

Embryonic Stem Cell Research An Ethical Dilemma
Article sidebar.
Main Article Content
Introduction
In November 1998, two teams of U.S. scientists confirmed successful isolation and growth of stems cells obtained from human fetuses and embryos. Since then, research that utilizes human embryonic cells has been a widely debated, controversial ethical issue. Human embryonic cells possess the ability to become stem cells, which are used in medical research due to two significant features. First, they are unspecialized cells, meaning they can undergo cell division and renew themselves even with long periods of inactivity. Secondly, stem cells are pluripotent, with the propensity to be induced to become specified tissue or any “organ-specific cells with special functions” depending on exposure to experimental or physiologic conditions, as well as undergo cell division and become cell tissue for different organs.
The origin of stem cells themselves encapsulates the controversy: embryonic stem cells, originate from the inner cell mass of a blastocyst, a 5-day pre-implantation embryo. The principal argument for embryonic stem cell research is the potential benefit of using human embryonic cells to examine or treat diseases as opposed to somatic (adult) stem cells. Thus, advocates believe embryonic stem cell research may aid in developing new, more efficient treatments for severe diseases and ease the pain and suffering of numerous people. However, those that are against embryonic stem cell research believe that the possibility of scientific benefits of research do not outweigh the immoral action of tampering with the natural progression of a fetal development and interfering with the human embryo’s right to live. In light of these two opposing views, should embryonic stem cells be used in research? It is not ethically permissible to destroy human embryonic life for medical progress.
Personhood and the Scientific Questionability of Embryonic Stem Cell Research
The ethics behind embryonic stem cell research are controversial because the criteria of ‘personhood’ is “notoriously unclear.” Personhood is defined as the status of being a person, entitled to “moral rights and legal protections” that are higher than living things that are not classified as persons. Thus, this issue touches on existential questions such as: When does life begin? and What is the moral status that an embryo possesses? There is a debate on when exactly life begins in embryonic development and when the individual receives moral status. For example, some may ascribe life starting from the moment of fertilization, others may do so after implantation or the beginning of organ function. However, since the “zygote is genetically identical to the embryo,” which is also genetically identical to the fetus, and, by extension, identical to the baby, inquiring the beginning of personhood can lead to an occurrence of the Sorites paradox, also acknowledged as “the paradox of the heap.”
The paradox of the heap arises from vague predicates in philosophy. If there is a heap of sand and a grain is taken away from that heap one by one, at what point will it no longer be considered a heap – what classifies it as a heap? The definition of life is similarly arbitrary. When, in the development of a human being, is an embryo considered a person with moral standing? The complexity of the ethics of embryonic stem cell research, like the Sorites paradox, demonstrates there is no single, correct way to approach a problem; thus, there may be multiple different solutions that are acceptable. Whereas the definition of personhood cannot be completely resolved on a scientific basis, it serves a central role in the religious, political, and ethical differences within the field of embryonic stem cell research. Some ethicists attempt to determine what or who is a person by “setting boundaries” (Baldwin & Capstick, 2007).
Utilizing a functionalist approach, supporters of embryonic stem cell research argue that to qualify as a person, the individual must possess several indicators of personhood, including capacity, self-awareness, a sense of time, curiosity, and neo-cortical function. Proponents argue that a human embryo lacks these criteria, thereby is not considered a person and thus, does not have life and cannot have a moral status. Supporters of stem cell research believe a fertilized egg is just a part of another person’s body until the cell mass can survive on its own as a viable human. They further support their argument by noting that stem cell research uses embryonic tissue before its implantation into the uterine wall. Researchers invent the term “pre-embryo” to distinguish a pre-implantation state in which the developing cell mass does not have the full respects of an embryo in later stages of embryogenesis to further support embryonic stem cell research. Based on this reductionist view of life and personhood, utilitarian advocates argue that the result of the destruction of human embryos to harvest stem cells does not extinguish a life. Further, scientists state that any harm done is outweighed by the potential alleviation of the suffering enduring by tremendous numbers of people with varying diseases. This type of reasoning, known as Bentham’s Hedonic (moral) calculus, suggests that the potential good of treating or researching new cures for ailments such as Alzheimer’s disease, Parkinson’s disease, certain cancers, etc. outweighs any costs and alleviate the suffering of persons with those aliments. Thus, the end goal of stem cell use justifies sacrificing human embryos to produce stem cells, even though expending life is tantamount to murder. Opponents of embryonic stem cell research would equate the actions done to destroy the embryos as killing. Killing, defined as depriving their victims of life, will therefore reduce their victims to mere means to their own ends. Therefore, this argument touches on the question: if through the actions of embryotic stem cell research is “morally indistinguishable from murder?” (Outka, 2013). The prohibition of murder extends to human fetuses and embryos considering they are potential human beings. And, because both are innocent, a fetus being aborted and an embryo being disaggregated are direct actions with the intention of killing. Violating the prohibition of murder is considered an intolerable end. We should not justify this evil even if it achieves good. Under the deontological approach, “whether a situation is good or bad depends on whether the action that brought it about was right or wrong,” hence the ends do not justify the means. Therefore, under this feeble utilitarian approach, stem cell research proceeds at the expense of human life than at the expense of personhood.
One can reject the asserted utilitarian approach to stem cell research as a reductionist view of life because the argument fails to raise ethical concerns regarding the destruction embryonic life for the possibility of developing treatments to end certain diseases. The utilitarian approach chooses potential benefits of stem cell research over the physical lives of embryos without regard to the rights an embryo possesses. Advocates of embryonic stem cell research claim this will cure diseases but there is a gap in literature that confirms how many diseases these cells can actually cure or treat, what diseases, and how many people will actually benefit. Thus, killing human embryos for the potentiality of benefiting sick people is not ethically not ethically permissible.
Where the argument of personhood is concerned, the development from a fertilized egg (embryo) to a baby is a continuous process. Any effort to determine when personhood begins is arbitrary. If a newborn baby is a human, then surely a fetus just before birth is a human; and, if we extend a few moments before that point, we would still have a human, and so on all the way back to the embryo and finally to the zygote. Although an embryo does not possess the physiognomies of a person, it will nonetheless become a person and must be granted the respect and dignity of a person. Thus, embryotic stem cell research violates the Principle of “Full Human Potential,” which states: “Every human being […] deserves to be valued according to the full level of human development, not according to the level of development currently achieved.” As technology advances, viability outside the womb inches ever closer to the point of inception, making the efforts to identify where life begins after fertilization ineffectual. To complicate matters, as each technological innovation arrives, stem-cell scientists will have to re-define the start of life as many times as there are new technological developments, an exhausting and never-ending process that would ultimately lead us back to moment of fertilization. Because an embryo possesses all the necessary genetic information to develop into a human being, we must categorically state that life begins at the moment of conception. There is a gap in literature that deters the formation of a clear, non-arbitrary indication of personhood between conception and adulthood. Considering the lack of a general consensus of when personhood begins, an embryo should be referred to as a person and as morally equivalent to a fully developed human being.
Having concluded that a human embryo has the moral equivalent of a fully-fledged human being, this field of research clearly violates the amiable rights of personhood, and in doing so discriminates against pre-born persons. Dr. Eckman asserts that “every human being has a right to be protected from discrimination.” Thus, every human, and by extension every embryo, has the right to life and should not be discriminated against their for “developmental immaturity.” Therefore, the field of embryonic stem cell research infringes upon the rights and moral status of human embryos.
Principle of Beneficence in Embryonic Stem Cell Research
The destruction of human embryos for research is not ethically permissible because the practice violates the principle of beneficence depicted in the Belmont Report, which outlines the basic ethical principles and guidelines owed to human subjects involved in research. Stem cell researchers demonstrate a lack of respect for the autonomy and welfare of the human embryos sacrificed in stem cell research.
While supporters of embryonic stem cell research under the utilitarian approach argue the potential benefits of the research, the utilitarian argument however violates the autonomy of the embryo and its human rights, as well as the autonomy of the embryo donors and those that are Pro-Life. Though utilitarian supporters argue on the basis of rights, they exclusively refer to the rights of sick individuals. However, they categorically ignore the rights of embryos that they destroy to obtain potential disease curing stem cells. Since an embryo is regarded as a human being with morally obligated rights, the Principle of Beneficence is violated, and the autonomy and welfare of the embryo is not respected due to the destruction of an embryo in stem cell research. Killing embryos to obtain stem cells for research fails to treat embryos as ends in an of themselves. Yet, every human ought to be regarded as autonomous with rights that are equal to every other human being. Thus, the welfare of the embryo is sacrificed due to lack of consent from the subject.
The Principle of Beneficence is violated when protecting the reproductive interests of women in infertility treatment, who are dependent on the donations of embryos to end their infertility. Due to embryonic stem cell research, these patients’ “prospects of reproductive success may be compromised” because there are fewer embryos accessible for reproductive purposes. The number of embryos necessary to become fully developed and undergo embryonic stem cell research will immensely surpass the number of available frozen embryos in fertility clinic, which also contributes to the lack of embryos available for women struggling with infertility. Therefore, the basis of this research violates women’s reproductive autonomy, thus violating the Principle of Beneficence.
It is also significant to consider the autonomy and welfare of the persons involved. The autonomous choice to donate embryos to research necessitates a fully informed, voluntary sanction of the patient(s), which poses difficulty due to the complexity of the human embryonic stem cell research. To use embryos in research, there must be a consensus of agreement from the mother and father whose egg and sperm produced the embryo. Thus, there has to be a clear indication between the partners who has the authority or custody of the embryos, as well as any “third party donors” of gametes that could have been used to produce the embryo because these parties’ intentions for those gametes may solely have been for reproductive measures only. Because the researchers holding “dispositional authority” over the embryos may exchange cell lines and its derivatives (i.e., genetic material and information) with other researchers, they may misalign interests with the persons whose gametes are encompassed within the embryo. This mismatch of intent raises complications in confidentiality and autonomy.
Lastly, more ethical complications arise in the research of embryonic stem cells because of the existence viable alternatives that to not destroy human embryos. Embryonic stem cells themselves pose as a higher health risk than adult stem cells. Embryonic stem cells have a higher risk of causing tumor development in the patient’s body once the cells are implanted due to their abilities to proliferate and differentiate. Embryonic stem cells also have a high risk of immunorejection, where a patient’s immune system rejects the stem cells. Since the embryonic stem cells are derived from embryos that underwent in vitro fertilization, when implanted in the body, the stem cell’s marker molecules will not be recognized by the patient’s body, resulting in the destruction of the stem cells as a defensive response to protect the body (Cahill, 2002). With knowledge of embryonic stem cells having higher complications than the viable adult stem cells continued use of embryonic stem cells violates the Principle of Beneficence not only for the embryos but for the health and safety of the patients treated with stem cells. Several adult stem cell lines (“undifferentiated cells found throughout the body”) exist and are widely used cell research. The use of adult stem cells represents research that does not treat human beings as means to themselves, thus, complying with the Principle of Beneficence. This preferable alternative considers the moral obligation to discover treatments, and cures for life threating diseases while avoiding embryo destruction.
It is not ethically permissible to destroy human embryonic life for medical progress due to the violations of personhood and human research tenets outlined in the Belmont Report. It is significant to understand the ethical implications of this research in order to respect the autonomy, welfare, beneficence, and basic humanity afforded to all parties involved. Although embryonic stem cell research can potentially provide new medical advancements to those in need, the harms outweigh the potential, yet ill-defined benefits. There are adult stem cell alternatives with equivalent viability that avoid sacrificing embryos. As society further progresses, humans must be cautious of compromising moral principles that human beings are naturally entitled to for scientific advancements. There are ethical boundaries that are crossed when natural processes of life are altered or manipulated. Though there are potential benefits to stem cell research, these actions are morally and ethically questionable. Thus, it is significant to uphold ethical standards when practicing research to protect the value of human life.
Shamblott, M. J., J. Axelman, S. Wang, E. M. Bugg, J. W. Littlefield, P. J. Donovan, P. D. Blumenthal, G. R. Huggins, and J. D. Gearhart. “Derivation of Pluripotent Stem Cells from Cultured Human Primordial Germ Cells.” Proceedings of the National Academy of Sciences 95, no. 23 (November 10, 1998): 13726–31. doi:10.1073/pnas.95.23.13726.
National Institutes of Health, U.S. Department of Health and Human Services. “Stem Cell Basics I.” Stem Cell Information , 2016. https://stemcells.nih.gov/info/basics/1.htm .
Kitwood, Thomas Marris., Clive Baldwin, and Andrea Capstick. Tom Kitwood on Dementia: A Reader and Critical Commentary . Maidenhead, Berkshire: McGraw-Hill/Open University Press, 2007.
University of Michigan. “Stem Cell Research: Frequently Asked Questions,” 2013. http://www.stemcellresearch.umich.edu/overview/faq.html#section2 .
EuroStemCell. “Origins, Ethics and Embryos: The Sources of Human Embryonic Stem Cells,” 2016. https://www.eurostemcell.org/origins-ethics-and-embryos-sources-human-embryonic-stem-cells .
Perry, David L. “Some Issues in Contemporary Neurological Science and Technology,” 2011. https://www.scu.edu/ethics/focus-areas/bioethics/resources/ethics-and-personhood/ .
Swirsky, E. “Week Fourteen Unit: Minute Paper 5 [Blackboard Assignment],” 2018.
O’Mathúna, DP. “Personhood in Bioethics and Biomedical Research.” Research Practitioner 7 (2006): 167–74.
Grobstein, C. “External Human Fertilization.” Scientific American 240, no. 6 (June 1979): 57–67.
Mastin, L. “Deontology,” 2009. https://www.philosophybasics.com/branch_deontology.html .
Spitzer, Robert. “Introduction and Principles of Ethics.” In Ten Universal Principles: A Brief Philosophy of the Life Issues , xi–xii, 1-3, 20-29. San Fransisco, CA: Ignatius Press, 2011. https://www.catholiceducation.org/en/religion-and-philosophy/philosophy/introduction-amp-principles-of-ethics.html .
Eckman, Jim. “Human Embryonic Stem Cell Research.” Issues In Perspective , 2011. https://graceuniversity.edu/iip/2011/05/14-2/ .; Eckman, Jim. “The Devaluing of Life in America.” Issues In Perspective , 2015. https://graceuniversity.edu/iip/2015/09/the-devaluing-of-life-in-america/ .
Outka, Gene (2009) "The Ethics of Embryonic Stem Cell Research and the Principle of "Nothing is Lost"," Yale Journal of Health Policy, Law, and Ethics : Vol. 9 : Iss. 3 , Article 7.
Curzer, Howard. “The Ethics Of Embryonic Stem Cell Research.” The Journal of Medicine and Philosophy 29, no. 5 (October 1, 2004): 533–62. doi:10.1080/03605310490514225.
Lo, Bernard, and Lindsay Parham. “Ethical Issues in Stem Cell Research.” Endocrine Reviews 30, no. 3 (May 2009): 204–13. doi:10.1210/er.2008-0031.
Hubbard, James. “Embryonic Stem-Cell Research: Experts Debate Pros and Cons.” The Survival Doctor , 2013. http://thesurvivaldoctor.com/2013/02/14/doctors-debate-embryonic-stem-cell-research-pros-and-cons/ .
Koch, Valerie Gutmann, Beth E. Roxland, Barbara Pohl, and Sarah K. Keech. “Contemporary Ethical Issues in Stem Cell Research.” In Stem Cells Handbook , 29–37. New York, NY: Springer New York, 2013. doi:10.1007/978-1-4614-7696-2_2.
Cahill, Lisa Sowle. "Holland, Suzanne, Karen Lebacqz, and Laurie Zoloth, Eds. The Human Embryonic Stem Cell Debate: Science, Ethics, and Public Policy." The National Catholic Bioethics Quarterly 2, no. 3 (2002): 559-62. doi:10.5840/ncbq20022344.
Devolder, Katrien. The Ethics of Embryonic Stem Cell Research . Oxford University Press, 2015. doi:10.1093/acprof:oso/9780199547999.001.0001.
ScienceDaily. “Adult Stem Cell,” 2018. https://www.sciencedaily.com/terms/adult_stem_cell.htm .
Article Details
- Utility Menu
GA4 tracking code

Examining the ethics of embryonic stem cell research

Following the recent passage by both houses of Congress of the Stem Cell Research Enhancement Act of 2007, which would permit federal funding of research using donated surplus embryonic stem cells from fertility clinics, the president has once again threatened a veto.
Because neither the House nor the Senate had sufficient votes to override a presidential veto, it appears unlikely this new bill will be enacted into law, further stalling the pace of this research. “This bill crosses a moral line that I and others find troubling,” stated Bush, following the Senate’s vote.
SCL : What are th e main arguments for and against embryonic stem cell research? MS : Proponents argue that embryonic stem cell research holds great promise for understanding and curing diabetes, Parkinson’s disease, spinal cord injury, and other debilitating conditions. Opponents argue that the research is unethical, because deriving the stem cells destroys the blastocyst, an unimplanted human embryo at the sixth to eighth day of development. As Bush declared when he vetoed last year’s stem cell bill, the federal government should not support “the taking of innocent human life.”
It is surprising that, despite the extensive public debate—in Congress, during the 2004 and 2006 election campaigns, and on the Sunday morning talk shows—relatively little attention has been paid to the moral issue at the heart of the controversy: Are the opponents of stem cell research correct in their claim that the unimplanted human embryo is already a human being, morally equivalent to a person?

“It is important to be clear about the embryo from which stem cells are extracted. It is not implanted and growing in a woman’s uterus. It is not a fetus. It has no recognizable human features or form. It is, rather, a blastocyst, a cluster of 180 to 200 cells, growing in a petri dish, barely visible to the naked eye.”
SCL : What are the contradictions in Bush’s stance? MS : Before we address that, it is important to be clear about the embryo from which stem cells are extracted. It is not implanted and growing in a woman’s uterus. It is not a fetus. It has no recognizable human features or form.
It is, rather, a blastocyst, a cluster of 180 to 200 cells, growing in a petri dish, barely visible to the naked eye. Such blastocysts are either cloned in the lab or created in fertility clinics. The bill recently passed by Congress would fund stem cell research only on excess blastocysts left over from infertility treatments.
The blastocyst represents such an early stage of embryonic development that the cells it contains have not yet differentiated, or taken on the properties of particular organs or tissues—kidneys, muscles, spinal cord, and so on. This is why the stem cells that are extracted from the blastocyst hold the promise of developing, with proper coaxing in the lab, into any kind of cell the researcher wants to study or repair.
The moral and political controversy arises from the fact that extracting the stem cells destroys the blastocyst. It is important to grasp the full force of the claim that the embryo is morally equivalent to a person, a fully developed human being.
For those who hold this view, extracting stem cells from a blastocyst is as morally abhorrent as harvesting organs from a baby to save other people’s lives. This is the position of Senator Sam Brownback, Republican of Kansas, a leading advocate of the right-to-life position. In Brownback’s view, “a human embryo . . . is a human being just like you and me; and it deserves the same respect that our laws give to us all.
If Brownback is right, then embryonic stem cell research is immoral because it amounts to killing a person to treat other people’s diseases.
SCL : What is the basis for the belief that personhood begins at conception? MS : Some base this belief on the religious conviction that the soul enters the body at the moment of conception. Others defend it without recourse to religion, by the following line of reasoning: Human beings are not things. Their lives must not be sacrificed against their will, even for the sake of good ends, like saving other people’s lives. The reason human beings must not be treated as things is that they are inviolable. At what point do humans acquire this inviolability? The answer cannot depend on the age or developmental stage of a particular human life. Infants are inviolable, and few people would countenance harvesting organs for transplantation even from a fetus.
Every human being—each one of us—began life as an embryo. Unless we can point to a definitive moment in the passage from conception to birth that marks the emergence of the human person, we must regard embryos as possessing the same inviolability as fully developed human beings.
SCL : By this line of reasoning, human embryos are inviolable and should not be used for research, even if that research might save many lives. MS : Yes, but this argument can be challenged on a number of grounds. First, it is undeniable that a human embryo is “human life” in the biological sense that it is living rather than dead, and human rather than, say, bovine.
But this biological fact does not establish that the blastocyst is a human being, or a person. Any living human cell (a skin cell, for example) is “human life” in the sense of being human rather than bovine and living rather than dead. But no one would consider a skin cell a person, or deem it inviolable. Showing that a blastocyst is a human being, or a person, requires further argument.
Some try to base such an argument on the fact that human beings develop from embryo to fetus to child. Every person was once an embryo, the argument goes, and there is no clear, non-arbitrary line between conception and adulthood that can tell us when personhood begins. Given the lack of such a line, we should regard the blastocyst as a person, as morally equivalent to a fully developed human being.
SCL : What is the flaw in this argument? MS : Consider an analogy: although every oak tree was once an acorn, it does not follow that acorns are oak trees, or that I should treat the loss of an acorn eaten by a squirrel in my front yard as the same kind of loss as the death of an oak tree felled by a storm. Despite their developmental continuity, acorns and oak trees differ. So do human embryos and human beings, and in the same way. Just as acorns are potential oaks, human embryos are potential human beings.
The distinction between a potential person and an actual one makes a moral difference. Sentient creatures make claims on us that nonsentient ones do not; beings capable of experience and consciousness make higher claims still. Human life develops by degrees.
SCL : Yet there are people who disagree that life develops by degrees, and believe that a blastocyst is a person and, therefore, morally equivalent to a fully developed human being. MS : Certainly some people hold this belief. But a reason to be skeptical of the notion that blastocysts are persons is to notice that many who invoke it do not embrace its full implications.
President Bush is a case in point. In 2001, he announced a policy that restricted federal funding to already existing stem cell lines, so that no taxpayer funds would encourage or support the destruction of embryos. And in 2006, he vetoed a bill that would have funded new embryonic stem cell research, saying that he did not want to support “the taking of innocent human life.”
“The distinction between a potential person and an actual one makes a moral difference. Sentient creatures make claims on us that nonsentient ones do not; beings capable of experience and consciousness make higher claims still. Human life develops by degrees.”
But it is a striking feature of the president’s position that, while restricting the funding of embryonic stem cell research, he has made no effort to ban it. To adapt a slogan from the Clinton administration, the Bush policy might be summarized as “don’t fund, don’t ban.” But this policy is at odds with the notion that embryos are human beings.
SCL : If Bush’s policy were consistent with his stated beliefs, how, in your opinion, would it differ from his current “don’t fund, don’t ban” policy? MS : If harvesting stem cells from a blastocyst were truly on a par with harvesting organs from a baby, then the morally responsible policy would be to ban it, not merely deny it federal funding.
If some doctors made a practice of killing children to get organs for transplantation, no one would take the position that the infanticide should be ineligible for federal funding but allowed to continue in the private sector. In fact, if we were persuaded that embryonic stem cell research were tantamount to infanticide, we would not only ban it but treat it as a grisly form of murder and subject scientists who performed it to criminal punishment.
SCL : Couldn’t it be argued, in defense of the president’s policy, that Congress would be unlikely to enact an outright ban on embryonic stem cell research? MS : Perhaps. But this does not explain why, if the president really considers embryos to be human beings, he has not at least called for such a ban, nor even called upon scientists to stop doing stem cell research that involves the destruction of embryos. In fact, Bush has cited the fact that “there is no ban on embryonic stem cell research” in touting the virtues of his “balanced approach.”
The moral oddness of the Bush “don’t fund, don’t ban” position confused even his spokesman, Tony Snow. Last year, Snow told the White House press corps that the president vetoed the stem cell bill because he considered embryonic stem cell research to be “murder,” something the federal government should not support. When the comment drew a flurry of critical press attention, the White House retreated. No, the president did not believe that destroying an embryo was murder. The press secretary retracted his statement, and apologized for having “overstated the president’s position.”
How exactly the spokesman had overstated the president’s position is unclear. If embryonic stem cell research does constitute the deliberate taking of innocent human life, it is hard to see how it differs from murder. The chastened press secretary made no attempt to parse the distinction. His errant statement that the president considered embryo destruction to be “murder” simply followed the moral logic of the notion that embryos are human beings. It was a gaffe only because the Bush policy does not follow that logic.
SCL : You have stated that the president’s refusal to ban privately funded embryonic stem cell research is not the only way in which his policies betray the principle that embryos are persons. How so? MS : In the course of treating infertility, American fertility clinics routinely discard thousands of human embryos. The bill that recently passed in the Senate would fund stem cell research only on these excess embryos, which are already bound for destruction. (This is also the position taken by former governor Mitt Romney, who supports stem cell research on embryos left over from fertility clinics.) Although Bush would ban the use of such embryos in federally funded research, he has not called for legislation to ban the creation and destruction of embryos by fertility clinics.
SCL : If embryos are morally equivalent to fully developed human beings, doesn’t it then follow that allowing fertility clinics to discard thousands of embryos is condoning mass murder? MS : It does. If embryos are human beings, to allow fertility clinics to discard them is to countenance, in effect, the widespread creation and destruction of surplus children. Those who believe that a blastocyst is morally equivalent to a baby must believe that the 400,000 excess embryos languishing in freezers in U.S. fertility clinics are like newborns left to die by exposure on a mountainside. But those who view embryos in this way should not only be opposing embryonic stem cell research; they should also be leading a campaign to shut down what they must regard as rampant infanticide in fertility clinics.
Some principled right-to-life opponents of stem cell research meet this test of moral consistency. Bush’s “don’t fund, don’t ban” policy does not. Those who fail to take seriously the belief that embryos are persons miss this point. Rather than simply complain that the president’s stem cell policy allows religion to trump science, critics should ask why the president does not pursue the full implications of the principle he invokes.
If he does not want to ban embryonic stem cell research, or prosecute stem cell scientists for murder, or ban fertility clinics from creating and discarding excess embryos, this must mean that he does not really consider human embryos as morally equivalent to fully developed human beings after all.
But if he doesn’t believe that embryos are persons, then why ban federally funded embryonic stem cell research that holds promise for curing diseases and saving lives?

Viterbi Conversations in Ethics

Stem Cells: A Case for the Use of Human Embryos in Scientific Research
Embryonic stem cells have immense medical potential. While both their acquisition for and use in research are fraught with controversy, arguments against their usage are rebutted by showing that embryonic stem cells are not equivalent to human lives. It is then argued that not using human embryos is unethical. Finally, an alternative to embryonic stem cells is presented.
INTRODUCTION
Embryonic stem cells have the potential to cure nearly every disease and condition known to humanity. Stem cells are nature’s Transformers. They are small cells that can regenerate indefinitely, waiting to transform into a specialized cell type such as a brain cell, heart cell or blood cell [1]. Most stem cells form during the earliest stages of human development, immediately when an embryo is formed. These cells, known as embryonic stem cells (ESCs), eventually develop into every single type of cell in the body. As the embryo develops, adult stem cells (ASCs) replace these all-powerful embryonic stem cells. ASCs can only become a number of different cells within their potency. This limited application means an adult mesenchymal stem cell cannot become a neural cell.
By harnessing the unique ability of embryonic stem cells to transform into functional cells, scientists can develop treatments for a number of diseases and injuries, according to the California Institute for Regenerative Medicine, a private organization which awards grants for stem cell research [1]. For example, scientists at the Cleveland Clinic converted ESCs into heart muscle cells and injected them into patients who suffered from heart attacks. The cells continued to grow and helped the patients’ hearts recover [2].
With this enormous potential to cure devastating diseases, including heart failure, spinal cord injuries and Alzheimer’s disease, governments and research organizations have the moral imperative to support and encourage embryonic stem cell research. President Barack Obama signed an executive order in 2009 loosening federal funding restrictions on stem cell research, saying, “We will aim for America to lead the world in the discoveries it one day may yield.” [3]. The National Institute of Health and seven state governments, including California, Maryland and New York, followed Obama’s lead by creating programs that offered over $5 billion in funding and other incentives to scientists and research institutions for stem cell research [4].
A MIRACLE CURE
Scientists believe that harnessing the capability of embryonic stem cells will unlock the cure for countless diseases. “I am very excited about embryonic stem cells,” said Dr. Dieter Egli, professor of developmental cell biology at Columbia University. “They will lead to unprecedented discoveries that will transform life. I have no doubt about it.” [5]. The results thus far are inspiring. In 2016, Kris Boesen, a 21-year-old college student from Bakersfield, California, suffered a severe spinal cord injury in a car accident that left him paralyzed from the neck down. In a clinical trial conducted by Dr. Charles Liu at the University of Southern California Keck School of Medicine, Boesen was injected with 10 million embryonic stem cells that transformed into nerve cells [6]. Three months after the treatment, Boesen regained the use of his arms and hands. He could brush his teeth, operate a motorized wheelchair, and live more independently. “All I’ve wanted from the beginning was a fighting chance,” he said. The power of stem cells made his wish possible [6].
Embryonic stem cell treatments may also cure type 1 diabetes. Type 1 diabetes, which affects 42 million worldwide, is an autoimmune disorder that results in the destruction of insulin-producing beta cells found in the pancreas [7]. ViaCyte, a company in San Diego, California, is developing an implant that contains replacement beta cells originating from embryonic stem cells [7]. The implant will preserve or replace the original beta cells to protect them from the patient’s immune system [7]. The company believes that if successful, this strategy will effectively cure type 1 diabetes. Patients with the disease will no longer have to closely monitor their blood sugar levels and inject insulin [7]. ViaCyte projects that an experimental version of this implant will become available by 2020 [7].
Ultimately, scientists believe they will grow complex organs using stem cells within the next decade [8]. Over 115,000 people in the United States need a life-saving organ donation, and an average of 20 people die every day due to the lack of available organs for transplant, according to the American Transplant Foundation [9]. Three-dimensional printing of entire organs derived from stem cells holds the most promise for solving the organ shortage crisis [8]. Researchers at the University of California, San Diego have successfully printed part of a functional liver [8]. While the printed liver is not ready for transplant, it still performs the functions of a normal liver. This has helped scientists reduce the need for often cruel and unethical animal testing. The scientists expose drugs to the printed liver and observe how it reacts. The liver’s response closely mimics that of a human being’s and no living animals are harmed in the process [8].
HUMAN CELLS OR HUMAN LIFE?
Research using embryonic stems cells provides an unprecedented understanding of human development and the potential to cure devastating diseases. However, stem cell research has generated controversy among religious organizations such as the Catholic Church as well as the “pro-life” movement [3]. That is because scientists harvest stem cells from embryos donated by fertility clinics. Opponents of embryonicstem cell research equate the destruction of an embryo to the murder of an innocent human being [10]. Pope Benedict XVI said that harvesting stem cells is “not only devoid of the light of God but is also devoid of humanity” [3]. However, this view does not reflect a reasonable understanding and interpretation of basic biology. Researchers typically harvest embryonic stem cells from an embryo five days after fertilization [1]. At this stage, the entire embryo consists of less than 250 cells, smaller than the tip of a pin. Of these cells, only 30 are embryonic stem cells, which cannot perform any human function [11]. For comparison, an adult has more than 72 trillion cells, each with a specialized function [3]. Therefore, this microscopic blob of cells in no way represents human life.
With no functional cells, there exist no characteristics of a human being. Fundamentalist Christians believe that the presence or absence of a heartbeat signifies the beginning and end of a human life [10]. However, at this stage there is no heart, not even a single heart cell [10]. Some contend that brain activity, or the ability to feel, defines a human being. Michael Gazzaniga, president of the Cognitive Neuroscience Institute at the University of California, Santa Barbara, explains in his book, The Ethical Brain, that the “fertilized egg is a clump of cells with no brain.” [12]. There is no brain nor nerve cells that could allow this cellular object to interact with its environment [12]. The only uniquely human feature of embryonic cells at this stage is that they contain human DNA. This means that a 5-day-old human embryo is effectively no different than the Petri dishes of human cells that have grown in laboratories for decades with no controversy or opposition. Therefore, if the cluster of cells in the earliest stage of a human embryo is considered a “human life,” a growing plate of skin cells must also be considered “human life.” Few would claim that a Petri dish of human cells is morally equivalent to a living human or any other animal. Why, then, would a microscopic collection of embryonic cells have the same moral status as an adult human?
The status of the human embryo comes from its potential to turn into a fully grown human being. However, the potential of this entity to become an individual does not logically mean that it has the same status as an individual who can think and feel. If this were true, virtually every cell grown in a laboratory would be subject to the same controversy. This is because scientists have developed technology to convert an ordinary cell such as a skin cell into an embryo [10]. Although this requires a laboratory with special conditions, the normal development of a human being also requires special conditions in the womb of the mother. Therefore, almost any cell could be considered a potential individual, so it is illogical to conclude that a cluster of embryonic cells deserves a higher moral status.
THE FATE OF UNUSED EMBRYOS
Hundreds of thousands of embryos are destroyed each year in a process known as in vitro fertilization (IVF), a popular procedure that helps couples have children [13]. Society has an ethical obligation to use these discarded embryos to make medical advancements rather than simply throw them in the trash for misguided ideological and religious reasons as opponents of embryonic stem cell research desire.
With IVF, a fertility clinician harvests sperm and egg cells from the parents and creates an embryo in a laboratory before implanting it in the woman’s womb. However, creating and implanting a single embryo is expensive and often leads to unsuccessful implantation. Instead, the clinician typically creates an average of seven embryos and selects the healthiest few to implant [13].
This leaves several unused embryos for every one implanted. The couple can pay a fee to preserve the unused embryos by freezing them or can donate them to another family. Otherwise, they are slated for destruction [14]. A 2011 study in the “Journal of the American Society for Reproductive Medicine” found that 19 percent of the unused embryos are discarded and only 3 percent are donated for scientific research [14]. Many of these embryos could never grow into a living person given the chance because they are not healthy enough to survive past early stages of development [14]. If a human embryo is already destined for destruction or has no chance of survival, scientists have the ethical imperative to use these embryos to research and develop medical treatments that could save lives. The modern version of the Hippocratic oath states, “I will apply, for the benefit of the sick, all measures which are required [to heal]” [10]. Republican Senator Orrin Hatch of Utah supports the pro-life movement, which recognizes early embryos as human individuals. However, even he favors using the leftover embryos for the greater good. “The morality of the situation dictates that these embryos, which are routinely discarded, be used to improve and save lives. The tragedy would be in not using these embryos to save lives when the alternative is that they would be discarded.” [3]
ALTERNATIVES TO EMBRYONIC STEM CELLS
Although scientists have used embryonic stem cells (ESCs) for promising treatments, they are not ideal, and scientists hope to eliminate the need for them. Primarily, ESCs come from an embryo with different DNA than the patient who will receive the treatment, meaning they are not autologous. ESCs are not necessarily compatible with everyone and could cause the immune system to reject the treatment [11]. The most promising alternative to ESCs are known as induced pluripotent stem cells. In 2008, scientists discovered a way to reprogram human skin cells to embryonic stem cells [15]. Scientists easily obtained these cells from a patient’s skin, converted them into the desired cell type, then transplanted them into the diseased organ without risk of immune rejection [15]. This eliminates any ethical concerns because no embryos are harvested or destroyed in the process. However, induced stem cells have their own risks. Recent studies have shown that they can begin growing out of control and turn into cancer [3]. Several of the first clinical trials with induced stem cells, including one aimed at curing blindness by regenerating a patient’s retinal cells, were halted because potentially cancerous mutations were detected [3].
Scientists believe that induced stem cells created in a laboratory will one day completely replace embryonic stem cells harvested from human embryos. However, the only way to create perfect replicas of ESCs is to thoroughly understand their structure and function. Scientists still do not completely understand how ESCs work. Why does a stem cell sometimes become a nerve cell, sometimes become a heart cell and other times regenerate to produce another stem cell? How can we tell a stem cell what type of cell to become? To develop a viable alternative to ESCs, scientists must first answer these questions with experiments on ESCs from human embryos. Therefore, extensive embryonic stem cell research today will eliminate the need for embryonic stem cells in the future.
The Biomedical Engineering Society Code of Ethics calls upon engineers to “use their knowledge, skills, and abilities to enhance the safety, health and welfare of the public.” [16] Stem cell research epitomizes this. Stem cells hold the cure for numerous diseases ranging from spinal cord injuries to organ failure and have the potential to transform modern medicine. Therefore, the donation of human embryos to scientific research falls within most conventional ethical frameworks and should be allowed with minimal restriction.
Because of widespread ignorance about the science behind stem cells, ill-informed opposition has prevented scientists from receiving the funding and support they need to save millions of lives. For example, George W. Bush’s religious opposition to stem cell research resulted in a 2001 law severely limiting government funding for such research [3]. Although most opponents of stem cell research compare the destruction of a human embryo to the death of a living human, the biology of these early embryos is no more human than a plate of skin cells in a laboratory. Additionally, all embryos sacrificed for scientific research would otherwise be discarded and provide no benefit to society. If society better understood the process and potential of embryonic stem cell research, more people would surely support it.
Within the next decade, stem cells will likely provide simple cures for diseases that are currently untreatable, such as Alzheimer’s disease and organ failure [1]. As long as scientists receive support for embryonic stem cell research, stem cell therapies will become commonplace in clinics and hospitals around the world. Ultimately, the fate of this new medical technology lies in the hands of the public, who must support propositions that will continue to allow and expand the impact of embryonic stem cell research.
By Jonathan Sussman, Viterbi School of Engineering, University of Southern California
ABOUT THE AUTHOR
At the time of writing this paper, Jonathan Sussman was a senior at the University of Southern California studying biomedical engineering with an emphasis in biochemistry. He was an undergraduate research assistant in the Graham Lab investigating proteomics of cancer cells and was planning to attend an MD/PhD program.
[1] “Stem Cell Information”, Stem Cell Basics , 2016. [Online]. Available at: https://stemcells.nih.gov/info/basics/3.htm [Accessed 11 Oct. 2018].
[2] Cleveland Clinic, “Stem Cell Therapy for Heart Disease | Cleveland Clinic”, 2017. [Online]. Available at: https://my.clevelandclinic.org/health/diseases/17508-stem-cell-therapy-for-heart-disease [Accessed 14 Oct. 2018].
[3] B. Lo and L. Parham, “Ethical Issues in Stem Cell Research”, Endocrine Reviews , 30(3), pp.204-213, 2009.
[4] G. Gugliotta, “Why Many States Now Have Stem Cell Research Programs”, 2015. [Online]. Available at: http://www.governing.com/topics/health-human-services/last-decades-culture-wars-drove-some-states-to-fund-stem-cell-research.html [Accessed 14 Oct. 2018].
[5] D. Cyranoski, “How human embryonic stem cells sparked a revolution”, Nature Journal , 2018. [Online]. Available at: https://www.nature.com/articles/d41586-018-03268-4 [Accessed 11 Oct. 2018].
[6] K. McCormack, “Young man with spinal cord injury regains use of hands and arms after stem cell therapy”, The Stem Cellar, 2016. [Online]. Available at: https://blog.cirm.ca.gov/2016/09/07/young-man-with-spinal-cord-injury-regains-use-of-hands-and-arms-after-stem-cell-therapy/ [Accessed 11 Oct. 2018].
[7] A. Coghlan, “First implants derived from stem cells to ‘cure’ type 1 diabetes”, New Scientist , 2017. [Online]. Available at: https://www.newscientist.com/article/2142976-first-implants-derived-from-stem-cells-to-cure-type-1-diabetes/ [Accessed 11 Oct. 2018].
[8] C. Scott, “University of California San Diego’s 3D Printed Liver Tissue May Be the Closest We’ve Gotten to a Real Printed Liver”, 3DPrint.com | The Voice of 3D Printing / Additive Manufacturing , 2018. [Online]. Available at: https://3dprint.com/118932/uc-san-diego-3d-printed-liver/ [Accessed 11 Oct. 2018].
[9] American Transplant Foundation, “Facts and Myths about Transplant”. [Online]. Available at: https://www.americantransplantfoundation.org/about-transplant/facts-and-myths/ [Accessed 11 Oct. 2018].
[10] A. Siegel, “Ethics of Stem Cell Research”, Stanford Encyclopedia of Philosophy , 2013. [Online]. Available at: https://plato.stanford.edu/entries/stem-cells/ [Accessed 11 Oct. 2018].
[11] I. Hyun, “Stem Cells – The Hastings Center”, The Hastings Center , 2018. [Online]. Available at: https://www.thehastingscenter.org/briefingbook/stem-cells/ [Accessed 11 Oct. 2018].
[12] M. Gazzaniga, “The Ethical Brain”, New York: Harper Perennial , 2006.
[13] M. Bilger, “Shocking Report Shows 2.5 Million Human Beings Created for IVF Have Been Killed | LifeNews.com”, LifeNews , 2016. [Online]. Available at: https://www.lifenews.com/2016/12/06/shocking-report-shows-2-5-million-human-beings-created-for-ivf-have-been-killed/ [Accessed 11 Oct. 2018].
[14] Harvard Gazette, “Stem cell lines created from discarded IVF embryos”, 2008. [Online]. Available at: https://news.harvard.edu/gazette/story/2008/01/stem-cell-lines-created-from-discarded-ivf-embryos/ [Accessed 11 Oct. 2018].
[15] K. Murray, “Could we make babies from only skin cells?”, CNN, 2017. [Online]. Available at: https://www.cnn.com/2017/02/09/health/embryo-skin-cell-ivg/index.html [Accessed 11 Oct. 2018].
[16] Biomedical Engineering Society, “Biomedical Engineering Society Code of Ethics”, 2004. [Online]. Available at: https://www.bmes.org/files/CodeEthics04.pdf [Accessed 11 Oct. 2018].
- Search Menu
- Sign in through your institution
- Advance articles
- Mini-reviews
- ESHRE Pages
- Editor's Choice
- Supplements
- Author Guidelines
- Submission Site
- Reasons to Publish
- Open Access
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Journals Career Network
- About Human Reproduction
- About the European Society of Human Reproduction and Embryology
- Editorial Board
- Self-Archiving Policy
- Dispatch Dates
- Contact ESHRE
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, what are (embryonic) stem cells, potential applications of hes cells and state‐of‐the‐art, ethical exploration, the status of hes cells, instrumental use of embryos, ethics of using surplus ivf embryos as a source of hes cells, therapeutic cloning, conclusions and recommendations, acknowledgements.
- < Previous
Human embryonic stem cells: research, ethics and policy
- Article contents
- Figures & tables
- Supplementary Data
Guido de Wert, Christine Mummery, Human embryonic stem cells: research, ethics and policy, Human Reproduction , Volume 18, Issue 4, April 2003, Pages 672–682, https://doi.org/10.1093/humrep/deg143
- Permissions Icon Permissions
The use of human embryos for research on embryonic stem (ES) cells is currently high on the ethical and political agenda in many countries. Despite the potential benefit of using human ES cells in the treatment of disease, their use remains controversial because of their derivation from early embryos. Here, we address some of the ethical issues surrounding the use of human embryos and human ES cells in the context of state‐of‐the‐art research on the development of stem cell based transplantation therapy.
Human embryonic stem cells (hES cells) are currently discussed not only by the biologists by whom they were discovered but also by the medical profession, media, ethicists, governments and politicians. There are several reasons for this. On the one hand, these ‘super cells’ have a major clinical potential in tissue repair, with their proponents believing that they represent the future relief or cure of a wide range of common disabilities; replacement of defective cells in a patient by transplantation of hES cell‐derived equivalents would restore normal function. On the other hand, the use of hES cells is highly controversial because they are derived from human pre‐implantation embryos. To date, most embryos used for the establishment of hES cell lines have been spare embryos from IVF, but the creation of embryos specifically for deriving hES cells is also under discussion. The most controversial variant of this is the transfer of a somatic cell‐nucleus from a patient to an enucleated oocyte (unfertilized egg) in order to produce hES cells genetically identical to that patient for ‘autologous’ transplantation (so‐called ‘therapeutic’ cloning); this may prevent tissue rejection.
The question ‘Can these cells be isolated and used and, if so, under what conditions and restrictions’ is presently high on the political and ethical agenda, with policies and legislation being formulated in many countries to regulate their derivation. The UK has been the first to pass a law governing the use of human embryos for stem cell research. The European Science Foundation has established a committee to make an inventory of the positions taken by governments of countries within Europe on this issue ( European Science Foundation, 2001 ).
In order to discuss the moral aspects of the isolation and use of hES cells, which is the aim of the present article, it is first essential to understand exactly what these cells are, where they come from, their intended applications and to define the ethical questions to be addressed.
‘Stem cells’ are primitive cells with the capacity to divide and give rise to more identical stem cells or to specialize and form specific cells of somatic tissues. Broadly speaking, two types of stem cell can be distinguished: embryonic stem (ES) cells which can only be derived from pre‐implantation embryos and have a proven ability to form cells of all tissues of the adult organism (termed ‘pluripotent’), and ‘adult’ stem cells, which are found in a variety of tissues in the fetus and after birth and are, under normal conditions, more specialized (‘multipotent’) with an important function in tissue replacement and repair.
hES cells are derived from the so‐called ‘inner cell mass’ of blastocyst stage embryos that develop in culture within 5 days of fertilization of the oocyte ( Thomson et al ., 1998 ; Reubinoff et al ., 2000 ). Although hES cells can form all somatic tissues, they cannot form all of the other ‘extraembryonic’ tissues necessary for complete development, such as the placenta and membranes, so that they cannot give rise to a complete new individual. They are therefore distinct from the ‘totipotent’ fertilized oocyte and blastomere cells deriving from the first cleavage divisions. hES cells are also immortal, expressing high levels of a gene called telomerase, the protein product of which ensures that the telomere ends of the chromosomes are retained at each cell division and the cells do not undergo senescence. The only other cells with proven pluripotency similar to that of ES cells are embryonic germ (EG) cells, which as their name implies, have been derived from ‘primordial germ cells’ that would ultimately form the gametes if the fetus had not been aborted. In humans, hEG cells were first established in culture in 1998, shortly after the first hES cells, from tissue derived from an aborted fetus ( Shamblott et al ., 1998 ). Biologically, hEG cells have many properties in common with hES cells ( Shamblott et al ., 2001 ).
In the adult individual, a variety of tissues have also been found to harbour stem cell populations. Examples include the brain, skeletal muscle, bone marrow and umbilical cord blood, although the heart, by contrast, contains no stem cells after birth (reviewed in McKay 1997 ; Fuchs and Segre, 2000 ; Watt and Hogan, 2000 ; Weissman et al ., 2000 ; Blau et al ., 2001 ; Spradling et al ., 2001 ). These adult stem cells have generally been regarded as having the capacity to form only the cell types of the organ in which they are found, but recently they have been shown to exhibit an unexpected versatility ( Ferrari et al ., 1998 ; Bjornson et al ., 1999 ; Petersen et al ., 1999 ; Pittenger et al ., 1999 ; Brazelton et al ., 2000 ; Clarke et al ., 2000 ; Galli et al ., 2000 ; Lagasse et al ., 2000 ; Mezey et al ., 2000 ; Sanchez‐Ramos et al ., 2000 ; Anderson et al ., 2001 ; Jackson et al ., 2001 ; Orlic et al ., 2001 ). Evidence is strongest in animal experiments, but is increasing in humans, that adult stem cells originating in one germ layer can form a variety of other derivatives of the same germ layer (e.g. bone marrow‐to‐muscle within the mesodermal lineage), as well as transdifferentiate to derivatives of other germ layers (e.g. bone marrow‐to‐brain between the mesodermal and ectodermal lineages). To what extent transdifferentiated cells are immortal or acquire appropriate function in host tissue remains largely to be established but advances in this area are rapid, particularly for multipotent adult progenitor cells (MAPCs) of bone marrow ( Reyes and Verfaillie, 2001 ). Answers to these questions with respect to MAPCs, in particular whether they represent biological equivalents to hES and can likewise be expanded indefinitely whilst retaining their differentiation potential, are currently being addressed ( Jiang et al . 2002 ; Schwartz et al ., 2002 ; Verfaillie, 2002 ; Zhao et al ., 2002 ). For other adult stem cell types, such as those from brain, skin or intestine ( Fuchs and Segre, 2000 ), this may remain unclear for the immediate future. Although the discussion here concerns hES cells and the use of embryos, the scientific state‐of‐the‐art on other types of stem cell is important in the context of the ‘subsidiarity principle’ (see below).
In theory, hES cells could be used for many different purposes ( Keller and Snodgrass, 1999 ). Examples in fundamental research on early human development are the causes of early pregnancy loss, aspects of embryonic ageing and the failure of pregnancy in older women (where genetic defects in the oocyte appear to be important). A second category might be toxicology, more specifically research on possible toxic effects of new drugs on early embryonic cells which are often more sensitive than adult cells (drug screening). The most important potential use of hES cells is, however, clinically in transplantation medicine, where they could be used to develop cell replacement therapies. This, according to most researchers in the field represents the real ‘home run’ and it is the ethics of using embryos in this aspect of medicine that will be discussed here. Examples of diseases caused by the loss, or loss of function, of only one or a limited number of cell types and which could benefit from hES cell‐based therapies include diabetes, Parkinson’s disease, stroke, arthritis, multiple sclerosis, heart failure and spinal cord lesions. Although it is known that hES cells are capable of generating neural, cardiac, skeletal muscle, pancreas and liver cells in teratocarcinomas in vivo in immunodeficient mice as well as in tissue culture, it would be an illusion to consider that cell‐therapies will have widespread application in the short term (i.e. within a couple of years). It is unfortunate that sensational treatment in the media, which implied the generation of whole organs from hES cells, initially left this impression so that the more realistic view emerging is already a disappointment to some patient groups. Nonetheless, a proper scientific evaluation of the therapeutic potential is being carried out in countries that allow the isolation and/or use of existing hES cells. The ethical questions here then also include whether the establishment of new hES cell lines can be justified, in the realisation that eventual therapies, based on either hES or adult stem cells are long‐term perspectives.
There are, at least in theory, various sources of hES cells. In most cases to date, these have been spare IVF embryos, although IVF embryos have been specifically created for the purpose of stem cell isolation ( Lanzendorf et al ., 2001 ). In one variant of ‘embryo creation’, it has even been reported that normally organized blastocysts develop from chimeras of two morphologically non‐viable embryos ( Alikani and Willadsen, 2002 ). The most revolutionary option would be the creation of embryos specifically for the purpose of isolating stem cells via ‘nuclear transfer’ (‘therapeutic cloning’). This option is purported to be the optimal medical use of hES technology since the nuclear DNA of the cells is derived from a somatic cell of a patient to receive the transplant, reducing the chances of tissue rejection (see Barrientos et al ., 1998 ; 2000). It is of note that the oocyte in this case is not fertilized, but receives maternal and paternal genomes from the donor cell nucleus. Since by some definitions an embryo is the result of fertilization of an oocyte by sperm, there is no absolute consensus that nuclear transfer gives rise to an embryo (see below).
The establishment of embryonic cell lines is becoming increasingly efficient, with up to 50% of spare IVF embryos that develop into blastocysts after thawing at the 8‐cell stage reported to yield cell lines. There are reports of efficiencies much lower than 50%, however, the quality of the donated embryos being an important determinant of success. Growth of the cell lines over extended periods and in some cases under defined conditions ( Xu et al ., 2001 ) has also been reported, but the controlled expansion and differentiation to specific cell types is an area where considerable research will be required before cell transplantation becomes clinical practice (for review, see Passier and Mummery, 2003 ). In addition, research will be required on how to deliver cells to the appropriate site in the patient to ensure that they survive, integrate in the host tissue and adopt appropriate function. These are the current scientific challenges that will have to be overcome before cell therapy becomes clinical practice; the problems are common to both hES and adult stem cells. The efficiency of establishing embryonic stem cell lines from nuclear transfer embryos is currently unknown, but expected to be lower than from IVF embryos.
In the following section, the status of hES cells is first considered. The questions of whether it is acceptable to use pre‐implantation embryos as a source of ES cells for research on cell transplantation therapy and if so, whether embryo use should be limited to spare embryos or may also include the creation of embryos via nuclear transfer (‘therapeutic cloning’), are then addressed.
What is the ontological status of hES cells? Should they be considered equivalent to embryos or not? Let us first consider the status of the ‘naked’, isolated inner cell mass (ICM; the source for deriving hES cell lines). The ICM is as it were the ‘essence’ of the pre‐implantation embryo, the precursor of the ‘embryo proper’. The isolated ICM, however, no longer has the potential to develop into a fetus and child, as trophoblast cells, necessary for implantation and nourishment of the embryo, and extra‐embryonic endoderm, are absent. It does not necessarily follow, though, that the isolated ICM is no longer an embryo—we suggest that the whole, isolated ICM could best be qualified as a disabled, ‘non‐viable’ embryo (even though it might, at least in theory, be ‘rescued’ by enveloping the ICM with sufficient trophoblast cells).
What, then, is the status of the individual cells from the ICM once isolated, and the embryonic stem cell lines derived from them? Should we consider these cells/cell lines to be non‐viable embryos too? We would argue that when the cells of the ICM begin to spread and grow in culture, the ICM disintegrates and the non‐viable embryo perishes. Some might argue that hES cells are embryos, because, although hES cells in themselves cannot develop into a human being, they might if they were ‘built into’ a cellular background able to make extra‐embryonic tissues necessary for implantation and nutrition of the embryo. At present this is only possible by ‘embryo reconstruction’ in which the ICM of an existing embryo is replaced by ES cells ( Nagy et al ., 1993 ). Commentators who, against this background, regard hES cells as equivalent to embryos, apparently take recourse to the opinion that any cell from which a human being could in principle be created, even when high technology (micromanipulation) would be required to achieve this, should be regarded as an embryo. An absurd implication of this ‘inclusive’ definition of an embryo is that one should then also regard all somatic cells as equivalent to embryos—after all, a somatic nucleus may become an embryo after nuclear transplantation in an enucleated oocyte. It is therefore unreasonable to regard hES cells as equivalent to embryos.
Research into the development of cell‐replacement therapy requires the instrumental use of pre‐implantation embryos from which hES cells are derived since current technology requires lysis of the trophectoderm and culture of the ICM; the embryo disintegrates and is thus destroyed. As has already been discussed extensively in the embryo‐research debate, considerable differences of opinion exist with regard to the ontological and moral status of the pre‐implantation embryo ( Hursthouse, 1987 ). On one side of the spectrum are the ‘conceptionalist’ view (‘the embryo is a person’) and the ‘strong’ version of the potentiality‐argument (‘because of the potential of the embryo to develop into a person, it ought to be considered as a person’). On the other side of the spectrum we find the view that the embryo (and even the fetus) as a ‘non‐person’ ought not to be attributed any moral status at all. Between these extremes are various intermediates. Here, there is a kind of ‘overlapping consensus’: the embryo has a real, but relatively low moral value. The most important arguments are the moderate version of the potentiality argument (‘the embryo deserves some protection because of its potential to become a person’) and the argument concerning the symbolic value of the embryo (the embryo deserves to be treated with respect because it represents the beginning of human life). Differences of opinion exist on the weight of these arguments (how much protection does the embryo deserve?) and their extent (do they apply to pre‐implantation embryos?). In view of the fact that up to 14 days of development, before the primitive streak develops and three germ layers appear, embryos can split and give rise to twins or two embryos may fuse into one, it may reasonably be argued that at these early stages there is in principle no ontological individuality; this limits the moral value of an embryo.
Pre‐implantation embryos are generally regarded from the ethical point of view as representing a single class, whereas in fact ∼50–60% of these embryos are aneuploid and mostly non‐viable. For non‐viable embryos, the argument of potentiality does not of course apply. Their moral status is thus only based on their symbolic value, which is already low in ‘pre‐individualized’ pre‐implantation embryos. The precise implications of this moral difference for the regulation of the instrumental use of embryos is, however, beyond the scope of the present article.
The view that research with pre‐implantation embryos should be categorically forbidden is based on shaky premises and would be difficult to reconcile with the wide social acceptance of contraceptive intrauterine devices. The dominant view in ethics is that the instrumental use of pre‐implantation embryos, in the light of their relative moral value, can be justified under certain conditions. The international debate focuses on defining these conditions.
Possible objections are connected to the principle of proportionality, the slippery slope argument, and the principle of subsidiarity.
Proportionality
It is generally agreed that research involving embryos should be related to an important goal, sometimes formulated as ‘an important health interest’ (the principle of proportionality). Opinions differ on how this should be interpreted and made operational. In a number of countries, research on pre‐implantation embryos is permitted provided it is related to human reproduction. Internationally, however, such a limitation is being increasingly regarded as too restrictive ( De Wert et al ., 2002 ). The isolation of hES cells for research into cell‐replacement therapies operates as a catalyst for this discussion. It is difficult to argue that research into hES cells is disproportional. If embryos may be used for research into the causes or treatment of infertility, then it is inconsistent to reject research into the possible treatment of serious invalidating diseases as being not sufficiently important. The British Nuffield Council on Bioethics ( Nuffield Council on Bioethics, 2000 ) also saw no reason for making a moral distinction between research into diagnostic methods or reproduction and research into potential cell therapies.
Even if one argued that there is a difference between the two types of research, research on cell therapy would, if anything, be more defensible than research on reproduction. One (in our opinion somewhat dubious) argument is to be found in McGee and Caplan (1999 ); here the suggestion is made that in using embryos for cell therapy, no embryos are actually sacrificed: ‘In the case of embryos already slated to be discarded after IVF, the use of stem cells may actually lend permanence to the embryo. Our point here is that the sacrifice of an early human embryo, whether it involves a human person or not, is not the same as the sacrifice of an adult because life of a 100‐cell embryo is contained in its cells nuclear DNA.’ In other words, the unique characteristic of an embryo is its DNA; by transplanting cells containing this DNA to a new individual, the DNA is preserved and the embryo therefore not sacrificed—a ‘win–win’ situation for both the embryo and cell transplant recipient. The implication is thus that the use of embryos for cell transplantation purposes is ethically preferable to disposing of them or using them in other (‘truly destructive’) types of research. This extreme genetic ‘reductionism’ is highly disputable and not convincing: the fact that embryos are actually sacrificed in research into cell therapy is masked. A second, more convincing, argument, that the instrumental use of embryos is in principle easier to justify for isolation of hES cells than, for example, research directed towards improving IVF, is that it has potentially far wider clinical implications. It therefore, unquestionably meets the proportionality requirement.
Slippery slope
The slippery slope argument can be considered as having two variants, one empirical and the other logical. The empirical version involves a prediction of the future: ‘Acceptance of practice X will inevitably lead to acceptance of (undesirable) practice Y. To prevent Y, X must be banned’. The logical version concerns the presumed logical implications resulting from the moral justification of X: ‘Justification of X automatically implies acceptance of (undesirable) practice Y’. In this context the problem often lies in the lack of precise definition of X: ‘The difficulty in making a conceptual distinction between X and Y that is sharp enough to justify X without at the same time justifying Y, is a reason to disallow X.’ Both versions of the argument play a role in the debate about the isolation of hES cells for research into cell replacement therapy. An example of the logical version is that acceptance of hES cells for the development of stem cell therapy for the treatment of serious disease automatically means there is no argument against acceptance of use, for example, for cosmetic rejuvenation (Nuffield Council on Bioethics, 2000). The main difficulty is, according to these critics, the ‘grey area’ between these two extremes. One answer to this objection is to consider each case individually rather than reject all cases out of hand. One could use the same objection for example against surgery, which can equally be used for serious as well as trivial treatments.
An example of the empirical version of the slippery slope argument is that the use of hES cells for the development of cell therapy would inevitably lead to applications in germ‐line gene therapy and in therapeutic cloning, then ultimately reproductive cloning. This version of the argument is unconvincing too; even if germ line gene therapy and therapeutic cloning would be categorically unacceptable, which is not self‐evident, it does not necessarily follow from this that the use of hES cells for cell‐therapy is unacceptable. The presumed automatism in the empirical version of the slippery slope argument is disputable.
Subsidiarity
A further condition for the instrumental use of embryos is that no suitable alternatives exist that may serve the same goals of the research. This is termed ‘the principle of subsidiarity’. Critics of the use of hES cells claim that at least three such alternatives exist, which have in common that they do not require the instrumental use of embryos: (i) xenotransplantation; (ii) human embryonic germ cells (hEG cells), and (iii) adult stem cells.
The question is not whether these possible alternatives require further research (this is, at least for the latter two, largely undisputed), but whether only these alternatives should be the subject of research. Is a moratorium for isolating hES cells required, or is it preferable to carry out research on the different options, including the use of hES cells, in parallel?
The answer to this question depends on how the principle of subsidiarity ought to be applied. Although the principle of subsidiarity is meant to express concern for the (albeit limited) moral value of the embryo, it is a sign of ethical one‐dimensionality to present every alternative, which does not use embryos, as a priori superior. For the comparative ethical analysis of hES cells from pre‐implantation embryos on the one hand, and the possible alternatives mentioned on the other, a number of relevant aspects should be taken into account. These include: the burdens and/or risks of the different options for the patient and his or her environment; the chance that the alternative options have the same (probably broad) applicability as hES cells from pre‐implantation embryos; and the time‐scale in which clinically useful applications are to be expected.
A basis for initiating a comparative ethical analysis is set out below:
(i) Xenotransplantation is viewed at present as carrying a risk, albeit limited, of cross‐species infections and an accompanying threat to public health. This risk is, at least for the time being, an ethical and safety threshold for clinical trials. Apart from that, the question may be raised from a perspective of animal ethics whether it is reasonable to breed and kill animals in order to produce transplants, when at the same time spare human embryos are available which would otherwise be discarded;
(ii) In principle, the use of hEG cells from primordial germ cells of dead fetuses seems from a moral perspective to be more acceptable than the instrumental use of living pre‐implantation embryos, provided that the decision to abort was not motivated by the use of fetal material for transplantation purposes. To date, however, hEG cells have been difficult to isolate and culture, with only one research group reporting success ( Shamblott et al ., 1998 ; 2001). In addition, research in mice suggests abnormal reprogramming of these cells in culture: chimeric mice generated between mouse (m)EG cells and pre‐implantation embryos develop abnormally while chimeras using mouse (m)ES cells develop as normally as non‐chimeric mice ( Steghaus‐Kovac, 1999 ; Surani, 2001 ). This makes the outcome of eventual clinical application of these cells difficult to predict in terms of health risks for the recipient.
(iii) Analysis of the developmental potential of adult stem cells is a rapidly evolving field of research, particularly in animal model systems. Experiments carried out within the last two years have demonstrated, for example, that bone marrow cells can give rise to nerve cells in mouse brain ( Mezey et al ., 2000 ), neural cells from mouse brain can turn into blood and muscle ( Bjornson et al ., 1999 ; Galli et al ., 2000 ), and even participate in the development of chimeric mouse embryos up to mid‐gestation ( Clarke et al ., 2000 ). Although apparently spectacular in demonstrating that neural stem cells from mice can form most cell types under the appropriate conditions, it is still unclear whether true plasticity in terms of function has been demonstrated or whether the cells simply ‘piggy‐back’ with normal cells during development. Published evidence of ‘plasticity’ in adult human stem cells is more limited, but recent evidence suggests that the MAPCs from bone marrow may represent a breakthrough ( Jiang et al ., 2002 ; Schwartz et al ., 2002 ;). They are accessible. Collection is relatively non‐destructive for surrounding tissue compared, for example, with the collection of neural stem cells from adult brain, although their numbers are low: 1 in 10 8 of these cells exhibit the ability to form populations of nerve, muscle and a number of other cell types and they only become evident after several months of careful culture. Clonal analysis has provided rigorous proof of plasticity: a single haematopoietic stem cell can populate a variety of tissues when injected into lethally irradiated mice ( Krause et al ., 2001 ) or into blastocyst stage embryos to generate chimeric embryos ( Jiang et al ., 2002 ). Nonetheless, there are potential hazards to using cells that have been cultured for long periods for transplantation and although MAPCs seem to have normal chromosomes, it is important to establish that the pathways governing cell proliferation are unperturbed. This is also true for hES cells. However, the powerful performance of mES cells in restoring function in a rat model for Parkinson’s disease ( Kim et al ., 2002 ), has not yet been matched by MAPCs. Bone marrow stem cells have been shown very recently to restore function to some extent in a mouse heart damaged by coronary ligation, an experiment that mimics the conditions of the human heart soon after infarction ( Orlic et al ., 2001 ). Although clinical restoration of function in a damaged organ is usually sought rather longer after the original injury than in these experiments, which were performed before scar tissue had formed, this approach will certainly be worth pursuing. An alternative, non‐invasive, haematopoietic stem cell source is umbilical cord blood. This is used clinically for transplantation as an alternative to bone marrow in patients for whom no bone marrow match is available. Cord blood contains precursors of a number of lineages but its pluripotency, or even multipotency, is far from proven. Nevertheless, the prospect of autologous transplantation of haematopoietic stem cells of bone marrow in the long term makes this an important research area in terms of alternatives to therapeutic cloning (see below).
Although studies with adult stem cells so far have been encouraging, Galli (2000 ), author of the first adult neural stem studies and much cited by advocates of the view that adult stem cells have a proven developmental potency equal to that of ES cells, himself disagrees entirely with this viewpoint (see Editorial, 2000 ). It has even been suggested that the results from adult stem cell research are being misinterpreted for political motives and ‘hints of the versatility of the adult cells have been over interpreted, overplayed and over hyped’ ( Vastag, 2001 ). Opponents of ES cell research are now heralding Verfaillie’s adult stem cells as proof that work on hES cells is no longer needed. However the stem cell research community and Verfaillie herself ( Vastag, 2002 ) have called for more research on both adult and embryonic stem cells. ES cells that can perform as powerfully as those described by Kim et al . (2002 ) in the rat Parkinson model make it far too early in the game for them to be discounted ( Editorial, 2002 ).
The question remains, however, should a moratorium be imposed on isolating hES cells for research in cell therapy in the light of the indisputably promising results from adult stem cell research? The lack of consensus arises largely from disagreement on interpretation of the subsidiarity principle. Against the restrictive viewpoint that research on hES cells may only take place if there is proof that adult stem cells are not optimally useful, there is the more permissive viewpoint that hES cell research may, and indeed should, take place so long it is unclear whether adult stem cells are complete or even partial alternatives.
On the basis of the following arguments, a less restrictive interpretation of the subsidiarity principle is morally justified. ( Stem Cell Research, 2000 ) To begin with, the most optimistic expectation is that only in the long run will adult stem cells prove to have equal plasticity and developmental potential as hES cells (and be as broadly applicable in the clinic), and there is a reasonable chance that this will never turn out to be the case. If hES cells from pre‐implantation embryos have more potential clinical applications in the short term, then the risk of a moratorium is that patients will be deprived of benefit. This in itself is a reason to forgo a moratorium—assuming that the health interests of patients overrule the relative moral value of pre‐implantation embryos. Secondly, the simultaneous development of different research strategies is preferable, considering that research on hES cells will probably contribute to speeding up and optimising clinical applications of adult stem cells. In particular, the stimuli to drive cells in particular directions of differentiation may be common to both cell types, while methods of delivery to damaged tissue are as likely to be common as complementary. A moratorium on hES cell research would remove the driving force behind adult stem cell research.
A final variant on adult stem cell sources concerns the use of embryonal carcinoma (EC) cells, a stem cell population found in tumours (teratocarcinomas) of young adult patients. These cells have properties very similar to hES cells. The results of a phase I (safety) trial using these cells in 11 stroke victims in the USA have recently been published and permission granted by the Food and Drug Administration (FDA) for a phase II trial (effectivity) ( Kondziolka et al ., 2000 ). The patients received neural cells derived from retinoic acid (vitamin A) treatment of teratocarcinoma stem cells. Although the scientific and ethical consensus is that these trials were premature in terms of potential risk of teratocarcinoma development at the transplant site, all patients survived with no obvious detrimental effects, no tumour formation and in two cases a small improvement in symptoms. After two years, the transplanted cells were still detectable by scanning ( Kondziolka et al ., 2000 ). Despite its controversial nature, this trial has nevertheless probably set a precedent for similar trials using neural derivatives of hES, the best controlled differentiation pathway of hES cells at the present time ( Reubinoff et al ., 2001 ; Zhang et al ., 2001 ). Proponents believe that such trials would be feasible even in the short term ( McKay, 1997 ). Neural differentiation of hEC cells is fairly easy to induce reproducibly but most other forms of differentiation are not; even if ultimately regarded as ‘safe’, hEC cells will not replace hES cells in terms of developmental potential and are therefore not regarded as an alternative.
In view of both the only relative moral value of pre‐implantation embryos and the uncertainties and risks of the potential alternative sources for the development of cell therapy, a moratorium for isolating human embryonic stem cells is unjustified.
Before discussing the ethical issues around ‘therapeutic cloning’, the term itself requires consideration. To avoid confusion, it has been proposed that the term ‘cloning’ be reserved for reproductive cloning and that ‘Nuclear transplantation to produce stem cells’ would be better terminology for therapeutic cloning ( NAS report, 2002 ; Vogelstein et al ., 2002 ). Others have pointed out the disadvantage of this alternative term, namely that it masks the fact that an embryo is created for instrumental use. More important in our opinion however, is that the use of the adverb ‘therapeutic’ suggests that hES cell therapy is already a reality: strictu sensu there can only be a question of therapeutic applications once clinical trials have started. In the phase before clinical trials, it is only reasonable to refer to research on nuclear transfer as ‘research cloning’ or ‘nuclear transplantation for fundamental scientific research’, aimed at future applications of therapeutic cloning.
Some consider this technology to be ethically neutral; they claim that the ‘construct’ produced is not a (pre‐implantation) embryo. Qualifications suggested for these constructs include: activated oocyte, ovasome, transnuclear oocyte cell, etc. ( Kiessling, 2001 ; Hansen, 2002 ) However, to restrict the definition of ‘embryo’ to the product of fertilization in the post‐Dolly era is a misleading anachronism. Although the purpose of therapeutic cloning is not the creation of a new individual and it is unlikely that the viability of the constructed product is equivalent to that of an embryo derived from sexual reproduction, it is not correct to say that an embryo has not been created.
The core of the problem is that here human embryos are created solely for instrumental use. Whether or not this can be morally justified—and if so, under what conditions—has already been an issue of debate for years in the context of the development of ‘assisted reproductive technologies’ (ART). Is it acceptable to create embryos for research, and if so, is therapeutic cloning morally acceptable too?
A preliminary question: is it justified to create embryos for research?
Article 18 of the European Convention on Human Rights and Biomedicine forbids the creation of embryos for all research purposes ( Council of Europe, 1996 ). However, this does not close the ethical and political debates in individual EU member states.
In the ‘classical’ normative debate on embryo research, two perspectives can be distinguished: a ‘fetalist’ perspective (focusing on the moral value of the embryo), and a ‘feminist’ perspective (with the interests of women, particularly candidate oocyte donors, playing a central role) ( Raymond, 1987 ). Both perspectives have a different outlook on the question of whether or not there is a decisive moral distinction between research with spare IVF embryos on the one hand, and creating embryos for research on the other. In other words: is the difference between these practices such that the former can be acceptable under specific conditions, and the latter absolutely not?
Fetalist perspective
Instrumentalization of the embryo is sometimes regarded as far greater and fundamentally different when it involves the creation of embryos for research purposes rather than the use of spare embryos. This difference, however, is just gradual. Not only is the embryo used completely instrumentally in both cases, the moral status is also identical. The difference is in the intention at fertilization, which, although a real difference, is relative. It is a misconception to think that in the context of regular IVF treatment every embryo is created as a ‘goal in itself’: the goal is the solution of involuntary childlessness and the loss of some embryos is a calculated risk beforehand.
Feminist perspective
From a feminist perspective, the creation of embryos for research should be evaluated critically in as far as it may require hormone treatment of a woman to obtain oocytes for research purposes: can this be morally justified when it requires unpleasant treatment of the donor with no benefit at all, or even a detrimental outcome, for her own state of health? A first objection is that women themselves become objects of instrumental use. Here, however, an analogy can be made with recruiting healthy research subjects. Relevant considerations concern whether or not the research serves an important goal, whether the burdens and risks to the subjects are proportional, and whether valid informed consent of the research subject/donor is given. The second objection is that the health risks to the women themselves are too high and the degree of discomfort disproportional. Difference of opinion exists, however, also among women, about the disproportionality of hormone treatment. There are, furthermore, several potential alternatives that do not require hormone treatment of healthy women. One involves the in‐vitro maturation (IVM) of immature oocytes after their isolation from dead donors or donors having ovaries removed for other reasons. IVM is successful in cattle and sheep (efficiency ∼40%), although it is, for the moment, much lower in humans.
In conclusion, from both a fetalist and a feminist perspective there is no overriding categorical objection against bringing pre‐implantation embryos into existence for instrumental use. If the research cannot be conducted using spare embryos and its importance for human health is beyond doubt, we believe the creation of embryos specifically for research is morally justified subject to the required oocytes being obtained in a morally sound way.
Ethics of therapeutic cloning
Can therapeutic cloning be morally acceptable? The principle of proportionality, the slippery slope, and the principle of subsidiarity enter the debate again, but in a slightly different way.
It is doubtful whether the principle of proportionality provides a convincing a‐priori objection against therapeutic cloning. If it is considered acceptable to create embryos for research aimed at improving ART (freezing of oocytes; IVM of oocytes, etc…), then it is inconsistent to reject therapeutic cloning beforehand as being disproportional. Maybe even some opponents of creating embryos for the improvement of ART can conditionally accept therapeutic cloning because of the important health interests of patients.
Slippery‐slope
A consequentialist objection (fashioned as a ‘slippery‐slope’ argument) is that therapeutic cloning will inevitably lead to reproductive cloning. This objection is not convincing; if reproductive cloning is categorically unacceptable (the debate on this issue is still ongoing), it is reasonable to prohibit this specific technology, and not to ban other, non‐reproductive, applications of cloning. A second objection that could be raised in this context is that the creation of embryos through cloning for the isolation of stem cells could in the long term be used to justify the initiation of pregnancy from these embryos and their use simply as a vehicle for generating sufficient cells of the required type for transplantation; the pregnancy would be interrupted the moment the appropriate developmental stage was reached ( Lanza et al ., 2002 ). Relevant questions here are: is this a realistic scenario in the human (or just science fiction), would it be unacceptable, and is it unavoidable?
In terms of being a realistic means of generating genetically identical (fetal) tissue for transplantation, it could theoretically be an option, but whether it would actually be useful would depend on the alternatives available at the time transplantation techniques themselves have been perfected to clinical applicability (see below).
In terms of moral acceptability, most people would consider pregnancy‐and‐abortion‐for‐transplantation to be far more difficult to justify than the creation of pre‐implantation embryos for instrumental use in vitro , firstly because of the higher moral status/symbolic value of the fetus, and secondly because of the significantly greater burden of pregnancy‐and‐abortion‐for‐transplantation for women. ( De Wert et al ., 2002 ) Even though many countries do forbid pregnancy‐for‐transplantation, it has been argued that it could be morally justified as a last resort, on the basis that sacrificing a fetus (a potential person) may be justified in order to rescue the life of a person.
Finally, in scrutinising the slippery slope argument, it is important to assess whether instrumental use of pre‐implantation embryos makes pregnancy‐for‐abortion unavoidable. Again, the apparent automatism is disputable: if we reject pregnancy‐for‐abortion as being unacceptable, we can continue its prohibition.
Taking these points for and against together, the slippery slope argument does not provide a convincing basis for banning therapeutic cloning.
Therapeutic cloning can only be morally acceptable if there are no good alternatives. It is important to note that therapeutic cloning strictu sensu is not likely to be short‐term prospect. Apart from unsolved technical difficulties with nuclear transfer itself in human oocytes ( Cibelli et al ., 2002 ), much basic research is still needed to determine whether the differentiation of hES cells can be controlled and sufficient cell numbers generated to be a useful therapy. This research can be done with spare IVF embryos. In this light, creation of embryos for therapeutic cloning is, in our opinion, premature. Although critics of this point of view could use our own argument that delay in the development of research cloning could, just as a moratorium on hES cell isolation and research, have negative consequences for patients, the evidence suggests that further optimization of the technology as such could take place in animals. We believe that the duration of any ‘delay’ in offering therapy to patients would not then be of real significance.
At the same time, research on potential alternatives for therapeutic cloning, which likewise avoid (or at least reduce) the problem of rejection but which do not involve the creation of human embryos for instrumental use, should be stimulated. For the comparative ethical analysis, it is again important to avoid the pitfall of one‐dimensionality. Possible alternative options include: (i) the use of adult cells, both stem cells and differentiated cells; (ii) making optimal use of spare embryos: embryo‐banks and immuno‐tolerance and (iii) the use of entities with an undetermined status: ‘hybrids’ and ‘parthenotes’.
Adult cells
Adult tissue is a potential source of two alternatives: stem cells, which may be induced to transdifferentiate by extracellular signals, and somatic cells (nuclei) which require direct reprogramming signals, for example from an oocyte after nuclear transfer, to adopt a new fate. Both sources will, however, require substantial research to become realistic alternatives. Until it has been shown that adult stem cells at some point re‐express ES cell markers we will never know if transdifferentiation or direct reprogramming are the same or not.
For direct reprogramming of somatic nuclei, new methods may be developed which do not require nuclear transfer to oocyte cytoplasm. Examples of current work in this area include the study of cellular hybrids derived from the fusion of (embryonic) stem cells with somatic or adult stem cells ( Surani, 2001 ; Terada et al ., 2002; Ying et al ., 2002 ). An understanding of the basic mechanisms underlying reprogramming is already being undertaken in mice, cattle and sheep and indeed, the creation of ‘Dolly’ re‐initiated a wave of research in nuclear reprogramming in mammals. The ultimate aim of this research in the context of cell transplantation therapy would be chemically‐induced nuclear re‐programming in the test‐tube to derive the required cell type, obviating the necessity for therapeutic cloning altogether. First evidence that this might be feasible demonstrated direct reprogramming of fibroblasts to neural cells and T‐cells in culture by temporary permeabilization of the fibroblasts to allow them to take up extracts of neural and T‐cells, respectively ( Hakelien et al ., 2002 ). In this sense, therapeutic cloning may be regarded, perhaps, as a temporary option; in the long term it will be replaced by a direct reprogramming alternative.
Research on direct reprogramming of adult somatic nuclei may ultimately require the creation of human embryos for instrumental use. In view of the importance of this research, both in terms of the contribution to the development of cell therapy and the potential ultimately to reduce the instrumental use of human embryos by developing an alternative for therapeutic cloning, this research would no doubt also meet the principle of proportionality.
Optimal use of spare embryos
Various strategies should be considered. Firstly, the generation of a bank of hES cell lines from a wide spectrum of genotypes is required to be able to offer a reasonable tissue match for every patient requiring a cellular transplant. Estimates of the number of independent cell lines that would actually be required for this vary greatly, from a few hundred to several thousand. Such a bank is already being discussed in the UK but could ultimately be established as a European resource. However, even very good tissue matches between donor and recipient require some degree of immunosuppressive therapy, which has long term negative side‐effects for patients, including increased risk of tumorigenesis
Secondly, there should be further development and application of ‘immunotolerance’ methodology. This may be particularly useful in combination with matching from an hES cell bank. The observation that patients receiving bone marrow transplants are more immunotolerant to other tissue transplantation from the same donor have led to the suggestion that immunotolerance may also be induced by initial injection of hES‐derived haematopoietic cells followed by the cell type of interest derived from the same hES cell line ( Kaufman et al ., 2001 ). The transplant may then be tolerated without being genetically identical, and lower doses or no immunosuppressives required. The combination of ‘near match’ with immunotolerance is probably a promising option.
For certain genetically based diseases, autologous transplantation may not always be appropriate since the transplanted tissue will bear the same genetic defect. Immunotolerance hES cell strategies may then be a particularly attractive or the only option. Should the success rates be very high, then attempts to create genetically identical transplantable tissue may become superfluous, not only for these, but for all patients. If, however, it works imperfectly or only for some patients, then therapeutic cloning may well remain an important option for the majority of all other patients.
Creating entities with an undefined status
Various alternative options raise classification problems, as the entities created to obtain cells have an undefined status. Firstly, transplanting the somatic nucleus of a patient into an enucleated animal oocyte. The logic behind this variant of therapeutic cloning is twofold: one, assuming that the ‘units’ thus created are not human embryos because only their nuclear but not mitochondrial DNA is human, advocates of this strategy argue that it circumvents the controversial issue of the instrumental use of human embryos. Two, a technical advantage of this approach would be that plenty of animal oocytes would be available; the feminist objection to creating human embryos for research would, of course, not apply.
It is not yet known whether this is a scientifically realistic option (whether hES cells can be effectively obtained following this approach). Animal research has so far been limited and not generally successful ( Barrientos et al ., 1998 ; 2001); polymorphic interspecies differences in mitochondrial DNA are thought to make such reconstructed zygotes non‐viable or prone to major developmental abnormalities. There are however, unvalidated reports of successful applications of the technique in China. The Donaldson Committee advocated a ban on this approach, but without any argumentation (Stem Cell Research, 2000). However, if this were a realistic option scientifically, then we believe that the issues involved deserve further ethical discussion. The major questions that should be addressed include: is the risk acceptable? As for xenotransplantation, there is also here the risk of cross‐species infection, although this may be extremely small, because the nuclear DNA of the animal, which may harbour viruses, is removed from the oocyte. Is it reasonable to argue that this ‘artificial combination’ should not be considered equivalent to a human embryo? Since the entire nuclear DNA is human, the reconstructed combination should, we think, be regarded as a human embryo. The procedure should thus not be presented as an ‘embryo saving’ variant of therapeutic cloning. However, only further in‐utero research with reconstructed animal embryos, for example embryos created by transplanting the somatic nucleus of a rat into an enucleated mouse oocyte, will provide a more definitive answer. Finally: in‐vitro research may well show that embryos obtained by transplanting a human somatic nucleus into an enucleated animal oocyte are non‐viable (like parthenotes, see below). The moral status of non‐viable pre‐implantation embryos, and more particularly, the question as to whether the conditions for research using non‐viable embryos may be more permissive than the conditions for using viable embryos, needs further debate (see earlier).
A second option may be the generation of parthenogenetic embryos for the isolation of hES cell lines. Here, an unfertilized (haploid) oocyte is treated chemically such that it becomes diploid, with two identical sets of the maternal chromosomes. These uniparental embryos are by definition gynogenetic and never result in viable offspring, because they fail to generate extra‐embryonic tissues. Nevertheless, in mice (see Boediono et al ., 1999 ) and in apes ( Cibelli et al ., 2002 ), parthenotes have been shown to develop to the blastocyst stage and yield cell lines with properties not distinguishable from ES cells derived from fertilized oocytes. However, in view of the fact that some genes are genomically imprinted, such that they are expressed only if inherited via the male germ line, ES cells derived from parthenotes may well be abnormal. First attempts at parthenogenesis in humans have not yielded hES cell lines ( Cibelli et al ., 2002 ). It is important to realise that such hES cell lines, if developed in humans, would only provide a tissue match for the oocyte donor, i.e. women of reproductive age. Although it has been speculated that two sets of male chromosomes could also be used in parthenotes, there is no evidence that this is a real option.
Cibelli and colleagues have referred to parthenogenesis as cloning. Whether this is correct depends on the timing of parthenogenesis: if initiated before the first complete meiotic division, then the procedure amounts to cloning (the same genotype as the female); if after the first meiotic division (ie recombination and loss of half) then it is not cloning. In this light, the experiments of Cibelli et al . (2002) would not qualify as cloning in the strict sense.
Some will certainly argue that the parthenote is not an embryo; parthenogenesis would then be classified as an ‘embryo‐saving’ strategy. As the parthenote undergoes the first divisions normally and is at these stages not distinguishable from embryos derived by normal fertilization, we would argue that it should be regarded as a non‐viable embryo. In the light of its non‐viability, the potentiality argument is not applicable. The moral status of parthenotes may therefore be regarded as very low, lower even than that of normal viable embryos at the same stage (see earlier). Thus, although not an ‘embryo‐saving alternative’, all other things being equal, parthenogenesis may be regarded as ethically preferable to the generation of viable embryos by fertilization or nuclear transfer (for instrumental use). In addressing the question of whether this research is premature given the current lack of proof that human ES cells are clinically useful as a source of transplantable cells, the lower moral status of parthenotes should be taken into account.
Regarding moral judgements as a ‘quasi stable equilibrium’ is particularly appropriate when applied to the ethics of isolating hES cells for research into cell replacement therapy. Stem cell research is highly dynamic, with many questions and ‘unknowns’. New insights into the effectiveness, risks and usefulness of the various alternatives may have immediate consequences for the ethical evaluation of the isolation of hES cells.
The status of the pre‐implantation embryo is the most sensitive and disputed point in the debate on isolation of hES cells for research. The dominant view in ethics, however, is that the moral status of the pre‐implantation embryo is relatively low and that the instrumental use of these embryos can be morally justified under some conditions.
The moral status of non‐viable pre‐implantation embryos is lower than the moral status of viable pre‐implantation embryos. The precise implications of this difference in moral status for the regulation of the instrumental use of embryos need further ethical scrutiny.
Both the principle of proportionality and a permissive interpretation of the principle of subsidiarity, make a moratorium on the isolation of hES cells unjustified.
Parallel research on alternatives is important and requires major support. Research on hES cells can provide an important impetus in this context.
The moral difference between research on surplus embryos and the creation of embryos for research is only gradual. A complete ban on creating embryos for instrumental use in research is morally unjustified.
A categorical ban on research on human therapeutic cloning is not justified, although the creation of embryos by cloning for the isolation of hES cells is, at the present time, premature. The necessary research can currently be carried out using animal embryos and surplus human IVF embryos.
Research into potential alternatives for therapeutic cloning, which does not require human embryos or which requires only the use of spare embryos, should be stimulated.
Banning the transplantation of a human somatic nucleus to an animal oocyte (as a variant of therapeutic cloning) is premature and morally unjustified.
The question whether therapeutic cloning should be allowed, becomes acute if research with spare embryos suggests that usable transplants can be obtained in vitro from hES cells and if the possible alternatives for therapeutic cloning are less promising or need more time for development than is currently expected. In that case, therapeutic cloning can be morally justified on the basis of both the principle of proportionality and the principle of subsidiarity.
We are grateful to Drs K.Lawson and J.Geraedts for comments on the manuscript.
Alikani, M. and Willadsen, S.M. ( 2002 ) Human blastocysts from aggregated mono‐nucleated cells of two or more non‐viable zygote‐derived embryos. Reprod. Biomed. Online , 5 , 56 –58.
Anderson, D.J., Gage, F.H. and Weissman, I.L. ( 2001 ) Can stem cells cross lineage boundaries? Nat. Med. , 7 , 393 –395.
Barrientos, A., Kenyon, L. and Moraes, C.T. ( 1998 ) Human xeno mitochondrial cybrids. Cellular models of mitochondrial complex I deficiency. J. Biol. Chem. , 273 , 14210 –14217.
Barrientos, A., Muller, S., Dey, R., Weinberg, J. and Moraes, C.T. ( 2000 ) Cytochrome c oxidase assembly in primates is sensitive to small evolutionary variations in amino acid sequence. Mol. Biol. Evol. , 17 , 1508 –1519.
Boediono, A., Suzuki, T., Li, L.Y. and Godke, RA. ( 1999 ). Offspring born from chimeras reconstructed from parthenogenetic and in vitro fertilized bovine embryos. Mol. Reprod. Dev. , 53 , 159 –170.
Bjornson, C.R., Rietze, R.L., Reynolds, B.A., Magli, M.C. and Vescovi, A.L. ( 1999 ) Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science , 283 , 534 –537.
Blau, H.M., Brazelton, T.R. and Weimann, J.M. ( 2001 ) The evolving concept of a stem cell. Entity of function? Cell , 105 , 829 –841.
Brazelton, T.R., Rossi, F.M., Keshet, G.I. and Blau, H.M. ( 2000 ) From marrow to brain: expression of neuronal phenotypes in adult mice. Science , 290 , 1775 –1779.
Cibelli, J.B., Grant, K.A., Chapman, K.B., Cunniff, K., Worst, T., Green, H.L., Walker, S.J., Gutin, P.H., Vilner, L., Tabar, V. et al . ( 2002 ) Parthenogenetic stem cells in nonhuman primates. Science , 295 , 819 .
Clarke, D.L., Johansson, C.B., Wilbertz, J., Veress, B., Nilsson, E., Karlstrom, H., Lendahl, U. and Frisen, J. ( 2000 ) Generalized potential of adult neural stem cells. Science , 288 , 1559 –1561.
Council of Europe ( 1996 ) Convention on Human Rights and Biomedicine. Strasburg.
De Wert, G., Berghmans, R., Boer, G.J., Andersen, S., Brambati, B., Carvalho, A.S., Dierickx, K.M., Elliston, S., Nunez, P., Osswald, W. et al . ( 2002 ) Ethical guidance on human embryonic and fetal tissue transplantation: A European overview. Med., Health Care and Philosophy , 5 , 79 –90.
Editorial ( 2002 ) Nature .
European Science Foundation ( 2001 ) Human stem cell research: scientific uncertainties and ethical dilemmas. European Science Foundation Policy Briefing , 14.
Ferrari, G., Cusella‐De Angelis, G., Coletta, M., Paolucci, E., Stornaiuolo, A., Cossu, G. and Mavilio, F. ( 1998 ) Muscle regeneration by bone marrow‐derived myogenic progenitors. Science , 279 , 1528 –1530.
Fuchs, E. and Segre, J.A. ( 2000 ) Stem cells: a new lease on life. Cell , 100 , 143 –155.
Galli, R., Borello, U., Gritti, A., Minasi, M.G., Bjornson, C., Coletta, M., Mora, M., De Angelis, M.G., Fiocco, R., Cossu, G. et al . ( 2000 ) Skeletal myogenic potential of human and mouse neural stem cells. Nat. Neurosci. , 3 , 986 –991.
Hakelien, A.M., Landsverk, H.B., Robl, J.M., Skallhegg, B.S. and Collas, P. ( 2002 ) Reprogramming fibroblasts to express T‐cell functions using cell extracts. Nat. Biotechnol. , 20 , 460 –466.
Hansen, J‐E.S. ( 2002 ) Embryonic stem cell production through therapeutic cloning has fewer ethical problems than stem cell harvest from surplus IVF embryos. J. Med. Ethics , 28 , 86 –88.
Human Fertilisation and Embryology Act ( 1990 ) HFEA, London
Hursthouse, R. ( 1987 ) Beginning lives . Blackwell, Oxford.
Jackson, K.A., Majka, S.M., Wang, H., Pocius, J., Hartley, C.J., Majesky, M.W., Entman, M.L., Michael, L.H., Hirschi, K.K. and Goodell, M.A. ( 2001 ) Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J. Clin. Invest. , 107 , 1395 –1402.
Jiang, Y., Jahagirdar, B.N., Reinhardt, R.L., Schwartz, R.E., Keene, C.D., Ortiz‐Gonzalez, X.R., Reyes, M., Lenvik, T., Lund, T., Blackstad, M. et al . ( 2002 ) Pluripotency of mesenchymal stem cells derived from adult bone marrow. Nature , 418 , 41 –49.
Kaufman, D.S., Hanson, E.T., Lewis, R.L., Auerbach, R. and Thomson, J.A. ( 2001 ) Hematopoietic colony forming cells derived human embryonic stem cells. Proc. Natl Acad. Sci. USA , 98 , 10716 –10721.
Keller, G. and Snodgrass, H.R. ( 1999 ) Human embryonic stem cells: The future is now. Nat. Med. , 5 , 151 –152.
Kiessling, A. ( 2001 ) In the stem‐cell debate, new concepts need new words. Nature , 413 , 453.
Kim, J‐H., Auerbach,J.M., Rodriguez‐Gomez, J.A., Velasco, I.Gavin, D., Lumelsky,N., Lee, S‐H., Nguyen,J., Sanchez‐Pernaute, R., Bankiewicz, K. and McKay, R. ( 2002 ) Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson′s disease. Nature , 418 , 50 ‐56
Kondziolka, D., Wechsler, L., Goldstein, S., Meltzer, C., Thulborn, K.R., Gebel, J., Jannetta, P., DeCesare, S., Elder, E.M., McGrogan, M. et al . ( 2000 ). Transplantation of cultured human neuronal cells for patients with stroke. Neurology , 55 , 565 –569.
Krause, D.S., Theise, N.D., Collector, M.I., Henegariu, O., Hwang, S., Gardner, R., Neutzel, S. and Sharkis, S.J.( 2001 ) Multi‐organ, multi‐lineage engraftment by a single bone marrow‐derived stem cell. Cell , 105 , 369 –377.
Lagasse, E., Connors, H., Al‐Dhalimy, M., Reitsma, M., Dohse, M., Osborne, L., Wang, X., Finegold, M., Weissman, I.L. and Grompe, M. ( 2000 ) Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Medicine , 6 , 1229 –1234.
Lanza, RP., Chung, H.Y., Yoo, J.J., Wettstein, P.J., Blackwell, C., Borson, N., Hofmeister, E., Schuch, G., Soker, S., Moraes, C.T. et al .( 2002 ) Generation of histocompatible tissues using nuclear transplantation. Nature Biotech ., advance online publication DOI: 10.1038/nbt703.
Lanzendorf, S.E., Boyd, C.A., Wright, D.L., Muasher, S., Oehninger, S. and Hodgen, G.D. ( 2001 ) Use of human gametes obtained from anonymous donors for the production of human embryonic stem cell lines. Fertil. Steril. , 76 , 132 –137.
McGee, G. and Caplan, A. ( 1999 ) The ethics and politics of small sacrifices in stem cell research. Kennedy Institute of Ethics J. , 9 , 151 –158.
McKay, R. ( 1997 ) Stem cells in the central nervous system. Science , 276 , 66 –71.
Mezey, E., Chandross, K.J., Harta, G., Maki, R.A. and McKercher, S.R. ( 2000 ) Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science , 290 , 1779 –1782.
Nagy, A., Rossant, J., Nagy, R., Abramow‐Newerly, W. and Roder, J.C. ( 1993 ) Derivation of completely cell culture‐derived mice from early‐passage embryonic stem cells. Proc. Natl Acad. Sci. USA , 90 , 8424 –8428.
NAS (2002) Board on Life Sci., National Research Council and Board on Neuroscience and Behavioural Health, Institute of Medicine. Stem cells and future of regenerative medicine. National Academy Press. www.nap.edu/books/0309076307/html .
Nuffield Council on Bioethics ( 2000 ). Stem Cell Therapy: the ethical issues. A discussion paper. April, 2000.
Orlic, D., Kajstura, J., Chimenti, S., Jakoniuk, I., Anderson, S.M., Li, B., Pickel, J., McKay, R., Nadal‐Ginard, B., Bodine, D.M. et al . ( 2001 ) Bone marrow cells regenerate infarcted myocardium. Nature , 401 , 701 –705.
Passier, R. and Mummery, C.L ( 2003 ) The origin and use of embryonic and adult stem cells in differentiation and tissue repair: review in Spotlight Issue on Cell Transplantation and Stem cells in the Cardiovascular system. Cardiovasc. Res. , in press.
Petersen, B.E., Bowen, W.C., Patrene, K.D., Mars ,W.M., Sullivan, A.K., Murase, N., Boggs, S.S., Greenberger, J.S. and Goff, J.P. ( 1999 ) Bone marrow as a potential source of hepatic oval cells. Science , 284 , 1168 –1170.
Pittenger, M.F., Mackay, A.M., Beck, S.C., Jaiswal, R.K., Douglas, R., Mosca, J.D., Moorman, M.A., Simonetti, D.W., Craig, S. and Marshak, D.R. ( 1999 ) Multilineage potential of adult human mesenchymal stem cells. Science , 284 , 143 –147.
Raymond, J.G. ( 1987 ) Fetalists and feminists: they are not the same. In Spallone, P. and Steinberg D.L. (eds), Made to Order: The Myth of Reproductive and Genetic Progress. Pergamon Press, Oxford, pp. 58 –65.
Reubinoff, B.E., Pera, M.F., Fong, C.Y., Trounson, A. and Bongso, A. ( 2000 ) Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nature Biotechnol. , 18 , 399 –404.
Reubinoff, B.E., Itsykson, P., Turetsky, T., Pera, M.F., Reinhartz, E., Itzik, A. and Ben‐Hur, T. ( 2001 ) Neural progenitors from human embryonic stem cells. Nature Biotechnol. , 19 , 1134 –1140.
Reyes, M. and Verfaillie, C.M. ( 2001 ) Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann. N.Y. Acad. Sci. , 938 , 231 –233.
Sanchez‐Ramos, J., Song, S., Cardozo‐Pelaez, F., Hazzi, C., Stedeford, T., Willing, A., Freeman, T.B., Saporta, S., Janssen, W., Patel, N. et al . ( 2000 ) Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. , 164 , 247 –256.
Schwartz, R.E., Reyes, M., Koodie, L., Blacksrad, M., Lund, T., Lenvik, T., Johnson, S., Hu, W‐H. and Verfaillie, C. ( 2002 ) Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte‐like cells. J. Clin. Invest. , 109 , 1291 –302.
Shamblott, M.J., Axelman, J., Wang, S., Bugg, E.M., Littlefield, J.W., Donovan, P.J., Blumenthal, P.D., Huggins, G.R. and Gearhart, J.D. ( 1998 ) Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl Acad. Sci. USA , 95 , 13726 –13731.
Shamblott, M.J., Axelman, J., Littlefield, J.W., Blumenthal, P.D., Huggins, G.R., Cui Y., Cheng, L. and Gearhart, J.D. ( 2001 ) Human embryonic germ cell derivatives express a broad range of developmentally distinct markers and proliferate extensively in vitro. Proc. Natl Acad. Sci. USA , 98 , 113 –118.
Spradling, A., Drummond‐Barbosa, D. and Kai, T. ( 2001 ) Stem cells find their niche. Nature , 414 , 98 –104.
Steghaus‐Kovac, S. ( 1999 ) Ethical loophole closing up for stem cell researchers. Science , 286 , 31.
Stem Cell Research ( 2000 ) Medical progress with responsibility. A report from the chief medical officer’s expert group reviewing the potential of developments in stem cell research and cell nuclear replacement to benefit human health . Department of Health, UK.
Stem cells show their muscle ( 2000 ). Editorial. Nature Neurosci ., 3 , 961 .
Surani, M.A. ( 2001 ) Re‐programming of genome function through epigenetic inheritance. Nature , 414 , 122 –128.
Terada, N., Hamazaki, T., Oka, M., Hoki, M., Masterlerz, D.M., Nakano, Y., Meyer, E.M., Morel, L., Petersen, B.E. and Scott, E.W. ( 2002 ) Bone marrow cell adopt the phenotype of other cells by spontaneous cell fusion. Nature , 416 , 542 –545.
Thomson, J.A., Itskovitz‐Eldor, J., Shapiro, S.S., Waknitz, M.A., Swiergiel, J.J., Marshall, V.S. and Jones, J.M. ( 1998 ) Embryonic stem cell lines derived from human blastocysts. Science , 282 , 1145 –1147.
Vastag, B. ( 2001 ) Many say adult stem cell reports overplayed. JAMA , 286 , 293 .
Vastag, B. ( 2002 ) New bioethics council offers no recommendations. JAMA , 287 , 2934 –2935.
Verfaillie, CM. ( 2002 ) Hematopoietic stem cells for transplantation. J. Nat. Immunol. , 3 , 314 –317.
Vogelstein, B., Alberts, B. and Shine, K. ( 2002 ) Please don’t call it cloning! Science , 295 , 1237.
Watt, F.M. and Hogan, B.L. ( 2000 ) Out of Eden: stem cells and their niches. Science , 287 , 1427 –1430.
Weissman, I.L. ( 2000 ) Stem cells: units of development, units of regeneration, and units in evolution. Cell , 100 , 157 –168.
Xu, C., Inokuma, M.S., Denham, J., Golds, K., Kundu ,P., Gold, J.D. and Carpenter, M.K. ( 2001 ) Feeder‐free growth of undifferentiated human embryonic stem cells. Nature Biotechnol. , 19 , 971 –974.
Ying, Q.L., Nichols, J., Evans, E.P. and Smith, A.G. ( 2002 ) Changing potency by spontaneous fusion. Nature , 416 , 545 –548.
Zhao, L.R., Duan, W.M., Reyes, M., Keene, C.D., Verfaillie, C.M. and Low, W.C. ( 2002 ). Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp. Neurol. , 174 , 11 –20.
Zhang, S.C., Wernig, M., Duncan, I.D., Brustle, O. and Thomson, J.A.( 2001 ) In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. , 19 , 1129 –1133.
| Month: | Total Views: |
|---|---|
| December 2016 | 1 |
| January 2017 | 171 |
| February 2017 | 1,500 |
| March 2017 | 1,170 |
| April 2017 | 1,084 |
| May 2017 | 750 |
| June 2017 | 467 |
| July 2017 | 597 |
| August 2017 | 598 |
| September 2017 | 1,139 |
| October 2017 | 1,645 |
| November 2017 | 2,083 |
| December 2017 | 8,778 |
| January 2018 | 8,533 |
| February 2018 | 8,951 |
| March 2018 | 10,734 |
| April 2018 | 9,553 |
| May 2018 | 8,886 |
| June 2018 | 6,825 |
| July 2018 | 5,720 |
| August 2018 | 7,373 |
| September 2018 | 8,448 |
| October 2018 | 9,047 |
| November 2018 | 10,957 |
| December 2018 | 8,946 |
| January 2019 | 7,194 |
| February 2019 | 8,164 |
| March 2019 | 9,951 |
| April 2019 | 8,540 |
| May 2019 | 7,917 |
| June 2019 | 6,011 |
| July 2019 | 6,438 |
| August 2019 | 6,926 |
| September 2019 | 6,759 |
| October 2019 | 4,764 |
| November 2019 | 3,270 |
| December 2019 | 2,229 |
| January 2020 | 1,943 |
| February 2020 | 2,477 |
| March 2020 | 2,435 |
| April 2020 | 2,431 |
| May 2020 | 1,215 |
| June 2020 | 1,334 |
| July 2020 | 979 |
| August 2020 | 1,056 |
| September 2020 | 1,558 |
| October 2020 | 1,901 |
| November 2020 | 1,846 |
| December 2020 | 975 |
| January 2021 | 1,028 |
| February 2021 | 1,051 |
| March 2021 | 1,450 |
| April 2021 | 1,301 |
| May 2021 | 1,014 |
| June 2021 | 816 |
| July 2021 | 423 |
| August 2021 | 633 |
| September 2021 | 1,097 |
| October 2021 | 1,237 |
| November 2021 | 1,358 |
| December 2021 | 917 |
| January 2022 | 854 |
| February 2022 | 904 |
| March 2022 | 1,247 |
| April 2022 | 1,141 |
| May 2022 | 902 |
| June 2022 | 567 |
| July 2022 | 372 |
| August 2022 | 462 |
| September 2022 | 823 |
| October 2022 | 884 |
| November 2022 | 1,000 |
| December 2022 | 550 |
| January 2023 | 589 |
| February 2023 | 630 |
| March 2023 | 766 |
| April 2023 | 519 |
| May 2023 | 467 |
| June 2023 | 245 |
| July 2023 | 218 |
| August 2023 | 284 |
| September 2023 | 471 |
| October 2023 | 632 |
| November 2023 | 510 |
| December 2023 | 485 |
| January 2024 | 496 |
| February 2024 | 504 |
| March 2024 | 937 |
| April 2024 | 565 |
| May 2024 | 505 |
| June 2024 | 336 |
| July 2024 | 216 |
| August 2024 | 271 |
| September 2024 | 176 |
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1460-2350
- Copyright © 2024 European Society of Human Reproduction and Embryology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

- Latest Additions
- For Cardiff Authors
- User Area Login
- How to Add Research
- Your Research Thesis
Legal and moral aspects of human embryonic stem cell research
| 2009. PhD Thesis, Cardiff University. |
| |
This thesis is concerned with two different aspects of human embryonic stem cell research: legal and moral. These are not two distinct areas: the law cannot regulate this controversial area of science without the input of morality. There is not one moral viewpoint on the use of human embryos in scientific research and as such this thesis discusses several different moral viewpoints before moving on to consider how the law takes into account these wide ranging and diverse stances. The science of human embryonic stem cell research is discussed briefly so as to ensure that the reader comprehends the science that the law is seeking to regulate and over which there is so much ethical debate. The majority of this thesis then considers the legal aspects of human embryonic stem cell research. The focus is upon the human embryo and human embryonic stem cell interface how the legislation which governs human embryo research has been used to subsequently regulate human embryonic stem cell research. The examination of the legal aspects of human embryonic stem cell research starts with a historical chapter on how the legislation came into force. This is necessary so as to understand how and why we regulate human embryonic stem cell research as we do and what the legislation does, before moving onto the finer detail. The role of research ethics committees, the HFEA and the UK Stem Cell Bank in human embryonic stem cell research are all analysed in depth, problem areas highlighted and solutions suggested. An analytical discussion of the reform process which led to the Human Fertilisation and Embryology Act 2008 is the last step in the examination of the regulation of human embryonic stem cell research. Finally comparisons are made to the State of California, USA which was the first US state to permissively fund stem cell research. The law stated is correct as of the 13th November 2008 when the Human Fertilisation and Embryology Act 2008 received Royal Assent.
| Item Type: | Thesis (PhD) |
|---|---|
| Status: | Unpublished |
| Schools: | Law |
| Subjects: | B Philosophy. Psychology. Religion > BJ Ethics K Law > K Law (General) Q Science > QH Natural history > QH426 Genetics |
| ISBN: | 9781303217869 |
| Funders: | CesaGen, Cardiff Law School, Charles Cole Travelling Scholarship |
| Date of First Compliant Deposit: | 30 March 2016 |
| Last Modified: | 25 Oct 2022 08:45 |
| URI: |
Cited times time in Google Scholar . View in Google Scholar
Cited times time in Web of Science . View in Web of Science .
Actions (repository staff only)
| Edit Item |

Downloads per month over past year
View more statistics

Human Embryonic Stem Cell Research (hESCR): How Novel Research Has Impacted on the Current Ethical and Legal Situation
There is great ethical debate regarding the complex area of human embryonic stem cell research (hESCR) because in order to access the cells, destruction of the embryo is required. Therefore many different opinions regarding the moral status of the human embryo have developed. The environment of hESCR is highly politicised and one of the few scientific fields that is prohibited in some jurisdictions. Within the EU there is a diverse legislative environment. In particular there is no specific legislation in Ireland despite both the Commission on Assisted Human Reproduction and the Irish Council for Bioethics calling for such legislation in 2005 and 2008 respectively. As a result scientists have had to develop their own policies and regulations while looking elsewhere for funding. The Irish Medical Council has had to draft guidelines for doctors highlighting the regulatory vacuum. Corporations, non-profit organisations and philanthropists have had to step into the regulatory and funding void created by governments internationally. Despite these restrictions, research in the area of stem cells is rapidly evolving and progressing especially in the past year with use of the CRISPR-Cas9 system in gene editing of human embryos. With the recent advances in human embryo culturing, the 14 day rule originally enacted in the UK's Human Fertilisation and Embryology Act in 1990 is being questioned with discussions for a possible extension past 14 days. There is a moral duty amongst the scientific and medical community to practise ethical research and this is especially true for hESCR but clear legislations and regulatory bodies are required to guide these pioneers of research through this contentious and provocative research.
First Supervisor
Published citation, degree name.
- MSc Healthcare Ethics and Law
Date of award
Usage metrics.

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 29 September 2021
The next frontier for human embryo research
- Elizabeth Svoboda 0
Elizabeth Svoboda is a science writer in San Jose, California.
You can also search for this author in PubMed Google Scholar
In a laboratory in Israel, an incubator drum spins on a bench. The two glass bottles attached to the drum contain mouse embryos, each the size of a grain of rice, with translucent, pulsing hearts.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Nature 597 , S15-S17 (2021)
doi: https://doi.org/10.1038/d41586-021-02625-0
This article is part of Nature Outlook: Stem cells , an editorially independent supplement produced with the financial support of third parties. About this content .
Aguilera-Castrejon, A. et al. Nature 593 , 119–124 (2021).
Article PubMed Google Scholar
Liu, X. et al. Nature 591 , 627–632 (2021).
Yu, L. et al. Nature 591 , 620–626 (2021).
Tan, T. et al. Cell 184 , 2020–2032 (2021).
Molè, M. A. et al. Nature Commun. 12 , 3679 (2021).
Shahbazi, M. N. et al. Nature Commun. 11 , 3987 (2020).
Download references
Related Articles

- Developmental biology
- Biotechnology

Temporal BMP4 effects on mouse embryonic and extraembryonic development
Article 18 SEP 24
Gasdermin D-mediated metabolic crosstalk promotes tissue repair
Article 11 SEP 24
Human embryo models are getting more realistic — raising ethical questions
News Feature 11 SEP 24
Two-factor authentication underpins the precision of the piRNA pathway

The brain aged more slowly in monkeys given a cheap diabetes drug
News 12 SEP 24
My identity was stolen by a predatory conference
Correspondence 17 SEP 24

Famed Pacific island’s population ‘crash’ debunked by ancient DNA
News 11 SEP 24
Artificial intelligence can help to make animal research redundant
Correspondence 10 SEP 24
Independent Researcher
Dallas, Texas (US)
The University of Texas Southwestern Medical Center (UT Southwestern Medical Center)
Head of the Translational Cancer Research Laboratory
Northwell Health has formed a strategic partnership with the NCI-designated Cancer Center at Cold Spring Harbor Laboratory (CSHL). The institutions...
Manhasset, New York
Tenure-Track Faculty Positions in Human Genetics
Director of the institute of cancer research and robert and janet perro endowed chair.
Chief Scientist
The International Center for Biosaline Agriculture(ICBA) is an international, non-profit agricultural research center established in 1999
Dubai (Emirate) (AE)
International Center for Biosaline Agriculture (ICBA)
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

- Simple Search
- Advanced Search
- Deposit an Item
- Deposit Instructions
- Instructions for Students
| Jorgensen, Victoria Lynn (2023) Dissertation (Ph.D.), California Institute of Technology. doi:10.7907/t1fe-3915. Mammalian development is a complex and highly regulated process by which a single cell, the totipotent zygote, gives rise to all lineages of the future organism. While incredible advancements have been made to study and understand the earliest events of our life, many questions are still unanswered. Moreover, the most precarious stage of development, implantation, remains a “black box” to researchers due to inaccessibility of the embryo within the uterus of the mother. In the last decade, however, the emergence of stem cell derived embryos represents an exciting alternative avenue to study these dynamic stages. During my PhD, I worked to establish two pre-implantation stem cell models, one in human and one in mouse, to better understand the earliest days of mammalian development. These models replicate the blastocyst stage of development; at this point in time the embryo is ready to implant into the uterus and contains all embryonic and extra-embryonic tissues needed to form the future organism: the epiblast, the hypoblast, and the trophectoderm. Beginning with my human model, I demonstrate the ability of a single cell type, expanded potential stem cells (EPSCs), to give rise to structures that replicate the natural blastocyst in size, morphology, and initiation of lineage segregation. Furthermore, these human blastocyst-like structures can undergo the very beginning of post-implantation remodeling by forming an epiblast rosette and initiating lumenogenesis. Nevertheless, single cell RNA-seq (scRNA-seq) analysis reveals that lineages are not fully committed in this model, perhaps explaining why development is limited in these structures up to about Day 7/8. In the context of my mouse model, I combine not one but three distinct cell types to generate blastocyst-like structures: 1) wildtype embryonic stem cells (ESCs) to form the epiblast, 2) trophoblast stem cells (TSCs) to form the trophectoderm, and 3) Gata4-inducible ESCs to form the primitive endoderm. Again, these structures mimic the natural mouse blastocyst in morphology and lineage segregation and demonstrate the ability to transition to post-implantation stages. Development of the three blastocyst lineages was further confirmed via global scRNA-seq analysis comparing our Gata4i-Blastoids to natural embryos; importantly, however, this analysis also showed that differentiation of the mural trophectoderm, the tissue responsible for uterine invasion, is lacking in our stem cell model and likely explains the inability for these blastoids to implant . Altogether, this dissertation explains key aspects of pre- to post-implantation development and highlights the incredible power of stem cell-derived embryos to self-organize into structures that closely mimic the natural embryo.
|












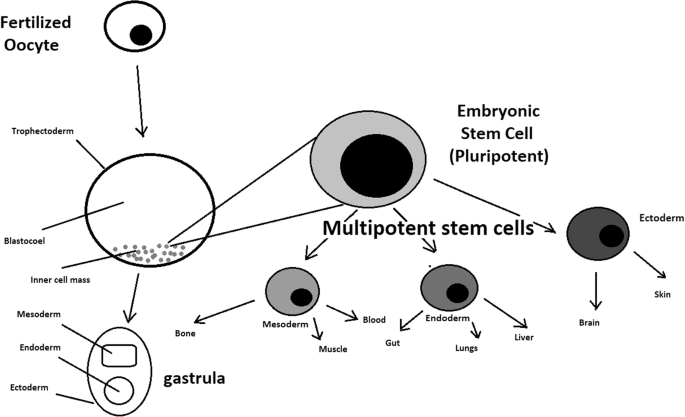
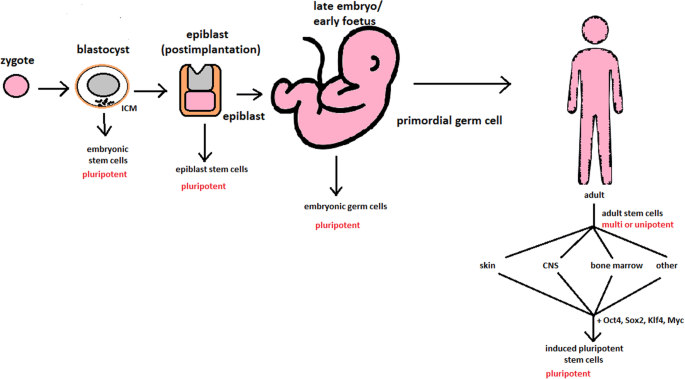
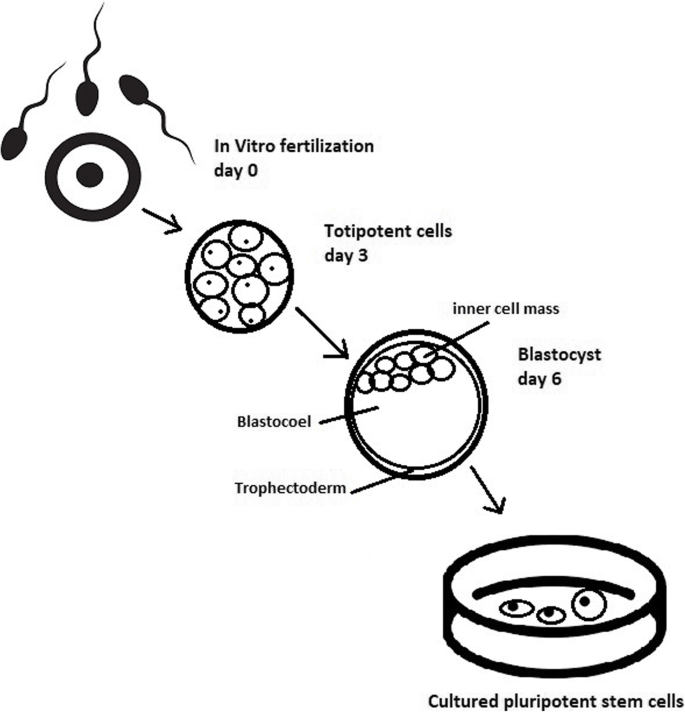
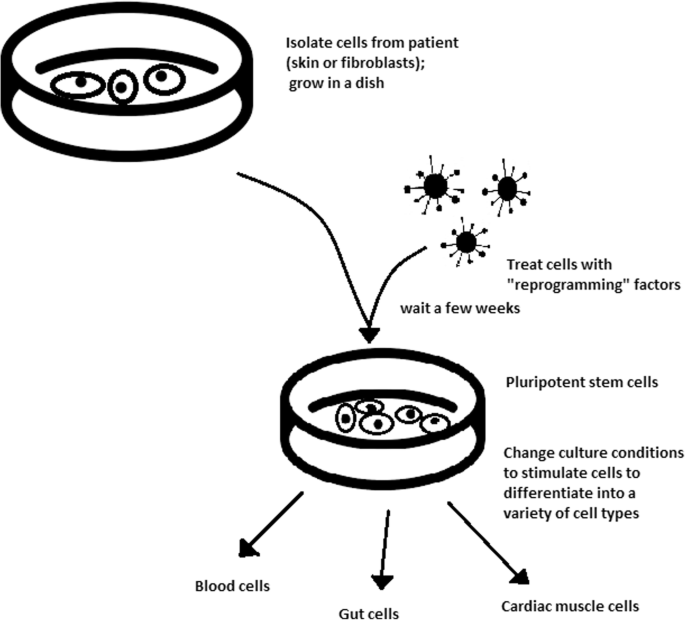
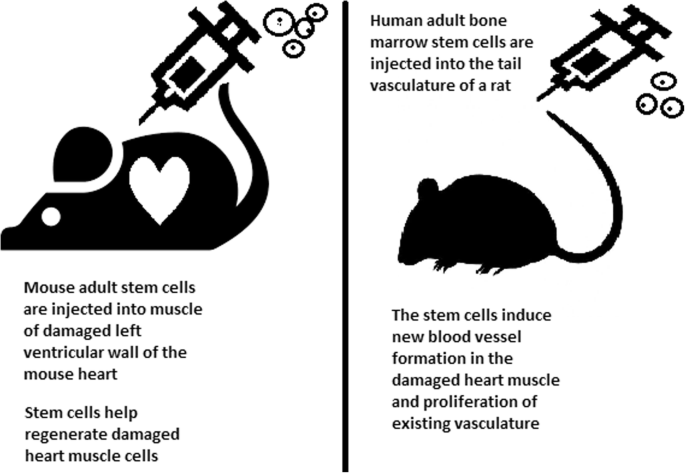
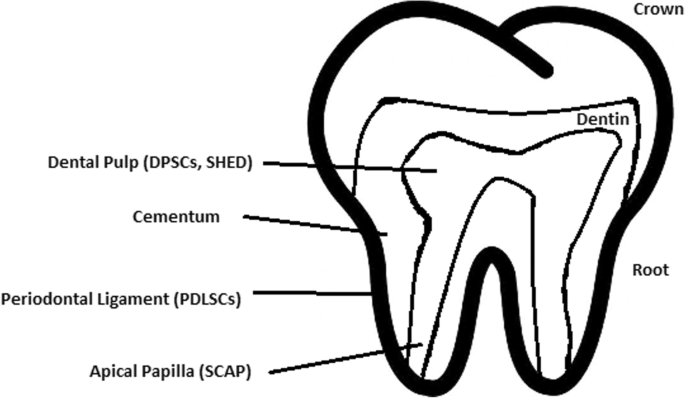
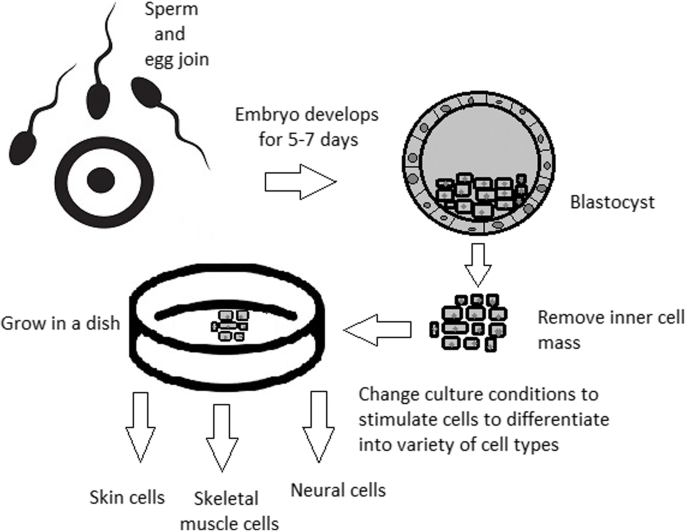




IMAGES
VIDEO
COMMENTS
Since then, research that utilizes human embryonic cells has been a widely debated, controversial ethical issue. Human embryonic cells possess the ability to become stem cells, which are used in medical research due to two significant features. First, they are unspecialized cells, meaning they can undergo cell division and renew themselves even ...
Introduction. The field of stem cell research has undergone a significant transformation with the advent of human embryonic stem cells (hESCs). Since their pioneering isolation in 1998, hESCs have been at the forefront of scientific inquiry due to their unique ability for self-renewal and pluripotency [1, 2].This comprehensive review article delves into the advancements, challenges, and ...
MS: Proponents argue that embryonic stem cell research holds great promise for understanding and curing diabetes, Parkinson's disease, spinal cord injury, and other debilitating conditions. Opponents argue that the research is unethical, because deriving the stem cells destroys the blastocyst, an unimplanted human embryo at the sixth to ...
Embryonic stem cells have immense medical potential. While both their acquisition for and use in research are fraught with controversy, arguments against their usage are rebutted by showing that embryonic stem cells are not equivalent to human lives. It is then argued that not using human embryos is unethical. Finally, an alternative to embryonic stem cells is presented.
The Ethics of Embryonic Stem Cell Research. KATRIEN DEVOLDER, 2015. Oxford, Oxford University Press 167 pp., £30 (hb) Human embryonic stem cell (hESC) research promises to enhance the way that we understand, prevent, and treat disease, potentially alleviating human suffering on a global scale. However it also involves the destruction of the ...
Sozen, B. et al. Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nat. Cell Biol. 20 , 979-989 (2018).
Abstract. The use of human embryos for research on embryonic stem (ES) cells is currently high on the ethical and political agenda in many countries. Despite the potential benefit of using human ES cells in the treatment of disease, their use remains controversial because of their derivation from early embryos.
regarding abortion are long and highly sophisticated; the proposed alternatives to. Embryonic Stem cell research as a whole hold promising results, but they have. drawbacks of their own as well, and these are part of the reason why other. countries outside of the U.S. are moving ahead in alternative forms of stem cell.
The Stem-Cell Debate At first glance, the case for federal funding of embryonic stem-cell research seems too obvious to need defending. Why should the government refuse to support research that hol...
This thesis is concerned with two different aspects of human embryonic stem cell research: legal and moral. These are not two distinct areas: the law cannot regulate this controversial area of science without the input of morality. There is not one moral viewpoint on the use of human embryos in scientific research and as such this thesis discusses several different moral viewpoints before ...
There is great ethical debate regarding the complex area of human embryonic stem cell research (hESCR) because in order to access the cells, destruction of the embryo is required. Therefore many different opinions regarding the moral status of the human embryo have developed. The environment of hESCR is highly politicised and one of the few scientific fields that is prohibited in some ...
Part of Nature Outlook: Stem cells. But ethical guidelines on human embryo use have halted most research into these phases of development — until now. This May, the International Society for ...
In the context of my mouse model, I combine not one but three distinct cell types to generate blastocyst-like structures: 1) wildtype embryonic stem cells (ESCs) to form the epiblast, 2) trophoblast stem cells (TSCs) to form the trophectoderm, and 3) Gata4-inducible ESCs to form the primitive endoderm.
Embryonic stem cells (ESCs) are found in the inner cell mass of the human blastocyst, an early stage of the developing embryo lasting from the 4th to 7th day after fertilization. In normal embryonic development, they disappear after the 7th day, and begin to form the three embryonic tissue layers. ESCs extracted from the inner cell mass during the blastocyst stage, however, can be cultured in ...
The Ethics Of Embryonic Stem Cell Research. Howard J. Curzer. Texas Tech University, Lubbock, TX, USA. In this article I rebut conservative objections to five phases of embryonic stem cell research. I argue that researchers using existing embryonic stem cell lines are not complicit in the past destruction of embryos because beneficiaries of ...
Embryonic Stem Cells (ESCs) ... In the last 50 years, more than 40,000 research papers have focused on stem-cell-based therapies. In this review study, we present a general overview of the translation of stem cell therapy from scientific ideas to clinical applications. Multiple mechanisms causing disease could be reversed by stem cells, due to ...
Human embryonic stem cells (hESCs) are cells derived from 5-day human embryos and are self-renewing cell lines that change into any type of cell in the body, a trait called pluripotency. hESCs have almost unlimited clinical and medical research potential. Despite the great therapeutic promise of hESC research, it comes with a controversial ethical debate due to its involvement with the ...
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and derivation.
Research on human embryonic stem cells, however, is controversial, given the diverse views held in our society about the moral and legal status of the early embryo. The controversy has encouraged provocative and conflicting claims both inside and outside the scientific community about the biology and biomedical potential of both adult and ...
Sydney Kossow, Creating a United Front: Harmonizing the United States Regulatory Policies Surrounding Human Embryonic Stem Cell Research, 25 SMU Sci. & Tech. L. Rev. 295 (2022) Stem cell therapy is an imperative development in science and medicine that is heavily regulated worldwide. With the potential to cure illnesses, help understand disease ...
Human embryonic stem (hES) cells are unique in their demonstrated potential to differentiate into all cell lineages. Reports by T. Wakayama et al. ("Differentiation of embryonic stem cell lines generated from adult somatic cells by nuclear transfer," 27 Apr., p. 740) and N. Lumelsky et al. ("Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic ...
While access to adult stem cells has certainly helped answer some questions in developmental biology, embryonic stem cell studies, in my opinion, truly transformed the research area. Just like the newborn that the embryo eventually forms into, these early-stage cells have the potential to choose any path of maturation.
The Executive Order states that the Secretary of Health and Human Services, through the Director of NIH, may support and conduct responsible, scientifically worthy human stem cell research, including human embryonic stem cell (hESC) research, to the extent permitted by law. These Guidelines implement Executive Order 13505, as it pertains to ...