Anencephaly
What is anencephaly.
Anencephaly is a condition present at birth that affects the formation of the brain and the skull bones that surround the head. Anencephaly results in only minimal development of the brain. Often, the brain lacks part or all of the cerebrum (the area of the brain that is responsible for thinking, vision, hearing, touch, and movement). There is no bony covering over the back of the head and there may also be missing bones around the front and sides of the head.

What causes anencephaly?
Anencephaly is a type of neural tube defect that occurs in about one of 10,000 pregnancies in the U.S. each year. The actual number is unknown because many of these births result in miscarriages. According to the CDC, one in every 4,859 newborns in the United States has this condition.
During pregnancy, the human brain and spine begin as a flat plate of cells, which rolls into a tube, called the neural tube. If all or part of the neural tube fails to close, leaving an opening, this is known as an open neural tube defect, or ONTD. This opening may be left exposed or covered with bone or skin.
Anencephaly and spina bifida are the most common ONTDs, while encephaloceles (where there is a protrusion of the brain or its coverings through the skull) are much rarer. Anencephaly occurs when the neural tube fails to close at the base of the skull, while spina bifida occurs when the neural tube fails to close somewhere along the spine.
ONTDs happen to couples without a prior family history of these defects in the vast majority of cases. ONTDs result from a combination of genes inherited from both parents, coupled with environmental factors. For this reason, ONTDs are considered multifactorial traits, meaning many factors , both genetic and environmental, contribute to their occurrence.
Once a child has been born with an ONTD in the family, the chance for an ONTD to happen again is increased by 4 to 10 percent. It is important to understand that the type of neural tube defect can differ the second time. For example, one child could be born with anencephaly, while the second child could have spina bifida.
What are the symptoms of anencephaly?
The following are the most common symptoms of anencephaly. However, each child may experience symptoms differently. Symptoms may include:
Absence of bony covering over the back of the head
Missing bones around the front and sides of the head
Folding of the ears
Cleft palate. A condition in which the roof of the child's mouth does not completely close, leaving an opening that can extend into the nasal cavity.
Congenital heart defects
Some basic reflexes, but without the cerebrum, there can be no consciousness and the baby cannot survive
The symptoms of anencephaly may resemble other problems or medical conditions. Always consult your child's doctor for a diagnosis.
How is anencephaly diagnosed?
The diagnosis of anencephaly may be made during pregnancy, or at birth by physical examination. The baby's head often appears flattened due to the abnormal brain development and missing bones of the skull.
Diagnostic tests performed during pregnancy to evaluate the baby for anencephaly include the following:
Alpha-fetoprotein. A protein produced by the fetus that is excreted into the amniotic fluid. Abnormal levels of alpha-fetoprotein may indicate brain or spinal cord defects, multiple fetuses, a miscalculated due date, or chromosomal disorders.
Amniocentesis. A test performed to determine chromosomal and genetic disorders and certain birth defects. The test involves inserting a needle through the abdominal and uterine wall into the amniotic sac to retrieve a sample of amniotic fluid.
Ultrasound (also called sonography). A diagnostic imaging technique that uses high-frequency sound waves and a computer to create images of blood vessels, tissues, and organs. Ultrasounds are used to view internal organs as they function, and to assess blood flow through various vessels.
Blood tests
Treatment of the newborn with anencephaly
There is no cure or standard medical treatment for anencephaly. Treatment is supportive.
Experiencing the loss of a child can be very traumatic. Grief counseling services are available to help you cope with the loss of your child.
Future pregnancies
Genetic counseling may be recommended by the doctor to discuss the risk of recurrence in a future pregnancy as well as vitamin therapy (a prescription for folic acid) that can decrease the recurrence for ONTDs. Extra folic acid, a B vitamin, if taken one to two months prior to conception and throughout the first trimester of pregnancy, has been found to decrease the reoccurrence of ONTDs for couples who have had a previous child with an ONTD. The CDC also recommends to avoid smoking and drinking alcohol during pregnancy.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Talking with Your Healthcare Provider
- Birth Defects Statistics
- Birth Defects Resources
- Birth Defects Awareness Month
- Living with Down Syndrome
- Conversation Tips
- Growth Charts for Down Syndrome
- Accessing NBDPS and BD-STEPS Data
- Birth Defects Awareness Month Social Media Resources
Related Topics:
- View All Home
- About Alcohol Use During Pregnancy
Anencephaly
- Anencephaly (an-en-sef-ah-lee) is a serious birth defect where a baby is born without parts of the brain and skull.
- Almost all babies born with anencephaly will die shortly after birth.
- Researchers estimate that about 1 in every 5,250 babies is born with anencephaly in the United States.
Anencephaly is a fatal condition where a baby is born without parts of the brain and skull. It is a type of neural tube defect (NTD). There is no known cure or standard treatment for anencephaly.
During early pregnancy, the neural tube develops into the baby's brain and spine. The upper part helps form the baby's brain and skull, and the lower part forms the spinal cord and back bones.
In anencephaly, the upper part of the neural tube does not close all the way. The baby is often born without the front part of the brain (forebrain) and the thinking and coordinating part of the brain (cerebrum). The remaining parts of the brain are often not covered by bone or skin.

Risk factors
Not all causes of anencephaly are known, but certain factors can affect the risk of a baby having anencephaly. This can include a change in the baby’s genes or a combination of genes and other factors.
Researchers have identified factors in pregnant people that might increase the risk for anencephaly:
- Low folate (vitamin B9) levels during early pregnancy 1
- Pre-existing health conditions, such as diabetes , that are not well controlled
- Certain medications, such as antiseizure medications
- Overheating (like getting in a hot tub) or fever
Importance of Folic Acid
Screening and diagnosis.
Anencephaly can be diagnosed during pregnancy or after the baby is born.
During pregnancy
During pregnancy, there are screening tests to check for birth defects and other conditions. Being pregnant with a baby with anencephaly can result in an abnormal result on a blood or serum screening test. Anencephaly can also be seen during an ultrasound exam.
After the baby is born
In some cases, anencephaly might not be diagnosed until after the baby is born. Anencephaly is immediately seen at birth.
Expected outcomes
There is no known cure or standard treatment for anencephaly. Pregnancy loss is high. Almost all babies born with anencephaly will die shortly after birth.
- Crider KS, Qi YP, Yeung LF, Mai CT, Head Zauche L, Wang A, Daniels K, Williams JL. Folic Acid and the Prevention of Birth Defects: 30 Years of Opportunity and Controversies. Annu Rev Nutr. 2022 Aug 22;42:423-452.
- Stallings, E. B., Isenburg, J. L., Rutkowski, R. E., Kirby, R. S., Nembhard, W.N., Sandidge, T., Villavicencio, S., Nguyen, H. H., McMahon, D. M., Nestoridi, E., Pabst, L. J., for the National Birth Defects Prevention Network. National population-based estimates for major birth defects, 2016–2020. Birth Defects Research. 2024 Jan;116(1), e2301.
Birth Defects
About one in every 33 babies is born with a birth defect. Although not all birth defects can be prevented, people can increase their chances of having a healthy baby by managing health conditions and adopting healthy behaviors before becoming pregnant.
For Everyone
Health care providers, public health.

Exencephaly – Anencephaly Sequence and its Sonographic Features
Anencephaly represents the most common neural tube defect. It’s incidence is approximately 1:1000 with female predominance (4:1) and geographical variability.
Introduction
Anencephaly represents the most common neural tube defect. It’s incidence is approximately 1:1000 with female predominance (4:1) and geographical variability. 1,2 The etiology of anencephaly closely mirrors that of spina bifida. The condition results from the failure of the rostral (cephalic) neuropore to close. Sonographic as well as pathologic evidence points to a close link between exencephaly (also frequently referred to as "acrania") and anencephaly. It has been proposed that the brain tissue of exencephalics may gradually degenerate due to the exposure to amniotic fluid in combination with mechanical trauma. This wearing down of the brain stroma produces the classic anencephalic features with flattened brain remnants behind the prominent orbits. This hypothesis is supported by animal studies, pathologic analysis of exencephalic brain stroma when compared with cerebrovasculosa 2 , as well as observations on ultrasonography combined with amniotic fluid cytology. 3
Ultrasound Findings
Reliable sonographic diagnosis of anencephaly is usually possible in early second trimester (10-14wks GA) 4 . Conventional 2D ultrasound is accurate in diagnosing anencephaly 5 and the sensitivity is virtually 100% after 14wks GA 6 . 3D sonography has been shown to be equally effective in detecting anencephaly. 7
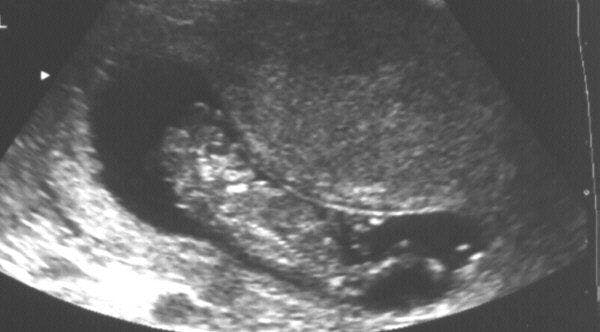
On ultrasound, the cranial vault (bony calvarium) is symmetrically absent. Rudimentary brain tissue (area cerebrovasculosa) is covered by a membrane, but not bone (Figure 1,2). This be seen protruding from the base of the skull in the early second trimester, and gradually degenerates until the appearance of the head is completely flattened behind the facial structures. Facial views reveal frog-like appearance with prominent bulging eyeballs (Figure 3,4). Associated polyhydramnios usually develops in the second trimester and is likely due to absent or ineffective fetal swallowing (Figure 3). High degree of fetal activity is often observed. 1,2,6
Metformin during pregnancy not linked to adverse birth outcomes
An analysis from Harvard shows there is no significantly increased newborn risk when continuing metformin to treat type 2 diabetes in pregnant women.

S1E4: Dr. Kristina Adams-Waldorf: Pandemics, pathogens and perseverance
This episode of Pap Talk by Contemporary OB/GYN features an interview with Dr. Kristina Adams-Waldorf, Professor in the Department of Obstetrics and Gynecology and Adjunct Professor in Global Health at the University of Washington (UW) School of Medicine in Seattle.

Survey: State abortion access impacted OB/GYN residency applications
National survey data presented at ACOG 2024 shows many medical students applying for OB/GYN residencies prioritized states with abortion access.

S1E3: Dr. Emily S. Miller: Placental pathology and MVM evidence
This episode of Pap Talk by Contemporary OB/GYN features an interview with Dr. Emily S. Miller, with Northwestern Medicine in Chicago.

Second trimester COVID-19 linked to increased preeclampsia risk
New ACOG 2024 data suggest SARS-CoV-2 in the early stages of pregnancy can lead to a higher likelihood of preeclampsia, as well as more severe disease.

Identifying gaps in syphilis treatment and prenatal care among pregnant individuals
Preventing congenital syphilis comes down to quick diagnosis and treatment of the infection in pregnancy, and the number of missed opportunities to do so in the United States continues to grow.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777


Anencephaly accounts for approximately one-half of all cases of spinal cord ( neural tube ) defects (Chescheir et al., 2003). The incidence of anencephaly in livebirths and stillbirths has been estimated as 0.3 per 1000 by the Centers for Disease Control (Medical Task Force on Anencephaly, 1990). Female fetuses are 3-4 times more commonly affected than males (Naidich et al., 1992). There is also an increased incidence of anencephaly in Hispanic women (Feuchtbaum et al., 1999). Some of the other risk factors include maternal diabetes prior to conception and maternal obesity (Mitchell, 2005). The most important environmental influence is diet. There is a well-documented protective effect of maternal folic acid supplementation starting at least 1 month prior to conception.
The major consideration as far as other potential diagnoses is to distinguish anencephaly from the presence of tissue fibers extending from the gestational sac ( amniotic bands ) that effectively amputate portions of the fetus. It is important to note that the skull defect associated with anencephaly always affects both sides ( symmetric ). With amniotic bands, there should be evidence of other defects, such as limb ( arms/legs ) or digital ( fingers/toes) amputations, abdominal wall defects, or spinal defects. Amniotic bands are often associated with there being little or no amniotic fluid around the fetus ( oligohydramnios ). In contrast, anencephaly is often associated with excess fluid ( polyhydramnios ).
Management Options and Outcomes
Of those fetuses with anencephaly, a small portion will die while still in the uterus ( intrauterine fetal demise or stillbirth ). Approximately 25% will have excessive amniotic fluid around the fetus ( polyhydramnios ). Polyhydramnios may cause extra stretching of the uterus resulting in preterm contractions. Sometimes, these patients require cesarean section because the fetus is breech. These infants will die in the immediate newborn period.
If prenatal screening is performed to detect spinal cord ( neural tube ) defects, the maternal serum alpha-fetoprotein level ( MSAFP ) will be significantly elevated in 90% of fetuses with anencephaly (Medical Task Force on Anencephaly, 1990). A detailed ultrasound examination is indicated for all patients with significantly elevated MSAFP levels. If there are no other abnormalities and the anencephaly is considered isolated, then a test to determine if there is a chromosomal abnormality may be recommended ( amniocentesis ). The chromosomes represent the instructions for how a fetus should form. It is quite likely that the chromosomes will be normal. Most fetuses with anencephaly deliver around 37 weeks of gestation (Melnick and Myrianthopoulos,1987). Because pregnancy with a fetus with anencephaly carries an increased medical risk for the mother, prospective parents may be offered the opportunity to terminate, especially if the diagnosis is made prior to 24 weeks of gestation. Cesarean delivery is indicated only for maternal health considerations or, perhaps, if the fetus is breech.
Candidacy for Fetal Treatment
There is no fetal intervention recommended for anencephaly.
Newborn Care
The diagnosis of anencephaly can be confirmed on physical examination when the following criteria are met: a large portion of the skull is absent, the scalp is absent over the skull defect, and a hemorrhagic, fibrotic mass of tissue is exposed to the environment. The newborn will seem unconscious because the upper brain portions ( cerebral hemispheres) are absent or not functioning. The parts that control the breathing, heartbeat and body temperature will be functional. The newborn has reflex responses to pain. Most newborns will die within the first few hours or days of life, though about 10% may live up to one week.
Surgical Management
Surgical treatment is not applicable in anencephaly.
Additional Information
The major issue in long-term outcome is the potential use of anencephalic fetuses or infants as organ donors. Difficulties exist, however, because of the traditional means of determining brain death for organ donors. For babies with anencephaly, the diagnosis of brain death depends on documentation of disappearance of previously existing brainstem functions ( i.e., breathing or spontaneous movements ), Most major organs from anencephalic infants are smaller than average for body size and have often not formed appropriately. Therefore, these organs might not be able to be donated.
A family history of spina bifida and/or anencephaly is one of the strongest risk factors for recurrence. Most cases of anencephaly have a recurrence risk of between 2% and 5% following a single case (Medical Task Force on Anencephaly, 1990). Some cases of anencephaly are associated with chromosomal abnormalities such as trisomies 13 and 18, and triploidy. For women who have previously had a fetus or infant affected with anencephaly, the Centers for Disease Control and Prevention (CDC) recommends increasing the intake of folic acid to 4000 mcg (4mg) per day beginning at least 1 month prior to conception (Committee on Genetics,1999).
A Difficult Diagnosis
The Fetal Health Foundation was founded by parents seeking hope for a fetal diagnosis. We try to provide accurate medical information, support, and hope. In the case of a diagnosis like this, we also want to offer resources that can help parents make the most of their time with their child.
Further reading and resources:
Read the story of our board member, Aran, meeting her daughter, Brianna Marie.
Read about memorial photography work of Now I Lay Me Down To Sleep. We are grateful for these volunteers.
Chescheir N. ACOG Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin. Neutral tube defects. Number 44, July 2003. Int J Gynaecol Obstet. 2003;83:123-133.
Committee on Genetics, American Academy of Pediatrics. Folic acid for the prevention of neural tube defects. Pediatrics. 1999;104:325-327.
Feuchtbaum LB, Currier RJ, Riggle S, Roberson M, Lorey FW, Cunningham GC. Neural tube defect prevalence (1990–1994): eliciting patterns by type of defect and maternal race/ethnicity. Genet Test. 1999;3:265-272.
Goldstein RB, Filly RA. Prenatal diagnosis of anencephaly: spectrum of sonographic appearances and distinction from the amniotic band syndrome. AJR Am J Roentgenol. 1988;151:547-550.
Medical Task Force on Anencephaly. The infant with anencephaly. N Engl J Med. 1990;332:669-674.
Melnick M, Myrianthopoulos NC. Studies in neural tube defects. II. Pathologic findings in a prospectively collected series of anencephalics. Am J Med Genet. 1987;26:797-810.
Mitchell LE. Epidemiology of neural tube defects. Am J Med Genet C Semin Med Genet. 2005;135C:88-94.
Naidich TP, Altman NR, Braffman BH, McLone DG, Zimmerman RA. Cephaloceles and related malformations. AJNR Am J Neuroradiol. 1992;13:655-690.
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

- Health Topics
- Drugs & Supplements
- Medical Tests
- Medical Encyclopedia
- About MedlinePlus
- Customer Support
Anencephaly
Description.
Anencephaly is a condition that prevents the normal development of the brain and the bones of the skull. This condition results when a structure called the neural tube fails to close during the first few weeks of embryonic development. The neural tube is a layer of cells that ultimately develops into the brain and spinal cord. Because anencephaly is caused by abnormalities of the neural tube, it is classified as a neural tube defect.
Because these nervous system abnormalities are so severe, almost all babies with anencephaly die before birth or within a few hours or days after birth.
Anencephaly is one of the most common types of neural tube defect, affecting about 1 in 1,000 pregnancies. However, most of these pregnancies end in miscarriage, so the prevalence of this condition in newborns is much lower. An estimated 1 in 10,000 infants in the United States is born with anencephaly.
Anencephaly is a complex condition that is likely caused by the interaction of multiple genetic and environmental factors. Some of these factors have been identified, but many remain unknown.
Changes in dozens of genes in individuals with anencephaly and in their mothers may influence the risk of developing this type of neural tube defect. The best-studied of these genes is MTHFR , which provides instructions for making a protein that is involved in processing the vitamin folate (also called vitamin B9). While a shortage (deficiency) of this vitamin is an established risk factor for neural tube defects, there are many factors that can contribute to folate deficiency. Changes in other genes related to folate processing and genes involved in the development of the neural tube have also been studied as potential risk factors for anencephaly. However, no genes appear to play a major role in causing the condition.
Researchers have also examined environmental factors that could contribute to the risk of anencephaly. Folate deficiency plays a significant role. Studies have shown that women who take supplements containing folic acid (the synthetic form of folate) before they get pregnant and very early in their pregnancy are significantly less likely to have a baby with a neural tube defect, including anencephaly. Other possible maternal risk factors for anencephaly include diabetes mellitus, obesity, exposure to high heat (such as a fever or use of a hot tub or sauna) in early pregnancy, and the use of certain anti-seizure medications during pregnancy. However, it is unclear how these factors may influence the risk of anencephaly.
Learn more about the gene associated with Anencephaly
Inheritance.
Most cases of anencephaly are sporadic, which means they occur in people with no history of the disorder in their family. A small percentage of cases have been reported to run in families; however, the condition does not have a clear pattern of inheritance. For parents who have had a child with anencephaly, the risk of having another affected child is increased compared with the risk in the general population.
Other Names for This Condition
- Anencephalia
- Anencephalus
- Aprosencephaly
- Congenital absence of brain
Additional Information & Resources
Genetic testing information.
Genetic and Rare Diseases Information Center
Patient support and advocacy resources.
- National Organization for Rare Disorders (NORD)

Clinical Trials
Catalog of genes and diseases from omim.
- NEURAL TUBE DEFECTS, FOLATE-SENSITIVE; NTDFS
- NEURAL TUBE DEFECTS, SUSCEPTIBILITY TO; NTD
- ANENCEPHALY 1; ANPH1
Scientific Articles on PubMed
- Au KS, Ashley-Koch A, Northrup H. Epidemiologic and genetic aspects of spina bifida and other neural tube defects. Dev Disabil Res Rev. 2010;16(1):6-15. doi: 10.1002/ddrr.93. Citation on PubMed or Free article on PubMed Central
- Bassuk AG, Kibar Z. Genetic basis of neural tube defects. Semin Pediatr Neurol. 2009 Sep;16(3):101-10. doi: 10.1016/j.spen.2009.06.001. Citation on PubMed
- Botto LD, Moore CA, Khoury MJ, Erickson JD. Neural-tube defects. N Engl J Med. 1999 Nov 11;341(20):1509-19. doi: 10.1056/NEJM199911113412006. No abstract available. Citation on PubMed
- Copp AJ, Greene ND. Genetics and development of neural tube defects. J Pathol. 2010 Jan;220(2):217-30. doi: 10.1002/path.2643. Citation on PubMed or Free article on PubMed Central
- Doudney K, Grinham J, Whittaker J, Lynch SA, Thompson D, Moore GE, Copp AJ, Greene ND, Stanier P. Evaluation of folate metabolism gene polymorphisms as risk factors for open and closed neural tube defects. Am J Med Genet A. 2009 Jul;149A(7):1585-9. doi: 10.1002/ajmg.a.32937. No abstract available. Citation on PubMed
- Greene ND, Stanier P, Copp AJ. Genetics of human neural tube defects. Hum Mol Genet. 2009 Oct 15;18(R2):R113-29. doi: 10.1093/hmg/ddp347. Citation on PubMed or Free article on PubMed Central
- Hickey SE, Curry CJ, Toriello HV. ACMG Practice Guideline: lack of evidence for MTHFR polymorphism testing. Genet Med. 2013 Feb;15(2):153-6. doi: 10.1038/gim.2012.165. Epub 2013 Jan 3. Erratum In: Genet Med. 2020 Dec;22(12):2125. doi: 10.1038/s41436-020-0843-0. Citation on PubMed
- Levin BL, Varga E. MTHFR: Addressing Genetic Counseling Dilemmas Using Evidence-Based Literature. J Genet Couns. 2016 Oct;25(5):901-11. doi: 10.1007/s10897-016-9956-7. Epub 2016 Apr 30. Citation on PubMed
- Obeidi N, Russell N, Higgins JR, O'Donoghue K. The natural history of anencephaly. Prenat Diagn. 2010 Apr;30(4):357-60. doi: 10.1002/pd.2490. Citation on PubMed
- Wilson RD; SOGC GENETICS COMMITTEE; SPECIAL CONTRIBUTOR. RETIRED: Prenatal screening, diagnosis, and pregnancy management of fetal neural tube defects. J Obstet Gynaecol Can. 2014 Oct;36(10):927-939. doi: 10.1016/S1701-2163(15)30444-8. Citation on PubMed
- Yan L, Zhao L, Long Y, Zou P, Ji G, Gu A, Zhao P. Association of the maternal MTHFR C677T polymorphism with susceptibility to neural tube defects in offsprings: evidence from 25 case-control studies. PLoS One. 2012;7(10):e41689. doi: 10.1371/journal.pone.0041689. Epub 2012 Oct 3. Citation on PubMed or Free article on PubMed Central
- Zhang T, Lou J, Zhong R, Wu J, Zou L, Sun Y, Lu X, Liu L, Miao X, Xiong G. Genetic variants in the folate pathway and the risk of neural tube defects: a meta-analysis of the published literature. PLoS One. 2013 Apr 4;8(4):e59570. doi: 10.1371/journal.pone.0059570. Print 2013. Citation on PubMed or Free article on PubMed Central

Genetics Home Reference has merged with MedlinePlus. Genetics Home Reference content now can be found in the "Genetics" section of MedlinePlus. Learn more
The information on this site should not be used as a substitute for professional medical care or advice. Contact a health care provider if you have questions about your health.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Skull Birth Defects: Anencephaly, Craniosynostosis, and Encephalocele
- Anencephaly
- Craniosynostosis
- Encephalocele
Skull birth defects are conditions that are present at birth. These birth defects were created as the fetus was still developing in a pregnant person's uterus. This overview will discuss three of the most common skull birth defects in babies: anencephaly , craniosynostosis , and encephalocele .
Verywell / Danie Drankwalter
What Is Anencephaly?
Anencephaly is a type of neural tube defect (NTD) in which a baby is born without parts of the brain and skull. Approximately 1 in every 4,600 babies in the United States is born with anencephaly.
The neural tube is formed very early in pregnancy. As it develops and closes, it helps form the brain, skull, spinal cord, and backbone.
If the upper part of the neural tube does not close all the way, anencephaly occurs. The baby is often born without the forebrain (the front part of the brain) and the cerebrum (the part of the brain responsible for thinking and coordination). Often, the rest of the brain is not covered by bone or skin.
Anencephaly develops during the third and fourth weeks of pregnancy, with the body of the fetus continuing to grow and form throughout the pregnancy.
The exact cause of anencephaly is not known, but it is believed to be caused by a combination of environmental factors, genetics, and nutrition during pregnancy. In most cases, there is no family history of it or other neural tube defects. In some instances, a familial pattern is suspected.
Some risk factors for anencephaly include:
- Lack of folic acid (at least 400 micrograms of folic acid is needed each day before and during pregnancy)
- Uncontrolled diabetes in pregnancy
- High body temperature (from a fever or using a hot tub or sauna during early pregnancy)
- Medications such as Dilantin (phenytoin), Tegretol (carbamazepine), and Depakote (valproic acid)
- Obesity before pregnancy
- Being around hazardous chemicals and materials
- Hispanic ethnicity of the pregnant person
Anencephaly is more common in girls rather than boys.
Anencephaly is associated with these conditions:
- Folding of the ears
- Cleft palate (a split in the roof of the mouth)
- Congenital heart defects
Anencephaly is often diagnosed before birth. If it is missed prenatally, it is immediately apparent through appearance when the baby is born.
Anencephaly may be diagnosed prenatally using tests and diagnostic tools such as:
Quad marker screen :
- It is a blood test.
- It checks for neural tube defects and genetic disorders.
- One of the tests is to look for higher levels of the protein alpha-fetoprotein (AFP), which is higher in the pregnant parent’s blood if anencephaly is present in the fetus.
Ultrasound :
- Uses sound waves to produce pictures of the fetus
- Used to look at the developing skull, brain, and spine
Fetal magnetic resonance imaging (MRI) :
- Produces images of tissues and bones using high-powered magnets
- Looks at the fetal brain and spine in greater detail
Amniocentesis :
- A small amount of fluid is removed using a thin needle inserted into the amniotic sac (the bag of fluid around the fetus).
- The fluid is checked for high levels of AFP and an enzyme called acetylcholinesterase , either of which may indicate a neural tube defect.
There is no treatment for anencephaly. Most pregnancies with anencephaly end in miscarriage or stillbirth. Babies who are born with anencephaly will almost always die within a few hours or a few days.
Treatment is supportive and involves helping parents and loved ones say goodbye and grieve their baby.
Folic Acid Can Reduce the Risk of Neural Tube Defects
There has been a 28% decline in pregnancies affected by neural tube defects since the U.S. began fortifying grains with folic acid. Neural tube defects often happen before a person knows they are pregnant, so it's important to get the recommended 400 micrograms of folic acid daily both before and during early pregnancy.
What Is Craniosynostosis?
A typical baby’s skull bones have spaces between them called sutures. They are filled with flexible material. These sutures stay open until around 2 years of age, to allow room for the baby's brain to grow. At around age 2, the sutures become bone and begin to "close" by joining together.
Craniosynostosis occurs when one or more of the sutures closes too early. This premature closure can limit or slow the growth of the baby’s brain. Approximately 1 in every 2,500 babies is born with craniosynostosis in the U.S.
Craniosynostosis can affect one, some, or all of the sutures. When a suture closes too early, the baby's head will stop growing in that area, but the rest of the skull will continue to grow. This may cause:
- An abnormally shaped skull
- Lack of room for the brain to grow to its proper size
- A buildup of pressure in the skull
Depending on how many sutures are affected and how much room there is, the brain may still grow to its usual size.
The types of craniosynostosis are determined by where the sutures close.
Sagittal craniosynostosis :
- Affects the suture on the top of the head
- Often causes a long, narrow head ( scaphocephaly )
Coronal craniosynostosis :
- Affects one of the coronal sutures (run from both ears to the top of the head)
- May cause a flat forehead and a broad head
Lambdoid craniosynostosis :
- Affects the suture along the back of the head
- May cause a flat back of the head ( plagiocephaly )
Metopic craniosynostosis :
- Affects the suture that runs from the top of the nose to the top of the forehead
- May cause a triangular head, with the narrow ridge at the midline of the forehead
If left untreated, craniosynostosis can lead to:
- Developmental delays
- Vision or eye movement disorders
- Breathing difficulties, especially if other bony abnormalities of the face are present
- Persistent head or facial deformities
The exact cause of craniosynostosis isn't known but may be the result of a combination of genes and other factors such as:
- Environmental contacts while pregnant
- Thyroid disease during pregnancy
- Use of fertility medications such as clomiphene citrate (Clomid)
- Premature birth
Craniosynostosis is usually diagnosed soon after birth, but it may be diagnosed later in life.
During a physical exam, the healthcare professional will look for symptoms such as:
- No fontanelle ("soft spot”) on the baby’s skull
- A raised firm ridge of bone on the baby's head
- Slow growth or no growth in the size of the baby’s head over time
An X-ray or computed tomography (CT) scan may be ordered to help confirm a suspected diagnosis of craniosynostosis.
Treatment depends on the severity of the condition. Some forms of craniosynostosis are mild and require little intervention, while others are more severe and may need more intensive treatments.
Treatments for craniosynostosis may include:
- Helmet therapy : A special medical helmet may be used to gently reshape the skull over time.
- Surgery : This may be necessary to reshape the skull, relieve pressure in the skull, and allow the baby’s brain room to grow and develop properly.
- Supportive therapies : Physical therapy, occupational therapy, and speech therapy may be necessary for some children with craniosynostosis.
What Is Encephalocele?
Encephalocele is a birth defect that happens when the neural tube does not close completely during pregnancy. Encephalocele occurs in approximately 1 in every 10,500 babies born in the U.S.
With encephalocele, part of the baby's brain and the membranes that cover it come through a hole in the skull. The protruding brain is covered in either skin or a thin membrane, creating a "sac."
The opening can be anywhere along the center of the skull from the nose to the back of the neck, but is usually at the back of the head, at the top of the head, or between the forehead and the nose.
Genetics may play a role in causing an encephalocele. Encephalocele occurs more frequently in families that have a history of neural tube defects (spina bifida and anencephaly).
More research is needed into the causes of encephalocele. As with other neural tube defects, 400 micrograms of folic acid taken daily before and during pregnancy is believed to help prevent encephalocele.
Symptoms and conditions that may occur with encephalocele include:
- Hydrocephalus (too much cerebrospinal fluid in parts of the brain)
- Microcephaly (a very small head)
- Vision problems
- Breathing problems (if there is a large encephalocele near the nose)
- Problems with swallowing
- Pain around the encephalocele
- Delayed growth and development
- Spasticity (high muscle tone) or other movement disorders
- Pituitary problems
- Differences with the bones in the skull and face
- Loss of strength in the arms and legs
- Uncoordinated use of muscles needed for movement (such as walking and reaching)
- Developmental delay
- Intellectual disability
Encephalocele is usually apparent at birth, but small encephaloceles (usually in the nose, sinuses, or forehead) may not be noticed right away.
Sometimes encephalocele is observed during a prenatal ultrasound. When this happens, fetal magnetic resonance imaging (MRI) may be ordered.
Treatment for encephalocele usually involves surgery (or several surgeries) to place the brain tissue and membranes back into the skull and repair the opening. If needed, a shunt may be put in to drain cerebrospinal fluid (CSF) from around the brain.
Once the encephalocele is treated, the child may need further or ongoing treatment for any conditions or symptoms caused by the encephalocele that remain.
Anencephaly, craniosynostosis, and encephalocele are three types of skull birth defects. They vary in whether they can be detected before birth, at birth, or later. Anencephaly cannot be treated and is fatal. Encephalocele usually requires surgery to repair. Craniosynostosis may require no intervention or need therapy or surgery.
Frequently Asked Questions
There are many types of birth defects that can cause abnormalities in the skull. Three of the most common are anencephaly, craniosynostosis, and encephalocele.
Skull deformities can be caused by a combination of genetics, a pregnant person's nutrition or general health, or their environmental exposures. A flat spot on one side or the back of a baby’s head, called plagiocephaly, can happen after birth due to sleep position. It is common, affecting about 10% of babies in the U.S.
Centers for Disease Control and Prevention. Facts about anencephaly .
Genetic and Rare Diseases Information Center. Anencephaly .
Golisano Children's Hospital. Anencephaly .
Flores AL, Cordero AM, Dunn M, et al. Adding folic acid to corn Masa flour: Partnering to improve pregnancy outcomes and reduce health disparities . Prev Med . 2018;106:26-30. doi:10.1016/j.ypmed.2017.11.003
Centers For Disease Control and Prevention. Facts about craniosynostosis .
Centers For Disease Control and Prevention. Facts about encephalocele .
Seattle Children's Hospital. Encephalocele .
Johns Hopkins Medicine. Craniofacial abnormalities .
By Heather Jones Jones is a freelance writer with a strong focus on health, parenting, disability, and feminism.

- Anencephaly
- Author: Robert G Best, PhD, FACMG; Chief Editor: Stephen L Nelson, Jr, MD, PhD, FAACPDM, FAAN, FAAP, FANA more...
- Sections Anencephaly
- Pathophysiology
- Epidemiology
- History and Physical Examination
- Lab Studies
- Imaging Studies
- Complications
- Patient Education
Anencephaly is a serious developmental defect of the central nervous system in which the brain and cranial vault are grossly malformed. The cerebrum and cerebellum are reduced or absent, but the hindbrain is present. Anencephaly is a part of the neural tube defect (NTD) spectrum. This defect results when the neural tube fails to close during the third to fourth weeks of development, leading to fetal loss, stillbirth, or neonatal death. [ 1 , 2 , 3 , 4 ]
Anencephaly, like other forms of NTDs, generally follows a multifactorial pattern of transmission, with interaction of multiple genes as well as environmental factors, although neither the genes nor the environmental factors are well characterized. In some cases, anencephaly may be caused by a chromosome abnormality, or it may be part of a more complex process involving single-gene defects or disruption of the amniotic membrane. Anencephaly can be detected prenatally with ultrasonography and may first be suspected as a result of an elevated maternal serum alpha-fetoprotein (MSAFP) screening test. Folic acid has been shown to be an efficacious preventive agent that reduces the potential risk of anencephaly and other NTDs by approximately two thirds. [ 5 , 6 , 7 , 8 ]
In the normal human embryo, the neural plate arises approximately 18 days after fertilization. During the fourth week of development, the neural plate invaginates along the embryonic midline to form the neural groove. The neural tube is formed as closure of the neural groove progresses from the middle toward the ends in both directions, with completion between day 24 for the cranial end and day 26 for the caudal end. Disruptions of the normal closure process give rise to NTDs. Anencephaly results from failure of neural tube closure at the cranial end of the developing embryo. Absence of the brain and calvaria may be partial or complete.
Most cases of anencephaly follow a multifactorial pattern of inheritance, with interaction of multiple genes as well as environmental factors. The specific genes that are most important in NTDs have not yet been identified, although genes involved in folate metabolism are believed to be important. One such gene, methylenetetrahydrofolate reductase ( MTHFR ), has been shown to be associated with the risk of NTDs. In 2007, a second gene, a membrane-associated signaling complex protein called VANGL1 , was also shown to be associated with the risk of neural tube defects. [ 9 ]
A variety of environmental factors appear to be influential in the closure of the neural tube. Most notably, folic acid and other naturally occurring folates have a strong preventive effect. Folate antimetabolites, maternal diabetes, maternal obesity, mycotoxins in contaminated corn meal, arsenic, and hyperthermia in early development have been identified as stressors that increase the risk of NTDs, including anencephaly.
Anencephaly is usually an isolated birth defect and not associated with other malformations or anomalies. The vast majority of isolated anencephaly cases are multifactorial in their inheritance pattern, implicating multiple genes interacting with environmental agents and chance events.
Inadequate folic acid
Adequate folic acid consumption during pregnancy is protective against anencephaly. [ 10 ] Exposure to agents that interfere with normal folate metabolism during the critical period of neural tube development (up to 6 weeks after last menstrual period) increases the likelihood of an NTD.
Valproic acid, an anticonvulsant, and other antimetabolites of folic acid have been shown to increase the chance of an NTD when exposure occurs in early development. While these induced NTDs are usually spina bifida, the chance of anencephaly is probably increased as well. [ 11 ]
Since the United States began fortifying grains with folic acid, there has been a 28% decline in pregnancies affected by neural tube defects (spina bifida and anencephaly). [ 12 ]
Insulin-dependent diabetes mellitus
Maternal type 1, or pregestational insulin-dependent diabetes mellitus (IDDM), confers a significant increase in the risk for NTDs, and it also delays production of alpha-fetoprotein (AFP) during pregnancy. [ 13 , 14 ] Maternal serum AFP is used as a screening test to detect NTDs, and adjustment of the expected values for AFP in maternal serum must be made if the patient is known to have IDDM. Presumably, well-controlled IDDM confers a lower risk for NTDs, while gestational diabetes does not appear to be associated with any significant increase in NTD risk. The degree of diabetic control is generally monitored using hemoglobin A1c levels.
Maternal hyperthermia
Maternal hyperthermia has been associated with an increased risk for NTD; therefore, pregnant women should avoid hot tubs and other environments that may induce transient hyperthermia. Similarly, maternal fever in early gestation also has been reported as a risk factor for anencephaly and other NTDs. [ 15 ]
While most NTDs are associated with a multifactorial model of inheritance, rare cases of NTDs are transmitted in an autosomal dominant or autosomal recessive manner in certain families. Such families may have children or fetuses with spina bifida, anencephaly, or other subtypes of NTDs. In families with a pedigree suggestive of autosomal dominant inheritance, reproduction is clearly only possible for the individuals with spina bifida, since death occurs early in the life of individuals with anencephaly.
Anencephaly may be associated with the unbalanced form of a structural chromosome abnormality in some families. In these cases, other malformations and birth defects that are not usually found in isolated cases of anencephaly may be present.
Amniotic band disruption sequence
Amniotic band disruption sequence is a condition resulting from rupture of the amniotic membranes. This can cause disruption of normally formed tissues during development, including the structures of the head and brain. Anencephaly caused by amniotic band disruption sequence is frequently distinguishable by the presence of remnants of the amniotic membrane. Recurrence risk for anencephaly caused by this mechanism is lower, and the risk is not modified by the use of folic acid.
Considerable geographical variation in neural tube defects (NTDs) rates exists, with noted hot spots in Guatemala, northern China, Mexico, and parts of the United Kingdom. Hispanic and non-Hispanic whites [ 16 ] are affected more frequently than women of African descent, and females are affected more frequently than males. Anencephaly is determined by the 28th day of conception and is therefore invariably present at the time of birth.
In the United States, about 1 in every 4600 babies is born with anencephaly. [ 12 ] The frequency during pregnancy is considerably higher than the birth prevalence, with estimates as high as 1 case per 1000 pregnancies. Such pregnancies often end in early pregnancy loss, spontaneous abortion, fetal death, or pregnancy termination.
Within the United States, South Carolina has historically reported the highest birth prevalence of NTDs, with a rate that has been approximately double that of the national average. The rate of NTDs in South Carolina has fallen dramatically following the introduction of aggressive campaigning for periconceptional folic acid supplementation, fortification of wheat flour, and increased periconceptional vitamin supplementation. [ 17 ] The reason for a higher occurrence of NTDs in South Carolina compared with other areas of the country is not known.
In 1990-1991, a cluster of NTDs was reported in Brownsville, Texas. [ 18 ] This primarily Hispanic population was targeted for surveillance as well as an intensive folic acid supplementation campaign directed at prevention of recurrences. Since that time, it has been generally accepted that the Hispanic population has an increased risk of anencephaly and other NTDs compared with other racial/ethnic populations in the United States, although the reasons have not been identified. [ 16 , 19 ]
In families that have previously experienced a pregnancy affected with anencephaly, the use of folic acid supplements at a dose 10 times higher than what is generally advised for the general population (4 mg/day vs 400 mcg/day) is recommended. In the South Carolina study, more than 300 pregnancies have been followed from women with a prior NTD-affected pregnancy who received the higher dose of folic acid supplements as part of the follow-up program with no recurrences of NTDs observed.
Study of NTDs in the United States by the Centers for Disease Control and Prevention shows a significant reduction of anencephaly and other NTDs following the introduction of fortification of wheat flour with folic acid. During the period of 1996-2001, there was a 23% decline in spina bifida and anencephaly combined, with spina bifida declining by 24% and anencephaly by 21%. [ 20 ]
Anencephaly is lethal in all cases because of the severe brain malformation that is present. A significant proportion of all anencephalic fetuses are stillborn or are aborted spontaneously.
The neonate's prognosis when born alive is exceptionally poor; death of a live child is unavoidable and most often occurs during the early neonatal period .
Anencephaly is readily apparent at birth because of the absence of the cranial vault and portions of the cerebrum and cerebellum. Facial structures are generally present and appear relatively normal. The cranial lesion occasionally is covered by skin, but usually it is not. When the lesion is covered with skin, prenatal screening using maternal serum alpha-fetoprotein (MSAFP) is ineffective. Babies are frequently stillborn, and spontaneous abortion during pregnancy is common. Although the features of anencephaly are readily evident, physical examination for anomalies not related directly to the anencephaly is indicated to evaluate the possible need for cytogenetic studies. When additional malformations are present, the likelihood of cytogenetic abnormalities is increased.
Maternal serum alpha-fetoprotein (MSAFP) screening during the second trimester of pregnancy is an effective screening tool for identification of the vast majority of cases of anencephaly in women with or without a positive family history or other risk factors for neural tube defects. [ 21 ]
Amniotic alpha-fetoprotein (AFAFP) testing during the late first trimester and second trimester of pregnancy is a diagnostic biochemical test for anencephaly. False positives from AFAFP can be excluded based on the results of testing for acetylcholinesterase (ACHE), which should be clearly positive for open anencephaly.
Laboratory studies are not performed postnatally in most cases of isolated anencephaly. Cytogenetic testing can exclude trisomy 13 as well as unbalanced structural chromosome abnormalities. [ 22 ]
Prenatal 2-dimensional ultrasonography has steadily improved over the years and has superseded maternal serum alpha-fetoprotein measurements as a screening tool. Since ossification of the cranial vault is not consistently apparent prior to the completion of the 12th week of pregnancy, anencephaly should not be diagnosed by ultrasonography any earlier than this.
In the first trimester, absent calvarium, reduced crown-rump length, absent or exposed neural tissue with lobular appearance (exencephaly), and absence of the normal head contour geometry with the orbits demarcating the upper border of the face (coronal view) are associated with anencephaly. Later in pregnancy, polyhydramnios may arise as a result of reduced swallowing of the amniotic fluid.
Postnatal MRI findings have included absence of the cranial vault, supretentorial structures, and the cerebellum. [ 23 ]
Treatment & Management
Because anencephaly is a lethal condition, heroic measures to extend the life of the infant are contraindicated. The physician and medical care team should focus on providing a supportive environment in which the family can come to terms with the diagnosis and make preparations for their loss.
Families who are not aware of the diagnosis of anencephaly prior to birth or for whom the diagnosis is still fresh probably will need extra emotional support and possibly grief counseling. Families who have had some time to adjust to the diagnosis prior to delivery and who have had an opportunity to begin the grieving process ahead of time may seem well prepared, but they also will need adequate time to grieve and come to closure. The presence of family, friends, or clergy may be helpful in many cases.
Families often want to hold the baby after delivery, even if the baby is stillborn, and families wanting photographs of the baby with the family are not unusual. A cap or head covering of some sort is useful to minimize the visual impact of the malformation. Some families want to see the lesion, and this may help to dispel mental pictures, which are often worse than the actual malformations. In most cases, direct personal contact with the baby may help the parents to actualize the medical information they have been given and may help in the process of grief resolution.
If parents have chosen a name for the baby, they may be comforted if the doctor refers to the baby by name.
Feelings of guilt are normal responses of parents of a baby with serious birth defects. The involvement of genetic counselors, if available, may be particularly useful to parents in this situation because of their experience in dealing with a wide range of birth defects.
With timely prenatal diagnosis of this lethal disorder, the option of pregnancy termination should be presented to the couple. For couples who elect to continue the pregnancy, the possibilities of preterm labor, polyhydramnios, failure to progress, and delayed onset of labor beyond term also should be discussed.
Families commonly inquire about organ donation after the diagnosis of anencephaly. This cannot practically be arranged without crossing the lines of ethical care. Patients should be affirmed in their desires to see something meaningful come from the tragedy of having a pregnancy affected with anencephaly.
Pregnancy care
All patients diagnosed prenatally with a fetus affected by anencephaly should be offered a consultation with a care provider who is skilled in delivering grave information and is knowledgeable about recurrence risk, prevention, screening, and diagnostic testing options for future pregnancies.
Although a geneticist or genetics counselor is an ideal source and may be best suited for exploring family history, an experienced maternal fetal medicine physician or properly trained obstetrician may provide requisite information, especially in regions of the United States where there are inadequate numbers of geneticists or genetic counselors. Specific information related to the management of an ongoing pregnancy should be discussed during this consultation.
Once a diagnosis of anencephaly is made, pregnancy management varies according to the gestational age at diagnosis. At pre-viable gestational ages, the option of pregnancy termination should be among those discussed. The gestational age limits for this procedure are state specific and subject to the training and skill of the physician available to perform the pregnancy termination.
When patients choose not to proceed with pregnancy termination or when the pregnancy has progressed to a viable gestational age such that pregnancy termination is no longer an option (except in rare locations throughout the United States), attention should be focused on whether the labor will be induced or spontaneous and, if the former, at what gestational age.
Due to the physical stresses of pregnancy compounded by the emotional stress of carrying a fetus with a lethal birth defect, or because of the identification of medical conditions (eg, preeclampsia ) that may complicate any pregnancy, labor induction may be considered.
Focused discussions directed at neonatal resuscitation efforts should be held in advance of labor. These discussions should include a discussion of neonatal procedures used to resuscitate neonates, the cost of these measures, and alternatives to aggressive resuscitation. It is often best to include a neonatologist in these discussions. Clear documentation of these discussions is warranted. When delivery is planned in a hospital setting, labor and delivery nurses, obstetric care providers, and pediatric/neonatal attendants should be informed of the patient’s wishes for her child.
Because of the lethal nature of this condition, tocolysis (medical management to reduce uterine contractions) in an effort to prevent preterm birth is not a reasonable option, nor is cesarean delivery.
The pregnancy management of a child with lethal and nonlethal birth defects can be complicated by available resources. In addition to having a wealth of experience in dealing with grieving patients, some delivering hospitals are vastly more experienced in the management of pregnancies complicated by known lethal fetal birth defects. For this reason we recommend that babies with anencephaly be delivered at such centers, when possible.
Consultations
Every couple with a child who has anencephaly should consult with a geneticist and/or a genetic counselor to obtain information regarding recurrence risks, prevention, screening, and diagnostic testing options for future pregnancies and to assess the family history. Ideally, a genetic counselor should be consulted prenatally and should remain involved, as needed, until the family comes to closure after the conclusion of the pregnancy. Genetic counselors are trained and are generally skillful in helping a family work through the complex psychosocial issues that are commonly encountered in a new diagnosis of anencephaly.
Folic acid supplementation and/or a folate-enriched diet prior to and during future pregnancies are recommended. Obtaining enough folates from diet alone to effectively prevent recurrences in future pregnancies is extremely difficult. The U.S. Public Health Service recommends that women capable of becoming pregnant consume 400 µg of folic acid daily for NTD prevention. [ 24 ]
The recurrence risk for NTDs, in general, is 2-4% in subsequent pregnancies. For families with multiple occurrences of NTDs, recurrence risks may be higher and must be determined on a case-by-case basis.
Folic acid supplementation has been shown to be an effective means of lowering recurrence risks for future pregnancies. For women who desire pregnancy and have had a child with an NTD with their current partner, supplementation with 4 mg of folic acid daily is indicated, beginning at least 3 months prior to conception.
For all other women and girls of reproductive age, regardless of family history, 0.4 mg (or 400 mcg) per day of folic acid supplementation is appropriate; this amount of folic acid is found in most over-the-counter multivitamins. Folic acid supplementation at these levels is estimated to prevent two thirds of both recurrent and new cases of NTD.
Increased folate intake also may be achieved through diet; however, the bioavailability of natural folates in foods is often lower than that of folic acid. In the United States, wheat flour is fortified with a small amount of folic acid, but it is not enough to achieve maximal preventive benefits against NTD for a woman with an average diet.
Because of the large number of pregnancies that are not actively planned and the early gestational age at which neural tube development occurs, folate supplementation should be encouraged for all girls, beginning at puberty, in order to establish this practice before entering the childbearing years.
Prenatal ultrasound and amniocentesis should be offered to any couple with a prior pregnancy affected with an NTD. Maternal serum prenatal screening with AFP is available throughout the United States and most developed countries for identification of NTDs. Positive serum screening should be followed with diagnostic testing to exclude the presence of NTDs. Since 90-95% of NTDs occur in families without a positive history, such screening is appropriate for all pregnant patients and should not be reserved only for those with a positive history.
Anencephaly cannot be treated in utero; thus, pregnancy termination is the only intervention available to prevent the birth of a child with anencephaly that has been diagnosed prenatally. Supportive care should be provided for families, irrespective of the option they choose.
Anencephaly is uniformly fatal. Polyhydramnios is a common complication during pregnancy, and patients may experience significant discomfort from the abdominal distention that accompanies this condition. Risk of preterm labor is increased.
Because the pituitary gland may be absent in persons with anencephaly, spontaneous precipitation of labor may be delayed; therefore, the risk of the pregnancy progressing into the postterm period is significant. Labor may need to be induced in these cases. The rate of abnormal fetal presentations during delivery is increased.
Parents of babies with anencephaly should be educated about preventive measures for future pregnancies. Consultation with a genetic counselor may be helpful.
A number of resources may assist families who are dealing with the loss of a child with anencephaly. These include the Spina Bifida Association of America (SBAA) and the March of Dimes (MOD) . Contact the SBAA at (800) 621-3141.
Families may wish to participate in one of several ongoing studies of anencephaly or NTDs as a part of the healing process.
Botto LD, Moore CA, Khoury MJ, Erickson JD. Neural-tube defects. N Engl J Med . 1999 Nov 11. 341(20):1509-19. [QxMD MEDLINE Link] .
Campbell LR, Dayton DH, Sohal GS. Neural tube defects: a review of human and animal studies on the etiology of neural tube defects. Teratology . 1986 Oct. 34(2):171-87. [QxMD MEDLINE Link] .
Russell SA, McHugo JM, Pilling D. Cranial abnormalities. Twining P, McHugo JM, Piling D. Textbook of Fetal Anomalies . 2nd ed. Churchill Livingstone Elsevier; 2007. 95-141.
Obeidi N, Russell N, Higgins JR, O'Donoghue K. The natural history of anencephaly. Prenat Diagn . 2010 Apr. 30(4):357-60. [QxMD MEDLINE Link] .
Oakley GP Jr. The scientific basis for eliminating folic acid-preventable spina bifida: a modern miracle from epidemiology. Ann Epidemiol . 2009 Apr. 19(4):226-30. [QxMD MEDLINE Link] .
Berry RJ, Li Z, Erickson JD, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med . 1999 Nov 11. 341(20):1485-90. [QxMD MEDLINE Link] .
Brent RL, Oakley GP, Md J. The unnecessary epidemic of folic acid-preventable spina bifida and anencephaly. Pediatrics . 2000 Oct. 106(4):825-7. [QxMD MEDLINE Link] .
Wilson RD, Genetics Committee, Wilson RD, Audibert F, Brock JA, et al. Pre-conception Folic Acid and Multivitamin Supplementation for the Primary and Secondary Prevention of Neural Tube Defects and Other Folic Acid-Sensitive Congenital Anomalies. J Obstet Gynaecol Can . 2015 Jun. 37 (6):534-52. [QxMD MEDLINE Link] .
Kibar Z, Torban E, McDearmid JR, Reynolds A, Berghout J, Mathieu M. Mutations in VANGL1 associated with neural-tube defects. N Engl J Med . 2007 Apr 5. 356(14):1432-7. [QxMD MEDLINE Link] .
Tinker SC, Devine O, Mai C, Hamner HC, Reefhuis J, Gilboa SM, et al. Estimate of the potential impact of folic acid fortification of corn masa flour on the prevention of neural tube defects. Birth Defects Res A Clin Mol Teratol . 2013 Oct. 97(10):649-57. [QxMD MEDLINE Link] .
Youngblood ME, Williamson R, Bell KN, Johnson Q, Kancherla V, Oakley GP Jr. 2012 Update on global prevention of folic acid-preventable spina bifida and anencephaly. Birth Defects Res A Clin Mol Teratol . 2013 Oct. 97(10):658-63. [QxMD MEDLINE Link] .
Mai CT, Isenburg JL, Canfield MA, Meyer RE, Correa A, Alverson CJ, et al. National population-based estimates for major birth defects, 2010-2014. Birth Defects Res . 2019 Nov 1. 111 (18):1420-1435. [QxMD MEDLINE Link] .
Salbaum JM, Kappen C. Neural tube defect genes and maternal diabetes during pregnancy. Birth Defects Res A Clin Mol Teratol . 2010 Aug. 88 (8):601-11. [QxMD MEDLINE Link] .
Gabbay-Benziv R, Reece EA, Wang F, Yang P. Birth defects in pregestational diabetes: Defect range, glycemic threshold and pathogenesis. World J Diabetes . 2015 Apr 15. 6 (3):481-8. [QxMD MEDLINE Link] .
Wang M, Wang ZP, Gong R, Zhao ZT. Maternal flu or fever, medications use in the first trimester and the risk for neural tube defects: a hospital-based case-control study in China. Childs Nerv Syst . 2013 Oct 26. [QxMD MEDLINE Link] .
Canfield MA, Ramadhani TA, Shaw GM, Carmichael SL, Waller DK, Mosley BS, et al. Anencephaly and spina bifida among Hispanics: maternal, sociodemographic, and acculturation factors in the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol . 2009 Jul. 85(7):637-46. [QxMD MEDLINE Link] .
Stevenson RE, Allen WP, Pai GS, Best R, Seaver LH, Dean J, et al. Decline in prevalence of neural tube defects in a high-risk region of the United States. Pediatrics . 2000 Oct. 106(4):677-83. [QxMD MEDLINE Link] .
Missmer SA, Suarez L, Felkner M, Wang E, Merrill AH Jr, Rothman KJ. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ Health Perspect . 2006 Feb. 114(2):237-41. [QxMD MEDLINE Link] .
Canfield MA, Marengo L, Ramadhani TA, Suarez L, Brender JD, Scheuerle A. The prevalence and predictors of anencephaly and spina bifida in Texas. Paediatr Perinat Epidemiol . 2009 Jan. 23(1):41-50. [QxMD MEDLINE Link] .
Mathews TJ, Honein MA, Erickson JD. Spina bifida and anencephaly prevalence--United States, 1991-2001. MMWR Recomm Rep . 2002 Sep 13. 51:9-11. [QxMD MEDLINE Link] .
Cameron M, Moran P. Prenatal screening and diagnosis of neural tube defects. Prenat Diagn . 2009 Apr. 29(4):402-11. [QxMD MEDLINE Link] .
Philipp T, Philipp K, Reiner A, et al. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod . 2003 Aug. 18(8):1724-32. [QxMD MEDLINE Link] .
Poretti A, Meoded A, Ceritoglu E, Boltshauser E, Huisman TA. Postnatal in-vivo MRI findings in anencephaly. Neuropediatrics . 2010 Dec. 41(6):264-6. [QxMD MEDLINE Link] .
Cordero AM, Crider KS, Rogers LM, Cannon MJ, Berry RJ. Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects: World Health Organization guidelines. MMWR Morb Mortal Wkly Rep . 2015 Apr 24. 64 (15):421-3. [QxMD MEDLINE Link] .
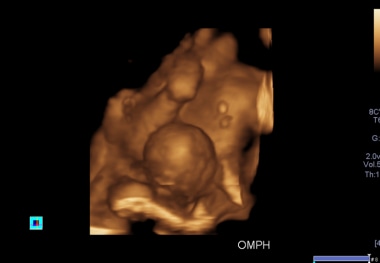
- A 3D antennal ultrasound scan shows an omphalocele in one of the conjoined twins, associated with anencephaly in the first trimester, allowing termination of pregnancy.

Contributor Information and Disclosures
Robert G Best, PhD, FACMG Professor of Biomedical Sciences and Associate Dean for Faculty Affairs, University of South Carolina School of Medicine, Greenville Robert G Best, PhD, FACMG is a member of the following medical societies: American College of Medical Genetics and Genomics , American Society of Human Genetics Disclosure: Nothing to disclose.
Anthony Romaine Gregg, MD Associate Professor, Director, Division of Maternal and Fetal Medicine, Medical Director, Division of Genetics, Medical Director, Genetics Counseling Program, Department of Obstetrics and Gynecology, University of South Carolina School of Medicine Anthony Romaine Gregg, MD is a member of the following medical societies: American Association for the Advancement of Science , American College of Medical Genetics and Genomics , American College of Obstetricians and Gynecologists , American Institute of Ultrasound in Medicine , American Medical Association , American Society of Human Genetics , Central Association of Obstetricians and Gynecologists , Society for Reproductive Investigation , Society for Maternal-Fetal Medicine , Society for the Study of Reproduction , Perinatal Research Society Disclosure: Nothing to disclose.
Nicholas Lorenzo, MD, CPE, MHCM, FAAPL Co-Founder and Former Chief Publishing Officer, eMedicine and eMedicine Health, Founding Editor-in-Chief, eMedicine Neurology; Founder and Former Chairman and CEO, Pearlsreview; Founder and CEO/CMO, PHLT Consultants; Former Chief Medical Officer, MeMD Inc Nicholas Lorenzo, MD, CPE, MHCM, FAAPL is a member of the following medical societies: Alpha Omega Alpha , American Academy of Neurology , American Association for Physician Leadership Disclosure: Nothing to disclose.
Stephen L Nelson, Jr, MD, PhD, FAACPDM, FAAN, FAAP, FANA Professor of Pediatrics, Neurology, Neurosurgery, and Psychiatry, Medical Director, Tulane Center for Autism and Related Disorders, Tulane University School of Medicine; Pediatric Neurologist and Epileptologist, Ochsner Hospital for Children; Professor of Neurology, Louisiana State University School of Medicine Stephen L Nelson, Jr, MD, PhD, FAACPDM, FAAN, FAAP, FANA is a member of the following medical societies: American Academy for Cerebral Palsy and Developmental Medicine , American Academy of Neurology , American Academy of Pediatrics , American Epilepsy Society , American Medical Association , American Neurological Association , Association of Military Surgeons of the US , Child Neurology Society , Southern Pediatric Neurology Society Disclosure: Nothing to disclose.
Edgar O Horger III, MD , Distinguished Professor Emeritus and Chair Emeritus, Department of Obstetrics and Gynecology, University of South Carolina School of Medicine
Edgar O Horger III, MD is a member of the following medical societies: Alpha Omega Alpha, American College of Obstetricians and Gynecologists, American Gynecological and Obstetrical Society, American Institute of Ultrasound in Medicine, American Medical Association, Association of Professors of Gynecology and Obstetrics, and South Carolina Medical Association.
Disclosure: Nothing to disclose.
Beth A Pletcher, MD Associate Professor, Co-Director of The Neurofibromatosis Center of New Jersey, Department of Pediatrics, University of Medicine and Dentistry of New Jersey
Beth A Pletcher, MD is a member of the following medical societies: American Academy of Pediatrics , American College of Medical Genetics , American Medical Association , and American Society of Human Genetics
James Stallworth, MD, Associate Professor of Pediatrics, University of South Carolina School of Medicine
James Stallworth, MD is a member of the following medical societies: Alpha Omega Alpha, Ambulatory Pediatric Association, American Academy of Pediatrics, Phi Beta Kappa, Society for Adolescent Medicine, and South Carolina Medical Association.
Francisco Talavera, PharmD, PhD, Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Reference Salary Employment
What would you like to print?
- Print this section
- Print the entire contents of
- Print the entire contents of article

- Neural Tube Defects
- Face and Brow Presentation
- Hydranencephaly Imaging
- Amniotic Band Syndrome (Streeter Dysplasia)
- Omphalocele Imaging
- Spinal Dysraphism (Neural Tube Defect) and Myelomeningocele Imaging
- Inpatient Hospitalization Costs Associated With Birth Defects Among Persons Aged <65 Years

- Drug Interaction Checker
- Pill Identifier
- Calculators

- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Chapter 13: Exencephaly/Acrania
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
- SONOGRAPHIC FINDINGS
- DIFFERENTIAL DIAGNOSIS
- ANTENATAL NATURAL HISTORY
- MANAGEMENT OF PREGNANCY
- FETAL INTERVENTION
- TREATMENT OF THE NEWBORN
- SURGICAL TREATMENT
- LONG-TERM OUTCOME
- GENETICS AND RECURRENCE RISK
- Full Chapter
- Supplementary Content
Rare fetal anomaly that is incompatible with survival.
Bones of the cranial vault are absent but facial structures and skull base are preserved. Residual brain tissue is present and floats free in amniotic fluid.
Likely to be the first trimester precursor to anencephaly. Now called fetal acrania–anencephaly sequence.
Incidence is 3 per 10,000 second trimester pregnancies.
Sonographic findings in the first trimester include: absent calcification of the cranial bones, lateral widening of the cerebral hemispheres (the “Mickey Mouse” sign), and echogenic amniotic fluid. Second trimester findings include free-floating disorganized brain tissue with preservation of the face.
Often associated with omphalocele, amniotic band syndrome, limb–body wall complex, and pentalogy of Cantrell.
Differential diagnosis includes acalvaria, massive meningoencephalocele, amniotic bands, limb–body wall complex, hypophosphatasia, and osteogenesis imperfecta type II.
Condition is uniformly fatal postnatally.
Recurrence risk depends on underlying etiology. If syndromic may have 25% to 50% recurrence risk. Otherwise, recurrence risk is 2% to 5%.
Preconceptual folic acid (4 mg/day) is recommended for subsequent pregnancies.
Exencephaly is a rare fetal anomaly that is incompatible with extrauterine life. In exencephaly, the bones of the cranial vault are absent (acrania), but the facial structures and the base of the skull are preserved ( Casellas et al., 1993 ). The terms exencephaly and acrania are used interchangeably in this chapter. Exencephaly is a precursor to anencephaly (the so-called fetal acrania-anencephaly sequence); it differs from anencephaly in that residual brain tissue is present and floating free in the amniotic fluid.
Exencephaly is frequently noted in animal teratogen studies. Human exencephaly appears to be confined to early gestation. Only rare reports exist of a third trimester diagnosis of an exencephalic fetus ( Wilkins-Haug and Freedman, 1991 ). Anencephaly, however, is more common in humans than in animals. The greater prevalence of anencephaly in humans is attributed to a longer gestational period, which presents the opportunity for destruction of the free-floating brain matter.
Exencephaly is due to the failure of the anterior neuropore to close during the 4th week of embryonic development. The underlying defect is due to a failure in mesenchymal migration ( Stagiannis et al., 1995 ). In pathologic studies, the exencephalic brain is noted to be covered by a highly vascular epithelial layer. In exencephaly, two relatively equivalent cerebral hemispheric remnants are present within a reddish mass of disorganized tissues, remnants of deep cerebral neural elements, blood vessels, fibrous tissues, and fluid-filled spaces ( Hendricks et al., 1988 ). The remaining brain has been termed the “anencephalic area cerebrovasculosa.” In exencephalic brain tissue, the gyri and sulci are shallow, flattened, and disorganized. All surfaces of the brain are highly vascular. The remaining central nervous system tissue is dysplastic, with little or no neuronal differentiation, and very little normal cortex ( Hendricks et al., 1988 ).
Get Free Access Through Your Institution
Pop-up div successfully displayed.
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Clin Diagn Res
- v.8(9); 2014 Sep
Anencephaly and its Associated Malformations
Ravikiran ashok gole.
1 Assistant Professor, Department of Anatomy, L.T.Medical College & General Hospital, College Building, 3 rd Floor, LTMMC & GH, Sion Hospital, Sion, Mumbai, India.
Pritee Madan Meshram
2 Assistant Professor, Department of Anatomy, L.T.Medical College & General Hospital, College Building, 3 rd Floor, LTMMC & GH, Sion Hospital, Sion, Mumbai, India.
Shanta Sunil Hattangdi
3 Professor and Head, Department of Anatomy, L.T.Medical College & General Hospital, College Building, 3 rd Floor, LTMMC & GH, Sion Hospital, Sion, Mumbai, India.
Introduction: Anencephaly is a serious neural tube defect in which parts of the brain and skull are not developed. But apart from this it is also associated with other malformations which are not related to neural tube in development.
Aim: The present study was undertaken to find the associated congenital malformations in western region of India and establish a aetiological correlation.
Materials and Methods: The study was conducted using 20 anencephalic fetuses.
Results: Nearly 80% of fetuses had associated malformations. Spina bifida was seen in 9 fetuses and cleft palate in 8. Female fetus with cleft palate had other severe associated gastrointestinal and skeletal malformation.
Conclusion: In cases of anencephaly other associated malfor–mations like spina bifida and cleft palate are commonly seen.
Introduction
Failure of closure of the cranial neuropore during the fourth week of development results in the abnormal vascularisation of the embryonic exencephalic brain [ 1 ]. The nervous tissue subsequently undergoes degeneration and brain remains as a spongy vascular mass with some hind brain structures [ 2 ]. Previously called as anencephaly (without brain), it is now called as Meroanencephaly as some functioning neural tissue is always present [ 3 ]. As Meroanencephaly is a lethal malformation the research on presence of other associated malformations has largely remained restricted. Ballantyne (1904)[ 4 ] as well as David and Illingworth [ 5 ] associated diapharagmatic hernia with anencephaly. British Perinatal Mortality Survey [ 6 ] of 1958 first stressed on registering the associated malformation in anencephalics. T.J.David [ 7 ] tabulated the associated malformations and found spina bifida to be the most common. Many researchers found that the most common associated malformations differed according to the geographical location [ 8 , 9 ]. According to David TJ cardiovascular defects were common in Lancashire while urinary tract defects were common in the Bristol [ 10 ]. Apart from CNS malformations gastrointestinal and skeletal abnormalities were the most common according to C Pandurang [ 11 ] in a study conducted in India.
Materials and Methods
The study was conducted between January 2013 to March 2014 in Lokmanya Tilak Municipal Medical College and general hospital which is a major tertiary care hospital for obstetrics in western India. Ethical clearance was obtained by the institutional ethical committee. Twenty anencephalic fetuses were dissected in the Anatomy department of the institute after obtaining informed consent. The cases originated from still birth, spontaneous abortion and therapeutic abortion. The gestational age was in the range of 16 to 34 weeks. There was no history of diabetes, obesity and infections in the mothers. There was no exposure to any teratogenic drugs. All mothers had received the recommended 0.5mg of folic acid supplementation. The findings were done by external examination, photography and internal examination. Internal examination was done for abdominal and genitourinary viscera only.
The results were recorded according to the following parameters.
Facial features: - Cleft lip, cleft palate.
CNS: - Spina bifida.
GIT: - Absence or underdeveloped organ.
Genito urinary system: - Hypospadias, penile hypoplasia, renal agenesis.
Skeletal system: - Clubbed foot, clubbed hands.
Associated malformations were seen in 16 out of 20 fetuses. There were 12 female and 8 male fetuses. Spina bifida was the most common anomaly followed by facial anomalies which included cleft lip and cleft palate. The details are given in [ Table/Fig-1 ].
[Table/Fig-1]:
Showing the distribution of number of cases for different anamoly
| Anomaly | No of cases | Percentage | Remarks |
|---|---|---|---|
| Spina bifida | 9 | 45 | Cervical region 7 cases Lumbar region 1 case. |
| Cleft Palate | 8 | 40 | 7 male 1 female |
| Cleft lip | 5 | 25 | All has associated cleft palate |
| Clubbed foot and clubbed hands | 7 | 35 | 5 males 2 females |
| Genital abnormalities | 2 cases | 10 | 2 males has Hypospadias No abnormality in females |
| Gastrointestinal system | 2 cases | 10 | 1 case gastroschisis. 1 case omphalocele. |
Anencephaly is associated with anomalies of not only central nervous system but other systems as well. Previous studies have mentioned a wide range for the percentage of fetus with associated malformation. Tan et al., [ 12 ] recorded 9.4 % while David TJ [ 10 ] recorded 84%. The present study recorded 80% fetus with associated malformations. There were more number of female fetuses (12 cases) as compared to males (8 cases). The percentage of female fetuses was 60%. All the previous studies have proved that the females outnumber males in cases of anencephaly. The female preponderance noticed by previous authors were David TJ [ 10 ] at 66%, Panduranga [ 11 ] at 56% Aruna [ 13 ] recorded 55%.
In the present study the commonest anomaly associated with anencephaly was spina bifida (9 cases). Associated malformations were more common in cases with spina bifida. Even though spina bifida is more common in lumbar region in general population, craniospinal rachischisis [ Table/Fig-2 ] of the cervical region was found to be commoner in anencephalic fetuse [ 14 ]. Craniospinal rachischisis is the most severe form of spina bifida cystica. In this defect the spine lies widely open and the neural plate has spread out on to the surface [ 15 ]. The thoracolumbar type spina bifida [ Table/Fig-3 ] was seen in only one case.

Fetus with craniospinal rachischisis and kyphosis of thoracic spine

Fetus with thoracolumbar spina bifida
Facial abnormalities like cleft palate were seen in 8 cases of which 7 were males and one female. Suggesting a correlation between cleft palate and male sex. Earlier studies have also reported cleft lip and palate to be more common in male anencephalic fetus [ 10 , 11 , 13 ]. Cleft lip was found in 5 cases in association with cleft palate.
The genital abnormalities were seen in two male cases in the form of Hypospadias. The remaining genital development was normal. There was no genital abnormality detected in females. Studies done by Tan et al.,[ 12 ], Nielsen et al.,[ 16 ] and Golalipour et al.,[ 9 ] did not find any abnormality in the genital system. David et al found 0.6% cases with abnormalities in the genital system and that too in male fetuses only. One female fetus which had marked deformities such as cleft lip, cleft palate, gastroschisis, clubbed feet and hands also showed normal genital development [ Table/Fig-4 ]. One case of omphalocele was seen [ Table/Fig-5 ]. No abnormality was detected in the urinary system.

Fetus with gastroschisis, cleft palate, cleft lip and clubbed feet

Fetus with omphalocele
Skeletal deformities like clubbed hands and clubbed feet were present in 5 males and 2 female fetuses. All the 5 male cases had associated cleft palate. Skeletal abnormalities found in previous studies were in the range of 1.7% by David TJ [ 10 ], 14.5% Vare et al.,[ 17 ] to 20 % by Tan et al.,[ 12 ]. The present study recorded a higher number of skeletal abnormalities at 35%.
The cause of anencephaly is still a disputed entity but the defect is failure of closure of rostral neuropore. Anencephaly, even though a defect of the neural tube can affect many other systems selectively [ 18 ]. The intake of 0.5mg of folic acid [ 19 ] during the course of pregnancy reduces the risk of anencephaly but does it have a similar effects on other anomalies is poorly understood. The presence of spina bifida can be correlated embryologically to the defect in closure of the neuropore. Cleft palate and lip might occur due to the defective neural crest cells. MTHFR gene has been associated with anencephaly and orofacial defects [ 20 ] but its increased frequency in males is poorly understood. Many studies have tried to find the genetic association of anencephaly. LD Botto [ 21 ] reported MTHFR gene located on chromosome 1 to be associated with neural tube defects. But EC Melvin [ 22 ] failed to reach a consensus on the involvement of p53, PAX3 and MTHFR gene in neural tube defects.
Anencephaly is common in females. Associated abnormalities were seen in 80% of cases in the present study. Commonest abnormality was spina bifida. Describing the associated malformations in anencephaly as described in present study is not only of academic and research interest but also helpful to radiologists for correct interpretation and diagnosis. The strong association between cleft palate and male fetus should be considered during the diagnosis. The presence of associated abnormality like spina bifida, cleft palate, clubbed foot, clubbed hands and gastroschisis points to the fact that anenchepaly consists of more than one aetiological entity. Studies are required at molecular level to find its association with other anomalies.
Financial or Other Competing Interests

COMMENTS
The most common neural tube defect (NTD) is anencephaly.1 Overall, the estimated prevalence of anencephaly is 3 per 10,000 births,2 although the incidence varies among different geographic regions, ethnic groups, and environmental exposures.2,3 This malformation is also referred to as acrania.
Anencephaly is a pathology of development characterized by a fetus that has no calvarium, with a lack of most or all of the fetus' brain tissue.[1] Anencephaly belongs to a collective group known as neural tube defects (NTD) and is a result of the neural tube failing to close in its rostral end during fetal development.[2] While the central nervous system (CNS) is developing in a fetus, the ...
Anencephaly [congenital absence of a major portion of the brain, skull, and scalp (Medical Task Force on Anencephaly, 1990)] is the most severe and single most common prenatally detected neural tube defect (Goldstein and Filly, 1988).Although the cerebral hemispheres can develop in this condition, any exposed brain tissue is subsequently destroyed (see Chapter 13).
Anencephaly and spina bifida are the most common ONTDs, while encephaloceles (where there is a protrusion of the brain or its coverings through the skull) are much rarer. ... Diagnostic tests performed during pregnancy to evaluate the baby for anencephaly include the following: Alpha-fetoprotein. A protein produced by the fetus that is excreted ...
Anencephaly is a fatal condition where a baby is born without parts of the brain and skull. It is a type of neural tube defect (NTD). There is no known cure or standard treatment for anencephaly. During early pregnancy, the neural tube develops into the baby's brain and spine. The upper part helps form the baby's brain and skull, and the lower ...
Anencephaly represents the most common neural tube defect. It's incidence is approximately 1:1000 with female predominance (4:1) and geographical variability. 1,2 The etiology of anencephaly closely mirrors that of spina bifida. The condition results from the failure of the rostral (cephalic) neuropore to close.
Anencephaly is characterized by an open defect in the calvaria and skin such that the cranial neural tube is exposed. It is a severe defect and is not compatible with survival. Nearly all liveborn infants with anencephaly die within the first few hours to days after birth. The clinical features, diagnosis, and postnatal management of ...
Anencephaly is a condition in which major portions of the brain, skull, and scalp are absent. It is the most severe and single most common prenatally detected spinal cord (neural tube) defect (Goldstein and Filly, 1988). Although the upper portions of the brain (the cerebral hemispheres) can develop in this condition, any exposed brain tissue ...
Anencephaly is a neural tube defect ... and so, it is classified as a lethal neural tube defect. Although stillbirth is a common outcome of fetal anencephaly, some affected fetuses are born alive with a rudimentary brain. ... Premature delivery (before 37 weeks of gestation) was the most common pregnancies outcome for the 53 fetuses, with a ...
Anencephaly is one of the most common types of neural tube defect, affecting about 1 in 1,000 pregnancies. However, most of these pregnancies end in miscarriage, so the prevalence of this condition in newborns is much lower. An estimated 1 in 10,000 infants in the United States is born with anencephaly.
Anencephaly is a type of neural tube defect (NTD) in which a baby is born without parts of the brain and skull. Approximately 1 in every 4,600 babies in the United States is born with anencephaly. The neural tube is formed very early in pregnancy. As it develops and closes, it helps form the brain, skull, spinal cord, and backbone.
Anencephaly is the absence of a major portion of the brain, skull, and scalp that occurs during embryonic development. [1] It is a cephalic disorder that results from a neural tube defect that occurs when the rostral (head) end of the neural tube fails to close, usually between the 23rd and 26th day following conception. [2] Strictly speaking, the Greek term translates as "without a brain" (or ...
The most common neural tube defect (NTD) is anenceph-aly.1 Overall, ... Exencephaly-anencephaly in a fetus at 11 6/7 weeks of gestation A, Sagittal view showing an irregularly shaped head. ... management: an unusual case presentation and systematic literature re-view. Pediatr Neurosurg 2015;50:204-9. 13. Seeds JW, Cefalo RC, Herbert WN.
Anencephaly is a severe malformation of the central nervous system (CNS), being one of the most common types of neural tube defects. It is defined as total or partial absence of the calvarium, with absence of the brain.Anencephaly has an incidence of 1 to 5 in every 1000 births, and the mortality rate is 100% during intrauterine life or within hours or days after birth.
anencephaly, acrania, lethal anomalies. Anencephaly is absence of the scalp, skull, cranial vault, and cerebral hemispheres. It is the most common neural tube defect and occurs in 1/1000 pregnancies.1 The condi-tion is incompatible with life, and management for liveborns is supportive only.
Overview. Anencephaly is a serious developmental defect of the central nervous system in which the brain and cranial vault are grossly malformed. The cerebrum and cerebellum are reduced or absent, but the hindbrain is present. Anencephaly is a part of the neural tube defect (NTD) spectrum. This defect results when the neural tube fails to close ...
Anencephaly is a serious birth defect that affects the fetal nervous system; it is therefore referred to as a neural tube defect. As a result of anencephaly, the brain and skull do not develop normally during pregnancy. Babies affected by anencephaly are missing large parts of the brain and skull. The parts of the baby's brain that remain are ...
The prenatal detection of anencephaly with ultrasound is high and reliable in the 10 - 14 weeks scan [8, 9]. In our study, the mean gestational age for the diagnosis of anencephaly was 18 weeks and the earliest gestational age was 11 weeks. Blaas et al reported that diagnosis of anencephaly could be made as early as the ninth week of pregnancy ...
Anencephaly is a defect in the closure of the neural tube during fetal development. A baby born with anencephaly is usually blind, deaf, unconscious and analgesia. It is one of the most common types of neural tube defect, after spina bifida, afecting approximately 1 in 1,000 pregnancies.
Exencephaly is frequently noted in animal teratogen studies. Human exencephaly appears to be confined to early gestation. Only rare reports exist of a third trimester diagnosis of an exencephalic fetus (Wilkins-Haug and Freedman, 1991). Anencephaly, however, is more common in humans than in animals.
Many researchers found that the most common associated malformations differed according to the geographical location ... Earlier studies have also reported cleft lip and palate to be more common in male anencephalic fetus [10,11,13]. Cleft lip was found in 5 cases in association with cleft palate. ... Anencephaly is common in females ...