 years.
Those on ChEIs – particularly two types called donepezil and galantamine – showed significantly slower cognitive decline than those being given memantine or neither treatment. There was also a reduced risk of death in the CHEI group, though this only held for the first year after DLB diagnosis.
While this was an observational study of existing records rather than a clinical trial , and therefore can't show cause and effect, it does indicate that it's worth continuing to investigate ChEIs as effective treatments for some DLB patients.
Around 10–15 percent of dementia cases are caused by DLB, which typically drives faster cognitive and functional decline than Alzheimer's , affecting sleep, behavior, cognition, movement, and bodily functions.
"This study demonstrates that the use of ChEIs in patients with DLB may reduce the risk of cognitive decline compared to memantine treatment or no treatment," write the researchers in their published paper.
"Future studies are needed to better elucidate these mechanisms."
In this sample at least, ChEIs made more of a difference than memantine. Neither treatment reduced the risk of major adverse cardiovascular events , which was somewhat surprising as ChEIs have shown to reduce risk in Alzheimer's patients.
The main job of ChEIs is to slow down the loss of the chemical acetylcholine ; it's made by neurons, is crucial for processing information in the brain, and gradually gets lost as Alzheimer's takes hold. It seems that this slowing down is beneficial for brains with DLB too, something further research can look into.
"There are currently no approved treatments for DLB, so doctors often use drugs for Alzheimer's disease, such as cholinesterase inhibitors and memantine, for symptom relief," says neurobiologist Hong Xu, from the Karolinska Institutet.
"However, the effectiveness of these treatments remains uncertain due to inconsistent trial results and limited long-term data."
The research has been published in Alzheimer's & Dementia: The Journal of the Alzheimer's Association .

Tom Murphy, Associated Press Tom Murphy, Associated Press
Leave your feedback
- Copy URL https://www.pbs.org/newshour/health/alzheimers-drug-shows-promise-in-early-results-of-study
Alzheimer’s drug shows promise in early results of study
Shares of Biogen and other drugmakers researching Alzheimer’s disease soared early Wednesday after Japan’s Eisai Co. said its potential treatment appeared to slow the fatal disease’s progress in a late-stage study.
Eisai announced results late Tuesday from a global study of nearly 1,800 people with early-stage Alzheimer’s.
The drugmaker said early results showed that its treatment, lecanemab, reduced patient clinical decline by 27% when compared to a placebo or fake drug after 18 months of the infused treatment. Patients were monitored using a scale that measures how they do in areas like memory, judgement, problem solving and personal care.
Eisai Co. Ltd. said it would discuss full results from the research at a conference in late November. It also plans to publish its findings in a peer-reviewed medical journal.
The company is already seeking an accelerated approval from the U.S. Food and Drug Administration, and the agency is expected to decide by early next year. Eisai and Biogen will co-promote the drug.
The initial results appear to be “quite robust” and will likely support regulatory approval, Mizuho Securities analyst Graig Suvannavejh said in a research note.
A statement from the Alzheimer’s Association called the findings the most encouraging to date for potential treatments of the underlying disease causes.
Alzheimer’s is a progressive neurological disease with no known cure. Long-standing treatments on the market just manage symptoms, and researchers don’t fully understand what causes the disease.
Last year, Biogen’s Aduhelm became the first new Alzheimer’s drug introduced in nearly two decades. But it has largely flopped after debuting with a price tag of $56,000 annually, which Biogen later slashed.
Doctors have been hesitant to prescribe it, given weak evidence that the drug slows the progression of Alzheimer’s. Insurers have blocked or restricted coverage over the drug’s high price tag and uncertain benefit. Like Aduhelm, lecanemab, which Eisai developed and ran through clinical trials, seeks to remove a protein called beta-amyloid from the brain.
But Eisai executives say lecanemab focuses more on floating clumps of the protein before it forms a plaque, which is what Aduhelm targets.
Eli Lilly and Co. also is developing a potential treatment, donanemab, that helps clear the protein.
Shares of Cambridge, Massachusetts-based Biogen Inc. jumped more than 50% in premarket trading Wednesday morning to top $300. The stock had largely tumbled since Aduhelm’s debut last year.
Shares of Indianapolis-based Eli Lilly and Co. were up 8%.
Support Provided By: Learn more
Educate your inbox
Subscribe to Here’s the Deal, our politics newsletter for analysis you won’t find anywhere else.
Thank you. Please check your inbox to confirm.

- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
September 28, 2022
New Alzheimer's drug shows promise in phase 3 clinical trial
Japanese drugmaker Eisai on Wednesday said its experimental drug lecanemab helped slow thinking declines among people in the early stages of Alzheimer's disease .
The findings from a phase 3 clinical trial have yet to be peer-reviewed in any medical journal. But according to a company news release , "lecanemab treatment met the primary endpoint and reduced clinical decline on the global cognitive and functional scale, CDR-SB, compared with placebo at 18 months, by 27%."
Given the largely disappointing rollout of Biogen's Alzheimer's drug, Aduhelm , last year, any drug which appears to help Alzheimer's patients is welcome. But expert reaction to the Eisai announcement was mixed.
In a statement , Dr. Joanne Pike, president of the Alzheimer's Association, called the results an "exciting major development."
"These are the most encouraging results in clinical trials treating the underlying causes of Alzheimer's to date," she said. "These results indicate lecanemab may give people more time at or near their full abilities to participate in daily life, remain independent and make future health care decisions."
But another expert offered a more muted response.
Dr. Alberto Espay, a neurologist at the University of Cincinnati College of Medicine, told NBC News that the benefit seen with lecanemab was "small" and added it might not translate to meaningful improvements for patients. Still, Espay believes "patients can view this [new development] with cautious optimism."
The new trial included almost 1,800 patients with early-stage Alzheimer's disease whose progress was tracked over 18 months. Investigators tracked cognition using what's known as the the CDR-SB scale , which Eisai said is "used to quantify the various severity of symptoms of dementia."
"Based on interviews of people living with Alzheimer's disease and family/caregivers, qualified healthcare professionals assess cognitive and functional performance in six areas: memory, orientation, judgment and problem-solving, community affairs, home and hobbies, and personal care," the company explained.
Patients enrolled in trials based in the United States were ethnically diverse, Eisai noted, with 25% of participants being either Black or Hispanic.
Compared to patients taking a placebo "dummy" pill, those who got lecanemab saw a "significant" slowing of cognitive decline as measured by the CDR-SB scale, Eisai reported. Noticeable changes in the rate of decline began as early as six months after taking the drug.
PET scans of the brains of patients who took lecanemab also showed noticeable declines in levels of the amyloid protein plaques , which have long been a hallmark of Alzheimer's disease, Eisai noted.
As to possible side effects, there was a slight uptick in drug users of cerebral hemorrhages known as "amyloid-related imaging abnormalities" (ARIA), with 21.3% of lecanemab users experiencing this issue, compared to 9.3% of those who took the placebo.
Lecanemab is a monoclonal antibody drug that's designed to target and help remove Alzheimer's-associated amyloid plaques in the brain. According to Eisai, in July the drug was placed on an "accelerated approval pathway and granted priority review" by the U.S. Food and Drug Administration. That may help speed the drug 's path to approval.
Eisai plans to present the full phase 3 clinical trial data in late November "at the Clinical Trials on Alzheimer's Congress [CTAD], and publish the findings in a peer-reviewed medical journal," the company said.
However, Alzheimer's patients and their caregivers have had their hopes raised in the past—only to be let down later.
Aduhelm also works by reducing amyloid plaques in the brain, but its approval by the FDA was contentious because amyloid reductions didn't seem to translate to significant improvements in daily function or any real slowing of disease.
Some scientists have begun to move away from an "amyloid-centered" approach to Alzheimer's treatment altogether.
Pike agreed that the full verdict on lecanemab is not yet in.
"We will know more at the end of November when the data behind these initial results will be public," she said. "We look forward to learning more at that time about participant safety and representation in the trials. It's also important to manage our own expectations that this treatment is not yet Food and Drug Administration [FDA]-approved and is not yet available in doctors' offices."
Still, Pike believes the new findings offer much needed hope.
"This is a major milestone for Alzheimer's disease treatments," she said. "It is a significant gain for people with the disease and their families, and it further positions us to advance our mission in new and exciting ways."
Copyright © 2022 HealthDay . All rights reserved.
Explore further
Feedback to editors

Team discovers role of ferroptosis in combating breast cancer resistance
9 hours ago

Metformin found to reduce organ aging in male monkeys

Researchers discover new target for treating heart failure: Protein kinase N
10 hours ago

RNA-sequencing study provides novel insights into chronic lymphocytic leukemia

Scientists discover potential cause of an enigmatic vascular disease primarily impacting women
11 hours ago

Key factors identified that can impact long-term weight loss in patients with obesity prescribed GLP-1 RA medications

Study finds 'supercharging' T cells with mitochondria enhances their antitumor activity

Using AI, researchers find e-cigarette brands are skirting the rules about health warning labels on Instagram

New therapy that targets and destroys tau tangles: A promising Alzheimer's disease treatment

Neoself-antigens found to induce autoimmune response in lupus
Related stories.

Experts encouraged by Alzheimer drug preliminary data
Sep 28, 2022

Medicare's strict Alzheimer's policy raises ante for drugmakers
Apr 11, 2022

Lilly to seek FDA approval for new Alzheimer's drug
Jun 25, 2021

New Alzheimer's drug delivers disappointing results
Jun 17, 2022

Biogen lays out awaited Alzheimer's drug data in obscure journal
Mar 17, 2022
FDA OKs marketing of new test to help diagnose Alzheimer's disease
May 9, 2022
Recommended for you

A new biomarker makes it easier to distinguish between Alzheimer's and primary tauopathy
Sep 12, 2024

Reductions seen in benzodiazepine dispensing when states relax medical, recreational cannabis access
Sep 11, 2024

Boosting brain protein levels may slow decline from Alzheimer's
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Election 2024
- Entertainment
- Newsletters
- Photography
- AP Investigations
- AP Buyline Personal Finance
- AP Buyline Shopping
- Press Releases
- Israel-Hamas War
- Russia-Ukraine War
- Global elections
- Asia Pacific
- Latin America
- Middle East
- Election results
- Google trends
- AP & Elections
- College football
- Auto Racing
- Movie reviews
- Book reviews
- Financial Markets
- Business Highlights
- Financial wellness
- Artificial Intelligence
- Social Media
Alzheimer’s drug shows promise in early results of study

FILE — The Biogen Inc., headquarters is shown March 11, 2020, in Cambridge, Mass. Shares of Biogen and other drugmakers researching Alzheimer’s disease soared early Wednesday, Sept. 28, 2022, after Japan’s Eisai Co. said its potential treatment appeared to slow the fatal disease’s progress in a late-stage study. Eisai announced results late Tuesday from a global study of nearly 1,800 people with early-stage Alzheimer’s (AP Photo/Steven Senne, File)

- Copy Link copied
Shares of Biogen and other drugmakers researching Alzheimer’s disease soared Wednesday after Japan’s Eisai Co. said its potential treatment appeared to slow the fatal disease in a late-stage study.
The drugmaker said early results showed that its treatment, lecanemab, reduced patient clinical decline by 27% when compared to a placebo or fake drug after 18 months of the infused treatment.
Eisai announced results late Tuesday from a global study of nearly 1,800 people with early-stage Alzheimer’s.
Patients were monitored using a scale that measures mental decline and their ability to do daily activities like getting dressed or feeding oneself.
Eisai Co. Ltd. said it would discuss full results from the research at a conference in late November. It also plans to publish findings in a peer-reviewed medical journal.
The company is already seeking an accelerated approval from the U.S. Food and Drug Administration, and the agency is expected to decide by early next year. Eisai and Biogen will co-promote the drug.
Researchers typically urge caution in evaluating a study until the full results are released. But the initial findings appear to be “quite robust” and will likely support regulatory approval, Mizuho Securities analyst Graig Suvannavejh said in a research note.
A statement from the Alzheimer’s Association called the findings the most encouraging to date for potential treatments of the underlying disease causes.
Some 6 million people in the U.S. and many more worldwide have Alzheimer’s, which gradually attacks areas of the brain needed for memory, reasoning, communication and basic daily tasks.
Alzheimer’s has no known cure. Long-standing treatments on the market just manage symptoms, and researchers don’t fully understand what causes the disease.
Last year, Biogen’s Aduhelm became the first new Alzheimer’s drug introduced in nearly two decades. But it has largely flopped after debuting with a price tag of $56,000 annually, which Biogen later slashed.
Doctors have been hesitant to prescribe it, given weak evidence that the drug slows the progression of Alzheimer’s. Insurers have blocked or restricted coverage due to concerns over the drug’s high price tag and uncertain benefit.
Earlier this year, the federal Medicare program imposed strict limits on who can get the drug, wiping out most of its potential U.S. market. Biogen announced afterward that it would stop most of its spending on the treatment.
Like Aduhelm, lecanemab, which Eisai developed, aims to clear a protein called beta-amyloid from the brain.
The protein forms a plaque that researchers believe is a contributor to Alzheimer’s. They also point to other potential factors like family history and chronic conditions such as diabetes.
Eisai executives say lecanemab focuses more on floating clumps of the protein before it forms the plaque, which is what Aduhelm targets.
Eli Lilly and Co. also is developing a potential treatment, donanemab, that targets the protein.
Shares of Cambridge, Massachusetts-based Biogen Inc. jumped 40% to close Wednesday at $276.61. The stock had largely tumbled since Aduhelm’s debut last year.
Shares of Indianapolis-based Eli Lilly and Co. rose 7.5%.

More From Forbes
Breakthrough reveals new treatment target for alzheimer’s.
- Share to Facebook
- Share to Twitter
- Share to Linkedin
Astrocyte brain cells joined to a capillary forming the blood-brain barrier.
Despite being the most prevalent neurodegenerative disease globally, Alzheimer’s has been notoriously challenging to prevent, let alone cure. The biggest hurdle for delivering drugs into the brain is the blood-brain barrier. These tiny blood vessels form tight junctions between brain cells to regulate the movement of molecules from the blood into the brain. The low permeability of the blood-brain barrier is crucial for keeping harmful pathogens from reaching the brain, but it also prevents synthesized drugs from having an effect. Now, researchers may have uncovered a promising treatment target for treating Alzheimer’s disease: insulin receptors in the blood-brain barrier.
Deficient insulin activity is most commonly associated with Type 2 diabetes. Insulin is primarily responsible for regulating blood sugar. Secreted by the pancreas, this hormone influences metabolism by targeting multiple cells around the body and triggering a signaling cascade. In Type 2 diabetes, the receptors that insulin stimulate become less sensitive, thus blunting the hormone’s effect. Over time, the pancreas attempts to compensate by producing more insulin, but the reduced efficacy of insulin-binding receptors limits the body’s ability to control blood sugar, leading to insulin resistance.
A growing body of evidence now shows that insulin resistance may also be implicated in Alzheimer’s disease. In post-mortem exams of human and animal brains with Alzheimer’s disease, changes in the brain’s insulin-binding receptors seem to correlate with learning and memory deficits. Other reports suggest that impaired insulin signaling may be associated with the accumulation of beta-amyloid and tau neurofibrillary tangles, which are characteristic of Alzheimer’s disease.
If insulin-binding receptors are impaired in Alzheimer’s, it is possible that drugs that target those receptors may be able to slow, or prevent, cognitive decline. The first question researchers had to answer, however, was where in the brain are these receptors located. To answer this question, the team of Quebec researchers, led by Manon Leclerc began their study by examining brain tissue samples from healthy individuals without dementia. They were surprised to find that there were no insulin-binding receptors in the brain itself. Rather, these receptors were dispersed throughout the blood-brain barrier. While other studies have found evidence of insulin receptors in brain cells, insulin’s inability to cross the blood-brain barrier enabled investigators to conclude that this hormone primarily influences the brain by binding to receptors lodged in those blood vessels. Insulin circulating in the bloodstream seems to act as a key that unlocks these receptors and triggers a signaling cascade capable of indirectly stimulating the brain.
When they examined brain samples from individuals with Alzheimer’s disease, however, the number of insulin-binding receptors found in the blood-brain barrier was considerably reduced. The researchers also observed this trend in mice models with genetic mutations associated with Alzheimer’s disease. Not only were there fewer insulin-binding receptors but the activation of these receptors was also blunted. Compared to healthy control mice, those with Alzheimer’s had impaired insulin-dependent signaling, even when exposed to high insulin levels.
Best High-Yield Savings Accounts Of 2024
Best 5% interest savings accounts of 2024.
Upon further analysis, they found that the prevalence of insulin-binding receptors positively correlated with key enzymes involved in clearing beta-amyloid plaques. This is an association that has also been reported in other studies. It has been speculated that beta-amyloid proteins may contribute to insulin resistance by (1) preventing insulin from binding and (2) promoting the degradation of these receptors in the blood-brain barrier. In light of this study’s findings, it seems that Alzheimer’s disease may correspond with fewer binding sites for insulin within the brain vasculature.
This presents a promising treatment approach for Alzheimer’s disease. Without having to cross the blood-brain barrier, drugs that target insulin-binding receptors within the blood vessels directly may be able to influence brain activity. However, it is still not well understood how insulin resistance contributes to cognitive decline, nor how stimulating these receptors would improve symptoms. Although additional studies are needed to understand the role of insulin in Alzheimer’s disease, this brings us one step closer to a future in which it becomes a manageable condition, much like Type 2 diabetes.
- Editorial Standards
- Reprints & Permissions
Join The Conversation
One Community. Many Voices. Create a free account to share your thoughts.
Forbes Community Guidelines
Our community is about connecting people through open and thoughtful conversations. We want our readers to share their views and exchange ideas and facts in a safe space.
In order to do so, please follow the posting rules in our site's Terms of Service. We've summarized some of those key rules below. Simply put, keep it civil.
Your post will be rejected if we notice that it seems to contain:
- False or intentionally out-of-context or misleading information
- Insults, profanity, incoherent, obscene or inflammatory language or threats of any kind
- Attacks on the identity of other commenters or the article's author
- Content that otherwise violates our site's terms.
User accounts will be blocked if we notice or believe that users are engaged in:
- Continuous attempts to re-post comments that have been previously moderated/rejected
- Racist, sexist, homophobic or other discriminatory comments
- Attempts or tactics that put the site security at risk
- Actions that otherwise violate our site's terms.
So, how can you be a power user?
- Stay on topic and share your insights
- Feel free to be clear and thoughtful to get your point across
- ‘Like’ or ‘Dislike’ to show your point of view.
- Protect your community.
- Use the report tool to alert us when someone breaks the rules.
Thanks for reading our community guidelines. Please read the full list of posting rules found in our site's Terms of Service.
- Share full article
Advertisement
Supported by
New Drug Approved for Early Alzheimer’s
The drug, Kisunla, made by Eli Lilly, is the latest in a new class of treatments that could modestly slow cognitive decline in initial stages of the disease but also carry safety risks.

By Pam Belluck
The Food and Drug Administration on Tuesday approved a new drug for Alzheimer’s disease , the latest in a novel class of treatments that has been greeted with hope, disappointment and skepticism.
The drug, donanemab, to be sold under the brand name Kisunla, was shown in studies to modestly slow the pace of cognitive decline in early stages of the disease. It also had significant safety risks, including swelling and bleeding in the brain.
Kisunla, made by Eli Lilly, is similar to another drug, Leqembi, approved last year. Both are intravenous infusions that attack a protein involved in Alzheimer’s, and both can slow the unfolding of dementia by several months. Both also carry similar safety risks. Leqembi, made by Eisai and Biogen, is given every two weeks; Kisunla is given monthly.
Kisunla has a significant difference that may appeal to patients, doctors and insurers: Lilly says patients can stop the drug after it clears the protein, amyloid, which clumps into plaques in the brains of people with Alzheimer’s.
“Once you’ve removed the target that you’re going after, you then can stop dosing,” said Anne White, an executive vice president of Lilly and president of its neuroscience division. She said that this could reduce the overall cost and inconvenience of the treatment as well as the risk of side effects.
The company said that 17 percent of patients receiving donanemab in the 18-month-long clinical trial were able to discontinue the drug at six months, 47 percent stopped within a year and 69 percent stopped within 18 months. Their cognitive decline continued to slow even after they stopped. The company is evaluating how long that slowing will continue past the duration of the trial, said Dr. John Sims, a medical director at Lilly.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .

Donanemab shows promise in slowing Alzheimer's disease
- Download PDF Copy
With yet a third new Alzheimer's drug expected to be approved by the Food and Drug Administration (FDA), the field is beginning to show progress in the fight to slow the disease.
But the drugs work best for those in the earliest stages of Alzheimer's, and other therapies will be needed to help those with advanced disease, according to Gil Rabinovici, MD, director of the UCSF Alzheimer's Disease Research Center.
This is likely "just the opening chapter in a new era of molecular therapies for Alzheimer's disease and related neurodegenerative disorders," Rabinovici wrote in a July 17, 2023, JAMA editorial that is being published along with the results of the latest drug, donanemab. Rabinovici was not involved in the trial.
Donanemab is a monoclonal antibody, like the two earlier Alzheimer's drugs, aducanumab (Aduhelm) and lecanemab (Leqembi). These drugs attack plaques in the brain that are made of a protein called amyloid. They disrupt cell function and lead to the rapid spread of another protein called tau. Both amyloid and tau contribute to the development of Alzheimer's disease.
The trial showed donanemab slowed cognitive decline by 35% compared with placebo in patients with low-to-intermediate levels of tau in the brain. These results are similar to those reported with Leqembi, which received FDA approval earlier this month. In the donanemab trial, patients also experienced a 40% lower risk of progressing from mild cognitive impairment to mild dementia, or from mild-to-moderate dementia.
Donanemab was better at removing amyloid plaques compared to Aduhelm and Leqembi. It reduced tau concentrations in the blood, but not in a key area of the brain.
While these results are encouraging, Rabinovici said an in-depth analysis still is needed to understand how these findings affect patient outcomes.
Not much benefit for those with more serious disease
Patients with more advanced disease showed little to no benefit compared to those who received the placebo. Together with the drug's potentially serious side effects, this should push experts to "aim higher in developing more impactful and safer treatments," wrote Rabinovici, who is affiliated with the UCSF Memory and Aging Center, departments of Neurology, Radiology and Biomedical Imaging, as well as the Weill Institute for Neurosciences.
Related Stories
- Distinct gut bacteria identified in octogenarians linked to aging and health
- Depression has a consistent mark in the brain even when symptoms are absent
- Scientists reveal how DNA methylation drives astrocytes to become stem cells, unlocking new potential for brain repair
Donanemab should be restricted to patients with low-to-intermediate levels of tau, which indicates mild disease. Other trials are evaluating how well monoclonal antibodies work in the earliest phase of the disease before symptoms appear.
Like the two other new Alzheimer's drugs, donanemab was associated with ARIA, amyloid-related imaging abnormalities that may include brain swelling and microbleeds. Serious ARIA occurred in 3.7% of patients, including three deaths. Risks were higher among patients with the APOE4 gene, which is related to an increased risk for Alzheimer's. For that reason, Rabinovici said, genetic testing should be recommended prior to monoclonal antibody treatment.
While ARIA has generally been managed safely in clinical trials, Rabinovici urged caution as these drugs enter into real-world practice. He suggested limiting access to patients with normal pre-treatment MRIs, repeating MRIs at regular intervals and stopping or suspending treatment when ARIA occurs.
Lack of racial and ethnic diversity was a major limitation of the trial. Just 8.6% of the 1,251 U. S. participants were non-white. Rabinovici said this raises ethical concerns about the "generalizability of results to populations at highest risk," noting studies that have shown higher rates of dementia in Black and Latino populations.
Given the anticipated high cost of donanemab and high patient demand, Rabinovici said it might make sense to limit the treatment duration to the time needed to clear amyloid plaques from the brain, which is the approach pioneered in the trial. He said this could "greatly enhance the feasibility of treatment for patients, clinicians, insurers and health systems."
University of California - San Francisco
Posted in: Drug Trial News | Medical Condition News
Tags: Aging , Alzheimer's Disease , Antibodies , Antibody , Blood , Brain , Cell , CT , Dementia , Drugs , Education , Food , Gene , Genetic , Health Systems , Imaging , Medicine , Monoclonal Antibody , Neurology , Placebo , Protein , Radiology , Research
Suggested Reading

Cancel reply to comment
- Trending Stories
- Latest Interviews
- Top Health Articles

How can microdialysis benefit drug development
Ilona Vuist
In this interview, discover how Charles River uses the power of microdialysis for drug development as well as CNS therapeutics.

Global and Local Efforts to Take Action Against Hepatitis
Lindsey Hiebert and James Amugsi
In this interview, we explore global and local efforts to combat viral hepatitis with Lindsey Hiebert, Deputy Director of the Coalition for Global Hepatitis Elimination (CGHE), and James Amugsi, a Mandela Washington Fellow and Physician Assistant at Sandema Hospital in Ghana. Together, they provide valuable insights into the challenges, successes, and the importance of partnerships in the fight against hepatitis.

Addressing Important Cardiac Biology Questions with Shotgun Top-Down Proteomics
In this interview conducted at Pittcon 2024, we spoke to Professor John Yates about capturing cardiomyocyte cell-to-cell heterogeneity via shotgun top-down proteomics.

Latest News

Newsletters you may be interested in
Your AI Powered Scientific Assistant
Hi, I'm Azthena, you can trust me to find commercial scientific answers from News-Medical.net.
A few things you need to know before we start. Please read and accept to continue.
- Use of “Azthena” is subject to the terms and conditions of use as set out by OpenAI .
- Content provided on any AZoNetwork sites are subject to the site Terms & Conditions and Privacy Policy .
- Large Language Models can make mistakes. Consider checking important information.
Great. Ask your question.
Azthena may occasionally provide inaccurate responses. Read the full terms .
While we only use edited and approved content for Azthena answers, it may on occasions provide incorrect responses. Please confirm any data provided with the related suppliers or authors. We do not provide medical advice, if you search for medical information you must always consult a medical professional before acting on any information provided.
Your questions, but not your email details will be shared with OpenAI and retained for 30 days in accordance with their privacy principles.
Please do not ask questions that use sensitive or confidential information.
Read the full Terms & Conditions .
Provide Feedback


- Latest news
- UCL in the media
- Services for media
- Student news
- Tell us your story

First ‘gene silencing’ drug for Alzheimer’s disease shows promise
25 April 2023
A world-first trial at UCL and UCLH has found a new genetic therapy for Alzheimer’s disease that is able to safely and successfully lower levels of the harmful tau protein known to cause the disease.
The trial, led by consultant neurologist Dr Catherine Mummery (UCL Queen Square Institute of Neurology & the National Hospital for Neurology and Neurosurgery), represents the first time that a ‘gene silencing’ approach has been taken in dementia and Alzheimer’s disease.
The approach uses a drug called BIIB080 (/ IONIS- MAPTRx ) , which is an antisense oligonucleaotide (used to stop RNA producing a protein), to ‘silence’ the gene coding for the tau protein – known as the microtubule-associated protein tau (MAPT) gene. This prevents the gene from being translated into the protein in a doseable and reversible way. It will also lower the production of that protein and alter the course of disease.
Further trials will be needed in larger groups of patients to determine whether this leads to clinical benefit, but the phase 1 results published in Nature Medicine - with results from 46 patients - are the first indication that this method has a biological effect.
There are currently no treatments targeting tau. The drugs aducanumab and lecanemab – recently approved for use in some situations by the FDA – target a separate disease mechanism in AD, the accumulation of amyloid plaques*.
The phase 1 trial looked at the safety of BIIB080, what it does in the body, and how well it targets the MAPT gene. It involved the UCL Dementia Research Centre, was supported by the NIHR UCLH Biomedical Research Centre, was supported by the NIHR UCLH Biomedical Research Centre, and took place at the Leonard Wolfson Experimental Neurology Centre at NHNN.
46 patients, with an average age of 66, were enrolled in the trial – which took place from 2017 to 2020. The trial looked at three doses of the drug, given by intrathecal injection (an injection into the nervous system via the spinal canal), compared with the placebo.
Results show that the drug was well tolerated, with all patients completing the treatment period and over 90% completing the post-treatment period.
Patients in both the treatment and placebo groups experienced either mild or moderate side effects - the most common being a headache after injection of the drug. However, no serious adverse events were seen in patients given the drug.
The research team also looked at two forms of the tau protein in the central nervous system (CNS) – a reliable indicator of disease – over the duration of the study.
They found a greater than 50% reduction in levels of total tau and phosphor tau concentration in the CNS after 24 weeks in the two treatment groups that received the highest dose of the drug.
Dr Mummery said: “We will need further research to understand the extent to which the drug can slow progression of physical symptoms of disease and evaluate the drug in older and larger groups of people and in more diverse populations.
“But the results are a significant step forward in demonstrating that we can successfully target tau with a gene silencing drug to slow – or possibly even reverse – Alzheimer’s disease, and other diseases caused by tau accumulation in the future.”
- Research in Nature Medicine
- Dr Cath Mummery’s academic profile
- UCL Queen Square Institute of Neurology
- UCL Brain Sciences
- National Hospital of Neurology & Neurosurgery
- * Lecanemab breakthrough
- Pathological phosphorylation (yellow) of Tau proteins (red-orange) leads to disintegration of microtubuli in the neuron axon an aggregation of the tau proteins. The transport of synaptic vesicles (orange-blue) is interrupted. Credit: selvanegra on iStock
Media contact
Poppy danby .
E: p.danby [at] ucl.ac.uk
Appointments at Mayo Clinic
Alzheimer's treatments: what's on the horizon.
Despite many promising leads, new treatments for Alzheimer's are slow to emerge.
Current Alzheimer's treatments temporarily improve symptoms of memory loss and problems with thinking and reasoning.
These Alzheimer's treatments boost the performance of chemicals in the brain that carry information from one brain cell to another. They include cholinesterase inhibitors and the medicine memantine (Namenda). However, these treatments don't stop the underlying decline and death of brain cells. As more cells die, Alzheimer's disease continues to progress.
Experts are cautious but hopeful about developing treatments that can stop or delay the progression of Alzheimer's. Experts continue to better understand how the disease changes the brain. This has led to the research of potential Alzheimer's treatments that may affect the disease process.
Future Alzheimer's treatments may include a combination of medicines. This is similar to treatments for many cancers or HIV / AIDS that include more than one medicine.
These are some of the strategies currently being studied.
Taking aim at plaques
Some of the new Alzheimer's treatments target clumps of the protein beta-amyloid, known as plaques, in the brain. Plaques are a characteristic sign of Alzheimer's disease.
Strategies aimed at beta-amyloid include:
Recruiting the immune system. Medicines known as monoclonal antibodies may prevent beta-amyloid from clumping into plaques. They also may remove beta-amyloid plaques that have formed. They do this by helping the body clear them from the brain. These medicines mimic the antibodies your body naturally produces as part of your immune system's response to foreign invaders or vaccines.
The U.S. Food and Drug Administration (FDA) has approved lecanemab (Leqembi) and donanemab (Kisunla) for people with mild Alzheimer's disease and mild cognitive impairment due to Alzheimer's disease.
Clinical trials found that the medicines slowed declines in thinking and functioning in people with early Alzheimer's disease. The medicines prevent amyloid plaques in the brain from clumping.
Lecanemab is given as an IV infusion every two weeks. Your care team likely will watch for side effects and ask you or your caregiver how your body reacts to the drug. Side effects of lecanemab include infusion-related reactions such as fever, flu-like symptoms, nausea, vomiting, dizziness, changes in heart rate and shortness of breath.
Donanemab is given as an IV infusion every four weeks. Side effects of the medicine may include flu-like symptoms, nausea, vomiting, headache and changes in blood pressure. Rarely, donanemab can cause a life-threatening allergic reaction and swelling.
Also, people taking lecanemab or donanemab may have swelling in the brain or may get small bleeds in the brain. Rarely, brain swelling can be serious enough to cause seizures and other symptoms. Also in rare instances, bleeding in the brain can cause death. The FDA recommends getting a brain MRI before starting treatment. The FDA also recommends periodic brain MRIs during treatment for symptoms of brain swelling or bleeding.
People who carry a certain form of a gene known as APOE e4 appear to have a higher risk of these serious complications. The FDA recommends testing for this gene before starting treatment.
If you take a blood thinner or have other risk factors for brain bleeding, talk to your healthcare professional before taking lecanemab or donanemab. Blood-thinning medicines may increase the risk of bleeds in the brain.
More research is being done on the potential risks of taking lecanemab and donanemab. Other research is looking at how effective the medicines may be for people at risk of Alzheimer's disease, including people who have a first-degree relative, such as a parent or sibling, with the disease.
The monoclonal antibody solanezumab did not show benefits for individuals with preclinical, mild or moderate Alzheimer's disease. Solanezumab did not lower beta-amyloid in the brain, which may be why it wasn't effective.
Preventing destruction. A medicine initially developed as a possible cancer treatment — saracatinib — is now being tested in Alzheimer's disease.
In mice, saracatinib turned off a protein that allowed synapses to start working again. Synapses are the tiny spaces between brain cells through which the cells communicate. The animals in the study experienced a reversal of some memory loss. Human trials for saracatinib as a possible Alzheimer's treatment are now underway.
Production blockers. These therapies may reduce the amount of beta-amyloid formed in the brain. Research has shown that beta-amyloid is produced from a "parent protein" in two steps performed by different enzymes.
Several experimental medicines aim to block the activity of these enzymes. They're known as beta- and gamma-secretase inhibitors. Recent studies showed that the beta-secretase inhibitors did not slow cognitive decline. They also were associated with significant side effects in those with mild or moderate Alzheimer's. This has decreased enthusiasm for the medicines.
Keeping tau from tangling
A vital brain cell transport system collapses when a protein called tau twists into tiny fibers. These fibers are called tangles. They are another common change in the brains of people with Alzheimer's. Researchers are looking at a way to prevent tau from forming tangles.
Tau aggregation inhibitors and tau vaccines are currently being studied in clinical trials.
Reducing inflammation
Alzheimer's causes chronic, low-level brain cell inflammation. Researchers are studying ways to treat the processes that lead to inflammation in Alzheimer's disease. The medicine sargramostim (Leukine) is currently in research. The medicine may stimulate the immune system to protect the brain from harmful proteins.
Researching insulin resistance
Studies are looking into how insulin may affect the brain and brain cell function. Researchers are studying how insulin changes in the brain may be related to Alzheimer's. However, a trial testing of an insulin nasal spray determined that the medicine wasn't effective in slowing the progression of Alzheimer's.
Studying the heart-head connection
Growing evidence suggests that brain health is closely linked to heart and blood vessel health. The risk of developing dementia appears to increase as a result of many conditions that damage the heart or arteries. These include high blood pressure, heart disease, stroke, diabetes and high cholesterol.
A number of studies are exploring how best to build on this connection. Strategies being researched include:
- Current medicines for heart disease risk factors. Researchers are looking into whether blood pressure medicines may benefit people with Alzheimer's. They're also studying whether the medicines may reduce the risk of dementia.
- Medicines aimed at new targets. Other studies are looking more closely at how the connection between heart disease and Alzheimer's works at the molecular level. The goal is to find new potential medicines for Alzheimer's.
- Lifestyle choices. Research suggests that lifestyle choices with known heart benefits may help prevent Alzheimer's disease or delay its onset. Those lifestyle choices include exercising on most days and eating a heart-healthy diet.
Studies during the 1990s suggested that taking hormone replacement therapy during perimenopause and menopause lowered the risk of Alzheimer's disease. But further research has been mixed. Some studies found no cognitive benefit of taking hormone replacement therapy. More research and a better understanding of the relationship between estrogen and cognitive function are needed.
Speeding treatment development
Developing new medicines is a slow process. The pace can be frustrating for people with Alzheimer's and their families who are waiting for new treatment options.
To help speed discovery, the Critical Path for Alzheimer's Disease (CPAD) consortium created a first-of-its-kind partnership to share data from Alzheimer's clinical trials. CPAD 's partners include pharmaceutical companies, nonprofit foundations and government advisers. CPAD was formerly called the Coalition Against Major Diseases.
CPAD also has collaborated with the Clinical Data Interchange Standards Consortium to create data standards. Researchers think that data standards and sharing data from thousands of study participants will speed development of more-effective therapies.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Treatments and research. Alzheimer's Association. https://www.alz.org/alzheimers-dementia/research_progress/treatment-horizon. Accessed March 23, 2023.
- Cummings J, et al. Alzheimer's disease drug development pipeline: 2022. Alzheimer's and Dementia. 2022; doi:10.1002/trc2.12295.
- Burns S, et al. Therapeutics of Alzheimer's disease: Recent developments. Antioxidants. 2022; doi:10.3390/antiox11122402.
- Plascencia-Villa G, et al. Lessons from antiamyloid-beta immunotherapies in Alzheimer's disease. Handbook of Clinical Neurology. 2023; doi:10.1016/B978-0-323-85555-6.00019-9.
- Brockmann R, et al. Impacts of FDA approval and Medicare restriction on antiamyloid therapies for Alzheimer's disease: Patient outcomes, healthcare costs and drug development. The Lancet Regional Health. 2023; doi:10.1016/j.lana. 2023.100467 .
- Wojtunik-Kulesza K, et al. Aducanumab — Hope or disappointment for Alzheimer's disease. International Journal of Molecular Sciences. 2023; doi:10.3390/ijms24054367.
- Can Alzheimer's disease be prevented? Alzheimer's Association. http://www.alz.org/research/science/alzheimers_prevention_and_risk.asp. Accessed March 23, 2023.
- Piscopo P, et al. A systematic review on drugs for synaptic plasticity in the treatment of dementia. Ageing Research Reviews. 2022; doi:10.1016/j.arr. 2022.101726 .
- Facile R, et al. Use of Clinical Data Interchange Standards Consortium (CDISC) standards for real-world data: Expert perspectives from a qualitative Delphi survey. JMIR Medical Informatics. 2022; doi:10.2196/30363.
- Imbimbo BP, et al. Role of monomeric amyloid-beta in cognitive performance in Alzheimer's disease: Insights from clinical trials with secretase inhibitors and monoclonal antibodies. Pharmacological Research. 2023; doi:10.1016/j.phrs. 2022.106631 .
- Conti Filho CE, et al. Advances in Alzheimer's disease's pharmacological treatment. Frontiers in Pharmacology. 2023; doi:10.3389/fphar. 2023.1101452 .
- Potter H, et al. Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer's disease. Alzheimer's and Dementia. 2021; doi:10.1002/trc2.12158.
- Zhong H, et al. Effect of peroxisome proliferator-activated receptor-gamma agonists on cognitive function: A systematic review and meta-analysis. Biomedicines. 2023; doi:10.3390/biomedicines11020246.
- Grodstein F. Estrogen and cognitive function. https://www.uptodate.com/contents/search. Accessed March 23, 2023.
- Mills ZB, et al. Is hormone replacement therapy a risk factor or a therapeutic option for Alzheimer's disease? International Journal of Molecular Sciences. 2023; doi:10.3390/ijms24043205.
- Custodia A, et al. Biomarkers assessing endothelial dysfunction in Alzheimer's disease. Cells. 2023; doi:10.3390/cells12060962.
- Overview. Critical Path for Alzheimer's Disease. https://c-path.org/programs/cpad/. Accessed March 29, 2023.
- Shi M, et al. Impact of anti-amyloid-β monoclonal antibodies on the pathology and clinical profile of Alzheimer's disease: A focus on aducanumab and lecanemab. Frontiers in Aging and Neuroscience. 2022; doi:10.3389/fnagi. 2022.870517 .
- Leqembi (approval letter). Biologic License Application 761269. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761269. Accessed July 7, 2023.
- Van Dyck CH, et al. Lecanemab in early Alzheimer's disease. New England Journal of Medicine. 2023; doi:10.1056/NEJMoa2212948.
- Leqembi (prescribing information). Eisai; 2023. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=761269. Accessed July 10, 2023.
- Kisunla (approval letter). Biologic License Application 761248. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process. Accessed July 9, 2024.
- Sims JR, et al. Donanemab in early symptomatic Alzheimer disease: The TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023; doi:10.1001/jama.2023.13239.
- Kisunla (prescribing information). Eli Lilly and Company; 2024. https://www.lilly.com/. Accessed July 3, 2024.
Products and Services
- Assortment of Products for Independent Living from Mayo Clinic Store
- A Book: Mayo Clinic on Alzheimer's Disease
- A Book: Day to Day: Living With Dementia
- A Book: Mayo Clinic on Healthy Aging
- Give today to find Alzheimer's cures for tomorrow
- Alzheimer's sleep problems
- Alzheimer's 101
- Understanding the difference between dementia types
- Alzheimer's disease
- Alzheimer's genes
- Alzheimer's drugs
- Alzheimer's prevention: Does it exist?
- Alzheimer's stages
- Antidepressant withdrawal: Is there such a thing?
- Antidepressants and alcohol: What's the concern?
- Antidepressants and weight gain: What causes it?
- Antidepressants: Can they stop working?
- Antidepressants: Side effects
- Antidepressants: Selecting one that's right for you
- Antidepressants: Which cause the fewest sexual side effects?
- Anxiety disorders
- Atypical antidepressants
- Caregiver stress
- Clinical depression: What does that mean?
- Corticobasal degeneration (corticobasal syndrome)
- Depression and anxiety: Can I have both?
- Depression, anxiety and exercise
- What is depression? A Mayo Clinic expert explains.
- Depression in women: Understanding the gender gap
- Depression (major depressive disorder)
- Depression: Supporting a family member or friend
- Diagnosing Alzheimer's
- Did the definition of Alzheimer's disease change?
- How your brain works
- Intermittent fasting
- Lecanemab for Alzheimer's disease
- Male depression: Understanding the issues
- MAOIs and diet: Is it necessary to restrict tyramine?
- Marijuana and depression
- Mayo Clinic Minute: 3 tips to reduce your risk of Alzheimer's disease
- Mayo Clinic Minute: Alzheimer's disease risk and lifestyle
- Mayo Clinic Minute: New definition of Alzheimer's changes
- Mayo Clinic Minute: Women and Alzheimer's Disease
- Memory loss: When to seek help
- Monoamine oxidase inhibitors (MAOIs)
- Natural remedies for depression: Are they effective?
- Nervous breakdown: What does it mean?
- New Alzheimers Research
- Pain and depression: Is there a link?
- Phantosmia: What causes olfactory hallucinations?
- Positron emission tomography scan
- Posterior cortical atrophy
- Seeing inside the heart with MRI
- Selective serotonin reuptake inhibitors (SSRIs)
- Serotonin and norepinephrine reuptake inhibitors (SNRIs)
- Sundowning: Late-day confusion
- Treatment-resistant depression
- Tricyclic antidepressants and tetracyclic antidepressants
- Video: Alzheimer's drug shows early promise
- Vitamin B-12 and depression
- Young-onset Alzheimer's
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Alzheimer s treatments What s on the horizon
5X Challenge
Thanks to generous benefactors, your gift today can have 5X the impact to advance AI innovation at Mayo Clinic.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 10 November 2023
AI that reads brain scans shows promise for finding Alzheimer’s genes
You can also search for this author in PubMed Google Scholar
Washington DC
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41586-023-03482-9
Lu, B. et al. Preprint at bioRxiv https://doi.org/10.1101/2020.08.18.256594 (2022).
Tosun, D. et al. Alzheimers Dement. https://doi.org/10.1002/alz.13447 (2023).
Article Google Scholar
Patel, K. et al. Preprint at medRxiv https://doi.org/10.1101/2022.12.10.22283302 (2022).
Download references
Reprints and permissions
Related Articles
Alzheimer’s drug trials plagued by lack of racial diversity
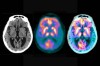
- Neuroscience
Brain region boosts avoidance of unpleasantness and pain — in mice
Research Highlight 12 SEP 24
Heteromeric amyloid filaments of ANXA11 and TDP-43 in FTLD-TDP Type C
Article 11 SEP 24
Brain-wide dynamics linking sensation to action during decision-making

Why does heart disease affect so many young South Asians?
News Feature 11 SEP 24
Rapa Nui’s population history rewritten using ancient DNA
News & Views 11 SEP 24

Famed Pacific island’s population ‘crash’ debunked by ancient DNA
News 11 SEP 24
Staff Scientist - Immunology
Staff Scientist- Immunology
Houston, Texas (US)
Baylor College of Medicine (BCM)
Institute for Systems Genetics, Tenure Track Faculty Positions
The Institute for Systems Genetics at NYU Langone Health has tenure track faculty positions (assistant professor level) at the new SynBioMed Center.
New York City, New York (US)
NYU Langone Health
Faculty Position
The Institute of Cellular and Organismic Biology (ICOB), Academia Sinica, Taiwan, is seeking candidates to fill multiple tenure-track faculty position
Taipei (TW)
Institute of Cellular and Organismic Biology, Academia Sinica
Postdoctoral Associate
Associate or senior editor, nature energy.
Job Title: Associate or Senior Editor, Nature Energy Location: New York, Jersey City, Philadelphia or London — Hybrid Working Application Deadline:...
Springer Nature Ltd
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Explore your options for easing the burden of student loan repayments with Savi.
AARP daily Crossword Puzzle
Hotels with AARP discounts
Life Insurance
AARP Dental Insurance Plans
AARP MEMBERSHIP
AARP Membership — $12 for your first year when you sign up for Automatic Renewal
Get instant access to members-only products, hundreds of discounts, a free second membership, and a subscription to AARP the Magazine.
- right_container
Work & Jobs
Social Security
- AARP en Español
- Membership & Benefits
- Members Edition
- AARP Rewards
- AARP Rewards %{points}%
Conditions & Treatments
Drugs & Supplements
Health Care & Coverage
Health Benefits

AARP Hearing Center
Advice on Tinnitus and Hearing Loss

Your Health
What to Know About Vaccines

Brain Health Resources
Tools and Explainers on Brain Health
25 Ways to Get a Flatter Stomach
Scams & Fraud
Personal Finance
Money Benefits

View and Report Scams in Your Area

AARP Foundation Tax-Aide
Free Tax Preparation Assistance
AARP Money Map
Get Your Finances Back on Track

How to Protect What You Collect
Small Business
Age Discrimination

Flexible Work
Freelance Jobs You Can Do From Home

AARP Skills Builder
Online Courses to Boost Your Career

31 Great Ways to Boost Your Career

ON-DEMAND WEBINARS
Tips to Enhance Your Job Search

Get More out of Your Benefits

When to Start Taking Social Security

10 Top Social Security FAQs

Social Security Benefits Calculator

Medicare Made Easy
Original vs. Medicare Advantage

Enrollment Guide
Step-by-Step Tool for First-Timers

Prescription Drugs
9 Biggest Changes Under New Rx Law

Medicare FAQs
Quick Answers to Your Top Questions
Care at Home
Financial & Legal
Life Balance

LONG-TERM CARE
Understanding Basics of LTC Insurance

State Guides
Assistance and Services in Your Area

Prepare to Care Guides
How to Develop a Caregiving Plan

End of Life
How to Cope With Grief, Loss
Recently Played
Word & Trivia
Atari® & Retro
Members Only
Staying Sharp
Mobile Apps
More About Games

Right Again! Trivia

Right Again! Trivia – Sports

Atari® Video Games

Throwback Thursday Crossword
Travel Tips
Vacation Ideas
Destinations
Travel Benefits

Camping and RV Ideas
Fun Camping and RV Journeys
Exploration
25 Great Ways to Explore

Train Travel
How to Find Great Train Deals

AARP National Park Guide
Travel to Pinnacles in California
Entertainment & Style
Family & Relationships
Personal Tech
Home & Living
Celebrities
Beauty & Style

TV for Grownups
Fall TV Preview

Kevin Costner’s Big Bet

Looking Back
Take Our ’80s Music Quiz

Sex & Dating
7 Dating Dos and 7 Don'ts

Get Happier
Creating Social Connections

Friends & Family
Veterinarians May Use AI to Treat Pets

Home Technology
What's Inside Your Smartphone

Virtual Community Center
Join Free Tech Help Events

Creative Ways to Store Your Pets Gear

Meals to Make in the Microwave

Wearing Shoes Inside: Pros vs. Cons
Driver Safety
Maintenance & Safety
Trends & Technology

AARP Smart Guide
How to Clean Your Car

We Need To Talk
Assess Your Loved One's Driving Skills

AARP Smart Driver Course

Building Resilience in Difficult Times

Tips for Finding Your Calm

Weight Loss After 50 Challenge

Cautionary Tales of Today's Biggest Scams

7 Top Podcasts for Armchair Travelers

Jean Chatzky: ‘Closing the Savings Gap’

Quick Digest of Today's Top News

AARP Top Tips for Navigating Life

Get Moving With Our Workout Series
You are now leaving AARP.org and going to a website that is not operated by AARP. A different privacy policy and terms of service will apply.
The Alzheimer's Drugs Showing Early Promise
Along with eli lily’s donanemab, researchers say several new therapies offer hope.

Hallie Levine,
Treatment for Alzheimer's disease, the devastating condition that affects more than 5 million Americans, has remained notoriously elusive for decades. Now, a small study released by drug company Eli Lilly offers at least a flicker of hope: It shows that the experimental drug donanemab may significantly slow patients’ cognitive decline.
The two-year study — which followed 272 people whose brain scans showed Alzheimer's — found that patients who took the drug had a 32 percent slower rate of decline than those who received a placebo. “It's very encouraging because this is the first time a drug of its kind has had positive results in early-stage trials,” says Lon Schneider, M.D., Della Martin chair in psychiatry and neuroscience at the Keck School of Medicine of the University of Southern California. The drug, known as a monoclonal antibody, works by binding to the hard plaque in the brain made from amyloid (a protein associated with Alzheimer's).

Get instant access to members-only products and hundreds of discounts, a free second membership, and a subscription to AARP the Magazine.
Though these initial findings are promising, Schneider says more data is needed. “It may have been everyone just had a small cognitive decline, in which case the results aren't as significant,” he says. (The drugmaker has said it will release this information shortly in a peer-reviewed clinical journal.)
But this isn't the only news Alzheimer's researchers are excited about. “There are several new drugs either close to getting FDA approval, or in development, that promise to really change the playing field when it comes to treatment of Alzheimer's disease,” says Marwan Sabbagh, M.D., director of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas. Here are some of the most promising contenders.

Join AARP today for $16 per year. Get instant access to members-only products and hundreds of discounts, a free second membership, and a subscription to AARP The Magazine.
ARTICLE CONTINUES AFTER ADVERTISEMENT
Aducanumab : This drug, up for FDA approval, is another monoclonal antibody similar to donanemab that binds to the hard amyloid plaques that are the hallmarks of Alzheimer's disease. “It will be a game changer if it's approved, because this will be the first drug shown to actually slow down the progression of Alzheimer's disease,” Sabbagh says. (Editor’s Note: Aducanumab, brand name Aduhelm, received FDA approval on June 7, 2021.)
Pimavanserin : This antipsychotic drug, already approved to treat hallucinations and delusions in people with Parkinson's disease, is under FDA review for the treatment of some of the behavioral and psychological symptoms of all dementias. “Research shows that it's very effective also in treating dementia-related psychosis or hallucinations,” Sabbagh says. “This is important because these sorts of episodes are the main reason patients with Alzheimer's get placed in memory care facilities. If caregivers can manage these symptoms, more people will be able to stay at home.”
AARP® Dental Insurance Plan administered by Delta Dental Insurance Company
Dental insurance plans for members and their families
Atuzaginstat : There's a growing body of evidence that the bacteria P. gingivalis (the cause of gum disease) can infect the brain and cause Alzheimer's disease. Atuzaginstat is in clinical trials to see if it can inactivate gingipains, the toxic proteins the bacteria release, which can damage healthy brain cells.
NDX-1017 : This drug — administered as a daily injectable — is a small molecule that improves the activity of hepatocyte growth factor (HGF), a protein found in your body's tissues, including your brain. It's thought that HGF can strengthen the synapses or connections between your brain cells, thus reversing some of the damage caused by Alzheimer's disease. Brain wave studies in patients show that the drug works quickly, causing changes in as little as eight days.
ALZ-801 : This medication offers an advantage over other drugs in clinical research, because you can take it orally rather than as an injection. Unlike monoclonal antibodies, which grab onto amyloid plaques and eliminate them, this drug attacks earlier in the process, blocking the amyloid from ever forming. Right now, studies are only in people with a high genetic risk of Alzheimer's who carry two copies of the APOE4 gene. But if this research proves successful, the drug will be tried on other groups in the early stages of Alzheimer's, Sabbagh says.
Lenalidomide (Revlimid) : Used to treat blood cancers such as leukemia or multiple myeloma, this medication is now being studied at the Cleveland Clinic for its potential to treat Alzheimer's. “Our early research has shown that it will inhibit amyloid plaques in the brains of mice,” Sabbagh says.
Faster Ways to Diagnose Alzheimer's Are Coming
Right now, most researchers diagnose Alzheimer's with a PET (positron emission tomography) scan, to measure amounts of amyloid deposits in the brain, or a spinal tap, to gauge amyloid levels in the fluid surrounding the brain and spinal cord.
But the procedures are expensive (making insurance coverage an issue), time-consuming and, with the spinal tap, potentially painful. That's one reason researchers are on the hunt for a better, cheaper and faster blood test . Arman Fesharaki-Zadeh, M.D., a behavioral neurologist and neuropsychiatrist at Yale Medicine, says that his and others’ blood-test research involves hunting for “abnormal versions of tau proteins,” which would also help in diagnosing the disease at earlier stages. “By the time many folks come to see me, they've progressed so far they can no longer qualify for clinical treatment trials , which is heartbreaking.”
But it can also be challenging to measure these sorts of biomarkers in blood, as they exist in very small concentrations, Schneider says. That's why researchers have turned to ultrasensitive tests, known as assays, to see if they can pick up minuscule amounts of biomarkers like amyloid and tau. A 2019 study in JAMA Neurology found that one such test, an immunoassay by Elecsys, was indeed able to pick up these markers in all stages of Alzheimer's disease. “The thought is eventually it could become a diagnostic screening tool, like other lab tests we do for other illnesses,” Schneider notes.
Hallie Levine is a contributing writer and an award-winning medical and health reporter. Her work has appeared in The New York Times, Consumer Reports, Real Simple, Health and Time , among other publications.
More on health

Your Brain Health After 50

Sanjay Gupta’s Prescription for Brain Health

New AARP Report Shows Power of Music on the Brain
Unlock Access to AARP Members Edition
Already a Member? Login
Benefits Recommended For You
SAVE MONEY WITH THESE LIMITED-TIME OFFERS
Experimental Alzheimer’s Drug, Donanemab, Shows Promise

August 9, 2023
An experimental drug for Alzheimer’s showed promise in slowing declines in thinking and memory skills when given early in the course of the disease, according to a new report. The findings are a reminder that researchers continue to investigate promising new drugs for Alzheimer’s, and that treatments for the disease may be most effective when given early in the course of the illness, when symptoms are milder and damage to the brain is less extensive.
Much of the attention around Alzheimer’s treatments has focused on new drugs like Leqembi, which was approved by the Food and Drug Administration earlier this year. But this new drug, called donanemab, showed similar benefits in helping to slow declines in cognition and the ability of people with Alzheimer’s disease to care for themselves.
The benefits were modest, and the drug is not a cure. In addition, the new drug also had some potentially serious side effects. But the results were encouraging and underscore the continued need for research into new therapies that can effectively treat Alzheimer’s disease.
Donanemab, like Leqembi, is what is known as a monoclonal antibody, a protein specially designed to recognize a specific target in the body. Both drugs target beta-amyloid, the toxic protein that builds up in the brains of those with Alzheimer’s disease. Toxic beta-amyloid forms amyloid aggregates and plaques, which are believed to disrupt normal brain signaling, and both monoclonal antibodies help to clear these build-ups. Also, like Leqembi, donanemab is given by infusion.
For the study, published in the journal JAMA, researchers studied 1,736 men and women in the early stages of Alzheimer’s disease, when declines in thinking and memory skills are somewhat modest. All had evidence of the telltale plaques of Alzheimer’s in their brains, as well as tau tangles, the spaghetti-like proteins that accumulate and clump inside neurons as the disease progresses.
After 18 months, donanemab slowed declines in thinking and memory skills by about four-and-a-half to seven-and-a-half months compared to study participants who were receiving a placebo drug. Benefits were most pronounced in those with the mildest symptoms, in people younger than 75, and in those with the lowest levels of tau in their brains. Patients who got the drug also showed clearing of the amyloid plaques in their brains.
The findings highlight earlier research showing that Alzheimer’s treatments may be most effective when given early in the course of the disease, when damage to the brain is less extensive. Therefore, early diagnosis and early treatment may be most effective for managing Alzheimer’s disease, many experts believe.
Donanemab and Leqembi have not been compared side-to-side in studies, and the design of this study was different than trials that were carried out to test Leqembi. So it is hard to say at this point which drug might be more effective.
Both drugs also carry the risk of swelling and bleeding in the brain, which can be life-threatening, so patients must be monitored closely. Doctors and patients must carefully weigh the benefits and risks of giving the drugs.
Importantly, neither drug can reverse or repair damage to the brain that has already occurred, and benefits are modest and may not be noticed by family members. But after nearly two decades in which no new Alzheimer’s drugs were available, these findings offer hope.
The current study was a Phase 3 trial, a late-stage study in a large number of patients. Lilly, the drug company that makes donanemab, is seeking approval from the Food and Drug Administration, which would make the drug available for doctors to prescribe.
By ALZinfo.org , The Alzheimer’s Information Site. Reviewed by Eric Schmidt, Ph.D., Fisher Center for Alzheimer’s Research Foundation at The Rockefeller University.
Source: John R. Sims, MD; Jennifer A. Zimmer, MD; Cynthis D Evans, PhD; et al: “Donanemab in Early Symptomatic Alzheimer Disease The TRAILBLAZER-ALZ 2 Randomized Clinical Trial.” JAMA July 17, 2023

Alzheimer's Articles
Add impact to your inbox.
" * " indicates required fields
Preserving Your Memory ® Magazine

- Name * First Last

WE CAN END ALZHEIMER'S
We support the pioneering research of the late Nobel Laureate Dr. Paul Greengard under the leadership of Dr. Nathaniel Heintz and his team as they continue pursuing the quest for a cure.

Connect with Us
Mailing Address: The Fisher Center for Alzheimer's Research Foundation FDR Station, PO Box 220 New York, NY 10150
1-800-ALZINFO (259-4636) Phone: 1-212-915-1328 Email: [email protected]
About Fisher
Information program, 7 clinical stages of alzheimer’s brochure.
FDA approves a second Alzheimer's drug that can modestly slow disease
It’s only the second drug that’s been convincingly shown to delay cognitive decline in patients, following last year’s approval of a similar drug from japanese drugmaker eisai., by matthew perrone | associated press • published july 3, 2024.
U.S. officials have approved another Alzheimer’s drug that can modestly slow the disease, providing a new option for patients in the early stages of the incurable, memory-destroying ailment.
The Food and Drug Administration approved Eli Lilly’s Kisunla on Tuesday for mild or early cases of dementia caused by Alzheimer’s. It’s only the second drug that’s been convincingly shown to delay cognitive decline in patients, following last year’s approval of a similar drug from Japanese drugmaker Eisai.
The delay seen with both drugs amounts to a matter of months — about seven months, in the case of Lilly’s drug. Patients and their families will have to weigh that benefit against the downsides, including regular IV infusions and potentially dangerous side effects like brain swelling.
24/7 New York news stream: Watch NBC 4 free wherever you are
Physicians who treat Alzheimer’s say the approval is an important step after decades of failed experimental treatments.
“I’m thrilled to have different options to help my patients,” said Dr. Suzanne Schindler, a neurologist at Washington University in St. Louis. “It’s been difficult as a dementia specialist — I diagnose my patients with Alzheimer’s and then every year I see them get worse and they progress until they die.”
Both Kisunla and the Japanese drug, Leqembi, are laboratory-made antibodies, administered by IV, that target one contributor to Alzheimer's — sticky amyloid plaque buildup in the brain. Questions remain about which patients should get the drugs and how long they might benefit.
Get Tri-state area news delivered to your inbox. Sign up for NBC New York's News Headlines newsletter.

Gena Rowlands has Alzheimer's, her son Nick Cassavetes says
A subset of Alzheimer's cases may be caused by two copies of a single gene, new research shows
The new drug's approval was expected after an outside panel of FDA advisors unanimously voted in favor of its benefits at a public meeting last month. That endorsement came despite several questions from FDA reviewers about how Lilly studied the drug, including allowing patients to discontinue treatment after their plaque reached very low levels.
Costs will vary by patient, based on how long they take the drug, Lilly said. The company also said a year’s worth of therapy would cost $32,000 — higher than the $26,500 price of a year’s worth of Leqembi.
The FDA’s prescribing information tells doctors they can consider stopping the drug after confirming via brain scans that patients have minimal plaque.
More than 6 million Americans have Alzheimer’s. Only those with early or mild disease will be eligible for the new drug, and an even smaller subset are likely to undergo the multi-step process needed to get a prescription.
The FDA approved Kisunla, known chemically as donanemab, based on results from an 18-month study in which patients given getting the treatment declined about 22% more slowly in terms of memory and cognitive ability than those who received a dummy infusion.
The main safety issue was brain swelling and bleeding, a problem common to all plaque-targeting drugs. The rates reported in Lilly's study — including 20% of patients with microbleeds — were slightly higher than those reported with competitor Leqembi. However, the two drugs were tested in slightly different types of patients, which experts say makes it difficult to compare the drugs' safety.
Kisunla is infused once a month compared to Leqembi’s twice-a-month regimen, which could make things easier for caregivers who bring their loved ones to a hospital or clinic for treatment.
“Certainly getting an infusion once a month is more appealing than getting it every two weeks,” Schindler said.
Lilly's drug has another potential advantage: Patients can stop taking it if they respond well.
In the company’s study, patients were taken off Kisunla once their brain plaque reached nearly undetectable levels. Almost half of patients reached that point within a year. Discontinuing the drug could reduce the costs and safety risks of long-term use. It's not yet clear how soon patients might need to resume infusions.
Logistical hurdles, spotty insurance coverage and financial concerns have all slowed the rollout of competitor Leqembi, which Eisai co-markets with U.S. partner Biogen. Many smaller hospitals and health systems aren’t yet setup to prescribe the new plaque-targeting Alzheimer's drugs.
First, doctors need to confirm that patients with dementia have the brain plaque targeted by the new drugs. Then they need to find a drug infusion center where patients can receive therapy. Meanwhile, nurses and other staff must be trained to perform repeated scans to check for brain swelling or bleeding.
“Those are all things a physician has to have set up," said Dr. Mark Mintun, who heads Lilly’s neuroscience division. "Until they get used to them, a patient who comes into their office will not be offered this therapy.”
This article tagged under:

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Large-scale study of brain proteins uncovers new clues to Alzheimer’s disease
Neuroscience Alzheimer's Disease Biomarkers
Through a large-scale analysis of tissue samples, NIA-funded researchers have discovered new disease-related changes in the brains of people who had died with Alzheimer’s disease. The study results, published in Nature Neuroscience , underscore the key role proteins may play in disease progression. Understanding these changes could help identify therapeutic targets and biomarkers for the disease.

For decades, scientists have known that much of the damage that underlies Alzheimer’s is ultimately carried out by changes in brain proteins. However, to get a better understanding of which changes may happen in the brains of most patients, scientists often measure the levels of ribonucleic acid (RNA) instead of proteins. Shaped like DNA, RNA often relays the genetic blueprints for proteins from a cell’s chromosomes to its protein-making machinery. Because RNA is easier to work with, scientists have relied on it as an indirect readout of global changes in protein levels when conducting large-scale studies, involving hundreds of brain samples. In this study, the researchers found that directly measuring protein levels on a large scale may reveal important clues to understanding Alzheimer’s that cannot be detected by analyzing RNA alone.
The research team — located at Emory University School of Medicine, part of the Accelerating Medicines Partnership® Program for Alzheimer’s Disease (AMP®-AD) Consortium — used advanced automated techniques to compare the levels of both proteins and RNA molecules in more than 1,000 brain tissue samples. The samples came from the postmortem brains of people who had Alzheimer’s, as well as from individuals who died from other, unrelated causes.
The team discovered several protein networks that may play an important role in Alzheimer’s. Specifically, they found 44 groups of proteins — termed “protein communities” — for which levels rose or fell in coordinated ways across the brains of people with Alzheimer’s, but not in a control group of people who had not had the disease.
Interestingly, only about half of these changes were observed when the researchers compared the protein communities to the corresponding RNA molecules. This finding suggests that there are important steps in the Alzheimer’s process that happen after a protein’s genetic blueprints are sent to a cell’s protein-making machinery.
Next, the researchers paid particular attention to two groups of protein communities that were not detected at the RNA level. One was associated with Mitogen-Activated Protein Kinase (MAPK) signaling and energy metabolism. Changes in these proteins appeared to be associated with changes in the thinking and memory problems seen during Alzheimer’s. The second community had changes that correlated to the early stages of the disease when amyloid and tau begin to accumulate in the brain.
The study results highlight the importance of directly analyzing proteins along with RNA to gain a more complete picture of how Alzheimer’s may damage the brain. The research team plans to use these results as a basis for developing new, more effective diagnostic tests and treatments for Alzheimer’s.
This research was supported in part by NIA grants RF1AG057471, RF1AG057470, R01AG01581, U54AG065187, U01AG061357, R01AG057911, R01AG061800, R01AG053960, RF1AG062181, P30AG10161, R01AG15819, R01AG17917, U01AG61356, RF1AG057440, U01AG046170, K08AG068604, P30AG19610, and R01AG056533.
These activities relate to NIA’s AD+ADRD Research Implementation Milestone 2. G, “Maximize the translational potential of genetics research by ensuring rapid and broad sharing of large-scale genetic/genomic data, similar to the open science, data sharing model of the AMP AD program and by supporting programs focused on: Understanding the mechanisms by which genetic variants (including ApoE) discovered through GWAS and sequencing studies influence AD risk; i ntegrating genomic data with other multi-omics data from brain, peripheral tissues, and iPSC cellular models from well-phenotyped cohorts; e stablishing the gene-basis and directionality for genetic variant association so gene networks can be annotated with causality as tools for drug discovery.”
Reference : Johnson, ECB, et al. Large-scale deep multi-layer analysis of Alzheimer’s disease brain reveals strong proteomic disease-related changes not observed at the RNA level . Nature Neuroscience . 2022;25(2):213-225. doi: 10.1038/s41593-021-00999-y.
nia.nih.gov
An official website of the National Institutes of Health

IMAGES
COMMENTS
In a recent breakthrough in Alzheimer's disease research, Auburn University scientists have studied a new drug, troriluzole, that can prevent brain changes leading to memory loss and cognitive decline in a mouse model of the disease. This study, recently published in the Journal of Neurochemistry, is the first to show how troriluzole can ...
New Alzheimer's drug shows promise in slowing disease but may include significant risks, study shows Japanese drugmaker Eisai and its U.S. partner, Biogen, had announced this fall that the drug ...
Research shows these blood tests which have been developed alongside powerful new amyloid and tau-clearing therapies, are now nearly as accurate gold-standard tests for measuring Alzheimer's ...
The drug, from pharmaceutical giant Eli Lilly, was able to slow Alzheimer's disease progression by 35 per cent in patients in the earliest stages of the disease. Across the whole study, there was ...
A recent study using focused ultrasound combined with a plaque-clearing drug found increased removal of amyloid plaque in the brain, offering new hope for treating Alzheimer's disease. The ...
A new study suggests that a treatment for Alzheimer's, the most common type of dementia, can also treat the second most common type - dementia with Lewy bodies (DLB).. The treatment is a cholinesterase inhibitor (ChEI): it's already been used with DLB patients, but up until now it was unclear how effective it was.This new research indicates it can indeed slow cognitive decline.
According to drugmakers Eisai and Biogen, a Phase 3 clinical study on a potential new Alzheimer's disease drug shows promise. The study findings show that the drug, lecanemab, reduced clinical decline of people with Alzheimer's disease by 27% compared with a placebo after 18 months of treatment.
Experimental Alzheimer's drug shows promise in injected-at-home form. An experimental version of Eisai Co's Alzheimer's drug that might be given in patients' homes exceeded the power of Leqembi ...
Health Sep 28, 2022 10:59 AM EDT. Shares of Biogen and other drugmakers researching Alzheimer's disease soared early Wednesday after Japan's Eisai Co. said its potential treatment appeared to ...
Japanese drugmaker Eisai on Wednesday said its experimental drug lecanemab helped slow thinking declines among people in the early stages of Alzheimer's disease. The findings from a phase 3 ...
The drugmaker said early results showed that its treatment, lecanemab, reduced patient clinical decline by 27% when compared to a placebo or fake drug after 18 months of the infused treatment. Eisai announced results late Tuesday from a global study of nearly 1,800 people with early-stage Alzheimer's. Patients were monitored using a scale ...
Eisai and Biogen said in a release that they plan to apply for U.S. approval of subcutaneous Leqembi by the end of March.
Now, researchers may have uncovered a promising treatment target for treating Alzheimer's disease: insulin receptors in the blood-brain barrier. Deficient insulin activity is most commonly ...
First 'gene silencing' drug for Alzheimer's disease shows promise. ScienceDaily . Retrieved September 9, 2024 from www.sciencedaily.com / releases / 2023 / 04 / 230425111215.htm
The drugmaker said early results showed that its treatment, lecanemab, reduced patient clinical decline by 27% when compared to a placebo or fake drug after 18 months of the infused treatment ...
July 2, 2024. The Food and Drug Administration on Tuesday approved a new drug for Alzheimer's disease, the latest in a novel class of treatments that has been greeted with hope, disappointment ...
Cancer drug could treat early-stage Alzheimer's disease, study shows Date: August 22, 2024 Source: Penn State Summary: A type of drug developed for treating cancer holds promise as a new treatment ...
APA. Chicago. Technical University of Munich (TUM). "Stopping and reversing Alzheimer's at an early stage." ScienceDaily. ScienceDaily, 14 August 2024. <www.sciencedaily.com / releases / 2024 / 08 ...
December 20, 2023. PHILADELPHIA - A "chaperone" molecule that slows the formation of certain proteins reversed disease signs, including memory impairment, in a mouse model of Alzheimer's disease, according to a study from researchers at the Perelman School of Medicine at the University of Pennsylvania. In the study, published in Aging ...
Like the two other new Alzheimer's drugs, donanemab was associated with ARIA, amyloid-related imaging abnormalities that may include brain swelling and microbleeds. Serious ARIA occurred in 3.7% ...
Assuming an Alzheimer's drug works, the effect "will be bigger the longer your trial happens," says Bart De Strooper, director of the UK Dementia Research Institute at University College London. And indeed, Biogen and Eisai noted that lecanemab failed to show a meaningful impact on cognition after 12 months, but did at 18 months.
A world-first trial at UCL and UCLH has found a new genetic therapy for Alzheimer's disease that is able to safely and successfully lower levels of the harmful tau protein known to cause the disease. ... First 'gene silencing' drug for Alzheimer's disease shows promise. ... The research team also looked at two forms of the tau protein ...
Other studies are looking more closely at how the connection between heart disease and Alzheimer's works at the molecular level. The goal is to find new potential medicines for Alzheimer's. Lifestyle choices. Research suggests that lifestyle choices with known heart benefits may help prevent Alzheimer's disease or delay its onset.
AI that reads brain scans shows promise for finding Alzheimer's genes. Machine-learning approach detects Alzheimer's disease with an accuracy of more than 90% — a potential boon for ...
The Alzheimer's Drugs Showing Early Promise. Treatment for Alzheimer's disease, the devastating condition that affects more than 5 million Americans, has remained notoriously elusive for decades. Now, a small study released by drug company Eli Lilly offers at least a flicker of hope: It shows that the experimental drug donanemab may ...
Immunotherapy for Alzheimer's disease shows promise in mouse study. ScienceDaily . Retrieved September 12, 2024 from www.sciencedaily.com / releases / 2024 / 04 / 240403170920.htm
Alzheimer's Drugs May Work in Whole New Way, Study Finds By Ernie Mundell HealthDay Reporter THURSDAY, Sept. 11, 2024 (HealthDay News) -- Two monoclonal antibody treatments to slow Alzheimer's ...
August 9, 2023. An experimental drug for Alzheimer's showed promise in slowing declines in thinking and memory skills when given early in the course of the disease, according to a new report. The findings are a reminder that researchers continue to investigate promising new drugs for Alzheimer's, and that treatments for the disease may be ...
U.S. officials have approved another Alzheimer's drug that can modestly slow the disease, providing a new option for patients in the early stages of the incurable, memory-destroying ailment.
The research team — located at Emory University School of Medicine, part of the Accelerating Medicines Partnership® Program for Alzheimer's Disease (AMP®-AD) Consortium — used advanced automated techniques to compare the levels of both proteins and RNA molecules in more than 1,000 brain tissue samples. The samples came from the ...