- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

U.S. Food and Drug Administration
- Search
- Menu
- News & Events
- FDA Newsroom
- Press Announcements
FDA Approves First Cellular Therapy to Treat Patients with Type 1 Diabetes
FDA News Release
Today, the U.S. Food and Drug Administration approved Lantidra, the first allogeneic (donor) pancreatic islet cellular therapy made from deceased donor pancreatic cells for the treatment of type 1 diabetes. Lantidra is approved for the treatment of adults with type 1 diabetes who are unable to approach target glycated hemoglobin (average blood glucose levels) because of current repeated episodes of severe hypoglycemia (low blood sugar) despite intensive diabetes management and education.
“Severe hypoglycemia is a dangerous condition that can lead to injuries resulting from loss of consciousness or seizures,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “Today’s approval, the first-ever cell therapy to treat patients with type 1 diabetes, provides individuals living with type 1 diabetes and recurrent severe hypoglycemia an additional treatment option to help achieve target blood glucose levels.”
Type 1 diabetes is a chronic autoimmune disease that requires lifelong care including requiring insulin, either through multiple daily injections or continuous infusion using a pump, every day to live. People with type 1 diabetes also perform blood glucose checks several times a day to guide the management of their diabetes.
Some people with type 1 diabetes have trouble managing the amount of insulin needed every day to prevent hyperglycemia (high blood sugar) without causing hypoglycemia. They may also develop hypoglycemia unawareness, where they are unable to detect their blood glucose is dropping and may not have a chance to treat themselves to prevent their blood glucose from further dropping. This makes it difficult to dose insulin. Lantidra provides a potential treatment option for these patients.
The primary mechanism of action of Lantidra is believed to be the secretion of insulin by the infused allogeneic islet beta cells. In some patients with type 1 diabetes, these infused cells can produce enough insulin, so the patient no longer needs to take insulin (by injections or pump) to control their blood sugar levels. Lantidra is administered as a single infusion into the hepatic (liver) portal vein. An additional infusion of Lantidra may be performed depending on the patient’s response to the initial dose.
The safety and effectiveness of Lantidra was evaluated in two non-randomized, single-arm studies in which a total of 30 participants with type 1 diabetes and hypoglycemic unawareness received at least one infusion and a maximum of three infusions. Overall, 21 participants did not need to take insulin for a year or more, with 11 participants not needing insulin for one to five years and 10 participants not needing insulin for more than five years. Five participants did not achieve any days of insulin independence.
Adverse reactions associated with Lantidra varied with each participant depending on the number of infusions they received and the length of time they were followed and may not reflect the rates observed in practice The most common adverse reactions included nausea, fatigue, anemia, diarrhea and abdominal pain. A majority of participants experienced at least one serious adverse reaction related to the procedure for infusing Lantidra into the hepatic portal vein and the use of immunosuppressive medications needed to maintain the islet cell viability. Some serious adverse reactions required discontinuation of immunosuppressive medications, which resulted in the loss of islet cell function and insulin independence. These adverse events should be considered when assessing the benefits and risks of Lantidra for each patient. Lantidra is approved with patient-directed labeling to inform patients with type 1 diabetes about benefits and risks of Lantidra.
The FDA granted approval of Lantidra to CellTrans Inc.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
June 25, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
Stem cell therapy could be breakthrough against type 1 diabetes
by Dennis Thompson

An experimental stem cell therapy can essentially cure type 1 diabetes by restoring insulin production in some patients, early clinical trial results show.
Seven out of 12 patients no longer needed daily insulin shots after receiving a full dose of the gene therapy, dubbed VX-800, researchers reported Friday at the American Diabetes Association's annual meeting in Orlando, FL.
Another two needed about 70% less insulin daily to keep their blood sugar stable, results show.
"This positive data adds to the growing body of evidence for VX-880's potential to revolutionize the treatment of type 1 diabetes," said researcher Dr. Piotr Witkowski, director of the pancreatic and islet transplant program at the University of Chicago.
People with type 1 diabetes aren't able to produce enough insulin to keep blood sugar levels stable. Type 1 diabetes occurs when the immune system mistakenly targets and attacks the islet cells in the pancreas that generate insulin.
VX-880 works by introducing fresh islet cells that have been derived from stem cells , with the aim that those cells will restore pancreatic function.
For the early-stage clinical trial, researchers recruited 12 people with poorly controlled type 1 diabetics. All had average hemoglobin A1C levels of 7.8%, a level at which there's an increased risk of complications from diabetes. They'd also experienced severe hypoglycemia two to four times in the prior year, and used about 40 units of insulin a day to try and stabilize their blood sugar.
A single infusion of VX-880 eliminated severe hypoglycemic events in all 12 patients and drove their hemoglobin A1C levels below 7%, results show.
The findings indicate that the VX-880 stem cell-derived islet cells function like a person's own islet cells, researchers concluded. The trial has been expanded to enroll a total of 37 participants, researchers said.
"We hope to see this treatment become a pivotal development in type 1 diabetes care," Witkowski said in a meeting news release.
Still, because these findings were presented at a medical meeting, they should be considered preliminary until published in a peer-reviewed journal.
© 2024 HealthDay . All rights reserved.
Explore further
Feedback to editors

Air pollution linked to higher risk of infertility in men
10 hours ago

Insulin and metformin combo aids diabetic foot ulcer healing, new study finds

Researchers advocate for tissue-engineering approach for arthritis relief
11 hours ago

Breaking the link between obesity and atrial fibrillation with a new cellular target
12 hours ago

Researchers reveal key LAG3 mechanisms that could enhance cancer immunotherapy

Banning friendships can backfire: Moms who 'meddle' make bad behavior worse

Heavy metal cadmium may be tied to memory issues for some

New study shows that 'super spikes' can increase track running speed by 2%

Keratin gene study pinpoints mutations associated with 'spindle hair'
13 hours ago

Study solves testosterone's paradoxical effects in prostate cancer
Related stories.

Inhaled insulin could help people with type 1 diabetes avoid needles
Jun 24, 2024

Biomimetic artificial islet model: A new way to control high blood sugar
May 28, 2024

Pancreas cells in people who have died show significant signs of stress
Apr 2, 2024
New process produces purer, safer pancreas stem cells for potential transplant
Nov 6, 2023
Clinical VX-880 trial improves blood sugar control in all treated patients, with three achieving insulin independence
Oct 3, 2023
Transplanting pancreatic islet cells under the skin to treat diabetes
Sep 8, 2020
Recommended for you

Beta cells alone can regulate blood sugar levels, study finds
Sep 3, 2024

No link found between popular diabetes medication and suicide

Mankai plant found to reduce post-meal sugar levels in diabetics
Aug 29, 2024

Certain diabetes drugs may reduce risk of dementia, Korean study reveals
Aug 28, 2024

New diabetes research links blood glucose levels and voice pitch
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.
New AI tool can diagnose cancer, guide treatment, predict patient survival
Blood test can warn women of risk decades before heart attack, stroke

Weight-loss drug linked to fewer COVID deaths

“When my son was diagnosed [with Type 1], I knew nothing about diabetes. I changed my research focus, thinking, as any parent would, ‘What am I going to do about this?’” says Douglas Melton.
Kris Snibbe/Harvard Staff Photographer
Breakthrough within reach for diabetes scientist and patients nearest to his heart
Harvard Correspondent
100 years after discovery of insulin, replacement therapy represents ‘a new kind of medicine,’ says Stem Cell Institute co-director Douglas Melton, whose children inspired his research
When Vertex Pharmaceuticals announced last month that its investigational stem-cell-derived replacement therapy was, in conjunction with immunosuppressive therapy, helping the first patient in a Phase 1/2 clinical trial robustly reproduce his or her own fully differentiated pancreatic islet cells, the cells that produce insulin, the news was hailed as a potential breakthrough for the treatment of Type 1 diabetes. For Harvard Stem Cell Institute Co-Director and Xander University Professor Douglas Melton, whose lab pioneered the science behind the therapy, the trial marked the most recent turning point in a decades-long effort to understand and treat the disease. In a conversation with the Gazette, Melton discussed the science behind the advance, the challenges ahead, and the personal side of his research. The interview was edited for clarity and length.
Douglas Melton
GAZETTE: What is the significance of the Vertex trial?
MELTON: The first major change in the treatment of Type 1 diabetes was probably the discovery of insulin in 1920. Now it’s 100 years later and if this works, it’s going to change the medical treatment for people with diabetes. Instead of injecting insulin, patients will get cells that will be their own insulin factories. It’s a new kind of medicine.
GAZETTE: Would you walk us through the approach?
MELTON: Nearly two decades ago we had the idea that we could use embryonic stem cells to make functional pancreatic islets for diabetics. When we first started, we had to try to figure out how the islets in a person’s pancreas replenished. Blood, for example, is replenished routinely by a blood stem cell. So, if you go give blood at a blood drive, your body makes more blood. But we showed in mice that that is not true for the pancreatic islets. Once they’re removed or killed, the adult body has no capacity to make new ones.
So the first important “a-ha” moment was to demonstrate that there was no capacity in an adult to make new islets. That moved us to another source of new material: stem cells. The next important thing, after we overcame the political issues surrounding the use of embryonic stem cells, was to ask: Can we direct the differentiation of stem cells and make them become beta cells? That problem took much longer than I expected — I told my wife it would take five years, but it took closer to 15. The project benefited enormously from undergraduates, graduate students, and postdocs. None of them were here for 15 years of course, but they all worked on different steps.
GAZETTE: What role did the Harvard Stem Cell Institute play?
MELTON: This work absolutely could not have been done using conventional support from the National Institutes of Health. First of all, NIH grants came with severe restrictions and secondly, a long-term project like this doesn’t easily map to the initial grant support they give for a one- to three-year project. I am forever grateful and feel fortunate to have been at a private institution where philanthropy, through the HSCI, wasn’t just helpful, it made all the difference.
I am exceptionally grateful as well to former Harvard President Larry Summers and Steve Hyman, director of the Stanley Center for Psychiatric Research at the Broad Institute, who supported the creation of the HSCI, which was formed specifically with the idea to explore the potential of pluripotency stem cells for discovering questions about how development works, how cells are made in our body, and hopefully for finding new treatments or cures for disease. This may be one of the first examples where it’s come to fruition. At the time, the use of embryonic stem cells was quite controversial, and Steve and Larry said that this was precisely the kind of science they wanted to support.
GAZETTE: You were fundamental in starting the Department of Stem Cell and Regenerative Biology. Can you tell us about that?
MELTON: David Scadden and I helped start the department, which lives in two Schools: Harvard Medical School and the Faculty of Arts and Science. This speaks to the unusual formation and intention of the department. I’ve talked a lot about diabetes and islets, but think about all the other tissues and diseases that people suffer from. There are faculty and students in the department working on the heart, nerves, muscle, brain, and other tissues — on all aspects of how the development of a cell and a tissue affects who we are and the course of disease. The department is an exciting one because it’s exploring experimental questions such as: How do you regenerate a limb? The department was founded with the idea that not only should you ask and answer questions about nature, but that one can do so with the intention that the results lead to new treatments for disease. It is a kind of applied biology department.
GAZETTE: This pancreatic islet work was patented by Harvard and then licensed to your biotech company, Semma, which was acquired by Vertex. Can you explain how this reflects your personal connection to the research?
MELTON: Semma is named for my two children, Sam and Emma. Both are now adults, and both have Type 1 diabetes. My son was 6 months old when he was diagnosed. And that’s when I changed my research plan. And my daughter, who’s four years older than my son, became diabetic about 10 years later, when she was 14.
When my son was diagnosed, I knew nothing about diabetes and had been working on how frogs develop. I changed my research focus, thinking, as any parent would, “What am I going to do about this?” Again, I come back to the flexibility of Harvard. Nobody said, “Why are you changing your research plan?”
GAZETTE: What’s next?
MELTON: The stem-cell-derived replacement therapy cells that have been put into this first patient were provided with a class of drugs called immunosuppressants, which depress the patient’s immune system. They have to do this because these cells were not taken from that patient, and so they are not recognized as “self.” Without immunosuppressants, they would be rejected. We want to find a way to make cells by genetic engineering that are not recognized as foreign.
I think this is a solvable problem. Why? When a woman has a baby, that baby has two sets of genes. It has genes from the egg, from the mother, which would be recognized as “self,” but it also has genes from the father, which would be “non-self.” Why does the mother’s body not reject the fetus? If we can figure that out, it will help inform our thinking about what genes to change in our stem cell-derived islets so that they could go into any person. This would be relevant not just to diabetes, but to any cells you wanted to transplant for liver or even heart transplants. It could mean no longer having to worry about immunosuppression.
Share this article
You might like.
Model uses features of a tumor’s microenvironment across 19 different cancer types
Findings support universal screening of three biomarkers, not just cholesterol

Large-scale study finds Wegovy reduces risk of heart attack, stroke
You want to be boss. You probably won’t be good at it.
Study pinpoints two measures that predict effective managers
Your kid can’t name three branches of government? He’s not alone.
Efforts launched to turn around plummeting student scores in U.S. history, civics, amid declining citizen engagement across nation
Good genes are nice, but joy is better
Harvard study, almost 80 years old, has proved that embracing community helps us live longer, and be happier
- Overview & Types
- Symptoms & Diagnosis
- Understanding Blood Sugar
- Diet & Exercise
- Better Living
- Complications
- Related Conditions
- Symptoms & Causes
- Diagnosis & Tests
- Prevention & Treatment
- Living & Managing
- Complications & Related Diseases
- Treating & Managing
- Complications & Related Conditions
- Gestational Diabetes
- Appointment Prep
- View Full Guide
Stem Cell Therapy Implant Shows Promise For Type 1 Diabetes
Dec. 11, 2023 – An experimental device containing millions of stem cells significantly reduced the need for insulin shots among people with type 1 diabetes , according to a new study – a treatment researchers say may someday provide a cure for the chronic, life-altering condition.
Researchers from the University of British Columbia and Vancouver Coastal Health used tiny implants filled with lab-grown pancreatic cells known as VC-02.
The study, published in the journal Nature Biotechnology , involved 10 people who at the start of the study could not produce insulin naturally. After 6 months with the implant, three of them showed significant improvement. Their bodies spent more time within the normal blood sugar range, reducing their need for external insulin.
“The hope is to get these cells strong enough to help stop requiring insulin injections all together,” said David Thompson, MD, principal investigator at the Vancouver trial site and clinical director of the Vancouver General Hospital Diabetes Centre. “I believe this is going to turn into a cure as soon as 2024.”
Type 1 diabetes is a condition in which the immune system destroys insulin-making cells in the pancreas, known as beta cells. Insulin is a hormone that regulates sugar in the blood. The condition – sometimes called juvenile diabetes – is most commonly diagnosed between the ages of 4 and 6 and in early puberty.
In the United States, people who are non-Hispanic and White are most likely to have type 1 diabetes, and it affects men and women at about the same rates. Having a close family member with the illness increases risk. About 1.24 million people in the United States live with type 1 diabetes; that number is expected to reach 5 million by 2050.
With type 1 diabetes, it's as if the body's insulin factory has shut down. People who have the disease need to take insulin from the start.
This differs from type 2 diabetes , in which the body doesn't use insulin properly. It can be managed with lifestyle changes, medications, and sometimes external insulin shots.
Until a century ago – when insulin was discovered – diabetes was a death sentence . A 14-year-old boy who lay dying from diabetes in a Toronto hospital was the first person to receive the new treatment in 1922. Within 24 hours, his high blood glucose levels dropped to near-normal levels.
“Insulin therapy for people with type 1 diabetes is better than it has ever been, but it's still not a cure,” Thompson said. “This is probably the first wave of a new era of medicine using cell therapy.”
The trial tested an experimental cell therapy developed by biotechnology company ViaCyte.
Thompson and his colleagues used devices implanted just beneath the skin, about the size of a small bandage. Unlike a glucose monitor – which is also inserted beneath the skin but only estimates blood glucose levels – the stem cell device delivers a steady supply of insulin to the body.
The trial builds off of a 2021 study that showed this approach could help the human body produce insulin. The latest study increased the number of devices for each person and improved the design to help the lab-grown cells survive.
All the people in the study started out with no insulin production and had surgery at sites in Vancouver, Belgium, and the U.S. to get up to 10 device implants each. After 6 months, three of them showed clear signs of insulin production that stayed steady throughout the yearlong study. One person in the study had showed notable improvement, spending more time in the target blood sugar range and reducing their need for extra daily insulin by 44%.
“Each device is like a miniature insulin-producing factory,” said co-author Timothy Kieffer, PhD, a professor with the departments of surgery and cellular and physiological sciences at the University of British Columbia, and past chief scientific officer of ViaCyte. The cells are “packaged into the device to essentially re-create the blood sugar-regulating functions of a healthy pancreas.”
A cure for type 1 diabetes would also mean preventing several other health complications related to the illness: blindness, kidney problems, limb loss, and even life-threatening blood sugar drops during sleep. Diabetes also significantly heightens the chances of having a heart attack or stroke.
The trial has two big limitations, said Robert Gabbay, MD, chief scientific and medical officer of the American Diabetes Association, who was not involved in the trial. Not only is it small, but the technology failed to normalize blood glucose levels, which is the goal.
But it shows promise, he said. Cell replacement therapies have previously faced a major barrier: The immune system attacks the implanted cells, requiring potentially harmful immunosuppressive drugs.
“This is particularly problematic for people with type I diabetes since the initial cause of type 1 is an autoimmune destruction of beta cells,” Gabbay said. “Placing beta cells sequestered from the immune system has been something that a number of investigative teams have worked on. This early study shows some proof of concept.”

Top doctors in ,
Find more top doctors on, related links.
- Diabetes Health Center Reference
- Diabetes Health Center Slideshows
- Diabetes Health Center Quizzes
- Diabetes Blogs
- Diabetes Health Center Videos
- Diabetes Health Center Medications
- Find an Endocrinologist
- Book: Take Control of Your Diabetes Risk
- General Diabetes
- Type 1 Diabetes
- Type 2 Diabetes
- Prediabetes
- Blood Sugar Control
- Diabetic Neuropathy
- Eye Problems Assessment
- Heart Disease
- Metabolic Syndrome
- Diabetes Overview
- Diabetes Symptoms
- Diabetes Causes
- Diabetes Diagnosis
- Diabetes Treatment

- Claim credits
- Plan your learning
- Browse journals and articles
- View sessions recordings
- Renew membership
- Submit Your Science!
- Find other members
- Become a member leader
- Get published
- Earn recognition
- Develop your career
- Promote your work
Stem-cell based therapy shows promise in treating high-risk type 1 diabetes
About Endocrine Society
Endocrinologists are at the core of solving the most pressing health problems of our time, from diabetes and obesity to infertility, bone health, and hormone-related cancers. The Endocrine Society is the world’s oldest and largest organization of scientists devoted to hormone research and physicians who care for people with hormone-related conditions.
The Society has more than 18,000 members, including scientists, physicians, educators, nurses, and students in 122 countries. To learn more about the Society and the field of endocrinology, visit our site at www.endocrine.org . Follow us on X (formerly Twitter) at @TheEndoSociety and @EndoMedia .
Colleen Williams Senior Communications Manager, Public Relations Phone: (202)-971-3611 [email protected]
Jenni Glenn Gingery Director, Communications and Media Relations Phone: (202)-971-3655 [email protected]
All News & Advocacy

Endocrine News Podcast

The Endocrine News podcast brings you the latest research and clinical advances from experts in the field, whether you are in your car, office, or out for a run.
Endocrine Society Journals
Our top-ranked peer-reviewed journals are among the first to publish major developments and discovery milestones.
For 100 years, the Endocrine Society has been at the forefront of hormone science and public health. Read about our history and how we continue to serve the endocrine community.
ViaCyte and CRISPR Introduce New Stem Cell Therapy for Type 1 Diabetes

In a collaboration between ViaCyte and CRISPR Therapeutics, a new clinical trial is investigating a stem cell therapy that may eventually help millions with type 1 diabetes .
Researchers have long sought a cell-based “cure” for type 1 diabetes, and in recent years, this goal has seemed more attainable. Since the condition results from the body’s own immune system destroying the insulin-producing beta cells in the islets of the pancreas, research has focused on finding a way to replace these non-functional cells with new and functional beta cells.
There are now two main methods for generating new and functional beta cells. People can undergo a surgical procedure to transplant healthy insulin-making cells (called beta cells) from a human donor into a person with type 1. Alternatively, researchers have now discovered ways to generate beta cells from stem cells , or cells that have not yet matured into one of the many types of cells in the human body. In 2021, Vertex performed the first successful beta cell transplant in a person with type 1 with functioning beta cells created from stem cells. In that same year, ViaCyte published the first peer-reviewed studies showing that its own stem cell therapy was successful in producing insulin in people with type 1 diabetes.
The drawback to these procedures, however, is that they require recipients to take medicine to suppress their immune system on a daily basis in order to stop the immune system from attacking the implanted cells. While both methods could be considered functional cures for type 1 diabetes, the need for permanent immunosuppressants is a limitation.
However, a new cell therapy developed at ViaCyte , in collaboration with CRISPR Therapeutics , may answer the call.
CRISPR gene-editing technology, often referred to simply as “CRISPR,” is a Nobel Prize-winning technology that allows researchers to alter the genetic code (or DNA) of a cell with extreme precision. CRISPR is one of the most common methods used to create genetically modified organisms, agricultural products, and certain medications.
With current stem cell treatments (like the cell therapy used in Vertex’s current clinical trial), when a person with type 1 receives a beta cell transplant, the implanted cells have their own unique immune “signature” in their genetic code, which is different from that of the recipient. This is why the body’s immune system thinks of the cells as foreign and dangerous, so it will target them for destruction in the same way that a person’s own beta cells are targeted for destruction when they have type 1.
By altering the genetic code of these implanted cells using the CRISPR technology, however, researchers may be able to create beta cells that avoid all recognition by the immune system, creating cells that might evade the attack from the immune system completely.
“This new product could provide a functional cure for type 1 diabetes,” said Dr. Howard Foyt, chief medical officer at ViaCyte. “By implanting these cells in the individual, the hope is that people can throw away their glucometers and insulin syringes, because these cells could provide all the insulin they need.”
According to ViaCyte , pre-clinical studies (which are done in test tubes or in animals, prior to being tested in humans) showed that these beta cell implants were well-tolerated, and had minimal side effects.
This therapy (called PEC-QT ) places genetically modified beta cells (that will hopefully be invisible to the recipient's immune system) into a pouch that is then implanted into the body. Blood vessels should be able to grow and penetrate the pouch coming into direct contact with the cells and giving them all the nutrients and oxygen they need.
“The advantage of having these cells in the pouch is that we can remove them all at once, unlike a typical islet cell transplant or cell infusion,” Foyt said. “So being designed for safety is a big advantage with this procedure. If someone does have an immune reaction, we can remove the cells.”
There are still several questions that need to be answered about these therapies, one of which is how often the pouch needs to be replaced.
“We refer to this as a potential ‘functional’ cure for type 1 diabetes because the device is not permanent; we know it will need to be replaced after a certain time,” Foyt said. “To an extent, because cells directly contact the blood, the pouch is akin to islet cell transplantation. If so, we could potentially see a lifespan of potentially five years, similar to islet transplants, and possibly as long as 10 years, but this question remains unanswered.”
Another therapy (called PEC-Encap ), now in Phase 2 clinical trials , places the modified beta cells into a pouch that blood vessels and immune cells cannot penetrate (a method called encapsulation), but that allows nutrients such as oxygen, glucose and other hormones to pass through the walls of the pouch.
While it will be several years before PEC-QT and other beta cell therapies hopefully become available for people with type 1 diabetes, the beginning of these clinical trials is another step toward the goal of finding a cure for type 1 diabetes.
On Feb. 2, ViaCyte and CRISPR Therapeutics announced that the first participant in the trial had received the PEC-QT implant. This marks the first gene-edited, stem cell-derived pancreatic cells to be implanted in a human that are specifically designed to evade the immune system in treating type 1 diabetes.
All in all, Foyt is optimistic that this therapy could eventually be used by a large portion of the type 1 population, if not people with insulin-requiring type 2 diabetes as well.
“This is the power of stem cells,” he said. “We have the capacity to create a seemingly unlimited supply of gene-edited stem cells to be used in developing this functional cure for type 1 diabetes.”
For more about the latest in the search for type 1 cures, stem cell therapy, or efforts to tackle immunosuppression, check out these articles:
- Hope Stems from Vertex’s New Therapy
- Type 1 Cures: A Sneak Peek into the Lab
- Type 1 Diabetes Research 2021: Science, Hope and Clinical Reality
- Open access
- Published: 08 July 2020
Current progress in stem cell therapy for type 1 diabetes mellitus
- Shuai Chen 1 ,
- Kechen Du 1 &
- Chunlin Zou ORCID: orcid.org/0000-0002-3308-5544 1
Stem Cell Research & Therapy volume 11 , Article number: 275 ( 2020 ) Cite this article
63k Accesses
81 Citations
28 Altmetric
Metrics details
Type 1 diabetes mellitus (T1DM) is the most common chronic autoimmune disease in young patients and is characterized by the loss of pancreatic β cells; as a result, the body becomes insulin deficient and hyperglycemic. Administration or injection of exogenous insulin cannot mimic the endogenous insulin secreted by a healthy pancreas. Pancreas and islet transplantation have emerged as promising treatments for reconstructing the normal regulation of blood glucose in T1DM patients. However, a critical shortage of pancreases and islets derived from human organ donors, complications associated with transplantations, high cost, and limited procedural availability remain bottlenecks in the widespread application of these strategies. Attempts have been directed to accommodate the increasing population of patients with T1DM. Stem cell therapy holds great potential for curing patients with T1DM. With the advent of research on stem cell therapy for various diseases, breakthroughs in stem cell-based therapy for T1DM have been reported. However, many unsolved issues need to be addressed before stem cell therapy will be clinically feasible for diabetic patients. In this review, we discuss the current research advances in strategies to obtain insulin-producing cells (IPCs) from different precursor cells and in stem cell-based therapies for diabetes.
Introduction
Diabetes mellitus (DM) is a group of chronic metabolic disorders characterized by hyperglycemia due to insufficient secretion of insulin or insulin resistance. DM is mainly divided into four categories: type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), gestational diabetes, and monogenic diabetes. Patients with T1DM need daily insulin injections because of the absolute insufficiency of endogenous insulin caused by autoimmune destruction of pancreatic β cells. Thus, type 1 diabetes is also known as insulin-dependent DM. Patients with type 2 diabetes may need exogenous insulin injections when oral medications cannot properly control the blood glucose levels. Diabetes without proper treatment can cause many complications. Acute complications include hypoglycemia, diabetic ketoacidosis, or hyperosmolar nonketotic coma (HHNC). Long-term complications include cardiovascular disease, diabetic nephropathy, and diabetic retinopathy [ 1 ]. Although hyperglycemia can be ameliorated by drugs or exogenous insulin administration, these treatments cannot provide physiological regulation of blood glucose. Therefore, the ideal treatment for diabetes should restore both insulin production and insulin secretion regulation by glucose in patients (Fig. 1 ).
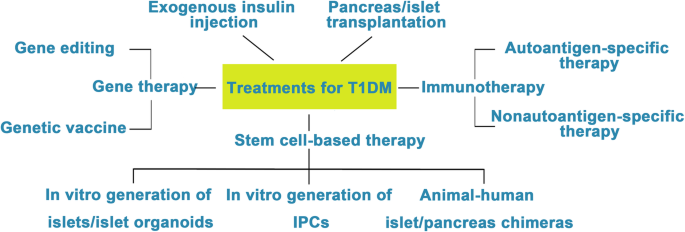
Attempts to cure T1DM. The discovery of insulin has enhanced the life span of T1DM patients, and successes in islet/pancreas transplantation have provided direct evidence for the feasibility of reestablishing β cells in vivo to treat T1DM. However, the restriction of a pancreas shortage has driven scientists to generate IPCs, and even whole pancreas, in vitro from hESCs, iPSCs, and adult stem cells. Studies focusing on the immune mechanism of T/B cell destruction in T1DM have made breakthroughs. Gene therapy has shown great promise as a potential therapeutic to treat T1DM, although its safety still needs to be confirmed in humans
Clinical pancreas or islet transplantation has been considered a feasible treatment option for T1DM patients with poor glycemic control. Dr. Richard Lillehei performed the first pancreas transplantation in 1966 [ 2 ]. Up until 2015, more than 50,000 patients (> 29,000 in the USA and > 19,000 elsewhere) worldwide had received pancreas transplantations according to the International Pancreas Transplant Registry (IPTR) [ 3 ]. Islet cell transplantation was first performed in 1974. However, efforts toward routine islet cell transplantation as a means for reversing type 1 diabetes have been hampered by limited islet availability and immune rejection. In 2000, Shapiro et al. reported that seven consecutive patients with type 1 diabetes attained sustained insulin independence after treatment with glucocorticoid-free immunosuppression combined with the infusion of adequate islet mass. Moreover, tight glycemic control and correction of glycated hemoglobin levels were observed in all seven patients. This treatment became known as the Edmonton protocol [ 4 ]. Over the past two decades, continuous improvements in islet isolation and immunosuppression have increased the efficiency of pancreatic islet transplant, and approximately 60% of patients with T1DM have achieved insulin independence 5 years after islet transplantation [ 3 , 5 , 6 , 7 , 8 ].
However, the worldwide shortage of pancreas donors in clinical islet transplantation remains a major challenge. Intensive studies have been conducted for the generation of IPCs or islet organoids in vitro since human pluripotent stem cells (hPSCs) have been anticipated for application in regenerative medicine. The sources for the generation of IPCs or islet organoids in vitro mainly include hPSCs (human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs)), adult stem cells, and differentiated cells from mature tissues that can be transdifferentiated into IPCs. Current strategies for generating IPCs are mainly based on approaches that mimic normal pancreas development. The obtained IPCs are supposed to express specific biological markers of normal β cells that identify a terminal differentiation status, such as MAFA (a basic leucine zipper transcription factor expressed in mature β cells and absent in pancreatic progenitors and other cell types), NEUROD1 (downstream factor of NGN3 expressed in most pancreatic endocrine cells, including β cells), and PDX1/NKX 6.1 (restricted coexpression in β cells), as well as key functional features of adult β cells, including glucose-stimulated insulin secretion (GSIS) and C-peptide secretion [ 9 , 10 , 11 , 12 , 13 , 14 ]. In addition, after implantation into DM patients or immunodeficient diabetic animals, these in vitro-generated IPCs or islet organoids should respond to changing blood glucose and produce sufficient insulin and finally reverse hyperglycemia.
In the last two decades, many protocols have been successfully designed for the generation of IPCs or islet organoids in vitro. In this review, we summarized the research progress in the generation of IPCs and islet organoids from hPSCs and adult stem cells and the new technological advances in stem cell-based therapy for T1DM.
Generating IPCs from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs)
ESCs are pluripotent cells isolated from the inner cell mass of a blastocyst, the early mammalian embryo that implants into the uterus. ESCs show the characteristics of infinite proliferative capacity and self-renewal and are able to differentiate into multiple types of adult cells in vitro [ 15 ]. iPSCs, which are reprogrammed from somatic cells, hold a similar capacity to proliferate and differentiate like ESCs. Hence, hPSCs provide a promising platform to produce in vitro insulin-secreting cells. Ethical issues in the applications of ESCs are still controversial due to their origins. In contrast, iPSCs are derived from adult somatic cells that have been reprogrammed back into an embryonic-like pluripotent state using Yamanaka factors [ 16 , 17 ]. During the last two decades, numerous methods to generate IPCs from hPSCs have been reported [ 9 , 10 , 11 , 12 , 18 , 19 , 20 , 21 , 22 ].
Ordinarily, the schemes for the generation of functional IPCs from hPSCs were based on imitating the in vivo development of the embryonic pancreas (Fig. 2 ). The pivotal stages of embryonic pancreas development include the development of the definitive endoderm (DE), primitive gut tube (PGT), pancreatic progenitor (PP), endocrine progenitor (EP), and hormone-expressing endocrine cells. By adding diverse cytokines (e.g., epidermal growth factor, bFGF) and signaling modulators (e.g., bone morphogenetic proteins, γ-secretase inhibitors) to each stage to activate or inhibit specific signaling pathways (e.g., Notch, Wnt) involved in the generation of adult β cells, the hPSC cell fate is manipulated into the β cell phenotype [ 18 , 20 , 23 ].
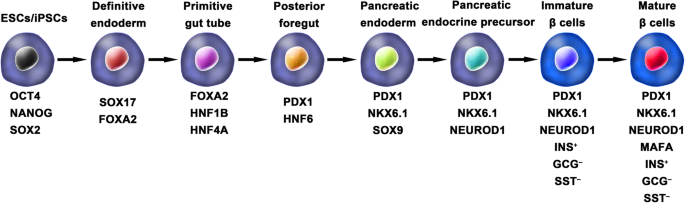
Generation of insulin-producing β cells from hPSCs. Schematic illustration of the differentiation protocol for generating insulin-producing β cells from hPSCs by mimicking the in vivo development of the embryonic pancreas. The key molecules of all key developmental stages of pancreatic islet β cells are illustrated
D’Amour et al. set up the first stepwise protocol to produce endocrine hormone-expressing cells that were able to synthesize and release multiple hormones from hESCs. However, at the final stage, the average percentage of insulin-positive cells in differentiated hES cell cultures was only 7.3%. Furthermore, these polyhormonal cells failed to respond to a high-glucose stimulus [ 18 ]. It is known that the fetal pancreas also possesses these characteristics, and previous studies demonstrated that fetal human pancreatic tissues could develop functionally after transplantation into animals [ 24 , 25 , 26 , 27 ]. Thus, the authors chose to determine whether these immature β cells derived from hESCs could mature into functional β cells under an in vivo environment. They generated pancreatic endoderm cells (similar to fetal 6- to 9-week pancreatic tissue) using an optimized protocol and then transplanted them into immunodeficient mice. The pancreatic endoderm cells successfully differentiated and matured into β-like cells in response to both fasting-induced hypoglycemia and glucose challenge and maintained normal glucose homeostasis for 3 months [ 28 ].
Similarly, the generation of IPCs from iPSCs is based on consecutive regulation of specific signaling pathways involved in pancreas development. Tateishi et al. first demonstrated that skin fibroblast-derived iPSCs were capable of producing islet-like clusters (ILCs) in vitro by mimicking the in vivo development of the pancreas. However, under high glucose stimulation (40 mM), the amount of C-peptide secreted by iPSC-derived ILCs and ESC-derived ILCs was only 0.3 ng/μg DNA and 0.15 ng/μg DNA, respectively [ 29 ].
Although the above studies have confirmed that hESCs and hiPSCs have the potential to differentiate into IPCs, this differentiation is done only cautiously owing to the low differentiation efficiency of protocols and the polyhormonal features of these β-like cells.
One of the breakthroughs comes from Rezania et al. in 2014, and the authors reported a more detailed protocol and generated mature and functional IPCs from hPSCs that were comparable to human β cells. The differentiation protocol was divided into 7 sequential stages, including definitive endoderm (stage 1), primitive gut hub (stage 2), posterior foregut (stage 3), pancreatic endoderm (stage 4), pancreatic endocrine precursors (stage 5), immature β cells (stage 6), and maturing β cells (stage 7). The obtained cells expressed key markers of mature β cells, such as MAFA, PDX1/NKX6.1, and INS, and showed functional similarities to human islets after transplantation in vivo. These β-like cells rapidly reversed hyperglycemia in STZ-diabetic mice by secreting C-peptide and insulin [ 20 ]. Nevertheless, the S7 (stage 7) cells were not equivalent to mature human β cells. S7 cells exhibited a very small and blunt response to high glucose stimulation, which differs from that of mature islet β cells. Moreover, a scalable suspension-based culture system developed by Paliuca et al. showed the possibility of generating large-scale stem cell-derived β cells (SC-β) [ 9 ]. Expression of NGN3 marks the initiation of endocrine differentiation. Previous studies have confirmed that inhibition of the Notch signaling pathway using γ secretase inhibitors or BMP inhibitors is essential for the induction of NGN3, followed by the addition of fibroblast growth factor 10 and keratinocyte growth factor (KGF), resulting in the robust generation of PDX1 + pancreatic progenitors and an increase in insulin expression in hPSC-derived progeny [ 9 , 20 ]. However, Russ et al. demonstrated that the use of BMP inhibitors promoted the precocious induction of endocrine differentiation in PDX1 + pancreatic progenitors and that omitting addition at pancreatic specification could successfully reduce the formation of polyhormonal cells. Subsequent exposure to retinoic acid and epidermal growth factors (EGF)/KGF cocktail efficiently induced the formation of PDX1 + /NKX6.1 + progenitor cells that differentiated into IPCs in vitro [ 10 ]. Recently, Yabe et al. reported that the addition of the selective glycogen synthase-kinase-3 β (GSK-3β) inhibitor (a substitute for Wnt3a; regarded as a key molecule for definitive endodermal induction from hPSCs) during definitive endodermal induction significantly decreased the death rate of endodermal cells [ 12 , 18 , 30 ]; further, spheroid formation of postendocrine progenitor cells rather than monolayer formation was crucial for generating IPCs from hiPSCs, which may be explained by the unique architecture of adult islets.
Among the above studies, the obtained cell population contains an average of 45% β cells, and the phenotypes of the remaining cells were unclarified. Identification of cell types that formed during differentiation is particularly important to improve the differentiated proportion of β cells. In a recent study, single-cell RNA sequencing in hPSCs undergoing in vitro β cell differentiation mapped a comprehensive description of cell production during stem-to-β cell differentiation [ 31 ]. Four distinct cell populations were isolated and identified from stem cell-derived islets, including SC-β cells, α-like polyhormonal cells, nonendocrine cells, and stem cell-derived enterochromaffin (SC-EC) cells. An in vitro study confirmed that α-like polyhormonal cells were transient toward SC-α cells and that nonendocrine cells were capable of generating exocrine cells (pancreatic acinar, mesenchymal and ductal cells). Additionally, CD49a was characterized as a surface marker of SC-β cells but not of adult islet β cells. Furthermore, SC-β cells could be purified up to 80% from SC islets using a scalable reaggregation method and magnetic sorting.
As patient-derived hiPSCs have been shown to provide tremendous advantages for studying the pathogenesis and pathophysiology of disease in vitro, studies on producing iPSCs from diabetic patients have generated great interest. Patient-specific iPSCs can overcome current obstacles in stem cell therapy, such as immune rejection and immune mismatch, and provide a platform to establish a personalized disease model to investigate pathogenic mechanisms and seek therapeutic methods for the disease. Maehr et al. successfully generated hiPSCs from skin fibroblasts of patients with T1DM (T1DM-specific iPSCs, DiPSCs). These DiPSCs resembled ESCs in the global gene expression profile and were capable of differentiating into pancreatic cell lineages, paving the path of generating T1DM SC-β cells and making autologous stem cell-derived pancreatic progeny transplantation for T1DM possible [ 32 ]. In 2015, Millman et al. confirmed that SC-β cells derived from DiPSCs functionally resembled adult islet β cells both in vivo and in vitro. GSIS tests showed that under high glucose stimulation (20 mM incubation for 30 min), T1DM and nondiabetic (ND) SC-β cells secreted 2.0 ± 0.4 and 1.9 ± 0.3 mIU of human insulin per 10 3 cells, respectively, and both of these cells functioned similarly to adult primary islets in a previous study. After transplantation into ND immunodeficient mice, the engraft function was evaluated by serum human insulin before and 30 min after an injection of glucose. At the early time point (2 weeks after transplantation), most engrafts responded to glucose and released more insulin after glucose injection, and the ratio of insulin secretion after glucose stimulation averaged 1.4 and 1.5 for T1DM and ND SC-β cells, respectively. The effects of these engrafts on insulin secretion were observed for several months. Of note, compared to the early time point, after 12–16 weeks, the human insulin content increased approximately 1.5 times after glucose stimulation [ 33 ]. It should be acknowledged that diversities exist among T1DM patients, and a larger number of specific stem cell lines from T1DM need to be developed for future clinical use. Although DiPSCs are an alternative source for cell replacement therapy for diabetes, some T1DM-specific stem cell lines have shown low efficiency in generating PDX1 + pancreatic progenitors [ 34 ]. Evaluated by flow cytometry, the number of IPCs derived from ND iPSCs (25–50.5%) was comparable to that of the β cells found in human primary islets, whereas the number of IPCs differentiated from T1DM iPSC lines was much lower (15.9%) [ 35 , 36 ]. Upon a strict differentiation protocol, pancreatic progenitors derived from T1DM iPSCs showed lower expression of PDX1 than ND iPSCs at a specific differentiation stage. Epigenetic changes resulting from dysmetabolism in T1DM might be responsible for the poor yield of β cells from T1DM iPSCs. Transient demethylation treatment of DE cells rescued the expression of PDX1 by inhibiting methyl group deposition on the cytosine residues of DNA and led to the differentiation of DE cells into IPCs [ 36 ]. The effect of demethylation on IPC differentiation has been shown to promote pancreatic progenitor induction rather than DE induction [ 37 ].
Generating pancreatic progenitors from ESCs and iPSCs
Pancreatic progenitors that coexpress specific markers indispensable for inducing a β-cell fate are a crucial cell state of differentiating hPSCs into β cells in vitro. Pancreatic and duodenal homeobox 1 (PDX1) transcription factor and NK6 homeobox transcription factor-related locus 1 (NKX6.1) have been considered to be the regulatory factors of differentiating DE into pancreatic progenitors [ 38 ]. Notably, high coexpression of PDX1 and NKX6.1 in pancreatic progenitors is essential for the efficient generation of mature and functional β cells [ 39 , 40 ].
Of note, the efficiency and safety of pancreatic progenitors that coexpress PDX1 and NKX6.1 for T1DM treatment are currently being evaluated in clinical trials by ViaCyte Company. Thus, elevating the production of hPSC-derived β cells, optimizing the in vitro differentiation protocols in multiple aspects, and generating a high population of PDX1 + /NKX6.1 + pancreatic progenitors are needed to accelerate the clinical trial. Multiple studies have been carried out to determine the appropriate cocktail of cytokines to mimic in vivo development [ 41 , 42 , 43 ]. Recently, Nostro et al. demonstrated that the combination of EGF and nicotinamide induced a higher production of NKX6.1 + pancreatic progenitors in adherent culture [ 44 ]. Importantly, the authors focused on the temporal window of foregut differentiation into the pancreatic endoderm and confirmed that the size of the NKX6.1 + population decreased with extended duration. Although previous studies have shown that the maintenance of cellular aggregation during the differentiation process could significantly elevate the efficiency of pancreatic progenitors [ 10 , 45 , 46 ], the impact of culture condition changes that affect the physical environment of cells on pancreatic progenitor differentiation is still less studied. Memon et al. showed that the generation of PDX1 + /NKX6.1 + pancreatic progenitors could be dramatically induced after dissociating and replating pancreatic endodermal cells at half density in monolayer culture [ 47 ]. Intriguingly, a novel NKX6.1 + /PDX1 − cell population that holds the potential to generate functional β cells was discovered, and the cell type was confirmed to be a new type of pancreatic progenitor cell by the same team [ 48 ].
Another important issue that needs to be resolved before hPSC-derived pancreatic progenitors can be used in the clinic is how the recipient’s in vivo environment affects the maturation and differentiation of these undifferentiated cells. Although many studies have highlighted the importance of the in vivo environment in promoting islet cell differentiation, the system mechanism regulating the response of the transplanted cells to the in vivo environment has not been well studied [ 9 , 20 , 21 ]. Most recently, Legøy et al. confirmed that short-term exposure of encapsulated pancreatic progenitors to an in vivo environment was beneficial for cell fate determination, as revealed by increased islet proteome characteristics [ 49 ]. These effects could be partially mediated by the levels of hepatocyte nuclear factor 1-α (HNF1A) and hepatocyte nuclear factor 4-α (HNF4A) in recipients.
Generating islet organoids/islets from ESCs and iPSCs
The pancreatic islet of Langerhans is comprised of α, β, δ, ε, and pancreatic polypeptide cells [ 46 , 50 ]. Many studies have highlighted the importance of reciprocal coordination and complementary interactions of different types of islet cells for glucose hemostasis [ 51 , 52 , 53 , 54 ]. Thus, it may be beneficial for producing whole islets or islet organoids rather than differentiating cells into a specific type.
Organoids are defined as 3D cultures maintained in vitro that can be generated from adult tissues or hPSCs and recapitulate the in vivo morphologies, cellular architecture and organ-specific functionality of the original tissue. Kim et al. developed islet-like organoids from hPSCs that showed a glucose response in vitro and in vivo [ 55 ]. Endocrine cells (ECs) were generated from hPSCs using a multistep protocol and expressed pancreatic hormones. Notably, dissociated ECs spontaneously formed islet-like spheroids, referred to as endocrine cell clusters (ECCs), under optimal 3D culture conditions in 24 h. The diameter of the ECCs was approximately 50–150 μm and contained 5 × 10 4 cells. ECCs consisted of several types of islet endocrine cells, apart from α cells, indicating that ECCs derived from hPSCs are partially similar to human adult islets. After high glucose stimulation (27.5 mM) for 1 h, ECCs showed increases in both insulin and C-peptide secretion, from 1.01 ± 0.22% up to 2.6 ± 0.21% and from 159.6 ± 20.01 pmol/L up to 336.3 ± 29.21 pmol/L, respectively. Additionally, ECCs exhibited intracellular Ca 2+ oscillation under a high glucose stimulus. Furthermore, a major breakthrough was that after ECCs were implanted into STZ-induced diabetic mice, normoglycemia was rapidly achieved within 3 days. In previous studies, transplanted hPSC-derived ECs took a long period (over 40 days) to normalize the glucose level in diabetic mice [ 9 , 10 , 20 , 28 ]. Therefore, this study suggested that it was promising to generate functional islet-like organoids from hPSCs and provided an alternative cell source for treating diabetes. Soon after that, based on a biomimetic 3D scaffold, islet organoids were successfully generated from hESCs [ 56 ]. The organoids contained all types of pancreatic cells (α, β, δ, and pancreatic polypeptide cells), specific markers of mature β cells as well as insulin secretory granules, which were characterized by a round electron-dense crystalline core surrounded by a distinctive large, clear halo. Insulin granules have been reported as an indication of mature β cells and a key participant in glucose homeostasis [ 36 , 57 ]. Generally, insulin granules in adult β cells were differentiated according to the shape and density of the core. Through transmission electron microscopy, insulin granules generally possess a characteristic “halo,” which is a product of glutaraldehyde fixation that does not exist in other endocrine granules. Many studies have reported remarkable insulin granules during the differentiation of hPSCs into IPCs [ 9 , 20 ]. Glucose loading experiments demonstrated that islet organoids exhibited a sharp increase in insulin secretion under high glucose conditions. Under the same glucose stimulation conditions (exposure from 5.5 mM to 25 mM), the 3D-induced cells had an insulin content that increased by seven-fold, whereas the 2D-induced cells had an insulin content that increased by 3.7-fold. These results suggested that 3D-induced IPCs are more sensitive to glucose stimulation due to their elevated maturity.
Fundamental studies of islet development during embryogenesis will promote optimization of protocols for differentiating hPSCs into 3D islet clusters or islet organoids. The traditional model of islet development is based on epithelial-mesenchymal transition (EMT) during the differentiation of pancreatic progenitors. However, this hypothesis was recently challenged by a study in which the dynamic changes in transcripts involved in islet formation were mapped [ 46 ]. Sharon et al. reported that along with EP differentiation, they maintained intact cell-to-cell adhesion and formed bud-like islet precursors (defined as peninsula-like structures) rather than undergoing EMT. Further in vitro generation of SC-β cells showed that the maintenance of cell adhesion could efficiently induce hESCs into peninsula-like structures. Importantly, these peninsula-like clusters could generate INS + and GCG + monohormonal cells after transplantation into SCID mice. This study provides a new framework for understanding islet embryogenesis and offers novel ideas to optimize the current protocols for the differentiation of SC-β cells.
Generating interspecific pancreatic chimeras from pancreatic stem cells (PSCs)
Interspecific chimeras, defined as organisms with cells originating from at least two different species, are able to produce organs completely consisting of donor-origin cells. Thus, human-animal chimeras have great potential for providing immune-compatible patient-specific human organs for transplantation.
In 2010, Kobayashi et al. successfully generated a functional rat pancreas in PDX1 −/− (pancreatogenesis knockout) mice via interspecies blastocyst complementation [ 58 ]. The rat iPSC-derived pancreas (rat M pancreas) in PDX1 −/− mice showed both exocrine and endocrine characteristics and expressed several pancreatic enzymes and hormones. In addition, outcomes from glucose tolerance testing (GTT) in adulthood indicated that endogenous insulin secretion was increased under high blood glucose, and glucose homeostasis was preserved. Recently, the same group reported the reverse experiment; mouse PSCs were injected into PDX1 −/− rat blastocysts to generate a pancreas (mouse R pancreas) the size of a rat pancreas with pancreatic cells primarily originating from mouse PSCs [ 59 ]. Most importantly, the isolated islets from the mouse R pancreas were subsequently injected into STZ-induced diabetic mice, and functional glucose-induced insulin secretion was successfully established in recipients for over 1 year. These data strongly supported the hypothesis that donor PSC-derived organs could be generated in a xenogeneic environment and provided the theoretical possibility of applying donor PSC-derived islets generated by animal-human interspecific blastocyst complementation in clinical trials. It is worth noting that rat M pancreases were the size of a rat pancreas, rather than the size of a mouse pancreas or an intermediate size, whereas mouse R pancreases were the size of a mouse pancreas. Thus, to adapt interspecific blastocyst complementation for patients, it seems necessary to generate organs in animals that are closer to humans in both size and evolutionary distance, such as sheep, pigs, and nonhuman primates (NHPs). Exogenic pancreases have been generated in vivo in transgenic cloned pigs by blastocyst complementation [ 60 ]. In this study, donor morula blastomeres derived from female cloned embryos were injected into the morula of male pancreatogenesis-disabled fetuses, and morphologically and functionally normal donor-derived pancreases were formed in adult chimeric pigs. Furthermore, PDX1 −/− sheep generated using CRISPR/Cas9 have been reported and can potentially serve as a host for interspecies organ generation [ 61 ]. However, blastocyst complementation has failed to generate chimeras in NHPs [ 62 ].
Differentiation of adult stem cells into IPCs
The search for adult pancreatic stem cells.
The adult pancreas consists of two unique parts: the exocrine pancreas and the endocrine pancreas, with unique morphology and function, respectively. The pancreas arises from two separate primordia along the dorsal and ventral surfaces of the posterior foregut. Lineage-tracing studies have demonstrated that all of the mature pancreatic cells were developed from PDX1 + /PTF1A + progenitor cells [ 63 , 64 ]. However, if there are detectable pancreatic stem cells in adult animal and human pancreases, how these cells participate in the regeneration of β cells is still under debate. The hypothesis was initially supported by histological observation of neogenesis occurring in adult rodent pancreatic ducts after pancreatic duct ligation (PDL) [ 65 ]. However, genetic lineage-tracing studies indicated that there was no contribution to endocrine regeneration during the adult life or after injury, and the major mechanism was enhanced replication by only preexisting β cells [ 63 , 66 , 67 ]. In 2007, supporting evidence comes from a study by Xu et al., in which NGN3 + (the earliest islet cell-specific transcription factor) endocrine precursors appeared in the ductal lining after PDL in mice and gave rise to all types of islet cells, including glucose-responsive β cells [ 68 ]. Additionally, increased proliferation and ectopic NGN3 + pancreatic progenitors were reported in experiments of α-to-β-cell reprogramming [ 69 , 70 ]. In conclusion, whether adult pancreatic stem cells exist in adulthood is unclear. Recent events in single-cell RNA sequencing are promising for mapping dynamic gene expression changes during the adult lifespan or after injury in animal and human pancreases, for constructing differentiation trajectories of pancreas/islet cells and for illustrating the mechanisms involved in β cell regeneration.
Pancreatic duct-derived stem cells
Theoretically, pancreatic duct epithelial cells possess a promising capacity for β cell generation because both originate from the same embryonic precursor [ 46 , 71 ]. Budding of β cells or new islets generated from ductal epithelium occurs during pancreatic regeneration in adults and has been reported [ 72 , 73 ]. Since then, studies have been designed to reprogram pancreatic ductal cells into β cells. Ramiya et al. isolated pancreatic ductal epithelial cells from prediabetic adult nonobese diabetic (NOD) mice, cultured them in vitro, and ensued the formation of ILCs that contained α, β, and δ cells. Subsequently, the blood glucose level of diabetic NOD mice was decreased from 400 to 180–220 mg/dl in 7 days [ 74 ]. Moreover, Bonner-Weir et al. demonstrated that the pancreatic ductal epithelium could expand and further differentiate into functional islet tissues in a Matrigel-based 3D culture system in vitro [ 75 ]. Further studies demonstrated that CK19 + nonendocrine pancreatic epithelial cells (NEPECs) can be differentiated into β cells in vitro [ 76 ].
Over the past two decades, attempts have been directed toward optimizing the protocols for generating IPCs from pancreas duct-derived stem cells. Since CA19-9 and CD133 were identified as specific membrane proteins of pancreas duct-derived stem cells, it became easier to purify these cells from the adult human pancreas [ 77 , 78 ]. It has been demonstrated that diverse growth factors (e.g., bFGF, EGF, and KGF) benefit the proliferation and differentiation of human pancreatic duct-derived stem cells [ 74 , 79 ]. Generally, epithelial cells show limited mitotic activity in vitro. Corritore et al. developed a differentiation protocol in which isolated human pancreatic duct cells from the pancreas were forced to undergo EMT to achieve a phenotypic change and allow them to extensively proliferate. After proliferation of these cells in vitro, pancreatic duct-derived cells differentiated into IPCs with a large array of specific marker expression and insulin secretion [ 78 ]. More recently, Zhang et al. reported that diabetic mice continuously administered gastrin and EGFs had accelerated transdifferentiation of SOX9 + duct cells into IPCs and consequently maintained blood glucose homeostasis [ 80 ].
Nestin-positive mesenchymal stem cells from islets
Nestin is an intermediate filament protein that is specifically expressed in neuronal and muscle precursor cells [ 81 , 82 ]. Recent studies have indicated that nestin-positive (nestin + ) cells resided in pancreatic islets and could differentiate into IPCs and islet-like cell clusters (Fig. 3 ), and now, nestin has been accepted as a critical pancreatic progenitor marker [ 83 , 84 ]. Zulewski et al. first demonstrated the existence of a distinct cell population within islets isolated from the human pancreas that express nestin, termed nestin-positive islet-derived progenitor cells (NIPs). These NIPs displayed features of stem cells and were able to generate cells with either pancreatic exocrine or endocrine phenotypes in vitro. Most importantly, the terminally differentiated cells were capable of secreting pancreatic hormones, such as insulin and glucagon [ 85 ]. Another study performed by the same group reported that NIPs also showed characteristics of bone marrow side population (SP) stem cells due to their coexpression of the ATP-binding cassette transporter ABCG2, which has been previously demonstrated to be a major component of the SP phenotype [ 85 , 86 , 87 ]. This was further supported by a study showing that NIPs isolated from a human fetal pancreas expressed ABCG2 and nestin [ 88 ]. Moreover, CD44, CD90, and CD147, which represent the phenotypes of bone marrow-derived mesenchymal stem cells, were also detected on NIPs. These data strongly indicated that NIPs have a high potential to become an alternative cell source for producing IPCs and islets in vitro. Huang et al. isolated and cultured NIPs from a human fetal pancreas. In this study, NIPs formed islet-like cell clusters (ICCs) in confluent cultures. Moreover, differentiation of ICCs from NIPs results in increased pancreatic islet-specific gene expression, along with a concomitant downregulation of ABCG2 and nestin. Additionally, the transplantation of ICCs reversed hyperglycemia in diabetic NOD-SCID mice [ 89 ].
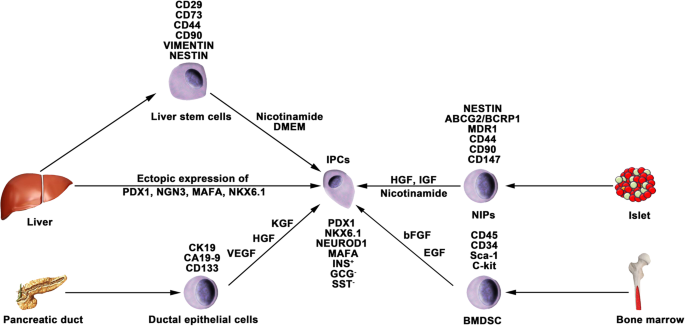
Generation of IPCs from adult stem cells. Adult pancreatic stem cells may be a potential source of IPCs. Functional IPCs have been generated from pancreatic ductal cells and NIPs isolated from adult islets. During embryogenesis, the liver and pancreas arise from common endoderm progenitors. Liver cells can transdifferentiate into IPCs by ectopic expression of pancreatic transcription factors. Additionally, a high pluripotent cell population termed HLSCs can also produce IPCs in vitro. Bone marrow-derived stem cells show the capacity to generate insulin cell clusters
The studies mentioned above about NIPs are based on rodent models. Nonhuman primate models often serve as an important bridge from laboratory research to clinical application; thus, generating pancreatic stem cells/progenitor cells from NHPs has led to great interest. Our previous study indicated that pancreatic progenitor cells existed in the adult pancreases of type 1 diabetic monkeys as well as in the pancreases of normal monkeys. The isolated pancreatic progenitor cells were able to proliferate in vitro and form ICCs in differentiation media. Furthermore, glucose-induced insulin and C-peptide secretion from the ICCs suggested that the ICCs functionally resembled primary islets [ 90 ]. In view of pathogenetic differences between STZ-induced diabetic monkeys and patients with T1DM, it still needs to be clarified whether NIPs also reside in T1DM patients.
Differentiation of bone marrow-derived stem cells (BMDSCs)
Several studies have reported that BMDSCs have the ability to differentiate into IPCs. Tang et al. reported that BMDSCs could spontaneously differentiate and form ICCs when continuously cultured with high glucose concentrations. The ICCs expressed multiple pancreatic lineage genes, including INS, GLUT2, glucose kinase, islet amyloid polypeptide, nestin, PDX-1, and PAX6, with β cell development. Moreover, ICCs could respond to glucose stimulation and release insulin and C-peptide in vitro, and following implantation into diabetic mice, hyperglycemia was reversed [ 91 ]. Since then, numerous studies have demonstrated the generation of IPCs from human and rat bone marrow stem cells (Fig. 3 ). However, the efficacy of BMDSC differentiation is low and highly variable with the current protocols. In particular, the quantity of insulin secreted by these cells was far from that secreted by adult β cells. Gabr and colleagues tested the efficiency of three differentiation protocols using immunolabeling, and the proportion of generated IPCs was modest (≈ 3%) in all protocols [ 92 ]. The expression of pancreatic-associated genes in generated IPCs was quite low compared to the expression in human islets. Optimizing differentiation protocols to upregulate the expression of specific genes by determining optimal molecules and culture conditions is crucial. Extracellular matrix proteins play a vital role in cell differentiation and proliferation. Laminin, one of the pancreatic extracellular matrices, has been confirmed to enhance the expression of insulin and promote the formation of ICCs from BMDSCs, whereas collagen type IV affects the expression of NEUROD1 and GCG [ 93 ]. Generally, differentiation of BMDSCs into IPCs is performed on nonadherent polymer surfaces and hydrogels. A recent study reported that 3D culture of BMDSCs on agar (a hydrogel-forming polysaccharide widely used in biomedical research) for 7 days followed by 2D culture of formed cellular clusters in high glucose media could enhance the production of IPCs from BMDSCs [ 94 ]. IPCs expressed INS genes at a 2215.3 ± 120.8-fold higher level than BMDSCs, whereas this fold change in previous studies was 1.2–2000-fold.
Differentiation of liver cells
The liver and pancreas originate from appendages of the upper primitive foregut endoderm. Later, separation of the liver and pancreas during organogenesis left both tissues with multipotent cells capable of generating both hepatic and pancreatic cell lineages. The common embryonic origin of the liver and pancreas raises the intriguing speculation that it may be possible to convert liver cells to pancreatic ECs (Fig. 3 ). Several studies have demonstrated that adult or fetal liver cells and biliary epithelial cells are capable of reprogramming into IPCs by inducing the expression of endocrine pancreatic-specific transcription factors [ 95 , 96 , 97 , 98 ]. The in vivo data showed that these hepatic cell-derived IPCs could ameliorate hyperglycemia upon implantation into diabetic mice. However, the efficiency of liver-to-pancreas reprogramming is still low, and the obtained IPCs are likely immature β-like cells. In addition, Herrera et al. isolated and characterized a population of human liver stem cells (HLSCs). HLSCs express both mesenchymal stromal cells (MSCs) and immature hepatocyte markers. In addition, HLSCs expressing nestin and vimentin are capable of differentiating into multiple cell lineages, including epithelial, endothelial, osteogenic, and islet-like structure (ILS) cells [ 99 ]. Later, Navarro-Tableros et al. confirmed that HLS-ILS cells expressed β cell transcription factors, such as NKX6.1, NKX6.3, and MAFA, and could respond to glucose loading by releasing C-peptide. Hyperglycemia was rapidly reversed in diabetic SCID mice after implantation [ 100 ]. These data suggest that HLSCs could be a novel potential resource for stem cell-based therapy for diabetes.
Encapsulation technique for stem cell therapy for T1DM
The encapsulation technique is based on a matrix that prevents immune cells, cytokines, and antibodies from reacting to grafts while allowing nutrient, oxygen, and signaling molecule diffusion. An appropriate encapsulation device is especially crucial for T1DM to prevent an autoimmune reaction against transplanted hPSC-derived pancreatic progeny, including allogenic grafts. Criteria to evaluate an encapsulation device should take many variables into consideration, including the biocompatibility, stability and permselectivity of the membrane, interaction with the bloodstream, availability of nutrients and oxygen, among others [ 101 , 102 , 103 ]. Studies have been performed to detect optimal materials to improve these properties and have mainly been developed for pancreatic islet transplantation.
Alginate, a scaffolding polysaccharide produced by brown seaweeds, has been widely employed by virtue of its biocompatibility [ 102 , 104 , 105 ]. Alginates are linear unbranched polymers containing β-(1 → 4)-linked d -mannuronic acid (M) and α-(1 → 4)-linked l -guluronic acid (G) residues and possess eminent gel-forming properties in the presence of polyvalent cations, such as Ca 2+ and Ba 2+ [ 103 , 106 , 107 , 108 ]. Earlier studies have confirmed that compared to nonencapsulated islets, encapsulated islets have significantly improved survival, long-term biocompatibility and function with the use of purified alginate [ 109 , 110 , 111 , 112 ]. Additionally, specific modifications to alginates trigger great interest, as they could circumvent the local immune response after transplantation of an allo- or xenograft. The incorporation of the chemokine CXCL2 with alginate microcapsules prevented allo- or xenoislet transplantation from immune reactions by establishing sustained local immune isolation [ 113 ]. Most recently, the same team confirmed that these modifications on alginates could also efficiently prolong the survival and function of hPSC-derived β cells and achieve long-term immunoprotection in immunocompetent mice with T1DM without systemic immunosuppression [ 114 ]. Of note, CXCL2 enhanced the GSIS activity of β cells, thus making it a crucial biomaterial to study for stem cell-based therapy for T1DM.
ViaCyte, leading the first and only islet cell replacement therapies derived from stem cells for diabetes, is testing for the safety and efficacy of its encapsulation devices PEC-Encap and PEC-Direct in clinical trials. The PEC-Encap is designed to fully contain hPSC-derived pancreatic progenitors in a semipermeable pouch so that vital nutrients and proteins can travel between the cells inside the device and the blood vessels, which grow along the outside of the device. In the case of PEC-Encap, the implanted cells were completely segregated from the recipients’ immune system. Another device called PEC-Direct allowed blood vessels to enter the device and directly interact with the implanted cells. Thus, immune suppression therapy was necessary for patients who received PEC-Direct, which made it suitable only for people with high-risk type 1 diabetes.
Immune modulation in stem cell therapy for T1DM
Human ESC/iPS-derived β cells have been proposed as a potential β cell replacement source for the treatment of T1DM. However, both the alloimmune and autoimmune responses remain a major problem for the wide application of cell replacement therapies for T1DM. Although massive efforts have been made in the progress of encapsulation technology, the engraftment of transplanted hPSC-derived pancreatic progenitors or β cells still faces challenges. The engraftments will certainly be destroyed by the recipient’s immune system if the encapsulation system is eliminated. Certain modulations of these encapsulated cells to circumvent autoimmune attack seem promising. Human leukocyte antigen (HLA) mismatching is the major molecular mechanism of immune rejection in allo- or xenografts [ 115 ]. Studies have proven that elimination of HLA-A genes by zinc-finger nucleases in hematopoietic stem cells could increase donor compatibility [ 116 , 117 ]. Likewise, knocking out the β2-microglobulin (B2M) gene, which abolishes all HLA class I molecules, or deleting HLA-A and HLA-B biallelically, retained one allele of HLA-C to allow the hPSC grafts to avoid T and NK cell attack [ 118 ]. Other protocols for immunosuppressive effects have been reported, such as targeted overexpression of PDL1-CTLA4Ig in β cells, which efficiently prevented the development of T1DM and allo-islet rejection, in turn promoting the survival of β cell mass [ 119 ]. Therefore, immune modulation strategies for hPSCs could be promising to overcome challenges associated with engraft rejection.
Clinical trials in stem cell therapy for T1DM
In the last few years, controlled clinical trials have been carried out to estimate the efficiency and safety of stem cell therapy for T1DM. It has been demonstrated that MSCs can ameliorate or reverse the manifestation of diabetes in animal models of T1DM. In 2014, Carlsson et al. confirmed that MSC treatment could preserve β cell functions in new-onset T1DM patients. Twenty adult patients (aged 18–40 years) with newly diagnosed (< 3 weeks) T1DM were enrolled and randomized to MSC treatment or to the control group and followed by a 1-year follow-up examination [ 120 ]. At the end of the clinical trial, mixed-meal tolerance tests (MMTTs) revealed that both C-peptide peak values and C-peptide significantly decreased in the treatment group. Of note, MSC treatment side effects were not observed during the follow-up examination. During January 2009 and December 2010, 42 patients aged 18–40 years with a history of T1DM for ≥ 2 years and ≤ 16 years were randomized into either the stem cell transplantation (umbilical cord MSCs in combination with autologous bone marrow mononuclear cells) or standard insulin care treatment groups [ 121 ]. A 1-year follow-up examination indicated that the C-peptide increased from 6.6 to 13.6 pmol/mL/180 min in treated patients, whereas it decreased from 8.4 to 7.7 pmol/mL/180 min in control groups; insulin increased from 1477.8 to 2205.5 mmol/mL/180 min in treated patients; and it decreased from 1517.7 to 1431.7 mmol/mL/180 min in control patients. Additionally, HbA 1c and fasting glycemia decreased in the treated groups and increased in the control subjects. Daily insulin requirements in the treated groups also decreased compared to those of the control groups. During the follow-up period, severe hypoglycemic events reported by patients were significantly decreased. Limitations of these studies could be a small sample size and the short follow-up period. Moreover, the treated patients did not achieve complete insulin independence. Even so, these results help to improve clinical trial outcomes in future large-scale trials.
Conclusions and perspectives
Stem cell-based therapy has been considered a promising potential therapeutic method for diabetes treatment, especially for T1DM. As mentioned in this review, major advances in research on the derivation of IPCs from hPSCs have improved our chance of reestablishing glucose-responsive insulin secretion in patients with T1DM. However, the clinical trial results of stem cell therapies for T1DM are still dissatisfactory [ 122 ], and many questions and technical hurdles still need to be solved. The major problems include the following four aspects: (1) how to generate more mature functional β-like cells in vitro from hPSCs; (2) how to improve the differentiation efficiency of IPCs from hPSCs; (3) how to protect implanted IPCs from autoimmune attack; (4) how to generate sufficient numbers of desired cell types for clinical transplantation; and (5) how to establish thorough insulin independence. Despite these obstacles, the application of stem cell-based therapy for T1DM represents the most advanced approach for curing type 1 diabetes.
Availability of data and materials
Not applicable.
Abbreviations
- Type 1 diabetes mellitus
- Insulin-producing cells
Diabetes mellitus
Type 2 diabetes mellitus
Hyperosmolar nonketotic coma
International Pancreas Transplant Registry
Human pluripotent stem cells
Human embryonic stem cells
Human induced pluripotent stem cells
MAF bZIP transcription factor A
Neuronal differentiation 1
Pancreatic and duodenal homeobox 1
NK6 homeobox transcription factor-related locus 1
Glucose-stimulated insulin secretion
Embryonic stem cells
Induced pluripotent stem cells
Definitive endoderm
Primitive gut tube
Pancreatic progenitor
Endocrine progenitor
Basic fibroblast growth factor
Islet-like clusters
Streptozocin
Stem cell-derived β cells
Neurogenin 3
Bone morphogenetic protein
Keratinocyte growth factor
Epidermal growth factors
Glycogen synthase-kinase-3 β
Stem cell-derived enterochromaffin
T1DM-specific iPSCs
Nondiabetic
Hepatocyte nuclear factor 1-α
Hepatocyte nuclear factor 4-α
Endocrine cells
Endocrine cell clusters
Epithelial-mesenchymal transition
Severe combined immunodeficiency
Pancreatic stem cells
Glucose tolerance testing
Nonhuman primates
Pancreas associated transcription factor 1a
Pancreatic duct ligation
Nonobese diabetic
Nonendocrine pancreatic epithelial cells
SRY-box transcription factor 9
Nestin-positive islet-derived progenitor cells
Side population
ATP binding cassette subfamily G member 2
Bone marrow-derived stem cells
Glucose transporter 2
Paired box 6
Human liver stem cells
Mesenchymal stromal cells
Islet-like structure
NK6 homeobox transcription factor-related locus 3
C-X-C motif chemokine ligand 2
Human leukocyte antigen
Major histocompatibility complex, class I, A
Major histocompatibility complex, class I, B
Major histocompatibility complex, class I, C
β2-microglobulin
Natural killer cell
Programmed cell death 1 ligand 1-cytotoxic T-lymphocyte antigen-4
Mixed-meal tolerance tests
Octamer-binding transcription factor-4
Nanog homeobox
SRY-box transcription factor 2
SRY-box transcription factor 17
Forkhead box A2
Hepatocyte nuclear factor 1-β
Hepatocyte nuclear factor 6
Somatostatin
Vascular endothelial growth factor
Hepatocyte growth factor
Insulin-like growth factor
Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–88.
CAS PubMed Google Scholar
Gruessner RW, Gruessner AC. The current state of pancreas transplantation. Nat Rev Endocrinol. 2013;9(9):555–62.
Lombardo C, et al. Update on pancreatic transplantation on the management of diabetes. Minerva Med. 2017;108(5):405–18.
PubMed Google Scholar
Shapiro AM, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8.
Niclauss N, et al. Beta-cell replacement: pancreas and islet cell transplantation. Endocr Dev. 2016;31:146–62.
Dean PG, et al. Pancreas transplantation. BMJ. 2017;357:j1321.
Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2017;13(5):268–77.
Rickels MR, Robertson RP. Pancreatic islet transplantation in humans: recent progress and future directions. Endocr Rev. 2019;40(2):631–68.
Pagliuca FW, et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159(2):428–39.
CAS PubMed PubMed Central Google Scholar
Russ HA, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34(13):1759–72.
Mu XP, et al. Enhanced differentiation of human amniotic fluid-derived stem cells into insulin-producing cells in vitro. J Diabetes Investig. 2017;8(1):34–43.
Yabe SG, et al. Efficient generation of functional pancreatic beta-cells from human induced pluripotent stem cells. J Diabetes. 2017;9(2):168–79.
Path G, et al. Stem cells in the treatment of diabetes mellitus - focus on mesenchymal stem cells. Metabolism. 2019;90:1–15.
Tao T, et al. Engineering human islet organoids from iPSCs using an organ-on-chip platform. Lab Chip. 2019;19(6):948–58.
Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7.
Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72.
Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20.
D'Amour KA, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24(11):1392–401.
Shim JH, et al. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50(6):1228–38.
Rezania A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32(11):1121–33.
Vegas AJ, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med. 2016;22(3):306–11.
Southard SM, Kotipatruni RP, Rust WL. Generation and selection of pluripotent stem cells for robust differentiation to insulin-secreting cells capable of reversing diabetes in rodents. PLoS One. 2018;13(9):e0203126.
PubMed PubMed Central Google Scholar
Zhang D, et al. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19(4):429–38.
Tuch BE. Reversal of diabetes by human fetal pancreas. Optimization of requirements in the hyperglycemic nude mouse. Transplantation. 1991;51(3):557–62.
Beattie GM, Butler C, Hayek A. Morphology and function of cultured human fetal pancreatic cells transplanted into athymic mice: a longitudinal study. Cell Transplant. 1994;3(5):421–5.
Hayek A, Beattie GM. Experimental transplantation of human fetal and adult pancreatic islets. J Clin Endocrinol Metab. 1997;82(8):2471–5.
Castaing M, et al. Ex vivo analysis of acinar and endocrine cell development in the human embryonic pancreas. Dev Dyn. 2005;234(2):339–45.
Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52.
Tateishi K, et al. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283(46):31601–7.
Kunisada Y, et al. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 2012;8(2):274–84.
Veres A, et al. Charting cellular identity during human in vitro beta-cell differentiation. Nature. 2019;569(7756):368–73.
Maehr R, et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A. 2009;106(37):15768–73.
Millman JR, et al. Generation of stem cell-derived beta-cells from patients with type 1 diabetes. Nat Commun. 2016;7:11463.
Chetty S, et al. A simple tool to improve pluripotent stem cell differentiation. Nat Methods. 2013;10(6):553–6.
Brissova M, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53(9):1087–97.
Manzar GS, Kim EM, Zavazava N. Demethylation of induced pluripotent stem cells from type 1 diabetic patients enhances differentiation into functional pancreatic beta cells. J Biol Chem. 2017;292(34):14066–79.
Wang Q, et al. Real-time observation of pancreatic beta cell differentiation from human induced pluripotent stem cells. Am J Transl Res. 2019;11(6):3490–504.
Al-Khawaga S, et al. Pathways governing development of stem cell-derived pancreatic beta cells: lessons from embryogenesis. Biol Rev Camb Philos Soc. 2018;93(1):364–89.
Rezania A, et al. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells. 2013;31(11):2432–42.
Taylor BL, Liu FF, Sander M. Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Rep. 2013;4(6):1262–75.
Mfopou JK, et al. Noggin, retinoids, and fibroblast growth factor regulate hepatic or pancreatic fate of human embryonic stem cells. Gastroenterology. 2010;138(7):2233–45 2245 e1–14.
Nostro MC, et al. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138(5):861–71.
Elham H, Mahmoud H. The effect of pancreas islet-releasing factors on the direction of embryonic stem cells towards Pdx1 expressing cells. Appl Biochem Biotechnol. 2018;186(2):371–83.
Nostro MC, et al. Efficient generation of NKX6-1+ pancreatic progenitors from multiple human pluripotent stem cell lines. Stem Cell Reports. 2015;4(4):591–604.
Toyoda T, et al. Cell aggregation optimizes the differentiation of human ESCs and iPSCs into pancreatic bud-like progenitor cells. Stem Cell Res. 2015;14(2):185–97.
Sharon N, et al. A peninsular structure coordinates asynchronous differentiation with morphogenesis to generate pancreatic islets. Cell. 2019;176(4):790–804 e13.
Memon B, et al. Enhanced differentiation of human pluripotent stem cells into pancreatic progenitors co-expressing PDX1 and NKX6.1. Stem Cell Res Ther. 2018;9(1):15.
Aigha II, et al. Differentiation of human pluripotent stem cells into two distinct NKX6.1 populations of pancreatic progenitors. Stem Cell Res Ther. 2018;9(1):83.
Legoy TA, et al. In vivo environment swiftly restricts human pancreatic progenitors toward mono-hormonal identity via a HNF1A/HNF4A mechanism. Front Cell Dev Biol. 2020;8:109.
Shahjalal HM, et al. Generation of pancreatic beta cells for treatment of diabetes: advances and challenges. Stem Cell Res Ther. 2018;9(1):355.
Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75:155–79.
Peiris H, et al. The beta-cell/EC axis: how do islet cells talk to each other? Diabetes. 2014;63(1):3–11.
Johnston NR, et al. Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metab. 2016;24(3):389–401.
Liu W, et al. Abnormal regulation of glucagon secretion by human islet alpha cells in the absence of beta cells. EBioMedicine. 2019;50:306–16.
Kim Y, et al. Islet-like organoids derived from human pluripotent stem cells efficiently function in the glucose responsiveness in vitro and in vivo. Sci Rep. 2016;6:35145.
Wang W, Jin S, Ye K. Development of islet organoids from H9 human embryonic stem cells in biomimetic 3D scaffolds. Stem Cells Dev. 2017;26(6):394–404.
Suckale J, Solimena M. The insulin secretory granule as a signaling hub. Trends Endocrinol Metab. 2010;21(10):599–609.
Kobayashi T, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142(5):787–99.
Yamaguchi T, et al. Interspecies organogenesis generates autologous functional islets. Nature. 2017;542(7640):191–6.
Matsunari H, et al. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci U S A. 2013;110(12):4557–62.
Vilarino M, et al. CRISPR/Cas9 microinjection in oocytes disables pancreas development in sheep. Sci Rep. 2017;7(1):17472.
Tachibana M, et al. Generation of chimeric rhesus monkeys. Cell. 2012;148(1–2):285–95.
Pan FC, et al. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development. 2013;140(4):751–64.
Fujitani Y. Transcriptional regulation of pancreas development and beta-cell function [Review]. Endocr J. 2017;64(5):477–86.
Peshavaria M, et al. Regulation of pancreatic beta-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes. 2006;55(12):3289–98.
Dor Y, et al. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–6.
Kopp JL, et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138(4):653–65.
Xu X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132(2):197–207.
Al-Hasani K, et al. Adult duct-lining cells can reprogram into beta-like cells able to counter repeated cycles of toxin-induced diabetes. Dev Cell. 2013;26(1):86–100.
Courtney M, et al. The inactivation of Arx in pancreatic alpha-cells triggers their neogenesis and conversion into functional beta-like cells. PLoS Genet. 2013;9(10):e1003934.
Lysy PA, Weir GC, Bonner-Weir S. Making beta cells from adult cells within the pancreas. Curr Diab Rep. 2013;13(5):695–703.
Bonner-Weir S, et al. Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans. 2008;36(Pt 3):353–6.
Carpino G, et al. Progenitor cell niches in the human pancreatic duct system and associated pancreatic duct glands: an anatomical and immunophenotyping study. J Anat. 2016;228(3):474–86.
Ramiya VK, et al. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000;6(3):278–82.
Bonner-Weir S, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A. 2000;97(14):7999–8004.
Hao E, et al. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med. 2006;12(3):310–6.
Lee J, et al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. Elife. 2013;2:e00940.
Corritore E, et al. Beta-cell differentiation of human pancreatic duct-derived cells after in vitro expansion. Cell Reprogram. 2014;16(6):456–66.
Hoesli CA, Johnson JD, Piret JM. Purified human pancreatic duct cell culture conditions defined by serum-free high-content growth factor screening. PLoS One. 2012;7(3):e33999.
Zhang M, et al. Growth factors and medium hyperglycemia induce Sox9+ ductal cell differentiation into beta cells in mice with reversal of diabetes. Proc Natl Acad Sci U S A. 2016;113(3):650–5.
Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4):585–95.
Zimmerman L, et al. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12(1):11–24.
Xie L, et al. Characterization of nestin, a selective marker for bone marrow derived mesenchymal stem cells. Stem Cells Int. 2015;2015:762098.
Bernal A, Arranz L. Nestin-expressing progenitor cells: function, identity and therapeutic implications. Cell Mol Life Sci. 2018;75(12):2177–95.
Kim M, et al. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8(1):22–8.
Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99(2):507–12.
Lechner A, et al. Nestin-positive progenitor cells derived from adult human pancreatic islets of Langerhans contain side population (SP) cells defined by expression of the ABCG2 (BCRP1) ATP-binding cassette transporter. Biochem Biophys Res Commun. 2002;293(2):670–4.
Zhang L, et al. Nestin-positive progenitor cells isolated from human fetal pancreas have phenotypic markers identical to mesenchymal stem cells. World J Gastroenterol. 2005;11(19):2906–11.
Huang H, Tang X. Phenotypic determination and characterization of nestin-positive precursors derived from human fetal pancreas. Lab Investig. 2003;83(4):539–47.
Zou C, et al. Isolation and in vitro characterization of pancreatic progenitor cells from the islets of diabetic monkey models. Int J Biochem Cell Biol. 2006;38(5–6):973–84.
Tang DQ, et al. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53(7):1721–32.
Gabr MM, et al. Generation of insulin-producing cells from human bone marrow-derived mesenchymal stem cells: comparison of three differentiation protocols. Biomed Res Int. 2014;2014:832736.
Pokrywczynska M, et al. Transdifferentiation of bone marrow mesenchymal stem cells into the islet-like cells: the role of extracellular matrix proteins. Arch Immunol Ther Exp. 2015;63(5):377–84.
CAS Google Scholar
Daryabor G, Shiri EH, Kamali-Sarvestani E. A simple method for the generation of insulin producing cells from bone marrow mesenchymal stem cells. In Vitro Cell Dev Biol Anim. 2019;55(6):462–71.
Sapir T, et al. Cell-replacement therapy for diabetes: generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci U S A. 2005;102(22):7964–9.
Zalzman M, Anker-Kitai L, Efrat S. Differentiation of human liver-derived, insulin-producing cells toward the beta-cell phenotype. Diabetes. 2005;54(9):2568–75.
Meivar-Levy I, Ferber S. Reprogramming of liver cells into insulin-producing cells. Best Pract Res Clin Endocrinol Metab. 2015;29(6):873–82.
Cerda-Esteban N, et al. Stepwise reprogramming of liver cells to a pancreas progenitor state by the transcriptional regulator Tgif2. Nat Commun. 2017;8:14127.
Herrera MB, et al. Isolation and characterization of a stem cell population from adult human liver. Stem Cells. 2006;24(12):2840–50.
Navarro-Tableros V, et al. Islet-like structures generated in vitro from adult human liver stem cells revert hyperglycemia in diabetic SCID mice. Stem Cell Rev Rep. 2019;15(1):93–111.
Opara EC, et al. Design of a bioartificial pancreas(+). J Investig Med. 2010;58(7):831–7.
Kepsutlu B, et al. Design of bioartificial pancreas with functional micro/nano-based encapsulation of islets. Curr Pharm Biotechnol. 2014;15(7):590–608.
Farney AC, Sutherland DE, Opara EC. Evolution of islet transplantation for the last 30 years. Pancreas. 2016;45(1):8–20.
O'Sullivan ES, et al. Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocr Rev. 2011;32(6):827–44.
Memon B, Abdelalim EM. Stem cell therapy for diabetes: beta cells versus pancreatic progenitors. Cells. 2020;9(2):283.
Kizilel S, Garfinkel M, Opara E. The bioartificial pancreas: progress and challenges. Diabetes Technol Ther. 2005;7(6):968–85.
Welman T, et al. Bioengineering for organ transplantation: progress and challenges. Bioengineered. 2015;6(5):257–61.
Hwang PT, et al. Progress and challenges of the bioartificial pancreas. Nano Converg. 2016;3(1):28.
de Vos P, et al. Long-term biocompatibility, chemistry, and function of microencapsulated pancreatic islets. Biomaterials. 2003;24(2):305–12.
Mallett AG, Korbutt GS. Alginate modification improves long-term survival and function of transplanted encapsulated islets. Tissue Eng Part A. 2009;15(6):1301–9.
Souza YE, et al. Islet transplantation in rodents. Do encapsulated islets really work? Arq Gastroenterol. 2011;48(2):146–52.
Rengifo HR, et al. Long-term survival of allograft murine islets coated via covalently stabilized polymers. Adv Healthc Mater. 2014;3(7):1061–70.
Chen T, et al. Alginate encapsulant incorporating CXCL12 supports long-term allo- and xenoislet transplantation without systemic immune suppression. Am J Transplant. 2015;15(3):618–27.
Alagpulinsa DA, et al. Alginate-microencapsulation of human stem cell-derived beta cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression. Am J Transplant. 2019;19(7):1930–40.
Williams RC, et al. The risk of transplant failure with HLA mismatch in first adult kidney allografts 2: living donors, summary, guide. Transplant Direct. 2017;3(5):e152.
Torikai H, et al. Toward eliminating HLA class I expression to generate universal cells from allogeneic donors. Blood. 2013;122(8):1341–9.
Torikai H, et al. Genetic editing of HLA expression in hematopoietic stem cells to broaden their human application. Sci Rep. 2016;6:21757.
Xu H, et al. Targeted disruption of HLA genes via CRISPR-Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell. 2019;24(4):566–78 e7.
El Khatib MM, et al. Beta-cell-targeted blockage of PD1 and CTLA4 pathways prevents development of autoimmune diabetes and acute allogeneic islets rejection. Gene Ther. 2015;22(5):430–8.
Carlsson PO, et al. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64(2):587–92.
Cai J, et al. Umbilical cord Mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care. 2016;39(1):149–57.
Hwang G, et al. Efficacies of stem cell therapies for functional improvement of the beta cell in patients with diabetes: a systematic review of controlled clinical trials. Int J Stem Cells. 2019;12(2):195–205.
Download references
Acknowledgements
We gratefully acknowledge the funding support from the National Key Research and Development Program of China (2016YFC1305703), the National Natural Science Foundation of China (81670750, 81971191, and 61627807), Guangxi Natural Science Foundation (2014GXNSFDA118030), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
Author information
Authors and affiliations.
Key Laboratory of Longevity and Ageing-Related Disease of Chinese Ministry of Education, Center for Translational Medicine and School of Preclinical Medicine, Guangxi Medical University, Nanning, 530021, Guangxi, China
Shuai Chen, Kechen Du & Chunlin Zou
You can also search for this author in PubMed Google Scholar
Contributions
CZ designed the concept. SC wrote the manuscript. SC and KD designed the figures. CZ revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Chunlin Zou .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Chen, S., Du, K. & Zou, C. Current progress in stem cell therapy for type 1 diabetes mellitus. Stem Cell Res Ther 11 , 275 (2020). https://doi.org/10.1186/s13287-020-01793-6
Download citation
Received : 29 January 2020
Revised : 19 June 2020
Accepted : 29 June 2020
Published : 08 July 2020
DOI : https://doi.org/10.1186/s13287-020-01793-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pancreatic islets
- Transplantation
Stem Cell Research & Therapy
ISSN: 1757-6512
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Scientists advance type 1 diabetes treatment with cutting-edge stem cell and gene editing technologies
- Download PDF Copy

Unlocking the potential of renewable β cells and precision gene editing, researchers aim to revolutionize diabetes care and bring us closer to a functional cure for type 1 diabetes.

In a recent study published in the journal Nature Reviews Endocrinology , researchers examined the progress in cell replacement therapies for type 1 diabetes mellitus (T1DM), focusing on generating replenishable β cells, improving transplantation methods, and addressing challenges related to immune modulation and clinical application.
T1DM affects 8.75 million people globally, approximately 1.52 million patients under 20. T1DM results from the autoimmune destruction of pancreatic β cells, consequent insulin insufficiency, and chronic hyperglycemia. Although glucose monitoring and insulin dosing help manage the disease, achieving optimal glycemic control remains challenging. Pancreatic islet or β cell transplantation offers a potential cure but faces challenges such as limited donor availability, poor cell engraftment, and the need for lifelong immunosuppression. Current research focuses on improving cell delivery, finding alternative cell sources, and reducing reliance on immunosuppression. In the present review, researchers discussed the current advancements in cell transplantation for T1DM, focusing on β cell generation, delivery technologies, immune modulation, relevant animal models, and the clinical translation of these therapies.
Renewable islet cell sources
The limited availability of donor islets has driven the development of stem cell-derived islets as a renewable source for T1DM therapy. These islets, generated from human pluripotent stem cells (hPSCs), show promise in clinical trials but remain challenged by functional immaturity, transcriptional identity issues, and the inability to control the ratio of β, α, and δ cells. During in vitro differentiation, a significant number of cells may acquire an unwanted identity, resembling serotonin-producing enterochromaffin cells, which complicates their application in T1DM therapy. While in vivo transplantation can enhance the function of these cells, optimizing in vitro production processes and ensuring safety, particularly concerning uncommitted cell types that could form tumors and ensuring genetic stability, remains crucial. Advances in scalable manufacturing, characterization protocols, and cryopreservation will be essential for the clinical adoption and accessibility of these therapies.
Cell delivery strategies
Pancreatic islet transplantation for T1DM involves strategies like microencapsulation and macroencapsulation to protect islet cells and enhance their function. Microencapsulation encloses cells in gel-like microspheres, allowing nutrient exchange while shielding them from immune attacks. However, challenges like inflammation and fibrotic overgrowth affect long-term viability. Macroencapsulation delivers larger cell doses in retrievable units but faces issues with oxygen supply and fibrosis. Open devices and scaffolds aim for direct vascularization of grafts to improve integration and function, using approaches like simultaneous implant-transplant methods, decellularized tissue scaffolds, and 3D-printed architectures. Prevascularization systems are also explored to establish a vascular network before cell transplantation, improving cell survival and reducing immune responses. Despite these innovations, ensuring adequate mass transfer of oxygen, glucose, and insulin within encapsulation devices and managing graft size and immune protection remain significant hurdles. While these approaches show promise, challenges remain in achieving long-term efficacy , minimizing immune rejection, and optimizing oxygen and nutrient delivery to transplanted cells.

Alternative immunoprotection methods
β cell replacement faces challenges distinct from non-autoimmune diseases, mainly due to the need to prevent autoimmunity recurrence. Current immunosuppressive therapies are effective but have severe side effects, including organ toxicity and increased infection risks. Emerging strategies focus on more targeted, less toxic immunomodulation. These include biomaterial-based localized drug delivery, islet co-delivery with immunomodulatory cells, and reducing islet graft immunogenicity through advanced gene editing techniques. Biomaterials can deliver immunomodulatory drugs directly to the transplant site, while co-delivery with cells like mesenchymal stem cells improves islet survival. Gene editing technologies, such as CRISPR–Cas9, are being utilized to engineer hypoimmune islet grafts by knocking down immunogenic markers or overexpressing protective signals. However, the long-term impact of these genetic modifications remains uncertain, and safety concerns persist regarding the potential for immune evasion by these modified cells.
Animal models
Animal models support the development of cell transplantation strategies and immunomodulatory interventions. Immunocompromised models, mainly using mice, allow for studying human islet engraftment without rejection. In contrast, immunocompetent models, such as rats, pigs, and non-human primates (NHPs), better mimic human immune responses critical for evaluating inflammatory and immune protection strategies. Humanized models, incorporating human immune components, provide a unique platform to assess the immunogenicity of β cell grafts and therapeutic interventions despite limitations such as graft-versus-host disease and shorter experimental timeframes. Pigs provide insights into islet transplantation due to their physiological similarities to humans, while NHPs serve as valuable translational models, contributing to understanding immune responses and developing new immunosuppressive strategies. Together, these models facilitate comprehensive assessments of therapeutic interventions for T1DM.
Clinical translation
Harmonizing preclinical testing protocols is vital for β cell replacement therapy development. Characterization of stem cell-derived β cells should include composition and functional assessments. Initial rodent studies must evaluate cell delivery and immune responses, with further validation in larger animal models. Development of a β cell replacement product involves renewable cell sources, effective delivery, and immune rejection prevention. The goal is to create a safe, reproducible product that restores glycemic control for over ten years without systemic immunosuppression.
Conclusion and outlook
In conclusion, cell transplantation for T1DM has evolved significantly, with stem cell-derived islets showing promise for clinical application. However, challenges related to cell composition, functional maturity, and long-term safety continue to be critical areas of focus. Collaborative consortia are accelerating progress by integrating complementary technologies. Innovations in renewable β cell sources, gene editing technology, and subcutaneous transplantation methods aim to improve cell delivery, immune modulation, and patient outcomes. The goal is to develop a widely applicable, practical, and accessible treatment that improves the quality of life for T1DM patients.
- Harnessing cellular therapeutics for type 1 diabetes mellitus: progress, challenges, and the road ahead. Grattoni, A. et al., Nature Reviews Endocrinology (2024), DOI: 10.1038/s41574-024-01029-0, https://www.nature.com/articles/s41574-024-01029-0
Posted in: Medical Science News | Medical Research News | Medical Condition News
Tags: Autoimmunity , Cas9 , Cell , Chronic , CRISPR , Diabetes , Diabetes Mellitus , Drug Delivery , Drugs , Efficacy , Endocrinology , Fibrosis , Gene , Genetic , Glucose , Hyperglycemia , Immunomodulatory , Immunosuppression , in vitro , in vivo , Inflammation , Insulin , Manufacturing , Mesenchymal Stem Cells , Oxygen , Preclinical , Preclinical Testing , Research , Serotonin , Stem Cells , Technology , Translation , Transplant , Type 1 Diabetes , Vascular

Dr. Sushama R. Chaphalkar
Dr. Sushama R. Chaphalkar is a senior researcher and academician based in Pune, India. She holds a PhD in Microbiology and comes with vast experience in research and education in Biotechnology. In her illustrious career spanning three decades and a half, she held prominent leadership positions in academia and industry. As the Founder-Director of a renowned Biotechnology institute, she worked extensively on high-end research projects of industrial significance, fostering a stronger bond between industry and academia.
Please use one of the following formats to cite this article in your essay, paper or report:
Chaphalkar, Sushama R.. (2024, September 05). Scientists advance type 1 diabetes treatment with cutting-edge stem cell and gene editing technologies. News-Medical. Retrieved on September 05, 2024 from https://www.news-medical.net/news/20240905/Scientists-advance-type-1-diabetes-treatment-with-cutting-edge-stem-cell-and-gene-editing-technologies.aspx.
Chaphalkar, Sushama R.. "Scientists advance type 1 diabetes treatment with cutting-edge stem cell and gene editing technologies". News-Medical . 05 September 2024. <https://www.news-medical.net/news/20240905/Scientists-advance-type-1-diabetes-treatment-with-cutting-edge-stem-cell-and-gene-editing-technologies.aspx>.
Chaphalkar, Sushama R.. "Scientists advance type 1 diabetes treatment with cutting-edge stem cell and gene editing technologies". News-Medical. https://www.news-medical.net/news/20240905/Scientists-advance-type-1-diabetes-treatment-with-cutting-edge-stem-cell-and-gene-editing-technologies.aspx. (accessed September 05, 2024).
Chaphalkar, Sushama R.. 2024. Scientists advance type 1 diabetes treatment with cutting-edge stem cell and gene editing technologies . News-Medical, viewed 05 September 2024, https://www.news-medical.net/news/20240905/Scientists-advance-type-1-diabetes-treatment-with-cutting-edge-stem-cell-and-gene-editing-technologies.aspx.
Suggested Reading

Cancel reply to comment
- Trending Stories
- Latest Interviews
- Top Health Articles

How can microdialysis benefit drug development
Ilona Vuist
In this interview, discover how Charles River uses the power of microdialysis for drug development as well as CNS therapeutics.

Global and Local Efforts to Take Action Against Hepatitis
Lindsey Hiebert and James Amugsi
In this interview, we explore global and local efforts to combat viral hepatitis with Lindsey Hiebert, Deputy Director of the Coalition for Global Hepatitis Elimination (CGHE), and James Amugsi, a Mandela Washington Fellow and Physician Assistant at Sandema Hospital in Ghana. Together, they provide valuable insights into the challenges, successes, and the importance of partnerships in the fight against hepatitis.

Addressing Important Cardiac Biology Questions with Shotgun Top-Down Proteomics
In this interview conducted at Pittcon 2024, we spoke to Professor John Yates about capturing cardiomyocyte cell-to-cell heterogeneity via shotgun top-down proteomics.

Latest News

Newsletters you may be interested in
Your AI Powered Scientific Assistant
Hi, I'm Azthena, you can trust me to find commercial scientific answers from News-Medical.net.
A few things you need to know before we start. Please read and accept to continue.
- Use of “Azthena” is subject to the terms and conditions of use as set out by OpenAI .
- Content provided on any AZoNetwork sites are subject to the site Terms & Conditions and Privacy Policy .
- Large Language Models can make mistakes. Consider checking important information.
Great. Ask your question.
Azthena may occasionally provide inaccurate responses. Read the full terms .
While we only use edited and approved content for Azthena answers, it may on occasions provide incorrect responses. Please confirm any data provided with the related suppliers or authors. We do not provide medical advice, if you search for medical information you must always consult a medical professional before acting on any information provided.
Your questions, but not your email details will be shared with OpenAI and retained for 30 days in accordance with their privacy principles.
Please do not ask questions that use sensitive or confidential information.
Read the full Terms & Conditions .
Provide Feedback
- Utility Menu
GA4 tracking code

It stands to reason that only by understanding the root causes of diseases like diabetes can we hope to develop effective therapies.
T1D is particularly challenging to study in human patients. By the time a patient is diagnosed with T1D, also known as juvenile diabetes, the destruction of insulin-producing beta cells by the immune system is nearly complete. Because of this, there is no way to discover what it was that led the person’s immune system to attack the beta cells in the first place. Even if it were possible to identify future T1D patients before the immune attack on beta cells began, disease onset and progression could not be studied in these individuals due to the inaccessibility of the pancreas – where beta cells are found – in a living person.
A New Model for Disease
Harvard Stem Cell Institute (HSCI) scientists are attempting an ambitious, long-term, and high-risk project to create the first animal model for T1D. Engineered mice will allow researchers to better understand the disease and increase the odds of developing effective therapies.

At the heart of the new T1D model will be transplanting the three human cell types that are the key components of T1D into mice. The T1D cell types are: beta cells; the immune cells that attack and destroy beta cells; and thymus cells, which educate the immune system by providing information about which cells should and should not be destroyed.
HSCI scientists will produce each of these cell types from patient-derived iPS cells while simultaneously raising genetically engineered mice that lack immune systems, allowing them to accept transplants of human cells. The researchers will transplant the T1D cell types into the mice, thereby creating a new T1D research model in which patient-derived cells can interact to generate the disease state.
A Collaborative Team
HSCI has assembled an inter-institutional team of experts in the stem cell and diabetes fields with a track record of ongoing and effective collaboration to build this model, giving us the best chance of success. Team members include Harvard Medical School, Boston Children’s Hospital, and the Immune Disease Institute.
This new T1D mouse model will allow HSCI investigators to test whether cells from a given T1D patient generate T1D in every case or whether particular environmental conditions might also be required in order to develop T1D. The model will also reveal whether all patients who present with T1D have the same disease, or whether there are different sub-types.
These are lengthy and expensive experiments and the project will take several years before major insights can be reached. But with a team of researchers now in place, even partial success would provide significant advances to biomedical research, and to the T1D community. For example, if our scientists are successful in producing beta cells from iPS cells, this would provide an unlimited source of urgently needed cells for transplantation therapy.
A new approach to T1D research is desperately needed. It makes no sense to spend any more precious research dollars on experiments that lead to insufficient results. HSCI’s project, while risky, has the potential to open up a new era in T1D research—one in which the disease is understood.
Making Beta Cells
HSCI scientists are also investigating how to treat diabetics with stem cell therapies. The goal is to create glucose-sensing, insulin-producing beta cells, the cells impacted in both type I and type II diabetes. The HSCI is approaching the problem in four ways.
Growing Cells
Our researchers have refined the process of making general pancreatic endocrine cells from embryonic stem cells so that it is more efficient. Using a unique bioreactor, it is possible to grow cells at a large enough scale to run extensive experiments and be effective therapeutically.
With this in place, researchers are now honing in on the final step of the process—turning pancreatic endocrine cells into beta cells. This last maturation step has taken place in the live mouse, but has not yet been accomplished in the lab setting. Once the process can be controlled, it will ensure that patients receive the right number and type of cells.
Direct Differentiation
A second approach is to turn other types of pancreatic cells into beta cells. HSCI Co-Director Douglas Melton, PhD, proved that this could be done by turning pancreatic acinar (digestive) cells in the live mouse into insulin-producing beta cells. Melton’s lab used a virus to make genetic modifications that could not be safely done in humans. However, the experiments proved that this type of “direct differentiation” was conceptually feasible. Scientists are now working with the same strategy on other closely related cell types, such as liver cells.
Reprogramming
A third approach is to take cells from patients who have diabetes, use reprogramming methods to create induced pluripotent stem cells (iPS cells) and then differentiate them into beta cells. Melton’s lab has already created many iPS cell lines from diabetics with different genetic backgrounds, but again, the final step of transforming iPS cells into beta cells is not yet ready for humans.
Self-Regulation
A fourth approach is not to turn other cells into beta cells but to get beta cells to make more of themselves. Thanks to work coming out of the Melton lab, it is now understood that beta cells in the pancreas do make more of themselves, albeit very slowly and at a low rate. This replication slows down even further with age.
These findings suggest that a possible strategy for type II diabetes and very early-diagnosed T1D is to increase the beta cell replication rate. Intrigued by the fact that the number of beta cells increases significantly in pregnant mammals, the Melton lab studied the differences in genes that are on or off before, during, and after pregnancy. The researchers identified several causal factors that led to the recent formation of a three-way joint development agreement between the lab, a biotechnology company, and a large pharmaceutical company to develop drugs that could affect this pathway. If successful, this development will result in a drug that can directly raise the number of beta cells by stimulating replication.
Disease Prevention
HSCI’s work to make functional beta cells is in motion, and while the reproducible production of beta cells from stem cells has not yet been achieved, our scientists have demonstrated that this is possible.
Encapsulation Devices
Parallel to this effort, and in planning for its success, HSCI investigators are now focusing attention on developing and testing devices for implantation, eventually in human subjects, that would protect functional beta cells from being attacked by the immune system.
Such devices would serve as receptacles for beta cells that would be transplanted into people, allowing the cells to read glucose levels and secrete insulin, while simultaneously preventing physical access by immune cells (T-cells). This is possible because glucose and insulin are both relatively small molecules, tiny in comparison to the size of T-cells.
In the longer term, HSCI researchers aim to make beta cells that are opaque or invisible to the immune attack, so that shielding them with an encapsulation device will not be necessary. But making an opaque beta cell is a complex challenge— one that is unlikely to be met in the next few years.
Encapsulation devices that have been developed to date by various labs and small manufacturers include those with alginate coating as well as devices that resemble “tea bags” made from high tech materials such as Teflon and Gore-Tex. In each of these configurations, the aim is to have a mesh or filter that allows sugar and insulin to easily pass through while blocking passage of the larger immune cells.
At this time, these devices are not routinely available, and those that have been created are expensive. A further problem that has slowed the development and testing of this approach has been the lack of a reproducible supply of human beta cells. Thus, existing devices have only been tested with human or rodent pancreatic tissue, which are inherently variable because of the state of the tissue donation and the method used for isolation. As such, there has been a distinct lack of robust testing of any device, most of which to date are high cost and in limited supply.
HSCI scientists will work to further develop and test encapsulation devices, in the first instance, using the immature beta cells that can now be derived from stem cells and mature following transplantation into animals. The focus of this work will be to explore the maturation and function of cells transplanted into devices.
- Blood Program
- Cancer Program
- Cardiovascular Program
- Kidney Program
- Nervous System Diseases Program
- Musculoskeletal Program
- Skin Program
- Aging & Fibrosis
- Stem Cells as Tools
- Early-Stage Research
- For Patients
Related Diabetes Stories:
Improving stem cell-derived pancreatic cells with genetic engineering, harvard university licenses technology to alkem, aiming to treat ischemic injury and vascular diseases, hsci receives grant from the crown prince of abu dhabi to help advance type 1 diabetes research, crosstalk between pancreatic cells may drive rare form of diabetes, new york times: a cure for type 1 diabetes for one man, it seems to have worked., a new therapy for treating type 1 diabetes.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Harnessing cellular therapeutics for type 1 diabetes mellitus: progress, challenges, and the road ahead
Affiliations.
- 1 Department of Nanomedicine, Houston Methodist Research Institute, Houston, TX, USA. [email protected].
- 2 Department of Surgery, Houston Methodist Hospital, Houston, TX, USA. [email protected].
- 3 Department of Radiation Oncology, Houston Methodist Hospital, Houston, TX, USA. [email protected].
- 4 Alberta Diabetes Institute, University of Alberta, Edmonton, Alberta, Canada.
- 5 Department of Surgery, University of Alberta, Edmonton, Alberta, Canada.
- 6 Diabetes Research Institute, University of Miami Miller School of Medicine, Miami, FL, USA.
- 7 Department of Biomedical Engineering, University of Miami, Miami, FL, USA.
- 8 Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, USA.
- 9 Department of Microbiology and Immunology, University of Miami Miller School of Medicine, Miami, FL, USA.
- 10 Woodruff School of Mechanical Engineering and Petit Institute for Bioengineering and Bioscience, Georgia Institute of Technology, Atlanta, GA, USA.
- 11 J. Crayton Pruitt Family Department of Biomedical Engineering, Herbert Wertheim College of Engineering, University of Florida, Gainesville, FL, USA.
- 12 Diabetes Institute, University of Florida, Gainesville, FL, USA.
- 13 Program in Molecular Medicine, Diabetes Center of Excellence, University of Massachusetts Chan Medical School, Worcester, MA, USA.
- 14 Department of Surgery, The University of Arizona, Tucson, AZ, USA.
- 15 Diabetes Research Institute, IRCCS Ospedale San Raffaele, Milan, Italy.
- 16 Department of Pediatrics, Ellis Fischel Cancer Center, School of Medicine, University of Missouri, Columbia, MO, USA.
- 17 Division of Endocrinology, Metabolism and Lipid Research, Washington University School of Medicine, St. Louis, MO, USA.
- 18 Department of Biomedical Engineering, Washington University in St. Louis, St. Louis, MO, USA.
- 19 Department of Cardiac Surgery, Boston Children's Hospital, Boston, MA, USA.
- 20 Department of Surgery, Harvard Medical School, Boston, MA, USA.
- 21 Harvard Stem Cell Institute, Cambridge, MA, USA.
- 22 Department of Surgery, University of Minnesota, Minneapolis, MN, USA.
- 23 Department of Veterinary Population Medicine, University of Minnesota, St. Paul, MN, USA.
- 24 Institute of Biomedical Engineering, University of Toronto, Toronto, Ontario, Canada.
- 25 Department of Chemical Engineering and Applied Chemistry, University of Toronto, Toronto, Ontario, Canada.
- 26 Department of Biological and Environmental Engineering, Cornell University, Ithaca, NY, USA.
- 27 Department of Bioengineering, Rice University, Houston, TX, USA.
- 28 University of California, San Francisco, Department of Bioengineering and Therapeutic Sciences, San Francisco, CA, USA.
- 29 Brown University, School of Engineering, Providence, RI, USA.
- 30 McEwen Stem Cell Institute, University Health Network, Toronto, ON, Canada.
- 31 Department of Physiology, University of Toronto, Toronto, ON, Canada.
- 32 Advocacy Department, Breakthrough T1D, Washington, DC, USA.
- 33 Department of Medicine, Columbia Center for Translational Immunology, Columbia University, New York, NY, USA.
- 34 Department of Microbiology and Immunology, Columbia University, New York, NY, USA.
- 35 Department of Surgery, Columbia University, New York, NY, USA.
- 36 Department of Pharmacology and Therapeutics, University of Florida, Gainesville, FL, USA.
- 37 UW Health Transplant Center, Madison, WI, USA.
- 38 Division of Transplantation, Department of Surgery, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
- 39 Diabetes Center, University of California San Francisco, San Francisco, CA, USA.
- 40 Department of Surgery, University of California San Francisco, San Francisco, CA, US.
- 41 Gladstone Institute of Genomic Immunology, University of California San Francisco, San Francisco, CA, USA.
- 42 Research Department, Breakthrough T1D, New York, NY, USA.
- 43 Research Department, Breakthrough T1D, New York, NY, USA. [email protected].
- 44 Vaccine and Immunotherapy Center, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA. [email protected].
- 45 Immunoendocrinology, Division of Medical Biology, Department of Pathology and Medical Biology, University of Groningen and University Medical Center Groningen, Groningen, Netherlands. [email protected].
- PMID: 39227741
- DOI: 10.1038/s41574-024-01029-0
Type 1 diabetes mellitus (T1DM) is a growing global health concern that affects approximately 8.5 million individuals worldwide. T1DM is characterized by an autoimmune destruction of pancreatic β cells, leading to a disruption in glucose homeostasis. Therapeutic intervention for T1DM requires a complex regimen of glycaemic monitoring and the administration of exogenous insulin to regulate blood glucose levels. Advances in continuous glucose monitoring and algorithm-driven insulin delivery devices have improved the quality of life of patients. Despite this, mimicking islet function and complex physiological feedback remains challenging. Pancreatic islet transplantation represents a potential functional cure for T1DM but is hindered by donor scarcity, variability in harvested cells, aggressive immunosuppressive regimens and suboptimal clinical outcomes. Current research is directed towards generating alternative cell sources, improving transplantation methods, and enhancing cell survival without chronic immunosuppression. This Review maps the progress in cell replacement therapies for T1DM and outlines the remaining challenges and future directions. We explore the state-of-the-art strategies for generating replenishable β cells, cell delivery technologies and local targeted immune modulation. Finally, we highlight relevant animal models and the regulatory aspects for advancing these technologies towards clinical deployment.
© 2024. Springer Nature Limited.
PubMed Disclaimer
- International Diabetes Federation. IDF Diabetes Atlas Reports: Type 1 Diabetes Estimates in Children and Adults (International Diabetes Federation, 2022).
- US Centers for Disease Control and Prevention. National Diabetes Statistics Report (CDC, 2024).
- Tonnies, T. et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2060: the SEARCH for diabetes in youth study. Diabetes Care 46, 313–320 (2023). - PubMed - DOI
- Syed, F. Z. Type 1 diabetes mellitus. Ann. Intern. Med. 175, ITC33–ITC48 (2022). - PubMed - DOI
- Ebekozien, O. et al. Longitudinal trends in glycemic outcomes and technology use for over 48,000 people with type 1 diabetes (2016-2022) from the T1D exchange quality improvement collaborative. Diabetes Technol. Ther. 25, 765–773 (2023). - PubMed - DOI
Publication types
- Search in MeSH
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Subscribe Digital Print

- Shohei Ohtani
- Special series: Boiling Point
Latest News
Today's print edition
Home Delivery
- Crime & Legal
- Science & Health
- More sports
- CLIMATE CHANGE
- SUSTAINABILITY
- EARTH SCIENCE
- Food & Drink
- Style & Design
- TV & Streaming
- Entertainment news
Kyoto University Hospital seeks to treat Type 1 diabetes using iPS cells
Kyoto University Hospital is looking to develop a treatment for severe Type 1 diabetes using induced pluripotent stem (iPS) cells reprogrammed to function as pancreatic islet cells, which produce the hormones that regulate glucose levels in the blood.
Pending the results of the clinical trial, researchers are hoping that a transplant treatment using iPS cell-derived pancreatic cells will be available in the 2030s.
In a time of both misinformation and too much information, quality journalism is more crucial than ever. By subscribing, you can help us get the story right.
With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.
Your subscription plan doesn't allow commenting. To learn more see our FAQ
- WHAT’S TRENDING
- Editors' Picks

SUSTAINABLE JAPAN

SPECIAL SUPPLEMENTS
Sponsored contents planned and edited by JT Media Enterprise Division.
広告出稿に関するおといあわせはこちらまで
SUPPLEMENTS BINDER

- Bridges by SMS
- Global Insight
- World Eye Reports
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 03 September 2024
Harnessing cellular therapeutics for type 1 diabetes mellitus: progress, challenges, and the road ahead
- Alessandro Grattoni ORCID: orcid.org/0000-0001-7888-422X 1 , 2 , 3 ,
- Gregory Korbutt 4 , 5 ,
- Alice A. Tomei 6 , 7 , 8 , 9 ,
- Andrés J. García ORCID: orcid.org/0000-0001-6602-2518 10 ,
- Andrew R. Pepper ORCID: orcid.org/0000-0002-2227-4446 5 ,
- Cherie Stabler 11 , 12 ,
- Michael Brehm ORCID: orcid.org/0000-0001-6813-3262 13 ,
- Klearchos Papas ORCID: orcid.org/0000-0002-4554-0911 14 ,
- Antonio Citro 15 ,
- Haval Shirwan ORCID: orcid.org/0000-0002-1657-9470 16 ,
- Jeffrey R. Millman 17 , 18 ,
- Juan Melero-Martin ORCID: orcid.org/0000-0002-4689-8149 19 , 20 , 21 ,
- Melanie Graham 22 , 23 ,
- Michael Sefton 24 , 25 ,
- Minglin Ma 26 ,
- Norma Kenyon 6 , 8 ,
- Omid Veiseh ORCID: orcid.org/0000-0003-1153-8079 27 ,
- Tejal A. Desai ORCID: orcid.org/0000-0003-3409-9208 28 , 29 ,
- M. Cristina Nostro ORCID: orcid.org/0000-0003-1166-8252 30 , 31 ,
- Marjana Marinac 32 ,
- Megan Sykes ORCID: orcid.org/0000-0002-4947-4376 33 , 34 , 35 ,
- Holger A. Russ 12 , 36 ,
- Jon Odorico ORCID: orcid.org/0000-0003-1096-464X 37 , 38 ,
- Qizhi Tang ORCID: orcid.org/0000-0001-7313-3574 39 , 40 , 41 ,
- Camillo Ricordi 6 , 8 ,
- Esther Latres 42 ,
- Nicholas E. Mamrak 42 na1 ,
- Jaime Giraldo 42 na1 ,
- Mark C. Poznansky ORCID: orcid.org/0000-0003-1344-7103 43 na1 &
- Paul de Vos 44 na1
Nature Reviews Endocrinology ( 2024 ) Cite this article
60 Altmetric
Metrics details
- Cell therapies
- Type 1 diabetes
Type 1 diabetes mellitus (T1DM) is a growing global health concern that affects approximately 8.5 million individuals worldwide. T1DM is characterized by an autoimmune destruction of pancreatic β cells, leading to a disruption in glucose homeostasis. Therapeutic intervention for T1DM requires a complex regimen of glycaemic monitoring and the administration of exogenous insulin to regulate blood glucose levels. Advances in continuous glucose monitoring and algorithm-driven insulin delivery devices have improved the quality of life of patients. Despite this, mimicking islet function and complex physiological feedback remains challenging. Pancreatic islet transplantation represents a potential functional cure for T1DM but is hindered by donor scarcity, variability in harvested cells, aggressive immunosuppressive regimens and suboptimal clinical outcomes. Current research is directed towards generating alternative cell sources, improving transplantation methods, and enhancing cell survival without chronic immunosuppression. This Review maps the progress in cell replacement therapies for T1DM and outlines the remaining challenges and future directions. We explore the state-of-the-art strategies for generating replenishable β cells, cell delivery technologies and local targeted immune modulation. Finally, we highlight relevant animal models and the regulatory aspects for advancing these technologies towards clinical deployment.
Stem cell-derived islets have advanced as a viable renewable source of cells for transplantation in type 1 diabetes mellitus (T1DM). Although these cells are being tested in the clinical setting, challenges remain to be addressed regarding cell safety, composition and function.
Genetic engineering of renewable β cells can reduce immunogenicity, lower metabolic needs and bolster hypoxia resistance. However, the effect on β cell performance requires further elucidation.
Local immunomodulation via in situ delivery of immunomodulatory molecules and adjuvant cells is emerging as a promising approach for abrogating the need for systemic immunosuppression in β cell transplantation.
Current preclinical results suggest that immunoprotected islet cell grafts in a retrievable subcutaneous site could restore normoglycaemia for at least 1 year or longer without systemic immunosuppression.
Despite the potential of new technologies, the development of cell therapy treatments must pragmatically focus on generating therapies that are not only effective and safe but also align with the real-world dynamics of patients’ lives and the capabilities of health-care systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
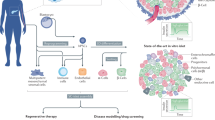
Engineering islets from stem cells for advanced therapies of diabetes
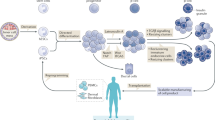
Emerging routes to the generation of functional β-cells for diabetes mellitus cell therapy

Targeting pancreatic β cells for diabetes treatment
International Diabetes Federation. IDF Diabetes Atlas Reports: Type 1 Diabetes Estimates in Children and Adults (International Diabetes Federation, 2022).
US Centers for Disease Control and Prevention. National Diabetes Statistics Report (CDC, 2024).
Tonnies, T. et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2060: the SEARCH for diabetes in youth study. Diabetes Care 46 , 313–320 (2023).
Article PubMed Google Scholar
Syed, F. Z. Type 1 diabetes mellitus. Ann. Intern. Med. 175 , ITC33–ITC48 (2022).
Ebekozien, O. et al. Longitudinal trends in glycemic outcomes and technology use for over 48,000 people with type 1 diabetes (2016-2022) from the T1D exchange quality improvement collaborative. Diabetes Technol. Ther. 25 , 765–773 (2023).
Article CAS PubMed Google Scholar
Wilson, L. M., Jacobs, P. G., Riddell, M. C., Zaharieva, D. P. & Castle, J. R. Opportunities and challenges in closed-loop systems in type 1 diabetes. Lancet Diabetes Endocrinol. 10 , 6–8 (2022).
Marfil-Garza, B. A. et al. Pancreatic islet transplantation in type 1 diabetes: 20-year experience from a single-centre cohort in Canada. Lancet Diabetes Endocrinol. 10 , 519–532 (2022).
Vantyghem, M. C., de Koning, E. J. P., Pattou, F. & Rickels, M. R. Advances in beta-cell replacement therapy for the treatment of type 1 diabetes. Lancet 394 , 1274–1285 (2019).
Article CAS PubMed PubMed Central Google Scholar
Butler, P. C. & Gale, E. A. Reversing type 1 diabetes with stem cell-derived islets: a step closer to the dream? J. Clin. Invest. 132 , e158305 (2022).
Paez-Mayorga, J. et al. Emerging strategies for beta cell transplantation to treat diabetes. Trends Pharmacol. Sci. 43 , 221–233 (2022).
Roberts, M. B. & Fishman, J. A. Immunosuppressive agents and infectious risk in transplantation: managing the “net state of Immunosuppression”. Clin. Infect. Dis. 73 , e1302–e1317 (2021).
Wehner, M. R. et al. Risks of multiple skin cancers in organ transplant recipients: a cohort study in 2 administrative data sets. JAMA Dermatol. 157 , 1447–1455 (2021).
Shapiro, A. M. J. & Verhoeff, K. A spectacular year for islet and stem cell transplantation. Nat. Rev. Endocrinol. 19 , 68–69 (2023).
Pagliuca, F. W. et al. Generation of functional human pancreatic beta cells in vitro. Cell 159 , 428–439 (2014).
Rezania, A. et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 32 , 1121–1133 (2014).
US National Library of Medicine. CinicalTrials.gov www.clinicaltrials.gov/study/NCT04786262 (2024).
Businesswire. Vertex presents positive, updated VX-880 results from ongoing phase 1/2 study in type 1 diabetes at the European Association for the Study of Diabetes 59th Annual Meeting. Businesswire www.businesswire.com/news/home/20231003786678/en/Vertex-Presents-Positive-Updated-VX-880-Results-From-Ongoing-Phase-12-Study-in-Type-1-Diabetes-at-the-European-Association-for-the-Study-of-Diabetes-59th-Annual-Meeting (2023).
Vertex Pharmaceuticals. Vertex provides pipeline and business updates in advance of upcoming investor meetings. Vertex news.vrtx.com/news-releases/news-release-details/vertex-provides-pipeline-and-business-updates-advance-upcoming (2024).
Hogrebe, N. J., Ishahak, M. & Millman, J. R. Developments in stem cell-derived islet replacement therapy for treating type 1 diabetes. Cell Stem Cell 30 , 530–548 (2023).
Augsornworawat, P. et al. Single-nucleus multi-omics of human stem cell-derived islets identifies deficiencies in lineage specification. Nat. Cell Biol. 25 , 904–916 (2023).
Zhu, H. et al. Understanding cell fate acquisition in stem-cell-derived pancreatic islets using single-cell multiome-inferred regulomes. Dev. Cell 58 , 727–743.e11 (2023).
Veres, A. et al. Charting cellular identity during human in vitro β-cell differentiation. Nature 569 , 368–373 (2019).
Balboa, D. et al. Functional, metabolic and transcriptional maturation of human pancreatic islets derived from stem cells. Nat. Biotechnol. 40 , 1042–1055 (2022).
Augsornworawat, P., Velazco-Cruz, L., Song, J. & Millman, J. R. A hydrogel platform for in vitro three dimensional assembly of human stem cell-derived islet cells and endothelial cells. Acta Biomater. 97 , 272–280 (2019).
Mahaddalkar, P. U. et al. Generation of pancreatic β cells from CD177 + anterior definitive endoderm. Nat. Biotechnol. 38 , 1061–1072 (2020).
Aghazadeh, Y. et al. GP2-enriched pancreatic progenitors give rise to functional beta cells in vivo and eliminate the risk of teratoma formation. Stem Cell Rep. 17 , 964–978 (2022).
Article CAS Google Scholar
Maxwell, K. G., Kim, M. H., Gale, S. E. & Millman, J. R. Differential function and maturation of human stem cell-derived islets after transplantation. Stem Cell Transl. Med. 11 , 322–331 (2022).
Ryan, E. A. et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes 51 , 2148–2157 (2002).
Markmann, J. F. et al. Insulin independence following isolated islet transplantation and single islet infusions. Ann. Surg. 237 , 741–749 (2003).
Article PubMed PubMed Central Google Scholar
Niebruegge, S. et al. Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol. Bioeng. 102 , 493–507 (2009).
Ismadi, M. Z. et al. Flow characterization of a spinner flask for induced pluripotent stem cell culture application. PLoS ONE 9 , e106493 (2014).
Hogrebe, N. J., Augsornworawat, P., Maxwell, K. G., Velazco-Cruz, L. & Millman, J. R. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat. Biotechnol. 38 , 460–470 (2020).
Paull, D. et al. Automated, high-throughput derivation, characterization and differentiation of induced pluripotent stem cells. Nat. Methods 12 , 885–892 (2015).
Millman, J. R., Tan, J. H. & Colton, C. K. Mouse pluripotent stem cell differentiation under physiological oxygen reduces residual teratomas. Cell Mol. Bioeng. 14 , 555–567 (2021).
Fong, C. Y., Gauthaman, K. & Bongso, A. Teratomas from pluripotent stem cells: a clinical hurdle. J. Cell Biochem. 111 , 769–781 (2010).
Laurent, L. C. et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 8 , 106–118 (2011).
Garber, K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat. Biotechnol. 33 , 890–891 (2015).
Taapken, S. M. et al. Karotypic abnormalities in human induced pluripotent stem cells and embryonic stem cells. Nat. Biotechnol. 29 , 313–314 (2011).
Merkle, F. T. et al. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature 545 , 229–233 (2017).
Rouhani, F. J. et al. Substantial somatic genomic variation and selection for BCOR mutations in human induced pluripotent stem cells. Nat. Genet. 54 , 1406–1416 (2022).
Roschke, A. V. & Kirsch, I. R. Targeting karyotypic complexity and chromosomal instability of cancer cells. Curr. Drug. Targets 11 , 1341–1350 (2010).
Muller, P. A. & Vousden, K. H. p53 mutations in cancer. Nat. Cell Biol. 15 , 2–8 (2013).
Astolfi, A. et al. BCOR involvement in cancer. Epigenomics 11 , 835–855 (2019).
Hirai, T., Yasuda, S., Umezawa, A. & Sato, Y. Country-specific regulation and international standardization of cell-based therapeutic products derived from pluripotent stem cells. Stem Cell Rep. 18 , 1573–1591 (2023).
International Society for Stem Cell Research. Guidelines for Stem Cell Research and Clinical Translation (International Society for Stem Cell Research, 2022).
US Food and Drug Administration. Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-based Products: Minimal Manipulation and Homologous Use (FDA, 2020).
Yamamoto, T. et al. Quality control for clinical islet transplantation: organ procurement and preservation, the islet processing facility, isolation, and potency tests. J. Hepatobiliary Pancreat. Surg. 16 , 131–136 (2009).
Ricordi, C. et al. Improved human islet isolation outcome from marginal donors following addition of oxygenated perfluorocarbon to the cold-storage solution. Transplantation 75 , 1524–1527 (2003).
Walker, S., Appari, M. & Forbes, S. Considerations and challenges of islet transplantation and future therapies on the horizon. Am. J. Physiol. Endocrinol. Metab. 322 , E109–E117 (2022).
Lee, K. Y. & Mooney, D. J. Alginate: properties and biomedical applications. Prog. Polym. Sci. 37 , 106–126 (2012).
Hu, S. et al. Toll-like receptor 2-modulating pectin-polymers in alginate-based microcapsules attenuate immune responses and support islet-xenograft survival. Biomaterials 266 , 120460 (2021).
Bennet, W., Groth, C. G., Larsson, R., Nilsson, B. & Korsgren, O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups. J. Med. Sci. 105 , 125–133 (2000).
van der Windt, D. J., Bottino, R., Casu, A., Campanile, N. & Cooper, D. K. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation 14 , 288–297 (2007).
Matsumoto, S. et al. Clinical porcine islet xenotransplantation under comprehensive regulation. Transpl. Proc. 46 , 1992–1995 (2014).
Huang, L. et al. Regulation of blood glucose using islets encapsulated in a melanin-modified immune-shielding hydrogel. ACS Appl. Mater. Interfaces 13 , 12877–12887 (2021).
Schaschkow, A. et al. Glycaemic control in diabetic rats treated with islet transplantation using plasma combined with hydroxypropylmethyl cellulose hydrogel. Acta Biomater. 102 , 259–272 (2020).
Kuwabara, R. et al. Extracellular matrix inclusion in immunoisolating alginate-based microcapsules promotes longevity, reduces fibrosis, and supports function of islet allografts in vivo. Acta Biomater. 158 , 151–162 (2023).
Elliott, R. B. et al. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation 14 , 157–161 (2007).
Harrington, S., Williams, J., Rawal, S., Ramachandran, K. & Stehno-Bittel, L. Hyaluronic acid/collagen hydrogel as an alternative to alginate for long-term immunoprotected islet transplantation. Tissue Eng. Part. A 23 , 1088–1099 (2017).
Medina, J. D. et al. Functionalization of alginate with extracellular matrix peptides enhances viability and function of encapsulated porcine islets. Adv. Healthc. Mater. 9 , e2000102 (2020).
Alagpulinsa, D. A. et al. Alginate-microencapsulation of human stem cell-derived β cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression. Am. J. Transpl. 19 , 1930–1940 (2019).
Chen, T. et al. Alginate encapsulant incorporating CXCL12 supports long-term allo- and xenoislet transplantation without systemic immune suppression. Am. J. Transpl. 15 , 618–627 (2015).
Sremac, M. et al. Preliminary studies of the impact of CXCL12 on the foreign body reaction to pancreatic islets microencapsulated in alginate in nonhuman primates. Transpl. Direct 5 , e447 (2019).
Zhang, Q. et al. Islet encapsulation: new developments for the treatment of type 1 diabetes. Front. Immunol. 13 , 869984 (2022).
Lew, B., Kim, I. Y., Choi, H. & Kim, K. K. Sustained exenatide delivery via intracapsular microspheres for improved survival and function of microencapsulated porcine islets. Drug. Deliv. Transl. Res. 8 , 857–862 (2018).
Vaithilingam, V. et al. Co-encapsulation and co-transplantation of mesenchymal stem cells reduces pericapsular fibrosis and improves encapsulated islet survival and function when allografted. Sci. Rep. 7 , 10059 (2017).
Cheng, Y., Liu, Y. F., Zhang, J. L., Li, T. M. & Zhao, N. Elevation of vascular endothelial growth factor production and its effect on revascularization and function of graft islets in diabetic rats. World J. Gastroenterol. 13 , 2862–2866, (2007).
Tomei, A. A. et al. Device design and materials optimization of conformal coating for islets of Langerhans. Proc. Natl Acad. Sci. USA 111 , 10514–10519 (2014).
Manzoli, V. et al. Immunoisolation of murine islet allografts in vascularized sites through conformal coating with polyethylene glycol. Am. J. Transpl. 18 , 590–603 (2018).
Stock, A. A. et al. Conformal coating of stem cell-derived islets for β cell replacement in type 1 diabetes. Stem Cell Rep. 14 , 91–104 (2020).
Desai, T. A. & Tang, Q. Islet encapsulation therapy – racing towards the finish line? Nat. Rev. Endocrinol. 14 , 630–632 (2018).
Scharp, D. W. & Marchetti, P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv. Drug. Deliv. Rev. 67-68 , 35–73 (2014).
Scharp, D. W. et al. Protection of encapsulated human islets implanted without immunosuppression in patients with type I or type II diabetes and in nondiabetic control subjects. Diabetes 43 , 1167–1170 (1994).
Chang, R. et al. Nanoporous immunoprotective device for stem-cell-derived β-cell replacement therapy. ACS Nano 11 , 7747–7757 (2017).
Ludwig, B. et al. Transplantation of human islets without immunosuppression. Proc. Natl Acad. Sci. USA 110 , 19054–19058 (2013).
An, D. et al. Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes. Proc. Natl Acad. Sci. USA 115 , E263–E272 (2018).
Hwang, D. G. et al. A 3D bioprinted hybrid encapsulation system for delivery of human pluripotent stem cell-derived pancreatic islet-like aggregates. Biofabrication 14 , 014101 (2021).
Article Google Scholar
Yang, K. et al. A therapeutic convection-enhanced macroencapsulation device for enhancing β cell viability and insulin secretion. Proc. Natl Acad. Sci. USA 118 , e2101258118 (2021).
Kharbikar, B. N., Chendke, G. S. & Desai, T. A. Modulating the foreign body response of implants for diabetes treatment. Adv. Drug. Deliv. Rev. 174 , 87–113 (2021).
Capuani, S., Malgir, G., Chua, C. Y. X. & Grattoni, A. Advanced strategies to thwart foreign body response to implantable devices. Bioeng. Transl. Med. 7 , e10300 (2022).
Avgoustiniatos, E. & Colton, C. in Principles of Tissue Engineering 1st edn (eds Lanza, R. P. et al.) 333–346 (Academic, 1997).
Colton, C. K. Oxygen supply to encapsulated therapeutic cells. Adv. Drug. Deliv. Rev. 67-68 , 93–110 (2014).
Papas, K. K., De Leon, H., Suszynski, T. M. & Johnson, R. C. Oxygenation strategies for encapsulated islet and beta cell transplants. Adv. Drug. Deliv. Rev. 139 , 139–156 (2019).
Wang, L. H. et al. An inverse-breathing encapsulation system for cell delivery. Sci. Adv. 7 , eabd5835 (2021).
Carlsson, P. O. et al. Transplantation of macroencapsulated human islets within the bioartificial pancreas βAir to patients with type 1 diabetes mellitus. Am. J. Transpl. 18 , 1735–1744 (2018).
Qin, T., Smink, A. M. & de Vos, P. Enhancing longevity of immunoisolated pancreatic islet grafts by modifying both the intracapsular and extracapsular environment. Acta Biomater. 167 , 38–53 (2023).
Valdes-Gonzalez, R. A. et al. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study. Eur. J. Endocrinol. 153 , 419–427 (2005).
Birmingham, K. Skepticism surrounds diabetes xenograft experiment. Nat. Med. 8 , 1047 (2002).
Dufrane, D., Goebbels, R. M. & Gianello, P. Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation 90 , 1054–1062 (2010).
Veriter, S. et al. Islets and mesenchymal stem cells co-encapsulation can improve subcutaneous bioartificial pancreas survival in diabetic primates. Xenotransplantation 18 , 276–276 (2011).
Google Scholar
Chendke, G. S. et al. Supporting survival of transplanted stem-cell-derived insulin-producing cells in an encapsulation device augmented with controlled release of amino acids. Adv. Biosyst. 3 , 1900086 (2019).
Lottes, A. E. et al. Navigating the regulatory pathway for medical devices – a conversation with the FDA, clinicians, researchers, and industry experts. J. Cardiovasc. Transl. Res. 15 , 927–943 (2022).
Businesswire. Vertex announces positive day 90 data for the first patient in the phase 1/2 clinical trial dosed with VX-880, a novel investigational stem cell-derived therapy for the treatment of type 1 diabetes. Businesswire www.businesswire.com/news/home/20211018005226/en/ (2021).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT02239354 (2022).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT05791201 (2024).
Moruzzi, N., Leibiger, B., Barker, C. J., Leibiger, I. B. & Berggren, P. O. Novel aspects of intra-islet communication: primary cilia and filopodia. Adv. Biol. Regul. 87 , 100919 (2023).
Burganova, G., Bridges, C., Thorn, P. & Landsman, L. The role of vascular cells in pancreatic beta-cell function. Front. Endocrinol. 12 , 667170 (2021).
Almaca, J., Weitz, J., Rodriguez-Diaz, R., Pereira, E. & Caicedo, A. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metab. 27 , 630–644.e4 (2018).
Bowers, D. T., Song, W., Wang, L. H. & Ma, M. Engineering the vasculature for islet transplantation. Acta Biomater. 95 , 131–151 (2019).
Yu, M. et al. Islet transplantation in the subcutaneous space achieves long-term euglycaemia in preclinical models of type 1 diabetes. Nat. Metab. 2 , 1013–1020 (2020).
Chua, C. Y. X. et al. Emerging immunomodulatory strategies for cell therapeutics. Trends Biotechnol. 41 , 358–373 (2023).
Forbes, S. et al. Human umbilical cord perivascular cells improve human pancreatic islet transplant function by increasing vascularization. Sci. Transl. Med. 12 , eaan5907 (2020).
Kinney, S. M., Ortaleza, K., Vlahos, A. E. & Sefton, M. V. Degradable methacrylic acid-based synthetic hydrogel for subcutaneous islet transplantation. Biomaterials 281 , 121342 (2022).
Weaver, J. D. et al. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci. Adv. 3 , e1700184 (2017).
Davalli, A. M. et al. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation 59 , 817–820 (1995).
Aghazadeh, Y. et al. Microvessels support engraftment and functionality of human islets and hESC-derived pancreatic progenitors in diabetes models. Cell Stem Cell 28 , 1936–1949.e8 (2021).
Wrublewsky, S. et al. Co-transplantation of pancreatic islets and microvascular fragments effectively restores normoglycemia in diabetic mice. NPJ Regen. Med. 7 , 67 (2022).
Wang, D. et al. Hyaluronic acid methacrylate/pancreatic extracellular matrix as a potential 3D printing bioink for constructing islet organoids. Acta Biomater. 165 , 86–101 (2023).
Tremmel, D. M. et al. A human pancreatic ECM hydrogel optimized for 3-D modeling of the islet microenvironment. Sci. Rep. 12 , 7188 (2022).
Citro, A. et al. Directed self-assembly of a xenogeneic vascularized endocrine pancreas for type 1 diabetes. Nat. Commun. 14 , 878 (2023).
Guyette, J. P. et al. Perfusion decellularization of whole organs. Nat. Protoc. 9 , 1451–1468 (2014).
Citro, A. et al. Biofabrication of a vascularized islet organ for type 1 diabetes. Biomaterials 199 , 40–51 (2019).
Song, W. et al. Engineering transferrable microvascular meshes for subcutaneous islet transplantation. Nat. Commun. 10 , 4602 (2019).
Salg, G. A. et al. Toward 3D-bioprinting of an endocrine pancreas: a building-block concept for bioartificial insulin-secreting tissue. J. Tissue Eng. 13 , 20417314221091033 (2022).
Liang, J. P. et al. Engineering a macroporous oxygen-generating scaffold for enhancing islet cell transplantation within an extrahepatic site. Acta Biomater. 130 , 268–280 (2021).
Dolgin, E. Diabetes cell therapies take evasive action. Nat. Biotechnol. 40 , 291–295 (2022).
Velazco-Cruz, L. et al. Acquisition of dynamic function in human stem cell-derived β cells. Stem Cell Rep. 12 , 351–365 (2019).
Capuani, S., Campa-Carranza, J. N., Hernandez, N., Chua, C. Y. X. & Grattoni, A. Modeling of a bioengineered immunomodulating microenvironment for cell therapy. Adv. Healthc. Mater. https://doi.org/10.1002/adhm.202304003 (2024).
Jang, S. B. et al. DAMP-modulating nanoparticle for successful pancreatic islet and stem cell transplantation. Biomaterials 287 , 121679 (2022).
Shapiro, A. M. J. et al. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep. Med. 2 , 100466 (2021).
Goswami, D. et al. Design considerations for macroencapsulation devices for stem cell derived islets for the treatment of type 1 diabetes. Adv. Sci. 8 , e2100820 (2021).
Pepper, A. R. et al. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat. Biotechnol. 33 , 518–523 (2015).
Farina, M. et al. Transcutaneously refillable, 3D-printed biopolymeric encapsulation system for the transplantation of endocrine cells. Biomaterials 177 , 125–138 (2018).
Paez-Mayorga, J. et al. Implantable niche with local immunosuppression for islet allotransplantation achieves type 1 diabetes reversal in rats. Nat. Commun. 13 , 7951 (2022).
Pepper, A. R. et al. Transplantation of human pancreatic endoderm cells reverses diabetes post transplantation in a prevascularized subcutaneous site. Stem Cell Rep. 8 , 1689–1700 (2017).
Pepper, A. R. et al. Posttransplant characterization of long-term functional hESC-derived pancreatic endoderm grafts. Diabetes 68 , 953–962 (2019).
Sernova Corp. Sernova provides recap of 2023 accomplishments and anticipated 2024 milestones. Sernova Corp www.sernova.com/press/release/?id=388 (2024).
Paez-Mayorga, J. et al. Enhanced in vivo vascularization of 3D-printed cell encapsulation device using platelet-rich plasma and mesenchymal stem cells. Adv. Healthc. Mater. 9 , e2000670 (2020).
Kuppan, P. et al. Co-transplantation of human adipose-derived mesenchymal stem cells with neonatal porcine islets within a prevascularized subcutaneous space augments the xenograft function. Xenotransplantation 27 , e12581 (2020).
Wassmer, C. H. et al. Bio-engineering of pre-vascularized islet organoids for the treatment of type 1 diabetes. Transpl. Int. 35 , 10214 (2021).
Rickels, M. R. & Robertson, R. P. Pancreatic islet transplantation in humans: recent progress and future directions. Endocr. Rev. 40 , 631–668 (2019).
Chang, C. A. et al. Curative islet and hematopoietic cell transplantation in diabetic mice without toxic bone marrow conditioning. Cell Rep. 41 , 111615 (2022).
Eledon Pharmaceuticals. Eledon reports updated data from ongoing phase 1b trial evaluating tegoprubart for prevention of rejection in kidney transplantation. Eledon Pharmaceuticals ir.eledon.com/news-releases/news-release-details/eledon-reports-updated-data-ongoing-phase-1b-trial-evaluating (2023).
Wisel, S. A. et al. A multi-modal approach to islet and pancreas transplantation with calcineurin-sparing immunosuppression maintains long-term insulin independence in patients with type I diabetes. Transpl. Int. 36 , 11367 (2023).
Anwar, I. J. et al. The anti-CD40L monoclonal antibody AT-1501 promotes islet and kidney allograft survival and function in nonhuman primates. Sci. Transl. Med. 15 , eadf6376 (2023).
Lee, K., Nguyen, V., Lee, K. M., Kang, S. M. & Tang, Q. Attenuation of donor-reactive T cells allows effective control of allograft rejection using regulatory T cell therapy. Am. J. Transpl. 14 , 27–38 (2014).
Cabello-Kindelan, C. et al. Immunomodulation followed by antigen-specific T reg infusion controls islet autoimmunity. Diabetes 69 , 215–227 (2020).
Yang, S. J. et al. Pancreatic islet-specific engineered T regs exhibit robust antigen-specific and bystander immune suppression in type 1 diabetes models. Sci. Transl. Med. 14 , eabn1716 (2022).
Marshall, G. P. et al. Biomaterials-based nanoparticles conjugated to regulatory T cells provide a modular system for localized delivery of pharmacotherapeutic agents. J. Biomed. Mater. Res. A 111 , 185–197 (2023).
Sicard, A. et al. Donor-specific chimeric antigen receptor T regs limit rejection in naive but not sensitized allograft recipients. Am. J. Transpl. 20 , 1562–1573 (2020).
Pierini, A. et al. T cells expressing chimeric antigen receptor promote immune tolerance. JCI Insight 2 , e92865 (2017).
Hu, M. et al. Low-dose interleukin-2 combined with rapamycin led to an expansion of CD4 + CD25 + FOXP3 + regulatory T cells and prolonged human islet allograft survival in humanized mice. Diabetes 69 , 1735–1748 (2020).
Yu, A. et al. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms supports the use of low-dose IL-2 therapy in type 1 diabetes. Diabetes 64 , 2172–2183 (2015).
Hering, B. J. et al. Factors associated with favourable 5 year outcomes in islet transplant alone recipients with type 1 diabetes complicated by severe hypoglycaemia in the Collaborative Islet Transplant Registry. Diabetologia 66 , 163–173 (2023).
Citro, A., Cantarelli, E., Pellegrini, S., Dugnani, E. & Piemonti, L. Anti-inflammatory strategies in intrahepatic islet transplantation: a comparative study in preclinical models. Transplantation 102 , 240–248 (2018).
Bachul, P. J. et al. Post-hoc analysis of a randomized, double blind, prospective study at the University of Chicago: additional standardizations of trial protocol are needed to evaluate the effect of a CXCR1/2 inhibitor in islet allotransplantation. Cell Transpl. 30 , 9636897211001774 (2021).
Ge, J. et al. Adjuvant conditioning induces an immunosuppressive milieu that delays alloislet rejection through the expansion of myeloid-derived suppressor cells. Am. J. Transpl. 23 , 935–945 (2023).
Velluto, D., Bojadzic, D., De Toni, T., Buchwald, P. & Tomei, A. A. Drug-integrating amphiphilic nanomaterial assemblies: 1. spatiotemporal control of cyclosporine delivery and activity using nanomicelles and nanofibrils. J. Control. Rel. 329 , 955–970 (2021).
Jamison, B. L. et al. Tolerogenic delivery of a hybrid insulin peptide markedly prolongs islet graft survival in the NOD mouse. Diabetes 71 , 483–496 (2022).
Kuppan, P. et al. Co-localized immune protection using dexamethasone-eluting micelles in a murine islet allograft model. Am. J. Transpl. 20 , 714–725 (2020).
Nguyen, T. T. et al. The impact of locally-delivered tacrolimus-releasing microspheres and polyethylene glycol-based islet surface modification on xenogeneic islet survival. J. Control. Rel. 336 , 274–284 (2021).
Nguyen, T. T. et al. Engineering “cell-particle hybrids” of pancreatic islets and bioadhesive FK506-loaded polymeric microspheres for local immunomodulation in xenogeneic islet transplantation. Biomaterials 221 , 119415 (2019).
Pathak, S. et al. Particulate-based single-dose local immunosuppressive regimen for inducing tolerogenic dendritic cells in xenogeneic islet transplantation. Adv. Healthc. Mater. 10 , e2001157 (2021).
Fan, Y., Zheng, X., Ali, Y., Berggren, P. O. & Loo, S. C. J. Local release of rapamycin by microparticles delays islet rejection within the anterior chamber of the eye. Sci. Rep. 9 , 3918 (2019).
Barra, J. M. et al. Localized cytotoxic T cell-associated antigen 4 and antioxidant islet encapsulation alters macrophage signaling and induces regulatory and anergic T cells to enhance allograft survival. Am. J. Transpl. 23 , 498–511 (2023).
Sremac, M. et al. Short-term function and immune-protection of microencapsulated adult porcine islets with alginate incorporating CXCL12 in healthy and diabetic non-human primates without systemic immune suppression: a pilot study. Xenotransplantation 30 , e12826 (2023).
Medina, J. D. et al. A hydrogel platform for co-delivery of immunomodulatory proteins for pancreatic islet allografts. J. Biomed. Mater. Res. A 110 , 1728–1737 (2022).
Li, Y. et al. Immunosuppressive PLGA TGF-β1 microparticles induce polyclonal and antigen-specific regulatory T cells for local immunomodulation of allogeneic islet transplants. Front. Immunol. 12 , 653088 (2021).
Skoumal, M. et al. Localized immune tolerance from FasL-functionalized PLG scaffolds. Biomaterials 192 , 271–281 (2019).
Coronel, M. M. et al. Immunotherapy via PD-L1-presenting biomaterials leads to long-term islet graft survival. Sci. Adv. 6 , eaba5573 (2020).
Polishevska, K. et al. Nanothin conformal coating with poly(N-vinylpyrrolidone) and tannic acid (PVPON/TA) preserves murine and human pancreatic islets function. Pharmaceutics 15 , 1137 (2023).
Gammon, J. M. et al. Engineering the lymph node environment promotes antigen-specific efficacy in type 1 diabetes and islet transplantation. Nat. Commun. 14 , 681 (2023).
Gooch, A. M., Chowdhury, S. S., Zhang, P. M., Hu, Z. M. & Westenfelder, C. Significant expansion of the donor pool achieved by utilizing islets of variable quality in the production of allogeneic “neo-islets”, 3-D organoids of mesenchymal stromal and islet cells, a novel immune-isolating biotherapy for type I diabetes. PLoS ONE 18 , e0290460 (2023).
Navaei-Nigjeh, M. et al. Microfluidically fabricated fibers containing pancreatic islets and mesenchymal stromal cells improve longevity and sustained normoglycemia in diabetic rats. Biofabrication 15 , 015013 (2022).
Lachaud, C. C. et al. Umbilical cord mesenchymal stromal cells transplantation delays the onset of hyperglycemia in the RIP-B7.1 mouse model of experimental autoimmune diabetes through multiple immunosuppressive and anti-inflammatory responses. Front. Cell Dev. Biol. 11 , 1089817 (2023).
Kenyon, N. S. et al. Extended survival versus accelerated rejection of nonhuman primate islet allografts: effect of mesenchymal stem cell source and timing. Am. J. Transpl. 21 , 3524–3537 (2021).
Wei, L. et al. Protective effect of mesenchymal stem cells on isolated islets survival and against hypoxia associated with the HIF-1α/PFKFB3 pathway. Cell Transpl. 31 , 9636897211073127 (2022).
Wang, X. et al. Engineered immunomodulatory accessory cells improve experimental allogeneic islet transplantation without immunosuppression. Sci. Adv. 8 , eabn0071 (2022).
Nguyen, T. T. et al. Engineering of hybrid spheroids of mesenchymal stem cells and drug depots for immunomodulating effect in islet xenotransplantation. Sci. Adv. 8 , eabn8614 (2022).
Sohni, A. & Verfaillie, C. M. Mesenchymal stem cells migration homing and tracking. Stem Cell Int. 2013 , 130763 (2013).
Mohammadi, M. R. et al. Exosome loaded immunomodulatory biomaterials alleviate local immune response in immunocompetent diabetic mice post islet xenotransplantation. Commun. Biol. 4 , 685 (2021).
Wang, L. et al. Engineered cytokine-primed extracellular vesicles with high PD-L1 expression ameliorate type 1 diabetes. Small 19 , e2301019 (2023).
Neshat, S. Y. et al. Improvement of islet engrafts via T reg induction using immunomodulating polymeric tolerogenic microparticles. ACS Biomater. Sci. Eng. 9 , 3522–3534 (2023).
Lebreton, F. et al. Mechanisms of immunomodulation and cytoprotection conferred to pancreatic islet by human amniotic epithelial cells. Stem Cell Rev. Rep. 18 , 346–359 (2022).
Huang, Q. et al. IL-10 producing type 2 innate lymphoid cells prolong islet allograft survival. EMBO Mol. Med. 12 , e12305 (2020).
Sackett, S. D. et al. Genetic engineering of immune evasive stem cell-derived islets. Transpl. Int. 35 , 10817 (2022).
Sackett, S. D., Rodriguez, A. & Odorico, J. S. The nexus of stem cell-derived beta-cells and genome engineering. Rev. Diabet. Stud. 14 , 39–50 (2017).
Forbes, S. et al. Islet transplantation outcomes in type 1 diabetes and transplantation of HLA-DQ8/DR4: results of a single-centre retrospective cohort in Canada. EClinicalMedicine 67 , 102333 (2024).
Hu, X. et al. Hypoimmune islets achieve insulin independence after allogeneic transplantation in a fully immunocompetent non-human primate. Cell Stem Cell 31 , 334–340.e5 (2024).
Parent, A. V. et al. Selective deletion of human leukocyte antigens protects stem cell-derived islets from immune rejection. Cell Rep. 36 , 109538 (2021).
Castro-Gutierrez, R., Alkanani, A., Mathews, C. E., Michels, A. & Russ, H. A. Protecting stem cell derived pancreatic beta-like cells from diabetogenic T cell recognition. Front. Endocrinol. 12 , 707881 (2021).
Santini-Gonzalez, J. et al. Human stem cell derived beta-like cells engineered to present PD-L1 improve transplant survival in NOD mice carrying human HLA class I. Front. Endocrinol. 13 , 989815 (2022).
Woodward, K. B. et al. Pancreatic islets engineered with a FasL protein induce systemic tolerance at the induction phase that evolves into long-term graft-localized immune privilege. Am. J. Transpl. 20 , 1285–1295 (2020).
Hu, X. et al. Hypoimmune induced pluripotent stem cells survive long term in fully immunocompetent, allogeneic rhesus macaques. Nat. Biotechnol. 42 , 413–423 (2024).
Shrestha, P. et al. Immune checkpoint CD47 molecule engineered islets mitigate instant blood-mediated inflammatory reaction and show improved engraftment following intraportal transplantation. Am. J. Transpl. 20 , 2703–2714 (2020).
Hu, X. et al. Human hypoimmune primary pancreatic islets avoid rejection and autoimmunity and alleviate diabetes in allogeneic humanized mice. Sci. Transl. Med. 15 , eadg5794 (2023).
Rao, J. S. et al. HLA-G1 + expression in GGTA1KO pigs suppresses human and monkey anti-pig T, B and NK cell responses. Front. Immunol. 12 , 730545 (2021).
Paul, P. K. et al. Islet allografts expressing a PD-L1 and IDO fusion protein evade immune rejection and reverse preexisting diabetes in immunocompetent mice without systemic immunosuppression. Am. J. Transpl. 22 , 2571–2585 (2022).
Yoshihara, E. et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature 586 , 606–611 (2020).
Lei, Y. et al. Neonatal islets from human PD-L1 transgenic pigs reduce immune cell activation and cellular rejection in humanized nonobese diabetic-scid IL2rγ null mice. Am. J. Transpl. 24 , 20–29 (2024).
Carvalho Oliveira, M. et al. Generating low immunogenic pig pancreatic islet cell clusters for xenotransplantation. J. Cell Mol. Med. 24 , 5070–5081 (2020).
Lorberbaum, D. S., Sarbaugh, D. & Sussel, L. Leveraging the strengths of mice, human stem cells, and organoids to model pancreas development and diabetes. Front. Endocrinol. 13 , 1042611 (2022).
Khosravi-Maharlooei, M. et al. Modeling human T1D-associated autoimmune processes. Mol. Metab. 56 , 101417 (2022).
Kottaisamy, C. P. D., Raj, D. S., Prasanth Kumar, V. & Sankaran, U. Experimental animal models for diabetes and its related complications – a review. Lab. Anim. Res. 37 , 23 (2021).
Walsh, N. C. et al. Humanized mouse models of clinical disease. Annu. Rev. Pathol. 12 , 187–215 (2017).
King, M., Pearson, T., Rossini, A. A., Shultz, L. D. & Greiner, D. L. Humanized mice for the study of type 1 diabetes and beta cell function. Ann. N. Y. Acad. Sci. 1150 , 46–53 (2008).
Shultz, L. D., Ishikawa, F. & Greiner, D. L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7 , 118–130 (2007).
Gerace, D. et al. Engineering human stem cell-derived islets to evade immune rejection and promote localized immune tolerance. Cell Rep. Med. 4 , 100879 (2023).
Vegas, A. J. et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 22 , 306–311 (2016).
Chen, Y. G., Mathews, C. E. & Driver, J. P. The role of NOD mice in type 1 diabetes research: lessons from the past and recommendations for the future. Front. Endocrinol. 9 , 51 (2018).
Burrack, A. L., Martinov, T. & Fife, B. T. T cell-mediated beta cell destruction: autoimmunity and alloimmunity in the context of type 1 diabetes. Front. Endocrinol. 8 , 343 (2017).
Serreze, D. V., Niens, M., Kulik, J. & DiLorenzo, T. P. in Mouse Models for Drug Discovery (Series Ed. Walker, J. M. Methods in Molecular Biology Vol. 1438) (eds Proetzel, G. & Wiles, M. V.) 137–151 (Springer, 2016).
Takaki, T. et al. HLA-A*0201-restricted T cells from humanized NOD mice recognize autoantigens of potential clinical relevance to type 1 diabetes. J. Immunol. 176 , 3257–3265 (2006).
Radenković, M., Stojanović, M. & Prostran, M. Experimental diabetes induced by alloxan and streptozotocin: the current state of the art. J. Pharmacol. Toxicol. Methods 78 , 13–31 (2016).
Furman, B. L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. 1 , e78 (2021).
Gvazava, I. G., Rogovaya, O. S., Borisov, M. A., Vorotelyak, E. A. & Vasiliev, A. V. Pathogenesis of type 1 diabetes mellitus and rodent experimental models. Acta Nat. 10 , 24–33 (2018).
Bortel, R. et al. Levels of Art2 + cells but not soluble Art2 protein correlate with expression of autoimmune diabetes in the BB rat. Autoimmunity 33 , 199–211 (2001).
Mordes, J. P., Bortell, R., Blankenhorn, E. P., Rossini, A. A. & Greiner, D. L. Rat models of type 1 diabetes: genetics, environment, and autoimmunity. ILAR J. 45 , 278–291 (2004).
Woda, B. A. & Padden, C. BioBreeding/Worcester (BB/Wor) rats are deficient in the generation of functional cytotoxic T cells. J. Immunol. 139 , 1514–1517 (1987).
Brehm, M. A., Powers, A. C., Shultz, L. D. & Greiner, D. L. Advancing animal models of human type 1 diabetes by engraftment of functional human tissues in immunodeficient mice. Cold Spring Harb. Perspect. Med. 2 , a007757 (2012).
Gonzalez, B. J., Creusot, R. J., Sykes, M. & Egli, D. How safe are universal pluripotent stem cells? Cell Stem Cell 26 , 307–308 (2020).
Rongvaux, A. et al. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu. Rev. Immunol. 31 , 635–674 (2013).
Brehm, M. A. et al. Human immune system development and rejection of human islet allografts in spontaneously diabetic NOD- Rag1 null IL2r γ null Ins2 Akita mice. Diabetes 59 , 2265–2270 (2010).
Tan, S. et al. Type 1 diabetes induction in humanized mice. Proc. Natl Acad. Sci. USA 114 , 10954–10959 (2017).
Ellis, C. E. et al. Human A2-CAR T cells reject HLA-A2 + human islets transplanted into mice without inducing graft-versus-host disease. Transplantation 107 , e222–e233 (2023).
CAS PubMed PubMed Central Google Scholar
Balboa, D., Iworima, D. G. & Kieffer, T. J. Human pluripotent stem cells to model islet defects in diabetes. Front. Endocrinol. 12 , 642152 (2021).
Doloff, J. C. et al. Identification of a humanized mouse model for functional testing of immune-mediated biomaterial foreign body response. Sci. Adv. 9 , eade9488 (2023).
Li, Y. et al. Humanized mice reveal new insights into the thymic selection of human autoreactive CD8 + T cells. Front. Immunol. 10 , 63 (2019).
Renner, S. et al. Permanent neonatal diabetes in INS C94Y transgenic pigs. Diabetes 62 , 1505–1511 (2013).
Pabst, R. The pig as a model for immunology research. Cell Tissue Res. 380 , 287–304 (2020).
Casu, A. et al. Metabolic aspects of pig-to-monkey ( Macaca fascicularis ) islet transplantation: implications for translation into clinical practice. Diabetologia 51 , 120–129 (2008).
Pepper, A. R. et al. Establishment of a stringent large animal model of insulin-dependent diabetes for islet autotransplantation: combination of pancreatectomy and streptozotocin. Pancreas 42 , 329–338 (2013).
Kin, T., Korbutt, G. S., Kobayashi, T., Dufour, J. M. & Rajotte, R. V. Reversal of diabetes in pancreatectomized pigs after transplantation of neonatal porcine islets. Diabetes 54 , 1032–1039 (2005).
Smink, A. M. et al. Successful islet transplantation into a subcutaneous polycaprolactone scaffold in mice and pigs. Transpl. Direct 9 , e1417 (2023).
Gibly, R. F. et al. Extrahepatic islet transplantation with microporous polymer scaffolds in syngeneic mouse and allogeneic porcine models. Biomaterials 32 , 9677–9684 (2011).
Kim, S. et al. Molecular and genetic regulation of pig pancreatic islet cell development. Development 147 , dev186213 (2020).
Knechtle, S. J., Shaw, J. M., Hering, B. J., Kraemer, K. & Madsen, J. C. Translational impact of NIH-funded nonhuman primate research in transplantation. Sci. Transl. Med. 11 , eaau0143 (2019).
Dehoux, J. P. & Gianello, P. The importance of large animal models in transplantation. Front. Biosci. 12 , 4864–4880 (2007).
Graham, M. L. & Schuurman, H. J. Validity of animal models of type 1 diabetes, and strategies to enhance their utility in translational research. Eur. J. Pharmacol. 759 , 221–230 (2015).
Fitch, Z. et al. Transplant research in nonhuman primates to evaluate clinically relevant immune strategies in organ transplantation. Transpl. Rev. 33 , 115–129 (2019).
Brennan, F. R. et al. Safety testing of monoclonal antibodies in non-human primates: case studies highlighting their impact on human risk assessment. MAbs 10 , 1–17 (2018).
Coe, T. M., Markmann, J. F. & Rickert, C. G. Current status of porcine islet xenotransplantation. Curr. Opin. Organ. Transpl. 25 , 449–456 (2020).
Graham, M. L. et al. Clinically available immunosuppression averts rejection but not systemic inflammation after porcine islet xenotransplant in cynomolgus macaques. Am. J. Transpl. 22 , 745–760 (2022).
Sykes, M. & Sachs, D. H. Progress in xenotransplantation: overcoming immune barriers. Nat. Rev. Nephrol. 18 , 745–761 (2022).
Kirk, A. D. Crossing the bridge: large animal models in translational transplantation research. Immunol. Rev. 196 , 176–196 (2003).
D’Amour, K. A. et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23 , 1534–1541 (2005).
D’Amour, K. A. et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24 , 1392–1401 (2006).
Nair, G. G. et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat. Cell Biol. 21 , 263–274 (2019).
Breakthrough T1D. Cures program research strategy. BreakthroughT1D.org https://www.breakthrought1d.org/wp-content/uploads/2024/05/Breakthrough-T1D-Research-Strategy-Cures-1.pdf (2024).
Lu, K., Brauns, T., Sluder, A. E., Poznansky, M. C. & Dogan, F. Combinatorial islet protective therapeutic approaches in β-cell transplantation: rationally designed solutions using a target product profile. FASEB Bioadv 5 , 287–304 (2023).
European Commission. Diabetes Reversing Implants with Enhanced Viability and Long-term Efficacy (CORDIS, 2015).
European Commission. Beta Cell Generation By Stem Cell-derived Implants In Diabetes (CORDIS, 2015).
Breakthrough T1D. Breakthrough T1D Beta Cell Replacement Consortium: sharing data and resources, saving time and money. BreakthroughT1D.org https://www.breakthrought1d.org/news-and-updates/jdrf-beta-cell-replacement-consortium-sharing-data-resources-saving-time-money/ (2019).
US Food and Drug Administration. FDA approves first cellular therapy to treat patients with type 1 diabetes. FDA www.fda.gov/news-events/press-announcements/fda-approves-first-cellular-therapy-treat-patients-type-1-diabetes (2023).
Download references
Acknowledgements
A.G. is supported by NIH NIDDK R01DK132104, R01DK133610, JDRF 2-SRA-2022-1224-S-B, JDRF 2-SRA-2021-1078-S-B, Vivian Smith Foundation and Men of Distinction. A.R.P. is supported through a JDRF Career Development Award (5-CDA-2020-945-A-N) and is a Canada Research Chair in Cell Therapies for Diabetes thanks to funding from the Canada Research Chairs Program. A.C. is supported by a grant from JDRF (3-SRA-2022-1155-S-B) and the Italian Ministry of Health (GR-2018-12366399). M.B. is a consultant for The Jackson Laboratory. J.R.M. was supported by the NIH (R01DK114233), JDRF (3-SRA-2023-1295-S-B), and the Edward J Mallinckrodt Foundation. H.A.R. is or was supported by NIDDK R01DK12044, NIDDK R01DK132387, NINDS 1R01NS122911, NIDDK/HIRN RRID: SCR_014393; UC24 DK104162, JDRF SRA 2-SRA-2023-1313-S-B and 3-SRA-2023-1367-S-B, and the Diabetes Research Connection. M.C.P. is supported by JDRF grants 2-SRA 2021 1075-S-B and 3-SRA 2023 1365-S-B, the VIC Innovation Fund and The Hill Family Foundation. F. Dogan helped to create Fig. 2 . The authors thank S. P. Rodgers and R. E. Whitehead for their support in finalizing the manuscript and figures.
Author information
These authors contributed equally: Nicholas E. Mamrak, Jaime Giraldo, Mark C. Poznansky, Paul de Vos.
Authors and Affiliations
Department of Nanomedicine, Houston Methodist Research Institute, Houston, TX, USA
Alessandro Grattoni
Department of Surgery, Houston Methodist Hospital, Houston, TX, USA
Department of Radiation Oncology, Houston Methodist Hospital, Houston, TX, USA
Alberta Diabetes Institute, University of Alberta, Edmonton, Alberta, Canada
Gregory Korbutt
Department of Surgery, University of Alberta, Edmonton, Alberta, Canada
Gregory Korbutt & Andrew R. Pepper
Diabetes Research Institute, University of Miami Miller School of Medicine, Miami, FL, USA
Alice A. Tomei, Norma Kenyon & Camillo Ricordi
Department of Biomedical Engineering, University of Miami, Miami, FL, USA
Alice A. Tomei
Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, USA
Department of Microbiology and Immunology, University of Miami Miller School of Medicine, Miami, FL, USA
Woodruff School of Mechanical Engineering and Petit Institute for Bioengineering and Bioscience, Georgia Institute of Technology, Atlanta, GA, USA
Andrés J. García
J. Crayton Pruitt Family Department of Biomedical Engineering, Herbert Wertheim College of Engineering, University of Florida, Gainesville, FL, USA
Cherie Stabler
Diabetes Institute, University of Florida, Gainesville, FL, USA
Cherie Stabler & Holger A. Russ
Program in Molecular Medicine, Diabetes Center of Excellence, University of Massachusetts Chan Medical School, Worcester, MA, USA
Michael Brehm
Department of Surgery, The University of Arizona, Tucson, AZ, USA
Klearchos Papas
Diabetes Research Institute, IRCCS Ospedale San Raffaele, Milan, Italy
Antonio Citro
Department of Pediatrics, Ellis Fischel Cancer Center, School of Medicine, University of Missouri, Columbia, MO, USA
Haval Shirwan
Division of Endocrinology, Metabolism and Lipid Research, Washington University School of Medicine, St. Louis, MO, USA
Jeffrey R. Millman
Department of Biomedical Engineering, Washington University in St. Louis, St. Louis, MO, USA
Department of Cardiac Surgery, Boston Children’s Hospital, Boston, MA, USA
Juan Melero-Martin
Department of Surgery, Harvard Medical School, Boston, MA, USA
Harvard Stem Cell Institute, Cambridge, MA, USA
Department of Surgery, University of Minnesota, Minneapolis, MN, USA
Melanie Graham
Department of Veterinary Population Medicine, University of Minnesota, St. Paul, MN, USA
Institute of Biomedical Engineering, University of Toronto, Toronto, Ontario, Canada
Michael Sefton
Department of Chemical Engineering and Applied Chemistry, University of Toronto, Toronto, Ontario, Canada
Department of Biological and Environmental Engineering, Cornell University, Ithaca, NY, USA
Department of Bioengineering, Rice University, Houston, TX, USA
Omid Veiseh
University of California, San Francisco, Department of Bioengineering and Therapeutic Sciences, San Francisco, CA, USA
Tejal A. Desai
Brown University, School of Engineering, Providence, RI, USA
McEwen Stem Cell Institute, University Health Network, Toronto, ON, Canada
M. Cristina Nostro
Department of Physiology, University of Toronto, Toronto, ON, Canada
Advocacy Department, Breakthrough T1D, Washington, DC, USA
Marjana Marinac
Department of Medicine, Columbia Center for Translational Immunology, Columbia University, New York, NY, USA
Megan Sykes
Department of Microbiology and Immunology, Columbia University, New York, NY, USA
Department of Surgery, Columbia University, New York, NY, USA
Department of Pharmacology and Therapeutics, University of Florida, Gainesville, FL, USA
Holger A. Russ
UW Health Transplant Center, Madison, WI, USA
Jon Odorico
Division of Transplantation, Department of Surgery, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA
Diabetes Center, University of California San Francisco, San Francisco, CA, USA
Department of Surgery, University of California San Francisco, San Francisco, CA, US
Gladstone Institute of Genomic Immunology, University of California San Francisco, San Francisco, CA, USA
Research Department, Breakthrough T1D, New York, NY, USA
Esther Latres, Nicholas E. Mamrak & Jaime Giraldo
Vaccine and Immunotherapy Center, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Mark C. Poznansky
Immunoendocrinology, Division of Medical Biology, Department of Pathology and Medical Biology, University of Groningen and University Medical Center Groningen, Groningen, Netherlands
Paul de Vos
You can also search for this author in PubMed Google Scholar
Contributions
A.G., N.E.M., J.G., M.C.P. and P.deV. researched data for the article, made a substantial contribution to discussion of content, wrote, and reviewed/edited the manuscript before submission. G.K., A.A.T., A.J.G., A.R.P., C.S., M.B., K.P., A.C., H.S., J.R.M., J.M.-M., M.G., M. Sefton, M. Ma, N.K., O.V., T.A.D., M.C.N., M. Marinac, M. Sykes, H.A.R., J.O. and Q.T. researched data for the article, made a substantial contribution to discussion of content and wrote the article. C.R. and E.L. wrote, and reviewed/edited the manuscript before submission.
Corresponding authors
Correspondence to Alessandro Grattoni , Jaime Giraldo , Mark C. Poznansky or Paul de Vos .
Ethics declarations
Competing interests.
A.G. is a co-founder of Continuity Biosciences LLC, and an inventor of intellectual property licensed by the same company. A.J.G. is an inventor of intellectual property related to technologies for cell therapy in T1DM owned in part by the Georgia Tech Research Corporation, is a co-founder, sits on the Board of Directors, and owns equity interest in iTolerance Inc. H.S. is an inventor on a patent licensed by iTolerance Inc, is a co-founder of the Company, and serves on the scientific advisory board of the Company. M. Ma is a co-founder and equity holder of AvantGuard and Persista Bio. J.O. is co-founder, owns stock equity, and serves on the scientific advisory board of Regenerative Medical Solutions Inc., is a clinical trial investigator for Vertex Pharmaceuticals Inc., and is a member of DSMB for Sernova Corp. J.R.M. is an inventor on related patents and patent applications, was employed at Sana Biotechnology, and has stocks and options in Sana Biotechnology. T.A.D. is a scientific founder of Encellin Inc., a cell therapy device company. K.P. discloses interest in Procyon Technologies LLC. M.C.N. has a sponsored research agreement with Universal Cells Inc., and a patent licensed to Sernova Corp. H.A.R. holds patents in the regenerative medicine space and served as SAB member of Sigilon Therapeutics, Prellis Biologics and consults or consulted for Sigilon Therapeutics, Eli Lilly, Minutia, Guidepoint Global, Axon Advisors and Tolerance Bio. C.R. is scientific adviser to Novo Nordisk, Vertex Pharma and iTolerance, and is a founding scientist of Lipogems International and AION Healthspan. M.C.P. is scientific founder of Vicapsys Life Sciences Inc. All other authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Endocrinology thanks Emmanuel Opara, Adrian Teo, Shareen Forbes for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Grattoni, A., Korbutt, G., Tomei, A.A. et al. Harnessing cellular therapeutics for type 1 diabetes mellitus: progress, challenges, and the road ahead. Nat Rev Endocrinol (2024). https://doi.org/10.1038/s41574-024-01029-0
Download citation
Accepted : 06 August 2024
Published : 03 September 2024
DOI : https://doi.org/10.1038/s41574-024-01029-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Talk to us about diabetes
0345 123 2399
customer support
Your top priorities for research into type 1 diabetes revealed

Hundreds of people with type 1 diabetes, their families and healthcare professionals have chosen their most pressing research priorities for type 1 diabetes. The top ten priorities will help to guide future type 1 diabetes research in the UK and Ireland to make sure it has the greatest possible benefit for people with the condition.
As the UK’s largest charitable funder of diabetes research, it’s critical that our research funds address the specific challenges and needs of people with diabetes and those who care for them.
It’s also critical that we make sure others - academics, healthcare professionals and other research funders – hear these views loud and clear and act upon them.
We work with the Priority Setting Partnership (PSP) initiative, run by the James Lind Alliance (JLA) and supported by the National Institute for Health Research (NIHR) , to help bring the views of people with real-life experience of diabetes into research.
Through surveys and workshops, this initiative finds and prioritises their most pressing concerns and questions that can be answered through research. Diabetes UK contributed to the first Type 1 Diabetes PSP in 2011, the Diabetes and Pregnancy PSP in 2020 , and led the Type 2 Diabetes PSP in 2017.
Your new top ten priorities
Since the last Type 1 Diabetes PSP in 2011 there's been some big changes in type 1 treatment and care , so the priorities were due an update. The latest Type 1 PSP – which condensed and whittled down nearly 3000 questions submitted by people affected by type 1 to a shortlist of the top ten – has just been published . And here they are:
1. Can the use of artificial intelligence or faster acting insulins help achieve fully closed loop insulin delivery?
2. Is time in range a better predictor of diabetes management and complications compared to HbA1c (an average reading of blood sugar over a 3-month period)?
3. What impact do hormonal phases such as the perimenstrual period and menopause play in glycaemic management and what treatments are most effective for managing glucose levels around these times?
4. What interventions are the most effective for reducing diabetes related distress and burnout?
5. What are the long-term implications of frequent hypoglycaemia on physical and mental health?
6. What impact does type 1 diabetes (including frequent low blood sugar) have on memory and cognition in older adults?
7. How can health care professionals better take into account the physical, psychological and social aspects of type 1 diabetes in clinics?
8. How can access to potential therapies like stem cell therapy, transplants and medications that modify the immune systems be improved so that everyone with type 1 diabetes can be guaranteed access?
9. Why do some people with type 1 diabetes become insulin resistant and does resistance increase with the number of years a person has diabetes and if so, why?
10. Can technology assist to accurately count carbohydrates without having to weigh or measure all foods and drink?
Dr Christine Newman, Lead Clinical Researcher at the Health Research Board Diabetes Collaborative Clinical Trial Network in Ireland who funded the PSP, emphasised the importance of these findings:
“This study is a powerful example of how Public and Patient Involvement can shape the future of healthcare. This work highlights the real-world challenges and unmet needs of adults living with Type 1 diabetes. By focusing on these top ten priorities, we can ensure that future research and healthcare services are aligned with what truly matters to those affected by the condition.”
We will use these top 10 research priorities in the decisions it makes about how research is funded, and they will inform the work of the Diabetes Research Steering Groups .
We will also publicise these priorities widely to researchers and organisations that fund diabetes research. The priorities could influence those who work in universities and academic institutions, government agencies or in industry.
Dr Elizabeth Robertson, Director of Research at Diabetes UK, explains:
“We need to make sure research that we fund has the greatest possible benefit for people with diabetes. Knowing the most important priorities of people living with or treating type 1 diabetes will help us direct funding to where it’s needed most.”
Share this Page
© The British Diabetic Association operating as Diabetes UK, a charity registered in England and Wales (no. 215199) and in Scotland (no. SC039136). A company limited by guarantee registered in England and Wales with (no.00339181) and registered office at Wells Lawrence House, 126 Back Church Lane London E1 1FH

Advertisement
Lab-grown stem cells could be a 'breakthrough' for cancer treatment
Stem cells made in the lab may one day aid cancer treatment by reducing our reliance on donors
By James Woodford
2 September 2024
Stem cells are produced by bone marrow and can turn into different types of blood cells
KATERYNA KON / SPL/ Alamy
Human blood stem cells have been made in a laboratory for the first time, which could significantly improve how we treat certain types of cancer .
The lab-grown cells have so far only been tested in mice, but when infused into the animals, the cells became functional bone marrow at similar levels to those seen after umbilical cord blood cell transplants.
A new understanding of how your blood type influences your health
Treating cancers such as leukaemia and lymphoma via radiation and chemotherapy can destroy the blood-forming cells in bone marrow. A stem cell transplant means that new, healthy bone marrow and blood cells can grow. Umbilical cords are a particularly rich source of stem cells, but donations are limited and the transplant can be rejected by the body.
The new method would allow researchers to produce stem cells from the actual patient, eliminating the supply issue and reducing the risk that their body would reject them.
First, human blood or skin cells were turned into so-called pluripotent stem cells through a process called reprogramming. “This involves temporarily turning on four genes, with the result that the patient cells revert to an early stage of development when they can become any cell in the body,” says Andrew Elefanty at the Murdoch Children’s Research Institute in Melbourne.
Sign up to our Health Check newsletter
Get the most essential health and fitness news in your inbox every Saturday.
The second stage involved turning the pluripotent cells into blood stem cells. “We first make thousands of small floating balls of cells, a few hundred cells in each ball, and direct them to change from being stem cells to sequentially become blood vessels and then blood cells,” says Elefanty. This process, called differentiation, takes about two weeks and makes millions of blood cells, he says.
These cells were then infused into mice that lacked an immune system and became functional bone marrow in up to 50 per cent of cases. This means it made the same cells that carry oxygen and fight infections as healthy human bone marrow does, says Elefanty. “It is this unique ability to make all the blood cell types for a prolonged period of time that defines the cells as blood stem cells,” he says.
We are finally improving prostate cancer diagnoses – here's how
Cases of prostate cancer are surging alarmingly around the world. Thankfully, we are developing more accurate tests that can catch the condition early
Abbas Shafiee at the University of Queensland in Brisbane says the work is a “magnificent breakthrough” towards new therapies for blood cancers. “It has not been done before and it has a lot of potential for the future.” But even once animal testing is complete, a lot of research in humans needs to be done before the approach can be used in clinics, he says.
Simon Conn at Flinders University in Adelaide, Australia, says a key advantage of the team’s approach is that it could be scaled up to produce “an essentially never-ending supply” of blood stem cells. But he adds that the rate of success and the diversity of the blood cells varied. “This suggests the treatment, even at the preclinical stage in mice, is not consistent, which will need to be addressed prior to any clinical trials in human patients,” he says.
Journal reference:
Nature Biotechnology DOI: 10.1038/s41587-024-02360-7
- stem cells /
Sign up to our weekly newsletter
Receive a weekly dose of discovery in your inbox! We'll also keep you up to date with New Scientist events and special offers.
More from New Scientist
Explore the latest news, articles and features
Stem cells grown in labs for experimental therapies pose a cancer risk
Subscriber-only

Wiping stem cells 'clean' could make them easier to produce
Genetically modified stem cells extend lifespan of mice by 20 per cent
Stem cells treat diabetes without triggering immune response in mice
Popular articles.
Trending New Scientist articles
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
The evaluation of selected trace elements in blood, serum and blood cells of type 2 diabetes patients with and without renal disorder.

1. Introduction
2. materials and methods, 3.1. results of chromium determination in whole blood, serum, and blood cells, 3.2. results of nickel determination in whole blood, serum, and blood cells, 3.3. results of cobalt determination in whole blood, serum, and blood cells, 3.4. results of manganese determination in whole blood, serum, and blood cells, 3.5. results of zinc determination in whole blood, serum, and blood cells, 3.6. results of assessment of elemental contents in the diet of diabetic and healthy people, 4. discussion, 4.1. chromium, 4.2. nickel, 4.3. cobalt, 4.4. manganese, 4.6. limitations, 5. conclusions, supplementary materials, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global. Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022 , 183 , 109119. [ Google Scholar ] [ CrossRef ]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and Type 2 Diabetes Mellitus: Connections in Epidemiology. Pathogenesis. and Treatments. Front. Endocrinol. 2023 , 14 , 1161521. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and Exercise in the Prevention and Treatment of Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2020 , 16 , 545–555. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ngala, R.A.; Awe, M.A.; Nsiah, P. The Effects of Plasma Chromium on Lipid Profile. Glucose Metabolism and Cardiovascular Risk in Type 2 Diabetes Mellitus. A Case—Control Study. PLoS ONE 2018 , 13 , e0197977. [ Google Scholar ] [ CrossRef ]
- Kazi, T.G.; Afridi, H.I.; Kazi, N.; Jamali, M.K.; Arain, M.B.; Jalbani, N.; Kandhro, G.A. Copper, Chromium, Manganese, Iron, Nickel, and Zinc Levels in Biological Samples of Diabetes Mellitus Patients. Biol. Trace Elem. Res. 2008 , 122 , 1–18. [ Google Scholar ] [ CrossRef ]
- Bjørklund, G.; Dadar, M.; Pivina, L.; Doşa, M.D.; Semenova, Y.; Aaseth, J. The Role of Zinc and Copper in Insulin Resistance and Diabetes Mellitus. Curr. Med. Chem. 2020 , 27 , 6643–6657. [ Google Scholar ] [ CrossRef ]
- Hansen, A.F.; Simić, A.; Åsvold, B.O.; Romundstad, P.R.; Midthjell, K.; Syversen, T.; Flaten, T.P. Trace Elements in Early Phase Type 2 Diabetes Mellitus—A Population-Based Study. The HUNT Study in Norway. J. Trace Elem. Med. Biol. 2017 , 40 , 46–53. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk. Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016 , 37 , 278–316. [ Google Scholar ] [ CrossRef ]
- Springmann, M.; Wiebe, K.; Mason-D’Croz, D.; Sulser, T.B.; Rayner, M.; Scarborough, P. Health and Nutritional Aspects of Sustainable Diet Strategies and Their Association with Environmental Impacts: A Global Modelling Analysis with Country-Level Detail. Lancet Planet. Health 2018 , 2 , e451–e461. [ Google Scholar ] [ CrossRef ]
- Toi, P.L.; Anothaisintawee, T.; Chaikledkaew, U.; Briones, J.R.; Reutrakul, S.; Thakkinstian, A. Preventive Role of Diet Interventions and Dietary Factors in Type 2 Diabetes Mellitus: An Umbrella Review. Nutrients 2020 , 12 , 2722. [ Google Scholar ] [ CrossRef ]
- Petroni, M.L.; Brodosi, L.; Marchignoli, F.; Sasdelli, A.S.; Caraceni, P.; Marchesini, G.; Ravaioli, F. Nutrition in Patients with Type 2 Diabetes: Present Knowledge and Remaining Challenges. Nutrients 2021 , 13 , 2748. [ Google Scholar ] [ CrossRef ]
- Jarosz, M.; Jarosz, M. Normy Żywienia Dla Populacji Polskiej—Nowelizacja ; Instytut Żywności i Żywienia: Warszawa, Poland, 2012; ISBN 978-83-86060-83-2. [ Google Scholar ]
- Reynolds, A.; Mitri, J. Dietary Advice For Individuals with Diabetes. In Endotext ; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com. Inc.: South Dartmouth, MA, USA, 2000. [ Google Scholar ]
- Ko, G.; Kalantar-Zadeh, K.; Goldstein-Fuchs, J.; Rhee, C. Dietary Approaches in the Management of Diabetic Patients with Kidney Disease. Nutrients 2017 , 9 , 824. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Beverly, E.A.; Bruemmer, D.; Collins, B.S.; Darville, A.; Ekhlaspour, L.; Hassanein, M.; et al. 5. Facilitating Positive Health Behaviors and Well-Being to Improve Health Outcomes: Standards of Care in Diabetes—2024. Diabetes Care 2024 , 47 , S77–S110. [ Google Scholar ] [ CrossRef ]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Borys, S.; Broncel, M.; Budzyński, A.; Cyganek, K.; Cypryk, K.; Cyranka, K.; Czupryniak, L.; Dzida, G.; et al. Standards of Care in Diabetes. The Position of Diabetes Poland—2024. Curr. Top. Diabetes 2024 , 3 , 1–348. [ Google Scholar ] [ CrossRef ]
- Reddy, S.; Anoop, S.; Jebasingh, F.K.; Dasgupta, R.; Joseph, M.; Saravanan, B.; Volena, R.; Mani, T.; Somasundaram, S.; Bhattacharji, S.; et al. Differentials in Dietary Intake of Macro and Micronutrients in Patients with Type 2 Diabetes and Foot Ulcers: Observations from a Pilot Study. Clin. Nutr. ESPEN 2022 , 47 , 170–176. [ Google Scholar ] [ CrossRef ]
- Anderson, R.A. Chromium Metabolism and Its Role in Disease Processes in Man. Clin. Physiol. Biochem. 1986 , 4 , 31–41. [ Google Scholar ]
- Vincent, J.B.; Lukaski, H.C. Chromium. Adv. Nutr. 2018 , 9 , 505–506. [ Google Scholar ] [ CrossRef ]
- Havel, P.J. A Scientific Review: The Role of Chromium in Insulin Resistance. Diabetes Educ. 2004 , 3 , 2–14. [ Google Scholar ]
- Zhao, F.; Pan, D.; Wang, N.; Xia, H.; Zhang, H.; Wang, S.; Sun, G. Effect of Chromium Supplementation on Blood Glucose and Lipid Levels in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2022 , 200 , 516–525. [ Google Scholar ] [ CrossRef ]
- Davies, S.; Howard, J.M.; Hunnisett, A.; Howard, M. Age-Related Decreases in Chromium Levels in 51.665 Hair. Sweat. and Serum Samples from 40.872 Patients—Implications for the Prevention of Cardiovascular Disease and Type II Diabetes Mellitus. Metabolism 1997 , 46 , 469–473. [ Google Scholar ] [ CrossRef ]
- Rajendran, K. Serum Chromium Levels in Type 2 Diabetic Patients and Its Association with Glycaemic Control. J. Clin. Diagn. Res. 2015 , 9 , OC05. [ Google Scholar ] [ CrossRef ]
- Eva, H.; Akter, Q.S.; Alam, M.K. Relationship between Glycemic Status and Serum Chromium Level with Type 2 Diabetes Mellitus. Mymensingh Med. J. MMJ 2020 , 29 , 183–186. [ Google Scholar ] [ PubMed ]
- Yakout, S.; Faqeeh, F.; Al-Attas, O.; Hussain, S.D.; Saadawy, G.M.; Alokail, M.S. Patterns of Essential Trace Elements (Cr. Mn. Ni. and Se) in Saudi Patients with Type 2 Diabetes Mellitus. Am. J. Transl. Res. 2022 , 14 , 8175. [ Google Scholar ] [ PubMed ]
- Chen, S.; Jin, X.; Shan, Z.; Li, S.; Yin, J.; Sun, T.; Luo, C.; Yang, W.; Yao, P.; Yu, K.; et al. Inverse Association of Plasma Chromium Levels with Newly Diagnosed Type 2 Diabetes: A Case-Control Study. Nutrients 2017 , 9 , 294. [ Google Scholar ] [ CrossRef ]
- Rükgauer, M.; Zeyfang, A. Chromium Determinations in Blood Cells. Biol. Trace Elem. Res. 2002 , 86 , 193–202. [ Google Scholar ] [ CrossRef ]
- Lin, C.-C.; Shih, C.-T.; Lee, C.-H.; Huang, Y.-L. Changes in Trace Elements During Early Stages of Chronic Kidney Disease in Type 2 Diabetic Patients. Biol. Trace Elem. Res. 2018 , 186 , 330–336. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Makhlough, A.; Makhlough, M.; Shokrzadeh, M.; Mohammadian, M.; Sedighi, O.; Faghihan, M. Comparing the Levels of Trace Elements in Patients With Diabetic Nephropathy and Healthy Individuals. Nephro-Urol. Mon. 2015 , 7 , e28576. [ Google Scholar ] [ CrossRef ]
- Chen, Y.W.; Yang, C.Y.; Huang, C.F.; Hung, D.Z.; Leung, Y.M.; Liu, S.H. Heavy Metals. Islet Function and Diabetes Development. Islets 2009 , 1 , 169–176. [ Google Scholar ] [ CrossRef ]
- Mansouri, B.; Rezaei, A.; Sharafi, K.; Azadi, N.; Pirsaheb, M.; Rezaei, M.; Nakhaee, S. Mixture Effects of Trace Element Levels on Cardiovascular Diseases and Type 2 Diabetes Risk in Adults Using G-Computation Analysis. Sci. Rep. 2024 , 14 , 5743. [ Google Scholar ] [ CrossRef ]
- Zhang, Y.; Cai, J.; Yao, Z.; Zhang, H.; Wang, Z.; Lei, J.; Guo, H. The Relationship between Plasma Nickel Concentrations and Type 2 Diabetes Mellitus Risk: A Protective Effect within a Specific Range. J. Trace Elem. Med. Biol. 2024 , 82 , 127362. [ Google Scholar ] [ CrossRef ]
- Cartañà, J.; Arola, L. Nickel-Induced Hyperglycaemia: The Role of Insulin and Glucagon. Toxicology 1992 , 71 , 181–192. [ Google Scholar ] [ CrossRef ]
- Serdar, M.; Bakir, F.; Hasimi, A.; Celik, T.; Akin, O.; Kenar, L.; Aykut, O.; Yildirimkaya, M. Trace and Toxic Element Patterns in Nonsmoker Patients with Noninsulin-Dependent Diabetes Mellitus. Impaired Glucose Tolerance. and Fasting Glucose. Int. J. Diabetes Dev. Ctries. 2009 , 29 , 35. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cao, J.; Vecoli, C.; Neglia, D.; Tavazzi, B.; Lazzarino, G.; Novelli, M.; Masiello, P.; Wang, Y.; Puri, N.; Paolocci, N.; et al. Cobalt-Protoporphyrin Improves Heart Function by Blunting Oxidative Stress and Restoring NO Synthase Equilibrium in an Animal Model of Experimental Diabetes. Front. Physiol. 2012 , 3 , 160. [ Google Scholar ] [ CrossRef ]
- Cao, B.; Fang, C.; Peng, X.; Li, X.; Hu, X.; Xiang, P.; Zhou, L.; Liu, H.; Huang, Y.; Zhang, Q.; et al. U-Shaped Association between Plasma Cobalt Levels and Type 2 Diabetes. Chemosphere 2021 , 267 , 129224. [ Google Scholar ] [ CrossRef ]
- Vasudevan, H.; McNeill, J.H. Chronic Cobalt Treatment Decreases Hyperglycemia in Streptozotocin-Diabetic Rats. BioMetals 2007 , 20 , 129–134. [ Google Scholar ] [ CrossRef ]
- Luan, F.; Chen, Y.; Xu, Y.; Jiang, X.; Liu, B.; Wang, Y. Associations between Whole Blood Trace Elements Concentrations and HbA1c Levels in Patients with Type 2 Diabetes. BioMetals 2022 , 35 , 1011–1022. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ohtomo, S.; Nangaku, M.; Izuhara, Y.; Takizawa, S.; Strihou, C.V.Y.D.; Miyata, T. Cobalt Ameliorates Renal Injury in an Obese. Hypertensive Type 2 Diabetes Rat Model. Nephrol. Dial. Transplant. 2007 , 23 , 1166–1172. [ Google Scholar ] [ CrossRef ]
- Aguirre, J.D.; Culotta, V.C. Battles with Iron: Manganese in Oxidative Stress Protection. J. Biol. Chem. 2012 , 287 , 13541–13548. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Du, S.; Wu, X.; Han, T.; Duan, W.; Liu, L.; Qi, J.; Niu, Y.; Na, L.; Sun, C. Dietary Manganese and Type 2 Diabetes Mellitus: Two Prospective Cohort Studies in China. Diabetologia 2018 , 61 , 1985–1995. [ Google Scholar ] [ CrossRef ]
- Gong, J.H.; Lo, K.; Liu, Q.; Li, J.; Lai, S.; Shadyab, A.H.; Arcan, C.; Snetselaar, L.; Liu, S. Dietary Manganese. Plasma Markers of Inflammation. and the Development of Type 2 Diabetes in Postmenopausal Women: Findings From the Women’s Health Initiative. Diabetes Care 2020 , 43 , 1344–1351. [ Google Scholar ] [ CrossRef ]
- Sanjeevi, N.; Freeland-Graves, J.; Beretvas, N.S.; Sachdev, P.K. Trace Element Status in Type 2 Diabetes: A Meta-Analysis. J. Clin. Diagn. Res. 2018 , 12 , OE01. [ Google Scholar ] [ CrossRef ]
- Koh, E.S.; Kim, S.J.; Yoon, H.E.; Chung, J.H.; Chung, S.; Park, C.W.; Chang, Y.S.; Shin, S.J. Association of Blood Manganese Level with Diabetes and Renal Dysfunction: A Cross-Sectional Study of the Korean General Population. BMC Endocr. Disord. 2014 , 14 , 24. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Walter, R.M.; Uriu-Hare, J.Y.; Olin, K.L.; Oster, M.H.; Anawalt, B.D.; Critchfield, J.W.; Keen, C.L. Copper. Zinc. Manganese. and Magnesium Status and Complications of Diabetes Mellitus. Diabetes Care 1991 , 14 , 1050–1056. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Al-Maroof, R.A.; Al-Sharbatti, S.S. Serum Zinc Levels in Diabetic Patients and Effect of Zinc Supplementation on Glycemic Control of Type 2 Diabetics. Saudi Med. J. 2006 , 27 , 344–350. [ Google Scholar ] [ PubMed ]
- Fernández-Cao, J.C.; Warthon-Medina, M.; Moran, V.H.; Arija, V.; Doepking, C.; Serra-Majem, L.; Lowe, N.M. Zinc Intake and Status and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2019 , 11 , 1027. [ Google Scholar ] [ CrossRef ]
- Marreiro, D.D.N.; Geloneze, B.; Tambascia, M.A.; Lerário, A.C.; Halpern, A.; Cozzolino, S.M.F. Effect of Zinc Supplementation on Serum Leptin Levels and Insulin Resistance of Obese Women. Biol. Trace Elem. Res. 2006 , 112 , 109–118. [ Google Scholar ] [ CrossRef ]
| Chromium [µg/L] | Manganese [µg/L] | Cobalt [µg/L] | Nickel [µg/L] | Zinc [mg/L] | |
|---|---|---|---|---|---|
| Declared content in the reference serum | 4.8 | 19.9 | 3.2 | 9.8 | 2.440 |
| Determined content in the reference serum | 4.20 ± 0.11 | 19.00 ± 0.03 | 3.2 ± 0.18 | 9.2 ± 0.31 | 2.742 ± 0.311 |
| Range of 95% compliance | 4.4–5.2 | 18.8–21.0 | 3.0–3.4 | 9.2–10.4 | 2.107–2.773 |
| Acceptable range | 4.0–5.6 | 17.7–22.1 | 2.8–3.6 | 8.6–11.0 | 1.774–3.106 |
| Element | Sample | Control | T2DM and GFR > 60 | T2DM and GFR < 60 | |
|---|---|---|---|---|---|
| Whole blood | 6.85 ± 2.80 | 7.32 ± 3.07 | |||
| Serum | 4.8 ± 3.09 | 4.97 ± 2.84 | |||
| Erythrocytes | 6.52 ± 3.05 | 8.017 ± 3.371 | |||
| Whole blood | 7.70 ± 3.31 | 7.90 ± 3.05 | |||
| Serum | 4.10 ± 3.10 | 5.30 ± 2.62 | |||
| Erythrocytes | 6.48 ± 3.23 | 7.50 ± 3.33 | |||
| Whole blood | 6.00 ± 2.29 | 6.50 ± 3.11 | |||
| Serum | 5.50 ± 3.08 | 4.50 ± 3.16 | 7.10 ± 5.10 | ||
| Erythrocytes | 6.56 ± 2.87 | 8.75 ± 3.43 | |||
| Whole blood | 4.1 ± 1.81 | ||||
| Serum | 10.75 ± 3.81 | 10.03 ± 3.21 | 9.31 ± 3.49 | ||
| Erythrocytes | 1 ± 0.345 | ||||
| Whole blood | 3.1 ± 1.37 | ||||
| Serum | 13.7 ± 4.23 | 15.7 ± 5.25 | 12.00 ± 4.31 | ||
| Erythrocytes | 1.00 ± 0.30 | ||||
| Whole blood | 5.1 ± 2.24 | 4.7 ± 2.47 | |||
| Serum | 7.80 ± 3.38 | ||||
| Erythrocytes | 1.00 ± 0.39 | 1.57 ± 1.44 | 10.34 ± 7.88 | ||
| Whole blood | 6.96 ± 2.84 | 7.37 ± 3.91 | 6.79 ± 4.39 | ||
| Serum | 1.20 ± 0.8 | 1.22 ± 0.64 | 1.42 ± 1.11 | ||
| Erythrocytes | 15.68 ± 6.36 | 16.67 ± 6.42 | 15.62 ± 7.60 | ||
| Whole blood | 7.02 ± 3.15 | 6.96 ± 3.33 | 6.78 ± 4.32 | ||
| Serum | 1.10 ± 0.90 | 1.30 ± 0.60 | 1.50 ± 1.30 | ||
| Erythrocytes | 16.71 ± 6.71 | 16.53 ± 5.99 | 16.04 ± 7.99 | ||
| Whole blood | 6.90 ± 2.53 | 7.94 ± 4.73 | 6.81 ± 4.57 | ||
| Serum | 1.30 ± 0.70 | 1.10 ± 0.70 | 1.20 ± 0.60 | ||
| Erythrocytes | 14.64 ± 6.01 | 16.88 ± 7.02 | 14.47 ± 6.52 | ||
| Whole blood | 42.95 ± 7.56 | 46.21 ± 8.34 | 40.95 ± 9.36 | ||
| Serum | 14 ± 6.11 | 16.43 ± 6.71 | |||
| Erythrocytes | 95.82 ± 27.12 | 104 ± 43.5 | 93.69 ± 39.94 | ||
| Whole blood | 42.1 ± 6.7 | 44.6 ± 8.3 | 41.2 ± 9.4 | ||
| Serum | 16.6 ± 6.0 | 17.3 ± 7.0 | 18.1 ± 7.4 | ||
| Erythrocytes | 99.5 ± 23.3 | 105.2 ± 44.5 | 97.1 ± 39.7 | ||
| Whole blood | 43.8 ± 8.4 | 48.5 ± 8.4 | 40.2 ± 9.4 | ||
| Serum | 11.4 ± 6.2 | 15.2 ± 6.2 | |||
| Erythrocytes | 92.1 ± 30.9 | 102.3 ± 42.1 | 84.5 ± 40.5 | ||
| Whole blood | 6.447 ± 3.441 | 4.563 ± 2.911 | 4.902 ± 3.000 | ||
| Serum | 3.544 ± 1.999 | ||||
| Erythrocytes | 6.447 ± 3.441 | 4.563 ± 2.911 | 4.902 ± 3.000 | ||
| Whole blood | 6.788 ± 3.771 | 4.933 ± 2.921 | 4.780 ± 3.002 | ||
| Serum | 3.513 ± 2.001 | ||||
| Erythrocytes | 11.31 ± 5.56 | 9.33 ± 6.45 | 8.97 ± 5.21 | ||
| Whole blood | 6.106 ± 3.111 | 4.040 ± 2.899 | 5.239 ± 2.998 | ||
| Serum | 3.575 ± 1.998 | 3.637 ± 2.322 | |||
| Erythrocytes | 8.96 ± 5.62 | 6.92 ± 4.45 | 7.05 ± 3.95 |
| Trace Element | Control | T2DM with or without CKD | p | ||
|---|---|---|---|---|---|
| Average | SD | Average | SD | ||
| Zn | 9.6 | 2.84 | 11.51 | 2.99 | |
| Mn | 4.16 | 1.48 | 6.29 | 1.72 | |
| Ni | 0.227 | 0.108 | 0.320 | 0.155 | |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Kosmalski, M.; Frankowski, R.; Leszczyńska, J.; Różycka-Kosmalska, M.; Pietras, T.; Majak, I. The Evaluation of Selected Trace Elements in Blood, Serum and Blood Cells of Type 2 Diabetes Patients with and without Renal Disorder. Nutrients 2024 , 16 , 2989. https://doi.org/10.3390/nu16172989
Kosmalski M, Frankowski R, Leszczyńska J, Różycka-Kosmalska M, Pietras T, Majak I. The Evaluation of Selected Trace Elements in Blood, Serum and Blood Cells of Type 2 Diabetes Patients with and without Renal Disorder. Nutrients . 2024; 16(17):2989. https://doi.org/10.3390/nu16172989
Kosmalski, Marcin, Rafał Frankowski, Joanna Leszczyńska, Monika Różycka-Kosmalska, Tadeusz Pietras, and Iwona Majak. 2024. "The Evaluation of Selected Trace Elements in Blood, Serum and Blood Cells of Type 2 Diabetes Patients with and without Renal Disorder" Nutrients 16, no. 17: 2989. https://doi.org/10.3390/nu16172989
Article Metrics
Article access statistics, supplementary material.
ZIP-Document (ZIP, 157 KiB)
Further Information
Mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Immunol
Hematopoietic Stem Cells in Type 1 Diabetes
Ida pastore.
1 Division of Endocrinology, ASST Fatebenefratelli-Sacco, Milan, Italy
2 International Center for T1D, Pediatric Clinical Research Center Romeo ed Enrica Invernizzi, DIBIC, Università di Milano, Milan, Italy
Moufida Ben Nasr
3 Nephrology Division, Boston Children’s Hospital and Transplantation Research Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
Andrea Mario Bolla
Anna maestroni, vera usuelli, cristian loretelli, andy joe seelam, ahmed abdelsalam, gian vincenzo zuccotti.
4 Department of Pediatrics, Buzzi Children’s Hospital, Milan, Italy
Francesca D’Addio
Paolo fiorina.
Despite the increasing knowledge of pathophysiological mechanisms underlying the onset of type 1 diabetes (T1D), the quest for therapeutic options capable of delaying/reverting the diseases is still ongoing. Among all strategies currently tested in T1D, the use of hematopoietic stem cell (HSC)-based approaches and of teplizumab, showed the most encouraging results. Few clinical trials have already demonstrated the beneficial effects of HSCs in T1D, while the durability of the effect is yet to be established. Investigators are also trying to understand whether the use of selected and better-characterized HSCs subsets may provide more benefits with less risks. Interestingly, ex vivo manipulated HSCs showed promising results in murine models and the recent introduction of the humanized mouse models accelerated the translational potentials of such studies and their final road to clinic. Indeed, immunomodulatory as well as trafficking abilities can be enhanced in genetically modulated HSCs and genetically engineered HSCs may be viewed as a novel “biologic” therapy, to be further tested and explored in T1D and in other autoimmune/immune-related disorders.
Introduction
Hematopoietic stem cells (HSCs) have been extensively used as an effective therapeutic approach in hematological malignancies and have demonstrated to be safe in human subjects ( 1 ). Over the last 10 years, several studies documented the extraordinary immunoregulatory properties of HSCs, which render them a potential useful tool in the fight for immune-mediated diseases ( 2 ). Despite being in limited number in the circulating blood of healthy individuals, HSCs are extremely potent and able to suppress the immune system response, as several in vitro and in vivo studies have shown ( 2 ). Based on these premises, the use of HSCs has been tested in numerous autoimmune diseases such as type 1 diabetes (T1D), multiple sclerosis (MS), systemic sclerosis, systemic lupus erythematosus and Chron’s disease, with relevant benefits ( 3 – 6 ). Indeed, HSCs may reset the immune response, thus reshaping the chronic derangement of the immune system to a more self-tolerant state ( 7 , 8 ). Interestingly, it has been also demonstrated that the bone marrow-derived and blood HSCs are altered in some autoimmune conditions such as T1D and MS, with HSCs being scanty in the circulation and often unable to exploit their immunoregulatory function ( 9 – 11 ). Here we are presenting major advances in the preclinical and clinical studies of HSCs in T1D. We report recent insights coming from novel T1D in vivo research and provide an update on the most relevant clinical studies that have been performed by using HSCs in human subjects with T1D. In this perspective, we envision to consider HCSs as a novel “biologic”, which can be personalized and modeled, as a novel relevant therapeutic option in T1D.
HSCs in Type 1 Diabetes: The Murine Scenario
The rationale behind the use of HSCs in autoimmune disease such as T1D has been extensively studied in the last decade by taking advantage of the NOD mouse model. This mouse spontaneously develops autoimmune diabetes at the age of 12–15 weeks, with severe hyperglycemia ( 12 , 13 ). However, signs of activation of the immune system against pancreatic islets are already visible at 8–10 weeks of age when the NOD mouse shows insulitis with an abundant T cell infiltrate ( 12 ). Over the last two decades, two major HSCs-based strategies have been pursued to prevent the onset of experimental autoimmune diabetes in murine models: (i) HSCs have been infused to induce mixed chimerism and to re-establish the peripheral deletion of autoreactive T cells, (ii) HSCs have been genetically engineered to reshape the immune reservoir and facilitate tolerance towards auto-antigens. The use of HSCs infusion was extremely successful in preventing diabetes onset in NOD mice through the induction of a mixed chimerism. Indeed, a deletion of autoreactive T cells generated at the thymus level ( 14 ) as well as the re-establishment of immune tolerance in the periphery were obtained. Furthermore, in the presence of a tolerogenic network between donor Regulatory T cells (Tregs) and host-donor dendritic cells (DCs), costimulatory pathways, particularly PDL-1, play a major role ( 15 ). However, the HSC-mediated chimerism, despite effective in reshaping the autoimmune response, requires the use of myeloablative agents/approaches, which may further limit translational applications ( 16 , 17 ). Given that common polymorphisms exist in MHC class II in T1D patients and in NOD mice, which confer a higher risk of developing T1D, genetically engineering of single HSCs to express the proper and protective MHC class II, held great promises in the new therapies in T1D ( 18 ). Indeed, the introduction of new protective MHC class II through lentiviral delivery in HSCs of NOD mice was able to prevent the onset of T1D, mainly through the deletion of autoreactive T cells which did not engage in the MHC class II-mediated response ( 19 , 20 ). While this approach was again limited by the need of immune ablation for the HSCs infusion, which is feasible in NOD mice but at high risk in humans, it paved the way for exploring genetic engineering of HSCs to better exploit their multiple properties in autoimmunity. Ex vivo genetic manipulation of NOD HSCs, to encode proinsulin and transgenically target MHC class II, successfully prevented T1D onset ( 21 , 22 ). Also, HSCs can be engineered for tolerogenic purposes such as those aimed at inducing tolerance to autoantigens or at replacing genetic alleles associated with increased disease susceptibility ( 23 ). In view of this, some studies explored whether HSCs in diabetic NOD mice are altered and might be fixed through genetic engineering or pharmacological modulation. Elevated levels of CXCL12 (SDF-1) in bone marrow-HSCs of NOD mice have been suggested to alter trafficking of HSCs and Tregs in the periphery, thus favoring the onset of T1D ( 24 ). The use of ADAM3100, which antagonizes the CXCL12 receptor SDF-1, was associated with increased mobilization of HSCs and T cells, and delayed onset of experimental autoimmune diabetes in NOD mice ( 24 ). Recently, a defect in PDL-1 expression has been demonstrated in HSCs of NOD mice, which was associated with a reduced immunomodulatory function ( 9 , 25 ). Genetic and pharmacological modulation of PDL-1 on HSCs restored the HSCs immunomodulatory properties, reset the immune balance and prevented the onset of T1D. In summary, all the aforementioned studies support the use of ex vivo manipulation of HSCs in the NOD mouse model as a successful tool to delay the onset of autoimmune diabetes. Genetic engineering of HSCs has been recently employed in a humanized mouse model in which ex vivo manipulated human HSCs successfully restored the development of functional Tregs and rescued the autoimmune IPEX syndrome ( 26 ). Recently, the introduction of the NOD-Rag1null IL2rγnull Ins2Akita (NRG-Akita) mouse, a humanized mouse model available in diabetes research which develops spontaneous hyperglycemia, fostered studies in the field ( 27 , 28 ). This model, in which human immune cells can be infused without being rejected, may be extremely useful in testing the potency of newly genetically engineered human HSCs in the diabetes prevention.
HSCs in Type 1 Diabetes: The Human Landscape
In the last 20 years, autologous hematopoietic stem cells transplantation (AHSCT) has been used in several clinical trials to treat refractory autoimmune disease such as multiple sclerosis (MS), systemic sclerosis (SSc), systemic lupus erythematosus (SLE), Crohn’s disease (CD), type 1 diabetes (T1D) and a range of other immune-mediated disorders ( 29 ). With regard to patients with T1D, the use of AHSCT obtained significant insulin independence and a well-preserved glycometabolic control in the short and mid-term follow-up ( Figure 1A and Table 1 ) ( 17 , 31 , 34 , 35 ). Also, an increase in C-peptide levels and C-peptide area under the curve (AUC) measurement were detectable in AHSCT-treated T1D patients as compared to baseline, and only minor adverse events were registered in the mid-term ( 25 , 36 ). A reduction of the T-helper-1 and T-helper-17 subsets was also observed in the short-term ( 37 ). Interestingly, a cost-effectiveness analysis conducted in patients with T1D undergoing AHSCT as compared to patients with T1D remaining on insulin therapy demonstrated that AHSCT provides some benefits over time depending on the duration of preserved glycated hemoglobin levels achieved with AHSCT, but overall being cost-effective for treatment of T1D if the AHSCT lasts from 3 to 8 years ( 38 ). Long-term follow-up analyses for AHSCT-treated T1D patients were only reported for a few studies, due to the high number of patients lost at follow-up and the worldwide spreading of the studies, which also accounted for a small sample size, missed randomization process, lack in standardized procedures and enrolment of a heterogenous patients’ population ( 39 ). Despite all these limitations, a minor percentage of relapse in the autoimmune disease was evident between 4 and 6 years of follow-up after the AHSCT, which varied among centers and lead few patients to resume insulin treatment ( 40 , 41 ). Moreover, a subgroup of AHSCT-treated patients exhibited a prolonged remission and remained insulin independent for more than 4 years, thus leading to hypothesize that the response to the AHSCT treatment may differ in patients with T1D ( 17 , 35 , 42 ). Indeed, Malmegrim and Colleagues demonstrated that a different immune profile exists between patients experiencing short and prolonged remission, with the latter exhibiting lower frequencies of effector-memory CD4 T cells and islet-specific autoreactive CD8 T cells, paralleled by a detectable expansion of immunoregulatory T cells ( 35 ). Moreover, the favorable outcome of AHSCT in T1D was also associated with a less islet-specific autoreactive immune profile at baseline, thus delineating a subgroup of patients with T1D who may benefit the most from an AHSCT-based strategy ( 43 , 44 ). This also emphasized the importance of the conditioning regimen, which may need to be employed in association with AHSCT in patients with a high level of autoimmune response. In summary, results of the use of HSC-based approach, primarily the AHSCT, in patients with T1D ( Table 1 ), suggest two major observations: (i) AHSCT treatment has to be limited to a subgroup of T1D patients and it requires high-level immunosuppression to obtain long-term effect, and (ii) the immune profile of T1D patients plays a central role in the achievement of long-term insulin-independence when using HSC-based strategies. Therefore, the infusion of a subset of HSCs, rather than the whole HSCs pool, such as in AHSCT, endowed with immunoregulatory properties may provide additional benefits in terms of balancing autoimmunity and achieving the proper clinical and metabolic outcomes.

Complete/partial remission of type 1 diabetes obtained with AHSCT in the long-term. Proposed genetic engineered HSC-based approach to target type 1 diabetes. (A) Proportion of patients with T1D undergoing AHSCT who achieved complete remission (insulin independence) and partial remission (low dose exogenous insulin requirement) at the latest timepoint analyzed within each clinical study registered in ClinicalTrials.gov available as publication. (B) Use of genetically engineered HSCs to target T1D: proposed approach. T1D, type 1 diabetes; AHSCT, autologous hematopoietic stem cell transplantation.
Table 1
Summary of main characteristics, clinical outcomes and results obtained in the clinical studies conducted in T1D and registered in ClinicalTrials.gov .
| Clinical Study | N of pts Follow-up Type of study | Clinical outcomes | Main results |
|---|---|---|---|
| Autologous Hematopoietic Stem Cell Transplantation for Early Onset Type 1 Diabetes (NCT00807651) | 28 pts T1D 3 years Monocentric prospective | EIR, HbA1c, C-peptide and anti-GAD level | Insulin independence: 53.6% Increased C-peptide level ( ) |
| Safety and Efficacy Study of Autologous Stem Cell Transplantation for Early Onset Type I Diabetes Mellitus (NCT00315133) | 23 pts T1D 5 years Monocentric prospective | C-peptide level Morbidity/mortality EIR changes HbA1c level | Insulin independent: 52% Low EIR: 35%; C-peptide AUC increase, HbA1c <7% ( ) |
| Hematopoietic Stem Cell Transplantation in Type 1 Diabetes Mellitus (NCT01121029) | 15 pts T1D 3 years Monocentric prospective | EIR C-peptide and HbA1C | Insulin independent: 44% HbA1c decrease: 2.3% Mortality: 0% ( ) |
| Efficacy and Safety Study of Autologous Hematopoietic Stem Cell Transplantation to Treat New Onset Type 1 Diabetes (NCT01341899) | 13 pts T1D 4 years Monocentric prospective | C-peptide and HbA1C Islet autoantibodies Immune profile Survival | 3/13 pts: no insulin 11/13 pts low EIR, reduced HbA1C low autoantibodies increased C-peptide ( ) |
| Stem Cell Mobilization (Plerixafor) and Immunologic Reset in Type 1 Diabetes (T1DM) (NCT03182426) | 60 pts T1D 2 years Monocentric prospective | C-peptide AUC, EIR HbA1C <7% Hypoglycemia Autoantibodies titer | Not available |
pts, patients; T1D, type 1 diabetes; AUC, area under the curve.
Conclusions and Future Directions
The use of HSCs has hold great promises in the treatment of autoimmune diabetes, however, in the last decade. The results obtained in clinical trials with the use of AHSCT in T1D suggest a potential novel approach to treat autoimmune diseases, despite all the aforementioned limitations. The use of a selected subset of HSCs endowed with immunoregulatory properties, without the need of additional immunosuppressive agents remains unexplored so far and deserves more investigation and testing from the scientific community. Patients with T1D who may benefit the most from this therapeutic approach need to be carefully identified, probably based on disease stages, degree of cellular and humoral autoimmune response, presence or not of diabetic ketoacidosis ( 17 ). The recent findings on the use of teplizumab in patients at risk for T1D ( 45 ) confirmed that immune ablation aimed at preventing T1D onset is a hot topic. HSCs, endowed with immunomodulatory properties, may offer a potent immunoregulatory effect without inducing T lymphocytes depletion, which is commonly observed with teplizumab. Indeed, several studies demonstrated that in absence of “healthy” HSCs central tolerance may be difficulty obtained. Autoimmune disorders, particularly type 1 diabetes, are associated with altered HSCs, which fail in exerting their immunomodulatory properties. Strategies aimed at targeting this defect successfully delayed diabetes onset in murine models. Feasibility and effectiveness in of the ex vivo manipulation and genetic engineering of HSCs are well-established in mouse models, while studies on safety for translational purposes are still required. In view of this, the use of humanized mouse model may accelerate the translation from murine experiments to human studies. The outstanding results collected in the past and ongoing clinical trials are encouraging in pursuing the research around the use of genetic engineered-HSCS in type 1 diabetes. Therefore, in our opinion, genetic modulation to reset HSCs physiological function, may find an interesting field of application not only in type 1 diabetes ( Figure 1B ) but in other autoimmune conditions too. Finally, in the era of the development of biologic therapy to treat immune-mediated diseases, we envision genetically engineered HSCs as a novel “biologic” agent and a “natural immunosuppressant” to be considered in the portfolio of alternative therapeutic options in type 1 diabetes and autoimmune diseases.
Author Contributions
IP and EA wrote the paper. AB, MB, AM, CL, VU, AS, and AA, collected clinical and preclinical data. GZ edited the paper. FD’A and PF conceived the idea, wrote and edited the paper. All authors contributed to the article and approved the submitted version.
FD is supported by SID Lombardia Grant and by EFSD/JDRF/Lilly Programme on Type 1 Diabetes Research 2019. PF is supported by the Italian Ministry of Health grant RF-2016-02362512 and by the Linea-2 2019 funding from Università di Milano. We thank the “Fondazione Romeo e Enrica Invernizzi” for extraordinary support.
Conflict of Interest
PF and MB hold a patent of modulated HSCs and founded Altheia Science.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.

IMAGES
VIDEO
COMMENTS
Type 1 diabetes mellitus (T1DM) is the most common chronic autoimmune disease in young patients and is characterized by the loss of pancreatic β cells; as a result, the body becomes insulin deficient and hyperglycemic. Administration or injection of exogenous insulin cannot mimic the endogenous insulin secreted by a healthy pancreas.
Promising early results show that longstanding Harvard Stem Cell Institute (HSCI) research may have paved the way for a breakthrough treatment of Type 1 diabetes. Utilizing research from the Melton Lab, Vertex Pharmaceuticals has developed VX-880, an investigational stem cell-derived, fully differentiated pancreatic islet cell replacement therapy for people with type 1 diabetes (T1D).
Keywords: type 1 diabetes mellitus, stem cell, β-cell, immunotolerance, ... In recent research, stem cell therapy has demonstrated itself as a rapidly expanding and potentially limitless source of β-cells to arrive at a cure for T1DM by reconstitution of immunotolerance and differentiation into islet β-cell clusters. As the immunosuppression ...
Metabolic engineering. Type 1 diabetes. For over two decades pluripotent stem cells have promised a renewable source of β cells to treat patients with type 1 diabetes. Major efforts to optimize ...
Español. Today, the U.S. Food and Drug Administration approved Lantidra, the first allogeneic (donor) pancreatic islet cellular therapy made from deceased donor pancreatic cells for the treatment ...
Type 1 diabetes mellitus (T1DM) is the most common chronic autoimmune disease in young patients and is characterized by the loss of pancreatic β cells; as a result, the body becomes insulin deficient and hyperglycemic. ... With the advent of research on stem cell therapy for various diseases, breakthroughs in stem cell-based therapy for T1DM ...
How stem cells could fix type 1 diabetes. Trials to replace the pancreatic β cells that are destroyed by this autoimmune disease are raising hopes of a cure. Encapsulated stem cell-derived islets ...
Glucose-Dependent Insulin Production and Insulin-Independence in Type 1 Diabetes from Stem Cell-Derived, Fully Differentiated Islet Cells—Updated Data from the VX-880 Clinical Trial ... the world's largest scientific meeting focused on diabetes research, prevention, and care, will be held in San Diego, CA on June 23-26. More than 12,000 ...
Type 1 diabetes (T1D) is particularly suited to this approach, as transplantation of the insulin-producing pancreatic β-cells could provide long-lasting therapy or even a cure.
Over the past decade, there had been progress in the development of cell therapy for insulin-dependent diabetes. Nevertheless, important hurdles that need to be overcome still remain. Protocols for the differentiation of pluripotent stem cells into pancreatic progenitors or fully differentiated β-cells have been developed. The resulting insulin-producing cells can control chemically induced ...
by Dennis Thompson. An experimental stem cell therapy can essentially cure type 1 diabetes by restoring insulin production in some patients, early clinical trial results show. Seven out of 12 ...
For Harvard Stem Cell Institute Co-Director and Xander University Professor Douglas Melton, whose lab pioneered the science behind the therapy, the trial marked the most recent turning point in a decades-long effort to understand and treat the disease. In a conversation with the Gazette, Melton discussed the science behind the advance, the ...
Dec. 11, 2023 - An experimental device containing millions of stem cells significantly reduced the need for insulin shots among people with type 1 diabetes, according to a new study - a ...
Developmental research on pancreas formation has informed in vitro differentiation of human pluripotent stem cells into functional islets. Although generating β cells from stem cells offers a potential cure for type 1 diabetes, several challenges remain, including protecting the cells from the immune system.
An investigative stem cell-based therapy called PEC-Direct, designed to act as a replacement pancreas, has the potential to provide blood sugar control in patients with high-risk type 1 diabetes, suggests a clinical study presented Saturday, June 11at ENDO 2022, the Endocrine Society's annual meeting in Atlanta, Ga.
Our goal is to cure diabetes. To do that, we have successfully developed ways to create new insulin-producing beta cells. This critical advance has enabled the first beta cell replacement clinical trial for type 1 diabetes. We are now focused on devising strategies to effectively protect beta cells from attack by the immune system.
On Feb. 2, ViaCyte and CRISPR Therapeutics announced that the first participant in the trial had received the PEC-QT implant. This marks the first gene-edited, stem cell-derived pancreatic cells to be implanted in a human that are specifically designed to evade the immune system in treating type 1 diabetes. All in all, Foyt is optimistic that ...
Type 1 diabetes mellitus (T1DM) is the most common chronic autoimmune disease in young patients and is characterized by the loss of pancreatic β cells; as a result, the body becomes insulin deficient and hyperglycemic. Administration or injection of exogenous insulin cannot mimic the endogenous insulin secreted by a healthy pancreas. Pancreas and islet transplantation have emerged as ...
Review Article: Harnessing cellular therapeutics for type 1 diabetes mellitus: progress, challenges, and the road ahead.Image Credit: Andrii Yalanskyi / Shutterstock. In a recent study published ...
Modern biomedical research is best at finding treatments for diseases that have relatively simple causes and well-understood genetic risk factors. Unfortunately, type 1 diabetes (T1D) has very complex genetics, with many genes each making relatively small, poorly understood contributions to disease risk.
Behind the headlines: Stem cell therapy breakthrough for type 1. 2021-10-26. Scientists in the United States have announced early results from a pioneering trial testing a new stem cell treatment designed to replace insulin-producing beta cells in people with type 1 diabetes. In a small clinical trial, which is still underway, the first person ...
Type 1 diabetes mellitus (T1DM) is a growing global health concern that affects approximately 8.5 million individuals worldwide. T1DM is characterized by an autoimmune destruction of pancreatic β cells, leading to a disruption in glucose homeostasis. Therapeutic intervention for T1DM requires a comp …
To accurately identify clinical trials of stem cell transplantation in DM patients, a search was performed using a combination of medical subject heading (MeSH) terms and text words: "diabetes mellitus, type 1" or "diabetes mellitus, type 2" and "stem cell transplantation" and "therapy" or "therapeutic use." Inclusion and Exclusion Criteria
A colony of human induced pluripotent stem (iPS) cells established from fibroblasts. Kyoto University Hospital is looking to develop a treatment for Type 1 diabetes using iPS cells.
Stem cell-derived islets have advanced as a viable renewable source of cells for transplantation in type 1 diabetes mellitus (T1DM). Although these cells are being tested in the clinical setting ...
Your source for the latest research news. Follow: ... Stem cell; Blood sugar; Diabetes mellitus type 1; ... Called Type 1 diabetes, this ... Print Email Share.
Since the last Type 1 Diabetes PSP in 2011 there's been some big changes in type 1 treatment and care, so the priorities were due an update. The latest Type 1 PSP - which condensed and whittled down nearly 3000 questions submitted by people affected by type 1 to a shortlist of the top ten - has just been published. And here they are: 1.
Human blood stem cells have been made in a laboratory for the first time, which could significantly improve how we treat certain types of cancer.. The lab-grown cells have so far only been tested ...
Research pertaining to diabetes has relatively neglected manganese despite its significant role in mitigating excessive oxidative stress and eliminating free radicals ... 2024. "The Evaluation of Selected Trace Elements in Blood, Serum and Blood Cells of Type 2 Diabetes Patients with and without Renal Disorder" Nutrients 16, no. 17: 2989. https ...
Despite the increasing knowledge of pathophysiological mechanisms underlying the onset of type 1 diabetes (T1D), the quest for therapeutic options capable of delaying/reverting the diseases is still ongoing. Among all strategies currently tested in T1D, the use of hematopoietic stem cell (HSC)-based approaches and of teplizumab, showed the most ...